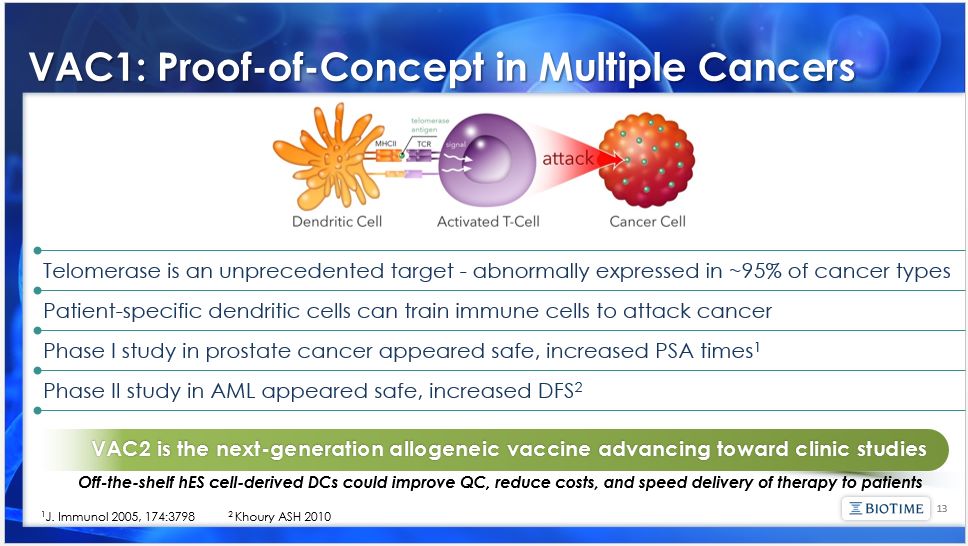
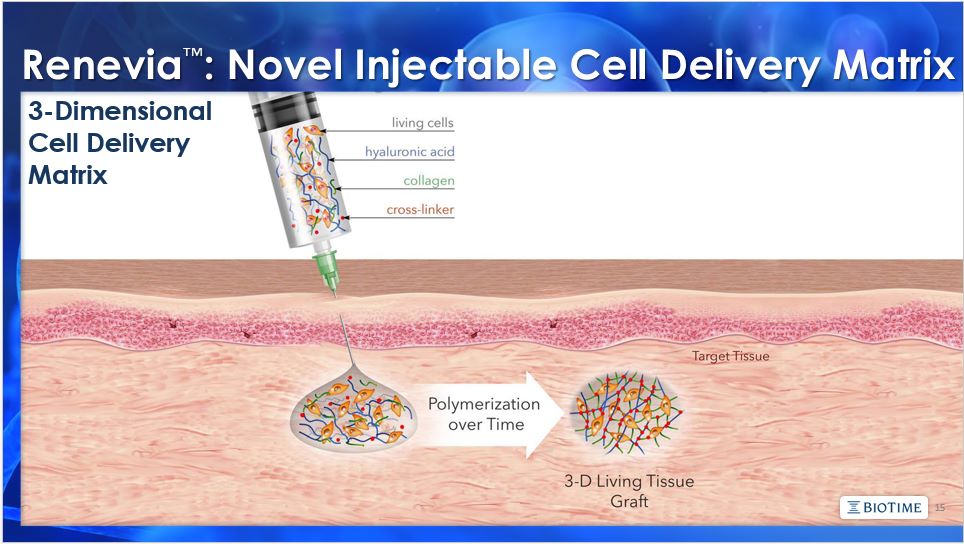
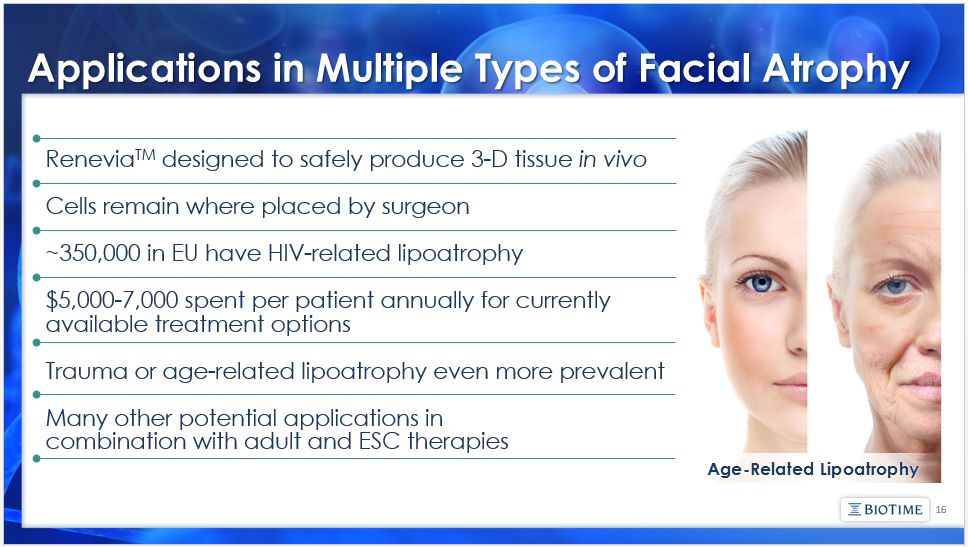
The matters discussed in this presentation include forward looking statements which are subject to various risks, uncertainties, and other factors that could cause actual results to differ materially from the results anticipated. Such risks and uncertainties include but are not limited to the success of BioTime in developing new stem cell products and technologies; results of clinical trials of BioTime products; the ability of BioTime and its licensees to obtain additional FDA and foreign regulatory approval to market BioTime products; competition from products manufactured and sold or being developed by other companies; the price of and demand for BioTime products; and the ability of BioTime to raise the capital needed to finance its current and planned operations. Any statements that are not historical fact (including, but not limited to statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. As actual results may differ materially from the results anticipated in these forward-looking statements they should be evaluated together with the many uncertainties that affect the business of BioTime and its subsidiaries, particularly those mentioned in the cautionary statements found in BioTime's Securities and Exchange Commission filings. BioTime disclaims any intent or obligation to update these forward-looking statements. 2 Safe Harbor Statement