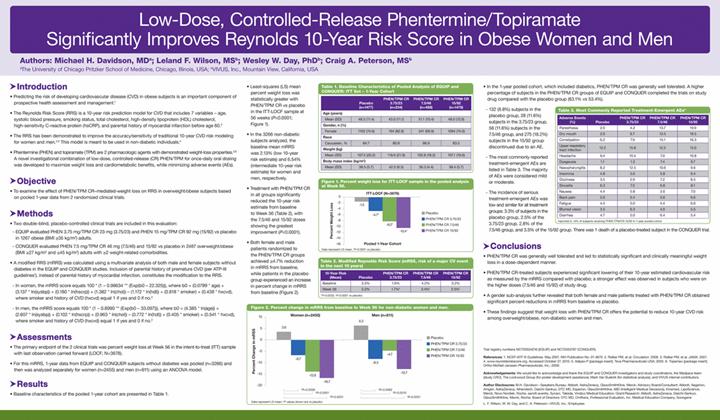
Below is a reproduction of the contents of the poster entitled “Low-Dose, Controlled-Release Phentermine/Topiramate Significantly Improves Reynolds 10-Year Risk Score in Obese Women and Men”.
Low-Dose, Controlled-Release Phentermine/Topiramate
Significantly Improves Reynolds 10-Year Risk Score in Obese Women and Men
Authors: Michael H. Davidson, MD(a); Leland F. Wilson, MS(b); Wesley W. Day, PhD(b); Craig A. Peterson, MS(b)
(a)The University of Chicago Pritzker School of Medicine, Chicago, Illinois, USA; (b)VIVUS, Inc., Mountain View, California, USA
· Introduction
· Predicting the risk of developing cardiovascular disease (CVD) in obese subjects is an important component of prospective health assessment and management.(1)
· The Reynolds Risk Score (RRS) is a 10-year risk prediction model for CVD that includes 7 variables - age, systolic blood pressure, smoking status, total cholesterol, high-density lipoprotein (HDL) cholesterol, high-sensitivity C-reactive protein (hsCRP), and parental history of myocardial infarction before age 60.(2)
· The RRS has been demonstrated to improve the accuracy/sensitivity of traditional 10-year CVD risk modeling for women and men.(2),(3) This model is meant to be used in non-diabetic individuals.(4)
· Phentermine (PHEN) and topiramate (TPM) are 2 pharmacologic agents with demonstrated weight-loss properties.(5),(6) A novel investigational combination of low-dose, controlled-release (CR) PHEN/TPM for once-daily oral dosing was developed to maximize weight loss and cardiometabolic benefits, while minimizing adverse events (AEs).
· Objective
· To examine the effect of PHEN/TPM CR–mediated-weight loss on RRS in overweight/obese subjects based on pooled 1-year data from 2 randomized clinical trials.
· Methods
· Two double-blind, placebo-controlled clinical trials are included in this evaluation:
· EQUIP evaluated PHEN 3.75 mg/TPM CR 23 mg (3.75/23) and PHEN 15 mg/TPM CR 92 mg (15/92) vs placebo in 1267 obese (BMI >35 kg/m2) adults.
· CONQUER evaluated PHEN 7.5 mg/TPM CR 46 mg (7.5/46) and 15/92 vs placebo in 2487 overweight/obese (BMI >27 kg/m2 and <45 kg/m2) adults with >2 weight-related comorbidities.
· A modified RRS (mRRS) was calculated using a multivariate analysis of both male and female subjects without diabetes in the EQUIP and CONQUER studies. Inclusion of parental history of premature CVD (per ATP-III guidelines(1)), instead of parental history of myocardial infarction, constitutes the modification to the RRS.
· In women, the mRRS score equals 100 * (1 – 0.98634 ** (Exp(b0 - 22.325))), where b0 = (0.0799 * age) + (3.137 * ln(sysbp)) + (0.180 * ln(hscrp)) + (1.382 * ln(chol)) – (1.172 * ln(hdl)) + (0.818 * smoker) + (0.438 * hxcvd), where smoker and history of CVD (hxcvd) equal 1 if yes and 0 if no.(3)
· In men, the mRRS score equals 100 * (1 – 0.8990 ** (Exp(b0 - 33.097))), where b0 = (4.385 * ln(age)) + (2.607 * ln(sysbp)) + (0.102 * ln(hscrp)) + (0.963 * ln(chol)) – (0.772 * ln(hdl)) + (0.405 * smoker) + (0.541 * hxcvd), where smoker and history of CVD (hxcvd) equal 1 if yes and 0 if no.(2)
· Assessments
· The primary endpoint of the 2 clinical trials was percent weight loss at Week 56 in the intent-to-treat (ITT) sample with last observation carried forward (LOCF; N=3678).
· For this mRRS, 1-year data from EQUIP and CONQUER subjects without diabetes was pooled (n=3266) and then was analyzed separately for women (n=2455) and men (n=811) using an ANCOVA model.
· Results
· Baseline characteristics of the pooled 1-year cohort are presented in Table 1.
Table 1. Baseline Characteristics of Pooled Analysis of EQUIP and CONQUER: ITT Set - 1-Year Cohort
| | Placebo
(n=1477) | | PHEN/TPM CR
3.75/23
(n=234) | | PHEN/TPM CR
7.5/46
(n=488) | | PHEN/TPM CR
15/92
(n=1479) | |
Age (years) | | | | | | | | | |
Mean (SD) | | 48.5 (11.4) | | 43.0 (11.1) | | 51.1 (10.4) | | 48.0 (12.0) | |
Gender, n (%) | | | | | | | | | |
Female | | 1102 (74.6) | | 194 (82.9) | | 341 (69.9) | | 1094 (74.0) | |
Race | | | | | | | | | |
Caucasian, % | | 84.7 | | 80.8 | | 86.9 | | 83.5 | |
Weight (kg) | | | | | | | | | |
Mean (SD) | | 107.5 (20.2) | | 118.6 (21.9) | | 102.8 (18.2) | | 107.1 (19.6) | |
Body mass index (kg/m2) | | | | | | | | | |
Mean (SD) | | 38.5 (5.7) | | 42.5 (6.5) | | 36.3 (4.4) | | 38.4 (5.7) | |
· Least-squares (LS) mean percent weight loss was statistically greater with PHEN/TPM CR vs placebo in the ITT-LOCF sample at 56 weeks (P<0.0001; Figure 1).

· In the 3266 non-diabetic subjects analyzed, the baseline mean mRRS was 2.13% (low 10-year risk estimate) and 6.54% (intermediate 10-year risk estimate) for women and men, respectively.
· Treatment with PHEN/TPM CR in all groups significantly reduced the 10-year risk estimate from baseline to Week 56 (Table 2), with the 7.5/46 and 15/92 doses showing the greatest improvement (P<0.0001).
Table 2. Modified Reynolds Risk Score (mRSS, risk of a major CV event in the next 10 years)
10-Year Risk
(Mean) | | Placebo | | PHEN/TPM CR
3.75/23 | | PHEN/TPM CR
7.5/46 | | PHEN/TPM CR
15/92 | |
Baseline | | 3.3 | % | 1.9 | % | 4.2 | % | 3.2 | % |
Week 56 | | 3.2 | % | 1.7 | %* | 3.4% | † | 2.5% | † |
*P=0.0032; †P<0.0001 vs placebo
· Both female and male patients randomized to the PHEN/TPM CR groups achieved >4.7% reduction in mRRS from baseline, while patients in the placebo group experienced an increase in percent change in mRRS from baseline (Figure 2).

· In the 1-year pooled cohort, which included diabetics, PHEN/TPM CR was generally well tolerated. A higher percentage of subjects in the PHEN/TPM CR groups of EQUIP and CONQUER completed the trials on study drug compared with the placebo group (63.1% vs 53.4%).
· 132 (8.8%) subjects in the placebo group, 28 (11.6%) subjects in the 3.75/23 group, 58 (11.6%) subjects in the 7.5/46 group, and 275 (18.2%) subjects in the 15/92 group discontinued due to an AE.
· The most commonly reported treatment-emergent AEs are listed in Table 3. The majority of AEs were considered mild or moderate.
Table 3. Most Commonly Reported Treatment-Emergent AEs*
Adverse Events
(%) | | Placebo | | PHEN/TPM CR
3.75/23 | | PHEN/TPM CR
7.5/46 | | PHEN/TPM CR
15/92 | |
Paresthesia | | 2.0 | | 4.2 | | 13.7 | | 19.9 | |
Dry mouth | | 2.9 | | 6.7 | | 13.5 | | 19.5 | |
Constipation | | 6.2 | | 7.9 | | 15.1 | | 16.3 | |
Upper respiratory tract infection | | 12.2 | | 15.8 | | 12.2 | | 13.0 | |
Headache | | 9.4 | | 10.4 | | 7.0 | | 10.8 | |
Dysgeusia | | 1.1 | | 1.3 | | 7.4 | | 9.7 | |
Nasopharyngitis | | 8.2 | | 12.5 | | 10.6 | | 9.6 | |
Insomnia | | 4.8 | | 5.0 | | 5.8 | | 9.4 | |
Dizziness | | 3.5 | | 2.9 | | 7.2 | | 8.5 | |
Sinusitis | | 6.3 | | 7.5 | | 6.8 | | 8.1 | |
Nausea | | 4.4 | | 5.8 | | 3.6 | | 7.0 | |
Back pain | | 5.0 | | 5.4 | | 5.6 | | 6.6 | |
Fatigue | | 4.4 | | 5.0 | | 4.4 | | 6.0 | |
Blurred vision | | 3.5 | | 6.3 | | 4.0 | | 5.5 | |
Diarrhea | | 4.7 | | 5.0 | | 6.4 | | 5.4 | |
*reported in >5% of subjects receiving PHEN TPM/CR 15/92 in 1-year pooled cohort
· The incidence of serious treatment-emergent AEs was low and similar for all treatment groups: 3.3% of subjects in the placebo group, 2.5% of the 3.75/23 group, 2.8% of the 7.5/46 group, and 3.5% of the 15/92 group. There was 1 death of a placebo-treated subject in the CONQUER trial.
· Conclusions
· PHEN/TPM CR was generally well tolerated and led to statistically significant and clinically meaningful weight loss in a dose-dependent manner.
· PHEN/TPM CR-treated subjects experienced significant lowering of their 10-year estimated cardiovascular risk as measured by the mRRS compared with placebo; a stronger effect was observed in subjects who were on the higher doses (7.5/46 and 15/92) of study drug.
· A gender sub-analysis further revealed that both female and male patients treated with PHEN/TPM CR obtained significant percent reductions in mRRS from baseline vs placebo.
· These findings suggest that weight loss with PHEN/TPM CR offers the potential to reduce 10-year CVD risk among overweight/obese, non-diabetic women and men.
Trial registry numbers NCT00554216 (EQUIP) and NCT0053787 (CONQUER).
References: 1. NCEP-ATP III Guidelines. May 2001. NIH Publication No. 01-3670. 2. Ridker PM, et al. Circulation. 2008. 3. Ridker PM, et al. JAMA. 2007. 4. www.reynoldsriskscore.org. Accessed October 27, 2010. 5. Adipex-P [package insert]. Teva Pharmaceuticals USA; 2005. 6. Topamax [package insert]. Ortho-McNeil-Janssen Pharmaceuticals, Inc.; 2009.
Acknowledgements: We would like to acknowledge and thank the EQUIP and CONQUER investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), Mark Van Buskirk (for statistical analysis), and VIVUS internal contributors.
Author Disclosures: M.H. Davidson–Speakers Bureau: Abbott, AstraZeneca, GlaxoSmithKline, Merck; Advisory Board/Consultant: Abbott, Aegerion, Amgen, AstraZeneca, Atherotech, Daiichi-Sankyo, DTC MD, Esperion, GlaxoSmithKline, iMD (Intelligent Medical Decisions), Kinemed, LipoScience, Merck, Novo Nordisk, Roche, sanofi-aventis, Synarc, Takeda, Vindico Medical Education; Grant/Research: Abbott, AstraZeneca, Daiichi-Sankyo, GlaxoSmithKline, Merck, Roche; Board of Directors: DTC MD, Omthera, Professional Evaluation, Inc. Medical Education Company, Sonogene
L. F. Wilson, W. W. Day, and C. A. Peterson–VIVUS, Inc.: Employees