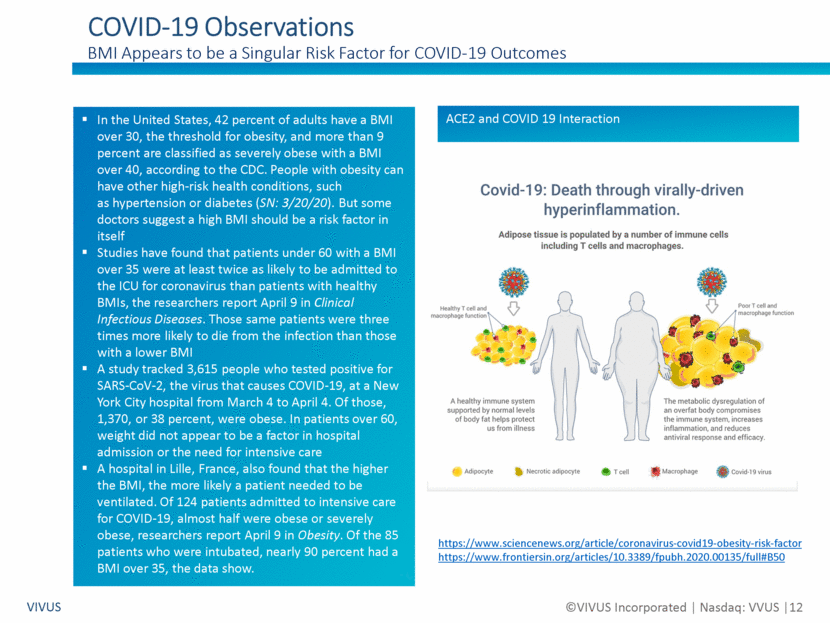
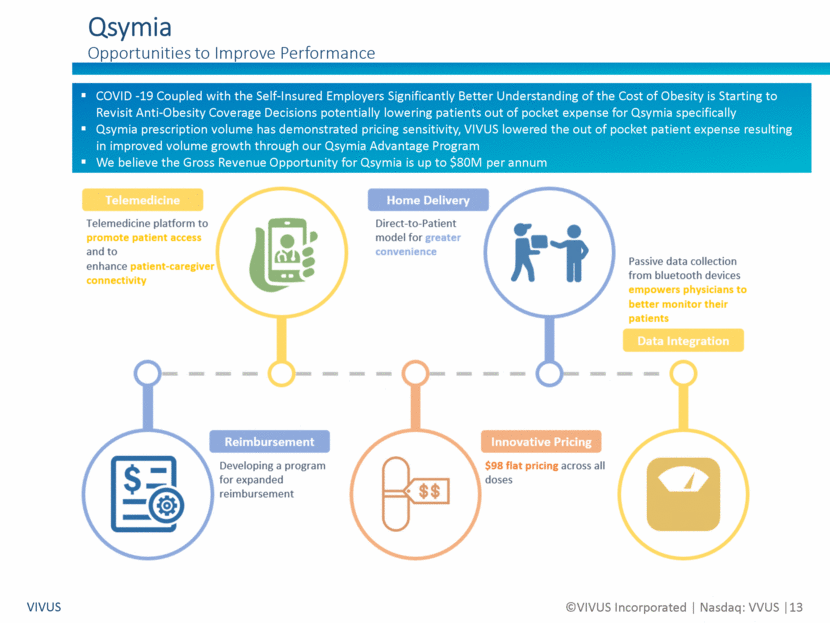
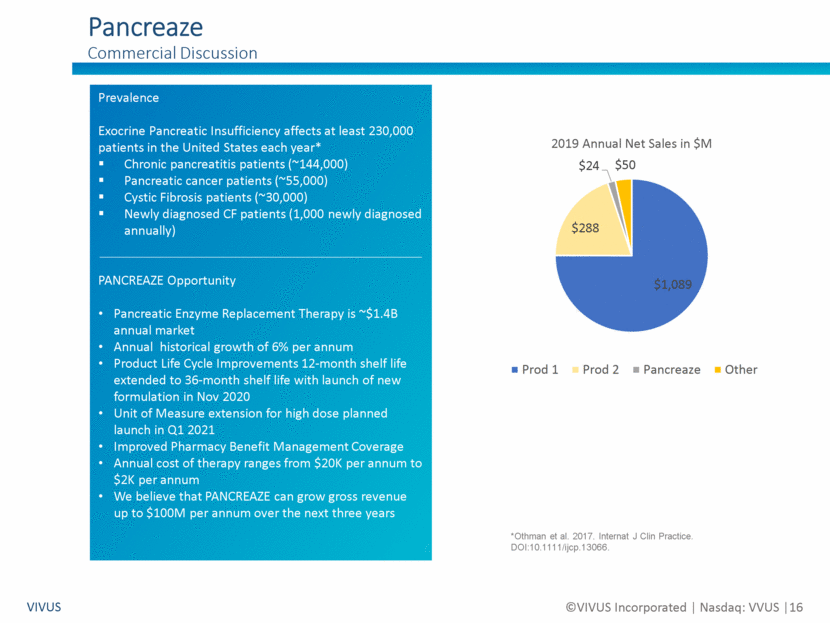
Forward Looking Statements Non-GAAP Financial Measures VIVUS ©VIVUS Incorporated Nasdaq: VVUS 2 Certain statements in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to risks, uncertainties and other factors, including risks and uncertainties related to our ability to execute on our business strategy to enhance long-term stockholder value; risks and uncertainties related to our ability to address our outstanding balance of the convertible notes due in May 2020, including our ability during the agreed upon 30-day grace period to work with IEH Biopharma LLC to restructure the outstanding principal amount of the convertible notes; risks and uncertainties related to the timing, strategy, structure and implementation of any restructuring transaction with IEH Biopharma LLC; risks and uncertainties related to a bankruptcy filing absent an agreement on a restructuring transaction in the short term; risks and uncertainties related to the effect of the recent coronavirus (COVID-19) outbreak on our business and the businesses of our partners; risks and uncertainties related to our liquidity and capital resources; risks and uncertainties related to our history of losses and variable quarterly results; risks and uncertainties related to our expected future revenues, operations and expenditures; risks and uncertainties related to the effectiveness of the VIVUS Health Platform, including its adoption by healthcare providers and its ability to improve patient outcomes and, if applicable, access to Qsymia® and PANCREAZE®; risks and uncertainties related to the timing, strategy, tactics and success of the marketing and sales of PANCREAZE, including our ability to improve patient access to PANCREAZE; risks and uncertainties related to our, or our current or potential partner’s, ability to successfully commercialize Qsymia, including our ability to improve patient and physician access to Qsymia; risks and uncertainties related to our ability to sell through the Qsymia retail pharmacy network and the Qsymia Advantage Program; risks and uncertainties related to the timing of initiation and completion of the post-approval clinical studies required as part of the approval of Qsymia by the U.S. Food and Drug Administration (“FDA”), including the Phase 4 post-marketing study of Qsymia in obese adolescents; risks and uncertainties related to the response from FDA to any data and/or information relating to post-approval clinical studies required for Qsymia; risks and uncertainties related to the impact of any possible future requirement to provide further analysis of previously submitted clinical trial data; risks and uncertainties related to the design and outcome of any clinical study required by FDA to expand the Qsymia label; risks and uncertainties related to our ability to work with FDA to significantly reduce or remove the requirements of the clinical post-approval cardiovascular outcomes trial; risks and uncertainties related to the failure to obtain FDA or foreign authority clearances or approvals and noncompliance with FDA or foreign authority regulations; risks and uncertainties related to our dialog with certain concerned member states in Europe relating to the pending decentralized Marketing Authorization Application, the timing and scope of the assessment by such Concerned Member State health authorities of our Marketing Authorization Application, and ultimately the decision of such Concerned Member State health authorities whether to grant Marketing Authorization for Qsymia in such EU countries; risks and uncertainties related to our ability to successfully develop or acquire a proprietary formulation of tacrolimus; risks and uncertainties related to our ability to identify, acquire and develop new product pipeline candidates; risks and uncertainties related to our ability to demonstrate through clinical testing the quality, safety, and efficacy of our current or future investigational drug candidates or approved products; risks and uncertainties related to the timing, strategy, tactics and success of the launches and commercialization of STENDRA/SPEDRA (avanafil) by our current or potential collaborators; risks and uncertainties related to our ability to successfully complete on acceptable terms, and on a timely basis, avanafil partnering discussions for territories under our license with MTPC in which we do not have a commercial collaboration; and risks and uncertainties related to the impact, if any, of changes to our Board of Directors and senior management team. These risks and uncertainties could cause actual results to differ materially from those referred to in these forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. Investors should read the risk factors set forth in VIVUS’ Form 10-K for the year ended December 31, 2019 as filed on March 3, 2020 and as amended on Form 10-K/A on April 29, 2020, and periodic reports filed with the Securities and Exchange Commission. VIVUS does not undertake an obligation to update or revise any forward-looking statements. Use of Non-GAAP Financial Measures We supplement our condensed consolidated financial statements presented on a GAAP basis by providing additional measures which are considered non-GAAP under applicable SEC rules, such as EBITDA and Enterprise Value. We believe that the disclosure of these non-GAAP measures provides investors with additional information that reflects the basis upon which our management assesses and operates our business. These non-GAAP financial measures are not in accordance with GAAP and should not be viewed in isolation or as a substitute for GAAP net loss and are not a substitute for, or superior to, measures of financial performance performed in conformity with GAAP.