
This presentation is copyright ©2006 by Alteon Inc.
Any duplication, use or distribution of this presentation is strictly prohibited without prior written authorization from Alteon Inc.
This presentation is copyright ©2006 by Alteon Inc.
Any duplication, use or distribution of this presentation is strictly prohibited without prior written authorization from Alteon Inc.
ALTEON
“The Anti-A.G.E.ing Company”
Breakthrough Medicines For Cardiovascular Aging and
Diabetic Complications
Rodman & Renshaw 3rd Annual Global Healthcare Conference
May 15, 2006
Kenneth I. Moch
President & CEO
Alteon Inc.
Noah Berkowitz, M.D., Ph.D.
President & CEO
HaptoGuard, Inc.

Safe Harbor Statement
Certain statements made in the course of this presentation may be forward-looking and involve a
number of risks and uncertainties, including, but not limited to:
Our technology and product development efforts (including the possibility that early clinical trial results
may not be predictive of results that will be obtained in large-scale testing or the possibility that any
clinical trials may not demonstrate sufficient safety and efficacy to obtain requisite approvals or result
in marketable products)
Anticipated operating losses and capital
Anticipated regulatory filing dates and clinical trial initiation dates
Our estimates regarding our capital requirements and our needs for additional financing
Our ability to obtain sufficient additional financing in near term
Uncertainties associated with obtaining and enforcing our patents and with the patent rights of others
Our selection and licensing of product candidates
Technological change and competition
Our ability to attract collaborative partners and other third parties with acceptable development,
regulatory and commercialization expertise
Our ability to form and maintain collaborative relationships, including those relating to the
development and commercialization of our product candidates
Other risks identified in Alteon’s filings with the Securities and Exchange Commission
Actual results, events or performance may differ materially. Alteon undertakes no obligation to publicly
release the result of any revision to these forward-looking statements that may be made to reflect events
or circumstances after the date hereof or to reflect the occurrence of unanticipated events.

Diabetes and Cardiovascular Synergy:
Merging Alteon and HaptoGuard
Deal Parameters
Technology Synergies
New Management Team
New Members of the Board
Renegotiated Agreement and Rights Granted to
Genentech
Small Transitional Financing
Detailed View of the Post-Merger Cardiovascular Product Pipeline
New Eyes on Alteon’s Alagebrium: CHF Patients With
Diastolic Dysfunction (Chronic)
Introducing HaptoGuard’s ALT-2074: A Pharmacogenomic
Approach to Post-MI M/M Reduction (Acute)
Diabetes and Cardiovascular Synergy
Alteon
HaptoGuard
Focus on novel
therapeutics for
cardiovascular
aging and diabetic
complications
Focus on novel
therapeutics for
inflammation in
cardiovascular
disease and diabetes

Alteon/HaptoGuard: Synergistic Technologies
With Two Phase 2 Compounds
A new company with a promising product pipeline focused on:
ALT-2074, HaptoGuard’s lead compound, a glutathione
peroxidase mimetic in development for reduction of mortality
in post-myocardial infarction patients with diabetes.
Alagebrium chloride (formally ALT-711), Alteon’s lead
compound, an Advanced Glycation End-product Crosslink
Breaker being developed for heart failure in diabetics with
diastolic dysfunction.

Alteon/HaptoGuard: A “Transforming Transaction”
A acquires all
outstanding H equity
H receives $5.3m
A common shares
(~22.5m)
G receives milestones
and royalties on
alagebrium
G receives ~13.5m
A common shares
upon conversion of
A preferred stock
G returns remaining
preferred stock, which
is cancelled
A
H
G
A sells 10.3 million
units of common stock
and warrants for ~$2.5m
G receives right of 1st
negotiation to H lead
compound
H receives A preferred
stock held by G valued
at $3.5m (= ~14.9m A
common shares)

Alteon/HaptoGuard: “The Deal”
A acquires all
outstanding H equity
H receives $5.3m
A common shares
(~22.5m)
A
H
G
A sells 10.3 million
units of common stock
and warrants for ~$2.5m
G receives right of 1st
negotiation to H lead
compound
H receives A preferred
stock held by G valued
at $3.5m (= ~14.9m A
common shares)
G receives milestones
and royalties on
alagebrium
G receives ~13.5m
A common shares
upon conversion of
A preferred stock
G returns remaining
preferred stock, which
is cancelled

Post-Merger Management Team
Upon shareholder approval, Alteon’s new management team will be as
follows:
Kenneth I. Moch, Chairman
Currently Chairman, President & CEO of Alteon
Noah Berkowitz, M.D., Ph.D., President & CEO
Currently President & CEO of HaptoGuard
Malcolm MacNab, M.D., Ph.D., Vice President of Clinical Development
Currently Chief Medical Officer of HaptoGuard
Howard B. Haimes, Ph.D, Executive Director, Preclinical Science
Currently Executive Director, Preclinical Science of Alteon

Post-Merger Board of Directors
From Alteon’s Current Board:
Kenneth I. Moch, Chairman - Director of Alteon since December 1998
President & CEO, Alteon; President & CEO, Biocyte Corporation; Mng.General Partner, Catalyst Ventures; VP,
The Liposome Company
Marilyn G. Breslow - Director of Alteon since June 1988
Former President/Analyst, W.P. Stewart; General Partner, Concord Partners; VP, Dillon, Read & Co.; Polaroid
Corp.; Peat Marwick
Thomas A. Moore - Director of Alteon since October 2001
Former President & CEO, Biopure; President & CEO, Nelson Communications; President, Procter & Gamble’s
Worldwide Prescription and OTC Healthcare Products
George M. Naimark, Ph.D - Director of Alteon since June 1999
President, Naimark & Barba; President, Naimark & Associates
From HaptoGuard’s Current Board:
Noah Berkowitz, M.D., Ph.D. - Director of HaptoGuard since November 2003
President & CEO, HaptoGuard; VP Clinical Development, IMPATH; Founder, Physician Choice
Mary Tanner - Director of HaptoGuard since January 2004
Principal and Founder, Life Sciences Partners; Senior Managing Director, Bear Stearns; Managing Director,
Lehman Brothers
Wayne P. Yetter - Director of HaptoGuard since August 2004
CEO Verispan; President and CEO, Odyssey Pharmaceuticals; Chairman & CEO, Synavant; CEO Astra
Merck; Executive at Pfizer, Merck, Novartis, IMS

Alteon Pro-forma Capitalization
Current Alteon Shares Outstanding
(including 4/06 Financing) 68.3
Genentech Common Shares upon
partial preferred stock conversion 13.5
HaptoGuard Shares
From Genentech (=$3.5 million) 14.9
From Alteon (=$5.3 million) 22.5
Total Shares Outstanding 119.2
Current Warrants and Options 11.5
New Financing Warrants 10.3
Fully Diluted Shares 141.0
Shares (Millions)

The Post-Transaction Alteon
Multi-product cardiovascular pipeline with focus
on patients with diabetes
Two distinct NCE’s in Phase 2 clinical trials
Additional management with highly complementary
cardiovascular/diabetes expertise
New Board members with extensive pharma and financing
expertise
Genentech overhang eliminated
New financing bridging towards shareholder vote

Proposed Transaction Calendar
Financing Mid-April 2006
Complete
Proxy Filed May 2006
SEC Review May-June 2006
Shareholder 3Q 2006
Vote
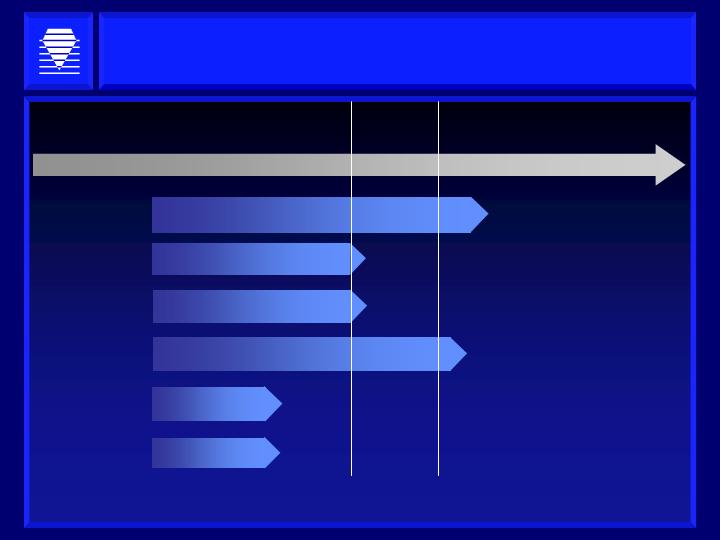
Post-Transaction Development Pipeline: Two Phase 2
Cardiovascular Compounds Plus Pipeline
Preclinical
Phase 1
Phase 2
Phase 3
NDA
Development Drugs/Indications
Alagebrium
Alagebrium
Alagebrium
ALT-2074
AGE Breakers
GPx Mimetics
Discovery
2nd Generation
Chronic Heart Failure
Nephropathy
Retinopathy
Acute Coronary Syndrome
Other
*
*
*Based on outcome of preclinical studies, may go directly to Phase 2
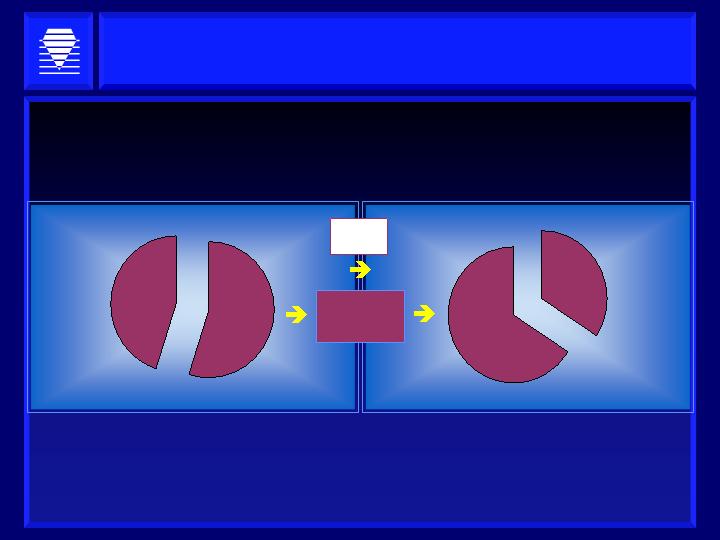
Segmenting Large Markets:
Cardiovascular Complications of Diabetes
-- Addressing Multi-billion Dollar Markets --
25- 44%
of Diabetic
Patients
Prevalence:
~5 Million (U.S.)
20-30%
Diabetic
Patients
Prevalence:
~13.9 Million (U.S.)
> $5 BILLION/YEAR
(Worldwide Estimate)
Sources: AHA; National Quality Measures Clearing House; Analyst Estimates
Alagebrium
Chronic Heart Failure
ALT-2074
Acute Coronary Syndrome
> $10 BILLION/YEAR
(Worldwide Estimate)
Mechanism
Markets
Management
Deal
Synergy

“The possibility of widespread coronary inflammation has
important implications for research and therapy. It
challenges the widely accepted hypothesis that a single
vulnerable plaque is responsible for the development of
coronary instability.”
July 2002: Widespread Coronary Inflammation in Unstable Angina
“Epidemiological and clinical studies have shown strong
and consistent relationships between markers of
inflammation and the risk of future cardiovascular events.”
2004: Inflammation as a Cardiovascular Risk Factor
Circulation, Journal of the American Heart Association
“The physiological processes of thrombosis and
inflammation should not be viewed in isolation because they
greatly influence each other.”
April 2005: New Links Between Inflammation and Thrombosis
Arteriosclerosis, Thrombosis, and Vascular Biology, Journal of
the American Heart Association
“ In addition, glycation of LDL and other lipoproteins is quite
common in diabetes, thus making the lipoproteins of
diabetic patients more susceptible to oxidation and more
atherogenic.”
Feb. 2006: Atherothrombosis, Inflammation and Diabetes
Sept. 2001: Role of Inflammatory Biomarkers in
Prediction of Coronary Heart Disease
“Early atherosclerosis has an inflammatory component
characterized by leucocytic infiltration of the vascular
endothelial wall.”
Inflammation in Chronic Heart Failure
and Acute Coronary Syndrome
The Lancet

Related Therapeutic Areas
Different Mechanisms of Action
Alagebrium
Targets Advanced Glycation
End Products (A.G.E.s)
Alagebrium breaks A.G.E.
Crosslinks
Restores structure and
function of tissues
ALT-2074
Lipid peroxides cause
inflammation
ALT-2074 metabolizes
lipid peroxides
Treats acute ischemic
injury
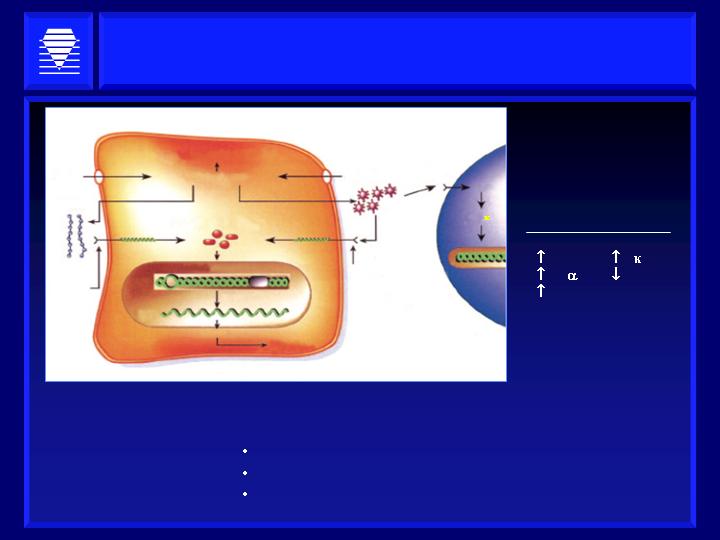
A.G.E.s Induce Inflammation
Results in Expression
of Growth Factors and
Cytokines
IL-1
TNF
TGFß
NFß
eNOS
Resulting Pathologies:
Vascular Stiffening
Chronic Heart Failure
Nephropathy
Source: Diabetes, Brownlee,
Vol. 54, June 2005
Intracellular protein glycation
AGE precursors
Glucose
Matrix
Intracellular transducers
Transcription factors
Glucose
DNA
Transcription
AGE
receptor
AGE
plasma
proteins
AGE
receptor
ROS
NF-ß
Macrophage
mesangial cell
mRNA
Proteins
Integrins
Endothelial cell
RNA
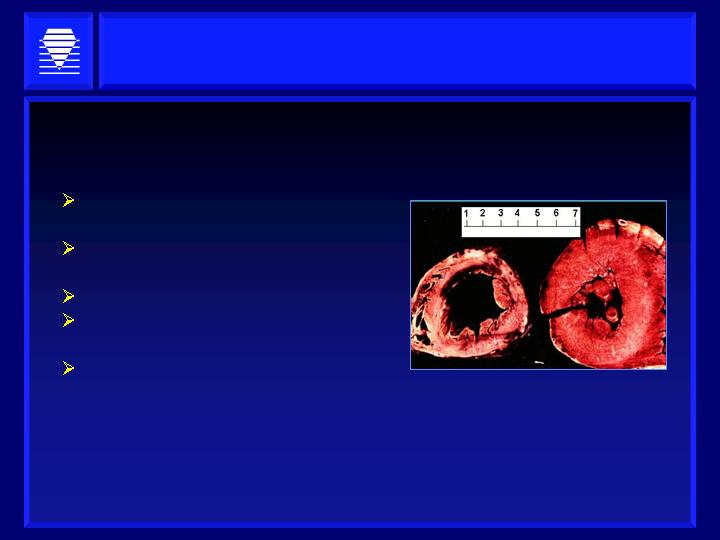
Impaired filling (elevated atrial
pressures)
Normal or impaired ejection
fraction
30-50% of all heart failure cases
70% of elderly heart failure
patients
No current therapy available
Alagebrium reverses ventricular and aortic stiffening associated
with diastolic dysfunction
Diastolic dysfunction in heart failure:
Source: William H. Luer, M.D.
Tulane School of Medicine
Rationale For Alagebrium in Heart Failure

Key Clinical Findings for Alagebrium
in Heart Failure
Meaningful reduction in left
ventricular mass (p=0.036), in
unprecedented timeframe
Marked improvement in initial
phase of left ventricular diastolic
filling (p=0.045)
Statistically significant
improvements in multiple QOL
measurements (p < 0.01)
Sickest patient population (class
III heart failure) benefited most
Source: Kitzman, Zile, et al; Presented as Poster at Society
of Geriatric Cardiology Annual Meeting, 2003
*D istensibility Improvement and
Remo deling in Diastolic Heart Failure
DIAMOND Study
Source: Thohan, Koerner, et al; Presented as Poster at the
American Heart Association Annual Meeting, 2005
Patients with Impaired Ejection Fraction and
Diastolic Dysfunction: Efficacy and
Safety Trial of Alagebrium
PEDESTAL Study
Improvements observed for:
Diastolic function (E/A, DT,
IVRT)
Hemodynamics (LAP, PASP)
LV remodeling (LAV, LVEDV,
LV mass)
NYHA score
No alterations in heart rate, blood
pressure or physical exam
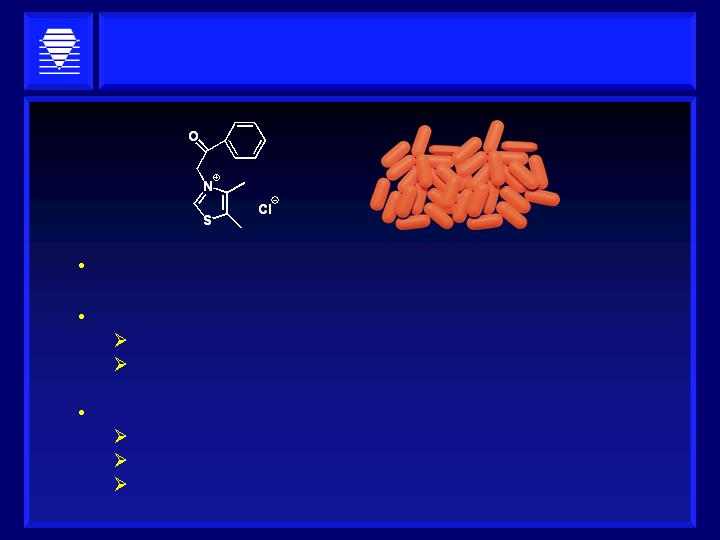
Alagebrium: A Novel “Therapeutic
Remodeling” Agent
Breaks A.G.E. Crosslinks
Phase 1 and 2 clinical trials in >1000 patients:
Safe and well tolerated
Encouraging Phase 2 data in CHF in 45 patients
Our Strategy:
Chronic heart failure indication
Diabetic patients only
HaptoGuard diagnostic test identifies highest risk
diabetic patients

Alagebrium: Phase 2b Study in High Risk
Diabetic Patients With Diastolic Heart Failure
Type Placebo Control, 3 arm
Screened with HaptoGuard
Test
# of Patients 200
Initiate 4Q 2006/1Q 2007
Duration 6 months dosing
First Interpretable Q1 2008
Results
Centers 20; U.S.; Target max 9 month
accrual
Endpoints Cardiac function, mass and
pressure, clinical endpoints
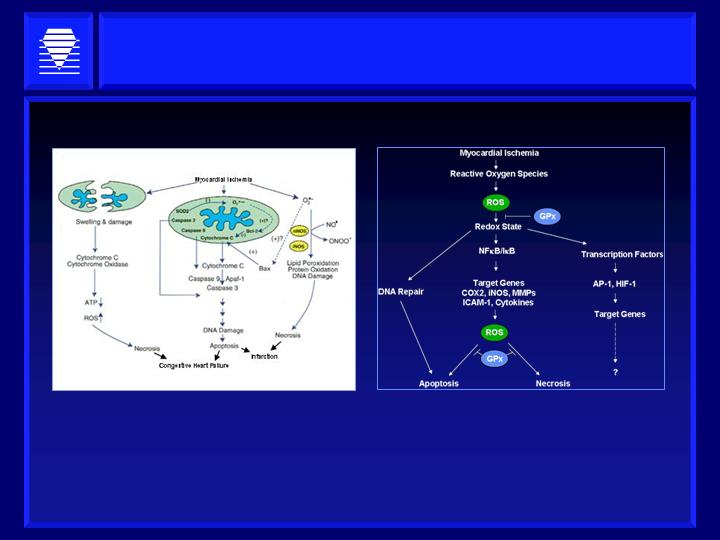
Source: Adapted from Pak H. Chan, J. Cereb Blood Flow Metab. Vol 22, No. 1, 2001
HaptoGuard Focus: Lipid
Hydroperoxides in Cardiovascular Diseases
Oxidized lipid peroxides stimulate multiple pathological
inflammatory and metabolic pathways
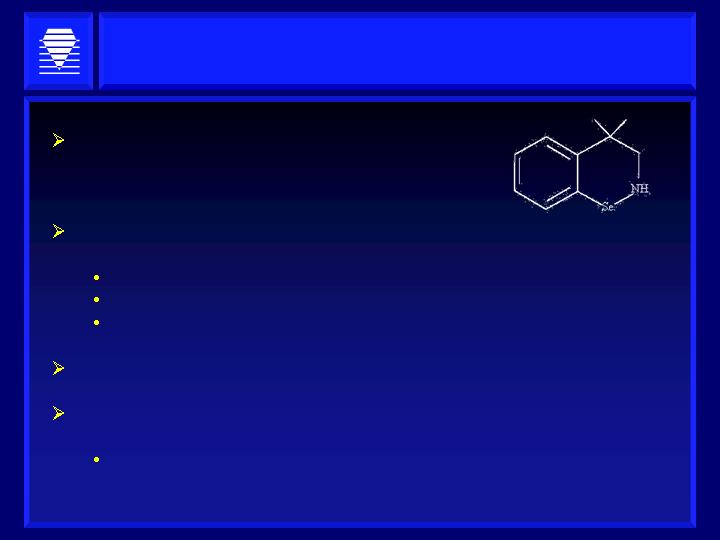
HaptoGuard’s Lead Compound
Metabolizes Oxidized Lipids
Orally Dosed Phase 2 Small Molecule
>50 patients in Phase 1 & 2 - anti-inflammation
indication
Novel Anti-Inflammatory Mechanism of Action
Glutathione Peroxidase (GPx) Mimetic
Metabolizes Lipid Peroxides
Decreases over-expression of key cytokines and messengers
Rapid Action
Restores Function
Acute, ischemia-reperfusion protection without hemodynamic
instability
Source: Diabetes 2005; 54: 2802-2806
HP 1-1
HP 2-2
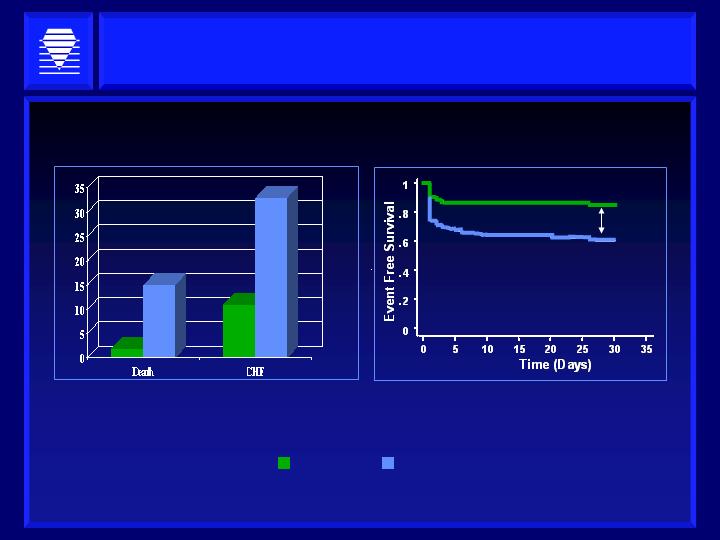
Haptoglobin Typing Predicts Clinical Event Rate
Obvious Consequences for Clinical Trials
Haptoglobin Type and 30 Days Post MI Events in Diabetics
HP 1-1
HP 2-2
1-1
1-1
2-2
2-2
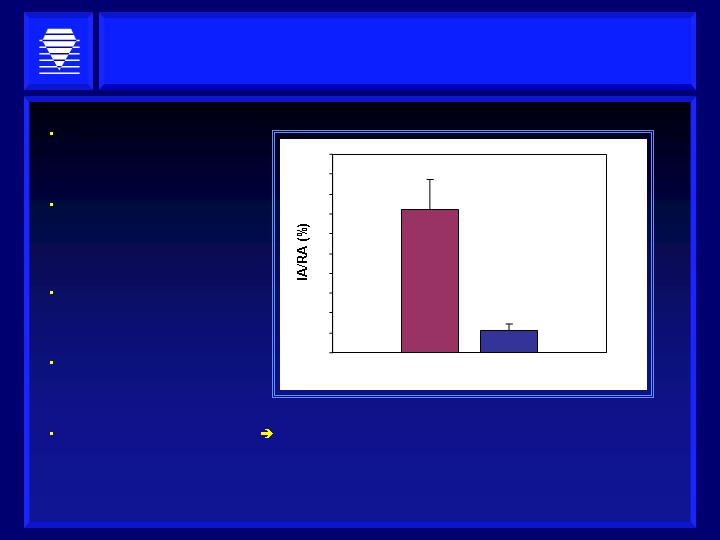
ALT-2074 Reduces MI Size in Hp 2-2 DM Mice
Mouse model for ischemia
reperfusion injury
(controlled heart attack)
High risk diabetic mice,
genetically engineered to
model the human
condition
Occlusion of the coronary
artery followed by
restoration of blood flow
Infarcts are represented
as Infarct Area/Area at
risk
0.5mg/kg to 5mg/kg of
ALT-2074 yielded similar
results
Approximately an 85% reduction in infarct size following
a single oral administration of ALT-2074
n=13 in each group
P=0.001
0
5
10
15
20
25
30
35
40
45
50
Placebo
ALT-2074

Type Placebo-controlled, 2 arm
Initiated May 1, 2006
First Interpretable Q4 2006
Results
# of Patients 60
Duration 5 days
Centers 5-10; Israel, Czech Republic
Endpoints Myocardial Damage (CK leak)
Holter, clinical events
ALT-2074: Phase 2 Study in
High Risk Diabetic Patients Undergoing PCI

Type Placebo Controlled, 3-4 arm
Initiate Q3 2006
First Interpretable Q1 2007
Results
# Patients 60-80
Duration 28 days
Centers 1-2; U.S.
Endpoints Safety and Dose Dependent
Changes in Inflammatory Markers
Status at Q1 2007 - Increased safety database; dose of
Phase 2b will be guided by anti-inflammatory marker results
ALT-2074: Multi-Dose Phase 2 Study in
High Risk Diabetic Patients
Anticipated 2006 Milestones
Q1 2006 ALT-2074 - ACC Presentation of Proprietary
Animal Model - Completed
Q2 2006 ALT-2074 - Initiate Phase 2 Study on Cardiac
Protection Following Angioplasty in ACS
Patients - Initiated May 1, 2006
Q3 2006 ALT-2074 - Initiate Phase 2 Anti-inflammatory
Biomarker Trial
Q4 2006/ Alagebrium - Initiate Phase 2b CHF Trial
Q1 2007
Q4 2006 ALT-2074 - Post Angioplasty Trial Results
Q1 2007 ALT-2074 - Anti-inflammatory Biomarker
Trial Results

ALTEON
This presentation is copyright 2006 by Alteon Inc.
Any duplication, use or distribution of this presentation is strictly prohibited without prior written authorization from Alteon Inc.
6 Campus Drive
Parsippany, NJ 07054
Tel: (201) 934-5000
Fax: (201) 934-8880
AMEX: ALT
www.alteon.com
TM
“The Anti-A.G.E.ing Company”




























