- BCRX Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
425 Filing
BioCryst Pharmaceuticals (BCRX) 425Business combination disclosure
Filed: 21 Jun 18, 12:00am
Combining Capabilities to Serve More Patients with Rare Diseases + Filed by BioCryst Pharmaceuticals, Inc. pursuant to Rule 425 Under the Securities Act of 1933 And Deemed Filed Pursuant to Rule 14a-12 Under the Securities Exchange Act of 1934 Subject Company: BioCryst Pharmaceuticals, Inc. Commission File No. of Subject Company: 000-23186
2 Additional Information and Where to Find It Additional Information and Where to Find It In connection with the proposed mergers, Nautilus Holdco, Inc. (“Holdco”) has filed with the U.S. Securities and Exchange Commission (the “SEC”), and the SEC has declared effective on May 23, 2018, a Post-Effective Amendment to the Registration Statement on Form S-4 (as may be amended from time to time, the “Registration Statement”) that includes the joint proxy statement of BioCryst Pharmaceuticals, Inc. (“BioCryst”) and Idera Pharmaceuticals, Inc. (“Idera”) and that also constitutes a prospectus of Holdco. BioCryst, Idera and Holdco may also file other documents with the SEC regarding the proposed transaction. This document is not a substitute for the definitive joint proxy statement/prospectus or Registration Statement or any other document that may be filed by each of BioCryst and Idera with the SEC. BEFORE MAKING ANY VOTING DECISION, IDERA’S AND BIOCRYST’S RESPECTIVE STOCKHOLDERS ARE URGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS IN ITS ENTIRETY AND ANY OTHER DOCUMENTS FILED BY EACH OF IDERA AND BIOCRYST WITH THE SEC IN CONNECTION WITH THE PROPOSED TRANSACTION OR INCORPORATED BY REFERENCE THEREIN BECAUSE THEY CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION AND THE PARTIES TO THE PROPOSED TRANSACTION. Investors and stockholders may obtain free copies of these materials and other documents filed with the SEC (when available) by BioCryst, Idera and Holdco through the website maintained by the SEC at www.sec.gov. Idera and BioCryst make available free of charge at www.iderapharma.com and www.biocryst.com, respectively (in the “Investors” section), copies of materials they file with, or furnish to, the SEC. Participants in the Solicitation This document does not constitute a solicitation of proxy, an offer to purchase or a solicitation of an offer to sell any securities. Idera, BioCryst and their respective directors, executive officers and certain employees and other persons may be deemed to be participants in the solicitation of proxies from the stockholders of Idera and BioCryst in connection with the proposed mergers. Security holders may obtain information regarding the names, affiliations and interests of Idera’s directors and officers in Idera’s Annual Report on Form 10-K for the fiscal year ended December 31, 2017, which was filed with the SEC on March 7, 2018 and its definitive proxy statement for the 2018 annual meeting of stockholders, which was filed with the SEC on May 22, 2018. Security holders may obtain information regarding the names, affiliations and interests of BioCryst’s directors and officers in BioCryst’s Annual Report on Form 10-K for the fiscal year ended December 31, 2017, and any amendments thereto, which was filed with the SEC on March 12, 2018 and its definitive proxy statement for the 2018 annual meeting of stockholders, which was filed with the SEC on May 10, 2018. Additional information about the interests of BioCryst’s directors and officers and Idera’s directors and officers in the proposed mergers can be found in the above-referenced Registration Statement. These documents may be obtained free of charge from the SEC’s website at www.sec.gov, Idera’s website at www.iderapharma.com and BioCryst’s website at www.biocryst.com.

Forward-Looking Statements 3 These materials contain forward-looking statements within the meaning of the federal securities laws, including Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements involve known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and are subject to risks and uncertainties, and important factors that could cause actual events or results to differ materially from Idera’s or BioCryst’s plans, estimates or expectations. Given these uncertainties, you should not place undue reliance on these forward-looking statements. With respect to the transactions contemplated by the merger agreement between Idera and BioCryst, these factors could include, but are not limited to: (i) Idera or BioCryst may be unable to obtain stockholder approval as required for the mergers; (ii) conditions to the closing of the mergers may not be satisfied; (iii) the mergers may involve unexpected costs, liabilities or delays; (iv) the effect of the announcement of the mergers on the ability of Idera or BioCryst to retain and hire key personnel and maintain relationships with patients, doctors and others with whom Idera or BioCryst does business, or on Idera’s or BioCryst’s operating results and business generally; (v) Idera’s or BioCryst’s respective businesses may suffer as a result of uncertainty surrounding the mergers and disruption of management’s attention due to the mergers; (vi) the outcome of any legal proceedings related to the mergers; (vii) Idera or BioCryst may be adversely affected by other economic, business, and/or competitive factors; (viii) the occurrence of any event, change or other circumstances that could give rise to the termination of the merger agreement; (ix) risks that the mergers disrupt current plans and operations and the potential difficulties in employee retention as a result of the mergers; (x) the risk that Idera or BioCryst may be unable to obtain governmental and regulatory approvals required for the transactions, or that required governmental and regulatory approvals may delay the transactions or result in the imposition of conditions that could reduce the anticipated benefits from the transactions contemplated by the merger agreement or cause the parties to abandon the transactions contemplated by the merger agreement; (xi) risks that the anticipated benefits of the mergers or other commercial opportunities may otherwise not be fully realized or may take longer to realize than expected; (xii) the impact of legislative, regulatory, competitive and technological changes; (xiii) risks relating to the value of the new holding company shares to be issued in the mergers; (xiv) expectations for future clinical trials, the timing and potential outcomes of clinical studies and interactions with regulatory authorities; (xv) the risk that the credit ratings of the combined company or its subsidiaries may be different from what the companies expect; (xvi) economic and foreign exchange rate volatility; (xvii) the continued strength of the medical and pharmaceutical markets; (xviii) the timing, success and market reception for Idera’s and BioCryst’s products; (xix) the possibility of new technologies outdating Idera’s or BioCryst’s products; (xx) continued support of Idera’s or BioCryst’s products by influential medical professionals; (xxi) reliance on and integration of information technology systems; (xxii) the risks associated with assumptions the parties make in connection with the parties’ critical accounting estimates and legal proceedings; (xxiii) the potential of international unrest, economic downturn or effects of currencies, tax assessments, tax adjustments, anticipated tax rates, raw material costs or availability, benefit or retirement plan costs, or other regulatory compliance costs; and (xxiv) other risks to the consummation of the mergers, including the risk that the mergers will not be consummated within the expected time period or at all. These risks, as well as other risks associated with the proposed mergers, are more fully discussed in the joint proxy statement/prospectus included in the Registration Statement filed with the SEC in connection with the proposed mergers. While the list of factors presented here is, and the list of factors presented in the Registration Statement are, considered representative, no such list should be considered a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward looking statements. Consequences of material differences in results as compared with those anticipated in the forward-looking statements could include, among other things, business disruption, operational problems, financial loss, legal liability to third parties and similar risks, any of which could have a material adverse effect on BioCryst’s or Idera’s consolidated financial condition, results of operations, credit rating or liquidity. Readers are urged to consider these factors carefully in evaluating these forward-looking statements, and not to place undue reliance on any forward-looking statements. Readers should also carefully review the risk factors described in other documents that Idera and BioCryst file from time to time with the SEC. The forward-looking statements in this document speak only as of the date of this document. Except as required by law, Idera and BioCryst assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.

Combination Creates Substantial Value 4 Complementary Assets and Platforms Enhance Market Opportunities and Accelerate Value Creation Maximizing Value and Market Potential Robust, Rare Disease Focused Pipeline Synergistic Discovery Engines Proven Clinical and Commercial Track Record Increased Financial Strength Creates a unique player in rare diseases, with scale and strengthened competitive position More opportunities for success through diversified late-stage pipeline, variety of early-stage programs and supporting assets Synergistic discovery engines with enhanced development opportunities, including through joint small molecule and oligo treatments Complementary leadership with best-in-class people, facilities and commercial know-how in rare diseases Increased financial strength and flexibility through significant cost synergies and opportunities to generate non-dilutive capital New Data Presented on Tilsotolimod at ASCO 2018
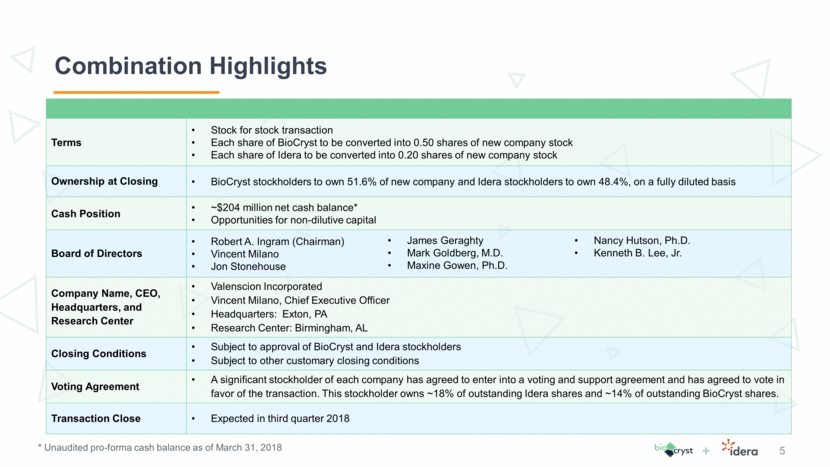
Terms Stock for stock transaction Each share of BioCryst to be converted into 0.50 shares of new company stock Each share of Idera to be converted into 0.20 shares of new company stock Ownership at Closing BioCryst stockholders to own 51.6% of new company and Idera stockholders to own 48.4%, on a fully diluted basis Cash Position ~$204 million net cash balance* Opportunities for non-dilutive capital Board of Directors Robert A. Ingram (Chairman) Vincent Milano Jon Stonehouse Company Name, CEO, Headquarters, and Research Center Valenscion Incorporated Vincent Milano, Chief Executive Officer Headquarters: Exton, PA Research Center: Birmingham, AL Closing Conditions Subject to approval of BioCryst and Idera stockholders Subject to other customary closing conditions Voting Agreement A significant stockholder of each company has agreed to enter into a voting and support agreement and has agreed to vote in favor of the transaction. This stockholder owns ~18% of outstanding Idera shares and ~14% of outstanding BioCryst shares. Transaction Close Expected in third quarter 2018 * Unaudited pro-forma cash balance as of March 31, 2018 Combination Highlights 5 James Geraghty Mark Goldberg, M.D. Maxine Gowen, Ph.D. Nancy Hutson, Ph.D. Kenneth B. Lee, Jr.
Creating a Leader in Innovative Rare Disease Therapies 6 Rare Disease Company with Strong Immuno-Oncology Assets Oligo Rare Disease Discovery Engine Recent Positive Data on Key Late-Stage Program Lead Candidate: Tilsotolimod PD-1 Refractory Melanoma Developing Oral Therapies for Life Threatening Rare Diseases Small Molecule Rare Disease Discovery Engine 2 Late-Stage Programs Lead Candidate: BCX7353 Prophylactic HAE Strengthened Scale and Competitive Position
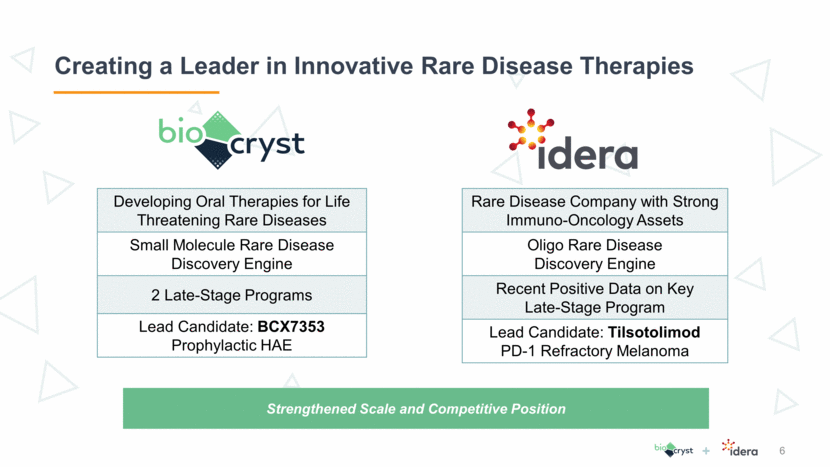
+ Lead optimization Pre-clinical Ph 1 Ph 2 Ph 3 Filed Approved STRATEGY: Discover and develop novel therapies for life-threatening, rare diseases Tilsotolimod – PD-1 Refractory Melanoma in combination with ipilimumab BCX7353 – HAE Prophylaxis (Capsule) BCX7353 – HAE Acute (Liquid) Tilsotolimod – Solid Tumor Monotherapy Second generation kallikrein inhibitors (HAE & Other Indications) BCX9250 BCX9499 Other rare diseases SUPPORTING ASSETS: Externally funded, potential for significant capital infusions RAPIVAB® (peramivir injection) IMO-9200 – Autoimmune Disease Galidesivir (broad spectrum antiviral) I.M. 3GA Candidate – Renal Target licensed to Seqirus, Shionogi and Green Cross licensed to Vivelix licensed to GSK Fibrodysplasia Ossificans Progressiva (FOP) – Orphan-Designation Orphan-Designation Robust Rare-Disease Focused Pipeline 7 Idera BioCryst
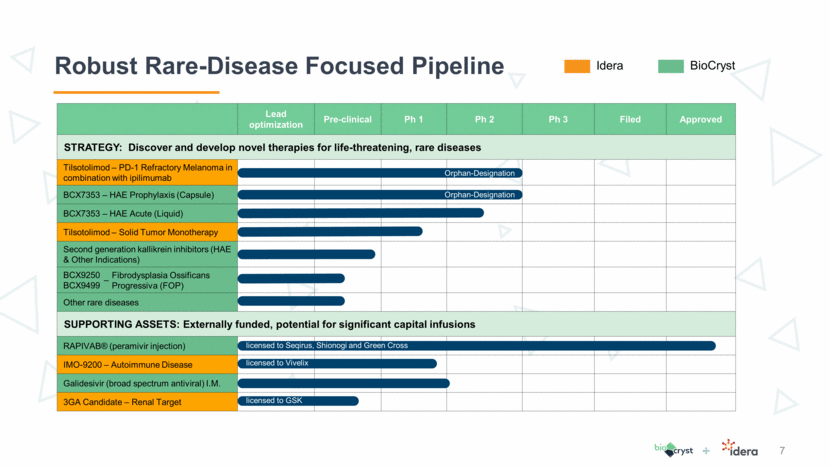
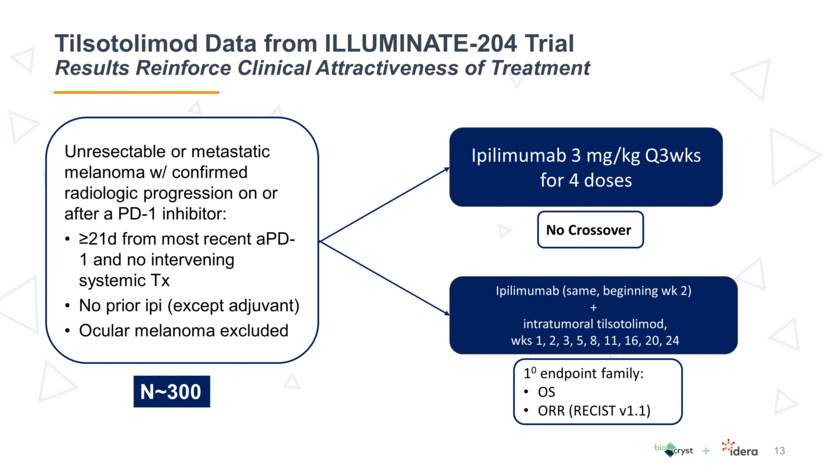
8 Tilsotolimod Data from ILLUMINATE-204 Trial Trial Objectives Assess preliminary clinical activity of tilsotolimod in combination with ipilimumab at the respective recommended Phase 2 dose (RP2D) in patients with metastatic melanoma that is not responsive to PD-1 inhibitor therapy, using Response Evaluation Criteria in Solid Tumors (RECIST v1.1) with a target of ORR of 35% Further assess the safety and tolerability of tilsotolimod in combination with ipilimumab Primary Objective Secondary Objective
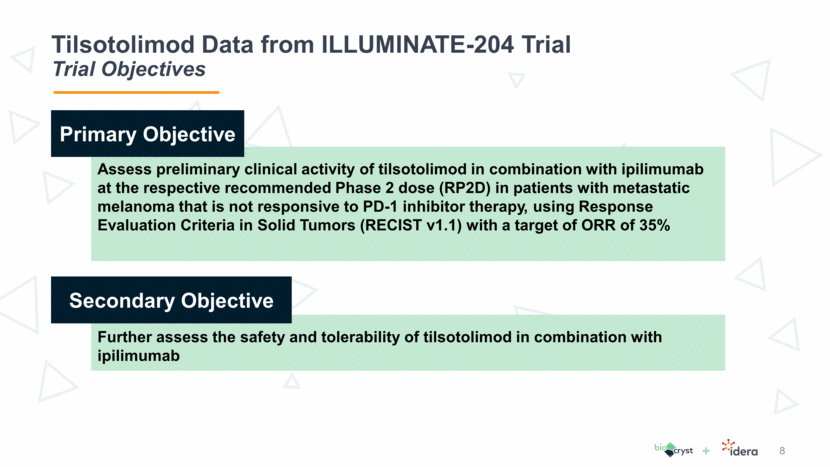
Tilsotolimod Data from ILLUMINATE-204 Trial 38.1% Response Rate / 71.4% Disease Control Rate 9
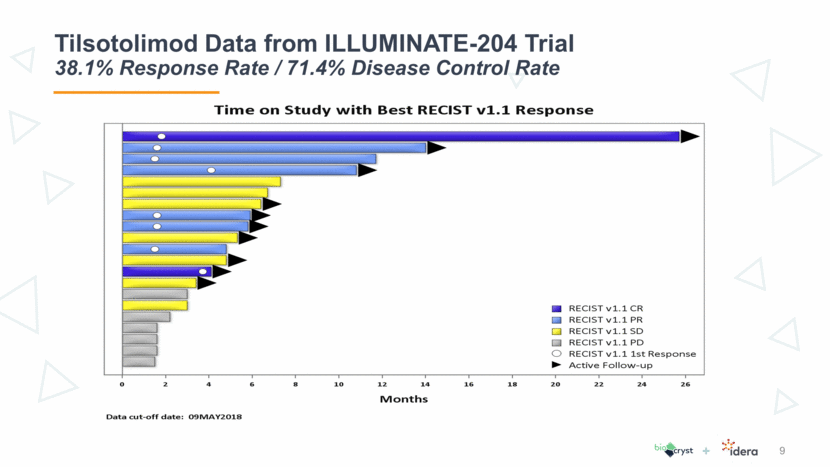
Tilsotolimod Data from ILLUMINATE-204 Trial 38.1% Response Rate / 71.4% Disease Control Rate 10
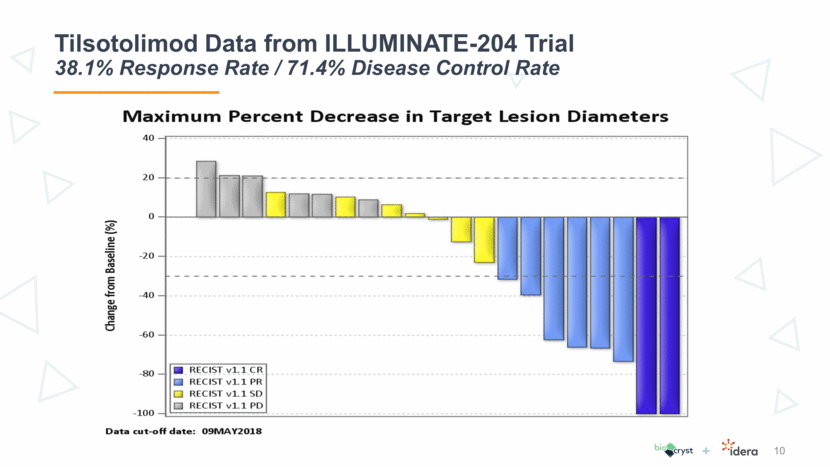
Tilsotolimod Data from ILLUMINATE-204 Trial Results Reinforce Clinical Attractiveness of Treatment 11 Tilsotolimod + ipilimumab revives the immune response in anti-PD-1-resistant tumors resulting in altering the tumor microenvironment and conversion of cold (noninflamed) to hot (inflamed) tumors This combination treatment has produced durable responses and demonstrates substantial disease control rate in this clinically challenging population, including subjects with Stage IV M1c disease and BRAF mutations The combination regimen is generally well tolerated and no synergistic toxicity was observed The toxicity profile was consistent with ipilimumab alone Six subjects (23%) had immune-related toxicities The current data led to an ongoing global randomized Phase 3 study comparing tilsotolimod plus ipilimumab to ipilimumab alone in the anti-PD-1 refractory melanoma population
Tilsotolimod Data from ILLUMINATE-204 Trial Phase 3 Asset with Real Utility in I/O Toolkit 12 Tilsotolimod data continues to be very clinically meaningful even after doubling the number of patients Response rate of 38.1% in Idera’s trial is approximately triple that of response rates of ipilimumab alone Tilsotolimod is the most advanced and has the best objective response rate, controllable disease rate and durability of response for all of the TLR9’s in PD-1 refractory melanoma Trial results create a treatment profile that is more attractive than BioCryst used in market research and to forecast the value Significantly larger data set and robust result demonstrate less risk and support value proposition of combined company
13 Tilsotolimod Data from ILLUMINATE-204 Trial Results Reinforce Clinical Attractiveness of Treatment Ipilimumab 3 mg/kg Q3wks for 4 doses Ipilimumab (same, beginning wk 2) + intratumoral tilsotolimod, wks 1, 2, 3, 5, 8, 11, 16, 20, 24 Unresectable or metastatic melanoma w/ confirmed radiologic progression on or after a PD-1 inhibitor: >21d from most recent aPD-1 and no intervening systemic Tx No prior ipi (except adjuvant) Ocular melanoma excluded 10 endpoint family: OS ORR (RECIST v1.1) No Crossover N~300
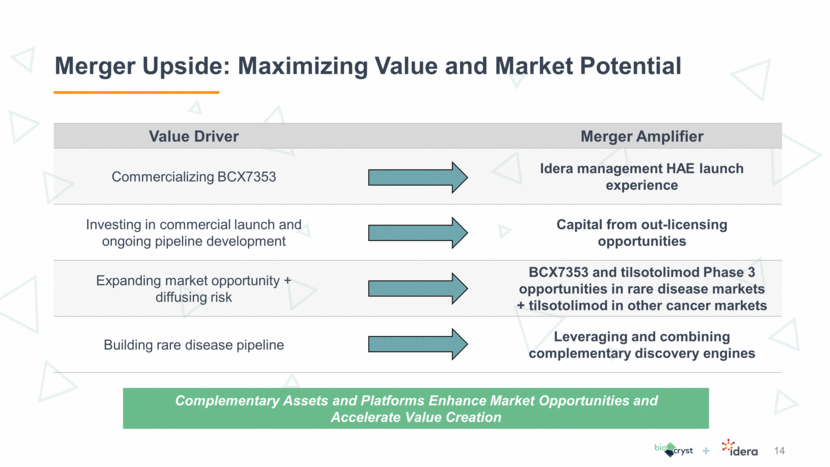
Merger Upside: Maximizing Value and Market Potential 14 Value Driver Merger Amplifier Commercializing BCX7353 Idera management HAE launch experience Investing in commercial launch and ongoing pipeline development Capital from out-licensing opportunities Expanding market opportunity + diffusing risk BCX7353 and tilsotolimod Phase 3 opportunities in rare disease markets + tilsotolimod in other cancer markets Building rare disease pipeline Leveraging and combining complementary discovery engines Complementary Assets and Platforms Enhance Market Opportunities and Accelerate Value Creation
Synergistic Discovery Engines with Enhanced Development Opportunities 15 KIDNEYS LIVER Idera’s oligonucleotide chemistry primarily targets the kidney and liver. BioCryst’s structure-based small molecule design combined with Idera’s oligo chemistry may enable delivery of oligo treatments to additional target organs. KIDNEYS LIVER HEART BRAIN

1st prophylactic treatment of HAE Grew to ~$400M in N.A. annual sales in 5 years Treatment of C. difficile-associated diarrhea (CDAD) Grew to ~$300M in annual sales Multiple global and U.S. rare disease launches Led launch for 5 global brands that drive ~70% of CSL’s current revenue Grew U.S. Hizentra and Privigen sales to >$1B Vincent Milano Chief Executive Officer Lynne Powell Chief Commercial Officer Clayton Fletcher VP, Strategy/ Bus. Development Dan Soland Chief Operating Officer Proven Rare Disease Clinical & Commercial Track Record William Sheridan, MB BS Chief Medical Officer Joanna Horobin, MB ChB Chief Medical Officer 16 >245 HAE patients dosed and studied CMOs clinical development/launch experience: Aranesp®, Enbrel®, Kineret®, Neulasta® and Sensipar® Taxotere® Bactroban®, Relafen®/ Reliflex® Lovenox®, Celectol®, Augmentin®, Timentin®, temocillin®.

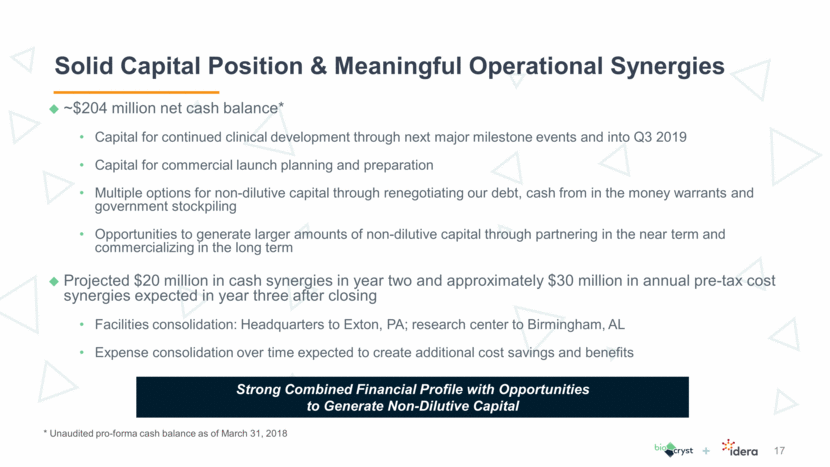
Solid Capital Position & Meaningful Operational Synergies 17 * Unaudited pro-forma cash balance as of March 31, 2018 Strong Combined Financial Profile with Opportunities to Generate Non-Dilutive Capital ~$204 million net cash balance* Capital for continued clinical development through next major milestone events and into Q3 2019 Capital for commercial launch planning and preparation Multiple options for non-dilutive capital through renegotiating our debt, cash from in the money warrants and government stockpiling Opportunities to generate larger amounts of non-dilutive capital through partnering in the near term and commercializing in the long term Projected $20 million in cash synergies in year two and approximately $30 million in annual pre-tax cost synergies expected in year three after closing Facilities consolidation: Headquarters to Exton, PA; research center to Birmingham, AL Expense consolidation over time expected to create additional cost savings and benefits
Engaged, well-advised Boards BioCryst and Idera Boards comprised of highly experienced directors with extensive industry knowledge BioCryst Board of Directors met numerous times over last two years to discuss value enhancing opportunities for BioCryst Both Boards retained financial and legal advisors to assist in the evaluation Reviewed alternative value enhancing strategies BioCryst and Idera Boards engaged in discussions with numerous potential partners BioCryst & Idera Boards Carefully Evaluated Strategic Options 18 Both Boards Determined Merger Made Strategic Sense and is a Unique Opportunity to Enhance Stockholder Value
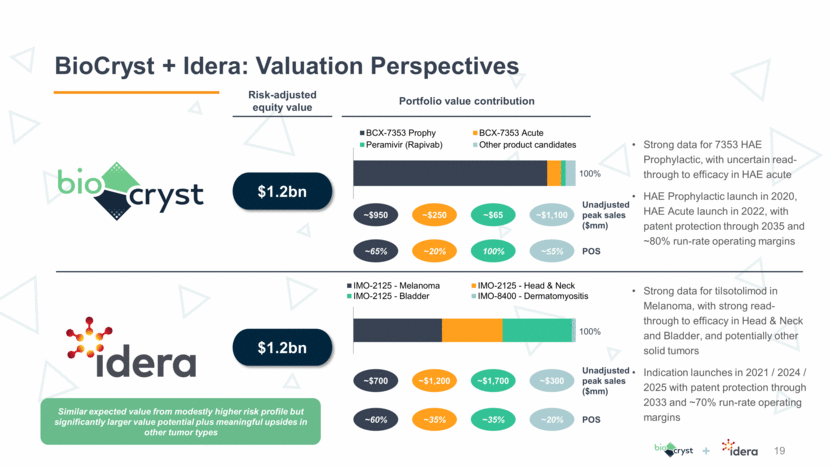
BioCryst + Idera: Valuation Perspectives 19 Similar expected value from modestly higher risk profile but significantly larger value potential plus meaningful upsides in other tumor types Risk-adjusted equity value $1.2bn $1.2bn Strong data for 7353 HAE Prophylactic, with uncertain read-through to efficacy in HAE acute HAE Prophylactic launch in 2020, HAE Acute launch in 2022, with patent protection through 2035 and ~80% run-rate operating margins Strong data for tilsotolimod in Melanoma, with strong read-through to efficacy in Head & Neck and Bladder, and potentially other solid tumors Indication launches in 2021 / 2024 / 2025 with patent protection through 2033 and ~70% run-rate operating margins Portfolio value contribution ~$950 ~$250 ~$65 ~$1,100 Unadjusted peak sales ($mm) ~65% ~20% 100% ~<5% POS ~$700 ~$1,200 ~$1,700 ~$300 ~60% ~35% ~35% ~20% Unadjusted peak sales ($mm) POS 100% 100% IMO-2125 - Melanoma IMO-2125 - Head & Neck IMO-2125 - Bladder IMO-8400 - Dermatomyositis BCX-7353 Prophy BCX-7353 Acute Peramivir (Rapivab) Other product candidates
Combination Creates Substantial Value 20 Complementary Assets and Platforms Enhance Market Opportunities and Accelerate Value Creation Maximizing Value and Market Potential Robust, Rare Disease Focused Pipeline Synergistic Discovery Engines Proven Clinical and Commercial Track Record Increased Financial Strength Creates a unique player in rare diseases, with scale and strengthened competitive position More opportunities for success through diversified late-stage pipeline, variety of early-stage programs and supporting assets Synergistic discovery engines with enhanced development opportunities, including through joint small molecule and oligo treatments Complementary leadership with best-in-class people, facilities and commercial know-how in rare diseases Increased financial strength and flexibility through significant cost synergies and opportunities to generate non-dilutive capital New Data Presented on Tilsotolimod at ASCO 2018
Appendix +

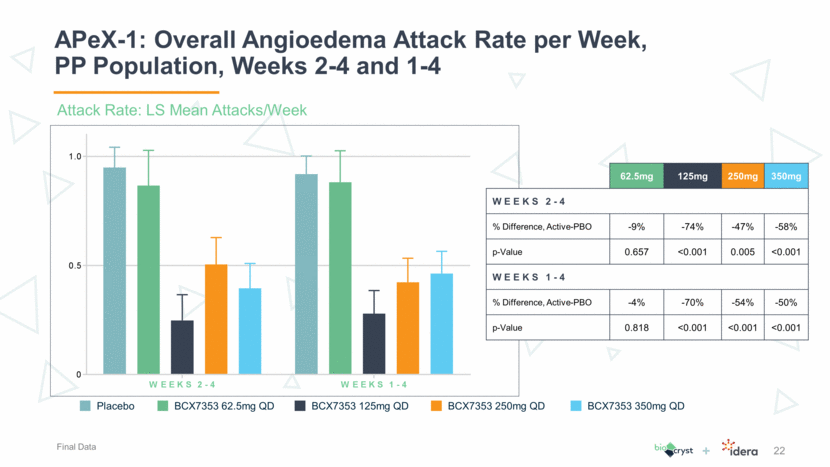
APeX-1: Overall Angioedema Attack Rate per Week, PP Population, Weeks 2-4 and 1-4 22 62.5mg 125mg 250mg 350mg WEEKS 2-4 % Difference, Active-PBO -9% -74% -47% -58% p-Value 0.657 <0.001 0.005 <0.001 WEEKS 1-4 % Difference, Active-PBO -4% -70% -54% -50% p-Value 0.818 <0.001 <0.001 <0.001 Attack Rate: LS Mean Attacks/Week Placebo BCX7353 62.5mg QD BCX7353 125mg QD BCX7353 250mg QD BCX7353 350mg QD Final Data W E E K S 2 - 4 W E E K S 1 - 4 0 0.5 1.0
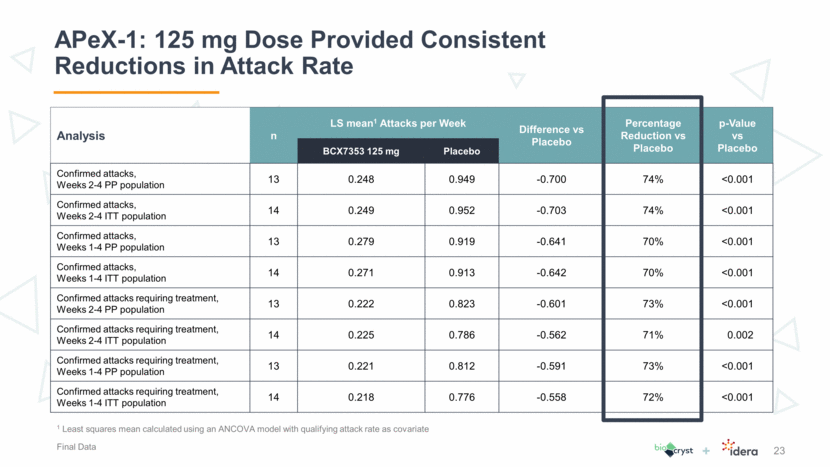
APeX-1: 125 mg Dose Provided Consistent Reductions in Attack Rate 23 1 Least squares mean calculated using an ANCOVA model with qualifying attack rate as covariate Final Data Analysis n LS mean1 Attacks per Week Difference vs Placebo Percentage Reduction vs Placebo p-Value vs Placebo BCX7353 125 mg Placebo Confirmed attacks, Weeks 2-4 PP population 13 0.248 0.949 -0.700 74% <0.001 Confirmed attacks, Weeks 2-4 ITT population 14 0.249 0.952 -0.703 74% <0.001 Confirmed attacks, Weeks 1-4 PP population 13 0.279 0.919 -0.641 70% <0.001 Confirmed attacks, Weeks 1-4 ITT population 14 0.271 0.913 -0.642 70% <0.001 Confirmed attacks requiring treatment, Weeks 2-4 PP population 13 0.222 0.823 -0.601 73% <0.001 Confirmed attacks requiring treatment, Weeks 2-4 ITT population 14 0.225 0.786 -0.562 71% 0.002 Confirmed attacks requiring treatment, Weeks 1-4 PP population 13 0.221 0.812 -0.591 73% <0.001 Confirmed attacks requiring treatment, Weeks 1-4 ITT population 14 0.218 0.776 -0.558 72% <0.001
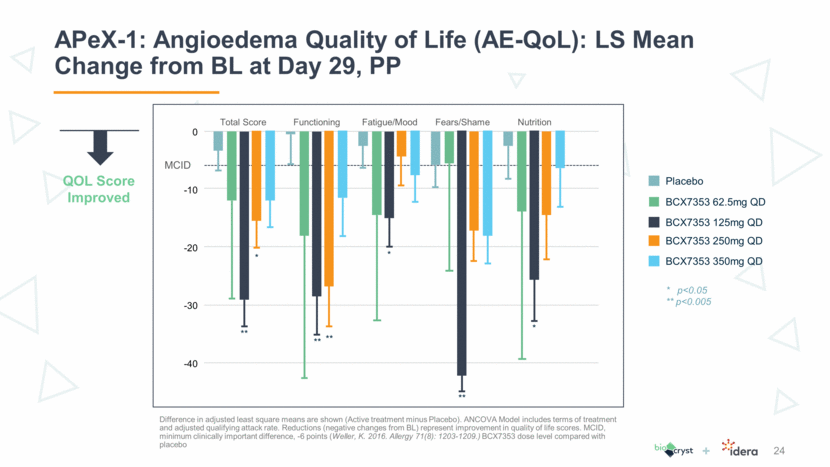
APeX-1: Angioedema Quality of Life (AE-QoL): LS Mean Change from BL at Day 29, PP 24 Difference in adjusted least square means are shown (Active treatment minus Placebo). ANCOVA Model includes terms of treatment and adjusted qualifying attack rate. Reductions (negative changes from BL) represent improvement in quality of life scores. MCID, minimum clinically important difference, -6 points (Weller, K. 2016. Allergy 71(8): 1203-1209.) BCX7353 dose level compared with placebo 0 -10 MCID Total Score Placebo BCX7353 62.5mg QD BCX7353 125mg QD BCX7353 250mg QD BCX7353 350mg QD * ** ** ** ** * * * p<0.05 ** p<0.005 QOL Score Improved -20 -30 -40 Functioning Fatigue/Mood Fears/Shame Nutrition
1 TEAE- treatment-emergent adverse event. 2 GI infection- investigator assessed as unrelated to study drug. Abdominal symptoms similar to several previous non-HAE-attack episodes occurring over past 3 years resulting in severe vomiting and diarrhea. Pt presented to ER and hospitalized for IV fluids and antiemetics as a precaution. No HAE acute attack meds given. Recovered and discharged the following day. Missed 1 day of dosing due to event. 3 Pre-existing liver disorder (improved from baseline, but persisting). Previously reported in 1st interim analysis. 4 n=1 Gastroenteritis with liver disorder (both assessed as related) (elevated ALT 1.9x ULN, GGT 5.4x ULN and ALP 1.6x ULN, with normal AST and bilirubin). Previously reported in 1st interim analysis. 5 n=1 Vomiting/abdominal cramps. Previously reported in 2nd interim analysis. APeX-1: Treatment-Emergent Adverse Event Summary 25 BCX7353 Category 62.5 mg N = 7 125 mg N = 14 250 mg N = 14 350 mg N = 18 Placebo N = 22 Subjects with any TEAE1, n (%) 4 (57) 7 (50) 11 (79) 14 (78) 15 ( 68.2) Subjects with any Serious AE, n (%) 0 0 1 (7)2 0 0 Subjects with Drug-Related Grade 3/4 AE, n (%) 0 0 0 1 (6) 0 Subjects with AE Leading to D/C from Study Drug, n (%) 0 0 0 3 (17) 0 Non-drug-related, n (%) 0 0 0 1 (6)3 0 Drug-related, n (%) 0 0 0 2 (11)4,5 0
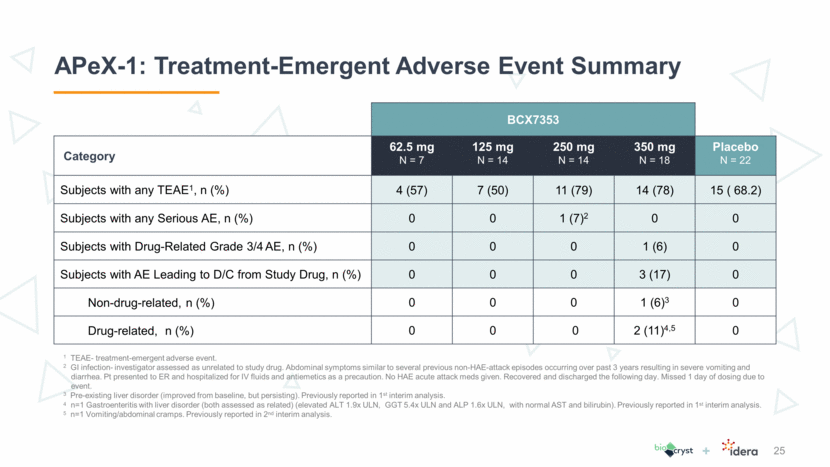
APeX-1: Exposure Comparisons of BCX7353 and SC C1INH 26 1 Longhurst, H. et al. N Engl J Med 376, 1131-1140 (2017). Box plots represent median, 25th and 75th percentiles, minimum and maximum values. CSL-830 and BCX7353 data are from distinct clinical trials and no head to head study has been conducted. CSL-830 Phase 3 Study BCX7353 APeX-1 & Phase 1 Study C1INH levels at baseline and after SC dosing with CSL-8301 BCX7353 plasma concentrations at 24 hours post-dose Baseline 40 IU/kg 60 IU/kg 10 100 2 4 8 16 32 CSL Phase 3 COMPACT study C1INH levels in COMPACT study % of normal mean Multiple of EC 50 Baseline 1 (observed) Trough during dosing 1 ( predicted from population PK ) 62.5 mg 125 mg 250 mg 350 mg 125 mg 250 mg 350 mg 500 mg 2 4 8 16 32 BCX7353 Trough Concentrations Phase 1 Study APeX-1 Multiple of EC 50 [geo. mean & SD] Oral daily dose of BCX7353
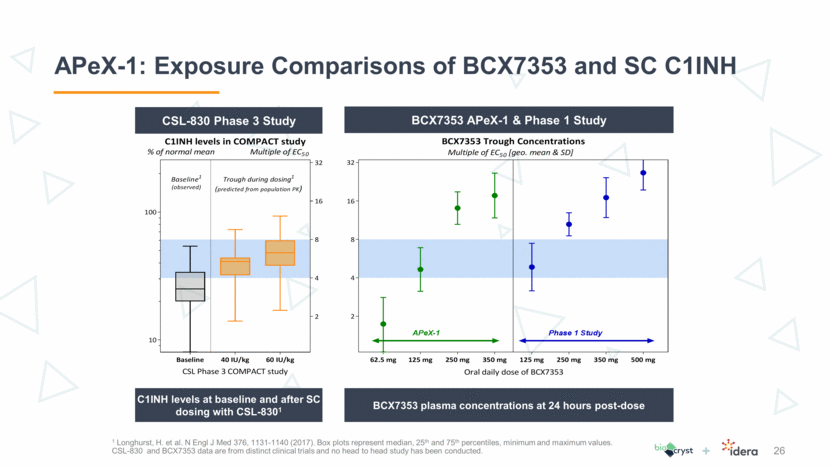
Predictable PK Supports 175 mg as Second Dose in Phase 3 27 Given the predictable PK of BCX7353, simulations are helpful in selecting an intermediate dose of BCX7353 to study that is between 125 mg and 250 mg. These simulations suggest a relatively small increase in dose above 125 mg should achieve a significant increase in the proportion of subjects achieving trough levels above the therapeutic target. These simulations suggest 175 mg dose should maintain trough drug levels > 4 x EC50 in > 90% of patients. Doses > 200 mg offer little additional increment in proportions achieving target level. Dose, mg QD % > 4 x EC50 % > 6 x EC50 % > 8 x EC50 Predicted Actual Predicted Actual Predicted Actual 62.5 -- 0 -- 0 -- 0 125 70 64 38 43 17 0 175 93 80 58 200 97 88 73 225 98 93 83 250 100 100 97 100 93 100
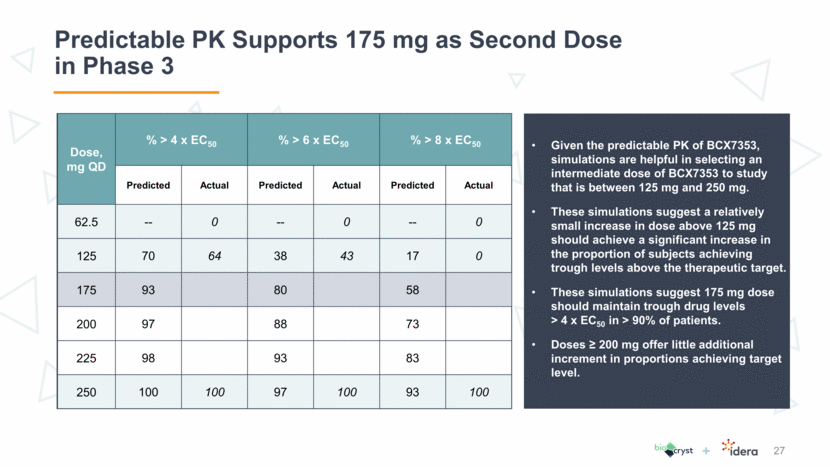
APeX-2: Phase 3 Trial Design 28 Primary endpoint at Week 24: Rate of Investigator-confirmed HAE attacks through entire treatment period Study powered at >90% to detect a >50% reduction in attack rate over placebo N 32 BCX7353 150 mg QD* Screening Visit Screening / Run-in Blinded Treatment 24 weeks N 32 BCX7353 110 mg QD* N 32 Placebo QD *Doses in Phase 2 APeX-1 were shown as the dihydrochloride salt: 150 mg = 175 mg dihydrochloride salt; 110 mg = 125 mg dihydrochloride salt Final analysis @ week 24
APeX-2: Phase 3 Trial Design – Safety Extension 29 N 32 BCX7353 150 mg QD* Screening / Run-in Blinded Treatment 24 weeks N 32 BCX7353 110 mg QD* N 32 Placebo QD BCX7353 150 mg QD BCX7353 110 mg QD 24 weeks Safety Extension *Doses in Phase 2 APeX-1 were shown as the dihydrochloride salt: 150 mg = 175 mg dihydrochloride salt; 110 mg = 125 mg dihydrochloride salt BCX7353 150 mg QD BCX7353 110 mg QD extension Screening Visit Final analysis @ week 24
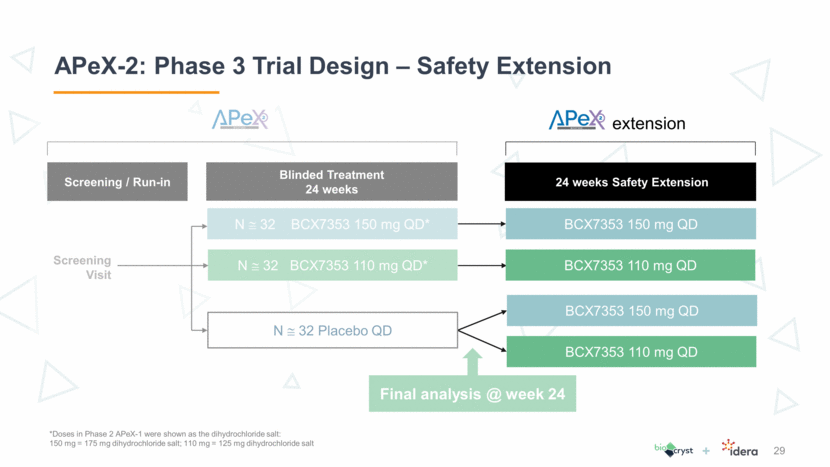
APeX-S: Long-term Safety Study Design 30 Endpoints: Long term safety of BCX7353 Durability of response Quality of Life APeX-1 subjects eligible N 80 BCX7353 150 mg QD 48 Weeks Treatment N 80 BCX7353 110 mg QD *Doses in Phase 2 APeX-1 were shown as the dihydrochloride salt: 150 mg = 175 mg dihydrochloride salt; 110 mg = 125 mg dihydrochloride salt Analyses as needed for regulatory submissions Safety database: Up to 100 subjects at each dose level Combination of APeX-2 extension and APeX-S
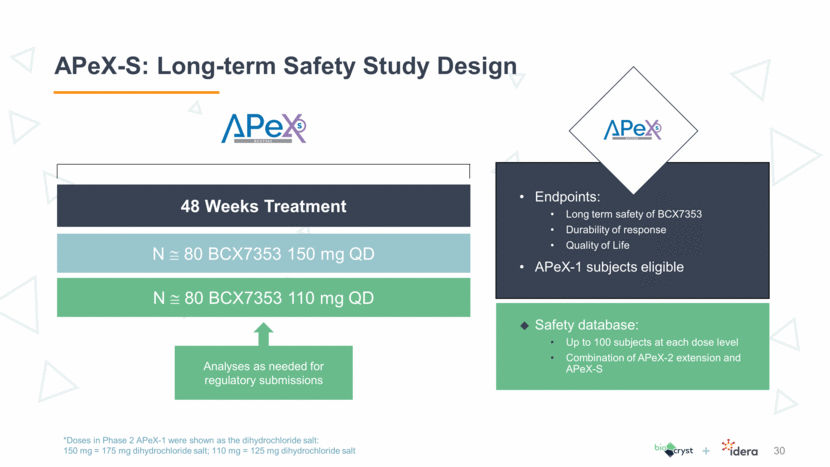
Phase 1/2 Study in Anti-PD-1 Refractory Melanoma 31 Phase 2 Expansion with Ipilimumab Enrolling Dosing: Tilsotolimod is given as a single intratumoral agent week 1,2,3,5,8,11,15,19,23 Ipilimumab and pembrolizumab are administered per label beginning week 2 Deep injections are permitted with interventional radiology guidance No need for infectious precautions
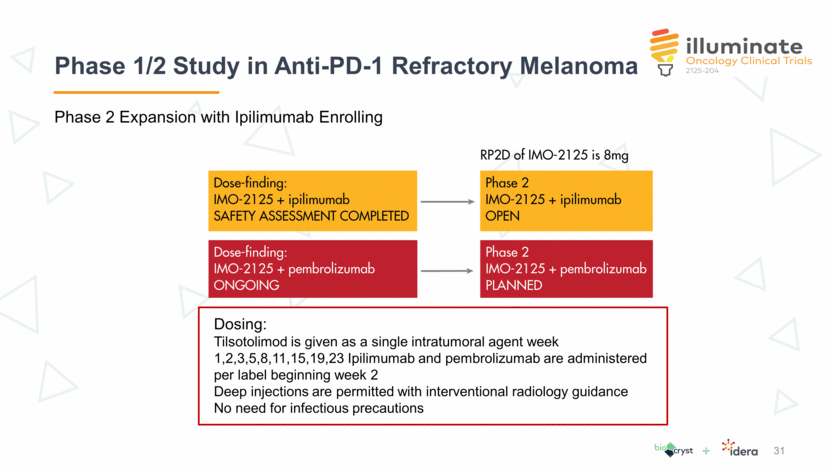
Time on Study: Best RECIST v1.1 Response and Largest Percentage Decrease in Target Lesions (8mg subjects) 32
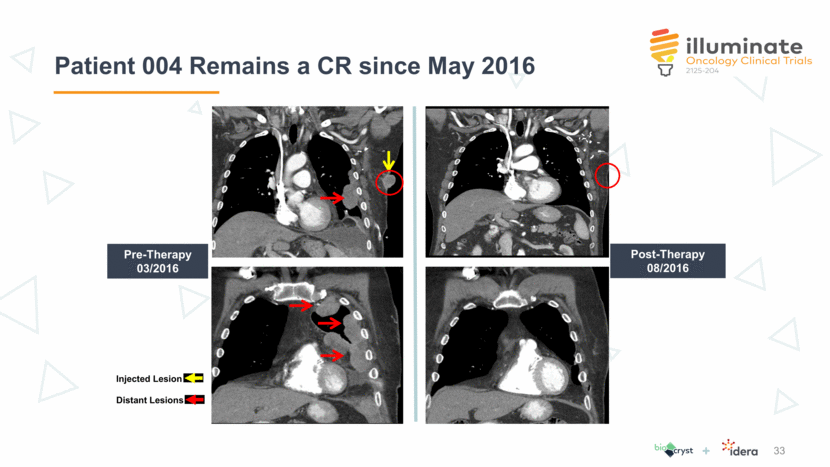
Post-Therapy 08/2016 Pre-Therapy 03/2016 Injected Lesion Distant Lesions Patient 004 Remains a CR since May 2016 33
Phase 1 Conclusions 34 The combination of tilsotolimod with ipilimumab was tolerable at all dose levels studied Dendritic cell activation, detectable within 24 hours of the first tilsotolimod injection, is evidence for target acquisition at the Recommended Phase 2 Dose (8mg) Tilsotolimod with ipilimumab showed clinical activity at the RP2D of 8mg in anti-PD-1 refractory melanoma 5 of 10 (50%) responded 7 of 10 (70%) experiencing disease control An additional PR of >1 year has been reported at 4mg Dose finding for tilsotolimod with pembrolizumab is ongoing, and one partial response (PR) has been seen
Phase 2 Expansion Update 35 Ipilimumab Combination Phase 2 Trial Expansion – Targeting approximately 60 patients with PD-1 refractory metastatic melanoma treated with 8mg 21 patients enrolled 10 Centers (5 sites currently enrolling) MD Anderson, Roswell Park, Vanderbilt, Huntsman, Uni. of Arizona Open label design Allows for periodic data updates Opportunistic engagements with regulatory authorities
Phase 3 Readiness (FPFV 1Q18) 36 Agreement with FDA and MHRA on design and path forward for regular and accelerated approval (one study) Fast Track Designation Granted by U.S. FDA in Q4 2017 Global trial (US, Can, EU, Aus) ~300 patients ~70 sites planned CMC work commenced1Q18 Commercial presentation of tilsotolimod will be used Regulatory filings underway Open U.S. IND CTA filings on track
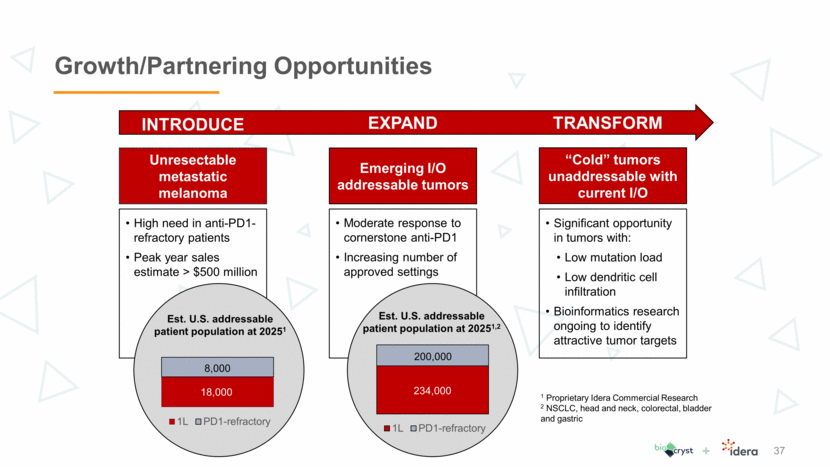
Growth/Partnering Opportunities 37 Expand Transform Unresectable metastatic melanoma High need in anti-PD1-refractory patients Peak year sales estimate > $500 million Emerging I/O addressable tumors “Cold” tumors unaddressable with current I/O Moderate response to cornerstone anti-PD1 Increasing number of approved settings Significant opportunity in tumors with: Low mutation load Low dendritic cell infiltration Bioinformatics research ongoing to identify attractive tumor targets Est. U.S. addressable patient population at 20251 1 Proprietary Idera Commercial Research 2 NSCLC, head and neck, colorectal, bladder and gastric 37 37 Est. U.S. addressable patient population at 20251,2 234,000 Introduce 18,000 8,000 1L PD1-refractory 200,000 1L PD1-refractory