Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
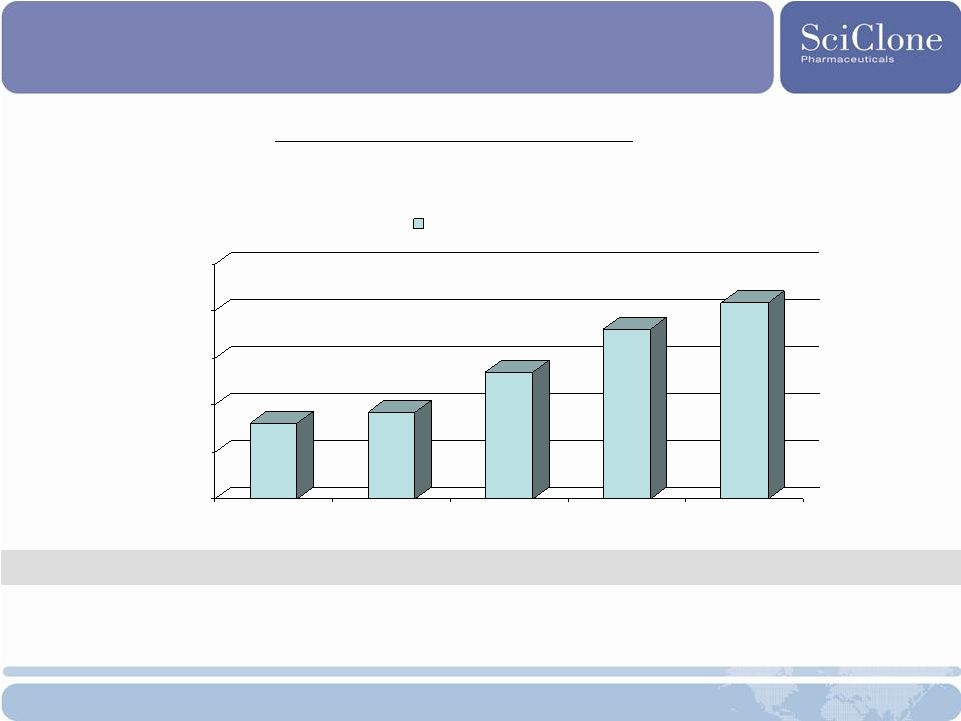
Filing tables
Filing exhibits
SCLN similar filings
- 6 May 10 Departure of Directors or Certain Officers
- 2 Mar 10 Departure of Directors or Certain Officers
- 2 Mar 10 Results of Operations and Financial Condition
- 11 Jan 10 Sciclone Provides 2009 Financial Update and Initial 2010 Sales Revenue Guidance
- 9 Nov 09 Pipeline Updates for the Third Quarter of 2009
- 6 Nov 09 Amendments to the Registrant's Code of Ethics, or Waiver of a Provision of the Code of Ethics
- 28 Oct 09 Sciclone Announces Corporate Restructuring and Workforce Reduction
Filing view
External links