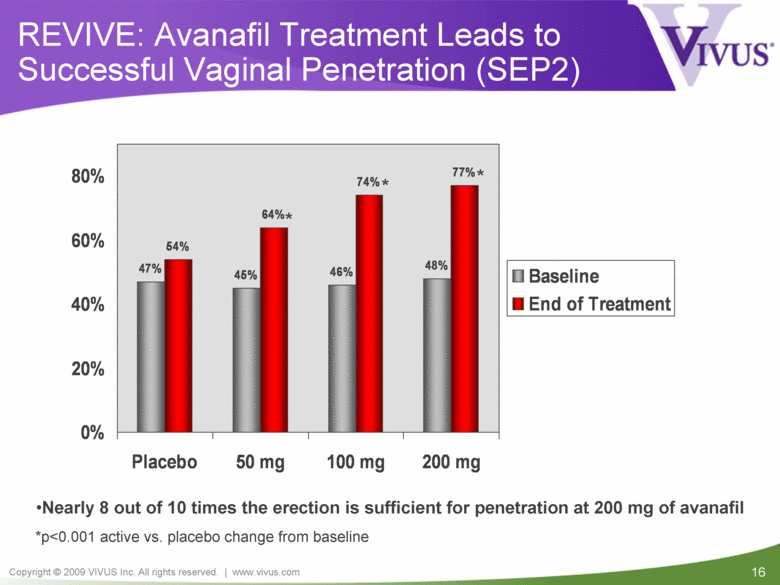
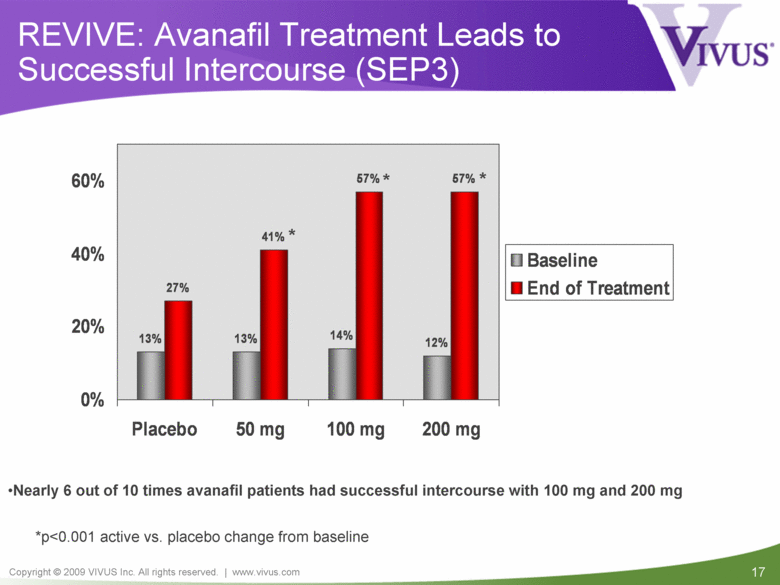
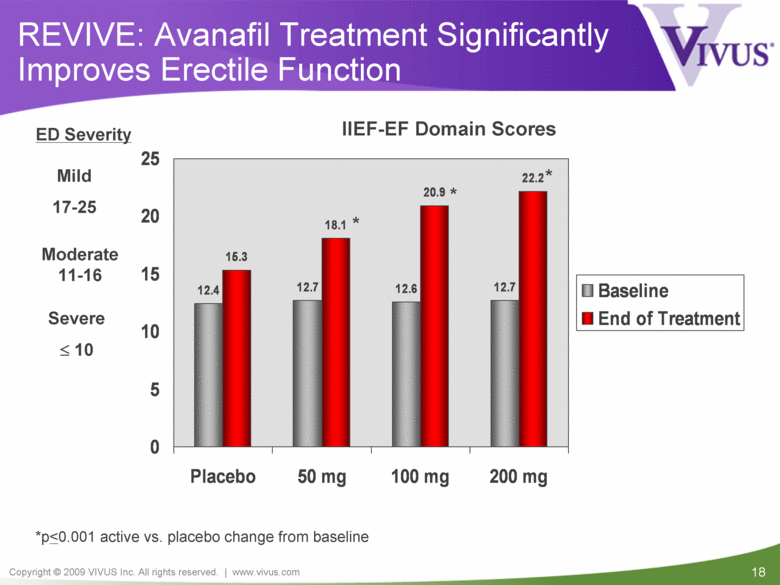
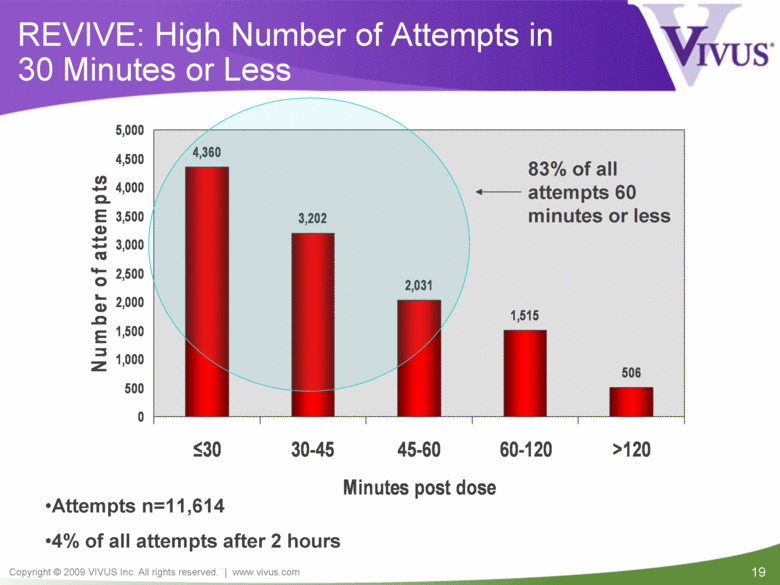
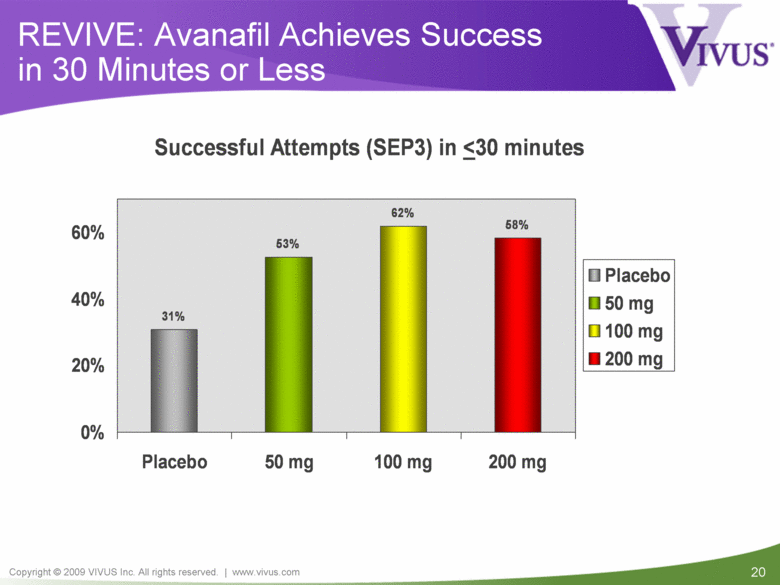
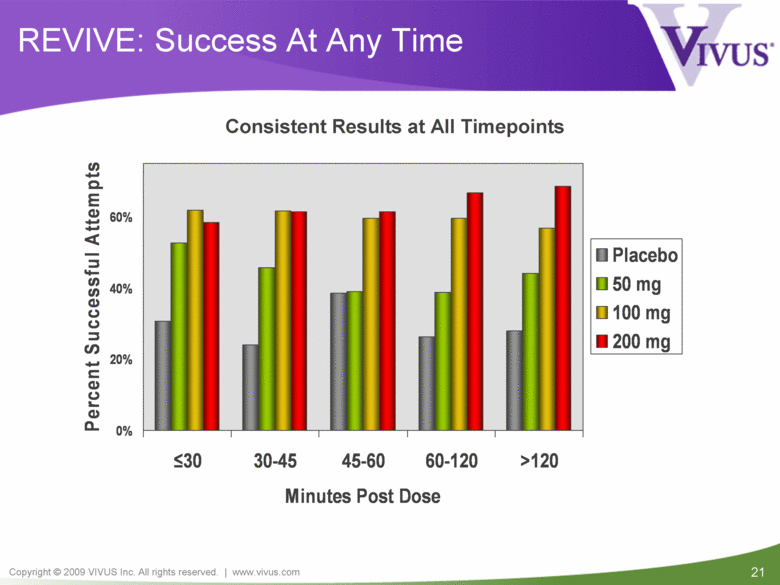
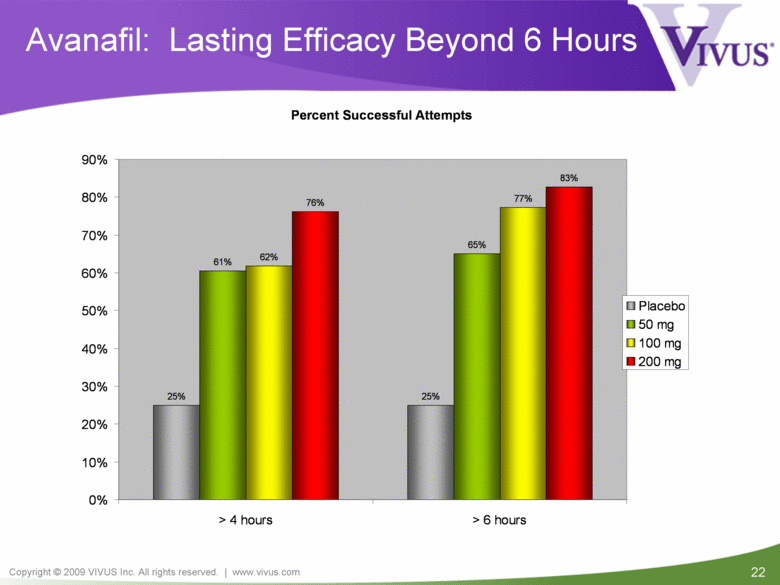
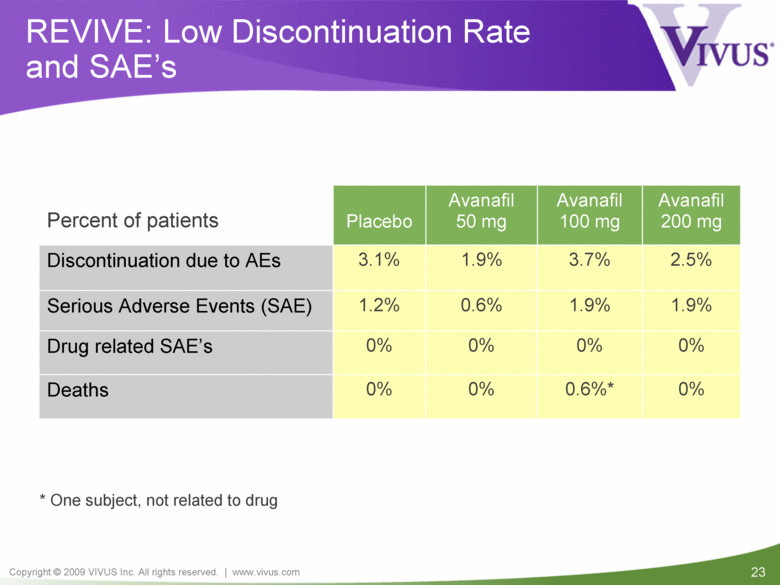
| VIVUS cautions you that statements included in this presentation that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests,“ "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the Company's current beliefs and expectations. These forward-looking statements include statements regarding the efficacy and safety of avanafil, the potential for, and timing of, an NDA submission for avanafil, the commercial and therapeutic potential of avanafil, and the potential to obtain regulatory approval for, and effectively treat erectile dysfunction with, avanafil. The inclusion of forward looking statements should not be regarded as a representation by VIVUS that any of its plans will be achieved. Actual results may differ from those set forth in this presentation due to the risk and uncertainties inherent in the VIVUS business, including, without limitation: additional analyses of data from the avanafil Phase 3 trials and any other clinical trials of avanafil may produce negative or inconclusive results, or may be inconsistent with previously announced results or previously conducted clinical trials; the FDA may not agree with the Company’s interpretation of efficacy and safety results; earlier clinical trials may not be predictive of future results; Avanafil may not receive regulatory approval on a timely basis or at all, and the FDA may require VIVUS to complete additional clinical, non-clinical or other requirements prior to the submission or the approval of NDAs for either product candidate; the potential for adverse safety findings relating to avanafil to delay or prevent regulatory approval or commercialization, or result in product liability claims, including serious adverse events that are not characterized by clinical investigators as possibly related to avanafil; the third parties on whom VIVUS relies to assist with the development programs for avanafil, including clinical investigators, contract laboratories, clinical research organizations and manufacturing organizations, may not successfully carry out their contractual duties or obligations or meet expected deadlines, and the quality or accuracy of the data or materials generated by such third parties may be of insufficient quality to include in the Company’s regulatory submissions; the ability of VIVUS and its licensors to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of its product candidates; VIVUS may be unable to enter a collaboration or partnership relating to avanafil for promotion to broader markets on attractive terms or at all; and other risks described in the Company's filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and VIVUS undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. Information included herein is based on the Company’s review and evaluation of the clinical data. All conclusions and determinations contained herein are subject to the Company’s further analysis of the clinical data. The ultimate determination of the safety and efficacy of avanafil will be made by the FDA and other relevant regulatory authorities Forward-Looking Statements |