Exhibit 99.1

PAR pharmaceutical companies
Company Update January 2014

Cautionary Note Regarding Forward-Looking Statements
This presentation includes forward-looking statements. These forward-looking statements generally can be identified by the use of words such as “anticipate,” “expect,” “plan,” “could,” “may,” “will,” “believe,” “estimate,” “forecast,” “goal,”
“project,” and other words of similar meaning. These forward-looking statements address various matters including our estimate of financial results for the fiscal year ended December 31, 2013, including select pro forma financial results and pro forma leverage ratios based on our estimate of financial results for the fiscal year ended December 31, 2013, estimated financial results of JHP for the fiscal year ended December 31, 2013 and an estimate of JHP’s future manufacturing excess capacity. Each forward-looking statement contained in this presentation is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. Applicable risks and uncertainties include, among others, finalizing the financial statements and completing the audits of the Company and JHP for the fiscal year ended December 31, 2013, and the risks identified under the heading “Risk Factors” in our Quarterly Report on Form 10-Q for the three months ended September 30, 2013, and filed with the Securities and Exchange Commission, as well as the other information we file with the SEC. We caution investors, potential investors, and others not to place considerable reliance on the forward-looking statements contained in this presentation. You are encouraged to read our filings with the SEC, available at www.sec.gov, for a discussion of these and other risks and uncertainties. The forward-looking statements in this presentation speak only as of the date of this document, and we undertake no obligation to update or revise any of these statements. Our business is subject to substantial risks and uncertainties, including those referenced above. Investors, potential investors, and others should give careful consideration to these risks and uncertainties.

Introduction
AGENDA Acquisition Rationale
Par Pharma
Financial Overview
2

Acquisition Rationale
Acquisition Rationale
Acquisition Rationale
Unique Opportunity to Enter Injectables are a high growth segment within the pharmaceutical market with total market growing to $12.2 billion in 2022E from $7.0 billion in 2012A,
Highly Attractive Injectables a CAGR of 5.7%
Market No material cost disadvantage for U.S. based injectable manufacturing, but an advantage from a regulatory and compliance perspective
Diverse Portfolio of Product portfolio includes 14 marketed branded and generic injectable products
Products with Strong
Market Share Stable and predictable contract manufacturing segment servicing top global pharmaceutical companies
Launch execution expertise with 9 products launched in 2012-2013
Robust Pipeline and Strong pipeline of 34 injectable products, with 17 filed Abbreviated New Drug Applications (“ANDA“s) with the FDA
Product Development JHP currently has 17 FTEs dedicated to R&D and expects to grow to 42 FTEs by 2018
Capabilities
In-house R&D team capable of filing 7-8 new ANDAs per year
• Significant investments in R&D infrastructure including a new, ~$10 million dollar state-of-the-art R&D facility
Sophisticated • Substantial capacity to support growth through 2018 with ~38% capacity utilization in 2013E and only 2/5 filling lines approaching full capacity by 2018
Manufacturing Facility with • Over $50 million of investment into the Rochester, MI manufacturing facility since 2008
Significant Excess Capacity
• Allows for strong operating leverage as incremental products come on line
• FDA scrutiny has increased over the last decade
Strong Compliance History • 45 audits and inspections since 2011; JHP has never received a warning letter
• In compliance with numerous international regulatory bodies
• Revenues increased from $92 million in 2011 to $145 million in 2013E due to growth of existing brands and new product launches
Strong Financial Profile • EBITDA increased from $22 million (24% margin) in 2011 to $40 million (28% margin) in 2013E mainly due to operating leverage, as products filled
excess capacity
• 2013E Free Cash Flow (1) conversion of ~59%
1 Defined as EBITDA minus Capital Expenditures.
4
JHP Overview
Acquisition Rationale
Business Description Segment Overview
Specialty healthcare company specializing in sterile Specialty Injectable Products Contract Manufacturing
injectable products • Established portfolio of 14 stable • Diversified portfolio of
Manufactures and sells branded aseptic injectable and growing specialty injectable well-respected
pharmaceuticals in hospital and clinical settings and also products branded pharma
provides contract manufacturing services for major – Core branded products provide customers
pharma companies stable growth profile • Mix of long-established
Products cover various therapeutic areas including – Generics generate ~27% of relationships and new
anesthesiology, diagnostics, endocrinology, 2013E revenues customers
gastroenterology, infectious disease, neurology and – Other stable products include • High customer
women’s health injectables, ophthalmics, and retention rate
Key brands include Adrenalin, Aplisol, Brevital, otics • Provides stable cash
Coly-Mycin M, Dantrium, Delestrogen, Pitocin and • Established pipeline of 34 flows to cover fixed
Pitressin products, 17 of which are filed with costs, with
Operates through one FDA approved manufacturing the FDA quality/compliance
facility, including a 171,000 square foot production – In-house capability to file 7–8 benefits through
building and warehouse, located in Rochester, MI ANDAs per year frequent customer
audits
Sells products across the US and JHP products reach 50% of revenues generated by key
more than 86 countries via their CMO customers brands
• Founded: 2007; Headquartered: Parsippany, NJ;
Employees: 483
% 2013E 77% 23%
Revenue
• Aplisol • Adrenalin
Major • Dantrium • Pitressin
Products • Brevital • Zoledronic Acid
5
JHP’s Specialty Pharmaceuticals Platform
Acquisition Rationale
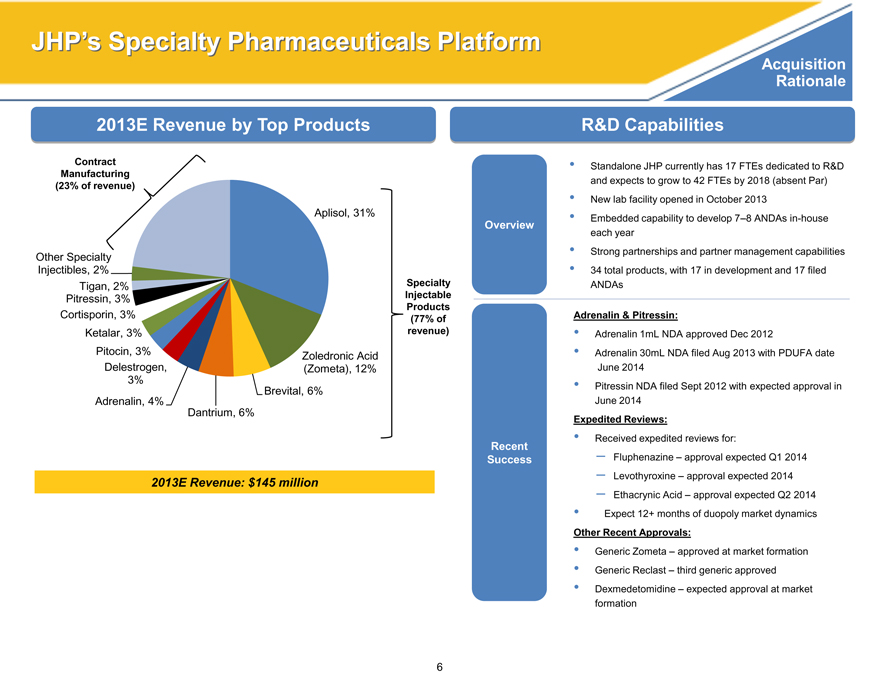
2013E Revenue by Top Products
Contract Manufacturing (23% of revenue)
Aplisol, 31%
Other Specialty Injectibles, 2%
Tigan, 2% Specialty Pitressin, 3% Injectable
Products
Cortisporin, 3% (77% of Ketalar, 3% revenue) Pitocin, 3% Zoledronic Acid Delestrogen, (Zometa), 12% 3% Brevital, 6% Adrenalin, 4% Dantrium, 6%
2013E Revenue: $145 million
R&D Capabilities
Standalone JHP currently has 17 FTEs dedicated to R&D
and expects to grow to 42 FTEs by 2018 (absent Par)
New lab facility opened in October 2013
Overview Embedded capability to develop 7–8 ANDAs in-house
each year
Strong partnerships and partner management capabilities
34 total products, with 17 in development and 17 filed
ANDAs
Adrenalin & Pitressin:
Adrenalin 1mL NDA approved Dec 2012
Adrenalin 30mL NDA filed Aug 2013 with PDUFA date
June 2014
Pitressin NDA filed Sept 2012 with expected approval in
June 2014
Expedited Reviews:
Received expedited reviews for:
Recent
Success – Fluphenazine – approval expected Q1 2014
– Levothyroxine – approval expected 2014
– Ethacrynic Acid – approval expected Q2 2014
Expect 12+ months of duopoly market dynamics
Other Recent Approvals:
Generic Zometa – approved at market formation
Generic Reclast – third generic approved
Dexmedetomidine – expected approval at market
formation
6
Sterile Injectables Opportunity
Acquisition Rationale
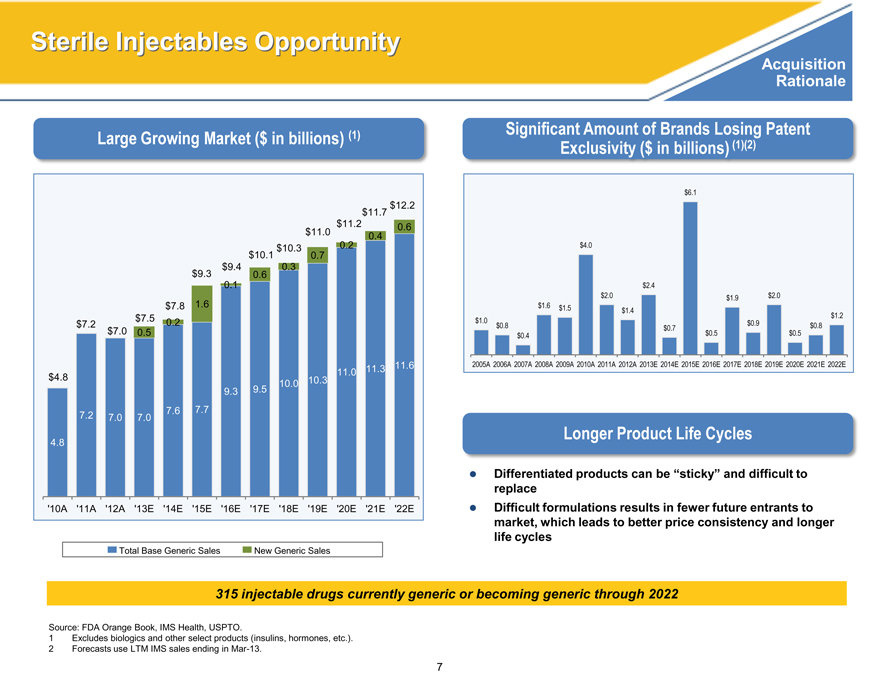
Large Growing Market ($ in billions) (1)
$12.2
$11.7
$11.2
0.6
$11.0
0.4
$10.3
0.2
$10.1
0.7
$9.4
0.3
$9.3
0.6
0.1
$7.8
1.6
$7.2
$7.5
0.2
$7.0
0.5
11.6
$4.8
10.3
11.0
11.3
9.3
9.5 10.0
7.2
7.0
7.0
7.6
7.7
4.8
‘10A
‘11A
‘12A
‘13E
‘14E
‘15E ‘16E ‘17E ‘18E ‘19E
‘20E
‘21E
‘22E
Total Base Generic Sales
New Generic Sales
Significant Amount of Brands Losing Patent
Exclusivity ($ in billions) (1)(2)
$6.1
$4.0
$2.4
$2.0 $1.9 $2.0
$1.6 $1.5
$1.4 $1.2
$1.0 $0.8 $0.9 $0.8
$0.7
$0.4 $0.5 $0.5
2005A 2006A 2007A 2008A 2009A 2010A 2011A 2012A 2013E 2014E 2015E 2016E 2017E 2018E 2019E 2020E 2021E 2022E
Longer Product Life Cycles
Differentiated products can be “sticky” and difficult to
replace
Difficult formulations results in fewer future entrants to
market, which leads to better price consistency and longer
life cycles
315 injectable drugs currently generic or becoming generic through 2022
Source: FDA Orange Book, IMS Health, USPTO.
1 Excludes biologics and other select products (insulins, hormones, etc.).
2 Forecasts use LTM IMS sales ending in Mar-13.
7
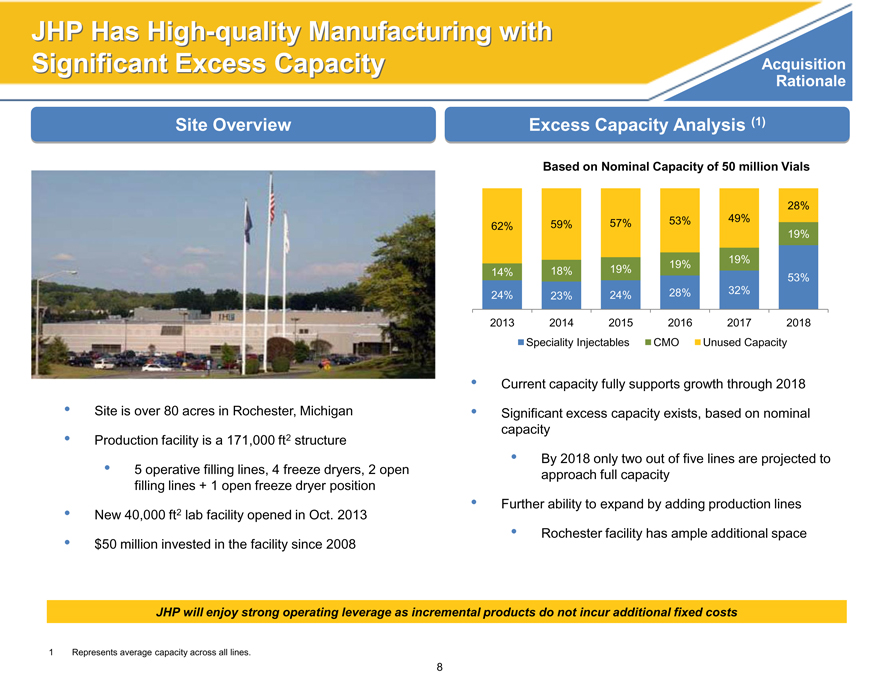
Acquisition
JHP has high-quality manufacturing with significant excess capacity
Rationale
Site overview
Excess Capacity Analysis (1)
Based on Nominal Capacity of 50 million Vials
28%
62% 59% 57% 53% 49%
19%
19% 19%
14% 18% 19% 53%
24% 23% 24% 28% 32%
2013 2014 2015 2016 2017 2018
Speciality Injectables CMO Unused Capacity
Current capacity fully supports growth through 2018
Significant excess capacity exists, based on nominal
capacity
By 2018 only two out of five lines are projected to
approach full capacity
Further ability to expand by adding production lines
Rochester facility has ample additional space
JHP will enjoy strong operating leverage as incremental products do not incur additional fixed costs
1 Represents average capacity across all lines.
8
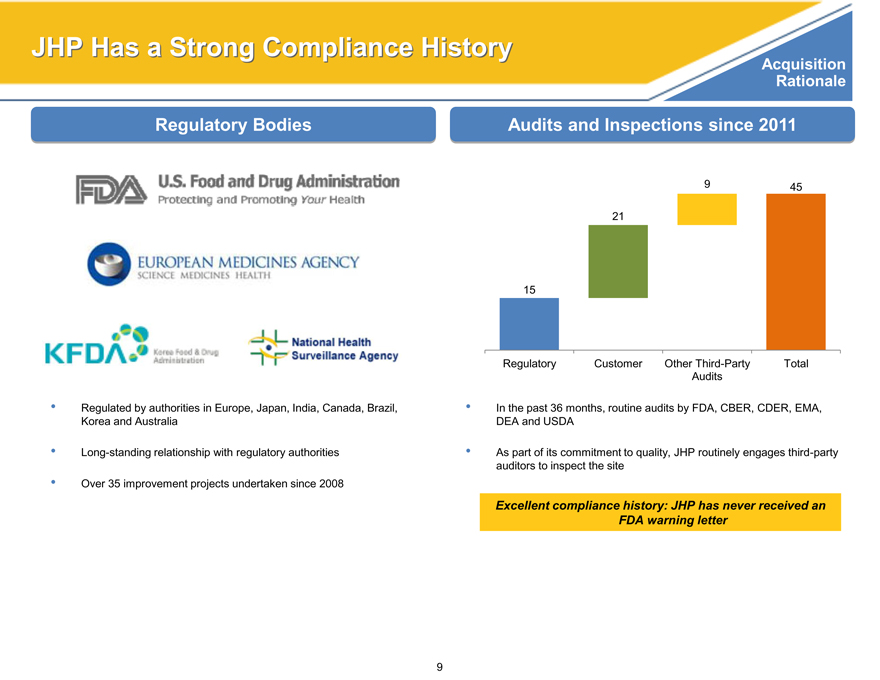
JHP Has a Strong Compliance History Acquisition
Rationale
Regulatory Bodies
Regulated by authorities in Europe, Japan, India, Canada, Brazil, Korea and Australia
Long-standing relationship with regulatory authorities
Audits and Inspections since 2011
9 45
21
15
Regulatory Customer Other Third-Party Total Audits
In the past 36 months, routine audits by FDA, CBER, CDER, EMA, DEA and USDA
As part of its commitment to quality, JHP routinely engages third-party auditors to inspect the site
Over 35 improvement projects undertaken since 2008
9
JHP’s Contract Manufacturing Platform
Acquisition Rationale
Contract Manufacturing Strategy
Consistent Business • Consistent business with long-term customer relationships
Which Covers • Absorbs excess capacity, but can be ramped down as capacity is needed for JHP’s own
Fixed Costs products
Maintains Quality • Frequent customer audits help maintain JHP’s quality and compliance standards
Standards • 6–10 customer audits each year, with customers holding JHP to very high standards
Opportunity to • Selectively use Contract Manufacturing opportunities to qualify and gain experience in new
Introduce New products and technologies
Capabilities • Training and learning can often be applied to future in-house products
Stable Customer Base • Customer base includes blue chip multinational pharmaceutical and biotech companies
10

Par Pharma
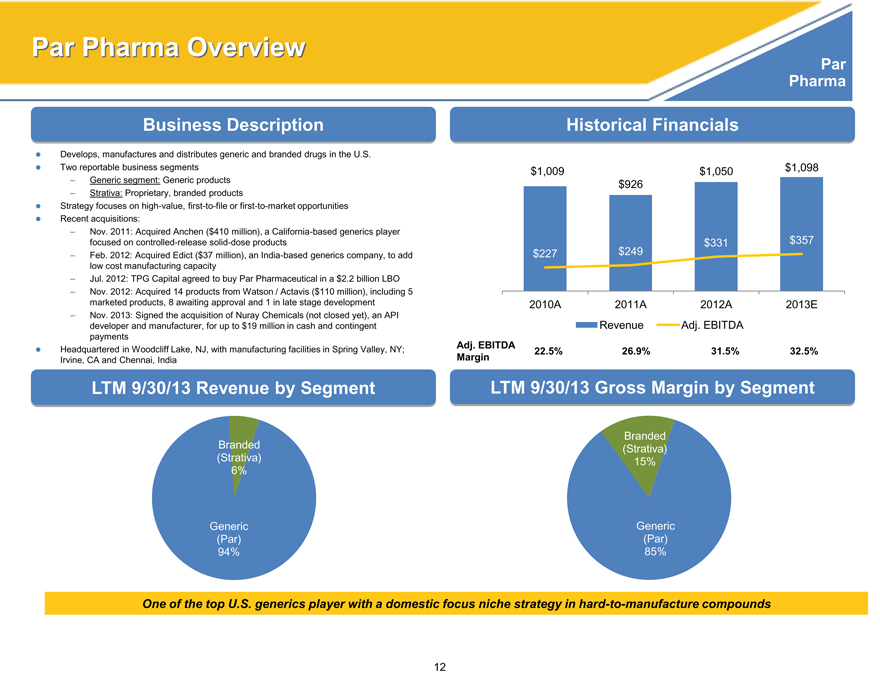
Par Pharma Overview
Par
Pharma
Business Description
Develops, manufactures and distributes generic and branded drugs in the U.S. Two reportable business segments
– Generic segment: Generic products
– Strativa: Proprietary, branded products
Strategy focuses on high-value, first-to-file or first-to-market opportunities Recent acquisitions:
– Nov. 2011: Acquired Anchen ($410 million), a California-based generics player focused on controlled-release solid-dose products
– Feb. 2012: Acquired Edict ($37 million), an India-based generics company, to add low cost manufacturing capacity
– Jul. 2012: TPG Capital agreed to buy Par Pharmaceutical in a $2.2 billion LBO
– Nov. 2012: Acquired 14 products from Watson / Actavis ($110 million), including 5 marketed products, 8 awaiting approval and 1 in late stage development
– Nov. 2013: Signed the acquisition of Nuray Chemicals (not closed yet), an API developer and manufacturer, for up to $19 million in cash and contingent payments Headquartered in Woodcliff Lake, NJ, with manufacturing facilities in Spring Valley, NY; Irvine, CA and Chennai, India
Historical Financials
$1,009 $1,050 $1,098
$ 926
$331 $357
$227 $ 249
2010A 2011A 2012A 2013E
Revenue Adj. EBITDA
Adj. EBITDA
Margin 22.5% 26.9% 31.5% 32.5%
LTM 9/30/13 Revenue by Segment
Branded
(Strativa)
6%
Generic
(Par)
94%
LTM 9/30/13 Gross Margin by Segment
Branded
(Strativa)
15%
Generic
(Par)
85%
One of the top U.S. generics player with a domestic focus niche strategy in hard-to-manufacture compounds
12
Unique Strategy and Competitive Position
Par
Pharma
Specializing in “niche,” high barrier-to-entry products: Difficult to manufacture and/or complex legal challenges; tend to be more profitable and long-lived products
Focus on first to file (FTF) or first to market (FTM): Leverages strong formulation capabilities to drive short- term profit and long-term market positioning
“Partner of choice”: Partner with large branded pharmaceutical companies and “big four” generics (who are often reticent to partner with one another)
Building a strong product pipeline: Pursuing 15-20 ANDAs / year
Manufacturing and product selection flexibilities through 3 facilities in NJ, California, and India
Strong track record of reliable supply and regulatory compliance
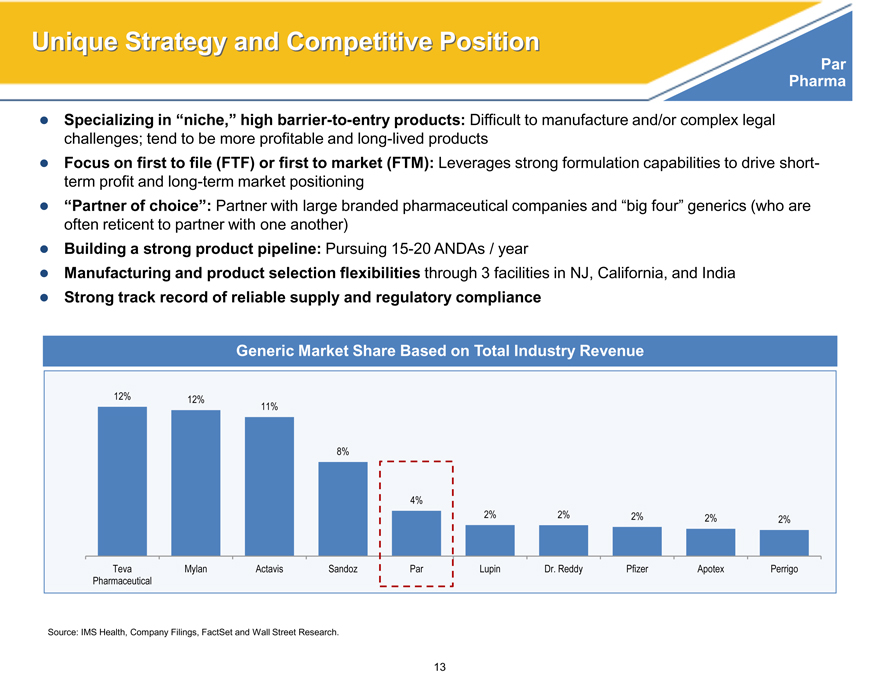
Generic Market Share Based on Total Industry Revenue
12% 12% 11%
8%
4%
2% 2% 2% 2% 2%
Teva Mylan Actavis Sandoz Par Lupin Dr. Reddy Pfizer Apotex Perrigo
Pharmaceutical
Source: IMS Health, Company Filings, FactSet and Wall Street Research.
13
Favorable Industry Dynamics and Volume Trends
Par
Pharma
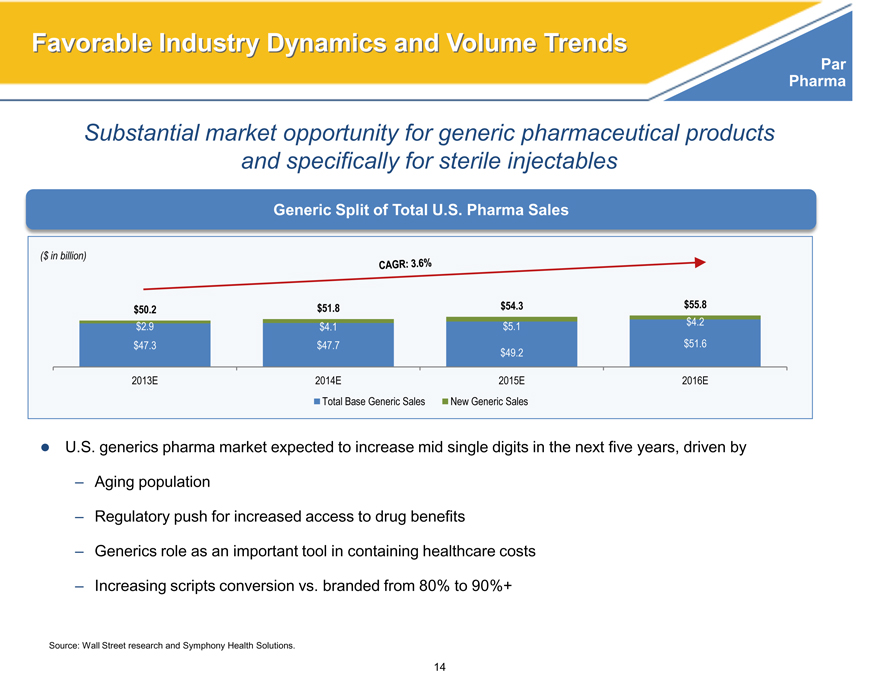
Substantial market opportunity for generic pharmaceutical products and specifically for sterile injectables
Generic Split of Total U.S. Pharma Sales
($ in billion)
$50.2 $51.8 $54.3 $55.8
$2.9 $4.1 $5.1 $4.2
$47.3 $47.7 $51.6
$49.2
2013E 2014E 2015E 2016E
Total Base Generic Sales New Generic Sales
U.S. generics pharma market expected to increase mid single digits in the next five years, driven by
– Aging population
– Regulatory push for increased access to drug benefits
– Generics role as an important tool in containing healthcare costs
– Increasing scripts conversion vs. branded from 80% to 90%+
Source: Wall Street research and Symphony Health Solutions.
14
Unique Strategy and Competitive Position
Par
Pharma
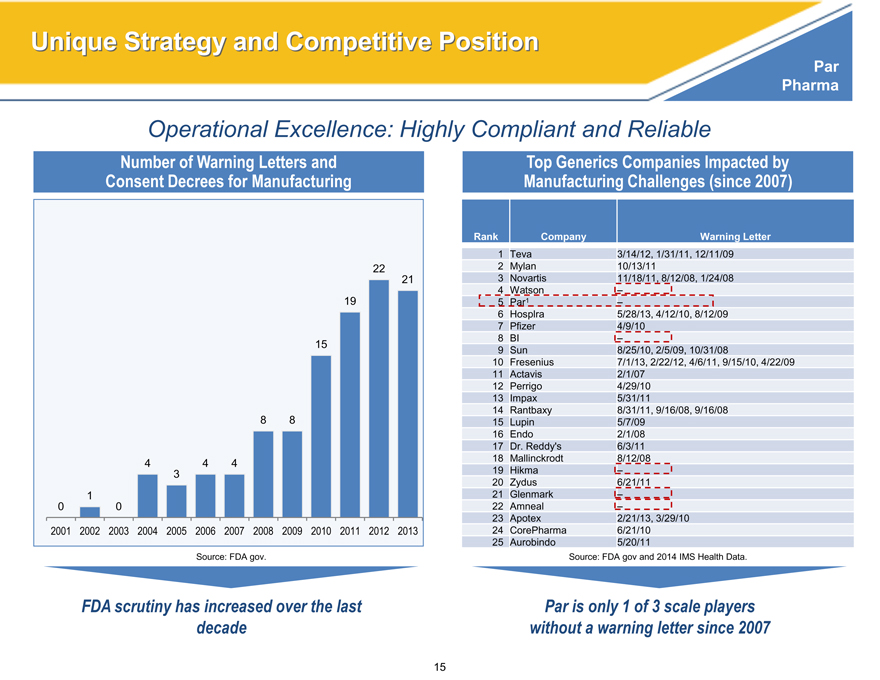
Operational Excellence: Highly Compliant and Reliable
Number of Warning Letters and
Consent Decrees for Manufacturing
22
21
19
15
8 8
4 4 4
3
1
0 0
2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013
Source: FDA gov.
FDA scrutiny has increased over the last
decade
Top Generics Companies Impacted by
Manufacturing Challenges (since 2007)
Rank Company Warning Letter
1 Teva 3/14/12, 1/31/11, 12/11/09
2 Mylan 10/13/11
3 Novartis 11/18/11, 8/12/08, 1/24/08
4 Watson –
5 Par1 –
6 Hosplra 5/28/13, 4/12/10, 8/12/09
7 Pfizer 4/9/10
8 BI –
9 Sun 8/25/10, 2/5/09, 10/31/08
10 Fresenius 7/1/13, 2/22/12, 4/6/11, 9/15/10, 4/22/09
11 Actavis 2/1/07
12 Perrigo 4/29/10
13 Impax 5/31/11
14 Rantbaxy 8/31/11, 9/16/08, 9/16/08
15 Lupin 5/7/09
16 Endo 2/1/08
17 Dr. Reddy’s 6/3/11
18 Mallinckrodt 8/12/08
19 Hikma –
20 Zydus 6/21/11
21 Glenmark –
22 Amneal –
23 Apotex 2/21/13, 3/29/10
24 CorePharma 6/21/10
25 Aurobindo 5/20/11
Source: FDA gov and 2014 IMS Health Data.
Par is only 1 of 3 scale players
without a warning letter since 2007
15
Well-positioned Portfolio of “Sticky,” Diverse
Products Par
Pharma
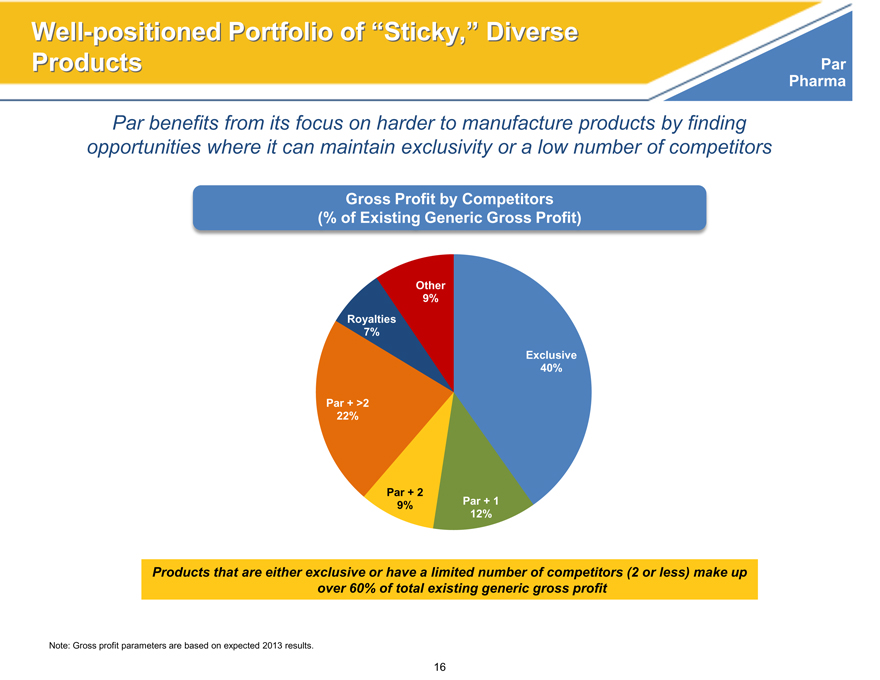
Par benefits from its focus on harder to manufacture products by finding opportunities where it can maintain exclusivity or a low number of competitors
Gross Profit by Competitors
(% of Existing Generic Gross Profit)
Other
9%
Royalties
7%
Exclusive
40%
Par + >2
22%
Par + 2
9%
Par + 1
12%
Products that are either exclusive or have a limited number of competitors (2 or less) make up over 60% of total existing generic gross profit
Note: Gross profit parameters are based on expected 2013 results.
16
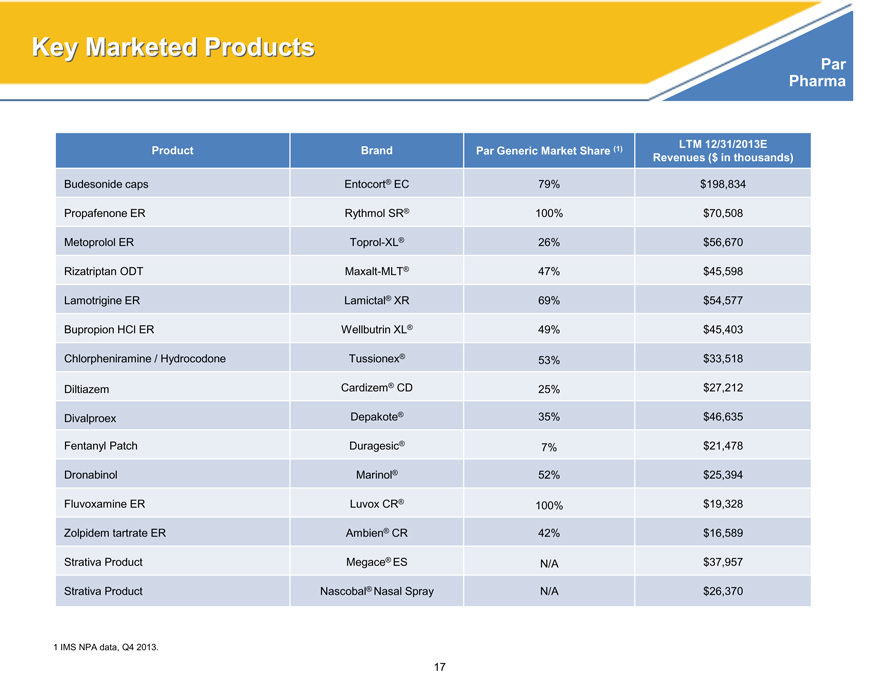
Key Marketed Products
Par
Pharma
Product Brand Par Generic Market Share (1) LTM 12/31/2013E
Revenues ($ in thousands)
Budesonide caps Entocort® EC 79% $198,834
Propafenone ER Rythmol SR® 100% $70,508
Metoprolol ER Toprol-XL® 26% $56,670
Rizatriptan ODT Maxalt-MLT® 47% $45,598
Lamotrigine ER Lamictal® XR 69% $54,577
Bupropion HCl ER Wellbutrin XL® 49% $45,403
Chlorpheniramine / Hydrocodone Tussionex® 53% $33,518
Diltiazem Cardizem® CD 25% $27,212
Divalproex Depakote® 35% $46,635
Fentanyl Patch Duragesic® 7% $21,478
Dronabinol Marinol® 52% $25,394
Fluvoxamine ER Luvox CR® 100% $19,328
Zolpidem tartrate ER Ambien® CR 42% $16,589
Strativa Product Megace® ES N/A $37,957
Strativa Product Nascobal® Nasal Spray N/A $26,370
1 IMS NPA data, Q4 2013.
17
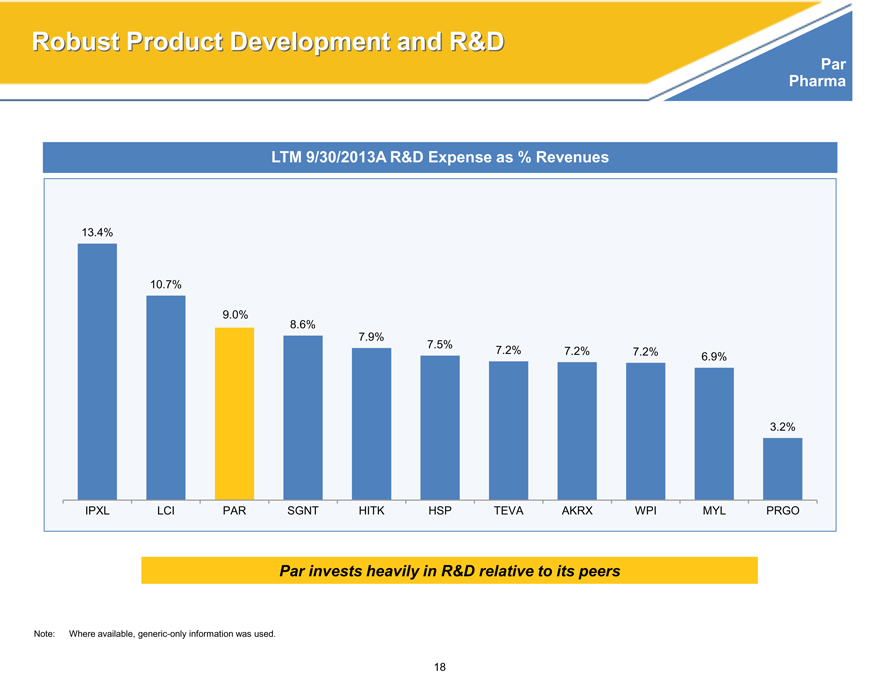
Robust Product Development and R&D
Par
Pharma
LTM 9/30/2013A R&D Expense as % Revenues
13.4%
10.7%
9.0%
8.6%
7.9%
7.5%
7.2% 7.2% 7.2% 6.9%
3.2%
IPXL LCI PAR SGNT HITK HSP TEVA AKRX WPI MYL PRGO
Par invests heavily in R&D relative to its peers
Note: Where available, generic-only information was used.
18
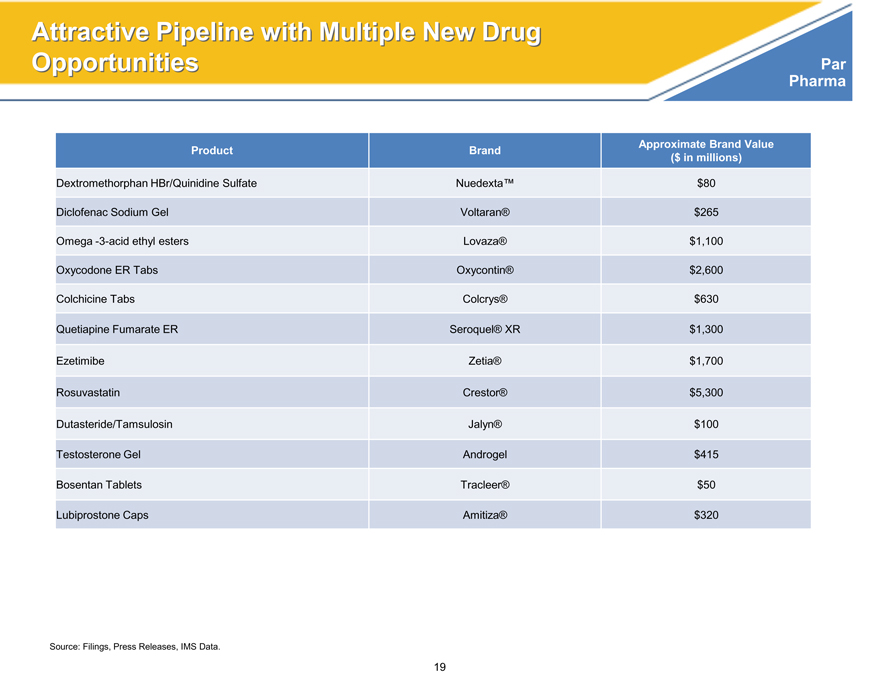
Attractive Pipeline with Multiple New Drug
Opportunities Par
Pharma
Product Brand Approximate Brand Value
($ in millions)
Dextromethorphan HBr/Quinidine Sulfate Nuedexta™ $80
Diclofenac Sodium Gel Voltaran® $265
Omega -3-acid ethyl esters Lovaza® $1,100
Oxycodone ER Tabs Oxycontin® $2,600
Colchicine Tabs Colcrys® $630
Quetiapine Fumarate ER Seroquel® XR $1,300
Ezetimibe Zetia® $1,700
Rosuvastatin Crestor® $5,300
Dutasteride/Tamsulosin Jalyn® $100
Testosterone Gel Androgel $415
Bosentan Tablets Tracleer® $50
Lubiprostone Caps Amitiza® $320
Source: Filings, Press Releases, IMS Data.
19
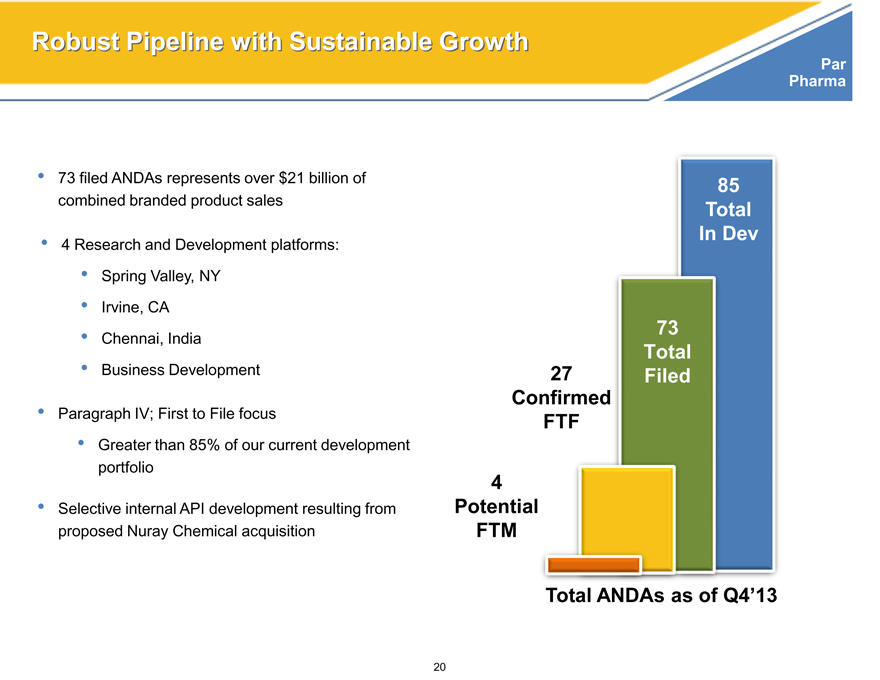
Robust Pipeline with Sustainable Growth
Par
Pharma
73 filed ANDAs represents over $21 billion of combined branded product sales
4 Research and Development platforms:
Spring Valley, NY
Irvine, CA
Chennai, India
Business Development
Paragraph IV; First to File focus
Greater than 85% of our current development portfolio
Selective internal API development resulting from proposed Nuray Chemical acquisition
85
Total
In Dev
73
Total
27 Filed
Confirmed
FTF
4
Potential
FTM
Total ANDAs as of Q4’13
20
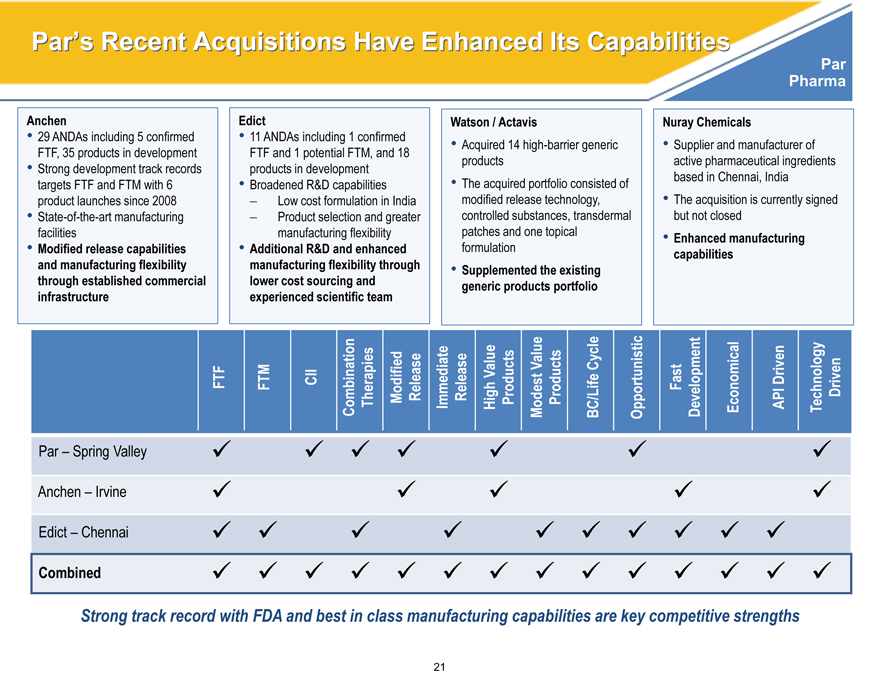
Par’s Recent Acquisitions Have Enhanced Its Capabilities
Par Pharma
Anchen Edict Watson / Actavis Nuray Chemicals
29 ANDAs including 5 confirmed 11 ANDAs including 1 confirmed Acquired 14 high-barrier generic Supplier and manufacturer of
FTF, 35 products in development FTF and 1 potential FTM, and 18
• Strong development track records products in development products active pharmaceutical ingredients
targets FTF and FTM with 6 • Broadened R&D capabilities • The acquired portfolio consisted of based in Chennai, India
product launches since 2008 – Low cost formulation in India modified release technology, • The acquisition is currently signed
State-of-the-art manufacturing – Product selection and greater controlled substances, transdermal but not closed
facilities manufacturing flexibility patches and one topical • Enhanced manufacturing
Modified release capabilities Additional R&D and enhanced formulation capabilities
and manufacturing flexibility manufacturing flexibility through • Supplemented the existing
through established commercial lower cost sourcing and generic products portfolio
infrastructure experienced scientific team
Value Value Cycle Fast Driven Driven
FTF FTM CII Combination Therapies Modified Release Immediate Release High Products Modest Products BC/Life Opportunistic Development Economical API Technology
Par – Spring Valley
Anchen – Irvine
Edict – Chennai
Combined
Strong track record with FDA and best in class manufacturing capabilities are key competitive strengths
21

Financial Overview
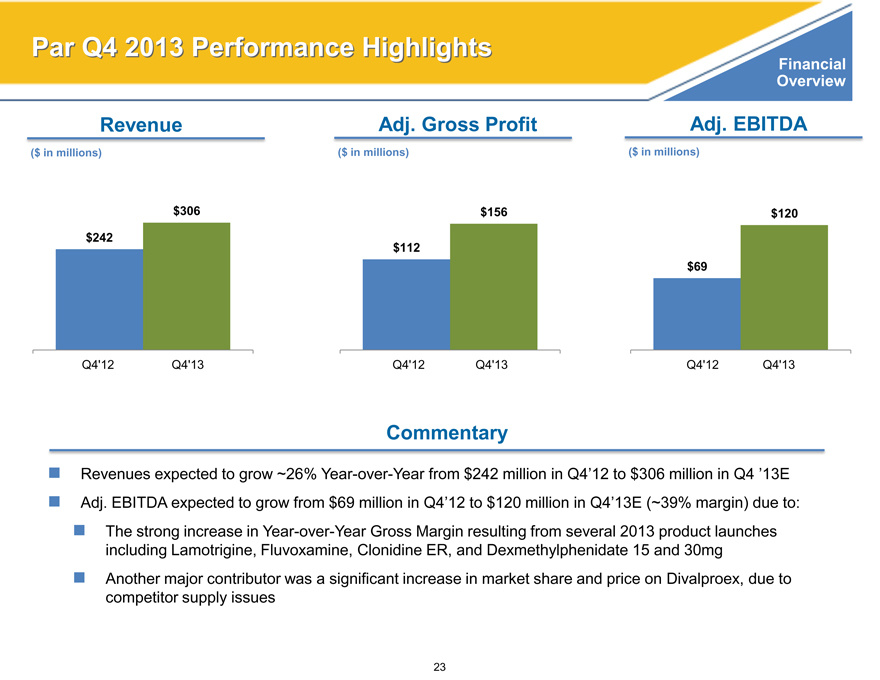
Par Q4 2013 Performance Highlights
Financial
Overview
Revenue Adj. Gross Profit Adj. EBITDA
($ in millions)($ in millions)($ in millions)
$306 $156 $120
$242
$112
$69
Q4’12 Q4’13 Q4’12 Q4’13 Q4’12 Q4’13
Commentary
Revenues expected to grow ~26% Year-over-Year from $242 million in Q4’12 to $306 million in Q4 ’13E Adj. EBITDA expected to grow from $69 million in Q4’12 to $120 million in Q4’13E (~39% margin) due to:
The strong increase in Year-over-Year Gross Margin resulting from several 2013 product launches including Lamotrigine, Fluvoxamine, Clonidine ER, and Dexmethylphenidate 15 and 30mg Another major contributor was a significant increase in market share and price on Divalproex, due to competitor supply issues
23
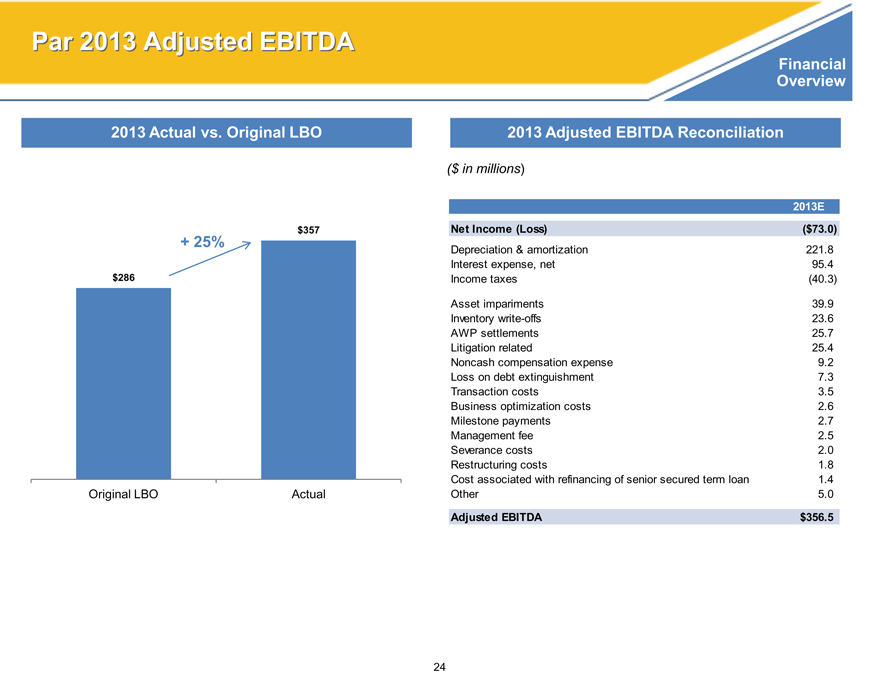
Par 2013 Adjusted EBITDA
Financial
Overview
2013 Actual vs. Original LBO
$357
+ 25%
$286
Original LBO Actual
2013 Adjusted EBITDA Reconciliation
($ in millions)
2013E
Net Income (Loss)($73.0)
Depreciation & amortization 221.8
Interest expense, net 95.4
Income taxes(40.3)
Asset impariments 39.9
Inventory write-offs 23.6
AWP settlements 25.7
Litigation related 25.4
Noncash compensation expense 9.2
Loss on debt extinguishment 7.3
Transaction costs 3.5
Business optimization costs 2.6
Milestone payments 2.7
Management fee 2.5
Severance costs 2.0
Restructuring costs 1.8
Cost associated with refinancing of senior secured term loan 1.4
Other 5.0
Adjusted EBITDA $356.5
24
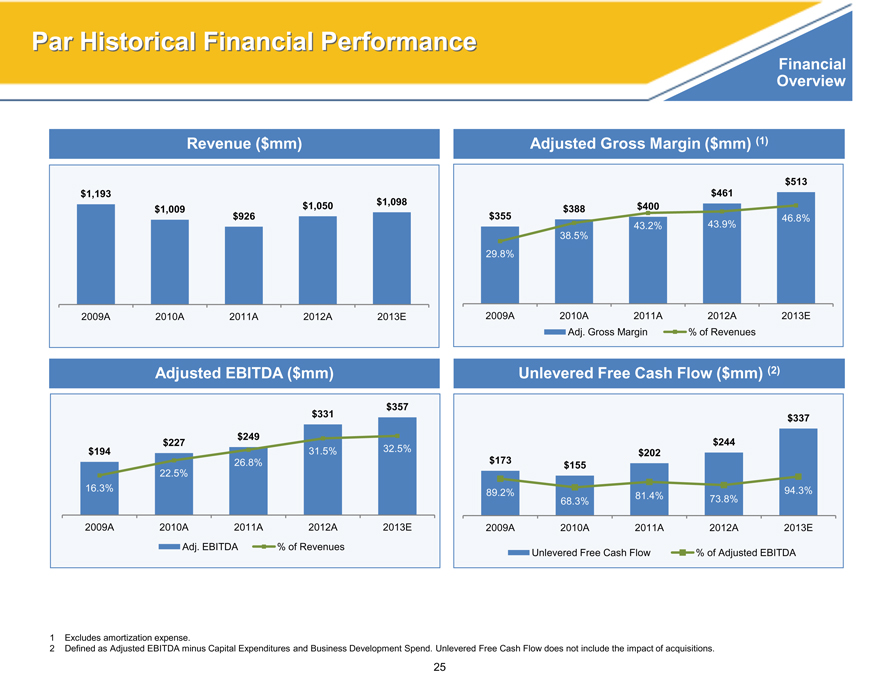
Par Historical Financial Performance
Financial
Overview
Revenue ($mm)
$1,193
$1,098
$1,009 $1,050
$926
2009A 2010A 2011A 2012A 2013E
Adjusted EBITDA ($mm)
$357
$331
$227 $249
$194 31.5% 32.5%
26.8%
22.5%
16.3%
2009A 2010A 2011A 2012A 2013E
Adj. EBITDA% of Revenues
Adjusted Gross Margin ($mm) (1)
$513
$461
$388 $400
$355 46.8%
43.2% 43.9%
38.5%
29.8%
2009A 2010A 2011A 2012A 2013E
Adj. Gross Margin% of Revenues
Unlevered Free Cash Flow ($mm) (2)
$337
$244
$202
$173 $155
89.2% 81.4% 94.3%
68.3% 73.8%
2009A 2010A 2011A 2012A 2013E
Unlevered Free Cash Flow% of Adjusted EBITDA
1 Excludes amortization expense.
2 Defined as Adjusted EBITDA minus Capital Expenditures and Business Development Spend. Unlevered Free Cash Flow does not include the impact of acquisitions.
25
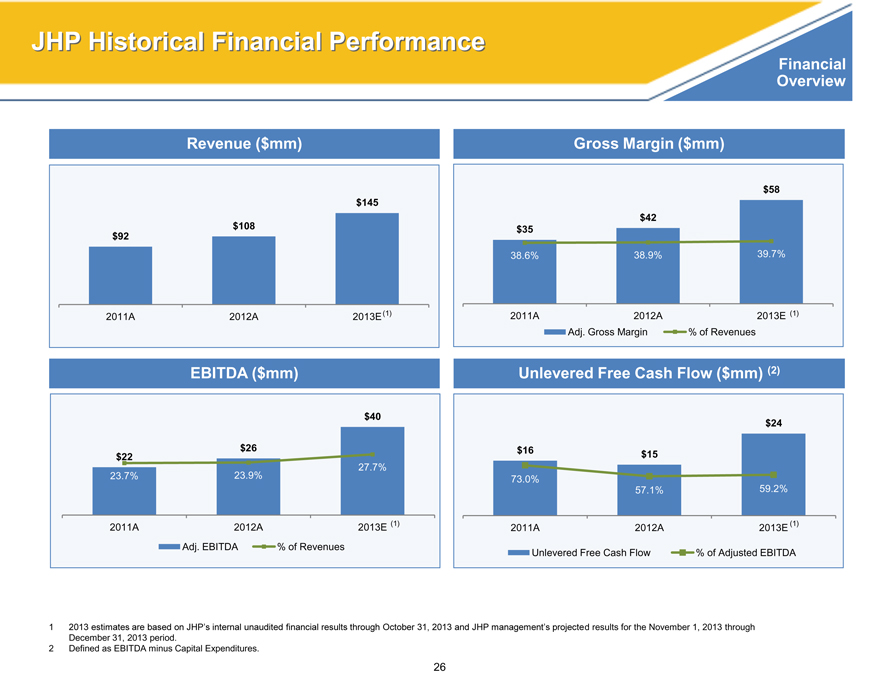
JHP Historical Financial Performance
Financial
Overview
Revenue ($mm)
$145
$108
$92
2011A 2012A 2013E (1)
EBITDA ($mm)
$40
$26
$22
27.7%
23.7% 23.9%
2011A 2012A 2013E (1)
Adj. EBITDA% of Revenues
Gross Margin ($mm)
$58
$42
$35
38.6% 38.9% 39.7%
2011A 2012A 2013E (1)
Adj. Gross Margin% of Revenues
Unlevered Free Cash Flow ($mm) (2)
$24
$16 $15
73.0%
57.1% 59.2%
2011A 2012A 2013E (1)
Unlevered Free Cash Flow% of Adjusted EBITDA
1 2013 estimates are based on JHP’s internal unaudited financial results through October 31, 2013 and JHP management’s projected results for the November 1, 2013 through
December 31, 2013 period.
2 Defined as EBITDA minus Capital Expenditures.
26

PAR pharmaceutical companies



























