SLIDES USED IN MEETINGS WITH PROSPECTIVE
INVESTORS AND STOCKHOLDERS OF
DENDREON CORPORATION AND OF
CORVAS INTERNATIONAL, INC. AND
MADE AVAILABLE ON JULY 7, 2003
AT WWW.DENDREON.COM AND WWW.CORVAS.COM
Filed by Dendreon Corporation
Pursuant to Rule 165 and Rule 425
under the Securities Act of 1933 and
deemed filed pursuant to Rule 14a-6
under the Securities Exchange Act of 1934
Subject Company: Corvas International, Inc.
Form S-4 File No. 333-104167
This filing relates to the proposed acquisition (“Acquisition”) by Dendreon Corporation (“Dendreon”) of Corvas International, Inc. (“Corvas”) pursuant to the terms of an Agreement and Plan of Merger, dated February 24, 2003, as amended on June 12, 2003 (the “Merger Agreement”), by and among Dendreon, Seahawk Acquisition, Inc., Charger Project LLC and Corvas. The Merger Agreement is on file with the Securities and Exchange Commission (“SEC”) as an exhibit to the Current Report on Form 8-K filed by Dendreon on February 25, 2003, and the Waiver and Amendment to the Merger Agreement, dated June 12, 2003, is on file with the SEC as an exhibit to Amendment No. 2 to the Registration Statement on Form S-4 filed by Dendreon on June 19, 2003, both of which are incorporated by reference into this filing.
Additional Information About the Acquisition and Where to Find It
Dendreon and Corvas have filed with the Securities and Exchange Commission (“SEC”) a Registration Statement on Form S-4, which contains a joint proxy statement/prospectus with respect to the Acquisition and other relevant materials. INVESTORS AND SECURITY HOLDERS OF DENDREON AND CORVAS ARE URGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS BECAUSE IT CONTAINS IMPORTANT INFORMATION ABOUT DENDREON, CORVAS AND THE ACQUISITION. The joint proxy statement/prospectus, including the annexes attached to, and the reports incorporated by reference in, the joint proxy statement/prospectus, and any other reports and documents filed by Dendreon or Corvas with the SEC, may be obtained free of charge at the SEC’s web site at www.sec.gov.
Investors and security holders may obtain free copies of the reports and documents filed with the SEC by Dendreon by directing a request to: Dendreon Corporation, 3005 First Avenue, Seattle, WA 98121, Attn: Investor Relations. Investors, and security holders may obtain free copies of the reports and documents filed with the SEC by Corvas by contacting Corvas Investor Relations at 3030 Science Park Road, San Diego CA 92121.
Dendreon, Corvas and their respective executive officers and directors may be deemed to be participants in the solicitation of proxies from the stockholders of Dendreon and Corvas in favor of the Acquisition. Information about the executive officers and directors of Dendreon and their ownership of Dendreon common stock is set forth in the joint proxy statement/prospectus. Information about the executive officers and directors of Corvas and their ownership of Corvas common stock is set forth in the joint proxy statement/prospectus and in Corvas’ Annual Report on Form 10-K, which was filed with the SEC on March 14, 2003. Certain directors and executive
officers of Corvas may have direct or indirect interests in the Acquisition due to securities holdings, pre-existing or future indemnification arrangements, vesting of options, or rights to severance payments if their employment is terminated following the Acquisition. Additional information regarding Dendreon, Corvas, and the interests of their respective executive officers and directors in the Acquisition is contained in the joint proxy statement/prospectus.
Investors and security holders are urged to read the joint proxy statement/prospectus, including the annexes attached to, and the reports incorporated by reference in, the joint proxy statement/prospectus, and any future report and document filed with the SEC by Dendreon and Corvas, before making any voting or investment decision with respect to the Acquisition.
Forward-looking Statements
Except for historical information contained herein, the slides contain forward-looking statements, including statements about the Acquisition and future financial and operating results of the combined company. These statements are based on management’s current expectations and beliefs and are subject to a number of risks and uncertainties, particularly those risks and uncertainties inherent in any acquisition transaction and the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, that could cause actual results to differ materially from those described in the forward-looking statements.
Statements of expected synergies, benefits, accretion, timing of closing, execution of integration plans and management and organizational structure are all forward-looking statements. Risks and uncertainties include the possibility that the market for the sale of certain products may not develop as expected; that development of the companies’ products, including potential cardiovascular and cancer products, may not proceed as planned; risks associated with completing ongoing clinical trials, including the rNAPc2 clinical trial for the treatment of patients with unstable angina and non-ST-segment elevation myocardial infarction; the risk that the results of one clinical trial will not be repeated in another clinical trial; the risk that results in preclinical studies may not be confirmed in clinical trials or that other preclinical studies will reveal adverse characteristics that preclude further development of a preclinical product candidate; the risk that the results of a clinical trial, including Phase 3 trials of Provenge, will not support applying for or approval of a biologics license by the FDA; the risk that the Acquisition does not close or that the companies may be required to modify aspects of the transaction to achieve regulatory approval; that prior to the closing of the Acquisition, the businesses of the companies, including the retention of key employees, suffer due to uncertainty; that the parties are unable to successfully execute their integration strategies or achieve planned synergies; risks related to Dendreon’s limited operating history; the risk that the companies may not secure or maintain relationships with collaborators; the companies’ dependence on intellectual property; and other risks and uncertainties that are described in the reports filed by Dendreon and Corvas with the SEC. Additional information on the risks and uncertainties that could affect the companies’ business, financial condition and results of operations are contained in their respective filings with the SEC, which are available at www.sec.gov.
The following are slides regarding the Acquisition used in presentations to prospective investors and stockholders of Dendreon and of Corvas and made available on July 7, 2003, by following the appropriate links, by Dendreon on its website at www.dendreon.com and by Corvas on its website at www.corvas.com/investor-relations.html.

Dendreon Corporation
Corvas International
Building a Premier Biotechnology Company

Forward-Looking Statement Disclaimer
This presentation includes forward-looking statements, including statements about Dendreon’s proposed acquisition of Corvas, post-closing business synergies and product opportunities, and product development and commercialization. Actual results may differ materially from those projected in the forward-looking statements. Factors that could cause actual results to differ materially from those in the forward-looking statements are contained in Dendreon’s filings with the SEC, including its Registration Statement on Form S-4 (Reg. No. 333-104167) under the caption “Risk Factors.”

Additional Information About the Acquisition and Where to Find It
Dendreon and Corvas have filed with the Securities and Exchange Commission (“SEC”) a Registration Statement on Form S-4, which contains a joint proxy statement/prospectus with respect to the combination and other relevant materials. INVESTORS AND SECURITY HOLDERS OF DENDREON AND CORVAS ARE URGED TO READ THE COMPANIES’ RELEVANT FILINGS WITH THE SEC, INCLUDING THE DEFINITIVE JOINT PROXY STATEMENT/PROSPECTUS, BECAUSE THEY CONTAIN IMPORTANT INFORMATION ABOUT DENDREON, CORVAS AND THE COMBINATION. The joint proxy statement/prospectus, including the annexes attached to, and the reports incorporated by reference in, the joint proxy statement/prospectus, and any other reports and documents filed by Dendreon or Corvas with the SEC, may be obtained free of charge at the SEC’s web site at www.sec.gov.
Investors and security holders may obtain free copies of the reports and documents filed with the SEC by Dendreon by directing a request to: Dendreon Corporation, 3005 First Avenue, Seattle, WA 98121, Attn: Investor Relations. Investors and security holders may obtain free copies of the reports and documents filed with the SEC by Corvas by contacting Corvas Investor Relations at 3030 Science Park Road, San Diego CA 92121.
Dendreon, Corvas and their respective executive officers and directors may be deemed to be participants in the solicitation of proxies from the stockholders of Dendreon and Corvas in favor of the combination. Information about the executive officers and directors of Dendreon and their ownership of Dendreon common stock is set forth in the joint proxy statement/prospectus. Information about the executive officers and directors of Corvas and their ownership of Corvas common stock is set forth in the joint proxy statement/prospectus and in Corvas’ Annual Report on Form 10-K, which was filed with the SEC on March 14, 2003. Certain directors and executive officers of Corvas may have direct or indirect interests in the combination due to securities holdings, pre-existing or future indemnification arrangements, vesting of options, or rights to severance payments if their employment is terminated following the combination. Additional information regarding Dendreon, Corvas, and the interests of their respective executive officers and directors in the combination is contained in the joint proxy statement/prospectus.

Dendreon Corporation
Developing targeted therapies for the treatment of cancer
| • | | Immunotherapeutic cancer programs |
-Provenge®, MylovengeTM, APC8024, Trp-p8, NY-ESO, CEA, MN, Telomerase
| • | | Monoclonal antibodies and small molecules |
-Trp-p8, DN1921, DN1924
| ��� | | Industry leading collaborators |
-Genentech, Inc., Kirin Brewery Co., Ltd.
 .
.
Momentum Continues in 2003
Corvas Acquisition
| • | | Stock for stock transaction |
| • | | Valued to $73 M, based on Dendreon closing stock price of $5.79 on 2/24/03, an 83% premium to Corvas’ stock price as of the same date |
| • | | Dendreon stockholders will own 71.6% and Corvas stockholders will own 28.4%, based on current capitalization |
| • | | Combination is expected to increase stockholder value by creating a more diversified and sustainable organization |
- Two Phase 3 programs
- Two Phase 2 programs
- One Phase 1 program

Combining Strengths
Technology
| • | | Broad oncology portfolio covering 4 platforms |
| • | | 5 ongoing clinical programs |
| • | | Enhanced intellectual property estate, 87 issued U.S. patents |
| • | | Small molecule, medicinal chemistry expertise |
Operations
| • | | Experienced management team |
| • | | Clinical development expertise |
| • | | Research and development expertise |
| • | | Collaborations with industry leaders |
Financial
| • | | Combined balance sheet of approximately $125M upon closing |
| • | | Resources to fuel Provenge development |
| • | | Resources to advance other clinical and preclinical programs |
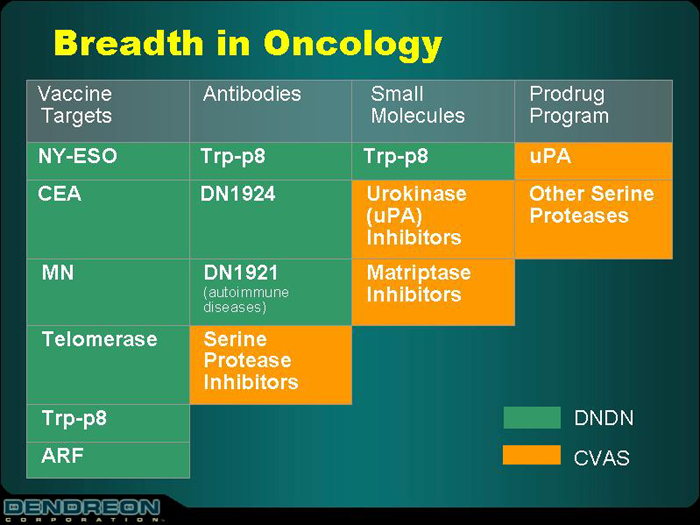
Breadth in Oncology
Vaccine Targets
| | Antibodies
| | Small Molecules
| | Prodrug Program
|
| NY-ESO | | Trp-p8 | | Trp-p8 | | uPA |
| | | |
| CEA | | DN1924 | | Urokinase (uPA) Inhibitors | | Other
Serine
Proteases |
| | | |
MN | | DN1921 (autoimmune
diseases) | | Matriptase Inhibitors | | |
| | | |
| Telomerase | | Serine
Protease
Inhibitors | | | | |
| Trp-p8 | | | | | | |
| | | |
| ARF | | | | | | |
DNDN
CVAS
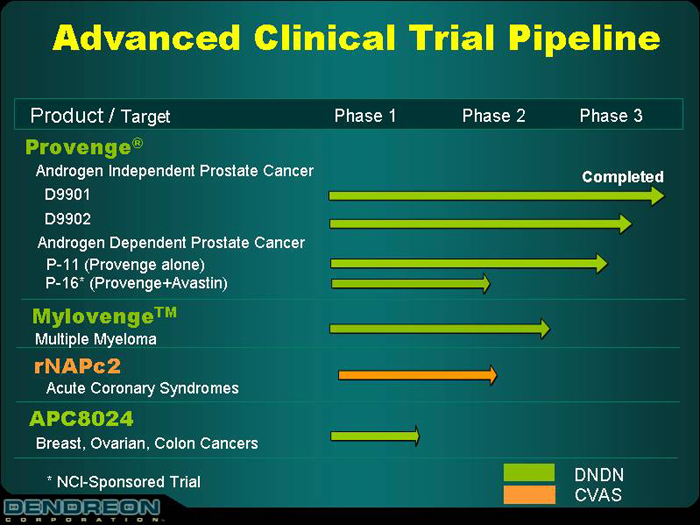
Advanced Clinical Trial Pipeline
Product/Target
| | Phase 1
| | Phase 2
| | Phase 3
|
Provenge® | | | | | | |
Androgen Independent Prostate Cancer | | | | | | Completed |
D9901 | | | | | | |
D9902 | | | | | | |
Androgen Dependent Prostate Cancer | | | | | | |
P-11 (Provenge alone) | | | | | | |
P-16 (Provenge+Avastin) | | | | | | |
Mylovenge™ | | | | | | |
Multiple Myeloma | | | | | | |
| | | |
rNAPc2 | | | | | | |
Acute Coronary Syndromes | | | | | | |
| | | |
APC8024 | | | | | | |
Breast, Ovarian, Colon Cancers | | | | | | |
| | | |
*NCI-Sponsored Trial | | | | | | |
DNDN
CVAS
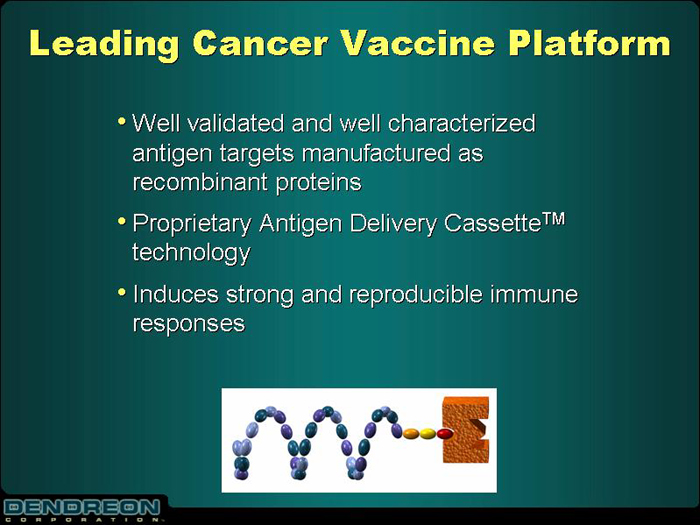
Leading Cancer Vaccine Platform
| • | | Well validated and well characterized antigen targets manufactured as recombinant proteins |
| • | | Proprietary Antigen Delivery CassetteTM technology |
| • | | Induces strong and reproducible immune responses |
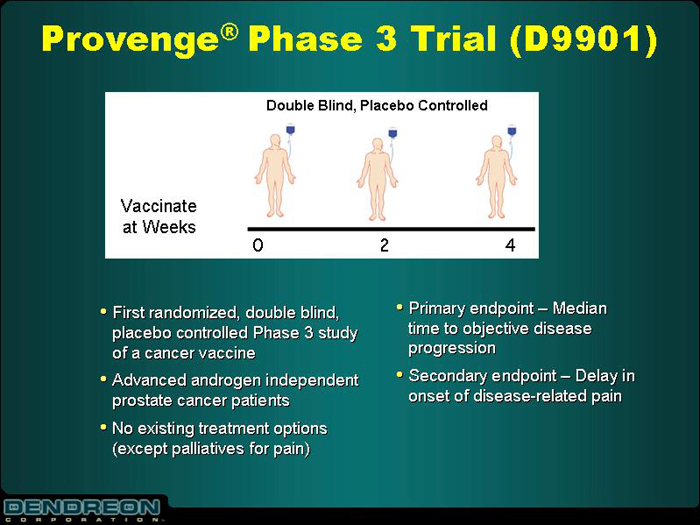
Provenge®Phase 3 Trial (D9901)
Double Blind, Placebo Controlled
Vaccinate at Weeks 0 2 4
• First randomized, double blind, placebo controlled Phase 3 study of a cancer vaccine | | • Primary endpoint – Median time to objective disease progression |
• Advanced androgen independent prostate cancer patients | | • Secondary endpoint – Delay in onset of disease-related pain |
• No existing treatment options (except palliatives for pain) | | |
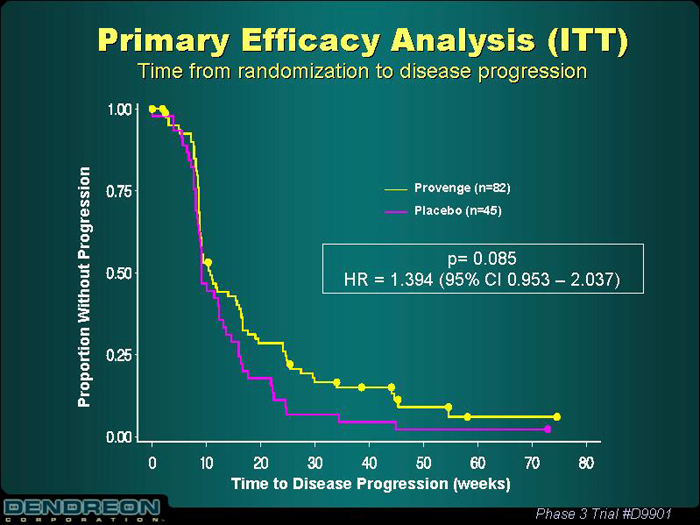
Primary Efficacy Analysis (ITT)
Time from randomization to disease progression
Provenge (n=82)
Placebo (n=45)
p=0.085
HR=1.394 (95% CI 0.953 - 2.037)
Proportion Without Progression
Time to Disease Progression (weeks)
Phase 3 Trial #D9901
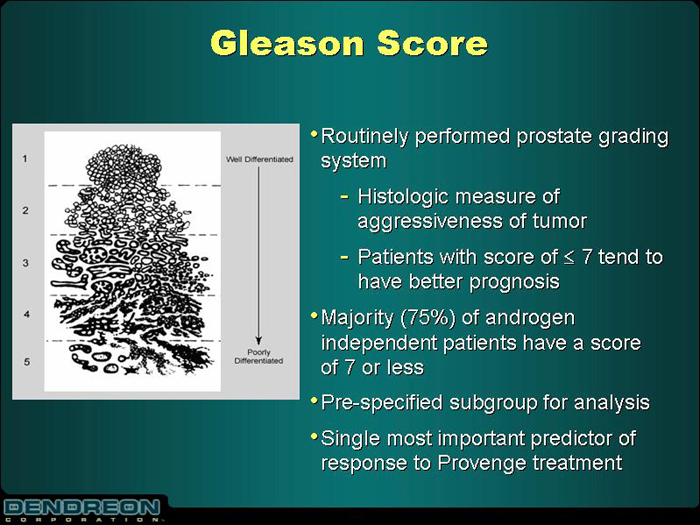
Gleason Score
| • | | Routinely performed prostate grading system |
- Histologic measure of aggressiveness of tumor
- Patients with score of£ 7 tend to have better prognosis
| • | | Majority (75%) of androgen independent patients have a score of 7 or less |
| • | | Pre-specified subgroup for analysis |
| • | | Single most important predictor of response to Provenge treatment |
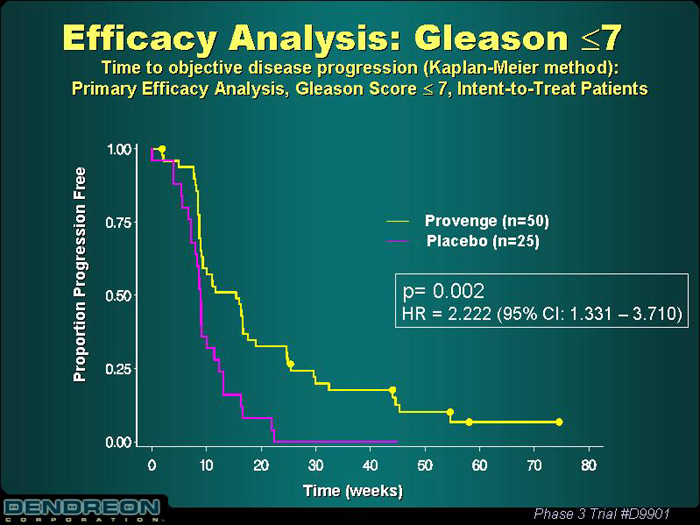
Efficacy Analysis: Gleason£ 7
Time to objective disease progression (Kaplan-Meier method):
Primary Efficacy Analysis, Gleason Score£ 7, Intent-to-Treat Patients
Provenge (n=50)
Placebo (n=25)
P=0.002
HR=2.222 (95% Cl: 1.331 – 3.710)
Proportion Progression Free
Time (weeks)
Phase 3 Trial #D9901
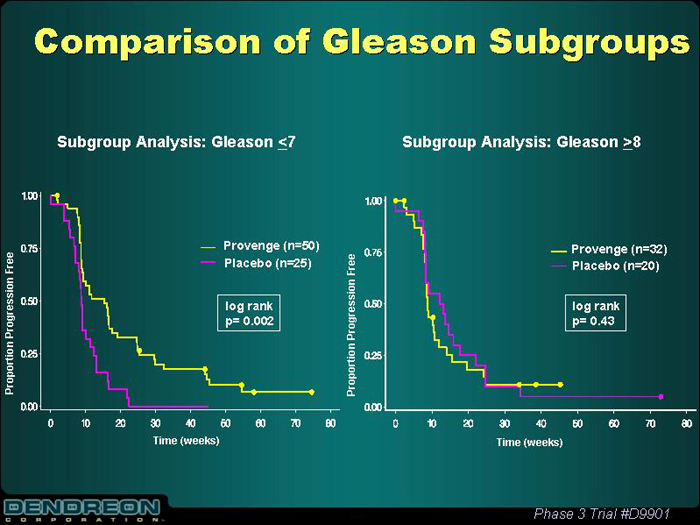
Comparison of Gleason Subgroups
| Subgroup Analysis: Gleason£ 7 | | Subgroup Analysis: Gleason ³ 8 |
Provenge (n=50) Placebo (n=25) | | Provenge (n=32) Placebo (n=20) |
| |
log rank p=0.002 | | log rank p=0.043 |
| |
Proportion Progression Free Time (weeks) | | Proportion Progression Free Time (weeks) |
Phase 3 Trial #D9901
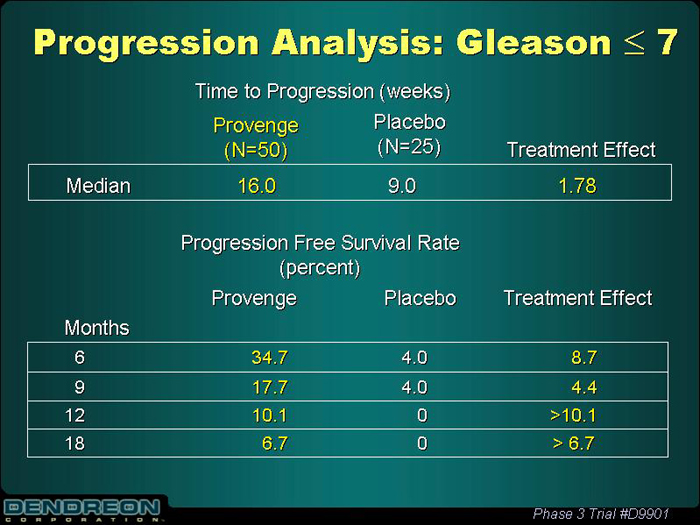
Progression Analysis: Gleason£ 7
Time to Progression (weeks)
| | | Provenge (N=50) | | Placebo (N=25) | | Treatment Effect |
| Median | | 16.0 | | 9.0 | | 1.78 |
Progression Free Survival Rate
(percent)
| Months | | Provenge | | Placebo | | Treatment Effect |
| 6 | | 34.7 | | 4.0 | | 8.7 |
| 9 | | 17.7 | | 4.0 | | 4.4 |
| 12 | | 10.1 | | 0 | | >10.1 |
| 18 | | 6.7 | | 0 | | > 6.7 |
Phase 3 Trial #D9901
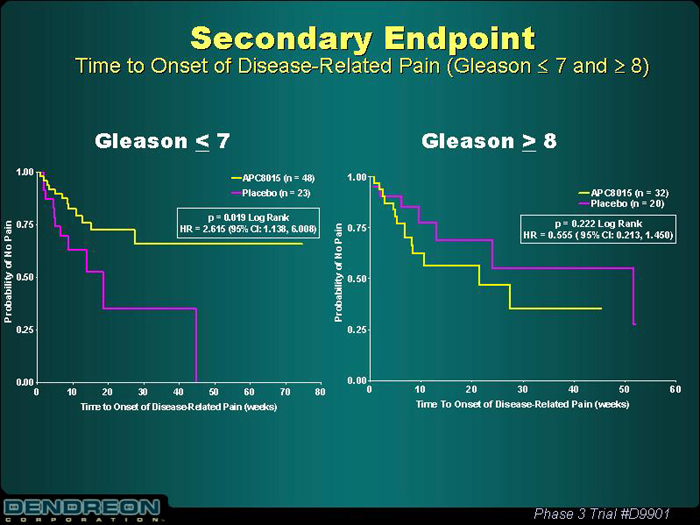
Secondary Endpoint
Time to Onset of Disease-Related Pain (Gleason£ 7 and³ 8)
Gleason£ 7 | | Gleason³ 8 |
[CHART] | | [CHART] |
Phase 3 Trial #D9901
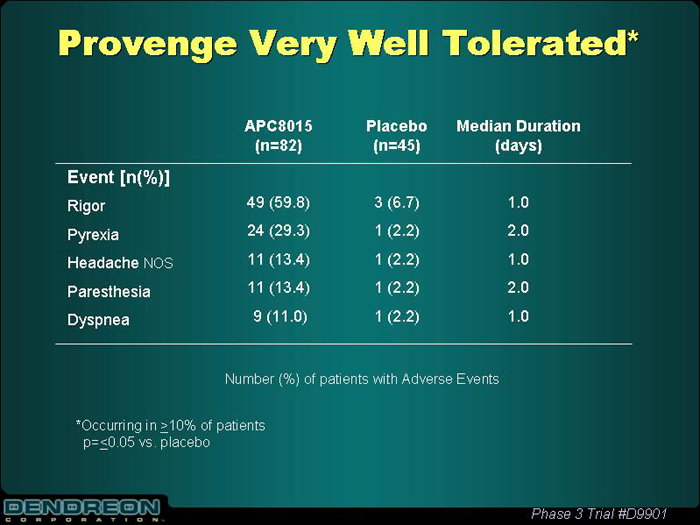
Provenge Very Well Tolerated*
| | | APC8015 (n=82) | | Placebo (n=45) | | Median Duration (days) |
Event [n(%)] | | | | | | |
Rigor | | 49 (59.8) | | 3 (6.7) | | 1.0 |
Pyrexia | | 24 (29.3) | | 1 (2.2) | | 2.0 |
Headache NOS | | 11 (13.4) | | 1 (2.2) | | 1.0 |
Paresthesia | | 11 (13.4) | | 1 (2.2) | | 2.0 |
Dyspnea | | 9 (11.0) | | 1 (2.2) | | 1.0 |
Number (%) of patients with Adverse Events
*Occurring in³10% of patients
p=£0.05 vs. placebo
Phase 3 Trial #D9901

Provenge Trial D9902B
| • | | Special Protocol Assessment received from FDA – D9902B will serve as basis for BLA application |
| • | | Trial enrolling AIPC patients with Gleason score£ 7 |
| • | | Approximately 275 patients will be enrolled into study to support BLA |

Provenge Collaboration Discussions
| | • | | Discussions progressing as planned |
| | • | | Large market, data and regulatory plan have driven interest |
| | • | | Will be structured as co-development and co-promotion agreement |
| | • | | Global agreement minus Asia and Oceania |

APC8024 Phase 1 Trials
Patient Population
| | • | | 18 patients enrolled; 16 evaluated |
| | • | | Advanced, metastatic breast cancer patients |
| | • | | Her-2 positive (2+, 3+) |
| | • | | Failed multiple courses of chemotherapy including 15/16 who failed Herceptin |
| | • | | Historical median time to progression 3-6 months |
Endpoints
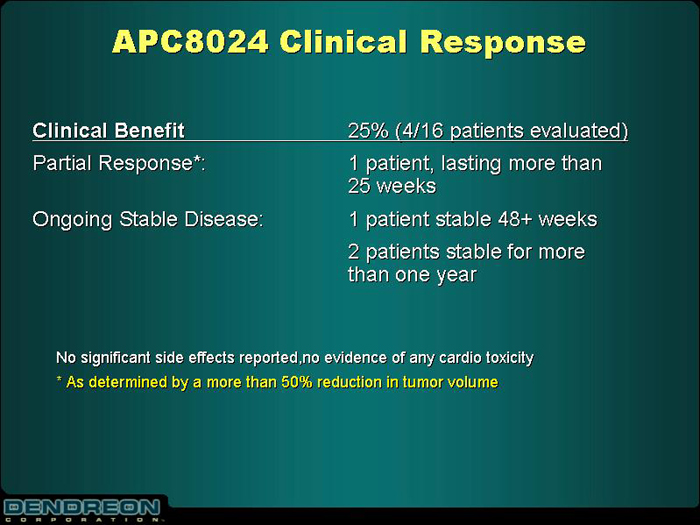
APC8024 Clinical Response
Clinical Benefit | | 25% (4/16 patients evaluated) |
| |
Partial Response*: | | 1 patient, lasting more than 25 weeks |
| |
Ongoing Stable Disease: | | 1 patient stable 48+ weeks |
| | | 2 patients stable for more than one year |
No significant side effects reported, no evidence of any cardio toxicity
* As determined by a more than 50% reduction in tumor volume

Dendreon & Genentech
Trp-p8 Collaboration
Trp-p8 Characteristics
| | • | | Trans-membrane voltage gated Ca2+ channel |
| | • | | Over-expressed in breast, prostate, lung and colon cancers as well as hyperplastic prostate |
| | • | | Multiple therapeutic opportunities |
- Vaccines
- Monoclonal antibodies
- Small molecules
References:
U.S. Patent 6,194,152: Prostate tumor polynucleotide compositions and methods of detection thereof
Published in Cancer Research 61, 3760-9, 2001
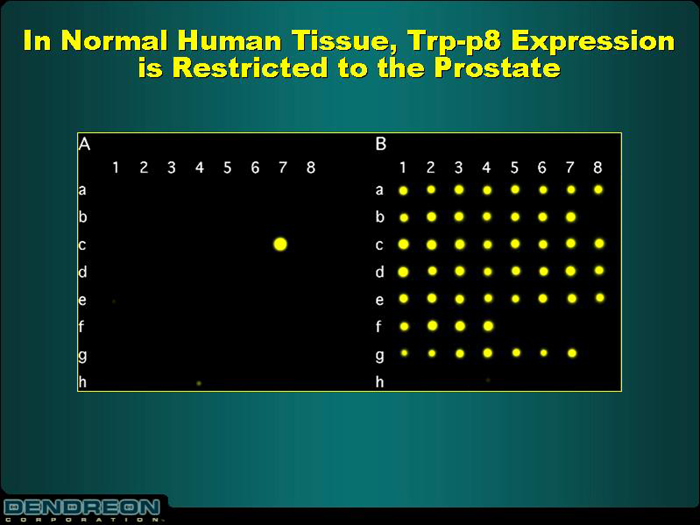
In Normal Human Tissue, Trp-p8 Expression
is Restricted to the Prostate
[CHART]
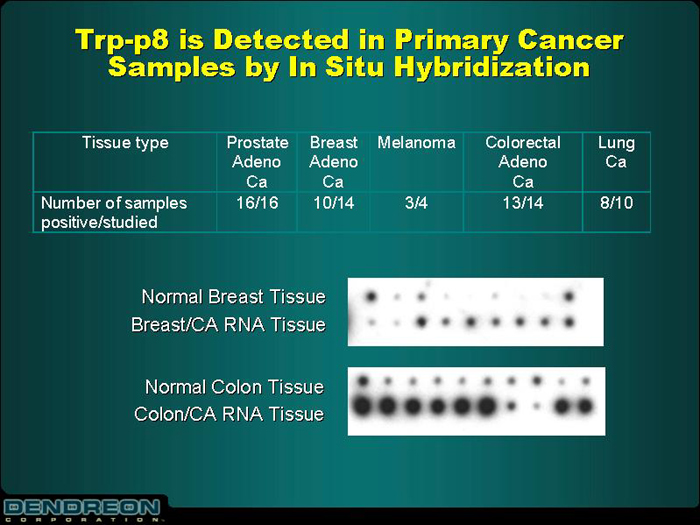
Trp-p8 is Detected in Primary Cancer
Samples by In Situ Hybridization
| Tissue Type | | Prostate Adeno Ca | | Breast Adeno Ca | | Melanoma | | Colorectal
Adeno Ca | | Lung Ca |
Number of samples positive/studied | | 16/16 | | 10/14 | | 3/4 | | 13/14 | | 8/10 |
| | | |
Normal Breast Tissue Breast/CA RNA Tissue | | [GRAPHIC] | | | | |
| | | |
Normal Colon Tissue Colon/CA RNA Tissue | | [GRAPHIC] | | | | |
Trp-p8 – Architecture

Genentech Trp-p8 Collaboration
| | • | | Structured as a co-development, co-promotion agreement with profit sharing rights in the U.S. |
| | • | | Significant upfront and milestone payments |
| | • | | Genentech responsible for manufacturing and funds majority of manufacturing and clinical trial costs |
| | • | | Leverage Corvas’ medicinal chemistry expertise for small molecule development |
| | • | | Dendreon maintains all development and commercialization rights in Asia and Oceania |

Collaboration Milestones
| | • | | Equity purchases and cash milestone payments |
- At deal signing
- Drug candidate identification
- IND filing
- Clinical trial milestones
- BLA submission
- BLA approvals
| | • | | Total milestone payments could exceed $110 Million |

Corvas Oncology Programs
Dendreon Corporation
Corvas International
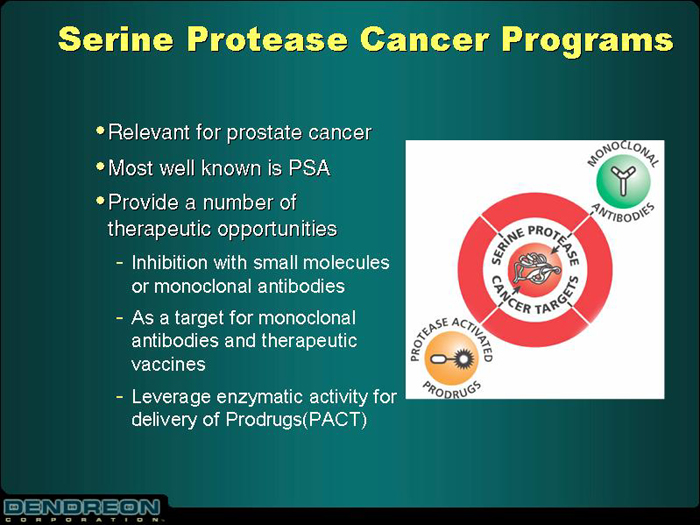
Serine Protease Cancer Programs
| | • | | Relevant for prostate cancer |
| | • | | Provide a number of therapeutic opportunities |
- Inhibition with small molecules or monoclonal antibodies
- As a target for monoclonal antibodies and therapeutic vaccines
- Leverage enzymatic activity for delivery of Prodrugs(PACT)
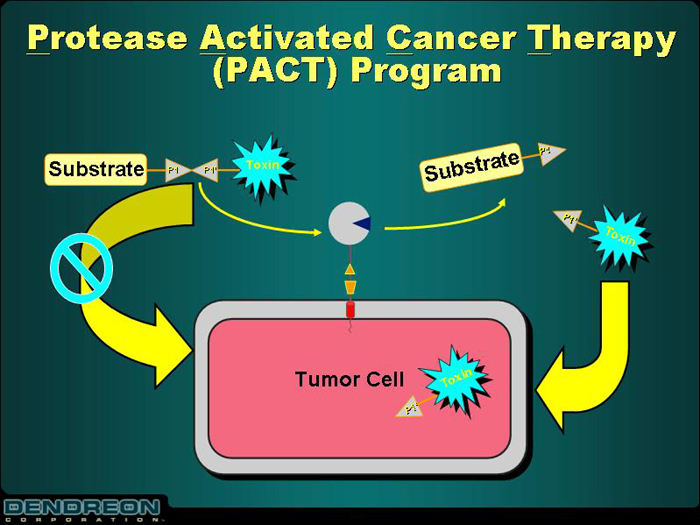
Protease Activated Cancer Therapy
(PACT) Program
[GRAPHIC]
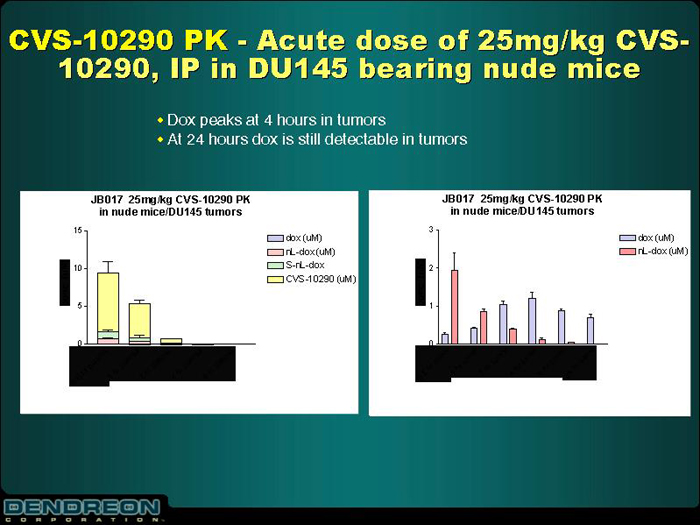
CVS-10290 PK—Acute dose of 25mg/kg CVS-10290, IP in DU145 bearing nude mice
| | • | | Dox peaks at 4 hours in tumors |
| | • | | At 24 hours dox is still detectable in tumors |
JB017 25mg/kg CVS-10290 PK in nude mice/DU145 tumors | | JB017 25mg/kg CVS-10290 PK in nude mice/DU145 tumors |
| [CHART] | | [CHART] |
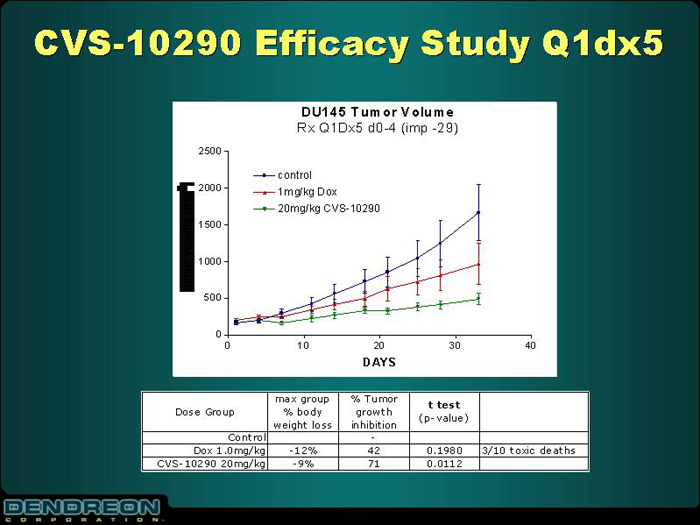
CVS-10290 Efficacy Study Q1dx5
DU145 Tumor Volume
Rx Q1 Dx5 d0-4 (imp -29)
[CHART]
Dose Group | | max group % body weight loss | | % Tumor growth inhibition | | t test (p-value) | | |
Control | | | | - | | | | |
Dox 1.0mg/kg | | -12% | | 42 | | 0.1980 | | 3/10 toxic deaths |
CVS-10290 20mg/kg | | -9% | | 71 | | 0.0112 | | |

Corvas Cardiovascular Program
Dendreon Corporation
Corvas International
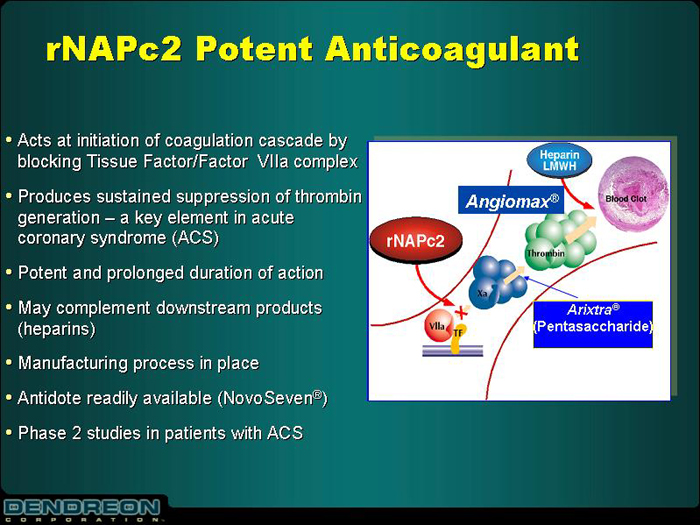
rNAPc2 Potent Anticoagulant
| | • | | Acts at initiation of coagulation cascade by blocking Tissue Factor/Factor VIIa complex |
| | • | | Produces sustained suppression of thrombin generation – a key element in acute coronary syndrome (ACS) |
| | • | | Potent and prolonged duration of action |
| | • | | May complement downstream products (heparins) |
| | • | | Manufacturing process in place |
| | • | | Antidote readily available (NovoSeven®) |
| | • | | Phase 2 studies in patients with ACS |
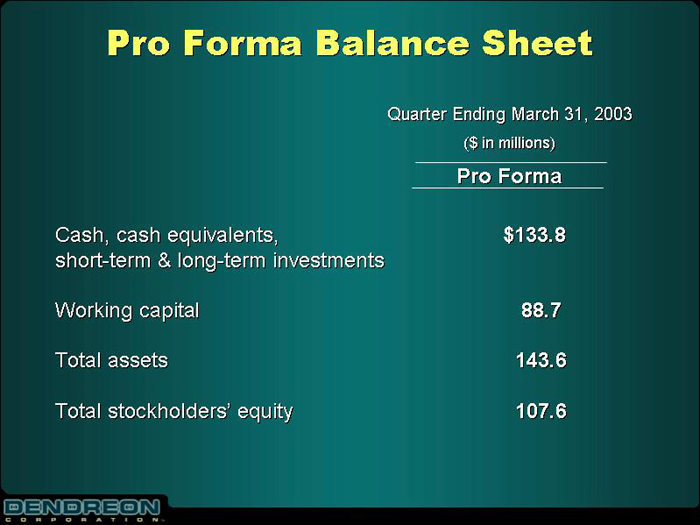
Pro Forma Balance Sheet
| | | Quarter Ending March 31, 2003 |
| | | ($ in millions) |
| | | Pro Forma |
Cash, cash equivalents, short-term & long-term investments | | $133.8 |
| |
Working capital | | 88.7 |
| |
Total assets | | 143.6 |
| |
Total stockholders’ equity | | 107.6 |

Expected Milestones and News
Flow
Type | | | | 2003 | | 2004 | | 2005 |
Clinical Data | | Presentations at upcoming scientific meetings. |
Regulatory | | | | Fast track designation — Provenge |
Preclinical Milestone | | | | | | Trp-p8 Mab lead selection and DNA milestone payment |
Clinical Trials | | | | | | Complete Phase II Mylovenge studies |
Clinical Trials | | | | | | Complete Phase I studies of APC8024, Design and begin Phase II studies |
Business Dev | | | | | | Announce Provenge collaboration agreement |
Business Dev | | | | | | Trp-p8 collaboration in Asia | | |
Clinical Trials | | | | | | Complete enrollment of Provenge hormone sensitive study (P-11) |
Clinical Trials | | | | | | Complete enrollment of D9902B – Pivotal Provenge study |
Clinical Trials | | | | | | Complete rNAPc2 part I/Phase II program |
Preclinical Milestone | | | | | | | | Trp-p8 small molecule lead and DNA milestone payment |
Clinical Trials | | | | | | | | IND CVS-10290 Pro-drug program |
Clinical Trials | | | | | | | | DN1924 Mab IND |
DNDN
CVAS

The New Dendreon
| | • | | Creating a top tier biotech company |
| | • | | Validated therapeutic cancer platform |
- Near-term market opportunities
- Significant clinical benefit demonstrated with Provenge®
| | • | | Broad portfolio includes vaccines, monoclonal antibodies, small molecules and prodrugs |
| | • | | Industry leading partners |
| | • | | Capital to invest in promising programs |

Dendreon Corporation
© Dendreon 2003




 .
.
































