Exhibit 99.1
Shareholder UpdateThe RegenerativeMedicine Revolution November 4, 2014
Safe Harbor Statement The matters discussed in this presentation include forward looking statements which are subject to various risks, uncertainties, and other factors that could cause actual results to differ materially from the results anticipated. Such risks and uncertainties include but are not limited to the success of BioTime in developing new stem cell products and technologies; results of clinical trials of BioTime products; the ability of BioTime and its licensees to obtain additional FDA and foreign regulatory approval to market BioTime products; competition from products manufactured and sold or being developed by other companies; the price of and demand for BioTime products; and the ability of BioTime to raise the capital needed to finance its current and planned operations. Any statements that are not historical fact (including, but not limited to statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. As actual results may differ materially from the results anticipated in these forward-looking statements they should be evaluated together with the many uncertainties that affect the business of BioTime and its subsidiaries, particularly those mentioned in the cautionary statements found in BioTime's Securities and Exchange Commission filings. BioTime disclaims any intent or obligation to update these forward-looking statements.
The Rise of the Aged Baby Boomers Source of data on projected growth of US population: US Census Bureau Investment Highlights
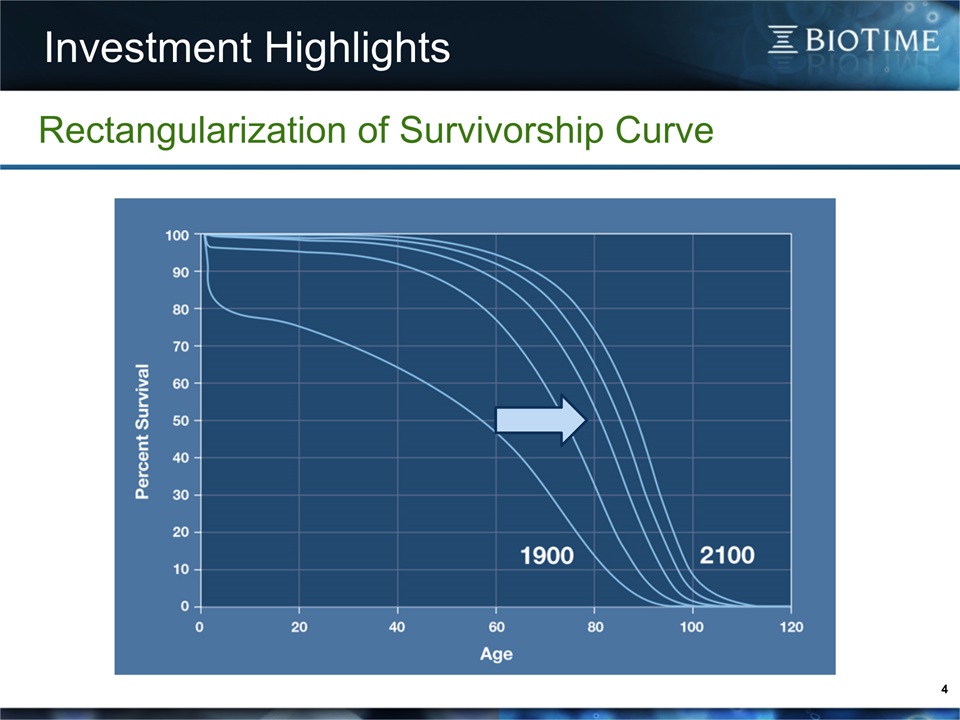
4 Rectangularization of Survivorship Curve Investment Highlights
5 Aging: The Rogue Wave Investment Highlights
6 The Tsunami of Chronic Age-Related Degenerative Disease Investment Highlights CDD due to lack of cellular regeneration80% Americans have CDD of aging50% of Americans have two CDDs95% costs in aging is CDD
Advent of Regenerative Medicine Recombinant DNA Monoclonal Antibodies Regenerative Medicine Advent: 1974: Gene cloning technology developedHurdle: 1976: moratorium on rDNA research Launch: 1989: EPO is first billion dollar product2014: products using rDNA technology are ubiquitous>140 clinical trials$75bn current global market Advent: 1975: Hybridoma technology developed Hurdle: HAMA responseLaunch: 1997: Rituximab is first billion-dollar product2014: 8 of the 20 best-selling biotechnology drugs are therapeutic monoclonal antibodies>200 clinical trials$44bn current global market Advent: 1998: Isolation of pluripotent stem cellsHurdle: 2001: U.S. federal funding restriction (reversed in 2009) 2010: First-in-human trial of OPC1 Future: First billion-dollar product 7 Biotech revolutions often take 15-20years from conception to commercialization
The Mission 8 To lead in the application of pluripotent stem cell technology for regenerative therapeutic applications in age-related degenerative disease.
9 Poised to commercialize one of the largest revolutions in medicineTargeting large markets in degen-erative diseases ($billion markets)Multiple clinical milestones in 2H2014Balance of near-term and longer-term products in developmentLeader in core pluripotent stem cell technology with >600 patents/ patent applications worldwideNo approval pathway for generics or biosimilars to our cell therapies The Technology Leader in Regenerative Medicine Investment Highlights Pluripotent Stem Cells OrthopedicsArthritisIVD diseaseTendon repair EndocrinologyDiabetesObesity NeurologySpinal cord injuryStrokeParkinsons CardiologyHeart failureIschemiaHypertension OphthalmologyMacular degeneration HematologyImmunotherapy OncolologyLung Cancer
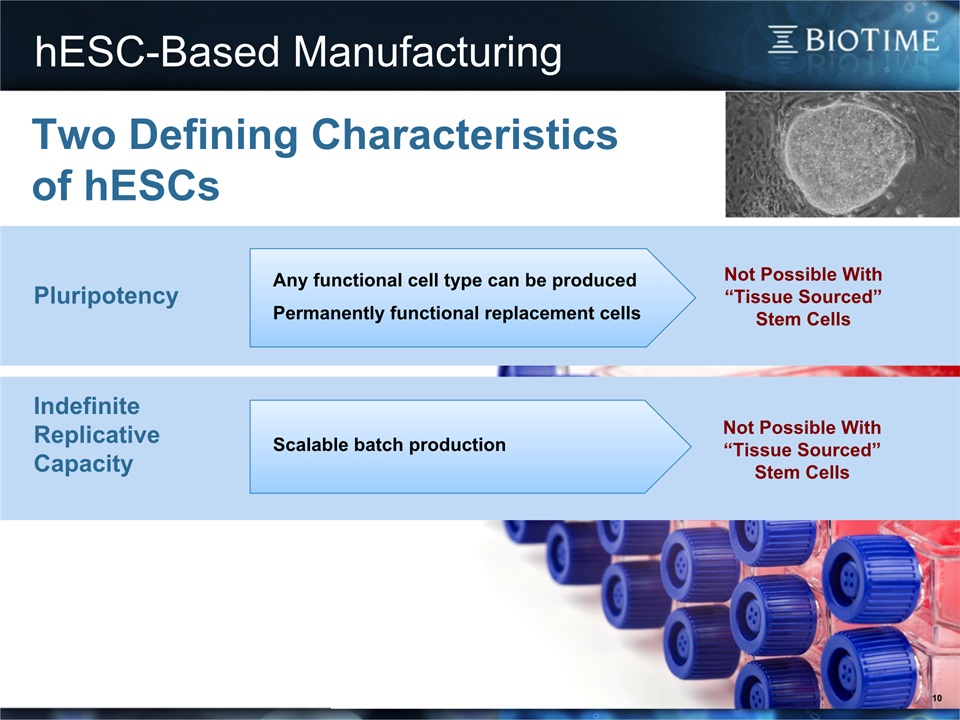
hESC-Based Manufacturing 10 Any functional cell type can be producedPermanently functional replacement cells Scalable batch production Pluripotency Indefinite Replicative Capacity Not Possible With “Tissue Sourced” Stem Cells Two Defining Characteristics of hESCs Not Possible With “Tissue Sourced” Stem Cells
Pluripotent vs. Adult Stem Cells 11 PluripotentStem Cells PluripotentStem Cells PluripotentStem Cells AdultStem Cells Pluripotent stem cells allow for the first time in history the capacity of medicine to produce all human cell types
Value of the Pluripotent Platform 12 Cell therapy: In growth phase – potentially long lifespan due to lack of regulatory pathway for generics or biosimilars Recombinant ProteinsTechnology in maturity phase Small MoleculesTechnology in decline Unique and proprietary Pluripotent Stem Cells Extensively characterized to Strict Regulatory Requirements. hESC MasterCell Bank Product Monoclonal AntibodiesTechnology in growing phase Pluripotent Cell TherapyTechnology in emerging phase
Safe and feasible in world’s first clinical trial of human embryonic stem cell-derived therapyFDA clearance for expansion of Phase I/IIa trial in cervical SCIInitiation of CIRM-funding of 3 yr $14M grantAsterias listed NYSE MKTFollow-on opportunities in MS, Stroke, other neurodegenerative diseases 13 OPC1: hESC-Derived Oligodendrocyte Progenitor Cells
Milestones –VAC2 & OpRegen 14 Complete Process Development of VAC2CRUK to fund Phase I/II clinical trial in Lung CAOpRegen IND Cleared for Phase I/IIa OpRegen (RPE Cells) VAC2 (Dendritic Cells)
Near-Term Strategic Products 15 HyStem Matrices
LifeMap Sciences, Inc. • More than 14 Million page views from more than 3,000 institutions world-wide, including academia, research hospitals, patent offices, and leading biotech and pharma• Premium services now being offered for a feeLifeMap Solutions developing mobile health applications 16 Integrated Biomedical Knowledgebase
Targeting High Medical Need OPC1 – Spinal cord injury, oligodendrocytes$20B annual economic impact of spinal cord injury in the U.S.Developed by Asterias Biotherapeutics (BioTime owns 70.6%)VAC2 – Cancer, telomerase immunotherapy $38B economic impact of non-small-cell lung cancer in the U.STargeted by Asterias Biotherapeutics (BioTime owns 70.6%)OpRegen – Dry AMD, retinal pigment epithelial cells>$5B annual spend on drug therapies for wet AMDCell Cure Neurosciences (BioTime owns 62.5%)Renevia – HyStem, device for lipoatrophy>3M HIV+ patients with lipoatrophyRenevia developed by BioTime (owns 100%)PanC-Dx – Cancer diagnostic, breast, lung, bladder CA$7B annual cost of mammography in the USTargeted by OncoCyte (BioTime owns 75.3%) 17
Balanced Strategy 18 Diversified, lower-cost, lower-risk strategyNot a bet on a single clinical programMultiple, growing revenue streams currentlySeven clinical phase programs by Q4 2014Internal focus on low-cost, low-risk, near-term programsPartnering strategy for select high-cost potential blockbustersSubsidiaries often invite outside investors, corporate partnersCapitalize on lack of generic or biosimilar pathways
BioTime – Investment Opportunity 19 Positioned as the technology leader in the coming regenerative medicine revolution
Asterias Biotherapeutics(NYSE MKT: AST)Leveraging Proprietary Regenerative Medicine Platforms to Address Significant Unmet Medical Needs
Forward Looking Statements Statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Asterias, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of Asterias, particularly those mentioned in the cautionary statements found in Asterias’ Registration Statement on Form S-1 and Prospectus filed with the Securities and Exchange Commission. Asterias disclaims any intent or obligation to update these forward-looking statements. 2
Two potential transformative platforms: 1) industry leading Pluripotent Stem Cells; 2) Allogeneic Dendritic Cell ImmunotherapyTwo products entering dose escalation clinical trialsPartnerships with leading scientific institutions provide validation and access to significant, non-dilutive capital (~$40 mil raised to date)Large potential target markets with significant unmet medical needsSignificant potential milestones during next 24 months 3 Key Investment Highlights
Regenerative medicine in emerging growth phase and approaching valuation inflection pointClinical development focus on de-risked, proof-of-concept initial product indicationsDeep, experienced management team with scientific and business expertise 4 Additional Investment Considerations
Successfully completed stock and warrant distribution to GERN shareholders, became publicly traded and listed on NYSE MKT under ticker “AST”Received FDA clearance for Phase 1/2a dose escalation trial of AST-OPC1 in complete cervical spinal cord injuryLaunched partnership with California Institute of Regenerative Medicine (CIRM) to initiate AST-OPC1 Phase 1/2a clinical trial Partnered with Cancer Research UK (CRUK) to develop AST-VAC2 immunotherapy product candidate for lung cancer 5 Recent Key Accomplishments
Asterias’ Programs are Built on Potentially TransformativeTechnology Platforms 6 Major scientific validation by top scientists in multiple countriesDe-risked by extensive nonclinical data & long term clinical safety data
Asterias’ Programs are Built on Potentially TransformativeTechnology Platforms 7 Major scientific validation by top scientists in multiple countriesDe-risked by clinical data for first generation, autologous product
Asterias’ Products Address Large Markets with Significant Unmet Medical Needs AST-OPC1 for Neurodegenerative Diseases Lead indication: Spinal Cord InjuryDevastating injury affecting 12,000 patients per year in US1No currently approved therapiesLifetime cost of care per patient of $2-4 mil1Healthcare costs to system of $14.5 bil per year in US alone, plus $5.5 bil in lost productivity2 8 1 National Spinal Cord Injury Statistical Center, Facts and Figures 20132 Berkowitz et al, Spinal Cord Injury: An Analysis of Medical and Social Costs, 1998
Asterias’ Products Address Large Markets with Significant Unmet Medical Needs AST-VAC2 (Cancer Immunotherapy) Unique mode of action in emerging field of Immune Cancer TherapeuticsCancer Immunotherapy market projected to reach $35 bil in annual revenues by 20241Partial HLA mismatch from allogeneic vaccine may serve as an adjuvant to enhance efficacy as compared to autologous vaccinesAST-VAC2 mechanism of action is likely synergistic with that of other immunotherapies such as immune checkpoint inhibitorsInitial indication of lung cancer selected due to high level of unmet medical need, proof of concept for sensitivity to immunotherapy 9 1 Kresge and Langreth, Bloomberg May 29, 2014
AST-OPC1: Oligodendrocyte Progenitor Cells 10 First hESC-Derived Product in Clinic Phase 1 successfully completedExtensive data in animal studies demonstrates:Engraftment over periods of at least 1 yearExtensive cavity filling and myelinationImproved locomotor functionNo observed toxicity or immunogenicityFDA clearance for P1/P2a POC study in complete cervical SCI (target initial commercial indication)Clear path to market - Endpoint measurement established by independent SCOPE initiative (Spinal Cord Outcomes Partnership Endeavor)1Up to $3 billion opportunity of peak annual US revenues for Asterias 1Steeves et al., Top Spinal Cord Inj Rehabil 2012; 18(1): 1-14
AST-OPC1 Phase 1 Trial in Complete Thoracic Injuries Five subjects received 2 mil AST-OPC1 cells, followed for 2-3 years Clean safety profile observed to date:No serious adverse events related to surgery, AST-OPC1, or immunosuppressionNo unexpected neurological changes to dateNo adverse changes on MRIMonitoring through one year shows no evidence of immune responses to AST-OPC1Potential evidence of biological activity:MRI results in 4 of 5 subjects are consistent with prevention of lesion cavity formation 11 Presented by Jane Lebkowski, ASGCT annual meeting, May 22, 2014Manuscript in preparation Feasible and Safe
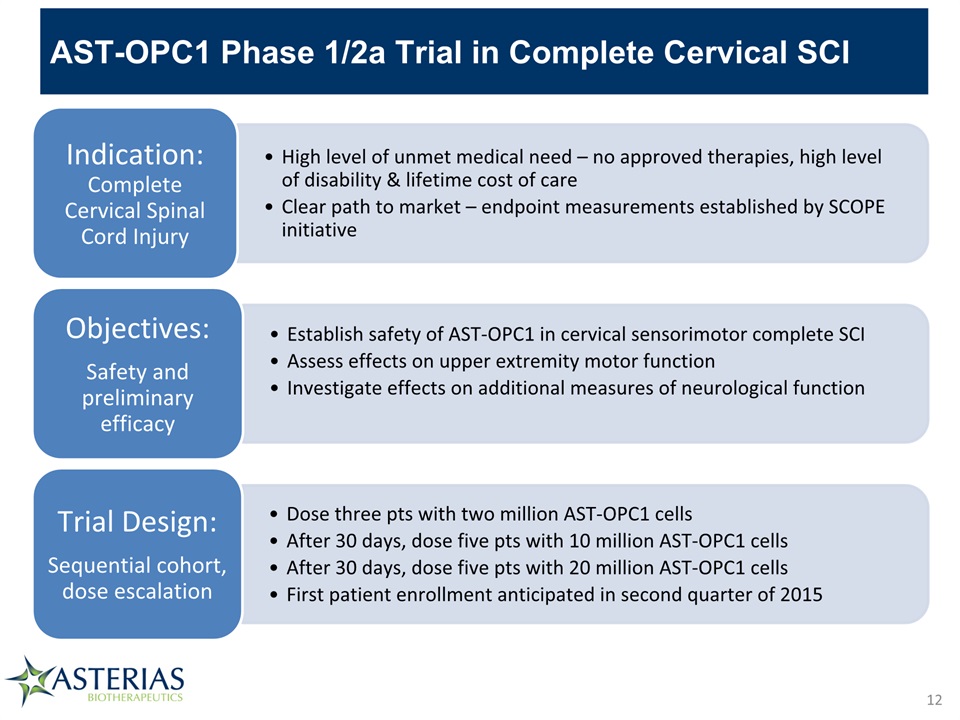
AST-OPC1 Phase 1/2a Trial in Complete Cervical SCI 12
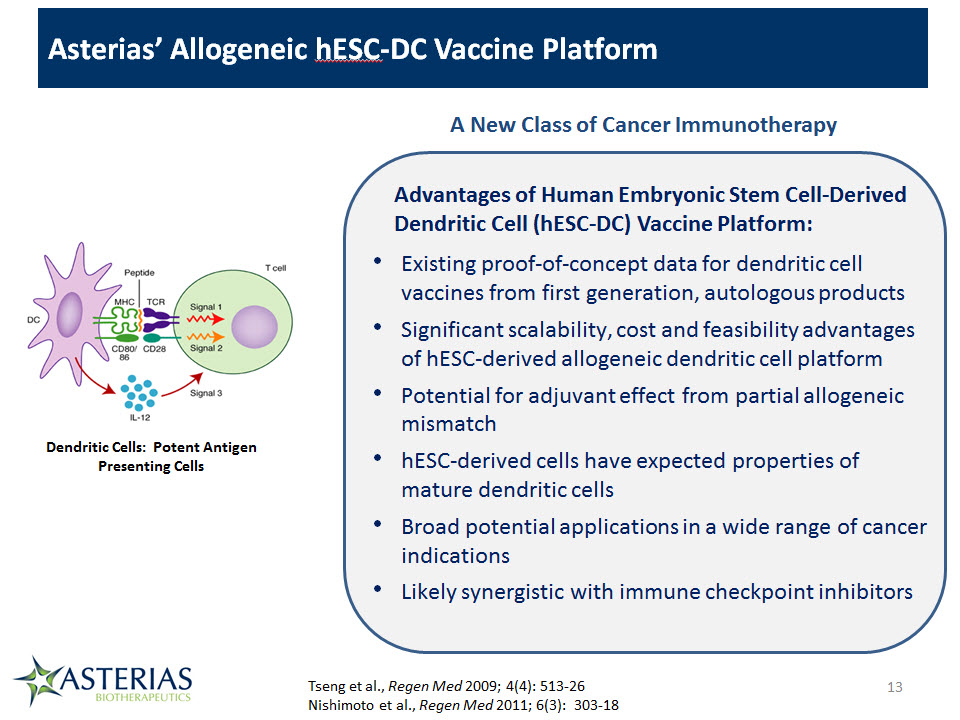
Asterias’ Allogeneic hESC-DC Vaccine Platform 13 A New Class of Cancer Immunotherapy Existing proof-of-concept data for dendritic cell vaccines from first generation, autologous productsSignificant scalability, cost and feasibility advantages of hESC-derived allogeneic dendritic cell platformPotential for adjuvant effect from partial allogeneic mismatchhESC-derived cells have expected properties of mature dendritic cellsBroad potential applications in a wide range of cancer indicationsLikely synergistic with immune checkpoint inhibitors Advantages of Human Embryonic Stem Cell-Derived Dendritic Cell (hESC-DC) Vaccine Platform: Dendritic Cells: Potent Antigen Presenting Cells Tseng et al., Regen Med 2009; 4(4): 513-26Nishimoto et al., Regen Med 2011; 6(3): 303-18
Proof of concept for Dendritic Cell Telomerase Immunotherapy from First Generation AST-VAC1 Product Prostate Cancer Acute Myelogenous Leukemia Phase 1, Single center (Duke) Phase 2, Multi-center Investigator-sponsored Industry-sponsored 20 patients 21 patients 95% Patients Develop Immune Responses to Telomerase 55% Patients Develop Immune Responses to Telomerase Highly Significant Increase in PSA Doubling Times Clearance of Circulating Immune Complexes Significant Increase in 12 Month DFS in High Risk Group (N=11) Compared to Published Historical Controls 14 Two Clinical Trials of Autologous AST-VAC1 Product Show that Dendritic Cell Based Immunotherapies Targeting Telomerase Can Stimulate Immune Responses and Clinical Activity J. Immunol 2005, 174: 3798Khoury, ASH 2010
Rationale for Telomerase 15 Telomerase Antigen:Expressed in >90% of human cancersRarely expressed in normal adult cellsPlays critical role in conferring replicative immortality to cancer cellsEvidence for immunogenicity from previous trials Hanahan and Weinberg, “The Hallmarks of Cancer”
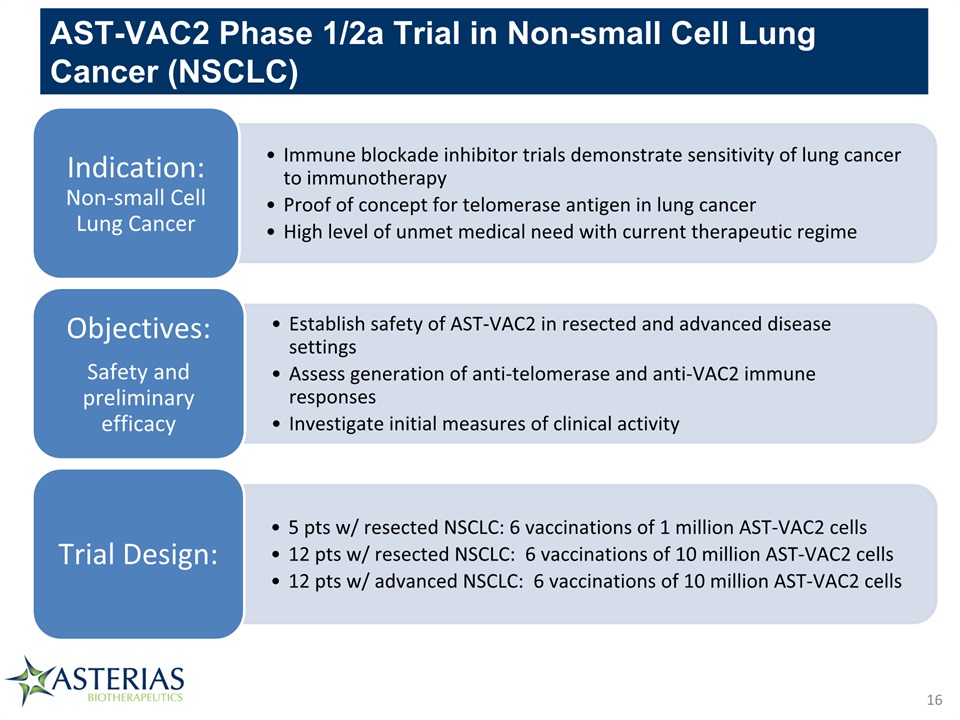
AST-VAC2 Phase 1/2a Trial in Non-small Cell Lung Cancer (NSCLC) 16
Partnerships Provide Validation and Non-dilutive Funding at Minimal Cost to AST Shareholders AST-OPC1 AST-VAC2 $14.3 Million Grant Includes funding for:Execution of Phase 1/2a studyProcess and assay development activities to prepare for pivotal trials and commercializationFacilities and indirect costsPotential follow-on grants to expand and accelerate trial Clinical Development Partnership Asterias performs scale-up and tech-transfer of AST-VAC2 manufacturing processCRUK provides personnel and funding for cGMP manufacturing, regulatory filing, Phase 1/2a trialAsterias has first option to reacquire program on preset, reasonable terms; majority revenue share on partner’s development if does not choose to reacquire Estimated ~$40 Million of Total Non-dilutive Funding Committed to Date 17
18 Asterias Milestones and Newsflow Next 24 Months 1H’15 2H’15 1H’16 Initiate AST-OPC1 P1/2a Trial 6 mo efficacy data from AST-OPC1 10M cell cohort Initiate AST-VAC2 P1/2a Trial MHRA Clearance for AST-VAC2 P1/2a Trial 30 day safety of 2M cell cohort; dose escalate to 10M cell cohort Complete AST-VAC2 process transfer 2H’16 Warrant exercise - $11.7M cash raised Signature of NGA; disbursement of CIRM funds begins Asterias Milestones and Newsflow Next 24 Months
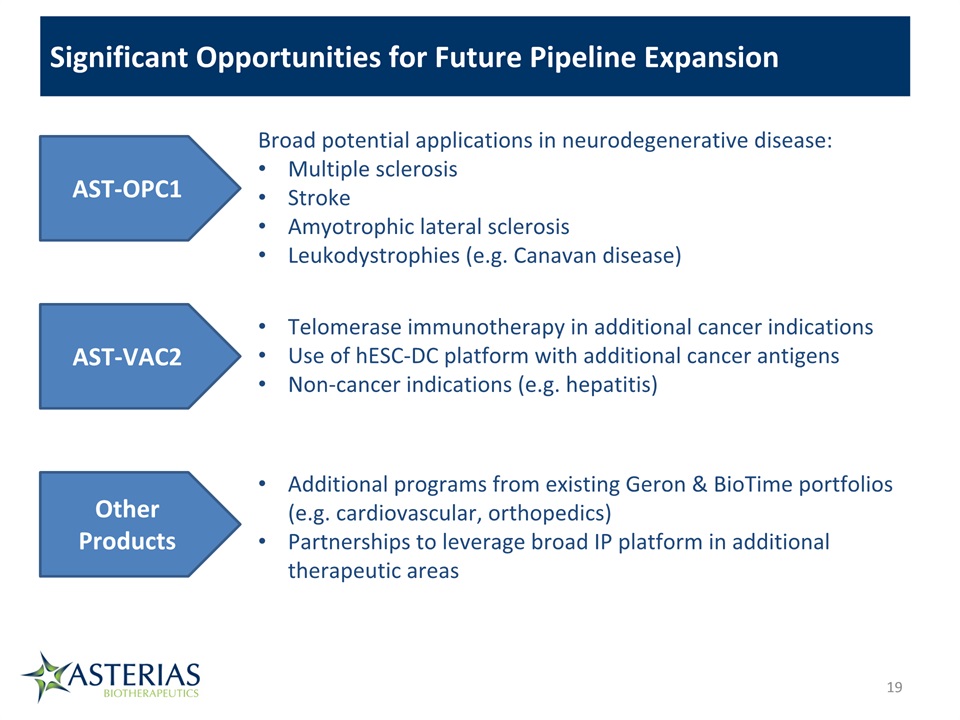
19 Significant Opportunities for Future Pipeline Expansion AST-OPC1 Broad potential applications in neurodegenerative disease:Multiple sclerosisStrokeAmyotrophic lateral sclerosisLeukodystrophies (e.g. Canavan disease) AST-VAC2 Telomerase immunotherapy in additional cancer indicationsUse of hESC-DC platform with additional cancer antigensNon-cancer indications (e.g. hepatitis) Other Products Additional programs from existing Geron & BioTime portfolios (e.g. cardiovascular, orthopedics)Partnerships to leverage broad IP platform in additional therapeutic areas
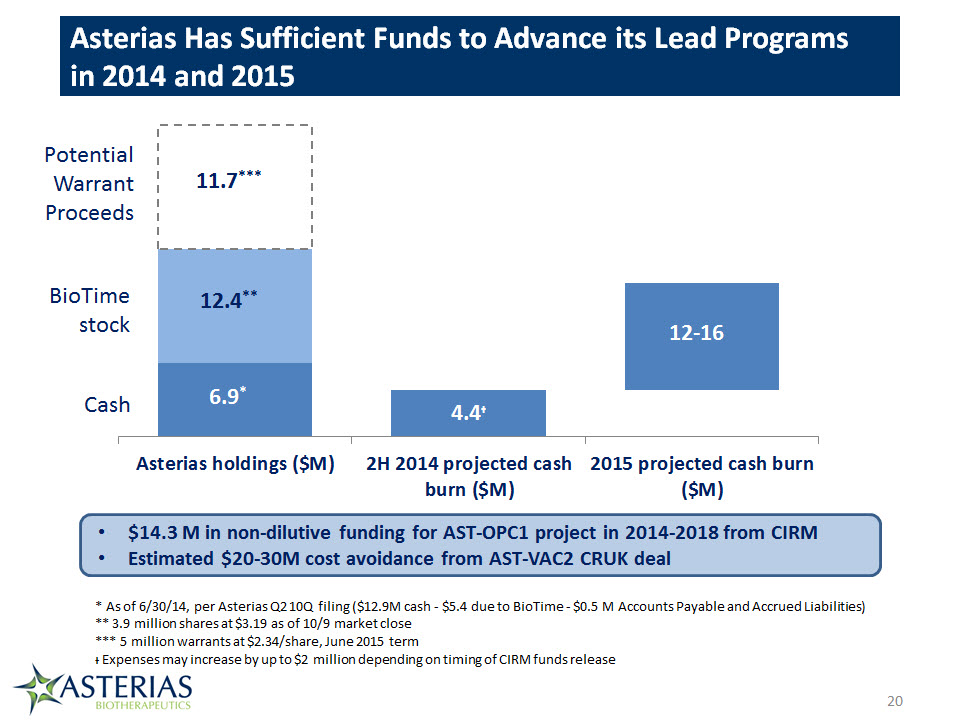
Asterias Has Sufficient Funds to Advance its Lead Programs in 2014 and 2015 20 6.9* 4.4ᵻ Cash 12.4** BioTimestock PotentialWarrantProceeds 11.7*** 12-16 $14.3 M in non-dilutive funding for AST-OPC1 project in 2014-2018 from CIRMEstimated $20-30M cost avoidance from AST-VAC2 CRUK deal * As of 6/30/14, per Asterias Q2 10Q filing ($12.9M cash - $5.4 due to BioTime - $0.5 M Accounts Payable and Accrued Liabilities)** 3.9 million shares at $3.19 as of 10/9 market close*** 5 million warrants at $2.34/share, June 2015 termᵻ Expenses may increase by up to $2 million depending on timing of CIRM funds release
Capital Structure Profile 21 Total 30.9 million outstanding shares valued at $130 mil ($4.20 per share) as of October 31, 2014Additional 5 million Warrants @ $2.34/warrant expiry June 2015 – held by George Karfunkel Trust and Broadwood Partners, LLPAdditional 3.5 million Warrants @ $5.00/warrant expiry September 2016 – held by BioTime and RomulusOption pool of 4.5 million shares for management and employees
Asterias Executive Team: Business Focused, with Best-in-Class Cell Therapy Product Development Experience Name Title Summary of experience Pedro Lichtinger Chief Executive Officer Former President & CEO, Optimer Pharmaceuticals. 25 year career at Pfizer including as President of Global Primary Care and Global Animal Health businesses Jane Lebkowski, PhD President of R&D 25 years experience in R&D of cell & gene therapies at Applied Immune Sciences, Rhone Poulenc Rorer, and Geron Katy Spink, PhD VP and Chief Operating Officer Former SVP, Cell Therapy Program Operations at Geron. Experience in biotech strategy, BD & program management and operations at Geron and McKinsey Ed Wirth, MD, PhD Chief Translational Officer 25 years experience in translational research of cell therapies and medical devices at University of Chicago, Geron, and InVivo Madelyn Marino VP, Quality 30 years experience in QA/QC for small molecules, biologics, cell & gene therapy at Amgen and Onyx Casey Case, PhD SVP Research and Nonclinical Development Former EVP Research at SanBio. Previous R&D leadership experience at Sangamo, Tularik, OSI 22 Broad expertise in pharma and biotech development throughout the product lifecycleTrack record of establishing value creating alliancesUnmatched expertise in development of cell therapies
Strong, Multilayer IP and Market Protection for Asterias’ Products 24 Confidential Additional market protection expected from:Lack of biosimilars pathway for cellular therapiesOrphan designations (7 year market exclusivity) for OPC1 in SCI, VAC2 in certain cancer indications Example U.S. issued patents: US 8,093,049: “Differentiation of Primate Pluripotent Stem Cells to Hematopoietic Lineage Cells”Asterias owned US 7,781,213: Composition of Matter Claims to hESC-derived Dendritic CellsExclusively licensed to Asterias from Isis Innovation, Ltd.US 6,440,735: Dendritic Cell Vaccine Containing Telomerase Reverse Transcriptase for the Treatment of CancerAsterias owned US 6,800,480: “Methods and Materials for the Growth of Primate-Derived Primordial Stem Cells in Feeder Free Culture”US 8,097,458: “Microcarrier Culture System for Rapid Expansion of Human Embryonic Stem Cells” Asterias Owned Covering AST-VAC2: Covering AST-OPC1: US 7,285,415: “Method of Producing Oligodendrocytes from Human Embryonic Stem Cells for Drug Screening or Treatment of SCI”US 7,579,188: “Oligodendrocytes Derived from Human Embryonic Stem Cells for Remyelination and Treatment of SCI”Exclusively licensed to Asterias from UC Irvine US 8,252,585: “Neural Progenitor Cell Populations”US 8,252,586: “Neural Cell Populations from Primate Pluripotent Stem Cells” Asterias Owned US 6,800,480: “Methods and Materials for the Growth of Primate-Derived Primordial Stem Cells in Feeder Free Culture”US 8,097,458: “Microcarrier Culture System for Rapid Expansion of Human Embryonic Stem Cells” Asterias Owned
AST-OPC1 Is Safe and Efficacious in Animal Models of SCI 25 Control AST-OPC1 Uninjured Injury + OPC1 Injury + Control Four restorative functions observed:Secretes factors to stimulate nerve growthIncreases myelination of axonsStimulates new blood vessels in injury siteReduces injury-related cavitation Improves locomotor function in rodent models of both thoracic and cervical SCI 28 Animal Studies; >3000 Rodents and PigsNo evidence of clinically significant safety concernsDelivery of target doses is feasible
Asterias Neurology Program AST-OPC1 Reduces Cavity Formation and Induces Persistent Myelination Myelinated Bundles of Nerve Fibers Traverse Injury 9 months vehicle hNucEC 1 mm 100 µm 50 µm Cavity forms in untreated SCI lesion Myelinated axons do not extend across cavity Brown: antibody to human nuclear antigen labels AST-OPC1; Blue: Eriochrome Cyanine stains myelin 9 months post-transplant with AST-OPC1 hNucEC 1 mm 100 µm 50 µm AST-OPC1 in SCI Lesion; Significantly Reduced Cavity Formation Robust AST-OPC1 survival (brown) Myelinated Fibers (blue) Rat Thoracic Spinal Cord Injury Model 26 Confidential 2.7
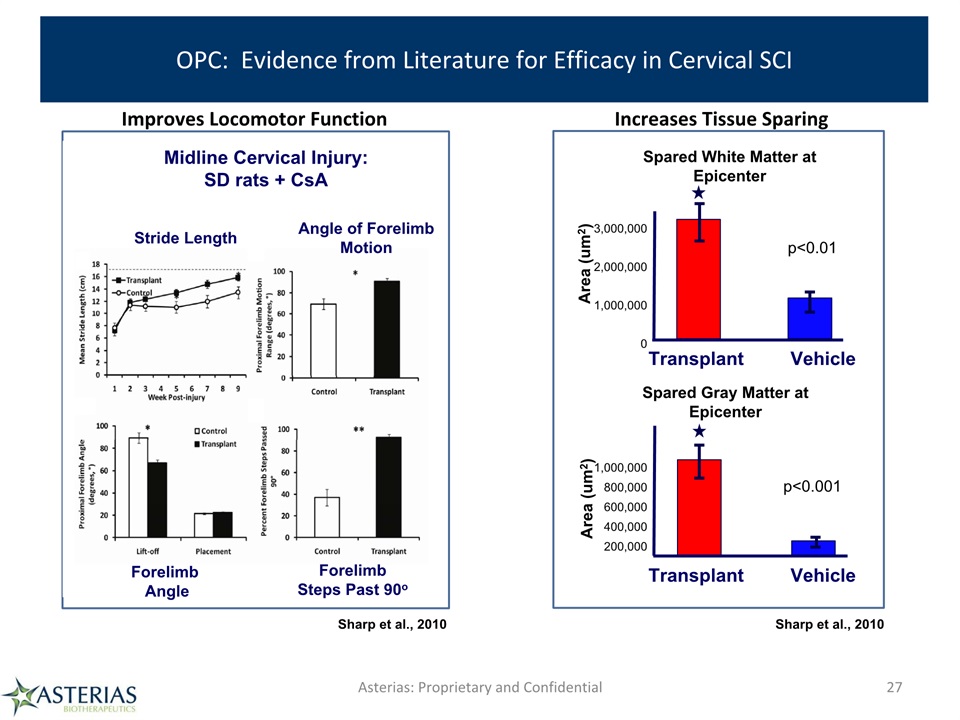
OPC: Evidence from Literature for Efficacy in Cervical SCI Asterias: Proprietary and Confidential 27 Transplant Vehicle Transplant Vehicle Spared White Matter at Epicenter Spared Gray Matter at Epicenter Area (um2) Area (um2) p<0.01 1,000,000800,000600,000400,000200,000 3,000,0002,000,0001,000,0000 p<0.001 Midline Cervical Injury: SD rats + CsA Stride Length Angle of Forelimb Motion Forelimb Angle Forelimb Steps Past 90o Sharp et al., 2010 Improves Locomotor Function Increases Tissue Sparing Sharp et al., 2010
Work of the SCOPE Consortium Has Defined Clinical Development Path in Complete Cervical Spinal Cord Injury 28 Target Product Profile for AST-OPC1 in Complete Cervical SCI: Increase of ≥20% in the percentage of patients regaining two or motor levels of function Enables powering of trial with only ~200 subjects Translates into clinically significant improvements in ability to self-care Steeves et al., Top Spinal Cord Inj Rehabil 2012; 18(1): 1-14
AST-OPC1 Improves Locomotor Recovery in SCI Rats Increased Weight BearingImproved HL-FL Coordination Improved Hind Paw Clearance Improved Trunk StabilityDecreased Tail Drag Increased Stride LengthDecreased Stride WidthDecreased Paw RotationDecreased Toe Spread 2.8 29 Confidential
Asterias has Rapidly Executed on Expanding Clinical Development of AST-OPC1 to Complete Cervical SCI Worked with FDA to define path to clinic for hESC-derived therapyPerformed >20 nonclinical studies to enable first in man testing of AST-OPC1Resolved two clinical holds to satisfaction of FDAEnrolled five subjects in AST-OPC1 trial in complete, thoracic SCIInitiated nonclinical studies to support expansion to cervical SCI2011: Announced decision to divest stem cell assets to focus on two Phase 2 oncology programs October 2013: completed acquisition of Geron stem cell assetsCompleted analysis and study reports for nonclinical studies in cervical SCIWrote clinical study report for thoracic SCI studyWorked with KOLs to establish clinical development plan for AST-OPC1Held meeting with FDA to discuss proposed Phase 1/2a studySecured $14.3M grant from CIRM to fund Ph 1/2a study in cervical SCISubmitted regulatory dossier seeking IND amendment for cervical studyAugust 2014: obtained FDA clearance for Phase 1/2a dose escalation POC study in complete cervical SCI Geron: Asterias:
Spinal Cord Injury Represents a Substantial Market OpportunityDue to A High Level of Unmet Need, Lack of Competing Therapies 31 Confidential Estimated Market Opportunity of $300M-$3B in U.S. aloneDriven by magnitude of impact on high lifetime costs of care for cervical ($3-4M) and thoracic ($2.3M) injury patientsDefined clinical endpoints with potential to support approval from <300 patient trialPotential for breakthrough therapy and orphan designation $50-$500M $100M-$1B $150M-$1.5B $300M-$3B
AST-VAC2 Has Expected Properties of Mature Dendritic Cells Confidential and Proprietary 32 Expresses Markers of Mature Dendritic CellsActivates T CellsMigrates in Response to InflammationCan be Cryopreserved and Irradiated Without Loss of Function g-IFN AST-VAC2 AST-GFP AST-VAC2 Stimulates Telomerase Specific T-cells
hESC-derived Mature DC PBM-derived Mature DC AST-VAC2 and PBM-derived mature DC show similar DC marker expression profiles Tseng et al, Regen Med. 2009 AST-VAC2 is Phenotypically Similar to PBM-Derived DC Confidential 4.2 33
hESC-DC Vaccines Are Likely to Be Synergistic With Immune Checkpoint Inhibitors 34 Immune Checkpoint Inhibitors Block Pathways that Tumors Use to Evade Immune Surveillance AST-VAC2 is the Only Allogeneic Dendritic Cell Cancer Immunotherapy That Educates the Immune System to Recognize Antigens on Tumor Cells Off-the Shelf ProductAvailable on DemandStimulates Immune Responses to TumorsCan Complement Immune Checkpoint InhibitorsPotential for adjuvant effect from allogeneic cells
POC Data from Phase 1/2a Study of AST-VAC2 in NSCLC Enables Broad Development of hESC-DC Platform 35 Demonstration of safety and immune responses in NSCLC study Demonstration of safety and immune responses in NSCLC study Phase 2 and pivotal trials of AST-VAC2 in NSCLC Expansion of AST-VAC2 into additional cancer indications Combination trials with immune checkpoint inhibitors Use of hESC-DC platform with other cancer antigens Enables: Rationale: Safety data in early and advanced disease Telomerase expressed in >95% of all cancersPlays critical role in proliferation & survival of cancer cells Potentially synergistic MOA DC platform can be used to present any antigen
Human embryonic stem cell –derived products for the treatment of retinal and neurodegenerative diseases Dr Charles Irving, CEO 04 Nov 2014 BIOTIME ANNUAL MEETING SHAREHOLDER UPDATE
Safe Harbor Statement 1 The matters discussed in this presentation include forward looking statements which are subject to various risks, uncertainties, and other factors that could cause actual results to differ materially from the results anticipated. Such risks and uncertainties include but are not limited to the success of BioTime in developing new stem cell products and technologies; results of clinical trials of BioTime products; the ability of BioTime and its licensees to obtain additional FDA and foreign regulatory approval to market BioTime products; competition from products manufactured and sold or being developed by other companies; the price of and demand for BioTime products; and the ability of BioTime to raise the capital needed to finance its current and planned operations. Any statements that are not historical fact (including, but not limited to statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. As actual results may differ materially from the results anticipated in these forward-looking statements they should be evaluated together with the many uncertainties that affect the business of BioTime and its subsidiaries, particularly those mentioned in the cautionary statements found in BioTime's Securities and Exchange Commission filings. BioTime disclaims any intent or obligation to update these forward-looking statements.
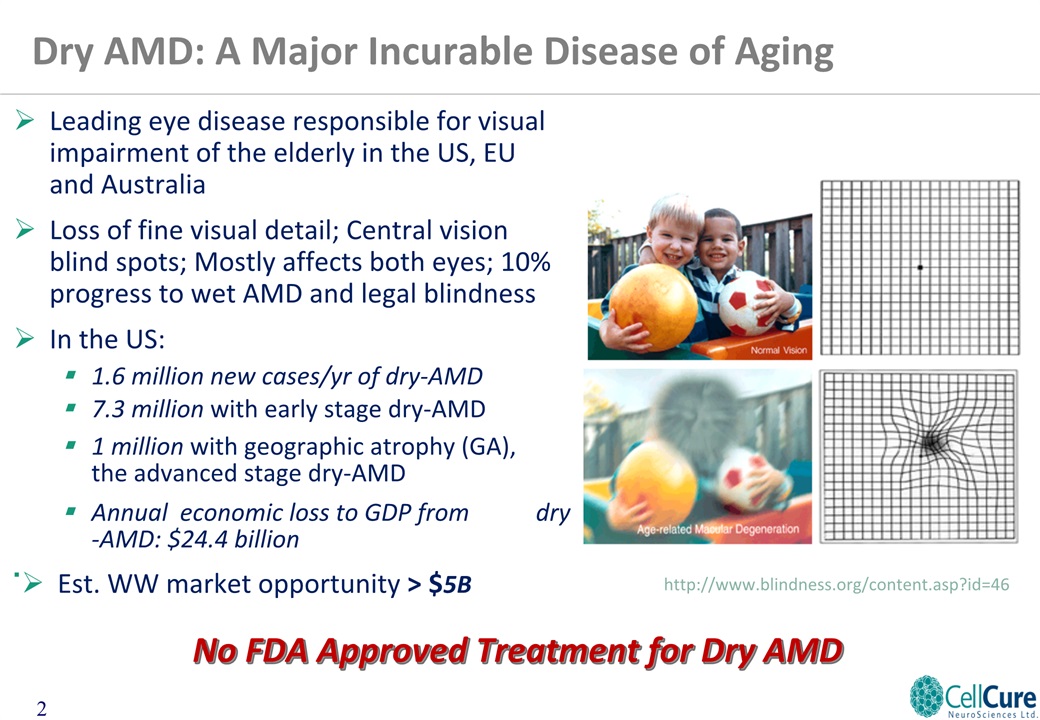
Leading eye disease responsible for visual impairment of the elderly in the US, EU and AustraliaLoss of fine visual detail; Central vision blind spots; Mostly affects both eyes; 10% progress to wet AMD and legal blindness In the US:1.6 million new cases/yr of dry-AMD7.3 million with early stage dry-AMD1 million with geographic atrophy (GA), the advanced stage dry-AMD Annual economic loss to GDP from dry-AMD: $24.4 billion http://www.blindness.org/content.asp?id=46 Dry AMD: A Major Incurable Disease of Aging Est. WW market opportunity > $5B No FDA Approved Treatment for Dry AMD
Types of Age-Related Degeneration Dry AMD (non-neovascular) is characterized by drusen and RPE changes which lead to central atrophy of photoreceptors. Geographic atrophy (GA) is the severe form of dry-AMD.No FDA approved therapy.Wet AMD (neovascular) can develop from dry-AMD. Pathological neovascularization leaks and/or bleeds in the macular area can occur rapidly and result in blindness. Can be treated with VEGF inhibitors, whose current estimated annual worldwide sales are greater than $7 billion
Photoreceptors depend on RPE cells for nourishment, recycling of visual pigment and waste disposal When RPE cells die, the photoreceptors also die and central vision is lost Geographic Atrophy Role of Retinal Pigment Epithelium (RPE) Cells in Dry AMD (RPE)
October ‘09 RPE RPE Transplantation Therapy: A Promising ApproachedThat Lacked a Reliable Source of RPE Cells Healthy RPE cells can replace old, dysfunctional RPEsHuman Embryonic Stem Cells (hESCs) could provide an unlimited supply of well-characterized RPE cells, if a method better than spontaneous differentiation was availableCell Cure’s new directed differentiation method produces homogenous batches of large numbers of pure and robust RPE cells
Production of RPE by Directed Differentiation of hESCsy Adherent Polygonal Pigmented Cell Culture Clinical Grade hESCsGrown on Cord Feeders Spheroid Bodies NicotinamideLow O2 Nicotinamide + Activin ALow O2 Adherent Culture (Pigmented and Non-Pigmented Areas) Selection of Pigmented Areas and Expansion
IP Status of Directed Differentiation Method Cell Cure has exclusive license from Hadasit Ltd.Publ. #: WO2008/129554; Publ. Date: 30-Oct-2008GrantedJapan – 5,395,058; Australia – 2008242106; China -200880020748.0; Israel -201600 AllowedEurope PendingUSA, Canada, Hong Kong, India, various divisionals
The BioTime family of companies has the world’s largest patent estate in pluripotency with >600 patents and patent applications worldwide.Examples of relevant IP:Feeder free culture of hES cellsDirected differentiation methodsNeuronal differentiation (NCAM positive lineages)License to WARF estateRPE-specific claims Title PCT Publication # Granted METHODS OF SELECTING RETINAL PIGMENTED EPITHELIAL CELLS (Cell Cure & Hadasit) WO2013114360 Stem Cell Derived Retinal Pigmented Epithelium Cells (Hadasit) WO2008129554 JP 5,395,058;AU 2008242106; CH 200880020748IL 201600 Stem Cells Culture System (Hadasit) WO2006070370 Embryonic stem cells and neural progenitor cells derived therefrom (ESI) WO0168815 AU 779694 US 7,011,828US 7,504,257 Methods of controlling differentiation of embryonic stem cells by culturing in the presence of BMP2 pathway antagonists (ESI) WO0198463 AU6570401US 7,112,437 Implanting neural progenitor cells derived from human embryonic stem cells (ESI) US 7,011,828 Generation of neural stem cells from undifferentiated hES (ESI) WO03104444 GB 2407821GB 2427616 Embryonic stem cells (ESI) WO0027995 IL 142748 Cell Cure’s In-licensed Patent Portfolio Some of Cell Cure’s Patent Families
Xeno-Free, Clinical-Grade, NIH -Registered hESCs: A Source for Manufacturing Xeno-free RPE Cells 102
Cell Cure’s RPE Cell Product for Dry AMD: OpRegen® OpRegen®: Cell replacement therapy for the dry form of age–related macular degeneration (dry-AMD) utilizing a suspension of RPE cells produced from human embryonic stem cells (hESCs). Cells: Allogeneic human RPE cells produced under cGMP conditions from NIH approved HAD-C 102 hESC line, using cGMP, xeno-free cells and reagentsIndication: Patients with geographic atrophy (GA), the severe dry-form AMDFormulation: Provided as cell suspension in BSS Plus; ready for injection Delivery Route: Subretinal injectionMechanism of Action: Integration into subretinal spaceand replacement of missing or diseased RPE cellsPlanned Clinical Trial: Phase I/IIa Study of OpRegen® in Progressive Dry-Form AMD with GAStudy Site: Hadassah Univ Medical Center, JerusalemStage of Development: FDA authorization received; Israel Ministry of Health approval is in process
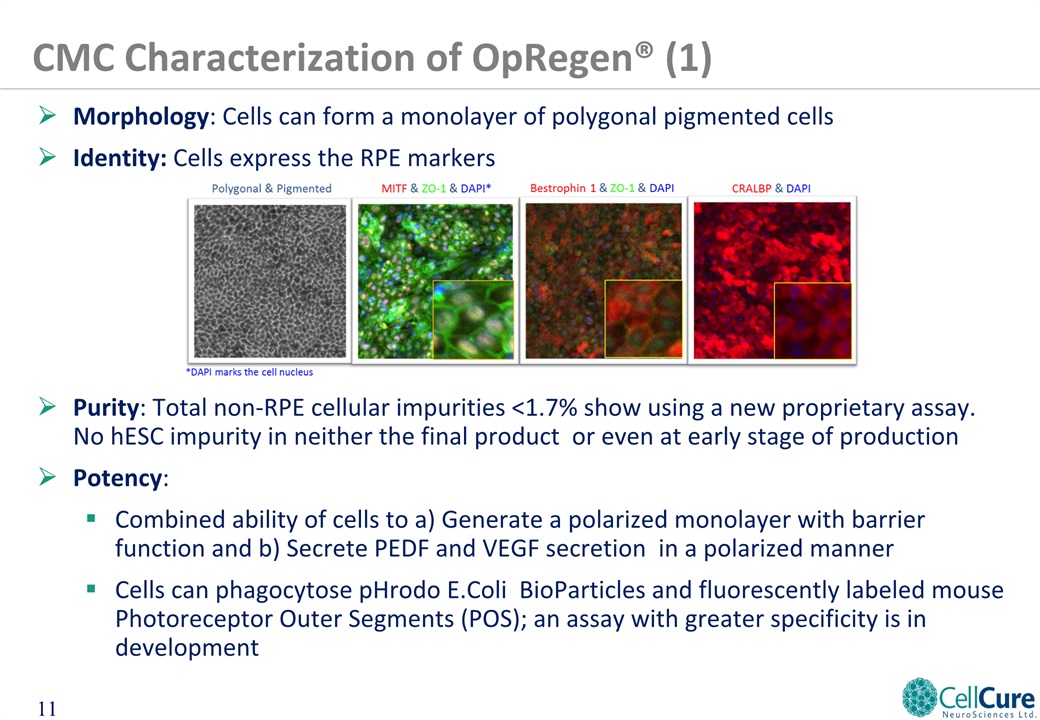
Morphology: Cells can form a monolayer of polygonal pigmented cellsIdentity: Cells express the RPE markersPurity: Total non-RPE cellular impurities <1.7% show using a new proprietary assay. No hESC impurity in neither the final product or even at early stage of productionPotency: Combined ability of cells to a) Generate a polarized monolayer with barrier function and b) Secrete PEDF and VEGF secretion in a polarized mannerCells can phagocytose pHrodo E.Coli BioParticles and fluorescently labeled mouse Photoreceptor Outer Segments (POS); an assay with greater specificity is in development CMC Characterization of OpRegen® (1)
OpRegen® Preclinical Studies Results Three independent CRO studies conducted: Safety/Biodistribution , Tumorigenicity and Spiking and EfficacyNo teratomas or human tumors found neither in 328 NOD/SCID mice nor in 180 RCS rats that received a maximal feasible dose of OpRegen®No teratomas found with OpRegen® spiked with up to 10% hESCs, which is 1000 -fold higher than the batch release criteria (< 0.01%)No product biodistribution from the contained subretinal treatment siteNo product-related mortality/morbidity and no product related ocular, clinical, body weight, macroscopic and microscopic adverse findings were found OpRegen® treated eyes rarely contained human proliferating cells (Ki67 immunostaining)OpRegen® cells survived over the lifetime of the NOD/SCID mouse (9 months)OpRegen® cells injected as a suspension organized into a monolayer within a few weeksOpRegen® cells rescued photoreceptors and mediated phagocytosis of RCS rat photoreceptor outer segments (POS) Optomotor functional studies in RCS rats demonstrated statistically significant, dose dependent functional rescue
Highlights of Preclinical Studies: Vision Rescue in RCS Rat Historical Data:
Highlights of Preclinical Studies: Long Term Graft Survival Long term (9 months –post transplant) engrafting of viable OpRegen® cells (pigmented cells stained positive for HuNu and PMEL17) in NOD/SCID subretinal space
OpRegen® cells are clustered at the place that the bleb was formed and then organize themselves into the monolayer structure found in healthy animals Human PMEL17Human Nuclei Highlights of Preclinical Studies: Cells Form Monolayer Human PMEL17Human Nuclei Human PMEL17Human Nuclei
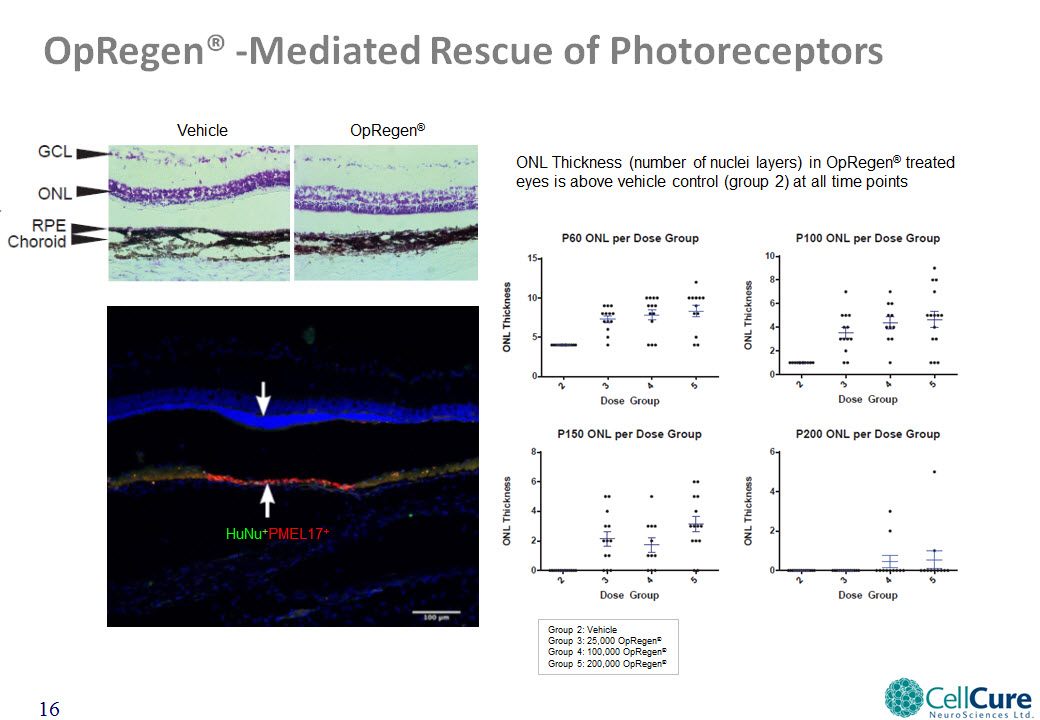
OpRegen® -Mediated Rescue of Photoreceptors Vehicle OpRegen® HuNu+PMEL17+ (OpRegen®) Rescued Photoreceptors; ONL ONL Thickness (number of nuclei layers) in OpRegen® treated eyes is above vehicle control (group 2) at all time points Group 2: VehicleGroup 3: 25,000 OpRegen®Group 4: 100,000 OpRegen®Group 5: 200,000 OpRegen®
OpRegen® Mediated Phagocytosis in Treated RCS Rats Rat RhodopsinHuman PMEL17 Rat RhodopsinHuman PMEL17 Rat rhodopsin outer segments (green) rest along the grafted cells (human PMEL17, red) and rat rhodopsin within transplanted OpRegen® cells (yellow)
Cell Cure Phase I/IIa Clinical Study (1) Phase I/IIa Dose Escalation Safety and Efficacy Study of OpRegen® Transplanted Subretinally in Patients with Advanced Dry-Form AMD (Geographic Atrophy)Open label, non-randomized, sequential, single center trialStudy Site: Hadassah University Medical Center, Jerusalem, IsraelDose and Administration: Single injection of 50,000-500,000 viable cells in 100-150 ml BSS+ delivered via a cannula through a small retinotomy into the subretinal space in the macular area along the border between areas of GA and the better preserved extra-foveal retina and RPE layer. Part 1Cohort 1: 3 Patients, BCVA 20/200 or less (legally blind), 50,000 OpRegen® cellsCohort 2: 3 Patients, BCVA 20/200 or less, 200,000 OpRegen® cellsCohort 3: 3 Patients, BCVA 20/200 or less, 500,000 OpRegen® cellsPart 2 Cohort 4: 6 Patients, BCVA 20/100, 500,000 OpRegen® cells
Primary Objective: Safety and tolerability of transplanted OpRegen®Secondary Objective: Initial exploration of the ability of transplanted OpRegen® to engraft, survive, and moderate disease progression Duration and Follow Up: Enrollment period plus 12 months post transplant follow-up; weekly during 1st month post-transplant, and then at 2, 3, 4, 6, 9, and 12 months post-transplant. Continued follow up at 15 months, 2, 3, 4, and 5 yrs post surgery.Immunotherapy:Topical steroidal and antibiotic treatmentSystemic Tacrolimus to 6 weeks post surgerySystemic Mycophenolate mofetil to 1 year post surgery. Phase I/IIa Clinical Study (2)
Selection of outcome measures based on an ongoing “Cohort Study” that tracks disease progression in patients, who might be candidates for the Phase I/II StudyVisual Acuity Rate of GA Progression over 6 and 12 monthsRetinal sensitivity to light in the engrafted regionsExtent and depth of central stomata A Cohort Study will provide quantitative assessments of disease progression patients prior to enrollment Phase I/IIa Clinical Study Efficacy Outcome Measures Fixation Map Sensitivity Map Microperimetry OCT Infrared Imaging
Company Overview Platform Technology Manufacturing of diverse cell products from xeno-free, GMP-grade human embryonic stem cellsLead product - OpRegen®, human Retinal Pigment Epithelium Cells History Founded in 2005 in Jerusalem, IsraelMajor investments by BioTime starting in 2010 Present Shareholdings:BioTime Inc (incl. ESI & Asterias) 62.5%Hadasit BioHoldings Ltd 21.2%Teva Pharmaceutical Industries Ltd 16.1%ManagementCharles S. Irving – CEOBenjamin Reubinoff – CSOMoshe Hukaylo – CFO
Joseph Wagner, Ph.D. Chief Executive OfficerOncoCyte CorporationShareholder UpdateNovember 4, 2014
OncoCyte Corporation 2 Our mission is to develop novel products for the diagnosis and treatment of cancer in order to improve both the quality and length of life of cancer patients Internally developed cancer gene discovery platformPlatform based on extensive microarray datasetMarker discovery principle based on similarity of gene expression in embryonic development and cancerScores of potential targets identified and IP filed – PanC-Dx™Multiple product opportunities Goal: Develop and market low-cost molecular diagnostic tests for major cancers with rapid adoption by initial users followed by widespread use in large patient populations
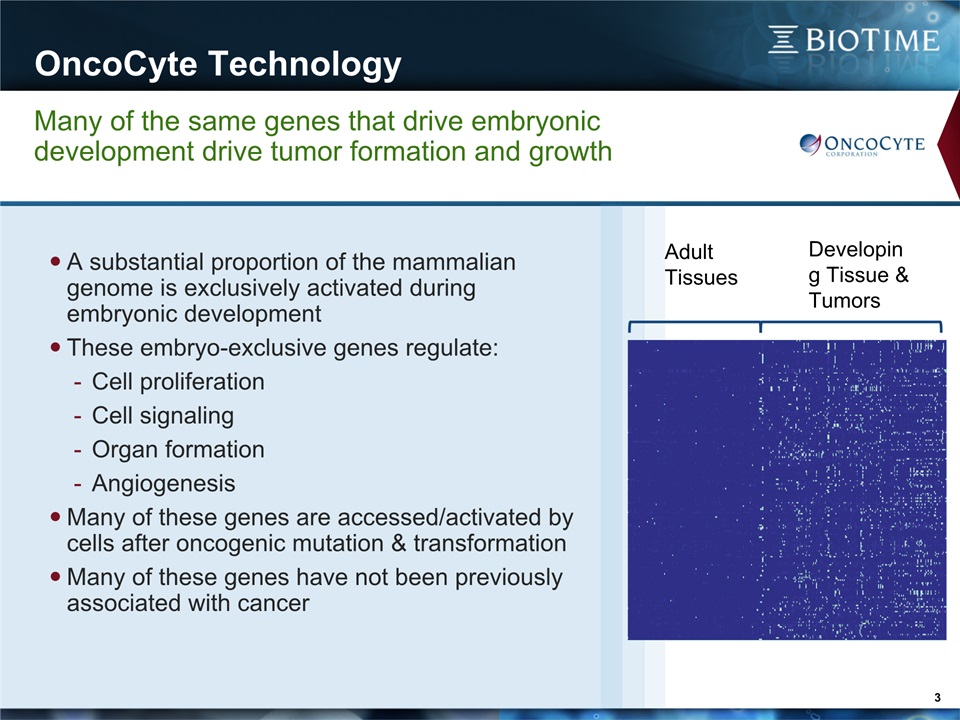
OncoCyte Technology 3 Many of the same genes that drive embryonic development drive tumor formation and growth A substantial proportion of the mammalian genome is exclusively activated during embryonic developmentThese embryo-exclusive genes regulate:Cell proliferationCell signalingOrgan formationAngiogenesisMany of these genes are accessed/activated by cells after oncogenic mutation & transformationMany of these genes have not been previously associated with cancer Developing Tissue & Tumors Adult Tissues
OncoCyte’s Product Development Path 4 Translating proprietary cancer marker platform into near-term revenue opportunities in oncology markets Cancer Marker Discovery (2011-):Assembled >700 sample microarray dataset Bioinformatics generated >3000 candidate markersProtection of Intellectual Property (2011-):Aggressive filings protecting all markers and all usesCancer Marker Validation (2012-):Verify gene expression using alternate methodologiesVerify protein expression in tumor tissueProof of Concept in Patient Samples (2013-):Verify gene/protein expression in retrospective clinical sample banksBuild candidate multiplex panels for large scale testingLarge Prospective Clinical Studies (2014-):Initiate larger studies in target patient populationsTest performance of multiplex panels using platform-neutral proprietary test kits
OncoCyte’s Business Formula 5 Designed to translate proprietary cancer marker platform into near-term revenue opportunities “Listen to Your Technology”“Listen to Your Customers”Create Scalable Business ModelEarly Revenues Fund Larger Trials
“Listen to Your Technology” 6 Technology platform defines product opportunities in subsectors of cancer diagnostics Potential products based on dataset structure:Screening diagnosticsRecurrence diagnosticsPotential products based on markers:Breast cancerColorectal/GI cancersBladder/urothelial cancersThyroid cancerLung cancerPotential products with low cost and ease of use:Blood-based testsUrine-based tests
“Listen to Your Customer” 7 Physician adoption is the key barrier to entry for cancer screening diagnostics User Will Adopt Tests That:Key Opinion Leaders supportResolve diagnostic dilemmasJustify the need for proceduresEliminate unnecessary proceduresHave reasonable cost/reimbursementUser Will Not Adopt Tests That:Create diagnostic ambiguityReplace proceduresCreate unnecessary costs Technology alone does not drive user adoption
OncoCyte Products: Business Strategy 8 How does OncoCyte identify and prioritize its products? Strategically develop diagnostic products that will be rapidly adopted and have short- and long-term revenue potentialFocus on developing products that:Fit with proprietary technologyAre simple to perform and interpretAre relatively low cost and easy to useAppeal to an immediate user baseHave long-term large market potentialAre based on protectable/enforceable IP
Product Development & Marketing Strategy 9 Create a scalable business model beginning with an immediate market with early adopters, successively expanding into larger opportunities $15M Initial Initial Use & Market:Early adopters with immediate needsCan be addressed rapidly with relatively low costComparable approach: Veracyte (VCYT)Expanded Use & Market:Larger markets and user basesRequires additional clinical validationFinal Target Use & Market:Addresses very large markets and tens of thousands of usersRequires partnerships to meet ultimate revenue goalsComparable approach: Exact Sciences (EXAS) Expansion Total Market
Key Driver: Limitations of Current Tests 10 Initial users/use will be to clarify diagnostic dilemmas due to uncertainties in imaging and other traditional tests Current Tests With Significant Limitations Include:Mammography for breast cancerUrine cytology for bladder cancer recurrence surveillanceLow-dose CT for lung cancer screening Definite Negative Definite Positive Indeterminate Test Score
Current PanC-Dx™ Product Programs 11 Simple, low cost cancer screens that address current unmet user needs in large patient markets Breast Cancer Diagnostic (BR-001):Blood-based screening diagnostic Panel of protein markersBladder Cancer Diagnostic (BL-002):Urine-based screening diagnostic for recurrencePanel of RNA markersLung Cancer Diagnostic (LN-003):Blood-based screening diagnostic for high-risk patientsPanel of RNA markers
12 Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-BR-001: Breast Cancer Product
13 ONC-BR-001: Breast Cancer Product Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier
ONC-BR-001: Breast Cancer Product 14 Blood-based screening diagnostic measures sera protein biomarkers using ELISA $15M $25M Initial Use & Market:Radiologist: Management of BIRADS 3-4 patients350K tests/year in USExpanded Use & Market:Radiologist: In conjunction with all diagnostic mammographyOncologist: Recurrence surveillance3M tests/year in USFinal Target Use & Market:PCP/Radiologists: In conjunction with or surrogate for screening mammography>30M tests/year in US $250M $2.5B
ONC-BR-001: Breast Cancer Clinical Study 15 Prospective, multisite study in diagnostic mammography patients Subjects:N = 600At time of diagnostic mammographyEndpoints:Primary: Correlation with BIRADS scoreSecondary: Correlation of biomarker with pathologySites:Up to 6 totalCurrently enrolling: Ron Korn, SMIL, Scottsdale, AZAbcodia providing additional samplesTiming:First subject enrolled early Jan 2014Complete study by end of 2014Data presentation at ACR & ASCO in May, 2015
16 Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-BL-002: Bladder Cancer Product
17 Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-BL-002: Bladder Cancer Product
ONC-BL-002: Bladder Cancer Diagnostic 18 Urine-based multiplex PCR recurrence diagnostic test $15M $15M Initial Use & Market:Pathologist: Resolution of indeterminate cytology during recurrence surveillance500K tests/year in USExpanded Use & Market:Urologist: All recurrence surveillance1.5M test/year in USFinal Target Use & Market:Urologist: Management of hematuria>5M tests/year in US $150M $500M
ONC-BL-002: Bladder Cancer Clinical Studies 19 Multisite clinical studies in setting of surveillance cytology testing or cystoscopy Subjects:N = 100/1200At time of surveillance cytology/cystoscopyEndpoints:Correlation of markers to cytology/cystoscopy resultsSites:Cytopathology: Johns HopkinsCystoscopy: Four large urology clinics in Texas, Ohio, Indiana and South CarolinaTiming:Cytopathology: Complete late 2014Cystoscopy: July 2015Data presentation at AUA & ASCO in May, 2015
20 Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-LN-003: Lung Cancer Product
21 Initial test use late in diagnostic tree with progressive data driving test use earlier and earlier ONC-LN-003: Lung Cancer Product
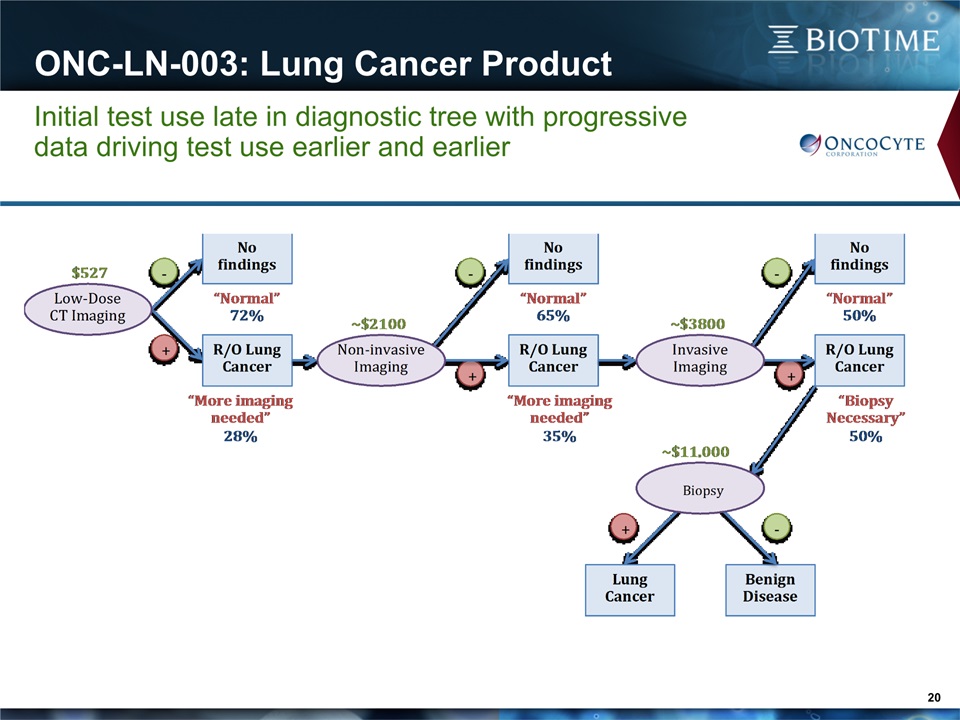
ONC-LN-003: Lung Cancer Product 22 Blood-based lung cancer screen for high-risk population Initial Use & Market:Radiologists/thoracic surgeons: Management of need-to-biopsy decision350K tests/year in USExpanded Use & Market:Radiologists/thoracic surgeons: Management of all LDCT+ patients1.75M tests/year in USFinal Target Use & Market:Radiologists/thoracic surgeons: Used in conjunction with/surrogate for LDCT in all high-risk patients>7M tests/year in US $125M $525M $25M
ONC-LN-003: Lung Cancer Clinical Study 23 Ongoing study in target population initiated by collaborators at the Wistar Institute Subjects:N = 600All high-risk, includes cancer-free, benign nodules, confirmed cancer patientsEndpoints:Primary: Correlation with diagnosisSecondary: Correlation with tumor origin, stage, gradeSites:6 total including NYU, Temple, Penn, ChristianaTiming:Study completed enrollment May 2014Sample analysis completed October 2014Initial data publication late 2014Study data presentations at ATS & ASCO 2015
Adoption Strategy 24 Superior product at lower cost to drive rapid adoption in initial markets, followed by widespread adoption KOL endorsementsDrive adoption within KOLs’ own practices as well as among other physiciansCan lead to medical society recommendationsStrong Clinical Trial DataSupports positive health economics and patient outcomesCost effectiveness of testsDrives user willingness to acquire additional dataAs test volume increases, adoption point becomes earlier as test accuracy gains credibility
Upcoming Development Milestones 25 Positive clinical trial results will drive commercialization efforts with first test launch in late 2015 Q4 2014: Completion of breast, bladder and lung clinical trial enrollment and data analysisPublication of lung trial results; submission of clinical study data for presentation at major oncology meetingsExpansion of executive management teamQ1 2015: Initiation of CLIA lab testing certificationQ2 2015: Presentation of breast, bladder and lung clinical trial at major oncology meetingsPublication of breast and bladder clinical trial data in peer-reviewed journalsQ3 2015: Assay validation completed for lead testCalifornia CLIA certification obtainedQ4 2015: Commercial launch of first diagnostic test to be announced at major oncology meeting
OncoCyte: Investment Highlights 26 Beginning with an immediate market with early adopters, expanding into large opportunities Broad platform of cancer markersCost-effective tests using standard methodsPotential for rapid initial adoption and broad eventual adoptionScalable business model:Significant revenue in initial marketsEarly revenues fund studies necessary for broader useVery large long-term potential revenue opportunity
HyStem® ProductDevelopment November 4, 2014Thomas I. Zarembinski, PhD MBASenior Director, New Product Development
Safe Harbor Statement 1 The matters discussed in this presentation include forward looking statements which are subject to various risks, uncertainties, and other factors that could cause actual results to differ materially from the results anticipated. Such risks and uncertainties include but are not limited to the success of BioTime in developing new stem cell products and technologies; results of clinical trials of BioTime products; the ability of BioTime and its licensees to obtain additional FDA and foreign regulatory approval to market BioTime products; competition from products manufactured and sold or being developed by other companies; the price of and demand for BioTime products; and the ability of BioTime to raise the capital needed to finance its current and planned operations. Any statements that are not historical fact (including, but not limited to statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. As actual results may differ materially from the results anticipated in these forward-looking statements they should be evaluated together with the many uncertainties that affect the business of BioTime and its subsidiaries, particularly those mentioned in the cautionary statements found in BioTime's Securities and Exchange Commission filings. BioTime disclaims any intent or obligation to update these forward-looking statements.
Introduction 2 Background on the need for HyStemCompetitive LandscapeStrategyMarketsProduct Pipeline & Development Status
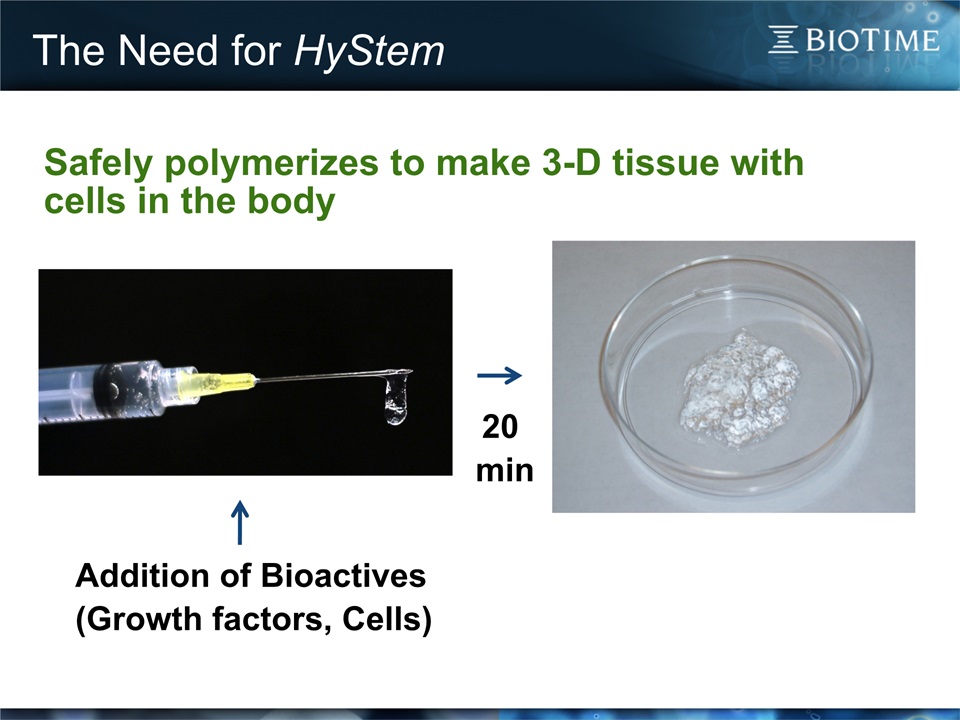
The Need for HyStem 3 + = UnprecedentedOpportunities inRegenerativeMedicine Cells Matrix • Cells need a matrix for attachment or they often die• An injectable matrix capable of polymerizing in vivo into 3-D tissue could revolutionize medicine
The Need for HyStem 4 The Elegance of Defined Minimal ECM One Component Two Components Multi-Component ReGlyde - Adhesion prevention Potential applications in arthritis Renevia – Cell transplants Premvia – Wound manage- ment Controlled growth factor release - Stroke - Cancer Prestwich, J. Cell. Biochem. 101, 1370 (2007) Courtesy of Dr. Glenn D. Prestwich
Two Component Matrix 5 Biocompatible & Biodegradable Carbohydrates Collagen Cell (Griffith and Schwartz, 2006)
The Need for HyStem 6 Addition of Bioactives (Growth factors, Cells) 20 min Safely polymerizes to make 3-D tissue with cells in the body
HyStem® Technology Strengths Strong IP – composition of matter & uses 2 U.S. patents issued with applications pending in the EU, Canada, Japan, and AustraliaEase of manufacture Modest capital investment - large gross margins (65% - 90%)Multiple products in rapidly emerging fields Stem cell applications, tissue engineering, regenerative medicine, and cell-based therapiesSimple – yet elegant technology Nearly 200 scientific publications demonstrating applications
Competitive Landscape 8 Animal-derivedHydrogels forResearch Solid matrices
9 pre-gelled dermal fillers can’t be mixed with cells and often don’t have the requisite attachment sites for cells. Dermal Fillers Viscosupplementation Competitive Landscape
Examples of Applications 10 Wu et al Ann Thorac Surg 2011
11 in a matrix Wu et al Ann Thorac Surg 2011 Examples of Applications
HyStem® Strategy 12 In the US - a medical device: HyStem® alone – CDRH as a Class II (510K) or Class III (PMA). HyStem® + drug – CDER as a combination new therapy. HyStem® + cells – CBER as a combination new therapy. In the EU - a medical device: HyStem® alone – Notified Body as a Class III. HyStem® + patient’s own “mm” cells – Notified Body as Class III HyStem® + cultured donor cells – European Medicines Agency
Quality Management Systems 13 Required for Regulatory Approval
HyStem® Product Pipeline 14 Partner Platform Early Pre-Clinical Formulation Development /IND-Enabling Studies Clinical development Regulatory Pathway Safety Efficacy Pivotal Marketed HyStem® In-House R&D Medical device in US & EU ReGlyde™ N/A N/A N/A Medical device in USClass II 510(k) Premvia™ 510(k) Cleared Medical device in USClass II 510(k) Renevia™ CE Mark as Class III medical device in EU University Collaborations Management of tendon healing post surgery. New formulations and formats – films, sponges Cell delivery matrix for treating subcutaneous lipoatrophies. Wound management – partial & full thickness, ulcers, and burns. Therapeutics in Developent
Premvia 15 Premvia™- an FDA-cleared device to treat dermal wounds and burns.
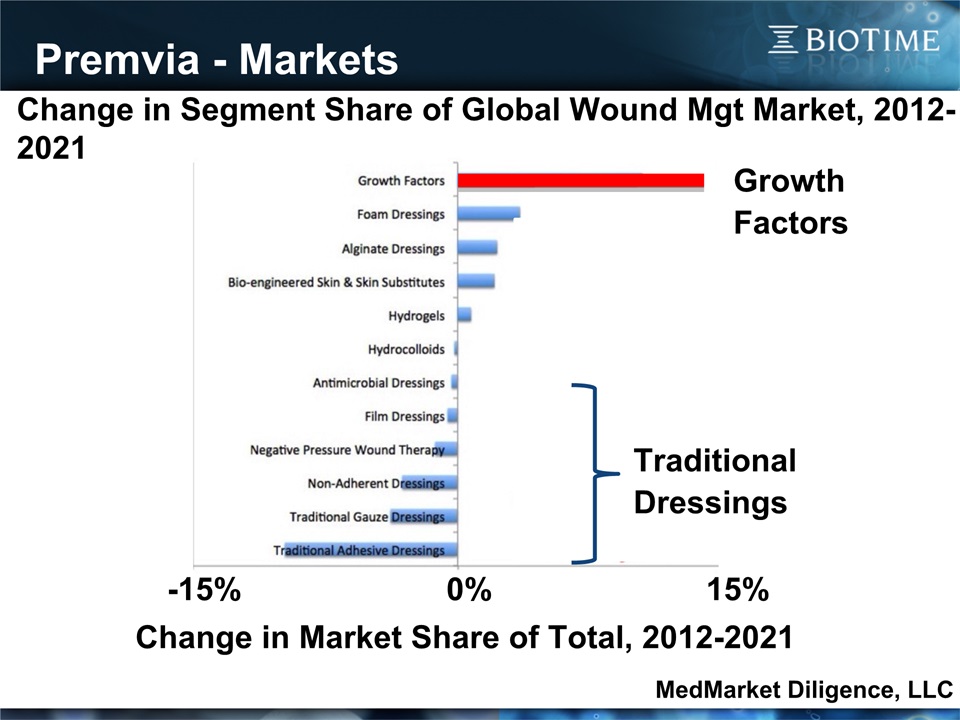
Premvia - Markets 16 Change in Segment Share of Global Wound Mgt Market, 2012-2021 MedMarket Diligence, LLC Change in Market Share of Total, 2012-2021 -15% 0% 15% Traditional Dressings Growth Factors MedMarket Diligence, LLC
17 Premvia Hydrogel + Bioactive
Premvia—510(k) Clearance 18
Renevia - Medical Need 19 ReneviaTM is expected to have numerous applications in multiple tissue types.BioTime initially is seeking a CE mark for use in HIV-associated lipoatrophy in combination with autologous fat cells.An estimated 33M people worldwide have HIV. Number on HIV treatment has tripled in five years – now ~10M, target of 15M by the end of 20151. An estimated 35-50% of patients on ARV therapy have lipoatrophy2.A greater number of people in the US have age-related lipoatrophy. Age-Related Lipoatrophy 1 GLOBAL UPDATE ON HIV TREATMENT 2013: RESULTS, IMPACT AND OPPORTUNITIES, WHO report in partnership with UNICEF and UNAIDS JUNE 2013 2 Source: AIDS Care Vol. 21, No. 5, May 2009, 664-671, VHA DIRECTIVE 2008-003, Veterans Health Administration, January 22, 2008
20 Indication: Renevia™ is a resorbable matrix to be used for the delivery of autologous adipose derived cells for the treatment of HIV-related facial lipoatrophy. Renevia™ serves as a temporary 3-dimentional matrix in which the implanted cells can attach, proliferate and differentiate into adipocytes. • Multicenter, randomized, controlled, single blind, trial • Treated vs. delayed treatment control, 25 - 92 subjects in each group with treatment effect measured a 1, 3, and 6 months • Primary endpoint: Increase in skin thickness as measured by ultrasound at 6 months • Secondary endpoint: Mid-face volume deficit score, Global Aesthetic Improvement Score • Two sites in Palma de Mallorca, Spain • Patients enrollment has begun ReneviaTM – Pivotal Clinical Trial
Primary Clinical Trial Site 21 …….The Stem Center, Palma de Mallorca, Spain Ramon Llull, MD, PhD - Medical Director
Summary of Accomplishments 22 Obtained ISO-13485 Certificate of Quality SystemsPremviaTM (Wound Management): Received 510(k) clearanceReneviaTM (Repair of Contour Defects): Pivotal Trial starting soon.
Thank You! Thomas I. Zarembinski, PhD MBASr. Director, New Product Developmenttzarembinski@biotimemail.com510-521-3390

lifemap-solutions.com © LifeMap Solutions Inc. 2014. Proprietary and confidential mHealth Industry Overview BioTime Shareholder Update November 4, 2014

Safe harbor statement The matters discussed in this presentation include forward looking statements which are subject to various risks, uncertainties, and other factors that could cause actual results to differ materially from the results anticipated. Such risks and uncertainties include but are not limited to the success of BioTime in developing new stem cell products and technologies; results of clinical trials of BioTime products; the ability ofBioTime and its licensees to obtain additional FDA and foreign regulatory approval to marketBioTime products; competition from products manufactured and sold or being developed by other companies; the price of and demand forBioTime products; and the ability of BioTime to raise the capital needed to finance its current and planned operations. Any statements that are not historical fact (including, but not limited to statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. As actual results may differ materially from the results anticipated in these forward-looking statements they should be evaluated together with the many uncertainties that affect the business of BioTimeand its subsidiaries, particularly those mentioned in the cautionary statements found in BioTime'sSecurities and Exchange Commission filings.BioTime disclaims any intent or obligation to update these forward-looking statements.

LifeMap Solutions: mission Working in partnership with the Icahn School of Medicine at Mount Sinai, LifeMap Solutions seeks to develop an mHealth platform that integrates disparate sources of information to generate an increasingly accurate picture of an individual’s health.
Key partner: Mount Sinai Top-20 hospital Top-20 research medical school 36,000 employees, including 6,200 physicians 170,000 admissions yearly 2.6M non-ER outpatient visits yearly 490,000 ER visits yearly Direct partner is Icahn Institute for Genomics and Multiscale Biology, run by Eric Schadt, Ph.D., founding director Source: US News and World Reports (http://health.usnews.com/health-news/best-hospitals/articles/2014/07/15/besthospitals- 2014-15-overview-and-honor-roll), NIH Funding (http://www.brimr.org/NIH_Awards/2013/SchoolOfMedicine_2013.xls)
Experienced team
mHealth overviewToday: mostly consumer apps and “wearables”
Major new entrants signal 2nd phase
Market growth is accelerating Source: Allied Market Research (http://www.alliedmarketresearch.com/mobile-health-market) Predicted Global mHealth Market Size (Billions USD)
Investment is accelerating Source: RockHealth Q3 2014 Update (http://rockhealth.com/2014/10/q3-funding-update-digital-health-rakes-3b/) Venture Funding of Digital Health Companies (2011 - Q3 2014)
Why now? Culture Ubiquity of smartphones “Quantified Self” movement Expectation of accelerating tech Technology Powerful processors High bandwidth High-quality sensors Big data analytics and management tools
Beneficiaries of mHealth Consumers Care Teams Direct Researchers Indirect Researchers All enabled by big data