Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE
SECURITIES EXCHANGE ACT OF 1934
SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2007
Commission file number 0-19941
MEDQUIST INC.
(Exact name of registrant as specified in its charter)
| New Jersey | 22-2531298 | |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) | |
| incorporation or organization) |
1000 Bishops Gate Blvd, Suite 300, Mount Laurel, NJ 08054-4632
(Address of principal executive offices)
(Address of principal executive offices)
Registrant’s telephone number, including area code:
(856) 206-4000
(856) 206-4000
Securities registered pursuant to Section 12(b) of the Act:
NONE
NONE
Securities registered pursuant to Section 12(g) of the Act:
Common Stock (no par value)
(Title of Class)
Common Stock (no par value)
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yeso Noþ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yeso Noþ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesþ Noo
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein and will not be contained, to the best of registrant’s knowledge, in definite proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large Accelerated Filero | Accelerated Filerþ | Non-Accelerated Filero (Do not check if a smaller reporting company) | Smaller reporting companyo |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yeso Noþ
The aggregate market value of the outstanding common stock held by non-affiliates of the registrant as of June 30, 2007, was $103,783,561. Such aggregate market value was computed by reference to the closing price of the common stock as reported on the “Pink Sheets” on June 30, 2007.
The number of registrant’s shares of common stock, no par value, outstanding as of February 29, 2008 was 37,543,893.
Documents incorporated by reference
Portions of the definitive Proxy Statement for the 2008 Annual Meeting of Shareholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
MedQuist Inc.
Annual Report on Form 10-K
Table of Contents
Annual Report on Form 10-K
Table of Contents
Table of Contents
PART I
Item 1.Business
General
MedQuist is the largest Medical Transcription Service Organization (MTSO) in the world, and a leader in technology enabled clinical documentation workflow. We service health systems, hospitals and large group medical practices throughout the U.S., and we employ approximately 6,000 skilled medical transcriptionists (MTs), making us the largest employer of MTs in the U.S. We believe our services and enterprise technology solutions — including mobile voice capture devices, speech recognition technologies, Web-based workflow platforms, and global network of MTs and editors — enable healthcare facilities to improve patient care, increase physician satisfaction, and lower operational costs.
We perform a substantial majority of our medical transcription services utilizing the DocQmenttm Enterprise Platform (DEP), our proprietary, web-based dictation and medical transcription management system. Our proprietary technologies enable our customers to efficiently manage the clinical documentation workflow.
Clinical Documentation Workflow
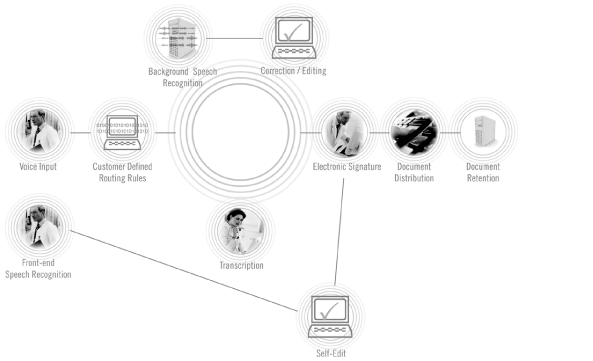
The clinical documentation workflow begins when physicians or other medical professionals dictate findings and plans of care into one of several input devices, including standard telephones, handheld devices or PC-based dictation stations. Once dictated, the voice files securely route through our DEP to either an MT or our speech recognition engine for conversion to text. The resulting draft report may then proceed to the editing and quality assurance process prior to being routed back to the physician or other medical professional for review, finalization, execution and incorporation into a patient’s medical record. Throughout this process, our DEP utilizes security measures that assist our customers with their compliance with privacy and security standards and regulations, including those adopted under the Health Insurance Portability and Accountability Act of 1996 (HIPAA), and the protection of the confidentiality of medical records. We also provide document retention services.
Industry Overview
As a provider of medical transcription technology and services, our revenues and growth are affected in part by certain trends in healthcare.
3
Table of Contents
Increased Spending and Demographic Factors in Healthcare Industry
Spending for healthcare in the U.S. has grown rapidly over the past few decades. According to estimates published by the Centers for Medicare & Medicaid Services (CMS) Office of the Actuary, the healthcare sector is growing faster than the overall economy. Healthcare spending increased as a share of U.S. gross domestic product from 13.3% in 2000 to 16% in 2005, as growth in healthcare spending outpaced economy-wide growth.
In 2005, healthcare spending in the U.S. was approximately $2 trillion. CMS estimates that by 2016 healthcare spending will have increased to $4.1 trillion, which will represent 19.6% of the projected U.S. gross domestic product. Demographic factors contribute to long-term growth projections in healthcare spending. According to the U.S. Census Bureau’s 2005 interim projections, there were approximately 35 million Americans aged 65 or older. The number of Americans aged 65 or older is expected to increase to approximately 40 million by 2010 and approximately 70 million by 2030, or 20% of the U.S. population. We believe that the aging of the U.S. population and the continuing growth in healthcare spending will increase demand for our products and services. Older age groups receive proportionately greater numbers of procedures, medical tests and treatments that require clinical documentation. We believe that the high demand for medical transcription services will also be sustained by the need of providers, third-party payors, consumers, regulators and health information networks to share electronic health information.
Competitive Environment
The healthcare industry is increasingly choosing to outsource services such as medical transcription. The medical transcription industry itself is highly fragmented and difficult to size based on a general lack of public market data and analysis. As such, we estimate that the U.S. medical transcription market is approximately $7 billion on an annual basis, including both outsourced services and medical transcription performed internally by healthcare providers. We believe that the top 10 medical transcription outsource providers based on revenues, of which we are the largest, account for less than 15% of the industry including in-house operations.
Although we are the leading provider of medical transcription services in the U.S., we experience competition from local, regional, national and international businesses. We believe that there are hundreds of companies in the U.S. and India currently performing medical transcription services for the U.S. market. There are currently two large service providers, one of which is us and the other of which is Spheris Inc., several mid-sized service providers with annual revenues of between $15 million and $100 million and hundreds of smaller, independent businesses with annual revenues of less than $15 million. We believe that a substantial portion of the medical transcription market is serviced internally by MTs directly employed by the healthcare providers.
We believe the outsourced portion of the medical transcription services market will increase due in part to healthcare providers seeking the following:
| • | reduction in overhead and other administrative costs; | ||
| • | improvement in the quality and speed of delivery of transcribed medical reports; | ||
| • | access to leading technologies, such as speech recognition technology, without development and investment risk; | ||
| • | expertise in implementing and managing a system tailored to the providers’ specific requirements; | ||
| • | access to skilled MTs; and | ||
| • | support for compliance with increasing governmental and industry mandated privacy and security requirements and electronic health record (EHR) initiatives. |
Our competitive position in the market is characterized primarily by the following factors:
| • | We are the leading medical transcription provider in the U.S., with a strong customer base, the largest pool of MTs and leading technologies capable of handling large volumes and complex workflows. As the largest medical transcription provider in the U.S., we are recognized as the leading brand in the industry. We have a well-established customer base, comprised of hundreds of health systems, hospitals and large group medical practices located throughout the U.S. We have an integrated, flexible and scalable technology platform that can be tailored to meet our customers’ needs. We are the largest employer of MTs and have the most widely deployed technology platform. As health systems, hospitals and large group medical practices increasingly seek to outsource their medical transcription and other clinical document workflow needs, we have the resources to quickly and efficiently provide our customers with comprehensive, scalable solutions. | ||
| • | We offer a comprehensive array of products and services.We offer a comprehensive array of products and services for |
4
Table of Contents
| dictation, medical transcription, speech recognition and electronic signature through a combination of remote and on-premise solutions. These solutions are designed to maximize the efficiency, accuracy and security of our customers’ documentation and coding processes while enhancing their patient care, accelerating their revenue cycle and lowering their costs. | |||
| • | We have a strong financial profile.We continue to maintain a substantial cash balance and have no debt. | ||
| • | We continue to face competition from MTSOs providing low cost services.Several low cost providers have emerged and aggressively moved into our market offering medical transcription services (performed both domestically and offshore) at prices significantly below our traditional price point causing some of our customers to switch to these providers, as well as resulting in a reduction in the prices we can charge our customers for our services. |
Business Strategy
We intend to maintain our position as the leading provider of medical transcription technology and services while transforming into a leading provider of complete clinical documentation workflow solutions. We plan to achieve this objective through the following strategies:
Retain and Expand Customer Relationships.We constantly seek to improve turnaround time, medical transcription quality and customer communications. We believe that advances in these areas improve retention of existing customers and increase our ability to secure new customers. We also have a strong, experienced sales team that focuses on customer executive level decision-makers to enhance sales opportunities for new and existing customers.
Enhance Profitability.We continue to identify ways to maximize the efficiencies of the organization through the following initiatives:
| • | expand our use of speech recognition which should increase our productivity; | ||
| • | realign our non-production workforce to match our current operational needs; and | ||
| • | reorganize our production workforce to optimize workflow. |
Leveraging our DEP.For medical transcription services that we perform on our platform, we completed in 2007 the migration of our outsourced medical transcription customers from disparate and older technology platforms to our DEP. We believe that the combination of standardization, advanced functionality and transparency with respect to service metrics provided by this platform will significantly increase our customers’ satisfaction and retention. Our commitment to our DEP allows us to accelerate the rate at which we can offer new functionalities to our customers. In addition, we currently offer and plan to expand the offering of our DEP to healthcare providers and other companies in the medical transcription industry for use as their medical dictation and transcription platform.
Expansion Into New Markets.We believe our breadth of products and services positions us to bridge the gap between traditional medical transcription and the EHR. The U.S. government has developed plans that call for all Americans to have an EHR by 2014.
We intend to leverage our market leading position and proprietary technologies to provide comprehensive solutions to our customers related to the management of health records. Historically, we have provided a combination of traditional medical transcription services, stand-alone products and other professional services to healthcare providers. We anticipate aggregating these existing services and products and new services and products into a more comprehensive clinical documentation workflow solution.
Exploration of Strategic Alternatives.In July 2007, we announced that, at the direction of our board of directors, we had engaged Bear, Stearns & Co. Inc. as our financial advisor to advise us and our board of directors on our strategic alternatives. On November 2, 2007, Koninklijke Philips Electronics N.V. (Philips), our majority shareholder, announced its intention to proceed with the sale of its approximate 69.6% ownership interest in us if a satisfactory price and other acceptable terms can be realized. On this same date and in light of Philips’ announcement, we announced that our board of directors, in connection with its previously disclosed review of strategic alternatives, is evaluating whether a sale of MedQuist is in the best interest of MedQuist and our shareholders. While such evaluation is continuing, there is no assurance that any sale transaction will occur as a result of such review.
Customer Base
We have a large and prestigious customer base. We believe that over 30% of non-federal acute care U.S. hospitals use at least one of our products or services. Additionally, we have the largest customer base of any medical transcription company in the U.S., currently serving over 1,500 hospital systems, clinics and large physician practices, including approximately 40% of hospitals with more than 500 licensed beds. We believe we are the medical transcription company of choice for large, complex hospitals and health systems in the U.S. due to our size, scale and scope. We provide services to entire healthcare systems as well as discrete departments within hospitals, such as health information management, emergency, radiology, pathology and cardiology departments and all types of clinic settings. None of our customers accounted for more than 10% of our annual net revenues in 2007.
5
Table of Contents
Products and Services
For the years ended December 31, 2007, 2006 and 2005, approximately 83.3%, 83.8% and 79.5%, respectively, of our net revenues were derived from our medical transcription technology and services. In addition, we also derive net revenues from, among other things, maintenance services, our front-end speech recognition solution, SpeechQ for Radiologytm, and our enterprise digital dictation management system, DocQment Ovation.
Medical Transcription Services
We provide health systems, hospitals and large group medical practices with comprehensive solutions to meet their medical transcription needs. As the largest medical transcription services provider, we employ approximately 6,000 skilled MTs. This scale allows us to continually offer our customers effective, standardized medical transcription services that meet organization-wide or departmental needs. We perform the vast majority of our medical transcription services utilizing our DEP. For our medical transcription customers that do not use our DEP, we provide medical transcription services directly into their platform. In addition, we also service a specialized area of the medical transcription market — specifically, radiology — whereby we retrieve voice files from, and transcribe directly into, customer-hosted transcription platforms.
In the clinical documentation workflow, we provide, in addition to medical transcription technology and services, digital dictation, speech recognition and electronic signature technologies.
Our DEP and flexible dictation solutions provide our customers with easy access to advanced technology and the confidence that medical reports will be completed quickly and accurately. Our DEP, which is a web-based dictation and transcription management system, integrates dictation capture, workflow management, speech recognition, medical transcription, and document distribution through multiple distinct yet integrated modules as follows:

Features and benefits of our DEP include the following:
Security and Scalability
| • | supports all standards required by HIPAA and other applicable laws and regulations | ||
| • | utilizes secure data centers with 24/7 system monitoring and maximum uptime | ||
| • | satisfies increased clinical document workflow demands through seamless scalability |
Cost Effectiveness
| • | provides one interface for health information systems | ||
| • | eliminates the costs and challenges of supporting multiple dictation and medical transcription systems for individual hospitals and departments | ||
6
Table of Contents
| • | allows for the centralized maintenance of all system hardware and software at our data centers | ||
| • | allows MTs and editors to work remotely | ||
| • | eliminates traditional phone charges and other overhead costs associated with home-based medical transcription |
Workflow
| • | allows viewing of medical reports on a real-time basis from multiple locations through a single and secure login, password and company identification | ||
| • | increases the level of our customers’ management control over medical transcription workflow across healthcare enterprises | ||
| • | reduces the amount of time reports spend in queue as well as MT downtime | ||
| • | takes transcribed text and matches it to user pre-defined templates using automatic post-transcription formatting |
Auditing and Reporting
| • | facilitates transparency in the billing process with detailed character count logs for every processed document | ||
| • | provides audit trails with detailed information regarding access to patient health information | ||
| • | offers quality and turnaround time reporting for tracking of service metrics |
Maintenance Services
We provide onsite maintenance, remote “break-fix” services, and application, hardware and software technical support for our products.
SpeechQ for Radiology
SpeechQ for Radiology is a flexible front-end speech recognition software application. It allows radiologists to dictate, edit and sign their reports in a single session or send them to an editor following dictation. SpeechQ for Radiology continuously learns from changes to a specific radiologist’s dictation made by either the radiologist or an editor, increasing the speech recognition accuracy for such radiologist with every edit. Powered by the same Philips SpeechMagictm speech engine used in our DEP, SpeechQ for Radiology is designed specifically for radiology and integrates with most radiology specific information systems providing a workflow that maximizes radiologists’ efficiency and significantly improves report turnaround time.
DocQment Ovation
DocQment Ovation is our latest web-based, enterprise digital voice capture and transport solution. DocQment Ovation creates opportunities to improve productivity by providing an enterprise view that allows medical transcription supervisors to easily manage MTs and voice files from a single dashboard instead of using multiple systems. DocQment Ovation was specifically engineered to be compatible with our previous generation dictation stations. An integral component of our growing technology portfolio, DocQment Ovation supports our end-to-end solution from dictation to billing. DocQment Ovation’s enterprise configuration options allow administrators to easily track work and share resources to get the right voice file to the right MTs at the right time.
Technological Capabilities
Research and Development
We continue to invest in our research and development capabilities to ensure we meet current and future customer requirements. Our proprietary software and hardware technologies support our medical transcription outsourced services. Our software capabilities enable us to operate a national service delivery model that includes nationwide multi-modal voice capture. Our expertise in the use of speech recognition enables us and our customers to achieve productivity gains and cost savings. We continue to work to enhance our speech recognition and
7
Table of Contents
editing technologies to achieve maximum productivity gains in the medical documentation process. Our DEP and technological expertise in the areas of work routing and work management support a nationwide, scalable model of medical transcription delivery. Our ability to focus on a single dictation and transcription management system, our DEP enables us to efficiently and effectively utilize our research and development resources.
We employ over 100 developers to conduct our research and development in five locations: Joplin, Missouri, Morgantown, West Virginia, Norcross, Georgia, Malvern, UK, and Hyderabad, India. Although we license a portion of our technology from third party vendors, a majority of our technological expertise resides in our development organization. Our development personnel have expertise across the breadth of our solutions including voice capture management, speech recognition and editing applications, medical transcription and electronic signature and distribution. All of our development teams follow the same rigorous development methodology which ensures repeatable, high quality and timely delivery of solutions.
Speech Recognition
A portion of our speech recognition technology is licensed from Philips Speech Recognition Systems GmbH (PSRS). We have integrated this technology into both SpeechQ for Radiology and our DEP which has provided us with productivity gains and streamlined workflows. In 2007 we significantly increased our use of automated speech recognition.
Sales and Marketing
We spend a significant portion of our sales and marketing resources on targeting healthcare facilities which are currently performing medical transcription in-house as well as those facilities that have already outsourced their medical transcription function, but are using a competitor.
In addition, we focus on retaining and expanding the business within our existing customer base. We use a direct sales force model of over 60 sales and account management associates throughout the U.S. This includes specialists for national accounts, technology and account management. In addition, we have an inside sales department that specializes in telesales and lead generation primarily for ancillary products to our existing customers including other medical transcription outsource companies.
To support our sales initiatives, we utilize various marketing programs to maintain and expand our brand. We promote our offerings regularly through:
| • | attending and sponsoring industry trade shows of national organizations such as the American Health Information Management Association, Healthcare Information and Management Systems Society, Association for Healthcare Documentation Integrity (formerly AAMT, American Association of Medical Transcription), Radiological Society of North America, and Society for Imaging Informatics in Medicine; | ||
| • | advertising in industry focused print and electronic trade journals; | ||
| • | demonstrating our thought leadership on industry topics and trends via webinars and participation in numerous state and regional trade show events; and | ||
| • | hosting a national user group events for existing customers to exchange product and market information. |
Service Delivery and Customer Support
We offer a wide range of customer support services through an expansive staff of customer-facing service personnel. The customer-facing relationship teams work with, and are supported by, our centrally managed customer service organization.
Centralized service delivery enhances workflow management, which we believe should result in improved levels of service and quality for our customers. This centralization coordinates the services of thousands of MTs on a nationwide level, facilitating superior capacity planning even when volume fluctuates. By applying streamlined processes and the highest standards nationwide, we are able to provide quick turnaround time and consistent quality documentation.
Our service and support organization is comprised of several smaller organizations, or teams, focused on delivering specific aspects of services. In addition to technical and product support, we offer implementation professional services, which provide our customers with complete implementation planning and services beginning with the initial scoping of system requirements through the customer acceptance phase of an implementation.
8
Table of Contents
Medical Transcriptionists
As the largest MTSO in the world, we employ approximately 6,000 skilled U.S.-based MTs, making us the largest employer of MTs in the U.S. In addition, we contract with MTs in Canada and have access to offshore MTs through our relationships with subcontractors. The size of our MT pool allows us to quickly and efficiently provide our customers with the labor resources necessary to implement comprehensive, scalable solutions. All of the MTs that we employ work from home, largely using computer hardware and telecommunications equipment that we provide, to access dictation files and transcribe reports utilizing the internet.
Recruitment
Working with a team of professional recruiters, we utilize multiple avenues to ensure that qualified MTs apply for employment opportunities with us. Regular advertisements and articles appear in trade journals and industry publications, and banner ads are placed on industry and trade websites. In addition, we participate in prominent local and national trade shows and work with premier medical transcription schools to offer top graduates an opportunity for employment with us.
Training
Substantially all of our new MTs participate in an on-line training program that includes both a company orientation as well as training on our DEP. In addition, those MTs that service specialized areas of the medical transcription market involving the direct transcription into customer-hosted medical transcription platforms receive platform-specific training. Prior to performing medical transcription services for our customers, each MT must demonstrate proficiency in the use of our DEP or the applicable customer-hosted platform.
With the emergence of speech recognition technology to produce draft transcribed reports, our MTs have an opportunity to become medical editors (MEs). Before they are eligible to edit the draft transcribed reports, MEs must be formally certified on DocQspeechtm, our DEP’s speech recognition module. Currently over 3,500 of our MTs have been cross trained as MEs.
Quality Assurance
Our automated technology routes reports with flagged quality issues to our quality assurance personnel for review prior to delivery to the customer. In addition, formal quality reviews are performed on a regular basis at both the individual MT and customer levels. We provide continuous feedback to the MT staff to increase learning and improve up-front quality. Our MTs participate in an on-going, comprehensive training program in order to maintain a high level of quality assurance.
Intellectual Property
We rely on a combination of copyright, patent, trademark, trade secret, and other intellectual property laws, nondisclosure agreements, license agreements, contractual provisions and other measures to protect our proprietary rights. We have a number of registered trademarks, including MedQuist®, and have current registrations of several domain names, including “www.medquist.com.”
Regulatory Matters
The provision of healthcare services, including the practice of medicine, is heavily regulated by federal and state statutes and regulations, by rules and regulations of state medical boards and state and local boards of health, and by codes established by various medical associations. Although many such laws, regulations and requirements do not directly apply to our operations, future laws and regulations related to the provision of medical transcription services may require us to restructure our operations in order to comply with such requirements.
Although many healthcare laws and regulations do not directly apply to our operations, our hospital and other healthcare provider customers must comply with a variety of requirements related to the handling of patient information, including HIPAA, which protects the privacy, confidentiality and security of protected health information (PHI). As part of the operation of our business, our customers provide us with certain PHI. The provisions of HIPAA require our customers to have agreements in place with us under which we are required to appropriately safeguard the PHI we create or receive on their behalf.
We have structured our operations to comply with these contractual requirements. We have designated a Chief Compliance Officer and have implemented appropriate safeguards related to the access, use and/or disclosure of PHI to help ensure the privacy and security of PHI consistent with our contractual requirements. We also are required to train personnel regarding HIPAA requirements. If we, or any of our MTs, are unable to maintain the privacy, confidentiality and security of the PHI that is entrusted to us, our customers could be subject
9
Table of Contents
to civil and criminal fines and sanctions and we could be found to have breached our contracts with our customers. Additionally, because all HIPAA standards are subject to interpretation and change, we cannot predict the future impact of HIPAA on our business and operations. Although it is not possible to anticipate the total effect of these regulations, we have made and continue to make investments in systems to support customer operations that are regulated by HIPAA.
Further, our customers are required to comply with HIPAA security regulations that require them to implement certain administrative, physical and technical safeguards to ensure the confidentiality, integrity and availability of electronic protected health information (EPHI). We are required by contract to protect the security of EPHI that we create, receive, maintain or transmit for our customers consistent with these regulations, including implementing administrative, physical and technical safeguards that reasonably and appropriately protect the confidentiality, integrity and availability of such EPHI. To comply with our contractual obligations, we may have to reorganize processes and invest in new technologies.
To the extent that the laws of the states in which we or our customers operate are more restrictive than HIPAA, we may have to incur additional costs to maintain compliance with any such applicable requirements.
Significant Events Over The Past Few Years
In November 2003, one of our employees raised allegations that we had engaged in improper billing practices. In response, our board of directors undertook an independent review of these allegations (Review). On March 16, 2004, we announced that we had delayed the filing of our Form 10-K for the year ended December 31, 2003 pending the completion of the Review. As a result of our noncompliance with the U.S. Securities and Exchange Commission’s (SEC) periodic disclosure requirements, our common stock was delisted from the NASDAQ National Market on June 16, 2004. In response to our customers’ concern over the public disclosure of certain findings from the Review, we made the decision in the fourth quarter of 2005 to take action to try to avoid litigation and preserve and solidify our customer business relationships by offering a financial accommodation to certain of our customers.
On July 5, 2007, we filed our Form 10-K for the year ended December 31, 2005 (2005 Form 10-K). The 2005 Form 10-K was our first periodic report covering the period after September 30, 2003. On August 31, 2007, we filed our Forms 10-Q for the quarters ended March 31, 2006, June 30, 2006 and September 30, 2006 as well as our Form 10-K for the year ended December 31, 2006. On October 4, 2007, we filed our Forms 10-Q for the quarters ended March 31, 2007 and June 30, 2007. On November 8, 2007, we timely filed our Form 10-Q for the quarter ended September 30, 2007.
Employees
As of February 29, 2008, we employed 7,512 people. Of these, 5,996 were MTs. Of our total work force, 3,956 were full-time employees and 3,556 were part-time employees.
Available Information
All periodic and current reports, registration statements, and other filings that we are required to file with the SEC, including our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) of the Securities Exchange Act of 1934 (Exchange Act), are available free of charge from the SEC’s website (www.sec.gov) or public reference room at 100 F Street N.E., Washington, DC 20549 (1-800-SEC-0330) or through our website at www.medquist.com. Such documents are available as soon as reasonably practicable after electronic filing of the material with the SEC. Copies of these reports (excluding exhibits) may also be obtained free of charge, upon written request to: Investor Relations, MedQuist Inc., 1000 Bishops Gate Boulevard, Suite 300, Mount Laurel, New Jersey 08054-4632. The website addresses included in this report are for identification purposes. The information contained therein or connected thereto are not intended to be incorporated into this report.
Item 1A.Risk Factors
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements that are based on current expectations, estimates, forecasts and projections about us, the industry in which we operate and other matters, as well as management’s beliefs and assumptions and other statements regarding matters that are not historical facts. These statements include, in particular, statements about our plans, strategies and prospects. For example, when we use words such as “projects,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “should,” “would,” “could,” “will,” “opportunity,” “potential” or “may,” variations of such words or other words that convey uncertainty of future events or outcomes, we are making forward-looking statements within the meaning of Section 27A of the Securities Act of 1933
10
Table of Contents
(Securities Act) and Section 21E of the Exchange Act. Our forward-looking statements are subject to risks and uncertainties. Actual events or results may differ materially from the results anticipated in these forward-looking statements as a result of a variety of factors. While it is impossible to identify all such factors, factors that could cause actual results to differ materially from those estimated by us include:
| • | each of the factors discussed in this Item 1A, Risk Factors as well as risks discussed elsewhere in this report; | ||
| • | each of the matters discussed in Item 3, Legal Proceedings; | ||
| • | difficulties relating to our significant management turnover; | ||
| • | our ability to recruit and retain qualified MTs and other employees; | ||
| • | the impact of our new services and products on the demand for our existing services and products; | ||
| • | our current dependence on medical transcription for substantially all of our business; | ||
| • | our ability to expand our customer base; | ||
| • | changes in law, including, without limitation, the impact HIPAA will have on our business; | ||
| • | infringement on the proprietary rights of others; | ||
| • | our ability to diversify into other businesses; | ||
| • | the results of our review of strategic alternatives, including an evaluation of whether a sale of MedQuist is in the best interests of MedQuist and our shareholders; | ||
| • | our ability to effectively integrate newly-acquired operations; | ||
| • | competitive pricing pressures in the medical transcription industry and our response to those pressures; and | ||
| • | general conditions in the economy and capital markets. |
Many of these factors are beyond our ability to predict or control. In addition, as a result of these and other factors, our past financial performance should not be relied on as an indication of future performance. The cautionary statements referred to in this section also should be considered in connection with any subsequent written or oral forward-looking statements that may be issued by us or persons acting on our behalf. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this report might not occur. Furthermore, we cannot guarantee future results, events, levels of activity, performance or achievements.
Set forth below are certain important risks and uncertainties that could adversely affect our results of operations or financial condition and cause our actual results to differ materially from those expressed in forward-looking statements made by us. Although we believe that we have identified and discussed below the key risk factors affecting our business, there may be additional risks and uncertainties that are not presently known or that are not currently believed to be significant that may adversely affect our performance or financial condition. More detailed information regarding certain risk factors described below is contained in other sections of this report.
We are subject to ongoing investigations, which could require us to pay substantial fines or other penalties or subject us to sanctions and we cannot predict the timing of developments in these matters.
The SEC is currently conducting a formal investigation of us related to our billing practices. We have been fully cooperating with the SEC since it opened its investigation in 2004. We have complied, and are continuing to comply, with information and document requests by the SEC.
On December 17, 2004, we received an administrative HIPAA subpoena for documents from the U.S. Department of Justice (DOJ). The subpoena seeks information primarily about our provision of medical transcription services to governmental and non-governmental customers. The information was requested in connection with a dual civil and criminal government investigation into whether we and others violated federal laws in connection with the provision of medical transcription services. We have been and intend to continue cooperating fully with the DOJ.
11
Table of Contents
On November 23, 2004 we received notice from the U.S. Department of Labor (DOL) of its commencement of a formal investigation into the administration of our 401(k) plan. We have been fully cooperating with the DOL since it opened its investigation in 2004. We have complied, and are continuing to comply, with information and document requests by the DOL.
We cannot predict when the investigations will be completed or the timing of any other developments, nor can we predict what the result of these matters may be. See Item 3, Legal Proceedings, for a further discussion of these matters.
Expenses incurred in connection with these matters (which include substantial fees for lawyers and other professional advisors) could adversely affect our financial position, results of operations and liquidity. We may be required to pay material judgments, fines, penalties or settlements or suffer other penalties, each of which could have a material adverse effect on our business, including the loss of customers, and our historical and future results of operations, financial condition and liquidity. The investigations may adversely affect our ability to obtain and/or increase the cost of obtaining directors’ and officers’ liability insurance and/or other types of insurance, which could have a material adverse affect on our ability to retain our current or obtain new senior management and directors. In addition, the findings and outcomes of the investigations described above may adversely affect the course of the civil litigation pending against us.
Several lawsuits have been filed against us involving our billing practices and other related matters and the outcome of these lawsuits may have a material adverse effect on our business, financial condition, results of operations and cash flows.
A number of lawsuits have been filed against us, as well as certain of our past and current officers and/or directors and current majority shareholder, relating to, among other things, allegations of violations of various common laws based on allegedly unlawful billing and payroll practices. Substantial damages or other monetary remedies assessed against us could have a material adverse effect on our business, financial condition, results of operations and cash flows. See Item 3, Legal Proceedings, for a further discussion of these matters.
The continuing time, effort, and expense relating to the allegations raised regarding our past billing practices and the development and implementation of improved internal controls and procedures may have an adverse effect on our business.
Our management team has spent considerable time and effort addressing the challenges of the various government investigations and extensive litigation we face as well as strengthening our accounting and internal controls and updating and developing accounting policies and procedures, disclosure controls and procedures, and corporate governance policies and procedures. To the extent these matters require continued management attention, our operations may be adversely affected.
Our ability to expand our business and properly service our customers depends on our ability to effectively manage our domestic production capacity, including our ability to recruit, train and retain qualified MTs and maintain high standards of quality service in our operations, which we may not be able to do.
Our success depends, in part, upon our ability to effectively manage our domestic production capacity including our ability to attract and retain qualified MTs who can provide accurate medical transcription. There is currently a shortage of qualified MTs in the U.S. and increased workflow has created industry-wide demand for quality MTs. As a result, competition for skilled MTs is intense. We have active programs in place to attract domestic MTs and to partner with offshore medical transcription service providers. However, this strategy may not alleviate any issues caused by the shortage. Because medical transcription is a skilled position in which experience is valuable, we require that our MTs have substantial experience or receive substantial training before being hired. Competition may force us to increase the compensation and benefits paid to our MTs, which could reduce our operating margins and profitability. In addition, failure to recruit and retain qualified MTs may have an adverse effect on our ability to service our customers, manage our production capacity and maintain our high standards of quality service. An inability to hire and retain a sufficient number of MTs could have a negative impact on our ability to grow.
We are reviewing strategic alternatives which could impact our operating results, our stock price and our business.
In June 2007 we engaged Bear, Stearns and Co. Inc. as our financial advisor to review our strategic alternatives. On November 2, 2007, Philips announced its intention to proceed with the sale of its approximate 69.6% ownership interest in us if a satisfactory price and other acceptable terms can be realized. On this same date and in light of Philips’ announcement, we announced that our board of directors, in connection with its previously disclosed review of strategic alternatives, is evaluating whether a sale of MedQuist is in the best interest of MedQuist and our shareholders. We are uncertain as to what impact the potential sales transaction by Philips or any other particular strategic alternative will have on our operating results, our stock price and our business if accomplished or whether any transaction will even occur as a result of this review. There can be no assurance that the exploration of strategic alternatives will result in any agreements or transactions. Other uncertainties and risks relating to our review of strategic alternatives include:
12
Table of Contents
| • | the review of strategic alternatives may disrupt our operations, affect morale and distract management which could have a material adverse effect on our operating results; | ||
| • | perceived uncertainties as to our future could have a negative impact on our relationships with customers and may result in the loss of business opportunities; | ||
| • | perceived uncertainties as to our future may result in increased difficulties in recruiting and retaining employees, particularly highly qualified employees; | ||
| • | the process of reviewing strategic alternatives may be more time consuming and expensive than we currently anticipate; | ||
| • | we may not be able to identify strategic alternatives that are worth pursuing; and | ||
| • | we may not be able to successfully achieve the benefits of any strategic alternative undertaken by us. |
If the electronic health records (EHR) companies produce solutions acceptable to large hospital systems for the creation of electronic clinical documentation that are not based on the conversion of voice to text, the overall demand for medical transcription services could be reduced.
EHR companies’ solutions for the collection of clinical data typically require physicians to directly enter and organize patient chart information through templates thereby eliminating or dramatically reducing the use of dictation or transcription. Although the EHR market is in the early stages of development and is rapidly evolving, a number of market entrants have introduced or developed products and services that are competitive with one or more components of the solutions we offer. We expect that additional companies will continue to enter this market. In new and rapidly evolving industries, there is significant uncertainty and risk as to the demand for, and market acceptance of, recently introduced solutions for the creation of electronic clinical documentation. In the event that such solutions are successful and gain wide acceptance, the overall demand for medical transcription services could be reduced. In the event that markets develop more quickly than expected, our business, financial condition and results of operations could be adversely affected.
We have experienced significant management turnover.
In the past few years, we have experienced a significant turnover in our senior management. This lack of management continuity, and the resulting lack of long-term history with us, could result in operational and administrative inefficiencies and added costs, could adversely impact our stock price and our customer relationships and may make recruiting for future management positions more difficult. In addition, we must successfully integrate any new management personnel that we hire within our organization in order to achieve our operating objectives, and changes in other key management positions may temporarily affect our financial performance and results of operations as new management becomes familiar with our business. Accordingly, our future financial performance will depend to a significant extent on our ability to motivate and retain key management personnel.
We have not complied with Section 404 of the Sarbanes-Oxley Act of 2002 (SOX) for our fiscal year ended December 31, 2004.
As directed by Section 404 of SOX (Section 404), the SEC adopted rules requiring certain public companies, including us, to include management’s assessment of the effectiveness of a public company’s internal control over financial reporting in its annual report on Form 10-K. In addition, the independent registered public accounting firms auditing certain public companies’ financial statements, including ours, must attest to the effective operation of such companies internal control over financial reporting. Although these requirements were first applicable to our annual report on Form 10-K for our fiscal year ending December 31, 2004, we were unable to comply with these requirements for such fiscal year. The time and resources expended in connection with the Review and management’s review of our historical customer billing, including the resulting changes in senior management, prevented us from completing our internal documentation, assessment and evaluation of our internal control over financial reporting, all of which are required to be undertaken to comply with Section 404. This correspondingly prevented our independent registered public accounting firm from commencing the required audit of our internal control over financial reporting as of December 31, 2004.
Since we determined that it would not be possible to complete either management’s assessment or an audit of our internal control over financial reporting as of December 31, 2004, our independent registered public accounting firm accordingly did not issue an opinion with respect to our internal control over financial reporting as of December 31, 2004. This failure to obtain an opinion does not comply with the SEC’s rules and regulations under Section 404, and this noncompliance, as well as our failure to provide the required Section 404 management assessment, has resulted in us being in violation of Section 13(a) under the Exchange Act. Section 13(a) establishes the
13
Table of Contents
general requirement that public companies must file with the SEC, in accordance with such rules and regulations as the SEC may prescribe, such information, documents and reports as the SEC may from time to time require for the protection of investors, including Forms 10-K and 10-Q.
In general, the SEC has broad authority under the Exchange Act to institute investigations, to seek injunctions, to seek monetary penalties, and to otherwise pursue enforcement actions for violations of Section 13(a), including a failure to file a Form 10-K or for the omission of necessary statements in a Form 10-K. Therefore, our failure to comply with the Section 404 requirements in our Form 10-K could potentially subject us to these same investigations, injunctions, penalties and enforcement actions. Section 404 is a relatively new legal requirement, and there is very little precedent establishing the consequences or appropriate response to a public company’s failure to comply with Section 404. Accordingly, although we have discussed our Section 404 noncompliance with the SEC, we cannot predict what action, if any, the SEC may take against us as a result of a failure to be compliant with our obligations under Section 404 or Section 13(a) of the Exchange Act.
Current and prospective investors, customers and employees may react adversely to the allegations concerning our billing practices.
Our future success depends in large part on the support of our current and future investors, customers and employees. The allegations concerning our past billing practices could cause current and future customers to lose confidence in us, which may affect their willingness to seek services from us. In addition, employees and prospective employees may factor in these considerations relating to our stability and the value of any equity incentives in their decision-making regarding employment opportunities.
We compete with many others in the market for medical transcription services which may result in lower prices for our services, reduced operating margins and an inability to maintain or increase our market share and expand our service offerings.
We compete with other outsourced medical transcription service companies in a very fragmented market that includes national, regional and local service providers, as well as service providers with global operations. These companies offer services that are similar to ours and compete with us for both customers and qualified MTs. We also compete with the in-house medical transcription staffs of our customers. While we attempt to compete on the basis of fast, predictable turnaround times and consistently high accuracy and document quality, all offered at a reasonable price, there can be no assurance that we will be able to compete effectively against our competitors or timely implement new products and services. Many of our competitors attempt to differentiate themselves by offering lower priced alternatives to our outsourced medical transcription services. Increased competition and cost pressures affecting the healthcare markets in general may result in lower prices for our services, reduced operating margins and the inability to maintain or increase our market share.
As technology evolves, including the continued refinement of speech recognition technology, health information technology providers may provide services that replace or reduce the need for medical transcription. Furthermore, companies that provide services complementary to medical transcription, such as electronic medical records, coding and billing, may expand the services they provide to include medical transcription. Current and potential competitors may have financial, technical and marketing resources that are greater than ours. As a result, competitors may be able to respond more quickly to evolving technological developments or changing customer needs or devote greater resources to the development, promotion or sale of their technology or services than we can. In addition, competition may increase due to consolidation of medical transcription companies. As a result of such consolidation, there may be a greater number of providers of medical transcription services with sufficient scale, service mix and financial resources to compete with us to provide services to larger, more complex organizations. Current and potential competitors may establish cooperative relationships with third parties to increase their ability to attract our current and potential customers.
We may pursue future transactions which could require us to incur debt and assume contingent liabilities and expenses, and we may not be able to effectively integrate new operations.
A significant portion of our historical growth has occurred through transactions, and we may pursue transactions in the future. Transactions involve risks that the combined businesses will not perform in accordance with expectations and that business judgments concerning the value, strengths and weaknesses of the combined businesses will prove incorrect. We cannot guarantee that if we decide to pursue future transactions, we will be able to identify attractive opportunities or successfully integrate any business or asset we combine with our existing business. Future transactions may involve high costs and may result in the incurrence of debt, contingent liabilities, interest expense, amortization expense or periodic impairment charges related to goodwill and other intangible assets as well as significant charges relating to integration costs.
We cannot guarantee that we will be able to successfully integrate any business we combine with our existing business or that any combined businesses will be profitable. The successful integration of new businesses depends on our ability to manage these new businesses effectively. The successful integration of future transactions may also require substantial attention from our senior
14
Table of Contents
management and the management of the combined businesses which could decrease the time that they have to service and attract customers. In addition, because we may actively pursue a number of opportunities simultaneously, we may encounter unforeseen expenses, complications and delays, including difficulties in employing sufficient staff and maintaining operational and management oversight. Our inability to complete the integration of any new businesses we combine with our existing business in a timely and orderly manner could reduce our net revenues and negatively impact our results of operations.
Our success will depend on our ability to adopt and integrate new technology into our DEP, to improve our production capabilities and expand the breadth of our service offerings, as well as our ability to address any unanticipated problems with our information technology systems, which we may not be able to do quickly, or at all.
Our ability to remain competitive in the medical transcription industry is based, in part, on our ability to develop and utilize technology in the services that we provide to our customers to improve our production capabilities and expand the breadth of our service offerings. Because our services are an integral part of our customers operations, we also must quickly address any unanticipated problems with our information technology systems that could cause an interruption in service or a decrease in our responsiveness to customers. Furthermore, as our customers advance technologically, we must be able to effectively integrate our DEP with their systems and provide advanced data collection technology. We plan to develop and integrate new technologies into our current service structure to give our customers high-quality and cost-effective services. We also may need to develop technologies to provide service systems comparable to those of our competitors as they develop new technology. If we are unable to effectively develop and integrate new technologies, we may not be able to expand our technology and service offerings or compete effectively with our competitors. In addition, if the cost of developing and integrating new technologies is high, we may not realize our expected return on investment.
Due to the critical nature of medical transcription to our customers’ operations, potential customers may be reluctant to outsource or change service providers as a result of the cost and potential for disruption in services which may inhibit our ability to attract new customers.
The up-front cost involved in changing medical transcription service providers or converting from an in-house medical transcription department to an outsourced provider may be significant. Many customers may prefer to remain with their current service provider or keep their medical transcription in-house rather than incur these costs or experience a potential disruption in services as a result of changing service providers. Also, as the maintenance of accurate medical records is a critical element of a healthcare provider’s ability to deliver quality care to its patients and to receive proper and timely reimbursement for the services it renders, potential customers may be reluctant to outsource such an important function.
If our intellectual property is not adequately protected, we may lose our market share to our competitors and be unable to operate our business profitably.
Our success depends, in part, upon our proprietary technology and our ability to license and renew third-party intellectual property. We regard some of the software underlying our services, including our DEP and interfaces, as proprietary, and we rely primarily on a combination of trade secrets, patent, copyright and trademark laws, confidentiality agreements, contractual provisions and technical measures to protect our proprietary rights. Despite our efforts to protect our proprietary rights, unauthorized parties may attempt to copy aspects of our intellectual property or obtain and use information that we regard as proprietary. There can be no assurance that our proprietary information will not be independently developed by competitors. There can be no assurance that the intellectual property we own or license will provide competitive advantages or will not be challenged or circumvented by our competitors.
We are dependent on third party speech recognition software incorporated in certain of our technologies, and impaired relations with such third party or the inability to enhance such third party software over time could harm our business.
We license speech recognition software from PSRS that we incorporate into our DEP and SpeechQ for Radiology. This license may not continue to be available on commercially reasonable terms or at all. Some of this technology would be difficult to replace. The loss of this license could significantly impact our business until we identify, license and integrate, or develop equivalent software. If we are required to enter into license agreements with third parties for replacement technology, we could face higher royalty payments and our products may lose certain attributes or features.
In addition, our products may be impacted if errors occur in the licensed software that we utilize. It may be more difficult for us to correct any defects in third-party software because the software is not within our control. Accordingly, our business could be adversely affected in the event of any errors in this software. There can be no assurance that these third-parties will continue to invest the appropriate levels of resources in their products and services to maintain and enhance the capabilities of their software.
15
Table of Contents
If we fail to comply with extensive contractual obligations and applicable laws and government regulations governing the handling of patient identifiable medical information, including those imposed on our customers in connection with HIPAA, we could suffer material losses or be negatively impacted as a result of our customers being subject to material penalties and liabilities.
As part of the operation of our business, our customers provide us with certain patient identifiable medical information. Although many regulatory and governmental requirements do not directly apply to our operations, our hospital and other healthcare provider customers must comply with a variety of requirements related to the handling of patient information, including laws and regulations protecting the privacy, confidentiality and security of PHI. Most of our customers are covered entities and, in many of our relationships, we function as a business associate. In particular, the provisions of HIPAA require our customers to have business associate agreements in place with a medical transcription company such as ours under which we are required to appropriately safeguard the PHI we create or receive on their behalf. Further, our customers are required to comply with HIPAA security regulations that require them to implement certain administrative, physical and technical safeguards to ensure the confidentiality, integrity and availability of EPHI. We are required by contract to protect the security of EPHI that we create, receive, maintain or transmit for our customers consistent with these regulations. To comply with our contractual obligations, we may have to reorganize processes and invest in new technologies. We also are required to train personnel regarding HIPAA requirements. If we, or any of our MTs or subcontractors, are unable to maintain the privacy, confidentiality and security of the PHI that is entrusted to us, our customers could be subject to civil and criminal fines and sanctions and we could be found to have breached our contracts with our customers. Additionally, because all HIPAA standards are subject to interpretation and change, we cannot predict the future impact of HIPAA on our business and operations. In the event that the standards and compliance requirements under HIPAA change or are interpreted in a way that requires any material change to the way in which we do business, our business, financial condition and results of operations could be adversely affected. To the extent that the laws of the states in which we or our customers operate are more restrictive than HIPAA, we may have to incur additional costs to maintain compliance with any such applicable requirements.
Proposed legislation and possible negative publicity may impede our ability to utilize global service capabilities.
Bills introduced in recent sessions of the U.S. Congress have sought to restrict the transmission of personally identifiable information regarding a U.S. resident to any foreign affiliate, subcontractor or unaffiliated third party without adequate privacy protections or without providing notice of the transmission and an opportunity to opt out. Some of the proposals would require patient consent. If enacted, these proposed laws would impose liability on healthcare businesses arising from the improper sharing or other misuse of personally identifiable information. Some proposals would create a private civil cause of action that would allow an injured party to recover damages sustained as a result of a violation of the new law. A number of states have also considered, or are in the process of considering, prohibitions or limitations on the disclosure of medical or other information to individuals or entities located outside of the U.S. Further, as a result of this negative publicity and concerns regarding the possible misuse of personally identifiable information, some of our customers have contractually limited our ability to use MTs located outside of the U.S.
Philips owns approximately 69.6% of our outstanding common stock, and its interests may conflict with the interests of our other shareholders.
Philips beneficially owns approximately 69.6% of our outstanding common stock. Philips has the ability to cause the election of all of the members of our board of directors, the appointment of new management and the approval of any action requiring the approval of our shareholders, including amendments to our certificate of incorporation and mergers or sales of substantially all of our assets. The directors elected by Philips will be able to make decisions affecting our capital structure, including decisions to issue additional capital stock, implement stock repurchase programs and declare dividends. Our interests and the interests of our affiliates, including Philips, could conflict with the interest of our other shareholders.
In November 2007, Philips announced its intention to proceed with the sale of its approximate 69.6% ownership interest in us if a satisfactory price and other acceptable terms can be realized. In light of Philips’ intention to proceed with the sale of its interest in us, our board of directors announced in November 2007 that it was evaluating whether a sale of MedQuist is in the best interests of MedQuist and our shareholders. There is no assurance that any sales transaction will occur as a result of this evaluation.
Our stock trades on the over-the-counter “Pink Sheets” market which may decrease the liquidity of our common stock.
On June 16, 2004, NASDAQ delisted our common stock because we were not able to file our periodic reports with the SEC in a timely manner. Since that time, our common stock has been traded on the over-the-counter “Pink Sheets” market (Pink Sheets) under the symbol “MEDQ.PK.” Broker-dealers often decline to trade in Pink Sheets stocks given that the market for such securities is often limited, the stocks are more volatile and the risks to investors are greater. Consequently, selling our common stock can be difficult because transactions can be delayed and security analyst and news media coverage of us may be reduced. These factors could result in lower prices and larger spreads in
16
Table of Contents
the bid and ask prices for shares of our common stock as well as lower trading volume. On November 13, 2007, we submitted an application for listing on NASDAQ, however, we cannot assure you that we will be successful in our application efforts. Investors should realize that they may be unable to sell shares of our common stock that they purchase. Accordingly, investors must be able to bear the financial risk of losing their entire investment in our common stock.
Item 1B.Unresolved Staff Comments
None.
Item 2.Properties
We currently do not own any real property. We currently lease approximately 39,000 square feet of office space in Mount Laurel, New Jersey which houses our corporate headquarters. This lease expires in 2014; however, we have the option to terminate the lease in 2011, subject to certain conditions, including the payment of a termination fee. We also lease approximately 38,000 square feet of office space in Norcross, Georgia for our sales, administrative and research and development functions. This lease expires in June 2008. We lease approximately 20,000 square feet for our call center in Marietta, Georgia and this lease expired on December 31, 2007. We are currently negotiating with the landlords to renew the Norcross and Marietta leases at commercially reasonable terms. The call center provides technical support and expertise to our customers and MTs. Other than our corporate headquarters, the Norcross facility and our call center, none of our other properties are material to our business. We believe that our corporate headquarters and other properties are suitable for their respective uses and are, in general, adequate for our present needs.
Item 3.Legal Proceedings
Governmental Investigations
The SEC is currently conducting a formal investigation of us relating to our billing practices. We have been fully cooperating with the SEC since it opened its investigation in 2004. We have complied, and are continuing to comply, with information and document requests by the SEC.
We also received an administrative HIPAA subpoena for documents from the DOJ on December 17, 2004. The subpoena sought information primarily about our provision of medical transcription services to governmental and non-governmental customers. The information was requested in connection with a government investigation into whether we and others violated federal laws in connection with the provision of medical transcription services. We have complied, and are continuing to comply, with information and document requests by the DOJ.
The DOL is currently conducting a formal investigation into the administration of our 401(k) plan. We have been fully cooperating with the DOL since it opened its investigation in 2004. We have complied, and are continuing to comply, with information and document requests by the DOL.
Developments relating to the SEC, DOJ and/or DOL investigations will continue to create various risks and uncertainties that could materially and adversely affect our business and our historical and future financial condition, results of operations and cash flows.
Shareholder Securities Litigation
A shareholder putative class action lawsuit was filed against us in the United States District Court District of New Jersey on November 8, 2004. The action, entitledWilliam Steiner v. MedQuist, Inc., et al., Case No. 1:04-cv-05487-FLW (Shareholder Putative Action), was filed against us and certain of our former officers, purportedly on behalf of an alleged class of all persons who purchased our common stock during the period from April 23, 2002 through November 2, 2004, inclusive (Securities Class Period). The complaint specifically alleged that defendants violated federal securities laws by purportedly issuing a series of false and misleading statements to the market throughout the Securities Class Period, which statements allegedly had the effect of artificially inflating the market price of our securities. The complaint asserted claims under Section 10(b) and 20(a) of the Exchange Act and Rule 10b-5, thereunder. Named as defendants, in addition to us, were our former President and Chief Executive Officer and our former Executive Vice President and Chief Financial Officer.
On August 16, 2005, a First Amended Complaint in the Shareholder Putative Class Action was filed against us in the United States District Court District of New Jersey. The First Amended Complaint named additional defendants, including certain current and former directors, certain of our former officers, our former and current external auditors and Philips. Like the original complaint, the First Amended Complaint asserted claims under Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 thereunder. The Securities
17
Table of Contents
Class Period of the original complaint was expanded 20 months to include the period from March 29, 2000 through June 14, 2004. Pursuant to an October 17, 2005 consent order approved by the Court, lead plaintiff Greater Pennsylvania Pension Fund filed a Second Amended Complaint on November 15, 2005. The Second Amended Complaint dropped Philips as a defendant, but alleged the same claims and the same purported class period as the First Amended Complaint. Plaintiffs sought unspecified damages. Pursuant to the provisions of the Private Securities Litigation Reform Act, discovery in the action was stayed pending the filing and resolution of the defendants’ motions to dismiss, which were filed on January 17, 2006, and which were fully briefed as of June 16, 2006. On September 29, 2006, the Court denied our motions to dismiss and the motion to dismiss of the individual defendants. In the same order, the Court granted the motion to dismiss filed by our former and current external auditors. On November 3, 2006, we filed our Answer denying the material allegations contained in the Second Amended Complaint. On March 23, 2007, we entered into a memorandum of understanding and a stipulation of settlement with the lead plaintiff in which we agreed to pay $7.75 million to settle all claims, throughout the class period, against all defendants in the action. On May 16, 2007, the Court issued an Order Preliminarily Approving Settlement and Providing for Notice. The Court conducted a final approval hearing and approved the settlement on August 15, 2007. Neither we nor any of the individuals named in the action has admitted to liability or any wrongdoing in connection with the settlement. On August 17, 2007, the Court entered final judgment and dismissed the case with prejudice.
Customer Litigation
A putative class action was filed in the United States District Court for the Central District of California. The action, entitled South Broward Hospital District, d/b/a Memorial Regional Hospital, et al. v. MedQuist, Inc. et al., Case No. CV-04-7520-TJH-VBKx, was filed on September 9, 2004 against us and certain of our present and former officials, purportedly on behalf of an alleged class of non-federal governmental hospitals and medical centers that the complaint claims were wrongfully and fraudulently overcharged for transcription services by defendants based primarily on our use of the AAMT line billing unit of measure. The complaint charged fraud, violation of the California Business and Professions Code, unjust enrichment, conversion, negligent supervision and violation of RICO. Plaintiffs seek damages in an unspecified amount, plus costs and interest, an injunction against alleged continuing illegal activities, an accounting, punitive damages and attorneys’ fees. Named as defendants, in addition to us, were one of our senior vice presidents, our former executive vice president of marketing and new business development, our former executive vice president and chief legal officer, and our former executive vice president and chief financial officer.
On December 20, 2004, we and the individual defendants filed motions to dismiss for lack of personal jurisdiction and improper venue, or in the alternative, to transfer the putative action to the United States District Court for the District of New Jersey. On February 2, 2005, plaintiffs filed a Second Amended Complaint both adding and deleting named plaintiffs in an attempt to keep the putative action in the United States District Court for the Central District of California. On March 30, 2005, the United States District Court for the Central District of California issued an order transferring the putative action to the United States District Court District of New Jersey and assigning Case No. 05-CV-2206-JBS-AMD.
On August 1, 2005, we and the individual defendants filed their respective Answers denying the material allegations contained in the Second Amended Complaint. On August 31, 2005, we and the individual defendants filed motions to dismiss the Second Amended Complaint for failure to state a claim and a motion to dismiss in favor of arbitration, or in the alternative, to stay pending arbitration. On December 12, 2005, the plaintiffs filed an Amendment to the Second Amended Complaint. On December 13, 2005, the Court issued an order requiring plaintiffs to file a Third Amended Complaint.
Plaintiffs filed the Third Amended Complaint on January 4, 2006. The Third Amended Complaint expands the claims made beyond issues arising from contracts based on AAMT line billing and beyond customers billed based on an AAMT line, alleging that we engaged in a scheme to inflate customers’ invoices without regard to the terms of individual contracts and even in the absence of any written contract. The Third Amended Complaint also limits plaintiffs’ claim for fraud in the inducement of the agreement to arbitrate to the three named plaintiffs whose contracts contain an arbitration provision and a subclass of similarly situated customers. On January 20, 2006 we and the individual defendants filed motions to dismiss the Third Amended Complaint for failure to state a claim and a motion to compel arbitration of all claims by the arbitration subclass and to stay the case in its entirety pending arbitration. On March 8, 2006 the Court held a hearing on these motions, and took the matter under submission. On March 30, 2007, the Court issued an order holding that plaintiffs could not make out a claim that we had violated the federal RICO statute, thus eliminating any claim against us for treble damages. The Court also found that plaintiffs could not make out a claim that we had engaged in any unfair or deceptive acts or practices in violation of state law, or that we had made any negligent misrepresentations to plaintiffs. In its ruling, the Court, without reaching a decision of whether any wrongdoing had occurred, allowed plaintiffs to proceed with their claims against us for fraud, unjust enrichment and an accounting. In its order, the Court denied our motion to compel arbitration regarding those customers whose contracts contained an agreement to arbitrate. We appealed that decision to the Third Circuit Court of Appeals, and we moved the District Court to stay the matter pending that appeal. The District Court heard oral argument on our motion to stay on May 30, 2007 and took the motion under submission.
18
Table of Contents
On December 18, 2007, the Third Circuit entered judgment denying our appeal and affirming the order of the District Court. That same day, the District Court entered an order denying our motion to stay pending appeal as moot and ordering all Initial Disclosures, all class-certification related discovery, and all briefing upon class-certification motion practice completed within three months unless enlarged for good cause. On February 21, 2008, the District Court entered a scheduling order pursuant to which all fact discovery related to class certification must be completed by April 15, 2008 and plaintiffs’ motion for class certification must be filed by April 30, 2008. All other fact discovery must be completed by July 31, 2008, and all expert discovery must be completed by October 31, 2008. Dispositive motions must filed by November 24, 2008. The parties exchanged Initial Disclosures and commenced discovery.
The parties participated in court-ordered mediation in late 2007, but no settlement was reached. The parties continued to explore the possibility of resolving the litigation before trial and have now reached agreement on settlement terms resolving all claims by the named plaintiffs. Under the parties’ agreement, we will make a lump sum payment of $7.5 million to resolve all claims by the individual named plaintiffs and certain other additional putative class members represented by plaintiffs’ counsel but not named in the action. Neither we, nor any of the individual defendants, will admit to any liability or any wrongdoing in connection with the settlement. The District Court has entered a consent order staying the action through April 18, 2008 to allow the parties to finalize the settlement. We anticipate that the parties will execute a final settlement agreement and the case will be dismissed with prejudice in its entirety within the next four to eight weeks. Because the settlement will not be on a class-wide basis, no class will be certified and thus there is no requirement to give notice.
Medical Transcriptionist Litigation
Hoffmann Putative Class Action
A putative class action lawsuit was filed against us in the United States District Court for the Northern District of Georgia. The action, entitled Brigitte Hoffmann, et al. v. MedQuist, Inc., et al., Case No. 1:04-CV-3452, was filed with the Court on November 29, 2004 against us and certain current and former officials, purportedly on behalf of an alleged class of current and former employees and statutory workers, who are or were compensated on a “per line” basis for medical transcription services (Class Members) from January 1, 1998 to the time of the filing of the complaint (Class Period). The complaint specifically alleged that defendants systematically and wrongfully underpaid the Class Members during the Class Period. The complaint asserted the following causes of action: fraud, breach of contract, demand for accounting, quantum meruit, unjust enrichment, conversion, negligence, negligent supervision, and RICO violations. Plaintiffs sought unspecified compensatory damages, punitive damages, disgorgement and restitution. On December 1, 2005, the Hoffmann matter was transferred to the United States District Court for the District of New Jersey. On January 12, 2006, the Court ordered this case consolidated with the Myers Putative Class Action discussed below. As set forth below, we believe that the claims asserted in the consolidated Myers Putative Class Action have no merit and intend to vigorously defend that action.
Force Putative Class Action
A putative class action entitled Force v. MedQuist Inc. and MedQuist Transcriptions, Ltd., Case No. 05-cv-2608-WSD, was filed against us on October 11, 2005, in the United States District Court for the Northern District of Georgia. The action was brought on behalf of a putative class of current and former employees who claim they are or were compensated on a “per line” basis for medical transcription services but were allegedly underpaid due to the actions of defendants. The named plaintiff asserted claims for breach of contract, quantum meruit, unjust enrichment, and for an accounting. Upon stipulation and consent of the parties, on February 17, 2006, the Force matter was ordered transferred to the United States District Court for the District of New Jersey. Subsequently, on April 4, 2006, the parties entered into a stipulation and consent order whereby the Force matter was consolidated with the Myers Putative Class Action discussed below, and the consolidated amended complaint filed in the Myers action on January 31, 2006 was deemed to supersede the original complaint filed in the Force matter. As set forth below, we believe that the claims asserted in the consolidated Myers Putative Class Action have no merit and intend to vigorously defend that action.
Myers Putative Class Action
A putative class action entitled Myers, et al. v. MedQuist Inc. and MedQuist Transcriptions, Ltd., Case No. 05-cv-4608 (JBS), was filed against us on September 22, 2005 in the United States District Court for the District of New Jersey. The action was brought on behalf of a putative class of our employee and independent contractor transcriptionists who claim that they contracted with us to be paid on a 65 character line, but were allegedly underpaid due to intentional miscounting of the number of characters and lines transcribed. The named plaintiffs asserted claims for breach of contract, unjust enrichment, and request an accounting.
The allegations contained in the Myers case are substantially similar to those contained in the Hoffmann and Force putative class actions and, as detailed above, the three actions have now been consolidated. A consolidated amended complaint was filed on January 31, 2006. In the consolidated amended complaint, the named plaintiffs assert claims for breach of contract, breach of the covenant of good faith and fair dealing, unjust enrichment and demand an accounting. On March 7, 2006 we filed a motion to dismiss all claims in the consolidated amended complaint. The motion was fully briefed and argued on August 7, 2006. The Court denied the motion on December 21, 2006. On January 19, 2007, we filed our answer denying the material allegations pleaded in the consolidated amended complaint.
19
Table of Contents
On May 17, 2007, the Court issued a Scheduling Order, ordering all pretrial fact discovery completed by October 30, 2007. The Court subsequently ordered plaintiffs to file their motion for class certification by December 14, 2007 and continued the date to complete fact discovery to January 14, 2007. On October 18, 2007, the Court heard oral argument on plaintiffs’ motion to compel further responses to written discovery regarding our billing practices. At the conclusion of the hearing, the Court denied plaintiffs’ motion, finding plaintiffs had not established that the billing discovery sought was relevant to the claims or defenses regarding transcriptionist pay alleged in their case. On December 14, 2007, plaintiffs filed their motion for class certification, identifying a proposed class of all our transcriptionists who were compensated on a per line basis for work completed on MedRite, MTS or DEP transcription platforms from November 29, 1998 to the present and alleging that the proposed class was underpaid by more than $80 million, not including interest.
On January 4, 2008, the Court entered a Consent Order, ordering our opposition to the motion for class certification to be filed by March 14, 2008, plaintiffs’ reply brief to be filed by May 14, 2008 and setting oral argument for June 2, 2008. No date has been set for trial. On January 9, 2008, the Court entered a Consent Order, extending the deadline for the parties to complete depositions of identified witnesses through February 15, 2008. We have now deposed each of the named plaintiffs and all witnesses who offered declarations in support of plaintiffs’ motion for class certification, and plaintiffs have deposed numerous MedQuist present and former employees. On February 8, 2008, plaintiffs indicated that they will seek leave to file an amended class certification brief to narrow their claims. On February 19, 2008, the parties exchanged their Initial Disclosures. Plaintiffs’ disclosures limit their damages estimate to $41 million related to alleged underpayment on the MedRite transcription platform; however, plaintiffs state that they are continuing to analyze potential undercounting and will supplement their damages claim. On March 10, 2008, plaintiffs moved for leave to file an amended motion for class certification dropping all allegations involving our DEP transcription platform and narrowing the claims asserted regarding the legacy MTS transcription platform. We did not oppose plaintiffs’ motion for leave. On March 11, 2008, the Court granted plaintiffs’ motion, ordering us to file our opposition to plaintiffs’ amended motion for class certification by April 4, 2008 and ordering plaintiffs to file their reply by May 23, 2008. The hearing date was not changed and oral argument remains set for June 2, 2008. We believe that the claims asserted in the consolidated actions have no merit and intend to vigorously defend the suit.
Shareholder Derivative Litigation
On October 4, 2005, we announced the dismissal with prejudice of a shareholder derivative action filed in United States District Court for the District of New Jersey. The suit, Rhoda Kanter (Plaintiff) v. Hans M. Barella et al. (Defendants), was filed on November 12, 2004 against Philips and 10 current and former members of our board of directors. We were named as a nominal defendant.
In a ruling dated September 21, 2005, the Court found plaintiff’s allegations that our board of directors breached their fiduciary duties to us to be insufficient. The plaintiff had alleged that for a period from 2001 through 2004, the Defendants violated their fiduciary duties by permitting artificial inflation of billing figures; failing to adequately ensure accurate and lawful billing practices; and failing to accurately report our true financial condition in our published financial statements. On October 3, 2005, plaintiff filed a motion for reconsideration of the Court’s order dismissing the action with prejudice. On November 16, 2005, the Court denied plaintiff’s motion for reconsideration. On December 13, 2005, plaintiff filed a Notice of Appeal with the United States Court of Appeals for the Third Circuit. Plaintiff’s appeal was fully briefed as of May 2006, and the Court of Appeals heard oral argument on the appeal on March 1, 2007. Plaintiff’s appeal was denied by the Court of Appeals on May 25, 2007.
Shareholder Litigation
Costa Brava Partnership III, L.P. Shareholder Litigation
On October 9, 2007, a single count Complaint and an Order to Show Cause were filed against us in the Superior Court of New Jersey, Chancery Division, Burlington County by one of our shareholders. The action, entitled Costa Brava Partnership III, L.P. v. MedQuist Inc. (Bur-C-0149-07), sought to compel us to hold an annual meeting of shareholders (Annual Meeting Claim).
On October 30, 2007, plaintiff requested access under New Jersey law to certain of our books and records. In response to plaintiff’s request, we voluntarily provided plaintiff with those books and records that we believed we were required to produce under New Jersey law. Thereafter, on November 9, 2007, plaintiff filed an Amended Complaint to assert a second claim to compel us to provide it with access to certain other books and records (Books and Records Claim). The Annual Meeting Claim and the Books and Records Claim sought equitable relief only.
In December 2007, we agreed to hold our annual meeting of shareholders on December 31, 2007. This resolved the Annual Meeting Claim. Prior to the annual meeting, we voluntarily produced to plaintiff certain additional books and records that plaintiff requested in the Books and Records Claim. Thereafter, on January 24, 2008, we filed an opposition to plaintiff’s Order to Show Cause to compel access to the remaining books and records. On February 4, 2008, plaintiff filed a reply brief. The Books and Records Claim has been briefed and the parties are
20
Table of Contents
waiting for the Court to schedule a hearing date to resolve the matter. We believe that the books and records requests at issue in the Books and Records Claim are burdensome and overbroad and intend to vigorously defend the action.
Kahn Putative Class Action
A shareholder putative class action lawsuit was filed against us in the Superior Court of New Jersey, Chancery Division, Burlington County. The action, entitled Alan R. Kahn v. Stephen H. Rusckowski, et al., Docket No. BUR-C-000007-08, was filed with the Court on January 22, 2008 against us, Philips and our four non-independent directors, Clement Revetti, Jr., Stephen H. Rusckowski, Gregory M. Sebasky and Scott Weisenhoff. Plaintiff purports to bring the action on his own behalf and on behalf of all current holders of our common stock. The complaint alleges that defendants breached their fiduciary duties of good faith, fair dealing, loyalty, and due care by purportedly agreeing to and initiating a process for our sale or a change of control transaction which will allegedly cause harm to plaintiff and the putative class. Plaintiff seeks damages in an unspecified amount, plus costs and interest, a judgment declaring that defendants breached their fiduciary duties and that any proposed transactions regarding our sale or change of control are void, an injunction preventing our sale or any change of control transaction that is not entirely fair to the class, an order directing us to appoint three independent directors to our board of directors and attorneys’ fees and expenses. We have not yet been required to file a responsive pleading. We believe that the claims asserted have no merit and intend to defend the case vigorously.
Reseller Arbitration Demand
On October 1, 2007, we received from counsel to nine current and former resellers of our products (Claimants), a copy of an arbitration demand filed by the Claimants, initiating an arbitration proceeding styled Diskriter, Inc., Electronic Office Systems, Inc., Milner Voice & Data, Inc., Nelson Systems, Inc., NEO Voice and Communications, Inc., Office Business Systems, Inc., Roach-Reid Office Systems, Inc., Stiles Office Systems, Inc., and Travis Voice and Data, Inc. v. MedQuist Inc. and MedQuist Transcriptions, Ltd. (filed on September 27, 2007, AAA, Case Number Not Yet Assigned). The arbitration demand purports to set forth claims for breach of contract; breach of covenant of good faith and fair dealing; promissory estoppel; misrepresentation; and tortuous interference with contractual relations. The Claimants allege that we breached our written agreements with the Claimants by: (i) failing to provide reasonable training, technical support, and other services; (ii) using the Claimants’ confidential information to compete against the Claimants; (iii) directly competing with the Claimants’ territories; and (iv) failing to make new products available to the Claimants. In addition, the Claimants’ allege that we made false oral representations that we: (i) would provide new products, opportunities and support to the Claimants; (ii) were committed to continuing to use Claimants; (iii) did not intend to create our own sales force with respect to the Claimants’ territory; and (iv) would stay out of Claimants’ territories and would not attempt to take over the Claimants business and relationships with the Claimants’ customers and end-users. The Claimants assert that they are seeking damages in excess of $24.3 million. The arbitrator has not yet set a date for us to formally respond to the arbitration demand. We deny all wrongdoing and intend to defend ourselves vigorously including asserting counterclaims against the Claimants as appropriate.
Anthurium Patent Litigation
On November 6, 2007, Anthurium Solutions, Inc. filed an action entitledAnthurium Solutions, Inc. v. MedQuist Inc., et al.,Civil Action No. 2-07CV-484, in the United States District Court for the Eastern District of Texas, alleging that we infringed and continue to infringe United States Patent No. 7,031,998 through our DEP transcription platform. The complaint also alleges patent infringement claims against Spheris Inc. and Arrendale Associates, Inc. The complaint seeks injunctive relief and unspecified damages, including enhanced damages and attorneys’ fees. We filed our answer on January 15, 2008 and counterclaimed seeking a declaratory judgment of non-infringement and invalidity. No scheduling order has been issued, and no pretrial dates have been set. Our investigation of the claims is ongoing, and we are awaiting plaintiffs’ preliminary infringement contentions. We believe that the claims asserted have no merit and intend to vigorously defend the suit.
Item 4.Submission Of Matters To A Vote Of Security Holders
We held our annual meeting of shareholders (Annual Meeting) on December 31, 2007. The purpose of the Annual Meeting was to elect six directors for terms of one year each. In addition to the nominees nominated by us, one of our shareholders, Costa Brava Partnership III, L.P. nominated six additional directors. The number of shares outstanding on our records as of the close of business on December 6, 2007, the record date for the Annual Meeting, was 37,487,323. There were 26,401,189 shares of company stock present at the meeting or by proxy. The results of voting at the Annual Meeting was as follows:
21
Table of Contents
| Name | Votes For | Votes Withheld | ||||||
Our Nominees: | ||||||||
| Brian O’Donoghue | 23,601,440 | 15,578 | ||||||
| Clement Revetti, Jr. | 23,601,292 | 15,726 | ||||||
| Stephen H. Rusckowski | 23,579,234 | 37,784 | ||||||
| Mark E. Schwarz | 23,579,382 | 37,636 | ||||||
| Gregory M. Sebasky | 23,601,292 | 15,726 | ||||||
| Scott M. Weisenhoff | 23,579,234 | 37,784 | ||||||
Costa Brava Nominees: | ||||||||
| Seth W. Hamot | 2,782,763 | 1,408 | ||||||
| Andrew R. Siegel | 2,782,763 | 1,408 | ||||||
| Douglas M. Gleason | 2,782,763 | 1,408 | ||||||
| Douglas E. Linton | 2,782,763 | 1,408 | ||||||
| Alok Mohan | 2,115,321 | 668,850 | ||||||
| Jay Scollins | 2,115,321 | 668,850 | ||||||
PART II
Item 5.Market For Registrant’s Common Equity, Related Shareholder Matters And Issuer Purchases Of Equity Securities
Our common stock is traded on the Pink Sheets under the symbol “MEDQ.PK”. Set forth below are the high and low closing bid quotations (as reported by the Pink Sheets LLC) for each quarter of 2006 and 2007 and the first quarter (through February 29, 2008) of 2008. The over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not necessarily reflect the prices for actual transactions.
| High | Low | |||||||
2006 | ||||||||
| First Quarter | $ | 13.94 | $ | 12.14 | ||||
| Second Quarter | $ | 14.90 | $ | 11.89 | ||||
| Third Quarter | $ | 12.45 | $ | 9.05 | ||||
| Fourth Quarter | $ | 13.65 | $ | 11.45 | ||||
2007 | ||||||||
| First Quarter | $ | 13.55 | $ | 9.90 | ||||
| Second Quarter | $ | 9.95 | $ | 7.80 | ||||
| Third Quarter | $ | 12.35 | $ | 9.05 | ||||
| Fourth Quarter | $ | 12.04 | $ | 8.70 | ||||
2008 | ||||||||
| First Quarter (through February 29, 2008) | $ | 10.75 | $ | 8.75 | ||||
Holders
As of February 29, 2008, the closing price of our common stock was $9.50 and we had 205 shareholders of record.
Dividends
We have never declared or paid any cash dividends on our common stock. We expect to retain any future earnings to fund operations and the continued development of our business and, therefore, do not anticipate paying any cash dividends in the foreseeable future.
22
Table of Contents
Item 6.Selected Financial Data
| For the Year Ended December 31, | ||||||||||||||||||||
| 2007 | 2006 | 2005 | 2004 | 2003 | ||||||||||||||||
| ($ in thousands, except per share data) | ||||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||
| Net Revenues | $ | 340,342 | $ | 358,091 | (1) | $ | 353,005 | (1)(2) | $ | 451,894 | (2) | $ | 484,762 | (2) | ||||||
| Net Income (loss) | $ | (15,206 | )(3) | $ | (16,942 | )(3) | $ | (111,632 | )(3)(4) | $ | 3,742 | (3)(5) | $ | 35,567 | ||||||
| Net Income (loss) per share — Basic | $ | (0.41 | ) | $ | (0.45 | ) | $ | (2.98 | ) | $ | 0.10 | $ | 0.96 | |||||||
| Net Income (loss) per share — Diluted | $ | (0.41 | ) | $ | (0.45 | ) | $ | (2.98 | ) | $ | 0.10 | $ | 0.94 | |||||||
| As of December 31, | ||||||||||||||||||||
| 2007 | 2006 | 2005 | 2004 | 2003 | ||||||||||||||||
Balance Sheet Data: | ||||||||||||||||||||
| Total assets | $ | 417,772 | $ | 441,139 | $ | 493,191 | $ | 540,934 | $ | 526,468 | ||||||||||
| Total non-current liabilities | $ | 17,294 | $ | 18,492 | $ | 18,534 | $ | 4,196 | $ | 3,339 | ||||||||||
| (1) | Reflects a reduction in net revenues of $10,402 and $57,678 in 2006 and 2005, respectively, related to accommodation offers to be made to certain of our customers. | |
| (2) | Reflects a reduction in net revenues related to a billing error of $133, $931 and $2,142 in 2005, 2004 and 2003, respectively. | |
| (3) | In 2007, 2006, 2005 and 2004, we recorded a charge, net of insurance recoveries, of $6,083, $13,001, $34,127 and $10,253, respectively, for costs associated with our review of certain of our historical line billings, our review of past allegations of improper billing practices, as well as legal fees and other costs associated with the matters described in Item 3, Legal Proceedings. | |
| (4) | In the fourth quarter of 2005, a valuation allowance of $56,808 was established against various domestic deferred tax assets. After consideration of all evidence, both positive and negative, management concluded that it was more likely than not that a majority of the domestic deferred tax assets would not be realized. | |
| (5) | In 2004, we recorded a goodwill impairment charge of $14,603 related to our former Solutions reporting unit. Due to reduced sales and margins, expected operating profits and cash flows were forecast lower than previously anticipated. |
Item 7.Management’s Discussion And Analysis Of Financial Condition And Results Of Operations
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our audited consolidated financial statements and related notes appearing elsewhere in this report. In addition to historical information, this discussion and analysis contains forward-looking statements based on current expectations that involve risks, uncertainties and assumptions, such as our plans, objectives, expectations and intentions set forth in the “Cautionary Statement Regarding Forward-Looking Statements,” which can be found in Item 1A, Risk Factors. Our actual results and the timing of events may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth in the “Risk Factors” section and elsewhere in this report.
Executive Overview
We are the largest MTSO in the world, and a leader in technology enabled clinical documentation workflow. We service health systems, hospitals and large group medical practices throughout the U.S., and we employ approximately 6,000 skilled MTs, making us the largest employer of MTs in the U.S. In the clinical documentation workflow, we provide, in addition to medical transcription technology and services, digital dictation, speech recognition and electronic signature services.
We were incorporated in New Jersey in 1984 and reorganized in 1987 as a group of outpatient healthcare businesses affiliated with a non-profit healthcare provider. In May 1994, we acquired our first medical transcription business. Through the date of this report, we have acquired over 50 companies. By the end of 1995, we had divested all of our non-medical transcription businesses.
In July 2000, Philips completed a tender offer in which it acquired approximately 60% of our outstanding common stock. Subsequent to the completion of the tender offer, Philips increased its ownership position and currently owns approximately 69.6% of our common stock.
In 2001, we acquired Speech Machines, a company based in the United Kingdom, whose technology has since developed into our DEP. In 2002, we began the process of migrating our customers to our DEP from our many disparate transcription platforms and completed this process in the first quarter of 2007. As a result of this process, we encountered customer attrition.
In July 2002, we acquired Lanier Healthcare, LLC (Lanier), which derived revenue largely from the sale and implementation of voice-capture and document management solutions and maintenance service of these products. In conjunction with the Lanier acquisition, we began operating in two segments: a Services segment, through which we provided our customers with medical transcription and coding reimbursement services, and a Solutions segment, which was comprised of the operations of Lanier. Effective January 1, 2005, we changed the way we review our financial performance and thus began operating in one segment for financial reporting purposes.
23
Table of Contents
We have devoted significant resources over the past few years to improving our fundamental business systems, including our corporate governance functions, financial controls, and operational infrastructure. In addition, during this period we also devoted a significant portion of our time and attention to matters outside the ordinary course of business such as cooperating with federal investigations, responding to ongoing legal proceedings and reviewing past allegations of improper billing practices. As our organization was focusing on these issues, we also pursued major operational initiatives to consolidate technology platforms, communicate actively with our customers, and restructure our business.
During this same period there have been several significant developments in the medical transcription industry, including:
| • | A shortage of qualified domestic MTs has increased the demand for outsourced medical transcription services by U.S.-based healthcare providers. This demand for qualified MTs, as well as budgetary pressures experienced by healthcare providers, has also caused many more U.S.-based healthcare providers to evaluate and consider the use of offshore medical transcription labor. | ||
| • | Several low cost providers have emerged and aggressively moved into our market offering medical transcription services (performed both domestically and offshore) at prices significantly below our traditional price point. While we believe the market for outsourced medical transcription continues to expand, the growing acceptance by customers of the use of offshore labor has further increased the competitive environment in the medical transcription industry. | ||
| • | Technological advances by us and our competitors can significantly reduce the length of time required to transcribe medical reports, in turn reducing the overall cost of medical transcription services. |
Although we remain the leading provider of medical transcription services in the U.S., we experience competition from many local, regional and national businesses. The medical transcription industry is highly fragmented, and we believe there are hundreds of companies in the U.S. performing medical transcription services. There are currently two large service providers, one of which is us and the other of which is Spheris Inc., several mid-sized service providers with annual revenues of between $15 million and $100 million and hundreds of smaller, independent businesses with annual revenues of less than $15 million.
We believe the outsourced portion of the medical transcription services market will increase due in part to healthcare providers seeking the following:
| • | reduction in overhead and other administrative costs; | ||
| • | improvement in the quality and speed of delivery of transcribed medical reports; | ||
| • | access to leading technologies, such as speech recognition technology, without any development and investment risk; | ||
| • | expertise in implementing and managing a medical transcription system tailored to the providers’ specific requirements; | ||
| • | access to skilled MTs; and | ||
| • | support for compliance with governmental and industry mandated privacy and security requirements and EHR initiatives. |
Although we believe the outsourced portion of the medical transcription services market continues to grow, in order to benefit from this trend we must overcome the following challenges: reverse recent market share decline, increase profit margins and continue to benefit from technological advances.
We evaluate our performance based upon the following factors:
| • | revenues; | ||
| • | operating income; | ||
| • | net income per share; | ||
| • | net cash provided by operating activities; and | ||
| • | days sales outstanding. |
24
Table of Contents
Our goal is to execute our strategy to yield growth in net revenues, operating income and net income per share.
Critical Accounting Policies, Judgments and Estimates
Management’s Discussion and Analysis (MD&A) is based in part upon our consolidated financial statements which have been prepared in accordance with generally accepted accounting principles in the U.S. (GAAP). We believe there are several accounting policies that are critical to understanding our historical and future performance as these policies affect the reported amounts of revenues and other significant areas that involve management’s judgments and estimates. These critical accounting policies and estimates have been discussed with our audit committee.
The preparation of our consolidated financial statements requires us to make estimates and judgments that affect our reported amounts of assets, liabilities, expenses and related disclosure of contingent liabilities. On an ongoing basis, we evaluate these estimates and judgments. We base these estimates on historical experience and on various other assumptions that are believed to be reasonable at such time, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may ultimately differ from these estimates. A critical accounting estimate meets two criteria: (1) it requires assumptions about highly uncertain matters, and (2) there would be a material effect on the financial statements from either using a different, although reasonable, amount within the range of the estimate in the current period or from reasonably likely period-to-period changes in the estimate. While there are a number of accounting policies, methods and estimates affecting our consolidated financial statements as addressed in Note 2 to our consolidated financial statements, areas that are particularly significant and critical include:
Valuation of Long-Lived and Other Intangible Assets and Goodwill.In connection with acquisitions, we allocate portions of the purchase price to tangible and intangible assets, consisting of acquired technologies, customer relationships, tradenames and non-compete agreements based on independent appraisals received after each acquisition, with the remainder allocated to goodwill. We assess the realizability of goodwill and intangible assets with indefinite useful lives at least annually, or sooner if events or changes in circumstances indicate that the carrying amount may not be recoverable. After our annual impairment test was completed in the second quarter of 2005, we changed our annual goodwill impairment test date to the fourth quarter of each fiscal year. This change in the testing date for impairment was designed to align the testing with our normal business process for updating our strategic plan and forecasts which are finalized in the fourth quarter of each fiscal year. We have determined that the reporting unit level is our sole operating segment. The test for goodwill is a two-step process:
| • | First, we compare the carrying amount of our reporting unit, which is the book value of our entire company, to the fair value of our reporting unit. If the carrying amount of our reporting unit exceeds its fair value, we have to perform the second step of the process. If not, no further testing is needed. | ||
| • | If the second part of the analysis is required, we allocate the fair value of our reporting unit to all assets and liabilities as if the reporting unit had been acquired in a business combination at the date of the impairment test. We then compare the implied fair value of our reporting unit’s goodwill to its carrying amount. If the carrying amount of our goodwill exceeds its fair value, we recognize an impairment loss in an amount equal to that excess. |
We review our long-lived assets, including amortizable intangibles, for impairment when events indicate that their carrying amount may not be recoverable. When we determine that one or more impairment indicators are present for an asset, we compare the carrying amount of the asset to net future undiscounted cash flows that the asset is expected to generate. If the carrying amount of the asset is greater than the net future undiscounted cash flows that the asset is expected to generate, we compare the fair value to the book value of the asset. If the fair value is less than the book value, we recognize an impairment loss. The impairment loss is the excess of the carrying amount of the asset over its fair value.
Some of the events that we consider as impairment indicators for our long-lived assets, including goodwill, are:
| • | our net book value compared to our market capitalization; | ||
| • | significant adverse economic and industry trends; | ||
| • | significant decrease in the market value of the asset; | ||
| • | the extent that we use an asset or changes in the manner that we use it; |
25
Table of Contents
| • | significant changes to the asset since we acquired it; and | ||
| • | other changes in circumstances that potentially indicate all or a portion of our company will be sold. |
Deferred income taxes.As of December 31, 2007, we had net deferred tax assets of $57.6 million prior to consideration of a valuation allowance. These deferred tax assets result primarily from expenses that have been recorded for book purposes but not yet recorded on tax returns and from net operating loss carry forwards. Deferred tax assets represent future tax benefits that we expect to be able to apply against future taxable income or that will result in future net operating losses that can be carried forward. Our ability to utilize the deferred tax assets is dependent upon our ability to generate future taxable income. To the extent that we believe it is more likely than not that all or a portion of the deferred tax asset will not be utilized, we record a valuation allowance against that asset. In making that determination we consider all positive and negative evidence and give stronger consideration to evidence that is objective in nature. Based on this analysis we determined that a valuation allowance would be provided against a portion of our deferred income tax assets in 2006 and 2007. No valuation allowance was established against deferred tax assets to the extent the asset could be benefited through the use of a net operating loss carry back or to the extent we have deferred tax liabilities as of the balance sheet date that will generate taxable income within the same period in which a deferred tax asset will reverse.
We will continue to evaluate the realizability of our deferred income tax assets in future periods and adjust the valuation allowance accordingly.
Commitments and contingencies.Other than an accrual of $7.5 million for the matter referenced under the caption “Customer Litigation” contained in Item 3, Legal Proceedings, as of December 31, 2007, we have not accrued for potential future settlements or adverse outcomes for the other items contained in Item 3, Legal Proceedings, since no matters were probable.
Revenue recognition.For the year ended December 31, 2007, approximately 83.3% of our net revenues were derived from our medical transcription technology and services. Medical transcription services revenues are recognized when there is persuasive evidence that an arrangement exists, the price is fixed or determinable, services are rendered and collectability is reasonably assured. These services are based on contracted rates. Medical transcription services revenues are net of estimates for customer credits. Historically, our estimates have been adequate. If actual results are higher or lower than our estimates, we would have to adjust our estimates and financial statements in future periods.
The remainder of our revenues is derived from the sale and implementation of voice-capture and document management products including software and implementation, training and maintenance services of these products. The application of the accounting guidelines requires judgment regarding the timing of the recognition of these revenues including: (i) whether a software arrangement includes multiple elements, and if so, whether vendor-specific objective evidence of fair value exists for those elements; (ii) whether customizations or modifications of the software are significant; and (iii) whether collection of the software fee is probable. Additionally, for certain contracts we recognize revenues using the percentage-of-completion method. Percentage-of-completion accounting involves estimates of the total costs to be incurred over the duration of the project.
Accounts receivable and allowance for doubtful accounts.Accounts receivable are recorded at the invoiced amount and do not bear interest. The carrying value of accounts receivable approximates fair value. The allowance for doubtful accounts is our best estimate of estimated losses resulting from the inability of our customers to make required payments and for service level credits offered to our customers. This allowance is used to state trade receivables at estimated net realizable value.
We estimate uncollectible amounts based upon our historical write-off experience, current customer receivable balances, aging of customer receivable balances, the customer’s financial condition and current economic conditions. Historically, these estimates have been adequate to cover our accounts receivable exposure.
We enter into medical transcription service arrangements which contain provisions for performance penalties in the event certain service levels, primarily related to turnaround time on transcribed reports, are not achieved. We reduce revenues for any performance penalties incurred and have included an estimate of such credits in our allowance for uncollectible accounts.
Product revenues for sales to end-user customers and resellers are recognized upon passage of title if all other revenue recognition criteria have been met. End-user customers generally do not have a right of return. We provide certain of our resellers and distributors with limited rights of return of our products. We reduce revenues for rights to return our product based upon our historical experience and have included an estimate of such credits in our allowance for uncollectible accounts.
26
Table of Contents
Accounting for consideration given to a customer.In response to customers’ concerns regarding historical billing matters, we offered financial accommodations to certain of our customers. Consideration given by a vendor to a customer is presumed to be a reduction of the selling price of the vendor’s services. Therefore, $10.4 million and $57.7 million of the authorized accommodation amount for our customers was characterized as a reduction of revenues in 2006 and 2005, respectively.
Basis of Presentation
Sources of Revenues
We derive revenues primarily from the provision of medical transcription services to health systems, hospitals and large group medical practices. Our customers are generally charged a rate times the volume of work that we transcribe or edit. In the clinical documentation workflow, we provide, in addition to medical transcription technology and services, maintenance services, digital dictation, speech recognition and electronic signature services. Our medical transcription revenues (excluding the impact of our customer accommodation program) have been declining over the past several years, as prices have declined and some customers have switched to alternative vendors. Our technology products and services revenues also declined over the past several years, as many products reached the end of their life and revenues from new products have not replaced the lost revenues.
As a result of our individual accommodation offers made to certain of our customers, net revenues for the years ended December 31, 2006 and 2005 were reduced by $10.4 million and $57.7 million, respectively.
Net revenues from customers in the U.S. were $335.1 million, $353.5 million and $348.4 million for the years ended December 31, 2007, 2006 and 2005, respectively. Net revenues from customers outside the U.S. were $5.2 million, $4.6 million and $4.6 million for the years ended December 31, 2007, 2006 and 2005, respectively.
Cost of Revenues
Cost of revenues includes compensation of MTs, other payroll costs (primarily related to operational and production management, quality assurance, quality control and customer and field service personnel), telecommunication and facility costs. Cost of revenues also includes the direct cost of technology products sold to customers. MT payroll cost is directly related to medical transcription revenues and is based on lines transcribed or edited multiplied by a specific rate. Therefore, MT costs trend directly in line with revenues. Fixed costs have been reduced though not at the same pace as net revenues.
Selling, General and Administrative (SG&A)
Our SG&A expenses include marketing and sales costs, accounting costs, information technology costs, professional fees, corporate facility costs, corporate payroll and benefits expenses.
Research and Development (R&D)
Our R&D expenses consist primarily of personnel and related costs, including salaries and employee benefits for software engineers and consulting fees paid to independent consultants who provide software engineering services to us. To date, our R&D efforts have been devoted to new products and services offerings and increases in features and functionality of our existing products and services.
Depreciation and amortization
Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets which range from two to seven years for furniture, equipment and software, and the lesser of the lease term or estimated useful life for leasehold improvements. Intangible assets are being amortized using the straight-line method over their estimated useful lives which range from three to 20 years.
Cost of investigation and legal proceedings, net
Cost of investigation and legal proceedings include legal fees incurred in connection with the SEC and DOJ investigations and proceedings and the defense of civil litigation matters described in Item 3, Legal Proceedings, litigation support consulting, and consulting services provided by Nightingale and Associates, LLC (Nightingale), net of insurance claims reimbursement. Howard Hoffmann serves as our non-employee President and Chief Executive Officer pursuant to the terms of an agreement between us and Nightingale. Our agreement with Nightingale also permits us to engage additional personnel employed by Nightingale to provide consulting services to us from time to time.
Shareholder Securities Litigation Settlement
Shareholder Securities Litigation Settlement represents the $7.75 million payment made pursuant to the memorandum of understanding and stipulation of settlement described under the caption “Shareholder Securities Litigation” in Item 3, Legal Proceedings.
27
Table of Contents
Consolidated Results of Operations
The following tables set forth our consolidated results of operations for the periods indicated below:
Comparison of Years Ended December 31, 2007 and 2006
| Years Ended December 31, | ||||||||||||||||||||||||
| 2007 | 2006 | |||||||||||||||||||||||
| % of Net | % of Net | |||||||||||||||||||||||
| ($ in thousands) | Amount | Revenues | Amount | Revenues | $ Change | % Change | ||||||||||||||||||
| Net revenues | $ | 340,342 | 100.0 | % | $ | 358,091 | 100.0 | % | $ | (17,749 | ) | (5.0 | %) | |||||||||||
| Operating costs and expenses: | ||||||||||||||||||||||||
| Cost of revenues | 260,879 | 76.7 | % | 280,273 | 78.3 | % | (19,394 | ) | (6.9 | %) | ||||||||||||||
| Selling, general and administrative | 62,288 | 18.3 | % | 53,675 | 15.0 | % | 8,613 | 16.0 | % | |||||||||||||||
| Research and development | 13,695 | 4.0 | % | 13,219 | 3.7 | % | 476 | 3.6 | % | |||||||||||||||
| Depreciation | 10,988 | 3.2 | % | 11,802 | 3.3 | % | (814 | ) | (6.9 | %) | ||||||||||||||
| Amortization of intangible assets | 5,511 | 1.6 | % | 5,829 | 1.6 | % | (318 | ) | (5.5 | %) | ||||||||||||||
| Cost of investigation and legal proceedings | 6,083 | 1.8 | % | 13,001 | 3.6 | % | (6,918 | ) | (53.2 | %) | ||||||||||||||
| Restructuring charges | 2,756 | 0.8 | % | 3,442 | 1.0 | % | (686 | ) | (19.9 | %) | ||||||||||||||
| Total operating costs and expenses | 362,200 | 106.4 | % | 381,241 | 106.5 | % | (19,041 | ) | (5.0 | %) | ||||||||||||||
| Operating loss | (21,858 | ) | (6.4 | %) | (23,150 | ) | (6.5 | %) | 1,292 | (5.6 | %) | |||||||||||||
| Equity in income of affiliated company | 625 | 0.2 | % | 874 | 0.2 | % | (249 | ) | (28.5 | %) | ||||||||||||||
| Interest income, net | 8,366 | 2.5 | % | 7,628 | 2.1 | % | 738 | 9.7 | % | |||||||||||||||
| Loss before income taxes | (12,867 | ) | (3.8 | %) | (14,648 | ) | (4.1 | %) | 1,781 | (12.2 | %) | |||||||||||||
| Income tax provision | 2,339 | 0.7 | % | 2,294 | 0.6 | % | 45 | 2.0 | % | |||||||||||||||
| Net loss | $ | (15,206 | ) | (4.5 | %) | $ | (16,942 | ) | (4.7 | %) | $ | 1,736 | (10.2 | %) | ||||||||||
Net revenues
Net revenues decreased $17.7 million, or 5.0%, to $340.3 million for the year ended December 31, 2007 compared with $358.1 million for the year ended December 31, 2006. Excluding a charge of $10.4 million in 2006 related to our customer accommodation program, revenues declined $28.1 million. This decrease was attributable primarily to:
| • | a decline in service revenues of $25.3 million resulting primarily from lower medical transcription volume and lower pricing to both new and existing customers. We believe the reduction in volume was the result primarily of customer losses to other outsourced medical transcription providers due to, among other things, price competition and our requirement that our medical transcription customers migrate from disparate and older technology platforms to our DEP; and | ||
| • | reduced sales and implementations of our technology products of $2.4 million as technology products sold in 2007 had longer implementation and customer acceptance periods. |
We continue to experience pricing pressures as our existing and prospective customers seek out opportunities to reduce costs, particularly through the utilization of offshore labor.
Cost of revenues
Cost of revenues decreased $19.4 million, or 6.9%, to $260.9 million for the year ended December 31, 2007 compared with $280.3 million for the year ended December 31, 2006. This decrease was attributable primarily to:
| • | reduced medical transcription payroll costs of $8.3 million related directly to the decrease in our service revenues as well as our increased use of speech recognition technology, which reduces the payroll costs associated with the production of revenues; |
28
Table of Contents
| • | decreased telecommunications costs of $3.8 million associated with both the decrease in our service revenues and the transition of customers from our non-DEP medical transcription platforms, which required MTs to access dictation using traditional phone lines, to our DEP, which allows MTs to access dictation through the internet; and | ||
| • | reduced other costs of $7.3 million resulting from headcount and facility reductions associated with restructuring actions taken to better align our overhead costs with our lower revenue levels. |
As a percentage of net revenues, cost of revenues decreased to 76.7% for the year ended December 31, 2007 from 78.3% for the same period in 2006, as a result largely of actions taken to reduce fixed costs at a faster pace than the reduction of our net revenues.
Selling, general and administrative
SG&A expenses increased $8.6 million, or 16.0%, to $62.3 million for the year ended December 31, 2007 compared with $53.7 million for the year ended December 31, 2006. This increase was due primarily to $4.3 million in higher legal fees for matters unrelated to the cost of investigation and legal proceedings; the reassignment of Nightingale services in 2007 to focus on operational matters of $2.9 million; professional fees incurred related to the evaluation of strategic alternatives of $1.9 million; and an increase in audit fees of $0.6 million related to the audit of our consolidated financial statements and the audit of our internal control over financial reporting. These increases were offset by decreases in all other SG&A expenses of $1.1 million. SG&A expenses as a percentage of net revenues were 18.3% for the year ended December 31, 2007 compared with 15.0% for the same period in 2006.
Research and development
R&D expenses increased $0.5 million, or 3.6%, to $13.7 million for the year ended December 31, 2007 compared with $13.2 million for the year ended December 31, 2006. This increase was due primarily to an increase in fees paid to Philips for enhancements related to our Speech Recognition software. R&D expenses as a percentage of net revenues were 4.0% for the year ended December 31, 2007 compared with 3.7% for the same period in 2006.
Depreciation
Depreciation expense decreased $0.8 million, or 6.9%, to $11.0 million for the year ended December 31, 2007 compared with $11.8 million for the year ended December 31, 2006. This decrease was attributable primarily to fixed assets reaching the end of their depreciable period. Depreciation expense as a percentage of net revenues was 3.2% for the year ended December 31, 2007 compared with 3.3% for the same period in 2006.
Amortization
Amortization of intangible assets decreased $0.3 million, or 5.5%, to $5.5 million for the year ended December 31, 2007 compared with $5.8 million for the year ended December 31, 2006. Amortization of intangible assets as a percentage of net revenues was 1.6% for both periods.
Cost of investigation and legal proceedings, net
Cost of investigation and legal proceedings, net decreased $6.9 million to $6.1 million for the year ended December 31, 2007 compared with $13.0 million for the year ended December 31, 2006. This decrease was due primarily to the realization of $15.4 million of insurance claim reimbursements in 2007 compared with $9.4 million for the year ended December 31, 2006. In addition, for the year ended December 31, 2007, $2.9 million of costs were charged to SG&A related to Nightingale’s services due to Nightingale’s focus on operational matters.
Restructuring charges
During 2007, we recorded a restructuring charge of $2.8 million comprised of $2.4 million for severance obligations and $0.3 million for non-cancelable leases related to the closure of offices. During 2006, we recorded a restructuring charge of $3.4 million comprised of $1.7 million for non-cancelable leases related to the closure of offices, $0.3 million for the write-off of property and equipment and $1.4 million for severance obligations.
29
Table of Contents
Interest income, net
Interest income, net reflects interest earned on cash and cash equivalent balances. Interest income, net increased $0.7 million, or 9.7%, to $8.4 million for the year ended December 31, 2007 compared with $7.6 million for the year ended December 31, 2006. This increase was attributable to higher interest rates earned in the 2007 period (5.0%) compared with the 2006 period (4.6%).
Income tax provision
The effective income tax rate for the year ended December 31, 2007 was 18.2% compared with an effective income tax rate of 15.7% for the year ended December 31, 2006. The difference in tax rates is related primarily to the decrease in the pre-tax loss from 2006 to 2007. After consideration of all evidence, both positive and negative, management concluded that it was more likely than not that a significant portion of the domestic deferred income tax assets would not be realized. In addition, various adjustments were recorded for the year ended December 31, 2007 including the reduction of the foreign valuation allowance and various adjustments related to state tax exposures.
Comparison of Years Ended December 31, 2006 and 2005
| Years Ended December 31, | ||||||||||||||||||||||||
| 2006 | 2005 | |||||||||||||||||||||||
| % of Net | % of Net | |||||||||||||||||||||||
| Amount | Revenues | Amount | Revenues | $ Change | % Change | |||||||||||||||||||
| ($ in thousands) | ||||||||||||||||||||||||
| Net revenues | $ | 358,091 | 100.0 | % | $ | 353,005 | 100.0 | % | $ | 5,086 | 1.4 | % | ||||||||||||
| Operating costs and expenses: | ||||||||||||||||||||||||
| Cost of revenues | 280,273 | 78.3 | % | 315,399 | 89.3 | % | (35,126 | ) | (11.1 | )% | ||||||||||||||
| Selling, general and administrative | 53,675 | 15.0 | % | 54,558 | 15.5 | % | (883 | ) | (1.6 | )% | ||||||||||||||
| Research and development | 13,219 | 3.7 | % | 9,784 | 2.8 | % | 3,435 | 35.1 | % | |||||||||||||||
| Depreciation | 11,802 | 3.3 | % | 17,099 | 4.8 | % | (5,297 | ) | (31.0 | )% | ||||||||||||||
| Amortization of intangible assets | 5,829 | 1.6 | % | 8,193 | 2.3 | % | (2,364 | ) | (28.9 | )% | ||||||||||||||
| Cost of investigation and legal proceedings | 13,001 | 3.6 | % | 34,127 | 9.7 | % | (21,126 | ) | (61.9 | )% | ||||||||||||||
| Shareholder securities litigation settlement | — | — | 7,750 | 2.2 | % | (7,750 | ) | (100.0 | )% | |||||||||||||||
| Impairment charges | — | — | 148 | 0.0 | % | (148 | ) | (100.0 | )% | |||||||||||||||
| Restructuring charges | 3,442 | 1.0 | % | 3,257 | 0.9 | % | 185 | 5.7 | % | |||||||||||||||
| Total operating costs and expenses | 381,241 | 106.5 | % | 450,315 | 127.6 | % | (69,074 | ) | (15.3 | )% | ||||||||||||||
| Operating loss | (23,150 | ) | (6.5 | )% | (97,310 | ) | (27.6 | )% | 74,160 | (76.2 | )% | |||||||||||||
| Equity in income of affiliated company | 874 | 0.2 | % | 500 | 0.1 | % | 374 | 74.8 | % | |||||||||||||||
| Interest income, net | 7,628 | 2.1 | % | 5,940 | 1.7 | % | 1,688 | 28.4 | % | |||||||||||||||
| Loss before income taxes | (14,648 | ) | (4.1 | )% | (90,870 | ) | (25.7 | )% | 76,222 | (83.9 | )% | |||||||||||||
| Income tax provision | 2,294 | 0.6 | % | 20,762 | 5.9 | % | (18,468 | ) | (88.9 | )% | ||||||||||||||
| Net loss | $ | (16,942 | ) | (4.7 | )% | $ | (111,632 | ) | (31.6 | )% | $ | 94,690 | (84.8 | )% | ||||||||||
Net revenues
Net revenues increased $5.1 million, or 1.4%, to $358.1 million for the year ended December 31, 2006 compared with $353.0 million for the year ended December 31, 2005. Excluding the charges for the customer accommodation program ($10.4 million in 2006 and $57.7 in 2005), service revenues decreased by $30.2 million in 2006 due to lower pricing to both new and existing customers and lower medical transcription volume. We believe the reduction in volume was the result primarily of customer losses to other outsourced medical transcription providers due to, among other things, price competition and our requirement that our medical transcription customers migrate from disparate and older technology platforms to our DEP. In addition, net revenues were also reduced by lower sales and implementations of our technology products of $12.0 million resulting primarily from the impact of certain technology products reaching the end of their life cycle.
Cost of revenues
Cost of revenues decreased $35.1 million, or 11.1%, to $280.3 million for the year ended December 31, 2006 compared with $315.4 million for the year ended December 31, 2005. This decrease was attributable primarily to:
| • | decreased telecommunications costs of $9.4 million associated with both the decrease in our medical transcription volume and the transition of customers from our non-DEP medical transcription platforms, which required MTs to access dictation using |
30
Table of Contents
| traditional phone lines, to our DEP, which allows MTs to access dictation through the internet; | |||
| • | reduced compensation costs of $8.4 million due to the decrease in our medical transcription volume as well as savings from our increased use of speech recognition technology; | ||
| • | reduced other costs of $8.1 million resulting primarily from decreased non-MT headcount; | ||
| • | reduced technology product costs of $3.4 million related directly to the reduction in our technology product revenues; | ||
| • | lower asset impairment costs of $0.2 million in 2006 compared with $3.9 million 2005; | ||
| • | reduced costs of $3.4 million resulting from facility closures; offset by | ||
| • | increased stock-based compensation expense of $1.3 million |
As a percentage of net revenues, cost of revenues decreased to 78.3% in 2006 from 89.3% in 2005 as a result primarily of higher customer accommodation charges in 2005 partially offset by lower medical transcription service rates in 2006 and the impact of fixed costs in 2006 not declining at the same pace as services revenues excluding customer accommodation charges.
Selling, general and administrative
SG&A expenses decreased $0.9 million, or 1.6%, to $53.7 million for the year ended December 31, 2006 compared with $54.6 million for the year ended December 31, 2005. This decline was attributable primarily to a decrease in bonus and commission expense of $3.0 million, $2.3 million associated with the separation and replacement of certain members of our management team in 2005 that did not recur in 2006, as well as reductions in sales and marketing expense of $1.3 million, human resources consultants of $0.9 million and all other miscellaneous SG&A expenses of $0.5 million. These decreases were offset by an increase in audit and outside consulting fees of $4.7 million related to the audit of our financial statements and the audit of our internal control over financial reporting, an increase in legal costs of $1.1 million due to higher than anticipated fees for certain matters, an increase of $0.7 million in insurance premiums as a result of insurance recoveries related to legal expenses and an increase in stock-based compensation expense of $0.6 million. SG&A expenses as a percentage of net revenues for 2006 were 15.0% compared with 15.5% for 2005.
Research & development
R&D expenses increased $3.4 million, or 35.1%, to $13.2 million for the year ended December 31, 2006 compared with $9.8 million for the year ended December 31, 2005. This increase was due primarily to an increase in compensation expense of $1.8 million due to an increase in headcount, higher professional fees for outside consultants of $0.6 million and higher miscellaneous expenses of $1.0 million. R&D expenses as a percentage of net revenues were 3.7% for the year ended December 31, 2006 compared with 2.8% for the year ended December 31, 2005.
Depreciation
Depreciation expense decreased $5.3 million, or 31.0%, to $11.8 million for the year ended December 31, 2006 compared with $17.1 million for the year ended December 31, 2005. This decrease was due primarily to a $4.1 million write-off of assets in the fourth quarter of 2005 and assets reaching the end of their depreciation period in 2006. Depreciation expense as a percentage of net revenues was 3.3% for the year ended December 31, 2006 compared with 4.8% for the year ended December 31, 2005.
Amortization
Amortization of intangible assets decreased $2.4 million, or 28.9%, to $5.8 million for the year ended December 31, 2006 compared with $8.2 million for the year ended December 31, 2005. This decrease was the result primarily of several assets reaching the end of their amortization period. Amortization of intangible assets as a percentage of net revenues was 1.6% for the year ended December 31, 2006 compared with 2.3% for the same period in 2005.
Cost of investigation and legal proceedings, net
These costs and expenses decreased $21.1 million, or 61.9%, to $13.0 million for the year ended December 31, 2006 compared with $34.1 million for the year ended December 31, 2005. This reduction in costs was the result of decreases in legal fees incurred in connection with the SEC and DOJ investigations and proceedings and the defense of civil litigation matters as well as litigation support consulting of $11.4 million, lower consulting services provided by Nightingale of $0.2 million and lower other miscellaneous costs of $0.1 million, as well as an insurance recovery on our legal expenses of $9.4 million.
31
Table of Contents
Shareholder securities litigation settlement
Shareholder securities litigation settlement for the year ended December 31, 2005 represents the $7.75 million payment made pursuant to the memorandum of understanding and stipulation of settlement described under the caption “Shareholder Securities Litigation” in Item 3, Legal Proceedings.
Restructuring charges
During the latter half of 2005, we implemented a restructuring plan based on a centralized national service delivery model to streamline our organizational and operational structure to better service our customers. The 2005 restructuring plan involved the consolidation of operating facilities and a related reduction in workforce. During 2006, we recorded a restructuring charge of $3.4 million comprised of $1.7 million for non-cancelable leases related to the closure of offices, $0.3 million for the write-off of property and equipment and $1.4 million for severance obligations. During 2005, we recorded a restructuring charge of $3.3 million comprised of $2.3 million for non-cancelable leases related to the closure of offices, $0.2 million for the write-off of property and equipment and $0.7 million of severance obligations.
As of December 31, 2006, $0.7 million of restructuring charges remained to be paid in future periods. The payment stream for non-cancelable leases will extend into 2009.
Interest income, net
Interest income, net reflects interest earned on cash and cash equivalent balances. Interest income, net increased $1.7 million, or 28.4%, to $7.6 million for the year ended December 31, 2006 compared with $5.9 million for the year ended December 31, 2005. This increase was attributable primarily to higher weighted average interest rates earned in 2006 (4.6%) compared to 2005 (3.1%), offset by a $28 million lower average cash balance in 2006 compared with 2005.
Income tax provision
The effective income tax rate for the year ended December 31, 2006 was 15.7% compared with an effective income tax rate of 22.8% for the year ended December 31, 2005. The difference in tax rates is related primarily to the decrease in the pre-tax book loss from 2005 to 2006 and the impact of the establishment of a valuation allowance against the deferred income tax assets in 2005. After consideration of all evidence, both positive and negative, management concluded that it was more likely than not that the deferred income tax assets would not be realized. In addition, adjustments were recognized for the year ended December 31, 2006 including the reduction of state tax expense for the expiration of statue of limitations with respect to uncertain tax positions, the recognition of tax benefits for Alternative Minimum Tax credits and adjustments for various exposures related to state taxes.
Liquidity and Capital Resources
As of December 31, 2007, we had net working capital of $133.2 million compared with $145.5 million as of December 31, 2006. Our principal source of liquidity is available cash on hand. Cash and cash equivalents declined $13.8 million to $161.6 million as of December 31, 2007 from $175.4 million as of December 31, 2006. This decline was driven primarily by purchases of property and equipment of $11.6 million, capitalized software of $2.0 million as well as cash used in operating activities of $0.3 million. The $0.3 million net cash used in operating activities reflects a $15.2 million net loss, a payment of $7.8 million related to the settlement of shareholders securities litigation, customer accommodation payments of $3.7 million as well as cash used in other operating activities of $5.8 million. These decreases were offset by depreciation and amortization expense of $16.5 million and insurance recoveries of $16.1 million.
We believe our existing cash and cash equivalents and cash to be generated from operations, if any, will be sufficient to finance our operations for the foreseeable future. However, if we fail to generate adequate cash flows from operations in the future due to an unexpected decline in our net revenues or due to increased cash expenditures in excess of the net revenues generated, then our cash balances may not be sufficient to fund our continuing operations without obtaining additional debt or equity. There are no assurances that sufficient funding from external sources will be available to us on acceptable terms, if at all. For instance, we may have increased cash expenditures relating to:
| • | the SEC, DOJ and DOL investigations and proceedings; and | ||
| • | the defense and resolution of the civil litigation matters. |
32
Table of Contents
Off-Balance Sheet Arrangements
We are not involved in any off-balance sheet arrangements that have or are reasonably likely to have a material current or future impact on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Contractual Obligations
The following table summarizes our obligations to make future payments under current contracts as of December 31, 2007 (in thousands):
| Payment Due By Period | ||||||||||||||||||||
| Less | ||||||||||||||||||||
| than | After | |||||||||||||||||||
| Total | 1 Year | 1-3 Years | 3-5 Years | 5 Years | ||||||||||||||||
| Operating Lease Obligations | $ | 15,732 | $ | 4,650 | $ | 9,440 | $ | 1,642 | $ | — | ||||||||||
| Purchase Obligations(1) | 21,424 | 5,650 | 14,827 | 947 | — | |||||||||||||||
| Severance and Other Guaranteed Payment Obligations(2) | 2,678 | 2,678 | — | — | — | |||||||||||||||
| Total Contractual Obligations | $ | 39,834 | $ | 12,978 | $ | 24,267 | $ | 2,589 | $ | — | ||||||||||
| (1) | Purchase obligations are for telecommunication contracts ($21,174) and other recurring purchase obligations ($250). | |
| (2) | Severance and Other Guaranteed Payment Obligations are comprised of severance payments ($1,493), employee retention payments ($785) and amounts owed to Nightingale ($400). |
We have agreements with certain of our named executive officers that provide for severance payments to the employee in the event the employee is terminated without cause. The maximum cash exposure under these agreements was approximately $1,207 as of December 31, 2007.
Recent Accounting Pronouncements
In September 2006, the Financial Accounting Standards Board (FASB) issued Statement 157,Fair Value Measurements, (Statement 157) which defines fair value, establishes guidelines for measuring fair value and expands disclosures regarding fair value measurements. Statement 157 does not require any new fair value measurements. The provisions of this statement are effective for fiscal years beginning after November 15, 2007. On February 12, 2008, the FASB delayed the effective date of Statement 157 for non-financial assets and liabilities until fiscal years beginning after November 15, 2008. We do not expect the adoption of Statement 157 to have a material impact on our consolidated financial statements.
In February 2007, the FASB issued Statement 159,The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of FASB Statement 115 (Statement 159) which permits entities to choose to measure eligible items at fair value at specified election dates. Unrealized gains and losses on items for which the fair value option has been elected will be reported in earnings at each subsequent reporting date. The following balance sheet items are within the scope of Statement 159:
| • | recognized financial assets and financial liabilities unless a special exception applies; | ||
| • | firm commitments that would otherwise not be recognized at inception and that involve only financial instruments; | ||
| • | non-financial insurance contracts; and | ||
| • | most financial instruments resulting from separation of an embedded non-financial derivative instrument from a non-financial hybrid instrument. |
Statement 159 will be effective for fiscal years beginning after November 2007 with early adoption possible but subject to certain requirements. We do not expect the adoption of Statement 159 to have a material impact on our consolidated financial statements.
In December 2007, the FASB issued Statement 141(R),Business Combinations(Statement 141R). Statement 141R establishes principles and requirements for how the acquirer of a business recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, and any noncontrolling interest in the acquiree. Statement 141R also provides guidance for recognizing and measuring the goodwill acquired in the business combination and determines what information to disclose to enable users of the financial statements to
33
Table of Contents
evaluate the nature and financial effects of the business combination. The guidance will become effective as of the beginning of our fiscal year beginning after December 15, 2008.
In December 2007, the FASB issued Statement No. 160,Noncontrolling Interests in Consolidated Financial Statements-an amendment of ARB No. 51(Statement 160). Statement 160 establishes accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. Statement 160 will become effective as of the beginning of our fiscal year beginning after December 15, 2008. We do not believe that Statement 160 will have a material effect on our consolidated financial statements.
Item 7A.Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily a result of fluctuations in interest rates. We do not hold or issue financial instruments for trading purposes.
Interest Rate Risk
We earn interest income from our balances of cash and cash equivalents. This interest income is subject to market risk related to changes in interest rates which affects primarily our investment portfolio. We invest in instruments that meet high credit quality standards as specified in our investment policy.
Management estimates that if the average yield of our investments decreased by 100 basis points, our interest income for the year ended December 31, 2007 would have decreased by approximately $1.7 million. The impact on our future interest income will depend largely on the gross amount of our investments and future changes in investment yields.
Item 8.Financial Statements And Supplementary Data
Our consolidated financial statements and supplementary data required by this item are attached to this report beginning on page F-1.
Item 9.Changes in And Disagreements With Accountants On Accounting And Financial Disclosure
None.
Item 9A.Controls And Procedures
(a) Evaluation of Disclosure Controls and Procedures
Our management team, under the supervision and with the participation of our principal executive officer and our principal financial officer, evaluated the effectiveness of the design and operation of our disclosure controls and procedures as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended (Exchange Act), as of the last day of the fiscal period covered by this report, December 31, 2007. The term disclosure controls and procedures means our controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to management, including our principal executive and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective as of December 31, 2007.
(b) Management’s Report on Internal Control over Financial Reporting as of December 31, 2007
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and Rule 15d-15(f) under the Exchange Act as a process designed by, or under the supervision of, the issuer’s principal executive and principal financial officers, or persons performing similar functions, and effected by the issuer’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial
34
Table of Contents
reporting and the preparation of financial statements for external purposes in accordance with GAAP and includes those policies and procedures that:
| • | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the issuer; | ||
| • | provide reasonable assurance that transactions are recorded as necessary to permit the preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the issuer are being made only in accordance with authorizations of management and directors of the issuer; and | ||
| • | provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of the issuer’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with existing policies or procedures may deteriorate.
In accordance with the internal control reporting requirements of the SEC, management completed an assessment of the adequacy of our internal control over financial reporting as of December 31, 2007. In making this assessment, management used the criteria set forth inInternal Control — Integrated Frameworkby the Committee of Sponsoring Organizations of the Treadway Commission (COSO). As a result of this assessment and based on the criteria in the COSO framework, management has concluded that, as of December 31, 2007, our internal control over financial reporting was effective. Our independent registered public accounting firm has issued an audit report on the effectiveness of our internal control over financial reporting. This report is included on page F-2 of our consolidated financial statements included as part of this Annual Report on Form 10-K.
(c) Changes in Internal Control Over Financial Reporting
As a result of initiatives that management undertook in 2007 with oversight from the Audit Committee to remediate known material weaknesses in our control over financial reporting as of December 31, 2006, we made the following material changes in our internal control over financial reporting throughout and during the year ended December 31, 2007.
| • | During the fourth quarter of fiscal 2007, we retained third party consultants to serve as technical resources with respect to accounting for certain selected accounting transactions; | ||
| • | During the third quarter of fiscal 2007, we expanded our disclosure committee to include our Chief Executive Officer, our outside litigation counsel and a representative of Nightingale who is assisting us with various projects at the direction of our Chief Executive Officer. During the fourth quarter of fiscal 2007, this process was further enhanced by extending internal control and disclosure certificates to a group of functional managers; | ||
| • | During the third quarter of fiscal 2007, we enhanced quarter-end procedures to timely identify and record outstanding liabilities; | ||
| • | During the first quarter of fiscal 2007, we established a formal accounting methodology and implemented formal, monthly review procedures performed by corporate accounting personnel prior to the recording of any post-contract customer support (PCS), hardware, software and implementation service revenues; and | ||
| • | During the first quarter of fiscal 2007, we centralized accounting related to PCS, implementation services and deferred revenue to our corporate headquarters. |
Item 9B.Other Information
None.
PART III
Item 10.Directors, Executive Officers and Corporate Governance
The information called for by this item is incorporated by reference to the portions of our definitive proxy statement entitled, “Election of Directors,” “Governance of the Company,” and “Section 16 (a) Beneficial Ownership Reporting Compliance.”
35
Table of Contents
Item 11.Executive Compensation
The information called for by this item is incorporated by reference to the portions of our definitive proxy statement entitled “Compensation Discussion and Analysis,” “Report of the Compensation Committee,” and “Compensation of our Named Executive Officers.”
Item 12.Security Ownership of Certain Beneficial Owners and Management
The information called for by this item is incorporated by reference to the portions of our definitive proxy statement entitled “Stock Ownership of Our Directors, Executive Officers and 5% Beneficial Owners,” “Compensation of Directors,” “Securities Authorized For Issuance Under Equity Compensation Plans” and “Compensation of our Named Executive Officers.”
Item 13.Certain Relationships and Related Transactions, and Director Independence
The information called for by this item is incorporated by reference to the portion of our definitive proxy statement entitled “Certain Relationships and Related Transactions.”
Item 14.Principal Accountant Fees and Services
The information called for by this item is incorporated by reference to the portion of our definitive proxy statement entitled “Fees Paid to Independent Registered Public Accounting Firm.”
PART IV
Item 15.Exhibits and Financial Statement Schedules
(a) Documents filed as part of this report:
(1) Financial Statements.The consolidated financial statements filed as part of this report are listed on the Index to Consolidated Financial Statements on page F-1.
(2) Financial Statement Schedules. Schedule II - Valuation and Qualifying Accounts filed as part of this report is listed on page F-35. All other financial statement schedules have been omitted here because they are not applicable, not required, or the information is shown in the consolidated financial statements or notes thereto.
(3) Exhibits.See (b) below.
(b) Exhibits:
| No. | Description | |
| 3.1(1) | Certificate of Incorporation of MedQuist Inc. (as amended) | |
| 3.2(1) | By-Laws of MedQuist Inc. (as amended) | |
| 4.1(1) | Specimen Stock Certificate | |
| 10.1*(1) | The MRC Group, Inc. Amended and Restated 1992 Stock Option Plan | |
| 10.2*(1) | 1992 Stock Option Plan of MedQuist Inc., as amended | |
| 10.3*(1) | Nonstatutory Stock Option Plan for Non-Employee Directors of MedQuist Inc. | |
| 10.4*(1) | MedQuist Inc. 2002 Stock Option Plan | |
| 10.5*(1) | Form of Award Agreement under the MedQuist Inc. 2002 Stock Option Plan | |
| 10.6*(1) | 1996 Employee Stock Purchase Plan | |
| 10.7*(1) | MedQuist Inc. Executive Deferred Compensation Plan | |
| 10.8*(1) | Separation Agreement and General Release dated June 28, 2007 by and between MedQuist Inc. and Frank Lavelle | |
| 10.9*(1) | Separation Agreement and General Release dated June 28, 2007 by and between MedQuist Inc. and Linda Reino |
36
Table of Contents
| No. | Description | |
| 10.10*(1) | Relocation Letter Agreement, dated as of April 26, 2006, between MedQuist Inc. and Adele T. Barbato | |
| 10.11*(1) | Letter Agreement, dated as of April 21, 2005, between MedQuist Inc. and Michael Clark | |
| 10.12*(1) | Letter Agreement, dated as of April 21, 2005, between MedQuist Inc. and Mark Sullivan | |
| 10.13*(1) | Employment Agreement, dated as of May 27, 2005, between MedQuist Inc. and Mark Ivie | |
| 10.14*(1) | Employment Agreement, dated as of June 2, 2005, between MedQuist Inc. and Kathleen Donovan | |
| 10.15*(1) | Employment Agreement, dated as of October 26, 2005, between MedQuist Inc. and R. Scott Bennett | |
| 10.16*(1) | Employment Agreement, dated as of August 10, 2006, between MedQuist Inc. and Linda Reino | |
| 10.17*(1) | Letter Agreement, dated as of November 10, 2006, by and between MedQuist Inc. and James Brennan | |
| 10.18*(4) | Retention and Strategic Transaction Bonus Agreements, dated September 20, 2007, with each of Kathleen Donovan, Mark Ivie and Scott Bennett | |
| 10.19*(4) | Retention and Strategic Transaction Bonus Agreements, dated September 20, 2007, with each of Mark Sullivan and Michael Clark | |
| 10.20*(1) | Engagement Letter, dated as of July 30, 2004, between MedQuist Inc. and Nightingale & Associates, LLC | |
| 10.20.1*(1) | Amendment to Engagement Letter, dated as of January 7, 2005, between MedQuist Inc. and Nightingale & Associates, LLC | |
| 10.20.2*(1) | Amendment to Engagement Letter, dated as of September 25, 2006 between MedQuist Inc. and Nightingale & Associates, LLC | |
| 10.20.3*(1) | Amendment to Engagement Letter, dated as of January 8, 2007, between MedQuist Inc. and Nightingale & Associates, LLC | |
| 10.20.4*(4) | Letter Agreement by and between MedQuist Inc. and Nightingale & Associates, LLC dated September 19, 2007 | |
| 10.20.5* | Letter Agreement by and between MedQuist Inc. and Nightingale & Associates, LLC dated March 14, 2008 | |
| 10.21(1) | Governance Agreement, dated as of May 22, 2000, between MedQuist Inc. and Koninklijke Philips Electronics N.V. | |
| 10.21.1(5) | Amendment to the Governance Agreement by and between MedQuist Inc. and Koninklijke Philips Electronics N.V. dated November 8, 2007 | |
| 10.21.2(9) | Amendment to the Governance Agreement by and between MedQuist Inc. and Koninklijke Philips Electronics N.V. dated March 12, 2008 | |
| 10.22(1) | Licensing Agreement, dated as of May 22, 2000, between MedQuist Inc. and Philips Speech Processing GmbH | |
| 10.22.1(1) | Amendment No. 1 to Licensing Agreement, dated as of January 1, 2002, between MedQuist Inc. and Philips Speech Processing GmbH | |
| 10.22.2#(1) | Amendment No. 2 to Licensing Agreement, dated as of December 10, 2002, between MedQuist Inc. and Philips Speech Processing GmbH | |
| 10.22.3#(1) | Amendment No. 3 to Licensing Agreement, dated as of August 10, 2003, between MedQuist Inc. and Philips Speech Processing GmbH | |
| 10.22.4#(1) | Amendment No. 4 to Licensing Agreement, dated as of September 1, 2004, between MedQuist Inc. and Philips Speech Processing GmbH | |
| 10.22.5#(1) | Amendment No. 5 to Licensing Agreement, dated as of December 30, 2005, between MedQuist Transcriptions, Ltd. and Philips Speech Recognition Systems GmbH f/k/a Philips Speech Processing GmbH | |
| 10.22.6#(1) | Amendment No. 6 to Licensing Agreement, dated as of February 13, 2007, between MedQuist Inc. and Philips Speech Recognition Systems GmbH f/k/a Philips Speech Processing GmbH | |
| 10.23#(1) | Amended and Restated OEM Supply Agreement dated September 21, 2007, between MedQuist Inc. and Philips Speech Recognition Systems GmbH f/k/a Philips Speech Processing GmbH | |
| 10.24(1) | Norcross, Georgia Office Lease Agreement dated as of September 6, 2002 | |
| 10.25(1) | Mt. Laurel, New Jersey Office Lease Agreement dated as of June 17, 2003 | |
| 10.26(1) | First Amendment to Mt. Laurel, New Jersey Office Lease Agreement dated as of August 26, 2003 | |
| 10.27(1) | Second Amendment to Mt. Laurel, New Jersey Office Lease Agreement dated as of November 30, 2003 |
37
Table of Contents
| No. | Description | |
| 10.28(1) | Third Amendment to Mt. Laurel, New Jersey Office Lease Agreement dated as of November 30, 2003 | |
| 10.29(1) | Confirmation of Lease Term regarding Mt. Laurel, New Jersey Office Lease dated as of August 10, 2006 | |
| 10.30(1) | Marietta, Georgia Office Lease Agreement dated as of September 6, 2002 | |
| 10.31(1) | First Amendment to Marietta, Georgia Office Lease Agreement dated March 24, 2006 | |
| 10.31.1(1) | Memorandum of Understanding dated March 23, 2007 by and among (i) MedQuist, Inc., Brian J. Kearns, David A. Cohen, John A. Donohoe, Ethan Cohen, John W. Quaintance, and Ronald F. Scarpone, and (ii) Greater Pennsylvania Carpenters Pension Fund | |
| 10.31.2(2) | Stipulation of Settlement dated March 23, 2007 by and among (i) MedQuist, Inc., Brian J. Kearns, David A. Cohen, John A. Donohoe, Ethan Cohen, John W. Quaintance, and Ronald F. Scarpone, and (ii) Greater Pennsylvania Carpenters Pension Fund | |
| 10.32*(1) | MedQuist Inc. Board of Directors Deferred Compensation Plan | |
| 10.33*(1) | Indemnification Agreement, dated as of July 15, 2005, between MedQuist Inc. and John Simmons | |
| 10.34*(1) | Indemnification Agreement, dated as of July 3, 2007 between MedQuist Inc. and John H. Underwood | |
| 10.35*(1) | Indemnification Agreement, dated as of July 3, 2007 between MedQuist Inc. and Richard H. Stowe | |
| 10.36*(3) | Form of Management Indemnification Agreement by and between MedQuist Inc. and Certain Officers | |
| 10.37*(7) | Indemnification Agreement, dated as of December 11, 2007 between MedQuist Inc. and Mark Schwarz | |
| 10.38*(7) | Indemnification Agreement, dated as of December 7, 2007 between MedQuist Inc. and Brian O’Donoghue | |
| 10.39*(8) | Indemnification Agreement, dated as of February 21, 2008 between MedQuist Inc. and Warren Pinckert | |
| 10.40(9) | Settlement Term Sheet dated March 10, 2008 by and among (i) MedQuist Inc. and (ii) Partners Healthcare System, Northbay Healthcare Group, Hospital Corporation of America, St. Lukes Regional Medical Center, Palisades Medical Center, Mt. Sinai Medical Center, Ascension Health Ministry, Bayonne Medical Center, Bon Secours Health System, Inc., South Broward Memorial Hospital District and University of Colorado, and all related or associated facilities | |
| 21(1) | Subsidiaries of MedQuist Inc. | |
| 23 | Consent of KPMG LLP | |
| 24 | Power of Attorney (included on the signature page hereto) | |
| 31.1 | Certification of Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2 | Certification of Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2 | Certification of Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| * | Management contract or compensatory plan or arrangement. | |
| # | Portions of this Exhibit were omitted and filed separately with the Secretary of the SEC pursuant to a request for confidential treatment that has been filed with the SEC. | |
| (1) | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2005 filed on July 5, 2007 | |
| (2) | Incorporated by reference to our Current Report on Form 8-K/A filed on August 24, 2007 | |
| (3) | Incorporated by reference to our Current Report on Form 8-K filed on August 28, 2007 | |
| (4) | Incorporated by reference to our Current Report on Form 8-K filed on September 25, 2007 | |
| (5) | Incorporated by reference to our Current Report on Form 8-K filed on November 19, 2007 |
38
Table of Contents
| (6) | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2007 filed on November 11, 2007 | |
| (7) | Incorporated by reference to our Current Report on Form 8-K filed on December 11, 2007 | |
| (8) | Incorporated by reference to our Current Report on Form 8-K filed on February 22, 2008 | |
| (9) | Incorporated by reference to our Current Report on Form 8-K filed on March 14, 2008 |
(c) Financial Statement Schedules.
None.
39
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Medquist Inc. | ||||||
| By: | /s/Howard S. Hoffmann | |||||
| President and Chief Executive Officer | ||||||
| Date: March 17, 2008 | ||||||
Pursuant to the requirements of the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Each person, in so signing also makes, constitutes, and appoints Howard S. Hoffmann and Kathleen E. Donovan, and each of them acting alone, as his or her true and lawful attorneys-in-fact, with full power of substitution, in his or her name, place, and stead, to execute and cause to be filed with the SEC any or all amendments to this report.
| Signature | Capacity | Date | ||
/s/Howard S. Hoffmann | President and Chief Executive Officer (Principal Executive Officer) | March 17, 2008 | ||
/s/Kathleen E. Donovan | Senior Vice President and Chief Financial Officer (Principal Financial Officer) | March 17, 2008 | ||
/s/James Brennan | Principal Accounting Officer, Controller and Vice President | March 17 , 2008 | ||
/s/ Brian O’Donoghue | Director | March 17, 2008 | ||
/s/ Warren e. pinckert, ii | Director | March 17, 2008 | ||
/s/Stephen H. Rusckowski | Non-Executive Chairman of the Board of Directors | March 17, 2008 | ||
/s/Clement Revetti, Jr | Director | March 17, 2008 | ||
/s/ Mark E. Schwarz | Director | March 17, 2008 | ||
/s/Gregory M. Sebasky | Director | March 17, 2008 | ||
/s/Scott M. Weisenhoff | Director | March 17, 2008 |
40
Table of Contents
Index to Consolidated Financial Statements
| F-2 | ||
| F-3 | ||
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-7 | ||
| F-8 | ||
| F-34 |
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
MedQuist Inc.:
MedQuist Inc.:
We have audited MedQuist Inc.’s internal control over financial reporting as of December 31, 2007 based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). MedQuist Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting as of December 31, 2007 (Item 9A(b)). Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, MedQuist Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of MedQuist Inc. as of December 31, 2007 and 2006 and the related consolidated statements of operations, shareholders’ equity and other comprehensive income, and cash flows for each of the years in the three-year period ended December 31, 2007, and our report dated March 17, 2008 expressed an unqualified opinion on those consolidated financial statements.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 17, 2008
March 17, 2008
F-2
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of MedQuist Inc.:
We have audited the accompanying consolidated balance sheets of MedQuist Inc. and subsidiaries as of December 31, 2007 and 2006, and the related consolidated statements of operations, shareholders’ equity and other comprehensive income, and cash flows for each of the years in the three-year period ended December 31, 2007. In connection with our audits of the consolidated financial statements, we also have audited financial statement Schedule II - - Valuation and Qualifying Accounts. These consolidated financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of MedQuist Inc. and subsidiaries as of December 31, 2007 and 2006, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2007, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Notes 3 and 14 to the consolidated financial statements, effective January 1, 2006, MedQuist Inc. and subsidiaries adopted the fair value method of accounting for stock-based compensation as required by Statement of Financial Accounting Standards No. 123(R),Share-Based Payment.
As discussed in Note 15 to the consolidated financial statements, effective January 1, 2007, MedQuist Inc. and subsidiaries adopted Financial Accounting Standards Board Interpretation No. 48,Accounting for Uncertainty in Income Taxes – an Interpretation of SFAS No. 109.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), MedQuist Inc.’s internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 17, 2008 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 17, 2008
March 17, 2008
F-3
Table of Contents
MedQuist Inc. and Subsidiaries
Consolidated Statements of Operations
(In thousands, except per share amounts)
| Years Ended December 31, | ||||||||||||
| 2007 | 2006 | 2005 | ||||||||||
| Net revenues | $ | 340,342 | $ | 358,091 | $ | 353,005 | ||||||
| Operating costs and expenses: | ||||||||||||
| Cost of revenues | 260,879 | 280,273 | 315,399 | |||||||||
| Selling, general and administrative | 62,288 | 53,675 | 54,558 | |||||||||
| Research and development | 13,695 | 13,219 | 9,784 | |||||||||
| Depreciation | 10,988 | 11,802 | 17,099 | |||||||||
| Amortization of intangible assets | 5,511 | 5,829 | 8,193 | |||||||||
| Cost of investigation and legal proceedings, net | 6,083 | 13,001 | 34,127 | |||||||||
| Shareholder securities litigation settlement | — | — | 7,750 | |||||||||
| Impairment charges | — | — | 148 | |||||||||
| Restructuring charges | 2,756 | 3,442 | 3,257 | |||||||||
| Total operating costs and expenses | 362,200 | 381,241 | 450,315 | |||||||||
| Operating loss | (21,858 | ) | (23,150 | ) | (97,310 | ) | ||||||
| Equity in income of affiliated company | 625 | 874 | 500 | |||||||||
| Interest income, net | 8,366 | 7,628 | 5,940 | |||||||||
| Loss before income taxes | (12,867 | ) | (14,648 | ) | (90,870 | ) | ||||||
| Income tax provision | 2,339 | 2,294 | 20,762 | |||||||||
| Net loss | $ | (15,206 | ) | $ | (16,942 | ) | $ | (111,632 | ) | |||
| Loss per share: | ||||||||||||
| Basic | $ | (0.41 | ) | $ | (0.45 | ) | $ | (2.98 | ) | |||
| Diluted | $ | (0.41 | ) | $ | (0.45 | ) | $ | (2.98 | ) | |||
| Weighted average shares outstanding: | ||||||||||||
| Basic | 37,488 | 37,484 | 37,484 | |||||||||
| Diluted | 37,488 | 37,484 | 37,484 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
MedQuist Inc. and Subsidiaries
Consolidated Balance Sheets
(In thousands)
| As of December 31, | ||||||||
| 2007 | 2006 | |||||||
Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 161,582 | $ | 175,412 | ||||
| Accounts receivable, net | 48,725 | 54,778 | ||||||
| Income tax receivable | 815 | 1,772 | ||||||
| Deferred income taxes | — | 298 | ||||||
| Other current assets | 7,920 | 8,352 | ||||||
| Total current assets | 219,042 | 240,612 | ||||||
| Property and equipment, net | 21,366 | 20,969 | ||||||
| Goodwill | 125,505 | 124,826 | ||||||
| Other intangible assets, net | 42,262 | 45,448 | ||||||
| Deferred income taxes | 2,712 | 2,378 | ||||||
| Other assets | 6,885 | 6,906 | ||||||
| Total assets | $ | 417,772 | $ | 441,139 | ||||
Liabilities and Shareholders’ Equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 12,754 | $ | 10,779 | ||||
| Accrued expenses | 18,989 | 28,812 | ||||||
| Accrued compensation | 14,826 | 15,558 | ||||||
| Customer accommodation and quantification | 18,459 | 24,777 | ||||||
| Deferred income tax liability — current | 4,783 | — | ||||||
| Deferred revenue | 16,023 | 15,202 | ||||||
| Total current liabilities | 85,834 | 95,128 | ||||||
| Deferred income taxes | 15,151 | 18,034 | ||||||
| Other non-current liabilities | 2,143 | 458 | ||||||
| Commitments and contingencies (Note 13) | ||||||||
| Shareholders’ equity: | ||||||||
| Common stock — no par value; authorized 60,000 shares; 37,544 and 37,484 shares issued and outstanding, respectively | 236,412 | 235,080 | ||||||
| Retained earnings | 72,876 | 87,693 | ||||||
| Deferred compensation | — | 332 | ||||||
| Accumulated other comprehensive income | 5,356 | 4,414 | ||||||
| Total shareholders’ equity | 314,644 | 327,519 | ||||||
| Total liabilities and shareholders’ equity | $ | 417,772 | $ | 441,139 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
MedQuist Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
| As of December 31, | ||||||||||||
| 2007 | 2006 | 2005 | ||||||||||
Operating activities: | ||||||||||||
| Net loss | $ | (15,206 | ) | $ | (16,942 | ) | $ | (111,632 | ) | |||
| Adjustments to reconcile net loss to cash (used in) provided by operating activities: | ||||||||||||
| Depreciation and amortization | 16,499 | 17,631 | 25,292 | |||||||||
| Equity in income of affiliated company | (625 | ) | (874 | ) | (500 | ) | ||||||
| Write-off and impairment of intangible assets | — | — | 148 | |||||||||
| Deferred income tax provision (benefit) | 1,878 | 5,225 | 36,655 | |||||||||
| Stock option expense | 565 | 2,117 | 37 | |||||||||
| Stock based compensation — Board Members | 150 | — | — | |||||||||
| Provision for doubtful accounts | 4,967 | 4,955 | 8,111 | |||||||||
| Asset writeoff charges | 168 | 767 | 4,096 | |||||||||
| Changes in operating assets and liabilities excluding effects of acquisitions: | ||||||||||||
| Accounts receivable | (1,359 | ) | 11,066 | (2,749 | ) | |||||||
| Income tax receivable | 957 | 19,889 | (15,981 | ) | ||||||||
| Other current assets | 431 | 1,666 | 1,132 | |||||||||
| Other non-current assets | 646 | 1,216 | 600 | |||||||||
| Accounts payable | 1,981 | 92 | (2,583 | ) | ||||||||
| Accrued expenses | (9,378 | ) | (9,366 | ) | 16,799 | |||||||
| Accrued compensation | (727 | ) | (5,537 | ) | 527 | |||||||
| Customer accommodation and quantification | (3,723 | ) | (21,121 | ) | 37,176 | |||||||
| Deferred revenue | 592 | (3,343 | ) | (3,737 | ) | |||||||
| Other non-current liabilities | 1,910 | (2,090 | ) | (1,142 | ) | |||||||
| Net cash (used in) provided by operating activities | (274 | ) | 5,351 | (7,751 | ) | |||||||
Investing activities: | ||||||||||||
| Purchase of property and equipment | (11,639 | ) | (8,191 | ) | (9,535 | ) | ||||||
| Capitalized software | (2,035 | ) | (58 | ) | (638 | ) | ||||||
| Net cash used in investing activities | (13,674 | ) | (8,249 | ) | (10,173 | ) | ||||||
Financing activities: | ||||||||||||
| Repayment of debt | — | — | (25 | ) | ||||||||
| Proceeds from exercise of stock options | 10 | — | — | |||||||||
| Net cash provided by (used in) financing activities | 10 | — | (25 | ) | ||||||||
| Effect of exchange rate changes on cash | 108 | 39 | 1 | |||||||||
| Net decrease in cash and cash equivalents | (13,830 | ) | (2,859 | ) | (17,948 | ) | ||||||
| Cash and cash equivalents — beginning of year | 175,412 | 178,271 | 196,219 | |||||||||
| Cash and cash equivalents — end of year | $ | 161,582 | $ | 175,412 | $ | 178,271 | ||||||
Supplemental cash flow information: | ||||||||||||
| Cash (recovered) paid for income taxes | $ | (451 | ) | $ | (22,381 | ) | $ | 162 | ||||
| Accommodation payments paid with credits | $ | 2,595 | $ | 980 | $ | — | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
MedQuist Inc. and Subsidiaries
Consolidated Statements of Shareholders’ Equity and Other Comprehensive Income
Years Ended December 31, 2007, 2006 and 2005
Years Ended December 31, 2007, 2006 and 2005
| Accumulated | ||||||||||||||||||||||||
| Other | Total | |||||||||||||||||||||||
| Common Stock | Retained | Deferred | Comprehensive | Shareholders’ | ||||||||||||||||||||
| Shares | Amount | Earnings | Compensation | Income | Equity | |||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
Balance, January 1, 2005 | 37,484 | $ | 232,926 | $ | 216,267 | $ | 332 | $ | 4,425 | $ | 453,950 | |||||||||||||
| Comprehensive loss: | ||||||||||||||||||||||||
| Net loss | — | — | (111,632 | ) | — | — | (111,632 | ) | ||||||||||||||||
| Foreign currency translation adjustments | — | — | — | — | (1,135 | ) | (1,135 | ) | ||||||||||||||||
| Total comprehensive loss | (112,767 | ) | ||||||||||||||||||||||
| Employee stock compensation | 37 | — | — | — | 37 | |||||||||||||||||||
Balance, December 31, 2005 | 37,484 | 232,963 | 104,635 | 332 | 3,290 | 341,220 | ||||||||||||||||||
| Comprehensive loss: | ||||||||||||||||||||||||
| Net loss | — | — | (16,942 | ) | — | — | (16,942 | ) | ||||||||||||||||
| Foreign currency translation adjustments | — | — | — | — | 1,124 | 1,124 | ||||||||||||||||||
| Total comprehensive loss | (15,818 | ) | ||||||||||||||||||||||
| Stock-based compensation expense | — | 2,117 | — | — | — | 2,117 | ||||||||||||||||||
Balance, December 31, 2006 | 37,484 | 235,080 | 87,693 | 332 | 4,414 | 327,519 | ||||||||||||||||||
| Comprehensive loss: | ||||||||||||||||||||||||
| Net loss | — | — | (15,206 | ) | — | — | (15,206 | ) | ||||||||||||||||
| Foreign currency translation adjustments | — | — | — | — | 942 | 942 | ||||||||||||||||||
| Total comprehensive loss | — | — | — | — | — | (14,264 | ) | |||||||||||||||||
| Stock-based compensation expense | — | 565 | — | — | — | 565 | ||||||||||||||||||
| Exercise of stock options | 4 | 10 | — | — | — | 10 | ||||||||||||||||||
| Adoption of FIN 48 | — | — | 389 | — | — | 389 | ||||||||||||||||||
| Deferred compensation-stock grants | 56 | 757 | — | (332 | ) | — | 425 | |||||||||||||||||
Balance, December 31, 2007 | 37,544 | $ | 236,412 | $ | 72,876 | $ | — | $ | 5,356 | $ | 314,644 | |||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
1. Description of Business
We are a provider of medical transcription technology and services which are integral to the clinical documentation workflow. We service health systems, hospitals and large group medical practices throughout the U.S. In the clinical documentation workflow, we provide, in addition to medical transcription technology and services, digital dictation, speech recognition and electronic signature services. We are a member of the Philips Group of Companies and collaborate with Philips Medical Systems in product development. On November 2, 2007, our majority shareholder, Koninklijke Philips Electronics N.V. (Philips), announced that it was going to proceed with the sale of its ownership interest in us if a satisfactory price and other acceptable terms can be realized. In addition, on November 2, 2007 we announced, in light of Philips’ announcement, that our board of directors, in connection with its previously disclosed review of our strategic alternatives, is evaluating whether a sale of us is in our best interests and the best interests of our shareholders.
2. Introductory Note
In November 2003, one of our employees raised allegations that we had engaged in improper billing practices. In response, our board of directors undertook an independent review of these allegations and engaged the law firm of Debevoise and Plimpton LLP, who in turn retained PricewaterhouseCoopers LLP, to assist in the review (Review). On March 16, 2004, we announced that we had delayed the filing of our 2003 annual report on Form 10-K pending completion of the Review. Subsequently, on March 25, 2004, we filed a Form 8-K detailing our determination that the Review would not be completed by the March 30, 2004 filing deadline for our 2003 Form 10-K. As a result of our noncompliance with the U.S. Securities and Exchange Commission’s (SEC) periodic disclosure requirements, our common stock was delisted from the NASDAQ National Market on June 16, 2004.
On July 30, 2004, we issued a press release entitled “MedQuist Announces Key Findings Of Independent Review Of Client Billing,” which announced certain findings in the Review regarding our billing practices (July 2004 Press Release). The Review found, among other things, that with respect to our medical transcription services contracts that called for billing based on the “AAMT line” billing unit of measure, we used ratios and formulae to help calculate the number of AAMT transcription lines for which our customers (AAMT Customers) were billed rather than counting each of the relevant characters to determine a billable line as provided for in the contracts. With respect to these contracts, our use of ratios and formulae to arrive at AAMT line counts was generally not disclosed to our AAMT Customers.
The AAMT line unit of measure was developed in 1993 by three medical transcription industry groups, including the American Association for Medical Transcription (AAMT), in an attempt to standardize industry billing practices for medical transcription services. Following the development of the AAMT line unit of measure, customers increasingly began to request AAMT line billing. Accordingly, we, along with other vendors in the medical transcription industry, began to incorporate the AAMT line unit of measure into certain customer contracts. The AAMT line definition provides that a “line” consists of 65 characters and defined the term “character” to include such things as macros and function keys as well as other information necessary for the final appearance and content of a document. However, these definitions turned out to be inherently ambiguous and difficult to apply in practice. As a result, the AAMT line was applied inconsistently throughout the medical transcription industry. In fact, no single set of AAMT characters was ever defined or agreed upon for this unit of measure, and it was eventually renounced by the groups responsible for its development.
The Review concluded that our rationale for using ratios and formulae to determine the number of AAMT transcription lines for billing was premised on a good faith attempt to adopt a consistent and commercially reasonable billing method given the lack of common standards in the industry and ambiguities inherent in the AAMT line definition. The Review concluded that the use of ratios and formulae within the medical transcription platform setups may have resulted in over billing and under billing of some customers. In addition, in some instances, customers’ ratios and formulae were adjusted without disclosure to the AAMT Customers. However, the Review found no evidence that the amounts we billed AAMT Customers were, in general, commercially unfair or inconsistent with what competitors would have charged. Moreover, it was noted in the Review that we have been able to attract and retain customers in a competitive market.
Following the issuance of the July 2004 Press Release, we began an extensive review of our historical AAMT line billing (Management’s Billing Assessment) and in August 2004 informed our current and former customers that we would be contacting them to discuss how they might have been impacted. In response, several former and current customers, including some of our largest customers, contacted us requesting, among other things, (i) an explanation of the billing methods employed by us for the customer’s account; (ii) an individualized review of the customer’s past billings, and/or (iii) a meeting with a member of our management team to discuss the July
F-8
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
2004 Press Release as it pertained to the customer’s particular account. Some customers demanded an immediate refund or credit to their account; others threatened to withhold payment on invoices and/or take their business elsewhere unless we timely responded to their information and/or audit requests.
In response to our customers’ concern over the July 2004 Press Release, we made the decision to take action to try to avoid litigation and preserve and solidify our customer business relationships by offering a financial accommodation to our AAMT Customers. See Note 4.
Disclosure of the findings of the Review, along with the delisting of our common stock, precipitated a number of governmental investigations and civil lawsuits. See Note 13.
3. Significant Accounting Policies
Principles of Consolidation
Our consolidated financial statements include the accounts of MedQuist Inc. and its subsidiary companies. All intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates and Assumptions in the Preparation of Consolidated Financial Statements
The preparation of our consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect amounts reported in our consolidated financial statements. Significant items subject to such estimates and assumptions include the carrying amount of property and equipment, valuation of long-lived and intangible assets and goodwill, valuation allowances for receivables, inventories and deferred income taxes, revenue recognition, stock-based compensation and commitments and contingencies. Actual results could differ from those estimates.
Revenue Recognition
We follow revenue recognition criteria outlined in Staff Accounting Bulletin (SAB) 101,Revenue Recognition in Financial Statements,as amended by SAB 104. The majority of our revenues are derived from providing medical transcription services. Revenues for medical transcription services are recognized when the services are rendered. These services are based on contracted rates. The remainder of our revenues are derived from the sale of voice-capture and document management products including software, hardware and implementation, training and maintenance service related to these products.
We recognize software and software-related revenues pursuant to the requirements of American Institute of Certified Public Accountants (AICPA) Statement of Position (SOP) 97-2Software Revenue Recognition(SOP 97-2), as amended by SOP 98-9,Software Revenue Recognition, With Respect to Certain Transactions, SOP 81-1,Accounting for Performance of Construction-type and Certain Production-type Contracts, Emerging Issues Task Force (EITF) 00-03Application of AICPA Statement of Position 97-2 to Arrangements That Include the Right to Use Software Stored on Another Entity’s Hardware, EITF 03-05Applicability of AICPA Statement of Position 97-2 to Non-Software Deliverables in an Arrangement Containing More-Than-Incidental Softwareand other authoritative accounting guidance.
We recognize software-related revenues using the residual method when vendor-specific objective evidence (VSOE) of fair value exists for all of the undelivered elements in the arrangement, but does not exist for one or more delivered elements. We allocate revenues to each undelivered element based on its respective fair value determined by the price charged when that element is sold separately or, for elements not yet sold separately, the price established by management if it is probable that the price will not change before the element is sold separately. We defer revenues for the undelivered elements and recognize the residual amount of the arrangement fee, if any, when the basic criteria in SOP 97-2 have been met.
Under SOP 97-2, provided that the arrangement does not involve significant production, modification, or customization of the software, revenues are recognized when all of the following four criteria have been met; persuasive evidence of an arrangement exists, delivery has occurred, the fee is fixed or determinable and collectability is probable.
F-9
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
If at the outset of an arrangement, we determine that the arrangement fee is not fixed or determinable, revenues are deferred until the arrangement fee becomes due and payable by the customer. If at the outset of an arrangement we determine that collectability is not probable, revenues are deferred until payment is received. Our license agreements typically do not provide for a right of return other than during the standard warranty period. If an arrangement allows for customer acceptance of the software or services, we defer revenues until the earlier of customer acceptance or when the acceptance rights lapse.
We separately market and sell hardware and software post contract customer support (PCS). PCS covers phone support, hardware parts and labor, software bug fixes and limited upgrades, if and when available. We do not commit to specific future software upgrades or releases. The contract period for PCS is generally one year. We recognize both hardware and software PCS on a straight line basis over the life of the underlying PCS contract. In some of our PCS contracts, we bill the customer prior to performing the services. As of December 31, 2007 and 2006, deferred PCS revenues of $11,494 and $12,235, respectively, are included in deferred revenues and $221 and $450, respectively, are included in non-current liabilities in the accompanying consolidated balance sheets.
Certain arrangements include multiple elements involving software, hardware and implementation, training, or other services that are not essential to the functionality of the software. VSOE for services does not exist. Since the undelivered elements are typically services, we recognize the entire arrangement fee ratably over the period during which the services are expected to be performed or the PCS period, whichever is longer, beginning with delivery of the software, provided that all other revenue recognition criteria in SOP 97-2 are met. The services are typically completed before the PCS term expires. As such, upon completion of the services, the difference between the VSOE of fair value for the remaining PCS period and the remaining unrecognized portion of the arrangement fee is recognized as revenue (i.e. the residual method), and the remaining deferred revenue is recognized ratably over the remaining PCS period, provided that all other revenue recognition criteria in SOP 97-2 are met.
Sales Taxes
We present taxes assessed by a governmental authority including sales, use, value added and excise taxes on a net basis and therefore the presentation of these taxes is excluded from our revenues and is included in accrued expenses in the accompanying consolidated balance sheets until such amounts are remitted to the taxing authorities.
Accounting for Consideration Given to a Customer
As a result of the Accommodation Analysis (which is described in Note 4), we offered financial accommodations to our customers. Pursuant to EITF Issue 01-9,Accounting for Consideration Given by a Vendor to a Customer (Including a Reseller of the Vendor’s Products) (EITF 01-9), consideration given by a vendor to a customer is presumed to be a reduction of the selling price of the vendor’s services and, therefore, should be characterized as a reduction of revenues when recognized in the vendor’s income statement. For the years ended December 31, 2007, 2006 and 2005, $0, $10,402 and $57,678, respectively, was recorded as a reduction of revenues related to the Accommodation Analysis.
Litigation and Settlement Costs
From time to time, we are involved in litigation, claims, contingencies and other legal matters. We record a charge equal to at least the minimum estimated liability for a loss contingency when both of the following conditions are met: (i) information available prior to issuance of the financial statements indicates that it is probable that an asset had been impaired or a liability had been incurred at the date of the financial statements and (ii) the range of the loss can be reasonably estimated. We expense legal costs, including those legal costs expected to be incurred in connection with a loss contingency, as incurred.
Services Provided by Independent Registered Public Accountant
Services provided by our independent registered public accounting firm are expensed as the services are provided and were $6,840, $6,429, and $1,254 for the years ended December 31, 2007, 2006 and 2005, respectively.
Restructuring Costs
A liability for restructuring costs associated with an exit or disposal activity is recognized and measured initially at fair value when the liability is incurred. We record a liability for severance costs when employees are notified that they are to be terminated and for future, non-cancellable operating lease costs when we vacate a facility.
Our estimates of future liabilities may change, requiring us to record additional restructuring charges or reduce the amount of liabilities recorded. At the end of each reporting period, we evaluate the remaining accrued restructuring charges to ensure their adequacy, that no excess accruals are retained and the utilization of the provisions are for their intended purposes in accordance with developed exit plans.
F-10
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
We periodically evaluate currently available information and adjust our accrued restructuring charges as necessary. Changes in estimates are accounted for as restructuring costs or credits in the period identified.
Research and Development Costs
Research and development costs are expensed as incurred.
Income Taxes
Deferred tax assets and liabilities are recorded for temporary differences between the tax basis of assets and liabilities and their reported amounts in the consolidated financial statements, using statutory tax rates in effect for the year in which the differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in our statements of operations in the period that includes the enactment date. A valuation allowance is recorded to reduce the carrying amounts of deferred tax assets unless it is more likely than not that such assets will be realized. Management considers various sources of future taxable income including projected book earnings, the reversal of deferred tax liabilities, and prudent and feasible tax planning strategies in determining the need for a valuation allowance.
Stock-Based Compensation
On January 1, 2006, we adopted the fair value recognition provisions of Financial Accounting Standards Board (FASB) Statement 123 (revised 2004),Share-Based Payment, (Statement 123(R)), using the modified prospective transition method which requires application of Statement 123(R) on the date of adoption and, therefore, we have not retroactively adjusted results from periods prior to 2006. Under the modified prospective transition method, compensation costs associated with share-based awards recognized in 2006 include compensation costs for all share-based payments granted prior to, but not yet vested as of January 1, 2006, based on the grant-date fair value previously estimated in accordance with the provisions of FASB Statement 123,Accounting for Stock-Based Compensation(Statement 123). Had we granted options in 2006, the compensation costs for those options would have been based on the grant-date fair value estimated in accordance with the provisions of Statement 123(R). In March 2005, the SEC issued SAB 107 (SAB 107) which provided supplemental guidance related to Statement 123(R). We have applied the provisions of SAB 107 in our adoption of Statement 123(R).
Statement 123(R) requires companies to estimate the fair value of stock options on the date of grant using an option pricing model. We use the Black-Scholes option pricing model to determine the fair value of our options. The determination of the fair value of stock based awards using an option pricing model is affected by a number of assumptions including expected volatility of the common stock over the expected term, the expected term, the risk free interest rate during the expected term and the expected dividends to be paid. The value of the portion of the award that is ultimately expected to vest is recognized as compensation expense over the requisite service periods.
Stock-based compensation expense related to employee stock options recognized under Statement 123(R) for 2007 and 2006 was $565 and $2,117 which was charged to selling, general and administrative expenses ($255 and $562), research and development expenses ($91 and $240) and cost of revenues ($219 and $1,315). Included in the $565 and $2,117 is $120 and $194, respectively, of expense related to options that were issued to certain executive officers when we became current in our periodic reporting obligations with the SEC in October 2007. As of December 31, 2007, total unamortized stock-based compensation cost related to non-vested stock options, net of expected forfeitures, was $1,371 which is expected to be recognized over a weighted-average period of 4.7 years.
Prior to the adoption of Statement 123(R), we accounted for stock-based awards to employees and directors using the intrinsic value method in accordance with Accounting Principles Board (APB) Opinion 25,Accounting for Stock Issued to Employees(APB 25), as allowed under Statement 123. Under the intrinsic value method, no compensation expense for employee stock options was recognized in our consolidated statements of operations because the exercise price of the stock options granted to employees was greater than or equal to the fair market value of the underlying stock at the date of grant.
F-11
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
The following table illustrates the pro forma effect on net loss and loss per share amounts for the year ended December 31, 2005 as if we had applied the fair-value recognition provisions of Statement 123 to stock-based employee compensation.
| Net loss | $ | (111,632 | ) | |
| Add: Stock-based employee compensation expense included in reported net loss | 37 | |||
| Deduct: Total stock-based employee compensation expense determined under fair-value based method for all awards | (3,487 | ) | ||
| Pro forma net loss | $ | (115,082 | ) | |
| Basic net loss per share: | ||||
| As reported | $ | (2.98 | ) | |
| Pro forma | $ | (3.07 | ) | |
| Diluted net loss per share: | ||||
| As reported | $ | (2.98 | ) | |
| Pro forma | $ | (3.07 | ) | |
We did not grant any options for the years ended December 31, 2006 and 2005.
Net Loss per Share
Basic net loss per share is computed by dividing net loss by the weighted average number of shares outstanding during each period. Diluted net loss per share is computed by dividing net loss by the weighted average shares outstanding, as adjusted for the dilutive effect of common stock equivalents, which consist only of stock options, using the treasury stock method.
The table below reflects basic and diluted net loss per share for the years ended December 31:
| 2007 | 2006 | 2005 | ||||||||||
| Net loss | $ | (15,206 | ) | $ | (16,942 | ) | $ | (111,632 | ) | |||
| Weighted average shares outstanding: | ||||||||||||
| Basic | 37,488 | 37,484 | 37,484 | |||||||||
| Effect of dilutive stock | — | — | — | |||||||||
| Diluted | 37,488 | 37,484 | 37,484 | |||||||||
| Net loss per share: | ||||||||||||
| Basic and diluted | $ | (0.41 | ) | $ | (0.45 | ) | $ | (2.98 | ) | |||
The computation of diluted net loss per share does not assume conversion, exercise or issuance of shares that would have an anti-dilutive effect on diluted net loss per share. During 2007, 2006 and 2005, we had a net loss. As a result, any assumed conversions would result in reducing the net loss per share and, therefore, are not included in the calculation. Shares having an anti-dilutive effect on net loss per share and, therefore, excluded from the calculation of diluted net loss per share, totaled 2,298 shares, 2,150 shares and 3,432 shares for the years ended December 31, 2007, 2006 and 2005, respectively.
Advertising Costs
Advertising costs are expensed as incurred and for the years ended December 31, 2007, 2006 and 2005 were $1,674, $1,903 and $2,098, respectively.
Cash and Cash Equivalents
We consider all highly liquid instruments with original maturities of three months or less to be cash equivalents. Our cash management and investment policies dictate that cash equivalents be limited to investment grade, highly liquid securities. We place our temporary cash investments with high-credit rated, quality financial institutions. Deposits held with banks may exceed the amount of insurance provided on such deposits. Consequently, our cash equivalents are subject to potential credit risk. As of December 31, 2007 and 2006, cash equivalents consisted of money market investments. The carrying value of cash and cash equivalents approximates fair value.
F-12
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recorded at the invoiced amount and do not bear interest. The carrying value of accounts receivable approximates fair value. The allowance for doubtful accounts is our best estimate for losses inherent in our accounts receivable portfolio. The sales return and allowance reserve is our best estimate of sales credits that will be issued related to our accounts receivable portfolio. These allowances are used to state trade receivables at estimated net realizable value.
We estimate uncollectible amounts based upon our historical write-off experience, current customer receivable balances, age of customer receivable balances, the customer’s financial condition and current economic conditions. Historically, these estimates have been adequate to cover our accounts receivable exposure.
We enter into medical transcription service arrangements which contain provisions for performance penalties in the event certain service levels, primarily related to turnaround time on transcribed reports, are not achieved. We reduce revenues for any performance penalties incurred and have included an estimate of such credits in our allowance for uncollectible accounts.
Product revenues for sales to end-user customers and resellers is recognized upon passage of title if all other revenue recognition criteria have been met. End-user customers generally do not have a right of return. We provide certain of our resellers and distributors with limited rights of return of our products. We reduce revenues for rights to return our product based upon our historical experience and have included an estimate of such credits in our allowance for doubtful accounts.
Inventories
Inventories, which are primarily comprised of finished goods, are stated at the lower of cost or market, with cost determined on a weighted-average basis. Inventories in excess of anticipated future demand or for obsolete products are reserved. As of December 31, 2007 and 2006, the net inventory balances were $2,011 and $2,608, respectively, and are included in other current assets in the accompanying consolidated balance sheets.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets which range from two to seven years for furniture, equipment and software, and the lesser of the lease term or estimated useful life for leasehold improvements. Repairs and maintenance costs are charged to expense as incurred while additions and betterments are capitalized. Gains or losses on disposals are charged to operations. Upon retirement, sale or other disposition, the related cost and accumulated depreciation are eliminated from the accounts and any gain or loss is included in operations.
Goodwill
Goodwill represents the excess of the aggregate purchase price over the fair value of the net assets acquired in a purchase business combination. Goodwill is reviewed for impairment on December 1 of each year.
The goodwill impairment test is a two-step test. Under the first step, the fair value of the reporting unit is compared with its carrying value (including goodwill). We consider three methods when determining fair value; the discounted cash flow method, the quoted price method and the public company method. Of these three methods, we assign the most significant weighting to the discounted cash flow method. If the fair value of the reporting unit is less than its carrying value, an indication of goodwill impairment exists for the reporting unit and the enterprise must perform step two of the impairment test. Under step two, an impairment loss is recognized for any excess of the carrying amount of the reporting unit’s goodwill over the implied fair value of that goodwill. The implied fair value of goodwill is determined by allocating the fair value of the reporting unit in a manner similar to a purchase price allocation, in accordance with FASB Statement 141,Business Combinations. The residual fair value after this allocation is the implied fair value of the reporting unit’s goodwill.
Software Development
We capitalize software development costs pursuant to the requirements of FASB Statement 86,Accounting for the Costs of Computer Software to be Sold, Leased, or Otherwise Marketed(Statement 86), for our software developed for sale and AICPA SOP 98-1,
F-13
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
Accounting for the Costs of Computer Software Developed or Obtained for internal Use(SOP 98-1), for our software developed for internal use.
Statement 86 specifies that costs incurred in creating a computer software product shall be charged to expense when incurred as research and development until technical feasibility has been established. Technical feasibility is established upon completion of a detail program design or, in its absence, completion of a working model. Thereafter, all software production costs shall be capitalized until the product is available for release to customers.
SOP 98-1 specifies that software costs incurred in the preliminary project stage should be expensed as incurred. Capitalization of costs should begin when the preliminary project stage is completed and management, with the relevant authority, authorizes and commits funding of the project and it is probable that the project will be completed and the software will be used to perform the function intended. Capitalization should cease no later than the point at which the project is substantially complete and ready for its intended use.
Capitalized software is reported at the lower of unamortized cost or net realizable value and is amortized over the product’s estimated economic life which is generally three years. As of December 31, 2007 and 2006, $2,343 and $485, respectively, of unamortized software development costs are included in other intangible assets in the accompanying consolidated balance sheets. For the years ended December 31, 2007, 2006 and 2005, software amortization expense was $360, $262 and $336, respectively.
Long-Lived and Other Intangible Assets
Long-lived assets, including property and equipment and purchased intangible assets subject to amortization, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. To determine the recoverability of long-lived assets, the estimated future undiscounted cash flows expected to be generated by an asset is compared to the carrying value of the asset. If the carrying value of the long-lived asset exceeds its estimated future undiscounted cash flows, an impairment charge is recognized in the amount by which the carrying value of the asset exceeds its fair value. Annually we evaluate the reasonableness of the useful lives of these assets.
Intangible assets include certain assets (primarily customer lists) obtained from business acquisitions and are being amortized using the straight-line method over their estimated useful lives which range from three to 20 years.
Foreign Currency Translation
Our operating subsidiaries in the United Kingdom and Canada use the local currency as their functional currency. We translate the assets and liabilities of those entities into U.S. dollars using the month-end exchange rate. We translate revenues and expenses using the average exchange rates prevailing during the reporting period. The resulting translation adjustments are recorded in accumulated other comprehensive income within shareholders’ equity. Gains and losses from foreign currency transactions are included in net loss and were not material for the years ended December 31, 2007, 2006 and 2005, respectively.
Business Enterprise Segments
We operate in one reportable operating segment which is medical transcription technology and services.
Concentration of Risk, Geographic Data and Enterprise-wide Disclosures
No single customer accounted for more than 10% of our net revenues in any period. There is no single geographic area of significant concentration other than the United States.
F-14
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
The following table summarizes the net revenues by the categories of our products and services as a percentage of our total net revenues.
| 2007 | 2006 | 2005 | ||||||||||
| Medical transcription | 83.3 | % | 83.8 | % | 79.5 | % | ||||||
| Products and related services | 4.7 | % | 4.6 | % | 8.1 | % | ||||||
| PCS | 8.2 | % | 8.5 | % | 9.3 | % | ||||||
| Other | 3.8 | % | 3.1 | % | 3.1 | % | ||||||
| Total | 100.0 | % | 100.0 | % | 100.0 | % | ||||||
Other includes medical coding, application service provider and other miscellaneous revenues.
Fair Value of Financial Instruments
Cash and cash equivalents, accounts receivable, accounts payable and accrued expenses are reflected in the accompanying consolidated balance sheets at carrying values which approximate fair value due to the short-term nature of these instruments and the variability of the respective interest rates where applicable.
Comprehensive Income/Loss
Comprehensive income is comprised of Net income and Other comprehensive income/loss.
Other comprehensive income/loss consists of foreign currency translation adjustments. Other comprehensive income/loss and comprehensive income are displayed separately in the Consolidated Statements of Shareholders’ Equity and Other Comprehensive Income.
Recent Accounting Pronouncements
In September 2006, the FASB issued Statement 157,Fair Value Measurements, (Statement 157) which defines fair value, establishes guidelines for measuring fair value and expands disclosures regarding fair value measurements. Statement 157 does not require any new fair value measurements. The provisions of this statement are effective for fiscal years beginning after November 15, 2007. On February 12, 2008, the FASB delayed the effective date of Statement 157 for non-financial assets and liabilities until fiscal years beginning after November 15, 2008. We do not expect the adoption of Statement 157 to have a material impact on our consolidated financial statements.
In February 2007, the FASB issued Statement 159,The Fair Value Option for Financial Assets and Financial Liabilities, including an amendment of FASB Statement 115(Statement 159) which permits entities to choose to measure eligible items at fair value at specified election dates. Unrealized gains and losses on items for which the fair value option has been elected will be reported in earnings at each subsequent reporting date. The following balance sheet items are within the scope of Statement 159:
| • | Recognized financial assets and financial liabilities unless a special exception applies; | ||
| • | Firm commitments that would otherwise not be recognized at inception and that involve only financial instruments; | ||
| • | Non-financial insurance contracts; and | ||
| • | Host financial instruments resulting from separation of an embedded non-financial derivative instrument from a non-financial hybrid instrument |
Statement 159 will be effective for fiscal years beginning after November 2007 with early adoption possible but subject to certain requirements. We do not expect the adoption of Statement 159 to have a material impact on our consolidated financial statements.
In December 2007, the FASB issued Statement 141(R),Business Combinations(Statement 141R). Statement 141R establishes principles and requirements for how the acquirer of a business recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, and any noncontrolling interest in the acquiree. Statement 141R also provides guidance for recognizing and measuring the goodwill acquired in the business combination and determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. Statement 141R will become effective as of the beginning of our fiscal year beginning after December 15, 2008.
F-15
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
In December 2007, the FASB issued Statement No. 160,Noncontrolling Interests in Consolidated Financial Statements-an amendment of ARB No. 51(Statement 160). Statement 160 establishes accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. Statement 160 will become effective as of the beginning of our fiscal year beginning after December 15, 2008. We do not believe that Statement 160 will have a material effect on our consolidated financial statements.
4. Customer Accommodation and Quantification
As discussed in Note 2, in connection with our decision to offer financial accommodations to our AAMT Customers, we analyzed our historical billing information and the available report-level data to develop individualized accommodation offers to be made to our AAMT Customers (Accommodation Analysis). This analysis took approximately one year to complete. The methodology utilized to develop the individual accommodation offers was designed to generate positive accommodation outcomes for our AAMT Customers. As such, the methodology was not a calculation of potential over billing nor was it intended as a measure of damages or a reflection of any admission of liability due and owed to our AAMT Customers. Instead, the Accommodation Analysis was a methodology that was developed to arrive at commercially reasonable and fair accommodation offers that would be acceptable to our AAMT Customers without negotiation.
In the fourth quarter of 2005, based on the Accommodation Analysis, our board of directors authorized management to make cash accommodation offers to AAMT customers in the aggregate amount of $65,413. In 2006, this amount was adjusted by a net additional amount of $1,157 based on a refinement of the Accommodation Analysis resulting in an aggregate amount of $66,570. By accepting our accommodation offer, an AAMT Customer must agree, among other things, to release us from any and all claims and liability regarding AAMT line and other billing related issues.
As part of this process, we also conducted an analysis in an attempt to quantify the economic consequences of potentially unauthorized adjustments to AAMT Customers’ ratios and formulae within the transcription platform setups (Quantification). This Quantification was calculated to be $9,835.
Of the authorized cash accommodation amount of $66,570, $1,157 and $57,678 were treated as consideration given by a vendor to a customer and accordingly recorded as a reduction in revenues in 2006 and 2005, respectively. The balance of $7,735 plus an additional $2,100 has been accounted for as a billing error associated with the Quantification resulting in a reduction of revenues in various reporting periods from 1999 to 2005.
The goal of our customer accommodation was to reach a settlement with our AAMT Customers. However, the Accommodation Analysis for certain AAMT Customers did not result in positive accommodation outcomes. For certain other customers, the Accommodation Analysis resulted in calculated cash accommodation offers that we believed were insufficient as a percentage of their historical AAMT line billing to motivate such customers to resolve their billing disputes with us. Therefore, in 2006 we modified our customer accommodation to enable us to offer this group of AAMT Customers credits for the purchase of future products and/or services from us over a defined period of time. On July 21, 2006, our board of directors authorized management to make credit accommodation offers up to an additional $8,676 beyond amounts previously authorized. During 2006, this amount was adjusted by a net additional amount of $569 based on a refinement of the Accommodation Analysis, resulting in an aggregate amount of $9,245. In connection with the credit accommodation offers we recorded a reduction in revenues and corresponding increase in accrued expenses of $9,245 in 2006.
The following is a summary of the financial statement activity related to the customer accommodation and the Quantification which is included as a separate line item in the accompanying consolidated balance sheets as of December 31, 2007 and 2006:
| 2007 | 2006 | |||||||
| Beginning balance | $ | 24,777 | $ | 46,878 | ||||
| Customer accommodation | — | 10,402 | ||||||
| Payments and other adjustments | (3,723 | ) | (31,523 | ) | ||||
| Credits | (2,595 | ) | (980 | ) | ||||
| Ending balance | $ | 18,459 | $ | 24,777 | ||||
F-16
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
5. Cost of Investigation and Legal Proceedings, net
For the years ended December 31, 2007, 2006 and 2005, we recorded a charge of $6,083, $13,001 and $34,127, respectively, for costs associated with the Review, Management’s Billing Assessment as well as defense and other costs associated with the SEC and U.S. Department of Justice (DOJ) investigations and civil litigation that we deemed to be unusual in nature. These costs are net of insurance claim reimbursements. We record insurance claims when the realization of the claim is probable. The following is a summary of the amounts recorded in the accompanying consolidated statements of operations:
| 2007 | 2006 | 2005 | ||||||||||
| Legal fees | $ | 18,678 | $ | 14,427 | $ | 20,858 | ||||||
| Other professional fees | 2,592 | 4,787 | 9,789 | |||||||||
| Nightingale and Associates, LLC (Nightingale) services | 197 | 3,005 | 3,207 | |||||||||
| Insurance recoveries and claims | (15,386 | ) | (9,409 | ) | — | |||||||
| Other | 2 | 191 | 273 | |||||||||
| Total | $ | 6,083 | $ | 13,001 | $ | 34,127 | ||||||
Other professional fees represent accounting and dispute analysis costs and document search and retrieval costs. In 2006, insurance recoveries and claims represent insurance recoveries ($8,702) and insurance claims ($707). The insurance claims were recorded in other current assets in the accompanying consolidated balance sheet as of December 31, 2006 and payment related to these claims was received in the first quarter of 2007. During 2007 we recorded ($15,386) in additional insurance recoveries and received payment of this entire amount in 2007.
6. Restructuring Charges
2007 Restructuring Plans
During the third quarter of 2007, we implemented a restructuring plan related to a reduction in workforce of 104 employees as a result of the refinement of our centralized national services delivery model. In addition, during the fourth quarter of 2007 we implemented a restructuring plan related to an additional reduction in workforce of 183 employees attributable to our efforts to reduce costs. We recorded $2,263 in severance charges related to the 2007 restructuring plans. The remaining restructuring costs are included in accrued expenses in the accompanying consolidated balance sheet as of December 31, 2007. The table below reflects the financial statement activity related to the 2007 restructuring plans for the year ended December 31, 2007:
| Initial charge | $ | 2,263 | ||
| Usage | (770 | ) | ||
| Balance as of December 31, 2007 | $ | 1,493 | ||
The remainder of payments related to the 2007 restructuring plans will be made in 2008.
2005 Restructuring Plan
During 2005, we implemented a restructuring plan (2005 Plan) based on the implementation of a centralized national service delivery model. The 2005 Plan involved the consolidation of operating facilities and a related reduction in workforce. The table below reflects the financial statement activity related to the 2005 Plan which is included in accrued expenses in the accompanying consolidated balance sheets as of December 31, 2007 and 2006:
| Non-Cancelable | ||||||||||||||||
| Total | Leases | Severance | Equipment | |||||||||||||
| Balance as of January 1, 2006 | $ | 2,050 | $ | 1,693 | $ | 357 | $ | — | ||||||||
| Additional charge | 3,442 | 1,653 | 1,447 | 342 | ||||||||||||
| Usage | (4,780 | ) | (2,698 | ) | (1,740 | ) | (342 | ) | ||||||||
| Balance as of December 31, 2006 | 712 | 648 | 64 | — | ||||||||||||
| Additional Charge | 493 | 322 | 146 | 25 | ||||||||||||
| Usage | (1,079 | ) | (844 | ) | (210 | ) | (25 | ) | ||||||||
| Balance as of December 31, 2007 | $ | 126 | $ | 126 | $ | — | $ | — | ||||||||
F-17
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
Payments related to the 2005 Plan were made in 2007 for severance and non-cancelable leases. The remainder of payments related to the 2005 Plan will be made by 2009 for non-cancelable leases.
7. Accounts Receivable
Accounts receivable consisted of the following as of December 31
| 2007 | 2006 | |||||||
| Trade accounts receivable | $ | 53,084 | $ | 59,272 | ||||
| Less: Allowance for doubtful accounts | (4,359 | ) | (4,494 | ) | ||||
| Accounts receivable, net | $ | 48,725 | $ | 54,778 | ||||
8. Property and Equipment
Property and equipment consisted of the following as of December 31:
| 2007 | 2006 | |||||||
| Computer equipment | $ | 27,610 | $ | 28,541 | ||||
| Communication equipment | 6,932 | 6,602 | ||||||
| Software | 20,889 | 18,002 | ||||||
| Furniture and office equipment | 1,650 | 1,586 | ||||||
| Leasehold improvements | 3,057 | 4,076 | ||||||
| Total property and equipment | 60,138 | 58,807 | ||||||
| Less: accumulated depreciation | (38,772 | ) | (37,838 | ) | ||||
| Property and equipment, net | $ | 21,366 | $ | 20,969 | ||||
During 2006, we recorded a write-off of $767 which was allocated between cost of revenues ($425) and restructuring charges related to the 2005 Plan ($342). In the fourth quarter of 2005, based upon an inventory of fixed assets, we recorded a write-off of $4,070 (original cost $29,116 less accumulated depreciation $25,046). This expense was allocated between cost of revenues ($3,851) and restructuring charges related to the 2005 Plan ($219). In addition, during 2005 approximately $50,832 in fully depreciated assets no longer in use were written off which had no impact on net loss.
9. Goodwill and Other Intangible Assets
Goodwill
The following table reflects the financial statement activity related to the carrying amount of goodwill as of December 31, 2007 and 2006:
| Balance as of January 1, 2006 | $ | 123,849 | ||
| Foreign currency adjustments | 977 | |||
| Balance as of December 31, 2006 | 124,826 | |||
| Foreign currency adjustments | 679 | |||
| Balance as of December 31, 2007 | $ | 125,505 | ||
The foreign currency adjustments reflect changes in the period-end currency rates of our foreign subsidiaries.
F-18
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
Other Intangible Assets
As of December 31, other intangible asset balances were:
| 2007 | ||||||||||||
| Accumulated | Net book | |||||||||||
| Cost | Amortization | Value | ||||||||||
| Customer lists | $ | 77,331 | $ | (37,412 | ) | $ | 39,919 | |||||
| Tradenames | 5,325 | (5,325 | ) | — | ||||||||
| Capitalized software | 4,815 | (2,472 | ) | 2,343 | ||||||||
| Total | $ | 87,471 | $ | (45,209 | ) | $ | 42,262 | |||||
| 2006 | ||||||||||||
| Accumulated | Net book | |||||||||||
| Cost | Amortization | Value | ||||||||||
| Customer lists | $ | 77,185 | $ | (32,654 | ) | $ | 44,531 | |||||
| Noncompete agreements | 4,559 | (4,559 | ) | — | ||||||||
| Tradenames | 5,325 | (4,893 | ) | 432 | ||||||||
| Capitalized software. | 2,597 | (2,112 | ) | 485 | ||||||||
| Total | $ | 89,666 | $ | (44,218 | ) | $ | 45,448 | |||||
The estimated useful life and the weighted average remaining lives of the intangible assets as of December 31, 2007 were as follows:
| Estimated | Weighted Average | |||||||
| Useful Life | Remaining Lives | |||||||
| Customer lists | 10 - 20 years | 10.7 years | ||||||
| Capitalized software | 3 years | 2.7 years | ||||||
Estimated annual amortization expense for intangible assets is as follows:
| 2008 | $ | 5,693 | ||
| 2009 | 5,479 | |||
| 2010 | 5,328 | |||
| 2011 | 4,584 | |||
| 2012 | 3,634 | |||
| Thereafter | 17,544 | |||
| Total | $ | 42,262 | ||
10. Contractual Obligations
Leases
Minimum rental payments under operating leases are recognized on a straight-line basis over the term of the lease, including any periods of free rent and landlord incentives. Rental expense for operating leases for the years ended December 31, 2007, 2006 and 2005 was $2,489, $4,089 and $5,710, respectively. Future minimum lease payments under non-cancelable operating leases (with initial or remaining lease terms in excess of one year) as of December 31, 2007 were:
| Total | Continuing | Restructuring | ||||||||||
| 2008 | $ | 4,650 | $ | 4,571 | $ | 79 | ||||||
| 2009 | 3,809 | 3,768 | 41 | |||||||||
| 2010 | 3,419 | 3,419 | — | |||||||||
| 2011 | 2,212 | 2,212 | — | |||||||||
| 2012 and thereafter | 1,642 | 1,642 | — | |||||||||
| Total minimum lease payments | $ | 15,732 | $ | 15,612 | $ | 120 | ||||||
F-19
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
Other Contractual Obligations
The following summarizes our other contractual obligations as of December 31, 2007:
| Severance and Other | ||||||||||||
| Total | Purchase | Guaranteed Payments | ||||||||||
| 2008 | $ | 8,328 | $ | 5,650 | $ | 2,678 | ||||||
| 2009 | 5,400 | 5,400 | — | |||||||||
| 2010 | 5,400 | 5,400 | — | |||||||||
| 2011 | 4,027 | 4,027 | — | |||||||||
| 2012 and thereafter | 947 | 947 | — | |||||||||
| Total | $ | 24,102 | $ | 21,424 | $ | 2,678 | ||||||
Purchase obligations represent telecommunication contracts ($21,174), and other recurring purchase obligations ($250). Severance and other guaranteed payments are comprised of severance payments ($1,493), employee retention payments ($785) and amounts owed to Nightingale ($400).
As of December 31, 2007, we had agreements with certain of our senior management that provided for severance payments in the event these individuals were terminated without cause. The maximum cost exposure related to these agreements was $1,207 as of December 31, 2007.
As of December 31, 2007, we had agreements with certain of our senior management other than our President and Chief Executive Officer that provided for payments to such individuals in the event we are able to successfully complete a strategic transaction and such individuals remained employed by us (or the successor company as the case may be) for the 90-day period immediately following the closing of the strategic transaction or such individuals experience an involuntary termination at any time during such 90-day period. The maximum cost exposure related to these agreements was $504 as of December 31, 2007.
As of December 31, 2007, we had an agreement with Nightingale that provided for a payment to Nightingale in the event we are able to successfully complete a strategic transaction and Mr. Hoffmann continues to serve as our President and Chief Executive Officer for the 90-day period immediately following the closing of a strategic transaction or Nightingale’s engagement with us (or any successor to our business), including the retention of Mr. Hoffmann as our President and Chief Executive Officer (or any successor to our business), is terminated upon the closing of a strategic transaction or during such 90-day period. The maximum cost exposure related to this agreement was $133 as of December 31, 2007.
11. Investment in A-Life Medical, Inc. (A-Life)
We have an investment in A-Life, a privately held entity which provides advanced natural language processing technology for the medical industry. Our investment is recorded under the equity method of accounting since we owned 33.6% of A-Life’s outstanding voting shares as of December 31, 2007 and 2006. The table below reflects the financial statement activity related to A-Life as of December 31, 2007 and 2006 that is recorded in other assets in the accompanying consolidated balance sheets.
| Balance as of January 1, 2006 | $ | 5,015 | ||
| Share in income | 874 | |||
| Balance as of December 31, 2006 | 5,889 | |||
| Share in income | 625 | |||
| Reclassification | (498 | ) | ||
| Balance as of December 31, 2007 | $ | 6,016 | ||
Our investment in A-Life included a note receivable plus accrued interest due from A-Life which matured on December 31, 2003. Prior to 2007, this note receivable and accrued interest had been recorded in other assets. In January 2008, A-Life paid us $1,250 to satisfy this note receivable and accrued interest in full, as well as all other disputes and claims between A-Life and us. Accordingly, we reclassified the note receivable and accrued interest balances to other current assets in the accompanying December 31, 2007 consolidated balance sheet.
12. Accrued Expenses
Accrued expenses consisted of the following as of December 31:
| 2007 | 2006 | |||||||
| Professional services | $ | 4,959 | $ | 4,938 | ||||
| Shareholder litigation settlement | — | 7,750 | ||||||
| Other | 14,030 | 16,124 | ||||||
| Total accrued expenses | $ | 18,989 | $ | 28,812 | ||||
No other individual accrued expense is in excess of 5% of total current liabilities.
13. Commitments and Contingencies
Governmental Investigations
The SEC is currently conducting a formal investigation of us relating to our billing practices. We have been fully cooperating with the SEC since it opened its investigation in 2004. We have complied, and are continuing to comply, with information and document requests by the SEC.
We also received an administrative HIPAA subpoena for documents from the DOJ on December 17, 2004. The subpoena sought information primarily about our provision of medical transcription services to governmental and non-governmental customers. The information was requested in connection with a government investigation into whether we and others violated federal laws in connection
F-20
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
with the provision of medical transcription services. We have complied, and are continuing to comply, with information and document requests by the DOJ.
The DOL is currently conducting a formal investigation into the administration of our 401(k) plan. We have been fully cooperating with the DOL since it opened its investigation in 2004. We have complied, and are continuing to comply, with information and document requests by the DOL.
Developments relating to the SEC, DOJ and/or DOL investigations will continue to create various risks and uncertainties that could materially and adversely affect our business and our historical and future financial condition, results of operations and cash flows.
Shareholder Securities Litigation
A shareholder putative class action lawsuit was filed against us in the United States District Court District of New Jersey on November 8, 2004. The action, entitledWilliam Steiner v. MedQuist, Inc., et al., Case No. 1:04-cv-05487-FLW (Shareholder Putative Action), was filed against us and certain of our former officers, purportedly on behalf of an alleged class of all persons who purchased our common stock during the period from April 23, 2002 through November 2, 2004, inclusive (Securities Class Period). The complaint specifically alleged that defendants violated federal securities laws by purportedly issuing a series of false and misleading statements to the market throughout the Securities Class Period, which statements allegedly had the effect of artificially inflating the market price of our securities. The complaint asserted claims under Section 10(b) and 20(a) of the Exchange Act and Rule 10b-5, thereunder. Named as defendants, in addition to us, were our former President and Chief Executive Officer and our former Executive Vice President and Chief Financial Officer.
On August 16, 2005, a First Amended Complaint in the Shareholder Putative Class Action was filed against us in the United States District Court District of New Jersey. The First Amended Complaint named additional defendants, including certain current and former directors, certain of our former officers, our former and current external auditors and Philips. Like the original complaint, the First Amended Complaint asserted claims under Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 thereunder. The Securities Class Period of the original complaint was expanded 20 months to include the period from March 29, 2000 through June 14, 2004. Pursuant to an October 17, 2005 consent order approved by the Court, lead plaintiff Greater Pennsylvania Pension Fund filed a Second Amended Complaint on November 15, 2005. The Second Amended Complaint dropped Philips as a defendant, but alleged the same claims and the same purported class period as the First Amended Complaint. Plaintiffs sought unspecified damages. Pursuant to the provisions of the Private Securities Litigation Reform Act, discovery in the action was stayed pending the filing and resolution of the defendants’ motions to dismiss, which were filed on January 17, 2006, and which were fully briefed as of June 16, 2006. On September 29, 2006, the Court denied our motions to dismiss and the motion to dismiss of the individual defendants. In the same order, the Court granted the motion to dismiss filed by our former and current external auditors. On November 3, 2006, we filed our Answer denying the material allegations contained in the Second Amended Complaint. On March 23, 2007, we entered into a memorandum of understanding and a stipulation of settlement with the lead plaintiff in which we agreed to pay $7,750 to settle all claims, throughout the class period, against all defendants in the action. We accrued the aforementioned $7,750 as of December 31, 2005. In April 2007, we paid the entire $7,750 into an escrow account for the eventual distribution to the plaintiffs. On May 16, 2007, the Court issued an Order Preliminarily Approving Settlement and Providing for Notice. The Court conducted a final approval hearing and approved the settlement on August 15, 2007. Neither we nor any of the individuals named in the action has admitted to liability or any wrongdoing in connection with the settlement. On August, 17, 2007, the Court entered final judgment and dismissed the case with prejudice.
Customer Litigation
A putative class action was filed in the United States District Court for the Central District of California. The action, entitled South Broward Hospital District, d/b/a Memorial Regional Hospital, et al. v. MedQuist, Inc. et al., Case No. CV-04-7520-TJH-VBKx, was filed on September 9, 2004 against us and certain of our present and former officials, purportedly on behalf of an alleged class of non-federal governmental hospitals and medical centers that the complaint claims were wrongfully and fraudulently overcharged for transcription services by defendants based primarily on our use of the AAMT line billing unit of measure. The complaint charged fraud, violation of the California Business and Professions Code, unjust enrichment, conversion, negligent supervision and violation of RICO. Plaintiffs seek damages in an unspecified amount, plus costs and interest, an injunction against alleged continuing illegal activities, an accounting, punitive damages and attorneys’ fees. Named as defendants, in addition to us, were one of our senior vice presidents, our former
F-21
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
executive vice president of marketing and new business development, our former executive vice president and chief legal officer, and our former executive vice president and chief financial officer.
On December 20, 2004, we and the individual defendants filed motions to dismiss for lack of personal jurisdiction and improper venue, or in the alternative, to transfer the putative action to the United States District Court for the District of New Jersey. On February 2, 2005, plaintiffs filed a Second Amended Complaint both adding and deleting named plaintiffs in an attempt to keep the putative action in the United States District Court for the Central District of California. On March 30, 2005, the United States District Court for the Central District of California issued an order transferring the putative action to the United States District Court District of New Jersey and assigning Case No.05-CV-2206-JBS-AMD.
On August 1, 2005, we and the individual defendants filed their respective Answers denying the material allegations contained in the Second Amended Complaint. On August 31, 2005, we and the individual defendants filed motions to dismiss the Second Amended Complaint for failure to state a claim and a motion to dismiss in favor of arbitration, or in the alternative, to stay pending arbitration. On December 12, 2005, the plaintiffs filed an Amendment to the Second Amended Complaint. On December 13, 2005, the Court issued an order requiring plaintiffs to file a Third Amended Complaint.
Plaintiffs filed the Third Amended Complaint on January 4, 2006. The Third Amended Complaint expands the claims made beyond issues arising from contracts based on AAMT line billing and beyond customers billed based on an AAMT line, alleging that we engaged in a scheme to inflate customers’ invoices without regard to the terms of individual contracts and even in the absence of any written contract. The Third Amended Complaint also limits plaintiffs’ claim for fraud in the inducement of the agreement to arbitrate to the three named plaintiffs whose contracts contain an arbitration provision and a subclass of similarly situated customers. On January 20, 2006 we and the individual defendants filed motions to dismiss the Third Amended Complaint for failure to state a claim and a motion to compel arbitration of all claims by the arbitration subclass and to stay the case in its entirety pending arbitration. On March 8, 2006 the Court held a hearing on these motions, and took the matter under submission. On March 30, 2007, the Court issued an order holding that plaintiffs could not make out a claim that we had violated the federal RICO statute, thus eliminating any claim against us for treble damages. The Court also found that plaintiffs could not make out a claim that we had engaged in any unfair or deceptive acts or practices in violation of state law, or that we had made any negligent misrepresentations to plaintiffs. In its ruling, the Court, without reaching a decision of whether any wrongdoing had occurred, allowed plaintiffs to proceed with their claims against us for fraud, unjust enrichment and an accounting. In its order, the Court denied our motion to compel arbitration regarding those customers whose contracts contained an agreement to arbitrate. We have appealed that decision to the Third Circuit Court of Appeals, and we moved the District Court to stay the matter pending that appeal. The District Court heard oral argument on our motion to stay on May 30, 2007 and took the motion under submission.
On December 18, 2007, the Third Circuit entered judgment denying our appeal and affirming the order of the District Court. That same day, the District Court entered an order denying our motion to stay pending appeal as moot and ordering all Initial Disclosures, all class-certification related discovery, and all briefing upon class-certification motion practice completed within three months unless enlarged for good cause. On February 21, 2008, the District Court entered a scheduling order pursuant to which all fact discovery related to class certification must be completed by April 15, 2008 and plaintiffs’ motion for class certification must be filed by April 30, 2008. All other fact discovery must be completed by July 31, 2008, and all expert discovery must be completed by October 31, 2008. Dispositive motions must be filed by November 24, 2008. The parties exchanged Initial Disclosures and commenced discovery.
The parties participated in court-ordered mediation in late 2007, but no settlement was reached. The parties continued to explore the possibility of resolving the litigation before trial, and have now reached agreement on settlement terms resolving all claims by the named plaintiffs. Under the parties’ agreement, we will make a lump sum payment of $7,537 to resolve all claims by the individual named plaintiffs and certain other additional putative class members represented by plaintiffs’ counsel but not named in the action. We have accrued the entire amount of this lump sum payment, $5,205 of which was accrued during 2005, in the accompanying consolidated balance sheet as of December 31, 2007. Neither we, nor any of the individual defendants, will admit to any liability or any wrongdoing in connection with the settlement. The District Court has entered a consent order staying the action through April 18, 2008 to allow the parties to finalize the settlement. We anticipate that the parties will execute a final settlement agreement and the case will be dismissed with prejudice in its entirety within the next four to eight weeks. Because the settlement will not be on a class-wide basis, no class will be certified and thus there is no requirement to give notice.
F-22
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
Medical Transcriptionist Litigation
Hoffmann Putative Class Action
A putative class action lawsuit was filed against us in the United States District Court for the Northern District of Georgia. The action, entitled Brigitte Hoffmann, et al. v. MedQuist, Inc., et al., Case No. 1:04-CV-3452, was filed with the Court on November 29, 2004 against us and certain current and former officials, purportedly on behalf of an alleged class of current and former employees and statutory workers, who are or were compensated on a “per line” basis for medical transcription services (Class Members) from January 1, 1998 to the time of the filing of the complaint (Class Period). The complaint specifically alleged that defendants systematically and wrongfully underpaid the Class Members during the Class Period. The complaint asserted the following causes of action: fraud, breach of contract, demand for accounting, quantum meruit, unjust enrichment, conversion, negligence, negligent supervision, and RICO violations. Plaintiffs sought unspecified compensatory damages, punitive damages, disgorgement and restitution. On December 1, 2005, the Hoffmann matter was transferred to the United States District Court for the District of New Jersey. On January 12, 2006, the Court ordered this case consolidated with the Myers Putative Class Action discussed below. As set forth below, we believe that the claims asserted in the consolidated Myers Putative Class Action have no merit and intend to vigorously defend that action.
Force Putative Class Action
A putative class action entitled Force v. MedQuist Inc. and MedQuist Transcriptions, Ltd., Case No. 05-cv-2608-WSD, was filed against us on October 11, 2005, in the United States District Court for the Northern District of Georgia. The action was brought on behalf of a putative class of current and former employees who claim they are or were compensated on a “per line” basis for medical transcription services but were allegedly underpaid due to the actions of defendants. The named plaintiff asserted claims for breach of contract, quantum meruit, unjust enrichment, and for an accounting. Upon stipulation and consent of the parties, on February 17, 2006, the Force matter was ordered transferred to the United States District Court for the District of New Jersey. Subsequently, on April 4, 2006, the parties entered into a stipulation and consent order whereby the Force matter was consolidated with the Myers Putative Class Action discussed below, and the consolidated amended complaint filed in the Myers action on January 31, 2006 was deemed to supersede the original complaint filed in the Force matter. As set forth below, we believe that the claims asserted in the consolidated Myers Putative Class Action have no merit and intend to vigorously defend that action.
Myers Putative Class Action
A putative class action entitled Myers, et al. v. MedQuist Inc. and MedQuist Transcriptions, Ltd., Case No. 05-cv-4608 (JBS), was filed against us on September 22, 2005 in the United States District Court for the District of New Jersey. The action was brought on behalf of a putative class of our employee and independent contractor transcriptionists who claim that they contracted with us to be paid on a 65 character line, but were allegedly underpaid due to intentional miscounting of the number of characters and lines transcribed. The named plaintiffs asserted claims for breach of contract, unjust enrichment, and request an accounting.
The allegations contained in the Myers case are substantially similar to those contained in the Hoffmann and Force putative class actions and, as detailed above, the three actions have now been consolidated. A consolidated amended complaint was filed on January 31, 2006. In the consolidated amended complaint, the named plaintiffs assert claims for breach of contract, breach of the covenant of good faith and fair dealing, unjust enrichment and demand an accounting. On March 7, 2006 we filed a motion to dismiss all claims in the consolidated amended complaint. The motion was fully briefed and argued on August 7, 2006. The Court denied the motion on December 21, 2006. On January 19, 2007, we filed our answer denying the material allegations pleaded in the consolidated amended complaint.
On May 17, 2007, the Court issued a Scheduling Order, ordering all pretrial fact discovery completed by October 30, 2007. The Court subsequently ordered plaintiffs to file their motion for class certification by December 14, 2007 and continued the date to complete fact discovery to January 14, 2007. On October 18, 2007, the Court heard oral argument on plaintiffs’ motion to compel further responses to written discovery regarding our billing practices. At the conclusion of the hearing, the Court denied plaintiffs’ motion, finding plaintiffs had not established that the billing discovery sought was relevant to the claims or defenses regarding transcriptionist pay alleged in their case. On December 14, 2007, plaintiffs filed their motion for class certification, identifying a proposed class of all our transcriptionists who were compensated on a per line basis for work completed on MedRite, MTS or DEP transcription platforms from November 29, 1998 to the present and alleging that the proposed class was underpaid by more than $80 million, not including interest.
On January 4, 2008, the Court entered a Consent Order, ordering our opposition to the motion for class certification to be filed by March 14, 2008, plaintiffs’ reply brief to be filed by May 14, 2008 and setting oral argument for June 2, 2008. No date has been set for trial. On January 9, 2008, the Court entered a Consent Order extending the deadline for the parties to complete depositions of identified witnesses
F-23
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
through February 15, 2008. We have now deposed each of the named plaintiffs and all witnesses who offered declarations in support of plaintiffs’ motion for class certification, and plaintiffs have deposed numerous MedQuist present and former employees. On February 8, 2008, plaintiffs indicated that they will seek leave to file an amended class certification brief to narrow their claims. On February 19, 2008, the parties exchanged their Initial Disclosures. Plaintiffs’ disclosures limit their damages estimate to $41 million related to alleged underpayment on the MedRite transcription platform; however, plaintiffs state that they are continuing to analyze potential undercounting and will supplement their damages claim. On March 10, 2008, plaintiffs’ moved for leave to file an amended motion for class certification dropping all allegations involving our DEP transcription platform and narrowing the claims asserted regarding the legacy MTS transcription platform. We did not oppose plaintiffs’ motion for leave. On March 11, 2008, the Court granted plaintiffs’ motion, ordering us to file our opposition to plaintiffs’ amended motion for class certification by April 4, 2008 and ordering plaintiffs to file their reply by May 23, 2008. The hearing date was not changed and oral argument remains set for June 2, 2008. We believe that the claims asserted in the consolidated actions have no merit and intend to vigorously defend the suit.
Shareholder Derivative Litigation
On October 4, 2005, we announced the dismissal with prejudice of a shareholder derivative action filed in United States District Court for the District of New Jersey. The suit, Rhoda Kanter (Plaintiff) v. Hans M. Barella et al. (Defendants), was filed on November 12, 2004 against Philips and 10 current and former members of our board of directors. We were named as a nominal defendant.
In a ruling dated September 21, 2005, the Court found plaintiff’s allegations that our board of directors breached their fiduciary duties to us to be insufficient. The plaintiff had alleged that for a period from 2001 through 2004, the Defendants violated their fiduciary duties by permitting artificial inflation of billing figures; failing to adequately ensure accurate and lawful billing practices; and failing to accurately report our true financial condition in our published financial statements. On October 3, 2005, plaintiff filed a motion for reconsideration of the Court’s order dismissing the action with prejudice. On November 16, 2005, the Court denied plaintiff’s motion for reconsideration. On December 13, 2005, plaintiff filed a Notice of Appeal with the United States Court of Appeals for the Third Circuit. Plaintiff’s appeal was fully briefed as of May 2006, and the Court of Appeals heard oral argument on the appeal on March 1, 2007. Plaintiff’s appeal was denied by the Court of Appeals on May 25, 2007.
Shareholder Litigation
Costa Brava Partnership III, L.P. Shareholder Litigation
On October 9, 2007, a single count Complaint and an Order to Show Cause were filed against us in the Superior Court of New Jersey, Chancery Division, Burlington County by one of our shareholders. The action, entitled Costa Brava Partnership III, L.P. v. MedQuist Inc. (Bur-C-0149-07), sought to compel us to hold an annual meeting of shareholders (Annual Meeting Claim).
On October 30, 2007, plaintiff requested access under New Jersey law to certain of our books and records. In response to plaintiff’s request, we voluntarily provided plaintiff with those books and records that we believed we were required to produce under New Jersey law. Thereafter, on November 9, 2007, plaintiff filed an Amended Complaint to assert a second claim to compel us to provide it with access to certain other books and records (Books and Records Claim). The Annual Meeting Claim and the Books and Records Claim sought equitable relief only.
In December 2007, we agreed to hold our annual meeting of shareholders on December 31, 2007. This resolved the Annual Meeting Claim. Prior to the annual meeting, we voluntarily produced to plaintiff certain additional books and records that plaintiff requested in the Books and Records Claim. Thereafter, on January 24, 2008, we filed an opposition to plaintiff’s Order to Show Cause to compel access to the remaining books and records. On February 4, 2008, plaintiff filed a reply brief. The Books and Records Claim has been briefed and the parties are waiting for the Court to schedule a hearing date to resolve the matter. We believe that the books and records requests at issue in the Books and Records Claim are burdensome and overbroad and intend to vigorously defend the action.
Kahn Putative Class Action
A shareholder putative class action lawsuit was filed against us in the Superior Court of New Jersey, Chancery Division, Burlington County. The action, entitled Alan R. Kahn v. Stephen H. Rusckowski, et al., Docket No. BUR-C-000007-08, was filed with the Court on January 22, 2008 against us, Philips and our four non-independent directors, Clement Revetti, Jr., Stephen H. Rusckowski, Gregory M. Sebasky and Scott Weisenhoff. Plaintiff purports to bring the action on his own behalf and on behalf of all current holders of our common stock. The complaint alleges that defendants breached their fiduciary duties of good faith, fair dealing, loyalty, and due care by
F-24
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
purportedly agreeing to and initiating a process for our sale or a change of control transaction which will allegedly cause harm to plaintiff and the putative class. Plaintiff seeks damages in an unspecified amount, plus costs and interest, a judgment declaring that defendants breached their fiduciary duties and that any proposed transactions regarding our sale or change of control are void, an injunction preventing our sale or any change of control transaction that is not entirely fair to the class, an order directing us to appoint three independent directors to our board of directors, and attorneys’ fees and expenses. We have not yet been required to file a responsive pleading. We believe that the claims asserted have no merit and intend to defend the case vigorously
Reseller Arbitration Demand
On October 1, 2007, we received from counsel to nine current and former resellers of our products (Claimants), a copy of an arbitration demand filed by the Claimants, initiating an arbitration proceeding styled Diskriter, Inc., Electronic Office Systems, Inc., Milner Voice & Data, Inc., Nelson Systems, Inc., NEO Voice and Communications, Inc., Office Business Systems, Inc., Roach-Reid Office Systems, Inc., Stiles Office Systems, Inc., and Travis Voice and Data, Inc. v. MedQuist Inc. and MedQuist Transcriptions, Ltd. (filed on September 27, 2007, AAA, Case Number Not Yet Assigned). The arbitration demand purports to set forth claims for breach of contract; breach of covenant of good faith and fair dealing; promissory estoppel; misrepresentation; and tortuous interference with contractual relations. The Claimants allege that we breached our written agreements with the Claimants by: (i) failing to provide reasonable training, technical support, and other services; (ii) using the Claimants’ confidential information to compete against the Claimants; (iii) directly competing with the Claimants’ territories; and (iv) failing to make new products available to the Claimants. In addition, the Claimants’ allege that we made false oral representations that we: (i) would provide new product, opportunities and support to the Claimants; (ii) were committed to continuing to use Claimants; (iii) did not intend to create our own sales force with respect to the Claimants’ territory; and (iv) would stay out of Claimants’ territories and would not attempt to take over the Claimants business and relationships with the Claimants’ customers and end-users. The Claimants assert that they are seeking damages in excess of $24.3 million. The arbitrator has not yet set a date for us to formally respond to the arbitration demand. We deny all wrongdoing and intend to defend ourselves vigorously including asserting counterclaims against the Claimants as appropriate.
Anthurium Patent Litigation
On November 6, 2007, Anthurium Solutions, Inc. filed an action entitledAnthurium Solutions, Inc. v. MedQuist Inc., et al.,Civil Action No. 2-07CV-484, in the United States District Court for the Eastern District of Texas, alleging that we infringed and continue to infringe United States Patent No. 7,031,998 through our DEP transcription platform. The complaint also alleges patent infringement claims against Spheris, Inc. and Arrendale Associates, Inc. The complaint seeks injunctive relief and unspecified damages, including enhanced damages and attorneys’ fees. We filed our answer on January 15, 2008 and counterclaimed seeking a declaratory judgment of non-infringement and invalidity. No scheduling order has been issued, and no pretrial dates have been set. Our investigation of the claims is ongoing, and we are awaiting plaintiffs’ preliminary infringement contentions. We believe that the claims asserted have no merit and intend to vigorously defend the suit.
Other Matters
From time to time, we have been involved in various claims and legal actions arising in the ordinary course of business. In our opinion, the outcome of such actions will not have a material adverse effect on our consolidated financial position, results of operations, liquidity or cash flows.
We provide certain indemnification provisions within our standard agreement for the sale of software and hardware (collectively, Products) to protect our customers from any liabilities or damages resulting from a claim of U.S. patent, copyright or trademark infringement by third parties relating to our Products. We believe that the likelihood of any future payout relating to these provisions is remote. Accordingly, we have not recorded any liability in our consolidated financial statements as of December 31, 2007 or 2006 related to these indemnification provisions.
We had insurance policies which provided coverage for certain of the matters related to the legal actions described herein. We received total insurance recoveries of $24,795 related to these policies (See Note 5). We do not expect to receive any additional insurance recoveries related to the legal actions described above.
F-25
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
14. Stock Option Plans
Our stock option plans provide for the granting of options to purchase shares of common stock to eligible employees (including officers) as well as to our non-employee directors. Options may be issued with the exercise prices equal to the fair market value of the common stock on the date of grant or at a price determined by a committee of our board of directors. Stock options vest and are exercisable over periods determined by the committee, generally five years, and expire no more than 10 years after the grant.
In July 2004, our board of directors affirmed our June 2004 decision to indefinitely suspend the exercise and future grant of options under our stock option plans. Ten former executives separated from us in 2005 and 2004. Notwithstanding the suspension, to the extent such executives held options that were vested as of their resignation date, such options remain exercisable for the post-termination period, generally 90 days, commencing on the date that the suspension is lifted for the exercise of options. This suspension was lifted on October 4, 2007. There were 704 shares that qualified for this post-termination exercise period. A summary of these remaining post-termination options as of December 31, 2007 is as follows:
| Options Exercisable | ||||||||||||
| Average | ||||||||||||
| Number of | Intrinsic | Exercise | ||||||||||
| Range of Exercise Prices | Shares | Value | Price | |||||||||
| $ 2.71-$10.00 | 31 | $ | 161 | $ | 5.71 | |||||||
| $10.01-$20.00 | 142 | — | $ | 15.04 | ||||||||
| $20.01-$70.00 | 512 | — | $ | 47.31 | ||||||||
| 685 | $ | 161 | ||||||||||
The extension of the life of the awards was recorded as a modification of the grants. Under APB 25, the modification created intrinsic value for vested stock if the market value of the stock on the date of termination exceeded the exercise price. Therefore, these grants required an immediate recognition of the compensation expense with an offsetting credit to common stock. We recorded a charge of $0, $0 and $37 for the years ended December 31, 2007, 2006 and 2005, respectively.
Information with respect to our common stock options is as follows:
| Weighted | ||||||||||||||||
| Weighted | Average | |||||||||||||||
| Shares | Average | Remaining | Aggregate | |||||||||||||
| Subject to | Exercise | Contractual | Intrinsic | |||||||||||||
| Options | Price | Life in Years | Value | |||||||||||||
| Outstanding, January 1, 2005 | 4,211 | $ | 29.27 | |||||||||||||
| Forfeited | (216 | ) | $ | 34.25 | ||||||||||||
| Canceled | (401 | ) | $ | 27.77 | ||||||||||||
| Outstanding, December 31, 2005 | 3,594 | $ | 28.80 | |||||||||||||
| Forfeited | (75 | ) | $ | 22.11 | ||||||||||||
| Canceled | (1,207 | ) | $ | 22.00 | ||||||||||||
| Outstanding, December 31, 2006 | 2,312 | $ | 32.57 | |||||||||||||
| Granted | 200 | $ | 11.20 | |||||||||||||
| Exercised | (4 | ) | $ | 2.71 | ||||||||||||
| Forfeited | (137 | ) | $ | 29.10 | ||||||||||||
| Canceled | (12 | ) | $ | 17.45 | ||||||||||||
| Outstanding, December 31, 2007 | 2,359 | $ | 31.08 | 3.0 | $ | 162 | ||||||||||
| Exercisable, December 31, 2007 | 2,108 | $ | 33.30 | 2.3 | $ | 162 | ||||||||||
| Options vested and expected to vest as of December 31, 2007 | 2,273 | $ | 31.64 | 2.8 | $ | 162 | ||||||||||
The aggregate intrinsic value is calculated using the difference between the closing stock price on the last trading day of 2007 and the option exercise price, multiplied by the number of in-the-money options.
Under the Black-Scholes option pricing model, input assumptions are determined at the time of option grant and are not adjusted during the life of that grant. The following are assumptions used in the 2007 option fair value calculations.
F-26
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
| Expected term (years) | 6.50 | |||
| Expected volatility | 61.6 | % | ||
| Dividend Yield | 0 | % | ||
| Expected risk free interest rate | 4.40 | % |
Significant assumptions required to estimate the fair value of stock options include the following:
| • | Expected term: The SEC Staff Accounting Bulletin No 107 “Simplified” method has been used to determine a weighted average expected term of options granted. | ||
| • | Expected volatility: We have estimated expected volatility based on the historical stock price volatility of a group of similar publicly traded companies. We believe that our historical volatility is not indicative of future volatility. |
The weighted average grant date fair value of options issued in 2007 was $7.03 per share.
A summary of outstanding and exercisable options as of December 31, 2007 is as follows:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Weighted | ||||||||||||||||||||
| Average | Weighted | Weighted | ||||||||||||||||||
| Remaining | Average | Average | ||||||||||||||||||
| Range of | Number | Contractual Life | Exercise | Number | Exercise | |||||||||||||||
| Exercise Prices | of Shares | (in years) | Price | of Shares | Price | |||||||||||||||
| $2.71-$10.00 | 31 | 1.8 | $ | 5.71 | 31 | $ | 5.71 | |||||||||||||
| $10.01-$20.00 | 728 | 5.0 | $ | 15.03 | 477 | $ | 16.37 | |||||||||||||
| $20.01-$30.00 | 830 | 3.0 | $ | 26.61 | 830 | $ | 26.61 | |||||||||||||
| $30.01-$40.00 | 218 | 1.2 | $ | 32.23 | 218 | $ | 32.23 | |||||||||||||
| $40.01-$70.00 | 552 | 1.1 | $ | 59.84 | 552 | $ | 59.84 | |||||||||||||
| 2,359 | 3.0 | $ | 31.08 | 2,108 | $ | 33.30 | ||||||||||||||
There were 200 and 0 options granted during 2007 and 2006. There were 4, 0 and 0 shares exercised in 2007, 2006 and 2005. The total fair value of shares vested during 2007 was $396.
As of December 31, 2007, there were 962 additional options available for grant under our stock option plans. When we became up to date in our reporting to the SEC in October 2007, certain executive officers, in accordance with their employment agreements, received an aggregate of 200 options with an exercise price equal to the then market value of our common stock on the date of grant. In 2005, $78 is included in the pro forma stock-based compensation amount depicted in Note 3 related to these options. In 2007 and 2006, $84 and $136, respectively, is included as selling, general and administrative expenses and $36 and $58 is included in research and development expenses in the accompanying consolidated statement of operations related to these options with the total of $120 and $194 included in common stock in the accompanying consolidated balance sheet as of December 31, 2006.
15. Income Taxes
The sources of loss before income taxes and the income tax provision for the years ended December 31, 2007, 2006 and 2005 are as follows:
| 2007 | 2006 | 2005 | ||||||||||
| Loss before income taxes: | ||||||||||||
| Domestic | $ | (13,557 | ) | $ | (15,302 | ) | $ | (93,021 | ) | |||
| Foreign | 690 | 654 | 2,151 | |||||||||
| Loss before income taxes | $ | (12,867 | ) | $ | (14,648 | ) | $ | (90,870 | ) | |||
| Current income tax provision: | ||||||||||||
| Federal | $ | 34 | $ | (3,615 | ) | $ | (16,171 | ) | ||||
| State and local | 194 | 291 | (151 | ) | ||||||||
| Foreign | 233 | 393 | 429 | |||||||||
| Current income tax provision | 461 | (2,931 | ) | (15,893 | ) | |||||||
F-27
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
| 2007 | 2006 | 2005 | ||||||||||
| Deferred income tax provision: | ||||||||||||
| Federal | 2,735 | 4,825 | 28,702 | |||||||||
| State and local | (499 | ) | (259 | ) | 7,830 | |||||||
| Foreign | (358 | ) | 659 | 123 | ||||||||
| Deferred income tax provision | 1,878 | 5,225 | 36,655 | |||||||||
| Income tax provision | $ | 2,339 | $ | 2,294 | $ | 20,762 | ||||||
The reconciliation of the statutory federal income tax rate to our effective income tax rate is as follows:
| 2007 | 2006 | 2005 | ||||||||||
| Statutory federal income tax rate | 35.0 | 35.0 | 35.0 | |||||||||
| State income taxes, net of federal tax effect | 3.6 | (2.5 | ) | 5.0 | ||||||||
| Valuation allowance | (53.0 | ) | (40.4 | ) | (62.5 | ) | ||||||
| Impact of foreign operations | (0.4 | ) | (1.1 | ) | (0.5 | ) | ||||||
| Adjustments to tax reserves | (0.6 | ) | 1.7 | 0.5 | ||||||||
| Permanent differences | (3.4 | ) | (7.1 | ) | (0.7 | ) | ||||||
| Tax law changes | 1.8 | — | — | |||||||||
| Other | (1.2 | ) | (1.3 | ) | 0.4 | |||||||
| Effective income tax rate | (18.2 | ) | (15.7 | ) | (22.8 | ) | ||||||
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets and liabilities as of December 31, 2007, 2006 and 2005 were as follows:
| 2007 | 2006 | 2005 | ||||||||||
Deferred tax assets: | ||||||||||||
| Foreign net operating loss carryforwards | $ | 2,733 | $ | 3,349 | $ | 3,022 | ||||||
| Domestic net operating loss carryforwards | 36,295 | 18,492 | 2,687 | |||||||||
| Accounts receivable | 1,673 | 1,846 | 1,964 | |||||||||
| Property and equipment | 1,917 | 1,975 | 1,285 | |||||||||
| Intangibles | 21,746 | 22,285 | 24,192 | |||||||||
| Employee compensation and benefit plans | 953 | 1,250 | 2,136 | |||||||||
| Deferred compensation | 617 | 446 | 954 | |||||||||
| Customer accommodation and quantification | 7,087 | 6,374 | 18,485 | |||||||||
| Accruals and reserves | 4,855 | 10,556 | 8,078 | |||||||||
| Other | 1,714 | 2,113 | 1,761 | |||||||||
| Total gross deferred tax assets | 79,590 | 68,686 | 64,564 | |||||||||
| Less: Valuation allowance | (74,500 | ) | (64,601 | ) | (58,039 | ) | ||||||
| Total deferred tax assets | 5,090 | 4,085 | 6,525 | |||||||||
Deferred tax liabilities: | ||||||||||||
| Intangibles | (21,377 | ) | (18,704 | ) | (15,947 | ) | ||||||
| Other | (935 | ) | (739 | ) | (919 | ) | ||||||
| Total deferred tax liabilities | (22,312 | ) | (19,443 | ) | (16,866 | ) | ||||||
| Net deferred tax liability | $ | (17,222 | ) | $ | (15,358 | ) | $ | (10,341 | ) | |||
As of December 31, 2007, we had federal net operating loss carry forwards of approximately $80,520 which will partially expire in 2027 and 2028.
As of December 31, 2007 and 2006, we had state net operating loss carry forwards of approximately $167,750 and $115,632, respectively, which will expire between 2008 and 2027. In addition, we have foreign net operating loss carry forwards of approximately $15,492, which do not expire. Utilization of the net operating loss carry forwards may be subject to an annual limitation in the event of a change in ownership in future years as defined by Section 382 of the Internal Revenue Code and similar state provisions.
In assessing the future realization of deferred taxes, we consider whether it is more likely than not that some portion or all of the deferred income tax assets will not be realized based on projections of our future taxable earnings. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible.
F-28
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
During 2007, we decreased our valuation allowance against our deferred tax assets generated in foreign tax jurisdictions from $1,803 to $1,217 based on management’s assessment of future earnings available to utilize these deferred tax assets.
In the fourth quarter of 2005, a valuation allowance of $56,808 was established against various domestic deferred tax assets. After consideration of all evidence, both positive and negative, management concluded that it was more likely than not that a majority of the domestic deferred tax assets would not be realized. In 2006 and 2007, we increased this valuation allowance to $62,798 and $73,313, respectively.
Domestic deferred tax assets were recognized to the extent that objective positive evidence existed with respect to their future utilization. The objective positive evidence included the potential to carry back any losses generated by the deferred tax assets in the future as well as income expected to be recognized due to the reversal of deferred tax liabilities as of December 31, 2007. In analyzing deferred tax liabilities as a source for potential income for purposes of recognizing deferred tax assets, the deferred tax liabilities related to excess book basis in goodwill over tax basis in goodwill were considered a source of future income for benefiting deferred tax assets with indefinite lives only due to the indefinite life and uncertainty of reversal of these liabilities during the same period as the non-indefinite life deferred tax assets.
Our consolidated income tax expense for the years ended December 31, 2007, 2006 and 2005 consists principally of an increase in deferred tax liabilities related to goodwill amortization deductions for income tax purposes during the applicable year as well as state and foreign income taxes.
Effective January 1, 2007, we adopted FASB Interpretation 48,Accounting for Uncertainty in Income Taxes — an interpretation of FASB Statement 109(FIN 48). FIN 48 prescribes, among other things, a recognition threshold and measurement attributes for the financial statement recognition and measurement of uncertain tax positions taken or expected to be taken in a company’s income tax return. FIN 48 utilizes a two-step approach for evaluating uncertain tax positions accounted for in accordance with FASB Statement 109,Accounting for Income Taxes. Step one,Recognition, requires a company to determine if the weight of available evidence indicates that a tax position is more likely than not to be sustained upon audit, including resolution of related appeals or litigation processes, if any. Step two,Measurement, is based on the largest amount of benefit, which is more likely than not to be realized on settlement with the taxing authority. We recorded a cumulative effect increase to retained earnings of $389 upon adoption.
In accordance with FIN 48, the total amount of unrecognized tax benefits as of December 31, 2007 was $5,614, which includes $581 of accrued interest related to unrecognized income tax benefits which we recognize as a component of the provision for income taxes. Of the $5,614 unrecognized tax benefits, $4,773 relates to tax positions which if recognized would impact the effective tax rate, not considering the impact of any valuation allowance. Of the $4,773, $2,859 is attributable to uncertain tax positions with respect to certain deferred tax assets which if recognized would currently be offset by a full valuation allowance due to the fact that at the current time it is more likely than not that these assets would not be recognized due to a lack of sufficient projected income in the future.
F-29
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
The following is a roll-forward of the changes in our unrecognized tax benefits:
| Total unrecognized tax benefits as of January 1, 2007 | $ | 5,002 | ||
| Gross amount of decreases in unrecognized tax benefits as a result of tax positions taken during the prior period | (64 | ) | ||
| Gross amount of increases in unrecognized tax benefits as a result of tax positions taken during the prior period | 10 | |||
| Gross amount of increases in unrecognized tax benefits as a result of tax positions taken during the current period | 252 | |||
| Amount of decreases in the unrecognized tax benefits relating to settlements with taxing authorities | (10 | ) | ||
| Reduction to unrecognized tax benefits as a result of a lapse of applicable statute of limitations | (157 | ) | ||
| Total unrecognized tax benefits as of December 31, 2007 | $ | 5,033 | ||
| Total unrecognized tax benefits that would impact the effective tax rate if recognized | $ | 4,773 | ||
| Total amount of interest and penalties recognized in the accompanying consolidated statement of operations for the year ended December 31, 2007 | $ | 141 | ||
| Total amount of interest and penalties recognized in the accompanying consolidated balance sheet as of December 31, 2007 | $ | 581 | ||
We file income tax returns in the U.S. federal jurisdiction, all U.S. states which require income tax returns and foreign jurisdictions. Due to the nature of our operations, no state or foreign jurisdiction is individually significant. With limited exceptions we are no longer subject to examination by the U.S. federal or states jurisdiction for years beginning prior to 2003. We are currently under federal tax audit for the tax years 2003 through 2006. We are no longer subject to examination by the UK federal jurisdiction for years beginning prior to 2005. We do have various state tax audits and appeals in process at any given time.
We anticipate decreases in unrecognized tax benefits of approximately $304 related to state statutes of limitations expiring during 2008. Our unrecognized tax benefits are expected to change in 2008. However, we do not anticipate any significant increases or decreases within the next twelve months.
16. Employee Benefit Plans
401(k) Plan
We maintain a tax-qualified retirement plan named the MedQuist 401(k) Plan (401(k) Plan) that provides eligible employees with an opportunity to save for retirement on a tax advantaged basis. Our 401(k) Plan allows eligible employees to contribute up to 25% of their annual eligible compensation on a pre-tax basis, subject to applicable Internal Revenue Code limits. Elective deferral contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participant’s directives. Employee elective deferrals are 100% vested at all times. Our 401(k) Plan provides that we may make a discretionary matching contribution to the participants in the 401(k) Plan. Our discretionary matching contribution, if any, shall be in an amount not to exceed 100% of the first 25% of a plan participant’s compensation contributed as pre-tax contributions to the 401(k) Plan. In our sole discretion, we may make descretionary matching contributions on a quarterly or annual basis. Historically we have matched 50% of each participant’s contribution, up to a maximum of 5% of each participant’s total annual compensation. Matching contributions are 33% vested after one year of service, 67% vested after two years of service and 100% vested after three years of service. The charge to operations for our matching contributions for the years ended December 31, 2007, 2006 and 2005 was $1,547, $1,432 and $1,380 respectively.
Executive Deferred Compensation Plan
We established the MedQuist Inc. Executive Deferred Compensation Plan (EDCP) in 2001. The EDCP, which is administered by the compensation committee of our board of directors, allowed certain members of management and highly compensated employees to defer a certain percentage of their income. Participants were permitted to defer compensation into an account in which proceeds were available either during or after termination of employment. The compensation committee authorized that certain contributions made to a retirement distribution account be matched with either shares of our common stock or cash. Participants were not entitled to receive matching contributions if they elected to make deferrals to an account into which proceeds are available during employment. Participants were able to defer up to 15% of their base salary (or such other maximum percentage as may be approved by the compensation committee) and 90% of their bonus (or such other maximum percentage as may be approved by the compensation committee). Distributions to a participant made pursuant to a retirement distribution account may be made to the participant upon the participant’s termination or attainment of age 65, as elected by the participant in their enrollment agreement. Distributions to a participant made pursuant to an in-service distribution account may be made at the election of the participant in their enrollment agreement, subject to certain exceptions. The balances in the EDCP are not funded, but are segregated, and participants in the EDCP are our general creditors. All amounts deferred in the EDCP increase or decrease based on hypothetical investment results of the participant’s selected investment alternatives. However, EDCP distributions are paid out of our funds rather than from a dedicated
F-30
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
investment portfolio. As of December 31, 2007 and 2006, the value of the assets held, managed and invested pursuant to the EDCP was $1,118 and $1,120, respectively, and is included in other current assets in the accompanying consolidated balance sheets. As of December 31, 2007 and 2006, the corresponding deferred compensation liability reflecting amounts due to employees was $637 and $827, respectively, and is included in accrued expenses in the accompanying consolidated balance sheets.
Effective January 1, 2005, the EDCP was suspended and no further contributions have been made.
Board of Directors Deferred Compensation Plan
Commencing on January 1, 2005, a portion of the compensation paid to our independent directors was deferred compensation in the form of common stock having a fair market value of $50 on the date of grant. Our board of directors postponed the granting of the deferred compensation awards for 2006 and 2007 until October 2007 which is when we became up to date with our periodic reporting obligations with the SEC. As of December 31, 2007 and 2006, $0 and $332, respectively, related to deferred compensation is included in the accompanying consolidated statements of shareholders’ equity and other comprehensive income. This plan was terminated in February 2008.
17. Related-Party Transactions
From time to time, we enter into transactions in the normal course of business with related parties. The audit committee of our board of directors has been charged with the responsibility of approving or satisfying all related party transactions other than those between us and Philips. In any situation where the audit committee sees fit to do so, any related party transaction, other than those between us and Philips, may be presented to disinterested members of our board of directors for approval or ratification.
In connection with Philips’ investment in us, we have entered into various agreements with Philips. All material transactions between Philips and us are reviewed and approved by the supervisory committee of our board of directors. The supervisory committee is comprised of directors’ independent from Philips. Listed below is a summary of our material agreements with Philips.
Licensing Agreement
In connection with Philips’ tender offer, we entered into a Licensing Agreement with Philips Speech Processing GmbH, an affiliate of Philips which is now known as Philips Speech Recognition Systems GmbH (PSRS), on May 22, 2000 (Licensing Agreement). The Licensing Agreement was subsequently amended by the parties as of January 1, 2002, February 23, 2003, August 10, 2003, September 1, 2004, December 30, 2005 and February 13, 2007.
Under the Licensing Agreement, we license from PSRS its SpeechMagic speech recognition and processing software, including any updated versions of the software developed by PSRS during the term of the License Agreement (Licensed Product), for use by us anywhere in the world. We pay a fee for use of this license based upon a per line fee for each transcribed line of text processed through the Licensed Product.
Upon the expiration of its initial term on June 28, 2005, the Licensing Agreement was renewed for an additional five year term.
In connection with the Licensing Agreement, we have a consulting arrangement with PSRS whereby PSRS assists us with the integration of its speech and transcription technologies.
OEM Supply Agreement
On September 21, 2007, we entered into an Amended and Restated OEM Supply Agreement (Amended OEM Agreement) with PSRS. The Amended OEM Agreement amends and restates a previous OEM Supply Agreement with PSRS dated September 23, 2004. In connection with the Amended OEM Agreement certain amounts paid to PSRS were capitalized in fixed assets and are being amortized over a three-year period.
Pursuant to the Amended OEM Agreement, we purchased a co-ownership interest in all rights and interests in and to SpeechQ for Radiology together with its components, including object and source code for the SpeechQ for Radiology application and the SpeechQ
F-31
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
for Radiology integration SDK (collectively, the Product), but excluding the SpeechMagic speech recognition and processing software, which we separately license from PSRS for a fee under the Licensing Agreement. Additionally, the Amended OEM Agreement provides that we shall receive, in exchange for a fee, the exclusive right in the United States, Canada and certain islands of the Caribbean (collectively the Exclusive Territory) to sell, service and deliver the Product. In addition, PSRS has agreed that for the term of the Amended OEM Agreement it will not release a front-end multi-user reporting solution (including one similar to the Product) in the medical market in the Exclusive Territory nor will it directly authorize or assist any of its affiliates to do so either; provided that the restriction does not prevent PSRS’s affiliates from integrating SpeechMagic within their general medical application products. The Amended OEM Agreement further provides that we shall make payments to PSRS for PSRS’ development of an interim version of the software included in the Product (Interim Version). Except for the Interim Version which we and PSRS will co-own, the Amended OEM Agreement provides that any improvements, developments or other enhancements either we or PSRS makes to the Product (collectively, Improvements) shall be owned exclusively by the party that developed such Improvement. Each party has the right to seek patent or other protection of the Improvements it owns independent of the other party.
The term of the Amended OEM Agreement extends through June 30, 2010 and will automatically renew for an additional three year term provided that we are in material compliance with the Amended OEM Agreement as of such date. If PSRS decides to discontinue all business relating to the Product in the Exclusive Territory on or after June 30, 2010, PSRS can effect such discontinuation by terminating the Amended OEM Agreement by providing us with six months’ prior written notice of such discontinuation, provided the earliest such notice can be delivered is June 30, 2010. Either party may terminate the Amended OEM Agreement for cause immediately in the event that a material breach by the other party remains uncured for more than 30 days following delivery of written notice or in the event that the other party becomes insolvent or files for bankruptcy.
Equipment Sales
We purchase dictation related equipment from Philips.
Insurance Coverage through Philips
We obtain all of our business insurance coverage (other than workers’ compensation) through Philips.
Purchasing Agreements
For the three years ended December 31, 2007 we entered into annual letter agreements with Philips Electronics North America Corporation (PENAC), an affiliate of Philips, to purchase products and services from certain suppliers under the terms of the prevailing agreements between such suppliers and PENAC. As of January 1, 2008, we are no longer a party to an agreement with PENAC to purchase the aforementioned products and services.
From time to time, we enter into other miscellaneous transactions with Philips including Philips purchasing certain products and implementation services from us. We recorded net revenues from sales to Philips of $0, $26 and $754 for the years ended December 31, 2007, 2006 and 2005, respectively.
Our consolidated balance sheets as of December 31, 2007 and 2006 reflect other assets related to Philips of $1,002 and $0, respectively, and accrued expenses related to Philips of $1,534 and $2,030, respectively.
Listed below is a summary of the expenses incurred by us in connection with the various Philips agreements noted above for the years ended December 31, 2007, 2006 and 2005. Charges related to these agreements are included in cost of revenues and selling, general and administrative expenses in the accompanying consolidated statements of operations.
| Years Ended December 31, | ||||||||||||
| 2007 | 2006 | 2005 | ||||||||||
| PSRS licensing | $ | 2,479 | $ | 2,390 | $ | 1,216 | ||||||
| PSRS consulting | — | 3 | 162 | |||||||||
| OEM agreement | 2,252 | 1,429 | 1,521 | |||||||||
| Dictation equipment | 854 | 878 | 1,238 | |||||||||
| Insurance | 1,794 | 1,601 | 957 | |||||||||
| PENAC | 40 | 30 | 54 | |||||||||
| Other | — | 42 | 248 | |||||||||
| Total | $ | 7,419 | $ | 6,373 | $ | 5,396 | ||||||
F-32
Table of Contents
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
(In thousands, except per share amounts)
On July 29, 2004, we entered into an agreement with Nightingale under which Nightingale agreed to provide interim chief executive services to us. On July 30, 2004, our board of directors appointed Howard S. Hoffmann to serve as our non-employee Chief Executive Officer (CEO). Mr. Hoffmann serves as the Managing Partner of Nightingale. With the departure of our former President in May 2007, our board of directors appointed Mr. Hoffmann to the additional position of President in June 2007. Mr. Hoffmann serves as our President and Chief Executive Officer pursuant to the terms of the agreement between us and Nightingale which was amended on March 14, 2008 (Amendment). The Amendment, among other things, extends the term of Mr. Hoffmann’s role as our President and Chief Executive Officer through August 1, 2008. Our board of directors is responsible for monitoring and reviewing the performance of Mr. Hoffmann on an ongoing basis. Our agreement with Nightingale also permits us to engage additional personnel employed by Nightingale to provide consulting services to us from time to time.
For the years ended December 31, 2007, 2006 and 2005, we incurred charges of $2,914, $3,005 and $3,207, respectively, for Nightingale services. From February 1, 2007 through December 31, 2007, the Nightingale charges were recorded in selling, general and administrative expenses in the accompanying consolidated statements of operations due to Nightingale’s focus on operational matters instead of the Review and Management’s Billing Assessment. Prior to February 1, 2007, charges related to Nightingale were recorded in cost of investigation and legal proceedings, net (see Note 5). As of December 31, 2007 and 2006, accrued expenses included $400 and $548, respectively, for amounts due to Nightingale for services performed.
See Note 11 for a discussion of our agreements with A-Life.
18. Quarterly Data (unaudited)
| 1st | 2nd | 3rd | 4th | |||||||||||||
| Quarter | Quarter | Quarter | Quarter | |||||||||||||
2006 | ||||||||||||||||
| Net revenues | $ | 96,014 | $ | 93,359 | $ | 82,096 | $ | 86,622 | ||||||||
| Net loss | $ | (8,471 | ) | $ | (2,416 | ) | $ | (2,104 | ) | $ | (3,951 | ) | ||||
| Net loss per share: | ||||||||||||||||
| Basic | $ | (0.23 | ) | $ | (0.06 | ) | $ | (0.06 | ) | $ | (0.11 | ) | ||||
| Diluted | $ | (0.23 | ) | $ | (0.06 | ) | $ | (0.06 | ) | $ | (0.11 | ) | ||||
| Weighted average shares outstanding: | ||||||||||||||||
| Basic | 37,484 | 37,484 | 37,484 | 37,484 | ||||||||||||
| Diluted | 37,484 | 37,484 | 37,484 | 37,484 | ||||||||||||
2007 | ||||||||||||||||
| Net revenues | $ | 89,066 | $ | 88,692 | $ | 82,518 | $ | 80,066 | ||||||||
| Net income (loss) | $ | (1,886 | ) | $ | 5,886 | $ | (8,935 | ) | $ | (10,271 | ) | |||||
| Net income (loss) per share: | ||||||||||||||||
| Basic | $ | (0.05 | ) | $ | 0.16 | $ | (0.24 | ) | $ | (0.27 | ) | |||||
| Diluted | $ | (0.05 | ) | $ | 0.16 | $ | (0.24 | ) | $ | (0.27 | ) | |||||
| Weighted average shares outstanding: | ||||||||||||||||
| Basic | 37,484 | 37,484 | 37,484 | 37,500 | ||||||||||||
| Diluted | 37,484 | 37,497 | 37,484 | 37,500 | ||||||||||||
F-33
Table of Contents
MedQuist Inc. and Subsidiaries
Schedule II — Valuation and Qualifying Accounts
(In thousands)
Schedule II — Valuation and Qualifying Accounts
(In thousands)
Allowance for Doubtful Accounts and Returns
| Balance at | Charges to | Doubtful | Balance at | |||||||||||||
| Beginning | Revenues and | Accounts | End of | |||||||||||||
| of Period | and Expenses | Written Off | Period | |||||||||||||
| December 31, 2005 | $ | 4,643 | 8,111 | (8,365 | ) | $ | 4,389 | |||||||||
| December 31, 2006 | $ | 4,389 | 4,955 | (4,850 | ) | $ | 4,494 | |||||||||
| December 31, 2007 | $ | 4,494 | 4,967 | (5,102 | ) | $ | 4,359 | |||||||||
Includes amounts written off to costs and expenses for bad debts of $(17), $552 and $540 for the years ended December 31, 2007, 2006 and 2005, respectively, and amounts charged to revenues for customer credits of $4,984, $4,403 and $7,571 for the years ended December 31, 2007, 2006 and 2005, respectively.
F-34
Table of Contents
EXHIBIT INDEX
| No. | Description | |
| 10.20.5 | Letter Agreement by and between MedQuist Inc. and Nightingale & Associates, LLC dated March 14, 2008 | |
| 23 | Consent of KPMG LLP | |
| 24 | Power of Attorney (included on the signature page hereto) | |
| 31.1 | Certification of Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2 | Certification of Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2 | Certification of Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |