UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________________________________
FORM 10-K
___________________________________________
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2020
OR
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | | | |
| For the transition period from to |
Commission File Number: 000-19756
___________________________________________
PDL BioPharma, Inc.
(Exact name of registrant as specified in its charter)
___________________________________________
| | | | | |
| Delaware | 94-3023969 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
59 Damonte Ranch Parkway, Suite B-375
Reno, Nevada 89521
(Address of principal executive offices)
Registrant’s telephone number, including area code
(775) 832-8500
932 Southwood Boulevard
Incline Village, Nevada 89451
(Former Address)
___________________________________________
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of Class | Trading Symbol | Name of Exchange on which Registered |
| None | N/A | N/A |
Securities registered pursuant to Section 12(g) of the Act: None
___________________________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ý No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ý No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| | | | | | | | | | | | | | |
Large accelerated filer ¨ | | | Accelerated filer ¨ | |
Non-accelerated filer ý | | | Smaller reporting company ☒ | |
| | | Emerging growth company ☐ | |
If an emerging growth company, indicated by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ¨ |
Indicate by check mark whether the registrant has filed a report and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ý
The aggregate market value of shares of common stock held by non-affiliates of the registrant, based on the closing sale price of a share of common stock on June 30, 2020 (the last business day of the registrant’s most recently completed second fiscal quarter), as reported on the Nasdaq Global Select Market, was $291,544,988.
As of January 6, 2021, the registrant had outstanding 114,515,806 shares of common stock.
PDL BIOPHARMA, INC.
2020 Form 10-K Annual Report
Table of Contents
| | | | | | | | | | | |
| PART I | | | |
| | | |
| Item 1 | | | |
| Item 1A | | | |
| Item 1B | | | |
| Item 2 | | | |
| Item 3 | | | |
| Item 4 | | | |
| | | |
| PART II | | | |
| | | |
| Item 5 | | | |
| Item 6 | | | |
| Item 7 | | | |
| Item 7A | | | |
| Item 8 | | | |
| Item 9 | | | |
| Item 9A | | | |
| Item 9B | | | |
| | | |
| PART III | | | |
| | | |
| Item 10 | | | |
| Item 11 | | | |
| Item 12 | | | |
| Item 13 | | | |
| Item 14 | | | |
| | | |
| PART IV | | | |
| | | |
| Item 15 | | | |
| Item 16 | | | |
| | | |
| |
PART I
Forward-looking Statements
This Form 10-K contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including any projections of earnings, revenues or other financial items, any statements of the plans and objectives of management for future operations, including any statements concerning the timing, implementation or success of our monetization strategy/plan of complete liquidation, any statements regarding future economic conditions or performance, and any statement of assumptions underlying any of the foregoing. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, forward-looking statements can be identified by the use of terminology such as “may,” “will,” “intends,” “plans,” “believes,” “targets,” “anticipates,” “expects,” “estimates,” “predicts,” “potential,” “continue” or “opportunity,” or the negative thereof or other comparable terminology. The forward-looking statements in this Form 10-K are only predictions. Although we believe that the expectations presented in the forward-looking statements contained herein are reasonable at the time of filing, there can be no assurance that such expectations or any of the forward-looking statements will prove to be correct. These forward-looking statements, including with regards to our plan of dissolution, are subject to inherent risks and uncertainties, including but not limited to the risk factors set forth below, and for the reasons described elsewhere in this Form 10-K. All forward-looking statements and reasons why results may differ included in this Form 10-K are made as of the date hereof. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
We own or have rights to certain trademarks, trade names, copyrights and other intellectual property used in our business, including PDL BioPharma, Inc. and the PDL logo, each of which is considered a registered trademark. All other company names, product names, trade names and trademarks included in this Form 10-K are trademarks, registered trademarks or trade names of their respective owners.
ITEM 1. BUSINESS
Overview
In this report all references to “PDL,” “we,” “us,” “our” or the “Company” mean collectively PDL BioPharma, Inc. and its subsidiaries, except where it is made clear that the term means only PDL BioPharma, Inc.
Throughout our history, our mission has been to improve the lives of patients by aiding in the successful development of innovative therapeutics and healthcare technologies. PDL BioPharma was founded in 1986 as Protein Design Labs, Inc. when it pioneered the humanization of monoclonal antibodies, enabling the discovery of a new generation of targeted treatments that have had a profound impact on patients living with different cancers as well as a variety of other debilitating diseases. In 2006, we changed our name to PDL BioPharma, Inc.
Historically, we generated a substantial portion of our revenues through the license agreements related to patents covering the humanization of antibodies, which we refer to as the Queen et al. patents. In 2012, and in anticipation of declining revenues from the Queen et al. patents, we began providing alternative sources of capital through royalty monetizations and debt facilities, and, in 2016, we began acquiring commercial-stage products and launching specialized companies dedicated to the commercialization of these products, first with our acquisition of branded prescription pharmaceutical drugs from Novartis AG, Novartis Pharma AG and Speedel Holding AG (collectively, “Novartis”) in 2016 and, in 2017, with the acquisition of LENSAR, Inc. (“LENSAR”), a medical device ophthalmology equipment manufacturing company. In 2019, we entered into a securities purchase agreement with Evofem Biosciences, Inc. (“Evofem”), pursuant to which we invested $60.0 million in a private placement of securities. These investments provided funding for Evofem’s pre-commercial activities for Phexxi®, its non-hormonal, on-demand prescription contraceptive gel for women.
Our Segments
Based on the nature of our investments entered into between 2012 through 2019 and further discussed below, our operations were structured in four segments designated as Medical Devices, Strategic Positions, Income Generating Assets, and Pharmaceutical.
Our Medical Devices segment consisted of revenue from the sale and lease of the LENSAR® Laser System, which included equipment, Patient Interface Devices (“PIDs”), procedure licenses, training, installation, warranty and maintenance agreements.
Our Strategic Positions segment consisted of an investment in Evofem (NASDAQ: EVFM). Our investment included shares of common stock and warrants to purchase additional shares of common stock.
Our Pharmaceutical segment consisted of revenue derived from branded prescription medicine products sold under the name Tekturna® and Tekturna HCT® in the United States, Rasilez® and Rasilez HCT® in the rest of the world and revenue generated from the sale of an authorized generic form of Tekturna in the United States (collectively, the “Noden Products”).
Our Income Generating Assets segment consisted of revenue derived from (i) notes and other long-term receivables, (ii) royalty rights and hybrid notes/royalty receivables, (iii) equity investments and (iv) royalties from issued patents in the United States and elsewhere covering the humanization of antibodies, which we refer to as the Queen et al. patents.
Financial information about our segments, including our revenues and net loss for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, and select long-lived assets as of December 31, 2020 and 2019, is included in our Consolidated Financial Statements and accompanying notes in this Form 10-K.
Liquidation and Dissolution Plan
In September 2019, we engaged financial and legal advisors and initiated a review of our strategy. This review was completed in December 2019. At such time, we disclosed that we planned to halt the execution of our growth strategy, cease making additional strategic transactions and investments and instead pursue a formal process to unlock the value of our portfolio by monetizing our assets and ultimately distributing net proceeds to stockholders (the “monetization strategy”). We further announced in December 2019 that we would explore a variety of potential transactions in connection with the monetization strategy, including a whole Company sale, divestiture of our assets, spin-offs of operating entities, merger opportunities or a combination thereof. Over the subsequent months, our board of directors (the “Board”) and management analyzed, together with our outside financial and legal advisors, how to best capture value pursuant to our monetization strategy and best return the significant intrinsic value of the assets in our portfolio to the stockholders.
In February 2020, the Board approved a plan of complete liquidation (the “Plan of Liquidation”) of our assets and passed a resolution to seek stockholder approval to dissolve our Company. At our Annual Meeting of Stockholders in August 2020, the proposal to liquidate and dissolve our Company pursuant to a plan of dissolution was approved by our stockholders. On November 5, 2020, our Board approved filing a certificate of dissolution with the Secretary of State of Delaware in January 2021 and proceeding to complete the dissolution process for our Company in accordance with the Delaware General Corporate Law. The filing of the certificate of dissolution occurred on January 4, 2021 and we closed our stock transfer books as of such date (the “Final Record Date”). After such time, we are not recording any further transfers of our common stock, except pursuant to the provisions of a deceased stockholder’s will, intestate succession, or by operation of law and we will not issue any new stock certificates, other than replacement certificates. In addition, we will not be issuing any shares of our common stock for the outstanding stock options. As a result of the closing of our transfer books, it is anticipated that distributions, if any, made in connection with the dissolution will be made pro rata to the stockholders of record as of the Final Record Date. In accordance with our dissolution plan, we completed the voluntary delisting process from the Nasdaq Stock Market exchange so that suspension of trading occurred before the market opened on December 31, 2020 and official delisting of our stock occurred on January 7, 2021. On January 8, 2021, we filed a Form 15 notifying the SEC of deregistration of our common stock under Section 12(g) of the Exchange Act and suspension of our duty to file reports under Sections 13 and 15(d) of the Exchange Act. We do not anticipate participating in Over-The-Counter (“OTC”) trading related to our stock or economic interests in our stock.
Pursuant to our monetization strategy, we explored a variety of potential transactions, including a whole Company sale, divestiture of assets, spin-offs of operating entities, merger opportunities or a combination thereof. In addition, we analyzed, and continue to analyze, optimal mechanisms for returning value to stockholders in a tax-efficient manner, including share repurchases, cash dividends and other distributions of assets. Despite the challenges of the 2019 novel coronavirus (“COVID-19”), we made significant progress in our monetization strategy during 2020, including monetizing most of our key assets and resolving a longstanding legal issue as follows:
•In May 2020, pursuant to our Plan of Liquidation we made a liquidation distribution of all of our common stock in Evofem to PDL stockholders of record as of the close of business on May 15, 2020 (the “Evofem Record Date”).
•In August 2020, we entered into a settlement agreement (the “Settlement Agreement”) with related entities of Defined Diagnostics, LLC (f/k/a Wellstat Diagnostics, LLC) (“Wellstat Diagnostics” and, together with such related entities, the “Wellstat Parties”) resolving previously reported litigation relating to loans made to Wellstat Diagnostics by us
•In August 2020, we sold three royalty interests related to third party sales of Kybella®, Zalviso®, and Coflex® to SWK Funding, LLC (“SWK”), a wholly owned subsidiary of SWK Holdings Corporation
•In September 2020, we completed the previously announced sale of our interest in Noden DAC and Noden USA
•In October 2020, pursuant to our Plan of Liquidation we completed the previously announced spin-off of LENSAR, our majority-owned medical device company, whereby we made a liquidation distribution of all of our shares of LENSAR common stock to our stockholders as of September 22, 2020 (the “LENSAR Record Date”)
•In December 2020, we entered into a Capital Provision Agreement with Epps Investments LLC (“Epps”) regarding our previously announced Settlement Agreement with the Wellstat Parties whereby we sold all remaining amounts owed to us under the Settlement Agreement for consideration received
The Settlement Agreement with the Wellstat Parties provided for the payment of $7.5 million upon the signing of the Settlement Agreement, which has been received, and either (1) $5.0 million by February 10, 2021 and $55.0 million by July 26, 2021; or (2) $67.5 million by July 26, 2021. Under the terms of the Settlement Agreement, failure by the Wellstat Parties to make payment in full by July 26, 2021, authorized us to record judgment against the Wellstat Parties for an amount of $92.5 million or such lesser amount as may be owed under the Settlement Agreement.
Pursuant to the Capital Provision Agreement with Epps we received $51.4 million on December 31, 2020 in exchange for 100% of the payments or other property or value received by PDL on or after the date of the Capital Provision Agreement pursuant to the Settlement Agreement.
The proceeds from the sale of the three royalty interests to SWK totaled $4.35 million, 90% of which was received at the closing of the transaction. The remaining 10% is currently held in escrow against certain potential contingencies and is to be released on the one-year anniversary of the closing, subject to the satisfaction of any such potential contingencies.
On July 30, 2020, we signed a definitive agreement for the sale of our interest in Noden DAC and Noden USA to CAT Capital Bidco Limited (“Stanley Capital”). In accordance with the terms of the agreement, we expect to receive consideration of up to $52.8 million. Stanley Capital made an initial cash payment to us of $12.2 million on the September 9, 2020 closing date. We are also entitled to recover $0.5 million related to value-added tax (“VAT”) for inventory purchases from Novartis. The agreement provides for an additional $33.0 million to be paid to us in twelve equal quarterly installments from January 2021 to October 2023, of which the first installment payment has been received. An additional $3.9 million will be paid in four equal quarterly installments from January 2023 to October 2023. The agreement also provides for the potential for additional contingent payments to us. We are entitled to receive $2.5 million upon Stanley Capital or any of its affiliates entering into a binding agreement for a specified transaction within one year of the closing date. We are also entitled to 50% of a license fee from a third party distributor within 10 days of receipt by Noden. Upon closing, we recorded a gain of $0.2 million. In connection with the closing of the transaction, the guaranty agreement between Novartis and us which guaranteed certain payments owed to Novartis by Noden was terminated.
We intend to pursue monetization of our remaining assets in a disciplined and cost-effective manner to maximize returns to stockholders. At the same time, we recognize that accelerating the timeline to complete our monetization process, while continuing to optimize asset value, could increase returns to stockholders due to reduced general and administrative expenses as well as provide for faster returns to stockholders. While we are cognizant that an accelerated timeline may provide greater and faster returns to our stockholders, we also recognize that the duration and extent of the public health issues related to the COVID-19 pandemic make it possible that the timing of the sale of all or substantially all of our remaining assets may require additional time to execute or for us to pursue alternatives to the sale of these assets. For example, if a suitable offer to purchase the remaining royalty assets is not received prior to completing the dissolution process, they could be placed in a liquidating trust. The available proceeds from either the ongoing collection of royalty income or from the sale of the royalty assets would ultimately be distributed to our stockholders. We will continue to assess the market for our remaining assets to determine the appropriate time to sell them or to opt for alternative paths to return their value to our stockholders.
Following is a discussion of our current and historical segments.
Medical Devices
LENSAR
LENSAR is a commercial-stage medical device company focused on designing, developing and marketing an advanced femtosecond laser system for the treatment of cataracts and the management of pre-existing or surgically induced corneal astigmatism. LENSAR’s femtosecond laser uses proprietary advanced imaging and laser technology to customize planning and treatments, allowing faster visual recovery and improved outcomes, as compared to conventional cataract surgery, a more manual procedure combined with ultrasound, referred to as phacoemulsification. LENSAR has developed the LENSAR® Laser System, which is the only femtosecond cataract laser built specifically for refractive cataract surgery. At spin-off, LENSAR had over 95 granted patents in the United States and the rest of the world and over 55 pending patent applications in the United States and the rest of the world.
As noted above, on October 1, 2020 all outstanding shares of LENSAR common stock held by us were distributed to our holders of common stock as of the LENSAR Record Date. On October 1, each of our stockholders received 0.075879 shares of LENSAR common stock for every one share of our common stock held by such holders, based on the number of outstanding shares on the LENSAR Record Date. LENSAR continues to own and operate its femtosecond laser system business following completion of the distribution. As of October 1, 2020 LENSAR became an independent, publicly traded company listed on the Nasdaq Stock Market under the symbol “LNSR”.
Strategic Positions
Evofem
We invested $60.0 million in Evofem in the second quarter of 2019, representing approximately a 27% ownership interest in the company as of March 31, 2020. The transaction was structured in two tranches. The first tranche comprised $30.0 million, which was funded on April 11, 2019. We invested an additional $30.0 million in a second tranche on June 10, 2019, alongside two existing Evofem stockholders, who each invested an additional $10.0 million. On May 21, 2020 we distributed all of our 13,333,334 shares of common stock of Evofem to our stockholders of record on the Evofem Record Date, which represented approximately 26.7% of the outstanding shares of Evofem common stock as of the close of business on May 15, 2020. As of December 31, 2020, we continued to hold the warrants to purchase up to 3,333,334 shares of Evofem common stock.
Evofem is a commercial-stage biopharmaceutical company committed to developing and commercializing innovative products to address unmet needs in women’s sexual and reproductive health. Evofem is leveraging its proprietary Multipurpose Vaginal pH Regulator (MVP-R™) platform for its first commercial product Phexxi® (L-lactic acid, citric acid and potassium bitartrate) for hormone-free birth control. On May 22, 2020 Phexxi® was approved by the U.S. Food and Drug Administration for the prevention of pregnancy in women who choose to use on demand methods for their contraceptive needs. On September 8, 2020, Phexxi® was commercially launched in the United States.
As of June 30, 2020, the Strategic Positions segment was classified as discontinued operations.
Pharmaceutical
Noden
On July 1, 2016, our subsidiary, Noden DAC, entered into an asset purchase agreement (“Noden Purchase Agreement”) whereby it purchased from Novartis the exclusive worldwide rights to manufacture, market, and sell the Noden Products and certain related assets and assumed certain related liabilities (the “Noden Transaction”). Noden DAC and Noden USA, together, and including their respective subsidiaries represented deployed capital of $191.2 million.
Tekturna (or Rasilez outside of the United States) contains aliskiren, a direct renin inhibitor, for the treatment of hypertension. While indicated as a first line treatment, it is more commonly used as a third line treatment in those patients who are intolerant of angiotensin-receptor blockers (“ARBs”) or angiotensin converting enzyme inhibitors (“ACEIs”). Studies indicate that approximately 12% of hypertension patients are ARB/ACEI intolerant. Tekturna and Rasilez are not indicated for use with ARBs and ACEIs in patients with diabetes or renal impairment and are contraindicated for use by pregnant women. In March 2019, we launched an authorized generic (“AG”) form of Tekturna, aliskiren hemifumarate 150 mg and 300 mg tablets in the United States with the same drug formulation as Tekturna. The AG is distributed by Prasco, LLC d/b/a Prasco Laboratories.
Tekturna HCT is a combination of aliskiren and hydrochlorothiazide, a diuretic, for the treatment of hypertension in patients not adequately controlled by monotherapy and as an initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals. It is not indicated for use with ACEIs and ARBs in patient with diabetes or renal impairment, or for use in patients with known anuria or hypersensitivity to sulfonamide derived drugs and is contraindicated for use by pregnant women.
On September 9, 2020, we sold 100% of our interests in our wholly owned subsidiaries Noden DAC and Noden USA to Stanley Capital.
As of March 31, 2020, the Pharmaceutical segment was classified as discontinued operations.
Income Generating Assets
The income generating assets included in continuing operations consist of (i) notes and other long-term receivables, (ii) equity investments and (iii) royalties from the Queen et. al patents.
Notes and Other Long-Term Receivables
We have entered into credit agreements with borrowers across the healthcare industry, under which we made available cash loans to be used by the borrower. Obligations under these credit agreements are typically secured by a pledge of substantially all the assets of the borrower and any of its subsidiaries. As of December 31, 2020, we had one note receivable transaction outstanding, CareView, which is summarized below:
CareView
Technology
CareView is a provider of products and on-demand application services for the healthcare industry by specializing in bedside video monitoring, archiving and patient care documentation systems and patient entertainment services.
Deal Summary
In June 2015, we entered into a credit agreement with CareView, whereby we made available to CareView up to $40.0 million in loans comprised of two tranches of $20.0 million each, subject to CareView’s attainment of specified milestones and under which we have a security interest in substantially all of CareView’s assets. In October 2015, we and CareView entered into an amendment of the credit agreement to modify certain definitions related to the first and second tranche milestones and we funded the first tranche of $20.0 million, net of fees, based on CareView’s attainment of the first milestone, as amended. The second $20.0 million tranche was not funded due to CareView’s failure to meet the funding milestone and we have no further funding obligation at this time. The outstanding borrowing under the credit agreement initially bore interest at the rate of 13.5% per annum payable quarterly in arrears. Principal repayment was to commence on the ninth quarterly interest payment date and continue in equal installments until final maturity of the loan in October 2020.
In February 2018, we entered into a modification agreement with CareView (the “February 2018 Modification Agreement”) whereby we agreed, effective as of December 28, 2017, to modify the credit agreement before remedies could otherwise have become available to us under the credit agreement in relation to certain obligations of CareView that would potentially not be met, including the requirement to make principal payments. Under the February 2018 Modification Agreement, we agreed that (i) a lower liquidity covenant would be applicable and (ii) principal repayment would be delayed for a period of up to December 31, 2018. In exchange for agreeing to these modifications, among other things, the exercise price of our warrants to purchase 4.4 million shares of common stock of CareView was reduced and, subject to the occurrence of certain events, CareView agreed to grant us additional equity interests. In each of September 2018, December 2018, May 2019, September 2019 and December 2019, we entered into amendments to the February 2018 Modification Agreement with CareView whereby we agreed to deferrals of principal repayments and interest payments. In the May 2019 amendment we also increased the interest rate to 15.5% and removed the liquidity covenant under the credit agreement. In January 2020 we agreed to a further amendment of the February 2018 Modification Agreement that deferred principal repayment and interest payments until April 30, 2020, which was conditioned upon CareView raising additional financing from third parties. Pursuant to further amendments to the February 2018 Modification Agreement, the Company has agreed to defer principal and interest payments until May 31, 2021.
Royalties from Queen et al. patents
We have been issued patents in the United States and elsewhere, covering the humanization of antibodies, which we refer to as our Queen et al. patents. Our Queen et al. patents, for which final patent expiry was in December 2014, covered, among other things, humanized antibodies, methods for humanizing antibodies, polynucleotide encoding in humanized antibodies and methods of producing humanized antibodies.
We previously entered into licensing agreements under our Queen et al. patents with numerous entities that are independently developing or have developed humanized antibodies. Under our licensing agreements, we are entitled to receive a flat-rate royalty based upon our licensees’ net sales of covered antibodies, although the royalties under these agreements have substantially ended.
Solanezumab is a Lilly-licensed humanized monoclonal antibody being tested in a study of older individuals who may be at risk of memory loss and cognitive decline due to Alzheimer’s disease. Lilly has characterized the study as an assessment of whether an anti-amyloid investigational drug in older individuals who do not yet show symptoms of Alzheimer's disease cognitive impairment or dementia can slow memory loss and cognitive decline. The study will also test whether solanezumab treatment can delay the progression of Alzheimer’s disease related brain injury on imaging and other biomarkers. If solanezumab is approved and commercialized pursuant to this clinical trial or another, we would be entitled to receive a royalty based on a "know-how" license for technology provided in the design of this antibody. The 2% royalty on net sales is payable for 12.5 years after the product's first commercial sale. The above described study is currently in Phase 3 testing with an estimated study completion date expected in January of 2023.
Competition
The underlying products associated with our income generating assets compete with existing products and are vulnerable to new branded or generic entrants in the marketplace.
Human Capital
As of December 31, 2020, we had 11 full-time employees managing our intellectual property, operations and other corporate activities, as well as performing certain essential functions of a public company. All of our employees were based in the United States. None of our employees are covered by a collective bargaining agreement, and we consider our relationship with our employees to be good. As a result of the dissolution process, our human capital resources objectives include, retaining, and incentivizing our management team and other employees as we continue our monetization strategy. For example, the Compensation Committee of the Board adopted a Wind Down Retention Plan in which our executive officers and other employees were eligible to participate. Under the Wind Down Retention Plan, participants have been eligible to earn a retention benefit in consideration for their continued employment with us as we continue our monetization strategy and dissolution process.
About PDL
We were incorporated under the laws of the state of Delaware in 1986 under the name Protein Design Labs, Inc. In 2006, we changed our name to PDL BioPharma, Inc. Our business previously included a biotechnology operation that was focused on the discovery and development of novel antibodies. We spun-off the operation to our stockholders as Facet Biotech Corporation (“Facet”) in December 2008. Our principal executive offices are located at 10585 Double R Boulevard, Reno, Nevada, 89521, (775) 832-8500, and our website address is www.pdl.com. The information in or accessible through our website is not incorporated into, and is not considered part of, this filing.
Available Information
We file electronically with the SEC our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that website is www.sec.gov.
We make available free of charge on or through our website at www.pdl.com our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and proxy statements, as well as amendments to these reports and statements, as soon as practicable after we have electronically filed such material with, or furnished them to, the SEC. You may
also obtain copies of these filings free of charge by calling us at (775) 832-8500. The information in or accessible through the SEC and our website is not incorporated into, and is not considered part of, this filing.
On January 8, 2021, we filed a Form 15 notifying the SEC of deregistration of our common stock under Section 12(g) of the Exchange Act and suspension of our duty to file reports under Sections 13 and 15(d) of the Exchange Act.
ITEM 1A. RISK FACTORS
RISK FACTORS
Set forth below and elsewhere in this Annual Report on Form 10-K and in other documents we file with the Securities and Exchange Commission (SEC) are descriptions of the risks and uncertainties that could cause our actual results to differ materially from the results contemplated by forward looking statements contained in this Annual Report on Form 10-K. You should carefully consider and evaluate all of the information included in this report, including the risk factors set forth. The following is not an exhaustive discussion of all of the risks facing our company. Additional risks not presently known to us or that we currently deem immaterial may impair our wind-down.
Risks Related to Our Dissolution
There can be no assurances as to the amount of distributions, if any, to be made to our stockholders.
We filed for dissolution in January of 2021 and intend to use the dissolution process under Delaware law to liquidate our remaining assets, settle claims and, if available, make liquidating distributions of cash or other property to our stockholders. However, our dissolution and the liquidation of our remaining assets will be subject to uncertainties, and it is possible that there will be no additional liquidating distribution made to our stockholders.
We intend to rely on the “safe harbor” procedures under Sections 280 and 281(a) of the Delaware General Corporation Law (the DGCL) to, among other things, obtain an order from the Delaware Court of Chancery (the Court Order) establishing the amount and form of security for pending claims for which the Company is a party, contingent or unmatured contract claims for which the holder declined the Company’s offer of a security, and unknown claims that, based on facts known to the Company, are likely to arise or become known within five years from the filing of the Certificate of Dissolution (or such longer period of time, not to exceed ten years, as the Delaware Court of Chancery may determine), and pay or make reasonable provision for our uncontested known claims and expenses and establish reserves for other claims as required by the Court Order and the DGCL. We expect to distribute all of our remaining assets in excess of the amount to be used by us to pay claims and fund the reserves required by the Court Order and pay our operating expenses through the completion of the dissolution and winding-down process to our stockholders. The Court Order will reflect the Delaware Court of Chancery’s own determination as to the amount and form of security reasonably likely to be sufficient to provide compensation for all known, contingent and potential future claims against us. There can be no assurances that the Delaware Court of Chancery will not require us to withhold additional amounts in excess of the amounts that we believe are sufficient to satisfy our potential claims and liabilities. Accordingly, stockholders may not receive any distributions of our remaining assets, if any, for a substantial period of time.
In addition, there are numerous factors that could impact the amount of the reserves to be determined by the Court Order, and consequently the amount of cash initially available for distribution, if any, to our stockholders following the filing of the Certificate of Dissolution, including without limitation:
•whether any claim is resolved or barred pursuant to Section 280 of the DGCL;
•unanticipated costs relating to the defense, satisfaction or settlement of existing or future lawsuits or other claims threatened against us;
•whether unforeseen claims are asserted against us, in which case we would have to defend or resolve such claims and/or be required to establish additional reserves to provide for such claims;
•the amount of time it will take us to liquidate all of our remaining non-cash assets, and the amount of any costs and expenses that may be incurred in connection therewith;
•the amount of cash collected from amounts due from previous asset sales, such as our sale of Noden to Stanley Capital;
•the time to complete any tax audits for tax years impacted by amounts claimed under the CARES Act;
•the value, if any, we are able to obtain for our remaining non-cash assets in our monetization process;
•whether our royalty assets, for the next couple of years, and afterwards if retained in a liquidation trust, continue to perform as expected; and
•whether any of the expenses incurred in the winding-down process, including expenses of required personnel and other operating expenses (including legal, accounting and other professional fees) necessary to dissolve and liquidate the Company, are more or less than our estimates.
Further, the amount of any distributable proceeds and our ability to make distributions to our stockholders depend on our ability to execute our monetization strategy for our remaining non-cash assets, which is subject to significant risks and uncertainties, as further discussed in other risk factors herein.
In addition, as we wind down, we will continue to incur costs to manage the remaining business, such as salaries and other employee compensation, rental payments, insurance, and taxes, contractual obligations to current and former employees (e.g., true-up payments to holders of stock options that vested prior to the filing for dissolution) and other legal, accounting, financial advisory and consultant fees, which will reduce any amounts available for distribution to our stockholders.
As a result of these and other factors, we cannot assure you as to any amounts to be distributed to our stockholders in dissolution.
Liquidating distributions to stockholders could be substantially reduced and/or delayed due to uncertainty regarding the resolution of potential tax claims, litigation matters and other unresolved contingent liabilities of the Company.
Whether any remaining assets of the Company can be used to make liquidating distributions to stockholders would depend on whether claims for which we have set aside reserves are resolved or satisfied at amounts less than such reserves and whether a need has arisen to establish additional reserves. For example, we are subject to tax audits by the California Franchise Tax Board that have yet to be resolved, and the timing for resolution of such audits is uncertain. We cannot assure stockholders that our liabilities can be resolved for less than the amounts we have reserved, that unknown liabilities that have not been accounted for will not arise or the timing with respect to resolution of any such liabilities or claims. As a result, we may continue to hold back funds and delay additional liquidating distributions to stockholders.
We cannot predict the timing of the distributions to stockholders.
Under the DGCL, before a dissolved corporation may make any distribution to its stockholders, it must pay or make reasonable provision to pay all of its claims and obligations, including all contingent, conditional or unmatured contractual claims known to the corporation. The precise amount and timing of any distributions to our stockholders will depend on and could be delayed or diminished due to many factors, including without limitation:
•whether a claim is resolved for more than the amount of reserve established for such claim pursuant to the Court Order;
•whether we are unable to resolve claims with creditors or other third parties, or if such resolutions take longer than expected;
•whether a creditor or other third party seeks an injunction against the making of additional distributions to stockholders on the basis that the amounts to be distributed are needed to satisfy our liabilities or other obligations to the extent not previously reserved for;
•whether due to new facts and developments, new claims, as the Board reasonably determines, require additional funds to be reserved for their satisfaction; and
•whether the expenses we incur in the winding-down process, including expenses of personnel required and other expenses (including legal, accounting and other professional fees) necessary to dissolve and liquidate the Company are more than anticipated.
As a result of these and other factors, it might take significant time to resolve these matters, and as a result we are unable to predict the timing of distributions, if any, made to our stockholders.
Our dissolution pursuant to the Plan of Dissolution may be disrupted and adversely impacted by the effects of natural disasters, political crises, public health crises, and other events outside of our control.
Natural disasters, such as adverse weather, fires, earthquakes, power shortages and outages, political crises, such as terrorism, war, political instability, or other conflict, criminal activities, public health crises, such as the COVID-19 pandemic and other disease epidemics and pandemics, and other disruptions or events outside of our control could negatively affect our operations and our ability to monetize our remaining assets or realize the estimated value of our net assets in liquidation. Any of these events may cause a delay in our ability to make distributions in dissolution, and may materially impact the amount of cash or value of other non-cash assets available to distribute to our stockholders, if any.
Our stockholders may be liable to our creditors for part or all of the amount received from us in our liquidating distributions if reserves are inadequate.
In dissolution we may establish a contingency reserve designed to satisfy any additional claims and obligations that may arise. Any contingency reserve may not be adequate to cover all of our claims and obligations. Under the DGCL, if we fail to create an adequate contingency reserve for payment of our expenses, claims and obligations, each stockholder could be held liable for payment to our creditors for claims brought during this three-year period after we filed the Certificate of Dissolution with the Secretary of State, up to the lesser of (i) such stockholder’s pro rata share of amounts owed to creditors in excess of the contingency reserve and (ii) the amounts previously received by such stockholder in dissolution from us and from any liquidating
trust or trusts. Accordingly, in such event, a stockholder could be required to return part or all of the distributions previously made to such stockholder in dissolution, and a stockholder could receive nothing from us under the Plan of Dissolution. Moreover, if a stockholder has paid taxes on amounts previously received, a repayment of all or a portion of such amounts received could result in a situation in which such repayment does not result in a commensurate refund of such taxes paid. As a result, while we intend to use the protections of DGCL Sections 280 and 281(a), including having a court order approve distributions, it is important for us to retain sufficient funds through our dissolution to pay the expenses and liabilities actually owed to our creditors because if we fail to do so, each stockholder could be held liable for the repayment to creditors out of the amounts previously distributed to such stockholder from us or from any liquidating trust or trusts, up to the full amount actually received by such stockholder in our dissolution.
The directors and officers of the Company will continue to receive benefits from the Company during the dissolution.
During the dissolution, we will continue to indemnify each of our current and former directors and officers to the extent permitted under the DGCL and the Company’s certificate of incorporation, bylaws and agreements as in effect at the time of the filing of the Certificate of Dissolution.
Further stockholder approval will not be required in connection with the implementation of our Plan of Dissolution, including for the sale or disposition of any of our remaining assets.
Our Plan of Dissolution provides that we may sell our remaining assets after dissolution, as necessary to affect our Plan of Dissolution. Under our Plan of Dissolution, we will not seek and are not required to seek additional stockholder authorization of any future asset sale. As a result, the Board may authorize actions in implementing the Plan of Dissolution, including the terms and prices for the sale or disposition of our remaining assets, with which our stockholders may not agree.
Our common stock ceased to be traded at the time of our dissolution.
We closed our stock transfer books of our common stock after our dissolution became effective at approximately 4:00 p.m., Eastern time on January 4, 2021 (the “Final Record Date”). Therefore, shares of our common stock are no longer freely transferable. As a result of the closing of the stock transfer books, all liquidating distributions from a liquidating trust, if any, or from us after the Final Record Date will be made pro rata to the stockholders of record as of the Final Record Date.
We have not and do not intend to record any assignments or transfers of our common stock after the Final Record Date, other than as required by will, intestate succession or operation of law. We have been informed that after the Final Record Date some shares of our common stock have been traded under contractual obligations between the seller and purchaser of the stock, who negotiate and rely on themselves with respect to the allocation of stockholder proceeds arising from ownership of the shares. We are not facilitating or participating in the trading in our common stock or interests in proceeds from our liquidation. Accordingly, trading in our stock is highly speculative and the market for our stock is highly illiquid. As we are no longer an operating company, the only value underlying the trading price of our shares is the right to receive further distributions, if any, as part of the liquidation process. Because of the difficulty in estimating the amount and timing of the liquidating distributions and due to the other risk factors discussed herein, the economic interests derived from our common stock may be subject to significant volatility and may trade above or below the amount of any future liquidating distribution that may be made.
The loss of key personnel could adversely affect our ability to efficiently dissolve, liquidate and wind down.
We intend to rely on a few individuals in key management roles to dissolve, liquidate our remaining assets and wind-down operations. Loss of one or more of these key individuals could hamper the efficiency or effectiveness of these processes.
Our remaining licensees, borrowers and royalty-agreement counterparties may be unable to maintain regulatory approvals for their products, or to obtain regulatory approvals or favorable pricing for new products, and they may voluntarily remove products from marketing and commercial distribution. Any of such events, whether due to safety issues or other factors, could limit our revenues or return on investment. or our ability to generate expected returns from the monetization of such assets, including their sale, and reduce resulting distributions to our stockholders in dissolution.
Our licensees, borrowers and royalty-agreement counterparties are subject to stringent regulation with respect to product safety and efficacy by various international, federal, state and local authorities. Even if our licensees’, borrowers’ and royalty-agreement counterparties’ products receive regulatory approval, they will remain subject to ongoing FDA and other international regulations including, but not limited to, obligations to conduct additional clinical trials or other testing, changes to the product label, new or revised regulatory requirements for manufacturing practices, written advisements to physicians and/or a product recall or
withdrawal. Our licensees, borrowers and royalty-agreement counterparties may not maintain necessary regulatory approvals for their existing products. Moreover, the current political environment in the United States is focused on potential reductions in pricing for pharmaceutical and other healthcare products, which may negatively impact any existing or new products from which our revenues would be derived. We are unable to control the pricing strategies used by our licensees, borrowers and royalty-agreement counterparties, and if they fail to use appropriate pricing strategies, or receive negative reactions to their pricing strategies, it could negatively impact our revenues or return on investment.
In addition, communications from government officials regarding pricing for pharmaceutical and other health care products could have a negative impact on the value of the assets we intend to monetize, even if such communications do not ultimately impact our borrowers’ and royalty-agreement counterparties’ products. The occurrence of adverse events reported by any borrower or royalty-agreement counterparty may result in the revocation of regulatory approvals or decreased sales of the applicable product due to a change in physicians’ willingness to prescribe, or patients’ willingness to use the applicable product. Our borrowers and royalty-agreement counterparties could also choose to voluntarily remove licensed products from marketing and commercial distribution. Any value we may receive upon a potential transaction in furtherance of our monetization strategy in dissolution could be materially and adversely affected.
The value of our remaining income generating royalty assets may be dependent on the actions of unrelated third parties, which may negatively impact the value we are able to realize in dissolution.
In connection with our income generating assets, we are dependent, to a large extent, on third parties to enforce certain rights for our benefit. For example, Assertio (formerly Depomed), as the licensor of certain patents, retains various rights, including the contractual right to audit its licensees and to ensure those licensees are complying with the terms of the underlying license agreements. Assertio also retained full responsibility to protect and maintain the intellectual property rights underlying the licenses. While we have contractual rights to require Assertio to take action regarding certain of these rights, because Assertio’s economic interest in the license agreements is limited, it may not enforce or protect those rights as it otherwise would have had it retained the full economic interest in the payments under the license agreements.
Our royalty-agreement counterparties face significant market pressures with respect to their products, and the amount of revenues from their pharmaceutical products or medical devices, or from our income generating assets that we receive are subject to various competitive and market factors, including generic competitors, that may be outside of our control.
Our royalty-agreement counterparties face competition from other pharmaceutical companies. The introduction of new competitive products, including generics, may result in lost market share for our royalty-agreement counterparties, reduced use of their products, lower prices and/or reduced sales, any of which could reduce our royalty revenues, and have a material adverse effect on any realized value we may obtain in connection with the evaluation of potential transactions to monetize such remaining assets in dissolution.
The amounts we are able to realize from any transaction in furtherance of our monetization of our remaining assets, and the amount of cash or other assets we are able to distribute our stockholders in dissolution, will depend on many factors, including the following:
•the timing and availability of generic product competition for our royalty-agreement counterparties’ products;
•potential challenges or design-arounds to product, use or manufacturing related patents which provide exclusivity for products and assets before their expiration by generic pharmaceutical manufacturers;
•the size of the market for our royalty-agreement counterparties’ products;
•the extent and effectiveness of the sales and marketing and distribution support for our royalty-agreement counterparties’ products;
•the existence of novel or superior products to our royalty-agreement counterparties’ products;
•the availability of reduced pricing and discounts applicable to our royalty-agreement counterparties’ products;
•stocking and inventory management practices related to our royalty-agreement counterparties’ products;
•limitations on indications for which our royalty-agreement counterparties’ products can be marketed; the competitive landscape for approved products and developing therapies that compete with royalty-agreement counterparties’ products;
•the ability of patients to be able to afford our royalty-agreement counterparties’ products or obtain healthcare coverage that covers those products;
•acceptance of, and ongoing satisfaction with, our royalty-agreement counterparties’ products by the care providers, patients receiving therapy and third-party payors; or
•the unfavorable outcome (or potential thereof) of any litigation relating to our royalty-agreement counterparties’ business practices or products.
For example, in mid-2019, Bausch Health announced expected price decreases on Glumetza, a royalty-bearing product under our Assertio Royalty Agreement. These price decreases could negatively affect revenues and thus our royalties. Due to the uncertainties caused by changes in pricing by third parties that are outside our control, including as a result of generic competition, we may not be able to accurately estimate the impact on royalties on such sales paid to us for Glumetza or any other product.
We are also aware of a number of approved generic extended-release metformin products, which could further negatively affect Glumetza revenues.
Any of these factors may have a material and adverse effect on our ability to realize significant value for our remaining royalty assets and negatively affect the amount of cash or other assets we are able to distribute to our stockholders in dissolution.
Risks Related to Taxes
We may not be able to realize certain expected tax benefits.
During 2020, we engaged in certain transactions that may result in the recognition of ordinary tax losses. Such losses, through provisions of the CARES Act, could generate meaningful tax benefits to the Company as the CARES Act permits taxpayers to carry back five years any net operating losses arising in a taxable year beginning in 2018, 2019 or 2020. In connection with our monetization process, we have executed transactions that result in ordinary tax losses that could be applied to prior tax years in which PDL was a substantial tax payor. There can be no assurance that such tax benefits will be realized as expected. For example, in February 2021, a letter was sent to congressional leaders signed by 120 members of Congress that proposed retroactively repealing the net operating loss carry back provisions of the CARES Act. If these provisions were retroactively repealed, it would likely have a material adverse effect on the amount of tax benefits that may be available to us under the current provisions of the CARES Act. Any failure to obtain such expected tax benefits under the CARES Act would likely reduce the funds available for distribution to our stockholders.
U.S. Stockholders may not be able to recognize a loss for U.S. federal income tax purposes until they receive a final distribution from us.
Distributions made pursuant to the Plan of Dissolution are intended to be treated as received by a U.S. stockholder in exchange for the U.S. stockholder’s shares of our common stock. Accordingly, the amount of any such distribution allocable to a block of shares of our common stock owned by the U.S. stockholder will reduce the U.S. stockholder’s tax basis in such shares, but not below zero. Any excess amount allocable to such shares will be taxable as capital gain. Such gain generally will be taxable as long-term capital gain if the shares have been held for more than one year. Any tax basis remaining in a share of our common stock following the final liquidating distribution by the Company will be treated as a capital loss. The deductibility of capital losses is subject to limitations. U.S. stockholders should consult their tax advisors as to the particular tax consequences of our dissolution for them, including the applicability of any U.S. federal, state, local and non-U.S. tax laws.
The tax treatment of any liquidating distribution may vary from stockholder to stockholder.
We have not requested a ruling from the IRS with respect to the anticipated tax consequences of our complete dissolution and liquidation, and we will not seek an opinion of counsel with respect to the anticipated tax consequences of any liquidating distributions. If any of the anticipated tax consequences prove to be incorrect, the result could be increased taxation at the corporate or stockholder level, thus reducing the benefit to our stockholders and us from our dissolution and liquidation. Tax considerations applicable to particular stockholders may vary with and be contingent on the stockholder’s individual circumstances. Stockholders should consult with their own tax advisors for tax advice on our dissolution and liquidation’s impact on their taxes.
General Risk Factors
Failure to protect our information technology infrastructure against cyber-based attacks, network security breaches, service interruptions or data corruption could materially disrupt our operations and adversely affect our business and operating results.
We rely on our information technology systems to effectively manage all business activities and data, including accounting, financial and legal functions, asset management and business development. Our information technology systems are vulnerable to damage or interruption from earthquakes, fires, floods and other natural disasters, terrorist attacks, power losses, computer system
or data network failures, security breaches, data corruption and cyber-based attacks, including malicious software programs or other attacks, which have been attempted against us in the past. In addition, a variety of our software systems are cloud-based data management applications, hosted by third-party service providers whose security and information technology systems are subject to similar risks.
The failure to protect either our or our service providers’ information technology infrastructure could disrupt our entire operation or result in our inability to manage and monetize our remaining assets, increased overhead costs, loss or misuse of proprietary or confidential information, intellectual property or sensitive or personal information, all of which could have a material adverse effect on our business and affect the amount of cash or other assets we may be able to distribute to our stockholders in dissolution.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We lease approximately 1,750 square feet of office space in Reno, Nevada, which serves as our corporate headquarters. The lease expires in December 2022. Previously, we leased approximately 5,900 square feet of office space in Incline Village, Nevada, which served as our corporate headquarters until December 31, 2020 when the lease was terminated.
In July 2006, we entered into two leases and a sublease for facilities in Redwood City, California, which formerly served as our corporate headquarters and cover approximately 450,000 square feet of office space. Under the amendments to the leases entered into in connection with the spin-off of Facet, Facet was added as a co-tenant under the leases. As a co-tenant, Facet is bound by all of the terms and conditions of the leases. We and Facet are jointly and severally liable for all obligations under the leases, including the payment of rental obligations. The guarantee runs through December 2021. We also entered into a Co-Tenancy Agreement with Facet in connection with the spin-off and the lease amendments under which we assigned to Facet all rights under the leases, including, but not limited to, the right to amend the leases, extend the lease terms or terminate the leases, and Facet assumed all of our obligations under the leases. Under the Co-Tenancy Agreement, we also relinquished any right or option to regain possession, use or occupancy of these facilities. Facet agreed to indemnify us for all matters associated with the leases attributable to the period after the spin-off date and we agreed to indemnify Facet for all matters associated with the leases attributable to the period before the spin-off date. In addition, in connection with the spin-off, we assigned the sublease to Facet. In April 2010, Abbott Laboratories acquired Facet and later renamed the entity AbbVie Biotherapeutics, Inc. (“AbbVie”). To date, AbbVie has satisfied all obligations under the Redwood City leases.
We believe that our existing facilities are adequate to meet our business requirements for the reasonably foreseeable future.
ITEM 3. LEGAL PROCEEDINGS
The information set forth in Note 25, Legal Proceedings, to the Consolidated Financial Statements included in Item 8, “Financial Statements and Supplementary Data” of this Annual Report is incorporated by reference herein.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
On December 8, 2020, we formally notified the Nasdaq Stock Market, Inc. of our intent to delist the Company's common stock from the Nasdaq Global Select Market ("Nasdaq"). We filed a Form 25 (Notification of Removal from Listing) with the SEC and Nasdaq relating to the voluntary delisting of our common stock on December 28, 2020 and suspended trading of our common stock prior to the opening of trading on December 31, 2020. We filed a certificate of dissolution with the Delaware Secretary of State on January 4, 2021 (the “Final Record Date”) and instructed our transfer agent to close our stock transfer books at the close of business on this date. PDL's common stock was delisted effective on January 7, 2021. On January 8, 2021, we filed a Form 15 notifying the SEC of our deregistration of our common stock under Section 12(g) of the Exchange Act and suspension of our duty to file reports under Sections 13 and 15(d) of the Exchange Act.
As of January 6, 2021, we had approximately 86 common stockholders of record. Most of our outstanding shares of common stock are held of record by one stockholder, Cede & Co., as nominee for the Depository Trust Company. Many brokers, banks and other institutions hold shares of common stock as nominees for beneficial owners that deposit these shares of common stock in participant accounts at the Depository Trust Company. The actual number of beneficial owners of our stock is likely significantly greater than the number of stockholders of record; however, we are unable to reasonably estimate the total number of beneficial owners.
Dividends
On May 5, 2020, our board of directors approved a distribution of all of the Company’s shares of common stock of Evofem Biosciences, Inc. (“Evofem”) via a special one-time dividend to PDL stockholders. The Evofem shares were distributed on May 21, 2020 to PDL shareholders of record as of the close of business on May 15, 2020 (the “Evofem Record Date”). Based on the shares of PDL common stock outstanding as of the close of business on the Evofem Record Date, PDL stockholders were entitled to receive 0.11591985 shares of Evofem common stock for each share of PDL common stock held.
On September 10, 2020, our board of directors approved a distribution of all of the Company’s shares of common stock of LENSAR via a special one-time dividend to PDL stockholders. The LENSAR shares were distributed on October 2, 2020 to PDL shareholders of record as of the close of business on September 22, 2020 (the “LENSAR Record Date”). Based on the shares of PDL common stock outstanding as of the close of business on the LENSAR Record Date, PDL stockholders were entitled to receive 0.075879 shares of LENSAR common stock for each share of PDL common stock held.
Equity Compensation Plan Information
See Part III, Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” for information regarding securities authorized for issuance under equity compensation plans.
Recent Sales of Unregistered Securities
There were no unregistered sales of equity securities during the period covered by this report.
Issuer purchases of Equity Securities
There were no repurchases of our common stock made by us in the three months ended December 31, 2020.
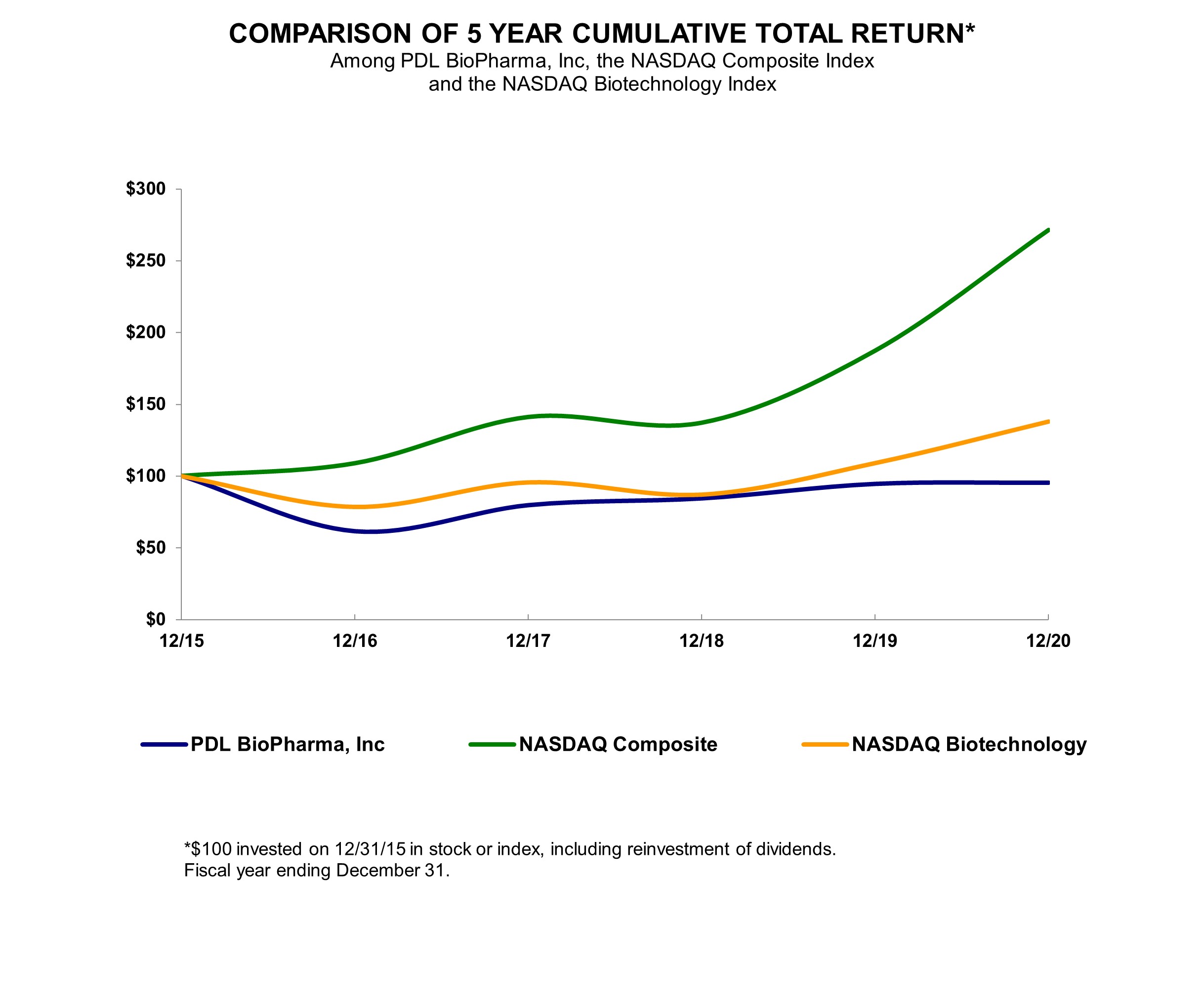
Comparison of Stockholder Returns
The line graph below compares the cumulative total stockholder return on our common stock between December 31, 2015, and December 31, 2020, with the cumulative total return of (i) the Nasdaq Biotechnology Index and (ii) the Nasdaq Composite Index over the same period. This graph assumes that $100.00 was invested on December 31, 2015, in our common stock at the closing sales price for our common stock on that date and at the closing sales price for each index on that date and that all dividends were reinvested. Stockholder returns over the indicated period should not be considered indicative of future stockholder returns and are not intended to be a forecast.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 12/31/2015 | | 12/31/2016 | | 12/31/2017 | | 12/31/2018 | | 12/31/2019 | | 12/31/2020 |
| PDL BioPharma, Inc. | $ | 100.00 | | | $ | 61.71 | | | $ | 79.76 | | | $ | 84.42 | | | $ | 94.46 | | | $ | 95.29 | |
| Nasdaq Composite Index | $ | 100.00 | | | $ | 108.87 | | | $ | 141.13 | | | $ | 137.12 | | | $ | 187.44 | | | $ | 271.64 | |
| Nasdaq Biotechnology Index | $ | 100.00 | | | $ | 78.65 | | | $ | 95.67 | | | $ | 87.19 | | | $ | 109.08 | | | $ | 137.90 | |
The information in this section shall not be deemed to be “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate it by reference in such filing.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
Reserved.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the Consolidated Financial Statements and related Notes included elsewhere in this Form 10-K.
Overview
Throughout our history, our mission has been to improve the lives of patients by aiding in the successful development of innovative therapeutics and healthcare technologies. PDL BioPharma was founded in 1986 as Protein Design Labs, Inc. when it pioneered the humanization of monoclonal antibodies, enabling the discovery of a new generation of targeted treatments that have had a profound impact on patients living with different cancers as well as a variety of other debilitating diseases. In 2006, we changed our name to PDL BioPharma, Inc.
Historically, we generated a substantial portion of our revenues through the license agreements related to patents covering the humanization of antibodies, which we refer to as the Queen et al. patents. In 2012, and in anticipation of declining revenues from the Queen et al. patents, we began providing alternative sources of capital through royalty monetization and debt facilities, and, in 2016, we began acquiring commercial-stage products and launching specialized companies dedicated to the commercialization of these products, first with our acquisition of branded prescription pharmaceutical drugs from Novartis in 2016 and, in 2017, with the acquisition of LENSAR, a medical device ophthalmology equipment manufacturing company. In 2019, we entered into a securities purchase agreement with Evofem pursuant to which we invested $60.0 million in a private placement of securities. These investments provided funding for Evofem’s pre-commercial activities for Phexxi®, its non-hormonal, on-demand prescription contraceptive gel for women.
Based on the nature of our investments entered into between 2012 through 2019 and further discussed below, our operations were structured in four segments designated as Medical Devices, Strategic Positions, Income Generating Assets, and Pharmaceutical.
Our Medical Devices segment consisted of revenue from the sale and lease of the LENSAR® Laser System, which included equipment, PIDs, procedure licenses, training, installation, warranty and maintenance agreements.
Our Strategic Positions segment consisted of an investment in Evofem (NASDAQ: EVFM). Our investment included shares of common stock and warrants to purchase additional shares of common stock.
Our Pharmaceutical segment consisted of revenue derived from the Noden Products.
Our Income Generating Assets segment consisted of revenue derived from (i) notes and other long-term receivables, (ii) royalty rights and hybrid notes/royalty receivables, (iii) equity investments and (iv) royalties from issued patents in the United States and elsewhere covering the humanization of antibodies, which we refer to as the Queen et al. patents.
Financial information about our segments, including our revenues and net loss for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, and select long-lived assets as of December 31, 2020 and 2019, is included in our Consolidated Financial Statements and accompanying notes in this Form 10-K.
In September 2019, we engaged financial and legal advisors and initiated a review of our strategy. This review was completed in December 2019. At such time, we disclosed that we planned to halt the execution of our growth strategy, cease making additional strategic transactions and investments and instead pursue a formal process to unlock the value of our portfolio by monetizing our assets and ultimately distributing net proceeds to stockholders (the “monetization strategy”). Pursuant to our monetization strategy, we did not expect to enter into any additional strategic investments. We further announced in December 2019 that we would explore a variety of potential transactions in connection with the monetization strategy, including a whole Company sale, divestiture of our assets, spin-offs of operating entities, merger opportunities or a combination thereof. Over the subsequent months, our board of directors (the “Board”) and management analyzed, together with our outside financial and legal advisors, how to best capture value pursuant to our monetization strategy and best return the significant intrinsic value of the assets in our portfolio to the stockholders.
In February 2020, the Board approved a plan of complete liquidation (the “Plan of Liquidation”) of our assets and passed a resolution to seek stockholder approval to dissolve our Company. At our Annual Meeting of Stockholders in August 2020, the proposal to liquidate and dissolve our Company pursuant to a plan of dissolution was approved by our stockholders. On November 5, 2020, our Board approved filing a certificate of dissolution with the Secretary of State of Delaware in January 2021
and proceeding to complete the dissolution process for our Company in accordance with the Delaware General Corporate Law. The filing of the certificate of dissolution occurred on January 4, 2021 and we closed our stock transfer books as of such date (the “Final Record Date”). After such time, we are not recording any further transfers of our common stock, except pursuant to the provisions of a deceased stockholder’s will, intestate succession, or by operation of law and we will not issue any new stock certificates, other than replacement certificates. In addition, we will not be issuing any shares of our common stock upon exercise of outstanding stock options. As a result of the closing of our transfer books, it is anticipated that distributions, if any, made in connection with the dissolution will be made pro rata to the stockholders of record as of the Final Record Date. In accordance with our dissolution plan, we completed the voluntary delisting process from the Nasdaq Stock Market exchange so that suspension of trading occurred before the market opened on December 31, 2020 and official delisting of our stock occurred on January 7, 2021. We do not anticipate participating in any OTC trading of our stock or economic rights in our stock.
Pursuant to our monetization strategy, we explored a variety of potential transactions, including a whole Company sale, divestiture of assets, spin-offs of operating entities, merger opportunities or a combination thereof. In addition, we analyzed, and continue to analyze, optimal mechanisms for returning value to stockholders in a tax-efficient manner, including share repurchases, cash dividends and other distributions of assets. Despite the challenges of COVID-19, we made significant progress in our monetization strategy during 2020, including monetizing most of our key assets and resolving a longstanding legal issue as follows:
•In May 2020, we made a liquidation distribution of all of our common stock in Evofem to our stockholders. As of December 31, 2020, we held warrants to purchase up to 3,333,334 shares of Evofem common stock with an exercise price of $6.38
•In August 2020, we entered into a settlement agreement (the “ Settlement Agreement”) with related entities of Defined Diagnostics, LLC (f/k/a Wellstat Diagnostics, LLC) ("Wellstat Diagnostics" and, together with such related entities, the "Wellstat Parties") resolving previously reported litigation relating to loans made to Wellstat Diagnostics by us
•In August 2020, we sold three royalty interests related to third party sales of Kybella®, Zalviso®, and Coflex®
•In September 2020, we completed the previously announced sale of our interest in Noden DAC and Noden USA
•In October 2020, we completed the previously announced spin-off of LENSAR whereby we made a liquidation distribution of all of our shares of LENSAR common stock to our stockholders as of September 22, 2020
•In December 2020, we entered into a Capital Provision Agreement with Epps Investments LLC (“Epps”) regarding our previously announced Settlement Agreement with the Wellstat Parties whereby we sold all remaining amounts owed to us under the Settlement Agreement for consideration received
The Settlement Agreement with the Wellstat Parties provided for the payment of $7.5 million upon the signing of the Settlement Agreement, which has been received, and either (1) $5.0 million by February 10, 2021 and $55.0 million by July 26, 2021; or (2) $67.5 million by July 26, 2021. Under the terms of the Settlement Agreement, failure by the Wellstat Parties to make payment in full by July 26, 2021, authorized us to record judgment against the Wellstat Parties for an amount of $92.5 million or such lesser amount as may be owed under the Settlement Agreement.
The Capital Provision Agreement with Epps provided for the payment of $51.4 million, which was received on December 31, 2020, in exchange for 100% of the payments or other property or value received by PDL on or after the date of the Capital Provision Agreement pursuant to the Settlement Agreement.
The proceeds from the sale of the three royalty interests totaled $4.35 million, 90% of which was received at the closing of the transaction. The remaining 10% is currently held in escrow against certain potential contingencies and is to be released on the one-year anniversary of the closing, subject to the satisfaction of any such potential contingencies.
On July 30, 2020, we signed a definitive agreement for the sale of our interest in Noden DAC and Noden USA to CAT Capital Bidco Limited (“Stanley Capital”). In accordance with the terms of the agreement, we expect to receive consideration of up to $52.8 million. Stanley Capital made an initial cash payment to us of $12.2 million on the September 9, 2020 closing date. We are also entitled to recover $0.5 million related to value-added tax (“VAT”) for inventory purchases from Novartis. The agreement provides for an additional $33.0 million to be paid to us in twelve equal quarterly installments from January 2021 to October 2023, of which the first installment payment has been received. An additional $3.9 million will be paid in four equal quarterly installments from January 2023 to October 2023. The agreement also provides for the potential for additional contingent payments to us. We are entitled to receive $2.5 million upon Stanley Capital or any of its affiliates entering into a binding agreement for a specified transaction within one year of the closing date. We are also entitled to 50% of a license fee from a third party distributor within 10 days of receipt by Noden. Upon closing, we recorded a gain of $0.2 million. In connection with the
closing of the transaction, the guaranty agreement between Novartis and us which guaranteed certain payments owed to Novartis by Noden was terminated.
We intend to pursue monetization of our remaining assets in a disciplined and cost-effective manner to maximize returns to stockholders. At the same time, we recognize that accelerating the timeline to complete our monetization process, while continuing to optimize asset value, could increase returns to stockholders due to reduced general and administrative expenses as well as provide for faster returns to stockholders. While we are cognizant that an accelerated timeline may provide greater and faster returns to our stockholders, we also recognize that the duration and extent of the public health issues related to the COVID-19 pandemic make it possible that the timing of the sale of all or substantially all of our remaining assets may require additional time to execute or for us to pursue alternatives to the sale of these assets. For example, if a suitable offer to purchase the remaining royalty assets is not received prior to completing the dissolution process, they could be ultimately placed in a liquidating trust. The available proceeds from either the ongoing collection of royalty income or from the sale of the royalty assets would ultimately be distributed to our stockholders. We will continue to assess the market for our remaining assets to determine the appropriate time to sell them or to opt for alternative paths to return their value to our stockholders.
Critical Accounting Policies and Significant Estimates
The preparation of financial statements and related disclosures in conformity with U.S. Generally Accepted Accounting Principles (“GAAP”) and the discussion and analysis of our financial condition and operating results require our management to make judgments, assumptions and estimates that affect the amounts reported in its Consolidated Financial Statements and accompanying notes. Note 2, Summary of Significant Accounting Policies, to the Consolidated Financial Statements included in this Form 10-K describes the significant accounting policies and methods used in the preparation of our Consolidated Financial Statements. Management bases its estimates on historical experience and on various other assumptions it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates and such differences may be material.
While our significant accounting policies are more fully described in the notes to our Consolidated Financial Statements appearing elsewhere in this Form 10-K, management believes that the following accounting policies related to the liquidation basis of accounting, assets and liabilities held for sale, discontinued operations, notes receivable and other long-term receivables, inventory, intangible assets, convertible notes, product revenue, royalty rights - at fair value, and income taxes are critical because they are both important to the portrayal of our financial condition and operating results, and they require management to make judgments and estimates about inherently uncertain matters.
Liquidation Basis of Accounting
As a result of the approval of the Company’s stockholders to pursue dissolution of the Company pursuant to a plan of dissolution, the Company’s basis of accounting transitioned, effective September 1, 2020, from the going concern basis of accounting (“Going Concern Basis”) to the liquidation basis of accounting (“Liquidation Basis”) in accordance with Generally Accepted Accounting Principles. Under the Liquidation Basis, the values of the Company's assets and liabilities include management's estimate of income to be generated from the remaining assets until the anticipated date of sale, estimated sales proceeds, estimates for operating expenses and expected amounts required to settle liabilities. The estimated liquidation values for assets derived from future revenue streams and asset sales and the settlement of estimated liabilities are reflected on the Consolidated Statement of Net Assets in Liquidation. The actual amounts realized could differ materially from the estimated amounts.
Assets Held for Sale
Under the Going Concern Basis, assets and liabilities are classified as held for sale and presented separately on the balance sheet when all of the following criteria for a plan of sale have been met: (1) management, having the authority to approve the action, commits to a plan to sell the assets; (2) the assets are available for immediate sale, in their present condition, subject only to terms that are usual and customary for sales of such assets; (3) an active program to locate a buyer and other actions required to complete the plan to sell the assets have been initiated; (4) the sale of the assets is probable and is expected to be completed within one year; (5) the assets are being actively marketed for a price that is reasonable in relation to their current fair value; and (6) actions required to complete the plan indicate that it is unlikely that significant changes to the plan will be made or the plan will be withdrawn. The Pharmaceutical segment, Strategic Positions segment and certain royalty right assets within the Income Generating Assets segment are classified as held for sale. The royalty right assets that were classified as held for sale are Assertio, Viscogliosi Brothers, University of Michigan, AcelRx, and Kybella. The assets and liabilities held for sale are included on the Company’s Consolidated Balance Sheet as of December 31, 2019, as Assets held for sale and Liabilities held for sale.
Discontinued Operations
Discontinued operations comprise those activities that were disposed of during the period or that were classified as held for sale at the end of the period, represent a separate major line of business or geographical area that can be clearly distinguished for operational and financial reporting purposes, and represent a strategic shift that has or will have a major effect on the Company’s operations and financial results. The profits and losses of the investments classified as held for sale, the Pharmaceutical segment, the Strategic Positions segment, and certain royalty assets noted above, are presented on the Consolidated Statements of Operations as discontinued operations. Depreciation and amortization of assets ceases upon designation as held for sale.
See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
Notes Receivable and Other Long-Term Receivables
Under the Going Concern Basis, we accounted for our notes receivable at amortized cost, net of unamortized origination fees, if any, and adjusted for any impairment losses. Interest was accreted or accrued to “Interest revenue” using the effective interest method. When and if supplemental payments are received from certain of these notes and other long-term receivables, an adjustment to the estimated effective interest rate was affected prospectively.
We evaluated the collectability of both interest and principal for each note receivable or loan to determine whether it is impaired. A note receivable or loan was considered to be impaired when, based on available information and events, we determined it is probable that it would be unable to collect amounts due according to the existing contractual terms. When a note receivable or loan was considered to be impaired, the amount of loss was calculated by comparing the carrying value of the financial asset to the value determined by discounting the expected future cash flows at the loan’s effective interest rate or to the estimated fair value of the underlying collateral, less costs to sell, if the loan was collateralized and we expected repayment to be provided solely by the collateral. Impairment assessments required significant judgments and were based on significant assumptions related to the borrower’s credit risk, financial performance, expected sales, and estimated fair value of the collateral.
We recorded interest on an accrual basis and recognized it as earned in accordance with the contractual terms of the applicable credit agreement, to the extent that the underlying note receivable or loan was not impaired and such amounts were expected to be collected. When a note receivable or loan became past due, or if management otherwise did not expect that principal, interest, and other obligations due would be collected in full, we generally placed the note receivable or loan on an impaired status and ceased recognizing interest income on that note receivable or loan. Any uncollected interest related to prior periods was reversed from income in the period that collection of the interest receivable is determined to be doubtful.
As of December 31, 2020, we had one note receivable investment with an aggregate carrying value of approximately $0.7 million, compared to two note receivable investments which we determined to be impaired as of December 31, 2019 with an aggregate carrying value and fair value of approximately $52.1 million and $57.3 million, respectively. We did not recognize any losses on extinguishment of notes receivable during the eight months ended August 31, 2020 or the years ended December 31, 2019 and 2018. There were no impairment losses on notes receivable for the eight months ended August 31, 2020. During the years ended December 31, 2019 and 2018, we recorded impairment losses of $10.8 million and $8.2 million, respectively, related to the CareView note receivable. For the eight months ended August 31, 2020 and the year ended December 31, 2019, we did not recognize any interest income for note receivable investments as all such note receivable investments were on an impaired status and no cash interest payments were received. For the year ended December 31, 2018, we recognized $2.3 million of interest revenue for the CareView note receivable investment as result of cash interest payments made during the fiscal year.
Inventory
Under the Going Concern Basis, inventory, which consisted of raw materials, work-in-process and finished goods, was stated at the lower of cost or net realizable value. We determined cost using the first-in, first-out method. Inventory levels were analyzed periodically and written down to their net realizable value if they had become obsolete, had a cost basis in excess of its expected net realizable value or were in excess of expected requirements. We analyzed current and future product demand relative to the remaining product shelf life to identify potential excess inventory. We built demand forecasts by considering factors such as, but not limited to, overall market potential, market share, market acceptance and patient usage. The Company classified inventory as current on the Consolidated Balance Sheet when the Company expected inventory to be consumed for commercial use within the next twelve months.
Intangible Assets
Under the Going Concern Basis, intangible assets with finite useful lives consisted primarily of acquired product rights and acquired technology and were amortized on a straight-line basis over their estimated useful lives (five to 20 years). The estimated useful lives associated with finite-lived intangible assets were consistent with the estimated lives of the associated products. Such assets were reviewed for impairment when events or circumstances indicated that the carrying value of an asset may not be recoverable. An impairment loss was recognized when estimated undiscounted future cash flows expected to result from the use of an asset and its eventual disposition were less than its carrying amount. The amount of any impairment loss was measured as the difference between the carrying amount and the fair value of the impaired asset.
Convertible Notes
Under the Going Concern Basis, we performed an assessment of all embedded features of a debt instrument to determine if (i) such features should be bifurcated and separately accounted for, and (ii) if bifurcation requirements are met, whether such features should be classified and accounted for as equity or debt instruments. If the embedded feature met the requirements to be bifurcated and accounted for as a liability, the fair value of the embedded feature is measured initially, included as a liability on the Consolidated Balance Sheet, and re-measured to fair value at each reporting period. Any changes in fair value were recorded in the Consolidated Statement of Operations. We monitored, on an ongoing basis, whether events or circumstances could give rise to a change in our classification of embedded features.
We issued $150.0 million of December 2021 Notes with an option to settle conversions by paying or delivering, as applicable, cash, shares of our common stock or a combination of cash and shares of our common stock, at our election. In accordance with accounting guidance for convertible debt instruments that may be settled in cash or other assets on conversion, we separated the principal balance between the fair value of the liability component and the common stock conversion feature using a market interest rate for a similar nonconvertible instrument at the date of issuance.
On September 17, 2019, we exchanged $86.1 million aggregate principal of December 2021 Notes for an identical aggregate original principal amount of December 2024 Notes, plus a cash payment of $70.00 for each $1,000 principal amount exchanged (the “September 2019 Exchange Transaction”). We issued the December 2024 Notes with an option to settle conversions by paying or delivering, as applicable, cash, shares of our common stock or a combination of cash and shares of our common stock, at our election. In accordance with accounting guidance for convertible debt instruments that may be settled in cash or other assets on conversion, we separated the principal balance between the fair value of the liability component and the common stock conversion feature using a market interest rate for a similar nonconvertible instrument at the date of issuance.
The September 2019 Exchange Transaction qualified as a debt extinguishment.
In accordance with the accounting guidance for an extinguishment of convertible debt instruments with a cash conversion feature, we were required to allocate the fair value of the consideration transferred between the liability component and the equity component. To calculate the fair value of the debt immediately prior to derecognition, the carrying value was recalculated in a manner that reflected the estimated market interest rate for a similar nonconvertible instrument at the date of issuance.
In connection with the September 2019 Exchange Transaction, we entered into a capped call transaction with a counterparty on similar terms and conditions as the capped call transaction entered into between the two parties when the December 2021 Notes were issued. We evaluated the capped call transaction under authoritative accounting guidance and determined that it should be accounted for as a separate transaction and classified as a net reduction to Additional paid-in capital within stockholders’ equity with no recurring fair value measurement recorded. Also with the September 2019 Exchange Transaction, we and the counterparty unwound a portion of the capped call entered into when the December 2021 Notes were issued as they were no longer scheduled to mature in 2021. The proceeds from the unwind of the capped call, which reflected the value of the options outstanding at the time of the September 2019 Exchange Transaction and the average share price of our common stock, were included as an increase to Additional paid-in capital within stockholders’ equity.
On December 12, 2019, we initiated the repurchase of $119.3 million in aggregate principal amount of our December 2021 and December 2024 convertible notes for $97.9 million in cash and 13.4 million shares of our common stock in privately negotiated transactions (the “December Exchange Transaction”). The closing of the December Exchange Transaction occurred on December 17, 2019. We determined that the repurchase of the principal amount should be accounted for as a partial extinguishment of the December 2021 Notes and December 2024 Notes and a loss on extinguishment was recorded at closing of the transaction. The loss on extinguishment included the derecognition of a proportional share of the deferred issuance costs. In connection with the December Exchange Transaction, we unwound a corresponding portion of the capped call related to the convertible notes and
repurchased 3.2 million shares of our common stock from the capped call counterparty. The common stock repurchased was reflected as a decrease to Retained earnings within stockholders’ equity. The proceeds from the capped call were included as an increase to Additional paid-in capital within stockholders’ equity. In furtherance of our monetization strategy, we expect to continue to repurchase or satisfy obligations relating to our convertible notes.
During the eight months ended August 31, 2020, the Company repurchased $5.4 million in aggregate principal amount of its December 2021 Notes and $10.5 million in aggregate principal amount of its December 2024 notes for cash.
In December 2020, the Company repurchased an additional $2.2 million par value of December 2021 Notes and $1.0 million par value of December 2024 Notes in a privately negotiated transactions for cash.
The estimated fair value of the liability components at the date of issuance for the December 2021 Notes and December 2024 Notes were determined using valuation models and are complex and subject to judgment. Significant assumptions within the valuation models included an implied credit spread, the expected volatility and dividend yield of our common stock and the risk-free interest rate for notes with a similar term.
Product Revenue
General
In accordance with ASC 606, revenue under the Going Concern Basis, was recognized from the sale of products and services when a customer obtained control of such promised products and services. The amount of revenue recognized reflected the consideration to which we expected to be entitled to receive in exchange for these products and services. A five-step model was utilized to achieve the core principle and includes the following steps: (1) identify the customer contract; (2) identify the contract’s performance obligations; (3) determine the transaction price; (4) allocate the transaction price to the performance obligations; and (5) recognize revenue when the performance obligations were satisfied.
The following is a description of principal activities, separated by reportable segments, from which we generated revenue.
Medical Devices
We principally generated revenue in our Medical Devices segment from the sale and lease of the LENSAR® Laser System, which included equipment, PIDs, procedure licenses, and training, installation, warranty and maintenance agreements.
For bundled packages, we accounted for individual products and services separately if they were distinct - i.e. if a product or service is separately identifiable from other promises in the bundled package and if the customer can benefit from it on its own or with other resources that are readily available to the customer. The LENSAR® Laser system, training and installation services were one performance obligation. All other elements are separate performance obligations. PIDs, procedure licenses, warranty and maintenance services were also sold on a stand-alone basis.
As we both sold and leased the LENSAR® Laser System, the consideration (including any discounts) was first allocated between lease and non-lease components and then allocated between the separate products and services based on their stand-alone selling prices. The stand-alone selling prices for the PIDs and procedure licenses were determined based on the prices at which we separately sold the PIDs and procedure licenses. The LENSAR® Laser System and warranty stand-alone selling prices were determined using the expected cost plus a margin approach.
For LENSAR® Laser System sales, we recognized revenue in product revenue when a customer took possession of the system. This usually occurred after the customer signs a contract, LENSAR installed the system, and LENSAR performed the requisite training for use of the system. For LENSAR® Laser System leases, we recognized revenue in Product revenue over the length of the lease in accordance with ASC Topic 840, Leases through December 31, 2018 and recognized Product revenue in accordance with ASC Topic 842, Leases, after January 1, 2019.
The LENSAR® Laser System requires both a PID and a procedure license to perform each procedure. We recognized revenue for PIDs in product revenue when the customer took possession of the PID. PIDs were sold by the case. We recognized revenue for procedure licenses in product revenue when a customer purchased a procedure license from the web portal. Typically, consideration for PIDs and procedure licenses was considered fixed consideration except for certain customer agreements that provided for tiered volume discount pricing which was considered variable consideration.
We offered an extended warranty that provided additional services beyond the standard warranty. We recognized revenue from the sale of extended warranties in product revenue over the warranty period. Customers had the option of renewing the warranty period, which was considered a new and separate contract.
Income Generating Assets
Royalty Rights - At Fair Value
Under the Going Concern Basis, we accounted for our investments in royalty rights at fair value with changes in fair value presented in earnings. The fair value of the investments in royalty rights was determined by using a discounted cash flow analysis related to the expected future cash flows to be received. For each arrangement, we are entitled to royalty payments based on revenue generated by the net sales of the product.
Under the Going Concern Basis, these assets are classified as Level 3 assets within the fair value hierarchy, as our valuation estimates utilize significant unobservable inputs, including estimates. Critical estimates may include probability and timing of future sales of the related products, product demand and market growth assumptions, inventory target levels, product approval and pricing assumptions. Factors that could cause a change in estimates of future cash flows include a change in estimated market size, market share of the products on which we receive royalties, a change in pricing strategy or reimbursement coverage, a delay in obtaining regulatory approval, changes to forecast volume and pricing as a result of generic competition, a change in dosage of the product, and a change in the number of treatments.
Under the Going Concern Basis, the changes in the estimated fair value from investments in royalty rights along with cash receipts in each reporting period are presented together on our Consolidated Statements of Operations as a component of revenue under the caption, “Royalty rights - change in fair value.” Realized gains and losses on Royalty Rights were recognized as they were earned and when collection was reasonably assured. Royalty Rights revenue was recognized over the respective contractual arrangement period. Transaction-related fees and costs were expensed as incurred.
Income Taxes
The provision for income taxes is determined using the asset and liability approach. Tax laws require items to be included in tax filings at different times than the items are reflected in the financial statements. A current liability is recognized for the estimated taxes payable for the current year. Deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. Deferred taxes are adjusted for enacted changes in tax rates and tax laws. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized.
We recognize tax benefits from uncertain tax positions only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the Consolidated Financial Statements from such positions are then measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. We adjust the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions. Any interest and penalties on uncertain tax positions are included within the tax provision.
The Coronavirus Aid, Relief, and Economic Security (“CARES”) Act was signed into law at the end of March 2020 and contains numerous forms of economic stimulus, including Small Business Association guaranteed loans and certain income tax provisions. The CARES Act, among other provisions, permits Net Operating Loss (“NOL”) carryovers and carrybacks to offset 100% of taxable income for taxable years beginning before 2021. In addition, the CARES Act allows NOLs incurred in 2018, 2019, and 2020 to be carried back to each of the five preceding taxable years to generate a refund of previously paid income taxes at the 35% corporate tax rate then in effect.
Recently Issued Accounting Standards
See Note 2, Summary of Significant Accounting Policies, to the Consolidated Financial Statements of this Form 10-K for a discussion of recently adopted accounting pronouncements and recently issued accounting pronouncements not yet adopted as of December 31, 2020.
Recent Developments
Dissolution
On January 4, 2021, we filed a Certificate of Dissolution with the State of Delaware and closed our stock transfer books. On January 7, 2021, we were formally de-listed from Nasdaq.
Convertible Senior Notes
In connection with the Fundamental Change Repurchase Right resulting from the suspension of trading of the Company’s common stock on Nasdaq prior to the opening of business on December 31, 2020, holders of 142 December 2021 Notes tendered their notes for repurchase, which occurred on February 17, 2021.
In connection with the Fundamental Make-Whole Change resulting from the suspension of trading of the Company’s common stock on Nasdaq prior to the opening of business on December 31, 2020, holders of 50 December 2021 Notes exercised their conversion rights. Such notes will be retired for cash.
CareView
As further discussed in Note 9, Notes and Other Long-Term Receivables, to the Consolidated Financial Statements included in Item 8, the first principal payment and the scheduled interest payment due December 31, 2018 and those that followed that were previously deferred until January 31, 2021 were subsequently deferred until May 31, 2021 under additional amendments.
Summary of 2020, 2019 and 2018 Financial Results
•Our net loss for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018 was $77.3 million, $70.4 million and $68.9 million, respectively and included net loss from discontinued operations of $34.9 million, $13.6 million and $36.1 million, respectively;
•At December 31, 2020, we had cash and cash equivalents of $126.8 million as compared with $169.0 million at December 31, 2019, excluding, as of December 31, 2019, cash and cash equivalents classified in assets held for sale of $24.5 million;
•At December 31, 2020, we had $386.9 million in net assets in liquidation;
•At December 31, 2019, we had $717.2 million in total assets, including $447.9 million classified as assets held for sale; and $123.9 million in total liabilities at December 31, 2019, including $31.2 million classified as liabilities held for sale.
Revenues
A summary of our revenues for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, is presented below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, | | Change from | | Year Ended December 31, | | Change from |
| (Dollars in thousands) | | 2020 | | 2019 | | Prior Year % | | 2018 | | Prior Year % |
| Revenues: | | | | | | | | | | |
Product revenue, net (1) | | $ | 10,946 | | | $ | 22,331 | | | (51) | % | | $ | 15,928 | | | 40 | % |
| Lease revenue | | 2,139 | | | 5,072 | | | (58) | % | | 5,903 | | | (14) | % |
| Service revenue | | 2,126 | | | 3,339 | | | (36) | % | | 2,821 | | | 18 | % |
| Royalty rights - change in fair value | | — | | | — | | | N/M | | (30) | | | N/M |
| Royalties from Queen et al. patents | | — | | | 9 | | | N/M | | 4,536 | | | (100) | % |
| Interest revenue | | — | | | — | | | N/M | | 2,337 | | | N/M |
| License and other | | 110 | | | (45) | | | 344 | % | | 533 | | | (108) | % |
| Total revenues | | $ | 15,321 | | | $ | 30,706 | | | (50) | % | | $ | 32,028 | | | (4) | % |
___________________
N/M Not meaningful
(1) Our Product revenue, net consisted entirely of revenue from our Medical Devices segment. We recorded Product revenue from our Medical Devices segment from our LENSAR product sales which include LENSAR® Laser Systems, PIDs, procedures, training, installation, warranty and maintenance services.
For the eight months ended August 31, 2020, compared to the year ended December 31, 2019
Our total revenues decreased by 50%, or $15.4 million, for the eight months ended August 31, 2020, when compared to the year ended December 31, 2019. The decrease was primarily due to:
•the shorter measurement period in the current year period due to the transition to the Liquidation Basis on September 1, 2020,
•a decrease in Product, Lease and Service revenues from our Medical Devices segment due to the COVID-19 pandemic reducing demand for elective surgical procedures in North America and the rest of the world, and
•no Queen et al. patent revenue in the current year period, partially offset by
•higher license and other revenue.
Revenue from our Medical Devices segment for the eight months ended August 31, 2020 was $15.2 million, a decrease of 51% compared to the year ended December 31, 2019. The decrease is attributable to lower net revenues in both North America and the rest of the world. The decrease was primarily driven by the impact of the COVID-19 pandemic on the Medical Devices segment and the associated decline in elective surgical procedures in addition to the shorter measurement period in the current year.
Revenue from our Income Generating Assets segment for the eight months ended August 31, 2020 was $0.1 million, an increase of $0.1 million when compared to the year ended December 31, 2019. The increase was primarily due to:
•higher license and other revenue, partially offset by
•decreasing royalties from the Queen et al. patents as the patents have expired.
For the year ended December 31, 2019, compared to December 31, 2018
Our total revenues decreased by 4%, or $1.3 million, for the year ended December 31, 2019, when compared to the year ended December 31, 2018. The decrease was primarily due to:
•lower Queen et al. patent revenue in the current period
•decreased interest revenue related to the CareView note receivable asset, and
•lower license and other revenue, partially offset by
•an increase in product revenue from sales of the LENSAR Laser Systems in our Medical Devices segment.
Revenue from our Medical Devices segment for the year ended December 31, 2019 was $30.7 million, an increase of 25% compared to the year ended December 31, 2018. The increase is attributable to higher net revenues in both North America and the rest of the world, with the majority of the increase outside of North America.
Revenue from our Income Generating Assets segment for the year ended December 31, 2019 was $(36,000), a decrease of 100%, or $7.4 million, when compared to the same period in 2018. The decrease was due to:
•the absence of royalties from the Queen et al. patents as the patents have expired,
•the absence of interest revenue recognized from our CareView note receivable in 2019, and
•lower license and other revenue.
The following table summarizes the percentage of our total revenues earned, which individually accounted for 10% or more of our total revenues for the eight months ended August 31, 2020 and one or more of the years ended December 31, 2019 and 2018:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| Product Name | | 2020 | | 2019 | | 2018 |
| | | | | | |
| | | | | | |
| Biogen | | — | % | | — | % | | 14 | % |
| LENSAR | | 99 | % | | 100 | % | | 77 | % |
Operating Expenses
A summary of our operating expenses for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018 is presented below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, | | Change from | | Year Ended December 31, | | Change from |
| (Dollars in thousands) | | 2020 | | 2019 | | Prior Year % | | 2018 | | Prior Year % |
| Costs of product revenue (excluding intangible amortization) | | $ | 6,626 | | | $ | 17,276 | | | (62) | % | | $ | 13,555 | | | 27 | % |
| Amortization of intangible assets | | 841 | | | 1,290 | | | (35) | % | | 1,294 | | | — | % |
| Severance and retention | | 24,713 | | | — | | | N/M | | — | | | N/M |
| General and administrative | | 29,695 | | | 38,334 | | | (23) | % | | 33,700 | | | 14 | % |
| Sales and marketing | | 3,322 | | | 6,806 | | | (51) | % | | 6,341 | | | 7 | % |
| Research and development | | 4,374 | | | 7,350 | | | (40) | % | | 2,759 | | | 166 | % |
| | | | | | | | | | |
| Asset impairment loss | | — | | | 10,768 | | | (100) | % | | 8,200 | | | 31 | % |
| | | | | | | | | | |
| | | | | | | | | | |
| Change in fair value of contingent consideration | | — | | | — | | | N/M | | 369 | | | N/M |
| Total operating expenses | | $ | 69,571 | | | $ | 81,824 | | | (15) | % | | $ | 66,218 | | | 24 | % |
| Percentage of total revenues | | 454 | % | | 266 | % | | | | 207 | % | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
___________________
N/M Not meaningful
For the eight months ended August 31, 2020, compared to the year ended December 31, 2019
Total operating expenses decreased by 15%, or $12.3 million for the eight months ended August 31, 2020, when compared to the year ended December 31, 2019. The decrease was primarily a result of:
•lower general and administrative expenses, primarily due to a shorter measurement period due to our transition to the Liquidation Basis,
•a decrease in research and development expenses in our Medical Devices segment primarily due to the exclusive licensing of intellectual property from a third party in 2019 for $3.5 million in cash,
•lower cost of product revenue, due to decreased sales in our Medical Devices segment, as previously discussed,
•lower sales and marketing expenses in our Medical Devices segment due to the impact of COVID-19, and
•a $10.8 million impairment loss on the CareView note receivable recorded in 2019 with no comparable adjustment in the current year period, partially offset by
•severance and retention recorded in the current year period with no corresponding expense in the prior year period, including provisions under our Wind Down Retention Plan, which, as a result of the adoption of the Plan of Liquidation in the first quarter of 2020, accelerated the vesting of outstanding stock awards for employees.
For the year ended December 31, 2019, compared to December 31, 2018
Total operating expenses increased by 24%, or $15.6 million for the year ended December 31, 2019, when compared to the year ended December 31, 2018. The increase was primarily a result of:
•an increase in research and development expenses in our Medical Devices segment primarily due to the exclusive licensing of intellectual property from a third party for $3.5 million in cash for use in developing its next generation technology,
•higher cost of product revenue, due to increased sales in our Medical Devices segment, with the majority of the increase related to increased system sales in 2019,
•an increase in our general and administrative expenses, as detailed below,
•an increase in our sales and marketing expenses in our Medical Devices segment, and
•a $10.8 million impairment loss on the CareView note receivable recorded in 2019 compared to the $8.2 million impairment loss on the CareView note receivable recorded in 2018, partially offset by
•a decline in the expense recorded for the change in fair value of contingent consideration.
General and administrative expenses for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018 by segment are summarized in the tables below:
| | | | | | | | | | | | | | | | | | | | | | |
| | | | Eight Months Ended August 31, 2020 |
| (in thousands) | | | | Medical Devices | | Income Generating Assets | | Total |
| Compensation | | | | $ | 2,464 | | | $ | 8,633 | | | $ | 11,097 | |
| Salaries and wages (including taxes) | | | | 1,666 | | | 4,468 | | | 6,134 | |
| Bonuses (including accruals) | | | | 410 | | | 1,890 | | | 2,300 | |
| Equity | | | | 388 | | | 2,275 | | | 2,663 | |
| Asset management | | | | — | | | 5,299 | | | 5,299 | |
| Business development | | | | — | | | 650 | | | 650 | |
| Accounting and tax services | | | | 2,221 | | | 4,156 | | | 6,377 | |
| Other professional services | | | | 369 | | | 2,365 | | | 2,734 | |
| Other | | | | 1,184 | | | 2,354 | | | 3,538 | |
| Total general and administrative | | | | $ | 6,238 | | | $ | 23,457 | | | $ | 29,695 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | Year Ended December 31, 2019 |
| (in thousands) | | | | Medical Devices | | Income Generating Assets | | Total |
| Compensation | | | | $ | 4,109 | | | $ | 16,656 | | | $ | 20,765 | |
| Salaries and wages (including taxes) | | | | 1,883 | | | 6,277 | | | 8,160 | |
| Bonuses (including accruals) | | | | 1,260 | | | 3,643 | | | 4,903 | |
| Equity | | | | 966 | | | 6,736 | | | 7,702 | |
| Asset management | | | | — | | | 2,041 | | | 2,041 | |
| Business development | | | | — | | | 1,282 | | | 1,282 | |
| Accounting and tax services | | | | 759 | | | 4,400 | | | 5,159 | |
| Other professional services | | | | 403 | | | 1,970 | | | 2,373 | |
| Other | | | | 1,713 | | | 5,001 | | | 6,714 | |
| Total general and administrative | | | | $ | 6,984 | | | $ | 31,350 | | | $ | 38,334 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | Year Ended December 31, 2018 |
| (in thousands) | | | | Medical Devices | | Income Generating Assets | | Total |
| Compensation | | | | $ | 3,627 | | | $ | 10,204 | | | $ | 13,831 | |
| Salaries and wages (including taxes) | | | | 1,871 | | | 6,193 | | | 8,064 | |
| Bonuses (including accruals) | | | | 991 | | | (203) | | | 788 | |
| Equity | | | | 765 | | | 4,214 | | | 4,979 | |
| Asset management | | | | — | | | 5,040 | | | 5,040 | |
| Business development | | | | — | | | 1,168 | | | 1,168 | |
| Accounting and tax services | | | | 39 | | | 4,288 | | | 4,327 | |
| Other professional services | | | | 825 | | | 1,921 | | | 2,746 | |
| Other | | | | 1,399 | | | 5,189 | | | 6,588 | |
| Total general and administrative | | | | $ | 5,890 | | | $ | 27,810 | | | $ | 33,700 | |
Non-operating Expense, Net
A summary of our non-operating expense, net, for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, is presented below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, | | Change from | | Year Ended December 31, | | Change from |
| (Dollars in thousands) | | 2020 | | 2019 | | Prior Year % | | 2018 | | Prior Year % |
| Interest and other income, net | | $ | 608 | | | $ | 6,030 | | | (90) | % | | $ | 6,065 | | | (1) | % |
| Interest expense | | (996) | | | (11,404) | | | (91) | % | | (12,157) | | | (6) | % |
| | | | | | | | | | |
| Gain on sale of intangible assets | | — | | | 3,476 | | | N/M | | — | | | N/M |
| | | | | | | | | | |
| (Loss) gain on investments | | (5,576) | | | — | | | N/M | | 764 | | | N/M |
| | | | | | | | | | |
| Loss on exchange and extinguishment of convertible notes | | (606) | | | (8,430) | | | (93) | % | | — | | | N/M |
| | | | | | | | | | |
| Total non-operating expense, net | | $ | (6,570) | | | $ | (10,328) | | | (36) | % | | $ | (5,328) | | | 94 | % |
___________________
N/M Not meaningful
For the eight months ended August 31, 2020, compared to the year ended December 31, 2019
Total non-operating expense, net, decreased by 36% from $10.3 million for the year ended December 31, 2019 to $6.6 million for the eight months ended August 31, 2020, primarily due to:
•lower interest expense in conjunction with the extinguishment of a substantial portion of our convertible notes, and
•a larger loss on the exchange and extinguishment of convertible notes in the prior period, partially offset by
•a decrease in interest and other income due to lower cash balances in the current period,
•a decrease in the value of our investment in AEON, and
•a gain recognized in the prior year on the sale of our Direct Flow Medical, Inc. (“Direct Flow Medical”) intangible assets.
For the year ended December 31, 2019, compared to December 31, 2018
Total non-operating expenses, net, increased by 94%, or $5.0 million for the year ended December 31, 2019, compared to the year ended December 31, 2018. Non-operating expense, net, increased due to:
•the loss on the exchange and extinguishment of a portion of our December 2021 Notes and December 2024 Notes, partially offset by
•the decrease in interest expense due to the repurchase of some of our convertible notes, and
•the gain recognized in 2019 on the sale of our Direct Flow Medical, Inc. intangible assets.
Income Taxes
Income tax benefit from continuing operations for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018 was $17.8 million, $4.4 million and $6.8 million, respectively, which resulted primarily from applying the federal statutory income tax rate to loss before income taxes from continuing operations. The tax rate of 29.2% for the eight months ended August 31, 2020 differs from the statutory tax rate of 21% primarily as a result of net operating loss carryback under the CARES Act. The tax rate of 7.2% in 2019 and 17.0% in 2018 differs from the statutory tax rate of 21% primarily as a result of the increase in our valuation allowance in both years and, for 2019, the increase in our unrecognized tax benefits.
During 2020, the amount of our unrecognized tax benefits increased by $1.5 million. The future impact of the unrecognized tax benefits of $85.8 million, if recognized, is comprised of $32.6 million, which would affect the effective tax rate, and $53.2 million, which would result in adjustments to deferred tax assets and our valuation allowance.
Estimated interest and penalties associated with unrecognized tax benefits increased our income tax expense in the Consolidated Statements of Operations by $1.0 million during the eight months ended August 31, 2020, $1.6 million during the year ended December 31, 2019 and $1.0 million during the year ended December 31, 2018. Interest and penalties associated with
unrecognized tax benefits accrued on the balance sheet were $11.2 million, $9.7 million and $8.0 million as of December 31, 2020, 2019 and 2018, respectively.
The Company’s U.S. federal income tax returns are subject to examination for the tax years 2017 forward, In general, our state and local income tax returns are subject to examination by tax authorities for tax years 2000 forward. We are currently under income tax examination by the State of California for tax years 2009 through 2018. The timing of the resolution of the income tax examination is highly uncertain, and the amount ultimately paid, if any, upon resolution of the issues raised by the taxing authority may differ materially from the amounts accrued for each year. We do not anticipate any material change to the amount of our unrecognized tax benefit over the next 12 months.
Assets held for sale and discontinued operations
The Strategic Positions segment, Pharmaceutical segment and the royalty right assets in the Income Generating Assets segment have been classified as held for sale and reported as discontinued operations. The operating results from discontinued operations are presented separately in the Company’s Consolidated Statements of Operations as discontinued operations. Components of amounts reflected in Loss from discontinued operations are as follows:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands) | | 2020 | | 2019 | | 2018 |
| Revenues | | | | | | |
| Product revenue, net | | $ | 29,479 | | | $ | 55,093 | | | $ | 80,796 | |
| Royalty rights - change in fair value | | (8,804) | | | (31,042) | | | 85,287 | |
| Total revenues | | 20,675 | | | 24,051 | | | 166,083 | |
| Operating expenses | | | | | | |
| Cost of product revenue (excluding intangible asset amortization and impairment) | | 17,576 | | | 36,343 | | | 34,906 | |
| Amortization of intangible assets | | 389 | | | 5,016 | | | 14,536 | |
| General and administrative | | 6,105 | | | 7,264 | | | 11,720 | |
| Sales and marketing | | 257 | | | 1,675 | | | 10,800 | |
| Research and development | | — | | | (41) | | | 196 | |
| Impairment of intangible assets | | — | | | 22,490 | | | 152,330 | |
| Change in fair value of anniversary payment and contingent consideration | | — | | | — | | | (42,000) | |
| Total operating expenses | | 24,327 | | | 72,747 | | | 182,488 | |
| Operating loss from discontinued operations | | (3,652) | | | (48,696) | | | (16,405) | |
| Non-operating expense (income), net | | | | | | |
| Equity affiliate - change in fair value | | (25,365) | | | 36,402 | | | — | |
| Loss on classification as held for sale | | (28,904) | | | — | | | — | |
| Total non-operating expense (income), net | | (54,269) | | | 36,402 | | | — | |
| Loss from discontinued operations before income taxes | | (57,921) | | | (12,294) | | | (16,405) | |
| Income tax (benefit) expense from discontinued operations | | (23,006) | | | 1,303 | | | 19,689 | |
| Loss from discontinued operations | | $ | (34,915) | | | $ | (13,597) | | | $ | (36,094) | |
The following tables provides a summary of activity with respect to our royalty rights assets in discontinued operations for the eight months ended August 31, 2020 and the year ended December 31, 2019:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, 2020 |
| | | | Change in | | |
| (in thousands) | | Cash Royalties | | Fair Value | | Total |
| Assertio | | $ | 29,926 | | | $ | (18,209) | | | $ | 11,717 | |
| VB | | 612 | | | (9,408) | | | (8,796) | |
| U-M | | 4,355 | | | (2,948) | | | 1,407 | |
| | | | | | |
| AcelRx | | 194 | | | (12,952) | | | (12,758) | |
| | | | | | |
| KYBELLA | | 42 | | | (416) | | | (374) | |
| | $ | 35,129 | | | $ | (43,933) | | | $ | (8,804) | |
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, 2019 |
| | | | Change in | | |
| (in thousands) | | Cash Royalties | | Fair Value | | Total |
| Assertio | | $ | 72,225 | | | $ | (45,699) | | | $ | 26,526 | |
| VB | | 966 | | | (518) | | | 448 | |
| U-M | | 5,664 | | | (5,197) | | | 467 | |
| | | | | | |
| AcelRx | | 307 | | | (57,428) | | | (57,121) | |
| KYBELLA | | 110 | | | (1,472) | | | (1,362) | |
| | $ | 79,272 | | | $ | (110,314) | | | $ | (31,042) | |
The adjustment to the fair value of the AcelRx royalty asset in the second quarter of 2019 was due to the slower than expected adoption of Zalviso® since its initial launch relative to our estimates and the increased variance noted between our forecast model and actual results in the second quarter of 2019. We engaged a third-party expert in the second quarter of 2019 to reassess the market and expectations for the product. Key findings from the third-party study included: the post-surgical PCA (Patient-Controlled Analgesia) market being smaller than previously forecasted; the higher price of the product relative to alternative therapies, the product not being used as a replacement for systemic opioids and the design of the delivery device, which is pre-filled for up to three days of treatment, which limited its use for procedures with anticipated shorter recovery times.
The adjustment to the fair value of the Assertio royalty asset in the fourth quarter of 2019 was due to a decrease in the sales forecast for the Assertio products. We engaged a third-party expert in the fourth quarter of 2019 to reassess the market and expectations for the royalty asset. Key findings from the third-party study included: an anticipated decrease in the Glumetza net sales forecast due to an accelerated shift in the channel mix resulting in a substantial decline in net selling prices, particularly in the fourth quarter of 2019 and beyond, as previously announced by Bausch Health and the delayed launch dates of the extended release products in the Assertio royalty asset portfolio outside of the United States.
Revenue from our Pharmaceutical segment for the eight months ended August 31, 2020 was $29.5 million, a decrease of 46% when compared to the year ended December 31, 2019. All revenues from our Pharmaceutical segment were derived from sales of the Noden Products. The decrease in revenue from our Pharmaceutical segment reflects a shorter measurement period as well as lower net revenues in the United States and the rest of the world. The decrease in revenue from our Pharmaceutical segment in the United States for the eight months ended August 31, 2020 reflects the introduction of our authorized generic of Tekturna and a third-party generic of aliskiren late in the first quarter of 2019. The decrease in revenue for the rest of the world is due to lower sales volume of Rasilez in certain territories.
Revenue from our Pharmaceutical segment for the year ended December 31, 2019 was $55.1 million, a decrease of 32% when compared to the same period in 2018. The decrease in revenue from our Pharmaceutical segment reflects lower net revenues in the United States and the rest of the world. In particular, the decrease in revenue from our Pharmaceutical segment in the United States for the year ended December 31, 2019 reflects the introduction of our authorized generic form of Tekturna and a third-party generic form of aliskiren during the year ended December 31, 2019. The decrease in revenue for the rest of the world is due to lower sales volume of Rasilez in certain territories. This increase in cost of goods sold, compared to the prior year is due to the higher percentage of authorized generic sales in the current period and costs associated with the amended Novartis supply
agreement. Sales and marketing expenses have decreased substantially while the portion of general and administrative expenses attributable to the Pharmaceutical segment decreased as well. Intangible assets amortization expense decreased after Noden’s intangible assets were impaired at June 30, 2018.
Expenses decreased in our Pharmaceutical segment by 70%, or $50.0 million for the eight months ended August 31, 2020, when compared to the year ended December 31, 2019. The decrease was primarily a result of:
•a shorter measurement period in the current year,
•lower cost of sales due to decreased sales in the current year period,
•lower amortization expense for the Noden intangible assets in 2020 resulting from the accounting for discontinued operations, and
•lower sales and marketing expenses.
Expenses decreased in our Pharmaceutical segment by 61%, or $110.4 million for the year ended December 31, 2019, when compared to the year ended December 31, 2018. The decrease was primarily a result of:
•a $22.5 million impairment of the Noden intangible asset in the current year compared to a $152.3 million impairment in 2018,
•lower amortization expense for the Noden intangible assets in 2019 resulting from the impairment recorded in 2018 due to the increased probability of a third-party generic form of aliskiren being launched in the United States,
•lower sales and marketing expenses reflecting the cost savings from the change in our marketing strategy to a non-personal promotion strategy for the Noden Products in anticipation of a launch of a third-party generic form of aliskiren. This non-personal promotion strategy was subsequently discontinued upon the launch of our authorized generic form of Tekturna in the first quarter of 2019, partially offset by,
•the favorable adjustment to the Noden acquisition related contingent consideration, which was first reduced in the second quarter of 2018 prompted by the increased probability of a third-party generic form of aliskiren being launched in the United States and subsequently eliminated in the fourth quarter of 2018 when the launch was imminent.
Net Loss per Share
Net loss per share for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, is presented below:
| | | | | | | | | | | | | | | | | |
| Eight Months Ended August 31, | | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| | | | | |
| Net loss per share - basic: | | | | | |
| Continuing operations | $ | (0.36) | | | $ | (0.48) | | | $ | (0.22) | |
| Discontinued operations | $ | (0.30) | | | $ | (0.11) | | | $ | (0.25) | |
| Net loss attributable to PDL’s shareholders per basic share | $ | (0.66) | | | $ | (0.59) | | | $ | (0.47) | |
| | | | | |
| Net loss per share - diluted: | | | | | |
| Continuing operations | $ | (0.36) | | | $ | (0.48) | | | $ | (0.22) | |
| Discontinued operations | $ | (0.30) | | | $ | (0.11) | | | $ | (0.25) | |
| Net loss attributable to PDL’s shareholders per diluted share | $ | (0.66) | | | $ | (0.59) | | | $ | (0.47) | |
Liquidity and Capital Resources
In February 2020, our Board approved the Plan of Liquidation. In August 2020, we received stockholder approval to dissolve our Company under Delaware law and in January 2021 we filed a certificate of dissolution in Delaware. Our liquidity and capital resource needs in dissolution primarily consist of managing the successful wind down of our business and distributing the remaining net proceeds to our stockholders,
We have previously financed our operations primarily through royalty and other license-related revenues, public and private placements of debt and equity securities, interest income on invested capital and cash generated from pharmaceutical and medical device product sales. During 2020, we also generated cash from the sale of several assets in our portfolio, including our
Pharmaceutical segment and certain royalty rights assets included in our Income Generating Assets’ segment. We plan to finance our operations in the near term primarily through existing cash, from our remaining royalty rights assets until such assets are sold or placed in a liquidating trust and from additional cash proceeds from the sale of one or more of the assets in our portfolio.
In addition, we expect to generate additional cash from the recovery under the CARES Act of previous taxes paid and from the collection of additional amounts owed from prior assets sales, primarily the sale of our Pharmaceutical segment.
In September 2020, we sold our interests in Noden DAC and Noden USA which comprised our Pharmaceutical segment. Upon closing, we were released of our guarantee to Novartis under Noden’s supply agreement. Under the terms of the sale of our interests in Noden DAC and Noden USA, we received proceeds of $12.2 million on September 9, 2020, the closing date of the transaction. We are also entitled to recover $0.5 million related to VAT for inventory purchases from Novartis. The agreement provides for an additional $33.0 million to be paid to the us in twelve equal quarterly installments from January 2021 to October 2023, of which the first installment has been received. An additional $3.9 million will be paid in four equal quarterly installments from January 2023 to October 2023. The agreement also provides for the potential for additional contingent payments to us. We are entitled to receive $2.5 million upon Stanley Capital or any of its affiliates entering into a binding agreement for a specified transaction within one year of the closing date. We are also entitled to 50% of a license fee from a third party distributor within 10 days of receipt by Noden.
Our future capital requirements primarily relate to the costs to wind down the Company, including expenses to manage the remaining business, such as salaries and other employee compensation, rental payments, insurance, and taxes, contractual obligations to current and former employees (e.g., true-up payments to holders of stock options that vested prior to the filing for dissolution) and other legal, accounting, financial advisory and consultant fees, which will reduce any amounts available for distribution to our stockholders.
On December 9, 2019, we announced that our Board authorized the repurchase of issued and outstanding shares of our common stock and convertible notes up to an aggregate value of $200.0 million pursuant to a share repurchase program. On December 16, 2019, we announced that our Board approved a $75.0 million increase to this repurchase program. Repurchases under this program were eligible to be made from time to time in the open market or in privately negotiated transactions and funded from our working capital. Common stock and convertible note repurchases were also eligible to be made under a trading plan under Rule 10b5-1, which would permit shares and convertible notes to be repurchased when we might otherwise be precluded from doing so because of self-imposed trading blackout periods or other regulatory restrictions. The 10b5-1 plan was terminated on May 31, 2020 in consideration of the impact and uncertainty introduced by the COVID-19 pandemic on our monetization process and no common stock was repurchased after this date. All shares of common stock repurchased under our repurchase program were retired and restored to authorized but unissued shares of common stock. All convertible notes repurchased under the program were retired as will any additional convertible notes that may be repurchased prior to their scheduled maturity on December 1, 2021.
Our debt service obligations consist of interest payments and repayment of our December 2021 Notes. As of December 31, 2020 approximately $2.3 million in aggregate principal amount of December 2021 Notes outstanding of which $1.9 million par value was repurchased prior to December 31, 2021 but pending settlement. We may continue to repurchase the remaining outstanding December 2021 Notes with cash on hand.
We had cash and cash equivalents in the aggregate of $126.8 million and $169.0 million at December 31, 2020 and 2019, respectively, representing a decrease of $42.2 million. The decrease was primarily attributable to the repurchase of the December 2021 Notes and December 2024 Notes and the repurchase of stock, partially offset by cash received from royalties, cash received from the sale of the Wellstat receivable (including the subsequent sale of the Settlement Agreement) and the sale of the Noden entities and certain royalty rights assets.
We believe that cash on hand and cash generated from the above-described sources, net of operating expenses, debt service and income taxes, will be sufficient to fund our wind-down operations until all net proceeds are distributed to our stockholders.
Off-Balance Sheet Arrangements
As of December 31, 2020, we did not have any off-balance sheet arrangements, as defined under SEC Regulation S-K Item 303(a)(4)(ii).
Contractual Obligations
The following table summarizes our contractual obligations and commercial commitments as of December 31, 2020:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Payments Due by Period |
| (in thousands) | | Less than 1 year | | 1-3 years | | 3-5 years | | Thereafter | | Total |
Operating leases 1 | | $ | 37 | | | $ | 38 | | | $ | — | | | $ | — | | | $ | 75 | |
Convertible notes 2 | | 412 | | | — | | | — | | | — | | | 412 | |
| | | | | | | | | | |
| Total contractual obligations | | $ | 449 | | | $ | 38 | | | $ | — | | | $ | — | | | $ | 487 | |
_____________________________
1 Amounts represent the lease for our offices in Reno, Nevada and do not include estimated remaining lease payments guaranteed under the Redwood City lease discussed below.
2 Amount represents estimated remaining principal and cash interest payments due on the December 2021 Notes and excludes amounts paid before December 31, 2020 for an unsettled trade totaling $1.9 million.
Our liability for uncertain tax positions was $35.3 million as of December 31, 2020, all of which has been excluded from the table above due to the uncertainty in the timing of the settlement of these positions.
The above table excludes any contractually committed wind down payments due to stock option holders discussed under Guarantees below as the amount and timing of such payments, if any, is uncertain.
Purchase Obligations
After the sale of Noden and the spin-off of LENSAR, we no longer have any purchase obligations and were released from all of our guarantees for purchase obligations.
Guarantees
Redwood City Lease Guarantee
In connection with the spin-off of Facet in December 2008, we entered into amendments to the leases for our former facilities in Redwood City, California, under which Facet was added as a co-tenant and a Co-Tenancy Agreement, under which Facet agreed to indemnify us for all matters related to the leases attributable to the period after the spin-off date. In April 2010, Abbott Laboratories acquired Facet and later renamed the entity AbbVie Biotherapeutics, Inc. (“AbbVie”). If AbbVie were to default under its lease obligations, we could be held liable by the landlord as a co-tenant, and, thus, we have in substance guaranteed the payments under the lease agreements for the Redwood City facilities. As of December 31, 2020, the total lease payments for the duration of the guarantee, which runs through December 2021, are approximately $11.3 million. For additional information regarding our lease guarantee, see Note 16, Commitments and Contingencies.
Wind Down Payments to Stock Option Holders
On December 21, 2019, the Compensation Committee of the Board adopted a Wind Down Retention Plan in which our executive officers and other employees who are participants in the Company’s Severance Plan are eligible to participate. The Wind Down Retention Plan provides for equitable adjustments to outstanding stock options held by participants to ensure such participants realize the same benefits provided to shareholders in the event one or more cash or other distributions become payable to shareholders. Consistent with the existing terms of the Equity Plan, in the event one or more cash or other distributions are paid to shareholders, the exercise price of outstanding stock options will be reduced on a dollar-for-dollar basis to reflect the per share value of such cash or other distributions. In the event that the Company declares cash or other distributions that, in the aggregate, exceed the difference between the exercise price of an outstanding stock option and the par value of the underlying shares ($0.01), the holder of such stock option will be entitled to receive from the Company a cash payment in an amount equal to the number of shares subject to such stock option multiplied by the per share amount of the cash or other distribution that exceeds the difference between exercise price of the outstanding option and the par value of the underlying shares (a “true-up payment”). As of January 4, 2021, the date we filed our Certificate of Dissolution, the Company was unable to issue stock for any purpose, including to cover the exercise of employee options, and as a result such options became unexercisable. For a full discussion regarding the Wind-Down Retention Plan, including benefits conferred on employee option holders post-dissolution, please see Item 11.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Sensitive Financial Instruments
Our exposure to market risk for changes in interest rates relates primarily to our excess cash investments and our convertible notes.
Our excess cash investments consist of Rule 2a-7 money market funds and had a fair value of approximately $51.2 million at December 31, 2020 and $131.3 million at December 31, 2019. Due to the short duration of these investments, with a maximum weighted average maturity of 60 days or less, if market interest rates were to increase or decrease by 1%, there would be no material impact on the fair value of our portfolio.
As of December 31, 2020, our convertible notes consisted of $2.3 million in principal of the December 2021 Notes with an annual fixed interest rate of 2.75%, of which $1.9 million par value was acquired before December 31, 2020 in a transaction that remained unsettled at year end. The aggregate fair value of our convertible notes was estimated to be $33.9 million at December 31, 2019. At December 31, 2019 our convertible notes consisted of the December 2021 Notes and the December 2024 Notes. The December 2024 Notes had an annual fixed interest rate of 2.75% and a principal accretion rate of 2.375% per year. Changes in interest rates do not affect interest expense on fixed rate debt. While changes in interest rates do not impact the amount of interest we pay, these obligations are subject to interest rate risk because changes in interest rates would affect the fair values of fixed rate debt.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index to Consolidated Financial Statements
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of PDL BioPharma, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of PDL BioPharma, Inc. and its subsidiaries (the “Company”) as of December 31, 2019, and the related consolidated statements of operations, of comprehensive (loss) income, of stockholders’ equity and of cash flows for each of the two years in the period ended December 31, 2019 and for the period from January 1, 2020 to August 31, 2020, and audited the consolidated statement of net assets in liquidation as of December 31, 2020, and the related consolidated statement of changes in net assets in liquidation for the period from September 1, 2020 to December 31, 2020, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019, the results of its operations and its cash flows for each of the two years in the period ended December 31, 2019 and for the period from January 1, 2020 to August 31, 2020, its net assets in liquidation as of December 31, 2020, and the changes in its net assets in liquidation for the period from September 1, 2020 to December 31, 2020 in conformity with accounting principles generally accepted in the United States of America applied on the bases described below.
Basis of Accounting
As discussed in Notes 2 and 3 to the consolidated financial statements, the stockholders of the Company approved a plan of liquidation on August 19, 2020, and the Company determined liquidation is imminent. As a result, the Company changed its basis of accounting on September 1, 2020 from the going concern basis to a liquidation basis. This matter is also discussed below as a critical audit matter.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Liquidation Basis of Accounting
As described above and in Notes 2 and 3 to the consolidated financial statements, as a result of the August 19, 2020 approval by the Company’s stockholders to file for dissolution pursuant to a plan of dissolution, it was determined that liquidation was imminent and the Company’s basis of accounting transitioned from the going concern basis of accounting to the liquidation basis of accounting on September 1, 2020, in accordance with generally accepted accounting principles. Under the liquidation basis,
the remeasurement of the Company's assets and liabilities include management's estimates and assumptions of: (i) income to be generated from the remaining assets until the anticipated date of sale, (ii) sales proceeds to be received for these assets at the time of sale, (iii) operating expenses to be incurred; and, (iv) amounts required to settle liabilities. The estimated liquidation values for assets derived from future revenue streams and asset sales and the settlement of liabilities are reflected on the consolidated statement of net assets in liquidation. Under the liquidation basis, the accounting estimates that require management’s most significant, difficult and subjective judgments include the determination that the liquidation was imminent, the estimated sales proceeds of assets, estimated settlement amounts of liabilities, the estimated revenue and operating expenses that are projected during dissolution, and discount rates. Additional significant estimates under the liquidation basis include the recognition and measurement of amounts recoverable under the Coronavirus Aid, Relief, and Economic Security (“CARES”) Act. The total effect of adoption of the liquidation basis of accounting was a $21,339 thousand increase from consolidated net equity as of August 31, 2020 to net assets in liquidation as of September 1, 2020. The changes in net assets and liabilities in liquidation from September 1, 2020 to December 31, 2020 was a reduction of $59,634 thousand.
The principal considerations for our determination that performing procedures relating to the Company’s adoption of the liquidation basis of accounting is a critical audit matter are the significant judgment by management when determining (i) the point at which liquidation was imminent, and (ii) remeasuring the values of assets and liabilities, which included significant assumptions related to sales proceeds of assets, settlement amounts of liabilities, revenue and operating expenses, discount rates and amounts recoverable under the CARES Act. This in turn led to a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating audit evidence related to (i) management’s judgments around applying the liquidation basis to the consolidated financial statements and determining the point at which liquidation was imminent, and (ii) the remeasurement of certain assets and liabilities. Also, the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included, among others, (i) testing management’s process for and evaluating management’s judgments around applying the adoption of the liquidation basis of accounting and the point at which liquidation was imminent; (ii) testing management’s process for developing the estimates and assumptions used in the remeasurement of certain assets and liabilities as of September 1, 2020 and December 31, 2020; (iii) testing the completeness and accuracy of the data used by management in the developing the estimates, and (iv) evaluating the reasonableness of the significant assumptions used by management for certain assets related to (1) the estimated sales proceeds, (2) amounts estimated to be recoverable under the CARES act, (3) the estimated revenue and operating expenses that are projected during dissolution and (4) the discount rates; for certain liabilities related to (1) the amounts estimated to be paid in settlement, (2) the estimated revenue and operating expenses that are projected during dissolution, and (3) the discount rates; and (v) evaluating the adequacy of the Company’s disclosures. The work of management’s specialist was used in performing the procedures to evaluate the reasonableness of enterprise values. Evaluating management’s assumptions related to the estimated revenue and operating expenses that are projected during dissolution, estimated proceeds from sales of certain assets, and the estimated amounts to be paid in settlement of certain liabilities involved evaluating whether the assumptions used by management were reasonable considering (i) the current and past performance of the Company; (ii) the consistency with external industry data; and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in evaluating the appropriateness of valuation methods and evaluating the reasonableness of the discount rates and amounts recoverable under the CARES Act.
| | | | | | | | | | | |
| | /s/ PricewaterhouseCoopers LLP | |
San Francisco, California
March 26, 2021
We have served as the Company’s auditor since 2014.
PDL BIOPHARMA, INC.
CONSOLIDATED STATEMENT OF NET ASSETS IN LIQUIDATION
(In thousands)
| | | | | | | | |
| | December 31, |
| | | 2020 |
| | (Note 2) |
| | | (Under Liquidation Basis of Accounting) |
| Assets | | |
| Current assets: | | |
| Cash and cash equivalents | | $ | 126,842 | |
| | |
| Receivables from asset sales | | 40,574 | |
| | |
| Royalty assets | | 220,023 | |
| Income tax receivable | | 91,753 | |
| Other assets | | 5,768 | |
| Total assets | | $ | 484,960 | |
| | |
| Liabilities | | |
| Current liabilities: | | |
| Accounts payable | | $ | 531 | |
| Uncertain tax positions | | 43,742 | |
| Compensation and benefit costs | | 9,337 | |
| Lease guarantee | | 10,700 | |
| Costs to sell assets | | 3,997 | |
| Other accrued liquidation costs | | 27,268 | |
| Convertible notes payable | | 2,466 | |
| Total liabilities | | $ | 98,041 | |
| | |
| Net assets in liquidation | | $ | 386,919 | |
See accompanying notes.
PDL BIOPHARMA, INC.
CONSOLIDATED BALANCE SHEET
(In thousands, except per share amounts)
| | | | | | | | | | |
| | | | December 31, |
| | | | 2019 |
| | | | (Note 2) |
| | | | (Under Going Concern Basis of Accounting) |
| Assets | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | | | $ | 168,982 | |
| | | | |
| | | | |
| Accounts receivable, net | | | | 6,559 | |
| Notes receivable | | | | 52,583 | |
| | | | |
| Inventory | | | | 8,061 | |
| Assets held for sale (Note 4) | | | | 70,366 | |
| Prepaid and other current assets | | | | 7,344 | |
| Total current assets | | | | 313,895 | |
| Property and equipment, net | | | | 2,560 | |
| | | | |
| | | | |
| | | | |
| | | | |
| Notes and other receivables, long-term | | | | 827 | |
| | | | |
| Intangible assets, net | | | | 13,186 | |
| | | | |
| Long-term assets held for sale (Note 4) | | | | 377,491 | |
| Other assets | | | | 9,247 | |
| Total assets | | | | $ | 717,206 | |
| | | | |
| Liabilities and Stockholders’ Equity | | | | |
| Current liabilities: | | | | |
| Accounts payable | | | | $ | 2,675 | |
| | | | |
| Accrued liabilities | | | | 11,923 | |
| | | | |
| Liabilities held for sale (Note 4) | | | | 31,095 | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| Total current liabilities | | | | 45,693 | |
| Convertible notes payable | | | | 27,250 | |
| | | | |
| Liabilities held for sale, long-term (Note 4) | | | | 120 | |
| Other long-term liabilities | | | | 50,865 | |
| Total liabilities | | | | 123,928 | |
| | | | |
| Commitments and contingencies (Note 16) | | | | 0 |
| | | | |
| Stockholders’ equity: | | | | |
| Preferred stock, par value $0.01 per share, 10,000 shares authorized; no shares issued and outstanding | | | | 0 | |
| Common stock, par value $0.01 per share, 350,000 shares authorized; 124,303 shares issued and outstanding at December 31, 2019 | | | | 1,243 | |
| Additional paid-in capital | | | | (78,875) | |
| | | | |
| | | | |
| Retained earnings | | | | 670,832 | |
| Total PDL’s stockholders’ equity | | | | 593,200 | |
| Noncontrolling interests | | | | 78 | |
| Total stockholders’ equity | | | | 593,278 | |
| Total liabilities and stockholders’ equity | | | | $ | 717,206 | |
See accompanying notes.
PDL BIOPHARMA, INC.
CONSOLIDATED STATEMENT OF CHANGES IN NET ASSETS IN LIQUIDATION
(In thousands)
| | | | | | | | |
| | (Note 2) |
| | (Under Liquidation Basis of Accounting) |
| Net assets in liquidation, at September 1, 2020 | | $ | 446,553 | |
| | |
| Changes in assets and liabilities in liquidation: | | |
| | |
| Decrease in liquidation value of royalty assets | | (12,696) | |
| Decrease in receivables from asset sales | | (8,328) | |
| Decrease in liquidation value of notes receivable | | (44,920) | |
| Decrease in other assets | | (70,976) | |
| Increase in income tax receivable | | 56,081 | |
| Decrease in estimated costs to sell assets | | 4,058 | |
| Increase in uncertain tax positions | | (4,385) | |
| Increase in accrued liquidation costs | | (12,911) | |
| Decrease in compensation and benefit costs | | 11,882 | |
| Decrease in other liabilities and accounts payable | | 22,561 | |
| Total changes in net assets in liquidation | | (59,634) | |
| | |
| Net assets in liquidation, at December 31, 2020 | | $ | 386,919 | |
See accompanying notes.
PDL BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| | | | | | | | | | | | | | | | | |
| Eight Months Ended August 31, | | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| (Under Going Concern Basis of Accounting) |
| Revenues | |
| Product revenue, net | $ | 10,946 | | | $ | 22,331 | | | $ | 15,928 | |
| Lease revenue | 2,139 | | | 5,072 | | | 5,903 | |
| Service revenue | 2,126 | | | 3,339 | | | 2,821 | |
| Royalty rights - change in fair value | 0 | | | 0 | | | (30) | |
| Royalties from Queen et al. patents | 0 | | | 9 | | | 4,536 | |
| Interest revenue | 0 | | | 0 | | | 2,337 | |
| License and other | 110 | | | (45) | | | 533 | |
| Total revenues | 15,321 | | | 30,706 | | | 32,028 | |
| Operating expenses | | | | | |
| Cost of product revenue (excluding intangible asset amortization) | 6,626 | | | 17,276 | | | 13,555 | |
| Amortization of intangible assets | 841 | | | 1,290 | | | 1,294 | |
| Severance and retention | 24,713 | | | 0 | | | 0 | |
| General and administrative | 29,695 | | | 38,334 | | | 33,700 | |
| Sales and marketing | 3,322 | | | 6,806 | | | 6,341 | |
| Research and development | 4,374 | | | 7,350 | | | 2,759 | |
| | | | | |
| | | | | |
| Asset impairment loss | 0 | | | 10,768 | | | 8,200 | |
| | | | | |
| | | | | |
| Change in fair value of anniversary payment and contingent consideration | 0 | | | 0 | | | 369 | |
| Total operating expenses | 69,571 | | | 81,824 | | | 66,218 | |
| Operating loss from continuing operations | (54,250) | | | (51,118) | | | (34,190) | |
| Non-operating expense, net | | | | | |
| Interest and other income, net | 608 | | | 6,030 | | | 6,065 | |
| Interest expense | (996) | | | (11,404) | | | (12,157) | |
| | | | | |
| Gain on sale of intangible assets | 0 | | | 3,476 | | | 0 | |
| | | | | |
| (Loss) gain on investments | (5,576) | | | 0 | | | 764 | |
| Loss on exchange and extinguishment of convertible notes | (606) | | | (8,430) | | | 0 | |
| | | | | |
| Total non-operating expense, net | (6,570) | | | (10,328) | | | (5,328) | |
| Loss before income taxes from continuing operations | (60,820) | | | (61,446) | | | (39,518) | |
| Income tax benefit from continuing operations | (17,780) | | | (4,352) | | | (6,753) | |
| Net loss from continuing operations | (43,040) | | | (57,094) | | | (32,765) | |
| Loss from discontinued operations before income taxes | (57,921) | | | (12,294) | | | (16,405) | |
| Income tax (benefit) expense of discontinued operations | (23,006) | | | 1,303 | | | 19,689 | |
| Loss from discontinued operations | (34,915) | | | (13,597) | | | (36,094) | |
| Net loss | (77,955) | | | (70,691) | | | (68,859) | |
| Less: Net loss attributable to noncontrolling interests | (659) | | | (280) | | | 0 | |
| Net loss attributable to PDL’s stockholders | $ | (77,296) | | | $ | (70,411) | | | $ | (68,859) | |
| | | | | |
| Net loss per share - basic: | | | | | |
| Continuing operations | $ | (0.36) | | | $ | (0.48) | | | $ | (0.22) | |
| Discontinued operations | (0.30) | | | (0.11) | | | (0.25) | |
| Net loss attributable to PDL’s shareholders per basic share | $ | (0.66) | | | $ | (0.59) | | | $ | (0.47) | |
| Net loss per share - diluted: | | | | | |
| Continuing operations | $ | (0.36) | | | $ | (0.48) | | | $ | (0.22) | |
| Discontinued operations | (0.30) | | | (0.11) | | | (0.25) | |
| Net loss attributable to PDL’s shareholders per diluted share | $ | (0.66) | | | $ | (0.59) | | | $ | (0.47) | |
| Weighted-average shares outstanding | | | | | |
| Basic | 118,001 | | | 118,631 | | | 145,669 | |
| Diluted | 118,001 | | | 118,631 | | | 145,669 | |
| | | | | |
See accompanying notes.
PDL BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
| | | | | | | | | | | | | | | | | | | | |
| | | Eight Months Ended August 31, | | Year Ended December 31, |
| | | 2020 | | 2019 | | 2018 |
| | (Under Going Concern Basis of Accounting) |
| Net loss | | $ | (77,955) | | | $ | (70,691) | | | $ | (68,859) | |
| | | | | | |
| Other comprehensive loss, net of tax | | | | | | |
| Change in unrealized losses on investments in available-for-sale securities: | | | | | | |
| Change in fair value of investments in available-for-sale securities, net of tax | | 0 | | | 0 | | | (578) | |
| Adjustment for net (gains) losses realized and included in net loss, net of tax | | 0 | | | 0 | | | (603) | |
Total change in unrealized losses on investments in available-for-sale securities, net of tax(a) | | 0 | | | 0 | | | (1,181) | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Comprehensive loss | | (77,955) | | | (70,691) | | | (70,040) | |
| Less: Comprehensive loss attributable to noncontrolling interests | | (659) | | | (280) | | | 0 | |
| Comprehensive loss attributable to PDL’s stockholders | | $ | (77,296) | | | $ | (70,411) | | | $ | (70,040) | |
___________________________________
(a) Net of tax of ($314) for the year ended December 31, 2018.
See accompanying notes.
PDL BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| PDL’s Stockholders Equity | | | | |
| Common Stock | | Treasury Stock | | Additional
Paid-In
Capital | | Retained Earnings | | Accumulated
Other Comprehensive
Income (Loss) | | Non-controlling Interest | | Total
Stockholders’ Equity |
| Shares | | Amount | | | | | |
| (Under Going Concern Basis of Accounting) |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Balance at December 31, 2017 | 153,774,756 | | | $ | 1,538 | | | $ | — | | | $ | (102,443) | | | $ | 945,614 | | | $ | 1,181 | | | $ | 0 | | | $ | 845,890 | |
| Issuance of common stock, net of forfeitures | (601,668) | | | (6) | | | — | | | 6 | | | 58 | | | — | | | — | | | 58 | |
| Stock-based compensation expense | — | | | — | | | — | | | 4,407 | | | — | | | — | | | — | | | 4,407 | |
| | | | | | | | | | | | | | | |
| Repurchase and retirement of common stock | (16,660,566) | | | (167) | | | (2,103) | | | — | | | (48,266) | | | — | | | — | | | (50,536) | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Comprehensive loss: | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | — | | | (68,859) | | | — | | | — | | | (68,859) | |
| Change in unrealized gains and losses on investments in available-for-sale securities, net of tax | — | | | — | | | — | | | — | | | — | | | (1,181) | | | — | | | (1,181) | |
| | | | | | | | | | | | | | | |
| Total comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (70,040) | |
| Balance at December 31, 2018 | 136,512,522 | | | 1,365 | | | (2,103) | | | (98,030) | | | 828,547 | | | 0 | | | 0 | | | 729,779 | |
| Issuance of common stock, net of forfeitures | 729,191 | | | 7 | | | — | | | (7) | | | 8 | | | — | | | — | | | 8 | |
| Stock-based compensation expense | — | | | — | | | — | | | 6,907 | | | — | | | — | | | — | | | 6,907 | |
| | | | | | | | | | | | | | | |
| Repurchase and retirement of common stock | (26,321,293) | | | (263) | | | 2,103 | | | — | | | (87,312) | | | — | | | — | | | (85,472) | |
| Transfer of subsidiary shares to non-controlling interest | — | | | — | | | — | | | 426 | | | — | | | — | | | 358 | | | 784 | |
| Exchange of convertible notes | — | | | — | | | — | | | (36,963) | | | — | | | — | | | — | | | (36,963) | |
| Issuance of common stock in connection with repurchase of convertible notes | 13,382,196 | | | 134 | | | — | | | 45,767 | | | — | | | — | | | — | | | 45,901 | |
| Capped call transactions | — | | | — | | | — | | | 3,025 | | | — | | | — | | | — | | | 3,025 | |
| Comprehensive loss: | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | — | | | (70,411) | | | — | | | (280) | | | (70,691) | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Total comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (70,691) | |
| Balance at December 31, 2019 | 124,302,616 | | | 1,243 | | | 0 | | | (78,875) | | | 670,832 | | | 0 | | | 78 | | | 593,278 | |
| Issuance of common stock, net of forfeitures | 2,131,730 | | | 22 | | | — | | | 438 | | | — | | | — | | | — | | | 460 | |
| Stock-based compensation expense | — | | | — | | | — | | | 15,527 | | | — | | | — | | | — | | | 15,527 | |
| | | | | | | | | | | | | | | |
| Repurchase and retirement of common stock | (12,322,988) | | | (124) | | | — | | | — | | | (39,245) | | | — | | | — | | | (39,369) | |
| Noncash liquidating distribution (0.11591985 shares of Evofem Biosciences, Inc. common stock distributed per share of the Company’s common stock ) | — | | | — | | | — | | | — | | | (64,400) | | | — | | | — | | | (64,400) | |
| Transfer of subsidiary shares to non-controlling interest | — | | | — | | | — | | | 683 | | | — | | | — | | | 100 | | | 783 | |
| | | | | | | | | | | | | | | |
| Extinguishment of convertible notes | — | | | — | | | — | | | (3,911) | | | — | | | — | | | — | | | (3,911) | |
| Capped call transactions | — | | | — | | | — | | | 801 | | | — | | | — | | | — | | | 801 | |
| Comprehensive loss: | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | — | | | (77,296) | | | — | | | (659) | | | (77,955) | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Total comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (77,955) | |
| Balance at August 31, 2020 | 114,111,358 | | | $ | 1,141 | | | $ | 0 | | | $ | (65,337) | | | $ | 489,891 | | | $ | 0 | | | $ | (481) | | | $ | 425,214 | |
See accompanying notes.
PDL BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | | | | | | |
| Eight Months Ended August 31, | | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| (Under Going Concern Basis of Accounting) |
| Cash flows from operating activities | | | | | |
| Net loss | $ | (77,955) | | | $ | (70,691) | | | $ | (68,859) | |
| Less: Loss from discontinued operations | (34,915) | | | (13,597) | | | (36,094) | |
| Net loss from continuing operations | (43,040) | | | (57,094) | | | (32,765) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Amortization of convertible notes conversion options and debt issuance costs | 622 | | | 7,237 | | | 7,609 | |
| Accreted interest on convertible note principal | 44 | | | 79 | | | 0 | |
| Amortization of intangible assets | 841 | | | 1,290 | | | 1,294 | |
| Amortization of right-of-use assets | 497 | | | 729 | | | 0 | |
| | | | | |
| Asset impairment loss | 0 | | | 10,768 | | | 8,200 | |
| Loss on investment | 5,576 | | | 0 | | | (764) | |
| Change in fair value of royalty rights - at fair value | 0 | | | 0 | | | 31 | |
| | | | | |
| Change in fair value of derivative assets | 110 | | | 46 | | | (33) | |
| Change in fair value of anniversary payment and contingent consideration | 0 | | | 0 | | | 369 | |
| Other amortization, depreciation and accretion of embedded derivative | 1,017 | | | 2,691 | | | 3,149 | |
| | | | | |
| | | | | |
| | | | | |
| Loss on exchange and extinguishment of convertible notes | 606 | | | 8,430 | | | 0 | |
| Gain on sale of intangible assets | 0 | | | (3,476) | | | 0 | |
| | | | | |
| Loss on disposal of property and equipment | 331 | | | 0 | | | 66 | |
| | | | | |
| | | | | |
| Stock-based compensation expense | 18,802 | | | 6,834 | | | 4,337 | |
| | | | | |
| | | | | |
| Deferred income taxes | (18,723) | | | (11,303) | | | 11,597 | |
| Changes in assets and liabilities: | | | | | |
| Accounts receivable | 3,344 | | | (1,686) | | | 234 | |
| | | | | |
| Prepaid and other current assets | (29,875) | | | 2,764 | | | (1,629) | |
| | | | | |
| Inventory | (8,062) | | | (4,744) | | | (889) | |
| Other assets | 306 | | | (165) | | | (2,142) | |
| Accounts payable | 371 | | | 109 | | | 796 | |
| | | | | |
| Accrued liabilities | 5,762 | | | 4,845 | | | (5,380) | |
| | | | | |
| Accrued income taxes | 0 | | | 0 | | | (28) | |
| | | | | |
| Other long-term liabilities | 1,030 | | | 4,967 | | | (462) | |
| Net cash used in operating activities - continuing operations | (60,441) | | | (27,679) | | | (6,410) | |
| Net cash provided by (used in) operating activities - discontinued operations | 28,318 | | | (4,765) | | | (7,015) | |
| Cash flows from investing activities | | | | | |
| | | | | |
| | | | | |
| | | | | |
| Settlement agreement cash received | 7,500 | | | 0 | | | 0 | |
| Payment of contingent consideration | 0 | | | 0 | | | (858) | |
| Proceeds from sales of available-for-sale securities | 0 | | | 0 | | | 4,116 | |
| | | | | |
| Proceeds from royalty rights - at fair value | 0 | | | 0 | | | 366 | |
| Purchase of intangible assets | 0 | | | (1,700) | | | 0 | |
| | | | | |
| Proceeds from the sale of intangible assets | 0 | | | 5,000 | | | 0 | |
| | | | | |
| | | | | |
| Purchase of property and equipment | (221) | | | (763) | | | (1,117) | |
| Net cash provided by investing activities - continuing operations | 7,279 | | | 2,537 | | | 2,507 | |
| Net cash provided by investing activities - discontinued operations | 38,966 | | | 19,273 | | | 54,197 | |
| Cash flows from financing activities | | | | | |
| | | | | |
| | | | | |
| Repurchase of convertible notes | (18,845) | | | (97,889) | | | 0 | |
| | | | | |
| Repayment of convertible notes | 0 | | | 0 | | | (126,447) | |
| | | | | |
| | | | | |
| Payment to exchange convertible notes | 0 | | | (7,451) | | | 0 | |
| | | | | |
| Capped call transactions | 801 | | | 3,025 | | | 0 | |
| | | | | |
| Payment of contingent consideration | 0 | | | (1,071) | | | 0 | |
| | | | | |
| Repurchase of Company common stock | (39,373) | | | (86,898) | | | (49,109) | |
| Cash dividends paid | 0 | | | (9) | | | (48) | |
| Proceeds from the exercise of stock options | 461 | | | 0 | | | 0 | |
| Net settlement of stock-based compensation awards | (3,462) | | | (143) | | | (232) | |
| | | | | |
| Net cash used in financing activities - continuing operations | (60,418) | | | (190,436) | | | (175,836) | |
| Net cash used in financing activities - discontinued operations | (359) | | | (69) | | | (119) | |
| Net decrease in cash and cash equivalents | (46,655) | | | (201,139) | | | (132,676) | |
| Cash and cash equivalents at beginning of period | 193,451 | | | 394,590 | | | 527,266 | |
| Cash and cash equivalents at end of period | 146,796 | | | 193,451 | | | 394,590 | |
| Less: Cash and cash equivalents of discontinued operations at end of period | 25,060 | | | 24,469 | | | 28,910 | |
| Cash and cash equivalents of continuing operations at end of period | $ | 121,736 | | | $ | 168,982 | | | $ | 365,680 | |
See accompanying notes
PDL BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS, continued
(In thousands)
| | | | | | | | | | | | | | | | | |
| Eight Months Ended August 31, | | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| (Under Going Concern Basis of Accounting) |
| Supplemental cash flow information for continuing and discontinued operations | | | | | |
| Cash (refunded) paid for income taxes | $ | (4) | | | $ | (2,689) | | | $ | 3,805 | |
| Cash paid for interest | $ | 298 | | | $ | 4,265 | | | $ | 6,654 | |
| Supplemental schedule of non-cash investing and financing activities for continuing and discontinued operations | | | | | |
| Noncash liquidating distribution | $ | 64,400 | | | $ | 0 | | | $ | 0 | |
| Convertible notes due December 2021 exchanged for convertible notes due December 2024 | $ | 0 | | | $ | 86,053 | | | $ | 0 | |
| Common stock used to settle convertible notes payable | $ | 0 | | | $ | 45,901 | | | $ | 0 | |
| Assets held for sale reclassified from other assets to intangible assets | $ | 0 | | | $ | 0 | | | $ | 1,811 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
See accompanying notes
PDL BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
1. Organization and Business
Throughout our history, the Company’s mission has been to improve the lives of patients by aiding in the successful development of innovative therapeutics and healthcare technologies. PDL BioPharma was founded in 1986 as Protein Design Labs, Inc. when it pioneered the humanization of monoclonal antibodies, enabling the discovery of a new generation of targeted treatments that have had a profound impact on patients living with different cancers as well as a variety of other debilitating diseases. In 2006, the Company changed its name to PDL BioPharma, Inc.
Historically, the Company generated a substantial portion of its revenues through the license agreements related to patents covering the humanization of antibodies, which it refers to as the Queen et al. patents. In 2012, the Company began providing alternative sources of capital through royalty monetization and debt facilities, and, in 2016, the Company began acquiring commercial-stage products and launching specialized companies dedicated to the commercialization of these products. In 2019, and as a further evolution of the Company’s strategy, it began to enter into strategic transactions involving innovative late clinical-stage or early commercial-stage therapeutics. Consistent with this strategy, on April 10, 2019, the Company entered into a securities purchase agreement with Evofem Biosciences, Inc. (“Evofem”), pursuant to which it invested $60.0 million in a private placement of securities structured in two tranches.
In September 2019, the Company engaged financial and legal advisors and initiated a review of its strategy. This review was completed in December 2019 at which time the Company announced that it decided to halt the execution of its growth strategy, cease additional strategic transactions and investments and instead pursue a formal process to unlock value by monetizing its assets and returning net proceeds to stockholders (the “monetization strategy”). Pursuant to the Company’s monetization strategy, the Company does not expect to enter into any additional strategic investments. The Company further announced in December 2019 that it would explore a variety of potential transactions in connection with the monetization strategy, including a whole Company sale, divestiture of assets, spin-offs of operating entities, merger opportunities or a combination thereof. Over the subsequent months, the Company’s Board of Directors (the “Board”) and management analyzed, together with outside financial and legal advisors, how to best capture value pursuant to the monetization strategy and best return the value of the assets in its portfolio to its stockholders.
During the first quarter of 2020, the Board approved a plan of complete liquidation (the “Plan of Liquidation”) and passed a resolution to seek stockholder approval to dissolve the Company as permitted by the General Corporation Law of the State of Delaware (the “DGCL”). The proposal was approved by stockholders on August 19, 2020 at the Company’s 2020 Annual Meeting of Stockholders. On November 5, 2020, our Board approved filing a Certificate of Dissolution with the Secretary of State of Delaware on January 4, 2021 (the “Final Record Date”) and proceeding to complete the dissolution process for the Company in accordance with the DGCL. The liquidation and dissolution process will take a minimum of three years. However, the timing may be extended due to circumstances such as pending litigation or other factors that may affect the ability of the Company to wind down its business. Upon filing the Certificate of Dissolution on the Final Record Date, the Company closed its stock transfer books, meaning it will not record any further transfers of its common stock after the Final Record Date, except pursuant to the provisions of a deceased stockholder’s will, intestate succession, or by operation of law and the Company will not issue any new stock certificates, other than replacement certificates. In addition, after the Final Record Date, the Company will not issue any shares of its common stock for outstanding stock options. As a result of the closing of our transfer books, it is anticipated that distributions, if any, made in connection with the dissolution will be made pro rata to the stockholders of record as of the Final Record Date. On January 7, 2021 the Company was formally delisted from the Nasdaq Stock Market exchange, and it does not anticipate participating in Over-The-Counter (“OTC”) trading. On January 8, 2021, the Company filed a Form 15 notifying the SEC of deregistration of its common stock under Section 12(g) of the Exchange Act and suspension of its duty to file reports under Sections 13 and 15(d) of the Exchange Act.
Pursuant to the Company’s monetization strategy, the Company explored a variety of potential transactions, including a whole Company sale, divestiture of assets, spin-offs of operating entities, merger opportunities or a combination. During the year ended December 31, 2020, the Company’s Pharmaceutical and Strategic Positions segments and the royalty right assets within the Income Generating Assets segment met the criteria to be classified as held for sale. Those investments are reported as discontinued operations on the Consolidated Statements of Operations for the eight months ended August 31, 2020 and for the years ended December 31, 2019, and 2018 and as Assets and Liabilities held for sale on the Consolidated Balance Sheet as of December 31, 2019.
Based on the composition of its investment portfolio, under the Going Concern Basis, the Company historically operated in 4 segments designated as Medical Devices, Strategic Positions, Pharmaceutical and Income Generating Assets. Those investments are reported as discontinued operations on the Consolidated Statements of Operations for the eight months ended August 31, 2020 and for the years ended December 31, 2019, and 2018. Following is a summary of the Company’s segments including those that have been classified as discontinued operations.
The Medical Devices segment consisted of revenue derived from the sale and lease of the LENSAR® Laser System made by the Company’s majority-owned subsidiary, LENSAR, Inc. (“LENSAR”), which may include equipment, Patient Interface Devices (“PIDs”), procedure licenses, training, installation, warranty and maintenance agreements. On October 1, 2020, the Company completed the previously announced spin-off of LENSAR into a new, independent publicly traded company, through a distribution in the form of a liquidation distribution of all outstanding shares of LENSAR common stock owned by the Company to holders of the Company’s common stock on a pro rata basis (the “Distribution”). The Distribution was made to the Company’s stockholders of record as of the close of business on September 22, 2020 (the “LENSAR Record Date”) and such stockholders received 0.075879 shares of LENSAR common stock for every one share of the Company’s common stock held as of close of business on the LENSAR Record Date. Prior to the Distribution, the Company owned approximately 81.5% of LENSAR common stock. Following the completion of the distribution, PDL does not own any equity interest in LENSAR. LENSAR became an independent public company whose stock is listed and trading under the symbol “LNSR” on the Nasdaq Stock Market.
The Strategic Positions segment consisted of an investment in Evofem. Evofem is a publicly-traded (NASDAQ: EVFM) clinical-stage biopharmaceutical company committed to developing and commercializing innovative products to address unmet needs in women's sexual and reproductive health. Evofem is leveraging its proprietary Multipurpose Vaginal pH Regulator (MVP-R™) platform to develop Phexxi® (L-lactic acid, citric acid and potassium bitartrate) for hormone-free birth control. On May 21, 2020 the Evofem common stock held within the Strategic Positions segment was distributed in the form of a liquidation distribution to the Company’s stockholders on a pro rata basis. As of December 31, 2020, the Company held warrants to purchase up to 3,333,334 shares of Evofem common stock at an exercise price of $6.38 per share.
Our Pharmaceutical segment consisted of revenue derived from branded prescription medicine products sold under the name Tekturna® and Tekturna HCT® in the United States and Rasilez® and Rasilez HCT® in the rest of the world and revenue generated from the sale of an authorized generic form of Tekturna in the United States (collectively, the “Noden Products”). The branded prescription Noden Products were acquired from Novartis AG, Novartis Pharma AG and Speedel Holding AG (collectively, “Novartis”) in July 2016 (the “Noden Transaction”) by the Company’s wholly-owned subsidiary, Noden Pharma DAC (“Noden DAC”). The Company, through its wholly-owned subsidiary, Noden Pharma USA Inc. (“Noden USA”) launched its authorized generic form of Tekturna in the United States in March 2019. In September 2020, the Company sold the Noden business, including its Noden Pharma USA, Inc. subsidiary (“Noden USA” and, together with Noden DAC, “Noden”) to a third-party.
Our Income Generating Assets segment consisted of revenue derived from (i) notes and other long-term receivables, (ii) royalty rights and hybrid notes/royalty receivables, (iii) equity investments and (iv) royalties from issued patents in the United States and elsewhere covering the humanization of antibodies, which we refer to as the Queen et al. patents.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying Consolidated Financial Statements of PDL Biopharma, Inc. and its subsidiaries (collectively, the “Company” or “PDL”) have been prepared in accordance with Generally Accepted Accounting Principles (United States) (“GAAP”).
Certain prior period amounts have been reclassified for consistency with the current period presentation. These reclassifications had no effect on the reported results of operations.
Liquidation Basis of Accounting
As a result of the August 19, 2020 approval by the Company’s stockholders to file for dissolution pursuant to a plan of dissolution, it was determined that liquidation was imminent and the Company’s basis of accounting transitioned, effective September 1, 2020, the beginning of the fiscal month following the approval (“Convenience Date”), from the going concern basis of accounting (“Going Concern Basis”) to the liquidation basis of accounting (“Liquidation Basis”) in accordance with GAAP. Under the Liquidation Basis, the remeasurement of the Company's assets and liabilities includes management's estimates and assumptions of: (i) income to be generated from the remaining assets until the anticipated date of sale; (ii) sales proceeds to be received for these assets at the time of sale; (iii) operating expenses to be incurred; and (iv) amounts required to settle liabilities.
The estimated liquidation values for assets derived from future revenue streams and asset sales and the settlement of liabilities are reflected on the Consolidated Statement of Net Assets in Liquidation. The actual amounts realized could differ materially from the estimated amounts.
Principles of Consolidation
The Consolidated Financial Statements include the accounts of the Company and its wholly-owned and majority-owned subsidiaries up to their date of sale or distribution. All significant intercompany balances and transactions have been eliminated upon consolidation.
A subsidiary is an entity in which the Company, directly or indirectly, controls more than one half of the voting power; has the power to appoint or remove the majority of the members of the board of directors; to cast a majority of votes at the meeting of the board of directors; or to govern the financial and operating policies of the investee under a statute or agreement among the stockholders or equity holders.
The Company applies the guidance codified in ASC 810, Consolidations, which requires certain variable interest entities to be consolidated by the primary beneficiary of the entity in which it has a controlling financial interest. The Company identifies an entity as a variable interest entity if either: (1) the entity does not have sufficient equity investment at risk to permit the entity to finance its activities without additional subordinated financial support, or (2) the entity’s equity investors lack the essential characteristics of a controlling financial interest. The Company performs ongoing qualitative assessments of its variable interest entities to determine whether the Company has a controlling financial interest in any variable interest entity and therefore is the primary beneficiary, and if it has the power to direct activities that impact the activities of the entity.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the Consolidated Financial Statements and accompanying Notes to the Consolidated Financial Statements. While such estimates and assumptions are not always unique to the basis of accounting being followed under GAAP, some are more applicable to the accounting basis being followed.
Under the Liquidation Basis, the accounting estimates that require management’s most significant, difficult and subjective judgments include: the determination that the liquidation was imminent; the estimated sales proceeds of our assets; estimated settlement amounts of our liabilities, the estimated revenue and operating expenses that are projected during dissolution and discount rates.
Significant estimates under the Going Concern Basis include: the valuation of royalty rights; product revenue recognition and allowances for customer rebates; the valuation of notes receivable and inventory; the assessment of recoverability of intangible assets and their estimated useful lives; the valuation and recognition of stock-based compensation; the valuation of warrants to acquire shares of common stock and the discount rates used in fair value measurements.
Additional significant estimates under both the Liquidation Basis and Going Concern Basis include the recognition and measurement of current and deferred income tax assets and liabilities, including amounts recoverable under the Coronavirus Aid, Relief, and Economic Security (“CARES”) Act and the amount of uncertain tax positions.
Furthermore, the impact on accounting estimates and judgments on the Company’s financial condition and results of operations due to the 2019 coronavirus (“COVID-19”) has introduced additional uncertainties. Actual results could differ materially from those estimates.
Segment Reporting
Under ASC 280, Segment Reporting, operating segments are defined as components of an enterprise about which separate financial information is available that is regularly evaluated by the entity’s chief operating decision maker, in deciding how to allocate resources and in assessing performance. Under the Going Concern Basis, the Company evaluated its operating segments in accordance with ASC 280, and had identified 4 reportable segments: Medical Devices, Strategic Positions, Pharmaceutical and Income Generating Assets.
Severance and retention
After the Company announced its monetization strategy, it recognized that its ability to execute on its plan and optimize returns to its stockholders depended to a large extent on its ability to retain the necessary expertise to effectively transact with respect to its assets. On December 21, 2019, the Compensation Committee of the Board adopted a Wind Down Retention Plan in which the Company’s executive officers and other employees who were participants in the Company’s Severance Plan were eligible to participate. Under the Wind Down Retention Plan, participants have been eligible to earn a retention benefit in consideration for their continued employment with the Company. The Wind Down Retention benefits are equivalent to previously disclosed compensation payments contemplated in connection with a change in control under the Company’s existing Severance Plan. Under the Wind Down Retention Plan, the Company has been obligated to pay a retention benefit to each participant upon termination of the participant’s employment with the Company either by the Company without cause or by the participant for good reason. The retention benefits are in lieu of (and not in addition to) any other severance compensation that were payable to the participant under the Company’s Severance Plan. In connection with the adoption of the Wind Down Retention Plan, a severance liability was being recorded over the remaining service period for the participating employees under the Going Concern Basis. Upon the adoption of the Liquidation Basis on September 1, 2020, all remaining estimated severance and retention costs were accrued. As of December 31, 2020, the Company has a remaining severance liability of $0.3 million, which is included in Compensation and benefit costs on the Company’s Consolidated Statement of Net Assets. Expenses associated with severance payments and accruals under the Going Concern Basis are reflected in Severance and retention on the Company’s Consolidated Statements of Operations for the eight months ended August 31, 2020.
The Wind Down Retention Plan also provides that, consistent with the existing terms of the Company’s Amended and Restated 2005 Equity Incentive Plan (the “Equity Plan”), the vesting of all outstanding equity awards held by participants as of the date the Wind Down Retention Plan was adopted are accelerated upon the earlier of: (i) a termination of the participant’s employment with the Company either by the Company without cause or by the participant for good reason or (ii) the consummation of a change in control (as defined in the Equity Plan) of the Company. In addition, the post-termination exercise period for all outstanding stock options are extended until their expiration date. In connection with the Board adopting the Plan of Liquidation in the first quarter of 2020, all of the outstanding and unvested stock options and restricted stock granted to the Company’s employees and executive officers, with the exception of certain outstanding awards under the 2016/20 Long-Term Incentive Plan, accelerated and vested under the change in control definition in the Equity Plan. The expense associated with the accelerated vesting, totaling $15.7 million, is reported as Severance and retention on the Company’s Consolidated Statements of Operations for the eight months ended August 31, 2020 under the Going Concern Basis.
Assets Held for Sale
Under the Going Concern Basis, assets and liabilities are classified as held for sale when all of the following criteria for a plan of sale have been met: (1) management, having the authority to approve the action, commits to a plan to sell the assets; (2) the assets are available for immediate sale, in their present condition, subject only to terms that are usual and customary for sales of such assets; (3) an active program to locate a buyer and other actions required to complete the plan to sell the assets have been initiated; (4) the sale of the assets is probable and is expected to be completed within one year; (5) the assets are being actively marketed for a price that is reasonable in relation to their current fair value; and (6) actions required to complete the plan indicate that it is unlikely that significant changes to the plan will be made or the plan will be withdrawn. When all of these criteria have been met, the assets and liabilities are classified as held for sale in the balance sheet. Assets classified as held for sale are reported at the lower of their carrying value or fair value less costs to sell. Depreciation and amortization of assets ceases upon designation as held for sale. The assets and liabilities held for sale are recorded on the Company’s Consolidated Balance Sheet as of December 31, 2019 as Assets held for sale and Liabilities held for sale, respectively.
Discontinued Operations
Under the Going Concern Basis, discontinued operations comprise those activities that were disposed of during the period or which were classified as held for sale at the end of the period, represent a separate major line of business or geographical area that can be clearly distinguished for operational and financial reporting purposes and represent a strategic shift that has or will have a major effect on the Company’s operations and financial results. The profits and losses are presented on the Consolidated Statements of Operations as discontinued operations. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
Cash Equivalents
The Company considers all highly liquid investments with initial maturities of three months or less at the date of purchase to be cash equivalents. The Company places its cash and cash equivalents with high credit quality financial institutions and, by policy, limits the amount of credit exposure in any one financial instrument.
Accounts Receivable
Under the Going Concern Basis the Company concluded that an allowance for doubtful accounts was not required as of December 31, 2019. On January 1, 2020 the Company adopted ASU No. 2016-13 on. See “Adopted Accounting Pronouncements” below for additional information.
Investments
As of December 31, 2020, the Company had an investment in a privately held company AEON BioPharma, Inc., including its ownership of Alphaeon 1, LLC, (formerly collectively referred to as “Alphaeon” and currently and collectively referred to as “AEON”). As of December 31, 2019, the Company’s investments were comprised of an investment in a privately held company and a publicly traded company, Evofem.
Under the Going Concern Basis, the Company’s investment in Evofem qualified for equity method accounting given its prior percentage ownership in Evofem and the ability to exercise significant influence. The Company elected the fair value method to account for its investment in Evofem as it believed it better reflected economic reality, the financial reporting of the investment and the current value of the asset. Changes in fair value of the Evofem equity investment are presented in the Consolidated Statements of Operations as discontinued operations. All shares of common stock of Evofem owned by PDL were distributed to the Company’s stockholders in May 2020. As of December 31, 2020, the Company held 3.3 million warrants convertible into Evofem common stock at an exercise price of $6.38 per share. Under the Going Concern Basis, the fair value of the warrants was estimated using recently quoted market prices of the underlying equity security and the Black-Scholes option pricing model. In addition to these inputs, under the Liquidation Basis, a discount is applied to the resulting value to reflect limited liquidity.
The Company’s equity security investment in AEON qualified to be measured at fair value under the Going Concern Basis, although the fair value of the investment was not readily determinable as AEON’s shares are not publicly traded. The Company evaluated the fair value of this investment by performing a qualitative assessment each reporting period. If the results of this qualitative assessment indicated that the fair value was less than the carrying value, the investment was written down to its fair value. This investment is included in Other assets on the Consolidated Statement of Net Assets as of December 31, 2020 and on the Company’s Consolidated Balance Sheet as Other long-term assets as of December 31, 2019.
Fair Value Measurements
The fair value of the Company’s financial instruments are estimates of the amounts that would be received if the Company were to sell an asset or the Company paid to transfer a liability in an orderly transaction between market participants at the measurement date or exit price. Under the Going Concern Basis, the assets and liabilities are categorized and disclosed in one of the following three categories:
Level 1 – based on quoted market prices in active markets for identical assets and liabilities;
Level 2 – based on observable inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are or can be corroborated by observable market data for substantially the full term of the assets or liabilities, and
Level 3 – based on unobservable inputs using management’s best estimate and assumptions when inputs are unavailable.
Notes Receivable and Other Long-Term Receivables
Under the Going Concern Basis, the Company accounted for its notes receivable at amortized cost, net of unamortized origination fees, if any, and adjusted for any impairment losses. Interest was accreted or accrued to “Interest revenue” using the effective interest method. When and if supplemental payments were received from certain of these notes and other long-term receivables, an adjustment to the estimated effective interest rate was affected prospectively.
The Company evaluated the collectability of both interest and principal for each note receivable and loan to determine whether it is impaired. A note receivable or loan was considered to be impaired when, based on current information and events, the Company determines it is probable that it will be unable to collect amounts due according to the existing contractual terms. When a note receivable or loan was considered to be impaired, the amount of loss was calculated by comparing the carrying value of the financial asset to the value determined by discounting the expected future cash flows at the loan’s effective interest rate or to the estimated fair value of the underlying collateral, less costs to sell, if the loan was collateralized and the Company expected repayment to be provided solely by the collateral. Impairment assessments required significant judgments and were based on significant assumptions related to the borrower’s credit risk, financial performance, expected sales, and estimated fair value of the collateral.
The Company recorded interest on an accrual basis and recognized it was earned in accordance with the contractual terms of the credit agreement, to the extent that such amounts were expected to be collected. When a note receivable or loan became past due, or if management otherwise did not expect that principal, interest, and other obligations due would be collected in full, the Company generally placed the note receivable or loan on an impaired status and ceased recognizing interest income on that note receivable or loan. Any interest payments received for notes receivable or loans on an impaired status were recognized as interest income on a cash basis.
The Company did not recognized any interest revenue for the CareView Communications, Inc. (“CareView”) note receivable while on impaired status. The last interest payments were received during the year ended December 31, 2018, when the Company recognized $2.3 million of interest revenue for the CareView note receivable as a result of cash interest payments made during the year.
As of December 31, 2020, the Company had 1 note receivable investment with a Liquidation Basis value of approximately $0.7 million. At December 31, 2019, under the Going Concern Basis, the Company had 2 note receivable investments, which were determined to be impaired, with a cumulative investment cost and fair value of approximately $52.1 million and $57.3 million, respectively, as of this date. During the eight months ended August 31, 2020 and the years ended December 31, 2019, and 2018, the Company did not recognize any losses on extinguishment of notes receivable.
There were 0 impairment losses on notes receivable for the eight months ended August 31, 2020. During the years ended December 31, 2019 and 2018, the Company recorded an impairment loss of $10.8 million and $8.2 million, respectively, related to the CareView note receivable. For additional information about the impairment loss recorded on the CareView note receivable, see Note 9, Notes and Other Long-Term Receivables.
Inventory
Under the Going Concern Basis, Inventory, which consisted of raw materials, work-in-process and finished goods, was stated at the lower of cost or net realizable value. The Company determined cost using the first-in, first-out method. Inventory levels were analyzed periodically and written down to their net realizable value if they had become obsolete, had a cost basis in excess of its expected net realizable value or were in excess of expected requirements. The Company analyzed current and future product demand relative to the remaining product shelf life to identify potential excess inventory. The Company built demand forecasts by considering factors such as, but not limited to, overall market potential, market share, market acceptance and patient usage. The Company classified inventory as current on the Consolidated Balance Sheet when the Company expected inventory to be consumed for commercial use within the next twelve months.
Intangible Assets
Under the Going Concern Basis, intangible assets with finite useful lives consisted primarily of customer relationships, acquired technology and trademarks. They were amortized on a straight-line basis over their estimated useful lives, ranging from five years to 20 years, which was consistent with the estimated lives of the associated products. Such assets were reviewed for impairment when events or circumstances indicated that the carrying value of an asset was not recoverable. An impairment loss was recognized when estimated undiscounted future cash flows expected to result from the use of an asset and its eventual disposition were less than its carrying amount. The amount of any impairment was measured as the difference between the carrying amount and the fair value of the impaired asset.
Property and Equipment
Under the Going Concern Basis, Property and equipment are stated at cost less accumulated depreciation. Depreciation was computed using the straight-line method over the following estimated useful lives:
| | | | | | | | |
| Leasehold improvements | | Lesser of useful life or term of lease |
| Manufacturing equipment | | 3-5 years |
| Computer and office equipment | | 3 years |
| Transportation equipment | | 3 years |
| Furniture and fixtures | | 7 years |
| Equipment under lease | | Greater of lease term or 5-10 years |
Convertible Notes
The Company has previously issued convertible notes with settlement features that allow the Company to settle the notes by paying or delivering, as applicable, cash, shares of the Company’s common stock or a combination of cash and shares of its common stock, at the Company’s election. In accordance with accounting guidance under the Going Concern Basis for convertible debt instruments that may be settled in cash or other assets on conversion, the Company separated the principal balance between the fair value of the liability component and the common stock conversion feature using a market interest rate for a similar nonconvertible instrument at the date of issuance.
Financing Costs Related to Long-term Debt
Under the Going Concern Basis, costs associated with obtaining long-term debt were deferred and amortized over the term of the related debt using the effective interest method. Such costs are presented as reductions from the carrying amount of the long-term debt liability, consistent with debt discounts, on the Company’s Consolidated Balance Sheet.
Revenue Recognition
The reported results under the Going Concern Basis reflected the application of ASC 606, Revenue from Contracts with Customers (“ASC 606”).
Policy Elections and Practical Expedients Taken
Upon the Company’s adoption of ASC 606, it elected the following practical expedients:
Taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction, that are collected by the Company from a customer, are excluded from revenue.
Shipping and handling costs associated with outbound freight after control over a product has transferred to a customer are accounted for as a fulfillment cost and are included in cost of product revenue.
The Company elected to apply the practical expedient that allows an entity to not adjust the promised amount of consideration in customer contracts for the effect of a significant financing component when the period between the transfer of product and services and payment of the related consideration is less than one year.
General
In accordance with ASC 606, revenue was recognized from the sale of products when a customer obtained control of promised products and services. The amount of revenue recognized reflects the consideration to which the Company expected to be entitled to receive in exchange for products and services. A five-step model was utilized to achieve the core principle and included the following steps: (1) identify the customer contract; (2) identify the contract’s performance obligations; (3) determine the transaction price; (4) allocate the transaction price to the performance obligations; and (5) recognize revenue when the performance obligations are satisfied.
The following is a description of principal activities - separated by reportable segments - from which the Company generated its revenue. For more detailed information about reportable segments, see Note 21, Segment Information.
Pharmaceutical
The Company’s Pharmaceutical segment consisted of revenue derived from sales of the Noden Products. Noden’s revenue is included in Loss from discontinued operations.
The agreement between Novartis and Noden DAC provided for various transition periods for development and commercialization activities relating to the Noden Products. For the period from July 1, 2016 through October 4, 2016, all of the Noden Products were distributed by Novartis under the terms of the Noden Purchase Agreement while transfers of the marketing authorization rights were pending. During this time, the Company presented revenue under the Novartis transition arrangement on a “net” basis and established a reserve for retroactive adjustment to the profit transfer with Novartis. As of the third quarter of 2018, Noden Pharma DAC completed the marketing authorization transfers for all territories.
In the United States, the duration of the profit transfer ran from July 1, 2016 through October 4, 2016. Beginning on October 5, 2016, Noden Pharma USA, Inc. distributed the Noden Products in the United States. At such time, the Company presented revenue for all sales in the United States on a “gross” basis, meaning product costs were reported separately and there was no fee to Novartis, and established a reserve for discounts and allowances further described below.
Initially, Novartis distributed the Noden Products on behalf of Noden DAC worldwide and Noden DAC received a profit transfer on such sales. Generally, the profit transfer to Noden DAC was defined as gross revenues less product cost and a low single-digit percentage fee to Novartis. The profit transfer terminated upon the transfer of the marketing authorization from Novartis to Noden DAC in each country. For the period from October 5, 2016 to August 31, 2017, Novartis continued to distribute the Noden Products outside of the United States. Beginning on September 1, 2017, Noden Pharma DAC began distributing the Noden Products to select countries outside the United States. Outside the United States, the profit transfer ended in the first quarter of 2018.
Except for the sales in certain countries outside of the United States preceding the final profit transfer that occurred in the first quarter of 2018, revenues of the Noden Products for the periods herein were recognized on a gross basis.
Noden USA launched an authorized generic of Tekturna in the United States in March 2019.
The Pharmaceutical segment principally generated revenue from products sold to wholesalers and distributors. Customer orders were generally fulfilled within a few days of receipt resulting in minimal order backlog. Contractual performance obligations were usually limited to transfer of the product to the customer. The transfer occurred either upon shipment or upon receipt of the product in certain countries outside the United States after considering when the customer obtained control of the product. In addition, in some countries outside of the United States, the Company sold product on a consignment basis where control was not transferred until the customer resold the product to an end user. At these points, customers were able to direct the use of and obtain substantially all of the remaining benefits of the product.
Sales to customers were initially invoiced at contractual list prices. Payment terms were typically 30 to 90 days based on customary practice in each country. Revenue was reduced from the list price at the time of recognition for expected chargebacks, discounts, rebates, sales allowances and product returns, which were collectively referred to as gross-to-net adjustments. These reductions were attributed to various commercial agreements, managed healthcare organizations and government programs such as Medicare, Medicaid, and the 340B Drug Pricing Program containing various pricing implications such as mandatory discounts, pricing protection below wholesaler list price and other discounts when Medicare Part D beneficiaries were in the coverage gap. These various reductions in the transaction price were estimated using either a most likely amount, in the case of prompt pay discounts, or expected value method for all other variable consideration and were reflected as liabilities and was settled through cash payments, typically within time periods ranging from a few months to one year. Significant judgment is required in estimating gross-to-net adjustments considering legal interpretations of applicable laws and regulations, historical experience, payer channel mix, current contract prices under applicable programs, unbilled claims, processing time lags and inventory levels in the distribution channel.
Customer Credits: The Company’s customers were offered various forms of consideration, including allowances, service fees and prompt payment discounts. The Company expected customers would earn prompt payment discounts and, therefore, the Company deducted the full amount of these discounts from total product sales when revenues were recognized. Service fees were also deducted from total product sales as they were earned.
Rebates and Discounts: Allowances for rebates included mandated discounts under the Medicaid Drug Rebate Program in the United States and mandated discounts in the European Union (“EU”) in markets where government-sponsored healthcare systems are the primary payers for healthcare. Rebates are amounts owed after the final dispensing of the product to a benefit plan participant and are based upon contractual agreements or legal requirements with public sector benefit providers. The accrual for rebates was based on negotiated discount rates and expected utilization as well as historical data. Estimates for expected utilization of rebates were based on data received from the customers. Rebates were generally invoiced and paid in arrears so that the accrual balance consists of an estimate of the amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known prior quarters’ unpaid rebates.
Chargebacks: Chargebacks are discounts that occur when certain contracted customers, which currently consist primarily of group purchasing organizations, Public Health Service institutions, non-profit clinics, and Federal government entities purchasing via the Federal Supply Schedule, purchased directly from the Company’s wholesalers. Contracted customers generally purchased the product at a discounted price. The wholesalers, in turn, charged back to the Company the difference between the price initially paid by the wholesalers to the Company and the discounted price paid by the contracted customers. In addition to actual chargebacks received, the Company maintained an accrual for chargebacks based on the estimated contractual discounts on products sold for which the chargeback had not been billed.
Medicare Part D Coverage Gap: Medicare Part D prescription drug benefit mandates manufacturers to fund 70% in 2020 and 2019 and 50% in 2018 of the Medicare Part D insurance coverage gap for prescription drugs sold to eligible patients. Estimates for the expected Medicare Part D coverage gap were based on historical invoices received and in part from data received from the Company’s customers. Funding of the coverage gap was generally invoiced and paid in arrears.
Co-payment Assistance: Patients who have commercial insurance and meet certain eligibility requirements may receive co-payment assistance. The Company accrues a liability for co-payment assistance based on actual program participation and estimates of program redemption using data provided by third-party administrators.
Returns: Returns were generally estimated and recorded based on historical sales and returns information. Products that exhibited unusual sales or return patterns due to dating, competition or other marketing matters were specifically investigated and analyzed as part of the accounting for sales returns.
Reserves for chargebacks, discounts, rebates, sales allowances and product returns are included within Liabilities held for sale in the Company’s Consolidated Balance Sheet as of December 31, 2019.
For licenses that were bundled with other promises, the Company utilized judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front license fees. The Company evaluated the measure of progress each reporting period and, if necessary, adjusted the measure of performance and related revenue recognition.
Medical Devices
The Medical Devices segment principally generated revenue from the sale and lease of the LENSAR® Laser System and the sale of other related products and services prior to the spin-off of LENSAR in October 2020.
For bundled packages, which included the sale or lease of a LENSAR® Laser System and provision of other products and services, the Company accounted for individual products and services separately if they were distinct—i.e. if a product or service was separately identifiable from other items in the bundled package and if the customer could benefit from it on its own or with other resources that are readily available to the customer. The LENSAR Laser System, training and installation services were one performance obligation. The other products and services, including PIDs, procedure licenses, and extended warranty services, which were either sold together with the LENSAR Laser System or on a standalone basis, were all accounted for as separate performance obligations. The transaction price of bundled packages was allocated to each performance obligation on a relative standalone selling price basis. Standalone selling prices were based on observable prices at which the Company separately sold the products or services. If a standalone selling price was not directly observable, the Company estimated the selling price using available observable information.
The Company recognized revenue as the performance obligations are satisfied by transferring control of the product or service to a customer, as described below.
Product Revenue. The Company recognized revenue for the sale of the following products at a point in time:
Equipment. The Company’s LENSAR Laser System sales were recognized as Product revenue when the Company transferred control of the system. This usually occurred after the customer signed a contract, LENSAR installed the system, and LENSAR performed the requisite training for use of the system for direct customers. LENSAR Laser System sales to distributors were recognized as revenue upon shipment as they did not require training and installation.
PID and Procedure Licenses. The LENSAR Laser System requires both a PID and a procedure license to perform each procedure. The Company recognized Product revenue for PIDs when the Company transferred control of the PID. The Company recognized Product revenue for procedure licenses at the point in time when control of the procedure license was transferred to the customer. A procedure license represents a one-time right to utilize the LENSAR Laser System surgical application in connection with a surgery procedure. For the sale of PIDs and procedure licenses, the Company had the option to offer volume discounts to certain customers. To determine the amount of revenue that should be recognized at the time control over these products transferred to the customer, the Company estimated the average per unit price, net of discounts.
Service Revenue. The Company offered an extended warranty that provided additional maintenance services beyond the standard limited warranty. The Company recognized Service revenue from the sale of extended warranties over the warranty period on a ratable basis as the Company stood ready to provide services as needed. Customers had the option of renewing the warranty period, which was considered a new and separate contract.
Lease Revenue. For LENSAR Laser System operating leases, the Company recognized lease revenue over the length of the lease in accordance with ASC Topic 840, Leases, through December 31, 2018 and recognized lease revenue in accordance with ASC Topic 842, Leases, after January 1, 2019. For additional information regarding accounting for leases, see the Leases section within this footnote below and Note 10, Leases.
Contract Costs
The Company offered a variety of commission plans to the Company’s salesforce. Certain compensation under these plans was earned by sales representatives solely as a result of obtaining a customer contract. These are considered incremental costs of obtaining a contract and were eligible for capitalization under ASC 340-40, Other Assets and Deferred Costs – Contracts with Customers, to the extent they were recoverable. For goods or services that were to be delivered over a period that was longer than one year, incremental costs of obtaining a contract were capitalized and expensed over the period the related revenue was recognized. The Company elected not to defer costs related to goods or services that were to be delivered over a period that was one year or less.
Significant Financing Component
The Company provided extended payment terms to certain customers that represented a significant financing component. The Company adjusted the amount of promised consideration for the time value of money using its discount rate and recognized interest income separate from the revenue recognized on contracts with customers.
Limited Warranty Obligations
The Company offered limited warranties on the Company’s products which provided the customer assurance that the product would function as the parties intended because it complied with agreed-upon specifications; therefore, these assurance-type warranties were not treated as a separate revenue performance obligation and were accounted for as guarantees under U.S. GAAP. The Company regularly reviewed its warranty liability and updated those balances based on historical warranty cost trends.
Income Generating Assets
Under the Going Concern Basis, for licenses of intellectual property, if the license to the Company’s intellectual property was determined to be distinct from the other performance obligations identified in the arrangement, the Company recognized revenues from non-refundable, up-front fees allocated to the license when the license was transferred to the customer and the customer was able to use and benefit from the license.
In January 2018, DFM, LLC, a wholly-owned subsidiary of the Company, granted an exclusive license related to certain Direct Flow Medical, Inc. assets in exchange for $0.5 million in cash and up to $2.0 million in royalty payments. The $0.5 million
payment was accounted for in accordance with ASC 606 under which the full cash payment was recognized as revenue in the first quarter of 2018 as DFM, LLC had fulfilled its performance obligation under the agreement. In September 2019, the remaining assets of DFM, LLC were sold for $5.0 million.
Queen et al. Royalty Revenues
Under the Company’s license agreements related to the Queen et al. patents, the Company received royalty payments based upon its licensees’ net sales of covered products. Under the Going Concern Basis, royalties qualify for the sales-and-usage exemption under ASC 606 as (i) royalties are based strictly on the sales-and-usage by the licensee; and (ii) a license of intellectual property is the sole or predominant item to which such royalties relate. Based on this exemption, these royalties were earned under the terms of a license agreement in the period the products were sold by the Company's partner and the Company had a present right to payment. Generally, under these agreements, the Company received royalty reports from its licensees approximately one quarter in arrears; that is, generally in the second month of the quarter after the licensee has sold the royalty-bearing product. The Company recognized royalty revenues when it could reliably estimate such amounts and collectability was reasonably assured. Under this accounting policy, the royalty revenues the Company reported were not based upon estimates, and such royalty revenues were typically reported in the same period in which the Company received payment from its licensees.
Although the last of the Queen et al. patents expired in December 2014, the Company has received royalties beyond expiration based on the terms of its licenses and its legal settlement. Under the terms of the legal settlement between Genentech, Inc. (“Genentech”) and the Company, the first quarter of 2016 was the last period for which Genentech paid royalties to the Company for Avastin®, Herceptin®, Xolair®, Perjeta® and Kadcyla®. Other products from the Queen et al. patent licenses, such as Tysabri®, entitled the Company to royalties following the expiration of its patents with respect to sales of licensed product manufactured prior to patent expiry in jurisdictions providing patent protection licenses. In November 2017, the Company was notified by Biogen, Inc. that product supply for Tysabri® that was manufactured prior to patent expiry and for which the Company would receive royalties, had been extinguished in the United States and was rapidly being reduced in other countries. As a result, royalties from product sales of Tysabri were consecutively lower in 2018 and 2019 during which time they ceased.
Royalty Rights - At Fair Value
Under the Going Concern Basis, the Company accounted for its investments in royalty rights at fair value with changes in fair value presented in earnings. The fair value of the investments in royalty rights was determined by using a discounted cash flow analysis related to the expected future cash flows to be received. These assets were classified as Level 3 assets within the fair value hierarchy, as the Company’s valuation estimates utilized significant unobservable inputs, including estimates as to the probability and timing of future sales of the related products. Transaction-related fees and costs were expensed as incurred.
Under the Going Concern Basis, the changes in the estimated fair value from investments in royalty rights along with cash receipts in each reporting period were presented together on the Company’s Consolidated Statements of Operations as Loss from discontinued operations before income taxes.
Under the Going Concern Basis, realized gains and losses on royalty rights were recognized as they were earned and when collection was reasonably assured. Royalty Rights revenue was recognized over the respective contractual arrangement period. Critical estimates included product demand and market growth assumptions, inventory target levels, product approval, pricing assumptions and the impact of competition from other branded or generic products. Factors that could cause a change in estimates of future cash flows included a change in estimated market size, a change in pricing strategy or reimbursement coverage, a delay in obtaining regulatory approval, a change in dosage of the product a change in the number of treatments and the entrants of new competitors or generic products.
For each arrangement, the Company is entitled to royalty payments based on revenue generated by the net sales of the product.
Research and Development
Under the Going Concern Basis, the Company expensed research and development costs as incurred. Research and development expenses consisted primarily of engineering, product development, clinical studies to develop and support the Company’s products, regulatory expenses, and other costs associated with products and technologies that were in development. Research and development expenses included employee compensation, including stock-based compensation, supplies, consulting, prototyping, testing, materials, travel expenses, and depreciation.
Foreign Currency Translation
Under the Going Concern Basis, the Company used the U.S. dollar predominately as the functional currency of its foreign subsidiaries. For foreign subsidiaries where the U.S. dollar was the functional currency, gains and losses from remeasurement of foreign currency balances into U.S. dollars are included in the Consolidated Statements of Operations. The aggregate net (losses) gains resulting from foreign currency transactions and remeasurement of foreign currency balances into U.S. dollars that were included in the Consolidated Statements of Operations amounted to a gain of $0.2 million and a loss of $0.5 million and $0.7 million for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, respectively.
Comprehensive Loss
Under the Going Concern Basis, comprehensive loss was comprised of net loss adjusted for other comprehensive loss, using the specific identification method, which included unrealized gains and losses on the Company’s investments in available-for-sale securities, net of tax, which are excluded from the Company’s net loss.
Income Taxes
The provision for income taxes is determined using the asset and liability approach. Tax laws require items to be included in tax filings at different times than the items are reflected in the Consolidated Financial Statements. A current liability is recognized for the estimated taxes payable for the current year. Deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. Deferred taxes are adjusted for enacted changes in tax rates and tax laws. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized.
The Company recognizes tax benefits from uncertain tax positions only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the consolidated financial statements from such positions are then measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. The Company adjusts the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions. Any interest and penalties on uncertain tax positions are included within the tax provision.
Leases
General
In February 2016, the FASB issued ASU No. 2016-02, Leases, that supersedes ASC 840, Leases. Subsequently, the FASB issued several updates to ASU No. 2016-02, codified in ASC Topic 842 (“ASC 842”). The Company adopted ASC 842, Leases, on January 1, 2019 using the modified retrospective method for all leases not substantially completed as of the date of adoption. The reported results for the eight months ended August 31, 2020 and the year ended December 31, 2019 reflect the application of ASC 842 guidance while the reported results for the year ended December 31, 2018 was prepared under the guidance of ASC 840, which is also referred to herein as “legacy GAAP” or the “previous guidance”. The cumulative impact of the adoption of ASC 842 was not material, therefore, the Company did not record any adjustments to retained earnings. As a result of adopting ASC 842, the Company recorded operating lease right-of-use (“ROU”) assets of $2.1 million and operating lease liabilities of $2.1 million, primarily related to corporate office leases, based on the present value of the future lease payments on the date of adoption. Changes to lessor accounting focused on conforming with certain changes made to lessee accounting and the recently adopted revenue recognition guidance. The adoption of ASC 842 did not materially change how the Company accounted for lessor arrangements.
Under the Going Concern Basis, the Company determined if an arrangement was a lease or contained an embedded lease at inception if it contained the right to control the use of an identified asset under a leasing arrangement with an initial term greater than 12 months. The Company determined whether a contract conveyed the right to control the use of an identified asset for a period of time if the contract contained both the right to obtain substantially all of the economic benefits from the use of the identified asset and the right to direct the use of the identified asset. The Company has lease arrangements with lease and non-lease components, which were accounted for separately.
Policy Elections and Practical Expedients Taken
For leases that commenced before the effective date of ASC 842, the Company elected the practical expedients to not reassess the following: (i) whether any expired or existing contracts contain leases; (ii) the lease classification for any expired or existing leases; and (iii) initial direct costs for any existing leases.
The Company adopted a policy of expensing short-term leases, defined as 12 months or less, as incurred.
The Company’s policy was to exclude from the consideration in a lessor contract all taxes assessed by a governmental authority that are both imposed on and concurrent with a specific lease revenue-producing transaction and collected by the Company from a lessee.
Lessee arrangements
Under the Going Concern Basis, lessee operating leases are included in Other assets, Accrued liabilities, and Other long-term liabilities in the Company’s Consolidated Balance Sheet. The Company does not have lessee financing leases.
Operating lease ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent its obligation to make lease payments arising from the lease. Under the Going Concern Basis, operating lease ROU assets and liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. The Company used the implicit rate when readily determinable at lease inception. As most of the Company’s leases do not provide an implicit rate, the Company used its incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The Company’s remaining lease terms may include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option. Lease expense for lease payments was recognized on a straight-line basis as operating expense in the Consolidated Statements of Operations over the lease term.
For lease arrangements with lease and non-lease components where the Company is the lessee, the Company separately accounted for lease and non-lease components, which consists primarily of taxes and common area maintenance costs. Non-lease components were expensed as incurred.
Lessor arrangements
The Company leased medical device equipment to customers in operating lease arrangements generated from its Medical Devices segment.
For lease arrangements with lease and non-lease components where the Company was the lessor, the Company allocated the contract’s transaction price (including discounts) to the lease and non-lease components on a relative standalone selling price basis using the Company’s best estimate of the standalone selling price of each distinct product or service in the contract. Lease elements generally included a LENSAR Laser System, while non-lease elements generally included extended warranty services, PIDs and procedure licenses. The stand-alone selling prices for the extended warranty services, PIDs and procedure licenses were determined based on the prices at which the Company separately sold such products and services. The LENSAR Laser System stand-alone selling prices were determined using the expected cost plus a margin approach. Allocation of the transaction price was determined at the inception of the lease arrangement. The Company’s leases primarily consisted of leases with fixed lease payments. For those leases with variable lease payments, the variable lease payment was typically based upon use of the leased equipment or the purchase of procedure licenses and PIDs used with the leased equipment. Non-lease components were accounted for under ASC 606. For additional information regarding ASC 606, see Note 20, Revenue from Contracts with Customers.
Some leases included options to extend the leases on a month-to-month basis if the customer does not notify the Company of the intention to return the equipment at the end of the lease term. The Company typically did not offer options to terminate the leases before the end of the lease term. A new contract was generated if a customer intended to continue using the equipment under the initial term and the new contract term was not included in the initial lease term.
In determining whether a transaction should be classified as a sales-type or operating lease, the Company considered the following criteria at lease commencement: (1) whether title of the system transfers automatically or for a nominal fee by the end of the lease term, (2) whether the present value of the minimum lease payments equals or exceeds substantially all of the fair value of the leased system, (3) whether the lease term is for the major part of the remaining economic life of the leased system, (4) whether the lease grants the lessee an option to purchase the leased system that the lessee is reasonably certain to exercise, and (5)
whether the underlying system is of such a specialized nature that it is expected to have no alternative use to the Company at the end of the lease term. If any of these criteria were met, the lease was classified as a sales-type lease. If none of these criteria are met the lease was classified as an operating lease. The Company does not have any sales-type leases.
For operating leases, rental income was recognized on a straight-line basis over the lease term. The cost of customer-leased equipment was recorded within Property and equipment, net in the accompanying Consolidated Balance Sheet and depreciated over the equipment’s estimated useful life. Depreciation expense associated with the leased equipment under operating lease arrangements was reflected in Cost of product revenue in the accompanying Consolidated Statements of Operations for the eight months ended August 31, 2020 and the year ended December 31, 2019. Some of the Company’s operating leases included a purchase option for the customer to purchase the leased asset at the end of the lease arrangement. The purchase price did not qualify as a bargain purchase option. The Company managed its risk on its investment in the equipment through pricing and the term of the leases. Lessees did not provide residual value guarantees on leased equipment. Equipment returned to the Company could be leased or sold to other customers. Initial direct costs were deferred and recognized over the lease term.
Adopted Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments - Credit Losses: Measurement of Credit Losses on Financial Instruments. The guidance amended the impairment model to utilize an expected loss methodology in place of the incurred loss methodology, resulting in more timely recognition of losses. The Company adopted ASU No. 2016-13 on January 1, 2020 using a modified retrospective approach. The adoption of this standard did not have a material impact on the Company’s consolidated financial statements. As a consequence of adopting ASU 2016-13, the Company’s accounts receivable accounting policy under the Going Concern Basis was updated, as follows:
Accounts and Notes Receivable
The Company makes estimates of the collectability of accounts receivable. In doing so, the Company analyzes historical bad debt trends, customer credit worthiness, current economic trends and changes in customer payment patterns when evaluating the adequacy of the allowance for credit losses. Amounts are charged off against the allowance for credit losses when the Company determines that recovery is unlikely and the Company ceases collection efforts. The Company applies the practical expedient for its collateral-dependent notes receivable. Estimated credit losses are based on the fair value of the collateral (less costs to sell, as applicable).
In April 2020, the FASB issued a staff question-and-answer document, “Topic 842 and Topic 840: Accounting for Lease Concessions Related to the Effects of the COVID-19 Pandemic” (the “COVID-19 Q&A”), to address certain frequently asked questions pertaining to lease concessions arising from the effects of the COVID-19 pandemic. Existing lease guidance requires entities to determine if a lease concession was a result of a new arrangement reached with the lessee (which would be addressed under the lease modification accounting framework) or if a lease concession was under the enforceable rights and obligations within the existing lease agreement (which would not fall under the lease modification framework). The COVID-19 Q&A clarifies that entities may elect to not evaluate whether lease-related relief granted in light of the effects of COVID-19 is a lease or obligations of the lease. This election is available for concessions that result in the total payments required by the modified contract being substantially the same or less than the total payments required by the original contract.
As a result of the COVID-19 pandemic, LENSAR entered into agreements with 23 customers through which LENSAR agreed to waive monthly rental and minimum monthly license fees ranging from one to four months for an aggregate of $0.9 million of revenue for the eight months ended August 31, 2020, consisting of $0.5 million in Product revenue, $0.3 million in Lease revenue and $0.1 million in Service revenue. In return for these concessions the related contracts were extended by the same number of months waived. No accounts receivable or notes receivable amounts were deemed uncollectible due to COVID-19 during the 2020 periods presented herein; however, the Company considered the effects of COVID-19 in estimating its credit losses for the period.
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement. The new guidance modifies disclosure requirements related to fair value measurement. The Company adopted ASU No. 2018-13 on January 1, 2020. The adoption did not have an effect on the Consolidated Financial Statements on the adoption date and no adjustment to prior year Consolidated Financial Statements was required.
In August 2018, the FASB issued ASU No. 2018-15, Intangibles-Goodwill and Other-Internal-Use Software. The new guidance reduces complexity for the accounting for costs of implementing a cloud computing service arrangement and aligns the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the
requirements for capitalizing implementation costs incurred to develop or obtain internal-use software (and hosting arrangements that include an internal use software license). The Company adopted ASU No. 2018-15 on January 1, 2020 using the prospective transition option. The adoption did not have an effect on the Consolidated Financial Statements on the adoption date and no adjustment to prior year Consolidated Financial Statements was required.
Recently Issued Accounting Pronouncements
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes: Simplifying the Accounting for Income Taxes. This guidance removes certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences. This guidance also clarifies and simplifies other areas of ASC 740. This ASU will be effective for public companies for fiscal years, and interim periods within those fiscal years beginning after December 15, 2020. Early adoption is permitted. Certain amendments in this update must be applied on a prospective basis, certain amendments must be applied on a retrospective basis, and certain amendments must be applied on a modified retrospective basis through a cumulative effect adjustment to retained earnings/(deficit) in the period of adoption. The Company does not expect this guidance to have a significant impact on its financial statements.
3. Net Assets in Liquidation
Upon adoption of the Liquidation Basis on September 1, 2020, the Company estimated the net assets in liquidation, which represents the expected future cash flows related to its remaining assets, liabilities and operating costs through dissolution. The actual cash inflows and outflows may differ materially from the estimated amounts.
| | | | | | | | |
| (in thousands) | | |
| Consolidated Net Equity, as of August 31, 2020 | | $ | 425,214 | |
| | |
| Effect of adopting the liquidation basis of accounting: | | |
Change in the estimated value of royalty rights (1) | | 13,770 | |
Change in the receivable from the sale of Noden (2) | | 9,056 | |
Increase in intangible assets (3) | | 28,702 | |
Change in the estimated value of other assets (4) | | (4,813) | |
Estimated liquidation and future operating costs (5) | | (25,376) | |
| Total effect of adopting the liquidation basis of accounting | | 21,339 | |
| | |
| Net assets in liquidation, as of September 1, 2020 | | $ | 446,553 | |
_______________
(1) The royalty rights consist of Assertio and University of Michigan (“U-M”). The Assertio royalties are valued using undiscounted estimated cash receipts until the estimated date of sale of June 30, 2021, plus a discounted value of the remaining estimated cash flows as an estimate of the expected cash consideration from the sale of these royalty rights at this time. The Company expects it will retain the royalty rights for the U-M royalty asset until its expiration in September 2022. As such, it is valued as the sum of its undiscounted cash receipts until the end of the agreement. Previously, under the Going Concern Basis, royalty rights were valued using discounted cash flow models, see Note 8, Fair Value Measurements.
| | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | August 31, 2020 | | September 1, 2020 | | Change |
| | (Going Concern Basis) | | (Liquidation Basis) | | |
| | | | | | |
| Assertio | | $ | 200,463 | | | $ | 211,626 | | | $ | 11,163 | |
| U-M | | 17,450 | | | 20,057 | | | 2,607 | |
| Total | | $ | 217,913 | | | $ | 231,683 | | | $ | 13,770 | |
(2) Adjustments reflect Liquidation Basis which does not discount future estimated cash receipts. Previously, under the Going Concern Basis we had estimated the fair value of Noden, as an asset held for sale, using a discounted cash flow model, see Note 8, Fair Value Measurements.
(3) The increase in intangible assets represents the difference between the existing assets and liabilities of LENSAR upon adoption of Liquidation Basis and its enterprise value that was distributed to PDL shareholders in the spin-off of LENSAR on October 1, 2020. The enterprise value was determined through an analysis of comparable public companies combined with cash flow forecasts.
(4) Adjustments to other assets include a liquidity discount for the Evofem warrants and the write-off of certain assets that will not be converted to cash such as prepaid expenses, fixed assets and right of use assets.
(5) Represents estimated future expenses related to operating the business through dissolution and settlement of future liabilities. Amounts include estimated compensation, legal and other professional fees, insurance, taxes, estimated costs to dispose of our assets and other miscellaneous expenses. Certain of the estimated costs to dispose of our assets had already been accrued under the Going Concern Basis and were presented net within our assets held for sale.
4. Discontinued Operations Classified as Assets Held for Sale
As a result of the above-described monetization strategy and subsequent efforts to monetize the Company’s key assets, representing a strategic shift in the operations of the Company, the assets held for sale and discontinued operations criteria were met for the Company’s royalty assets (included in the Income Generating Assets segment) and its Noden subsidiaries (Pharmaceutical segment) during the first quarter of 2020. The discontinued operations criteria were met for the Company’s investment in Evofem (Strategic Positions segment) during the second quarter of 2020 after the Company distributed all of its shares of common stock of Evofem to the Company’s stockholders. The historical financial results of the investment in Evofem, royalty assets and Noden are reflected in the Company’s consolidated financial statements as discontinued operations for all Going Concern Basis periods presented herein, and assets and liabilities were retrospectively reclassified as assets and liabilities held for sale.
On July 30, 2020, the Company signed a definitive agreement for the sale of the Company’s interest in Noden DAC and Noden USA to CAT Capital Bidco Limited (“Stanley Capital”). In accordance with the terms of the agreement, the Company expects to receive consideration of up to $52.8 million. Stanley Capital made an initial cash payment to the Company of $12.2 million on September 9, 2020, the closing date of the transaction. The Company is also entitled to recover $0.5 million related to value-added tax (“VAT”) for inventory purchases from Novartis. The agreement provides for an additional $33.0 million to be paid to the Company in 12 equal quarterly installments from January 2021 to October 2023. An additional $3.9 million will be paid in 4 equal quarterly installments from January 2023 to October 2023. The agreement also provides for the potential for additional contingent payments to the Company. The Company is entitled to receive $2.5 million upon Stanley Capital or any of its affiliates entering into a binding agreement for a specified transaction within one year of the closing date. The Company is also entitled to 50% of a license fee from a third party distributor within 10 days of receipt by Noden. Upon closing, the Company recorded a gain of $0.2 million. In connection with the closing of the transaction, the guaranty agreement between Novartis and the Company, which guaranteed certain payments owed to Novartis by Noden, was terminated. As of December 31, 2020, under the Liquidation Basis, the remaining receivable from the sale of Noden was $39.4 million and is included in “Receivables from asset sales” in the Statement of Net Assets in Liquidation. Refer to Note 3, Net Assets in Liquidation, for a discussion of the valuation of the Noden asset sale receivable under the Liquidation Basis.
On August 31, 2020, the Company announced the signing and closing of a definitive agreement for the sale of its royalty interests for Kybella®, Zalviso®, and Coflex® to SWK Funding, LLC, a wholly owned subsidiary of SWK Holdings Corporation, for $4.35 million in cash.
Components of amounts reflected in Loss from discontinued operations are as follows:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands) | | 2020 | | 2019 | | 2018 |
| Revenues | | | | | | |
| Product revenue, net | | $ | 29,479 | | | $ | 55,093 | | | $ | 80,796 | |
| Royalty rights - change in fair value | | (8,804) | | | (31,042) | | | 85,287 | |
| Total revenues | | 20,675 | | | 24,051 | | | 166,083 | |
| Operating expenses | | | | | | |
| Cost of product revenue (excluding intangible asset amortization and impairment) | | 17,576 | | | 36,343 | | | 34,906 | |
| Amortization of intangible assets | | 389 | | | 5,016 | | | 14,536 | |
| General and administrative | | 6,105 | | | 7,264 | | | 11,720 | |
| Sales and marketing | | 257 | | | 1,675 | | | 10,800 | |
| Research and development | | 0 | | | (41) | | | 196 | |
| Impairment of intangible assets | | 0 | | | 22,490 | | | 152,330 | |
| Change in fair value of anniversary payment and contingent consideration | | 0 | | | 0 | | | (42,000) | |
| Total operating expenses | | 24,327 | | | 72,747 | | | 182,488 | |
| Operating loss from discontinued operations | | (3,652) | | | (48,696) | | | (16,405) | |
| Non-operating (expense) income, net | | | | | | |
| Equity affiliate - change in fair value | | (25,365) | | | 36,402 | | | 0 | |
| Loss on classification as held for sale | | (28,904) | | | 0 | | | 0 | |
| Total non-operating (expense) income, net | | (54,269) | | | 36,402 | | | 0 | |
| Loss from discontinued operations before income taxes | | (57,921) | | | (12,294) | | | (16,405) | |
| Income tax (benefit) expense from discontinued operations | | (23,006) | | | 1,303 | | | 19,689 | |
| Loss from discontinued operations | | $ | (34,915) | | | $ | (13,597) | | | $ | (36,094) | |
| | | | | | |
| | | | | | |
The carrying amounts of the major classes of assets reported as “Assets held for sale” on the Company’s Consolidated Balance Sheet consisted of the following:
| | | | | | | | | | |
| (in thousands) | | | | December 31, 2019 |
| | | | |
| Cash and cash equivalents | | | | $ | 24,469 | |
| Accounts receivable, net | | | | 6,993 | |
| Inventory | | | | 31,712 | |
| Prepaid and other current assets | | | | 7,192 | |
| Property and equipment, net | | | | 2,960 | |
| Royalty rights - at fair value | | | | 266,196 | |
| Investment in equity affiliate | | | | 82,267 | |
| Intangible assets, net | | | | 10,112 | |
| Other assets | | | | 15,956 | |
| Total assets held for sale | | | | $ | 447,857 | |
The carrying amounts of the major classes of liabilities reported as “Liabilities held for sale” on the Company’s Consolidated Balance Sheet consisted of the following:
| | | | | | | | | | |
| (in thousands) | | | | December 31, 2019 |
| | | | |
| Accounts payable | | | | $ | 14,695 | |
| Accrued liabilities | | | | 16,400 | |
| Other long-term liabilities | | | | 120 | |
| Total liabilities held for sale | | | | $ | 31,215 | |
5. Investment in Evofem Biosciences, Inc.
On April 10, 2019, the Company entered into a securities purchase agreement with Evofem and two other purchasers, pursuant to which the Company purchased $60.0 million of Evofem securities in a private placement. The transaction was structured in two tranches.
The first tranche closed on April 11, 2019, pursuant to which the Company invested $30.0 million to purchase 6,666,667 shares of Evofem common stock at $4.50 per share and was also issued warrants to purchase up to 1,666,667 shares of Evofem common stock. The warrants are exercisable beginning six months after the issuance date for a period of seven years from the issuance date at an exercise price of $6.38 per share.
The second tranche closed on June 10, 2019, pursuant to which the Company invested an additional $30.0 million to purchase an additional 6,666,667 shares of Evofem common stock at $4.50 per share and was also issued warrants to purchase up to an additional 1,666,667 shares of Evofem common stock with the same terms as the warrants issued in the first tranche.
On May 21, 2020, the Company announced that, pursuant to its Plan of Liquidation, it had completed a liquidation distribution of all 13,333,334 shares of common stock of Evofem it owned to the Company’s stockholders, which represented approximately 26.7% of the outstanding shares of Evofem common stock as of the close of business on May 15, 2020. The distribution was recorded as a noncash distribution of $64.4 million, reducing retained earnings.
Following the distribution, the Company continues to hold warrants to purchase up to 3,333,334 shares of Evofem common stock. As of December 31, 2020, the Evofem warrants were valued under the Liquidation Basis at $1.8 million and are classified as “Other assets” on the Company’s Consolidated Statement of Net Assets. The Evofem common stock and the Evofem warrants are included in “Long-term assets held for sale” on the Company’s December 31, 2019 Consolidated Balance Sheet. See to Note 3, Net Assets in Liquidation and Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
For the eight months ended August 31, 2020, the Company recorded a loss of $25.4 million on its investment in Evofem included in Loss from discontinued operations before income taxes on the Company’s Consolidated Statement of Operations, of which $17.9 million was related to the realized loss from the change in fair value of the Evofem common stock and $7.5 million was related to the unrealized loss from the change in fair value of the Evofem warrants.
For the year ended December 31, 2019, the Company had an unrealized gain of $36.4 million on its investment in Evofem, of which $31.6 million was related to the change in fair value of the Evofem common stock and $4.8 million was related to the change in fair value of the Evofem warrants.
6. Cash and Cash Equivalents
As of December 31, 2020 and 2019, the Company had invested its excess cash balances primarily in cash and money market funds. The fair values of cash equivalents approximate their carrying values due to the short-term nature of such financial instruments.
The following table summarizes the Company’s cash and cash equivalents by significant investment category reported as cash and cash equivalents on the Consolidated Statement of Net Assets and the Consolidated Balance Sheet as of December 31, 2020 and 2019, respectively:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | | | | | | | | | December 31, 2020 | | December 31, 2019 (1) (2) | | | | | | |
| | | | | | | | | | (Liquidation Basis) | | (Going Concern Basis) | | | | | | |
| Cash | | | | | | | | | | $ | 75,681 | | | $ | 37,718 | | | | | | | |
| Money market funds | | | | | | | | | | 51,161 | | | 131,264 | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| Total | | | | | | | | | | $ | 126,842 | | | $ | 168,982 | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
________________
(1) The amounts exclude $24.5 million of cash at Noden classified as held for sale as of December 31, 2019. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
(2) The table above includes amounts held by LENSAR as of December 31, 2019. LENSAR was spun-off on October 1, 2020.
The Company recognized approximately $0.8 million of gains on sales of available-for-sale securities in the year ended December 31, 2018. As of December 31, 2020 and 2019, the Company did not have any available-for-sale securities.
7. Inventories
Inventories under the Going Concern Basis consisted of the following:
| | | | | | | | | | |
| (in thousands) | | | | December 31, 2019 |
| | | | |
| Raw materials | | | | $ | 3,739 | |
| Work in process | | | | 1,170 | |
| Finished goods | | | | 3,152 | |
Total inventories (1) (2) | | | | $ | 8,061 | |
________________
(1) The amounts exclude $31.7 million of inventory at Noden classified as held for sale as of December 31, 2019. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information. Noden was sold in September 2020.
(2) The table above includes amounts held by LENSAR as of December 31, 2019. LENSAR was spun-off on October 1, 2020.
8. Fair Value Measurements
The fair value of the Company’s financial instruments under the Going Concern Basis are estimates of the amounts that would be received if the Company were to sell an asset or pay to transfer a liability (exit price) in an orderly transaction between market participants at the measurement date. The assets and liabilities are categorized and disclosed in one of the following three categories:
Level 1 – based on quoted market prices in active markets for identical assets and liabilities;
Level 2 – based on observable inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and
Level 3 – based on unobservable inputs using management’s best estimate and assumptions when inputs are unavailable.
Assets/Liabilities Measured and Recorded at Fair Value on a Recurring Basis
The following table presents the fair value of the Company’s financial instruments measured at fair value on a recurring basis by level within the valuation hierarchy under the Going Concern Basis:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | December 31, 2019 |
| (in thousands) | | | | | | | | | | Level 1 | | Level 2 | | Level 3 | | Total |
| Financial assets: | | | | | | | | | | | | | | | | |
| Money market funds | | | | | | | | | | $ | 131,264 | | | $ | 0 | | | $ | 0 | | | $ | 131,264 | |
Corporate securities (1) | | | | | | | | | | 82,267 | | | 0 | | | 0 | | | 82,267 | |
Warrants (2) | | | | | | | | | | 0 | | | 14,152 | | | 0 | | | 14,152 | |
Royalty rights - at fair value (3) | | | | | | | | | | 0 | | | 0 | | | 266,196 | | | 266,196 | |
| Total | | | | | | | | | | $ | 213,531 | | | $ | 14,152 | | | $ | 266,196 | | | $ | 493,879 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
___________________
(1) Corporate securities are classified as “Long-term assets held for sale” on the December 31, 2019 Consolidated Balance Sheet.
(2) Warrants consist of Evofem warrants, which are classified as “Long-term assets held for sale” and CareView Communications, Inc. (“CareView”) warrants, classified as “Other assets” on the Consolidated Balance Sheet as of December 31, 2019.
(3) Royalty rights are classified as “Long-term assets held for sale” on the Consolidated Balance Sheet as of December 31, 2019.
Money Market Funds - The fair values of cash equivalents approximate their carrying values due to the short-term nature of such financial instruments.
Corporate Securities - Corporate securities consisted of common stock shares of Evofem. For additional information, see Note 4, Discontinued Operations Classified as Assets Held for Sale, and Note 5, Investment in Evofem Biosciences, Inc.
Warrants - Warrants consist of rights to purchase shares of common stock in Evofem and CareView, see Note 4, Discontinued Operations Classified as Assets Held for Sale, Note 5, Investment in Evofem Biosciences, Inc., and Note 9, Notes and Other Long-Term Receivables. The fair value of the warrants is based upon recently quoted market prices of the underlying equity security and the Black-Scholes option pricing model and adjusted by an estimated discount to sell.
Royalty Rights - At Fair Value
During the quarter ended March 31, 2020, it was determined that the Company’s royalty rights assets met the criteria as an asset held for sale, see Note 4, Discontinued Operations Classified as Assets Held for Sale. Assets classified as held for sale are reported at the lower of their carrying value or fair value less costs to sale under the Going Concern Basis. The Company historically accounted for such royalty rights assets at fair value, which, as discussed below, primarily reflected the expected future cash to be received but did not consider the expected costs to sell the assets. The Company’s royalty rights assets are comprised of several separate and distinct royalty rights.
Assertio (Depomed) Royalty Agreement
On October 18, 2013, the Company entered into the Royalty Purchase and Sale Agreement (the “Assertio Royalty Agreement”) with Assertio Therapeutics, Inc. (formerly known as Depomed, Inc.), and Depo DR Sub, LLC (together, “Assertio”), whereby the Company acquired the rights to receive royalties and milestones payable on sales of five Type 2 diabetes products licensed by Assertio in exchange for a $240.5 million cash payment. Total consideration was $241.3 million, which was comprised of the $240.5 million cash payment to Assertio and $0.8 million in transaction costs.
The rights acquired included Assertio’s royalty and milestone payments accruing from and after October 1, 2013: (a) from Santarus, Inc., which was subsequently acquired by Salix Pharmaceuticals, Inc., which itself was acquired by Valeant Pharmaceuticals International, Inc. (“Valeant”), which, in July 2018, changed its name to Bausch Health Companies Inc. (“Bausch Health”) with respect to sales of Glumetza (metformin HCL extended-release tablets) in the United States; (b) from Merck & Co., Inc. with respect to sales of Janumet® XR (sitagliptin and metformin HCL extended-release tablets); (c) from Janssen Pharmaceutica N.V. with respect to potential future development milestones and sales of its approved fixed-dose combination of Invokana® (canagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor) and extended-release metformin tablets, marketed as Invokamet XR®; (d) from Boehringer Ingelheim and Eli Lilly (“Lilly”) and Company with respect to
potential future development milestones and sales of the investigational fixed-dose combinations of drugs and extended-release metformin subject to Assertio’s license agreement with Boehringer Ingelheim, including its approved products, Jentadueto XR® and Synjardy XR®; and (e) from LG Chem, Ltd. and Bausch Health for sales of extended-release metformin tablets in Korea and Canada, respectively.
The Company determined that its royalty purchase interest in Depo DR Sub, LLC represented a variable interest in a variable interest entity. However, the Company did not have the power to direct the activities of Depo DR Sub, LLC that most significantly impact Depo DR Sub, LLC’s economic performance and was not the primary beneficiary of Depo DR Sub, LLC; therefore, Depo DR Sub, LLC was not subject to consolidation by the Company.
On August 2, 2018, PDL Investment Holding, LLC (“PDLIH”), a wholly-owned subsidiary of the Company and assignee from the Company under the Assertio Royalty Agreement, entered into an amendment to the Assertio Royalty Agreement with Assertio. Pursuant to the amendment, PDLIH purchased all of Assertio’s remaining interests in royalty and milestone payments payable on sales of Type 2 diabetes products licensed by Assertio for $20.0 million. Prior to the amendment, the Assertio Royalty Agreement provided that the Company would have received all royalty and milestone payments due under license agreements between Assertio and its licensees until the Company received payments equal to two times the cash payment it made to Assertio, or approximately $481.0 million, after which all net payments received by Assertio would have been shared equally between the Company and Assertio. Following the amendment, the Assertio Royalty Agreement provides that the Company will receive all royalty and milestone payments due under the license agreements between Assertio and its licensees. After the amendment, the Company elected to continue to follow the fair value option and carry the financial asset at fair value.
The Assertio Royalty Agreement terminates on the third anniversary following the date upon which the later of the following occurs: (a) October 25, 2021, or (b) at such time as no royalty payments remain payable under any license agreement and each of the license agreements has expired by its terms.
As of December 31, 2018, in conjunction with the amendment described above, the Company was provided the power to direct the activities of Depo DR Sub, LLC and is the primary beneficiary of Depo DR Sub, LLC; therefore, Depo DR Sub, LLC is subject to consolidation by the Company. As of December 31, 2020, Depo DR Sub, LLC did not have any assets or liabilities of value for consolidation with the Company.
The financial asset acquired represents a single unit of accounting. Under the Going Concern Basis, this financial asset is classified as a Level 3 asset as of December 31, 2019 within the fair value hierarchy, as the Company’s valuation utilized significant unobservable inputs, including estimates as to the probability and timing of future commercialization for products not yet approved by regulatory agencies outside of the United States. The estimated fair value was determined by using a discounted cash flow analysis related to the expected amount and timing of future cash flows to be generated by each licensed product. The discounted cash flows were based upon expected royalties from sales of licensed products over approximately a nine-year period. Significant judgment is required in selecting appropriate discount rates. The discount rates utilized range from 10% to 24%.
As of December 31, 2020, under the Liquidation Basis, the expected cash realizable value of the Assertio royalty asset was $204.5 million and included in the “Royalty assets” in the Statement of Net Assets in Liquidation. Refer to Note 3, Net Assets in Liquidation, for a discussion of the valuation of Assertio under the Liquidation Basis.
Viscogliosi Brothers Royalty Agreement
On June 26, 2014, the Company entered into a Royalty Purchase and Sale Agreement (the “VB Royalty Agreement”) with Viscogliosi Brothers, LLC (“VB”) whereby VB conveyed to the Company the right to receive royalties payable on sales of a spinal implant that received pre-market approval from the FDA held by VB and commercialized by Paradigm Spine, LLC (“Paradigm Spine”) in exchange for a $15.5 million cash payment, less fees. Paradigm Spine was acquired in March 2019 by RTI Surgical Holdings, Inc.
The royalty rights acquired included royalties accruing from and after April 1, 2014. Under the terms of the VB Royalty Agreement, the Company was eligible to receive all royalty payments due to VB pursuant to certain technology transfer agreements between VB and Paradigm Spine until the Company received payments equal to 2.3 times the cash payment made to VB, after which all rights to receive royalties would be returned to VB. VB’s ability to repurchase the royalty right for a specified amount expired on June 26, 2018.
In August 2020, the Company sold the royalty rights to a third-party for $4.2 million. NaN gain or loss was recognized on the date of sale due to an adjustment to the royalty rights fair value in the prior quarter that was informed by bids received.
University of Michigan Royalty Agreement
On November 6, 2014, the Company acquired a portion of all royalty payments of the U-M worldwide royalty interest in Cerdelga® (eliglustat) for $65.6 million pursuant to the Royalty Purchase and Sale Agreement with U-M (the “U-M Royalty Agreement”). Under the terms of the U-M Royalty Agreement, the Company receives 75% of all royalty payments due under the U-M license agreement with Genzyme Corporation, a Sanofi company (“Genzyme”) until expiration of the licensed patents, excluding any patent term extension. Cerdelga, an oral therapy for adult patients with Gaucher disease type 1, was developed by Genzyme. Cerdelga was approved in the United States in August 2014, in the European Union (“EU”) in January 2015, and in Japan in March 2015. In addition, marketing applications for Cerdelga are under review by other regulatory authorities. While marketing applications have been approved in the United States, the EU and Japan, national pricing and reimbursement decisions are delayed in some countries.
The estimated fair value of the royalty right at December 31, 2019, was determined by using a discounted cash flow analysis related to the expected future cash flows to be received. Under the Going Concern Basis, this asset was classified as a Level 3 asset as the Company’s valuation utilized significant unobservable inputs, including estimates as to the probability and timing of future sales of the licensed product. The estimated fair value of the financial asset acquired was determined by using a discounted cash flow analysis related to the expected amount and timing of future cash flows. The discounted cash flow was based upon expected royalties from sales of licensed product over approximately a three-year period. Significant judgment is required in selecting the appropriate discount rate. The discount rate utilized was approximately 12.8%.
As of December 31, 2020, under the Liquidation Basis, the expected cash realizable value of the Cerdelga royalty asset was $15.5 million and included in the “Royalty assets” in the Statement of Net Assets in Liquidation. Refer to Note 3, Net Assets in Liquidation, for a discussion of the valuation of the U-M Royalty Agreement under the Liquidation Basis.
AcelRx Royalty Agreement
On September 18, 2015, the Company entered into a royalty interest assignment agreement (the “AcelRx Royalty Agreement”) with ARPI LLC, a wholly-owned subsidiary of AcelRx Pharmaceuticals, Inc. (“AcelRx”), whereby the Company acquired the rights to receive a portion of the royalties and certain milestone payments on sales of Zalviso® (sufentanil sublingual tablet system) in the EU, Switzerland and Australia by AcelRx’s commercial partner, Grünenthal, in exchange for a $65.0 million cash payment. Under the terms of the AcelRx Royalty Agreement, the Company was eligible to receive 75% of all royalty payments and 80% of the first four commercial milestone payments due under AcelRx’s license agreement with Grünenthal until the earlier to occur of (i) receipt by the Company of payments equal to three times the cash payments made to AcelRx and (ii) the expiration of the licensed patents. Zalviso received marketing approval by the European Commission in September 2015. Grünenthal launched Zalviso in the second quarter of 2016 and the Company started to receive royalties in the third quarter of 2016. On May 15, 2020, AcelRx received notice that the product marketer of Zalviso, Grünenthal GmbH, would exercise its right to terminate the license agreement with AcelRx, effective as of 180 days from the date of the notice. AcelRx is obligated to use commercially reasonable efforts to find a new license agreement under the terms no less favorable than those in the license with Grünenthal.
In August 2020, the Company sold the asset to a third-party for 0 consideration. NaN gain or loss was recognized on the date of sale, due to an adjustment to the royalty rights fair value in the prior quarter that resulted from the notification that the license agreement was terminated by the marketer of the product.
Kybella Royalty Agreement
On July 8, 2016, the Company entered into a royalty purchase and sales agreement with an individual, whereby the Company acquired that individual’s rights to receive certain royalties on sales of KYBELLA® by Allergan plc in exchange for a $9.5 million cash payment and up to $1.0 million in future milestone payments based upon product sales targets. The Company started to receive royalty payments during the third quarter of 2016.
In August 2020, the Company sold the asset to a third-party for $0.2 million. NaN gain or loss was recognized on the date of sale, due to an adjustment to the royalty rights fair value in the prior quarter that was informed by bids received.
The following tables summarize the changes in Level 3 Royalty Right Assets and the gains and losses included in earnings for the eight months ended August 31, 2020 under the Going Concern Basis:
| | | | | | | | | | | | | | | | | | | | |
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) - Royalty Right Assets |
| | | | | | |
| (in thousands) | | | Royalty Rights
- At Fair Value |
| Fair value as of December 31, 2019 | | | $ | 266,196 | |
| | | | | | |
| | | | |
| | | | |
| Total net change in fair value for the period | | | |
| | Change in fair value of royalty rights - at fair value | | (8,804) | | |
| | Cash received from royalty rights | | (35,129) | | |
| | | Total net change in fair value for the period | | | (43,933) | |
| Sale of royalty rights | | | (4,350) | |
| | | | | | |
| Fair value as of August 31, 2020 | | | $ | 217,913 | |
The table above does not include the aggregate remaining estimated cost to sell the royalty right assets of $4.6 million.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) - Royalty Right Assets |
| | Fair Value as of | | Sale of | | Royalty Rights - | | Fair Value as of |
| (in thousands) | | December 31, 2019 | | Royalty Rights (1) | | Change in Fair Value | | August 31, 2020 (2) |
| Assertio | | $ | 218,672 | | | $ | 0 | | | $ | (18,209) | | | $ | 200,463 | |
| VB | | 13,590 | | | (4,182) | | | (9,408) | | | 0 | |
| U-M | | 20,398 | | | 0 | | | (2,948) | | | 17,450 | |
| | | | | | | | |
| AcelRx | | 12,952 | | | 0 | | | (12,952) | | | 0 | |
| | | | | | | | |
| KYBELLA | | 584 | | | (168) | | | (416) | | | 0 | |
| | $ | 266,196 | | | $ | (4,350) | | | $ | (43,933) | | | $ | 217,913 | |
_______________
(1) In August 2020 the Company sold the royalty rights to VB, AcelRx, and KYBELLA to a third-party.
(2) Excludes the aggregate remaining estimated costs to sell of $4.6 million.
Gains and losses from changes in Level 3 assets are included in earnings under the Going Concern Basis and are presented as “Royalty rights - change in fair value” as follows:
| | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands) | | 2020 | | 2019 |
| | | | |
| | | | |
| Total change in fair value for the period included in earnings for royalty right assets held at the end of the reporting period | | $ | (8,804) | | | $ | (31,042) | |
| | | | |
| | | | |
Assets/Liabilities Measured and Recorded at Fair Value on a Nonrecurring Basis
The Company remeasures the fair value of certain assets and liabilities upon the occurrence of certain events. Such assets consist of long-lived assets, including property and equipment and intangible assets and the shares of AEON common stock, received in connection with the loans made to LENSAR by the Company prior to its acquisition of LENSAR.
The Company’s carrying value of the 1.7 million shares of AEON common stock as of December 31, 2019 under the Going Concern Basis was $6.6 million based on an estimated per share value of $3.84, which was established by a valuation performed when the shares were acquired. The value of the Company’s investment in AEON is not readily determinable as AEON’s shares are not publicly traded. Under the Going Concern Basis, the Company evaluated the fair value of this investment by performing a qualitative assessment each reporting period. If the results of this qualitative assessment indicate that the fair value was less than
the carrying value, the investment is written down to its fair value. Based on additional financial information received from AEON, the Company performed an analysis on August 31, 2020 and concluded the investment was impaired and wrote it down to $1.0 million. The loss is reported as Loss on investment on the Company’s Consolidated Statement of Operations for the eight months ended August 31, 2020. This investment is included in Other Assets on the Company’s Consolidated Statement of Net Assets as of September 30, 2020 and as Other long-term assets on the December 31, 2019 Consolidated Balance Sheet. For additional information on the AEON investment, see Note 9, Notes and Other Long-Term Receivables. As of December 31, 2020, under the Liquidation Basis, the expected cash realizable value of the AEON asset was $1.0 million and included in “Other assets” on the Statement of Net Assets in Liquidation. Refer to Note 3, Net Assets in Liquidation, for a discussion of the valuation of AEON under the Liquidation Basis.
During the quarter ended March 31, 2020, it was determined that Noden met the criteria as an asset held for sale. As a result of the Company’s analysis of the fair value of Noden, the Company recorded a loss on classification as held for sale of $6.7 million during the quarter ended March 31, 2020 of which $1.8 million related to the estimated costs to sell Noden and $4.9 million related to the difference in carrying value versus fair value. The fair value calculation was made using a discounted cash flow model, utilizing a discount rate of approximately 19%, and included level 3 inputs. During the quarter ended June 30, 2020, the Company recorded an additional loss of $16.8 million related primarily to the difference in carrying value and fair value. The reduction in fair value reflected lower estimated sales proceeds as informed by the Company’s sales process. At June 30, 2020, the fair value calculation was made using a discounted cash flow model, utilizing a discount rate of approximately 17%, and included level 3 inputs. For information on the sale of the business in September 2020, see Note 4, Discontinued Operations Classified as Assets Held for Sale.
Assets/Liabilities Not Subject to Fair Value Recognition
The following tables present the fair value of assets and liabilities not subject to fair value recognition by level within the valuation hierarchy:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | December 31, 2019 |
| (in thousands) | | | | | | | | Carrying Value | | Fair Value
Level 2 | | Fair Value
Level 3 |
| Assets: | | | | | | | | | | | | |
| Wellstat Diagnostics note receivable | | | | | | | | $ | 50,191 | | | $ | — | | | $ | 55,389 | |
| Hyperion note receivable | | | | | | | | 1,200 | | | 0 | | | 1,200 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| CareView note receivable | | | | | | | | 690 | | | 0 | | | 690 | |
| Total | | | | | | | | $ | 52,081 | | | $ | 0 | | | $ | 57,279 | |
| Liabilities: | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| December 2021 Notes | | | | | | | | $ | 16,950 | | | $ | 20,978 | | | $ | 0 | |
| December 2024 Notes | | | | | | | | 10,300 | | | 12,953 | | | 0 | |
| | | | | | | | | | | | |
| Total | | | | | | | | $ | 27,250 | | | $ | 33,931 | | | $ | 0 | |
There were 0 impairment losses on notes receivable in the eight months ended August 31, 2020. During the years ended December 31, 2019 and 2018 the Company recorded impairment losses of $10.8 million and $8.2 million, respectively, for the note receivable with CareView.
The Company had 2 notes receivable assets as of December 31, 2019 under the Going Concern Basis. The notes receivable were classified under the Going Concern Basis as Level 3 in the fair value hierarchy as the Company’s valuations utilized significant unobservable inputs, including estimates of future revenues, discount rates, expectations about settlement, terminal values, required yield and the value of underlying collateral. The Company engages third-party valuation experts when deemed necessary to assist in evaluating its investments and the related inputs needed to estimate the fair value of certain investments.
As of December 31, 2019 under the Going Concern Basis, the estimated fair value of the CareView note receivable was determined using a liquidation analysis. A liquidation analysis considers the asset side of the balance sheet and adjusts the value in accordance with the relative risk associated with the asset and the probable liquidation value. The asset recovery rates varied by asset. As of December 31, 2019 under the Going Concern Basis, the estimated fair value of the Wellstat Diagnostics note receivable was determined by using an asset approach and discounted cash flow model related to the underlying collateral and adjusted to consider estimated costs to sell the asset.
The Company’s liabilities not subject to fair value recognition under the Going Concern Basis consist of its 2021 and 2024 convertible notes. The fair values of the Company’s convertible senior notes were determined using quoted market pricing and were classified as Level 2 in the fair value hierarchy.
The following table represents significant unobservable inputs used in determining the estimated fair value of the Wellstat Diagnostics note receivable investment under the Going Concern Basis:
| | | | | | | | | | | | | | | | | | | | | | |
| Asset | | Valuation
Technique | | Unobservable
Input | | December 31,
2019 | | |
| | | | | | | | |
| Wellstat Diagnostics | | | | | | | | |
| Wellstat Guarantors intellectual property | | Income Approach | | | | | | |
| | | | Discount rate | | 12% | | |
| | | | Undiscounted royalty amount | | $21 million | | |
| Settlement Amount | | Income Approach | | | | | | |
| | | | Discount rate | | 15% | | |
| | | | Undiscounted settlement amount | | $28 million | | |
| Real Estate Property | | Market Approach | | | | | | |
| | | | Annual appreciation rate | | 0% | | |
| | | | Estimated realtor fee | | 6% | | |
| | | | Undiscounted market value | | $16 million | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
9. Notes and Other Long-Term Receivables
Notes and other long-term receivables included the following significant agreements:
Wellstat Diagnostics Note Receivable and Credit Agreement and Related Litigation
On November 2, 2012, the Company and Wellstat Diagnostics entered into a $40.0 million credit agreement pursuant to which the Company was to accrue quarterly interest payments at the rate of 5% per annum (payable in cash or in kind). In addition, the Company was to receive quarterly royalty payments based on a low double-digit royalty rate of Wellstat Diagnostics’ net revenues, generated by the sale, distribution or other use of Wellstat Diagnostics’ products, if any, commencing upon the commercialization of its products. A portion of the proceeds of the $40.0 million credit agreement were used to repay certain notes receivable which Wellstat Diagnostics entered into in March 2012.
In January 2013, the Company was informed that, as of December 31, 2012, Wellstat Diagnostics had used funds contrary to the terms of the credit agreement and breached Sections 2.1.2 and 7 of the credit agreement. The Company sent Wellstat Diagnostics a notice of default on January 22, 2013, and accelerated the amounts owed under the credit agreement. In connection with the notice of default, the Company exercised one of its available remedies and transferred approximately $8.1 million of available cash from a bank account of Wellstat Diagnostics to the Company and applied the funds to amounts due under the credit agreement. On February 28, 2013, the parties entered into a forbearance agreement whereby the Company agreed to refrain from exercising additional remedies for 120 days. During such forbearance period, the Company provided approximately $1.3 million to Wellstat Diagnostics to fund ongoing operations of the business. During the year ended December 31, 2013, approximately $8.7 million was advanced pursuant to the forbearance agreement.
On August 15, 2013, the Company entered into an amended and restated credit agreement with Wellstat Diagnostics. The Company determined that the new agreement should be accounted for as a modification of the existing agreement.
Except as otherwise described herein, the material terms of the amended and restated credit agreement were substantially the same as those of the original credit agreement, including quarterly interest payments at the rate of 5% per annum (payable in cash or in kind). In addition, the Company was to continue to receive quarterly royalty payments based on a low double-digit royalty rate of Wellstat Diagnostics’ net revenues. However, pursuant to the amended and restated credit agreement: (i) the principal amount was reset to approximately $44.1 million, which was comprised of approximately $33.7 million original loan principal and interest, $1.3 million term loan principal and interest and $9.1 million forbearance principal and interest; (ii) the specified internal rates of return increased; (iii) the default interest rate was increased; (iv) Wellstat Diagnostics’ obligation to provide certain financial
information increased in frequency to monthly; (v) internal financial controls were strengthened by requiring Wellstat Diagnostics to maintain an independent, third-party financial professional with control over fund disbursements; (vi) the Company waived the existing events of default; and (vii) the owners and affiliates of Wellstat Diagnostics were required to contribute additional capital to Wellstat Diagnostics upon the sale of an affiliate entity. The amended and restated credit agreement had an ultimate maturity date of December 31, 2021 (but has subsequently been accelerated as described below).
In June 2014, the Company received information from Wellstat Diagnostics showing that it was generally unable to pay its debts as they became due, constituting an event of default under the amended and restated credit agreement.
On August 5, 2014, the Company delivered a notice of default to Wellstat Diagnostics, which accelerated all obligations under the amended and restated credit agreement and demanded immediate payment in full in an amount equal to approximately $53.9 million, (which amount, in accordance with the terms of the amended and restated credit agreement, included an amount that, together with interest and royalty payments already made to the Company, would generate a specified internal rate of return to the Company), plus accruing fees, costs and interest, and demanded that Wellstat Diagnostics protect and preserve all collateral securing its obligations.
On August 7, 2014, the Company delivered a notice to each of the guarantors of Wellstat Diagnostics’ obligations to the Company (collectively, the “Wellstat Diagnostics Guarantors”) under the credit agreement, which included a demand that the guarantors remit payment to the Company in the amount of the outstanding obligations. The guarantors include certain affiliates and related companies of Wellstat Diagnostics, including Wellstat Therapeutics and Wellstat Diagnostics’ stockholders.
On September 24, 2014, the Company filed an ex-parte petition for appointment of a receiver with the Circuit Court of Montgomery County, Maryland, which was granted on the same day. Wellstat Diagnostics remained in operation during the period of the receivership with incremental additional funding from the Company. On May 24, 2017, Wellstat Diagnostics transferred substantially all of its assets to the Company pursuant to a credit bid. The credit bid reduced the outstanding balance of the loan by an immaterial amount.
On September 4, 2015, the Company filed in the Supreme Court of New York a motion for summary judgment in lieu of complaint which requested that the court enter judgment against certain of the Wellstat Diagnostics Guarantors for the total amount due on the Wellstat Diagnostics debt, plus all costs and expenses including lawyers’ fees incurred by the Company in enforcement of the related guarantees. On September 23, 2015, the Company filed in the same court an ex parte application for a temporary restraining order and order of attachment of the Wellstat Diagnostics Guarantor defendants’ assets. Although the court denied the Company’s request for a temporary restraining order at a hearing on September 24, 2015, it ordered that assets of the Wellstat Diagnostics Guarantor defendants should be held in status quo ante and only used in the normal course of business.
On July 29, 2016, the Supreme Court of New York granted the Company’s motion for summary judgment and held that the Wellstat Diagnostics Guarantor defendants are liable for all “Obligations” owed by Wellstat Diagnostics to the Company.
After appeal by the Wellstat Diagnostics Guarantor defendants on February 14, 2017, the Appellate Division of the Supreme Court of New York reversed on procedural grounds a portion of the Memorandum of Decision granting the Company summary judgment in lieu of complaint, but affirmed the portion of the Memorandum of Decision denying the Wellstat Diagnostics Guarantor defendants’ motion for summary judgment in which they sought a determination that the guarantees had been released. As a result, the proceeding was remanded to the Supreme Court of New York to proceed on the Company’s claims as a plenary action. On June 21, 2017, the Supreme Court of New York ordered the Company to file a Complaint, which was filed by the Company on July 20, 2017. The Wellstat Diagnostics Guarantors filed their answer on August 9, 2017, including counterclaims against the Company alleging breach of contract, breach of fiduciary duty, and tortious interference with prospective economic advantage.
On October 14, 2016, the Company sent a notice of default and reference to foreclosure proceedings to certain of the Wellstat Diagnostics Guarantors which were not defendants in the New York action, but which are owners of real estate assets over which a deed of trust in favor of the Company securing the guarantee of the loan to Wellstat Diagnostics had been executed. On March 2, 2017, the Company sent a second notice to foreclose on the real estate assets, and noticed the sale for March 29, 2017. The sale was taken off the calendar by the trustee under the deed of trust and was not re-scheduled. On March 6, 2017, the Company sent a letter to the Wellstat Diagnostics Guarantors seeking information in preparation for a UCC Article 9 sale of some or all of the intellectual property-related collateral of the Wellstat Diagnostics Guarantors. The Wellstat Diagnostics Guarantors did not respond to the Company’s letter, but on March 17, 2017, filed an order to show cause with the Supreme Court of New York to enjoin the Company’s sale of the real estate or enforcing its security interests in the Wellstat Diagnostics Guarantors’ intellectual property during the pendency of any action involving the guarantees at issue. On February 6, 2018, the Supreme Court of New
York issued an order from the bench which enjoins the Wellstat Diagnostics Guarantors from selling, encumbering, removing, transferring or altering the collateral pending the outcome of the proceedings before it. The Supreme Court of New York also issued an order precluding the Company from foreclosing on certain of the Wellstat Diagnostics Guarantors’ collateral pending the outcome of the proceedings before it. In September of 2018, discovery in the New York action was completed. Summary judgment motions were filed by Wellstat Diagnostics and the Company in 2018 and a hearing was held on May 22, 2019. On September 11, 2019, the Supreme Court of New York granted the Company’s summary judgment motion, the court holding that the guarantees executed by the Wellstat Diagnostics Guarantors are valid and enforceable, and that the Wellstat Diagnostics Guarantors are liable for the amount owed under the loan agreement. The court ordered a damages inquest before a special referee to calculate the amount owed under the loan agreement between Wellstat Diagnostics and the Company. On September 12, 2019, the Wellstat Diagnostics Guarantors filed a notice of appeal in relation to the court’s decision. On September 17, 2019, the Wellstat Diagnostics Guarantors requested a stay of the enforcement of the New York Supreme Court’s decision pending their appeal of the decision, which was denied on November 21, 2019. A damages hearing was scheduled to begin before a judicial hearing officer on December 17, 2019. At the request of the judicial hearing officer, the parties agreed to mediate their dispute prior to the commencement of the damages hearing. As a result, no decision was made by the hearing officer with respect to the amount of damages owed to the Company.
In an unrelated litigation, Wellstat Therapeutics filed a lawsuit against BTG International, Inc. for breach of contract (the “BTG Litigation”). In September 2017, the Delaware Chancery Court found in favor of Wellstat Therapeutics and awarded a judgment of $55.8 million in damages, plus interest. In October 2017, the Company filed a motion with the Supreme Court of New York requesting a pre-judgement attachment of the award. In June 2018, the Delaware Supreme Court largely affirmed the September 2017 decision of the Delaware Chancery Court, including the $55.8 million awarded in judgment. In August of 2018, in a letter to the Company’s counsel, Wellstat Diagnostics Guarantors’ counsel confirmed that the Wellstat Diagnostics Guarantors would preserve the BTG Litigation judgment award proceeds consistent with the New York Court’s prior directions.
On October 22, 2015, certain of the Wellstat Diagnostics Guarantors filed a separate complaint against the Company in the Supreme Court of New York seeking a declaratory judgment that certain contractual arrangements entered into between the parties subsequent to Wellstat Diagnostics’ default, and which relate to a split of proceeds in the event that the Wellstat Diagnostics Guarantors voluntarily monetize any assets that are the Company’s collateral, is of no force or effect. This case was joined for all purposes, including discovery and trial, and consolidated with the then pending case filed by the Company. The Wellstat Diagnostic Guarantors filed a summary judgment motion with regard to this case, which was also heard by the court at the hearing on May 22, 2019. The court, in its September 11, 2019 decision, denied in its entirety the Wellstat Diagnostics Guarantors’ motion for summary judgment.
Effective April 1, 2014, and as a result of the event of default, the Company determined the loan to be impaired and it ceased to accrue interest revenue. At that time and under previous Going Concern reporting periods, it was determined that an allowance on the carrying value of the note was not necessary, as the Company believed the value of the collateral securing Wellstat Diagnostics’ obligations exceeded the carrying value of the asset and was sufficient to enable the Company to recover the carrying value.
In August 2020, the Company entered into a settlement agreement (the “Settlement Agreement”) with Samuel J. Wohlstadter, Nadine H. Wohlstadter, Hyperion Catalysis International, Wellstat Vaccines, LLC, Wellstat ImmunoTherapeutics, LLC, Wellstat BioCatalysis, LLC, Wellstat AVT Investment, LLC, Wellstat Biologics Corporation, Wellstat Management Company, LLC, Wellstat Ophthalmics Corporation, Wellstat Therapeutics Corporation, Wellstat Therapeutics EU Limited, Duck Farm, Inc., Hebron Valley Farms, Inc., HVF, Inc., Hyperion Catalysis EU Limited, NHW, LLC, and SJW Properties, Inc., together with their respective successors and assigns, (collectively the “Wellstat Parties”), and Defined Diagnostics, LLC (f/k/a Wellstat Diagnostics, LLC) resolving all claims in litigation relating to loans made to Wellstat Diagnostics by the Company. Wellstat paid the company an amount of $7.5 million upon the signing of the Settlement Agreement and must pay either (1) $5.0 million by February 10, 2021 and $55.0 million by July 26, 2021; or (2) $67.5 million by July 26, 2021. If the Wellstat Parties fail to make payment in full by July 26, 2021 as required under the terms of the Settlement Agreement, the Company is authorized to record and confess judgment against the Wellstat Parties for an amount of $92.5 million or such lesser amount as may be owed under the Settlement Agreement as of that date. The $7.5 million initial signing payment was received by the Company in August 2020. The Company’s Settlement Agreement with the Wellstat Parties also settled its disputes with Hyperion (a Wellstat Diagnostics Guarantor).
On December 11, 2020, the Company sold its rights in the Settlement Agreement to Epps Investments LLC (“Epps”). Under the terms of the Agreement, Epps paid the Company approximately $51.4 million in exchange for 100% of the awards, damages, recoveries, judgments or other property or value awarded to or received by the Company on or after the date of the Agreement pursuant to or as a result of (i) the Settlement Agreement, and (ii) the underlying claims resolved by the Settlement Agreement.
The Company agreed to reimburse Epps for certain expenses related to the Agreement. Pursuant to the agreement, the Company granted Epps a security interest in the Company’s interest in certain collateral, as further described in the Agreement, including the Settlement Agreement and the underlying claims resolved by the Settlement Agreement, as security for the prompt payment of the Company’s obligations under the Agreement.
Avinger Credit and Royalty Agreement
On April 18, 2013, the Company entered into a credit agreement with Avinger, Inc. (the “Avinger Credit and Royalty Agreement”). Under the terms of the Avinger Credit and Royalty Agreement, the Company received a low, single-digit royalty on Avinger’s net revenues until April 2018. Commencing in October 2015, after Avinger repaid $21.4 million pursuant to its note payable to the Company prior to its maturity date, the royalty on Avinger’s net revenues was reduced by 50%, subject to certain minimum payments from the prepayment date until April 18, 2018. The Company accounted for the royalty rights in accordance with the fair value option. As of April 18, 2018, there were no further obligations owed to the Company.
Direct Flow Medical Credit Agreement
On November 5, 2013, the Company entered into a credit agreement with Direct Flow Medical, Inc. (“Direct Flow Medical”) under which the Company agreed to provide up to $50.0 million to Direct Flow Medical. Of the $50.0 million available to Direct Flow Medical, the first tranche of $35.0 million, net of fees, was funded by the Company at the close of the transaction.
On November 10, 2014, the Company and Direct Flow Medical agreed to an amendment to the credit agreement to permit Direct Flow Medical to borrow an additional $15.0 million (in a second tranche) upon receipt by Direct Flow Medical of a specified minimum amount of proceeds from an equity offering prior to December 31, 2014. In exchange, the parties amended the credit agreement to provide for additional fees associated with certain liquidity events, such as a change of control or the consummation of an initial public offering, and granted the Company certain board of director observation rights. On November 19, 2014, upon Direct Flow Medical satisfying the amended tranche two milestone, the Company funded the $15.0 million second tranche to Direct Flow Medical, net of fees.
Outstanding borrowings under tranche one bore interest at the rate of 15.5% per annum, payable quarterly in arrears, until the occurrence of the second tranche. Upon occurrence of the borrowing of this second tranche, the interest rate applicable to all loans under the credit agreement was decreased to 13.5% per annum, payable quarterly in arrears.
Under the terms of the credit agreement, Direct Flow Medical’s obligation to repay loan principal commenced on the twelfth interest payment date, September 30, 2016. The principal amount outstanding at commencement of repayment was required to be repaid in equal installments until final maturity of the loans. The loans were scheduled to mature on November 5, 2018. The obligations under the credit agreement were secured by a pledge of substantially all of the assets of Direct Flow Medical and any of its subsidiaries.
On December 21, 2015, Direct Flow Medical and the Company entered into a waiver to the credit agreement in anticipation of Direct Flow Medical being unable to comply with the liquidity covenant and make interest payments due under the credit agreement, which was subsequently extended on January 14, 2016, and further delayed the timing of the interest payments through the period ending September 30, 2016 while Direct Flow Medical sought additional financing to operate its business.
On January 28, 2016, the Company funded an additional $5.0 million to Direct Flow Medical in the form of a short-term secured promissory note.
On February 26, 2016, the Company and Direct Flow Medical entered into the fourth amendment to the credit agreement that, among other things, (i) converted the $5.0 million short-term secured promissory note into a loan under the credit agreement with substantially the same interest and payment terms as the existing loans, (ii) added a conversion feature whereby the $5.0 million loan would convert into equity of Direct Flow Medical upon the occurrence of certain events and (iii) provided for a second $5.0 million convertible loan tranche commitment, to be funded at the option of the Company. The commitment for the second tranche was not funded and has since expired. In addition, (i) the Company agreed to waive the liquidity covenant and delay the timing of the unpaid interest payments until September 30, 2016 and (ii) Direct Flow Medical agreed to issue to the Company a specified amount of warrants to purchase shares of convertible preferred stock on the first day of each month for the duration of the waiver period at an exercise price of $0.01 per share.
On July 15, 2016, the Company and Direct Flow Medical entered into the fifth amendment and limited waiver to the credit agreement. The Company funded an additional $1.5 million to Direct Flow Medical in the form of a note with substantially the
same interest and payment terms as the existing loans and a conversion feature whereby the $1.5 million loan would convert into equity of Direct Flow Medical upon the occurrence of certain events. In addition, Direct Flow Medical agreed to issue to the Company warrants to purchase shares of convertible preferred stock at an exercise price of $0.01 per share.
On September 12, 2016, the Company and Direct Flow Medical entered into the sixth amendment and limited waiver to the credit agreement under which the Company funded an additional $1.5 million to Direct Flow Medical in the form of a note with substantially the same interest and payment terms as the existing loans. In addition, Direct Flow Medical agreed to issue to the Company a specified amount of warrants to purchase shares of convertible preferred stock at an exercise price of $0.01 per share.
On September 30, 2016, the Company and Direct Flow Medical entered into a waiver to the credit agreement where the parties agreed, among other things, to (i) delay payment on all overdue interest payments until October 31, 2016, (ii) waive the initial principal repayment until October 31, 2016 and (iii) continue to waive the liquidity requirements until October 31, 2016. Further, Direct Flow Medical agreed to issue to the Company a specified amount of warrants to purchase shares of convertible preferred stock at an exercise price of $0.01 per share.
On October 31, 2016, the Company agreed to extend the waivers described above until November 30, 2016 and on November 14, 2016, the Company advanced an additional $1.0 million loan while Direct Flow Medical continued to seek additional financing.
On November 16, 2016, Direct Flow Medical advised the Company that its potential financing source had modified its proposal from an equity investment to a loan with a substantially smaller amount and under less favorable terms. Direct Flow Medical shut down its operations in December 2016 and in January 2017 made an assignment for the benefit of creditors. The Company then initiated foreclosure proceedings, resulting in the Company obtaining ownership of most of the Direct Flow Medical assets through the Company’s wholly-owned subsidiary, DFM, LLC. The assets were held for sale and carried at the lower of carrying amount or fair value, less estimated selling costs, which was primarily based on supporting data from market participant sources, and valid offers from third parties.
At December 31, 2016, the Company completed an impairment analysis and concluded that the situation qualified as a troubled debt restructuring and recognized an impairment loss of $51.1 million.
In January 2017, the Company started to actively market the asset held for sale. On January 23, 2017, the Company and DFM, LLC entered into an Intellectual Property Assignment Agreement with Hong Kong Haisco Pharmaceutical Co., Limited (“Haisco”), a Chinese pharmaceutical company, whereby Haisco acquired former Direct Flow Medical clinical, regulatory and commercial information and intellectual property rights exclusively in China for $7.0 million. The Company, through DFM, LLC, also sold Haisco certain manufacturing equipment for $450,000 and collected $692,000 on outstanding Direct Flow Medical accounts receivable during the year ended December 31, 2017.
On January 6, 2018, DFM, LLC and HaisThera Advisors Co., Limited (“HaisThera”) entered into a license agreement whereby DFM, LLC granted HaisThera an exclusive license to develop, manufacture and commercialize percutaneously implanting stentless aortic valves in the EU. The consideration for the license agreement was $500,000 upfront and up to $2.0 million in royalty payments. In August 2019, the remaining assets of DFM, LLC were sold for $5.0 million.
kaléo Note Purchase Agreement
On April 1, 2014, the Company entered into a note purchase agreement with Accel 300, LLC (“Accel 300”), a wholly-owned subsidiary of kaléo, Inc. (“kaléo”), pursuant to which the Company acquired $150.0 million of secured notes due 2029 (the “kaléo Note”). The kaléo Note was issued pursuant to an indenture between Accel 300 and U.S. Bank, National Association, as trustee, and was secured by 20% of net sales of its first approved product, Auvi-Q® (epinephrine auto-injection, USP) (known as Allerject® in Canada) and 10% of net sales of kaléo’s second proprietary auto-injector based product, EVZIO (naloxone hydrochloride injection ) (the “kaléo Revenue Interests”), and a pledge of kaléo’s equity ownership in Accel 300.
On September 21, 2017, the Company entered into an agreement (the “kaléo Note Sale Agreement”) with MAM-Kangaroo Lender, LLC, a Delaware limited liability company (the “kaléo Purchaser”), pursuant to which the Company sold its entire interest in the kaléo Note.
Pursuant to the kaléo Note Sale Agreement, the kaléo Purchaser paid to the Company an amount equal to all of the then outstanding principal, a premium of 1% of such amount and accrued interest under the kaléo Note, for an aggregate cash purchase price of $141.7 million, subject to an 18-month escrow holdback of $1.4 million against certain potential contingencies. The escrow period ended on March 20, 2019 and the escrow agent released the entire $1.4 million to the Company.
CareView Credit Agreement
On June 26, 2015, the Company entered into a credit agreement with CareView, under which the Company made available to CareView up to $40.0 million in loans comprised of two tranches of $20.0 million each, subject to CareView’s attainment of specified milestones relating to the placement of CareView Systems. On October 7, 2015, the Company and CareView entered into an amendment of the credit agreement to modify certain definitions related to the first and second tranche milestones and the Company funded the first tranche of $20.0 million, net of fees, based on CareView’s attainment of the first milestone, as amended. The second $20.0 million tranche was not funded due to CareView’s failure to achieve the related funding milestones and there is no additional funding obligation due from the Company. Outstanding borrowings under the credit agreement initially bore interest at the rate of 13.5% per annum and are payable quarterly in arrears.
As part of the original credit agreement, the Company received a warrant to purchase approximately 4.4 million shares of common stock of CareView at an exercise price of $0.45 per share. The Company has accounted for the warrant as derivative asset with an offsetting credit as debt discount. Under the Going Concern Basis, the warrant was market to market for changed in fair value each reporting period.
In connection with the October 2015 amendment of the credit agreement, the Company and CareView also agreed to amend the warrant to purchase common stock agreement by reducing the warrant’s exercise price from $0.45 to $0.40 per share.
In February 2018, the Company entered into a modification agreement with CareView (the “February 2018 Modification Agreement”) whereby the Company agreed, effective December 28, 2017, to modify the credit agreement before remedies could otherwise have become available to the Company under the credit agreement in relation to certain obligations of CareView that would potentially not be met, including the requirement to make principal payments. Under the February 2018 Modification Agreement, the Company agreed that (i) a lower liquidity covenant would be applicable and (ii) principal repayment would be delayed until December 31, 2018. In exchange for agreeing to these modifications, among other things, the exercise price of the Company’s warrants to purchase 4.4 million shares of common stock of CareView was repriced from $0.40 to $0.03 per share and, subject to the occurrence of certain events, CareView agreed to grant the Company additional equity interests. As a result of the February 2018 Modification Agreement, the Company determined the loan to be impaired and it ceased to accrue interest revenue effective October 1, 2017.
In September 2018, the Company entered into an amendment to the February 2018 Modification Agreement with CareView whereby the Company agreed, effective as of September 28, 2018, that a lower liquidity covenant would be applicable. In December 2018, the Company further modified the loan by agreeing that (i) a lower liquidity covenant would be applicable, (ii) the first principal payment would be deferred until January 31, 2019, and (iii) the scheduled interest payment due December 31, 2018 would be deferred until January 31, 2019. In December 2018, and in consideration of the further modification to the credit agreement, the Company completed an impairment analysis and determined that the note was impaired and recorded an impairment loss of $8.2 million. The principal repayment and interest payment were subsequently deferred until May 15, 2019 under additional amendments. In May 2019, and in consideration of additional capital raised by CareView, the Company further modified the loan by agreeing that (i) the first principal and interest payments would be deferred until September 30, 2019 (ii) the remaining liquidity covenant would be removed, and (iii) the interest rate would be increased to 15.5%. Pursuant to further amendments to the February 2018 Modification Agreement in September 2019, December 2019, January 2020, April 2020, September 2020, November 2020 and January 2021, the Company agreed to defer principal and interest payments until May 31, 2021.
In December 2019, and in consideration of the further modification to the credit agreement and February 2018 Modification Agreement, the Company updated its impairment analysis and determined that an additional impairment was necessary and recorded an impairment loss of $10.8 million.
As of December 31, 2020, under the Liquidation Basis, the expected cash realizable value of the CareView note receivable and warrant was $0.8 million and included in “Other assets” on the Statement of Net Assets in Liquidation. Refer to Note 3, Net Assets in Liquidation, for a discussion of the valuation of the warrants under the Liquidation Basis.
10. Leases
Lessee arrangements
As of December 31, 2020, the Company has an operating lease for corporate offices. The Company’s operating lease has a remaining lease term of two years, with an option to extend the lease for up to six months.
Prior to the sale of Noden and the spin-off of LENSAR, the Company also included operating leases for their corporate offices and certain equipment.
The components of lease expense from continuing operations under the Going Concern Basis were as follows:
| | | | | | | | | | | | | | |
| | Eight Months Ended | | Year Ended |
| (in thousands) | | August 31, 2020 | | December 31, 2019 |
| | | | |
| Operating lease cost | | $ | 532 | | | $ | 760 | |
| Short-term lease cost | | 49 | | | 79 | |
| Total lease cost | | $ | 581 | | | $ | 839 | |
Supplemental cash flow information related to leases for continuing operations is as follows:
| | | | | | | | | | | | | | |
| | Eight Months Ended | | Year Ended |
| (in thousands) | | August 31, 2020 | | December 31, 2019 |
| | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | |
| Operating cash flows from operating leases | | $ | 522 | | | $ | 762 | |
| Right-of-use-assets obtained in exchange for lease obligations: | | | | |
| Operating leases | | $ | 3,320 | | | $ | 2,055 | |
The following table presents the lease balances relating to continuing operations within the Consolidated Balance Sheet, weighted-average remaining lease term, and weighted-average discount rates related to the Company’s operating leases (in thousands):
| | | | | | | | | | | | | | |
| Operating Leases | | Classification | | December 31, 2019 |
| | | | |
| Operating lease ROU assets | | Other assets | | $ | 1,359 | |
| | | | |
| Operating lease liabilities, current | | Accrued liabilities | | $ | 760 | |
| Operating lease liabilities, long-term | | Other long-term liabilities | | 634 | |
| Total operating lease liabilities | | Total operating lease liabilities | | $ | 1,394 | |
| | | | |
| Weighted-average remaining lease term | | 1.9 years |
| Weighted-average discount rate | | 6.5 | % |
_______________
Operating leases above exclude right of use assets and liabilities of $0.3 million classified as held for sale in the Consolidated Balance Sheet as of December 31, 2019.
Maturities of operating lease liabilities as of December 31, 2020 are as follows (in thousands):
| | | | | | | | |
| Fiscal Year | | Amount |
| | |
| 2021 | | $ | 37 | |
| 2022 | | 38 | |
| 2023 | | 0 | |
| 2024 | | 0 | |
| 2025 | | 0 | |
| Thereafter | | 0 | |
| Total operating lease payments | | 75 | |
| Less: imputed interest | | 0 | |
| Total operating lease liabilities | | $ | 75 | |
Lessor arrangements
The Company had operating leases for medical device equipment generated from its Medical Devices segment. The Company’s leases had remaining lease terms of less than one to four years, some of which included options to extend the leases on a month-to-month basis if the customer did not notify the Company of the intention to return the equipment at the end of the lease term. The Company typically did not offer options to terminate the leases before the end of the lease term. The Medical Devices segment was spun-off on October 1, 2020.
The components of lease income under the Going Concern Basis were as follows:
| | | | | | | | | | | | | | | | | | | | |
| | | | Eight Months Ended | | Year Ended |
| (in thousands) | | Classification | | August 31, 2020 | | December 31, 2019 |
| | | | | | |
| | | | | | |
| | | | |
| | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Operating lease income | | Lease revenue | | $ | 2,139 | | | $ | 5,072 | |
Under the Going Concern Basis, and prior to the spin-off of LENSAR, Equipment under lease was stated at cost less accumulated depreciation and was classified as Property and equipment, net on the Consolidated Balance Sheet. Depreciation was computed using the straight-line method over an estimated useful life of the greater of the lease term or five years to ten years. Equipment under lease was as follows:
| | | | | | | | | | |
| (in thousands) | | | | December 31, 2019 |
| | | | |
| Equipment under lease | | | | $ | 6,652 | |
| Less accumulated depreciation | | | | (5,231) | |
| Equipment under lease, net | | | | $ | 1,421 | |
Depreciation expense on equipment under lease amounted to $1.0 million, $2.1 million and $2.7 million for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, respectively.
11. Property and Equipment
Property and equipment, net under the Going Concern Basis consisted of the following:
| | | | | | | | | | |
| (in thousands) | | | | December 31, 2019 |
| Leasehold improvements | | | | $ | 350 | |
| Manufacturing equipment | | | | 1,550 | |
| Computer and office equipment | | | | 9,101 | |
| Furniture and fixtures | | | | 136 | |
| Equipment under lease | | | | 6,652 | |
| Transportation equipment | | | | 67 | |
| Total | | | | 17,856 | |
| Less accumulated depreciation | | | | (16,040) | |
| Construction in progress | | | | 744 | |
Property and equipment, net (1) (2) | | | | $ | 2,560 | |
________________
(1) The amounts above exclude $3.0 million of Property and Equipment at Noden classified as held for sale as of December 31, 2019. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
(2) The amounts above include amounts held by LENSAR which was spun-off on October 1, 2020.
Depreciation expense on property and equipment amounted to $1.0 million, $2.7 million and $3.1 million for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, respectively.
12. Intangible Assets
Noden
On June 8, 2018, Noden DAC entered into a Settlement Agreement (the “Settlement Agreement”) with Anchen Pharmaceuticals, Inc. and its affiliates (“Anchen”) to resolve the patent litigation relating to infringement of U.S. Patent No. 8,617,595 (the “‘595 Patent”) based on their submission of an Abbreviated New Drug Application (“ANDA”) seeking authorization from the FDA to market a generic version of aliskiren, the active ingredient in the Tekturna and Tekturna HCT drug. Under the Settlement Agreement, Anchen, the sole ANDA filer of which the Company is aware, agreed to not commercialize its generic version of aliskiren prior to March 1, 2019. Per the Settlement Agreement, Anchen may commercialize their formulation of aliskiren, but is not permitted to commercialize a copy of Tekturna.
Accordingly, under the Going Concern Basis, management evaluated the ongoing value of the Noden DAC asset group based upon the probability of Anchen’s market entry of a generic version of aliskiren in the United States and the associated cash flows and conducted a test for impairment. Due to the increased probability of a generic version of aliskiren being launched in the United States, the Company revised its estimates of future cash flows and as a result of this analysis, determined that the sum of undiscounted cash flows was not greater than the carrying value of the assets. Therefore, the Company performed a discounted cash flow analysis to estimate the fair value of the asset group in accordance with ASC 360, Impairment or Disposal of Long-lived Assets. The cash flows used in this analysis were those expected to be generated by market participants, discounted to reflect an appropriate amount of risk, which was determined to be 21%. The Company concluded that the Noden DAC acquired product rights and customer relationship long-lived assets, with a carrying amount of $192.5 million, were no longer recoverable and wrote them down to their estimated fair value of $40.1 million, resulting in an impairment charge of $152.3 million in the second quarter of 2018. This write-down is included in Loss from discontinued operations before income taxes in the Consolidated Statement of Operations and Net cash used in operating activities - discontinued operations in the Consolidated Statement of Cash Flows for the year ended December 31, 2018.
At December 31, 2019, due to the Company’s monetization strategy and updated forecasts for Noden, the Company revised its estimates of future cash flows and as a result of this analysis, determined that the sum of undiscounted cash flows was not greater than the carrying value of the assets. Therefore, the Company performed a discounted cash flow analysis to estimate the fair value of the asset group in accordance with ASC 360. The cash flows used in this analysis were those expected to be generated by market participants, discounted to reflect an appropriate amount of risk, which was determined to be 19%. The Company concluded that the Noden DAC acquired product rights and customer relationship long-lived assets, with a carrying amount of
$32.6 million, were no longer recoverable and wrote them down to their estimated fair value of $10.1 million, resulting in an impairment charge of $22.5 million in the fourth quarter of 2019. This write-down is included in Loss from discontinued operations before income taxes in the Consolidated Statement of Operations and Net cash used in operating activities - discontinued operations in the Consolidated Statement of Cash Flows for the year ended December 31, 2019.
During the fourth quarter of 2019, while performing its impairment analysis on its Noden intangible assets, the Company identified an error in the 2018 impairment charge recorded on its Noden intangible assets, which resulted in a $10.5 million overstatement of the 2018 impairment charge. As of December 31, 2018, the net carrying value of the intangible asset was understated by $9.8 million with a corresponding overstatement of net loss for the year ended December 31, 2018. This prior year impairment expense error was corrected as an out of period adjustment in 2019 in connection with the further impairment of the intangible asset to $10.1 million.
Based on an analysis of Accounting Standards Codification (“ASC”) 250, Accounting Changes and Error Corrections (“ASC 250”), Staff Accounting Bulletin 99, Materiality (“SAB 99”) and Staff Accounting Bulletin 108, Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements (“SAB 108”), the Company determined that these errors were immaterial to the previously issued annual and interim financial statements. The amount of the intangible assets and accumulated amortization were corrected as of December 31, 2019.
LENSAR
In April 2019, LENSAR acquired certain intellectual property from a third-party for $2.0 million in cash and obligations to pay a $0.3 million milestone payment and royalties upon the completion of certain events, which were met prior to December 31, 2019.
In September 2019, LENSAR exclusively licensed certain intellectual property from a third-party for $3.5 million in cash for use in research and development activities. The amount was immediately expensed and is included in Research and development expense in the Consolidated Statement of Operations for the year ended December 31, 2019.
LENSAR was spun-off on October 1, 2020.
The components of intangible assets as of December 31, 2019 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2019 | | |
| (in thousands) | | Gross Carrying Amount | | Accumulated Amortization | | Net Carrying Amount | | | | | | |
| Finite-lived intangible assets: | | | | | | | | | | | | |
| | | | | | | | | | | | |
Customer relationships 1, 3 | | $ | 4,045 | | | $ | (884) | | | $ | 3,161 | | | | | | | |
Acquired technology 1, 2, 4 | | 11,500 | | | (1,741) | | | 9,759 | | | | | | | |
Acquired trademarks 1 | | 570 | | | (304) | | | 266 | | | | | | | |
Total 5 | | $ | 16,115 | | | $ | (2,929) | | | $ | 13,186 | | | | | | | |
_______________
1 The Company acquired certain intangible assets as part of its acquisition of LENSAR in May 2017. These assets were being amortized on a straight-line basis over a weighted-average period of 15 years. The intangible assets for customer relationships were being amortized using a double-declining method of amortization as such method better represents the economic benefits to be obtained.
2 The Company acquired certain intangible assets as part of the foreclosure on certain of Direct Flow Medical assets. In August 2019, the Company sold the DFM, LLC intangible assets for $5.0 million in cash and a single-digit percentage of any net final award received as part of the acquirer’s monetization process using the intangible assets. Prior to the sale, these intangible assets were being amortized on a straight-line basis over a weighted-average period of 10 years.
3 LENSAR acquired certain intangible assets for customer relationships from Precision Eye Services, which were being amortized using a double-declining method over a period of 20 years.
4 LENSAR acquired certain intangible assets from a third-party in 2019, which were being amortized on a straight-line basis over a period of 15 years.
5 The Company acquired certain intangible assets as part of the Noden transaction. Those intangible assets are excluded from the table above and included in “Assets held for sale” as of December 31, 2019. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
Amortization expense related to our continuing operations for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018 was $0.8 million, $1.3 million and $1.3 million, respectively.
13. Accrued Liabilities
Accrued liabilities under the Going Concern Basis consisted of the following:
| | | | | | | | | | |
| (in thousands) | | | | December 31, 2019 |
| | | | |
| Accrued rebates, chargebacks and other revenue reserves | | | | $ | 5 | |
| Deferred revenue | | | | 959 | |
| Compensation | | | | 6,823 | |
| Interest | | | | 70 | |
| | | | |
| | | | |
| | | | |
| | | | |
| Legal | | | | 921 | |
| Other | | | | 3,145 | |
Total (1) (2) | | | | $ | 11,923 | |
________________
(1) The amounts above exclude $16.4 million of accrued liabilities at Noden classified as held for sale as of December 31, 2019. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
(2) The amounts above include amounts held by LENSAR which was spun-off on October 1, 2020.
Upon adoption of the Liquidation Basis, the Company accrued for all estimated cash expenditures. These expenses include $9.3 million in accrued compensation and benefit costs, $9.0 million in deferred tax liabilities (see Note 23, Income Taxes, for additional information), $4.0 million in estimated costs to dispose of the Assertio asset and $7.3 million in estimated future expenses related to operating the business through dissolution and settlement of future liabilities. Amounts include estimated compensation, legal and other professional fees, insurance, taxes, estimated costs to dispose of our assets and other miscellaneous expenses. For additional information, see Note 3, Net Assets in Liquidation.
14. Convertible Senior Notes
February 2018 Notes
On February 12, 2014, the Company issued $300.0 million in aggregate principal amount, at par, of the 4.0% Convertible Senior Notes due February 1, 2018 (the “February 2018 Notes”) Notes in an underwritten public offering, for net proceeds of $290.2 million. The February 2018 Notes were due February 1, 2018, and the Company paid interest at 4.0% on the February 2018 Notes semiannually in arrears on February 1 and August 1 of each year, beginning August 1, 2014. A portion of the proceeds from the February 2018 Notes, net of amounts used for purchased call option transactions and provided by the warrant transactions described below, were used to redeem $131.7 million of the Company’s 2.975% Convertible Senior Notes due February 17, 2016.
In accordance with the accounting guidance for convertible debt instruments that may be settled in cash or other assets on conversion, the Company was required to separately account for the liability component of the instrument in a manner that reflected the market interest rate for a similar nonconvertible instrument at the date of issuance. As a result, the Company separated the principal balance of the February 2018 Notes between the fair value of the debt component and the fair value of the common stock conversion feature. Using an assumed borrowing rate of 7.0%, which represented the estimated market interest rate for a similar nonconvertible instrument available to the Company on the date of issuance, the Company recorded a total debt discount of $29.7 million, allocated $19.3 million to additional paid-in capital and allocated $10.4 million to deferred tax liability. The discount was being amortized to interest expense over the term of the February 2018 Notes and increased interest expense during the term of the February 2018 Notes from the 4.0% cash coupon interest rate to an effective interest rate of 6.9%.
In connection with the issuance of the February 2018 Notes, the Company entered into purchased call option transactions with 2 hedge counterparties. The Company paid an aggregate amount of $31.0 million for the purchased call options with terms substantially similar to the embedded conversion options in the February 2018 Notes. The purchased call options covered, subject to anti-dilution and certain other customary adjustments substantially similar to those in the February 2018 Notes, approximately 13.8 million shares of the Company’s common stock. Outstanding purchased call options expired on February 1, 2018.
In addition, the Company sold to the hedge counterparties warrants exercisable, on a cashless basis, for the sale of rights to receive shares of common stock underlying the February 2018 Notes at a strike price of $10.3610 per share, which represented a
premium of approximately 30% over the last reported sale price of the Company’s common stock of $7.97 on February 6, 2014. The Company received an aggregate amount of $11.4 million for the sale from the two counterparties.
The purchased call options and warrants were considered indexed to the Company stock, required net-share settlement and met all criteria for equity classification at inception and in subsequent periods. The purchased call options cost of $31.0 million less deferred taxes of $10.8 million, and the $11.4 million received for the warrants, were recorded as adjustments to additional paid-in capital.
On November 20, 2015, the Company’s agent initiated the repurchase of $53.6 million in aggregate principal amount of its February 2018 Notes for $43.7 million in cash in four open market transactions. The closing of these transactions occurred on November 30, 2015. It was determined that the repurchase of the principal amount should be accounted for as a partial extinguishment of the February 2018 Notes. As a result, a gain on extinguishment of $6.5 million was recorded at closing of the transaction. The $6.5 million gain on extinguishment included the de-recognition of a proportional share of the original issuance discount of $3.1 million, outstanding deferred issuance costs of $0.9 million and agent fees of $0.1 million. In connection with this repurchase of the February 2018 Notes, the Company unwound a corresponding portion of the purchased call options related to the notes. As a result of this unwinding, the Company received $0.3 million in cash. The payments received have been recorded as an increase to additional paid-in-capital. In addition, the Company unwound a corresponding portion of the warrants issued in connection with the notes for $0.2 million in cash, payable by the Company. The payments have been recorded as a decrease to additional paid-in-capital.
On November 22, 2016, the Company repurchased $120.0 million in aggregate principal amount of its February 2018 Notes for approximately $121.5 million in cash (including $1.5 million of accrued interest) in open market transactions. It was determined that the repurchase of the principal amount be accounted for as an extinguishment. The extinguishment included the de-recognition of a proportional share of the original issuance discount of $4.3 million and outstanding deferred issuance costs of $1.3 million. In connection with the repurchase of the February 2018 Notes, the Company unwound a corresponding portion of the purchased call options. The transaction did not result in any cash payments between the parties. In addition, the Company and the counterparties agreed to unwind a corresponding portion of the warrants, which also did not result in any cash payments between the parties.
On February 1, 2018, upon maturity of the February 2018 Notes, the Company repaid a total cash amount of $129.0 million to the custodian, The Bank of New York Mellon Trust Company, N.A., which was comprised of $126.4 million in principal amount and $2.6 million in accrued interest, to retire the February 2018 Notes.
Interest expense for the February 2018 Notes on the Company’s Consolidated Statements of Operations was as follows:
| | | | | | | | | | | | |
| (in thousands) | | | | | | Year Ended December 31, 2018 |
| | | | | | |
| Contractual coupon interest | | | | | | $ | 422 | |
| Amortization of debt issuance costs | | | | | | 88 | |
| Amortization of debt discount | | | | | | 293 | |
| Total | | | | | | $ | 803 | |
December 2021 Notes
On November 22, 2016, the Company issued $150.0 million in aggregate principal amount, at par, of 2.75% Convertible Senior Notes due December 1, 2021 (the “December 2021 Notes”) in an underwritten public offering, for net proceeds of $145.7 million. The December 2021 Notes are due December 1, 2021, and the Company pays interest at 2.75% on the December 2021 Notes semiannually in arrears on June 1 and December 1 of each year, beginning June 1, 2017. A portion of the proceeds from the December 2021 Notes, net of amounts used for the capped call transaction described below, was used to extinguish $120.0 million of the February 2018 Notes.
In September 2019, the Company entered into privately negotiated exchange agreements with certain holders of approximately $86.1 million aggregate principal amount of outstanding December 2021 Notes. The Company exchanged $86.1 million aggregate principal of December 2021 Notes for an identical principal amount of 2.75% Convertible Senior Notes due December 1, 2024 (the “December 2024 Notes”), plus a cash payment of $70.00 for each $1,000 principal amount tendered (“September
2019 Exchange Transaction”). See “December 2024 Notes” below. The terms of the remaining December 2021 Notes remained unchanged.
The September 2019 Exchange Transaction qualified as a debt extinguishment and the Company recognized a loss on exchange of the convertible notes of $3.9 million, which is included in Non-operating income (expense), net in the Consolidated Statement of Operations for the year ended December 31, 2019.
Upon the occurrence of a fundamental change, as defined in the indenture entered into in connection with the December 2021 Notes (the “December 2021 Notes Indenture”), holders have the option to require the Company to repurchase their December 2021 Notes at a purchase price equal to 100% of the principal, plus accrued interest.
Prior to the delisting of its common stock, the December 2021 Notes were convertible into PDL common stock upon the occurrence of specified corporate events as described in the December 2021 Notes Indenture or under either of the following circumstances at any time prior to the close of business on the business day immediately preceding June 1, 2021 (or at any time beginning on June 1, 2021 until the close of business on the second scheduled trading day immediately preceding the stated maturity):
•During any fiscal quarter (and only during such fiscal quarter) commencing after the fiscal quarter ended June 30, 2017, if the last reported sale price of Company common stock for at least 20 trading days (whether or not consecutive), in the period of 30 consecutive trading days, ending on, and including, the last trading day of the immediately preceding fiscal quarter, exceeds 130% of the conversion price for the notes on each applicable trading day; or
•During the five business-day period immediately after any five consecutive trading-day period, which the Company refers to as the measurement period, in which the trading price per $1,000 principal amount of notes for each trading day of that measurement period was less than 98% of the product of the last reported sale price of Company common stock and the conversion rate for the notes for each such trading day.
The December 2021 Notes remain convertible upon the occurrence of specified corporate events.
The initial conversion rate for the December 2021 Notes was 262.2951 shares of the Company’s common stock per $1,000 principal amount of December 2021 Notes, which was equivalent to an initial conversion price of approximately $3.81 per share of common stock, subject to adjustments upon the occurrence of certain specified events as set forth in the December 2021 Notes Indenture, the following of which occurred in 2020:
•Upon the distribution by the Company of its stock in Evofem to the PDL stockholders on May 21, 2020, the conversion rate for the December 2021 Notes increased from 262.2951 to 316.5801 shares of the Company’s common stock per $1,000 principal amount of December 2021 Notes equating to a conversion price of $3.16 per share of common stock.
•Upon the stockholders’ approval of the dissolution of the Company on August 19, 2020, a Fundamental Make-Whole Change, as defined in the December 2021 Notes Indenture, was triggered and the conversion rate for the December 2021 Notes was temporarily increased by 26.5297 to 343.1098 shares of the Company’s common stock for those bondholders who elected to convert their bonds during the conversion period.
•Upon the spin-off of the Company’s majority-owned subsidiary, LENSAR, on October 1, 2020, the conversion rate increased for the December 2021 Notes from 316.5801 to 410.6268 shares of the Company’s common stock per $1,000 principal amount of December 2021 Notes equating to a conversion price of $2.44 per share of common stock.
•As the adjustment to the conversion rate resulting from the spin-ff of LENSAR occurred during the fundamental make-whole period, the temporary increase to the conversion rate of 26.5297 shares of the Company’s common stock associated with the stockholders’ approval of the dissolution of the Company was further increased by 7.8812 shares of the Company’s common stock for the applicable portion of the observation period for those bondholders who elected to convert their bonds during the conversion period.
•Upon the suspension of trading of the Company’s common stock on the Nasdaq prior to the opening of business on December 31, 2020, a Fundamental Make-Whole Change occurred on December 31, 2020 resulting in a temporary increase to the conversion rate of 34.7383 shares of the Company’s common stock during the conversion period.
In accordance with the accounting guidance for convertible debt instruments that may be settled in cash or other assets on conversion, upon the issuance of the December 2021 Notes in November 2016 the Company was required to separately account for the liability component of the instrument in a manner that reflects the market interest rate for a similar nonconvertible instrument at the date of issuance. As a result, the Company separated the principal balance of the December 2021 Notes between the fair value of the debt component with the remainder of the consideration being allocated to the equity component. Using an assumed borrowing rate of 9.5%, which represented the estimated market interest rate for a similar nonconvertible instrument
available to the Company on the date of issuance, the Company recorded a debt discount of $4.3 million, allocated $23.8 million to Additional paid-in capital for the conversion feature and allocated $12.8 million to deferred tax liability.
The debt discount, including the conversion feature and issuance costs allocated to debt, which remained after amortization and the effect of the September 2019 Exchange Transaction, was being amortized to interest expense over the term of the December 2021 Notes until the adoption of the Liquidation Basis and increased, until such time, interest expense for the December 2021 Notes from the 2.75% cash coupon interest rate to an effective interest rate of 9.7%.
On December 17, 2019, the Company repurchased $44.8 million in aggregate principal amount of its December 2021 Notes for $39.9 million in cash and 3.5 million shares of its common stock in privately negotiated transactions (the “December 2019 Exchange Transaction”). It was determined that the repurchase of the principal amount should be accounted for as a partial extinguishment of the December 2021 Notes. As a result, a loss on extinguishment of $2.5 million was recorded at closing of the transaction. The loss on extinguishment included the de-recognition of a proportional share of the original issuance discount of $0.3 million and outstanding deferred issuance costs of less than $0.1 million.
The approval of the Plan of Dissolution by the Company’s stockholders in August 2020, provided for a Fundamental Change Repurchase Right, as defined in the December 2021 Notes Indenture, to each holder of the December 2021 Notes which would require the Company, at the holder’s option, to repurchase for cash on September 29, 2020 such holder’s notes, or any portion of the principal amount thereof, equal to $1,000 or an integral multiple of $1,000. No holders tendered their notes for repurchase under this Fundamental Change Repurchase Right.
The approval of the Plan of Dissolution by the Company’s stockholders also initiated a Fundamental Make-Whole Change, as noted above, allowing the holders of the December 2021 Notes to exercise their conversion rights during the conversion period for a cash amount equal to the conversion rate as defined in the December 2021 Notes Indenture (the “September 2020 Conversion”). Holders of $11.2 million par value of December 2021 Notes exercised their conversion right for an aggregate cash amount of $12.0 million. Such notes were repurchased and retired prior to December 31, 2020.
As a result of the suspension of trading of the Company’s common stock on the Nasdaq prior to the opening of business on December 31, 2020, a Fundamental Change occurred on December 31, 2020, the first day the Company’s common stock was no longer quoted and traded on a national securities exchange, and accordingly provided for a Fundamental Change Repurchase Right, which requires the Company, at the holder’s option, to repurchase for cash on February 17, 2021 such holder’s notes, or any portion of the principal amount thereof, equal to $1,000 or an integral multiple of $1,000. See Note 26, Subsequent Events, for further information.
The suspension of trading of the Company’s common stock also initiated a Fundamental Make-Whole Change allowing the holders of the notes to exercise their conversion rights until the close of business on February 16, 2021 for a cash amount equal to the conversion rate as defined in the December 2021 Notes Indenture (the “2021 Conversion”). See Note 26, Subsequent Events, for further information.
In December 2020, the Company repurchased an additional $2.2 million par value of December 2021 Notes in separate privately negotiated transactions for an aggregate amount of $2.3 million, including interest (the “December 2020 Repurchases”). As of December 31, 2020, $1.9 million par value of these notes remained outstanding pending final settlement, the offset of which is recorded as “Other Assets” on the Company’s Consolidated Statement of Net Assets as of December 31, 2020.
The carrying value and unamortized discount of the December 2021 Notes were as follows:
| | | | | | | | | | | | | | |
| (in thousands) | | December 31, 2020 | | December 31, 2019 |
| | (Liquidation Basis) | | (Going Concern Basis) |
| | | | |
| Principal amount of the December 2021 Notes | | $ | 2,330 | | | $ | 19,170 | |
| Unamortized discount of liability component under Going Concern Basis | | 0 | | | (2,220) | |
| Expected settlement premium under Liquidation Basis | | 132 | | | 0 | |
| Net carrying value of the December 2021 Notes | | $ | 2,462 | | | $ | 16,950 | |
Interest expense for the December 2021 Notes included in the Company’s Consolidated Statements of Operations was as follows:
| | | | | | | | | | | | | | | | | | | |
| Eight Months Ended | | Year Ended December 31, |
| (in thousands) | August 31, 2020 | | 2019 | | 2018 | | |
| | | | | | | |
| Contractual coupon interest | $ | 281 | | | $ | 3,390 | | | $ | 4,125 | | | |
| Amortization of debt issuance costs | 5 | | | 64 | | | 76 | | | |
| Amortization of debt discount | 39 | | | 459 | | | 542 | | | |
| Amortization of conversion feature | 544 | | | 5,973 | | | 6,611 | | | |
| Total | $ | 869 | | | $ | 9,886 | | | $ | 11,354 | | | |
As of December 31, 2020, the December 2021 Notes are convertible.
Capped Call Transaction
In connection with the offering of the December 2021 Notes, the Company entered into a privately-negotiated capped call transaction with an affiliate of the underwriter of such issuance. The aggregate cost of the capped call transaction was $14.4 million. The capped call transaction is generally expected to reduce the potential dilution upon conversion of the December 2021 Notes and/or partially offset any cash payments the Company is required to make in excess of the principal amount of converted December 2021 Notes in the event that the market price per share of the Company’s common stock, as measured under the terms of the capped call transaction, is greater than the strike price of the capped call transaction. This initially corresponds to the approximate $3.81 per share conversion price of the December 2021 Notes and is subject to anti-dilution adjustments substantially similar to those applicable to the conversion rate of the December 2021 Notes. The cap price of the capped call transaction was initially $4.88 per share and is subject to certain adjustments under the terms of the capped call transaction. Both the per share conversion price and cap price were adjusted for the above-noted changes to the conversion rate of the December 2021 Notes that occurred during 2020. The Company will not be required to make any cash payments to the option counterparty upon the exercise of the options that are a part of the capped call transaction, but the Company will be entitled to receive from it an aggregate amount of cash and/or number of shares of the Company’s common stock, based on the settlement method election chosen for the related convertible senior notes, with a value equal to the amount by which the market price per share of the Company’s common stock, as measured under the terms of the capped call transaction, is greater than the strike price of the capped call transaction during the relevant valuation period under the capped call transaction, with such number of shares of the Company’s common stock and/or amount of cash subject to the cap price.
The Company evaluated the capped call transaction under authoritative accounting guidance and determined that it should be accounted for as a separate transaction and classified as a net reduction to additional paid-in capital within stockholders’ equity with no recurring fair value measurement recorded.
In connection with the September 2019 Exchange Transaction, the Company unwound a portion of the capped call entered into when the December 2021 Notes were issued, as they were no longer scheduled to mature in 2021. This generated proceeds to the Company of $0.9 million. The $0.9 million proceeds from the unwind of the capped call, which reflected the value of the options outstanding at the time of the September 2019 Exchange Transaction and the average share price of the Company’s common stock were included as an increase to Additional paid-in capital within stockholders’ equity.
In connection with the December 2019 Exchange Transaction, the Company unwound a corresponding portion of the capped call related to the notes and repurchased 1.6 million shares of its common stock from the counterparty. The Company paid the capped call counterparty $3.1 million, representing $5.6 million for the common stock repurchased from the counterparty, net of $2.5 million owed from the counterparty to the Company for unwinding the capped call. The common stock repurchased was reflected as a decrease to Retained earnings within stockholders’ equity. The proceeds from the capped call were included as an increase to Additional paid-in capital within stockholders’ equity.
In connection with the September 2020 Conversion and the December 2020 Repurchases, the Company unwound a corresponding portion of the capped call which generated aggregate proceeds to the Company of $1.3 million.
As of December 31, 2020, under the Liquidation Basis, the estimated cash expenditures of the December 2021 Notes was $2.5 million, this includes principal, interest and other payments expected to repurchase or convert the notes to cash. For additional information, see Note 3, Net Assets in Liquidation.
December 2024 Notes
On September 17, 2019, in connection with the September 2019 Exchange Transaction, the Company exchanged $86.1 million aggregate principal of December 2021 Notes for an identical aggregate original principal amount of December 2024 Notes, plus a cash payment of $70.00 for each $1,000 principal amount exchanged, totaling approximately $6.0 million. The December 2024 Notes were scheduled to mature on December 1, 2024. The Company paid interest at 2.75% on the December 2024 Notes semiannually in arrears on June 1 and December 1 of each year, beginning December 1, 2019. The original principal of the December 2024 Notes was expected to accrete at a rate of 2.375% per year (“Accretion Interest”) commencing September 17, 2019 through the maturity of the December 2024 Notes. The accreted principal amount of the December 2024 Notes was payable in cash upon maturity and is included in “Other long-term liabilities” on the Company’s Consolidated Balance Sheet as of December 31, 2019.
Upon the occurrence of a fundamental change, as defined in the indenture entered into in connection with the December 2024 Notes (the “December 2024 Notes Indenture”), holders have the option to require the Company to repurchase their December 2024 Notes at a purchase price equal to 100% of the accreted principal amount of such December 2024 Notes, plus accrued interest on the original principal amount thereon.
Prior to the delisting of its common stock, the December 2024 Notes were convertible into PDL common stock upon the occurrence of specified corporate events as described in the December 2024 Notes Indenture or under either of the following circumstances at any time prior to the close of business on the business day immediately preceding June 1, 2024 (or at any time beginning on June 1, 2024 until the close of business on the second scheduled trading day immediately preceding the stated maturity):
•During any fiscal quarter (and only during such fiscal quarter) commencing after the fiscal quarter ended December 31, 2019, if the last reported sale price of Company common stock for at least 20 trading days (whether or not consecutive), in the period of 30 consecutive trading days, ending on, and including, the last trading day of the immediately preceding fiscal quarter, exceeds 130% of the conversion price for the notes on each applicable trading day; or
•During the five business-day period immediately after any five consecutive trading-day period, which the Company refers to as the measurement period, in which the trading price per $1,000 original principal amount of notes for each trading day of that measurement period was less than 98% of the product of the last reported sale price of Company common stock and the conversion rate for the notes for each such trading day.
In accordance with the terms of the December 2024 Notes Indenture, the Company had the right, but not the obligation, to redeem all or any portion of the December 2024 Notes that is equal to $1,000 original principal amount or an integral multiple of $1,000 prior to their scheduled maturity on a redemption date beginning on or after December 1, 2021 and on or before the 60th scheduled trading day before December 1, 2024, for a cash purchase price equal to the redemption price, but only if the last reported sale price of Company common stock exceeds 128% of the conversion price for the December 2024 Notes on (i) each of at least 20 trading Days (whether or not consecutive) during the 30 consecutive trading days ending on, and including, the trading day immediately before the redemption notice date for such redemption; and (ii) the trading day immediately before such redemption notice date. The redemption price for the December 2024 Notes called for redemption is equal to the then accreted principal amount of such December 2024 Notes plus accrued but unpaid interest on the original principal amount thereon. The calling of any December 2024 Notes for redemption would constitute a make-whole fundamental change with respect to such notes, entitling the holders who convert such December 2024 Notes called for redemption prior to the applicable redemption date to receive an increase in the applicable conversion rate, as described in the December 2024 Notes Indenture.
The initial conversion rate for the December 2024 Notes was 262.2951 shares of the Company’s common stock per $1,000 original principal amount of December 2024 Notes, which was equivalent to an initial conversion price of approximately $3.81 per share of common stock, subject to adjustments upon the occurrence of certain specified events as set forth in the December 2024 Notes Indenture, the following of which occurred in 2020:
•Upon the distribution by the Company of its stock in Evofem to the PDL stockholders on May 21, 2020, the conversion rate for the December 2024 Notes increased from 262.2951 to 316.5801 shares of the Company’s common stock per $1,000 principal amount of December 2024 Notes equating to a conversion price of $3.16 per share of common stock.
•Upon the stockholders’ approval of the dissolution of the Company on August 19, 2020, a Fundamental Make-Whole Change, as defined in the December 2024 Notes Indenture, was triggered and the conversion rate for the December 2024 Notes was temporarily increased by 62.2203 to 378.8004 shares of the Company’s common stock during the conversion period.
•Upon the spin-off of the Company’s majority-owned subsidiary, LENSAR, On October 1, 2020 the conversion rate increased for the December 2024 Notes from 316.5801 to 410.6268 shares of the Company’s common stock per $1,000 principal amount of December 2024 Notes equating to a conversion price of $2.44 per share of common stock.
In accordance with the accounting guidance for an extinguishment of convertible debt instruments with a cash conversion feature, the Company was required to allocate the fair value of the consideration transferred between the liability component and the equity component. To calculate the fair value of the debt immediately prior to derecognition, the carrying value was recalculated in a manner that reflected the estimated market interest rate for a similar nonconvertible instrument at the date of issuance. Using an assumed borrowing rate of 7.05% the Company calculated the fair value of the debt representing the amount allocated to the liability component of the December 2024 Notes with the remainder of the consideration allocated to the equity conversion feature, to reflect the reacquisition of the embedded conversion option. The conversion feature together with the fees allocated to the debt are accounted for as a debt discount. As a result of the September 2019 Exchange Transaction, the Company recorded a total debt discount of $9.4 million, which included the cash conversion feature of $8.1 million and the debt issuance fees of $1.3 million, charged $5.5 million to Additional paid-in capital ($13.5 million charge to Additional paid-in capital representing the reduction to the 2021 equity component, partially offset by the $8.1 million allocated to equity for the 2024 notes) and recorded $1.2 million to deferred tax liability. The net amount charged to Additional paid-in capital represents the difference between the consideration paid for the September 2019 Exchange Transaction and the fair value of the convertible debt prior to the extinguishment.
The Accretion Interest and debt discount, including the conversion feature and issuance costs allocated to debt, were being amortized to interest expense over the term of the December 2024 Notes until the adoption of the Liquidation Basis and increased, until such time, interest expense for the December 2024 Notes from the 2.75% cash coupon interest rate to an effective interest rate of 7.5%.
On December 17, 2019, in connection with the December 2019 Exchange Transaction, the Company repurchased $74.6 million in aggregate principal amount of its December 2024 Notes for $58.0 million in cash and 9.9 million shares of its common stock in privately negotiated transactions. It was determined that the repurchase of the principal amount should be accounted for as a partial extinguishment of the December 2024 Notes. As a result, a loss on extinguishment of $2.1 million was recorded at closing of the transaction. The loss on extinguishment included the de-recognition of a proportional share of the deferred issuance costs of $1.1 million.
The approval of the Plan of Dissolution by the Company’s stockholders in August 2020 provided for a Fundamental Change Repurchase Right, as defined in the December 2024 Notes Indenture, to each holder of the December 2024 Notes which would require the Company, at the holder’s option, to repurchase for cash on September 29, 2020 such holder’s notes, or any portion of the principal amount thereof, equal to $1,000 or an integral multiple of $1,000. No holders tendered their notes for repurchase under this Fundamental Change Repurchase Right.
The approval of the Plan of Dissolution by the Company’s stockholders also initiated a Fundamental Make-Whole Change allowing the holders of the December 2024 Notes to exercise their conversion rights during the conversion period for a cash amount equal to the conversion rate as defined in the December 2024 Notes Indenture (the “September 2020 Conversion”). No holders exercised their conversion right for the December 2024 Notes.
In December 2020, the Company repurchased $1.0 million par value of December 2024 Notes in a privately negotiated transaction for an aggregate amount of $1.1 million, including interest.
There were no December 2024 Notes outstanding as of December 31, 2020.
The carrying value, accretion and unamortized discount of the December 2024 Notes under the Going Concern Basis were as follows:
| | | | | | | | | | | | |
| (in thousands) | | | | December 31, 2019 | | |
| | | | | | |
| Principal amount of the December 2024 Notes | | | | $ | 11,500 | | | |
| Unamortized discount of liability component | | | | (1,200) | | | |
| Net carrying value of the December 2024 Notes | | | | $ | 10,300 | | | |
Interest expense for the December 2024 Notes included in the Company’s Consolidated Statement of Operations was as follows:
| | | | | | | | | | | |
| Eight Months Ended | | Year Ended |
| (in thousands) | August 31, 2020 | | December 31, 2019 |
| | | |
| Contractual coupon interest | $ | 49 | | | $ | 598 | |
| Accretion Interest on outstanding principal | 42 | | 517 | |
| Amortization of debt issuance costs | 6 | | 53 | |
| Amortization of conversion feature | 29 | | 350 | |
| Total | $ | 126 | | | $ | 1,518 | |
Capped Call Transaction
In connection with the issuance of the December 2024 Notes in the September 2019 Exchange Transaction, the Company entered into a privately-negotiated capped call transaction with an affiliate of the underwriter of such issuance. The aggregate cost of the capped call transaction was $4.5 million. The capped call transaction is generally expected to reduce the potential dilution upon conversion of the December 2024 Notes and/or partially offset any cash payments the Company is required to make in excess of the principal amount of converted December 2024 Notes in the event that the market price per share of the Company’s common stock, as measured under the terms of the capped call transaction, is greater than the strike price of the capped call transaction. This initially corresponds to the approximate $3.81 per share conversion price of the December 2024 Notes and is subject to anti-dilution adjustments substantially similar to those applicable to the conversion rate of the December 2024 Notes. The cap price of the capped call transaction was initially $4.88 per share and is subject to certain adjustments under the terms of the capped call transaction. Both the per share conversion price and cap price were adjusted for the above-noted changes to the conversion rate of the December 2024 Notes that occurred during 2020. The Company will not be required to make any cash payments to the option counterparty upon the exercise of the options that are a part of the capped call transaction, but the Company will be entitled to receive from it an aggregate amount of cash and/or number of shares of the Company’s common stock, based on the settlement method election chosen for the related convertible senior notes, with a value equal to the amount by which the market price per share of the Company’s common stock, as measured under the terms of the capped call transaction, is greater than the strike price of the capped call transaction during the relevant valuation period under the capped call transaction, with such number of shares of the Company’s common stock and/or amount of cash subject to the cap price.
The Company evaluated the capped call transaction under authoritative accounting guidance and determined that it should be accounted for as separate transaction from the debt as it was entered into with a separate counterparty and does not relate to the same risk. The $4.5 million premium for the capped call was classified as a reduction to Additional paid-in capital within stockholders’ equity and will not be subject to recurring fair value measurement.
In connection with the December 2019 Exchange Transaction, the Company unwound a corresponding portion of the capped call related to the notes and repurchased 1.6 million shares of its common stock from the counterparty. The Company paid the capped call counterparty $1.2 million, representing $5.4 million for the common stock repurchased from the counterparty, net of $4.2 million owed from the counterparty to the Company for unwinding the capped call. The common stock repurchased was reflected as a decrease to Retained earnings within stockholders’ equity. The proceeds from the capped call were included as an increase to Additional paid-in capital within stockholders’ equity.
In connection with the repurchase of the December 2024 Notes in December 2020, the Company unwound a corresponding portion of the capped call which generated aggregate proceeds to the Company of $0.1 million.
As of December 31, 2020, the future minimum principal payments under the December 2021 and December 2024 Notes were:
| | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | December 2021 Notes | | December 2024 Notes | | Total |
| | | | | | |
| 2021 | | $ | 405 | | | $ | 0 | | | $ | 405 | |
| 2022 | | 0 | | | 0 | | | 0 | |
| 2023 | | 0 | | | 0 | | | 0 | |
| 2024 | | 0 | | | 0 | | | 0 | |
| 2025 | | 0 | | | 0 | | | 0 | |
| Thereafter | | 0 | | | 0 | | | 0 | |
| Total | | $ | 405 | | | $ | 0 | | | $ | 405 | |
15. Other Long-Term Liabilities
Other long-term liabilities under the Going Concern Basis consisted of the following:
| | | | | | | | | | |
| (in thousands) | | | | December 31,2019 |
| | | | |
| Uncertain tax positions | | | | $ | 37,574 | |
| Deferred tax liability | | | | 1,571 | |
| Accrued lease liability | | | | 10,700 | |
| | | | |
| | | | |
| | | | |
| | | | |
| Other | | | | 1,020 | |
Total (1) (2) | | | | $ | 50,865 | |
________________
(1) The amounts above exclude $0.1 million of Other long-term liabilities at Noden classified as held for sale as of December 31, 2019. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
(2) The amounts above include amounts held by LENSAR which was spun-off on October 1, 2020.
16. Commitments and Contingencies
Lease Guarantee
In connection with the spin-off by the Company of Facet Biotech Corporation (“Facet”), the Company entered into amendments to the leases for the Company’s former facilities in Redwood City, California, under which Facet was added as a co-tenant, and a Co-Tenancy Agreement, under which Facet agreed to indemnify the Company for all matters related to the leases attributable to the period after the spin-off date. In April 2010, Abbott Laboratories acquired Facet and later renamed the entity AbbVie Biotherapeutics, Inc. (“AbbVie”). If AbbVie were to default under its lease obligations, the Company could be held liable by the landlord as a co-tenant and, thus, the Company has in substance guaranteed the payments under the lease agreements for the Redwood City facilities. As of December 31, 2020, the total lease payments for the duration of the guarantee, which runs through December 2021 and is non-extendible, are approximately $11.3 million.
The Company prepared a discounted, probability weighted cash flow analysis to calculate the estimated fair value of the lease guarantee as of the spin-off. The Company was required to make assumptions regarding the probability of Facet’s default on the lease payment, the likelihood of a sublease being executed and the times at which these events could occur. These assumptions are based on information that the Company received from real estate brokers and the then-current economic conditions, as well as expectations of future economic conditions. The fair value of this lease guarantee was charged to Additional paid-in capital upon the spin-off and any future adjustments to the carrying value of the obligation will also be recorded in Additional paid-in capital.
The Company has recorded a liability of $10.7 million as of December 31, 2020 and 2019, related to this guarantee.
Wind Down Payments to Stock Option Holders
The Wind Down Retention Plan provides for equitable adjustments to outstanding stock options held by participants to ensure such participants realize the same benefits provided to shareholders in the event one or more cash or other distributions become payable to shareholders. Consistent with the existing terms of the Equity Plan, in the event one or more cash or other distributions
are paid to shareholders, the exercise price of outstanding stock options will be reduced on a dollar-for-dollar basis to reflect the per share value of such distributions. In the event that the Company declares cash or other distributions that, in the aggregate, exceed the difference between the exercise price of an outstanding stock option and the par value of the underlying shares ($0.01), the holder of such stock option will be entitled to receive from the Company a cash payment in an amount equal to the number of shares subject to such stock option multiplied by the per share amount of the cash or other distribution that exceeds the difference between exercise price of the outstanding option and the par value of the underlying shares (a “true-up payment”). A true-up payment is also paid with respect to a post-dissolution cash or other distributions with respect to a stock-option that was not exercised prior to dissolution in the same fashion as provided above. True-up payments are to be made on the same date that cash or other distributions are paid or made to the Company’s stockholders. As of December 31, 2020, the Company has 11,141,051 stock options outstanding at a weighted average adjusted exercise price of $1.87. As of January 4, 2021, the date we filed our Certificate of Dissolution, the Company was unable to issue stock for any purpose, including to cover the exercise of employee options, and as a result such options became unexercisable.
Purchase Obligations
After the sale of Noden and the spin-off of LENSAR, the Company no longer has any purchase obligations and the Company was released from all of its guarantees for purchase obligations.
Escrow Receivable
SWK Royalty Asset Escrow
The proceeds from the sale of the three royalty interests to SWK totaled $4.35 million, 90% of which was received at the closing of the transaction. The remaining 10% or $435 thousand, included in “Receivables from asset sales” in the Statement of Net Assets in Liquidation, is currently held in escrow against certain potential contingencies and is to be released on the one-year anniversary of the closing, subject to the satisfaction of any such potential contingencies.
17. Stockholders’ Equity
Dividends
On May 5, 2020, our Board, pursuant to the Plan of Liquidation, approved a distribution of all of the Company’s shares of common stock of Evofem via a liquidation distribution to PDL stockholders. The Evofem shares were distributed on May 21, 2020 to PDL shareholders of record as of the close of business on May 15, 2020 (the “Evofem Record Date”). Based on the shares of PDL common stock outstanding as of the close of business on the Evofem Record Date, PDL stockholders were entitled to receive 0.11591985 shares of Evofem common stock for each share of PDL common stock held.
On September 10, 2020, the Company’s Board, pursuant to the Plan of Liquidation, approved a distribution of all of the Company’s shares of common stock of LENSAR via a liquidation distribution to PDL stockholders. The LENSAR shares were distributed on October 2, 2020 to PDL shareholders of record as of the close of business on September 22, 2020 (the “LENSAR Record Date”). Based on the shares of PDL common stock outstanding as of the close of business on the LENSAR Record Date, PDL stockholders were entitled to receive 0.075879 shares of LENSAR common stock for each share of PDL common stock held.
Stock Repurchase Program
On March 1, 2017, the Company announced that its Board authorized the repurchase through March 2018 of issued and outstanding shares of the Company’s common stock having an aggregate value of up to $30.0 million pursuant to a share repurchase program. The repurchases under the share repurchase program were made from time to time in the open market or in privately negotiated transactions and were funded from the Company’s working capital. All shares of common stock repurchased under the Company’s share repurchase program were retired and restored to authorized but unissued shares of common stock at June 30, 2017. The Company repurchased 13.3 million shares of its common stock under the share repurchase program during the fiscal year ended December 31, 2017 for an aggregate purchase price of $30.0 million, or an average cost of $2.25 per share, including trading commissions.
On September 25, 2017, the Company announced that its Board authorized the repurchase of issued and outstanding shares of the Company’s common stock having an aggregate value of up to $25.0 million pursuant to a share repurchase program. The repurchases under the share repurchase program were made from time to time in the open market or in privately negotiated
transactions and were funded from the Company’s working capital. All shares of common stock repurchased under this share repurchase program were retired and restored to authorized but unissued shares of common stock. The Company repurchased 8.7 million shares of its common stock under the share repurchase program during the fiscal year ended December 31, 2018, for an aggregate purchase price of $25.0 million, or an average cost of $2.86 per share, including trading commissions.
On September 24, 2018, the Company announced that its Board authorized the repurchase of issued and outstanding shares of the Company’s common stock having an aggregate value of up to $100.0 million pursuant to a share repurchase program. Repurchases under this share repurchase program were made from time to time in the open market or in privately negotiated transactions and funded from the Company’s working capital. All shares of common stock repurchased under this repurchase program were retired and restored to authorized but unissued shares of common stock at July 31, 2019. The Company repurchased 31.0 million shares of its common stock under this share repurchase program for an aggregate purchase price of $100.0 million, or an average cost of $3.22 per share, including trading commissions.
On December 9, 2019, the Company announced that its Board authorized the repurchase of issued and outstanding shares of the Company's common stock and convertible notes up to an aggregate value of $200 million. On December 16, 2019, the Company announced that its Board approved a $75 million increase to the aforementioned $200 million repurchase program to acquire outstanding PDL common stock and convertible notes. Repurchases under the new repurchase program were made from time to time in the open market or in privately negotiated transactions and funded from the Company’s working capital. All shares of common stock repurchased under the Company’s repurchase program were retired and restored to authorized but unissued shares of common stock. All convertible notes repurchased under the program were retired. During the year ended December 31, 2019, the Company repurchased $44.8 million in aggregate principal amount of 2021 Convertible Notes and $74.6 million in aggregate principal amount of 2024 Convertible Notes for consideration consisting of a cash payment of $97.9 million and the issuance of 13.4 million shares of the Company’s common stock. During the eight months ended August 31, 2020, the Company repurchased $5.4 million in aggregate principal amount of 2021 Convertible Notes and $10.5 million in aggregate principal amount of 2024 Convertible Notes for cash payments totaling $18.8 million. During the eight months ended August 31, 2020, the Company repurchased 12.3 million shares of its common stock under the share repurchase program for an aggregate purchase price of $39.4 million, or an average cost of $3.20 per share, including trading commissions.
Upon the approval by the Company’s stockholders 2020 to seek dissolution in August 2020 during the Annual Shareholder Meeting, holders of $11.2 million par value of December 2021 Notes exercised their conversion right for an aggregate amount of $12.0 million. Such notes were repurchased entirely for cash and were retired prior to December 31, 2020. In December 2020, the Company repurchased an additional $2.2 million par value of December 2021 Notes in separate privately negotiated transactions for an aggregate amount of $2.3 million, including interest and repurchased $1.0 million par value of December 2024 Notes in a privately negotiated transaction for an aggregate amount of $1.1 million, including interest.
As of December 31, 2020, the Company had repurchased 12.3 million shares of its common stock under the share repurchase program for an aggregate purchase price of $39.4 million, or an average cost of $3.20 per share, including trading commissions.
18. Accumulated Other Comprehensive Income
Under the Going Concern Basis, comprehensive income is comprised of net loss and other comprehensive loss. The Company includes unrealized net gains (losses) on investments held in its available-for-sale securities in other comprehensive loss, and presents the amounts net of tax. The Company’s other comprehensive loss is included in the Company’s Consolidated Statements of Comprehensive Loss.
The balance of “accumulated other comprehensive loss,” net of tax, was as follows:
| | | | | | | | | | | | | | | | | | |
| (in thousands) | | Unrealized gains
(losses) on
available-for-
sale securities | | | | | | Total Accumulated
Other
Comprehensive
Income |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Balance at December 31, 2017 | | $ | 1,181 | | | | | | | $ | 1,181 | |
| | | | | | | | |
| Activity for the year ended December 31, 2018 | | (1,181) | | | | | | | (1,181) | |
| Ending Balance at December 31, 2018 | | 0 | | | | | | | 0 | |
| | | | | | | | |
| Activity for the year ended December 31, 2019 | | 0 | | | | | | | 0 | |
| Ending Balance at December 31, 2019 | | 0 | | | | | | | 0 | |
| | | | | | | | |
| Activity for the eight months ended August 31, 2020 | | 0 | | | | | | | 0 | |
| Ending Balance at August 31, 2020 | | $ | 0 | | | | | | | $ | 0 | |
19. Stock-Based Compensation
The Company previously granted restricted stock awards and stock options pursuant to a stockholder approved stock-based incentive plan.
The following table summarizes the Company’s stock option and restricted stock award compensation expense during the eight months ended August 31, 2020 and years ended December 31, 2019 and 2018:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| Stock-based Compensation | | 2020 | | 2019 | | 2018 |
| (in thousands) | | | | | | |
Employees and directors (1) | | $ | 18,802 | | | $ | 6,834 | | | $ | 4,337 | |
| | | | | | |
| | | | | | |
_______________
(1) Stock option and restricted stock award compensation expense from discontinued operations are excluded from the table above.
The fair value of each stock option grant was estimated on the date of grant using the Black-Scholes option-pricing model. Expected volatility was based on the historical volatility of the Company’s common stock over the estimated expected life of the options. The expected term represents the period of time the options were expected to be outstanding. The expected term is based on the “simplified method” as defined by the SEC Staff Accounting Bulletin No. 110 (Topic 14.D.2). The Company used the “simplified method” due to the lack of sufficient historical exercise data to provide a reasonable basis upon which to otherwise estimate the expected life of the options. The risk-free rate was based on yields on U.S. Treasury securities with a maturity similar to the estimated expected term of the options. The fair value of restricted stock awards was based on the closing price of the Company’s common stock on the grant date.
The fair value of the Company’s stock options granted in the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018, were estimated assuming no expected dividends and the following weighted-average assumptions:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| | 2020 | | 2019 | | 2018 | | |
| | | | | | | | | | | | |
| Range of expected term (in years) | | 1.0 | | 3.5 | - | 6.1 | | 3.5 | - | 6.0 | | | | |
| Range of risk-free interest rate | | 0.1% | | 1.5% | - | 3.0% | | 2.7% | - | 3.0% | | |
| Expected volatility | | 55% | | 40% | | 40% | | |
Stock-Based Incentive Plans
On February 7, 2020, the Board approved the Plan of Liquidation, which accelerated the vesting of a significant portion of the Company’s outstanding equity awards pursuant to provisions in the Wind Down Retention Plan. The Wind Down Retention Plan further provides for equitable adjustments to outstanding stock options held by participants to ensure such participants realize the same benefits provided to shareholders in the event one or more cash or other distributions become payable to shareholders. Consistent with the existing terms of the Equity Plan, in the event one or more cash or other distributions are paid to shareholders, the exercise price of outstanding stock options will be reduced on a dollar-for-dollar basis to reflect the per share value of such distribution. In the event that the Company declares cash or other distributions that, in the aggregate, exceed the difference between the exercise price of an outstanding stock option and the par value of the underlying shares ($0.01), the holder of such stock option will be entitled to receive from the Company a cash payment in an amount equal to the number of shares subject to such stock option multiplied by the per share amount of the cash or other distribution that exceeds the difference between exercise price of the outstanding option and the par value of the underlying shares (a “true-up payment”). A true-up payment is also paid with respect to a post-dissolution cash or other distributions with respect to a stock-option that was not exercised prior to dissolution in the same fashion as provided above. True-up payments are to be made on the same date that cash or other distributions are paid or made to the Company’s stockholders. In May 2020, in accordance with this provision and in conjunction with the Evofem distribution, the exercise price of the outstanding option awards was decreased by $0.58 per share. In October 2020, in accordance with this provision and in conjunction with the LENSAR distribution, the exercise price of the outstanding option awards was decreased by $0.78 per share. As of December 31, 2020, the Company has 11,141,051 stock options outstanding at a weighted average adjusted exercise price of $1.87. As of January 4, 2021, the date we filed our Certificate of Dissolution, the Company was unable to issue stock for any purpose, including to cover the exercise of employee options, and as a result such options became unexercisable.
2005 Equity Incentive Plan
The Company had one active stock-based incentive plan under which it previously granted stock-based awards to the Company’s employees, directors and non-employees.
Under the Company’s Amended and Restated 2005 Equity Incentive Plan effective June 8, 2018 (the “2005 Equity Incentive Plan”), the Company was authorized to issue a variety of incentive awards, including stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance share and performance unit awards, deferred compensation awards and other stock-based or cash-based awards. As of December 31, 2020, awards granted under the 2005 Equity Incentive Plan consisted of stock options and restricted stock awards. There were no other grants of any other award types under the 2005 Equity Incentive Plan. Upon the Company’s filing for dissolution in January 2021, the Company is no longer authorized to issue equity awards under the 2005 Equity Incentive Plan.
In June 2018, the Company’s stockholders approved an amendment and restatement of the 2005 Equity Incentive Plan that increased the number of shares available for grant by 15,000,000 to 26,200,000. The number of shares of common stock authorized for issuance, shares of common stock issued upon exercise of options or grant of restricted stock awards, shares of common stock subject to outstanding awards and shares available for grant under this plan as of December 31, 2020, are as follows:
| | | | | | | | | | | | | | | | | | | | | | |
| Title of Plan | | Total Shares of Common Stock Authorized | | Total Shares of Common Stock Issued | | | | Total Shares of Common Stock Available for Grant |
| | | | | | | | |
| 2005 Equity Incentive Plan | | 26,200,000 | | | 17,731,795 | | | | | 8,468,205 | |
Stock Options
The following table summarizes the option activity under the 2005 Equity Incentive Plan for the year ended December 31, 2020:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Options | | Weighted-Average Adjusted Exercise Price | | Weighted-Average Remaining Contractual Term (Years) | | Aggregate Intrinsic Value |
| | (in thousands) | | | | (in thousands) |
| Outstanding at beginning of year | | 11,652 | | | $ | 1.76 | | | 8.5 | | $ | 3,473 | |
| Granted | | 630 | | | $ | 2.78 | | | | | |
| Exercised | | (1,375) | | | $ | 1.84 | | | | | |
| Forfeited | | (728) | | | $ | 3.08 | | | | | |
| Outstanding at end of year | | 10,180 | | | $ | 1.87 | | | 7.8 | | $ | 6,298 | |
| | | | | | | | |
| Exercisable at end of year | | 10,180 | | | $ | 1.87 | | | 7.8 | | $ | 6,298 | |
Options to purchase common stock generally vested over a 3 or 4-year period and were generally granted for a term of 10 years.
The weighted-average grant-date fair value of options granted during the year ended December 31, 2020 was $0.78 per share. The intrinsic value of options exercised during the year ended December 31, 2020 was $1.2 million and the Company received $0.7 million in cash related to the exercise of the option awards. In conjunction with the dissolution process, all outstanding options were fully vested in the year ended December 31, 2020.
Restricted Stock Awards
Restricted stock has the same rights as other issued and outstanding shares of the Company’s common stock, including, in some cases, the right to accrue dividends, which are held in escrow until the award vests. The compensation expense related to these awards was determined using the fair market value of the Company’s common stock on the date of the grant, and the compensation expense was recognized ratably over the vesting period. Under the Company’s restricted stock plans, restricted stock awards typically vested over one to five years and compensation expense associated with these awards was recognized on a straight-line basis over the vesting period. In addition to service requirements, vesting of restricted stock awards may have been subject to the achievement of specified performance goals set by the Compensation Committee. If the performance goals were not met, no compensation expense was recognized and any previously recognized compensation expense was reversed.
The following table summarizes the restricted stock award activity under the 2005 Equity Incentive Plan for the year ended December 31, 2020:
| | | | | | | | | | | |
| 2020 |
| Number of shares | | Weighted-average grant-date fair value per share |
| (in thousands) | | |
| Unvested at beginning of year | 933 | | | $ | 3.56 | |
| Awards granted | 3,044 | | | $ | 3.10 | |
| Awards vested | (2,872) | | | $ | 3.14 | |
| Withheld related to net settlement | (1,089) | | | $ | 3.39 | |
| Forfeited | (16) | | | $ | 3.22 | |
| Unvested at end of year | 0 | | | $ | 0 | |
The total fair value of restricted stock awards vested during the years ended December 31, 2020, 2019 and 2018 was approximately $9.0 million, $1.4 million and $2.1 million, respectively.
The weighted-average grant date fair value for restricted stock awards granted under the 2005 Equity Incentive Plan for the years end December 31, 2020, 2019 and 2018 was $3.10, $3.62 and $2.61, respectively.
At December 31, 2020, there was 0 unrecognized compensation expense related to stock options or restricted stock awards granted under the 2005 Equity Incentive Plan.
Inducement Award Agreements
On September 12, 2017, the Company granted 961,000 shares of common stock in the form of a non-statutory inducement stock option grant pursuant to a non-statutory inducement stock option agreement and granted 240,200 shares of our common stock in the form of an inducement restricted stock grant pursuant to an inducement restricted stock agreement. These inducement awards were not granted under the 2005 Equity Incentive Plan.
Inducement Stock Option Activity
As of December 31, 2020, all stock options awarded under the non-statutory inducement stock option agreement were outstanding and exercisable. All compensation costs related to these options have been fully recognized.
Inducement Restricted Stock
In the year ended December 31, 2020, the remaining 80,067 shares of restricted stock awarded under the non-statutory inducement restricted stock agreement were vested. The total fair value of the restricted stock awards vested during the year ended December 31, 2020 was approximately $0.3 million.
Compensation expense associated with unvested restricted stock awards was recognized on a straight-line basis over the vesting period. At December 31, 2020, there was 0 unrecognized compensation expense related to restricted stock awards granted under the non-statutory inducement restricted stock agreement.
20. Revenue from Contracts with Customers
Disaggregation of Revenue
The Company disaggregated its revenue from contracts with customers by segment and geographic location as the Company believed it best depicted how the nature, amount, timing and uncertainty of its revenue and cash flows were affected by economic factors. In the following table, revenue is disaggregated by segment and primary geographical market for the eight months ended August 31, 2020 and the year ended December 31, 2019:
| | | | | | | | | | | | | | | | | | | | | | | |
| Eight Months Ended August 31, 2020 | | Year Ended December 31, 2019 |
| (in thousands) | Medical Devices | | Pharmaceutical (1) | | Medical Devices | | Pharmaceutical (1) |
| | | | | | | |
| Primary geographical markets: | | | | | | | |
| North America | $ | 6,656 | | | $ | 10,093 | | | $ | 10,155 | | | $ | 26,034 | |
| Europe | 2,078 | | | 13,008 | | | 3,438 | | | 22,816 | |
| Asia | 4,137 | | | 6,378 | | | 11,536 | | | 6,243 | |
| Other | 200 | | | 0 | | | 433 | | | 0 | |
Total revenue from contracts with customers (2) | $ | 13,071 | | | $ | 29,479 | | | $ | 25,562 | | | $ | 55,093 | |
_______________
(1) The revenue from the Company’s Pharmaceutical segment for the eight months ended August 31, 2020 and the year ended December 31, 2019 is included in Loss from discontinued operations. For additional information, see Note 4, Discontinued Operations Classified as Assets held for sale.
(2) The table above does not include lease revenue from the Company’s Medical Devices segment of $2.1 million and $5.2 million for the eight months ended August 31, 2020 and the year December 31, 2019, respectively. For additional information, see Note 10, Leases.
Contract Balances
The following table provides information about receivables, contract assets and contract liabilities from contracts with customers:
| | | | | | | | | | |
| (in thousands) | | | | December 31, 2019 |
| | | | |
| Receivables, net | | | | $ | 10,377 | |
| Contract assets | | | | $ | 3,512 | |
| Contract liabilities | | | | $ | 4,024 | |
Receivables, Net—Receivables, net, included amounts billed and due from customers. The amounts due are stated at their net estimated realizable value and are classified as current or noncurrent based on the timing of when the Company expects to receive payment. The Company maintained an allowance for doubtful accounts to provide for the estimated amount of receivables that would not be collected. The allowance was based upon an assessment of customer creditworthiness, historical payment experience, the age of outstanding receivables and collateral to the extent applicable. Receivables, net for our Pharmaceutical segment were classified as a current asset and included in Assets held for sale. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
Contract Assets—The Company’s contract assets represented revenue recognized for performance obligations completed before an unconditional right to payment exists, and therefore invoicing or associated reporting from the customer regarding the computation of the net product sales had not yet occurred. The Company’s contract assets were only attributable to the Pharmaceutical segment, and as such classified contract assets in Assets held for sale on the Company’s Consolidated Balance Sheet.
| | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | Medical Devices | | Pharmaceutical | | Total |
| | | | | | |
| Contract assets at December 31, 2019 | | $ | 0 | | | $ | 3,512 | | | $ | 3,512 | |
| Contract assets recognized | | 0 | | | (6,730) | | | (6,730) | |
| Payments received | | 0 | | | 8,562 | | | 8,562 | |
| Contract assets disposed of | | 0 | | | (5,344) | | | (5,344) | |
| Contract assets at December 31, 2020 | | $ | 0 | | | $ | 0 | | | $ | 0 | |
Contract Liabilities—The Company’s contract liabilities consisted of deferred revenue for products sold to customers for which the performance obligation had not been completed by the Company. The Company classified deferred revenue as current or noncurrent based on the timing of when it expected to recognize revenue. The noncurrent portion of deferred revenue was included in Other long-term liabilities on the Company’s Consolidated Balance Sheet. The Pharmaceutical segment deferred revenue was classified as a current liability and included in Liabilities held for sale on the Company’s Consolidated Balance Sheet.
| | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | Medical Devices | | Pharmaceutical | | Total |
| | | | | | |
| Contract liabilities at December 31, 2019 | | $ | 1,075 | | | $ | 2,949 | | | $ | 4,024 | |
| Additions | | 658 | | | 1,659 | | | 2,317 | |
| Amounts recognized in revenue | | (851) | | | (888) | | | (1,739) | |
| Contract liabilities disposed of | | (882) | | | (3,720) | | | (4,602) | |
| Contract liabilities at December 31, 2020 | | $ | 0 | | | $ | 0 | | | $ | 0 | |
21. Segment Information
Information regarding the Company’s segments for the eight months ended August 31, 2020 and the year ended December 31, 2019 is as follows:
| | | | | | | | | | | | | | |
| Revenues by segment | | Eight Months Ended | | Year Ended |
| (in thousands) | | August 31, 2020 | | December 31, 2019 |
| | | | |
| Medical Devices | | $ | 15,211 | | | $ | 30,742 | |
| Strategic Positions | | 0 | | | 0 | |
| Pharmaceutical | | 0 | | | 0 | |
| Income Generating Assets | | 110 | | | (36) | |
| Total revenues | | $ | 15,321 | | | $ | 30,706 | |
________________
The table above excludes revenues related to discontinued operations. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
| | | | | | | | | | | | | | |
| (Loss) income by segment | | Eight Months Ended | | Year Ended |
| (in thousands) | | August 31, 2020 | | December 31, 2019 |
| | | | |
| Medical Devices | | $ | (4,454) | | | $ | (5,230) | |
| Strategic Positions | | (15,723) | | | 28,758 | |
Pharmaceutical (1) | | (15,855) | | | (19,048) | |
Income Generating Assets (1) | | (37,217) | | | (74,891) | |
| Total | | (73,249) | | | (70,411) | |
Change in fair value of warrants not allocated to segments (2) | | (4,047) | | | 0 | |
| Total net loss | | $ | (77,296) | | | $ | (70,411) | |
________________
(1) The (Loss) income by segment presented above includes amounts related to both continuing and discontinued operations. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
(2) The change in fair value of warrants not allocated to segments presented above includes the amounts related to the change in fair value of the Evofem warrants after the distribution of the Evofem common stock to PDL stockholders on May 21, 2020. The Strategic Positions segment ceased to be a reporting segment as of this date.
| | | | | | | | | | | |
| Long-lived assets by segment | | | | | Year Ended |
| (in thousands) | | | | | December 31, 2019 |
| | | | | |
| Medical Devices | | | | | $ | 2,435 | |
| Strategic Positions | | | | | 0 | |
Pharmaceutical (1) | | | | | 2,960 | |
| Income Generating Assets | | | | | 125 | |
| Total long-lived assets | | | | | $ | 5,520 | |
________________
(1) The amounts above include Property and Equipment in the Pharmaceutical segment classified as Assets held for sale. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
The operations for the Medical Devices segment were primarily located in the United States and the operations for the Pharmaceutical segment were primarily located in Italy, Ireland and the United States.
22. Concentration of Credit Risk
Product Line Concentration
The percentage of total revenue recognized, which individually accounted for 10% or more of the Company’s total revenues in one or more of the periods presented below, was as follows:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands) | | 2020 (1) | | 2019 (1) | | 2018 (1) |
| | | | | | |
| | | | | | |
| | | | | | |
| Biogen | | 0 | % | | 0 | % | | 14 | % |
| LENSAR | | 99 | % | | 100 | % | | 77 | % |
| | | | | | |
| | | | | | |
| | | | | | |
________________
(1) The amounts above exclude product sales in our Pharmaceutical segment and royalty rights classified as held for sale in the Income Generating Assets segment, each of which is included in the Statements of Operations as Loss from discontinued operations. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
Total revenues by geographic area are based on the country of domicile of the counterparty to the agreement are as follows:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands) | | 2020 | | 2019 | | 2018 |
| | | | | | |
| United States | | $ | 6,656 | | | $ | 15,151 | | | $ | 21,434 | |
| Europe | | 2,078 | | | 3,438 | | | 2,451 | |
| Rest of world | | 4,337 | | | 12,117 | | | 8,143 | |
Total revenues (1) | | $ | 13,071 | | | $ | 30,706 | | | $ | 32,028 | |
________________
(1) The amounts above exclude product sales in our Pharmaceutical segment and royalty rights held for sale in the Income Generating Assets segment, each of which is included in the Statements of Operations as Loss from discontinued operations. See Note 4, Discontinued Operations Classified as Assets Held for Sale, for additional information.
One customer accounted for more than 10% of accounts receivable, net as of December 31, 2019.
23. Income Taxes
For financial reporting purposes, Loss before income taxes from continuing operations includes the following components:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands) | | 2020 | | 2019 | | 2018 |
| United States | | $ | (60,820) | | | $ | (61,446) | | | $ | (39,518) | |
| Foreign | | 0 | | | 0 | | | 0 | |
| Total | | $ | (60,820) | | | $ | (61,446) | | | $ | (39,518) | |
The provision for income taxes from continuing operations for the eight months ended August 31, 2020 and the years ended December 31, 2019 and 2018 consisted of the following:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands) | | 2020 | | 2019 | | 2018 |
| Current income tax (benefit) expense | | | | | | |
| Federal | | $ | (14,316) | | | $ | 3,750 | | | $ | (271) | |
| State | | 1,051 | | | 2,046 | | | 1,033 | |
| Foreign | | 0 | | | 0 | | | 0 | |
| Total current | | (13,265) | | | 5,796 | | | 762 | |
| Deferred income tax (benefit) expense | | | | | | |
| Federal | | (4,340) | | | (10,978) | | | (7,932) | |
| State | | (175) | | | 830 | | | (615) | |
| Foreign | | 0 | | | 0 | | | 1,032 | |
| Total deferred | | (4,515) | | | (10,148) | | | (7,515) | |
| Total provision | | $ | (17,780) | | | $ | (4,352) | | | $ | (6,753) | |
A reconciliation of the income tax benefit from continuing operations computed using the U.S. statutory federal income tax rate compared to the income tax benefit for income from continuing operations included in the Consolidated Statements of Operations is as follows:
| | | | | | | | | | | | | | | | | | | | |
| | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands) | | 2020 | | 2019 | | 2018 |
| Tax at U.S. statutory rate on loss before income taxes | | $ | (12,772) | | | $ | (12,904) | | | $ | (8,299) | |
| Change in valuation allowance | | (3,762) | | | 3,613 | | | 875 | |
| State taxes | | 656 | | | 2,446 | | | (397) | |
| | | | | | |
| | | | | | |
| Change in uncertain tax positions | | 0 | | | 1,513 | | | 809 | |
| | | | | | |
| | | | | | |
| | | | | | |
| True-ups | | 0 | | | 249 | | | (27) | |
| CARES Act refund | | (5,406) | | | 0 | | | 0 | |
| Other | | 3,504 | | | 731 | | | 286 | |
| Total | | $ | (17,780) | | | $ | (4,352) | | | $ | (6,753) | |
Deferred tax assets and liabilities are determined based on the differences between financial reporting and income tax bases of assets and liabilities, as well as net operating loss carryforwards and are measured using the enacted tax rates and laws in effect
when the differences are expected to reverse. The significant components of the Company’s net deferred tax assets and liabilities from continuing operations are as follows:
| | | | | | | | | | | | | | |
| (in thousands) | | December 31, 2020 | | December 31, 2019 |
| | (Liquidation Basis) | | (Going Concern Basis) |
| Deferred tax assets: | | | | |
| Net operating loss carryforwards | | $ | 3,345 | | | $ | 3,602 | |
| Research and other tax credits | | 1,448 | | | 1,448 | |
| ASC 740-10 Reserve | | 6,455 | | | 8,419 | |
| Stock-based compensation | | 2,513 | | | 1,758 | |
| Accruals | | 84 | | | 1,388 | |
| | | | |
| Debt modifications | | 6,030 | | | 7,189 | |
| | | | |
| Capital loss carryforward | | 2,947 | | | 1,213 | |
| Other | | 51 | | | 1,145 | |
| Total deferred tax assets | | 22,873 | | | 26,162 | |
| Valuation allowance | | (21,105) | | | (7,465) | |
| Total deferred tax assets, net of valuation allowance | | 1,768 | | | 18,697 | |
| Deferred tax liabilities: | | | | |
| | | | |
| Debt modifications | | 0 | | | (308) | |
| Intangible assets | | (21,673) | | | (19,649) | |
| Other | | (16) | | | (311) | |
| | | | |
| Total deferred tax liabilities | | (21,689) | | | (20,268) | |
| Net deferred tax liabilities | | $ | (19,921) | | | $ | (1,571) | |
The CARES Act, among other provisions, permits Net Operating Loss (“NOL”) carryovers and carrybacks to offset 100% of taxable income for taxable years beginning before 2021. In addition, the CARES Act allows NOLs incurred in 2018, 2019, and 2020 to be carried back to each of the five preceding taxable years to generate a refund of previously paid income taxes at the 35% corporate tax rate then in effect. The Company was a significant taxpayer in the earlier eligible carryback years and expects that the NOL carryback provision of the CARES Act will result in a material cash benefit as a result of the 2020 ordinary tax losses generated and, to a lesser degree, for the 2019 tax year. The expected cash benefit to be received for ordinary losses incurred in 2020 totals $91.8 million. The Company received a refund of $2.1 million associated with 2019 ordinary losses that were carried back to an eligible carryback year.
As of December 31, 2020 and 2019, the Company had federal NOL carryforwards of $103.3 million and $108.6 million, respectively. As of December 31, 2020 and 2019, the Company also had state NOL carryforwards of $63.7 million and $63.9 million, respectively, excluding $215.5 million of California NOLs available to offset assessments, if any, resulting from the current audit by the California Franchise Tax Board (the “CA FTB”). The federal and state NOL carryforwards will begin expiring in the year 2023, if not utilized. As of December 31, 2020 and 2019, the Company had $2.2 million of federal tax credits that will begin expiring in the year 2025, if not utilized. As of December 31, 2020 and 2019, the Company had $19.3 million of state tax credit carryforwards that do not expire.
Utilization of the federal and state NOL and tax credit carryforwards may be subject to a substantial annual limitation due to the “change in ownership” provisions of the Internal Revenue Code of 1986. The annual limitation may result in the expiration of NOLs and credits before utilization. Of the Company’s $103.3 million of federal NOL carryforwards as of December 31, 2020, $26.9 million are subject to an annual limitation of $1.8 million for each of the years ending December 31, 2021 to 2022, and $1.3 million for the year ending December 31, 2023. As of December 31, 2020, the Company estimates that at least $22.0 million of federal NOL carryforwards will expire unutilized and none of the state NOLs will expire unutilized. Tax attributes acquired from LENSAR may be subject to separate return limitations that may limit the corporation’s ability to use the acquired NOLs and credits. Furthermore, under the Tax Cuts and Job Act of 2017 (the ‘2017 Tax Act’), although the treatment of tax losses generated in taxable years ending before December 31, 2017 has not changed, tax losses generated in taxable years beginning after December 31, 2017 may only be utilized to offset 80% of taxable income annually. This change may require the Company to pay additional federal income taxes in future years if additional losses are generated post 2017.
As of December 31, 2020, the Company determined that it was more likely than not that certain deferred tax assets from continuing operations would not be realized in the near future and had a $21.1 million valuation allowance against deferred tax assets. The net change in total valuation allowance for each of the years ending December 31, 2020 and 2019, was an increase of $13.6 million and $5.8 million, respectively. $2.9 million of the valuation allowance at December 31, 2020, is related to capital losses that have limited carryforward utilization. The Company does not have an expectation of future capital gains against which such losses could be utilized and as such determined that it was more likely than not that such deferred tax assets would not be realized. $18.2 million of the valuation allowance at December 31, 2020 is related to federal and state deferred tax assets that the Company determined it was more likely than not would be realized.
The 2017 Tax Act significantly changed the existing U.S. corporate income tax laws by, among other things, lowering the corporate tax rate (from a top rate of 35% to a flat rate of 21%), implementing elements of a territorial tax system, and imposing a one-time deemed repatriation transition tax on cumulative undistributed foreign earnings, for which the Company had not previously paid U.S. taxes.
A reconciliation of the Company’s unrecognized tax benefits, excluding accrued interest and penalties, for 2020, 2019 and 2018 is as follows:
| | | | | | | | | | | | | | | | | | | | |
| | December 31, |
| (in thousands) | | 2020 | | 2019 | | 2018 |
| Balance at the beginning of the year | | $ | 84,213 | | | $ | 80,783 | | | $ | 79,179 | |
| Increases related to tax positions from prior fiscal years | | 0 | | | 3,927 | | | 1,604 | |
| | | | | | |
| Increases related to tax positions taken during current fiscal year | | 8,412 | | | 0 | | | 0 | |
| Decreases related to tax positions from prior fiscal years | | (6,867) | | | (497) | | | 0 | |
| Balance at the end of the year | | $ | 85,758 | | | $ | 84,213 | | | $ | 80,783 | |
The future impact of the unrecognized tax benefit of $85.8 million, if recognized, is as follows: $32.6 million would affect the effective tax rate and $53.2 million would result in adjustments to deferred tax assets and valuation allowances. The Company periodically evaluates its exposures associated with our tax filing positions. The Company is currently under audit by the CA FTB. The timing of the audit resolution and the amount to be ultimately paid (if any) is uncertain. The outcome of the audit could result in the payment of tax amounts that differ from the amounts the Company has reserved for uncertain tax positions for the periods under audit resulting in incremental expense or a reversal of the Company’s reserves in a future period. At this time, the Company does not anticipate a material change in the unrecognized tax benefits related to the California FTB audit that would affect the effective tax rate, deferred tax assets or valuation allowances over the next 12 months.
Estimated interest and penalties associated with unrecognized tax benefits increased our income tax expense in the Consolidated Statements of Operations by $1.0 million during the eight months ended August 31, 2020, $1.6 million during the year ended December 31, 2019 and $1.0 million during the year ended December 31, 2018. Interest and penalties associated with unrecognized tax benefits accrued on the statement of net assets and balance sheet were $11.2 million, $9.7 million, and $8.0 million as of December 31, 2020, 2019 and 2018, respectively.
The Company’s U.S. federal income tax returns are subject to examination for the tax years 2017 forward, In general, the Company’s state and local income tax returns are subject to examination by tax authorities for tax years 2000 forward. The Company is currently under income tax examination by the CA FTB for the tax years 2009 through 2018.
24. Net Loss per Share
| | | | | | | | | | | | | | | | | |
| Net Loss per Basic and Diluted Share | Eight Months Ended August 31, | | Year Ended December 31, |
| (in thousands, except per share amounts) | 2020 | | 2019 | | 2018 |
| Numerator | | | | | |
| | | | | |
| | | | | |
| Net loss from continuing operations | $ | (43,040) | | | $ | (57,094) | | | $ | (32,765) | |
| Net loss from discontinued operations | $ | (34,915) | | | $ | (13,597) | | | $ | (36,094) | |
| Loss attributable to the PDL’s stockholders used to compute net loss per basic and diluted share | $ | (77,296) | | | $ | (70,411) | | | $ | (68,859) | |
| | | | | |
| Denominator | | | | | |
| Total weighted-average shares used to compute net loss attributable to PDL's stockholders, per basic share | 118,001 | | | 118,631 | | | 145,669 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Shares used to compute net loss attributable to PDL’s stockholders, per diluted share | 118,001 | | | 118,631 | | | 145,669 | |
| | | | | |
| Net loss per share - basic | | | | | |
| Continuing operations | $ | (0.36) | | | $ | (0.48) | | | $ | (0.22) | |
| Discontinued operations | $ | (0.30) | | | $ | (0.11) | | | $ | (0.25) | |
| Net loss attributable to PDL’s stockholders per basic share | $ | (0.66) | | | $ | (0.59) | | | $ | (0.47) | |
| | | | | |
| Net loss per share - diluted | | | | | |
| Continuing operations | $ | (0.36) | | | $ | (0.48) | | | $ | (0.22) | |
| Discontinued operations | $ | (0.30) | | | $ | (0.11) | | | $ | (0.25) | |
| Net loss attributable to PDL’s stockholders per diluted share | $ | (0.66) | | | $ | (0.59) | | | $ | (0.47) | |
The Company computes net loss per diluted share using the sum of the weighted-average number of common and common equivalent shares outstanding common equivalent shares used in the computation of net loss per diluted share include shares that may be issued pursuant to outstanding stock options and restricted stock awards in each case, on a weighted-average basis for the period they were outstanding, including, if applicable, the underlying shares using the treasury stock method.
The February 2018 Notes that were repaid on February 1, 2018, the December 2021 Notes and the December 2024 Notes allow, or previously allowed, for the settlement entirely or partially in cash, and are accounted for under the treasury stock method. Under the treasury stock method, the shares issuable upon conversion of the notes are not included in the calculation of diluted earnings per share except to the extent that the conversion value of the notes exceeds their principal amount. The effect of which, for diluted earnings per share purposes, is that only the number of shares of common stock that would be necessary to settle such excess, if the Company elected to settle such excess in shares, are included in the computation.
December 2021 Notes and December 2024 Notes Capped Call Potential Dilution
In November 2016, the Company issued $150.0 million in aggregate principal of the December 2021 Notes. In September 2019, the Company entered into the September 2019 Exchange Transaction through which it exchanged a portion of the December 2021 Notes for the December 2024 Notes. Both the December 2021 Notes and the December 2024 Notes provide in certain situations for the conversion of the outstanding principal amount into shares of the Company’s common stock at a predefined conversion rate. In conjunction with the issuance of the December 2021 Notes and the issuance of the December 2024 Notes pursuant to the September 2019 Exchange Transaction, the Company entered into capped call transactions, with a hedge counterparty. The capped call transactions are expected generally to reduce the potential dilution, and/or offset, to an extent, the cash payments the Company may choose to make in excess of the principal amount, upon conversion of the December 2021 Notes or the December 2024 Notes. The Company has excluded the capped call transaction from the net loss per diluted share computation as such securities would have an anti-dilutive effect and those securities should be considered separately rather than in the aggregate in determining whether their effect on net loss per diluted share would be dilutive or anti-dilutive. For additional information regarding the conversion rates and the capped call transactions related to the Company’s December 2021 Notes and December 2024 Notes; see Note 14, Convertible Senior Notes.
Anti-Dilutive Effect of Restricted Stock Awards and Stock Options
For the eight months ended August 31, 2020, and years ended December 31, 2019 and 2018, the Company excluded approximately 0.2 million, 1.0 million, and 1.1 million shares, respectively, underlying restricted stock awards, calculated on a weighted-average basis, from the Company’s net loss per diluted share calculations because their effect was anti-dilutive.
For the eight months ended August 31, 2020, and years ended December 31, 2019 and 2018, the Company excluded approximately 11.9 million, 11.2 million and 3.9 million shares underlying outstanding stock options, respectively, calculated on a weighted-average basis, from the Company’s net loss per diluted share calculations because their effect was anti-dilutive.
25. Legal Proceedings
Wellstat Litigation
On September 4, 2015, the Company filed in the Supreme Court of New York a motion for summary judgment in lieu of complaint which requested that the court enter judgment against Wellstat Diagnostics Guarantors for the total amount due on the Wellstat Diagnostics debt, plus all costs and expenses including lawyers’ fees incurred by the Company in enforcement of the related guarantees. On July 29, 2016, the court issued its Memorandum of Decision granting the Company’s motion for summary judgment and denying the Wellstat Diagnostics Guarantors’ cross-motion for summary judgment seeking a determination that they were no longer liable under the guarantees. The Supreme Court of New York held that the Wellstat Diagnostics Guarantors are liable for all “Obligations” owed by Wellstat Diagnostics to the Company. It did not set a specific dollar amount due, but ordered that a judicial hearing officer or special referee be designated to determine the amount of the Obligations owing, and awarded the Company its attorneys’ fees and costs in an amount to be determined. On July 29, 2016, the Wellstat Diagnostics Guarantors filed a notice of appeal from the Memorandum of Decision to the Appellate Division of the Supreme Court of New York. On February 14, 2017, the Appellate Division reversed the summary judgment decision of the Supreme Court in the Company’s favor, but affirmed the denial of the Wellstat Diagnostics Guarantors’ cross-motion for summary judgment. The Appellate Division determined that the action was inappropriate for summary judgment pursuant to New York Civil Practice Law & Rules section 3213 on procedural grounds, but specifically made no determination regarding whether the Company was entitled to a judgment on the merits. Pursuant to this decision, the action was remanded to the Supreme Court for further proceedings on the merits. The proceeding has been conducted as a plenary proceeding, with both parties having the opportunity to take discovery and file dispositive motions in accordance with New York civil procedure. On September 11, 2019, the Supreme Court of New York granted the Company’s summary judgment motion, the court holding that the guarantees executed by the Wellstat Diagnostics Guarantors are valid and enforceable, and that the Wellstat Diagnostics Guarantors are liable for the amount owed under the loan agreement. The court ordered a damages hearing before a special referee to calculate the amount owed under the loan agreement between Wellstat Diagnostics and the Company. On September 12, 2019, the Wellstat Diagnostics Guarantors filed a notice of appeal of the Supreme Court of New York’s decision on summary judgment. On September 17, 2019, the Wellstat Diagnostics Guarantors requested a stay of the enforcement of the New York Supreme Court’s decision pending their appeal of the decision, which was denied on November 21, 2019. A damages hearing was scheduled to begin before a judicial hearing officer on December 17, 2019. At the request of the judicial hearing officer, the parties agreed to mediate their dispute prior to the commencement of the damages hearing. As a result, no decision was made by the hearing officer with respect to the amount of damages owed to the Company.
On August 11, 2020, PDL entered into a Settlement and Mutual Release Agreement with all parties including Samuel J. Wohlstadter, Nadine H. Wohlstadter, Hyperion Catalysis International, Wellstat Vaccines, LLC, Wellstat ImmunoTherapeutics, LLC, Wellstat BioCatalysis, LLC, Wellstat AVT Investment, LLC, Wellstat Biologics Corporation, Wellstat Management Company, LLC, Wellstat Ophthalmics Corporation, Wellstat Therapeutics Corporation, Wellstat Therapeutics EU Limited, Duck Farm, Inc., Hebron Valley Farms, Inc., HVF, Inc., Hyperion Catalysis EU Limited, NHW, LLC, and SJW Properties, Inc., together with their respective successors and assigns, (collectively the “Wellstat Parties”), and Defined Diagnostics, LLC (f/k/a Wellstat Diagnostics, LLC) (“Diagnostics”), resolving all litigation between the Company and the Wellstat Parties (the “Settlement Agreement”).
Under the terms of the Settlement Agreement, the parties agreed that the Wellstat Parties would pay an amount of $7.5 million on the signing of the Settlement Agreement and either (1) $5.0 million by February 10, 2021 and $55.0 million by July 26, 2021; or (2) $67.5 million by July 26, 2021. Further under the terms of the Settlement Agreement, upon payment of either $5.0 million prior to April 21, 2021 or completion of the payment of $67.5 million by July 26, 2021, the Company will transfer to Diagnostics on an “as is” and “where is” basis certain assets currently owned by the Company which were acquired through the Company’s credit bid in 2017 for the assets of Diagnostics. If the Wellstat Parties or Diagnostics fail to make payment in full by July 26, 2021
as required under the terms of the Settlement Agreement, the Company is authorized to record judgment against the Wellstat Parties for an amount of $92.5 million or such lesser amount as may be owed under the Settlement Agreement as of that date. The foregoing description is qualified in its entirety by reference to the full text of the Settlement Agreement, a copy of which is expected to be filed with the Securities and Exchange Commission. On December 11, 2020, the Company sold its rights to the settlement agreement to a third-party. For additional information on the sale, see Note 9, Notes and Other Long-Term Receivables.
Glumetza Class Action Antitrust Litigation
On September 18, 2019, the City of Providence filed a civil antitrust suit on behalf of a putative class of payors in the Northern District of California against Bausch Health Companies, Inc., Salix Pharmaceuticals, Inc., Santarus, Inc., Assertio Therapeutics, Inc., Lupin Pharmaceuticals, Inc. and the Company, inter alia, alleging that a patent settlement agreement between Assertio and Lupin unlawfully restrained competition in an alleged market for Glumetza and its AB-rated generic equivalents sold in the United States. The plaintiffs claim that the settlement agreement violated the federal Sherman Act and various state antitrust laws. The Company was a named defendant by certain End Payor Plaintiffs (EPPs) due to its purchase from Assertio in 2013 of a royalty asset based on sales of Glumetza. On January 21, 2020, the EPPs voluntarily dismissed their claims against the Company, without prejudice. The Company agreed to toll the running of statute of limitations for a limited period of time and to respond to certain discovery requests, subject to reasonable objections, which time period has elapsed.
Noden Pharma DAC v Anchen Pharmaceuticals, Inc. et al
On June 12, 2017, Noden Pharma DAC filed a complaint against Anchen and Par Pharmaceutical (“Par”) for infringement of U.S. Patent No. 8,617,595 based on their submission of an ANDA seeking authorization from the FDA to market a generic version of Tekturna® aliskiren hemifumarate tablets, 150 mg and 300 mg, in the United States. Noden Pharma DAC’s suit triggered a 30-month stay of FDA approval of that application under the Hatch Waxman Act. Par filed a counterclaim seeking a declaratory judgment that their proposed generic version of Tekturna HCT® aliskiren hemifumarate hydrochlorothiazide tablets (150 mg eq. base/12.5 mg HCT, 150 mg eq. base/25 mg HCT, 300 mg eq. base/12.5 mg HCT, and 300 mg eq. base/25 mg HCT), described in a separate ANDA submitted by Par to FDA, alleging noninfringement of U.S. Patent No. 8,618,172 (“the ‘172 Patent”), also owned by Noden Pharma DAC. This case was litigated in the United States District Court for the District of Delaware. In March of 2018, the Parties filed a joint stipulation of dismissal of the defendants’ counterclaim seeking a declaratory judgment of non-infringement of the ‘172 Patent. In the stipulation, Anchen and Par agreed that they will not seek, or otherwise join or assist in, any post-grant review, including inter partes review, of the ‘172 patent or U.S. Patent No. 9,023,893. The defendants further stipulated that they will not seek marketing approval of Par’s ANDA or submit any other ANDA seeking approval to market aliskiren hemifumarate hydrochlorothiazide prior to the expiration of the ‘172 Patent in July of 2028. Both the ‘172 Patent and the ‘893 Patent are listed in the Orange Book for Tekturna HCT. On June 8, 2018, Noden and Anchen entered into the Settlement Agreement. Under the Settlement Agreement, the parties agreed to file a stipulation of dismissal with the court to facilitate dismissal of the litigation in its entirety, with prejudice. In the Settlement Agreement, Noden granted Anchen a non-exclusive, royalty free, fully paid up and non-transferable license to manufacture and commercialize in the United States a generic version of aliskiren which is described in Anchen’s ANDA, and Anchen agreed not to commercialize its generic version of aliskiren prior to March 1, 2019. The license grant excludes certain formulations covered by the ‘595 Patent which closely relate to the commercial formulation of Tekturna marketed by Noden. The Settlement Agreement includes a release by each party for liabilities associated with the litigation and an acknowledgment from Anchen that the ‘595 Patent claims are valid and enforceable.
Other Legal Proceedings
From time to time, the Company is involved in lawsuits, arbitrations, claims, investigations and proceedings, consisting of intellectual property, commercial, employment and other matters, which arise in the ordinary course of business. The Company makes provisions for liabilities when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Such provisions are reviewed at least quarterly and adjusted to reflect the impact of settlement negotiations, judicial and administrative rulings, advice of legal counsel, and other information and events pertaining to a particular case. Litigation is inherently unpredictable. If any unfavorable ruling were to occur in any specific period, there exists the possibility of a material adverse impact on the results of the Company’s operations of that period and on its cash flows and liquidity.
26. Subsequent Events
Dissolution
In January 2021, the Company filed a certificate of dissolution in Delaware and will proceed to wind-down and dissolve the Company in accordance with Delaware General Corporate Law. Also in January 2021, the Company’s common stock was formally delisted by Nasdaq.
Convertible Senior Notes
In connection with the Fundamental Change Repurchase Right resulting from the suspension of trading of the Company’s common stock on Nasdaq prior to the opening of business on December 31, 2020, holders of 142 December 2021 Notes tendered their notes for repurchase, which occurred on February 17, 2021.
In connection with the Fundamental Make-Whole Change resulting from the suspension of trading of the Company’s common stock on Nasdaq prior to the opening of business on December 31, 2020, holders of 50 December 2021 Notes exercised their conversion rights. Such notes will be retired for cash.
CareView
The first principal payment and the scheduled interest payment due December 31, 2018 that were previously deferred until January 31, 2021 were subsequently deferred until May 31, 2021 under additional amendments.
27. Quarterly Financial Data (Unaudited)
| | | | | | | | | | | | | | | | | | | | | | |
| | | | Two Months Ended | | Three Months Ended |
| (in thousands, except per share amounts) | | | | August 31,
2020 | | June 30,
2020 | | March 31,
2020 |
| | | | (Under Going Concern Basis of Accounting) |
| Total revenues | | | | $ | 4,115 | | | $ | 5,211 | | | $ | 5,995 | |
| Net loss from continuing operations | | | | $ | (10,852) | | | $ | (12,929) | | | $ | (19,259) | |
| Net income (loss) from discontinued operations | | | | $ | 15,236 | | | $ | (37,399) | | | $ | (12,752) | |
| Net income (loss) attributable to PDL’s stockholders | | | | $ | 4,398 | | | $ | (49,971) | | | $ | (31,723) | |
| Net loss from continuing operations per basic share | | | | $ | (0.10) | | | $ | (0.11) | | | $ | (0.15) | |
| Net income (loss) from discontinued operations per basic share | | | | $ | 0.14 | | | $ | (0.32) | | | $ | (0.11) | |
| Net loss from continuing operations per diluted share | | | | $ | (0.10) | | | $ | (0.11) | | | $ | (0.15) | |
| Net income (loss) from discontinued operations per diluted share | | | | $ | 0.14 | | | $ | (0.32) | | | $ | (0.11) | |
| Net income (loss) per basic share | | | | $ | 0.04 | | | $ | (0.43) | | | $ | (0.26) | |
| Net income (loss) per diluted share | | | | $ | 0.04 | | | $ | (0.43) | | | $ | (0.26) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended |
| (in thousands, except per share amounts) | | December 31,
2019 | | September 30,
2019 | | June 30,
2019 | | March 31,
2019 |
| | (Under Going Concern Basis of Accounting) |
| Total revenues | | $ | 8,521 | | | $ | 8,031 | | | $ | 7,458 | | | $ | 6,696 | |
| Net loss from continuing operations | | $ | (28,817) | | | $ | (11,811) | | | $ | (8,016) | | | $ | (8,450) | |
| Net (loss) income from discontinued operations | | $ | (26,011) | | | $ | (6,155) | | | $ | 3,502 | | | $ | 15,067 | |
| Net (loss) income attributable to PDL’s stockholders | | $ | (54,888) | | | $ | (17,784) | | | $ | (4,419) | | | $ | 6,680 | |
| Net loss from continuing operations per basic share | | $ | (0.25) | | | $ | (0.10) | | | $ | (0.07) | | | $ | (0.07) | |
| Net (loss) income from discontinued operations per basic share | | $ | (0.23) | | | $ | (0.06) | | | $ | 0.03 | | | $ | 0.12 | |
| Net loss from continuing operations per diluted share | | $ | (0.25) | | | $ | (0.10) | | | $ | (0.07) | | | $ | (0.07) | |
| Net (loss) income from discontinued operations per diluted share | | $ | (0.23) | | | $ | (0.06) | | | $ | 0.03 | | | $ | 0.12 | |
| Net (loss) income per basic share | | $ | (0.48) | | | $ | (0.16) | | | $ | (0.04) | | | $ | 0.05 | |
| Net (loss) income per diluted share | | $ | (0.48) | | | $ | (0.16) | | | $ | (0.04) | | | $ | 0.05 | |
In the fourth quarter of 2020, the Company determined that a $3.0 million tax benefit from discontinued operations was omitted from the consolidated statement of operations for the two-month period ending August 31, 2020. This error resulted from an incorrect tax calculation related to the sale of the Noden business. To correct for this error, the Company recorded an out-of-period adjustment to increase Income tax receivable and record a tax benefit of $3.0 million as of and for the three months ended December 31, 2020. The Company determined that the error and out-of-period correction were not material to any of the Company’s interim period financial statements.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
The Company’s management has evaluated, with the participation of the chief executive officer and the chief financial officer, the effectiveness of the Company’s disclosure controls and procedures (as defined in Rule 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (Exchange Act)) as of the end of the period covered by this report. Based on this evaluation, management concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2020.
Management’s Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in the Exchange Act Rules 13a-15(f) and 15d-15(f). The Company’s management, including the chief executive officer and chief financial officer, conducted an evaluation of the effectiveness of the Company’s internal control over financial reporting based on the Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on the results of this evaluation, the Company’s management concluded that internal control over financial reporting was effective as of December 31, 2020.
This annual report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to the rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this Annual Report.
Changes in Internal Control over Financial Reporting
During the quarter ended December 31, 2020, there were no changes in the Company’s internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information Regarding Directors
Certain information with respect to the current directors on the Board of Directors (“Board”) of the Company is set forth below. There are no family relationships among any of our directors or executive officers.
As previously disclosed, on August 19, 2020, at the 2020 Annual Meeting of Stockholders, the stockholders of the Company, upon the recommendation of the Board, approved amendments to the Company’s Restated Certificate of Incorporation (the “Certificate”) in order to phase out the classification of the Board and to provide for the annual election of all directors, as described below. The amendments to the Certificate (the “Declassification Amendment”) became effective upon filing with the Office of the Secretary of State of the State of Delaware (the “Delaware Secretary of State”) on August 26, 2020. Specifically, the Declassification Amendment provides for the annual election of directors beginning at the 2021 annual meeting of stockholders, and that the declassification of the Board will be phased in over a period of three years. Beginning with the 2023 annual meeting of stockholders, the declassification of the Board will be complete and all directors will be subject to annual election for one year terms.
Directors Continuing in Office until the 2021 Annual Meeting of Stockholders
John P. McLaughlin, age 69, was first appointed a director of the Company in October 2008. Mr. McLaughlin was our Chief Executive Officer from December 2008 until his retirement in December 2018. From November 2008 to December 2008 he served as a Senior Advisor to the Company. He was the Chief Executive Officer and a director of Anesiva, Inc., formerly known as Corgentech, Inc., a publicly-traded biopharmaceutical company, from January 2000 to June 2008. From December 1997 to September 1999, Mr. McLaughlin was President of Tularik Inc., a biopharmaceutical company. From September 1987 to December 1997, Mr. McLaughlin held a number of senior management positions at Genentech, Inc., a biopharmaceutical company, including Executive Vice President and General Counsel. From January 1985 to September 1987, Mr. McLaughlin was a partner at a Washington, D.C. law firm specializing in food and drug law. Prior to that, Mr. McLaughlin served as counsel to various subcommittees of the United States House of Representatives, where he drafted numerous measures that became Food and Drug Administration laws. Mr. McLaughlin co-founded and served as Chairman of the Board of Eyetech Pharmaceuticals, Inc., a publicly-traded biopharmaceutical company subsequently bought by OSI Pharmaceuticals, Inc., co-founded and served as a director of Peak Surgical, Inc., a private medical device company, until it was acquired by Medtronic in 2011, served as a director of AxoGen, Inc., a publicly-traded biopharmaceutical company until 2014, served as a director of Adverum Biotechnologies, Inc., a publicly-traded biopharmaceutical company, until 2016 and served as a director of Seattle Genetics, Inc. (now Seagen Inc.), a publicly-traded biopharmaceutical company, until 2016. Currently, he serves on the board of directors of Rockwell Medical Inc. and LENSAR, Inc. He received a B.A. from the University of Notre Dame and a J.D. from Catholic University of America.
Mr. McLaughlin possesses a strong understanding of the biotechnology industry and has experience in development and commercialization of antibodies, corporate licensing and patent litigation that the Company values.
Dominique Monnet, age 62, was first appointed as a director of the Company in December 2018. Mr. Monnet joined the Company in September 2017 as our President and was promoted to President and Chief Executive Officer effective December 31, 2018. Before joining the Company, Mr. Monnet served as senior vice president and chief marketing officer of Alexion Pharmaceuticals from May 2014 to October 2015 where he was responsible for commercial operations in the United States and Latin America and oversaw new products and global business operations functions. From August 2013 to May 2014 he was a managing director at Biotech Advisors International, LLC, a biotechnology consulting firm. Prior to that, from July 2002 through July 2013, he was a senior executive at Amgen Inc. (“Amgen”) where he served in a number of key commercial leadership positions in the United States and internationally. Most recently he acted as vice president and general manager for Amgen’s Inflammation Business Unit from August 2011 until July 2013, where he was responsible for accelerating the growth of the Enbrel® franchise in the highly competitive U.S. market. Prior to this, he served as vice president and head of Amgen’s Global Marketing and Commercial Development, where he led the marketing strategies and global launches of new products across a range of therapeutic areas. From July 2002 through 2006, Mr. Monnet was based in Zug, Switzerland, where he served as Amgen’s vice president of International Marketing and Business Operations, building Amgen’s international commercial capability and leading the creation of its successful international franchises in oncology and nephrology. Before joining Amgen, Mr. Monnet held positions of increasing responsibility in line commercial management and global marketing over 19 years at Schering-Plough - including General Manager of its affiliate in the UK and Republic of Ireland - Ciba-Geigy and Alza Corporation. Mr. Monnet holds a business degree from EDHEC Business School in Lille, France, and an M.B.A. from INSEAD in Fontainebleau, France.
Mr. Monnet brings the Board a strong understanding of the pharmaceutical and biotechnology industries and experience in the commercialization of pharmaceutical products. In addition, Mr. Monnet provides strategic guidance to our management team and the Board.
Directors Continuing in Office until the 2022 Annual Meeting
Alan Bazaar, age 51, was appointed as a director of the Company in February 2020. Mr. Bazaar is currently the Chief Executive Officer of Hollow Brook Wealth Management LLC, a position he has held since November 2013, where he is responsible for firm-wide operations, investment research, and portfolio management. Mr. Bazaar served as a director of Hudson Global Inc. from June 2015 to May 2019 and a director of Sparton Corp. from May 2016 until the completion of its sale in March 2019. Mr. Bazaar served as a director of LoJack Corporation from March 2015 until the completion of its sale in March 2016. Mr. Bazaar was formerly a director of NTS, and served from December 2012 until the completion of its sale in June 2014. From 2004 until April 2008, Mr. Bazaar served as a director of Media Sciences International, Inc., which manufactured and distributed business color printer supplies and industrial ink applications in the United States. From July 1999 until December 2009, Mr. Bazaar was a Managing Director and Portfolio Manager at Richard L. Scott Investments, LLC where he co-managed the public equity portfolio and was responsible for all elements of due diligence. Previously, Mr. Bazaar served as a director of Airco Industries, Inc., a privately held manufacturer of aerospace products, and was employed by Arthur Andersen LLP in the Assurance and Financial Buyer’s Practices group and in the Business Fraud and Investigation Services Unit. Mr. Bazaar received an undergraduate degree in History from Bucknell University and a Master of Business Administration from the Stern School of Business at New York University. He is a Certified Public Accountant (inactive).
Mr. Bazaar brings significant financial, operational and transactional experience to the Board from his roles as both a chief executive officer and a director on the boards of several public companies.
Natasha A. Hernday, age 48, was first elected as a director of the Company in June 2019. Ms. Hernday has served as a member of the leadership team of Seagen Inc. (“Seagen”), a publicly-traded biotechnology company, since January 2011. She is currently its Executive Vice President, Corporate Development. At Seagen, Ms. Hernday built and led the business development team responsible for sourcing, evaluating and negotiating licensing deals, acquisitions and partnerships. From July 1994 until January 2011, Ms. Hernday served in various roles of increasing responsibility at Amgen, including Director, Mergers & Acquisitions and Director, Out-Partnering. Currently, she serves on the board of directors of Xoma Corporation and Alpine Immune Sciences, Inc. Ms. Hernday received her B.A. in microbiology from the University of California at Santa Barbara and her M.B.A. from Pepperdine University.
Ms. Hernday provides approximately 25 years of biotechnology experience to the Board with particular expertise in corporate development and corporate strategy. Her experience and perspective are extremely valuable to the Board and the Company’s leadership team.
Directors Continuing in Office until the 2023 Annual Meeting
Elizabeth G. O’Farrell, age 57, was first appointed as a director of the Company in June 2018, and currently serves as the Chairperson of the Board. Ms. O’Farrell previously served 24 years with Eli Lilly and Company (“Eli Lilly”), most recently as Chief Procurement Officer from 2012 to 2017, until her retirement. At Eli Lilly, she advanced through various executive management positions, including Senior Vice President, Policy and Finance; Senior Vice President, Finance; Chief Financial Officer, Lilly USA; Chief Financial Officer, Lilly Canada; and General Auditor. Before joining Eli Lilly, Ms. O’Farrell was an accountant with Boise Cascade Office Products and auditor at Whipple & Company and Price Waterhouse. Currently, she serves on the board of directors of Geron Corporation, Inhibikase Therapeutics, Inc. and LENSAR, Inc. Ms. O’Farrell served as a board member of the YMCA of Greater Indianapolis from 2006 until 2017, including as its chairperson from 2014 to 2016. She is a member of the Finance Committee of the United Way of Brevard, in Brevard County, FL., and previously served on the Boards of the Washington Township Schools Foundation and Keep Indianapolis Beautiful. Ms. O’Farrell holds a B.S. in accounting with honors and an M.B.A. in management information systems, both from Indiana University.
Ms. O’Farrell provides the Board with extensive experience as a senior executive of a major pharmaceutical company with global operations. In addition, Ms. O’Farrell has been determined by the Board to be an “audit committee financial expert” (as defined in applicable SEC rules) for the Company.
Information Regarding Executive Officers
Certain information with respect to our current executive officers is set forth below. Under the Bylaws, each executive officer is appointed annually by the Board, and each holds office until such officer resigns, is removed, is otherwise disqualified to serve or such officer’s successor is elected and qualified. There are no family relationships among any of our directors or executive officers.
| | | | | | | | | | | | | | |
| Name | | Age | | Position |
| Dominique Monnet | | 62 | | President and Chief Executive Officer |
| Christopher Stone | | 56 | | Vice President, General Counsel and Secretary |
| Edward Imbrogno | | 56 | | Vice President, Chief Financial Officer and Chief Accounting Officer |
Dominique Monnet, please see discussion under “Directors Continuing in Office until the 2021 Annual Meeting of Stockholders” for biographical information about Mr. Monnet.
Christopher Stone joined the Company in February 2009 as our Vice President, General Counsel and Secretary. He brings more than 30 years of legal experience to the role. Before joining PDL, Mr. Stone served as Vice President of Legal Affairs and Corporate Secretary at LS9, an advanced biofuels development company, where his work included a focus on intellectual property protection and licensing. Prior to that time, he was Vice President of Intellectual Assets USA at Danisco A/S, a global producer of food ingredients, enzymes and bio-based solutions. From 1994 to 2005, Mr. Stone was with Genencor International, a biotechnology company that was acquired by Danisco in 2005, most recently as Vice President of Intellectual Property and General Patent Counsel. At Genencor, he handled all intellectual property matters, including developing and implementing an overall strategy for its domestic and international patent estate of approximately 3,700 patents and patent applications, and managed multiple litigation and interference proceedings and numerous European patent oppositions. Mr. Stone received a J.D. from the National Law Center at George Washington University and a B.S. in Biochemistry from the University of Massachusetts. He is an active member of the District of Columbia Bar, Nevada Bar (company counsel) and California Bar, and was admitted to practice before the United States Patent & Trademark Office in 1992.
Edward Imbrogno joined the Company in October 2018 as our Vice President, Finance. In July 2019, he was appointed as Chief Accounting Officer, in October 2019, he was appointed as Acting Chief Financial Officer and in March 2020, he was appointed Chief Financial Officer. From May 2017 to October 2018, Mr. Imbrogno was Senior Director and Corporate Controller for BioDelivery Sciences International, Inc., a Nasdaq-listed specialty pharmaceutical company. From June 2016 to April 2017, Mr. Imbrogno provided accounting and financial reporting consulting services. From September 2013 to May 2014, Mr. Imbrogno was Vice President, Financial Reporting for International Lease Finance Corporation, the world’s largest independent aircraft lessor, until its acquisition by AerCap Holdings N.V. Mr. Imbrogno remained with AerCap through March 2016 assisting with post-acquisition financial reporting transition, primarily at AerCap’s headquarters in Amsterdam, Netherlands. From 2006 to 2013, he was Director, Accounting for Amgen, a Nasdaq-listed multinational biopharmaceutical company. Prior to Amgen, Mr. Imbrogno held positions at several companies with increasing financial accounting and reporting responsibilities. Mr. Imbrogno began his career in public accounting with Ernst & Young LLP culminating as an Audit Manager where he provided audit and related financial services to public and private companies. Mr. Imbrogno holds a B.S. in accounting from Pennsylvania State University and an M.B.A. from Wake Forest University. He is a licensed Certified Public Accountant and is a member of the American Institute of Certified Public Accountants.
Code of Ethics
The Company has adopted a Code of Business Conduct (the “Conduct Code”) that applies to all officers, directors and employees. A stockholder can obtain a copy of the Code of Conduct from the Company without charge, by requesting it by telephone at (775) 832-8500 or in writing to the following address:
PDL BioPharma, Inc.
59 Damonte Ranch Parkway, Suite B-375
Reno, Nevada 89521
Candidates for Nomination
Before we filed a certificate of dissolution with the Secretary of State of Delaware and also voluntarily delisted our common stock from the Nasdaq Global Stock Market in January 2021, the Nominating and Governance Committee of the Board had adopted a policy to evaluate any recommendation for director nominee proposed by a stockholder, and our Bylaws also permit stockholders to nominate directors for consideration at an annual meeting, subject to certain conditions. Other than the Board disbanding the Nominating and Governance Committee and assuming its duties, there have been no changes to these procedures since the Company’s disclosures of these procedures in its definitive proxy statement for the 2020 annual meeting of stockholders filed with the SEC on July 7, 2020.
Audit Committee Information
The Audit Committee of the Board was established by the Board in accordance with Section 3(a)(58)(A) of the Exchange Act to oversee the Company’s corporate accounting and financial reporting processes and audits of our financial statements. As of December 31, 2020, the Audit Committee was comprised of Mr. Gryska, Ms. O’Farrell and Mr. Bazaar. Mr. Gryska was the chairperson of the Audit Committee until his resignation from the Board on December 31, 2020. Following the Company’s delisting from Nasdaq in January 2021, the Audit Committee was disbanded, at which point the Board assumed the duties of the Audit Committee.
The Board determined that all members of the Audit Committee were independent directors in 2020 as defined in the Nasdaq qualification standards and by Section 10A of the Exchange Act. In addition, Mr. Gryska and Ms. O’Farrell each were determined by the Board to be an “audit committee financial expert” as defined by applicable SEC rules.
ITEM 11. EXECUTIVE COMPENSATION
COMPENSATION DISCUSSION AND ANALYSIS
Overview
This Compensation Discussion and Analysis describes our compensation program as it relates to our named executive officers set forth below in “Executive Officer Compensation-Summary Compensation Table.” Our named executive officers for 2020 include Dominique Monnet, our President and Chief Executive Officer; Christopher Stone, our Vice President, General Counsel and Secretary; Edward Imbrogno, our Vice President, Chief Financial Officer and Chief Accounting Officer; and Jill Jene, our former Vice President, Business Development.
We present our Compensation Discussion and Analysis in the following sections:
| | | | | | | | | | | |
| 1. | Executive Summary. In this section, we describe certain aspects of our business, highlight our 2020 corporate performance, and summarize certain governance aspects of our executive compensation program. | p. | |
| | | |
| 2. | Executive Compensation Program Philosophy, Objectives and Process. In this section, we describe our executive compensation philosophy and objectives and the process the Compensation Committee follows in deciding how to compensate our named executive officers and the material elements of our executive compensation program. | p. | |
| | | |
| 3. | Compensation Program Elements. In this section, we present a brief overview of the specific elements of our compensation program and a detailed discussion and analysis of the Compensation Committee’s specific decisions about the compensation of our named executive officers for fiscal year 2020. | p. | |
| | | |
| 4. | Other Executive Compensation Matters. In this section, we summarize our other compensation policies, review the accounting and tax treatment of compensation and the relationship between our compensation program and risk. | p. | |
This Compensation Discussion and Analysis contains forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. The actual compensation programs that we adopt in the future may differ materially from currently planned programs as summarized in this discussion.
Executive Summary
Fiscal 2020 Business Overview
In September of 2019, our Board and management decided to initiate a strategic review process of the Company’s business and growth strategy with the assistance of certain independent financial advisors, during which we suspended our growth strategy. In December of 2019, we announced that the Board had completed the strategic review process and, as a result, we decided to halt the execution of our growth strategy, cease additional strategic investments and pursue a formal process to unlock value by monetizing our assets and returning net proceeds to our stockholders.
Over the subsequent months, the Board and management analyzed, together with outside financial and legal advisors, how to best capture value pursuant to our monetization strategy and best return the significant intrinsic value of the assets in our portfolio to our stockholders. In February 2020, the Board adopted a plan of complete liquidation (the “Plan of Liquidation”). Adopting the Plan of Liquidation was an important first step for any distributions to our stockholders pursuant to that plan to qualify as liquidating distributions, which are eligible for potentially preferable tax treatment under U.S. federal tax law. There can be no assurance any such distributions will in fact be made or that they will ultimately be determined to be liquidating distributions.
Pursuant to our monetization strategy and Plan of Liquidation, we explored a variety of potential transactions, including a whole Company sale, divestiture of assets, spin-offs of operating entities, merger opportunities or a combination thereof. In addition, we analyzed, and continue to analyze, optimal mechanisms for returning value to stockholders in a tax-efficient manner, including share repurchases, cash dividends and other distributions of assets. Despite the challenges of COVID-19, we made significant progress in our monetization strategy during 2020, including monetizing most of our key assets and resolving a longstanding legal issue, as further discussed below.
In May 2020 we distributed all of the common stock of Evofem Biosciences, Inc. held by us to our stockholders.
In August 2020, we entered into a settlement agreement with the Wellstat Parties (the “Wellstat Settlement Agreement”) resolving previously reported litigation relating to loans we made to Wellstat Diagnostics. Pursuant to the Settlement Agreement we received $7.5 million upon the signing, and the Settlement Agreement further provided for additional payments of either (1) $5.0 million by February 10, 2021 and $55.0 million by July 26, 2021; or (2) $67.5 million by July 26, 2021. If the Wellstat Parties fail to make payment in full by July 26, 2021, we were authorized to record judgment against the Wellstat Parties for an amount of $92.5 million or such lesser amount as may be owed under the Settlement Agreement.
Also in August 2020, the Company sold its royalty interests for Kybella®, Zalviso®, and Coflex® to SWK Funding, LLC, a wholly owned subsidiary of SWK Holdings Corporation, for $4.35 million in cash.
In September 2020, the Company closed the sale of its previously wholly-owned subsidiaries, Noden Pharma DAC and Noden Pharma USA, Inc. to CAT Capital Bidco Limited, an indirect, wholly-owned subsidiary of Stanley Capital Limited for consideration of up to $52.8 million, including a payment at closing of $12.2 million. The Company is also entitled to (i) recover $0.5 million related to value-added tax for inventory purchases from Novartis, (ii) an additional $33.0 million to be paid in twelve equal quarterly installments from January 2021 to October 2023, (iii) an additional $3.9 million to be paid in four equal quarterly installments from January 2023 to October 2023 and (iv) additional contingent payments of up to $3.2 million based on the occurrence of certain events.
In October 2020, the Company completed the previously announced spin-off of its subsidiary LENSAR, Inc. into a new, independent publicly traded company, through a distribution of all outstanding shares of LENSAR common stock owned by the Company to its stockholders on a pro rata basis.
In December 2020, the Company entered into a Capital Provision Agreement with Epps Investments LLC (“Epps”) by which it sold all rights to awards, damages, recoveries, judgments or other property or value awarded to or received by the Company pursuant to or as a result of the Wellstat Settlement Agreement or any underlying claim resolved by such settlement agreement for approximately $51.3 million (the “Wellstat Settlement Sale”).
In early January 2021, in connection with our Plan of Dissolution, we filed a certificate of dissolution with the Secretary of State of Delaware and also voluntarily de-listed our common stock from the Nasdaq Global Stock Market. Also in January 2021, we filed a Form 15 notifying the SEC of deregistration of our common stock under Section 12(g) of the Exchange Act and suspension of our duty to file reports under Sections 13 and 15(d) of the Exchange Act.
Fiscal 2020 Executive Compensation
Emphasis on “At Risk”, Performance Based Compensation
The Compensation Committee is focused on linking both cash and equity compensation to Company performance and making a significant portion of such compensation variable or “at risk.” “At risk” pay is tied to the achievement of corporate goals, individual objectives and/or stock price performance. Since 2014, all cash and equity compensation earned by executive officers has been “at risk” under the annual bonus and long-term incentive plans devised by the Compensation Committee; the only component of compensation not “at risk” has been base salary.
Key 2020 Compensation Decisions Affecting our Chief Executive Officer:
•Base Salary and Target Annual Bonus for Chief Executive Officer Position in 2020: For 2020, Mr. Monnet received a salary of $650,875, an increase of 2.5% in comparison to his annual salary in 2019, and had a target bonus equal to 80% of his annual salary, which was unchanged from 2019.
•Target Annual Bonus Payout for Chief Executive Officer in 2020: For 2020, Mr. Monnet received an annual bonus payout equal to 100% of his target bonus based on the Company's performance relative to the corporate objectives established by the Compensation Committee for 2020 annual bonus purposes.
Changes to Annual Bonus Plan
For the Company’s 2020 annual bonus plan, the Compensation Committee eliminated the individual goal scoring portion for executive officers (other than the Company’s chief executive officer, whose goals have always been based 100% on corporate goals) and the Company’s employees. The Compensation Committee determined that, in order to appropriately align each participant’s incentives with the Plan of Liquidation, all payments under the 2020 annual bonus plan should be based solely on the achievement of the corporate goals under the plan with no individual goal component. In addition, the corporate goals were designed to be aligned with the Plan of Liquidation.
Wind Down Retention Plan
After we announced our strategy to monetize our assets and distribute net proceeds to our stockholders, we recognized that our ability to execute on our monetization plan and optimize returns to our stockholders depended to a large extent on our ability to retain the necessary expertise to effectively transact with respect to our assets. Due to the unique diverse nature of our assets, we believe that effectively transacting our assets requires a significant level of expertise and engagement. Accordingly, on December 21, 2019, the Compensation Committee of the Board adopted a Wind Down Retention Plan in which the Company’s executive officers and other key employees were eligible to participate. We believe that the Wind Down Retention Plan was a necessary component for both efficiently implementing our monetization strategy and also to fully optimize asset value and subsequent stockholder returns.
Under the Wind Down Retention Plan, participants were eligible to earn a retention benefit in consideration for their continued employment with the Company. The Wind Down Retention benefits were equivalent to previously disclosed compensation payments contemplated in connection with a change in control under the Company’s existing Severance Plan. For our Chief Executive Officer, the retention benefit was a lump sum cash payment equal to three times the sum of his base salary and target bonus, plus an amount equal to the cost of 12 months of health insurance continuation under COBRA. For other named executive officers, the retention benefit was equal to two times the sum of their base salary and target bonus, plus an amount equal to the cost of 12 months of health insurance continuation under COBRA. Under the Wind Down Retention Plan, payment of the retention benefit to any participant would occur upon termination of the participant’s employment with the Company either by the Company without cause or by the participant for good reason. The retention benefit, if paid, would be in lieu of (and not in addition to) any other severance compensation that could become payable to the participant under the Company’s Severance Plan. The Wind Down Retention Plan, during its term, superseded any inconsistent terms or duplicative benefits that would apply from the Company’s preexisting severance program.
The Wind Down Retention Plan also provided that, consistent with the existing terms of the Amended and Restated 2005 Equity Incentive Plan (the “Equity Plan”), the vesting of all outstanding equity awards held by participants would be accelerated upon the earlier of: (i) a termination of the participant’s employment with the Company either by the Company without cause or by the participant for good reason or (ii) the consummation of a change in control (as defined in the Equity Plan) of the Company. In addition, the post-termination exercise period for all outstanding stock options will be extended until their expiration date.
In February 2020, a change in control was triggered under the Wind Down Retention Plan and the Equity Plan by the Board’s adoption of the Plan of Liquidation, which resulted in the vesting of substantially all equity awards held by our named executive officers.
The Wind Down Retention Plan further provides for equitable adjustments to outstanding stock options held by participants to ensure such participants realize the same benefits provided to stockholders in the event one or more cash or other distributions become payable to stockholders. Consistent with the existing terms of the Equity Incentive Plan, in the event one or more cash or other distributions are paid to stockholders, the exercise price of outstanding stock options will be reduced on a dollar-for-dollar basis to reflect the per share value of such distributions. In the event that the Company declares cash or other distributions that, in the aggregate, exceed the difference between the exercise price of an outstanding stock option and the par value of the underlying shares ($0.01), the holder of such stock option will be entitled to receive from the Company a cash payment in an amount equal to the number of shares subject to such stock option multiplied by the per share amount of the cash or other distributions that exceeds the difference between exercise price of the outstanding option and the par value of the underlying shares (a “true-up payment”). A true-up payment is also paid with respect to a post-dissolution cash or other distributions with respect to a stock-option that was not exercised prior to dissolution in the same fashion as provided above. True-up payments are to be made on the same date that cash or other distributions are paid or made to the Company’s stockholders.
The terms of the Wind Down Retention Plan provide that the plan may not be amended or terminated without the written consent of each affected participant.
Given the previously disclosed decision to file for dissolution on January 4, 2021 and in order to realize certain potential tax benefits for the Company, the Compensation Committee and the Board approved the accelerated payment of the retention benefits under the Wind Down Retention Plan in December 2020.
Stockholder Engagement and Response to 2020 Say-on-Pay Vote
In July 2020, we reached out to our top 25 stockholders, who in the aggregate owned approximately 70% of our outstanding common stock at such time, to determine if such stockholders had any issues or questions regarding the voting proposals in the 2020 proxy statement. An overwhelming majority of the votes cast (above 91%) at our annual meeting held in August 2020 were in favor of each of the proposals, including the “say-on-pay” proposal. In reviewing our executive compensation program, the Compensation Committee considered, among other things, the 2020 vote results and other feedback we received from stockholders.
Selected Compensation Governance Highlights
Our executive compensation program consists of an array of compensation governance features and controls. Below we summarize certain executive compensation-related practices that were in effect during 2020 and that we believe serve our stockholders’ long-term interests.
| | | | | | | | |
| What We Do |
| ü | | The Compensation Committee is comprised solely of independent directors. |
| ü | | We structure a substantial portion of officer pay opportunities in the form of “at-risk” performance-based compensation. |
| ü | | Our executive officers’ annual cash bonus is 100% attributable to the achievement of corporate goals set by the Compensation Committee and ratified by the Board and is fully at risk of non-payment in the event of unsatisfactory performance, thereby putting a substantial portion of our executive officers’ total annual cash compensation (comprised of base salary and an annual cash bonus opportunity) at risk and tied to the Company’s annual performance. |
| ü | | We conduct an annual say-on-pay vote. |
| ü | | We seek input from, listen to and respond to stockholders. |
| ü | | We have adopted a clawback policy to prevent executive officers involved in certain wrongful conduct from unjustly benefiting from such conduct, and to remove the financial incentives to engage in such conduct. |
| ü | | We enforce robust stock ownership guidelines for executive officers and directors. |
| ü | | We strictly prohibit our directors and executive officers from “short sales,” hedging and other monetization transactions (such as zero-cost collars and forward sale contracts), holding the Company’s securities in margin accounts and pledging the Company’s securities as collateral for loans. |
| ü | | The Compensation Committee retained an independent compensation consultant. |
| | |
What We Do Not Do |
| �� | | We do not provide gross-up tax payments for our named executive officers. |
| û | | We do not provide guaranteed bonuses. |
| û | | We do not re-price underwater awards and do not provide discount stock options or stock appreciation rights. |
Executive Compensation Program Philosophy, Objectives and Process
Philosophy and Objectives
The Compensation Committee has structured our executive compensation program to take into account our unique business model, leveraged headcount and location. In 2020, given our monetization strategy adopted by our Board, the Compensation Committee structured executive compensation to incentivize our executives to maximize net proceeds under the Plan of Liquidation for purposes of maximizing distributions to be made to the Company’s stockholders.
The Compensation Committee structured our executive compensation program in a manner that it believes does not promote inappropriate risk taking by our executive officers, and encourages them to take a balanced approach, focused on achieving our corporate goals and enhancing stockholder return. A more complete discussion regarding the risk assessment process can be found at “Risk Assessment of Compensation Policies” above.
Process
When making executive compensation program decisions, the Compensation Committee reviewed: (i) the Company’s competitive market compensation data, (ii) our performance against our corporate goals and objectives, (iii) our performance relative to our comparator companies, and (iv) the unique circumstances of the Company, most significantly, the monetization strategy adopted by our Board at the end of 2019. To accomplish these reviews, the Compensation Committee engaged Board Advisory to provide advice on competitive market practice and recommendations for structuring our named executive officers’ compensation for fiscal year 2020. Board Advisory reports, and is accountable, to the Compensation Committee, and may not conduct any other work for us without the authorization of the Compensation Committee. Board Advisory did not provide any services to us in 2020 beyond its engagement as an advisor to the Compensation Committee on compensation matters. After review and consultation with Board Advisory, the Compensation Committee has determined that Board Advisory is independent and there is no conflict of interest resulting from retaining Board Advisory currently or during the year ended December 31, 2020. In reaching these conclusions, the Compensation Committee considered the factors set forth in Exchange Act Rule 10C-1 and Nasdaq Stock Market (“Nasdaq”) listing standards.
Our chief executive officer aided the Compensation Committee by providing annual recommendations regarding the compensation of all executive officers, other than himself. Each named executive officer, in turn, participates in an annual performance review with the chief executive officer to provide input about his or her contributions to the Company’s success for the period being assessed. The performance of our chief executive officer and executive team as a group is reviewed annually by the Compensation Committee.
Comparator Companies
Due to the Company’s unique business model and the monetization strategy adopted by the Board, the Compensation Committee found it difficult to establish an appropriate group of peer companies for the purposes of evaluating our compensation practices. The Compensation Committee, in consultation with Board Advisory, determined that companies in the pharmaceutical industry remained the most appropriate peers for executive compensation comparison purposes in 2020 because (i) all of our revenues, income generating assets and products were derived from the healthcare industry and (ii) it is the sector from which we primarily drew our existing management. To that end, the Compensation Committee directed Board Advisory to analyze companies in the pharmaceutical industry with the same Global Industry Classification Standard code, or GICS code, and revenues between 0.4x and 2.5x the Company’s annual revenue, or between $56 million to $350 million, and in particular comparable small, profitable pharmaceutical companies, to recommend a peer group for purposes of analyzing the Company’s compensation practices.
The list of comparator companies is reviewed and updated annually as the previous year’s companies may no longer fit the most appropriate parameters for the Company. The list of comparator companies was selected based in part on the Company’s size in terms of revenues based on information available to the Compensation Committee in the fall of 2019. The following list of comparator companies for 2020 compensation decisions demonstrates the Compensation Committee’s focus on selecting companies relatively similar in industry sector and size (after taking into account that the Company is unique in its business model and circumstances):
| | | | | |
| Amphastar Pharmaceuticals, Inc. | Ironwood Pharmaceuticals, Inc. |
| ANI Pharmaceuticals, Inc. | Ligand Pharmaceuticals Inc. |
| Avadel Pharmaceuticals plc | Osmotica Pharmaceuticals plc |
| Collegium Pharmaceutical, Inc. | Pacira BioSciences, Inc. |
| Corcept Therapeutics, Inc. | Retrophin, Inc. |
| Eagle Pharmaceuticals, Inc. | Spectrum Pharmaceuticals, Inc. |
| Halozyme Therapeutics, Inc. | Supernus Pharmaceuticals, Inc. |
| Innoviva, Inc. | Teligent, Inc. |
| Intersect ENT, Inc. | Vanda Pharmaceuticals Inc. |
Competitive Market Data
Each year, Board Advisory surveys the compensation practices of the comparator companies to assess the competitiveness of our compensation programs. Although we maintain the peer group for executive compensation and performance reference purposes, the comparator company compensation data is limited to publicly available information and therefore does not necessarily provide comparisons for all officers. By contrast, survey data has the advantage of including data on executive positions beyond what is available in public filings but may not be specific to the selected companies in the peer group. In light of this, Board Advisory analyzed (i) the compensation levels of the comparator companies referenced above and (ii) the 2019 Radford Global Life Sciences Survey for biotechnology companies with annual revenues in a range between $50 million and less than $499 million (the “2019 Radford Compensation Survey”). With respect to the survey data presented to the Compensation Committee, the identities of the individual companies included in the survey were not provided to the Compensation Committee, and the Compensation Committee did not refer to individual compensation information for such companies.
We believe that by utilizing both publicly available comparator company data and the survey data, we are able to develop the best set of robust competitive data reasonably available for use in making compensation decisions. The Compensation Committee, when making compensation adjustments to the named executive officers, reviews the publicly available comparator company group data and the survey data to ensure that, following any compensation adjustment, the total compensation of named executive officers falls within our guidelines.
The Compensation Committee uses the 50th percentile of comparator companies as a benchmark, or starting point, for decisions regarding target total direct compensation (comprised of base salary, annual bonus opportunity and long-term incentive opportunity) of the Company’s executive officers. However, in order to attract and retain the executive talent necessary to lead the Company, the Compensation Committee does not establish compensation levels based solely on benchmarking. The Compensation Committee instead relies on the judgment of its members in making compensation decisions regarding base salaries, target bonus levels and long-term equity incentive awards after reviewing our performance and carefully evaluating each named executive officer’s performance during the year, leadership qualities, business responsibilities, career with our Company,
current compensation arrangements and long-term potential to enhance stockholder value. The Compensation Committee does not guarantee that any executive will receive a specific market-derived compensation level.
Qualifications and Unique Company Existence
In addition to analyzing market compensation and performance data, the Compensation Committee considers each individual’s qualifications, experience and contribution to the Company when making compensation decisions, as well as our unique business model and the difficulty in locating experienced and qualified executive officer candidates willing to relocate to the remote area surrounding our headquarters. The only named executive officer not required to be located proximate to our headquarters in Nevada was our former Vice President of Business Development whose responsibilities include extensive travels and is domiciled in the San Francisco Bay Area, close to key stakeholders and potential target companies.
No Delegation of Compensation Decisions
The Compensation Committee has not delegated any of its exclusive power to determine matters of executive compensation and benefits. Our chief executive officer and an independent compensation consultant assist the Compensation Committee by presenting proposals and recommendations to the Compensation Committee, information on the Company and individual performance of our named executive officers and management’s perspective and recommendations on compensation matters (our chief executive officer recuses himself from that portion of the Compensation Committee meetings involving deliberation and decision-making pertaining to his own compensation). The Compensation Committee reports to the Board on the major items covered at each Compensation Committee meeting.
Compensation Program Elements
In 2020, the annual compensation payable to our named executive officers was comprised of five primary elements which were designed together to motivate our named executive officers to achieve our strategic goals. These five elements were: (i) base salary, (ii) annual cash bonus, (iii) long-term incentive compensation, (iv) wind-down retention benefits, and (v) employee benefits.
In addition, we provide other limited perquisites and benefits, but such perquisites and benefits are generally available to all of our employees on the same terms as to our named executive officers.
Each element, and why we pay it, is discussed below.
Base Salary
Base salary is the fundamental, fixed element of our named executive officers’ compensation and the foundation for each named executive officer’s total compensation.
Base salaries are reviewed annually and may be adjusted by the Compensation Committee to take into account the individual performance of the named executive officer as well as that of the Company as a whole.
For fiscal year 2020, the Compensation Committee reviewed the base salary and performance of each of our named executive officers, the performance of the Company and Board Advisory’s analysis and summary of the market practice of the comparator companies. Based on this review, the Compensation Committee determined to increase the base salary of the named executive officers by 2.5% for 2020.
The fiscal year 2020 base salaries for our named executive officers are set forth in the table below:
| | | | | | | | | | | | | | | | | | | | |
| Name | | Title | | 2020
Base Salary | | % Increase or Decrease
from 2019
Base Salary |
| Dominique Monnet | | President and Chief Executive Officer | | $ | 650,875 | | | 2.5% |
| Christopher Stone | | Vice President, General Counsel and Secretary | | $ | 509,927 | | | 2.5% |
| Dr. Jill Jene | | Former Vice President, Business Development | | $ | 358,955 | | (1) | 2.5% |
| Edward Imbrogno | | Vice President, Chief Financial Officer and Chief Accounting Officer | | $ | 354,650 | | | 2.5% |
_____________________
(1) Dr. Jene separated from the Company, effective August 15, 2020.
Annual Cash Bonus
The second component of our named executive officers’ total compensation is the annual cash bonus.
Fiscal Year 2020 Annual Bonus Evaluation
For fiscal year 2020, the Compensation Committee established the 2020 Annual Bonus Plan. The 2020 Annual Bonus Plan is comprised of cash compensation that is entirely at risk, depending on performance. In the event of underperformance, the Compensation Committee may elect to award no bonus. In the event of performance that exceeds the Compensation Committee’s performance expectations, the bonus amount is capped at a maximum of 200% of the target amount.
As part of the 2020 Annual Bonus Plan, the Compensation Committee reviewed, and recommended to the Board for approval, the Company’s corporate goals for 2020 that would be measured in determining the bonuses of our named executive officers for fiscal year 2020.
Given the monetization strategy adopted by our Board in late 2019 and the importance of maximizing net proceeds from the strategy that could be distributed to our stockholders, for 2020, the Compensation Committee determined to eliminate the individual performance goals for our named executive officers, making 100% of the payments under the 2020 Annual Bonus plan subject to achievement of corporate goals.
In connection with the annual cash bonus, the Compensation Committee reviewed the target bonus (as a percentage of base salary) of each named executive officer and Board Advisory’s analysis and summary of the market practice of the comparator companies.
The target and maximum bonus (as a percentage of base salary) and the ratio of corporate to individual goals for each named executive officer are set forth in the table below:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | | Title | | 2019 Target
Bonus | | 2020 Target
Bonus | | 2020 Maximum
Bonus |
| Dominique Monnet | | President and Chief Executive Officer | | 80% | | 80% | | 160% |
| Christopher Stone | | Vice President, General Counsel and Secretary | | 75% | | 75% | | 150% |
| Jill Jene | | Former Vice President, Business Development | | 75% | | 75% | | 150% |
| Edward Imbrogno | | Vice President, Chief Financial Officer and Chief Accounting Officer | | 75% | | 75% | | 150% |
Corporate Goals
For 2020, the Compensation Committee determined the corporate goals should be aligned with our monetization strategy and Plan of Liquidation. To that end, the Compensation Committee developed the corporate goals keeping in mind the two most likely outcomes of the monetization strategy -- either the monetization of individual assets or the sale of the entire company that would
be approved by our stockholders. Our corporate goals for 2020 and the relative weight ascribed to them are set forth in the table below:
| | | | | | | | | | | |
| 2020 Corporate Goals | | Weight |
| Alternative 1: | | |
| Maximize proceeds from asset monetization | | 75% |
| Sale of royalty assets | | |
| Monetization of LENSAR | | |
| Monetization of Evofem shares | | |
| Sale of Noden business | | |
| Execute monetization in a cost and tax efficient manner | | 10% |
| Finalize Plan of Liquidation to enable tax efficient dividend distributions | | |
| Control expenses not to exceed 2020 G&A budget | | |
| Complete monetization of key assets (royalties, LENSAR, Evofem shares, Noden) by December 31, 2020 and/or be in position to file for dissolution by year end | | 15% |
| | | |
| Total | | | 100% |
| | | |
| OR | | | |
| | | |
| Alternative 2: | | |
| Sell whole company | | 100% |
At the time the Compensation Committee set the goals for 2020, it believed that each of the 2020 annual bonus plan goals was achievable, but only with significant effort. The Compensation Committee monitored the achievement of the 2020 corporate goals throughout the year.
2020 Performance Evaluations and Bonus Amounts
In December 2020, the Compensation Committee evaluated the Company's performance against the 2020 corporate goals. The Compensation Committee determined that 100% of the 2020 corporate goals established for the 2020 Annual Bonus Plan had been achieved, as further described below. The Compensation Committee based its decision on the following factors:
•Maximize proceeds from asset monetization. The Company entered into several transactions in 2020 whereby it was able to sell its Noden business, certain of its royalty assets and distribute its ownership of LENSAR and Evofem directly to its stockholders. While the Company was not able to sell its Assertio royalty asset, this was offset by potential tax benefits that the Company will likely be able to realize under the CARES Act and the receipt of approximately $59 million in relation to its Wellstat dispute between the initial $7.5 million received upon the signing of the Wellstat Settlement Agreement and the approximately $51.4 received for the Wellstat Settlement Sale. The Compensation Committee used its discretion to conclude that the Company had performed at a level of 75% versus the target level of 75%.
•Execute monetization in a cost and tax efficient manner. The Board adopted the Plan of Liquidation in February 2020, which should enable stockholders to realize tax efficient distributions. The Company’s G&A expenses for 2020 were consistent with the 2020 budget. Based on the substantial achievement of the goal, the Compensation Committee concluded that the Company had performed at a level of 10% versus the target level of 10%.
•Complete monetization of key assets (royalties, LENSAR, Evofem shares, Noden) by December 31, 2020 and/or be in position to file for dissolution by year end. The Company completed the monetization process for the assets that needed to be divested prior to filing for dissolution. The Board was able to make a determination to delist the Company’s stock from trading on Nasdaq on December 31, 2020 and file for dissolution with the State of Delaware on January 4, 2021. Based on the substantial achievement of the goal, the Compensation Committee concluded that the Company had performed at a level of 15% versus the target level of 15%.
Following this review, the Compensation Committee approved bonus payments to each of our named executive officers based on the above determinations:
| | | | | | | | | | | | | | |
| Name | | Title | | 2020 Annual
Bonus |
| Dominique Monnet | | President and Chief Executive Officer | | $ | 520,700 | |
| Christopher Stone | | Vice President, General Counsel and Secretary | | $ | 382,445 | |
Jill Jene(1) | | Former Vice President, Business Development | | $ | 167,708 | |
| Edward Imbrogno | | Vice President, Chief Financial Officer and Chief Accounting Officer | | $ | 265,988 | |
___________________
(1) Because she separated from the Company on August 15, 2020, Dr. Jene received a pro-rated portion of her bonus under the 2020 Annual Bonus Plan.
Long-Term Incentives
The third component of our compensation strategy is long-term incentive awards. In 2020, our long-term incentive award program was comprised solely of restricted stock awards, which we believe best aligned the interests of management and stockholders in relation to the Company’s monetization strategy. Specifically, our named executive officers received the following restricted stock awards in 2020:
| | | | | | | | | | | | | | |
| Name | | Title | | Number of Shares of Restricted Stock Granted in 2020 |
| Dominique Monnet | | President and Chief Executive Officer | | 1,233,766 |
| Christopher Stone | | Vice President, General Counsel and Secretary | | 422,078 |
| Jill Jene | | Former Vice President, Business Development | | 194,805 |
| Edward Imbrogno | | Vice President, Chief Financial Officer and Chief Accounting Officer | | 292,208 |
The restricted stock awards were granted in January 2020 and vest over a three-year period, with 40% vesting on the first anniversary of the grant date and 2.5% vesting monthly thereafter. The vesting of all restricted stock is subject to: (i) continuous service with the Company as of such vesting date and (ii) accelerated vesting in the event of a change of control.
In February 2020, in connection with the Board adopting the Plan of Liquidation, all of the stock options and restricted stock granted to our employees and executive officers accelerated and vested under the change in control definition in the Equity Plan, other than the outstanding awards under the 2016/20 LTIP (as discussed below).
2016/20 LTIP Payments in 2020
Under our long-term incentive plans in place prior to 2018, each named executive officer was granted awards consisting of: (i) restricted stock and (ii) a cash payment.
Subject to the acceleration provisions set forth in the long-term incentive plans described below, each cash award and restricted stock award vested at the times specified in each plan, provided that the named executive officer remains employed by the Company through such date. In 2020, one long-term incentive plan was still effective, the 2016/20 long-term incentive plan (the “2016/20 LTIP”). Mr. Stone was the only remaining named executive officer that was a participant in the 2016/20 LTIP. Under such long-term incentive plan, in addition to remaining employed by the Company through the applicable vesting dates, the Company must meet minimum performance goals described over the applicable performance periods for the restricted stock and cash awards to vest and/or be paid.
Dividend payments and other distributions made on the restricted stock during the vesting periods of the restricted stock will accrue through the vesting periods and will be paid, plus interest, to the named executive officer upon vesting of the restricted stock award. If the minimum performance goals for the long-term incentive plan’s restricted stock awards are not met, the accrued dividend payments and other distributions will be forfeited.
In December 2020, the Compensation Committee determined, that consistent with other outstanding equity grants, the 2016/20 LTIP should vest and be paid in accordance with the 66% performance achievement level that was awarded with respect to the initial vesting period. Upon vesting, Mr. Stone received the awards set forth in the table below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | | Title | | Target Cash | |
Cash
Awarded | | Target Value of Restricted Stock Award at Grant(1) | | Target Number of Shares Underlying Restricted Stock Award at Grant(2) | | Number of Shares Vested under Restricted Stock Award |
| Christopher Stone | | Vice President, General Counsel and Secretary | | $ | 255,154 | | | $ | 168,372 | | | $ | 109,332 | | | 33,954 | | 22,410 |
| | | | | | | | |
| (1) | Target Value of Restricted Stock Award at Grant is the value of the restricted stock on the date granted assuming 100% of the award is achieved. The realized value of such restricted stock may be more or less based on: (i) the value of our common stock when the restricted stock actually vests over the life of the 2016/20 LTIP and (ii) the number of shares that actually vest. |
| (2) | A price of $3.22 per share was used to determine the initial number of shares granted in 2016, which reflected the closing price of the Company’s shares on January 26, 2016, as per the terms of the 2016/20 LTIP. |
Wind Down Retention Benefits
As discussed in more detail above, our Wind Down Retention Plan enabled participants to a cash retention payment in consideration for their continued employment with the Company. For our Chief Executive Officer, the cash retention payment was a lump sum cash payment equal to three times the sum of his base salary and target bonus, plus an amount equal to the cost of 12 months of health insurance continuation under COBRA. For other named executive officers, the cash retention payment was equal to two times the sum of their base salary and target bonus, plus an amount equal to the cost of 12 months of health insurance continuation under COBRA. The cash retention payment was in lieu of (and not in addition to) any other severance compensation that could become payable to the participant.
Given the previously disclosed decision to file for dissolution on January 4, 2021 and in order to realize certain potential tax benefits for the Company, the Compensation Committee and the Board approved the accelerated payment of the cash retention payment under the Wind Down Retention Plan in December 2020. In 2020, the following cash retention payments were paid to our named executive officers:
| | | | | | | | | | | | | | |
| Name | | Title | | Wind-Down Retention Benefits Paid in 2020 |
| Dominique Monnet | | President and Chief Executive Officer | | $ | 3,514,725 | |
| Christopher Stone | | Vice President, General Counsel and Secretary | | $ | 1,784,745 | |
| Jill Jene | | Former Vice President, Business Development | | $ | 1,266,443 | |
| Edward Imbrogno | | Vice President, Chief Financial Officer and Chief Accounting Officer | | $ | 1,241,275 | |
Dr. Jene’s benefits under the Wind Down Retention Plan were paid in August 2020 upon her separation from the Company.
Employee Benefits
The final component of the Company’s compensation strategy is the inclusion of certain employee benefits. We provide our employees, including our named executive officers, with customary benefits, including medical, dental, vision and life insurance coverage, short-term and long-term disability coverage and the ability to participate in our 401(k) plan, which provides for a Company matching contribution up to certain limits. The costs of our insurance coverage benefits are largely borne by us; however, employees pay portions of the premiums for some of these benefits. We think that these benefits are of the type customarily offered to employees by our peer group and in our industry.
This element of compensation is intended to provide assurance of financial support in the event of illness or injury and encourage retirement savings through a 401(k) plan.
All Other Compensation
Due to the Company’s unique business model, the difficulty in locating experienced and qualified executive officer candidates in the remote area surrounding the Company’s headquarters and the fact that in most cases we require our named executive officers to be located proximate to the Company’s headquarters in Nevada, the Compensation Committee decided to provide housing assistance in 2020 to Mr. Monnet ($4,000 per month) and Mr. Imbrogno ($4,000 per month). We generally do not offer any other perquisites to our named executive officers.
Other Executive Compensation Matters
Stock Ownership Guidelines
The Board has determined that ownership of our common stock by our officers promotes a focus on long-term growth and aligns the interests of our officers with those of our stockholders. As a result, the Board has adopted stock ownership guidelines stating that our chief executive officer and our other five most-highly-compensated officers (based on annual base salary), should maintain certain minimum ownership levels of our common stock.
Our stock ownership guidelines require the following levels of ownership among our named executive officers not later than five years after the date the person is initially appointed to the applicable position:
| | | | | | | | |
| Title | | Ownership Threshold |
| Chief Executive Officer | | Three times (3x) base salary |
| Chief Financial Officer and General Counsel | | One times (1x) base salary |
| Other Executives | | 50% of base salary |
As of December 31, 2020, all of our named executive officers were in compliance with this requirement.
The Board is permitted, in its discretion, to waive the application of our stock ownership guidelines to any covered individual if it determines that, as a result of the individual’s personal circumstances, application of our stock ownership guidelines would result in a hardship.
Hedging and Pledging Prohibition
Our Trading Compliance Policy prohibits our directors, officers, and other individuals designated as having access to material non-public information, both during their service with us and following their termination of service, from engaging in the following transactions: (i) short sales in our securities, (ii) hedging transactions, including zero-cost collars and forward sale contracts, that limit economic losses from holding our securities, (iii) holding our securities in a margin account, and (iv) pledging our securities. Other employees are strongly discouraged from engaging in these transactions. We prohibit and/or discourage our directors, officers and employees from engaging in hedging transactions because it is our view that such transactions detract from the alignment of our directors, officers and employees with our stockholders, signal to the market that individuals engaging in these transactions have no confidence in us or in our short-term prospects, and may reduce applicable individual’s incentive to improve our performance.
Clawback Policy
The Board has adopted a policy for recoupment of incentive compensation, or the clawback policy. The Board adopted the clawback policy to prevent executive officers involved in certain wrongful conduct from unjustly benefiting from that conduct, and to remove the financial incentives to engage in such conduct. The clawback policy generally requires an executive officer who is involved in wrongful conduct that results in a restatement of the Company’s financial statements to repay to the Company up to the full amount of any incentive compensation based on the financial statements that were subsequently restated. Incentive compensation includes bonuses or awards under the Company’s annual cash bonus plans, long-term incentive plans and equity incentive plans.
Tax and Accounting Considerations
In determining executive compensation, the Compensation Committee also considers, among other factors, the possible tax consequences to the Company and to our executive officers. The Compensation Committee has determined that our interests are best served in certain circumstances by providing compensation that is not deductible under Section 162(m) of the Code and,
accordingly, may grant such compensation that may be subject to the $1,000,000 annual limit on deductibility, including base salary, annual cash bonuses and long-term incentive awards.
Sections 280G and 4999 of the Code provide that executive officers, persons who hold significant equity interests and certain other highly-compensated service providers may be subject to an excise tax if they receive payments or benefits in connection with a change in control of the Company that exceeds certain prescribed limits, and that the Company (or a successor) may forfeit a deduction on the amounts subject to this additional tax. Further, Section 409A of the Code imposes certain additional taxes on service providers who enter into certain deferred compensation arrangements that do not comply with the requirements of Section 409A of the Code. We have not agreed to pay any named executive officer a “gross-up” or other reimbursement payment for any tax liability that he or she might owe as a result of the application of Section 280G, 4999 or 409A of the Code.
The Compensation Committee also considers the accounting consequences to the Company of different compensation decisions and the impact of certain arrangements on stockholder dilution. However, neither of these factors by themselves will compel a particular compensation decision.
Compensation Committee Report
Following the Company’s delisting from Nasdaq in January 2021, the Compensation Committee was disbanded, at which point the Board assumed the duties of the Compensation Committee. The Board has reviewed and discussed with management the Compensation Discussion and Analysis contained in this Annual Report on Form 10-K. Based on this review and discussion, the Board has decided to include the Compensation Discussion and Analysis in this Annual Report on Form 10-K for the fiscal year ended December 31, 2020.
Respectfully Submitted By:
The Board
Elizabeth O’Farrell (chairperson)
Alan Bazaar
Natasha Hernday
John McLaughlin
Dominique Monnet
Summary Compensation Table
The compensation earned by our chief executive officer, chief financial officer, the other individual serving as an executive officer as of the end of 2020 and our former vice president, business development (the named executive officers) for the last three fiscal years is set forth in the table below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and Title | | Year | | Salary | | Bonus | | Stock Awards(1) | | Option Awards(2) | | Non-Equity
Incentive Plan
Compensation | | All Other
Compensation | | Total |
| Dominique Monnet | | 2020 | | $ | 650,875 | | | $ | — | | | $ | 3,800,000 | | | $ | — | | | $ | 520,700 | | (3) | $ | 3,574,125 | | (4) | $ | 8,545,700 | |
| President and Chief | | 2019 | | $ | 635,000 | | | $ | — | | | $ | 1,678,000 | | | $ | 3,822,000 | | | $ | 508,000 | | | $ | 58,000 | | | $ | 6,701,000 | |
| Executive Officer | | 2018 | | $ | 525,000 | | | $ | — | | | $ | — | | | $ | 1,568,740 | | | $ | 346,500 | | | $ | 58,000 | | | $ | 2,498,240 | |
| | | | | | | | | | | | | | | | |
| Edward Imbrogno | | 2020 | | $ | 354,650 | | | $ | — | | | $ | 900,000 | | | $ | — | | | $ | 265,988 | | (3) | $ | 1,300,675 | | (5) | $ | 2,821,313 | |
| Vice President, Chief Financial Officer and Chief Accounting Officer | | 2019 | | $ | 294,200 | | | $ | — | | | $ | 82,500 | | | $ | 167,500 | | | $ | 211,943 | | | $ | 58,000 | | | $ | 814,143 | |
| | | | | | | | | | | | | | | | |
| Christopher Stone | | 2020 | | $ | 509,927 | | | $ | — | | | $ | 1,300,000 | | | $ | — | | | $ | 550,817 | | (6) | $ | 1,796,145 | | (7) | $ | 4,156,889 | |
| Vice President, General | | 2019 | | $ | 497,490 | | | $ | — | | | $ | — | | | $ | 1,300,000 | | | $ | 645,129 | | | $ | 10,000 | | | $ | 2,452,619 | |
| Counsel and Secretary | | 2018 | | $ | 483,000 | | | $ | — | | | $ | — | | | $ | 1,137,555 | | | $ | 500,940 | | | $ | 10,000 | | | $ | 2,131,495 | |
| | | | | | | | | | | | | | | | |
| Jill Jene | | 2020 | | $ | 224,347 | | | $ | — | | | $ | 600,000 | | | $ | — | | | $ | 167,708 | | (3) | $ | 1,277,843 | | (8) | $ | 2,269,898 | |
| Former Vice President, Business Development | | 2019 | | $ | 350,200 | | | $ | — | | | $ | — | | | $ | 600,000 | | | $ | 280,707 | | | $ | 10,000 | | | $ | 1,240,907 | |
| 2018 | | $ | 210,139 | | | $ | 50,000 | | (9) | $ | — | | | $ | 400,000 | | | $ | 108,000 | | | $ | 3,063 | | | $ | 771,202 | |
_______________
(1)Amounts in this column represent the grant date fair value of restricted stock awards granted in the relevant fiscal year, calculated in accordance with FASB ASC Topic 718. The amounts shown disregard estimated forfeitures. Assumptions used in the calculation of these amounts for awards are included in Note 19 to the Consolidated Financial Statements included in this Annual Report on Form 10-K.
(2)Amounts in this column represent the grant date fair value of stock options granted in the relevant fiscal year, calculated in accordance with FASB ASC Topic 718. The amounts shown disregard estimated forfeitures. Assumptions used in the calculation of these amounts are included in Note 19 to the Consolidated Financial Statements included in this Annual Report on Form 10-K.
Mr. Stone was granted stock options on August 29, 2017, subject to the approval of the stockholders of the Company. As a result, pursuant to SEC rules and FASB ASC Topic 718, since the stock option grants were subject to stockholder approval, the grant date of such awards for purposes of FASB ASC Topic 718 and SEC disclosure rules was June 8, 2018, the date such option grants were approved by the stockholders. This date was also used to determine the grant date fair value of these stock options for purposes of FASB ASC Topic 718, which was $777,555. In addition, during 2018 Mr. Stone was granted additional stock options with a grant date fair value of $360,000. Assumptions used in the calculation of these amounts are included in Note 19 to the Consolidated Financial Statements included in this Annual Report on Form 10-K.
(3)Consists of a payment under the 2020 Annual Bonus Plan.
(4)Consists of: (a) matching contributions we made to our 401(k) plan - $11,400, (b) the housing allowance paid to Mr. Monnet - $48,000 and (c) retention benefits under the Wind Down Retention Plan - $3,514,725.
(5)Consists of: (a) matching contributions we made to our 401(k) plan - $11,400, (b) the housing allowance paid to Mr. Imbrogno - $48,000 and (c) retention benefits under the Wind Down Retention Plan - $1,241,275.
(6)Consists of: (a) payment under the 2020 Annual Bonus Plan - $382,445 and (b) the performance-based cash payment under the 2016/20 LTIP - $168,372.
(7)Consists of: (a) matching contributions we made to our 401(k) plan - $11,400 and (b) retention benefits under the Wind Down Retention Plan - $1,813,646.
(8)Consists of: (a) matching contributions we made to our 401(k) plan - $11,400 and (b) retention benefits under the Wind Down Retention Plan - $1,266,443.
(9)Consists of a signing bonus paid in connection with Dr. Jene’s employment with the Company.
Grants of Plan-Based Awards During 2020
The following table lists each grant of plan-based awards made by the Company during 2020 to our named executive officers:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Estimated Future Payouts Under Non-Equity Incentive Plan Awards ($)(1) | | Estimated Future Payouts Under Equity Incentive Plan Awards (#)(2) | | All Other Stock Awards: Number of Shares of Stock or Units (#) | | Grant Date Fair Value of Stock Awards ($)(3) |
| Name | | Approval Date | | Grant Date | | Threshold | | Target | | Maximum | | Threshold | | Target | | |
| Dominique Monnet | | 1/8/2020 | | 1/8/2020 | | — | | — | | — | | — | | 1,233,766 | | | — | | $ | 3,800,000 | |
| | — | | — | | — | | $ | 520,700 | | | $ | 1,041,400 | | | — | | — | | | — | | |
| Edward Imbrogno | | 1/8/2020 | | 1/8/2020 | | — | | — | | — | | — | | 292,208 | | | — | | $ | 900,000 | |
| | — | | — | | — | | $ | 265,988 | | | $ | 531,976 | | | — | | — | | | — | | |
| Christopher Stone | | 1/8/2020 | | 1/8/2020 | | — | | — | | — | | — | | 422,078 | | | — | | $ | 1,300,000 | |
| | — | | — | | — | | $ | 382,445 | | | $ | 764,890 | | | — | | — | | | — | | |
| Jill Jene | | 1/8/2020 | | 1/8/2020 | | — | | — | | — | | — | | 194,805 | | | — | | $ | 600,000 | |
| | — | | — | | — | | $ | 269,216 | | | $ | 538,432 | | | — | | — | | | — | | |
_______________________
(1)The amounts in the below columns relate to the Company’s 2020 Annual Bonus Plan. Actual amounts paid in December 2020 under the 2020 Annual Bonus Plan were based on the Compensation Committee’s review of corporate performance and are included in the “Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table.
(2)Reflects shares of restricted stock granted to our named executive officers in 2020. 40% of the restricted stock was scheduled to vest on the first anniversary of the grant date, with 2.5% vesting monthly thereafter. In February 2020, in connection with the Board adopting the Plan of Liquidation, all of the restricted stock accelerated and vested under the change in control definition in the Equity Plan.
(3)Amounts in this column represent the grant date fair value of restricted stock granted in 2020, calculated in accordance with FASB ASC Topic 718. The amounts shown disregard estimated forfeitures. Assumptions used in the calculation of these amounts are included in Note 19 to the Consolidated Financial Statements included in this Annual Report on Form 10-K.
Outstanding Equity Awards at December 31, 2020
The following table sets forth information concerning stock options held by the named executive officers as of December 31, 2020 (there are no outstanding restricted stock awards as of such date):
| | | | | | | | | | | | | | | | | | | | | |
| Name | | Number of Securities Underlying Unexercised Options (#) Exercisable(1) | | Option Exercise Price ($)(2) | | Option Expiration Date | |
| Dominique Monnet | | 961,000 | | $1.85 | | 9/10/2027 |
| | 1,479,944 | | $1.16 | | 9/25/2028 | |
| | 2,450,000 | | $2.36 | | 3/28/2029 | |
| Edward Imbrogno | | 288,611 | | $1.32 | | 12/20/2028 | |
| | 108,766 | | $2.33 | | 3/25/2029 | |
| Christopher Stone | | 700,500 | | $1.58 | | 8/28/2027 |
| | 339,623 | | $1.16 | | 9/25/2028 |
| | 833,333 | | $2.36 | | 3/28/2029 |
| Jill Jene | | 384,615 | | $2.36 | | 3/28/2029 |
_____________________
(1)All stock options vested in February 2020 per the terms of the Equity Plan.
(2)Represents stock option exercise price, as adjusted on a dollar-for-dollar basis to reflect the per share value of the distributions of LENSAR common stock and Evofem common stock that occurred in 2020.
Option Exercises and Stock Vested in 2020
The following table sets forth (i) the number of options exercised by the named executive officers during 2020 and (ii) restricted shares granted to our named executive officers that vested during 2020:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Options Exercised | | Stock Awards | |
| Name | | Number of Options Exercised (#) | | Value Realized on Exercise ($) | | Number of Shares Acquired on Vesting (#) | | Value Realized on Vesting ($) | |
| Dominique Monnet | | — | | $ | — | | | 1,764,908 | | | $ | 6,194,827 | | (1) |
| Edward Imbrogno | | — | | $ | — | | | 314,565 | | | $ | 1,104,123 | | (1) |
| Christopher Stone | | — | | $ | — | | | 444,488 | | | $ | 1,538,191 | | (2) |
| Jill Jene | | 377,358 | | $ | 407,547 | | (3) | 194,805 | | | $ | 683,766 | | (1) |
________________
(1)Value based on the “closing” price of the shares on the vesting date, February 7, 2020 ($3.51).
(2)Value based on the “closing” price of the shares on (i) the vesting date, February 7, 2020 ($1,481,494) with respect to 422,078 shares and (ii) the vesting date December 2, 2020 ($56,697) with respect to 22,410 shares.
(3)Value based on the difference between the exercise price ($1.16) and the “closing” price of the shares on the date of exercise, November 5, 2020 ($2.24).
Potential Payments upon Termination or Change in Control
Severance Arrangements with Named Executive Officers
Wind Down Retention Plan
After we announced our strategy to monetize our assets and distribute net proceeds to our stockholders, on December 21, 2019, the Compensation Committee of the Board adopted the Wind Down Retention Plan in which the Company’s executive officers and other employees who are participants in the Company’s Severance Plan are eligible to participate. All payments and other benefits paid out under the Wind Down Retention Plan are in lieu of, and not in addition to, the payments under the Severance Plan. For a further description of the Wind Down Retention Plan, see "--Wind Down Retention Plan" above.
Potential Payments Upon Termination or Change in Control
The terms of the Wind Down Retention Plan superseded any other severance arrangements with respect to any cash retention payments to be paid upon termination or a Change in Control. In addition, all outstanding equity awards vested in February and December of 2020 under the Wind Down Retention Plan. There are no further cash retention payments payable to any named executive officers as a result of termination or a Change in Control as of December 31, 2020 under the Wind Down Retention Plan or any other severance arrangements between the Company and any executive officer.
Jill Jene Separation Agreement
On August 15, 2020, we entered into a Confidential Severance Agreement and Release of All Claims with Jill Jene, Ph.D., our former Vice President, Business Development, which agreement is a condition under the Wind Down Retention Plan for the payment of the cash retention payment. Under the severance agreement, Dr. Jene agreed to a general release of claims in our favor and a covenant not to sue, among other promises. In consideration for her release of claims and covenants, Dr. Jene received a one-time payment of $1,266,443. In addition, Dr. Jene was retained as a consultant for the four-month period following her resignation, which was effective as of August 15, 2020.
CEO Pay Ratio
As required by Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act, and Item 402(u) of Regulation S-K, we are providing the following information regarding the relationship of the annual total compensation of our employees and the annual total compensation of Dominique Monnet, our President and Chief Executive Officer (our “CEO”) during 2020. The pay ratio included in this information is a reasonable estimate calculated in a manner that is intended to be consistent with Item 402(u) of Regulation S-K.
For 2020, our last completed fiscal year:
•the median of the annual total compensation of all employees of our company (other than our CEO) was $710,082; and
•the annual total compensation of our CEO, as reported in the Summary Compensation Table included above, was $8,545,700.
Based on this information, for 2020, the ratio of the median of the total compensation of all employees of the Company to the annual total compensation of our CEO during 2020, was 1 to 12.
Determining the Median Employee
We determined that, as of December 31, 2020, our employee population consisted of ten individuals (not including our CEO), with all of these individuals located in the United States. Our employee workforce on such date consisted of full-time employees only.
For purposes of measuring the compensation of our employees, we selected total annual cash compensation for 2020 as the most appropriate measure of compensation, which was consistently applied to all our employees included in the calculation. We did not make any cost-of-living adjustments in identifying the “median employee”. With respect to the total annual compensation of the “median employee,” we identified and calculated the elements of such employee’s compensation for fiscal year 2020 in accordance with the requirements of Item 402(c)(2)(x) of Regulation S-K, resulting in annual total compensation of $710,082.
Compensation Committee Interlocks and Insider Participation
During the year ended December 31, 2020, each of Mr. Bazaar, Ms. O’Farrell, Ms. Hernday and Mr. Sandman served on the Compensation Committee for at least a portion of the year. None of the members of the Compensation Committee was an officer or an employee of the Company at any time during 2020. None of our executive officers serve as a member of the board of directors or compensation committee of any other entity that has one or more executive officers serving as a member of the Board or Compensation Committee. Our chief executive officer assists the Compensation Committee by presenting proposals and recommendations to the Compensation Committee, information on Company and individual performance of the named executive officers and management’s perspective and recommendations on compensation matters. Our chief executive officer recuses himself from that portion of the Compensation Committee meetings involving deliberation and decision making of his own compensation.
COMPENSATION OF OUR DIRECTORS
The Board has established its compensation policy for outside directors, which was most recently amended in April 2020, primarily in consultation with the Compensation Committee’s current compensation consultant, Board Advisory. Members of the Board who are also employees of the Company are not entitled to any compensation with respect to their service as Board members.
Cash Compensation
Prior to April 1, 2020, each outside director received amounts under a retainer based on a per annum rate of $100,000, except for the Chairperson who received a retainer based on a per annum rate of $115,000, for his or her service on the Board. Each outside director also received annual cash retainers for service on Board committees, as follows:
•Each member of the Audit Committee received a retainer of $17,500 per year, except for the chairperson of the Audit Committee who received a retainer of $30,000 per year, for his or her service on the Audit Committee.
•Each member of the Compensation Committee received a retainer of $15,000 per year, except for the chairperson of the Compensation Committee who received a retainer of $22,500 per year, for his or her service on the Compensation Committee.
•Each member of the Litigation Committee received a retainer of $10,000 per year, except for the chairperson of the Litigation Committee who received a retainer of $20,000 per year, for his or her service on the Litigation Committee.
•Each member of the Nominating and Governance Committee received a retainer of $2,500 per year, except for the chairperson of the Nominating and Governance Committee who received a retainer of $5,000 per year, for his or her service on the Nominating and Governance Committee.
Effective April 1, 2020, the Board reduced the retainer from $100,000 per year to $50,000 per year, applicable to all outside directors, for service on the Board. It also increased the retainer for membership and chairpersonship of the Nominating and Governance Committee to $5,000 per year and $10,000 per year, respectively, to be aligned with the 50th percentile of the Company’s peer group. In addition, in connection with the Cost Committee that was formed in February 2020, the Board established a retainer of $5,000 per year for each member’s service and $10,000 per year for the chairperson’s service.
All cash compensation paid to outside directors for their service on the Board and its committees is paid on a quarterly basis in arrears.
We also reimburse our directors for their reasonable travel expenses for Board and committee meetings, although there were no in-person Board or committee meetings requiring travel expenses in 2020.
Equity Compensation
We provide our outside directors with equity awards as a portion of their total compensation to ensure that our outside directors own common stock in the Company and their interests are aligned with our stockholders. In 2020, given the change in the Company’s strategy to liquidate its assets and a cooperation agreement with one of the Company’s stockholders, the Compensation Committee and the Board revised the structure of equity compensation for our outside directors. The Board agreed to align its equity compensation in form and amount with the 50th percentile of the Company’s peer group. In doing so, it increased the amount of the annual equity compensation to awards with a grant date value of $187,500, which value was granted 50% in the form of restricted stock awards and 50% in the form of stock options.
Such grants were made to each current outside director annually after the conclusion of our annual general meeting of stockholders in August 2020. Each grant of restricted stock or stock options, as the case may be, was scheduled to vest on the first anniversary of the grant date so long as the director continues to serve on the Board on the vesting date. In the case of restricted stock, during the vesting period, our outside directors have the right to vote their restricted stock and to receive any dividends or distributions paid on their restricted stock, except that dividends or distributions are accumulated and paid on the earlier of the vesting of the underlying stock in accordance with the vesting conditions of the original award or March 15th of the year following the payment of such dividend or distribution to all stockholders. Such grants were also subject to acceleration upon the date the Board approved the filing of a certificate of dissolution with the State of Delaware, which occurred on November 5, 2020.
Any outstanding stock options held by directors are subject to equitable adjustments to ensure such directors realize the same benefits provided to stockholders in the event one or more cash or other distributions become payable to stockholders. Consistent with the existing terms of the Equity Incentive Plan, in the event one or more cash or other distributions are paid to stockholders,
the exercise price of outstanding stock options will be reduced on a dollar-for-dollar basis to reflect the per share value of such distributions. I the event that the Company declares cash or other distributions that, in the aggregate, exceed the difference between the exercise price of an outstanding stock option and the par value of the underlying shares ($0.01), the holder of such stock option will be entitled to receive from the Company a cash payment in an amount equal to the number of shares subject to such stock option multiplied by the per share amount of the cash or other distributions that exceeds the difference between exercise price of the outstanding option and the par value of the underlying shares (a “true-up payment”). A true-up payment shall be paid with respect to a post-dissolution cash or other distributions with respect to a stock-option that was not exercised prior to dissolution.
2020 Compensation of Directors
In 2020, our outside directors who served on the Board during 2020 earned the compensation set forth in the table below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Director | | Fees Earned | | Stock Awards(1) | | Option Awards(1) | | All Other Compensation | | Total |
| Elizabeth O’Farrell | | $ | 128,750 | | | $ | 93,750 | | | $ | 93,750 | | | $ | — | | | $ | 316,250 | |
Alan Bazaar(2) | | $ | 58,885 | | | $ | 117,111 | | $ | 117,111 | | | $ | — | | | $ | 293,107 | |
David W. Gryska(3) | | $ | 96,875 | | | $ | 93,750 | | | $ | 93,750 | | | $ | — | | | $ | 284,375 | |
| Natasha Hernday | | $ | 85,377 | | | $ | 93,750 | | | $ | 93,750 | | | $ | — | | | $ | 272,877 | |
| John P. McLaughlin | | $ | 70,000 | | | $ | 93,750 | | | $ | 93,750 | | | $ | 97,500 | | (4) | $ | 355,000 | |
Paul W. Sandman(5) | | $ | 77,527 | | | $ | — | | | $ | — | | | $ | — | | | $ | 77,527 | |
Shlomo Yanai(6) | | $ | 30,834 | | | $ | — | | | $ | — | | | $ | 10,000 | | (7) | $ | 40,834 | |
_______________
(1)Amounts in this column represent the grant date fair value of the stock options and restricted stock granted to our outside directors, as determined in accordance with FASB ASC Topic 718. The amounts shown disregard estimated forfeitures. Assumptions used in the calculation of these amounts for awards are included in Note 19 to the Consolidated Financial Statements included in this Annual Report on Form 10-K.
As of December 31, 2020, Ms. O’Farrell held 244,721 outstanding stock options; Messrs. Gryska and Bazaar and Ms. Hernday held 118,671 outstanding stock options; Mr. McLaughlin held 2,166,821 outstanding stock options and Mr. Sandman held 126,050 outstanding stock options.
(2)Mr. Bazaar was appointed to the Board in February 2020 pursuant to the Engine Cooperation Agreement.
(3)Mr. Gryska resigned from the Board effective December 31, 2020.
(4)Includes the following compensation for Mr. McLaughlin: (i) $60,000 earned under a consulting agreement for services provided in connection with the transition after he retired as the Company’s chief executive officer at the end of 2018 and (ii) $37,500 in connection with his service as a director for LENSAR, Inc., while it was a subsidiary of the Company.
(5)Mr. Sandman resigned from the Board effective August 19, 2020.
(6)Mr. Yanai resigned from the Board effective April 30, 2020.
(7)Fees paid to Mr. Yanai under a consulting agreement for services provided in connection with certain monetization strategies after his resignation.
Stock Ownership Guidelines for Directors
The Board has determined that ownership of our common stock by our officers and directors promotes a focus on long-term growth and aligns the interests of our officers and directors with those of our stockholders. As a result, the Board adopted stock ownership guidelines stating that our outside directors should maintain certain minimum ownership levels of our common stock.
Our stock ownership guidelines require that each outside director should own shares of common stock with a value of at least three times the annual cash retainer we pay to the outside director not later than five years after the date the person initially becomes an outside director. As of December 31, 2020, all of our outside directors are in compliance with this requirement or are on track to be compliant within the compliance period.
The Board is permitted, in its discretion, to waive the application of our stock ownership guidelines to any covered individual if it determines that, as a result of the individual’s personal circumstances, application of the ownership guidelines would result in a hardship. No such waivers were approved in 2020 for any of our officers or directors.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plan Information
Following the filing of the certificate of dissolution with the Secretary of State of Delaware in January 2021, the Company can no longer issue any equity securities, including through any equity compensation plans. The following table provides information on our equity compensation plans as of December 31, 2020.
As of December 31, 2020, we had one active stock-based incentive plan in place under which equity awards were outstanding or shares of our common stock were authorized for issuance and stand-alone inducement awards to one employee, detailed as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Plan Category | | Number of securities to be issued upon exercise of outstanding options, warrants and rights | | Weighted-average exercise price of outstanding options, warrants and rights | | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
| | (a) | | (b) | | | (c) |
| Equity compensation plans approved by security holders | | 10,180,051 | | (1) | | $ | 1.87 | | (2) | | 8,468,205 | |
Equity compensation plans not approved by security holders (3) | | 961,000 | | | | $ | 1.85 | | (2) | | — | |
_________________
(1)Represents outstanding stock options as of December 31, 2020 under the Amended and Restated 2005 Equity Incentive Plan.
(2)Represents weighted-average stock option exercise price, as adjusted on a dollar-for-dollar basis to reflect the per share value of the distributions of LENSAR common stock and Evofem common stock that occurred in 2020.
(3)As an inducement material to the decision by Dominique Monnet to accept employment with the Company, the Compensation Committee approved the grant of inducement awards to Mr. Monnet as employment inducement awards pursuant to Nasdaq Listing Rule 5635(c)(4). Effective September 11, 2017, Mr. Monnet was granted stock options to purchase 961,000 shares of the Company’s common stock at an exercise price equal to the closing price per share of the Company’s common stock on the date of grant. All of the options vested in February 2020 upon the Board’s adoption of the Plan of Liquidation. For a description of the inducement awards to Mr. Monnet, please see Note 19 to the Consolidated Financial Statements included in this Annual Report on Form 10-K.
Security Ownership of Certain Beneficial Owners and Management
On December 28, 2020, the Company filed a Form 25 with the SEC notifying the SEC of its intent to voluntarily withdraw its common stock from listing and registration on an exchange. Effective before the market opened on December 31, the Company requested Nasdaq to suspend trading in its common stock in anticipation of filing for dissolution. On January 4, 2020 in accordance with Section 275 of the DGCL and the Plan of Dissolution, the Company filed a certificate of dissolution with the Secretary of State of Delaware, closed its stock transfer books and discontinued recording transfers of its Common Stock. On January 7, 2020, the Company’s common stock was formally delisted from the Nasdaq Global Select Market. From and after the effectiveness of the filing for dissolution on January 4, 2021, the Company’s stockholders have only such rights and obligation as are provided under the DGCL for stockholders of a dissolved corporation.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Procedures for Approval of Related Person Transactions
The Audit Committee is responsible for reviewing and approving all related person transactions, including transactions with executive officers and directors, for potential conflicts of interests or other improprieties. Under SEC rules, related person transactions are those transactions where we are or may be a party and the amount involved exceeds $120,000, and where any of our directors or executive officers or any other related person had or will have a direct or indirect material interest, excluding, among other things, compensation arrangements with respect to employment and Board membership. The Audit Committee
would approve a related person transaction if it determined that the transaction was in the Company’s best interests and on terms no less favorable than terms generally available to an unaffiliated third-party under the same or similar circumstances.
The Audit Committee has adopted a stringent written policy whereby the Audit Committee will review for approval all related person transactions where the amount involved is anticipated to exceed $25,000. Our directors are required to disclose to the Board any potential conflict of interest or personal interest in a transaction that the Board or the Company is considering. Our executive officers are required to disclose any related person transaction to our Compliance Officer who would notify the Audit Committee of the transaction. We poll our directors regularly, but no less frequently than annually, with respect to related person transactions and their service as an officer or director of other entities.
Any director involved in a related person transaction that is being reviewed or approved must recuse himself or herself from participation in any related deliberation or decision. All related person transactions anticipated to exceed $25,000 are reviewed in advance of the transaction being completed.
Related Person Transactions
There were no transactions in 2020 and there is not any currently proposed transaction where we were or are to be a party and the amount involved exceeded $120,000, and where any of our directors or executive officers or any other related person had or will have a direct or indirect material interest, other than the compensation paid to our executive officers with respect to their employment relationship with us and compensation paid to our outside directors for their service as members of the Board, which compensation is disclosed above in “Item 11. Executive Compensation.”
Independence of Directors
As required under the Nasdaq listing standards, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by the board of directors. The Board consults with the Company’s counsel to ensure that the Board’s determinations are consistent with relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in the applicable Nasdaq listing standards, as in effect from time to time.
Prior to the Company’s voluntary delisting from Nasdaq, consistent with these considerations, after review of all relevant transactions or relationships between each director, or any of his or her family members, and the Company, its senior management and its independent auditors, the Board affirmatively determined that the following five directors were independent directors within the meaning of the applicable Nasdaq listing standards during their service on the Board after January 1, 2020: Ms. O’Farrell, Mr. Bazaar, Ms. Hernday, Mr. Gryska and Mr. Sandman. In making these determinations, the Board found that none of these directors or nominees for director had a material or other disqualifying relationship with the Company. Mr. McLaughlin, the Company’s former Chief Executive Officer, and Mr. Monnet, the Company’s President and Chief Executive Officer, were not independent directors by virtue of their employment history with the Company.
The Board also determined that each member of each of the Compensation Committee, the Nominating and Governance Committee and the Audit Committee was independent during 2020.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Principal Independent Registered Public Accounting Firm Fees and Services
The aggregate fees billed to the Company for the fiscal years ended December 31, 2020 and 2019, by PricewaterhouseCoopers LLP, the Company’s independent registered public accounting firm:
| | | | | | | | | | | | | | |
| (in thousands) | | 2020 | | 2019 |
| Fee Category | | | | |
Audit Fees(1) | | $ | 4,330 | | | $ | 2,812 | |
Audit-related Fees(2) | | — | | | — | |
Tax Fees(3) | | — | | | — | |
All Other Fees(4) | | 3 | | | 2 |
| Total Fees | | $ | 4,333 | | | $ | 2,814 | |
_______________
(1)Audit fees consist of fees for professional services rendered for the audit of our consolidated financial statements, attestation services surrounding the effectiveness of our internal control environment and review of the interim condensed consolidated financial statements included in quarterly reports. It also includes services that normally would be provided in connection with statutory and regulatory filings or engagements and services that generally only the principal auditor reasonably can provide to a client, such as comfort letters, attestation services (except those not required by statute or regulation), procedures related to audit of income tax provisions and related reserves, consents and assistance with and review of documents filed with the SEC.
(2)Audit-related fees consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of our consolidated financial statements and are not reported under “Audit Fees.”
(3)Tax fees consist of fees for tax compliance, tax advice and tax planning.
(4)All other fees include any fees billed that are not audit, audit related or tax fees. In 2020 and 2019, these fees included a license to an accounting research database and an accounting disclosure checklist.
The Audit Committee pre-approves all audit services provided by the independent registered public accounting firm and permissible non-audit services in excess of a certain de minimis amount provided by the independent registered public accounting firm. These services may include audit services, audit-related services, tax services and other services. The Audit Committee has a policy for the pre-approval of services provided by the independent registered public accounting firm. Under the policy, any pre-approval is detailed as to the particular service or category of services and includes an estimate of the related fees. The Audit Committee may delegate pre-approval authority to one or more of its members. Such a member must report any decisions to the Audit Committee at the next scheduled meeting. During fiscal years 2020 and 2019, the Audit Committee approved all of the fees described above.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)The following documents are filed as part of this Annual Report on Form 10-K:
(1)Financial Statements - See Index to Consolidated Financial Statements at Item 8 of this Annual Report on Form 10-K.
(2)Financial Statement Schedules
The financial statement schedules are omitted because the information is not applicable, not required under the instructions, or the information requested is set forth in our Consolidated Financial Statements or related notes thereto.
(3)Exhibits required by Item 601 of Regulation S-K
The information required by this Section (a)(3) of Item 15 is set forth on the exhibit index that precedes the Signatures page of this Annual Report on Form 10-K.
ITEM 16. FORM 10-K SUMMARY
None.
EXHIBIT INDEX
| | | | | |
Exhibit
Number | Exhibit Title |
| |
| 2.1 | |
| |
| 2.2 | |
| |
| 3.1 | Restated Certificate of Incorporation effective March 23, 1993 (incorporated by reference to Exhibit 3.1 to Annual Report on Form 10-K filed March 31, 1993) |
| |
| 3.2 | |
| |
| 3.3 | |
| |
| 3.4 | |
| |
| 3.5 | |
| |
| 3.6 | |
| |
| 3.7 | |
| |
| 4.1 | |
| |
| 4.2 | |
| |
| 4.3 | |
| |
| 4.4 | |
| |
| 4.5 | |
| |
| 4.6 | |
| |
| 4.7 | |
| |
| 4.8# | |
| |
| 10.1* | |
| |
| 10.2 | |
| |
| | | | | |
| 10.3 | |
| |
| 10.4 | |
| |
| 10.5 | |
| |
| 10.6 | |
| |
| 10.7 | |
| |
| 10.8 | |
| |
| 10.9* | |
| |
| 10.10 | |
| |
| 10.11 | |
| |
| 10.12 | |
| |
| 10.13 | |
| |
| 10.14 | |
| |
| 10.15 | |
| |
| 10.16 | |
| |
| 10.17 | |
| |
| 10.18 | |
| |
| 10.19 | |
| |
| 10.20 | |
| |
| 10.21* | |
| |
| 10.22 | |
| |
| 10.23 | |
| |
| | | | | |
| 10.24 | |
| |
| 10.25 | |
| |
| 10.26 | |
| |
| 10.27* | |
| |
| 10.28 | |
| |
| 10.29* | |
| |
| 10.30 | |
| |
| 10.31* | |
| |
| 10.32 | |
| |
| 10.33 | |
| |
| 10.34 | |
| |
| 10.35 | |
| |
| 10.36 | |
| |
| 10.37 | |
| |
| 10.38* | |
| |
| 10.39* | |
| |
| 10.40* | |
| |
| 10.41 | |
| |
| 10.42* | |
| |
| 10.43* | |
| |
| 10.44* | |
| | | | | |
| |
| 10.45* | |
| |
| 10.46 | |
| |
| 10.47* | |
| |
| 10.48* | |
| |
| 10.49 |
|
| |
| 10.50* | |
| |
| 10.51* | |
| |
| 10.52† | |
| |
| 10.53* | |
| |
| 10.54 | |
| |
| 10.55 | |
| |
| 10.56* | |
| |
| 10.57* | |
| |
| 10.58* | |
| |
| 10.59* | |
| |
| 10.60* | |
| |
| 10.61* | |
| |
| 10.62* | |
| |
| 10.63* | |
| |
| 10.64* | |
| |
| 10.65* | |
| |
| 10.66 | |
| |
| 10.67* | |
| |
| 10.68 | |
| | | | | |
| |
| 10.69 | Settlement and Mutual Release Agreement among the Company, Samuel J. Wohlstadter, Nadine H. Wohlstadter, Hyperion Catalysis International, Wellstat Vaccines, LLC, Wellstat Immuno Therapeutics, LLC, Wellstat BioCatalysis, LLC, Wellstat AVT Investment, LLC, Wellstat Biologics Corporation, Wellstat Management Company, LLC, Wellstat Ophthalmics Corporation, Wellstat Therapeutics Corporation, Wellstat Therapeutics EU Limited, Duck Farm, Inc., Hebron Valley Farms, Inc., HVF, Inc., Hyperion Catalysis EU Limited, NHW, LLC, and SJW Properties, Inc., and Defined Diagnostics, LLC (f/k/a Wellstat Diagnostics, LLC, effective as of August 11, 2020 (incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed November 13, 2020) |
| |
| 10.70 | |
| |
| 10.71* | |
| |
| 10.72 | |
| |
| 10.73 | |
| |
| 10.74 | |
| |
| 10.75# | |
| |
| 21.1# | |
| |
| 31.1# | |
| |
| 31.2# | |
| |
| 32.1#+ | |
| |
| 101.INS | XBRL Instance Document |
| |
| 101.SCH | XBRL Taxonomy Extension Schema |
| |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase |
| |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase |
| |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase |
| |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase |
____________________________________
| | | | | |
| # | Filed herewith. |
| |
| * | Management contract or compensatory plan or arrangement. |
| |
| † | Portions of this exhibit have been redacted in compliance with Regulation S-K Item 601(b)(10). The omitted information is not material and would likely cause competitive harm to the Company if publicly disclosed. |
| + | The certifications attached as Exhibit 32.1 accompany this Annual Report on Form 10-K pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed “filed” by the Registrant for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| PDL BIOPHARMA, INC. | |
| | | |
| By: | | /S/ DOMINIQUE MONNET | |
| | (Dominique Monnet) | |
| | President and Chief Executive Officer | |
| | | |
| Date: | March 26, 2021 | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | |
| Signature | Title | Date |
| | |
| /S/ DOMINIQUE MONNET | President and Chief Executive Officer (Principal Executive Officer) | March 26, 2021 |
| (Dominique Monnet) | | |
| | |
| /S/ EDWARD A. IMBROGNO | Vice President and Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | March 26, 2021 |
| (Edward A. Imbrogno) | | |
| | |
| /S/ ALAN BAZAAR | Director | March 26, 2021 |
| (Alan Bazaar) | | |
| | |
| /S/ NATASHA A. HERNDAY | Director | March 26, 2021 |
| (Natasha A. Hernday) | | |
| | |
| /S/ JOHN P. MCLAUGHLIN | Director | March 26, 2021 |
| (John P. McLaughlin) | | |
| | |
| /S/ ELIZABETH O’FARRELL | Director | March 26, 2021 |
| (Elizabeth O’Farrell) | | |