QuickLinks -- Click here to rapidly navigate through this documentSCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934
| Filed by the Registrant ý |
Filed by a Party other than the Registrant o |
Check the appropriate box: |
o |
|
Preliminary Proxy Statement |
o |
|
Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
ý |
|
Definitive Proxy Statement |
o |
|
Definitive Additional Materials |
o |
|
Soliciting Material under Rule 14a-12
|
Amylin Pharmaceuticals, Inc. |
(Name of Registrant as Specified In Its Charter) |
|
(Name of Person(s) Filing Proxy Statement if Other Than the Registrant) |
| | | | | |
| Payment of Filing Fee (Check the appropriate box) |
ý |
|
No fee required. |
o |
|
Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | | 1. | | Title of each class of securities to which transaction applies:
|
| | | 2. | | Aggregate number of securities to which transaction applies:
|
| | | 3. | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined):
|
| | | 4. | | Proposed maximum aggregate value of transaction:
|
| | | 5. | | Total fee paid:
|
o |
|
Fee paid previously with preliminary materials. |
o |
|
Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
|
|
1. |
|
Amount Previously Paid:
|
| | | 2. | | Form, Schedule or Registration Statement No.:
|
| | | 3. | | Filing Party:
|
| | | 4. | | Date Filed:
|
Amylin Pharmaceuticals, Inc.
9360 Towne Centre Drive
San Diego, CA 92121
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON MAY 5, 2004
DEAR STOCKHOLDER:
NOTICE IS HEREBY GIVEN that the Annual Meeting of Stockholders ofAmylin Pharmaceuticals, Inc., a Delaware corporation, will be held on Wednesday, May 5, 2004 at 10:00 a.m. local time at our facilities located at 9360 Towne Centre Drive, San Diego, California 92121 for the following purposes:
- •
- to elect directors to serve for the ensuing year and until their successors are elected
- •
- to approve an increase in the aggregate number of shares of our common stock authorized for issuance under the 2001 Employee Stock Purchase Plan by 750,000 shares
- •
- to ratify the selection of Ernst & Young LLP as our independent auditors for the fiscal year ending December 31, 2004
- •
- to transact such other business as may properly come before the Annual Meeting or any adjournment or postponement thereof
The foregoing items of business are more fully described in the proxy statement accompanying this Notice.
The Board of Directors has fixed the close of business on March 12, 2004 as the record date for the determination of stockholders entitled to vote at the Annual Meeting and at any adjournment or postponement thereof. If you are unable to attend the Annual Meeting, you may listen to a webcast of it on our website,www.amylin.com.
| | | By Order of the Board of Directors |
|
|
 |
| | | Ginger L. Graham
President and Chief Executive Officer |
San Diego, California
April 12, 2004
ALL STOCKHOLDERS ARE CORDIALLY INVITED TO ATTEND THE MEETING IN PERSON. WHETHER OR NOT YOU EXPECT TO ATTEND THE MEETING, PLEASE VOTE VIA TELEPHONE, INTERNET OR MAIL. IF YOU VOTE VIA TELEPHONE OR INTERNET, USE THE INSTRUCTIONS ON THE ENCLOSED PROXY CARD. IF YOU CHOOSE TO VOTE VIA MAIL, PLEASE COMPLETE, DATE, SIGN AND RETURN THE ENCLOSED PROXY AS PROMPTLY AS POSSIBLE IN ORDER TO ENSURE YOUR REPRESENTATION AT THE MEETING. A RETURN ENVELOPE (WHICH IS POSTAGE PREPAID IF MAILED IN THE UNITED STATES) IS ENCLOSED FOR THAT PURPOSE. EVEN IF YOU HAVE GIVEN YOUR PROXY, YOU MAY STILL VOTE IN PERSON IF YOU ATTEND THE MEETING. PLEASE NOTE, HOWEVER, THAT IF YOUR SHARES ARE HELD OF RECORD BY A BROKER, BANK OR OTHER NOMINEE AND YOU WISH TO VOTE AT THE MEETING, YOU MUST OBTAIN FROM THE RECORD HOLDER A PROXY ISSUED IN YOUR NAME.
|
Amylin Pharmaceuticals, Inc.
9360 Towne Centre Drive
San Diego, CA 92121
PROXY STATEMENT
FOR ANNUAL MEETING OF STOCKHOLDERS
May 5, 2004
INFORMATION CONCERNING SOLICITATION AND VOTING
GENERAL
The enclosed proxy is solicited on behalf of the Board of Directors (the "Board") of Amylin Pharmaceuticals, Inc., a Delaware corporation ("Amylin" or the "Company"), for use at our Annual Meeting of Stockholders to be held on Wednesday, May 5, 2004 at 10:00 a.m. local time (the "Annual Meeting"), or at any adjournment or postponement thereof, for the purposes set forth below and in the accompanying Notice of Annual Meeting. The Annual Meeting will be held at our offices located at 9360 Towne Centre Drive, San Diego, California 92121. If you are unable to attend the Annual Meeting, you may listen to a webcast of it on our website,www.amylin.com. We intend to mail this proxy statement and accompanying proxy card on or about April 12, 2004, to all stockholders entitled to vote at the Annual Meeting.
SOLICITATION
We will bear the entire cost of solicitation of proxies, including preparation, assembly, printing and mailing of this proxy statement, the proxy card and any additional information furnished to stockholders. Copies of solicitation materials will be furnished to banks, brokerage houses, fiduciaries and custodians holding in their names shares of our common stock beneficially owned by others to forward to such beneficial owners. We may reimburse persons representing beneficial owners of our common stock for their costs of forwarding solicitation materials to such beneficial owners. Original solicitation of proxies by mail may be supplemented by telephone, telegram or personal solicitation by directors, officers or other employees of Amylin. No additional compensation will be paid to directors, officers or other employees for such services. In addition, we have retained Georgeson Shareholder Communications Inc. to assist in the distribution of proxy materials and solicitation of votes for a fee of $6,000, plus reimbursement of out-of-pocket expenses.
VOTING RIGHTS AND OUTSTANDING SHARES
Only holders of record of our common stock at the close of business on March 12, 2004 will be entitled to notice of and to vote at the Annual Meeting. At the close of business on March 12, 2004, we had outstanding and entitled to vote 93,847,728 shares of our common stock.
Each holder of record of our common stock on the record date will be entitled to one vote for each share held on all matters to be voted upon at the Annual Meeting.
A quorum of stockholders is necessary to hold a valid meeting. A quorum will be present if at least a majority of the outstanding shares are represented by votes at the Annual Meeting or by proxy. All votes will be tabulated by the inspector of election appointed for the Annual Meeting, who will separately tabulate affirmative and negative votes, abstentions and broker non-votes. Abstentions will
1
be counted towards the tabulation of votes cast on proposals presented to the stockholders and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether a proposal has been approved.
VOTING VIA THE INTERNET OR BY TELEPHONE
Stockholders may grant a proxy to vote their shares by means of the enclosed proxy card, by telephone or on the Internet. The law of Delaware, under which we are incorporated, specifically permits electronically transmitted proxies, provided that each such proxy contains or is submitted with information from which the inspector of election can determine that such proxy was authorized by the stockholder.
Please refer to your proxy card to determine if you are eligible to vote either by telephone or via the Internet.
DELIVERY OF THIS PROXY STATEMENT
We have adopted "householding," a procedure under which stockholders of record who have the same address will receive only one copy of our annual report and proxy statement unless one or more of these stockholders notifies us that they wish to continue receiving individual copies. This procedure saves printing and postage costs by reducing duplicative mailings. Stockholders who participate in householding will continue to receive separate proxy cards. Beneficial stockholders can request information about householding from their banks, brokers, other holders of record, or our Investor Relations Department. If you participate in householding and wish to receive a separate copy of our 2003 annual report and proxy statement, or if you wish to receive separate copies of future annual reports and proxy statements, please call us at 858-552-2200, extension 7299 or write to: Investor Relations, 9360 Towne Centre Drive, Suite 110, San Diego, California 92121. We will deliver the requested documents to you promptly upon your request.
REVOCABILITY OF PROXIES
Any person giving a proxy pursuant to this solicitation has the power to revoke it at any time before it is voted. It may be revoked by filing with our Secretary at our principal executive office, 9360 Towne Centre Drive, Suite 110, San Diego, California 92121, a written notice of revocation or a duly executed proxy bearing a later date, or it may be revoked by attending the Annual Meeting and voting in person. Attendance at the Annual Meeting will not, by itself, revoke a proxy.
FUTURE STOCKHOLDER PROPOSALS
The deadline for submitting a stockholder proposal for inclusion in our proxy statement and form of proxy for our 2005 annual meeting of stockholders pursuant to Rule 14a-8 promulgated by the Securities and Exchange Commission, or SEC, is December 23, 2004. Stockholder proposals submitted outside the processes of Rule 14a-8 will also be considered untimely if submitted after December 23, 2004. Stockholders are also advised to review our Bylaws, which contain additional requirements with respect to the advance notice provisions for stockholder proposals and director nominations.
2
PROPOSAL 1
ELECTION OF DIRECTORS
There are nine nominees for the nine Board positions presently authorized. Each director to be elected will hold office until the next annual meeting of stockholders and until a successor is elected and has qualified, or until such director's earlier death, resignation or removal. Each nominee listed below is currently a director of Amylin, with eight directors having been previously elected by the stockholders and one director, Mr. Joseph P. Sullivan, having been elected by the Board. Mr. Sullivan was recommended as a director nominee by one of our independent directors.
Directors are elected by a plurality of the votes present in person or represented by proxy and entitled to vote. The candidates receiving the highest number of affirmative votes of the shares entitled to be voted will be elected directors of Amylin. Shares represented by executed proxies will be voted, if authority to do so is not withheld, for the election of the nine nominees named below. In the event that any nominee should be unavailable for election as a result of an unexpected occurrence, such shares will be voted for the election of such substitute nominee as management may propose. Each person nominated for election has agreed to serve if elected and management has no reason to believe that any nominee will be unable to serve.
THE BOARD OF DIRECTORS RECOMMENDS
A VOTE IN FAVOR OF EACH NAMED NOMINEE.
NOMINEES
The names of the nominees and certain information about them as of March 1, 2004 are set forth below:
Name
| | Age
| | Position
|
|---|
| Ginger L. Graham(4) | | 48 | | President, Chief Executive Officer and Director |
| Joseph C. Cook, Jr.(4) | | 62 | | Chairman of the Board |
| Vaughn D. Bryson(1)(3) | | 65 | | Director |
| Howard E. Greene, Jr.(2)(3)(4) | | 61 | | Director |
| Terrance H. Gregg(1)(3) | | 55 | | Director |
| Jay S. Skyler, M.D.(3) | | 57 | | Director |
| Joseph P. Sullivan(2)(3)(4) | | 61 | | Director |
| Thomas R. Testman(2)(3)(4) | | 67 | | Director |
| James N. Wilson(1)(3) | | 60 | | Director |
- (1)
- Member of the Compensation and Human Resources Committee.
- (2)
- Member of the Audit Committee.
- (3)
- Member of the Nominating and Governance Committee.
- (4)
- Member of the Finance Committee.
Ms. Graham was named President and Chief Executive Officer effective September 1, 2003. Ms. Graham has served as a director since November 1995 and currently serves on the Finance Committee. She previously served on the Audit Committee and the Nominating and Governance Committee. From April 2002 until June 2003, Ms. Graham served as Advisor to the President for Guidant Corporation, a medical technology company. From February 2000 until April 2002, Ms. Graham served as Group Chairman, Office of the President with responsibility for global geographically based operations. Prior to this role, Ms. Graham served as President of the Vascular Intervention Group and Vice President, Guidant. In 1993, Ms. Graham was named President and CEO of Advanced Cardiovascular Systems (ACS). Prior to joining ACS, she held various positions with Eli
3
Lilly and Company from 1979 to 1992 including sales, marketing and strategic planning positions. She serves on the board of directors of Millennium Pharmaceuticals, Inc., the Harvard Business School Health Advisory Board, the Advisory Board for the Kellogg Center for Executive Women, and the University of California, San Diego Health Sciences Advisory Board. Ms. Graham received an M.B.A. from Harvard University.
Mr. Cook has been our Chairman of the Board since March 1998. He currently serves on our Finance Committee. He served as Chief Executive Officer from March 1998 until September 2003. From 1994 to 1998, Mr. Cook served as a member of our Board and a consultant to us. Mr. Cook is a founder and serves as Chairman of the Board of Microbia, Inc., a privately held biotechnology company. He also serves as a director of Corcept Therapeutics, Inc., a privately held biotechnology company. Mr. Cook is also a founder of Mountain Group Capital, LLC, Clinical Products, Inc., Cambrian Associates, LLC, and Mountain Ventures, Inc. Mr. Cook also serves on the boards of the American Diabetes Research Foundation, the Advisory Board of the College of Engineering, University of Tennessee and the Board of Trustees for Louisville Presbyterian Theological Seminary. Mr. Cook retired as a Group Vice President of Eli Lilly & Company in 1993 after more than 28 years of service. Mr. Cook received a B.S. in Engineering from the University of Tennessee.
Mr. Bryson has served as a director since July 1999 and serves on the Compensation and Human Resources Committee and the Nominating and Governance Committee. Mr. Bryson was a thirty-two year employee of Eli Lilly & Company and retired as its President and Chief Executive Officer in 1993. He was Executive Vice President from 1986 until 1991, and served as a member of Eli Lilly's board of directors from 1984 until his retirement in 1993. Mr. Bryson was Vice Chairman of Vector Securities International from April 1994 to 1996. Mr. Bryson is President of Clinical Products, Inc., which develops and markets medical foods for people with diabetes and obesity. He serves on the board of directors of AtheroGenics, Inc., ICOS Corporation and Chiron Corporation. Mr. Bryson received a B.S. in Pharmacy from the University of North Carolina and completed the Sloan Program at the Stanford University Graduate School of Business.
Mr. Greene is our co-founder and has served as a director since our inception in September 1987. Mr. Greene serves on the Audit Committee, the Nominating and Governance Committee, and the Finance Committee. Mr. Greene is an entrepreneur who has participated in the founding and/or management of eleven medical technology companies over two decades, including three companies for which he served as chief executive officer. From September 1987 to July 1996, Mr. Greene served as our Chief Executive Officer. He was a full-time employee of Amylin from September 1989 until September 1996, and a part-time employee until March 1998. From October 1986 until July 1993, Mr. Greene was a founding general partner of Biovest Partners, a seed venture capital firm. He was Chief Executive Officer of Hybritech from March 1979 until its acquisition by Eli Lilly & Company in March 1986, and he was co-inventor of Hybritech's patented monoclonal antibody assay technology. Prior to joining Hybritech, he was an executive with the medical diagnostics division of Baxter Healthcare Corporation from 1974 to 1979 and a consultant with McKinsey & Company from 1967 to 1974. He is Chairman of the Board of Epimmune, Inc. and a director of Biosite Incorporated. Mr. Greene received an M.B.A. from Harvard University.
Mr. Gregg has served as a director since October 2001 and serves on the Compensation and Human Resources Committee and the Nominating and Governance Committee. Mr. Gregg currently serves as a senior advisor to the diabetes business of Medtronic, Inc., a medical technology company. In July 2002, Mr. Gregg retired as Vice President of Medtronic and as President of Medtronic MiniMed, positions he had held since August 2001. Mr. Gregg previously served as President and Chief Operating Officer of Minimed Inc. from October 1996 until its acquisition by Medtronic in August 2001. Mr. Gregg joined Minimed as Vice President of Regulatory Affairs and Clinical Research in September 1994 and in 1995 was promoted to Executive Vice President, Operations. Prior to joining Minimed, Mr. Gregg spent the preceding nine years as Vice President of Governmental Affairs for
4
Ioptex Research, the ophthalmic surgical products subsidiary of Smith & Nephew, plc. Prior to joining Ioptex Research, Mr. Gregg was responsible for Regulatory Affairs, Clinical Research and Quality Assurance for divisions of Allergan, Inc. Mr. Gregg serves on the board of directors of Ocular Sciences, Inc., a manufacturer of contact lenses, and Vasogen, Inc., a developer of immune modulation therapies for treatment of various diseases. Mr. Gregg is also an Ambassador to the President of the University of Southern California, and serves as the Chairman of the American Diabetes Association Research Foundation Board. Mr. Gregg received a B.S. in Zoology from Colorado State University.
Dr. Skyler has served as a director since August 1999 and serves on the Nominating and Governance Committee. He is Professor of Medicine, Pediatrics and Psychology, Director of the Division of Endocrinology, Diabetes and Metabolism and Director of the General Clinical Research Center at the University of Miami in Florida, where he has been employed since 1976. He is also Study Chairman for the National Institute of Diabetes & Digestive & Kidney Diseases of the Type 1 Diabetes TrialNet clinical trial network, and serves on the board of directors of Dexcom, Inc. Dr. Skyler has served as President of the American Diabetes Association, and as Vice President of the International Diabetes Federation. Dr. Skyler serves on the editorial board of diabetes and general medicine journals. He received his B.S. from Pennsylvania State University, his M.D. from Jefferson Medical College, and completed postdoctoral studies at Duke University Medical Center.
Mr. Sullivan has served as a director since September 2003 and serves on the Audit Committee, the Nominating and Governance Committee, and the Finance Committee. Mr. Sullivan is currently Chairman of the Board of Advisors of RAND Health and Vice Chairman of the Board of the UCLA Medical Center. From 2000 to 2003, Mr. Sullivan served as Chairman, Chief Executive Officer and a director of Protocare, Inc. From 1993 until November 1999, he served as Chairman, Chief Executive Officer and a director of American Health Properties, Inc. For the previous twenty years, Mr. Sullivan was an investment banker with Goldman Sachs. Mr. Sullivan also currently serves on the board of directors of SCCI, Inc. (a private long-term acute care hospital company), Covenant Care, Inc. (a private nursing home company), and Health Care Property Investors, Inc. (a real estate investment trust). Mr. Sullivan received his M.B.A. from Harvard Graduate School of Business Administration and his J.D. from the University of Minnesota Law School.
Mr. Testman has served as a director since December 2002 and serves on the Audit Committee, the Nominating and Governance Committee, and the Finance Committee. Mr. Testman is a former managing partner of Ernst & Young, LLP where, during his tenure from 1962 to 1992, he served as managing partner of both Health Care Services and Management Consulting Services for the West Coast and national practices. He also served as an area managing partner for the audit and tax practice. Mr. Testman currently serves on the board of directors of Endocare, Inc. and is Chairman of the Board of Specialty Laboratories, Inc. He formerly served as a director at MiniMed Inc., ChromaVision Medical Systems, Inc., Peregrine Pharmaceuticals, Inc. and Nichols Institute. He also serves on the board of four privately held companies. He received an M.B.A. from Trinity University and is a certified public accountant (retired).
Mr. Wilson has served as a director since March 2002 and serves on the Compensation and Human Resources Committee and the Nominating and Governance Committee. He is a director and Chairman of the Board of Corcept Therapeutics Incorporated. From 1996 to 2001, Mr. Wilson was Chairman of the Board of Amira Medical, Inc. From 1990 to 1994, Mr. Wilson served as President and Chief Operating Officer of Syntex Corporation. Prior to 1990, he served in various senior management positions, including Chief Executive Officer for Neurex Corporation and LifeScan, Inc. Mr. Wilson serves on the board of directors of the American Diabetes Association Research Foundation, the Palo Alto Medical Foundation, A Stepping Stone Foundation (pre-school education) and the Insight Prison Project (rehabilitation for San Quentin inmates). Mr. Wilson received his B.A. and his M.B.A. from the University of Arizona.
5
BACKGROUND OF EXECUTIVES NOT LISTED ABOVE
The names of and certain information regarding our executives as of March 1, 2004 are set forth below:
Name
| | Age
| | Position
|
|---|
| Daniel M. Bradbury | | 42 | | Chief Operating Officer |
| Alain D. Baron, M.D | | 50 | | Senior Vice President, Clinical Research |
| Martin R. Brown | | 57 | | Senior Vice President, Operations |
| Joann L. Data, M.D., Ph.D. | | 59 | | Senior Vice President, Regulatory Affairs and Quality Assurance |
| Dwayne M. Elwood | | 56 | | Senior Vice President, Marketing |
| Orville G. Kolterman, M.D. | | 56 | | Senior Vice President, Clinical Affairs |
| Craig A. Eberhard | | 44 | | Vice President, Sales |
| Mark G. Foletta | | 43 | | Vice President, Finance and Chief Financial Officer |
| Michael R. Hanley, Ph.D. | | 52 | | Vice President, Discovery Research |
| Joni Harvey | | 49 | | Vice President, Quality Assurance |
| Lloyd A. Rowland | | 47 | | Vice President, Legal, General Counsel and Secretary |
| Michael D. Step | | 44 | | Vice President, Corporate Development |
| Gregg Stetsko, Ph.D | | 47 | | Vice President, Product Development |
| Andrew A. Young, M.D., Ph.D. | | 51 | | Vice President and Senior Research Fellow |
Mr. Bradbury, one of our executive officers, has served as our Chief Operating Officer since June 2003, and previously served as Executive Vice President since June 2000. He previously served as Senior Vice President, Corporate Development from April 1998 to June 2000 and as Vice President of Marketing from June 1995 to April 1998. From July 1994 to May 1995, Mr. Bradbury, a native of the United Kingdom, served as Director of Marketing for Amylin Europe Limited. Prior to joining us, Mr. Bradbury was employed by SmithKline Beecham Pharmaceuticals from September 1984 to July 1994, where he held a number of positions, most recently as Associate Director, Anti-Infectives in the Worldwide Strategic Product Development Division. He is a director of Illumina, Inc. and Peninsula Pharmaceuticals, Inc. Mr. Bradbury is a member of the Royal Pharmaceutical Society of Great Britain and serves on the Advisory Council of the Keck Graduate Institute and the University of California-San Diego Leadership Council. He received a Bachelor of Pharmacy from Nottingham University and a Diploma in Management Studies from Harrow and Ealing Colleges of Higher Education.
Dr. Baron, one of our executive officers, has served as our Senior Vice President, Clinical Research since June 2002. He previously served as Vice President, Clinical Research since December 1999. Dr. Baron has been clinical Professor of Medicine at the University of California, San Diego, and Clinical VA Staff Physician at the VA Medical Center, San Diego, since 2001. From 1989 to 2000, Dr. Baron worked for the Indiana University School of Medicine, where he served as Professor of Medicine and Director, Division of Endocrinology and Metabolism. Earlier, Dr. Baron held academic and clinical positions in the Division of Endocrinology and Metabolism at the University of California, San Diego, and the Veterans Administration Medical Center in San Diego. He is the recipient of several prestigious awards for his research in diabetes and vascular disease, including the 1996 Outstanding Clinical Investigator Award from the American Federation for Medical Research, several awards from the American Diabetes Association, and is a current National Institutes of Health MERIT award recipient. He earned his M.D. from the Medical College of Georgia, Augusta, and completed postdoctoral studies at the University of California, San Diego.
Mr. Brown, one of our executive officers, has served as Senior Vice President, Operations since March 2000. Mr. Brown previously served as Vice President, Operations from October 1998 to March 2000, and as Senior Director, Information Technology from May 1994 to October 1998. Prior to joining us, Mr. Brown was Director, Information Systems, Europe, for Eli Lilly from 1989 to 1993.
6
From 1988 to 1989, Mr. Brown was Director, Information Systems for the Medical Devices and Diagnostics Division of Eli Lilly; he served as Director, Information Systems of IVAC Corporation, one of the seven companies in that division, from 1983 to 1988. Mr. Brown received a B.S. in Commerce and Engineering and an M.B.A. in Operations Research from Drexel University.
Dr. Data, one of our executive officers, has served as Senior Vice President, Regulatory Affairs and Quality Assurance since August 1999. From 1996 to 1999, Dr. Data served as an officer of CoCensys, most recently as Executive Vice President, Product Development and Regulatory Affairs. From 1990 to 1996, Dr. Data held several positions at The Upjohn Company, most recently as Corporate Vice President for Pharmaceutical Regulatory Affairs and Project Management. Previously, she held a number of positions at Hoffmann-La Roche, including Vice President of Clinical Research and Development. Dr. Data is a director of Stressgen Biotechnology Company. She earned her M.D. from Washington University School of Medicine and her Ph.D. in Pharmacology from Vanderbilt University.
Mr. Elwood, one of our executive officers, has served as Senior Vice President, Marketing since January 2003. Prior to joining us, Mr. Elwood served as a consultant to various pharmaceutical companies and other companies regarding pharmaceutical industry matters from November 2001 to January 2003. He served as Chief Commercial Officer at Corixa Corporation from December 2000, when Corixa acquired Coulter Pharmaceuticals, Inc., to November 2001. Mr. Elwood served in various positions at Coulter from 1997 until its acquisition by Corixa, including as Chief Commercial Officer beginning in January 1999, and Senior Vice President, Marketing and Sales beginning in July 1997. Earlier, Mr. Elwood served as Executive Director, New Product Development from 1990 to 1995, and Vice President, New Product Development from January 1995 to 1997 for Ortho-McNeil Pharmaceutical, a division of Johnson & Johnson. From 1983 to 1990, Mr. Elwood served in various positions at Bristol-Myers Squibb Company. He received his B.S. in Business Administration, with a special emphasis in Marketing and Accounting, from California State University.
Dr. Kolterman, one of our executive officers, has served as Senior Vice President, Clinical Affairs since February 1997. Dr. Kolterman previously served as Vice President, Medical Affairs from July 1993 to February 1997 and Director, Medical Affairs from May 1992 to July 1993. From 1983 to May 1992, he was Program Director of the General Clinical Research Center and Medical Director of the Diabetes Center, at the University of California, San Diego Medical Center. Since 1989, he has been Adjunct Professor of Medicine at UCSD. From 1978 to 1983, he was Assistant Professor of Medicine in the Endocrinology and Metabolism Division at the University of Colorado School of Medicine, Denver. He was a member of the Diabetes Control and Complications Trial Study Group and presently serves as a member of the Epidemiology of Diabetes Intervention and Complications Study. He is also a past-president of the California Affiliate of the American Diabetes Association. Dr. Kolterman earned his M.D. from Stanford University School of Medicine.
Mr. Eberhard, one of our executive officers, has served as Vice President, Sales since May 2003. Prior to joining us, Mr. Eberhard was Regional Vice President, Sales, at Pharmacia Corporation, for which he had worked for 21 years. During his career with Pharmacia Corporation and its related pre-merger companies, he held positions in sales, sales management, corporate training, sales operations, and managed care before assuming the Vice President, Sales position. Mr. Eberhard received his B.S. in Biology from the California Lutheran University.
Mr. Foletta, one of our executive officers, has served as Vice President, Finance and Chief Financial Officer since March 2000. Mr. Foletta previously served as a Principal of Triton Group Management, Inc. from 1997 to 2000. From 1986 to 1997, Mr. Foletta held a number of management positions with Intermark, Inc. and Triton Group Ltd., the most recent of which was Senior Vice President, Chief Financial Officer and Corporate Secretary. From 1982 to 1986, Mr. Foletta was with Ernst & Young, most recently serving as an Audit Manager. Mr. Foletta earned his B.A. in Business
7
Economics from the University of California, Santa Barbara. Mr. Foletta is a certified public accountant.
Dr. Hanley has served as Vice President, Discovery Research since October 2003. He has been a member of our Scientific Advisory Board since 1992, and recently served as a senior scientific advisor for us. Prior to joining us, Dr. Hanley held faculty positions at Imperial College, London, the Medical Research Council Laboratories, Cambridge, and the University of California at Davis, where he was Professor of Biological Chemistry. Dr. Hanley has served on advisory or review panels for the National Institutes of Health, the Medical Research Council and Wellcome Trust of Great Britain, and for the governments of Australia, Singapore, New Zealand, Hong Kong, Denmark and Japan. From 1997 to 2003, Dr. Hanley was a senior consultant for healthcare investors in the venture capital and banking communities and for biotechnology companies such as Cell Therapeutics, Zymogenetics, Elan Pharmaceuticals, and Chiron Corporation. Dr. Hanley has also set-up and directed research programs in privately-held start-ups, such as Chemocentryx, PsychoGenics, and most recently Harvard-based Resolvyx Pharmaceuticals. He received his B.S. in Biochemistry and his Ph.D. in Molecular Biology from the University of California, Berkeley.
Ms. Harvey has served as Vice President, Quality Assurance since July 2003. Ms. Harvey previously served in various positions at Alliance Pharmaceutical Corp. from November 2000 to June 2003, including most recently as Vice President, Operations. Prior to joining Alliance, she was with Oliver Wight Americas, serving as an ERP consultant to various pharmaceutical and electronic component companies. Ms. Harvey was with Molecular Biosystems, Inc. from 1988 to 2000, where she held various management positions, including most recently Vice President, Operations. In addition, she held both manufacturing and quality management positions at Baxter Hyland Division between 1980 and 1988. Ms. Harvey received her B.S. in Microbiology from California State University Long Beach.
Mr. Rowland, one of our executive officers, has served as our Vice President, Legal, General Counsel and Secretary since September 2001. Prior to joining us, Mr. Rowland served in various positions at Alliance Pharmaceutical Corp., including as Vice President beginning in May 1999, Secretary beginning in May 1998 and General Counsel and Assistant Secretary beginning in 1993. Earlier, Mr. Rowland served as Vice President and Senior Counsel, Finance and Securities, at Imperial Savings Association for four years. For the previous eight years, he was engaged in the private practice of corporate law with the San Diego, California law firm of Gray, Cary, Ames & Fry, and the Houston, Texas law firm of Bracewell & Patterson. He received a J.D. from Emory University.
Mr. Step has served as Vice President, Corporate Development since August 2002 and has been a member of our Corporate Development group since October 2001. Mr. Step joined us in July 2000, and until October 2001, served as Executive Director of Commercial Operations where he was responsible for developing the sales function for our future commercialization plans. Prior to serving in this position, he was the Senior Director of Business Development at Dura Pharmaceuticals from 1998 until 2000. From 1996 through 1998, Mr. Step served as Associate Director of Business Development and Strategic Planning at Hoffmann-La Roche. From 1993 to 1996, he served in various sales and management roles for Syntex Labs and Roche Labs. He received his B.A. in Political Science from Vanderbilt University and his M.B.A. in Marketing from the University of Southern California.
Dr. Stetsko was appointed Vice President, Product Development in July 2002. Dr. Stetsko previously served as Executive Director of Preclinical and Product Development from September 2000 to July 2002. Prior to joining us, from September 1999 to September 2000 he was an independent consultant providing regulatory, quality assurance and product development support to biotech companies. From November 1994 to September 1999, Dr. Stetsko was responsible for product development at Ligand Pharmaceuticals, most recently as the Senior Director of Pharmaceutical and Analytical Development. From February 1987 to October 1994, he held a number of management positions at Sterling Winthrop, most recently Associate Director of Pharmaceutical Sciences. Prior to
8
employment at Sterling Winthrop, from June 1983 to January 1987, Dr. Stetsko was a senior research scientist at Sandoz, Ltd. He received his B.S. in Pharmacy from the University of Rhode Island and his Ph.D. in Industrial and Physical Pharmacy from Purdue University.
Dr. Young has served as Vice President, Research since October 1998 and as Senior Research Fellow since March 2002. From 1989 to 1998, he held a number of positions in our Physiology Department, most recently as Vice President, Physiology. Prior to joining us in 1989, Dr. Young was a lecturer in the Department of Physiology at the University of Auckland, New Zealand and a part-time general medical practitioner. From 1984 to 1987, Dr. Young was a Clinical Research Scientist at the National Institutes of Health in Phoenix, Arizona, where he studied insulin resistance and diabetes. He received his M.B., Ch.B. (M.D.) and his Ph.D. in Physiology from the University of Auckland, New Zealand.
CORPORATE GOVERNANCE MATTERS
The Board, upon the recommendation of the Nominating and Governance Committee, has adopted Corporate Governance Guidelines, a copy of which can by found on the corporate governance section of our web site,www.amylin.com. These Guidelines are intended to enhance the functioning of the Board and its committees, promote the interests of our stockholders and establish a common set of expectations as to how the Board, its various committees and individual directors should perform their functions.
Our independent directors meet in executive sessions at each regularly scheduled Board meeting without any member of management present. The Chair of the Nominating and Governance Committee serves as chair of these executive sessions.
COMMUNICATIONS WITH THE BOARD OF DIRECTORS
Stockholders who wish to communicate with the Board may do so by writing to the Board of Directors, 9360 Towne Centre Drive, Suite 110, San Diego, California 92121. The Nominating and Governance Committee has established procedures for the handling of communications from stockholders and directed our Corporate Secretary to act as their agent in processing any communications received. All communications that relate to matters that are within the scope of responsibilities of the Board and its committees are to be forwarded by our Corporate Secretary to our independent directors. Communications that relate to matters that are within the responsibility of one of our Board committees are also to be forwarded by our Corporate Secretary to the chair of the appropriate committee. Communications that relate to ordinary business matters that are not within the scope of the Board's responsibilities are to be sent to the appropriate member of management. Solicitations, junk mail and obviously frivolous or inappropriate communications are not to be forwarded, but will be made available to any non-management director who wishes to review them.
BOARD COMMITTEES AND MEETINGS
During the year ended December 31, 2003, the Board met four times. The Board has an Audit Committee, a Compensation and Human Resources Committee, a Nominating and Governance Committee, and a Finance Committee. Each committee operates pursuant to a written charter, copies of which can be found on the corporate governance section of our web site,www.amylin.com. Each of our Board committees performs an annual self-performance evaluation, which evaluation includes a comparison of the performance of such committee with the requirements of its charter. The performance evaluation also includes a recommendation to the Board of any improvements to the committee's charter deemed necessary or desirable by such committee.
Each of our Board committees has the full power and authority to discharge its duties and responsibilities, including the authority to select, retain, terminate, and approve the fees and other
9
retention terms of special counsel or other experts or consultants, as it deems appropriate, without seeking approval of the Board or our management.
The Audit Committee assists the Board in fulfilling its oversight responsibilities for financial matters and ensuring the integrity of our financial statements. The Audit Committee has the sole authority to appoint, retain or terminate our independent auditors; approves all audit and, in advance, all non-audit services to be provided to us by the independent auditors; oversees the independence of the independent auditors; evaluates the independent auditors' performance; oversees and evaluates our accounting and financial controls; receives and considers the independent auditors' comments as to accounting and financial controls; discusses with management and the independent auditors the results of the annual audit and our annual financial statements; discusses with management and the independent auditors, as applicable, the results of the independent auditors' interim review of our quarterly financial statements, as well as our earnings press releases; and approves all related-party transactions that are required to be disclosed by applicable laws, rules or regulation. As of the date of this proxy statement, the Audit Committee is composed of three directors, Mr. Greene, Mr. Sullivan and Mr. Testman, each of whom is independent as defined by the rules of the Nasdaq Stock Market, Inc. The Board has determined that Mr. Testman is an audit committee financial expert. Members of the Audit Committee do not receive any compensation from Amylin other than for Board or committee service. The Audit Committee met eight times in 2003. The Board recently amended the Audit Committee's written Audit Committee Charter as part of our overall enhancement of our corporate governance. A copy of the amended Audit Committee Charter is attached to this proxy statement as Appendix A.
The Compensation and Human Resources Committee assists the Board in fulfilling its responsibilities in connection with the compensation of our directors, officers, employees and consultants. It performs this function by establishing and overseeing the administration of our compensation policies for executives; reviewing and approving strategies for attracting, developing and motivating management and employees; and by recommending to the Board the approval of compensation plans and programs, including various incentive compensation, retirement and other benefit plans; and administering or overseeing approved plans or programs. The Compensation and Human Resources Committee also develops a succession plan for our Chief Executive Officer and other key executives and produces an annual report on executive compensation included in this proxy statement. The Compensation and Human Resources Committee conducts annual reviews of the performance of our Chief Executive Officer and establishes her compensation. In consultation with management, the Compensation and Human Resources Committee recommends to the Board annual goals for Amylin to serve as guidance in making awards under our incentive compensation plans and makes recommendations to the Board for our overall performance of those goals. As of the date of this proxy statement, the Compensation and Human Resources Committee is composed of three directors, Mr. Bryson, Mr. Gregg and Mr. Wilson, each of whom are independent directors as defined by the rules promulgated by the Nasdaq Stock Market, Inc., are outside directors as defined by Rule 162(m) of the Code, and are non-employee directors as defined by Rule 16b-3 promulgated by the SEC under the Securities and Exchange Act. The Compensation and Human Resources Committee met seven times in 2003.
The Nominating and Governance Committee administers the process for determining the selection of candidates for the Board; assesses the composition, operations and performance of the Board and the performance and independence of each director; develops and recommends to the Board corporate governance guidelines and periodically reviews and assesses these guidelines and their application and recommends any changes deemed appropriate to the Board for its consideration; oversees and administers our corporate governance functions on behalf of the Board; oversees and administers compliance matters, to the extent such activities are not delegated to other committees; recommends any changes considered appropriate in the authority, operations, charter, number or membership of the
10
Board or any committee; evaluates the need and, if necessary, develops and institutes a plan or program for the continuing education of our directors; and oversees and reviews with management and the Board the adequacy of, and monitors compliance with, our Guidelines for Shared Business Conduct and related conduct and ethics policies. In addition to its Board nominating role, the Nominating and Governance Committee assists the Board in working to assure that Amylin operates with proper corporate governance principles and practices. As of the date of this proxy statement, the Nominating and Governance Committee is composed of all of our independent directors: Messrs. Bryson, Greene, Gregg, Skyler, Sullivan, Testman and Wilson. During 2003, the Nominating and Governance Committee met one time.
The Finance Committee was formed in early 2004 to assist the Board in matters relating to our capital-raising and other financing activities. The Finance Committee considers the ongoing financing needs of Amylin, considers alternative financing mechanisms available to Amylin, makes recommendations to the Board regarding the implementation of appropriate financing mechanisms, and undertakes any other duties or responsibilities expressly delegated to the Finance Committee by the Board from time to time. The Finance Committee charter requires that it consists of at least three (3) directors, one of whom shall be our Chief Executive Officer. All members of the Finance Committee are financially literate and have previous experience with the financing of companies in the United States generally, including securities financing. As of the date of this proxy statement, the Finance Committee is composed of Messrs. Cook, Greene, Sullivan and Testman, and Ms. Graham.
During the year ended December 31, 2003, all of our directors attended at least 75% of the meetings of the Board and of the committees on which they served that were held during the period for which they were a director or committee member. In addition, each of our directors attended our 2003 Annual Meeting of Stockholders, and our Corporate Governance Guidelines require each director to attend our annual stockholder meetings absent an irreconcilable conflict.
The Nominating and Governance Committee is responsible for determining the Board's slate of director nominees for election to our Board and the individuals to fill vacancies on our Board occurring between annual meetings of stockholders. The Nominating and Governance Committee will, at least on an annual basis, consider the mix of skills and experience that the then-current directors bring to the Board to assess whether the Board has the necessary membership and resources to perform its oversight function effectively. The qualifications of any non-incumbent director candidates brought to the attention of the Nominating and Governance Committee by directors, management, stockholders or third parties will be evaluated from time to time in light of the Nominating and Governance Committee's determination of the Board's needs, and under the same criteria as set forth below. Stockholders wishing to suggest candidates to the Nominating and Governance Committee for consideration as directors must submit a written notice to our Board, who will provide it to the Nominating and Governance Committee. The address for our Board can be found in this proxy statement under the caption "Communications with the Board of Directors" or in the corporate governance section of our website atwww.amylin.com. Our Bylaws set forth the procedures a stockholder must follow to nominate candidates for director. Certain elements of these procedures are described in this proxy statement under the caption "Future Stockholder Proposals." The Nominating and Governance Committee does not distinguish between nominees suggested by stockholders and other nominees.
In evaluating the suitability of potential candidates for Board membership, the Nominating and Governance Committee takes into account many factors, including whether the potential nominee meets requirements for independence; the individual's personal qualities and characteristics, accomplishments and reputation in the business community; the potential candidate's current knowledge and contacts in the communities in which Amylin does business and in Amylin's industry or
11
other industries relevant to Amylin's business; the individual's ability and willingness to commit adequate time to Board and committee matters; the fit of the individual's skills and personality with those of other directors and potential directors in building a Board that is effective and responsive to the needs of Amylin; and the need for the Board to have a diversity of viewpoints, background, experience and other factors. The Nominating and Governance Committee has not established any specific minimum qualification standards for nominees to the Board.
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities Exchange Act of 1934 (the "Exchange Act") requires our directors and executive officers, and persons who beneficially own more than 10% of our common stock, to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities. Officers, directors and greater than 10% stockholders are required by regulations of the SEC to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us and written representations that no other reports were required, during the fiscal year ended December 31, 2003, all of our officers, directors and greater than 10% beneficial owners complied with the Section 16(a) filing requirements except as described below.
Due to a single clerical oversight by Amylin, a Form 4 representing the grant of a stock option was filed late for each of Messrs. Bryson, Greene, Gregg, Testman, Wilson, Bradbury, Brown, Cook, Eberhard, Elwood, Foletta, and Rowland, Ms. Graham, and Drs. Skyler, Baron, Data and Kolterman, and a Form 4 representing the grant of phantom stock was filed late for each of Messrs. Bryson, Greene, Gregg, Testman and Wilson, and Ms. Graham and Dr. Skyler. In addition, a Form 4 representing a sale pursuant to a Rule 10b5-1 Plan was filed late for Mr. Greene, a Form 4 representing two purchases was filed late for Mr. Gregg, and a Form 5 representing a gift by Mr. Bradbury in 2003 was filed late in 2004.
12
PROPOSAL 2
APPROVAL OF AMENDMENT OF THE
2001 EMPLOYEE STOCK PURCHASE PLAN
At our 2001 Annual Meeting of Stockholders, our stockholders approved our 2001 Employee Stock Purchase Plan (the "2001 Purchase Plan"). In February 2004, the Board amended our 2001 Purchase Plan, subject to stockholder approval. The amendment increased the number of shares of our common stock reserved for issuance under the 2001 Purchase Plan from 400,000 to 1,150,000. The amendment also permits the Board to specify that participants in an offering under the 2001 Purchase Plan, as amended, may elect to have payroll deductions relating to the offering made prior to the offering's commencement. An offering expired under the 2001 Purchase Plan on January 31, 2004, and the Board's Compensation and Human Resources Committee, which administers the 2001 Purchase Plan, determined prior to that time that the subsequent offering under the 2001 Purchase Plan that otherwise would have commenced on February 1, 2004 would not occur. The Compensation and Human Resources Committee made this determination because the remaining number of shares of common stock reserved for issuance under the 2001 Purchase Plan at the expiration of the offering that expired on January 31, 2004 was 61,226, and the Compensation and Human Resources Committee considered it in Amylin's and its stockholders' best interests to delay commencing another offering under the 2001 Purchase Plan until the share reserve had been increased to a level that better reflected anticipated participation in future offerings. The Compensation and Human Resources Committee authorized a new offering under the 2001 Purchase Plan, as amended, that is subject to stockholder approval of this increase in shares of our common stock available for issuance under the 2001 Purchase Plan. That offering commences on the date our stockholders approve the amended 2001 Purchase Plan and ends on July 31, 2006. Potential eligible participants in that offering were permitted, under the terms and subject to stockholder approval of the 2001 Purchase Plan, as amended, to authorize payroll deductions for the offering effective as of February 1, 2004.
Stockholders are requested in this Proposal 2 to approve the 2001 Purchase Plan, as amended. The affirmative vote of the holders of a majority of the shares present in person or represented by proxy and entitled to vote at the Annual Meeting will be required to approve the 2001 Purchase Plan, as amended. Abstentions will be counted toward the tabulation of votes cast and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether Proposal 2 has been approved.
THE BOARD OF DIRECTORS RECOMMENDS
A VOTE IN FAVOR OF PROPOSAL 2.
The essential features of the 2001 Purchase Plan, as amended, are outlined below.
The purpose of the 2001 Purchase Plan, as amended, is to provide a means by which our employees may be given an opportunity to purchase our common stock through payroll deductions, to assist us in retaining the services of our employees, to secure and retain the services of new employees, and to provide incentives for such persons to exert maximum efforts for our success. As of March 1, 2004, approximately all of our approximately 550 employees were eligible to participate in the 2001 Purchase Plan, as amended.
The rights to purchase our common stock granted under the 2001 Purchase Plan, as amended, are intended to qualify as options issued under an "employee stock purchase plan" as that term is defined in Section 423(b) of the Internal Revenue Code of 1986, as amended, or the Code.
13
The 2001 Purchase Plan provides that the Board is responsible for administering the 2001 Purchase Plan, as amended, and has the final power to construe and interpret both the 2001 Purchase Plan, as amended, and the rights granted under it. The Board has the power, subject to the provisions of the 2001 Purchase Plan, as amended, to determine when and how rights to purchase our common stock will be granted, the provisions of each offering of such rights (which need not be identical), and whether employees of any subsidiary of Amylin will be eligible to participate in the 2001 Purchase Plan, as amended.
The 2001 Purchase Plan also provides that the Board may delegate administration of the 2001 Purchase Plan, as amended, to a committee. Accordingly, the Board has delegated administration of the 2001 Purchase Plan, as amended, to the Compensation and Human Resources Committee of the Board. As used below with respect to the 2001 Purchase Plan, as amended, the"Board" refers to the Compensation and Human Resources Committee and to the Board.
The 2001 Purchase Plan, as amended, is implemented by offerings of rights to all eligible employees from time to time as determined by the Board. The first offering approved by the Compensation and Human Resources Committee under the 2001 Purchase Plan, as amended, will begin on the date our stockholders approve the 2001 Purchase Plan, as amended, and end on July 31, 2006, and be divided into five "shorter" purchase periods ending on July 31, 2004 and every six months thereafter. We anticipate that subsequent offerings will generally be 24 months long and be divided into four shorter "purchase periods," each approximately six months in length.
Any person who is customarily employed more than 20 hours per week and five months per calendar year by Amylin on the first day of an offering or who becomes such during an offering is eligible to participate in that offering, except that, for the current offering approved by the Compensation and Human Resources Committee under the 2001 Purchase Plan, as amended, only individuals who otherwise qualify and who are employed from February 1, 2004 through the date our stockholders approve the 2001 Purchase Plan, as amended, will be eligible to participate in that offering at its commencement.
No employee is eligible to participate in the 2001 Purchase Plan, as amended, if, immediately after the grant of purchase rights, the employee would own, directly or indirectly, stock possessing 5% or more of the total combined voting power or value of all classes of stock of Amylin or of any subsidiary of Amylin (including any stock which such employee may purchase under all outstanding rights and options). In addition, no employee may purchase more than $25,000 worth of our common stock (determined at the fair market value of the shares at the time such rights are granted in accordance with the Code) under all employee stock purchase plans of Amylin and its affiliates for each calendar year in which such rights are outstanding.
Eligible employees enroll in the 2001 Purchase Plan, as amended, by delivering to Amylin, within the time period specified by the Board, an agreement authorizing payroll deductions of up to 15% of such employees' total compensation during the offering.
During 2003, shares of our common stock were purchased for the persons and groups of persons set forth below in the amounts and at the weighted average prices per share under the 2001 Purchase Plan as follows: Mr. Cook purchased 4,239 shares at a weighted average price per share of $7.225; Ms. Graham and Mr. Bradbury did not purchase any shares; Dr. Baron purchased 1,852 shares at a
14
weighted average price per share of $7.225; Mr. Brown purchased 4,741 shares at a weighted average price per share of $7.225; Dr. Data purchased 487 shares at a weighted average price per share of $7.225; Dr. Kolterman purchased 1,119 shares at a weighted average price per share of $7.225; all executive officers as a group purchased 19,932 shares at a weighted average price per share of $7.26; and all employees as a group purchased 166,739 shares at a weighted average price per share of $8.30.
Generally the purchase price per share at which shares of our common stock are sold in an offering under the 2001 Purchase Plan, as amended, is the lower of (i) 85% of the fair market value of a share of our common stock on the first day of the offering or the applicable offering date or (ii) 85% of the fair market value of a share of our common stock on the last day of the applicable "purchase period."
The purchase price of the shares is accumulated by payroll deductions before or during the offering. At any time during the offering, a participant in the offering may begin, increase, reduce or terminate his or her payroll deductions as the Board provides in the offering. All payroll deductions made for a participant are credited to his or her account under the 2001 Purchase Plan, as amended, and deposited with our general funds. A participant may not make additional payments into such account.
In connection with offerings made under the 2001 Purchase Plan, as amended, the Board may specify a maximum number of shares of our common stock an employee may be granted the right to purchase and the maximum aggregate number of shares of our common stock that may be purchased pursuant to such offering by all participants. If the aggregate number of shares to be purchased upon exercise of rights granted in the offering would exceed the maximum aggregate number of shares of our common stock available, the Board would make a pro rata allocation of available shares in a uniform and equitable manner. Unless the employee's participation is discontinued, his or her right to purchase shares is exercised automatically at the end of the purchase period at the applicable purchase price. See "Withdrawal" below.
While each participant in the 2001 Purchase Plan, as amended, is required to sign an agreement authorizing payroll deductions, the participant may withdraw from a given offering by terminating his or her payroll deductions and by delivering to Amylin a notice of withdrawal. The 2001 Purchase Plan, as amended, provides that a participant may withdraw from an offering at any time, unless the offering provides otherwise.
Upon any withdrawal from an offering by the employee, we will distribute to the employee his or her accumulated payroll deductions without interest, less any accumulated deductions previously applied to the purchase of shares of our common stock on the employee's behalf during such offering, and such employee's interest in the offering will be automatically terminated. The employee is not entitled to again participate in that offering. However, an employee's withdrawal from an offering will not have any effect upon such employee's eligibility to participate in subsequent offerings under the 2001 Purchase Plan, as amended.
15
Rights granted pursuant to any offering under the 2001 Purchase Plan, as amended, terminate immediately upon cessation of an employee's employment for any reason, and we will distribute to such employee all of his or her accumulated payroll deductions, without interest, less any accumulated deductions previously applied to the purchase of shares of our common stock on the employee's behalf during such offering.
Rights granted under the 2001 Purchase Plan, as amended, are not transferable otherwise than by will or the laws of descent and distribution and may be exercised only by the person to whom such rights are granted.
DURATION, AMENDMENT AND TERMINATION
The Board may suspend or terminate the 2001 Purchase Plan, as amended, at any time. The Board also may amend the 2001 Purchase Plan, as amended, at any time. Any amendment of the 2001 Purchase Plan, as amended, must be approved by the stockholders to the extent stockholder approval is necessary for the 2001 Purchase Plan, as amended, to satisfy the requirements of Section 423 of the Code, other applicable laws or regulations, or the rules promulgated by the Nasdaq Stock Market, Inc.
Rights granted before amendment or termination of the 2001 Purchase Plan, as amended, will not be altered or impaired by any amendment or termination of the 2001 Purchase Plan, as amended, without the consent of the employee to whom such rights were granted, unless doing so is necessary to comply with any laws or governmental regulations or necessary to ensure that the 2001 Purchase Plan, as amended, and the rights granted thereunder comply with Section 423 of the Code.
In the event of a disposition of all or substantially all of the assets of Amylin or specified types of mergers of Amylin, the surviving or acquiring corporation either will assume the rights under the 2001 Purchase Plan, as amended, or substitute similar rights, or the exercise date of any ongoing offering will be accelerated such that participants' accumulated payroll deductions will be used to purchase shares of our common stock in the offering immediately prior to any such event.
Subject to this Proposal 2 being approved by our stockholders, an aggregate of 1,150,000 shares of our common stock is reserved for issuance under the 2001 Purchase Plan. If rights granted under the 2001 Purchase Plan, as amended, expire, lapse or otherwise terminate without being exercised, the shares of our common stock not purchased under such granted rights again become available for issuance under the 2001 Purchase Plan, as amended.
Rights granted under the 2001 Purchase Plan, as amended, are intended to qualify for favorable federal income tax treatment associated with rights granted under an employee stock purchase plan which qualifies under provisions of Section 423 of the Code.
A participant will be taxed on amounts withheld for the purchase of shares of our common stock as if such amounts were actually received. Other than this, no income will be taxable to a participant until disposition of the acquired shares, and the method of taxation will depend upon the holding period of the acquired shares.
16
If the stock is disposed of at least two years after the first date a participant was eligible to participate in the offering period and at least one year after the stock is transferred to the participant, then the lesser of (i) the excess of the fair market value of the stock at the time of such disposition over the purchase price or (ii) an amount equal to 15% of the fair market value of the stock as of the beginning of the offering period in which the participant purchased the stock will be treated as ordinary income. Any further gain or any loss will be taxed as a long-term capital gain or loss. Such capital gains currently are generally subject to lower tax rates than ordinary income.
If the stock is sold or disposed of before the expiration of either of the holding periods described above, then the excess of the fair market value of the stock on the date it was purchased by the applicable participant over the purchase price will be treated as ordinary income at the time of such disposition. The balance of any gain will be treated as capital gain. Even if the stock is disposed of for less than its fair market value on the date it was purchased, the same amount of ordinary income is attributed to the participant, and a capital loss is recognized equal to the difference between the sales price and the fair market value of the stock on such purchase date. Any capital gain or loss will be short-term or long-term, depending on how long the stock has been held.
There are no federal income tax consequences to Amylin by reason of the grant or exercise of rights under the 2001 Purchase Plan, as amended. We are entitled to a deduction to the extent amounts are taxed as ordinary income to a participant (subject to the requirement of reasonableness and the satisfaction of tax reporting obligations).
EQUITY COMPENSATION PLAN INFORMATION
The following table sets forth information regarding all of our equity compensation plans as of December 31, 2003 (in thousands, except per share amounts):
Plan Category
| | Number of securities to
be issued upon exercise
of outstanding options,
warrants and rights
| | Weighted average
exercise price of
outstanding options,
warrants and rights
| | Number of securities
remaining available for
issuance under equity
compensation plans,
(excluding securities
reflected in first column)
|
|---|
| Equity compensation plans approved by securityholders | | 10,570 | | $ | 13.01 | | 4,750 |
| Equity compensation plans not approved by securityholders | | 9 | | $ | 6.58 | | — |
| | |
| |
| |
|
| Total | | 10,579 | | $ | 13.00 | | 4,750 |
| | |
| |
| |
|
We had the following equity compensation plans in effect as of December 31, 2003 that were adopted with the approval of our stockholders: the 1991 Stock Option Plan, the 2001 Equity Incentive Plan, the 2001 Employee Stock Purchase Plan, the 1994 Non-Employee Directors' Stock Option Plan, the 2003 Non-Employee Directors' Stock Option Plan and the Non-Employee Directors' Deferred Compensation Plan.
Our stockholders did not approve the individual compensation arrangement entered into in January 1995 with Mr. Cook, who served as our Chairman and Chief Executive Officer from 1998 to 2003, and continues to serve as our Chairman. From 1994 to 1998, Mr. Cook served as a consultant to us under various consulting agreements pursuant to which he received cash compensation and was granted non-qualified stock options. In connection with one of his consulting agreements with us entered into in January 1995, we also entered into a phantom stock unit agreement with Farview Management Co., L.P., a consulting firm of which Mr. Cook is a general partner. Pursuant to the phantom stock agreement, Farview received 9,000 phantom stock units, each representing the right to receive cash or shares of our common stock. The phantom stock agreement provides that on the date Mr. Cook ceases to be a consultant to or director of our company, we will pay Farview the fair market value of the phantom stock units in cash or shares of our common stock, at our election. The fair market value of each phantom stock unit is to be determined based on the closing price per share of our common stock on the Nasdaq National Market on the last trading day prior to the date that Mr. Cook ceases to be a consultant to or director of our company.
17
PROPOSAL 3
RATIFICATION OF SELECTION OF INDEPENDENT AUDITORS
The Audit Committee of the Board of Directors has selected Ernst & Young llp as our independent auditors for the fiscal year ending December 31, 2004 and has further directed that management submit the selection of independent auditors for ratification by the stockholders at the Annual Meeting. Ernst & Young llp has audited our financial statements since our inception in 1987. Representatives of Ernst & Young llp are expected to be present at the Annual Meeting, will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Stockholder ratification of the selection of Ernst & Young llp as our independent auditors is not required by our Bylaws or otherwise. However, the Audit Committee of the Board is submitting the selection of Ernst & Young llp to the stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, the Audit Committee will reconsider whether or not to retain Ernst & Young LLP. Even if the selection is ratified, the Audit Committee in its discretion may direct the appointment of different independent auditors at any time during the year if it determines that such a change would be in the best interest of Amylin and its stockholders.
The affirmative vote of the holders of a majority of the shares present in person or represented by proxy and entitled to vote at the Annual Meeting will be required to ratify the selection of Ernst & Young llp. Abstentions will be counted toward the tabulation of votes cast and will have the same effect as negative votes. Broker non-votes are counted towards a quorum, but are not counted for any purpose in determining whether Proposal 3 has been approved.
Audit Fees. During the fiscal years ended December 31, 2003 and 2002, the aggregate fees billed by Ernst & Young llp for professional services rendered for the audit of our financial statements for such fiscal years, reviews of our interim financial statements and in connection with statutory and regulatory filings or engagements were $222,996 and $139,543, respectively.
Audit-Related Fees. During the fiscal years ended December 31, 2003 and 2002, the aggregate fees billed by Ernst & Young llp for assurance and related services that were reasonably related to the performance of the audit or review of our financial statements that are not included under "Audit Fees" above were $24,000 and $24,115, respectively. These fees were for services related to Ernst & Young LLP's audit of our 401(k) plan and consultations related to accounting for one of our collaborations.
Tax Fees. During the fiscal years ended December 31, 2003 and 2002, the aggregate fees billed by Ernst & Young llp for professional services rendered for tax compliance and tax preparation were $18,000 and $15,750, respectively, and for tax advice and tax planning were $0 and $4,625, respectively. These fees for tax advice and tax planning were for consultations regarding the application of various provisions of the Code.
All Other Fees. During the fiscal years ended December 31, 2003 and 2002, no other fees were billed by Ernst & Young llp.
The Audit Committee has determined that the rendering of all non-audit services by Ernst & Young llp is compatible with maintaining the auditor's independence. Our Audit Committee Charter provides that the Audit Committee will approve all audit and pre-approve all non-audit services to be provided to us by our independent auditors. The Audit Committee pre-approved all audit or non-audit services provided by our independent auditors during 2003. A copy of our Audit Committee Charter is attached to this proxy statement as Appendix A.
THE BOARD OF DIRECTORS RECOMMENDS
A VOTE IN FAVOR OF PROPOSAL 3.
18
SECURITY OWNERSHIP OF
CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the ownership of our common stock as of March 1, 2004 by: (i) each nominee for director; (ii) each of the executive officers named in the Summary Compensation Table below; (iii) all our executive officers and directors as a group; and (iv) all those known by us to be beneficial owners of more than five percent of our common stock.
Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentages are based on 93,837,899 shares outstanding on March 1, 2004, adjusted as required by rules promulgated by the SEC. Except as shown otherwise in the table, the address of each stockholder listed is in care of Amylin at 9360 Towne Centre Drive, San Diego, California 92121.
| | Beneficial Ownership
|
|---|
Beneficial Owner(1)
| | Number of
Shares
| | Shares Issuable
Pursuant to
Options Exercisable
Within 60 Days of
March 1, 2004
| | Percent of
Total
|
|---|
Allen Andersson(2)
62 Sparhawk Lane
North Conway, NH 03860 | | 9,066,093 | | — | | 9.7% |
Capital Research & Management Co.(3)
333 South Hope Street
Los Angeles, CA 90071 | | 8,174,500 | | — | | 8.7 |
Putnam, LLC(3)
One Post Office Square
Boston, MA 02109 | | 5,477,443 | | — | | 5.8 |
FMR Corp.(3)
82 Devonshire Street
Boston, MA 02109 | | 5,025,800 | | — | | 5.4 |
| Alain D. Baron | | 259,921 | | 254,500 | | * |
| Daniel M. Bradbury(4) | | 430,793 | | 402,171 | | * |
| Martin R. Brown | | 397,328 | | 253,606 | | * |
| Vaughn D. Bryson(5) | | 128,013 | | 0 | | * |
| Joseph C. Cook, Jr.(6) | | 2,553,742 | | 1,440,324 | | 2.7 |
| Ginger L. Graham | | 158,600 | | 83,000 | | * |
| Howard E. Greene, Jr.(7) | | 2,139,867 | | 43,000 | | 2.3 |
| Terrance H. Gregg | | 45,000 | | 40,000 | | * |
| Orville G. Kolterman(8) | | 569,244 | | 502,389 | | * |
| Jay S. Skyler | | 160,704 | | 61,029 | | * |
| Joseph P. Sullivan | | 20,000 | | 20,000 | | * |
| Thomas R. Testman | | 32,000 | | 32,000 | | * |
| James N. Wilson(9) | | 107,000 | | 40,000 | | * |
| All executive officers and directors as a group (18 persons)(4)(5)(6)(7)(8)(9) | | 7,784,250 | | 3,842,675 | | 8.0 |
- *
- Less than one percent.
- (1)
- This table is based upon information supplied by officers, directors and principal stockholders and Schedules 13D, 13F and 13G filed with the SEC. Includes shares of our common stock issuable
19
pursuant to options and other rights to purchase shares of our common stock exercisable within 60 days of March 1, 2004.
- (2)
- This amount includes 2,602,779 shares held by Susan Riecken, Mr. Andersson's spouse.
- (3)
- Derived from information contained on the Form 13G filed by the stockholder for the period ended December 31, 2003.
- (4)
- Includes 18,118 shares held by the Bradbury Family Trust #3, of which trust Mr. Bradbury serves as a co-trustee, and shares voting and dispositive power. Does not include 473 shares held by the Bradbury Gift Trust, of which Mr. Bradbury's minor children are beneficiaries.
- (5)
- Includes 106,013 shares held by the Vaughn D. Bryson Irrevocable Trust, U-A 1/14/99, of which Mr. Bryson is the sole beneficiary, and 1,000 shares of unvested common stock that were subject to a repurchase right in favor of Amylin.
- (6)
- Includes 100,752 shares owned by Farview Management, L.P., as to which Mr. Cook shares voting and dispositive power with his wife, and 1,659 shares held by Charis Foundation, a charitable organization of which Mr. Cook is the President. Mr. Cook disclaims beneficial ownership of the shares held by Charis Foundation.
- (7)
- Includes 81,969 shares held by The Greene Children's Trust, of which Mr. Greene is a trustee and with respect to which he shares voting and dispositive power with his wife, Arlene Greene, 2,014,898 shares held in The Greene Family Trust, of which Mr. Greene is a trustee and with respect to which he also shares voting and dispositive power with his wife.
- (8)
- Includes 4,050 shares owned by Dr. Kolterman's children and 473 shares held by the Bradbury Gift Trust, of which trust Dr. Kolterman serves as a trustee and holds voting and dispositive power.
- (9)
- Includes 55,000 shares held by the James & Pamela Wilson Trust, of which Mr. Wilson may act as sole trustee and with respect to which he holds voting and dispositive power, 8,000 shares held by the James & Pamela Wilson Family Partnership, of which Mr. Wilson may act as general partner and with respect to which he holds voting and dispositive power, 2,000 shares held by the Scott West Irrevocable Trust, of which Mr. Wilson is the trustee and with respect to which he holds voting and dispositive power, and 2,000 shares held by the Taylor West Irrevocable Trust, of which Mr. Wilson is the trustee and with respect to which he holds voting and dispositive power.
EXECUTIVE COMPENSATION
COMPENSATION OF DIRECTORS
Prior to April 2003, each of our non-employee directors received an annual retainer of $10,000 payable in equal quarterly installments. In addition, effective beginning in the third quarter of 2002, each non-employee director received $9,000 per year, payable quarterly, for serving as committee chairman of the Audit or Compensation and Human Resources Committee and $6,000 per year, payable quarterly, for serving on the Audit or Compensation and Human Resources Committee, except if he or she served as chairman of the applicable committee. In April 2003, the Board implemented a revised compensation arrangement pursuant to which non-employee directors received an annual retainer of $20,000 payable in equal quarterly installments, plus $12,000 per year, payable quarterly, for serving as committee chairman for the Audit, Compensation and Human Resources or Nominating and Governance Committees and $8,000 per year, payable quarterly, for serving on the Audit or Compensation and Human Resources Committees, unless he or she is the chairman of the applicable committee. In February 2004, the Board implemented a revised compensation arrangement pursuant to which non-employee directors receive an annual retainer of $30,000 payable in equal quarterly installments, plus, payable in equal quarterly installments, $20,000 per year for serving as the chairman of the Audit Committee, $10,000 per year for serving as the chairman of other committees, and $5,000
20
per year for serving as Audit Committee members other than the chairman. Pursuant to a Non-Employee Directors' Deferred Compensation Plan (the "Directors' Deferral Plan"), non-employee directors may elect, on an annual basis, to defer all or a portion of their cash compensation as directors in a deferred stock account pursuant to which the fees are credited in the form of phantom shares of the our common stock, based on the market price of the stock at the time the fees are earned. Deferred amounts are valued according to fluctuations in the fair market value of our common stock. When a participant ceases serving as a director, the participant will be entitled to receive the value of his or her account in cash and/or in the form of our common stock, either in a single, lump-sum payment or in equal annual installments, as determined by us in our sole discretion.
Non-employee directors may also elect to defer all or a portion of their cash compensation through our 2001 Deferred Compensation Plan (the "DC Plan"). The DC Plan is an unfunded plan designed for the purpose of providing deferred compensation to a select group of our management and highly compensated executives, including officers and non-employee directors, in accordance with the Employee Retirement Income Security Act of 1974 ("ERISA"). In February 2003, we amended our DC Plan to permit non-employee directors to select our common stock as an investment alternative for deferred amounts contributed to the DC Plan. Participants receiving distributions will be taxed at ordinary income tax rates in the year of distribution. We will be entitled to a deduction at the time when, and in the amount that, the participant recognizes ordinary income.
All non-employee directors chose to defer all of their cash compensation for 2003 through the Directors' Deferral Plan or the DC Plan. In 2003, $97,000 was deferred into the Directors' Deferral Plan and $82,500 was deferred into the DC Plan.
Until 2003, each of our non-employee directors received stock option grants pursuant to our 1994 Non-Employee Directors' Stock Option Plan (the "1994 Non-Employee Directors' Plan"). The 1994 Non-Employee Directors' Plan provided that upon a non-employee director's initial election to the Board, he or she received a stock option to purchase 20,000 shares of our common stock. This grant had an exercise price equal to the fair market value of our common stock on the date of grant and vested over a period of four years, with one-quarter of each option becoming exercisable one year following the date of grant and the remainder becoming exercisable in equal daily increments over a three-year period. The 1994 Non-Employee Directors' Plan also provided that, effective as of each annual meeting of stockholders, each non-employee director elected at such meeting was granted an option to purchase 8,000 shares of our common stock, with the option vesting in equal monthly installments over 12 months and having an exercise price equal to the fair market value of our common stock on the date of grant. Options ceased being granted under the 1994 Non-Employee Directors' Plan when our stockholders approved our 2003 Non-Employee Directors' Stock Option Plan (the "2003 Non-Employee Directors' Plan") at our 2003 Annual Meeting.
In April 2003 the Board, and in May 2003, our stockholders, approved our 2003 Non-Employee Directors' Plan, which provides for automatic grants of stock options to our non-employee directors. These automatic grants consist of options to purchase 20,000 shares of our common stock upon non-employee directors being initially appointed or elected to the Board and options to purchase 12,000 shares of our common stock upon non-employee directors being elected as directors of Amylin at annual meetings of stockholders. Options granted pursuant to the automatic grants to non-employee directors upon their initial appointment or election to the Board vest, so long as those directors' service with Amylin or its affiliates continues, over a period of four years, with one-quarter of each such option becoming exercisable one year following the date of grant and the remainder becoming exercisable in equal daily increments over a three-year period. Options automatically granted to non-employee directors at annual meetings of stockholders vest, so long as those directors' service with Amylin or its affiliates continues, in equal monthly installments over the course of the following 12 months. The options automatically granted to non-employee directors pursuant to the 2003 Non-Employee Directors'
21
Plan, like the options granted under the 1994 Non-Employee Directors' Plan, have an exercise price equal to the fair market value of our common stock on the date of the grant.
During 2003, under the 2003 Non-Employee Directors' Plan, we granted options covering an aggregate of 84,000 shares to seven non-employee directors elected at the 2003 annual meeting of stockholders, at an exercise price per share of $19.24, which was the fair market value of our common stock on the date of grant. Additionally, during 2003 an option for 20,000 shares was granted to one new director under our 2003 Non-Employee Directors' Plan at an exercise price per share of $28.60, which was the fair market value of our common stock on the date of grant. As of March 1, 2004, options for 526,000 shares of our common stock had been exercised under the 1994 Non-Employee Directors' Plan and options for 12,000 shares of our common stock had been exercised under our 2003 Non-Employee Directors' Plan.
The members of the Board are also eligible for reimbursement for their expenses incurred in connection with attendance at Board meetings.
COMPENSATION OF EXECUTIVE OFFICERS
Summary of Compensation
The following table shows, for the years ended December 31, 2001, 2002 and 2003, compensation awarded or paid to, or earned by, our current Chief Executive Officer, our previous Chief Executive
22
Officer, and our other four most highly compensated executive officers for the year ended December 31, 2003 (the "Named Executive Officers"):
Summary Compensation Table
| |
| |
| |
| |
| | Long Term
Compensation
Awards
| |
|
|---|
| | Annual Compensation
| |
|
|---|
| | Number
of Shares
Underlying
Options
| |
|
|---|
Name and Principal Position
| | Year
| | Salary
| | Bonus(1)
| | Other Annual
Compensation(2)
| | All Other
Compensation(3)
|
|---|
Ginger L. Graham
President and Chief Executive Officer(4) | | 2003
2002
2001 | | $
| 195,112
—
— | | $
| —
—
— | | $
| 31,488(5)
—
— | | 512,000
8,000
3,000 | | $
| 21
—
— |
Joseph C. Cook, Jr.
Chairman of the Board of Directors(4) |
|
2003
2002
2001 |
|
|
403,239
475,000
475,000 |
|
|
475,000
—
250,000 |
|
|
131,200(5)
185,043(5)
189,307(5) |
|
250,000
60,000
140,000 |
|
|
5,835
6,040
5,292 |
Daniel M. Bradbury
Chief Operating Officer |
|
2003
2002
2001 |
|
|
335,000
329,167
300,000 |
|
|
131,667
—
— |
|
|
—
—
— |
|
100,000
36,000
70,000 |
|
|
6,035
5,540
5,289 |
Alain D. Baron
Senior Vice President, Clinical Research |
|
2003
2002
2001 |
|
|
275,000
270,867
250,200 |
|
|
108,347
—
— |
|
|
—
30,577(5)
— |
|
65,000
37,000
50,000 |
|
|
7,029
5,534
5,115 |
Martin R. Brown
Senior Vice President, Operations |
|
2003
2002
2001 |
|
|
275,000
270,867
250,200 |
|
|
108,347
—
— |
|
|
—
—
— |
|
65,000
22,000
65,000 |
|
|
7,029
5,534
59,506(6) |
Orville G. Kolterman
Senior Vice President, Clinical Affairs |
|
2003
2002
2001 |
|
|
275,000
270,867
250,200 |
|
|
108,347
—
— |
|
|
—
—
— |
|
65,000
27,000
65,000 |
|
|
7,029
6,034
5,037 |
- (1)
- Amounts are listed in the year in which bonus was actually paid, however, such bonus is awarded for corporate and individual performance in the previous year.
- (2)
- As permitted by rules promulgated by the SEC, no amounts are shown for any executive officer where the amounts constitute perquisites not exceeding the lesser of 10% of salary plus bonus or $50,000.
- (3)
- Except as set forth in footnote 6 below with respect to Mr. Brown, all amounts consist of (a) matching contributions made by us in the form of our common stock under our 401(k) plan and represents the fair market value of our common stock on the calculation date multiplied by the number of shares, and (b) amounts paid as premiums for term life insurance.
- (4)
- Ms. Graham became our President and Chief Executive Officer on September 1, 2003, and Mr. Cook served as Chief Executive Officer until such date.
- (5)
- Represents certain expenses paid by us or reimbursed to employee for certain living expenses, associated travel expense and tax gross-ups related thereto.
- (6)
- Includes $54,222 representing a special bonus paid to Mr. Brown to cover certain expenditures by Mr. Brown and an associated tax gross-up.
23
STOCK OPTION GRANTS AND EXERCISES
We grant options to our executive officers under our 2001 Equity Incentive Plan (the "Incentive Plan") and formerly granted them under our 1991 Stock Option Plan (the "1991 Stock Option Plan"). As of March 1, 2004, options to purchase a total of 3,313,275 shares of our common stock were outstanding under the 1991 Stock Option Plan. As of March 1, 2004, options to purchase a total of 6,964,166 shares of our common stock were outstanding under the Incentive Plan and an additional 4,061,394 shares of our common stock remained available for grant under the Incentive Plan. The 1991 Stock Option Plan has expired and no additional options may be granted thereunder.
The following tables show, for the year ended December 31, 2003, certain information regarding options granted to, exercised by, and held at year end by, the Named Executive Officers:
OPTION GRANTS IN 2003
Individual Grants
| |
| |
|
|---|
| | Potential Realizable Value
at Assumed Annual Rates
of Stock Price Appreciation
for Option Term(3)
|
|---|
| | Number of
Securities
Underlying
Options
Granted(1)
| | Percentage of
Total Options
Granted To
Employees in
2003(2)
| |
| |
|
|---|
Name
| | Exercise
Price per
Share
| | Expiration Date
|
|---|
| | 5%
| | 10%
|
|---|
| Ginger L. Graham | | 12,000
500,000(4) | | *
13.95% | | $
| 19.24
23.00 | | 5/14/2013
6/09/2013 | | $
| 145,199
7,232,288 | | $
| 367,963
18,328,038 |
| Joseph C. Cook, Jr. | | 250,000 | | 6.98 | | | 18.85 | | 5/12/2013 | | | 2,963,666 | | | 7,510,511 |
| Daniel M. Bradbury | | 100,000 | | 2.79 | | | 18.85 | | 5/12/2013 | | | 1,185,466 | | | 3,004,205 |
| Alain D. Baron | | 65,000 | | 1.81 | | | 18.85 | | 5/12/2013 | | | 770,553 | | | 1,952,734 |
| Martin R. Brown | | 65,000 | | 1.81 | | | 18.85 | | 5/12/2013 | | | 770,553 | | | 1,952,733 |
| Orville G. Kolterman | | 65,000 | | 1.81 | | | 18.85 | | 5/12/2013 | | | 770,553 | | | 1,952,733 |
- *
- Less than 1%.
- (1)
- Except as set forth in footnote 4 below, such options generally vest according to the following schedule: 25% vest one year from the date of grant and the remainder vest monthly over the following three years, subject to acceleration of vesting in the event of a change in control of Amylin.
- (2)
- Based on options to purchase 3,584,133 shares of our common stock granted to employees, including Named Executive Officers, during the year ended December 31, 2003.
- (3)
- Calculated on the assumption that the market value of the underlying stock increases at the stated values, compounded annually. The total appreciation of the options over their 10-year terms at 5% and 10% is 63% and 159% respectively.
- (4)
- Includes 350,000 shares having the vesting schedule set forth in footnote 1 above and 150,000 shares having the following Amylin performance-based conditions of exercisability: 50,000 shares vest on commercial launch of our drug candidate, SYMLIN®; 50,000 shares vest upon acceptance by the United States Food and Drug Administration of our New Drug Application for our drug candidate, exenatide; and 50,000 shares vest upon commercial launch of exenatide.
24
AGGREGATE OPTION EXERCISES IN 2003, AND YEAR-END OPTION VALUES
| |
| |
| | Number of
Securities Underlying
Unexercised Options
at Year-End
| |
| |
|
|---|
| |
| |
| | Value of Unexercised
In-the-Money Options
at Year-End
|
|---|
| | Number of
Shares
Acquired
on Exercise
| |
|
|---|
Name
| | Value
Realized
|
|---|
| | Exercisable
| | Unexercisable
| | Exercisable
| | Unexercisable
|
|---|
| Ginger L. Graham | | 0 | | $ | 0 | | 78,000 | | 505,000 | | $ | 1,009,610 | | $ | 14,900 |
| Joseph C. Cook, Jr. | | 62,000 | | | 1,312,263 | | 1,048,004 | | 352,320 | | | 16,798,127 | | | 2,150,100 |
| Daniel M. Bradbury | | 6,000 | | | 107,850 | | 242,374 | | 155,704 | | | 3,405,964 | | | 1,036,097 |
| Alain D. Baron | | 0 | | | 0 | | 137,349 | | 117,151 | | | 1,977,288 | | | 897,977 |
| Martin R. Brown | | 52,000 | | | 1,036,130 | | 145,249 | | 108,357 | | | 1,961,669 | | | 793,539 |
| Orville G. Kolterman | | 49,000 | | | 724,901 | | 397,955 | | 111,690 | | | 6,540,313 | | | 827,769 |
EMPLOYMENT, SEVERANCE AND CHANGE OF CONTROL ARRANGEMENTS
From 1994 to 1998, Mr. Cook, who served as our Chief Executive Officer from 1998 until September 1, 2003 and continues to serve as our Chairman of the Board, served as a consultant to Amylin under various consulting agreements under which Mr. Cook received cash compensation and was granted non-qualified stock options. In connection with one of his consulting agreements with us in January 1995, we also entered into a phantom stock unit agreement with Farview Management Co., L.P., a consulting firm of which Mr. Cook is a general partner. Pursuant to the phantom stock agreement, Farview received 9,000 phantom stock units, representing the right to receive cash or shares of our common stock. The phantom stock agreement provides that on the date Mr. Cook ceases to be a consultant to or director of Amylin, we will pay Farview the fair market value of the phantom stock units in cash or shares of our common stock, at our election. The fair market value of each phantom stock unit will be determined based on the closing price of a share of our common stock on The Nasdaq National Market on the last trading day prior to the date that Mr. Cook ceases to be a consultant to or director of Amylin.
In March 1998, Mr. Cook accepted a position as our Chairman of the Board and Chief Executive Officer. In connection with his appointment, we entered into an agreement with Mr. Cook pursuant to which his annual salary was set at $375,000. Under the terms of his agreement, Mr. Cook was eligible to receive an annual merit bonus of up to $250,000, payable upon the achievement of goals and milestones to be set by the Compensation and Human Resources Committee. In addition, Mr. Cook's agreement provided that we would reimburse Mr. Cook or pay for certain living expenses, associated travel expenses and tax gross-up payments related thereto. Mr. Cook was also granted an option to purchase an aggregate of 500,000 shares of our common stock under the 1991 Stock Option Plan at an exercise price of $2.656 per share. The option vested as follows: 200,000 of the option shares vested in equal monthly installments over the twelve months following Mr. Cook's employment start date; and the remaining 300,000 option shares vested in equal monthly installments over the following thirty-six months. Mr. Cook's salary was raised to $475,000 per annum in March 2000 and in March 2003 was raised to $500,000 per annum. In 2001, Mr. Cook and the Compensation Committee agreed that he would thereafter participate in our annual bonus program for all employees and officers rather than the bonus program under his employment agreement. Mr. Cook and the Compensation Committee believed this would better align his interests with our other officers and employees.
Mr. Cook's employment agreement also provided that the outstanding non-qualified stock options granted to Mr. Cook in connection with his consulting arrangements with us which were vested as of his employment start date will remain outstanding and exercisable in accordance with their terms for so long as Mr. Cook remains employed by Amylin and for twelve months thereafter, or for such longer period as is provided under the terms of those options.
25
Ginger Graham became our President and Chief Executive Officer on September 1, 2003. Ms. Graham has served as a member of our Board since November 1995. Her employment agreement provides for an annual salary of $500,000 and she is eligible to participate, with other full-time employees, in our cash bonus program. In addition, Ms. Graham received a stock option grant to purchase 500,000 shares of our common stock at an exercise price of $23.00 per share, the fair market value of our common stock on the date the option was granted. The option vests in the following manner: (i) 350,000 shares vest over four (4) years, with twenty-five percent (25%), or 87,500, of these shares vesting on September 1, 2004, and the remaining 262,500 shares vest thereafter on a monthly basis over a three year period; (ii) 50,000 shares vest upon the commercial launch of our drug candidate, SYMLIN®; (iii) 50,000 shares vest upon United States Food and Drug Administration, or FDA, acceptance of a New Drug Application for our drug candidate, exenatide; and (iv) 50,000 shares vest upon commercial launch of exenatide. In addition, under the terms of her employment agreement, we reimburse Ms. Graham or pay for certain living expenses, associated travel expenses and tax gross-up payments related thereto.
In February 2001, we adopted the Change in Control Employee Severance Benefit Plan (the "Change in Control Plan"). The Change in Control Plan provides designated employees of Amylin or certain affiliates, if any, who hold the position of vice president, or any position senior to vice president, with certain benefits in the event such employee ceases employment with Amylin without cause or under certain specified circumstances and within 90 days prior to, or within 13 months following specified change of control transactions. An eligible employee will receive continuation of salary for 18 months (24 months in the case of the president, chief executive officer or chief operating officer) in normal regular monthly installments and any bonus such employee would otherwise have received under our annual cash bonus plan then in effect, subject to an employee only being entitled to these benefits if he or she does not have a separate individual agreement with us regarding change of control or severance benefits (other than any agreement regarding equity incentive plans or arrangements, which are not superseded by the Change in Control Plan). As of the date of this proxy statement, none of our Named Executive Officers had separate agreements with us regarding change of control or severance benefits that supersede the Change in Control Plan.
2001 Equity Incentive Plan
The Board adopted the Incentive Plan to provide a means by which our employees, directors and consultants may be given an opportunity to purchase stock in Amylin, to assist us in retaining the services of such persons, to secure and retain the services of persons capable of filling our open positions and to provide incentives for these persons to exert maximum efforts for our success. The Incentive Plan allows us to grant awards to acquire our common stock through incentive stock options, non-incentive or non-qualified stock options, stock bonuses, and rights to acquire restricted stock. Incentive stock options granted under the Incentive Plan are intended to qualify as "incentive stock options" within the meaning of Section 422 of the Code. At our 2003 Annual Meeting of Stockholders, our stockholders approved an increase in the number of shares of our common stock issuable pursuant to the Incentive Plan to 11,500,000. As of March 1, 2004, options to purchase a total of 6,964,166 shares of our common stock were outstanding under the Incentive Plan and an additional 4,061,394 shares remained available for grant under the Incentive Plan.
The Board has delegated the responsibility for administering the Incentive Plan to the Compensation and Human Resources Committee. Under the Incentive Plan, the Compensation and Human Resources Committee provides for the grant of stock options to eligible employees. The Compensation and Human Resources Committee determines the provisions of each option granted, including the number of shares to be granted to each person and the time such option may be exercised. The exercise price of incentive stock options may not be less than the fair market value of our common stock on the date of the option grant. The exercise price of nonqualified stock options
26
may not be less than 85% of the fair market value of our common stock on the date of grant. Such options generally vest according to the following schedule: 25% vest one year from the date of grant and the remainder vest monthly over the following three years. From time to time we have granted options having alternative vesting schedules for specified business purposes, and we may do so in the future. Certain options granted under the Incentive Plan also are immediately exercisable, which is commonly referred to as an "early exercise" feature, but are subject to our right to repurchase unvested shares on termination of the optionholders' service with Amylin.
The Incentive Plan provides that, in the event of a dissolution or liquidation of Amylin, then all outstanding awards under the Incentive Plan shall terminate immediately prior to such dissolution or liquidation. The Incentive Plan further provides that, in the event of a sale, lease or other disposition of all or substantially all of the assets of Amylin or specified types of mergers or consolidations (each, a "corporate transaction"), any surviving or acquiring corporation shall either assume awards outstanding under the Incentive Plan or substitute similar awards for those outstanding under the Incentive Plan. If any surviving corporation declines to assume awards outstanding under the Incentive Plan or to substitute similar awards, then, with respect to participants whose service has not terminated as of the time of such corporate transaction, the vesting and the time during which such awards may be exercised will be accelerated in full, and all outstanding awards will terminate if the participant does not exercise such awards at or prior to the corporate transaction.
In addition, options granted to officers under the Incentive Plan have included, and it is expected that options granted to officers under the Incentive Plan will continue to include, certain change in control provisions. Pursuant to these provisions, if within 90 days prior to, or within 13 months following, the effective date of certain specified change in control transactions, an officer ceases employment with Amylin without cause or under certain other specified circumstances, then generally the vesting and exercisability of the options an officer holds that were issued under the Incentive Plan shall accelerate in full or any reacquisition or repurchase right of Amylin acquired pursuant to any early exercise of such options, if permitted, shall lapse in full. The acceleration of an option in the event of an acquisition, change in control or similar corporate event may be viewed as an anti-takeover provision, which may have the effect of discouraging a proposal to acquire or otherwise obtain control of Amylin.
1991 Stock Option Plan
The 1991 Stock Option Plan expired in 2001 and no shares remain available for future grant; however, stock options issued prior to its expiration remain outstanding. The 1991 Stock Option Plan provided for the grant of both incentive and non-incentive stock options. Incentive stock options are intended to qualify as "incentive stock options" within the meaning of Section 422 of the Code. As of March 1, 2004, options to purchase a total of 3,313,275 shares of our common stock were outstanding under the 1991 Stock Option Plan.
Under the 1991 Stock Option Plan, the Board or Compensation and Human Resources Committee provided for the grant of stock options to eligible employees. The Board or Compensation and Human Resources Committee determined certain provisions of each option granted, including the number of shares to be granted to each person and the time such option may be exercised. The exercise price of incentive stock options may not be less than the fair market value of our common stock on the date of the option grant. The exercise price of nonqualified stock options may not be less than 50% of the fair market value of our common stock on the date of grant. Such options generally vest according to the following schedule: 25% vest one year from the date of grant and the remainder vest daily over the following three years. However, in October 1998 we granted certain options that vested according to the following alternative schedule: 25% vested six months from the date of grant and the remainder vested daily over the following 18 months, and from time to time we have utilized alternative vesting
27
schedules. Certain options granted under the 1991 Stock Option Plan also are immediately exercisable but are subject to our right to repurchase unvested shares on termination of employment.
Substantially all of the options granted under the 1991 Stock Option Plan provide generally that the shares subject to those options shall immediately vest in full in the event of a change in control of Amylin. For those options under the 1991 Stock Option Plan, generally a "change in control" is defined as: (i) any merger, acquisition, consolidation, reorganization or other similar transaction pursuant to which our stockholders immediately prior to such merger, consolidation, reorganization or other similar transaction do not, immediately thereafter, own more than 50% of the outstanding voting securities of the resulting entity or (ii) our liquidation or dissolution or any sale of all or substantially all of our assets.
Compensation Committee Interlocks and Insider Participation
During the year ended December 31, 2003, Vaughn D. Bryson, Terrance H. Gregg and James N. Wilson served as members of our Compensation and Human Resources Committee. No member of the Compensation and Human Resources Committee was at any time during 2003 or previously an officer or employee of Amylin or its subsidiary. None of our executive officers serve as a member of the board of directors or compensation committee of any entity which has one or more executive officers serving as a member of the Board or our Compensation and Human Resources Committee.
28
REPORT OF THE COMPENSATION AND HUMAN RESOURCES COMMITTEE OF THE
BOARD OF DIRECTORS ON EXECUTIVE COMPENSATION
The Compensation and Human Resources Committee of the Board is composed solely of directors who are independent directors pursuant to rules of the Nasdaq Stock Market, Inc., non-employee directors pursuant to applicable SEC regulations and outside directors pursuant to the regulations of the Internal Revenue Service. The Compensation and Human Resources Committee is responsible for establishing and administering our executive compensation arrangements. In 2004, the name of the Compensation Committee was changed to the Compensation and Human Resources Committee.
Compensation Objectives and Implementation
The objectives of our executive compensation arrangements are to attract and retain the services of key management and to align the interests of our executives with those of our stockholders. The Compensation and Human Resources Committee endeavors to accomplish these objectives by:
- •
- Establishing compensation arrangements that are adequate to attract and retain the services of key management personnel and that deliver compensation commensurate with our performance, as measured against the achievement of operating, financial and strategic objectives and taking into account competitive compensation practices in the industry.
- •
- Providing significant equity-based incentives for executives to ensure that they are motivated over the long-term to respond to our business challenges and opportunities as owners rather than solely as employees.
Compensation Mix and Measurement
A significant portion of our annual executive compensation program is determined on the basis of corporate performance. Our executive compensation mix generally consists of a salary which in the Compensation and Human Resources Committee's opinion is adequate under the circumstances to retain the services of the executive, a cash bonus based on Amylin's performance and individual performance and stock options that are intended to provide long-term incentives tied to increases in the value of our common stock. All of our employees, including executives, are eligible to participate in our 2001 Purchase Plan and 401(k) plan. All of our full-time employees were or are also generally eligible to participate in the our cash bonus program, our expired 1991 Stock Option Plan, our expired 1991 Employee Stock Purchase Plan (the "1991 Purchase Plan") and our Incentive Plan.
Salary. Salary is targeted at competitive levels within the biotechnology industry. For the purpose of establishing these levels, we compare ourself to a selected group of biotechnology companies in stages of development similar to ours. The selected group of companies included in the salary survey is not necessarily the same companies included in the market indices included in the performance graph in this proxy statement. Although the compensation survey referred to above and the market indices included in the performance graph are broad and include companies in related industries, the survey and indices were created for different purposes and accordingly are not comparable.
For 2003, the Compensation and Human Resources Committee established target total compensation levels applicable to each executive officer based on data generated in the survey. The Compensation and Human Resources Committee made its target salary determinations subjectively after considering the competitive nature of the biotechnology industry and our need to attract and retain talented executive officers. The Compensation and Human Resources Committee also considers the level of responsibility, experience and contributions of each executive officer and sets each officer's salary taking into account the target compensation, recent corporate performance (based on the factors discussed above) and the Compensation and Human Resources Committee's evaluation of individual performance. For 2003, the salaries of three executive officers were increased. The salaries of the other
29
executive officers were not changed since they were generally at or near the median target total compensation levels determined through the survey.
Annual Cash Bonus. The Compensation and Human Resources Committee evaluates our corporate performance based on achievement of established strategic, scientific and financial goals. In 1997, we implemented a cash bonus program for officers and full-time employees based on corporate and individual performance. Beginning in 2001, our annual cash bonus program was revised to provide greater bonus differentiation based on individual performance, however, corporate performance remains the primary determinant for the bonus and all officers and other full-time employees remain eligible. No bonuses were paid for 2001 under this program. In 2002, we achieved important corporate performance goals and, in early 2003, the Compensation and Human Resources Committee authorized cash bonuses for officers and other employees who performed well individually in 2002. In 2003, we achieved many of our corporate performance goals and, in early 2004, the Compensation and Human Resources Committee authorized cash bonuses for officers and other employees who performed well individually in 2003. The corporate objectives for the year 2004 have been established and approved by the Compensation and Human Resources Committee and the Board.
Long-Term Incentives. Long-term incentives are provided to executives through our equity incentive program, which consists primarily of the Incentive Plan, the 2001 Purchase Plan, the expired 1991 Stock Option Plan, and the expired 1991 Purchase Plan. All of our employees are generally eligible to participate in these plans.
Stock options under both the 1991 Stock Option Plan and the Incentive Plan have a term of 10 years and are generally tied to the market valuation of our common stock, which provides an additional incentive for employees to increase stockholder value. In addition, stock options are generally subject to vesting over four years, with vesting tied to continued employment. Employees receive value from these stock options only if strategic goals are achieved and our common stock appreciates accordingly. This component is intended to retain and motivate executives to improve long-term stockholder value.
Option grant levels to executive officers are subjectively determined by the Compensation and Human Resources Committee after considering stock option grant data taken from the compensation survey referred to above, as well as the level of responsibility, experience and contributions of each executive officer. Generally, the Compensation and Human Resources Committee expects to grant options to executive officers annually as part of the performance review process for each officer. In determining the size of individual grants, the Compensation and Human Resources Committee also considers the number of shares subject to options previously granted to each executive officer, including the number of such shares that have vested and that remain unvested.
Additional long-term incentives are provided through the expired 1991 Purchase Plan and the 2001 Purchase Plan.
401(k) Matching Contribution. Since 1997, the Board has approved a discretionary 401(k) matching contribution for all 401(k) plan participants. The match has historically been equal to 50% of a participant's contributions to the plan each year up to a maximum of 3% of a participant's salary. The match has historically been made in the form of our common stock. Matching contributions are subject to a vesting schedule based on years of service with Amylin. All of our employees are generally eligible to participate in the 401(k) plan.
Chief Executive Officer Compensation
Joseph C. Cook, Jr. served as our Chief Executive Officer from March 1998 to September 2003. In March 2000, his salary was raised to $475,000 per annum and was adjusted to $500,000 per annum in March 2003. In 2001, Mr. Cook and the Compensation and Human Resources Committee agreed that
30
he would thereafter participate in our annual cash bonus program for all employees and officers rather than the bonus program under his employment agreement. Mr. Cook and the Compensation and Human Resources Committee believed this would better align his interests with our other officers and employees. In 2003, the Compensation and Human Resources Committee authorized a bonus to Mr. Cook of $475,000 for corporate and individual performance in 2002 and, in early 2004, the Compensation and Human Resources Committee authorized a bonus to Mr. Cook of $241,943 for corporate and individual performance in 2003. In addition, under the terms of his employment agreement, we reimburse Mr. Cook or pay for certain living expenses, associated travel expenses and tax gross-up payments related thereto.
Ginger Graham became our President and Chief Executive Officer on September 1, 2003. Ms. Graham has served as a member of our Board since November 1995. Her employment agreement provides for an annual salary of $500,000 and she is eligible to participate, with other full-time employees, in our cash bonus program. In addition, Ms. Graham received a stock option grant to purchase 500,000 shares of our common stock at an exercise price of $23.00 per share, the fair market value of our common stock on the date the option was granted. The option vests in the following manner: (i) 350,000 shares vest over four (4) years, with twenty-five percent (25%), or 87,500, of these shares vesting on September 1, 2004, and the remaining 262,500 shares vest thereafter on a monthly basis over a three year period; (ii) 50,000 shares vest upon the commercial launch of our drug candidate, SYMLIN®; (iii) 50,000 shares vest upon FDA acceptance of a New Drug Application for our drug candidate exenatide; and (iv) 50,000 shares vest upon commercial launch of exenatide. In 2003, Ms. Graham received $195,112 in salary. In early 2004, the Compensation and Human Resources Committee authorized a bonus to Ms. Graham of $117,067 for corporate and individual performance in 2003. In addition, under the terms of her employment agreement, we reimburse Ms. Graham or pay for certain living expenses, associated travel expenses and tax gross-up payments related thereto. Ms. Graham's salary is reviewed annually by the Compensation and Human Resources Committee on the same basis as for the other executives mentioned above.
COMPENSATION AND HUMAN RESOURCES COMMITTEE
Vaughn D. Bryson
Terrance H. Gregg
James N. Wilson
31
REPORT OF THE AUDIT COMMITTEE OF THE BOARD OF DIRECTORS
The Audit Committee reviews our corporate accounting and financial reporting process on behalf of the Board and operates under a written charter approved by the Board. The Charter was amended in 2004 and a copy of it is attached to this proxy statement as Appendix A. Management has the primary responsibility for the financial statements and the reporting process, including our internal control over financial reporting.
In this context, the Audit Committee has met and held discussions with management and our independent auditors. Management represented to the Audit Committee that our consolidated financial statements were prepared in accordance with generally accepted accounting principles, and the Audit Committee has reviewed and discussed the consolidated financial statements with management and the independent auditors. The Audit Committee discussed with the independent auditors matters required to be discussed by Statement on Auditing Standards No. 61 (Codification of Statements on Auditing Standards, AU § 380).
In addition, the Audit Committee and the independent auditors discussed the auditor's independence from Amylin and its management, including the matters in the written disclosures required by the Independence Standards Board Standard No. 1 (Independence Discussion With Audit Committees).
The Audit Committee discussed with our independent auditors the overall scope and plans for their audit. The Audit Committee meets with the independent auditors, with and without management present, to discuss the results of their examinations, the evaluations of our internal control over financial reporting, and the overall quality of our financial reporting.
In reliance on the reviews and discussions referred to above, the Audit Committee recommended to the Board, and the Board approved, that our audited financial statements be included in our Annual Report on Form 10-K for the year ended December 31, 2003, for filing with the SEC.
THE AUDIT COMMITTEE:
Howard E. Greene, Jr.
Joseph P. Sullivan
Thomas R. Testman
32
PERFORMANCE MEASUREMENT COMPARISON(1)
The following graph compares total stockholder returns of Amylin for the past 5 years to two indices: the Nasdaq CRSP Total Return Index for the Nasdaq Stock Market (U.S. companies) (the "Nasdaq-US") and the Nasdaq Pharmaceutical Index (the "Nasdaq-Pharmaceutical"). The total return for our common stock and for each index assumes the reinvestment of dividends, although dividends have never been declared on our common stock, and is based on the returns of the component companies weighted according to their capitalizations as of the end of each monthly period. The Nasdaq-US tracks the aggregate price performance of equity securities of U.S. companies traded on the Nasdaq National Market System (the "NMS"). The Nasdaq-Pharmaceutical tracks the aggregate price performance of equity securities of pharmaceutical companies traded on the NMS. During the period indicated, our common stock was traded on the NMS and was a component of both the Nasdaq-US and the Nasdaq-Pharmaceutical, except that from February 1, 1999 through February 9, 2000, our common stock was traded on the Nasdaq SmallCap Market. Our common stock was relisted on the NMS on February 10, 2000.
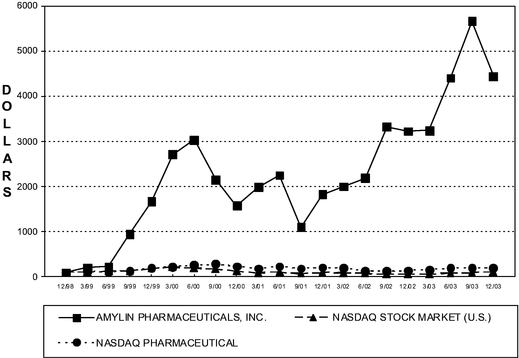
| | 12/31/98
| | 12/31/99
| | 12/31/00
| | 12/31/01
| | 12/31/02
| | 12/31/03
|
|---|
| Amylin Pharmaceuticals, Inc. | | 100.00 | | 1668.80 | | 1575.00 | | 1828.00 | | 3228.00 | | 4444.00 |
| Nasdaq Stock Market (U.S.) | | 100.00 | | 186.20 | | 126.78 | | 96.96 | | 68.65 | | 108.18 |
| Nasdaq Pharmaceutical | | 100.00 | | 195.32 | | 234.54 | | 214.66 | | 141.50 | | 203.84 |
- (1)
- The material in this section is not "soliciting material," is not deemed filed with the SEC and is not to be incorporated by reference in any filing of Amylin under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
- (2)
- Shows the cumulative total return on investment, including reinvestment of dividends, assuming an investment of $100 in each of our common stock, the Nasdaq-US and the Nasdaq-Pharmaceutical on December 31, 1998.
33
CERTAIN TRANSACTIONS
Mr. Cook owns in excess of 10% of Cambrian Associates LLC ("Cambrian"), a company that provides professional consulting services to biotech and pharmaceutical companies. In 2003 Cambrian was paid $260,220 for such services by Amylin and is continuing to provide such services in 2004. Pursuant to an agreement between Mr. Cook and Cambrian, Mr. Cook is not to receive compensation as a result of our relationship with Cambrian.
In April 2000, we extended a loan in the amount of $300,000 to Dr. Alain D. Baron, one of our executive officers, in connection with Dr. Baron's relocating to San Diego, California and purchasing a residence. The loan bore interest at 5% compounded annually, was secured by Dr. Baron's residence, and would have become due in a single payment upon the earliest to occur of the forty-fifth day following termination of Dr. Baron's employment with Amylin, the sale of the residence, or the fifth anniversary of the loan agreement. The largest outstanding balance under the loan in 2003 was $348,413.90. Dr. Baron repaid this loan in full in 2003.
Dr. Jay S. Skyler, one of our directors, has an ongoing consulting arrangement with us pursuant to which he has been granted five stock options to purchase an aggregate of up to 40,000 shares of our common stock. Two of the five options are each for 5,000 shares of our common stock, and the remaining three options are each for 10,000 shares of our common stock. The options have exercise prices per share of $0.938 and $1.344 for the two options for 5,000 shares of our common stock, and $1.031, $9.41 and $11.5625, respectively, for the three options for 10,000 shares of our common stock. The exercise prices of the options are equal to the fair market value of our common stock on the respective dates of grant. The three options for 10,000 shares of our common stock vest according to a schedule based on the amount of services provided by Dr. Skyler to us under the consulting arrangement. As of December 31, 2003 the option for 10,000 shares of our common stock at an exercise price of $1.031 per share was fully vested and had been fully exercised, the option for 10,000 shares of our common stock at an exercise price of $11.5625 had vested with respect to 8,029 shares, the remaining option for 10,000 shares had not vested, and each of the two options for 5,000 shares of our common stock was fully vested and had been fully exercised.
OTHER MATTERS
The Board of Directors knows of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the meeting, it is the intention of the persons named in the accompanying proxy to vote on such matters in accordance with their best judgment.
| | | By Order of the Board of Directors |
|
|
 |
| | | Ginger L. Graham
President and Chief Executive Officer |
April 12, 2004
A copy of our Annual Report on Form 10-K for the year ended December 31, 2003 filed with the SEC is available without charge upon written request to: Investor Relations, Amylin Pharmaceuticals, Inc., 9360 Towne Centre Drive, Suite 110, San Diego, California 92121.
34
APPENDIX A
AMYLIN PHARMACEUTICALS, INC.
AUDIT COMMITTEE CHARTER
The purpose of the audit committee (the "Committee") of the Board of Directors ("Board") of Amylin Pharmaceuticals, Inc. (the "Company") is to act on behalf of and provide assistance to the Board in fulfilling its oversight responsibility to the shareholders and others relating to: (i) the integrity of the Company's financial statements; (ii) the financial reporting process; (iii) the systems of internal accounting and financial controls; (iv) the performance of the Company's independent auditors ("the Auditors"); (v) the Auditors' qualifications and independence; and (vi) other duties established by the Bylaws of the Company and all applicable laws, rules and regulations, including rules promulgated by The NASDAQ Stock Market, Inc. (collectively, the "Requirements"). It is the intent of the Board to comply with the Requirements applicable to this Committee and the charter. To the extent any Requirements are added or amended, this charter shall be deemed to incorporate such additions or amendments.
The Committee shall consist of at least three (3) directors. All members of the Committee shall be "independent directors." (i.e., those directors who neither are officers or employees of the Company or its' subsidiaries nor have a relationship which, in the opinion of the Board, would interfere with exercise of independent judgment in carry out the responsibilities of a director). Members shall be "independent" under the rules of The NASDAQ Stock Market, Inc. and shall meet any other applicable qualifications established by the Requirements. Members of the Committee shall not receive any compensation from the Company other than for Board or committee service. All Committee members shall be financially literate and able to read and understand financial statements, and at least one member shall be a "financial expert," as defined by applicable Requirements.
Members shall be appointed by the Board, and shall serve at the pleasure of the Board and for such term or terms as the Board may determine.
The Board shall designate one member of the Committee as its chair. The majority of the members shall constitute a quorum. The Committee shall meet in person or telephonically at least four times a year and at other times as deemed necessary or desirable by the Committee or its chair. The Committee may also take action by unanimous written consent.
In discharging its role, the Committee is empowered to investigate any matter brought to its attention with full access to all books, records, facilities, and personnel of the Company. In so doing, it is the responsibility of the Committee to maintain unrestricted and open communication among the Committee, the Auditors, and management of the Company.
The Committee shall endeavor to assure that the Committee and the Company operate in accordance with all applicable Requirements on an on-going basis. The primary responsibility of the Committee is to oversee the Company's financial reporting process on behalf of the Board and report the results of their activities to the Board. For purposes of clarification, management is responsible for the preparation, presentation, and integrity of the Company's financial statements and for the appropriateness of the accounting principles and reporting policies that are used by the Company. The
A-1
Auditors are responsible for auditing the Company's financial statements and for reviewing the Company's unaudited interim financial statements.
The Committee should discuss with management and the Auditors the overall corporate "tone" for quality financial reporting, sound business risk practices, and ethical financial behavior. The Committee shall develop and implement procedures, as it deems appropriate, to enable it to accomplish the tasks outlined in this charter. The procedures may be modified from time to time by the Committee to address any issues, concerns or Requirements at the Committee's discretion.
The Committee shall be the sole authority for the appointment and termination (subject, if applicable, to shareholder ratification), compensation, and oversight of the Auditors; provided that the primary auditing partner for its Auditors shall be rotated in accordance with any applicable Requirements. The Committee shall approve all audit services provided by the Auditors and shall approve in advance all non-audit services performed by the Auditors. The Committee shall not engage the Auditors to perform specific non-audit services proscribed by any Requirement. At least annually, the Committee shall receive and review a written report from the Auditors delineating all relationships between the Auditors and the Company, to consider and discuss with the Auditors any disclosed relationships or services that could affect the Auditors' objectivity and independence, and to assess and otherwise take appropriate action to oversee the independence of the Auditors.
The Committee shall discuss with the Auditors the overall scope and plans for their respective audits, including the adequacy of staffing and compensation. The Committee shall review management's assertion on its assessment of the effectiveness of internal controls as of the end of the each fiscal year and the Auditors' report on management's assertion. The Committee shall discuss with management and the Auditors the adequacy and effectiveness of the accounting and financial controls, including the Company's policies and procedures to assess, monitor, and manage business risk.
On a periodic basis, the Committee shall meet separately with management and the Auditors to discuss issues and concerns warranting Committee attention. The Committee shall provide sufficient opportunity for the Auditors to meet privately with the members of the Committee. The Committee shall review with the Auditors any audit problems or difficulties and management's response.
The Committee shall review, upon completion of the annual audit, the financial statements to be included in the Company's Annual Report on Form 10-K.
The Committee shall discuss with the Auditors and management results of the annual audit, including the Auditors' assessment of the quality, not just acceptability, of accounting principles, the reasonableness of significant judgments and estimates (including material changes in estimates), and the adequacy of disclosures in the financial statements and any other written matters required to be communicated to the Committee by the Auditors under Statement on Auditing Standards No. 61 or any other Requirements.
The Committee shall discuss with management and the Auditors the results of the Auditors' review of the Company's quarterly financial statements, prior to public disclosure of quarterly financial information, if practicable, or filing with the Securities and Exchange Commission of the Company's Quarterly Report on Form 10-Q, and any other matters required to be communicated to the Committee by the Auditors under Statement on Auditing Standards No. 61 or any other Requirements. The chair of the Committee may represent the entire Committee for purposes of this discussion.
The Committee shall review and discuss with management and the Auditors, as appropriate, the Company's disclosures contained under the caption "Management's Discussion and Analysis of Financial Condition and Results of Operations" in its periodic reports to be filed with the Securities and Exchange Commission.
A-2
The Committee shall review and discuss with management and the Auditors, as appropriate, earnings press releases as well as the substance of financial information and earnings guidance provided to analysts and ratings agencies.
The Committee shall discuss with management procedures for the receipt, retention and treatment of complaints received by the Company regarding accounting, internal accounting controls, or auditing matters, including the confidential and anonymous submission by employees of concerns regarding questionable accounting or auditing matters.
The Committee shall review and approve all related-party transactions that are required to be disclosed by applicable Requirements.
The Committee may delegate approval authority to a member of the Committee. The decisions of any Committee member to whom approval authority is delegated shall be presented to the full Committee at its next scheduled meeting.
The Committee shall produce the following reports and provide them to the Board.
1. An annual report of the Committee for inclusion in the Company's annual proxy statement in accordance with Requirements.
2. An annual performance evaluation of the Committee, which evaluation must compare the performance of the Committee with the requirements of this charter. The performance evaluation should also evaluate the adequacy of the charter and recommend to the Board any improvements to this charter deemed necessary or desirable by the Committee. The performance evaluation by the Committee shall be conducted in such manner as the Committee deems appropriate. The report to the Board may take the form of an oral report by the chair of the Committee or any other member of the Committee designated by the Committee to make this report.
3. A summary of the actions at each Committee meeting, which shall be presented to the Board for review and presented to the Secretary of the Company for inclusion in the Company's minute books.
The Committee shall have full power and authority to discharge its duties and responsibilities, including the authority to select, retain, terminate, and approve the fees and other retention terms of special counsel or other experts or consultants, as it deems appropriate, without seeking approval of the Board or management.
A-3
AMYLIN PHARMACEUTICALS, INC.
PROXY SOLICITED BY THE BOARD OF DIRECTORS
FOR THE ANNUAL MEETING OF STOCKHOLDERS TO BE HELD ON MAY 5, 2004
The undersigned hereby appoints Ginger L. Graham, Mark G. Foletta and Daniel M. Bradbury, and each of them, as attorneys and proxies of the undersigned, with full power of substitution, to vote all of the shares of stock of Amylin Pharmaceuticals, Inc. (the "Company") which the undersigned may be entitled to vote at the Annual Meeting of Stockholders of the Company to be held at the Company's facilities, located at 9360 Towne Centre Drive, Suite 110, San Diego, California, 92121, on Wednesday, May 5, 2004, at 10:00 a.m., local time, and at any and all continuations, adjournments or postponements thereof, with all powers that the undersigned would possess if personally present, upon and in respect of the matters on the reverse side and in accordance with the following instructions, with discretionary authority as to any and all other matters that may properly come before the meeting.
UNLESS A CONTRARY DIRECTION IS INDICATED, THIS PROXY WILL BE VOTED FOR ALL NOMINEES LISTED IN PROPOSAL 1 AND FOR PROPOSALS 2 AND 3, AS MORE SPECIFICALLY DESCRIBED IN THE PROXY STATEMENT. IF SPECIFIC INSTRUCTIONS ARE INDICATED, THIS PROXY WILL BE VOTED IN ACCORDANCE THEREWITH.
(Continued on other side)
AMYLIN PHARMACEUTICALS, INC.
c/o AMERICAN STOCK TRANSFER
59 MAIDEN LANE
NEW YORK, NY 10038 | | VOTE BY INTERNET —www.proxyvote.com
Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 P.M. Eastern Time the day before the cut-off date or meeting date. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. |
| | | VOTE BY PHONE — 1-800-690-6903
Use any touch-tone telephone to transmit your voting instructions up until 11:59 P.M. Eastern Time the day before the cut-off date or meeting date. Have your proxy card in hand when you call and then follow the instructions. |
| | | VOTE BY MAIL
Mark, sign, and date your proxy card and return it in the postage-paid envelope we have provided or return it to Amylin Pharmaceuticals, Inc., c/o ADP, 51 Mercedes Way, Edgewood, NY 11717. |
THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED.
DETACH AND RETURN THIS PORTION ONLY
AMYLIN PHARMACEUTICALS, INC.
The Board of Directors recommends a vote for the nominees for director listed below.
| Proposal 1: | To elect directors to hold office until the next Annual Meeting of Stockholders and until their successors are elected. | | For
All
o | | Withhold
All
o | | For All
Except
o | | To withhold authority to vote for any nominee(s), mark "For All Except" and write the nominee(s)' number(s) on the line below. |
| | | | | | | | | | | | | | |
| Nominees: | 01) Vaughn D. Bryson, 02) Joesph C. Cook, Jr., 03) Ginger L. Graham, 04) Howard E. Greene, Jr., 05) Terrance H. Gregg, 06) Jay S. Skyler, 07) Joseph P. Sullivan, 08) Thomas R. Testman and 09) James N. Wilson | | | | | | | |
|
The Board of Directors recommends a vote for Proposal 2 |
|
For |
|
Against |
|
Abstain |
Proposal 2: |
To approve an increase in the aggregate number of shares of Common Stock authorized for issuance under the Company's 2001 Employee Stock Purchase Plan by 750,000 shares. |
|
o |
|
o |
|
o |
| | | | | | | | | | | | | | |
The Board of Directors recommends a vote for Proposal 3. |
|
|
|
|
|
|
Proposal 3: |
To ratify the selection of Ernst & Young LLP as independent auditors of the Company for its fiscal year ending December 31, 2004. |
|
o |
|
o |
|
o |
Please sign exactly as your name appears hereon. If the stock is registered in the names of two or more persons, each should sign. Executors, administrators, trustees, guardians and attorneys-in-fact should add their titles. If signer is a corporation, please give full corporate name and have a duly authorized officer sign, stating title. If signer is a partnership, please sign in partnership name by authorized person.
Please vote, date and promptly return this proxy in the enclosed return envelope which is postage prepaid if mailed in the United States.
Signature [PLEASE SIGN WITHIN BOX] | |
Date | |
Signature [Joint Owners] | |
Date |
|
|
|
|
|
|
|
QuickLinks
INFORMATION CONCERNING SOLICITATION AND VOTINGPROPOSAL 1 ELECTION OF DIRECTORSPROPOSAL 2 APPROVAL OF AMENDMENT OF THE 2001 EMPLOYEE STOCK PURCHASE PLANEQUITY COMPENSATION PLAN INFORMATIONPROPOSAL 3 RATIFICATION OF SELECTION OF INDEPENDENT AUDITORSSECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENTEXECUTIVE COMPENSATIONCOMPENSATION OF EXECUTIVE OFFICERSSTOCK OPTION GRANTS AND EXERCISESREPORT OF THE COMPENSATION AND HUMAN RESOURCES COMMITTEE OF THE BOARD OF DIRECTORS ON EXECUTIVE COMPENSATIONREPORT OF THE AUDIT COMMITTEE OF THE BOARD OF DIRECTORSCERTAIN TRANSACTIONSOTHER MATTERSAPPENDIX A AMYLIN PHARMACEUTICALS, INC. AUDIT COMMITTEE CHARTERAMYLIN PHARMACEUTICALS, INC. PROXY SOLICITED BY THE BOARD OF DIRECTORS FOR THE ANNUAL MEETING OF STOCKHOLDERS TO BE HELD ON MAY 5, 2004