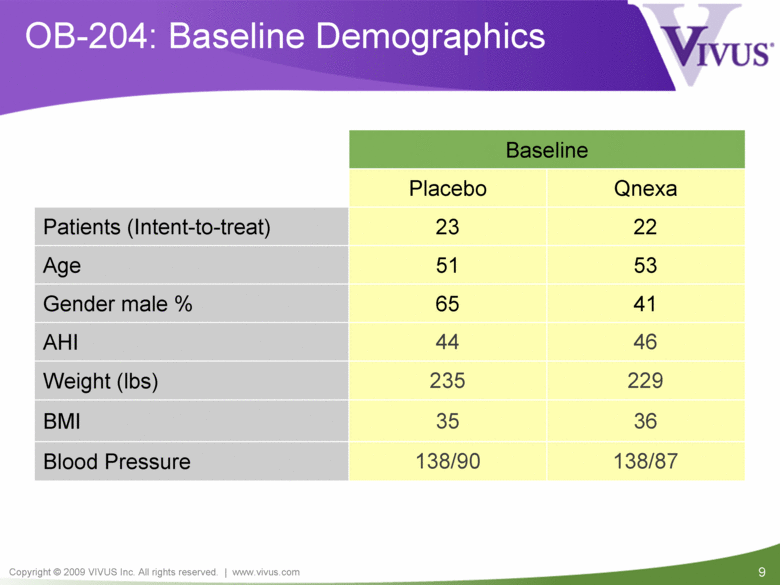
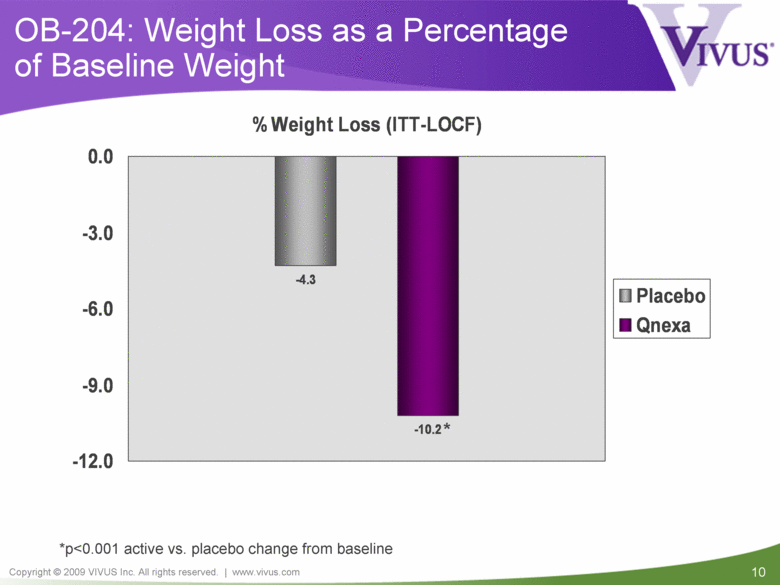
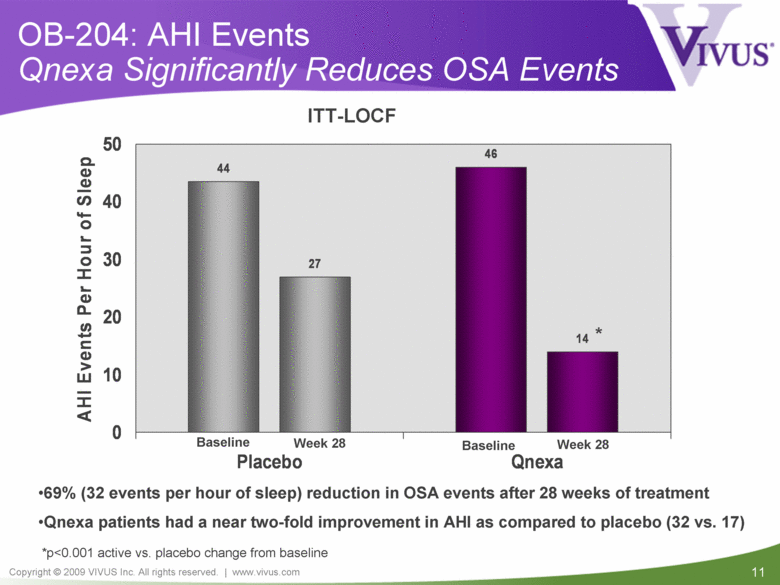
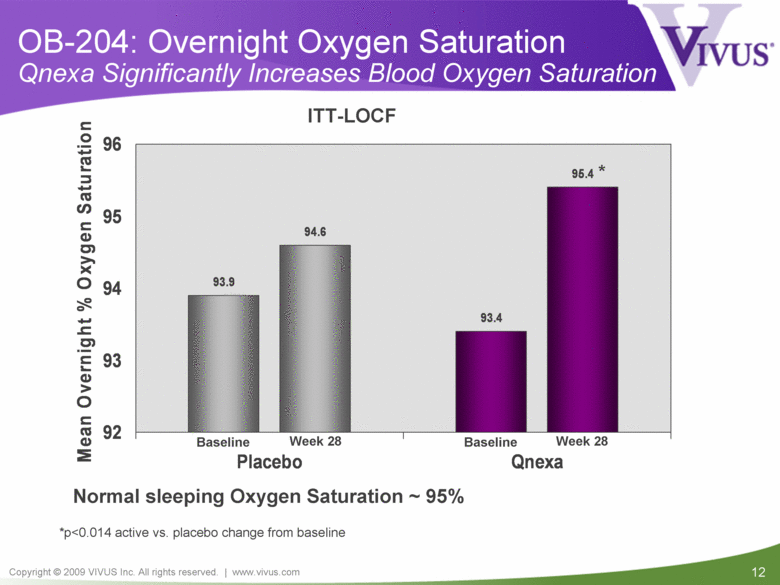
| VIVUS cautions you that statements included in this presentation that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests,“ "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on VIVUS’ current beliefs and expectations. These forward-looking statements include statements regarding the efficacy and safety of Qnexa, the potential for, and timing of, any NDA submission for Qnexa, the commercial and therapeutic potential of Qnexa, and the potential to obtain regulatory approval for, and effectively treat obstructive sleep apnea (OSA) with, Qnexa. The inclusion of forward looking statements should not be regarded as a representation by VIVUS that any of its plans will be achieved. Actual results may differ from those set forth in this presentation due to the risk and uncertainties inherent in VIVUS’ business, including, without limitation: additional analyses of data from the Qnexa Phase 3 obesity trials, the results of this single center study in OSA and any other clinical trials of Qnexa may produce negative or inconclusive results, or may be inconsistent with previously announced results or previously conducted clinical trials; the FDA may not agree with VIVUS’ interpretation of efficacy and safety results; earlier clinical trials may not be predictive of future results; Qnexa may not receive regulatory approval on a timely basis or at all, and the FDA may require VIVUS to complete additional clinical, non-clinical or other requirements prior to the submission or the approval of NDAs for a product candidate; the potential for adverse safety findings relating to Qnexa to delay or prevent regulatory approval or commercialization, or result in product liability claims, including serious adverse events that are not characterized by clinical investigators as possibly related to Qnexa; the third parties on whom VIVUS relies to assist with the development programs for Qnexa, including clinical investigators, contract laboratories, clinical research organizations and manufacturing organizations, may not successfully carry out their contractual duties or obligations or meet expected deadlines, and the quality or accuracy of the data or materials generated by such third parties may be of insufficient quality to include in VIVUS’ regulatory submissions; the ability of VIVUS and its licensors to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of its product candidates; VIVUS may be unable to enter a collaboration or partnership relating to Qnexa for promotion to broader markets on attractive terms or at all; and other risks described in VIVUS’ filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and VIVUS undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. Information included herein is based on VIVUS’ review and evaluation of the clinical data. All conclusions and determinations contained herein are subject to VIVUS’ Qnexa analysis of the clinical data. The ultimate determination of the safety and efficacy of Qnexa will be made by the FDA and other relevant regulatory authorities. Forward-Looking Statements |