Exhibit 99.1
Gilead to Acquire FortySeven March 2, 2020

This communication contains forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, related to Gilead, Forty Seven and the acquisition of Forty Seven by Gilead that are subject to risks, uncertainties and other factors. All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including all statements regarding the intent, belief or current expectation of the companies’ and members of their senior management team. Forward-looking statements include, without limitation, statements regarding the business combination and related matters, prospective performance and opportunities, post-closing operations and the outlook for the companies’ businesses, including, without limitation, the ability of Gilead to advance Forty Seven’s product pipeline, including magrolimab, FSI-174 and FSI-189; regulatory approval of magrolimab, FSI-174 and FSI-189 on a timely basis; the anticipated timing of clinical data; the possibility of unfavorable results from clinical trials; filings and approvals relating to the transaction; the expected timing of the completion of the transaction; the ability to complete the transaction considering the various closing conditions; difficulties or unanticipated expenses in connection with integrating the companies; and any assumptions underlying any of the foregoing. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties and are cautioned not to place undue reliance on these forward-looking statements. Actual results may differ materially from those currently anticipated due to a number of risks and uncertainties. Risks and uncertainties that could cause the actual results to differ from expectations contemplated by forward-looking statements include: uncertainties as to the timing of the tender offer and merger; uncertainties as to Forward Looking Statements h ow many of Forty Seven’s stockholders will tender their stock in the offer; the possibility that competing offers will be made; the possibility that various closing conditions for the transaction may not be satisfied or waived, including that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of the transaction; the effects of the transaction on relationships with employees, other business partners or governmental entities; the difficulty of predicting the timing or outcome of FDA approvals or actions, if any; the impact of competitive products and pricing; other business effects, including the effects of industry, economic or political conditions outside of the companies’ control; transaction costs; actual or contingent liabilities; and other risks and uncertainties detailed from time to time in the companies’ periodic reports filed with the U.S. Securities and Exchange Commission (the “SEC”), including current reports on Form 8-K, quarterly reports on Form 10-Q and annual reports on Form 10-K, as well as the Schedule 14D-9 to be filed by Forty Seven and the Schedule TO and related tender offer documents to be filed by Gilead and Toro Merger Sub, Inc., a wholly owned subsidiary of Gilead. All forward-looking statements are based on information currently available to Gilead and Forty Seven, and Gilead and Forty Seven assume no obligation and disclaim any intent to update any such forward-looking statements. 2

The tender offer described in this communication has not yet commenced. This communication is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares of Forty Seven, nor is it a substitute for any tender offer materials that Gilead, its acquisition company or Forty Seven will file with the SEC. A solicitation and an offer to buy shares of Forty Seven will be made only pursuant to an offer to purchase and related materials that Gilead intends to file with the SEC. At the time the tender offer is commenced, Gilead will file a Tender Offer Statement on Schedule TO with the SEC, and Forty Seven will file a Solicitation/Recommendation Statement on Schedule 14D-9 with the SEC with respect to the tender offer. FORTY SEVEN’s STOCKHOLDERS AND OTHER INVESTORS ARE URGED TO READ THE TENDER OFFER MATERIALS (INCLUDING AN OFFER TO PURCHASE, A RELATED LETTER OF TRANSMITTAL AND CERTAIN OTHER TENDER OFFER DOCUMENTS) AND THE SOLICITATION/RECOMMENDATION STATEMENT BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION WHICH SHOULD BE READ CAREFULLY BEFORE ANY DECISION IS MADE WITH RESPECT TO THE TENDER OFFER. The Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, will be sent to all stockholders of Forty Seven at no expense to them. The Tender Offer Statement and the Solicitation/Recommendation Statement will be made available for free at the SEC's web site at www.sec.gov. Additional copies may be obtained for free by contacting Gilead or Forty Seven. Free copies of these materials and certain other offering documents will be made available by Gilead by mail to Gilead Sciences, Inc., 333 Lakeside Drive, Foster City, CA 94404, attention: Investor Relations, by phone at 1-800-GILEAD-5 or 1-650-574-3000, or by directing requests for such materials to the information agent for the offer, which will be named in the Tender Offer Statement. Copies of the documents filed with the SEC by Forty Seven will be available free of charge under the “Investors” section of Forty Seven’s internet website at ir.fortyseveninc.com. Additional Information and Where to Find It In addition to the Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, Gilead and Forty Seven file annual, quarterly and current reports, proxy statements and other information with the SEC. Gilead’s and Forty Seven’s filings with the SEC are also available for free to the public from commercial document-retrieval services and at the website maintained by the SEC at www.sec.gov. 3

Three Pillars of Gilead’s Next Chapter + + 4 Existing Pipeline Opportunities Durable Core Business Strategy to Drive Additional Growth
Strategy to Drive Additional Growth 5 Strategy to Drive Additional Growth ++ Existing Pipeline Opportunities Durable Core Business
Gilead To Acquire Forty Seven • Forty Seven’s lead program and scientific expertise bolster Gilead’s strategic focus on expanding its pipeline and expertise in immuno-oncology beyond cell therapy • Magrolimab has the potential to be a foundational asset for Gilead’s immuno-oncology pipeline based on promising results in Phase 1b/2 clinical studies for various cancers, including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) • This deal is consistent with our strategy to pursue small and medium-sized bolt-on acquisitions that bring the best scientific innovation and talent available to Gilead • Forty Seven has a highly innovative and experienced team of individuals with expertise in a promising area of oncology, and we look forward to welcoming the team to Gilead when the transaction closes • Magrolimab, FSI-174 and FSI-189 diversify Gilead’s oncology portfolio and will help drive long-term shareholder value 6

Forty Seven Overview • Forty Seven is headquartered in Menlo Park, California and has ~65 employees • Founded in 2015 by leading scientists at Stanford who discovered that blocking CD47 (a key signaling molecule that is overexpressed on cancer cells), renders tumors susceptible to destruction by macrophages • The company’s lead program, magrolimab, is an anti-CD47 mAb that is currently in Phase 1b/2 clinical studies with >400 patients with cancer treated to date - In December 2019, Forty Seven presented promising efficacy and safety data from its ongoing Phase 1b trial in untreated patients with MDS and AML - Magrolimab has Fast Track designation in four hematologic malignancies: MDS, AML, relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma 7

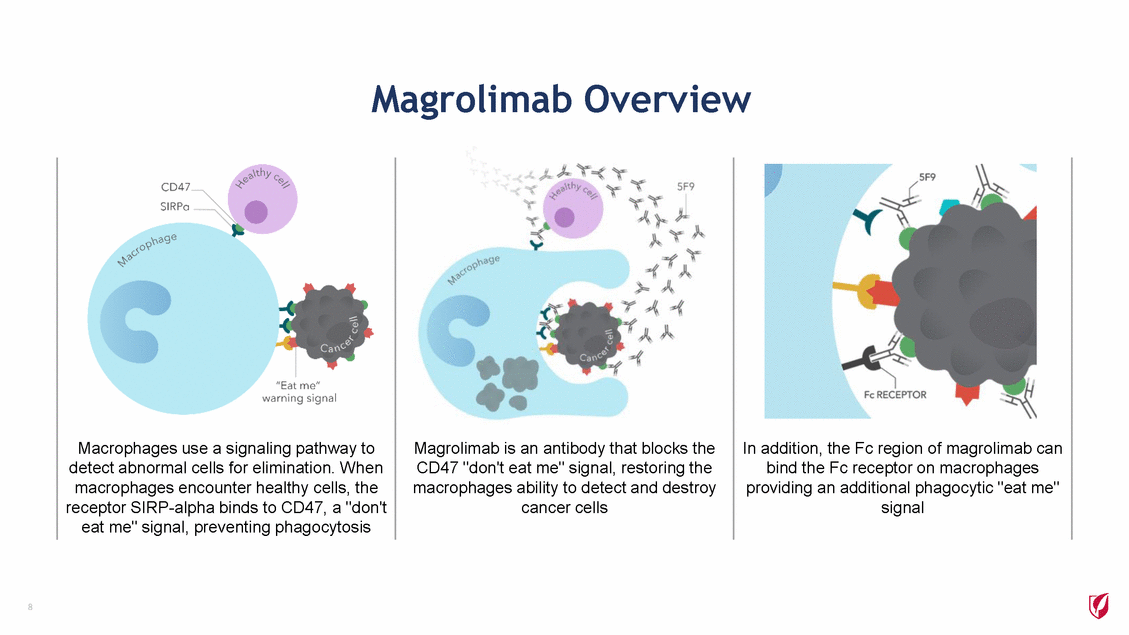
Magrolimab Overview 8 Macrophages use a signaling pathway to detect abnormal cells for elimination. When macrophages encounter healthy cells, the receptor SIRP-alpha binds to CD47, a "don't eat me" signal, preventing phagocytosis Magrolimab is an antibody that blocks the CD47 "don't eat me" signal, restoring the macrophages ability to detect and destroy cancer cells In addition, the Fc region of magrolimab can bind the Fc receptor on macrophages providing an additional phagocytic "eat me" signal
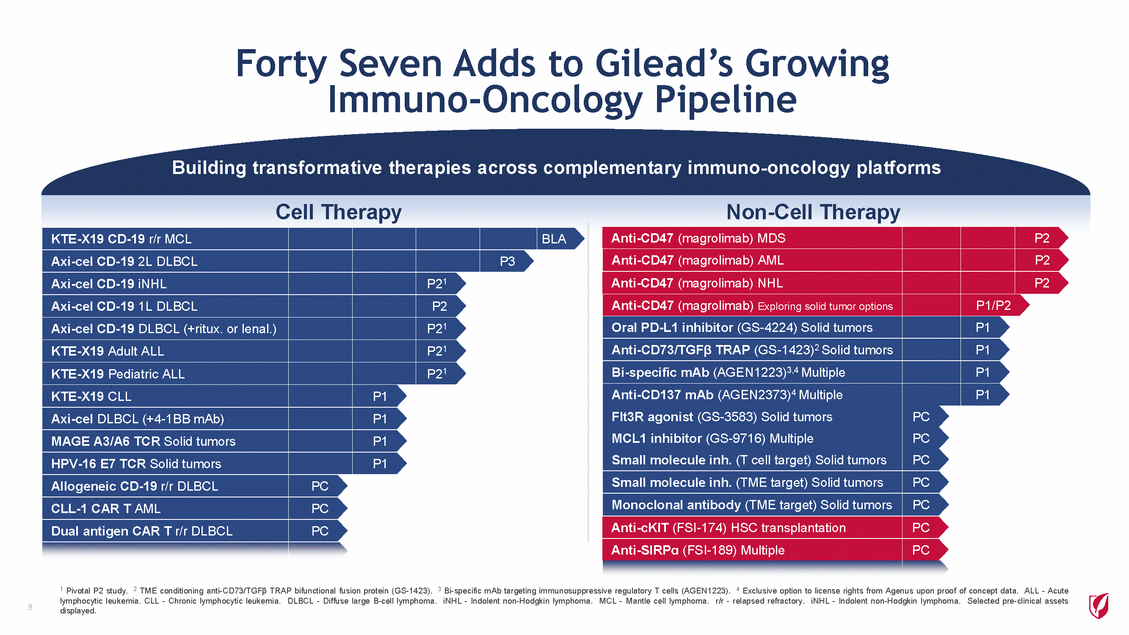
Forty Seven Adds to Gilead’s Growing Immuno-Oncology Pipeline Building transformative therapies across complementary immuno-oncology platforms Cell Therapy Non-Cell Therapy Anti-CD47 (magrolimab) MDS Anti-CD47 (magrolimab) AML P2 P2 KTE-X19 CD-19 r/r MCL Axi-cel CD-19 2L DLBCL BLA P3 Axi-cel CD-19 iNHL Axi-cel CD-19 1L DLBCL Axi-cel CD-19 DLBCL (+ritux. or lenal.) KTE-X19 Adult ALL KTE-X19 Pediatric ALL KTE-X19 CLL Axi-cel DLBCL (+4-1BB mAb) MAGE A3/A6 TCR Solid tumors HPV-16 E7 TCR Solid tumors Allogeneic CD-19 r/r DLBCL CLL-1 CAR T AML Dual antigen CAR T r/r DLBCL P21 P2 P21 P21 P21 Anti-CD47 (magrolimab) NHL Anti-CD47 (magrolimab) Exploring solid tumor options Oral PD-L1 inhibitor (GS-4224) Solid tumors Anti-CD73/TGF TRAP (GS-1423)2 Solid tumors Bi-specific mAb (AGEN1223)3,4 Multiple Anti-CD137 mAb (AGEN2373)4 Multiple Flt3R agonist (GS-3583) Solid tumors MCL1 inhibitor (GS-9716) Multiple Small molecule inh. (T cell target) Solid tumors Small molecule inh. (TME target) Solid tumors Monoclonal antibody (TME target) Solid tumors Anti-cKIT (FSI-174) HSC transplantation Anti-SIRP (FSI-189) Multiple P2 P1/P2 P1 P1 P1 P1 P1 P1 P1 P1 PC PC PC PC PC PC PC PC PC PC 1 Pivotal P2 study. 2 TME conditioning anti-CD73/TGF TRAP bifunctional fusion protein (GS-1423). 3 Bi-specific mAb targeting immunosuppressive regulatory T cells (AGEN1223). 4 Exclusive option to license rights from Agenus upon proof of concept data. ALL - Acute lymphocytic leukemia. CLL - Chronic lymphocytic leukemia. DLBCL - Diffuse large B-cell lymphoma. iNHL - Indolent non-Hodgkin lymphoma. MCL - Mantle cell lymphoma. r/r - relapsed refractory. iNHL - Indolent non-Hodgkin lymphoma. Selected pre-clinical assets displayed. 9
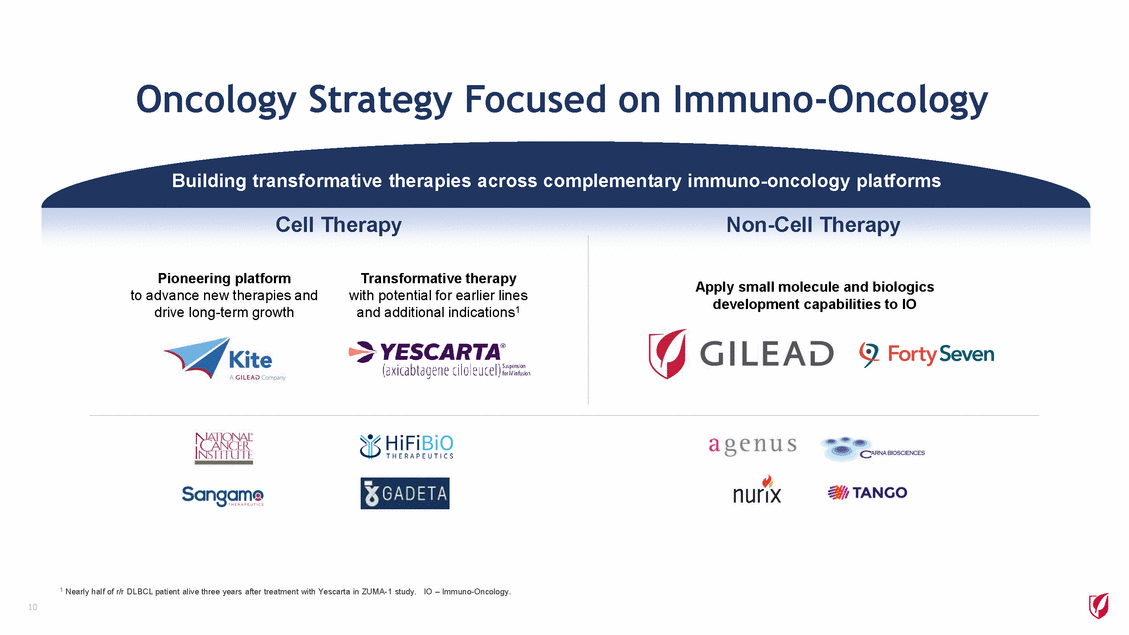
Oncology Strategy Focused on Immuno-Oncology Building transformative therapies across complementary immuno-oncology platforms Cell Therapy Non-Cell Therapy Pioneering platform Transformative therapy Apply small molecule and biologics development capabilities to IO to advance new therapies and drive long-term growth with potential for earlier lines and additional indications1 1 Nearly half of r/r DLBCL patient alive three years after treatment with Yescarta in ZUMA-1 study. IO – Immuno-Oncology. 10
Transaction Details • Gilead to acquire Forty Seven for $95.50 per share in cash for a total purchase price of $4.9 billion - Acquisition will be entirely financed with existing cash - The program is expected to be dilutive for the next several years given magrolimab’s clinical stage of development • Transaction unanimously approved by the Boards of Directors of both companies • The acquisition is expected to close during the second quarter, subject to regulatory approvals and other customary closing conditions 11

Three Pillars of Gilead’s Next Chapter + + Well positioned to maximize near-term opportunities and achieve long-term success 12 Existing Pipeline Opportunities Durable Core Business Strategy to Drive Additional Growth
THANK YOU
