UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2009
Commission file number 0-19941
MEDQUIST INC.
(Exact name of registrant as specified in its charter)
| | | |
New Jersey
(State or other jurisdiction of
incorporation or organization) | | 22-2531298
(I.R.S. Employer Identification No.) |
1000 Bishops Gate Blvd, Suite 300, Mount Laurel, NJ 08054-4632
(Address of principal executive offices)
Registrant’s telephone number, including area code:
(856) 206-4000
Securities registered pursuant to Section 12(b) of the Act:
| | | |
| Title of each class | | Name of each exchange on which registered |
| Common Stock, no par value per share | | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yeso Noþ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yeso Noþ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesþ Noo
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yeso Noo
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein and will not be contained, to the best of registrant’s knowledge, in definite proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check One):
| | | | | | | |
| Large Accelerated Filero | | Accelerated Filerþ | | Non-Accelerated Filero(Do not check if a smaller reporting company) | | Smaller reporting companyo |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yeso Noþ
The aggregate market value of the outstanding common stock held by non-affiliates of the registrant as of June 30, 2009, was $68,730,000. Such aggregate market value was computed by reference to the closing price of the common stock as reported on the Global Market of The NASDAQ Stock Market LLC .
The number of registrant’s shares of common stock, no par value, outstanding as of February 26, 2010 was 37,555,893.
Documents incorporated by reference
Portions of the definitive Proxy Statement for the 2010 Annual Meeting of Shareholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
MedQuist Inc.
Annual Report on Form 10-K
Table of Contents
| | | | | |
| | | Page | |
| | | | |
| | | | | |
| | | 3 | |
| | | 15 | |
| | | 20 | |
| | | 20 | |
| | | 20 | |
| | | 24 | |
| | | | | |
| | | | |
| | | | | |
| | | 24 | |
| | | 24 | |
| | | 25 | |
| | | 38 | |
| | | 38 | |
| | | 38 | |
| | | 38 | |
| | | 39 | |
| | | | | |
| | | | |
| | | | | |
| | | 40 | |
| | | 40 | |
| | | 40 | |
| | | 40 | |
| | | 40 | |
| | | | | |
| | | | |
| | | | | |
| | | 40 | |
| Amendment No. 7 to Licensing Agreement, dated as of November 10, 2009, between MedQuist Inc. and Nuance Communications, Inc. as the successor-in-interest to Philips Speech Recognition Systems GmbH Third Amended and Restated OEM Supply Agreement dated November 10, 2009, between MedQuist Inc. and Nuance Communications, Inc. as the successor-in-interest to Philips Speech Recognition Systems GmbH Licensing Agreement by and between Nuance Communications, Inc. and MedQuist Inc. dated November 10, 2009 | | | | |
| Consent of KPMG LLP | | | | |
| Certification of Chief Executive Officer | | | | |
| Certification of Chief Financial Officer | | | | |
| Certification of Chief Executive Officer, pursuant to Section 906 | | | | |
| Certification of Chief Financial Officer, pursuant to Section 906 | | | | |
2
PART I
Item 1.Business
General
We are a leading provider of technology-enabled clinical documentation services. We offer health systems, hospitals, and group medical practices integrated solutions for voice capture, speech recognition, medical transcription, document management and clinical documentation improvement using natural language processing, as well as coding services. Our solutions are designed to facilitate electronic access to clinical information by healthcare providers and to improve overall revenue cycle performance and adoption of the Electronic Health Record (EHR). We perform our services utilizing the DocQmenttm Enterprise Platform (DEP), our proprietary clinical documentation workflow management system. We believe our services and enterprise technology solutions — including mobile voice capture devices, speech recognition technologies, Web-based workflow platforms, and global network of medical transcriptionists (MTs) and medical editors (MEs) — enable health systems, hospitals and group medical to improve patient care, increase physician satisfaction and lower operational costs while facilitating their migration toward increased adoption and utilization of the EHR.
We service our customer base 24 hours a day, seven days a week, using skilled, English-speaking MTs, MEs and medical coders located primarily in the United States and India. We believe our clinical documentation workforce (MTs, MEs and medical coders), which includes employees as well as subcontractors, is the largest of any company providing these services.
Our customers use our solutions to efficiently manage changes in clinical documentation volume across their enterprise while reducing the operating and capital expenses associated with maintaining in-house transcription capabilities. Our customers range from large hospitals and health systems with complex workflows and a large number of users to smaller hospitals and large group medical practices. Our customers can use our total solution of technology and clinical documentation services, or they can use our technology with transcription performed by either third party transcription service providers or their in house medical transcriptionist staff.
3

The clinical documentation workflow begins when physicians or other medical professionals dictate findings and plans of care into one of several input devices, including standard telephones, handheld devices, or PC-based dictation launched from EHR systems. Once dictated, the voice files securely route through our DEP to either (i) our speech recognition engine for conversion to text and then an ME for editing or (ii) if not eligible for processing through our speech recognition engine, to an MT for transcription. The resulting draft report may then proceed to the quality assurance process prior to being routed back to the physician or other medical professional for review, authentication, distribution to other practioners indicated by the dictating physician and incorporation into a patient’s health record. Throughout this process, our DEP utilizes and maintains security measures that establish our compliance with and assist our customers in their compliance with privacy and security standards and regulations, including those adopted under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the Health Information Technology for Economic and Clinical Health (HITECH) Act, which was enacted as part of the American Recovery and Reinvestment Act of 2009, and the protection of the confidentiality of health records. We also provide document retention services.
Industry Overview
As a provider of technology enabled clinical documentation services, our revenues and growth are affected in part by certain trends in healthcare. Healthcare providers are under increasing pressure to efficiently manage their operations and provide electronic access to patient information. In April 2009, the American Hospital Association (AHA) estimated that eight out of 10 hospitals had cut administrative expenses in an effort to address economic concerns. Despite cutbacks, AHA estimated that 43% of hospitals experienced negative operating margin during the first quarter of 2009. In addition, AHA estimated that 36% of hospitals reported an increase in days in accounts receivable and 59% reported a decrease in cash on hand.
4
Competitive Environment
Integrated solutions for voice capture, speech recognition, medical transcription, document management and clinical documentation improvement using natural language processing, as well as coding services, are central to the clinical documentation and revenue cycle processes of healthcare providers. The medical transcription industry itself is highly fragmented and difficult to size based on a general lack of public market data and analysis. We estimate that the U.S. medical transcription market is approximately $7 billion on an annual basis, including both outsourced services and medical transcription performed internally by healthcare providers. We estimate that the top 10 medical transcription outsource providers based on revenues, of which we are the largest, account for less than 15% of the market, which includes in-house medical transcription. We believe that there are hundreds of companies in the U.S. and India currently performing medical transcription services for the U.S. market. There are currently six large transcription service providers, one of which is us and the others are Spheris Inc., Transcend Services, Inc., and Webmedex, Inc., Nuance Communications, Inc.’s transcription subsidiary (Focus Infomatics, Inc.) and CBay Systems & Services, Inc., each of which have annual revenues of approximately $50 million, as well several mid-sized service providers with annual revenues of between $15 million and $50 million and hundreds of smaller, independent businesses with annual revenues of less than $15 million. We believe that a substantial portion of the medical transcription market is serviced internally by MTs directly employed by the healthcare providers. We also substantially compete against speech recognition system vendors, Nuance Communications, Inc. and Multimodal Technologies, Inc., who have products marketed to displace and eliminate transcription services and EHR companies, like Epic Systems Corporation, Cerner Corporation, McKesson Corporation, Medical Information Technology, Inc., Eclipsys Corporation, and Allscripts-Misys Healthcare Solutions, Inc., who have alternative methods of capturing clinical documentation such as template, and point and click type applications.
While certain technologies and EHR adoption over time may displace certain clinical documentation worktypes (e.g. Progress Note, Radiology Report, Consultaton, etc.), we believe demand for clinical documentation services and the various different types of documents needed to be digitized for EHR adoption will continue to increase due in part to the following factors:
| | • | | Healthcare providers seeking: |
| | o | | means to efficiently and effectively capture clinical documentation that currently is not otherwise digital and needs to be converted to a digital format for use in the EHR; |
| |
| | o | | reduction in overhead and other administrative costs, driven by current and projected general U.S. economic conditions; |
| |
| | o | | improvement in the quality and speed of delivery of transcribed medical reports; |
| |
| | o | | access to leading technologies, such as speech recognition technology, without development and investment risk; |
| |
| | o | | expertise in implementing and managing a system tailored to the providers’ specific requirements; |
| |
| | o | | access to skilled MTs, MEs and medical coders; |
| |
| | o | | automated solutions to produce accurate clinical documentation due to concerns about medical errors, patient safety and litigation; and |
| |
| | o | | support for compliance with increasing governmental and industry mandated privacy and security requirements and EHR initiatives. |
| | • | | The growing and aging population in the United States will continue to require more medical tests, treatments and procedures that require documentation; |
| |
| | • | | Technological advances have significantly reduced medical transcription turnaround times and costs; |
| |
| | • | | Clinical documentation services enable healthcare providers to maximize the amount of time physicians spend on patient care, while reducing the disruption in daily work activities often associated with using electronic health records; and |
| |
| | • | | Federal and state laws increasingly encourage or require digital record keeping and Congress approved $19.5 billion of incentives for healthcare providers to adopt EHRs in February 2009 as part of its economic stimulus package and the HITECH Act. |
Our competitive position in the market is characterized primarily by the following factors:
| | • | | We are a leading provider of technology-enabled clinical documentation services in the U.S., with a strong customer base, the largest pool of worldwide MTs and MEs, and leading technologies capable of handling large volumes and complex workflows.As a leading provider of technology-enabled clinical documentation services in the U.S., we are recognized as a leading brand in the industry. We have a well-established customer base, comprised of hundreds of health systems, hospitals and large group medical practices located throughout the U.S. We have an integrated, flexible and scalable technology platform that can be tailored to meet our customers’ needs. We are the largest employer of U.S.-based MTs and MEs, utilize |
5
| | | | the largest supply of offshore medical transcription and medical editing labor, and have the most widely deployed technology platform. As health systems, hospitals and large group medical practices increasingly seek to outsource their medical transcription and other clinical document workflow needs, we have the resources to quickly and efficiently provide our customers with comprehensive and scalable global solutions. |
| | • | | We offer a comprehensive array of products and services.We offer a comprehensive array of products and services for voice capture, speech recognition, medical transcription, document management, clinical documentation improvement using natural language processing, coding and electronic signature through a combination of remote and on-premise solutions. These solutions are designed to maximize the efficiency, accuracy and security of our customers’ documentation and coding processes while enhancing their patient care, accelerating their revenue cycle and lowering their costs. |
| |
| | • | | We continue to face competition from medical transcription outsource providers providing low cost services.Several low cost medical transcription providers have emerged and aggressively moved into our market offering medical transcription services (performed both domestically and offshore) at prices significantly below our traditional price point causing some of our customers to switch to these providers, as well as resulting in a reduction in the prices we can charge our customers for our services. |
| |
| | • | | We see increasing competition from EHR and speech recognition vendors working to displace or replace traditional transcription services with alternative methods of documentation. There are several large well capitalized healthcare software and service IT vendors who sell into the clinical documentation market non-transcription methods of documentation such as front-end speech recognition or template-based point-and-click documentation systems at the point of care that otherwise may replace a dictated physician note. |
Transcription labor services
We believe that a majority of medical transcription services in our market are currently performed “in-house” by healthcare facilities. Such in-house medical transcription providers, as well as many outsourced medical transcription service organizations (MTSOs), are unable to deliver clinical documentation in a cost-effective manner. Unlike us, many of the in-house medical transcription providers and MTSOs lack sophisticated technology, the ability to handle complex workflows or a low-cost global labor force. Moreover, in-house transcription services and outsourced transcription vendors generally do not have the process expertise and technology needed to offer end-to-end solutions for clinical documentation, such as creating structured documentation for storage in an EHR. In-house providers and many outsourced MTSOs may also have difficulty handling sudden increases in transcription volume or demands for rapid turnaround.
Clinical documentation technology solutions
Standalone clinical documentation technology solutions present an alternative to basic medical transcription services that lack sophisticated technology. These standalone solutions, however, frequently do not offer a full range of products and services and are limited to dictation capture or voice-to-text solutions. A combination of both services and technology with control over the end-to-end process of generating clinical documentation, from voice capture to coding, helps to effectively manage the process and reduce the time from patient encounter to bill creation and submission to payors.
Direct-input EHR systems
Another alternative to medical transcription is technology that allow physicians to directly input data into patient EHRs. While these template-based systems allow data to be added immediately to EHRs, they often limit the range of narrative that can included by physicians. As a result, it may not be possible to document the details of a patient encounter as completely as voice capture and transcription would allow. In addition, direct-input EHR systems frequently require more time than dictation to put a comparable amount of data into a patient’s health record, placing a greater burden on physicians and other healthcare providers, thereby lowering their EHR adoption rate.
Business Strategy
Our goal is to expand our position as a leading provider of technology-enabled clinical documentation services in the United States. Going forward, our goal is to expand the scope of our services to current customers and continue to attract business from new customers. Key elements of our strategy include the following:
6
Expansion Into New Markets.We believe our breadth of products and services positions us to bridge the gap between traditional medical transcription and the EHR. The U.S. government has developed plans that call for all Americans to have an EHR by 2014. We believe that the U.S. government mandate of adoption of the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) coding classification will generate new opportunities for our services. In addition, we believe that commitment of President Obama’s administration to fund aspects of the adoption of electronic health records will provide additional opportunities for us.
Enhance Profitability.We continue to identify ways to maximize the efficiencies of our organization through the following initiatives:
| | • | | expand our use of speech recognition, which should continue to increase our productivity; |
| |
| | • | | realign our non-production workforce to match our current operational needs; |
| |
| | • | | use technology where applicable to automate manual processes; |
| |
| | • | | reorganize our production workforce to optimize workflow; and |
| |
| | • | | where contractually permitted, increase our use of lower cost offshore labor resources. |
Leveraging our DEP.For clinical documentation services that we perform on our platform (DEP), we believe that the combination of standardization, HIPAA compliance, other advanced functionality and transparency with respect to service metrics provided by this platform significantly increases our customers’ satisfaction and retention. Our DEP allows us to accelerate the rate at which we can offer new functionalities to our customers. In addition, we offer our DEP to healthcare providers and other companies in the clinical documentation industry for use as their medical dictation and transcription platform.
We intend to leverage our market leading position and proprietary technologies to provide comprehensive solutions to our customers related to the management of health records in connection with their migration to EHRs and improvements to their revenue cycles. Historically, we have provided a combination of traditional medical transcription services, stand-alone products and other professional services to healthcare providers. We aggregate these existing services and products, and anticipate adding new services and products, into a more comprehensive clinical documentation workflow solution.
| | • | | Expand existing customer relationships.We have developed a large, long-term and growing customer base. Beyond customer retention, we seek to sell complementary solutions to our existing customers and to sell our solutions into more of our customers’ functions and divisions, which we believe will more deeply integrate our solutions into our customers’ organizations. By selling to additional facilities within an existing customer enterprise or integrated delivery network (IDN), we attempt to leverage the existing customer contract and volume-based aggregate pricing. Our individual, stand-alone hospitals are typically members or participants of larger healthcare systems. By leveraging the existing experience, satisfaction and embedded technology footprint, we believe we can expand our relationship to the enterprise level and create organic same store-type growth. Through the increased use of technology and global processing ability, we seek to improve service performance, including turnaround time, performance reporting and clinical documentation quality, and reduce the cost to our customers. We actively work with our customers to find ways in which we can meet their needs more efficiently by adding value and reducing costs. We also have a strong, experienced sales team that continues to focus on customer executive level decision-makers to enhance sales opportunities for new and existing customers. In addition, our sales team focuses on customer communication to enhance sales opportunities for our integrated offering, which alleviates the need for our customers to purchase services from multiple sources, potentially saving customers time, money and integration challenges. |
| | • | | Grow our customer base.We target a wide range of customers, from large hospitals and health systems with complex workflows and a large number of users to smaller hospitals and large group medical practices. We believe that our ability to scale and customize solutions to specific customer requirements makes us valuable by allowing our products and services to change in accordance with a customer’s needs. Our global infrastructure allows us to offer a strong value proposition for customers of various sizes. This flexibility allows our customers to grow with us as their needs evolve. We are executing an aggressive sales and marketing plan and developing new channels to market our solutions to potential customers. We have identified the top 250 IDNs and aligned specific sales and account management focus for relationship development and |
7
| | | | coverage around our enterprise scalable solutions. As part of our efforts to increase national coverage, we intend to expand our relationships with physician organizations, hospital organizations and group purchasing organizations. |
| | • | | Be the low-cost, high-value provider of choice for clinical documentation and revenue cycle management solutions.We work constantly to identify areas where we can reduce our costs while maintaining the quality of our products and services. Our productivity is increasing due to our increased use of offshore labor, speech recognition technology and automation of workflows for both our U.S. and offshore workforces. We plan to continue to invest in these areas. We also continually refine our workflow model by aligning the needs of our customers with our worldwide operational capacities. |
| |
| | • | | Develop and acquire next-generation technology to enhance the solutions offered to our customers.We seek to differentiate our solutions through our use of sophisticated technology. We are now able to deliver an end-to-end automated solution for our radiology customers that integrates speech recognition technology with a customer’s existing radiology picture archiving and communication systems (PACS) and radiology information systems (RIS), allowing physicians to condense their billing time. We are expanding this technology beyond radiology to cover additional medical specialties. In addition, we are developing natural language processing technology to extract key information (such as medications, allergies and progress notes) from a transcript and put this information into a structured document ready for integration into a patient’s EHR. Our analytical tools and reporting capabilities enable customers to demonstrate to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) compliance with certain quality and control reporting obligations, most significantly their control over their clinical documentation function, which we provide. In addition, our analytical tools allow our customers to more efficiently manage their use of our solutions by providing them with key usage data, such as volume, service delivery, cost per physician, and physician compliance. |
| |
| | • | | Help our customers realize the adoption of and the full value of their EHR investments. Our clinical documentation solutions are designed to work with traditional health records as well as a wide range of EHR systems. We have integrations with all of the major EHR systems on the market today and are actively seeking to expand these relationships. As a result, we expect that funding provided by the HITECH Act to encourage the adoption of EHR by healthcare providers will offer growth opportunities for us. In particular, we are focused on enhancing our solutions to incorporate technologies that can automatically extract key information from physicians’ notes created outside the EHR for reformatting within certain components of the host EHR system. For example, the extracted information can be used to produce administrative and management reports or other types of structured documentation lists for updates or inclusion in a customer’s EHR. We believe that information extraction and other technologies under development will help our customers realize the full value associated with their EHR systems. |
| |
| | • | | Pursue strategic acquisitions that will improve our competitive position, grow our customer base or expand our technological capabilities.We seek appropriate opportunities to expand our business and augment our existing capabilities and customer base through strategic acquisitions. We focus on areas with high potential for cost consolidation and will also consider opportunistic targets to build our capabilities in clinical documentation technology and services and revenue cycle management or to acquire customers. We may consider opportunities in the United States, India and elsewhere. As of the date of this filing, we are actively pursuing the acquisition of the domestic assets of Spheris Inc., which filed for Chapter 11 bankruptcy protection on February 3, 2010 (see Subsequent Events included under Item 5 of this Form 10-K). |
Customer Base
We have a large and prestigious customer base. We believe that over 30% of non-federal acute care U.S. hospitals use at least one of our products or services. Additionally, we have the largest customer base of any provider of technology-enabled clinical documentation services in the U.S., currently serving over 1,500 hospital systems, clinics and large group medical practices, including approximately 40% of hospitals with more than 500 licensed beds. We believe we are the provider of choice of technology-enabled clinical documentation services for large, complex hospitals and health systems in the U.S. due to our technology, historical service performance, scale and scope. We provide services to entire healthcare systems as well as discrete departments within hospitals, such as health information management, emergency, radiology, and cardiology departments and all types of clinic settings. None of our customers accounted for more than 5% of our annual net revenues for the years ended December 31, 2009, 2008 or 2007.
8
Products and Services
For the years ended December 31, 2009, 2008 and 2007, approximately 85.7%, 84.9% and 83.3%, respectively, of our net revenues were derived from our medical transcription technology and services. In addition, we also derive net revenues from, among other things, maintenance services, our front-end speech recognition solution, SpeechQtm, our coding services, and our enterprise digital dictation management system, DocQvoice. Our technology-enabled clinical documentation services allow our customers to document patient encounters efficiently and accurately for inclusion in health records. Our service offerings cover the entire clinical documentation process, from dictation capture, routing and transcription to returning complete documents to multiple print destinations or back to the customer’s EHR system. In addition to the services described below, we also offer a complete suite of digital dictation systems, handheld devices and personal digital assistants designed to meet the needs of hospitals, enterprise-wide environments, departments and small facilities.
Clinical Documentation Services
We provide health systems, hospitals and large group medical practices with comprehensive solutions to meet their clinical documentation needs. As a leading provider of technology-enabled clinical documentation services, we utilize over 10,000 skilled and qualified MTs and MEs worldwide, including the employment of approximately 4,600 MTs and MEs in the United States. All of the transcription and editing work performed for us outside of North America is performed pursuant to an agreement with CBay Systems & Services, Inc., a wholly-owned subsidiary of CBay Inc., our majority shareholder. This scale allows us to continually offer our customers consistent, effective and standardized clinical documentation services that meet enterprise-wide or departmental needs. We perform the vast majority of our clinical documentation services utilizing our DEP. For our clinical documentation customers that do not use our DEP technology or services, we also service a specialized area of the clinical documentation market — specifically, radiology - where we retrieve voice files from, and transcribe directly into, customer-hosted transcription platforms.
In the clinical documentation workflow, in addition to medical transcription technology and services, we provide, digital dictation, speech recognition, advanced document distribution, comprehensive management reporting and physician’s electronic signature technologies.
DocQmenttmEnterprise Platform
DocQmenttm Enterprise Platform (DEP) is our proprietary, web-based dictation and clinical documentation management system. Our proprietary technologies enable our customers to efficiently manage their clinical documentation workflow, which begins when physicians or other medical professionals dictate findings and plans of care into one of several input devices, including standard telephones, handheld devices or PC-based dictation stations. The voice files then securely route through DEP to either (i) our speech recognition engine for conversion to text and then to a medical editor for editing or (ii) if not eligible for processing through our speech recognition engine, to an MT for transcription. The resulting draft report may then proceed to the quality assurance process prior to being routed back to the physician or other medical professional for review, authentication, distribution to other practitioners indicated by the dictating physician and incorporation into a patient’s health record. Throughout this process, DEP utilizes and maintains security measures and audit trails that establish our compliance with and assist our customers in their compliance with privacy and security standards and regulations and the protection of the confidentiality of health records. Our DEP and flexible dictation solutions provide our customers with easy access to advanced technology and the confidence that medical reports will be completed quickly and accurately. Our DEP integrates dictation capture, workflow management, speech recognition, medical transcription and editing, physician authentication and document distribution through multiple distinct yet integrated modules as follows:
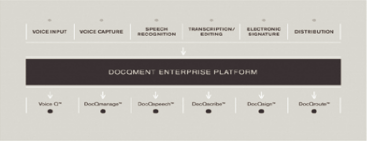
9
Features and benefits of our DEP include the following:
Security and Scalability
| | • | | supports requirements of the HITECH Act, HIPAA and other applicable laws and regulations |
| |
| | • | | provides detailed role-based job-level audit capabilities |
| |
| | • | | utilizes secure data centers with 24/7 system monitoring and maximum uptime |
| |
| | • | | satisfies increased clinical document workflow demands through seamless scalability |
Cost Effectiveness
| | • | | provides one interface for health information systems, thereby reducing IT and administrative support |
| |
| | • | | eliminates the costs and challenges of supporting multiple dictation and clinical documentation systems |
| |
| | • | | allows for the centralized maintenance of all system hardware and software at our data centers |
| |
| | • | | allows MTs and MEs to work remotely from anywhere in the world |
| |
| | • | | eliminates traditional phone charges and certain other overhead costs associated with home-based medical transcription |
Workflow
| | • | | increases and improves EHR usage and adoption through integration at point of care, providing multiple input methods and choices, allowing the documentation process to fit into a physician’s workflow |
| |
| | • | | allows secure, web-based viewing of medical reports on a real-time basis from multiple locations through a single and secure login, password and company identification |
| |
| | • | | increases the level of our customers’ management control over clinical documentation workflow across healthcare enterprises |
| |
| | • | | reduces the amount of time reports spend in queue as well as MT and ME downtime |
| |
| | • | | takes transcribed text and matches it to user pre-defined templates using automatic post-transcription formatting |
Customer HIPAA and Internal Control Auditing and Reporting
| | • | | facilitates transparency in the billing process with detailed character count logs for every processed document |
| |
| | • | | provides audit trails with detailed information regarding access to patient health information |
| |
| | • | | offers quality and turnaround time reporting for tracking of service metrics |
Products and Related Maintenance Services
We provide onsite maintenance, remote “break-fix” services, and application, hardware and software technical support for our products.
SpeechQ™
SpeechQ™is our flexible front-end speech recognition software application. It allows physicians in all medical disciplines to dictate, edit and sign their reports in a single session or send them to an editor following dictation. The SpeechQ™technology continuously learns from changes to a specific physician’s dictation made by either the physician or an editor, increasing the speech recognition accuracy for such physician with every edit. Powered by the same SpeechMagictm speech engine used in our DEP, our award-winning SpeechQ for Radiology™offering is designed specifically for radiology, and integrates with most radiology specific
10
information systems providing a workflow that is designed to maximize radiologists’ efficiency and significantly improve report turnaround time. In 2009, SpeechQ for Radiology™advanced its position in the marketplace through integrations with third-party applications to provide critical results reporting and search capabilities not otherwise available in a stand-alone product. In 2009, we introduced our SpeechQ for General Medicine™solution based on our strong success in radiology and increasing customer demand for real-time interactive speech recognition in many other hospital departments. SpeechQ for General Medicine™enables residents, attending and hospital physicians in high-volume dictation specialties such as family practice, orthopedics, cardiology, surgery, and many others to realize the benefits of this technology. In addition, within an acute care setting, many report types such as Progress Notes, History & Physicals, Procedure Notes, and Consultations lend themselves to interactive real-time speech recognition workflow. In real-time speech recognition, physicians dictate and self-complete reports in one sequence, bypassing the transcription process. The results include dramatic reductions in cost and turnaround time, while preserving physician preference for detailed narrative documentation.
DocQvoice
DocQvoice is our web-based, enterprise digital voice capture and transport solution attached at the front end of DEP for premise-based voice capture. DocQvoice creates opportunities to improve productivity by providing an enterprise view that allows our customers’ health information management supervisors to easily manage in-house MTs and MEs, MTSOs and voice files from a single dashboard instead of using multiple systems. DocQvoice was specifically engineered to be compatible with our previous generation dictation stations. An integral component of our growing technology portfolio, DocQvoice supports our end-to-end solution from dictation to billing. DocQvoice’s enterprise configuration options allow administrators to easily track work and share resources to get voice files to the correct MTs and MEs.
Coding & Revenue Cycle Services and Technology
We offer coding workflow technology and services to improve our customers’ reimbursement, compliance and revenue cycle, and which further supports our end-to-end solution from dictation to billing. Utilizing CodeRunner™, our internet-based coding workflow technology, our medical coders provide coding services to some of the largest and most prestigious healthcare institutions in the industry. We provide total departmental outsourcing of coding, on-site temporary assistance and remote-coding services through both VPN-enabled access to customer systems, as well as through CodeRunner™. Commencing in 2009, we offer complete recovery audit and consulting (RAC) services, as well as traditional coding audit services. We see this business as a natural complement to our strong footprint within health information management and see very similar workflow and technology challenges that can be met with our technology-enabled services approach to coding.
Technological Capabilities
Research and Development
We continue to invest in our research and development capabilities to ensure we meet current and future customer requirements. Our proprietary software and hardware technologies support our clinical documentation outsourced services. Our software capabilities enable us to operate a global service delivery model that provides our customers with multi-input voice capture. Our expertise in the scalable use of speech recognition enables us and our customers to achieve productivity gains and cost savings. We continue to work to enhance our speech recognition and editing technologies to achieve maximum productivity gains in the medical documentation process. Our DEP and technological expertise in the areas of work routing and work management support a global, scalable model of clinical documentation delivery. Our ability to focus on a single clinical documentation management system, our DEP, enables us to efficiently and effectively utilize our research and development resources.
We currently have a development staff of approximately 100 covering multiple disciplines, including engineering, quality assurance, program management and database administration, to conduct our research and development in five locations: Joplin, Missouri, Norcross, Georgia, Morgantown, West Virginia, Malvern, UK, and Hyderabad, India. Although we license a portion of our technology from partners, by far the majority of our technological expertise resides in our development organization. Our development personnel have expertise that covers the entire breadth of our solutions, including voice capture management, speech recognition and editing applications, medical transcription, electronic signature, document distribution and coding, and the following technology areas: n-tier and service oriented architectures, web-based clients, high volume transactional databases, data warehouses, web services and seamless third party integrations. All of our development teams follow the same rigorous development methodology which ensures repeatable, high quality and timely delivery of highly available, mission-critical solutions. Our research and
11
development expenses were approximately $9.6 million, $15.8 million and $13.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
Technology
We provide solutions using sophisticated proprietary and licensed technology systems and technology-enabled services and develop new software both through in-house development and by outsourcing to third-party developers. We regularly review our Internet-based technologies and enhance them through research and development. We believe that our technology systems are scalable to service additional customers without significant additional capital investment by us. We believe that our technology-enabled model has allowed us to reduce our operating costs.
We currently operate data centers in Alpharetta, Georgia and Norcross, Georgia. In addition, we operate an emergency backup location in Philadelphia, Pennsylvania, which provides our clients access to their critical data and functionality in the event of a failure at our primary data centers. Our data centers are maintained and supported by third-parties at their respective locations. The services provided by our data centers and disaster recovery service providers are generally commercially available at comparable rates from other service providers. Our mission-critical workflow solutions are hosted by us and accessed using high-speed Internet connections or private network connections. We use commercially available hardware and a combination of proprietary and commercially available software to provide our solutions.
Speech Recognition
A portion of our speech recognition technology is licensed from Nuance Communications, Inc. (Nuance). We have integrated this technology into SpeechQ for Radiology™, SpeechQ for General Medicine™, and our DEP, which has provided us with productivity gains and streamlined workflows. In 2009 we continued to significantly increase our use of automated speech recognition.
Sales and Marketing
We spend a significant portion of our sales and marketing resources on targeting healthcare facilities, which are currently performing medical transcription in-house, as well as those facilities that have already outsourced their medical transcription function, but are using a competitor. Additionally, as more and more healthcare providers look to aggregate their purchasing power and make group or enterprise decisions, our sales and marketing efforts are targeting agreements with these larger and more complex groups and enterprises, also referred to as IDNs.
In addition, we focus on retaining and expanding the business within our existing customer base. We use a direct sales force model of over 80 sales and customer care associates throughout the U.S. This includes specialists for enterprise executive sales, large account management, specific target market specialists for technology and services and account management. In addition, we have an inside sales department that specializes in telesales and lead generation primarily for ancillary products to our existing customers including other medical transcription outsource companies.
To support our sales initiatives, we utilize various marketing programs to maintain and expand our brand. We promote our offerings regularly through:
| | • | | attending and sponsoring industry trade shows of national organizations such as the American Health Information Management Association (AHIMA), Healthcare Information and Management Systems Society (HIMSS), Association for Healthcare Documentation Integrity (AHDI) , Radiological Society of North America, Society (RSNA) for Imaging Informatics in Medicine, and Medical Transcription Industry Alliance (MTIA) ; |
| |
| | • | | advertising in industry focused print and electronic trade journals; and |
| |
| | • | | demonstrating our thought leadership on industry topics and trends via speaking engagements, work group participation, committee memberships and leadership, webinars within all of the noted associations above, and participation in numerous state and regional trade show events. |
12
Service Delivery and Customer Support Services
We offer a wide range of customer support services through an expansive staff of customer-facing service personnel. The customer-facing relationship teams work with, and are supported by, our centrally managed customer service organization.
Our centralized service delivery enhances workflow management and has resulted in improved levels of service and quality for our customers. This centralization coordinates the services of thousands of MTs domestically and internationally, facilitating superior real-time capacity planning and demand management when volume fluctuates or other environmental factors impact performance. By applying streamlined processes and the highest standards worldwide, we are able to provide a spectrum of quick turnaround time and quality documentation consistently in the most complex of healthcare environments, 24 x 7 x 365.
Our service and support organization is comprised of several smaller organizations, or teams, focused on delivering specific aspects of services. In addition to the customer-facing (level one) local or telephone support, we provide more complex (level two) technical, application and product support, as well as implementation professional services, which provide our customers with complete implementation planning and services beginning with the initial scoping of system requirements through the customer acceptance phase of an implementation.
Medical Transcriptionists and Medical Editors
As a leading provider of technology-enabled clinical documentation services, we utilize over 10,000 skilled and qualified MTs and MEs worldwide, including the employment of approximately 4,600 MTs and MEs in the United States. All of the transcription and editing work performed for us outside of North America is performed pursuant to an agreement with CBay Systems & Services, Inc., a wholly-owned subsidiary of CBay Inc., our majority shareholder. The size of our global MT and ME pool allows us to quickly and efficiently provide our customers with the labor resources necessary to implement comprehensive, scalable solutions. All of the U.S.-based MTs and MEs that we employ work from home, largely using computer hardware and telecommunications equipment that we provide, to access dictation files and transcribe reports utilizing the Internet.
Recruitment
Working with a team of professional recruiters, we utilize multiple avenues to ensure that qualified MTs and MEs apply for employment opportunities with us. Regular advertisements and articles appear in trade journals and industry publications, and banner ads are placed on industry and trade websites. In addition, we participate in prominent local and national trade shows and work with premier trade schools to offer top graduates an opportunity for employment with us.
Training
Our MTs and MEs participate in an on-line training program that includes both a company orientation, as well as training on our DEP. In addition, those MTs and MEs that service specialized areas of the clinical documentation market involving the direct transcription into customer-hosted medical transcription platforms receive platform-specific training. Prior to performing clinical documentation services for our customers, each MT and ME must demonstrate proficiency in the use of our DEP or the applicable customer-hosted platform.
With the emergence of speech recognition technology to produce draft transcribed reports, all MTs who work on the DEP have an opportunity to become medical editors (MEs). Before they are eligible to edit the draft speech-recognized processed reports, MEs must be formally certified on DocQspeechtm, our DEP’s speech recognition module, to ensure the most efficient means to edit the reports.
In 2009, we introduced a new classification of employee that converges traditional transcription, editing and quality assurance, the Master Medical Editor (MME). By demonstrating a superior knowledge and competency in both the domain of clinical knowledge as well as proficiency within DEP, an MMEs performs both a production and quality assurance role. Our DEP routes work on a just-in-time basis for the MME to perform either function. This classification adds another interesting step in the career path for our MTs and MEs.
Quality Assurance
Our automated technology routes reports with flagged text to MMEs for review prior to delivery to the customer. In addition, formal quality reviews are performed on a regular basis at the individual MT and ME level. We provide continuous feedback to MTs and MEs working on our DEP to increase learning and improve up-front quality.
13
Intellectual Property
We rely on a combination of copyright, patent, trademark, trade secret, and other intellectual property laws, nondisclosure agreements, license agreements, contractual provisions and other measures to protect our proprietary rights. We have a number of registered trademarks, including MedQuist®, and have current registrations of several domain names, including “www.medquist.com.”
Regulatory Matters
Current HIPAA Privacy Standards and Security Standards
The provision of healthcare services, including the practice of medicine, is heavily regulated by federal and state statutes and regulations, by rules and regulations of state medical boards and state and local boards of health, and by codes established by various medical associations. Although many such laws, regulations and requirements do not directly apply to our operations, future laws and regulations related to the provision of medical transcription services may require us to restructure our operations in order to comply with such requirements. We and our hospital and other healthcare provider customers must comply with a variety of requirements related to the handling of patient information, including HIPAA, which establishes a set of national privacy and security standards for protecting the privacy, confidentiality and security of protected health information (PHI). Under HIPAA, health plans, healthcare clearinghouses, healthcare providers (sometimes referred to as “covered entities” for purposes of HIPAA) and their “business associates” must meet certain standards in order to protect individually identifiable health information. As part of the operation of our business, our customers provide us with certain PHI. We are a business associate to most of our customers for purposes of HIPAA. The provisions of HIPAA require our customers to have agreements (referred to as “business associate agreements” for purposes of HIPAA) in place with us under which we are required to appropriately safeguard the PHI we create or receive on their behalf. As a business associate we also have statutory and regulatory obligations under HIPAA.
The Health Information Technology for Economic and Clinical Health Act (HITECH Act), which was enacted into law on February 17, 2009 as part of the American Recovery and Reinvestment Act of 2009 (ARRA), enhances and strengthens the HIPAA Privacy and Security Standards and makes certain provisions of HIPAA applicable to “business associates” of covered entities. Currently, we are bound by business associate agreements with covered entities that require us to use and disclose PHI in a manner consistent with HIPAA in providing services to those covered entities. As of February 17, 2010, some provisions of HIPAA apply directly to us. In addition, the HITECH Act creates new security breach notification requirements. The direct applicability of the new HIPAA Privacy and Security provisions will require us to incur additional costs and may restrict our business operations. In addition, these new provisions will result in additional regulations and guidance issued by the United States Department of Health and Human Services and will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our customers.
We have structured our operations to comply with these regulatory and contractual requirements. We have designated a Chief Compliance Officer and have implemented appropriate safeguards related to the access, use and/or disclosure of PHI to help ensure the privacy and security of PHI consistent with our regulatory and contractual requirements. We also are required to train personnel regarding HIPAA requirements. If we, or any of our domestic MTs or our offshore medical transcription subcontractors, are unable to maintain the privacy, confidentiality and security of the PHI that is entrusted to us, we and our customers could be subject to civil and criminal fines and sanctions and we could be found to have breached our contracts with our customers. Additionally, because all HIPAA standards are subject to interpretation and change, we cannot predict the future impact of HIPAA on our business and operations. Although it is not possible to anticipate the total effect of these regulations, we have made and continue to make investments in systems to support customer operations that are regulated by HIPAA.
Further, we and our customers are required to comply with HIPAA security regulations that require us and them to implement certain administrative, physical and technical safeguards to ensure the confidentiality, integrity and availability of electronic protected health information (EPHI). We are required by regulation and contract to protect the security of EPHI that we create, receive, maintain or transmit for our customers consistent with these regulations, including implementing administrative, physical and technical safeguards that reasonably and appropriately protect the confidentiality, integrity and availability of such EPHI. To comply with our regulatory and contractual obligations, we may have to reorganize processes and invest in new technologies.
As of February 17, 2010, we are directly subject to HIPAA’s criminal and civil penalties for breaches of our privacy and security obligations.
14
Other Restrictions Regarding Confidentiality, Privacy and Security of Health Information
In addition to HIPAA, numerous other state and federal laws govern the collection, dissemination, use, access to, confidentiality and security of PHI. In addition, Congress and some states are considering new laws and regulations that further protect the privacy and security of medical records or medical information. In many cases, these state laws are not preempted by the HIPAA Privacy and Security Standards and may be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our customers.
These laws at the state or federal level, or new interpretations of these laws, could create liability for us, could impose additional operational requirements on our business, could affect the manner in which we use and transmit patient information and could increase our cost of doing business. In addition, to the extent that the laws of the states in which we or our customers operate are more restrictive than HIPAA, we may have to incur additional costs to maintain compliance with any such applicable requirements. Claims of violations of privacy rights or contractual breaches, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business.
Employees
As of February 12, 2010, we employed 5,382 people. Of these, 4,571 were MTs and MEs. Of our total work force, 3,163 were full-time employees and 2,219 were part-time employees.
Available Information
All periodic and current reports, registration statements, and other filings that we are required to file with the SEC, including our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) of the Securities Exchange Act of 1934 (Exchange Act), are available free of charge from the SEC’s website (www.sec.gov) or public reference room at 100 F Street N.E., Washington, DC 20549 (1-800-SEC-0330) or through our website at www.medquist.com. Such documents are available as soon as reasonably practicable after electronic filing of the material with the SEC. Copies of these reports (excluding exhibits) may also be obtained free of charge, upon written request to: Investor Relations, MedQuist Inc., 1000 Bishops Gate Boulevard, Suite 300, Mount Laurel, New Jersey 08054-4632. The website addresses included in this report are for identification purposes. The information contained therein or connected thereto are not intended to be incorporated into this report.
Item 1A.Risk Factors
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements that are based on current expectations, estimates, forecasts and projections about us, the industry in which we operate and other matters, as well as management’s beliefs and assumptions and other statements regarding matters that are not historical facts. These statements include, in particular, statements about our plans, strategies and prospects. For example, when we use words such as “projects,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “should,” “would,” “could,” “will,” “opportunity,” “potential” or “may,” variations of such words or other words that convey uncertainty of future events or outcomes, we are making forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 (Securities Act) and Section 21E of the Exchange Act. Our forward-looking statements are subject to risks and uncertainties. Actual events or results may differ materially from the results anticipated in these forward-looking statements as a result of a variety of factors. While it is impossible to identify all such factors, factors that could cause actual results to differ materially from those estimated by us include:
| | • | | each of the factors discussed in this Item 1A, Risk Factors as well as risks discussed elsewhere in this report; |
| |
| | • | | each of the matters discussed in Item 3, Legal Proceedings; |
| |
| | • | | our ability to recruit and retain qualified MTs, MEs and other employees; |
| |
| | • | | changes in law, including, without limitation, the impact HIPAA will have on our business; |
| |
| | • | | the impact of our new services and products on the demand for our existing services and products; |
15
| | • | | our increased dependence on speech recognition technology, which we license, but do not own; |
| |
| | • | | our current dependence on medical transcription for a significant portion of our technology-enabled clinical documentation services; |
| |
| | • | | our ability to expand our customer base; |
| |
| | • | | infringement on the proprietary rights of others; |
| |
| | • | | our ability to diversify into other businesses; |
| |
| | • | | our increased dependence on CBay Systems & Services, Inc. as the exclusive provider of medical transcription performed in Asia, the current location of all our medical transcription and medical editing work performed outside of North America; |
| |
| | • | | our ability to effectively integrate newly-acquired operations; |
| |
| | • | | competitive pricing and service feature pressures in the clinical documentation industry and our response to those pressures; |
| |
| | • | | difficulties relating to our significant management turnover; and |
| |
| | • | | general conditions in the economy and capital markets. |
Many of these factors are beyond our ability to predict or control. In addition, as a result of these and other factors, our past financial performance should not be relied on as an indication of future performance. The cautionary statements referred to in this section also should be considered in connection with any subsequent written or oral forward-looking statements that may be issued by us or persons acting on our behalf. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this report might not occur. Furthermore, we cannot guarantee future results, events, levels of activity, performance, or achievements.
Set forth below are certain important risks and uncertainties that could adversely affect our results of operations or financial condition and cause our actual results to differ materially from those expressed in forward-looking statements made by us. Although we believe that we have identified and discussed below the key risk factors affecting our business, there may be additional risks and uncertainties that are not presently known or that are not currently believed to be significant that may adversely affect our performance or financial condition. More detailed information regarding certain risk factors described below is contained in other sections of this report.
Our ability to expand our business and properly service our customers depends on our ability to effectively manage our domestic production capacity and our only offshore transcription labor partner (CBay Systems & Services, Inc.), including our ability to recruit, train and retain qualified MTs and MEs and maintain high standards of quality service in our operations, which we may not be able to do.
Our success depends, in part, upon our ability to effectively manage our domestic production capacity including our ability to attract and retain qualified MTs and MEs who can provide accurate medical transcription. There is currently a shortage of qualified MTs and MEs in the U.S., and an increasing need for more real-time turnaround of transcribed reports has created industry-wide demand for quality MTs and MEs. As a result, competition for skilled MTs and MEs is intense. We have active programs in place to attract domestic MTs and MEs. We must also effectively manage our only current offshore transcription labor partner, CBay Systems & Services, Inc.. However, this strategy may not alleviate any issues caused by the shortage. Because medical transcription is a skilled position in which experience is valuable, we require that our MTs and MEs have substantial experience or receive substantial training before being hired. Competition may force us to increase the compensation and benefits paid to our MTs and MEs, which could reduce our operating margins and profitability. In addition, failure to recruit and retain qualified MTs and MEs may have an adverse effect on our ability to service our customers, manage our production capacity and maintain our high standards of quality service. An inability to hire and retain a sufficient number of qualified MTs and MEs in the U.S., could have a negative impact on our ability to grow.
If the electronic health records (EHR) companies produce solutions acceptable to large hospital systems for the creation of electronic clinical documentation that are not based on the conversion of voice to text, the overall demand for medical
16
transcription services could be reduced.
EHR companies’ solutions for the collection of clinical data typically require physicians to directly enter and organize patient information through templates thereby eliminating or dramatically reducing the use of dictation or transcription. Although the EHR market is in the early stages of development and is rapidly evolving, a number of market entrants have introduced or developed products and services that are competitive with one or more components of the solutions we offer. We expect that additional companies will continue to enter this market. In new and rapidly evolving industries, there is significant uncertainty and risk as to the demand for, and market acceptance of, recently introduced solutions for the creation of electronic clinical documentation. In the event that such solutions are successful and gain wide acceptance, the overall demand for medical transcription services could be reduced. In the event that markets develop more quickly than expected, our business, financial condition and results of operations could be adversely affected.
Our success will depend on our ability to support existing technologies as well as to adopt and integrate new technology into our DEP, to improve our production capabilities and expand the breadth of our service offerings, as well as our ability to address any unanticipated problems with our information technology systems, which we may not be able to do quickly, or at all.
Our ability to remain competitive in the clinical documentation industry is based, in part, on our ability to develop, utilize and support technology in the services that we provide to our customers to improve our production capabilities and expand the breadth of our service offerings. As our customers advance technologically, we must be able to effectively integrate our DEP with their systems and provide advanced data collection technology. We plan to develop and integrate new technologies into our current service structure to give our customers high-quality and cost-effective services. We also may need to develop technologies to provide service systems comparable to those of our competitors as they develop new technology. If we are unable to effectively develop and integrate new technologies, we may not be able to expand our technology and service offerings or compete effectively with our competitors. In addition, if the cost of developing and integrating new technologies is high, we may not realize our expected return on investment.
Our business, particularly our DEP and our speech recognition software, depends on the continuous, effective, reliable, and secure operation of our computer hardware, software, and Internet applications and related tools and functions.
A substantial portion of our business involves the transfer of large amounts of data to and from our DEP via the Internet. Also, we rely on a global enterprise software system to operate and manage our business. Our DEP, and its underlying technologies, are designed to operate and to be accessible by our customers 24 hours a day, seven days a week. Network and information systems, the Internet and other technologies are critical to our business activities. Substantially all of our transcription services are dependent upon the use of network and information systems, including the use of our DEP and our speech recognition software, which is licensed from a third party. We have periodically experienced short term outages with our DEP that have not significantly disrupted our business.
We may experience interruption at our data centers or client support facilities.
We perform data center and/or hosting services for certain clients, including the storage of critical patient and administrative data. We have invested in reliability features such as multiple power feeds, multiple backup generators, high-availability server and storage systems and redundant telecommunications lines, as well as technical and physical security safeguards, and structured our operations to reduce the likelihood of disruptions. Periodic risk assessments are conducted to ensure additional risks are identified and appropriately mitigated. However, complete failure of all local public power and backup generators, impairment of all telecommunications lines, a “concerted denial of service cyber attack”, damage (environmental, accidental, intentional or pandemic) to the buildings, the equipment inside the buildings housing our data centers, the client data contained therein and/or the personnel trained to operate such facilities could cause a disruption in operations and negatively impact clients who depend on us for data center and system support services. Any interruption in operations at our data centers and/or client support facilities could damage our reputation, cause us to lose existing clients, hurt our ability to obtain new clients, result in revenue loss, create potential liabilities for our clients and us and increase insurance and other operating costs.
Due to the critical nature of clinical documentation to our customers’ operations, potential customers may be reluctant to outsource or change service providers as a result of the cost and potential for disruption in services, which may inhibit our ability to attract new customers.
The up-front cost involved in changing clinical documentation service providers or converting from an in-house medical transcription department to an outsourced provider may be significant. Many customers may prefer to remain with their current
17
service provider or keep their medical transcription in-house rather than incur these costs or experience a potential disruption in services as a result of changing service providers. Also, as the maintenance of accurate medical records is a critical element of a healthcare provider’s ability to deliver quality care to its patients and to receive proper and timely reimbursement for the services it renders, potential customers may be reluctant to outsource such an important function.
If our intellectual property is not adequately protected, we may lose our market share to our competitors and be unable to operate our business profitably.
Our success depends, in part, upon our proprietary technology and our ability to license and renew third-party intellectual property. We regard some of the software underlying our services, including our DEP and interfaces, as proprietary, and we rely primarily on a combination of trade secrets, patent, copyright and trademark laws, confidentiality agreements, contractual provisions and technical measures to protect our proprietary rights. Despite our efforts to protect our proprietary rights, if our intellectual property is not adequately protected, unauthorized parties may attempt to copy aspects of our intellectual property or obtain and use information that we regard as proprietary causing us to lose market share and rendering us unable to operate our business profitably. There can be no assurance that our proprietary information will not be independently developed by competitors. There can be no assurance that the intellectual property we own or license will provide competitive advantages or will not be challenged or circumvented by our competitors.
We are dependent on third party speech recognition software incorporated in certain of our technologies, and impaired relations with such third party or the inability to enhance such third party software over time could harm our business.
We license speech recognition software from Nuance Communications, Inc. (Nuance) that we incorporate into our DEP, SpeechQ for Radiology™ and SpeechQ for General Medicine™. Nuance is one of our competitors. Our ability to continue to sell and support SpeechQ for Radiology™ and SpeechQ for General Medicine™ depends on continued support from Nuance. In November 2009, we entered into extensions of the agreements pursuant to which we license speech recognition software from Nuance. The terms of these agreements extend until June 30, 2015. Thereafter, upon written notice to Nuance, we have the right to extend the agreements with Nuance for two successive terms of five years each (each a Renewal Term) on the same terms (except pricing terms) and conditions the applicable agreement in effect at the end of the then-current initial term or Renewal Term. Nuance may terminate the agreements in the event of certain defaults by us.
If the licenses were to become unavailable on commercially reasonable terms or at all, some of this technology would be difficult to replace. The loss of these licenses could significantly impact our business until we identify, license and integrate, or develop equivalent software. If we are required to enter into license agreements with third parties for replacement technology, we could face higher royalty payments and our products may lose certain attributes or features.
In addition, our speech recognition software may be impacted if errors occur in the software. It may be more difficult for us to correct any defects in the software because the software is not within our control. Accordingly, our business could be adversely affected in the event of any errors in the software. There can be no assurance that Nuance will continue to invest the appropriate levels of resources in the software to maintain and enhance the capabilities of the software.
We compete with many others in the market for clinical documentation services which may result in lower prices for our services, reduced operating margins and an inability to maintain or increase our market share and expand our service offerings.
We compete with other outsourced clinical documentation service companies in a very fragmented market that includes national, regional and local service providers, as well as service providers with global operations. These companies offer services that are similar to ours and compete with us for both customers and qualified MTs and MEs. We also compete with the in-house medical transcription staffs of our customers. While we attempt to compete on the basis of fast, predictable turnaround times and consistently high accuracy and document quality, all offered at a reasonable price, there can be no assurance that we will be able to compete effectively against our competitors or timely implement new products and services. Many of our competitors attempt to differentiate themselves by offering lower priced alternatives to our outsourced medical transcription services. Increased competition and cost pressures affecting the healthcare markets in general may result in lower prices for our services, reduced operating margins and the inability to maintain or increase our market share.
As technology evolves, including the continued refinement of speech recognition technology, health information technology providers may provide services that replace, or reduce the need for medical transcription. Furthermore, companies that provide
18
services complementary to medical transcription, such as electronic medical records, coding and billing, may expand the services they provide to include medical transcription. Current and potential competitors may have financial, technical and marketing resources that are greater than ours. As a result, competitors may be able to respond more quickly to evolving technological developments or changing customer needs or devote greater resources to the development, promotion or sale of their technology or services than we can. In addition, competition may increase due to consolidation of medical transcription companies. As a result of such consolidation, there may be a greater number of providers of medical transcription services with sufficient scale, service mix and financial resources to compete with us to provide services to larger, more complex organizations. Current and potential competitors may establish cooperative relationships with third parties to increase their ability to attract our current and potential customers.
We may pursue future transactions, including the proposed acquisition of certain assets of Spheris Inc., which could require us to incur debt and assume contingent liabilities and expenses, and we may not be able to effectively integrate new operations.
A significant portion of our historical growth has occurred through transactions, and we may pursue transactions in the future. Transactions involve risks that the combined businesses will not perform in accordance with expectations and that business judgments concerning the value, strengths and weaknesses of the combined businesses will prove incorrect. We cannot guarantee that if we acquire Spheris or decide to pursue other future transactions, we will be able to identify attractive opportunities or successfully integrate Spheris or such other future businesses or assets with our existing business. In addition, we cannot guarantee the ability to retain customers of Spheris or such other future businesses or assets we acquire. Future transactions may involve high costs and may result in the incurrence of debt, contingent liabilities, interest expense, amortization expense or periodic impairment charges related to goodwill and other intangible assets as well as significant charges relating to integration costs.
We cannot guarantee that we will be able to successfully integrate any future transaction with our existing business or that any combined businesses will be profitable. The successful integration of new businesses depends on our ability to manage these new businesses effectively. The successful integration of future transactions may also require substantial attention from our senior management and the management of the combined businesses, which could decrease the time that they have to service and attract customers. In addition, because we may actively pursue a number of opportunities simultaneously, we may encounter unforeseen expenses, complications and delays, including difficulties in employing sufficient staff and maintaining operational and management oversight. Our inability to complete the integration of any future transaction with our existing business in a timely and orderly manner could reduce our net revenues and negatively impact our results of operations.
If we fail to comply with extensive contractual obligations and applicable laws and government regulations governing the handling of patient identifiable medical information, including those imposed on us and our customers in connection with HIPAA, we could suffer material losses or be negatively impacted as a result of our and/or our customers’ exposure to material penalties and liabilities under HIPAA as amended by the HITECH Act.
As part of the operation of our business, our customers provide us with certain patient identifiable medical information. Although many regulatory and governmental requirements do not directly apply to our operations, we and our hospital and other healthcare provider customers must comply with a variety of requirements related to the handling of patient information, including laws and regulations protecting the privacy, confidentiality and security of PHI. Most of our customers are covered entities under HIPAA and, in many of our relationships, we function as a business associate. In particular, the provisions of HIPAA require our customers to have business associate agreements in place with a clinical documentation company such as ours under which we are required to appropriately safeguard the PHI we create or receive on their behalf. Further, we and our customers are required to comply with HIPAA security regulations that require us and them to implement certain administrative, physical and technical safeguards to ensure the confidentiality, integrity and availability of EPHI. We are required by regulation and contract to protect the security of EPHI that we create, receive, maintain or transmit for our customers consistent with these regulations. To comply with our regulatory and contractual obligations, we may have to reorganize processes and invest in new technologies. We also are required to train personnel regarding HIPAA requirements. If we, or any of our MTs or subcontractors, are unable to maintain the privacy, confidentiality and security of the PHI that is entrusted to us, we and/or our customers could be subject to civil and criminal fines and sanctions and we could be found to have breached our contracts with our customers.
We are bound by business associate agreements with covered entities that require us to use and disclose PHI in a manner consistent with HIPAA in providing services to those covered entities. The HITECH Act, which was enacted into law on February 17, 2009 as part of the ARRA, enhances and strengthens the HIPAA Privacy and Security Standards and makes certain provisions applicable to “business associates” of covered entities. As of February 17, 2010, some provisions of HIPAA apply directly to us. In addition, the HITECH Act creates new security breach notification requirements The direct applicability of the new HIPAA Privacy and Security provisions will require us to incur additional costs and may restrict our business operations. In addition, these new provisions will
19
result in additional regulations and guidance issued by the United States Department of Health and Human Services and will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our customers.
As of February 17, 2010, we are directly subject to HIPAA’s criminal and civil penalties for breaches of our privacy and security obligations.
Proposed legislation and possible negative publicity may impede our ability to utilize global service capabilities.
Certain stated laws that have recently been enacted and bills introduced in recent sessions of the U.S. Congress have sought to restrict the transmission of personally identifiable information regarding a U.S. resident to any foreign affiliate, subcontractor or unaffiliated third party without adequate privacy protections or without providing notice of the transmission and an opportunity to opt out. Some of the proposals would require patient consent. If enacted, these proposed laws would impose liability on healthcare businesses arising from the improper sharing or other misuse of personally identifiable information. Some proposals would create a private civil cause of action that would allow an injured party to recover damages sustained as a result of a violation of the new law. A number of states have also considered, or are in the process of considering, prohibitions or limitations on the disclosure of medical or other information to individuals or entities located outside of the U.S. Further, as a result of this negative publicity and concerns regarding the possible misuse of personally identifiable information, some of our customers have contractually limited our ability to use MTs located outside of the U.S.
CBaySystems Holdings beneficially owns approximately 69.5% of our outstanding common stock, and its interests may conflict with the interests of MedQuist and our other shareholders.
CBaySystems Holdings beneficially owns approximately 69.5% of our outstanding common stock. CBaySystems Holdings has the ability to cause the election of all of the members of our board of directors, the appointment of new management and the approval of actions requiring the approval of our shareholders, including amendments to our certificate of incorporation and mergers or sales of substantially all of our assets. The directors elected by CBaySystems Holdings will be able to make decisions affecting our capital structure, including decisions to issue additional capital stock, implement stock repurchase programs and declare dividends. The interests of CBaySystems Holdings could conflict with our interests and the interests of our other shareholders.
Our debt obligations include restrictive covenants which may restrict our operations or otherwise adversely affect us.
Under the Credit Agreement which we entered into in August 2009, we must abide by certain financial and other restrictive covenants that, among other things, require us to maintain certain minimum levels of EBITDA and a minimum fixed charge coverage ratio. Upon a breach of any of the covenants in the Credit Agreement the lender could declare us to be in default of the Credit Agreement and could further require any outstanding borrowings under the Credit Agreement to be immediately due and payable, and terminate all commitments to extend further credit. We currently do not have any borrowings outstanding under the Credit Agreement.
Item 1B.Unresolved Staff Comments
None.
Item 2.Properties
We currently do not own any real property. We currently lease approximately 33,000 square feet of office space in Mount Laurel, New Jersey which houses our corporate headquarters. This lease expires in 2014; however, we have the option to terminate the lease in 2011, subject to certain conditions, including the payment of a termination fee. We also lease approximately 21,000 square feet of office space in Norcross, Georgia for our sales, administrative and research and development functions. This lease expires in July 2014. We closed our leased facility in Marietta, Georgia in December 2009. Other than our corporate headquarters and Norcross facility, none of our other properties are material to our business. We believe that our corporate headquarters and other properties are suitable for their respective uses and are, in general, adequate for our present needs.
Item 3.Legal Proceedings
20
Customer Litigation
Kaiser Litigation
On June 6, 2008, plaintiffs Kaiser Foundation Health Plan, Inc., Kaiser Foundation Hospitals, The Permanente Medical Group, Inc., Kaiser Foundation Health Plan of the Mid-Atlantic States, Inc., and Kaiser Foundation Health Plan of Colorado (collectively, Kaiser) filed suit against MedQuist Inc. and MedQuist Transcriptions, Ltd. (collectively, MedQuist) in the Superior Court of the State of California in and for the County of Alameda. The action is entitled Foundation Health Plan Inc., et al v. MedQuist Inc. et al., Case No. CV-078-03425 PJH. The complaint asserts five causes of action, for common law fraud, breach of contract, violation of California Business and Professions Code section 17200, unjust enrichment, and a demand for an accounting. More specifically, Kaiser alleges that we fraudulently inflated the payable units of measure in medical transcription reports generated by us for Kaiser pursuant to the contracts between the parties. The damages alleged in the complaint include an estimated $7 million in compensatory damages, as well as punitive damages, attorneys’ fees and costs, and injunctive relief. We contend that we did not breach the contracts with Kaiser, or commit the fraud alleged, and we intend to defend the suit vigorously. The parties participated in private mediation on July 24, 2008, but the case was not settled. We removed the case to the United States District Court for the Northern District of California, and we filed motions to dismiss Kaiser’s complaint and to transfer venue of the case to the United Stated District Court for the District of New Jersey. Kaiser stipulated to transfer, and the case was transferred to the United States District Court for the District of New Jersey on or about August 26, 2008.
The parties exchanged initial disclosures on October 6, 2008 and appeared before the court for an initial scheduling conference on October 14, 2008. Kaiser’s initial disclosures claim damages, including compensatory damages, punitive damages, and prejudgment interest, in excess of $12 million. Following the scheduling conference, the court ordered the parties to mediation. The parties participated in court-ordered mediation on February 27, 2009 but the case was not settled. The court heard argument on our motion to dismiss on March 19, 2009. On April 8, 2009, the court entered an order denying our motion to dismiss, except that our motion to dismiss plaintiffs’ claim under the fraudulent prong of the California Unfair Competition Law was granted. The court issued a scheduling order on April 17, 2009, setting a pretrial schedule. We filed our answer to plaintiff’s complaint on April 23, 2009.
Kaiser served an initial set of discovery requests on May 7, 2009. On May 20, 2009, the court temporarily stayed discovery to address allegations of ethical misconduct by Kaiser’s trial counsel, Greenberg Traurig, LLP. On July 1, 2009, the court granted us leave to conduct limited written discovery regarding Greenberg Traurig’s alleged ethical misconduct and continued the temporary stay of all other discovery. On July 14, 2009, we moved for sanctions against Greenberg Traurig for breach of the New Jersey Rules of Professional Conduct, and we moved to stay the litigation pending resolution of the motion for sanctions. Discovery is presently stayed pending resolution of our motion to stay. No hearing date has been set for any of the pending motions. On February 24, 2010, the court granted our motion to stay pending resolution of the motion for sanctions and entered a scheduling order setting oral arguments on the pending motion for sanctions for March 16, 2010.
Kahn Putative Class Action
On January 22, 2008, Alan R. Kahn, one of our shareholders, filed a shareholder putative class action lawsuit against us, Koninklijke Philips Electronics N.V. (Philips), our former majority shareholder, and four of our former non-independent directors, Clement Revetti, Jr., Stephen H. Rusckowski, Gregory M. Sebasky and Scott Weisenhoff. The action, entitled Alan R. Kahn v. Stephen H. Rusckowski, et al., Docket No. BUR-C-000007-08, was venued in the Superior Court of New Jersey, Chancery Division, Burlington County. In the action, plaintiff purports to bring the action on his own behalf and on behalf of all current holders of our common stock. The original complaint alleged that defendants breached their fiduciary duties of good faith, fair dealing, loyalty, and due care by purportedly agreeing to and initiating a process for our sale or a change of control transaction which will allegedly cause harm to plaintiff and members of the putative class. Plaintiff sought damages in an unspecified amount, plus costs and interest, a judgment declaring that defendants breached their fiduciary duties and that any proposed transactions regarding our sale or change of control are void, an injunction preventing our sale or any change of control transaction that is not entirely fair to the class, an order directing us to appoint three independent directors to our board of directors, and attorneys’ fees and expenses.
On June 12, 2008, plaintiff filed an amended class action complaint against us, eight of our current and former directors, and Philips in the Superior Court of New Jersey, Chancery Division. In the amended complaint, plaintiff alleged that our current and former directors breached their fiduciary duties of good faith, fair dealing, loyalty, and due care by not providing our public shareholders with the opportunity to decide whether they wanted to participate in a share purchase offer with non-party CBaySystems Holdings Ltd. (CBaySystems Holdings) that would have allowed the public shareholders to sell their shares of our common stock for an amount above market price. Plaintiff further alleged that CBaySystems Holdings made the share purchase offer to Philips and that Philips breached its fiduciary duties by accepting CBaySystems Holdings’ offer. Based on these allegations, plaintiff sought
21
declaratory, injunctive, and monetary relief from all defendants. Plaintiff claimed that we were only named as a party to the litigation for purposes of injunctive relief.
On July 14, 2008, we moved to dismiss plaintiff’s amended class action complaint, arguing (1) that plaintiff’s amended class action complaint did not allege that we engaged in any wrongdoing which supported a breach of fiduciary duty claim and (2) that a breach of fiduciary duty claim is not legally cognizable against a corporation. Plaintiff filed an opposition to our motion to dismiss on July 21, 2008.
On November 21, 2008, the Court granted our motion and the motions filed by the other defendants and dismissed plaintiff’s amended class action complaint with prejudice. On December 31, 2008, plaintiff filed an appeal of the trial court’s dismissal order with the New Jersey Appellate Division. Thereafter, the parties briefed all the issues raised in plaintiff’s appeal. In our opposition brief, we opposed all the arguments plaintiff raised with respect to the dismissal of the claims against us.
On September 24, 2009, the Appellate Division held oral argument on the issues that are the subject of plaintiff’s appeal. We are now waiting for the Appellate Division to issue a decision.
Reseller Arbitration Demand
On October 1, 2007, we received from counsel to nine current and former resellers of our products (Claimants), a copy of an arbitration demand filed by the Claimants, initiating an arbitration proceeding styled Diskriter, Inc., Electronic Office Systems, Inc., Milner Voice & Data, Inc., Nelson Systems, Inc., NEO Voice and Communications, Inc., Office Business Systems, Inc., Roach-Reid Office Systems, Inc., Stiles Office Systems, Inc., and Travis Voice and Data, Inc. v. MedQuist Inc. and MedQuist Transcriptions, Ltd. (collectively MedQuist) (filed on September 27, 2007, AAA, 30-118-Y-00839-07). The arbitration demand purports to set forth claims for breach of contract; breach of covenant of good faith and fair dealing; promissory estoppel; misrepresentation; and tortious interference with contractual relations. The Claimants allege that we breached our written agreements with the Claimants by: (i) failing to provide reasonable training, technical support, and other services; (ii) using the Claimants’ confidential information to compete against the Claimants; (iii) directly competing with the Claimants’ territories; and (iv) failing to make new products available to the Claimants. In addition, the Claimants allege that we made false oral representations that we: (i) would provide new product, opportunities and support to the Claimants; (ii) were committed to continuing to use Claimants; (iii) did not intend to create our own sales force with respect to the Claimants’ territory; and (iv) would stay out of Claimants’ territories and would not attempt to take over the Claimants business and relationships with the Claimants’ customers and end-users. The Claimants assert that they are seeking damages in excess of $24.3 million. We also moved to dismiss MedQuist Inc. as a party to the arbitration since MedQuist Inc. is not a party to the Claimants’ agreements, and accordingly, has never agreed to arbitration. The AAA initially agreed to rule on these matters, but then decided to defer a ruling to the panel of arbitrators selected pursuant to the parties’ agreements (Panel). In response, we informed the Panel that a court, not the Panel, should rule on these issues. When it appeared that the Panel would rule on these issues, we initiated a lawsuit in the Superior Court of DeKalb County (the Court) and requested an injunction enjoining the Panel from deciding these issues. The Court denied the request, and indicated that a new motion could be filed if the Panel’s ruling was adverse to MedQuist Inc. or MedQuist Transcriptions, Ltd. On May 6, 2008, the Panel dismissed MedQuist Inc. as a party, but ruled against our opposition to a consolidated arbitration. We asked the Court to stay the arbitration in order to review that decision. The Court initially granted the stay, but later lifted the stay. The Court did not make any substantive rulings regarding consolidation, and in fact, left that decision and others to the assigned judge, who was unable to hear those motions. Accordingly, until further order of the Court, the arbitration will proceed forward.
We filed an answer and counterclaim in the arbitration, which generally denied liability. In the lawsuit, the defendants filed a motion to dismiss alleging that our complaint failed to state an actionable claim for relief. On July 25, 2008, we filed our response which opposed the motion to dismiss in all respects. On September 10, 2008, the Court heard argument on defendants’ motion to dismiss. The Court did not issue a decision, but rather, took the matter under advisement.
During discovery in the arbitration, Claimants repeatedly modified the individual damage claims and asserted two alternative damage theories. Claimants did not specify what the two alternative damage theories were, but stated that they were seeking alternative damage amounts for each Claimant. The Panel issued a Revised Scheduling Order, which tentatively scheduled the arbitration to begin in February 2010
On February 19, 2010, the parties reached agreement on settlement terms resolving all claims by the Claimants. Under the proposed settlement, we will pay Claimants $500,000 to resolve all claims, the parties will exchange mutual releases and the arbitration and related state court litigation will be dismissed with prejudice. The parties are in the process of documenting the settlement agreement. We have accrued the entire amount of this settlement as of December 31, 2009.
22
SEC Investigations of Former Officers
With respect to our historical billing practices, the SEC is pursuing civil litigation against our former chief financial officer, whose employment with us ended in July 2004. Pursuant to our bylaws, we have indemnification obligations for the legal fees for our former chief financial officer. In February 2010, one of our current employees, who was our former controller but who does not currently serve in a senior management or financial reporting oversight role, reached a settlement with the SEC in connection with the civil litigation that the SEC had brought against him in connection with our historical billing practices.
23
Item 4.Reserved
PART II
Item 5.Market For Registrant’s Common Equity, Related Stockholder Matters And Issuer Purchases Of Equity Securities
Our common stock began trading on the Global Market of The NASDAQ Stock Market LLC under the ticker symbol “MEDQ” effective on July 17, 2008. Prior to that, our common stock traded on the Pink Sheets under the symbol “MEDQ.PK”. Set forth below are the high and low closing bid quotations for those periods our stock was traded on the Pink Sheets (as reported by the Pink Sheets LLC) and the high and low sales prices for those periods our stock was quoted on NASDAQ (as reported by NASDAQ) for each quarter of 2008 and 2009 and the first quarter (through February 26, 2010) of 2010. The over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not necessarily reflect the prices for actual transactions.
| | | | | | | | | |
| | | High | | Low |
2008 | | | | | | | | |
| First Quarter | | $ | 10.75 | | | $ | 8.05 | |
| Second Quarter | | $ | 9.50 | | | $ | 6.60 | |
| Third Quarter | | $ | 8.00 | | | $ | 4.35 | |
| Fourth Quarter | | $ | 4.97 | | | $ | 1.92 | |
2009 | | | | | | | | |
| First Quarter | | $ | 2.75 | | | $ | 0.87 | |
| Second Quarter | | $ | 6.88 | | | $ | 2.12 | |
| Third Quarter | | $ | 9.32 | | | $ | 5.04 | |
| Fourth Quarter | | $ | 7.89 | | | $ | 5.74 | |
2010 | | | | | | | | |
| First Quarter (through February 26, 2010) | | $ | 7.99 | | | $ | 6.52 | |
Holders
On February 26, 2010, the closing price of our common stock (as reported by NASDAQ) was $7.86 and we had 438 shareholders of record.
Dividends
On July 14, 2008, we announced a dividend of $2.75 per share of our common stock which was paid on August 4, 2008 to shareholders of record as of the close of business on July 25, 2008. This resulted in the use of approximately $103.3 million of cash. On September 2, 2009, we announced a dividend of $1.33 per share of our common stock which was paid on September 15, 2009 to shareholders of record as of the close of business on September 9, 2009. This resulted in the use of approximately $49.9 million of cash. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, and other factors that our board of directors may deem relevant.
Item 6.Selected Financial Data
| | | | | | | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, |
| | | 2009 | | 2008 | | 2007 | | 2006 | | 2005 |
| | | ($ in thousands, except per share data) |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
| Net Revenues | | $ | 307,200 | | | $ | 326,853 | | | $ | 340,342 | | | $ | 358,091 | (1) | | $ | 353,005 | (1) |
| | | | | | | | | | | | | | | | | | | | | |
| Net Income (loss) | | $ | 23,291 | (2)(4) | | $ | (68,795 | )(2)(5) | | $ | (15,206 | )(2) | | $ | (16,942 | )(2) | | $ | (111,632 | )(2)(3) |
| Net Income (loss) per share — Basic | | $ | 0.62 | | | $ | (1.83 | ) | | $ | (0.41 | ) | | $ | (0.45 | ) | | $ | (2.98 | ) |
| Net Income (loss) per share — Diluted | | $ | 0.62 | | | $ | (1.83 | ) | | $ | (0.41 | ) | | $ | (0.45 | ) | | $ | (2.98 | ) |
24
| | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, |
| | | 2009 | | 2008 | | 2007 | | 2006 | | 2005 |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
| Total assets | | $ | 174,661 | (6) | | $ | 202,205 | (5)(6) | | $ | 417,772 | | | $ | 441,139 | | | $ | 493,191 | |
| Total non-current liabilities | | $ | 5,088 | | | $ | 2,832 | | | $ | 17,294 | | | $ | 18,492 | | | $ | 18,534 | |
| | |
| (1) | | Reflects a reduction in net revenues of $10,402 and $57,678 in 2006 and 2005, respectively, related to accommodation offers made to certain of our customers. |
| |
| (2) | | In 2009, 2008, 2007, 2006 and 2005, we recorded a charge, net of insurance recoveries, of $14,843, $19,738, $6,083, $13,001 and $34,127, respectively, for costs associated with our review of certain of our historical line billings, our review of past allegations of improper billing practices, as well as legal fees, settlement costs and other costs associated with these matters. |
| |
| (3) | | In the fourth quarter of 2005, a valuation allowance of $56,808 was established against various domestic deferred tax assets. After consideration of all evidence, both positive and negative, management concluded that it was more likely than not that a majority of the domestic deferred tax assets would not be realized. |
| |
| (4) | | In 2009, we recorded charges of $1,263 related to costs related to the proposed Spheris acquisition. |
| |
| (5) | | In 2008, we recorded a goodwill impairment charge of $82,233 (see Note 7 to our financial statements included under Item 8 of this Form 10-K) and recognized a deferred tax benefit of $18,470 primarily related to the reversal of deferred tax liabilities associated with indefinite life intangible assets. |
| |
| (6) | | In 2009 and 2008, we paid cash dividends of $1.33 and $2.75 per share, respectively, of our common stock which resulted in the use of approximately $49,949 and $103,279, respectively, of cash. |
Item 7.Management’s Discussion And Analysis Of Financial Condition And Results Of Operations
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our audited consolidated financial statements and related notes appearing elsewhere in this report. In addition to historical information, this discussion and analysis contains forward-looking statements based on current expectations that involve risks, uncertainties and assumptions, such as our plans, objectives, expectations and intentions set forth in the “Cautionary Statement Regarding Forward-Looking Statements,” which can be found in Item 1A, Risk Factors. Our actual results and the timing of events may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth in the “Risk Factors” section and elsewhere in this report.
Executive Overview
We are a leading provider of technology-enabled clinical documentation services. We offer health systems, hospitals, and group medical practices integrated solutions for voice capture, speech recognition, medical transcription, document management and clinical documentation improvement using natural language processing, as well as coding services. Our solutions are designed to facilitate electronic access to clinical information by healthcare providers and to improve overall revenue cycle performance and adoption of the Electronic Health Record (EHR). We perform our services utilizing the DocQmenttm Enterprise Platform (DEP), our proprietary clinical documentation workflow management system. We believe our services and enterprise technology solutions — including mobile voice capture devices, speech recognition technologies, Web-based workflow platforms, and global network of medical transcriptionists (MTs) and medical editors (MEs) — enable to improve patient care, increase physician satisfaction and lower operational costs while facilitating their migration toward increased adoption and utilization of the EHR.
25
Significant developments in the clinical documentation industry include:
| | • | | A shortage of qualified domestic MTs and MEs has increased the demand for offshore medical transcription services by healthcare providers. This demand for qualified MTs and MEs, combined with budgetary pressures experienced by healthcare providers nationwide, has also caused many more healthcare providers to evaluate and consider the use of offshore medical transcription services. |
| |
| | • | | Several low cost providers have emerged and aggressively moved into our market offering medical transcription services (performed both domestically and offshore) at prices significantly below our traditional price point. While we believe the market for outsourced medical transcription continues to expand, the growing acceptance of utilizing offshore labor by healthcare providers has further increased the competitive environment in the medical transcription industry. |
| |
| | • | | Technological advances including speech recognition products reduce the length of time required to transcribe medical reports, in turn reducing the overall cost of medical transcription services. |
| |
| | • | | One of our competitors, Spheris Inc. filed for bankruptcy protection and we have offered to purchase the domestic assets of Spheris, Inc. See Subsequent Events in this section. |
| |
| | • | | Growing market penetration of EHR and certain other technologies have eliminated or shortened the length of certain worktypes produced through transcription. |
Although we remain the leading provider of medical transcription services in the U.S., we experience competition from many other providers at the local, regional and national level. The medical transcription industry remains highly fragmented, with hundreds of small companies in the U.S. performing medical transcription services.
We believe the outsourced portion of the medical transcription services market will increase due in part to the majority of healthcare providers seeking the following:
| | • | | Reductions in overhead and other administrative costs; |
| |
| | • | | Improvements in the quality and delivery speed of transcribed medical reports; |
| |
| | • | | Access to leading technologies, such as speech recognition technology, without development and investment risk; |
| |
| | • | | Implementation and management of a medical transcription system tailored to the providers’ specific requirements; |
| |
| | • | | Access to skilled MTs and MEs; |
| |
| | • | | Solutions for compliance with governmental and industry mandated privacy and security requirements; and |
| |
| | • | | Product offerings that interface with EHR initiatives. |
We evaluate our operating results based upon the following factors:
| | • | | Revenues; |
| |
| | • | | Operating income; |
| |
| | • | | Net income per share; |
| |
| | • | | Adjusted earnings before interest, taxes, depreciation and amortization; |
| |
| | • | | Net cash provided by operating activities; and |
| |
| | • | | Days sales outstanding. |
Our goal is to execute our strategy to yield growth in net revenues, operating income, cash flow and net income per share.
26
Network and information systems, the Internet and other technologies are critical to our business activities. Substantially all of our transcription services are dependent upon the use of network and information systems, including the use of our DEP and our license to use speech recognition secured from a third party. If information systems including the Internet or our DEP are disrupted, we could face a significant disruption of services provided to our customers. We have periodically experienced short term outages with our DEP, which have not significantly disrupted our business and we have an active disaster recovery program in place for our information systems and our DEP.
Critical Accounting Policies, Judgments and Estimates
Management’s Discussion and Analysis (MD&A) is based in part upon our consolidated financial statements, which have been prepared in accordance with generally accepted accounting principles in the U.S. (GAAP). We believe there are several accounting policies that are critical to understanding our historical and future performance, as these policies affect the reported amounts of revenues and other significant areas that involve management’s judgments and estimates. These critical accounting policies and estimates have been discussed with our audit committee.
The preparation of our consolidated financial statements requires us to make estimates and judgments that affect our reported amounts of assets, liabilities, expenses and related disclosure of contingent liabilities. On an ongoing basis, we evaluate these estimates and judgments. We base these estimates on historical experience and on various other assumptions that are believed to be reasonable at such time, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other independent sources. Actual results may ultimately differ from these estimates. A critical accounting estimate must meet two criteria: (1) it requires assumptions about highly uncertain matters, and (2) there would be a material effect on the financial statements from either using a different, although reasonable, amount within the range of the estimate in the current period or from reasonably likely period-to-period changes in the estimate. While there are a number of accounting policies, methods and estimates affecting our consolidated financial statements as addressed in Note 2 to our consolidated financial statements, areas that are particularly significant and critical include:
Valuation of Long-Lived and Other Intangible Assets and Goodwill:In connection with acquisitions, we allocate portions of the purchase price to tangible and intangible assets, consisting primarily of acquired technologies, and customer relationships, based on independent appraisals received after each acquisition, with the remainder allocated to goodwill. We assess the realizability of goodwill and intangible assets with indefinite useful lives at least annually, or sooner if events or changes in circumstances indicate that the carrying amount may not be recoverable. We have determined that the reporting unit level is our sole operating segment.
We review our long-lived assets, including amortizable intangibles, for impairment when events indicate that their carrying amount may not be recoverable. When we determine that one or more impairment indicators are present for an asset, we compare the carrying amount of the asset to net future undiscounted cash flows that the asset is expected to generate. If the carrying amount of the asset is greater than the net future undiscounted cash flows that the asset is expected to generate, we then compare the fair value to the book value of the asset. If the fair value is less than the book value, we recognize an impairment loss. The impairment loss is the excess of the carrying amount of the asset over its fair value.
Some of the events that we consider as impairment indicators for our long-lived assets, including goodwill, are:
| | • | | our net book value compared to our fair value; |
| |
| | • | | significant adverse economic and industry trends; |
| |
| | • | | significant decrease in the market value of the asset; |
| |
| | • | | the extent that we use an asset or changes in the manner that we use it; |
| |
| | • | | significant changes to the asset since we acquired it; and |
| |
| | • | | other changes in circumstances that potentially indicate all or a portion of the company will be sold. |
27
Deferred income taxes.Deferred tax assets represent future tax benefits that we expect to be able to apply against future taxable income or that will result in future net operating losses that we can carry forward to use against future taxable earnings. Our ability to utilize the deferred tax assets is dependent upon our ability to generate future taxable income. To the extent that we believe it is more likely than not that all or a portion of the deferred tax asset will not be utilized, we record a valuation allowance against that asset. In making that determination we consider all positive and negative evidence and give stronger consideration to evidence that is objective in nature.
Commitments and contingencies.We routinely evaluate claims and other potential litigation to determine if a liability should be recorded in the event it is probable that we will incur a loss and can estimate the amount of such loss.
Revenue recognition.We recognize medical transcription services revenues when there is persuasive evidence that an arrangement exists, the price is fixed or determinable, services have been rendered and collectability is reasonably assured. These services are recorded using contracted rates and are net of estimates for customer credits. Historically, our estimates have been adequate. If actual results are higher or lower than our estimates, we would have to adjust our estimates and financial statements in future periods.
We recognize the remainder of our revenues from the sale and implementation of voice-capture and document management products including software and implementation, training and maintenance services related to these products. The application of the accounting guidelines requires judgment regarding the timing of the recognition of these revenues including: (i) whether a software arrangement includes multiple elements, and if so, whether vendor-specific objective evidence of fair value exists for those elements; (ii) whether customizations or modifications of the software are significant; and (iii) whether collection of the software fee is probable. Additionally, for certain contracts we recognize revenues using the percentage-of-completion method. Percentage-of-completion accounting involves estimates of the total costs to be incurred over the duration of the project.
Accounts receivable and allowance for doubtful accounts.Accounts receivable are recorded at the invoiced amount and do not bear interest. The carrying value of accounts receivable approximates fair value. The allowance for doubtful accounts is our best estimate of potential losses resulting from the inability of our customers to make required payments due. This allowance is used to state trade receivables at estimated net realizable value.
We estimate uncollectible amounts based upon our historical write-off experience, current customer receivable balances, aging of customer receivable balances, the customer’s financial condition and current economic conditions. Historically, our estimates have been adequate to provide for our accounts receivable exposure.
Additionally, we enter into medical transcription service contracts that may contain provisions for performance penalties in the event we do not meet certain required service levels, primarily related to turnaround time on transcribed reports. We reduce revenues for any such performance penalties and service level credits incurred and have included an estimate of such penalties and credits in our allowance for uncollectible accounts.
We recognize product revenues for sales to end-user customers and resellers upon passage of title if all other revenue recognition criteria have been met. End-user customers generally do not have a right of return. We provide certain of our resellers and distributors with limited rights of return of our products. We reduce revenues for rights to return our product based upon our historical experience and have included an estimate of such credits in our allowance for uncollectible accounts.
Customer Accommodation Program.In response to customers’ concerns regarding historical billing matters, we established a plan to offer financial accommodations to certain of our customers during 2005 and 2006 and recorded the related liability. In 2008 we reached an agreement on customer litigation resolving all claims by the named parties. Since then we have not made additional offers.
We are unable to predict how many customers, if any, may accept the outstanding accommodation offers on the terms proposed by us, nor are we able to predict the timing of the acceptance (or rejection) of any outstanding accommodation offers. Until any offers are accepted, we may withdraw or modify the terms of the accommodation program or any outstanding offers at any time. In addition, we are unable to predict how many future offers, if made, will be accepted on the terms proposed by us. We regularly evaluate whether to proceed with, modify or withdraw the accommodation program or any outstanding offers.
28
Basis of Presentation
Sources of Revenues
We derive revenues primarily from providing medical transcription services to health systems, hospitals and large group medical practices. Our customers are generally charged a rate times the volume of work that we transcribe or edit. In the clinical documentation workflow, we provide, in addition to medical transcription technology and services, maintenance services, digital dictation, speech recognition and electronic signature services.
Net revenues from customers in the U.S. were $301.1 million, $321.0 million and $335.1 million for the years ended December 31, 2009, 2008 and 2007, respectively. Net revenues from customers outside the U.S. were $6.1 million, $5.8 million and $5.2 million for the years ended December 31, 2009, 2008 and 2007, respectively.
Cost of Revenues
Cost of revenues includes compensation of our U.S.-based employee MTs and our subcontractor MTs, other production costs (primarily related to operational and production management, quality assurance, quality control and customer and field service personnel), and telecommunication and facility costs. Cost of revenues also includes the direct cost of technology products sold to customers. MT costs are directly related to medical transcription revenues and are based on lines transcribed or edited multiplied by a specific rate.
Selling, General and Administrative (SG&A)
Our SG&A expenses include marketing and sales costs, accounting costs, information technology costs, professional fees, corporate facility costs, corporate payroll and benefits expenses.
Research and Development (R&D)
Our R&D expenses consist primarily of personnel and related costs, including salaries and employee benefits for software engineers and consulting fees paid to independent consultants who provide software engineering services to us. To date, our R&D efforts have been devoted to new products and services offerings and increases in features and functionality of our existing products and services.
Depreciation and amortization
Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets, which range from two to seven years for furniture, equipment and software, and the lesser of the lease term or estimated useful life for leasehold improvements. Intangible assets are being amortized using the straight-line method over their estimated useful lives which range from three to 20 years.
Legal Matters
Cost of legal proceedings and settlements, net includes settlement of claims, ongoing litigation, and associated legal and other professional fees incurred.
Consolidated Results of Operations
The following tables set forth our consolidated results of operations for the periods indicated below:
Comparison of Years Ended December 31, 2009 and 2008
29
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | | | | | |
| | | 2009 | | | 2008 | | | | | | | |
| | | | | | | % of Net | | | | | | | % of Net | | | | | | | |
| ($ in thousands) | | Amount | | | Revenues | | | Amount | | | Revenues | | | $ Change | | | % Change | |
| Net revenues | | $ | 307,200 | | | | 100.0 | % | | $ | 326,853 | | | | 100.0 | % | | $ | (19,653 | ) | | | (6.0 | %) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of revenues | | | 206,265 | | | | 67.1 | % | | | 230,375 | | | | 70.5 | % | | | (24,110 | ) | | | (10.5 | %) |
| Selling, general and administrative | | | 33,441 | | | | 10.9 | % | | | 47,520 | | | | 14.5 | % | | | (14,079 | ) | | | (29.6 | %) |
| Research and development | | | 9,604 | | | | 3.1 | % | | | 15,848 | | | | 4.8 | % | | | (6,244 | ) | | | (39.4 | %) |
| Depreciation | | | 9,504 | | | | 3.1 | % | | | 11,950 | | | | 3.7 | % | | | (2,446 | ) | | | (20.5 | %) |
| Amortization of intangible assets | | | 6,168 | | | | 2.0 | % | | | 5,554 | | | | 1.7 | % | | | 614 | | | | 11.1 | % |
| Cost of legal proceedings and settlements, net | | | 14,843 | | | | 4.8 | % | | | 19,738 | | | | 6.0 | % | | | (4,895 | ) | | | (24.8 | %) |
| Acquisition related charges | | | 1,263 | | | | 0.4 | % | | | — | | | | — | | | | 1,263 | | | | n.a. | |
| Goodwill impairment charge | | | — | | | | — | | | | 82,233 | | | | 25.2 | % | | | (82,233 | ) | | | (100.0 | %) |
| Restructuring charges | | | 2,727 | | | | 0.9 | % | | | 2,055 | | | | 0.6 | % | | | 672 | | | | 32.7 | % |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total operating costs and expenses | | | 283,815 | | | | 92.4 | % | | | 415,273 | | | | 127.1 | % | | | (131,458 | ) | | | (31.7 | %) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating income (loss) | | | 23,385 | | | | 7.6 | % | | | (88,420 | ) | | | (27.1 | %) | | | 111,805 | | | | (126.4 | %) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Equity in income of affiliated company | | | 2,015 | | | | 0.7 | % | | | 236 | | | | 0.1 | % | | | 1,779 | | | | 753.8 | % |
| Other income | | | — | | | | — | | | | 438 | | | | 0.1 | % | | | (438 | ) | | | (100.0 | %) |
| Interest income (expense), net | | | (134 | ) | | | (0.0 | %) | | | 2,438 | | | | 0.7 | % | | | (2,572 | ) | | | (105.5 | %) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Income (loss) before income taxes | | | 25,266 | | | | 8.2 | % | | | (85,308 | ) | | | (26.1 | %) | | | 110,574 | | | | (129.6 | %) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Income tax provision (benefit) | | | 1,975 | | | | 0.6 | % | | | (16,513 | ) | | | (5.1 | %) | | | 18,488 | | | | (112.0 | %) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) | | $ | 23,291 | | | | 7.6 | % | | $ | (68,795 | ) | | | (21.0 | %) | | $ | 92,086 | | | | (133.9 | %) |
| | | | | | | | | | | | | | | | | | | |
Net revenues
Net revenues recorded for the year ended December 31, 2009 were $307.2 million, a decline of $19.7 million or 6.0% when compared to the prior year net revenue amount of $326.9 million.
The revenue decline was primarily due to:
| | • | | lower prices for our transcription service related revenues of $12.9 million, net of revenues realized from higher year over year transcription volume; |
| |
| | • | | declining product and companion maintenance revenues totaling $6.8 million largely related to customers not renewing maintenance contracts for legacy systems. |
Pricing for our transcription services remains under pressure as many customers seek us to reduce their costs by using more offshore labor and increasing productivity with expanded use of speech recognition.
Cost of revenues
Cost of revenues declined by $24.1 million, or 10.5%, to $206.3 million for the year ended December 31, 2009 when compared to the prior year amount of $230.4 million. The decline was attributable to the following:
| | • | | staffing reductions that reduced costs by $7.6 million resulting from restructuring actions taken by management to align our operating costs to better compete in a lower price environment for our services; |
| |
| | • | | a $13.5 million reduction in medical transcription costs related to our increased use of speech recognition technology, and our increased use of offshore labor to supplement our domestic workforce capacity; |
| |
| | • | | lower product costs of $1.5 million as a result of lower product and maintenance related sales and services; |
| |
| | • | | other reductions of $0.3 million, net; and |
| |
| | • | | a $1.2 million reversal of an accrual due to the lapsing of the statute of limitations. |
30
Selling, general and administrative
SG&A expenses decreased by $14.1 million, or 29.6%, to $33.4 million for the year ended December 31, 2009 compared with $47.5 million for the year ended December 31, 2008. This decrease was the result of company-wide cost reduction initiatives that included a $3.7 million decrease in compensation costs due to reductions in workforce and a $6.6 million decrease in legal and other professional fees. The remaining cost savings covered employee stock option related expenses of $0.8 million, a reduction in employee retention costs resulting from the change in control of our majority shareholder of $0.5 million, lower advertising and marketing costs of $0.6 million, lower rent expenses of $0.4 million, reduced bad debt expense of $0.4 million, lower travel and entertainment of $0.3 million, and a decrease in all other SG&A categories of $0.8 million. SG&A expenses in total as a percentage of net revenues were 10.9% for the year ended December 31, 2009 compared with 14.5% for in the year 2008.
Research and development
R&D expenses decreased by $6.2 million, or 39.4%, to $9.6 million for the year ended December 31, 2009 compared with $15.8 million for the year ended December 31, 2008. This decrease was attributable to reduced compensation expense of $3.3 million; a decrease in consulting expense of $1.1 million; an increase in the amount capitalized for software development of $0.6 million; a decrease of $0.4 million in stock option compensation as a result of immediate vesting of previously unvested stock options due to the change in control of our majority shareholder; a decrease in retention bonus for certain key employees during the change in control of $0.4 million; and a decrease in all other R&D expenses of $0.4 million. R&D expenses as a percentage of net revenues were 3.1% for the year ended December 31, 2009 compared with 4.8% for the same period in 2008.
Depreciation
Depreciation expense for the year ended December 31, 2009 decreased by $2.4 million, or 20.5%, to $9.5 million, compared with $11.9 million for the year ended December 31, 2008. The lower depreciation expense was principally the result of reduced capital spending in 2009 and 2008. Depreciation expense as a percentage of net revenues was 3.1% for the year ended December 31, 2009 compared with 3.7% for the same period in 2008.
Amortization
Amortization of intangible assets increased $0.6 million, or 11.1%, to $6.2 million for the year ended December 31, 2009 compared with $5.6 million for the year ended December 31, 2008. The higher amortization costs in 2009 were the result of software capitalization in 2009 and 2008. Amortization of intangible assets as a percentage of net revenues was 2.0% for the year ended December 31, 2009 compared with 1.7% for the same period in 2008.
Legal Matters
For the year ended December 31, 2009, legal and professional fees were $8.5 million and settlements were $6.3 million. The settlements related to the Anthurium litigation and reseller arbitration. For the year ended December 31, 2008, legal and professional fees were $12.3 million and settlement costs were $7.4 million. The settlement was for the settlement of the Department of Justice investigation related to our historical billing practices.
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| Legal and professional fees | | $ | 8,593 | | | $ | 12,313 | |
| Settlements | | | 6,250 | | | | 7,425 | |
| | | | | | | |
| Total | | $ | 14,843 | | | $ | 19,738 | |
| | | | | | | |
Restructuring charges
For the year ended December 31, 2009, restructuring charges included $2.4 million for employee related severance obligations and $0.3 million for non-cancelable leases related to the closure or consolidation of offices. For the year ended December 31, 2008, we had a restructuring charge of $2.1 million for employee severance obligations.
31
Interest income (expense), net
Interest income (expense), net reflects interest earned on cash and cash equivalent balances in banks offset by costs related to the Credit Agreement. Interest income (expense), net decreased $2.6 million, or 105.5%, to ($0.1) million for the year ended December 31, 2009 compared with $2.4 million for the year ended December 31, 2008. This decrease was due to the amortization of deferred financing costs and lower interest rates in the 2009 period as compared to 2008, and lower average cash balance in the 2009 period ($37.3 million) as compared to 2008 ($110.5 million).
Income tax provision
The effective income tax rate for the year ended December 31, 2009 was 7.8% compared with an effective income tax benefit rate of 19.4% for the year ended December 31, 2008. The 2009 tax expense includes an increase in the deferred tax liabilities associated with indefinite life intangible assets related to goodwill and an increase in the deferred tax liability associated with our investment in A-Life. After consideration of all evidence, both positive and negative, management concluded again in 2009, that it was more likely than not that a significant portion of the domestic deferred income tax assets would not be realized. In addition, various adjustments were recorded for the year ended December 31, 2009 including the reduction of the foreign valuation allowance and various adjustments related to state tax exposures.
Subsequent events
On February 2, 2010, we and our majority shareholder, CBay Inc. (CBay and, together with us, the Purchasers), entered into a “stalking horse” Stock and Asset Purchase Agreement (the APA) with Spheris, Inc. and certain of its affiliates (collectively, the Sellers), under which we have agreed to purchase substantially all of the domestic business of Spheris Inc., and CBay has agreed to purchase all of the outstanding shares of Spheris India Private Limited (together, the Assets), subject to the terms and conditions therein. Each of the Sellers is a debtor in a Chapter 11 case before the United States Bankruptcy Court for the District of Delaware (the Bankruptcy Court).
The Bankruptcy Court for the District of Delaware has issued an order approving bidding procedures naming us and CBay as the stalking horse bidders. Spheris filed for Chapter 11 bankruptcy protection on February 3, 2010, after entering into the APA with us and CBay to purchase substantially all of Spheris’ assets in a sale under Section 363 of the United States Bankruptcy Code. The court order was entered in accordance with the timeline provided for by the purchase agreement, and among other things, provides for an auction of Spheris’ assets on April 13, 2010, and the payment of certain transaction expenses and payment of a breakup fee in the amount of $2.1 million by Spheris to us and CBay in certain circumstances if we are not the winning bidders at the auction. Pending a successful conclusion of this process, we anticipate this transaction will close by mid-to-late April 2010.
Under the terms of the Agreement, Purchasers have agreed, absent any higher or otherwise better bid, to acquire the Assets from the Sellers for approximately $75.2 million in cash plus the assumption of specified liabilities of the domestic business of Spheris and the liabilities of Spheris India Private Limited. We have deposited $7.5 million into escrow which will be credited to the purchase price on the completion of the acquisition of the Assets. If the Agreement is terminated, the deposit will be returned to us unless the purchaser termination fee described below is payable, in which event the deposit will be retained by the Sellers and credited to the purchaser termination fee. If the Bankruptcy Court approves the APA and the APA is later terminated for certain reasons, including because the Sellers enter into a competing transaction, the Sellers may be required to pay the Purchasers a termination fee equal to $2.1 million, plus Purchasers’ reasonable transaction expenses. In addition, if the APA is terminated by Sellers for certain reasons, including because the Purchasers refuse to complete the acquisition in breach of the APA, we are required to pay the Sellers a termination fee equal to the difference between $15 million and the amount of the deposit and interest thereon.
The completion of the acquisition is subject to a number of conditions, including the performance by each party of its obligations under the Agreement, and the absence of a material adverse change in the Sellers’ business since the date of the APA, among other conditions which are set forth in the APA.
In connection with the acquisition, MedQuist is exploring debt financing options.
32
Consolidated Results of Operations
The following tables set forth our consolidated results of operations for the periods indicated below:
Comparison of Years Ended December 31, 2008 and 2007
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | | | | | |
| | | 2008 | | | 2007 | | | | | | | |
| | | | | | | % of Net | | | | | | | % of Net | | | | | | | |
| ($ in thousands) | | Amount | | | Revenues | | | Amount | | | Revenues | | | $ Change | | | % Change | |
| Net revenues | | $ | 326,853 | | | | 100.0 | % | | $ | 340,342 | | | | 100.0 | % | | $ | (13,489 | ) | | | (4.0 | %) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of revenues | | | 230,375 | | | | 70.5 | % | | | 260,879 | | | | 76.7 | % | | | (30,504 | ) | | | (11.7 | %) |
| Selling, general and administrative | | | 47,520 | | | | 14.5 | % | | | 62,288 | | | | 18.3 | % | | | (14,768 | ) | | | (23.7 | %) |
| Research and development | | | 15,848 | | | | 4.8 | % | | | 13,695 | | | | 4.0 | % | | | 2,153 | | | | 15.7 | % |
| Depreciation | | | 11,950 | | | | 3.7 | % | | | 10,988 | | | | 3.2 | % | | | 962 | | | | 8.8 | % |
| Amortization of intangible assets | | | 5,554 | | | | 1.7 | % | | | 5,511 | | | | 1.6 | % | | | 43 | | | | 0.8 | % |
| Cost of legal proceedings and settlements, net | | | 19,738 | | | | 6.0 | % | | | 6,083 | | | | 1.8 | % | | | 13,655 | | | | 224.5 | % |
| Goodwill impairment charge | | | 82,233 | | | | 25.2 | % | | | — | | | | — | | | | 82,233 | | | | n.a. | |
| Restructuring charges | | | 2,055 | | | | 0.6 | % | | | 2,756 | | | | 0.8 | % | | | (701 | ) | | | (25.4 | %) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total operating costs and expenses | | | 415,273 | | | | 127.1 | % | | | 362,200 | | | | 106.4 | % | | | 53,073 | | | | 14.7 | % |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating loss | | | (88,420 | ) | | | (27.1 | %) | | | (21,858 | ) | | | (6.4 | %) | | | (66,562 | ) | | | 304.5 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Equity in income of affiliated company | | | 236 | | | | 0.1 | % | | | 625 | | | | 0.2 | % | | | (389 | ) | | | (62.2 | %) |
| Other income | | | 438 | | | | 0.1 | % | | | — | | | | — | | | | 438 | | | | n.a. | |
| Interest income, net | | | 2,438 | | | | 0.7 | % | | | 8,366 | | | | 2.5 | % | | | (5,928 | ) | | | (70.9 | %) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss before income taxes | | | (85,308 | ) | | | (26.1 | %) | | | (12,867 | ) | | | (3.8 | %) | | | (72,441 | ) | | | 563.0 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Income tax provision (benefit) | | | (16,513 | ) | | | (5.1 | %) | | | 2,339 | | | | 0.7 | % | | | (18,852 | ) | | | (806.0 | %) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (68,795 | ) | | | (21.0 | %) | | $ | (15,206 | ) | | | (4.5 | %) | | $ | (53,589 | ) | | | 352.4 | % |
| | | | | | | | | | | | | | | | | | | |
Net revenues
Net revenues decreased $13.5 million, or 4.0%, to $326.9 million for the year ended December 31, 2008 compared with $340.3 million for the year ended December 31, 2007. This decrease was attributable primarily to:
| | • | | a decline in service revenues of $10.2 million resulting primarily from lower medical transcription volume and lower pricing to both new and existing customers. We believe the reduction in volume was the result primarily of customer losses to other outsourced medical transcription providers due to, among other things, price competition or, in the area of radiology, replacement of our transcription services with speech recognition technology offerings of our competitors; and |
| |
| | • | | reduced sales of our technology products of $3.3 million due primarily to reduced maintenance contracts. |
Cost of revenues
Cost of revenues decreased $30.5 million, or 11.7%, to $230.4 million for the year ended December 31, 2008 compared with $260.9 million for the year ended December 31, 2007. This decrease was attributable primarily to:
| | • | | headcount reductions associated with restructuring actions taken to better align our overhead costs with our lower revenue levels, and increased use of offshore labor to supplement our domestic capacity, reduced our costs by $12.8 million; |
| |
| | • | | reduced medical transcription revenues resulted in a reduction of $15.0 million of payroll costs; and |
| • | | reduced product and maintenance revenue resulted in lower technology costs of $2.7 million. |
As a percentage of net revenues, cost of revenues decreased to 70.5% for the year ended December 31, 2008 from 76.7% for the same period in 2007, as a result largely of actions taken to reduce fixed costs at a faster pace than the reduction of our net revenues.
33
Selling, general and administrative
SG&A expenses decreased $14.8 million, or 23.7%, to $47.5 million for the year ended December 31, 2008 compared with $62.3 million for the year ended December 31, 2007. This decrease was due primarily to expenses in 2007 which did not repeat in 2008 including audit fees of $4.7 million related to our consolidated financial statements and our internal control over financial reporting for years 2003 through 2007; a reduction of compensation expense of $3.9 million as a result of reductions in workforce; a decrease in legal fees of $2.6 million; $1.1 million associated with the separation of certain members of our executive management; $1.4 million related to the expiration of our agreement with a consulting company that provided interim chief executive officer services to us; $0.6 million for insurance premiums in 2007 triggered by our receipt of certain levels of insurance recovery; and a reduction of other expenses of $1.3 million. These decreases were offset by an increase of $0.8 million of stock option compensation as a result of immediate vesting of previously unvested stock options due to the change in control of our majority shareholder. SG&A expenses as a percentage of net revenues were 14.5% for the year ended December 31, 2008 compared with 18.3% for the same period in 2007.
Research and development
R&D expenses increased $2.2 million, or 15.7%, to $15.9 million for the year ended December 31, 2008 compared with $13.7 million for the year ended December 31, 2007. This increase was due to higher staffing costs associated with additional investments in our DEP of $1.3 million; an increase of $0.4 million of stock option compensation as a result of immediate vesting of previously unvested stock options due to the change in control of our majority shareholder; an increase in retention bonuses of $0.3 million for certain key employees due to the change in control of our majority shareholder; and an increase in all other R&D expenses of $0.2 million. R&D expenses as a percentage of net revenues were 4.8% for the year ended December 31, 2008 compared with 4.0% for the same period in 2007.
Depreciation
Depreciation expense increased $1.0 million, or 8.8%, to $12.0 million for the year ended December 31, 2008 compared with $11.0 million for the year ended December 31, 2007. This increase was attributable primarily to assets that were purchased in the 2007 period related to technology for production system enhancements and R&D enhancements. Depreciation expense as a percentage of net revenues was 3.7% for the year ended December 31, 2008 compared with 3.2% for the same period in 2007.
Amortization
Amortization of intangible assets increased $0.1 million, or 0.8%, to $5.6 million for the year ended December 31, 2008 compared with $5.5 million for the year ended December 31, 2007. Amortization of intangible assets as a percentage of net revenues were 1.7% for the year ended December 31, 2008 compared with 1.6% for the same period in 2007.
Legal Matters
Cost of settlements and legal proceedings, net increased $13.7 million, or 224.5%, to $19.7 million for the year ended December 31, 2008 compared with $6.1 million for the year ended December 31, 2007. This increase was due primarily to the 2007 realization of $15.4 million of insurance claim reimbursements; an increase of $7.4 million due to the settlement of all claims related to the consolidated medical transcriptionists putative class action and the DOJ Investigation; and a decrease in legal fees of $9.2 million.
| | | | | | | | | |
| | | 2008 | | | 2007 | |
| Legal and professional fees | | $ | 12,313 | | | $ | 21,469 | |
| Insurance recovery | | | — | | | | (15,386 | ) |
| Settlements | | | 7,425 | | | | — | |
| | | | | | | |
| Total | | $ | 19,738 | | | $ | 6,083 | |
| | | | | | | |
Impairment Charges
Valuation of Long-Lived and Other Intangible Assets and Goodwill.
34
In connection with acquisitions, we allocate portions of the purchase price to tangible and intangible assets, consisting primarily of acquired technologies, and customer relationships, agreements based on independent appraisals received after each acquisition, with the remainder allocated to goodwill. We assess the realizability of goodwill and intangible assets with indefinite useful lives at least annually, or sooner if events or changes in circumstances indicate that the carrying amount may not be recoverable. We have determined that the reporting unit level is our sole operating segment.
We tested goodwill for impairment during the first three quarters of 2008 and determined that the fair value of the reporting unit substantially exceeded the carrying value based upon the capitalization. In the fourth quarter, which included our annual impairment testing date in December, we determined our fair value using a combination of our market capitalization based on market price per share for approximately the 60 days before December 31, 2008, and a discounted cash flow analysis. Determining fair value requires the exercise of significant judgment, including judgment about appropriate discount rates, perpetual growth rates, the amount and timing of expected future cash flows, as well as relevant comparable company earnings multiples for the market-based approach. The cash flows employed in the discounted cash flow analyses are based on our internal business model for 2009 and, for years beyond 2009, the growth rates we used are an estimate of the future growth in the industry in which we participate. The discount rates used in the Discounted Cash Flow analyses are intended to reflect the risks inherent in the future cash flows of the reporting unit and are based on an estimated cost of capital, which we determined based on our estimated cost of capital relative to our capital structure. In addition, the market-based approach utilizes comparable company public trading values, research analyst estimates and, where available, values observed in private market transactions. Our analysis indicated that the reporting unit fair value was below our book value. The test for impairment of goodwill is a two-step process:
| | • | | First, we compare the carrying amount of our reporting unit, which is the book value of our entire company, to the fair value of our reporting unit. If the carrying amount of our reporting unit exceeds its fair value, we have to perform the second step of the process. If not, no further testing is needed. In the fourth quarter of 2008 we determined that the carrying amount of our reporting unit exceeded the fair value and accordingly performed the second step in the analysis. |
| |
| | • | | If the second part of the analysis is required, we allocate the fair value of our reporting unit to all assets and liabilities as if the reporting unit had been acquired in a business combination at the date of the impairment test. We then compare the implied fair value of our reporting unit’s goodwill to its carrying amount. If the carrying amount of our goodwill exceeds its implied fair value, we recognize an impairment loss in an amount equal to that excess. In the fourth quarter of 2008, the carrying value of goodwill exceeded its implied fair value and accordingly we recorded a non cash, pre-tax impairment charge of $82.2 million. |
We review our long-lived assets, including amortizable intangibles, for impairment when events indicate that their carrying amount may not be recoverable. When we determine that one or more impairment indicators are present for an asset, we compare the carrying amount of the asset to net future undiscounted cash flows that the asset is expected to generate. If the carrying amount of the asset is greater than the net future undiscounted cash flows that the asset is expected to generate, we compare the fair value to the book value of the asset. If the fair value is less than the book value, we recognize an impairment loss. The impairment loss is the excess of the carrying amount of the asset over its fair value.
Some of the events that we consider as impairment indicators for our long-lived assets, including goodwill, are:
| | • | | our net book value compared to our fair value; |
| |
| | • | | significant adverse economic and industry trends; |
| |
| | • | | significant decrease in the market value of the asset; |
| |
| | • | | the extent that we use an asset or changes in the manner that we use it; |
| |
| | • | | significant changes to the asset since we acquired it; and |
| |
| | • | | other changes in circumstances that potentially indicate all or a portion of the company will be sold. |
During 2008 we reviewed the carrying value of our long lived assets other than goodwill and determined that the carrying amounts of such assets was less than the undiscounted cash flows and accordingly no impairment charge was recorded.
35
Restructuring charges
For the year ended December 31, 2008, we recorded a restructuring charge of $2.0 million for severance obligations. For the year ended December 31, 2007, we recorded a restructuring charge of $2.8 million comprised of $2.4 million for severance obligations and $0.4 million for non-cancelable leases related to the closure of offices.
Interest income, net
Interest income, net reflects interest earned on cash and cash equivalent balances. Interest income, net decreased $5.9 million, or 70.9%, to $2.4 million for the year ended December 31, 2008 compared with $8.4 million for the year ended December 31, 2007. This decrease was attributable to lower interest rates earned in the 2008 period (2.5%) compared with the 2007 period (5.0%); and a decrease in the average cash balance in 2008 of $57.0 million.
Income tax provision
The effective income tax rate for the year ended December 31, 2008 was an income tax benefit rate of 19.4% compared with an effective income tax provision rate of 18.2% for the year ended December 31, 2007. The 2008 tax benefit includes the reversal of approximately $18.5 million of deferred tax liabilities associated with indefinite life intangible assets related to goodwill which was impaired in 2008. After consideration of all evidence, both positive and negative, management concluded again in 2008, that it was more likely than not that a significant portion of the domestic deferred income tax assets would not be realized. In addition, various adjustments were recorded for the year ended December 31, 2007 including the reduction of the foreign valuation allowance and various adjustments related to state tax exposures.
Liquidity and Capital Resources
As of December 31, 2009, we had working capital of $19.7 million compared with $39.5 million as of December 31, 2008. Our principal sources of liquidity include cash generated from operations, available cash on hand and our credit facility. Cash and cash equivalents declined $14.7 million to $25.2 million as of December 31, 2009 from $39.9 million as of December 31, 2008. This decrease included cash provided by operating activities of $44.6 million offset by cash used to purchase property and equipment of $4.9 million, cash used for software development activities and other investments of $3.4 million, cash dividend of $49.9 million, and cash used of $1.2 million for fees and expenses related to our line of credit facility.
In August 2009 we entered into a five-year $25 million revolving credit agreement (Credit Agreement) with Wells Fargo Foothill, LLC. Subject to certain terms and conditions, the Credit Agreement provides committed revolving funding through August 2014 and includes an option whereby we can increase its maximum credit to $40 million. The amount available for borrowings is based upon a percentage of eligible accounts receivable. There are reserves established which limit the amounts that can be available. At December 31, 2009, $21.0 million was available under the Credit Agreement. The Credit Agreement is a working capital facility that may be used for general corporate purposes. The Credit Agreement enables us to borrow funds in U.S. dollars, at variable interest rates. The Credit Agreement provides the lender a security interest in and against significantly all of our assets. Under the Credit Agreement we agreed to certain covenants customarily found in such agreements including, but not limited to, financial covenants requiring us to maintain certain minimum levels of EBITDA and a minimum fixed charge coverage ratio. At December 31, 2009, we were in compliance with the financial covenants of the Credit Agreement. At December 31, 2009, there were no borrowings outstanding under the Credit Agreement.
We believe our existing cash and cash equivalents combined with cash expected to be generated from operations and cash available under the Credit Agreement will be sufficient to finance our operations for the foreseeable future.
On February 2, 2010, we and our majority shareholder, CBay Inc. (CBay and, together with us, the Purchasers), entered into a “stalking horse” Stock and Asset Purchase Agreement (the APA) with Spheris, Inc. and certain of its affiliates (collectively, the Sellers), under which we have agreed to purchase substantially all of the domestic business of Spheris Inc., and CBay has agreed to purchase all of the outstanding shares of Spheris India Private Limited (together, the Assets), subject to the terms and conditions therein. Each of the Sellers is a debtor in a Chapter 11 case before the United States Bankruptcy Court for the District of Delaware (the Bankruptcy Court).
The Bankruptcy Court for the District of Delaware has issued an order approving bidding procedures naming us and CBay as the stalking horse bidders. Spheris filed for Chapter 11 bankruptcy protection on February 3, 2010, after entering into the APA with us
36
and CBay to purchase substantially all of Spheris’ assets in a sale under Section 363 of the United States Bankruptcy Code. The court order was entered in accordance with the timeline provided for by the purchase agreement, and among other things, provides for an auction of Spheris’ assets on April 13, 2010, and the payment of certain transaction expenses and payment of a breakup fee in the amount of $2.1 million by Spheris to us and CBay in certain circumstances if we are not the winning bidders at the auction. Pending a successful conclusion of this process, we anticipate this transaction will close by mid-to-late April 2010.
Under the terms of the Agreement, Purchasers have agreed, absent any higher or otherwise better bid, to acquire the Assets from the Sellers for approximately $75.2 million in cash plus the assumption of specified liabilities of the domestic business of Spheris and the liabilities of Spheris India Private Limited. We have deposited $7.5 million into escrow which will be credited to the purchase price on the completion of the acquisition of the Assets. If the Agreement is terminated, the deposit will be returned to us unless the purchaser termination fee described below is payable, in which event the deposit will be retained by the Sellers and credited to the purchaser termination fee. If the Bankruptcy Court approves the APA and the APA is later terminated for certain reasons, including because the Sellers enter into a competing transaction, the Sellers may be required to pay the Purchasers a termination fee equal to $2.1 million, plus Purchasers’ reasonable transaction expenses. In addition, if the APA is terminated by Sellers for certain reasons, including because the Purchasers refuse to complete the acquisition in breach of the APA, we are required to pay the Sellers a termination fee equal to the difference between $15 million and the amount of the deposit and interest thereon.
The completion of the acquisition is subject to a number of conditions, including the performance by each party of its obligations under the Agreement, and the absence of a material adverse change in the Sellers’ business since the date of the APA, among other conditions which are set forth in the APA.
In connection with the acquisition, MedQuist is exploring debt financing options.
Off-Balance Sheet Arrangements
We are not involved in any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Contractual Obligations
The following table summarizes our obligations to make future payments under current contracts as of December 31, 2009 (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | Payment Due By Period | |
| | | | | | | Less | | | | | | | | | | | | |
| | | | | | | than | | | | | | | | | | | After | |
| | | Total | | | 1 Year | | | 1-3 Years | | | 3-5 Years | | | 5 Years | |
| Operating Lease Obligations | | $ | 15,757 | | | $ | 3,645 | | | $ | 7,356 | | | $ | 4,756 | | | $ | — | |
| Purchase Obligations(1) | | | 10,869 | | | | 8,666 | | | | 2,203 | | | | — | | | | — | |
| Severance and Other Guaranteed Payment Obligations(2) | | | 2,000 | | | | 2,000 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| Total Contractual Obligations | | $ | 28,626 | | | $ | 14,311 | | | $ | 9,559 | | | $ | 4,756 | | | $ | — | |
| | | | | | | | | | | | | | | | |
| | |
| (1) | | Purchase obligations are for telecommunication contracts ($9,619), software development ($1,000) and other recurring purchase obligations ($250). |
| |
| (2) | | Severance and Other Guaranteed Payment Obligations are comprised of severance payments ($1,750) and other guaranteed payments of ($250). |
We have agreements with certain of our named executive officers that provide for severance payments to the employee in the event the employee is terminated without cause. The maximum cash exposure under these agreements was approximately $1.9 million as of December 31, 2009.
Recent Accounting Pronouncements
In June 2009, the Financial Accounting Standards Board (FASB) issued, “The FASB Accounting Standards Codification TM and the Hierarchy of Generally Accepted Accounting Principles. This establishes the Codification as the source of authoritative GAAP recognized by the FASB to be applied by nongovernmental entities. Rules and interpretive releases of the SEC under federal securities
37
laws are also sources of authoritative GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority.
In September 2009, the FASB ratified two consensuses affecting revenue recognition:
The first consensus,Revenue Recognition—Multiple-Element Arrangements, sets forth requirements that must be met for an entity to recognize revenue from the sale of a delivered item that is part of a multiple-element arrangement when other items have not yet been delivered. One of those current requirements is that there be objective and reliable evidence of the standalone selling price of the undelivered items, which must be supported by either vendor-specific objective evidence (VSOE) or third-party evidence (TPE).
This consensus eliminates the requirement that all undelivered elements have VSOE or TPE before an entity can recognize the portion of an overall arrangement fee that is attributable to items that already have been delivered. In the absence of VSOE or TPE of the standalone selling price for one or more delivered or undelivered elements in a multiple-element arrangement, entities will be required to estimate the selling prices of those elements. The overall arrangement fee will be allocated to each element (both delivered and undelivered items) based on their relative selling prices, regardless of whether those selling prices are evidenced by VSOE or TPE or are based on the entity’s estimated selling price. Application of the “residual method” of allocating an overall arrangement fee between delivered and undelivered elements will no longer be permitted.
The second consensus,Software-Revenue Recognitionaddresses the accounting for transaction involving software to exclude from its scope tangible products that contain both software and non-software and not-software components that function together to deliver a products functionality.
The Consensuses are effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. We are evaluating the potential impact of these requirements on our financial statements.
Item 7A.Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily a result of fluctuations in interest rates. We do not hold or issue financial instruments for trading purposes.
Interest Rate Risk
We earn interest income from our balances of cash and cash equivalents. This interest income is subject to market risk related to changes in interest rates, which affects primarily our investment portfolio. We invest in instruments that meet high credit quality standards, as specified in our investment policy.
Management estimates that if the average yield of our investments decreased by 20 basis points, our interest income for the year ended December 31, 2009 would have decreased by approximately $0.1 million to $0.0 million. The impact on our future interest income will depend largely on the gross amount of our investments and future changes in investment yields.
There are no material changes in our exposure to interest rate risk as compared to our exposure to interest rate risk for the year ended December 31, 2008.
Item 8.Financial Statements And Supplementary Data
Our consolidated financial statements and supplementary data required by this item are attached to this report beginning on page F-1.
Item 9.Changes in And Disagreements With Accountants On Accounting And Financial Disclosure
None.
Item 9A.Controls And Procedures
(a) Evaluation of Disclosure Controls and Procedures
38
Our management team, under the supervision and with the participation of our principal executive officer and our principal financial officer, evaluated the effectiveness of the design and operation of our disclosure controls and procedures as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended (Exchange Act), as of the last day of the fiscal period covered by this report, December 31, 2009. The term disclosure controls and procedures means our controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to management, including our principal executive and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Based on this evaluation, our principal executive officer and our principal financial officer concluded that, our disclosure controls and procedures were effective as of December 31, 2009.
(b) Management’s Report on Internal Control over Financial Reporting as of December 31, 2009
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and Rule 15d-15(f) under the Exchange Act as a process designed by, or under the supervision of, the issuer’s principal executive and principal financial officers, or persons performing similar functions, and effected by the issuer’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP and includes those policies and procedures that:
| | • | | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the issuer; |
| |
| | • | | provide reasonable assurance that transactions are recorded as necessary to permit the preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the issuer are being made only in accordance with authorizations of management and directors of the issuer; and |
| |
| | • | | provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of the issuer’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with existing policies or procedures may deteriorate.
In accordance with the internal control reporting requirements of the SEC, management completed an assessment of the adequacy of our internal control over financial reporting as of December 31, 2009. In making this assessment, management used the criteria set forth inInternal Control — Integrated Frameworkby the Committee of Sponsoring Organizations of the Treadway Commission (COSO). As a result of this assessment and based on the criteria in the COSO framework, management has concluded that, as of December 31, 2009, our internal control over financial reporting was effective. Our independent registered public accounting firm has issued an audit report on the effectiveness of our internal control over financial reporting as of December 31, 2009. This report is included on page F-2 of our consolidated financial statements included as part of this Annual Report on Form 10-K.
(c) Changes in Internal Control Over Financial Reporting
There have been no changes in internal control over financial reporting for the quarter ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B.Other Information
We held our annual meeting of shareholders (Annual Meeting) on December 17, 2009. The purpose of the Annual Meeting was to elect eight directors for terms of one year each. The number of shares outstanding as of the close of business on November 17, 2009, the record date for the Annual Meeting, was 37,555,893. There were 35,770,243 shares of company stock present at the meeting or by proxy. The results of voting at the Annual Meeting were as follows:
39
| | | | | | | | | |
| Name | | Votes For | | | Votes Withheld | |
Nominees: | | | | | | | | |
| Robert Aquilina | | | 34,297,381 | | | | 1,472,862 | |
| Frank Baker | | | 34,396,024 | | | | 1,374,219 | |
| Peter E. Berger | | | 34,395,792 | | | | 1,374,451 | |
| John F. Jastrem | | | 35,272,823 | | | | 497,420 | |
| Colin J. O’Brien | | | 35,272,923 | | | | 497,320 | |
| Warren E. Pinckert II | | | 35,169,531 | | | | 600,712 | |
| Michael Seedman | | | 34,396,024 | | | | 1,374,219 | |
| Andrew E. Vogel | | | 35,561,251 | | | | 208,992 | |
PART III
Item 10.Directors, Executive Officers and Corporate Governance
The information called for by this item is incorporated by reference to the portions of our definitive proxy statement entitled, “Election of Directors,” “Governance of the Company,” “Report of the Audit Committee” and “Section 16 (a) Beneficial Ownership Reporting Compliance.”
Item 11.Executive Compensation
The information called for by this item is incorporated by reference to the portions of our definitive proxy statement entitled “Compensation Discussion and Analysis,” “Report of the Compensation Committee,” and “Compensation of our Named Executive Officers.”
Item 12.Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information called for by this item is incorporated by reference to the portions of our definitive proxy statement entitled “Stock Ownership of Our Directors, Executive Officers and 5% Beneficial Owners,” “Compensation of Directors,” “Securities Authorized For Issuance Under Equity Compensation Plans” and “Compensation of our Named Executive Officers.”
Item 13.Certain Relationships and Related Transactions, and Director Independence
The information called for by this item is incorporated by reference to the portion of our definitive proxy statement entitled “Certain Relationships and Related Transactions.”
Item 14.Principal Accountant Fees and Services
The information called for by this item is incorporated by reference to the portion of our definitive proxy statement entitled “Fees Paid to Independent Registered Public Accounting Firm.”
PART IV
Item 15.Exhibits and Financial Statement Schedules
(a) Documents filed as part of this report:
(1) Financial Statements.The consolidated financial statements filed as part of this report are listed on the Index to Consolidated Financial Statements on page F-1.
(2) Financial Statement Schedules.Schedule II — Valuation and Qualifying Accounts filed as part of this report is listed on page F-30. All other financial statement schedules have been omitted here because they are not applicable, not required, or the information is shown in the consolidated financial statements or notes thereto.
(3) Exhibits.See (b) below.
40
(b) Exhibits:
| | | |
| No. | | Description |
| 3.1(1) | | Certificate of Incorporation of MedQuist Inc. (as amended) |
| | | |
| 3.2(6) | | Second Amended and Restated By-Laws, as amended, of MedQuist Inc. |
| | | |
| 4.1(1) | | Specimen Stock Certificate |
| | | |
| 10.1*(1) | | 1992 Stock Option Plan of MedQuist Inc., as amended |
| | | |
| 10.2*(1) | | Nonstatutory Stock Option Plan for Non-Employee Directors of MedQuist Inc. |
| | | |
| 10.3*(1) | | MedQuist Inc. 2002 Stock Option Plan |
| | | |
| 10.4*(1) | | Form of Award Agreement under the MedQuist Inc. 2002 Stock Option Plan |
| | | |
| 10.5*(1) | | 1996 Employee Stock Purchase Plan |
| | | |
| 10.6*(1) | | MedQuist Inc. Executive Deferred Compensation Plan |
| | | |
| 10.7*(1) | | Letter Agreement, dated as of April 21, 2005, between MedQuist Inc. and Michael Clark |
| | | |
| 10.8*(1) | | Letter Agreement, dated as of April 21, 2005, between MedQuist Inc. and Mark Sullivan |
| | | |
| 10.10*(1) | | Letter Agreement, dated as of November 10, 2006, by and between MedQuist Inc. and James Brennan |
| | | |
| 10.11*(5) | | Letter Agreement by and between MedQuist Inc. and Nightingale & Associates, LLC dated March 14, 2008 |
| | | |
| 10.12(1) | | Licensing Agreement, dated as of May 22, 2000, between MedQuist Inc. and Philips Speech Processing GmbH |
| | | |
| 10.12.1(1) | | Amendment No. 1 to Licensing Agreement, dated as of January 1, 2002, between MedQuist Inc. and Philips Speech Processing GmbH |
| | | |
| 10.12.2#(1) | | Amendment No. 2 to Licensing Agreement, dated as of December 10, 2002, between MedQuist Inc. and Philips Speech Processing GmbH |
| | | |
| 10.12.3#(1) | | Amendment No. 3 to Licensing Agreement, dated as of August 10, 2003, between MedQuist Inc. and Philips Speech Processing GmbH |
| | | |
| 10.12.4#(1) | | Amendment No. 4 to Licensing Agreement, dated as of September 1, 2004, between MedQuist Inc. and Philips Speech Processing GmbH |
| | | |
| 10.12.5#(1) | | Amendment No. 5 to Licensing Agreement, dated as of December 30, 2005, between MedQuist Transcriptions, Ltd. and Philips Speech Recognition Systems GmbH f/k/a Philips Speech Processing GmbH |
| | | |
| 10.12.6#(1) | | Amendment No. 6 to Licensing Agreement, dated as of February 13, 2007, between MedQuist Inc. and Philips Speech Recognition Systems GmbH f/k/a Philips Speech Processing GmbH |
| | | |
| 10.12.7 | | Amendment No. 7 to Licensing Agreement, dated as of November 10, 2009, between MedQuist Inc. and Nuance Communications, Inc. as the successor-in-interest to Philips Speech Recognition Systems GmbH |
41
| | | |
| No. | | Description |
| 10.13## | | Third Amended and Restated OEM Supply Agreement dated November 10, 2009, between MedQuist Inc. and Nuance Communications, Inc. as the successor-in-interest to Philips Speech Recognition Systems GmbH |
| | | |
| 10.14(1) | | Mt. Laurel, New Jersey Office Lease Agreement dated as of June 17, 2003 |
| | | |
| 10.14.1(1) | | First Amendment to Mt. Laurel, New Jersey Office Lease Agreement dated as of August 26, 2003 |
| | | |
| 10.14.2(1) | | Second Amendment to Mt. Laurel, New Jersey Office Lease Agreement dated as of November 30, 2003 |
| | | |
| 10.14.3(1) | | Third Amendment to Mt. Laurel, New Jersey Office Lease Agreement dated as of November 30, 2003 |
| | | |
| 10.14.4(1) | | Confirmation of Lease Term regarding Mt. Laurel, New Jersey Office Lease dated as of August 10, 2006 |
| | | |
| 10.15*(1) | | MedQuist Inc. Board of Directors Deferred Compensation Plan |
| | | |
| 10.16*(2) | | Form of Management Indemnification Agreement by and between MedQuist Inc. and Certain Officers |
| | | |
| 10.16.1*(7) | | First Amendment to the Form of Management Indemnification Agreement by and between MedQuist Inc. and Certain Officers |
| | | |
| 10.17*(4) | | Indemnification Agreement, dated as of February 21, 2008 between MedQuist Inc. and Warren Pinckert |
| | | |
| 10.18*(8) | | Employment Agreement by and between Peter Masanotti and MedQuist Inc., dated September 3, 2008 |
| | | |
| 10.19#(9) | | Transcription Services Agreement by and between MedQuist Transciptions, Ltd. and CBay Systems & Services, Inc. dated April 3, 2009 |
| | | |
| 10.20*(10) | | Indemnification Agreement dated November 21, 2008 between MedQuist Inc. and Peter Masanotti |
| | | |
| 10.21*(11) | | Amended and Restated Stock Option Agreement by and between Peter Masanotti and MedQuist Inc., dated March 2, 2009 |
| | | |
| 10.22*(9) | | Employment Agreement by and between Alan Gold and MedQuist Inc. dated February 26, 2009 |
| | | |
| 10.23*(12) | | Employment Agreement by and between Dominick Golio and MedQuist Inc. dated April 9, 2009 |
| | | |
| 10.24*(9) | | Employment Agreement by and between Kevin Piltz and MedQuist Inc. dated May 18, 2009 |
| | | |
| 10.25(9) | | Settlement and License Agreement by and between Anthurium Solutions, Inc. and MedQuist Inc. dated June 19, 2009 |
| | | |
| 10.26*(13) | | MedQuist Inc. Long-Term Incentive Plan adopted on August 27, 2009 |
| | | |
| 10.27(13) | | Credit Agreement by and among MedQuist Inc. and its subsidiaries, and Wells Fargo Foothill, LLC as the arranger and administrative agent and lender dated August 31, 2009 |
| | | |
| 10.28(14) | | Services Agreement by and between MedQuist Inc. and CBay Inc. dated September 19, 2009 |
| | | |
| 10.29## | | Licensing Agreement by and between Nuance Communications, Inc. and MedQuist Inc. dated November 10, 2009 |
42
| | | |
| No. | | Description |
| 10.30(15) | | Transcription Services Subcontracting Agreement by and between MedQuist Inc. and CBay Systems & Services, Inc. dated March 31, 2009 |
| | | |
| 21(1) | | Subsidiaries of MedQuist Inc. |
| | | |
| 23 | | Consent of KPMG LLP |
| | | |
| 24 | | Power of Attorney (included on the signature page hereto) |
| | | |
| 31.1 | | Certification of Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 31.2 | | Certification of Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 32.1 | | Certification of Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 32.2 | | Certification of Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | |
| * | | Management contract or compensatory plan or arrangement. |
| |
| # | | Portions of this Exhibit were omitted and filed separately with the Secretary of the SEC pursuant to an order for confidential treatment from SEC. |
| |
| ## | | Portions of this Exhibit were omitted and filed separately with the Secretary of the SEC pursuant to a request for confidential treatment that has been filed with the SEC. |
| |
| (1) | | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2005 filed on July 5, 2007 |
| |
| (2) | | Incorporated by reference to our Current Report on Form 8-K filed on August 28, 2007 |
| |
| (3) | | Incorporated by reference to our Current Report on Form 8-K filed on September 25, 2007 |
| |
| (4) | | Incorporated by reference to our Current Report on Form 8-K filed on February 22, 2008 |
| |
| (5) | | Incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2007 filed on March 17, 2008 |
| |
| (6) | | Incorporated by reference to our Current Report on Form 8-K filed on July 15, 2008 |
| |
| (7) | | Incorporated by reference to our Current Report on Form 8-K filed on August 25, 2008 |
| |
| (8) | | Incorporated by reference to our Current Report on Form 8-K filed on September 9, 2008 |
| |
| (9) | | Incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2009 filed on July 30, 2009 |
| |
| (10) | | Incorporated by reference to our Current Report on Form 8-K filed on November 28, 2008 |
43
| | |
| (11) | | Incorporated by reference to our Current Report on Form 8-K filed on March 6, 2009 |
| |
| (12) | | Incorporated by reference to our Current Report on Form 8-K filed with the SEC on April 15, 2009 |
| |
| (13) | | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 9, 2009 |
| |
| (14) | | Incorporated by reference to our Current Report on Form 8-K filed with the SEC on September 24, 2009 |
| |
| (15) | | Incorporated by reference to our Current Report on Form 8-K filed on April 6, 2009 |
44
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| | MedQuist Inc.
| |
| | By: | /s/Peter Masanotti | |
| | | Peter Masanotti | |
| | | President and Chief Executive Officer | |
| |
Date: March 11, 2010
Pursuant to the requirements of the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Each person, in so signing also makes, constitutes, and appoints Peter Masanotti and Dominick Golio, and each of them acting alone, as his or her true and lawful attorneys-in-fact, with full power of substitution, in his or her name, place, and stead, to execute and cause to be filed with the SEC any or all amendments to this report.
| | | | | |
| Signature | | Capacity | | Date |
| | | | | |
/s/Peter Masanotti Peter Masanotti | | President and Chief Executive Officer (Principal Executive Officer) | | March 11, 2010 |
| | | | | |
/s/Dominick Golio Dominick Golio | | Chief Financial Officer (Principal Financial Officer) | | March 11, 2010 |
| | | | | |
/s/James Brennan James Brennan | | Principal Accounting Officer, Controller and Vice President (Principal Accounting Officer) | | March 11, 2010 |
| | | | | |
/s/Robert Aquilina Robert Aquilina | | Non-Executive Chairman of the Board of Directors | | March 11, 2010 |
| | | | | |
/s/Frank Baker Frank Baker | | Director | | March 11, 2010 |
| | | | | |
/s/Peter E. Berger Peter E. Berger | | Director | | March 11, 2010 |
| | | | | |
/s/John F. Jastrem John F. Jastrem | | Director | | March 11, 2010 |
| | | | | |
/s/Colin J. O’Brien Colin J. O’Brien | | Director | | March 11, 2010 |
| | | | | |
/s/Warren E. Pinckert, ii Warren E. Pinckert II | | Director | | March 11, 2010 |
| | | | | |
/s/Michael Seedman Michael Seedman | | Director | | March 11, 2010 |
| | | | | |
/s/Andrew E. Vogel Andrew E. Vogel | | Director | | March 11, 2010 |
45
Index to Consolidated Financial Statements
| | | |
| | F-2 |
| | F-3 |
| | F-4 |
| | F-5 |
| | F-6 |
| | F-7 |
| | F-8 |
| | F-33 |
Report of Independent Registered Public Accounting Firm on Internal Control Over Financial Reporting
The Board of Directors and Shareholders of MedQuist Inc.:
We have audited MedQuist Inc.’s internal control over financial reporting as of December 31, 2009 based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). MedQuist Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting as of December 31, 2009. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, MedQuist Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of MedQuist Inc. and subsidiaries as of December 31, 2009 and 2008 and the related consolidated statements of operations, shareholders’ equity and other comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2009, and our report dated March 11, 2010 expressed an unqualified opinion on those consolidated financial statements.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 11, 2010
F-2
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of MedQuist Inc.:
We have audited the accompanying consolidated balance sheets of MedQuist Inc. and subsidiaries (the Company) as of December 31, 2009 and 2008, and the related consolidated statements of operations, shareholders’ equity and other comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2009. In connection with our audits of the consolidated financial statements, we also have audited financial statement Schedule II — Valuation and Qualifying Accounts. These consolidated financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of MedQuist Inc. and subsidiaries as of December 31, 2009 and 2008, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2009, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), MedQuist Inc.’s internal control over financial reporting as of December 31, 2009, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 11, 2010 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 11, 2010
F-3
MedQuist Inc. and Subsidiaries
Consolidated Statements of Operations
(In thousands, except per share amounts)
| | | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
| Net revenues | | $ | 307,200 | | | $ | 326,853 | | | $ | 340,342 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating costs and expenses: | | | | | | | | | | | | |
| Cost of revenues | | | 206,265 | | | | 230,375 | | | | 260,879 | |
| Selling, general and administrative | | | 33,441 | | | | 47,520 | | | | 62,288 | |
| Research and development | | | 9,604 | | | | 15,848 | | | | 13,695 | |
| Depreciation | | | 9,504 | | | | 11,950 | | | | 10,988 | |
| Amortization of intangible assets | | | 6,168 | | | | 5,554 | | | | 5,511 | |
| Cost of legal proceedings and settlements, net | | | 14,843 | | | | 19,738 | | | | 6,083 | |
| Acquistion related charges | | | 1,263 | | | | — | | | | — | |
| Goodwill impairment charge | | | — | | | | 82,233 | | | | — | |
| Restructuring charges | | | 2,727 | | | | 2,055 | | | | 2,756 | |
| | | | | | | | | | |
| Total operating costs and expenses | | | 283,815 | | | | 415,273 | | | | 362,200 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Operating income (loss) | | | 23,385 | | | | (88,420 | ) | | | (21,858 | ) |
| | | | | | | | | | | | | |
| Equity in income of affiliated company | | | 2,015 | | | | 236 | | | | 625 | |
| Other income | | | — | | | | 438 | | | | — | |
| Interest income (expense), net | | | (134 | ) | | | 2,438 | | | | 8,366 | |
| | | | | | | | | | |
| Income (loss) before income taxes | | | 25,266 | | | | (85,308 | ) | | | (12,867 | ) |
| | | | | | | | | | | | | |
| Income tax provision (benefit) | | | 1,975 | | | | (16,513 | ) | | | 2,339 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Net income (loss) | | $ | 23,291 | | | $ | (68,795 | ) | | $ | (15,206 | ) |
| | | | | | | | | | |
| Net income (loss) per share: | | | | | | | | | | | | |
| Basic | | $ | 0.62 | | | $ | (1.83 | ) | | $ | (0.41 | ) |
| | | | | | | | | | |
| Diluted | | $ | 0.62 | | | $ | (1.83 | ) | | $ | (0.41 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Weighted average shares outstanding: | | | | | | | | | | | | |
| Basic | | | 37,556 | | | | 37,549 | | | | 37,488 | |
| | | | | | | | | | |
| Diluted | | | 37,556 | | | | 37,549 | | | | 37,488 | |
| | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
MedQuist Inc. and Subsidiaries
Consolidated Balance Sheets
(In thousands)
| | | | | | | | | |
| | | As of December 31, | |
| | | 2009 | | | 2008 | |
Assets | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 25,216 | | | $ | 39,918 | |
| Accounts receivable, net | | | 43,627 | | | | 50,374 | |
| Income tax receivable | | | 772 | | | | 154 | |
| Other current assets | | | 4,940 | | | | 8,053 | |
| | | | | | | |
| Total current assets | | | 74,555 | | | | 98,499 | |
| |
| Property and equipment, net | | | 11,772 | | | | 15,785 | |
| Goodwill | | | 40,813 | | | | 40,545 | |
| Other intangible assets, net | | | 36,307 | | | | 39,877 | |
| Deferred income taxes | | | 1,396 | | | | 1,204 | |
| Other assets | | | 9,818 | | | | 6,295 | |
| | | | | | | |
| | | | | | | | | |
| Total assets | | $ | 174,661 | | | $ | 202,205 | |
| | | | | | | |
| | | | | | | | | |
Liabilities and Shareholders’ Equity | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 8,687 | | | $ | 7,487 | |
| Accrued expenses | | | 22,848 | | | | 24,049 | |
| Accrued compensation | | | 12,432 | | | | 11,204 | |
| Deferred income taxes | | | 4 | | | | 651 | |
| Deferred revenue | | | 10,854 | | | | 15,630 | |
| | | | | | | |
| Total current liabilities | | | 54,825 | | | | 59,021 | |
| | | | | | | | | |
| Deferred income taxes | | | 3,240 | | | | 799 | |
| Other non-current liabilities | | | 1,848 | | | | 2,033 | |
| | | | | | | |
| | | | | | | | | |
| Commitments and contingencies (Note 12) | | | | | | | | |
| | | | | | | | | |
| Shareholders’ equity: | | | | | | | | |
| Common stock — no par value; authorized 60,000 shares; 37,556 and 37,556 shares issued and outstanding, respectively | | | 237,848 | | | | 237,907 | |
| Retained earnings (deficit) | | | (125,854 | ) | | | (99,198 | ) |
| Accumulated other comprehensive income | | | 2,754 | | | | 1,643 | |
| | | | | | | |
| |
| Total shareholders’ equity | | | 114,748 | | | | 140,352 | |
| | | | | | | |
| |
| Total liabilities and shareholders’ equity | | $ | 174,661 | | | $ | 202,205 | |
| | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
MedQuist Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
| | | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2009 | | | 2008 | | | 2007 | |
Operating activities: | | | | | | | | | | | | |
| Net income (loss) | | | 23,291 | | | $ | (68,795 | ) | | $ | (15,206 | ) |
| Adjustments to reconcile net income (loss) to cash provided by (used in) operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | 15,672 | | | | 17,504 | | | | 16,499 | |
| Equity in income of affiliated company | | | (2,015 | ) | | | (236 | ) | | | (625 | ) |
| Goodwill impairment charge | | | — | | | | 82,233 | | | | — | |
| Deferred income tax (benefit) provision | | | 1,857 | | | | (17,091 | ) | | | 1,878 | |
| Stock option expense | | | 193 | | | | 1,427 | | | | 565 | |
| Stock based compensation — Board Members | | | — | | | | — | | | | 150 | |
| Provision for doubtful accounts | | | 2,306 | | | | 3,073 | | | | 4,967 | |
| Loss on disposal of property and equipment | | | 133 | | | | 571 | | | | 168 | |
| Changes in operating assets and liabilities excluding effects of acquisitions: | | | | | | | | | | | | |
| Accounts receivable | | | 4,529 | | | | (5,781 | ) | | | (1,359 | ) |
| Income tax receivable | | | (616 | ) | | | 661 | | | | 957 | |
| Insurance receivable | | | — | | | | — | | | | 707 | |
| Other current assets | | | 3,391 | | | | (154 | ) | | | (276 | ) |
| Other assets | | | 25 | | | | 134 | | | | 646 | |
| Accounts payable | | | 1,038 | | | | (5,557 | ) | | | 1,981 | |
| Accrued expenses | | | (1,198 | ) | | | (12,701 | ) | | | (13,101 | ) |
| Accrued compensation | | | 1,192 | | | | (3,559 | ) | | | (727 | ) |
| Deferred revenue | | | (4,939 | ) | | | (272 | ) | | | 592 | |
| Other non-current liabilities | | | (307 | ) | | | (211 | ) | | | 1,910 | |
| | | | | | | | | | |
| Net cash provided by (used in) operating activities | | | 44,552 | | | | (8,754 | ) | | | (274 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
Investing activities: | | | | | | | | | | | | |
| Purchase of property and equipment | | | (4,932 | ) | | | (6,574 | ) | | | (11,639 | ) |
| Proceeds from sale of investments | | | — | | | | 692 | | | | — | |
| Capitalized software | | | (2,582 | ) | | | (3,411 | ) | | | (2,035 | ) |
| Investment in affiliated company | | | (852 | ) | | | — | | | | — | |
| | | | | | | | | | |
| Net cash used in investing activities | | | (8,366 | ) | | | (9,293 | ) | | | (13,674 | ) |
| | | | | | | | | | |
| | | | | | | | | | | | | |
Financing activities: | | | | | | | | | | | | |
| Dividends paid | | | (49,949 | ) | | | (103,279 | ) | | | — | |
| Debt issuance costs | | | (1,201 | ) | | | — | | | | — | |
| Proceeds from exercise of stock options | | | — | | | | 68 | | | | 10 | |
| | | | | | | | | | |
| Net cash provided by (used in) financing activities | | | (51,150 | ) | | | (103,211 | ) | | | 10 | |
| | | | | | | | | | |
| |
| Effect of exchange rate changes | | | 262 | | | | (406 | ) | | | 108 | |
| | | | | | | | | | |
| |
| Net decrease in cash and cash equivalents | | | (14,702 | ) | | | (121,664 | ) | | | (13,830 | ) |
| |
| Cash and cash equivalents — beginning of year | | | 39,918 | | | | 161,582 | | | | 175,412 | |
| | | | | | | | | | |
| |
| Cash and cash equivalents — end of year | | $ | 25,216 | | | $ | 39,918 | | | $ | 161,582 | |
| | | | | | | | | | |
| |
Supplemental cash flow information: | | | | | | | | | | | | |
| | | | | | | | | | |
| Cash (recovered) paid for income taxes | | $ | 234 | | | $ | 210 | | | $ | (451 | ) |
| | | | | | | | | | |
| Accommodation payments paid with credits | | $ | 103 | | | $ | 740 | | | $ | 2,595 | |
| | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
MedQuist Inc. and Subsidiaries
Consolidated Statements of Shareholders’ Equity and Other Comprehensive Income (Loss)
Years ended December 31, 2009, 2008 and 2007
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | | | | | | | | | Retained | | | | | | | Other | | | Total | |
| | | Common Stock | | | Earnings | | | Deferred | | | Comprehensive | | | Shareholders' | |
| | | Shares | | | Amount | | | (Deficit) | | | Compensation | | | Income (Loss) | | | Equity | |
| | | |
Balance, January 1, 2007 | | | 37,484 | | | $ | 235,080 | | | $ | 87,693 | | | $ | 332 | | | $ | 4,414 | | | $ | 327,519 | |
| Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | (15,206 | ) | | | — | | | | — | | | | (15,206 | ) |
| Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | — | | | | 942 | | | | 942 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (14,264 | ) |
| Stock-based compensation expense | | | — | | | | 565 | | | | — | | | | — | | | | — | | | | 565 | |
| Exercise of stock options | | | 4 | | | | 10 | | | | — | | | | — | | | | — | | | | 10 | |
| Adoption of FIN 48 | | | — | | | | — | | | | 389 | | | | — | | | | — | | | | 389 | |
| Deferred compensation — stock grants | | | 56 | | | | 757 | | | | — | | | | (332 | ) | | | — | | | | 425 | |
| | | |
Balance, December 31, 2007 | | | 37,544 | | | $ | 236,412 | | | $ | 72,876 | | | $ | — | | | $ | 5,356 | | | $ | 314,644 | |
| | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | (68,795 | ) | | | — | | | | — | | | | (68,795 | ) |
| Foreign currency translation adjustments | | | — | | | | — | | | | — | | | | — | | | | (3,713 | ) | | | (3,713 | ) |
| Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (72,508 | ) |
| Stock-based compensation expense | | | — | | | | 1,427 | | | | — | | | | — | | | | — | | | | 1,427 | |
| Cash dividend | | | — | | | | — | | | | (103,279 | ) | | | — | | | | — | | | | (103,279 | ) |
| Exercise of stock options | | | 12 | | | | 68 | | | | — | | | | — | | | | — | | | | 68 | |
| | | |
Balance, December 31, 2008 | | | 37,556 | | | $ | 237,907 | | | $ | (99,198 | ) | | $ | — | | | $ | 1,643 | | | $ | 140,352 | |
| | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | — | | | | — | | | | 23,291 | | | | — | | | | — | | | | 23,291 | |
| Foreign currency translation adjustments | | | — | | | | — | | | | 2 | | | | — | | | | 1,111 | | | | 1,113 | |
| Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | 24,404 | |
| Stock-based compensation expense | | | — | | | | 193 | | | | — | | | | — | | | | — | | | | 193 | |
| Cash dividend | | | — | | | | — | | | | (49,949 | ) | | | — | | | | — | | | | (49,949 | ) |
| Dilution of affiliated company | | | — | | | | (252 | ) | | | — | | | | — | | | | — | | | | (252 | ) |
| | | |
Balance, December 31, 2009 | | | 37,556 | | | $ | 237,848 | | | $ | (125,854 | ) | | $ | — | | | $ | 2,754 | | | $ | 114,748 | |
| | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(In thousands, except per share amounts)
1. Description of Business
We are a leading provider of technology-enabled clinical documentation services. We offer health systems, hospitals, and group medical practices integrated solutions for voice capture, speech recognition, medical transcription, document management and clinical documentation improvement using natural language processing, as well as coding services. Our solutions are designed to facilitate electronic access to clinical information by healthcare providers and to improve overall revenue cycle performance and adoption of the Electronic Health Record (EHR). We perform our services utilizing the DocQmenttm Enterprise Platform (DEP), our proprietary clinical documentation workflow management system. We believe our services and enterprise technology solutions — including mobile voice capture devices, speech recognition technologies, Web-based workflow platforms, and global network of medical transcriptionists (MTs) and medical editors (MEs)— enable health systems, hospitals and group medical to improve patient care, increase physician satisfaction and lower operational costs while facilitating their migration toward increased adoption and utilization of the EHR.
Majority Owner
On August 6, 2008, CBaySystems Holdings Limited (CBaySystems Holdings), a company that is publicly traded on the AIM market of the London Stock Exchange with a portfolio of investments in medical transcription, which includes a company that competes in the medical transcription market, healthcare technology, and healthcare financial services, acquired a 69.5% ownership interest in us from Koninklijke Philips Electronics N.V. (Philips).
The Company’s consolidated financial statements do not include any components of purchase accounting, which was recorded at the CBaySystems Holdings reporting level, and are presented on the historical basis of accounting.
We paid cash dividends of $1.33 per share in 2009 and $2.75 per share in 2008.
2. Significant Accounting Policies
Principles of Consolidation
Our consolidated financial statements include the accounts of MedQuist Inc. and its subsidiary companies. All intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates and Assumptions in the Preparation of Consolidated Financial Statements
The preparation of our consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect amounts reported in our consolidated financial statements. Significant items subject to such estimates and assumptions include the carrying amount of property and equipment, valuation of long-lived and intangible assets and goodwill, valuation allowances for receivables and deferred income taxes, revenue recognition, stock-based compensation and commitments and contingencies. Actual results could differ from those estimates.
Revenue Recognition
The majority of our revenues are derived from providing medical transcription services. Revenues for medical transcription services are recognized when the services are rendered. These services are based on contracted rates. The remainder of our revenues is derived from the sale of voice-capture and document management products including software, hardware and implementation, training and maintenance service related to these products.
We recognize software-related revenues using the residual method when vendor-specific objective evidence (VSOE) of fair value exists for all of the undelivered elements in the arrangement, but does not exist for one or more delivered elements. We allocate revenues to each undelivered element based on its respective fair value determined by the price charged when that element is sold separately or, for elements not yet sold separately, the price established by management if it is probable that the price will not change before the element is sold separately. We defer revenues for the undelivered elements.
F-8
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Provided that the arrangement does not involve significant production, modification, or customization of the software, revenues are recognized when all of the following four criteria have been met: persuasive evidence of an arrangement exists, delivery has occurred, the fee is fixed or determinable and collectability is probable.
If at the outset of an arrangement, we determine that the arrangement fee is not fixed or determinable, revenues are deferred until the arrangement fee becomes due and payable by the customer. If at the outset of an arrangement we determine that collectability is not probable, revenues are deferred until payment is received. Our license agreements typically do not provide for a right of return other than during the standard warranty period. If an arrangement allows for customer acceptance of the software or services, we defer revenues until the earlier of customer acceptance or when the acceptance rights lapse.
We separately market and sell hardware and software post contract customer support (PCS). PCS covers phone support, hardware parts and labor, software bug fixes and limited upgrades, if and when available. We do not commit to specific future software upgrades or releases. The contract period for PCS is generally one year. We recognize both hardware and software PCS on a straight line basis over the life of the underlying PCS contract. In some of our PCS contracts, we bill the customer prior to performing the services. As of December 31, 2009 and 2008, deferred PCS revenues of $7,678 and $9,702, respectively, are included in deferred revenues and $177 and $340, respectively, are included in non-current liabilities in the accompanying consolidated balance sheets.
Certain arrangements include multiple elements involving software, hardware and implementation, training, or other services that are not essential to the functionality of the software. VSOE for services does not exist. Since the undelivered elements are typically services, we recognize the entire arrangement fee ratably over the period during which the services are expected to be performed or the PCS period, whichever is longer, beginning with delivery of the software, provided that all other revenue recognition criteria are met. The services are typically completed before the PCS term expires. As such, upon completion of the services, the difference between the VSOE of fair value for the remaining PCS period and the remaining unrecognized portion of the arrangement fee is recognized as revenue (i.e. the residual method), and the remaining deferred revenue is recognized ratably over the remaining PCS period.
Sales Taxes
We present taxes assessed by a governmental authority including sales, use, value added and excise taxes on a net basis and therefore the presentation of these taxes is excluded from our revenues and is included in accrued expenses in the accompanying consolidated balance sheets until such amounts are remitted to the taxing authorities.
Litigation and Settlement Costs
From time to time, we are involved in litigation, claims, contingencies and other legal matters. We record a charge equal to at least the minimum estimated liability for a loss contingency when both of the following conditions are met: (i) information available prior to issuance of the financial statements indicates that it is probable that an asset had been impaired or a liability had been incurred at the date of the financial statements and (ii) the range of the loss can be reasonably estimated. We expense legal costs, including those legal costs expected to be incurred in connection with a loss contingency, as incurred.
Restructuring Costs
A liability for restructuring costs associated with an exit or disposal activity is recognized and measured initially at fair value when the liability is incurred. We record a liability for severance costs when the obligation is attributable to employee service already rendered, the employees’ rights to those benefits accumulate or vest, payment of the compensation is probable and the amount can be reasonably estimated. We record a liability for future, non-cancellable operating lease costs when we vacate a facility.
Our estimates of future liabilities may change, requiring us to record additional restructuring charges or reduce the amount of liabilities recorded. At the end of each reporting period, we evaluate the remaining accrued restructuring charges to ensure their adequacy, that no excess accruals are retained and the utilization of the provisions are for their intended purposes in accordance with developed exit plans.
We periodically evaluate currently available information and adjust our accrued restructuring charges as necessary. Changes in estimates are accounted for as restructuring costs or credits in the period identified.
F-9
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Research and Development Costs
Research and development costs are expensed as incurred.
Income Taxes
Deferred tax assets and liabilities are recorded for temporary differences between the tax basis of assets and liabilities and their reported amounts in the consolidated financial statements, using statutory tax rates in effect for the year in which the differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in our statements of operations in the period that includes the enactment date. A valuation allowance is recorded to reduce the carrying amounts of deferred tax assets unless it is more likely than not that such assets will be realized. Management considers various sources of future taxable income including projected book earnings, the reversal of deferred tax liabilities, and prudent and feasible tax planning strategies in determining the need for a valuation allowance.
Stock-Based Compensation
We estimate the fair value of stock options on the date of grant using an option pricing model. We use the Black-Scholes option pricing model to determine the fair value of our options. The determination of the fair value of stock based awards using an option pricing model is affected by a number of assumptions including expected volatility of the common stock over the expected term, the expected term, the risk free interest rate during the expected term and the expected dividends to be paid. The value of the portion of the award that is ultimately expected to vest is recognized as compensation expense over the requisite service periods.
Stock-based compensation expense related to employee stock options recognized for 2009, 2008 and 2007 was $193, $1,427 and $565 which was charged to selling, general and administrative expenses ($193, $1,010 and $255), research and development expenses ($0, $405 and $91) and cost of revenues ($0, $12 and $219), respectively. Included in the $193 and $1,427 is $0 and $1,339, respectively, of expense related to stock options that were issued to certain executive officers when we became current in our periodic reporting obligations with the Securities and Exchange Commission (SEC) in October 2007. As of December 31, 2009, total unamortized stock-based compensation cost related to non-vested stock options, net of expected forfeitures, was $340 which is expected to be recognized over a weighted-average period of 1.8 years.
We did not grant any stock options for the year ended December 31, 2009.
Net Income (Loss) per Share
Basic net income (loss) per share is computed by dividing net income (loss) by the weighted average number of shares outstanding during each period. Diluted net income (loss) per share is computed by dividing net income (loss) by the weighted average shares outstanding, as adjusted for the dilutive effect of common stock equivalents, which consist only of stock options, using the treasury stock method.
The table below reflects basic and diluted net income (loss) per share for the years ended December 31:
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| Net income (loss) | | $ | 23,291 | | | $ | (68,795 | ) | | $ | (15,206 | ) |
| | | | | | | | | | |
| Weighted average shares outstanding: | | | | | | | | | | | | |
| Basic | | | 37,556 | | | | 37,549 | | | | 37,488 | |
| Effect of dilutive stock | | | — | | | | — | | | | — | |
| | | | | | | | | | |
| Diluted | | | 37,556 | | | | 37,549 | | | | 37,488 | |
| | | | | | | | | | |
| Net income (loss) per share: | | | | | | | | | | | | |
| Basic | | $ | 0.62 | | | $ | (1.83 | ) | | $ | (0.41 | ) |
| Diluted | | $ | 0.62 | | | $ | (1.83 | ) | | $ | (0.41 | ) |
The computation of diluted net income (loss) per share does not assume conversion, exercise or issuance of shares that would have an anti-dilutive effect on diluted net loss per share. During 2008 and 2007, we had a net loss. As a result, any assumed conversions would result in reducing the net loss per share and, therefore, are not included in the calculation. Shares having an anti-dilutive effect on net loss per share and, therefore, excluded from the calculation of diluted net loss per share, totaled 1,501 and 2,298 shares for the years ended December 31, 2008 and 2007, respectively.
F-10
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Advertising Costs
Advertising costs are expensed as incurred and for the years ended December 31, 2009, 2008 and 2007 were $613, $1,256 and $1,674, respectively.
Cash and Cash Equivalents
We consider all highly liquid instruments with original maturities of three months or less to be cash equivalents. Our cash management and investment policies dictate that cash equivalents be limited to investment grade, highly liquid securities. We place our temporary cash investments with high-credit rated, quality financial institutions. Deposits held with banks may exceed the amount of insurance provided on such deposits. Consequently, our cash equivalents are subject to potential credit risk. As of December 31, 2009 and 2008, cash equivalents consisted of money market investments. The carrying value of cash and cash equivalents approximates fair value.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recorded at the invoiced amount and do not bear interest. The carrying value of accounts receivable approximates fair value. The allowance for doubtful accounts is our best estimate for losses inherent in our accounts receivable portfolio. The sales return and allowance reserve is our best estimate of sales credits that will be issued related to our accounts receivable portfolio. These allowances are used to state trade receivables at estimated net realizable value.
We estimate uncollectible amounts based upon our historical write-off experience, current customer receivable balances, age of customer receivable balances, the customer’s financial condition and current economic conditions. Historically, these estimates have been adequate to cover our accounts receivable exposure.
We enter into medical transcription service arrangements which contain provisions for performance penalties in the event certain service levels, primarily related to turnaround time on transcribed reports, are not achieved. We reduce revenues for any performance penalties incurred and have included an estimate of such credits in our allowance for uncollectible accounts.
Product revenues for sales to end-user customers and resellers are recognized upon passage of title if all other revenue recognition criteria have been met. End-user customers generally do not have a right of return. We provide certain of our resellers and distributors with limited rights of return of our products. We reduce revenues for rights to return our product based upon our historical experience and have included an estimate of such credits in our allowance for doubtful accounts.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets which range from two to seven years for furniture, equipment and software, and the lesser of the lease term or estimated useful life for leasehold improvements. Repairs and maintenance costs are charged to expense as incurred while additions and betterments are capitalized. Gains or losses on disposals are charged to operations. Upon retirement, sale or other disposition, the related cost and accumulated depreciation are eliminated from the accounts and any gain or loss is included in operations.
Valuation of Long-Lived and Other Intangible Assets and Goodwill.
In connection with acquisitions, we allocate portions of the purchase price to tangible and intangible assets, consisting primarily of acquired technologies, and customer relationships, agreements based on independent appraisals received after each acquisition, with the remainder allocated to goodwill. We assess the realizability of goodwill and intangible assets with indefinite useful lives at least annually, or sooner if events or changes in circumstances indicate that the carrying amount may not be recoverable. We have determined that the reporting unit level is our sole operating segment. (See note 7 for goodwill impairment charge recorded in 2008)
Software Development
Software costs incurred in creating a computer software product are charged to expense when incurred as research and development until technical feasibility has been established. Technical feasibility is established upon completion of a detail program
F-11
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
design or, in its absence, completion of a working model. Thereafter, all software production costs are capitalized until the product is available for release to customers.
Software costs for internal use software incurred in the preliminary project stage are expensed as incurred. Capitalization of costs begins when the preliminary project stage is completed and management, with the relevant authority, authorizes and commits funding of the project and it is probable that the project will be completed and the software will be used to perform the function intended. Capitalization ceases no later than the point at which the project is substantially complete and ready for its intended use.
Capitalized software is reported at the lower of unamortized cost or net realizable value and is amortized over the product’s estimated economic life which is generally three years. As of December 31, 2009 and 2008, $5,866 and $4,802, respectively, of unamortized software development costs are included in other intangible assets in the accompanying consolidated balance sheets. For the years ended December 31, 2009, 2008 and 2007, software amortization expense was $1,452, $835 and $360, respectively.
Long-Lived and Other Intangible Assets
Long-lived assets, including property and equipment and purchased intangible assets subject to amortization, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. To determine the recoverability of long-lived assets, the estimated future undiscounted cash flows expected to be generated by an asset is compared to the carrying value of the asset. If the carrying value of the long-lived asset exceeds its estimated future undiscounted cash flows, an impairment charge is recognized in the amount by which the carrying value of the asset exceeds its fair value. Annually we evaluate the reasonableness of the useful lives of these assets.
Intangible assets include certain assets (primarily customer lists) obtained from business acquisitions and are being amortized using the straight-line method over their estimated useful lives which range from three to 20 years.
Foreign Currency Translation
Our operating subsidiaries in the United Kingdom and Canada use the local currency as their functional currency. We translate the assets and liabilities of those entities into U.S. dollars using the month-end exchange rate. We translate revenues and expenses using the average exchange rates prevailing during the reporting period. The resulting translation adjustments are recorded in accumulated other comprehensive income within shareholders’ equity. Gains and losses from foreign currency transactions are included in net gain (loss) and were not material for the years ended December 31, 2009, 2008 and 2007, respectively.
Business Enterprise Segments
We operate in one reportable operating segment which is medical transcription technology and services.
Concentration of Risk, Geographic Data and Enterprise-wide Disclosures
No single customer accounted for more than 10% of our net revenues in any period. There is no single geographic area of significant concentration other than the United States.
The following table summarizes the net revenues by the categories of our products and services as a percentage of our total net revenues.
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| Medical transcription | | | 85.7 | % | | | 84.9 | % | | | 83.3 | % |
| Products and related services | | | 3.7 | % | | | 3.6 | % | | | 4.7 | % |
| PCS | | | 6.4 | % | | | 7.6 | % | | | 8.2 | % |
| Other | | | 4.2 | % | | | 3.9 | % | | | 3.8 | % |
| | | | | | | | | | |
| Total | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % |
| | | | | | | | | | |
Other includes medical coding, application service provider and other miscellaneous revenues.
F-12
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Fair Value of Financial Instruments
Cash and cash equivalents, accounts receivable, accounts payable and accrued expenses are reflected in the accompanying consolidated balance sheets at carrying values which approximate fair value due to the short-term nature of these instruments and the variability of the respective interest rates where applicable.
Comprehensive Income (Loss)
Comprehensive income (loss) is comprised of Net income (loss) and Other comprehensive income (loss). Other comprehensive income (loss) consists of foreign currency translation adjustments. Other comprehensive income (loss) and comprehensive income (loss) are displayed separately in the Consolidated Statements of Shareholders’ Equity and Other Comprehensive Loss.
Acquisition related costs
We expense costs incurred in reviewing potential acquisitions in the period incurred. This includes legal, investment banking, accounting, tax and other consulting, as well as any internal costs.
Reclassification
Certain reclassifications were made to prior period financial statements to conform to the current presentation. Expenses and charges of $3,335 for the year ended December 31, 2008 related to the Anthurium, patent claim, reseller arbitration and shareholder lawsuits had previously been included in Selling, General and Administrative expenses. Due to the significant amount of such expenses in 2009, such costs are now included in the line item Cost of legal proceedings and settlements, net. This had no effect on operating income, net income, or earnings per share. On the balance sheet, we previously disclosed customer accommodations on a separate line item on the balance sheet, and now include this within accrued expenses, with disclosure in the footnote as permitted by SEC rules.
Recent Accounting Pronouncements
In June 2009, the FASB issued, “The FASB Accounting Standards Codification TM and the Hierarchy of Generally Accepted Accounting Principles. This establishes the Codification as the source of authoritative GAAP recognized by the FASB to be applied by nongovernmental entities. Rules and interpretive releases of the SEC under federal securities laws are also sources of authoritative GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. This is effective for financial statements issued for interim and annual periods ending after September 15, 2009.
In September 2009, the FASB ratified two consensuses affecting revenue recognition:
The first consensus,Revenue Recognition—Multiple-Element Arrangements, sets forth requirements that must be met for an entity to recognize revenue from the sale of a delivered item that is part of a multiple-element arrangement when other items have not yet been delivered. One of those current requirements is that there be objective and reliable evidence of the standalone selling price of the undelivered items, which must be supported by either vendor-specific objective evidence (VSOE) or third-party evidence (TPE).
This consensus eliminates the requirement that all undelivered elements have VSOE or TPE before an entity can recognize the portion of an overall arrangement fee that is attributable to items that already have been delivered. In the absence of VSOE or TPE of the standalone selling price for one or more delivered or undelivered elements in a multiple-element arrangement, entities will be required to estimate the selling prices of those elements. The overall arrangement fee will be allocated to each element (both delivered and undelivered items) based on their relative selling prices, regardless of whether those selling prices are evidenced by VSOE or TPE or are based on the entity’s estimated selling price. Application of the “residual method” of allocating an overall arrangement fee between delivered and undelivered elements will no longer be permitted.
The second consensus,Software-Revenue Recognitionaddresses the accounting for transaction involving software to exclude from its scope tangible products that contain both software and non-software and not-software components that function together to deliver a products functionality.
The Consensuses are effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. We are evaluating the potential impact of these requirements on our financial statements.
F-13
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
3. Cost of Legal Proceedings and Settlements, net
For the years ended December 31, 2009, 2008 and 2007, we recorded charges of $14,843, $19,738 and $6,083, respectively, for costs associated with legal proceedings and settlements. These costs are net of insurance claim reimbursements. We record insurance claims when the realization of the claim is probable. The following is a summary of the amounts recorded in the accompanying consolidated statements of operations:
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| Legal fees | | $ | 8,533 | | | $ | 11,729 | | | $ | 18,678 | |
| Other professional fees | | | 60 | | | | 584 | | | | 2,592 | |
| Insurance recoveries and claims | | | — | | | | — | | | | (15,386 | ) |
| Other, including settlements | | | 6,250 | | | | 7,425 | | | | 199 | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Total | | $ | 14,843 | | | $ | 19,738 | | | $ | 6,083 | |
| | | | | | | | | | |
Other professional fees represent accounting and dispute analysis costs and document search and retrieval costs. In 2007, insurance recoveries and claims represent insurance recoveries ($4,243) and insurance claims ($11,143). The insurance claims were recorded in other current assets and payment related to these claims was received in the third quarter of 2007. We do not expect to receive any additional insurance recoveries related to these claims in the future. The 2009 Other, including settlements, amount was primarily for the settlement amount of $5,750 related to the Anthurium patent claim which was settled and paid in June 2009 and $500 for the reseller arbitration. The 2008 Other, including settlements, amount of $7,425 was for the settlements of all claims related to the consolidated medical transcriptionists putative class action and the Department of Justice (DOJ) investigation.
4. Restructuring Charges
2009 Restructure Plan
During the third and fourth quarters of 2009, as a result of management’s continued planned process improvement and technology development investments we committed to an exit and disposal plan which includes projected employee severance for planned reduction in headcount. Because of plan development in late 2009 and execution of the plan over multiple quarters in 2009 and 2010, not all personnel affected by the plan know of the plan or its impact. The plan includes costs of $2.5 million for employee severance and $0.3 million for vacating operating leases. The table below reflects the financial statement activity related to the 2009 restructuring plan and is included in accrued expenses in the accompanying consolidated balance sheets:
| | | | | |
| Beginning Balance | | $ | — | |
| Charge | | | 2,810 | |
| Cash Paid | | | (746 | ) |
| | | | |
| Balance as of December 31, 2009 | | $ | 2,064 | |
| | | | |
We expect this to be paid in 2010.
2008 Restructure Plan
During the fourth quarter of 2008, we implemented a restructuring plan related to a reduction in workforce in order to better align costs with revenues. We recorded $2,135 in severance charges related to the 2008 restructuring plan. The remaining restructuring costs are included in accrued expenses in the accompanying consolidated balance sheet as of December 31, 2009. The table below reflects the financial statement activity related to the 2008 restructuring plan for the year ended December 31, 2009:
| | | | | | | | | |
| | | Year Ended | | | Year Ended | |
| | | December 31, 2009 | | | December 31, 2008 | |
| Beginning balance | | $ | 1,323 | | | $ | — | |
| Charge (Reversal) | | | (83 | ) | | | 2,135 | |
| Cash paid | | | (1,240 | ) | | | (812 | ) |
| | | | | | | |
| Ending balance | | $ | — | | | $ | 1,323 | |
| | | | | | | |
5. Accounts Receivable
F-14
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Accounts receivable consisted of the following as of December 31:
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| Trade accounts receivable | | $ | 46,786 | | | $ | 55,176 | |
| Less: Allowance for doubtful accounts | | | (3,159 | ) | | | (4,802 | ) |
| | | | | | | |
| Accounts receivable, net | | $ | 43,627 | | | $ | 50,374 | |
| | | | | | | |
6. Property and Equipment
Property and equipment consisted of the following as of December 31:
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| Computer equipment | | $ | 32,404 | | | $ | 27,140 | |
| Communication equipment | | | 5,447 | | | | 5,665 | |
| Software | | | 18,743 | | | | 19,888 | |
| Furniture and office equipment | | | 1,357 | | | | 1,590 | |
| Leasehold improvements | | | 2,703 | | | | 3,067 | |
| | | | | | | |
| Total property and equipment | | | 60,654 | | | | 57,350 | |
| Less: accumulated depreciation | | | (48,882 | ) | | | (41,565 | ) |
| | | | | | | |
| | | | | | | | | |
| Property and equipment, net | | $ | 11,772 | | | $ | 15,785 | |
| | | | | | | |
During 2008 we recorded a write-off of $571 which was allocated between cost of revenues ($91) and selling, general and administrative expenses ($480). In addition, approximately $7,786 in fully depreciated assets no longer in use were written off in 2008 which had no impact on net loss.
7. Goodwill and Other Intangible Assets
In connection with acquisitions, we allocate portions of the purchase price to tangible and intangible assets, consisting primarily of acquired technologies, and customer relationships, agreements based on independent appraisals received after each acquisition, with the remainder allocated to goodwill. We assess the realizability of goodwill and intangible assets with indefinite useful lives at least annually, or sooner if events or changes in circumstances indicate that the carrying amount may not be recoverable. We have determined that the reporting unit level is our sole operating segment.
Goodwill
The changes in the carrying amount of goodwill for the year ended December 31, 2009 and 2008 are as follows:
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| Balance as of January 1, | | | | | | | | |
| Goodwill | | $ | 122,778 | | | $ | 125,505 | |
| Accumulated impairment losses | | | (82,233 | ) | | | — | |
| | | | | | | |
| | | | 40,545 | | | | 125,505 | |
| Impairment loss | | | — | | | | (82,233 | ) |
| | | | | | | | | |
| Foreign currency adjustments | | | 268 | | | | (2,727 | ) |
| Balance at December 31, | | | | | | | | |
| | | | | | | |
| Goodwill | | | 123,046 | | | | 122,778 | |
| Accumulated impairment losses | | | (82,233 | ) | | | (82,233 | ) |
| | | | | | | |
| | | | | | | | | |
| Goodwill at December 31, | | $ | 40,813 | | | $ | 40,545 | |
| | | | | | | |
We tested goodwill for impairment during the fourth quarter of 2009 and determined that the fair value of the reporting unit substantially exceeded the carrying value based upon the market capitalization. In the fourth quarter of 2008, which included our annual impairment testing date in December, we determined our fair value using a combination of our market capitalization based on market price per share for approximately the 60 days before December 31, 2008, and a discounted cash flow analysis. Determining fair value requires the exercise of significant judgment, including judgment about appropriate discount rates, perpetual growth rates, the amount and timing of expected future cash flows, as well as
F-15
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
relevant comparable company earnings multiples for the market-based approach. The cash flows employed in the discounted cash flow analyses were based on our internal business model for 2009 and, for years beyond 2009 the growth rates we used were an estimate of the future growth in the industry in which we participate. The discount rates used in the discounted cash flow analyses are intended to reflect the risks inherent in the future cash flows of the reporting unit and are based on an estimated cost of capital, which we determined based on our estimated cost of capital relative to our capital structure. In addition, the market-based approach utilizes comparable company public trading values, research analyst estimates and, where available, values observed in private market transactions. Our analysis indicated that the reporting unit fair value was below our book value. The test for impairment of goodwill is a two-step process:
| | • | | First, we compare the carrying amount of our reporting unit, which is the book value of our entire company, to the fair value of our reporting unit. If the carrying amount of our reporting unit exceeds its fair value, we have to perform the second step of the process. If not, no further testing is needed. In the fourth quarter of 2008 we determined that the carrying amount of our reporting unit exceeded the fair value and accordingly performed the second step in the analysis. |
| |
| | • | | If the second part of the analysis is required, we allocate the fair value of our reporting unit to all assets and liabilities as if the reporting unit had been acquired in a business combination at the date of the impairment test. We then compare the implied fair value of our reporting unit’s goodwill to its carrying amount. If the carrying amount of our goodwill exceeds its implied fair value, we recognize an impairment loss in an amount equal to that excess. In the fourth quarter of 2008, the carrying value of goodwill exceeded its implied fair value and accordingly we recorded a non cash, pre-tax impairment charge of $82.2 million. |
Other Intangible Assets
We review our long-lived assets, including amortizable intangibles, for impairment when events indicate that their carrying amount may not be recoverable. When we determine that one or more impairment indicators are present for an asset, we compare the carrying amount of the asset to net future undiscounted cash flows that the asset is expected to generate. If the carrying amount of the asset is greater than the net future undiscounted cash flows that the asset is expected to generate, we compare the fair value to the book value of the asset. If the fair value is less than the book value, we recognize an impairment loss. The impairment loss is the excess of the carrying amount of the asset over its fair value.
Some of the events that we consider as impairment indicators for our long-lived assets, including goodwill, are:
| | • | | our net book value compared to our fair value; |
| |
| | • | | significant adverse economic and industry trends; |
| |
| | • | | significant decrease in the market value of the asset; |
| |
| | • | | the extent that we use an asset or changes in the manner that we use it; |
| |
| | • | | significant changes to the asset since we acquired it; and |
| |
| | • | | other changes in circumstances that potentially indicate all or a portion of the company will be sold. |
During 2009 and 2008 we reviewed the carrying value of our long lived assets other than goodwill and determined that the carrying amounts of such assets was less than the undiscounted cash flows and accordingly no impairment charge was recorded.
As of December 31, other intangible asset balances were:
| | | | | | | | | | | | | |
| | | 2009 | |
| | | | | | | Accumulated | | | Net book | |
| | | Cost | | | Amortization | | | Value | |
| Customer lists | | $ | 77,277 | | | $ | (46,836 | ) | | $ | 30,441 | |
| Capitalized software | | | 10,625 | | | | (4,759 | ) | | | 5,866 | |
| | | | | | | | | | |
| Total | | $ | 87,902 | | | $ | (51,595 | ) | | $ | 36,307 | |
| | | | | | | | | | |
F-16
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
| | | | | | | | | | | | | |
| | | 2008 | |
| | | | | | | Accumulated | | | Net book | |
| | | Cost | | | Amortization | | | Value | |
| Customer lists | | $ | 77,145 | | | $ | (42,070 | ) | | $ | 35,075 | |
| Capitalized software | | | 8,110 | | | | (3,308 | ) | | | 4,802 | |
| | | | | | | | | | |
| Total | | $ | 85,255 | | | $ | (45,378 | ) | | $ | 39,877 | |
| | | | | | | | | | |
The estimated useful life and the weighted average remaining lives of the intangible assets as of December 31, 2009 were as follows:
| | | | | |
| | | Estimated | | Weighted Average |
| | | Useful Life | | Remaining Lives |
| Customer lists | | 10-20 years | | 8.4 years |
| Capitalized software | | 3 years | | 1.8 years |
Estimated annual amortization expense for intangible assets is as follows:
| | | | | |
| 2010 | | $ | 7,198 | |
| 2011 | | | 6,657 | |
| 2012 | | | 4,879 | |
| 2013 | | | 2,919 | |
| 2014 | | | 2,851 | |
| Thereafter | | | 11,803 | |
| | | | |
| Total | | $ | 36,307 | |
| | | | |
8. Contractual Obligations
Leases
Minimum rental payments under operating leases are recognized on a straight-line basis over the term of the lease, including any periods of free rent and landlord incentives. Rental expense for operating leases for the years ended December 31, 2009, 2008 and 2007 was $2,991, $3,201 and $2,489, respectively. Future minimum lease payments under non-cancelable operating leases which expire in 2014 (with initial or remaining lease terms in excess of one year) as of December 31, 2009 were:
| | | | | |
| | | Total | |
| 2010 | | $ | 3,645 | |
| 2011 | | | 3,666 | |
| 2012 | | | 3,690 | |
| 2013 | | | 3,389 | |
| 2014 | | | 1,367 | |
| | | | |
| Total minimum lease payments | | $ | 15,757 | |
| | | | |
Other Contractual Obligations
The following summarizes our other contractual obligations as of December 31, 2009:
| | | | | | | | | | | | | |
| | | | | | | | | | | Severance and Other | |
| | | Total | | | Purchase | | | Guaranteed Payments | |
| 2010 | | $ | 10,666 | | | | 8,666 | | | | 2,000 | |
| 2011 | | | 2,203 | | | | 2,203 | | | | — | |
| 2012 | | | — | | | | — | | | | — | |
| | | | | | | | | | |
| Total | | $ | 12,869 | | | | 10,869 | | | | 2,000 | |
| | | | | | | | | | |
F-17
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Purchase obligations represent telecommunication contracts ($9,619), software development ($1,000) and other recurring purchase obligations ($250). Severance and other guaranteed payments are comprised of severance payments ($2,000).
As of December 31, 2009, we had agreements with certain of our senior management that provided for severance payments in the event these individuals were terminated without cause. The maximum cost exposure related to these agreements was $1,887 as of December 31, 2009.
9. Investment in A-Life Medical, Inc. (A-Life)
As of December 31, 2009 and December 31, 2008, we had an investment of $8,864 and $6,252 respectively, in A-Life, a privately held entity which provides advanced natural language processing technology for the medical industry, representing 32% of the outstanding ownership. Our investment is recorded under the equity method of accounting. For the year ended December 31, 2009, our investment increased by $2,015 related to our share of A-Life’s net income, primarily related to a gain resulting from an acquisition, additional cash investments in A-Life of $852 offset by $255 for a dilution which was recorded in Shareholders’ equity in our consolidated balance sheet. Our investment in A-Life is recorded in Other assets in the accompanying consolidated balance sheets.
| | | | | |
| Balance as of January 1, 2008 | | $ | 6,016 | |
| Share in income | | | 236 | |
| | | | |
| Balance as of December 31, 2008 | | | 6,252 | |
| Additional cash investments | | | 852 | |
| Share in income | | | 2,015 | |
| Dilution | | | (255 | ) |
| | | | |
| Balance as of December 31, 2009 | | $ | 8,864 | |
| | | | |
10. Accrued Expenses
Accrued expenses consisted of the following as of December 31:
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| Customer Accommodations | | $ | 11,635 | | | $ | 12,055 | |
| Other (No item exceeds 5% of current liabilities) | | | 11,213 | | | | 11,994 | |
| | | | | | | |
| Total accrued expenses | | $ | 22,848 | | | $ | 24,049 | |
| | | | | | | |
In November 2003, one of our employees raised allegations that we had engaged in improper billing practices. In response, our board of directors undertook an independent review of these allegations (Review). In response to our customers’ concern over the public disclosure of certain findings from the Review, we took action in the fourth quarter of 2005 to avoid unnecessary litigation, which preserved and solidified our customer business relationships by offering a financial accommodation to certain of our customers.
In connection with our decision to offer financial accommodations to certain of our customers (Accommodation Customers), we analyzed our historical billing information and the available report-level data (Management’s Billing Assessment) to develop individualized accommodation offers to be made to Accommodation Customers (Accommodation Analysis). Based on the Accommodation Analysis, our board of directors authorized management to make cash or credit accommodation offers to Accommodation Customers in the aggregate amount of $75,818. By accepting our accommodation offer, the customer agreed, among other things, to release us from any and all claims and liability regarding the billing related issues. We are unable to predict how many customers, if any, may accept the outstanding accommodation offers on the terms proposed by us, nor are we able to predict the timing of the acceptance (or rejection) of any outstanding accommodation offers. Until any offers are accepted, we may withdraw or modify the terms of the accommodation program or any outstanding offers at any time. In addition, we are unable to predict how many future offers, if made, will be accepted on the terms proposed by us. We regularly evaluate whether to proceed with, modify or withdraw the accommodation program or any outstanding offers.
F-18
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
The following is a summary of the financial statement activity related to the customer accommodation.
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| Beginning balance | | $ | 12,055 | | | $ | 18,459 | |
| Payments and other adjustments | | | (317 | ) | | | (5,664 | ) |
| Credits | | | (103 | ) | | | (740 | ) |
| | | | | | | |
| Ending balance | | $ | 11,635 | | | $ | 12,055 | |
| | | | | | | |
11. Commitments and Contingencies
Customer Litigation
Kaiser Litigation
On June 6, 2008, plaintiffs Kaiser Foundation Health Plan, Inc., Kaiser Foundation Hospitals, The Permanente Medical Group, Inc., Kaiser Foundation Health Plan of the Mid-Atlantic States, Inc., and Kaiser Foundation Health Plan of Colorado (collectively, Kaiser) filed suit against MedQuist Inc. and MedQuist Transcriptions, Ltd. (collectively, MedQuist) in the Superior Court of the State of California in and for the County of Alameda. The action is entitled Foundation Health Plan Inc., et al v. MedQuist Inc. et al., Case No. CV-078-03425 PJH. The complaint asserts five causes of action, for common law fraud, breach of contract, violation of California Business and Professions Code section 17200, unjust enrichment, and a demand for an accounting. More specifically, Kaiser alleges that we fraudulently inflated the payable units of measure in medical transcription reports generated by us for Kaiser pursuant to the contracts between the parties. The damages alleged in the complaint include an estimated $7 million in compensatory damages, as well as punitive damages, attorneys’ fees and costs, and injunctive relief. We contend that we did not breach the contracts with Kaiser, or commit the fraud alleged, and we intend to defend the suit vigorously. The parties participated in private mediation on July 24, 2008, but the case was not settled. We removed the case to the United States District Court for the Northern District of California, and we filed motions to dismiss Kaiser’s complaint and to transfer venue of the case to the United Stated District Court for the District of New Jersey. Kaiser stipulated to transfer, and the case was transferred to the United States District Court for the District of New Jersey on or about August 26, 2008.
The parties exchanged initial disclosures on October 6, 2008 and appeared before the court for an initial scheduling conference on October 14, 2008. Kaiser’s initial disclosures claim damages, including compensatory damages, punitive damages, and prejudgment interest, in excess of $12 million. Following the scheduling conference, the court ordered the parties to mediation. The parties participated in court-ordered mediation on February 27, 2009 but the case was not settled. The court heard argument on our motion to dismiss on March 19, 2009. On April 8, 2009, the court entered an order denying our motion to dismiss, except that our motion to dismiss plaintiffs’ claim under the fraudulent prong of the California Unfair Competition Law was granted. The court issued a scheduling order on April 17, 2009, setting a pretrial schedule. We filed our answer to plaintiff’s complaint on April 23, 2009.
Kaiser served an initial set of discovery requests on May 7, 2009. On May 20, 2009, the court temporarily stayed discovery to address allegations of ethical misconduct by Kaiser’s trial counsel, Greenberg Traurig, LLP. On July 1, 2009, the court granted us leave to conduct limited written discovery regarding Greenberg Traurig’s alleged ethical misconduct and continued the temporary stay of all other discovery. On July 14, 2009, we moved for sanctions against Greenberg Traurig for breach of the New Jersey Rules of Professional Conduct, and we moved to stay the litigation pending resolution of the motion for sanctions. Discovery is presently stayed pending resolution of our motion to stay. No hearing date has been set for any of the pending motions. On February 24, 2010, the court granted our motion to stay pending resolution of the motion for sanctions and entered a scheduling order setting oral arguments on the pending motion for sanctions for March 16, 2010.
Kahn Putative Class Action
On January 22, 2008, Alan R. Kahn, one of our shareholders, filed a shareholder putative class action lawsuit against us, Koninklijke Philips Electronics N.V. (Philips), our former majority shareholder, and four of our former non-independent directors, Clement Revetti, Jr., Stephen H. Rusckowski, Gregory M. Sebasky and Scott Weisenhoff. The action, entitled Alan R. Kahn v. Stephen H. Rusckowski, et al., Docket No. BUR-C-000007-08, was venued in the Superior Court of New Jersey, Chancery Division, Burlington County. In the action, plaintiff purports to bring the action on his own behalf and on behalf of all current holders of our common stock. The original complaint alleged that defendants breached their fiduciary duties of good faith, fair dealing, loyalty, and due care by purportedly agreeing to and initiating a process for our sale or a change of control transaction which will allegedly cause harm to plaintiff and members of the putative class. Plaintiff sought damages in an unspecified amount, plus costs and interest, a judgment declaring that defendants breached their fiduciary duties and that any proposed transactions regarding our sale or change of control are void, an injunction preventing our sale or any change of control transaction that is not entirely fair to the class, an order directing us to appoint three independent directors to our board of directors, and attorneys’ fees and expenses.
F-19
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
On June 12, 2008, plaintiff filed an amended class action complaint against us, eight of our current and former directors, and Philips in the Superior Court of New Jersey, Chancery Division. In the amended complaint, plaintiff alleged that our current and former directors breached their fiduciary duties of good faith, fair dealing, loyalty, and due care by not providing our public shareholders with the opportunity to decide whether they wanted to participate in a share purchase offer with non-party CBaySystems Holdings Ltd. (CBaySystems Holdings) that would have allowed the public shareholders to sell their shares of our common stock for an amount above market price. Plaintiff further alleged that CBaySystems Holdings made the share purchase offer to Philips and that Philips breached its fiduciary duties by accepting CBaySystems Holdings’ offer. Based on these allegations, plaintiff sought declaratory, injunctive, and monetary relief from all defendants. Plaintiff claimed that we were only named as a party to the litigation for purposes of injunctive relief.
On July 14, 2008, we moved to dismiss plaintiff’s amended class action complaint, arguing (1) that plaintiff’s amended class action complaint did not allege that we engaged in any wrongdoing which supported a breach of fiduciary duty claim and (2) that a breach of fiduciary duty claim is not legally cognizable against a corporation. Plaintiff filed an opposition to our motion to dismiss on July 21, 2008.
On November 21, 2008, the Court granted our motion and the motions filed by the other defendants and dismissed plaintiff’s amended class action complaint with prejudice. On December 31, 2008, plaintiff filed an appeal of the trial court’s dismissal order with the New Jersey Appellate Division. Thereafter, the parties briefed all the issues raised in plaintiff’s appeal. In our opposition brief, we opposed all the arguments plaintiff raised with respect to the dismissal of the claims against us.
On September 24, 2009, the Appellate Division held oral argument on the issues that are the subject of plaintiff’s appeal. We are now waiting for the Appellate Division to issue a decision.
Reseller Arbitration Demand
On October 1, 2007, we received from counsel to nine current and former resellers of our products (Claimants), a copy of an arbitration demand filed by the Claimants, initiating an arbitration proceeding styled Diskriter, Inc., Electronic Office Systems, Inc., Milner Voice & Data, Inc., Nelson Systems, Inc., NEO Voice and Communications, Inc., Office Business Systems, Inc., Roach-Reid Office Systems, Inc., Stiles Office Systems, Inc., and Travis Voice and Data, Inc. v. MedQuist Inc. and MedQuist Transcriptions, Ltd. (collectively MedQuist) (filed on September 27, 2007, AAA, 30-118-Y-00839-07). The arbitration demand purports to set forth claims for breach of contract; breach of covenant of good faith and fair dealing; promissory estoppel; misrepresentation; and tortious interference with contractual relations. The Claimants allege that we breached our written agreements with the Claimants by: (i) failing to provide reasonable training, technical support, and other services; (ii) using the Claimants’ confidential information to compete against the Claimants; (iii) directly competing with the Claimants’ territories; and (iv) failing to make new products available to the Claimants. In addition, the Claimants allege that we made false oral representations that we: (i) would provide new product, opportunities and support to the Claimants; (ii) were committed to continuing to use Claimants; (iii) did not intend to create our own sales force with respect to the Claimants’ territory; and (iv) would stay out of Claimants’ territories and would not attempt to take over the Claimants business and relationships with the Claimants’ customers and end-users. The Claimants assert that they are seeking damages in excess of $24.3 million. We also moved to dismiss MedQuist Inc. as a party to the arbitration since MedQuist Inc. is not a party to the Claimants’ agreements, and accordingly, has never agreed to arbitration. The AAA initially agreed to rule on these matters, but then decided to defer a ruling to the panel of arbitrators selected pursuant to the parties’ agreements (Panel). In response, we informed the Panel that a court, not the Panel, should rule on these issues. When it appeared that the Panel would rule on these issues, we initiated a lawsuit in the Superior Court of DeKalb County (the Court) and requested an injunction enjoining the Panel from deciding these issues. The Court denied the request, and indicated that a new motion could be filed if the Panel’s ruling was adverse to MedQuist Inc. or MedQuist Transcriptions, Ltd. On May 6, 2008, the Panel dismissed MedQuist Inc. as a party, but ruled against our opposition to a consolidated arbitration. We asked the Court to stay the arbitration in order to review that decision. The Court initially granted the stay, but later lifted the stay. The Court did not make any substantive rulings regarding consolidation, and in fact, left that decision and others to the assigned judge, who was unable to hear those motions. Accordingly, until further order of the Court, the arbitration will proceed forward.
We filed an answer and counterclaim in the arbitration, which generally denied liability. In the lawsuit, the defendants filed a motion to dismiss alleging that our complaint failed to state an actionable claim for relief. On July 25, 2008, we filed our response which opposed the motion to dismiss in all respects. On September 10, 2008, the Court heard argument on defendants’ motion to dismiss. The Court did not issue a decision, but rather, took the matter under advisement.
During discovery in the arbitration, Claimants repeatedly modified the individual damage claims and asserted two alternative damage theories. Claimants did not specify what the two alternative damage theories were, but stated that they were seeking damage theories. Claimants did not specify what the two alternative damage theories were, but stated that they were seeking
F-20
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
alternative damage amounts for each Claimant. The Panel issued a Revised Scheduling Order, which tentatively scheduled the arbitration to begin in February 2010.
On February 19, 2010, the parties reached agreement on settlement terms resolving all claims by the Claimants. Under the proposed settlement, we will pay Claimants $500,000 to resolve all claims, the parties will exchange mutual releases and the arbitration and related state court litigation will be dismissed with prejudice. The parties are in the process of documenting the settlement agreement. We have accrued the entire amount of this settlement as of December 31, 2009.
SEC Investigations of Former Officers
With respect to our historical billing practices, the Securities and Exchange Commission (SEC) is pursuing civil litigation against our former chief financial officer, whose employment with us ended in July 2004. Pursuant to our bylaws, we have indemnification obligations for the legal fees for our former chief financial officer. In February 2010, one of our current employees, who was our former controller but who does not currently serve in a senior management or financial reporting oversight role, reached a settlement with the SEC in connection with the civil litigation that the SEC had brought against him in connection with our historical billing practices.
12. Stock Option Plans
Our stock option plans provide for the granting of options to purchase shares of common stock to eligible employees (including officers) as well as to our non-employee directors. Options may be issued with the exercise prices equal to the fair market value of the common stock on the date of grant or at a price determined by a committee of our board of directors. Stock options vest and are exercisable over periods determined by the committee, generally five years, and expire no more than 10 years after the grant.
In July 2004, our board of directors affirmed our June 2004 decision to indefinitely suspend the exercise and future grant of options under our stock option plans. Ten former executives separated from us in 2005 and 2004. Notwithstanding the suspension, to the extent such executives held options that were vested as of their resignation date, such options remained exercisable for the post-termination period, generally 90 days, commencing on the date that the suspension was lifted for the exercise of options. There were 704 shares that qualified for this post-termination exercise period. The suspension was lifted on October 4, 2007 and all but 154 of these options terminated on February 1, 2008. In July 2008, 12 of the 154 options were exercised for an aggregate exercise amount of $68. As of December 31, 2009, there are no options outstanding related to the ten former executives.
Information with respect to our common stock options is as follows:
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Weighted | | | | |
| | | | | | | Weighted | | | Average | | | | |
| | | Shares | | | Average | | | Remaining | | | Aggregate | |
| | | Subject to | | | Exercise | | | Contractual | | | Intrinsic | |
| | | Options | | | Price | | | Life in Years | | | Value | |
| Outstanding, January 1, 2007 | | | 2,312 | | | $ | 32.57 | | | | | | | | | |
| Granted | | | 200 | | | $ | 11.20 | | | | | | | | | |
| Exercised | | | (4 | ) | | $ | 2.71 | | | | | | | | | |
| Forfeited | | | (137 | ) | | $ | 29.10 | | | | | | | | | |
| Canceled | | | (12 | ) | | $ | 17.45 | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding, December 31, 2007 | | | 2,359 | | | $ | 31.08 | | | | | | | | | |
| Granted | | | 296 | | | $ | 11.20 | | | | | | | | | |
| Exercised | | | (12 | ) | | $ | 2.71 | | | | | | | | | |
| Forfeited | | | (827 | ) | | $ | 29.10 | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Outstanding, December 31, 2008 | | | 1,816 | | | $ | 23.34 | | | | | | | | | |
| Granted | | | — | | | $ | — | | | | | | | | | |
| Exercised | | | — | | | $ | — | | | | | | | | | |
| Forfeited | | | (553 | ) | | $ | 22.57 | | | | | | | | | |
| | | | | | | | | | | | | |
| Outstanding, December 31, 2009 | | | 1,263 | | | $ | 24.47 | | | | 3.3 | | | $ | — | |
| | | | | | | | | | | | | |
| Exercisable, December 31, 2009 | | | 1,065 | | | $ | 27.47 | | | | 2.3 | | | $ | — | |
| | | | | | | | | | | | | |
| Options vested and expected to vest as of December 31, 2009 | | | 1,263 | | | $ | 24.47 | | | | 3.3 | | | $ | — | |
| | | | | | | | | | | | | |
F-21
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
The aggregate intrinsic value is calculated using the difference between the closing stock price on the last trading day of 2009 and the option exercise price, multiplied by the number of in-the-money options. As of December 31, 2009, no options were in the money.
There were no options granted or exercised in 2009. There were 296 options granted and 12 options exercised in 2008. In the first quarter of 2009, we modified the options previously granted in the third quarter of 2008 to our Chief Executive Officer. The grant price was increased from $4.85 per share to $8.25 per share by a committee of our Board of Directors. There was no incremental cost of the modification. We estimated fair value for the modified option grant as of the date of the modification by applying the Black-Scholes option pricing valuation model. The application of this model involves assumptions that are judgmental and sensitive in the determination of compensation expense. The key assumptions used in determining the fair value of the options modified in the first quarter of 2009 were:
| | | | | |
| Expected term (years) | | | 5.92 | |
| Expected volatility | | | 54.5 | % |
| Dividend Yield | | | 0 | % |
| Expected risk free interest rate | | | 3.25 | % |
Significant assumptions required to estimate the fair value of stock options include the following:
| | • | | Expected term: The SEC Staff Accounting Bulletin No. 107 “Simplified” method has been used to determine a weighted average expected term of options granted. |
| |
| | • | | Expected volatility: We have estimated expected volatility based on the historical stock price volatility of a group of similar publicly traded companies. We believe that our historical volatility is not indicative of future volatility. |
The weighted average grant date fair value of options modified in the first quarter of 2009 was $1.97 per share.
A summary of outstanding and exercisable options as of December 31, 2009 is as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding | | | Options Exercisable | |
| | | | | | | Weighted | | | | | | | | | | | |
| | | | | | | Average | | | Weighted | | | | | | | Weighted | |
| | | | | | | Remaining | | | Average | | | | | | | Average | |
| Range of | | Number | | | Contractual Life | | | Exercise | | | Number | | | Exercise | |
| Exercise Prices | | of Shares | | | (in years) | | | Price | | | of Shares | | | Price | |
| $2.71-$10.00 | | | 296 | | | | 8.8 | | | $ | 8.25 | | | | 98 | | | $ | 8.25 | |
| $10.01-$20.00 | | | 260 | | | | 2.4 | | | $ | 17.13 | | | | 260 | | | $ | 17.13 | |
| $20.01-$30.00 | | | 514 | | | | 1.6 | | | $ | 26.41 | | | | 514 | | | $ | 26.41 | |
| $30.01-$40.00 | | | 36 | | | | 0.9 | | | $ | 32.29 | | | | 36 | | | $ | 32.29 | |
| $40.01-$70.00 | | | 157 | | | | 0.4 | | | $ | 59.13 | | | | 157 | | | $ | 59.13 | |
| | | | | | | | | | | | | | | | |
| | | | 1,263 | | | | 3.3 | | | $ | 24.47 | | | | 1,065 | | | $ | 27.47 | |
| | | | | | | | | | | | | | | | |
There were 0, 296 and 200 options granted during 2009, 2008 and 2007 respectively. There were 0, 12 and 4 shares exercised in 2009, 2008 and 2007.
The total fair value of shares vested during 2009 was $193. The change in ownership which occurred on August 6, 2008 was a change in control as defined in the employment agreements for certain option holders. This resulted in the immediate vesting of previously unvested stock options. All previously unamortized stock option compensation expense related to such stock options was recognized as of August 6, 2008 resulting in a charge of approximately $1,060. Also recorded in 2008 was stock option compensation expense of $367 not related to the change in control.
As of December 31, 2009, there were 969 additional options available for grant under our stock option plans. When we became current in our reporting to the SEC in October 2007, certain executive officers, in accordance with their employment agreements, received an aggregate of 200 options with an exercise price equal to the then market value of our common stock on the date of grant.
F-22
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
For the years ended December 31, 2009, 2008 and 2007, $0, $937 and 84, respectively, was included as selling, general and administrative expenses and $0, $402 and $36 was included in research and development expenses in the accompanying consolidated statements of operations related specifically to these options granted to certain executive officers.
On August 27, 2009, our board of directors, upon the recommendation of its compensation committee, approved the MedQuist Inc. Long-Term Incentive Plan. The Incentive Plan is designed to encourage and reward the creation of long-term equity value by certain members of our senior management team. The executives and key employees will be selected by the Compensation Committee and will be eligible to participate in the Incentive Plan. No amounts have been accrued in 2009 under the Incentive Plan as no awards have been made as of December 31, 2009.
13. Income Taxes
The sources of income (loss) before income taxes and the income tax provision (benefit) for the years ended December 31, 2009, 2008 and 2007 are as follows:
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| Income (Loss) before income taxes: | | | | | | | | | | | | |
| Domestic | | $ | 24,314 | | | $ | (83,876 | ) | | $ | (13,557 | ) |
| Foreign | | | 952 | | | | (1,432 | ) | | | 690 | |
| | | | | | | | | | |
| Income (Loss) before income taxes | | $ | 25,266 | | | $ | (85,308 | ) | | $ | (12,867 | ) |
| | | | | | | | | | |
| Current income tax provision (benefit): | | | | | | | | | | | | |
| Federal | | $ | (660 | ) | | $ | 107 | | | $ | 34 | |
| State and local | | | 131 | | | | 216 | | | | 194 | |
| Foreign | | | 647 | | | | 255 | | | | 233 | |
| | | | | | | | | | |
| Current income tax provision (benefit) | | | 118 | | | | 578 | | | | 461 | |
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| Deferred income tax provision (benefit): | | | | | | | | | | | | |
| Federal | | | 1,724 | | | | (15,694 | ) | | | 2,735 | |
| State and local | | | 235 | | | | (2,379 | ) | | | (499 | ) |
| Foreign | | | (102 | ) | | | 982 | | | | (358 | ) |
| | | | | | | | | | |
| Deferred income tax provision (benefit) | | | 1,857 | | | | (17,091 | ) | | | 1,878 | |
| | | | | | | | | | |
| Income tax provision (benefit) | | $ | 1,975 | | | $ | (16,513 | ) | | $ | 2,339 | |
| | | | | | | | | | |
The reconciliation of the statutory federal income tax rate to our effective income tax rate is as follows:
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| Statutory federal income tax rate | | | 35.0 | % | | | 35.0 | % | | | 35.0 | % |
| State income taxes, net of federal tax effect | | | 0.9 | | | | 3.9 | | | | 3.6 | % |
| Valuation allowance | | | (29.8 | ) | | | (10.4 | ) | | | (53.0 | ) |
| Good will impairment | | | — | | | | (7.6 | ) | | | — | |
| Impact of foreign operations | | | 3.2 | | | | (0.4 | ) | | | (0.4 | ) |
| Adjustments to tax reserves | | | (0.3 | ) | | | 0.2 | | | | (0.6 | ) |
| Permanent differences | | | (0.9 | ) | | | (0.3 | ) | | | (3.4 | ) |
| Tax law changes | | | — | | | | — | | | | 1.8 | |
| Other | | | (0.3 | ) | | | (1.0 | ) | | | (1.2 | ) |
| | | | | | | | | | |
| Effective income tax rate | | | 7.8 | % | | | 19.4 | % | | | (18.2 | )% |
| | | | | | | | | | |
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets and liabilities as of December 31, 2009, 2008 and 2007 were as follows:
F-23
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
Deferred tax assets: | | | | | | | | | | | | |
| Foreign net operating loss carry forwards | | $ | 1,957 | | | $ | 1,892 | | | $ | 2,733 | |
| Domestic net operating loss carry forwards | | | 48,397 | | | | 47,694 | | | | 36,295 | |
| Accounts receivable | | | 1,226 | | | | 1,859 | | | | 1,673 | |
| Property and equipment | | | 2,020 | | | | 1,563 | | | | 1,917 | |
| Intangibles | | | 12,784 | | | | 20,976 | | | | 21,746 | |
| Employee compensation and benefit plans | | | 1,555 | | | | 1,149 | | | | 953 | |
| Deferred compensation | | | — | | | | 168 | | | | 617 | |
| Customer accommodation | | | 4,515 | | | | 4,668 | | | | 7,087 | |
| Accruals and reserves | | | 2,805 | | | | 3,613 | | | | 4,855 | |
| Other | | | 2,554 | | | | 2,129 | | | | 1,714 | |
| | | | | | | | | | |
| Total gross deferred tax assets | | | 77,813 | | | | 85,711 | | | | 79,590 | |
| Less: Valuation allowance | | | (74,641 | ) | | | (81,785 | ) | | | (74,500 | ) |
| | | | | | | | | | |
| Total deferred tax assets | | | 3,172 | | | | 3,926 | | | | 5,090 | |
| | | | | | | | | | |
Deferred tax liabilities: | | | | | | | | | | | | |
| Intangibles | | | (3,925 | ) | | | (2,907 | ) | | | (21,377 | ) |
| Other | | | (1,095 | ) | | | (1,265 | ) | | | (935 | ) |
| | | | | | | | | | |
| Total deferred tax liabilities | | | (5,020 | ) | | | (4,172 | ) | | | (22,312 | ) |
| | | | | | | | | | |
| Net deferred tax liability | | $ | (1,848 | ) | | $ | (246 | ) | | $ | (17,222 | ) |
| | | | | | | | | | |
As of December 31, 2009, we had federal net operating loss carry forwards of approximately $107,141 which will begin to expire in 2026 and 2028.
As of December 31, 2009 and 2008, we had state net operating loss carry forwards of approximately $225,074 and $213,777, respectively, which will expire between 2010 and 2029. In addition, we have foreign net operating loss carry forwards of approximately $11,297, which do not expire. Utilization of the net operating loss carry forwards will be subject to an annual limitation in future years as a result of the change in ownership as defined by Section 382 of the Internal Revenue Code and similar state provisions. We performed an analysis on the annual limitation as a result of the ownership change that occurred in 2008. As a result of this analysis, the annual limitation in future years may not prevent us from using the federal NOL carry forwards before their respective expiration periods.
In assessing the future realization of deferred taxes, we consider whether it is more likely than not that some portion or all of the deferred income tax assets will not be realized based on projections of our future taxable earnings. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible.
During 2009, we decreased our valuation allowance against our deferred tax assets generated in foreign tax jurisdictions from $1,316 to $1,310 based on management’s assessment of future earnings available to utilize these deferred tax assets.
After consideration of all evidence, both positive and negative, management concluded that it was more likely than not that a majority of the domestic deferred tax assets would not be realized. As of December 31, 2009, this valuation allowance has been decreased from $80,469 to $73,331.
Domestic deferred tax assets were recognized to the extent that objective positive evidence existed with respect to their future utilization. The objective positive evidence included the potential to carry back any losses generated by the deferred tax assets in the future as well as income expected to be recognized due to the reversal of deferred tax liabilities as of December 31, 2009. In analyzing deferred tax liabilities as a source for potential income for purposes of recognizing deferred tax assets, the deferred tax liabilities related to excess book basis in goodwill over tax basis in goodwill and book basis over tax basis in our investment in A-Life were considered a source of future income for benefiting deferred tax assets with indefinite lives only due to the indefinite life and uncertainty of reversal of these liabilities during the same period as the non-indefinite life deferred tax assets.
F-24
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Our consolidated income tax expense for the year ended December 31, 2009 consisted principally of an increase in deferred tax liabilities related to goodwill amortization deductions for income tax purposes during the applicable year as well as state and foreign income taxes. Our consolidated income tax benefit for the year ended December 31, 2008 consisted primarily of the reversal of approximately $18.5 million of certain deferred tax liabilities associated with indefinite life intangible assets related to goodwill which was impaired in 2008 offset by state and foreign income tax expense. Our consolidated income tax expense for the year ended December 31, 2007 consisted principally of an increase in deferred tax liabilities related to goodwill amortization deductions for income tax purposes during the applicable year as well as state and foreign income taxes.
The total amount of unrecognized tax benefits as of December 31, 2009 was $5,187 which includes $357 of accrued interest related to unrecognized income tax benefits which we recognize as a component of the provision for income taxes. Of the $5,187 unrecognized tax benefits, $4,495 relates to tax positions which if recognized would impact the effective tax rate, not considering the impact of any valuation allowance. Of the $4,495, $3,576 is attributable to uncertain tax positions with respect to certain deferred tax assets which if recognized would currently be offset by a full valuation allowance due to the fact that at the current time it is more likely than not that these assets would not be recognized due to a lack of sufficient projected income in the future.
The following is a roll-forward of the changes in our unrecognized tax benefits:
| | | | | |
| Total unrecognized tax benefits as of January 1, 2009 | | $ | 4,907 | |
| Gross amount of decreases in unrecognized tax benefits as a result of tax positions taken during the prior period | | | (9 | ) |
| Gross amount of increases in unrecognized tax benefits as a result of tax positions taken during the prior period | | | — | |
| Gross amount of increases in unrecognized tax benefits as a result of tax positions taken during the current period | | | 62 | |
| | | | | |
| Amount of decreases in the unrecognized tax benefits relating to settlements with taxing authorities | | | — | |
| Reduction to unrecognized tax benefits as a result of a lapse of applicable statute of limitations | | | (130 | ) |
| | | | |
| Total unrecognized tax benefits as of December 31, 2009 | | $ | 4,830 | |
| | | | |
| | | | | |
| Total unrecognized tax benefits that would impact the effective tax rate if recognized | | $ | 4,495 | |
| | | | |
| Total amount of interest and penalties recognized in the accompanying consolidated statement of operations for the year ended December 31, 2009 | | $ | (147 | ) |
| | | | |
| Total amount of interest and penalties recognized in the accompanying consolidated balance sheet as of December 31, 2009 | | $ | 357 | |
| | | | |
We file income tax returns in the U.S. federal jurisdiction, all U.S. states which require income tax returns and foreign jurisdictions. Due to the nature of our operations, no state or foreign jurisdiction is individually significant. With limited exceptions we are no longer subject to examination by the U.S. federal or states jurisdiction for years prior to 2007. The Internal Revenue Service concluded its federal tax audit for the tax years 2003 through 2006 with no material adjustments. We are no longer subject to examination by the UK federal jurisdiction for years prior to 2007. We do have various state tax audits and appeals in process at any given time.
We anticipate decreases in unrecognized tax benefits of approximately $126 related to state statutes of limitations expiring during 2010. Our unrecognized tax benefits are expected to change in 2010. We are currently in the process of negotiating with certain jurisdictions to resolve specific issues related to tax positions taken in prior periods.
14. Employee Benefit Plans
401(k) Plan
We maintain a tax-qualified retirement plan named the MedQuist 401(k) Plan (401(k) Plan) that provides eligible employees with an opportunity to save for retirement on a tax advantaged basis. Our 401(k) Plan allows eligible employees to contribute up to 25% of their annual eligible compensation on a pre-tax basis, subject to applicable Internal Revenue Code limits. Elective deferral contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participant’s directives. Employee elective deferrals are 100% vested at all times. Our 401(k) Plan provides that we may make a discretionary matching contribution to the participants in the 401(k) Plan. Our discretionary matching contribution, if any, shall be in an amount not to exceed 100% of the first 25% of a plan participant’s compensation contributed as pre-tax contributions to the 401(k) Plan. In our sole discretion, we may make discretionary matching contributions on a quarterly or annual basis. Historically we have
F-25
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
matched 50% of each participant’s contribution, up to a maximum of 5% of each participant’s total annual compensation. Matching contributions are 33% vested after one year of service, 67% vested after two years of service and 100% vested after three years of service. We did not match the employee contributions for the years ended December 31, 2009 and 2008. The charge to operations for our matching contributions for the year ended December 31, 2007 was $1,547.
Executive Deferred Compensation Plan
We established the MedQuist Inc. Executive Deferred Compensation Plan (EDCP) in 2001. The EDCP, which is administered by the compensation committee of our board of directors, allowed certain members of management and highly compensated employees to defer a certain percentage of their income. Participants were permitted to defer compensation into an account in which proceeds were available either during or after termination of employment. The compensation committee authorized that certain contributions made to a retirement distribution account be matched with either shares of our common stock or cash. Participants were not entitled to receive matching contributions if they elected to make deferrals to an account into which proceeds are available during employment. Participants were able to defer up to 15% of their base salary (or such other maximum percentage as may be approved by the compensation committee) and 90% of their bonus (or such other maximum percentage as may be approved by the compensation committee). Distributions to a participant made pursuant to a retirement distribution account may be made to the participant upon the participant’s termination or attainment of age 65, as elected by the participant in their enrollment agreement. Distributions to a participant made pursuant to an in-service distribution account may be made at the election of the participant in their enrollment agreement, subject to certain exceptions. The balances in the EDCP are not funded, but are segregated, and participants in the EDCP are our general creditors. All amounts deferred in the EDCP increase or decrease based on hypothetical investment results of the participant’s selected investment alternatives. However, EDCP distributions are paid out of our funds rather than from a dedicated investment portfolio. As of December 31, 2009 and 2008, the value of the assets held, primarily insurance contracts, managed and invested pursuant to the EDCP was $0 and $787, respectively, and is included in other current assets in the accompanying consolidated balance sheets. As of December 31, 2009 and 2008, the deferred compensation liability reflecting amounts due to employees was $0 and $237, respectively, and is included in accrued expenses in the accompanying consolidated balance sheets.
Effective January 1, 2005, the EDCP was suspended and no further contributions have been made. In the third quarter of 2009 we terminated the plan and distributed plan assets to participants and liquidated the remaining plan assets.
15. Related-Party Transactions
From time to time, we enter into transactions in the normal course of business with related parties. Prior to August 6, 2008, Koninklijke Philips Electronics N.V. (Philips) owned approximately 69.5% ownership interest in MedQuist. This ownership interest was sold to CBaySystems Holdings on August 6, 2008 (CBaySystems Holdings Purchase). Accordingly Philips ceased to be a related party on that date and CBaySystems Holdings (and affiliated entities) commenced to be a related party on that date. The Audit Committee of our board of directors has been charged with the responsibility of approving or ratifying all related party transactions other than those which were entered into between us and Philips prior to August 6, 2008. In any situation where the Audit Committee sees fit to do so, any related party transaction, other than those entered into between us and Philips prior to August 6, 2008, are presented to disinterested members of our board of directors for approval or ratification. All of the agreements with CBay Systems & Services, Inc. and CBay Inc. described below have been reviewed and approved by our Audit Committee.
On September 15, 2008, we entered into a transcription services agreement (the 2008 TSA) with CBay Systems & Services, Inc. (CBay Systems), a wholly-owned subsidiary of CBaySystems Holdings, pursuant to which we outsource certain medical transcription services to CBay Systems. The 2008 TSA was terminated by mutual written agreement on October 26, 2009. For the years ended December 31, 2009 and 2008, we incurred expenses of $958 and $283, respectively, which were recorded in Cost of revenues.
On April 3, 2009, we entered into a transcription services agreement (Transcription Services Agreement) with CBay Systems, pursuant to which we outsource certain medical transcription services to CBay Systems. The Transcription Services Agreement will expire on April 16, 2012 unless sooner terminated by either party. Under the Transcription Services Agreement, we pay CBay Systems a per line fee based on each transcribed line of text processed and the specific type of service provided. CBay Systems will perform its services using our DocQment Enterprise Platform and will be held to certain performance standards and quality guidelines set forth in the agreement. The specific services to be performed will be set forth in order forms delivered by us to CBay Systems from time to time during the term of the Transcription Services Agreement. For the year ended December 31, 2009 we incurred expenses of $5,840, which were recorded in Cost of revenues. The Transcription Services Agreement terminated by agreement of the parties on March 9, 2010 when we entered into the Sales & Services Agreement with CBay Systems referenced immediately below.
F-26
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
On March 9, 2010, we entered into the Sales & Services Agreement with CBay Systems, pursuant to which we outsource certain medical transcription services to CBay Systems. Under the Sales & Services Agreement, CBay Systems has discontinued its new customer marketing efforts for transcription services to hospitals in North America and has become the exclusive supplier to us of Asian based transcription production services, which are either performed directly by CBay Systems or through a network of third party suppliers managed by CBay Systems. Under the Sales & Services Agreement, we pay CBay Systems a per line fee based on each transcribed line of text processed and the specific type of service provided. The Sales & Services Agreement will expire on March 10, 2015, and thereafter shall automatically renew for two additional five year terms, unless either party provides the other with written notice of its election to terminate the agreement at the end of the initial term or at the end of a renewal term, provided such notice is provided to the other party, not earlier than 12 months nor less than six months prior to the end of the initial term or the applicable renewal term. Either we or CBay may terminate the Sales & Services Agreement if the other party materially breaches any term of the agreement, or in the event CBay Systems and its affiliates (other than us or our subsidiaries) cease to control, directly or indirectly, the power to vote at least thirty percent (30%) of the issued and outstanding common equity of us. The Sales & Services Agreement will be filed as an exhibit to our Quarterly Report onForm 10-Q for the quarter ending March 31, 2010, with portions omitted and filed separately with the SEC pursuant to a request for confidential treatment.
On March 31, 2009, we entered into a transcription services subcontracting agreement (Subcontracting Agreement) with CBay Systems, pursuant to which CBay Systems will subcontract certain medical transcription, editing and related services to us. Under the Subcontracting Agreement, we will provide the medical transcription, editing and related services to CBay Systems using labor located within the United States using our DocQment Enterprise Platform. The specific services to be performed will be set forth in order forms delivered by CBay Systems to us from time to time during the term of the Subcontracting Agreement. We will receive 98% of the net monthly fees collected by CBay Systems from its customers for the services provided by us. For the year ended December 31, 2009, we recorded revenue of $1,483 under the terms of the Subcontracting agreement.
On September 19, 2009, we entered into a services agreement (Management Services Agreement) with CBay Inc. (CBay), a wholly-owned subsidiary of CBay Systems, pursuant to which certain senior executives and directors of CBay render to us, upon the request of our Chief Executive Officer, certain advisory and consulting services in relation to the affairs of us and our subsidiaries. The Management Services Agreement will remain in effect until the earliest to occur of (i) December 31, 2009, if either party gives notice of termination of the Services Agreement to the other party no later than December 1, 2009, (ii) the end of any calendar quarter subsequent to December 31, 2009, if either party gives notice of termination of the Services Agreement to the other party no later than thirty (30) days prior to the end of such calendar quarter, (iii) such time as CBay or its affiliates (other than the us and our subsidiaries) control, directly or indirectly, the power to vote less than thirty percent (30%) of the issued and outstanding common equity of the MedQuist, and (iv) such earlier date as we and CBay may mutually agree upon in writing. The Management Services Agreement provides that, in consideration of the management services rendered by CBay to us since July 1, 2009 and to be rendered by CBay to us pursuant to the Management Services Agreement, we will pay CBay a quarterly services fee equal to $350, which shall be payable in arrears. For the years ended December 31, 2009 and 2008, we incurred services expenses with CBay Inc. of $1,398 and $0, respectively, which has been recorded in Selling, general and administrative expense.
As of December 31, 2009 and December 31, 2008, Accounts payable and Accrued expenses included $1,362 and $665, respectively, for amounts due to CBay. As of December 31, 2009 and December 31, 2008, Accounts receivable included $710 and $0, respectively, for amounts due from CBay Systems.
Listed below is a summary of our material agreements with Philips.
Licensing Agreement
We entered into a Licensing Agreement with Philips Speech Recognition Systems GmbH f/k/a Philips Austria GmbH, Philips Speech Processing, a Republic of Austria corporation and an affiliate of Philips (PSRS) on May 22, 2000 (Licensing Agreement). The Licensing Agreement was subsequently amended by the parties as of January 1, 2002, February 23, 2003, August 10, 2003, September 1, 2004, December 30, 2005 and February 13, 2007. During 2008, our competitor, Nuance Communications, Inc. (Nuance) purchased PSRS. Nuance is successor-in-interest to PSRS. On November 10, 2009, we entered into an amendment to the Licensing Agreement with Nuance.
Under the Licensing Agreement, we license from Nuance (previously PSRS) its SpeechMagic speech recognition and processing software, including any updated versions of the software developed by PSRS/Nuance during the term of the Licensing Agreement (Licensed Product), for use by us anywhere in the world. We pay a fee for use of the Licensed Product based upon a per line fee for each transcribed line of text processed through the Licensed Product. Upon the expiration of its initial term on June 28, 2005, the Licensing Agreement was renewed for an additional five year term. Nuance may terminate the Licensing Agreement for cause immediately in the event that we:
F-27
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
| | • | | default in any payment due to Nuance and the default continues for a period of 30 business days after written notice to us; |
| |
| | • | | fail to perform any material obligation, duty or responsibility or are in default with any material term or condition of the Licensing Agreement and the default continues for a period of 30 business days after written notice to us; or |
| |
| | • | | become insolvent or file for bankruptcy. |
We may terminate the Licensing Agreement for cause immediately in the event that Nuance:
| | • | | fails to perform any material obligation, duty or responsibility or is in default with any material term or condition of the Licensing Agreement and the default continues for a period of 30 business days after written notice to Nuance; or |
| |
| | • | | becomes insolvent or files for bankruptcy. |
Upon the expiration of its initial term on June 28, 2005, the Licensing Agreement was renewed for an additional five year term. As part of the Philips’ sale of its majority interest in us to CBay, PSRS waived, through June 30, 2011, its right to provide prior to June 30, 2011 a two year advance notice to terminate the Licensing Agreement. This waiver was conditioned upon a similar waiver from us which we have provided. The November 10, 2009 amendment to the Licensing Agreement that we entered into with Nuance extends the term of the Licensing Agreement until June 30, 2015. Thereafter, upon written notice to Nuance, we have the right to renew the Licensing Agreement for two successive terms of five years each (each a Renewal Term) on the same terms (except pricing terms) and conditions of the Licensing Agreement in effect at the end of the then-current initial term or Renewal Term. To the extent new pricing terms will apply to a Renewal Term, such terms must be determined and agreed to in writing by us and Nuance in advance of the start of such Renewal Term. In addition, any new pricing terms must be based upon Nuance’s then-current standard market prices.
In connection with the Licensing Agreement, we have a consulting arrangement with PSRS whereby PSRS assists us with the integration of its speech and transcription technologies.
Philips, as discussed above, is no longer a related party to us as of August 6, 2008. However, the Licensing Agreement is still in force.
OEM Supply Agreement
On September 21, 2007, we entered into an Amended and Restated OEM Supply Agreement with PSRS. The Amended and Restated OEM Supply Agreement amends and restates a previous OEM Supply Agreement with PSRS dated September 23, 2004. In connection with the Amended and Restated OEM Supply Agreement certain amounts paid to PSRS were capitalized in fixed assets and are being amortized over a three-year period. As part of the Philips’ sale of its majority interest in us to CBay, we entered into a Second Amended and Restated OEM Supply Agreement with PSRS dated as of October 1, 2008 which clarified certain provisions of the Amended and Restated OEM Supply Agreement (the Second Amended OEM Agreement and, together with the Amended and Restated OEM Supply Agreement, the Amended OEM Agreement). During 2008, our competitor, Nuance Communications, Inc. (Nuance) purchased PSRS. Nuance is successor-in-interest to PSRS.
Pursuant to the Amended OEM Agreement, we purchased a co-ownership interest in all rights and interests in and to SpeechQ for Radiology together with its components, including object and source code for the SpeechQ for Radiology application and the SpeechQ for Radiology integration SDK (collectively, the Product), but excluding the SpeechMagic speech recognition and processing software, which we separately licensed from PSRS pursuant to the terms and conditions of the Licensing Agreement, (other than pricing which was set forth in the Second Amended and Restated OEM Supply Agreement). SpeechQ for Radiology is our front-end speech recognition software application. Additionally, the Amended OEM Agreement provides that we shall receive, in exchange for a fee, the exclusive right in the United States, Canada and certain islands of the Caribbean (collectively the Exclusive Territory) to sell, service and deliver the Product. The Amended OEM Agreement further provided that we make payments to PSRS for PSRS’ development of an interim version of the software included in the Product (Interim Version). Except for the Interim Version which we and PSRS co-own, the Amended OEM Agreement provided that any improvements, developments or other enhancements either we or PSRS made to the Product (collectively, Improvements) would be owned exclusively by the party that developed such Improvement. Each party has the right to seek patent or other protection of the Improvements it owns independent of the other party. Either party may terminate the Amended OEM Agreement for cause immediately in the event that a material breach by the other party remains uncured for more than 30 days following delivery of written notice or in the event that the other party becomes insolvent or files for bankruptcy.
F-28
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
The term of the Amended OEM Agreement extended through June 30, 2010. On November 10, 2009, we entered into a Third Amended and Restated OEM Supply Agreement with Nuance, which clarified certain provisions of the Amended OEM Agreement and made the following substantive modifications to the Amended OEM Agreement:
| | • | | The termination date was extended to June 30, 2015 from June 30, 2010. In addition, upon written notice to Nuance, we have the right to renew the Third Amended and Restated OEM Supply Agreement for two successive terms of five years each (each an “OEM Renewal Term”) on the same terms (except pricing terms) and conditions of the Third Amended and Restated OEM Supply Agreement in effect at the end of the then-current initial term or OEM Renewal Term. To the extent new pricing terms will apply to an OEM Renewal Term, such terms must be determined and agreed to in writing by us and Nuance in advance of the start of such OEM Renewal Term. In addition, any new pricing terms must be based upon Nuance’s then-current standard market prices. |
| |
| | • | | Nuance is required to provide the services necessary to maintain the current code base of the Product until the earlier of: (i) the end of the term of the Third Amended OEM Agreement; or (ii) our termination of Nuance’s maintenance and support obligations. |
| |
| | • | | We are permitted to make modifications to the Product to use and market it in medical specialties other than radiology. |
We now license the SpeechMagic speech recognition and processing software for the Product - which we previously separately licensed pursuant to the terms and conditions of the Licensing Agreement, (other than pricing which was set forth in the Second Amended and Restated OEM Supply Agreement) — pursuant to a separate licensing agreement that we entered into with Nuance on November 10, 2009 (the SpeechMagic for SpeechQ Licensing Agreement). The term of the SpeechMagic for SpeechQ Licensing Agreement extends to June 30, 2015 and, thereafter, we have the right to renew the agreement for two (2) successive terms of five (5) years each in the same manner as the Third Amended OEM Agreement. The SpeechMagic for SpeechQ Licensing Agreement only applies to our licensing of the SpeechMagic speech recognition and processing software for the Product. The Licensing Agreement described above still remains in effect and applies to our other uses of the SpeechMagic speech recognition and processing software.
Equipment Purchases
We purchased certain dictation related equipment from Philips.
Insurance Coverage
Prior to the closing of the CBaySystems Holdings Purchase on August 6, 2008, we obtained all of our business insurance coverage (other than workers’ compensation) through Philips.
Other
From time to time prior to the CBaySystems Holdings Purchase, we entered into other miscellaneous transactions with Philips including Philips purchasing certain products and implementation services from us. We recorded net revenues from sales to Philips of $39 for the year ended December 31, 2008.
Listed below is a summary of the expenses incurred by us in connection with the various Philips agreements noted above for the years ended December 31, 2008 and 2007, respectively. Philips ceased being a related party on August 6, 2008. Charges related to these agreements are included in Cost of revenues and selling, general and administrative expenses in the accompanying consolidated statements of operations.
| | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2008* | | | 2007 | |
| PSRS licensing | | $ | 2,070 | | | $ | 2,479 | |
| PSRS consulting | | | — | | | | — | |
| OEM agreement | | | 1,645 | | | | 2,252 | |
| Dictation equipment | | | 586 | | | | 854 | |
| Insurance | | | 399 | | | | 1,794 | |
| PENAC | | | — | | | | 40 | |
| CBay Transaction | | | (172 | ) | | | — | |
| Other | | | (39 | ) | | | — | |
| | | | | | | |
| Total | | $ | 4,489 | | | $ | 7,419 | |
| | | | | | | |
F-29
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
| | |
| * | | Philips ceased being a related party on August 6, 2008. |
On July 29, 2004, we entered into an agreement with Nightingale and Associates, LLC (Nightingale) under which Nightingale agreed to provide interim chief executive officer services to us. On July 30, 2004, our board of directors appointed Howard S. Hoffmann to serve as our non-employee chief executive officer. Mr. Hoffmann served as the Managing Partner of Nightingale. With the departure of our former president in May 2007, our board of directors appointed Mr. Hoffmann to the additional position of president in June 2007.
Mr. Hoffmann served as our president and chief executive officer pursuant to the terms of the agreement between us and Nightingale which was amended on March 14, 2008 (Amendment). The Amendment, among other things, extended the term of Mr. Hoffmann’s role as our president and chief executive officer through August 1, 2008. Our agreement with Nightingale also permitted us to engage additional personnel employed by Nightingale to provide consulting services to us from time to time. Mr. Hoffman’s service as president and chief executive officer and the related engagement of Nightingale terminated consensually on June 10, 2008.
For the years ended December 31, 2009, 2008 and 2007, we incurred charges of $0, $1,073 and $2,914, respectively, for Nightingale services. From February 1, 2007 through December 31, 2008, the Nightingale charges were recorded in selling, general and administrative expenses in the accompanying consolidated statements of operations due to Nightingale’s focus on operational matters instead of the Review and Management’s Billing Assessment. Prior to February 1, 2007, charges related to Nightingale were recorded in cost of legal proceedings and settlements, net (see Note 3). As of December 31, 2009 and 2008 accrued expenses included $0 and $0, respectively, for amounts due to Nightingale for services performed.
16. Financial Instruments
Effective January 1, 2008, we adopted Fair Value accounting for financial assets and financial liabilities. This did not have a material impact on our financial position, results of operations and cash flows. The statement establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad categories. Level 1: Quoted market prices in active markets for identical assets or liabilities that the company has the ability to access. Level 2: Observable market based inputs or unobservable inputs that are corroborated by market data such as quoted prices, interest rates and yield curves. Level 3: Inputs are unobservable data points that are not corroborated by market data. At December 31, 2008, we held one financial asset, our Executive Deferred Compensation Plan assets (EDCP) included in Other current assets. We measured the fair value of our EDCP on a recurring basis using Level 2 (significant other observable) inputs. The adoption did not have an impact on the basis for measuring the fair value of these items. In the third quarter of 2009 we terminated the plan and distributed plan assets to participants and liquidated the remaining plan assets. Accordingly there were no financial instruments as defined as of December 31, 2009.
The following table presents our fair value hierarchy for those financial assets measured at fair value on a recurring basis in our consolidated balance sheets as of December 31, 2008:
| | | | | | | | | | | | | | | | | |
| | | | | | | Quoted Prices In | | | | | | | |
| | | | | | | Active Markets for | | | Significant Other | | | Significant | |
| | | December 31, | | | Identical | | | Observable | | | Unobservable | |
| | | 2008 | | | Assets (Level 1) | | | Inputs (Level 2) | | | Inputs (Level 3) | |
| Assets | | | | | | | | | | | | | | | | |
| Executive Deferred Compensation Plan assets | | $ | 787 | | | $ | — | | | $ | 787 | | | $ | — | |
| | | | | | | | | | | | | |
17. Credit Agreement
In August 2009, we entered into a five-year $25 million revolving credit agreement (the Credit Agreement) with Wells Fargo Foothill, LLC. Subject to certain terms and conditions, the Credit Agreement provides committed revolving funding through August 2014 and includes an option whereby we can increase its maximum credit to $40 million. The amount available for borrowings is based upon a percentage of eligible accounts receivable. Under the agreement, there are reserves established which limit the amounts that can be available. At December 31, 2009, $21.0 million was available under the Credit Agreement. The Credit Agreement is a working capital facility that may be used for general corporate purposes. The Credit Agreement enables us to borrow funds in U.S. dollars, at variable interest rates. The Credit Agreement provides the lender a security interest in and against significantly all of our assets. Under the Credit Agreement we agreed to certain covenants customarily found in such agreements including, but not limited to,
F-30
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
financial covenants requiring us to maintain certain minimum levels of EBITDA and a minimum fixed charge coverage ratio. At December 31, 2009, we were in compliance with the financial covenants of the agreement. At December 31, 2009 there were no borrowings outstanding under the Credit Agreement.
18. Subsequent Events
Stock and Asset Purchase Agreement with Spheris, Inc.
On February 2, 2010, we and our majority shareholder, CBay Inc. ( CBay and, together with us, Purchasers), entered into a “stalking horse” Stock and Asset Purchase Agreement (Agreement) with Spheris, Inc. and certain of its affiliates (collectively, the Sellers), under which we have has agreed to purchase substantially all of the domestic business of Sellers, and CBay has agreed to purchase all of the outstanding shares of Spheris India Private Limited (together, the Assets), subject to the terms and conditions therein. Each of the Sellers is a debtor in a Chapter 11 case before the United States Bankruptcy Court for the District of Delaware (Bankruptcy Court).
The United States Bankruptcy Court for the District of Delaware has issued an order approving bidding procedures naming us, along with CBay Inc., our majority owner, the stalking horse bidders in the Chapter 11 bankruptcy case of Spheris Inc. Spheris filed for Chapter 11 bankruptcy protection on February 3, 2010, after entering into a purchase agreement with us and CBay to purchase substantially all of Spheris’ assets in a sale under Section 363 of the United States Bankruptcy Code. The court order was entered in accordance with the timeline provided for by the purchase agreement, and among other things, provides for an auction of Spheris’ assets on April 13, 2010, and the payment of certain transaction expenses and payment of a breakup fee in the amount of $2.1 million by Spheris to us and CBay in certain circumstances if we are not the winning bidders at the auction. Pending a successful conclusion of this process, we anticipate this transaction will close by mid-to-late April 2010.
Under the terms of the Agreement, Purchasers have agreed, absent any higher or otherwise better bid, to acquire the Assets from the Sellers for approximately $75.2 million in cash plus the assumption of specified liabilities of the domestic business of Spheris and the liabilities of Spheris India Private Limited. We have has deposited $7.5��million into escrow which will be credited to the purchase price on the completion of the acquisition of the Assets. If the Agreement is terminated, the deposit will be returned to us unless the purchaser termination fee described below is payable, in which event the deposit will be retained by the Sellers and credited to the purchaser termination fee. If the Agreement is terminated for certain reasons, including because the Sellers enter into a competing transaction, the Sellers may be required to pay the Purchasers a termination fee equal to $2.1 million, plus Purchasers’ reasonable transaction expenses. In addition, if the Agreement is terminated by Sellers for certain reasons, including because the Purchasers refuse to complete the acquisition in breach of the Agreement, we are required to pay the Sellers a termination fee equal to the difference between $15 million and the amount of the deposit and interest thereon.
The completion of the acquisition is subject to a number of conditions, including the performance by each party of its obligations under the Agreement, and the absence of a material adverse change in the Sellers’ business since the date of the Agreement and other conditions which are set forth in the Agreement.
In connection with the acquisition, we are exploring debt financing options.
Sales and Services Agreement with CBay Systems & Services, Inc.
On March 9, 2010, we entered into the Sales & Services Agreement, pursuant to which we outsource certain medical transcription services to CBay Systems. Under the Sales & Services Agreement, CBay Systems has discontinued its new customer marketing efforts for transcription services to hospitals in North America and has become the exclusive supplier to us of Asian based transcription production services, which are either performed directly by CBay Systems or through a network of third party suppliers managed by CBay Systems. Under the Sales & Services Agreement, we pay CBay Systems a per line fee based on each transcribed line of text processed and the specific type of service provided. The Sales & Services Agreement will expire on March 10, 2015, and thereafter shall automatically renew for two additional five year terms, unless either party provides the other with written notice of its election to terminate the agreement at the end of the initial term or at the end of a renewal term, provided such notice is provided to the other party, not earlier than 12 months nor less than six months prior to the end of the initial term or the applicable renewal term. Either we or CBay may terminate the Sales & Services Agreement if the other party materially breaches any term of the agreement, or in the event CBay Systems and its affiliates (other than us or our subsidiaries) cease to control, directly or indirectly, the power to vote at least thirty percent (30%) of the issued and outstanding common equity of us. The Sales & Services Agreement will be filed as an exhibit to our Quarterly Report onForm 10-Q for the quarter ending March 31, 2010, with portions omitted and filed separately with the SEC pursuant to a request for confidential treatment.
F-31
MedQuist Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
19. Quarterly Data (unaudited)
| | | | | | | | | | | | | | | | | |
| | | 1st | | | 2nd | | | 3rd | | | 4th | |
| | | Quarter | | | Quarter | | | Quarter | | | Quarter | |
2009 | | | | | | | | | | | | | | | | |
| Net revenues | | $ | 78,944 | | | $ | 77,471 | | | $ | 76,836 | | | $ | 73,949 | |
| | | | | | | | | | | | | |
| Net income | | $ | 6,854 | | | $ | 836 | (3) | | $ | 9,704 | | | $ | 5,897 | (4) |
| | | | | | | | | | | | | |
| Net income per share: | | | | | | | | | | | | | | | | |
| Basic | | $ | 0.18 | | | $ | 0.02 | | | $ | 0.26 | | | $ | 0.16 | |
| Diluted | | $ | 0.18 | | | $ | 0.02 | | | $ | 0.26 | | | $ | 0.16 | |
| | | | | | | | | | | | | | | | | |
| Weighted average shares outstanding: | | | | | | | | | | | | | | | | |
| Basic | | | 37,556 | | | | 37,556 | | | | 37,556 | | | | 37,556 | |
| Diluted | | | 37,556 | | | | 37,556 | | | | 37,560 | | | | 37,556 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
2008 | | | | | | | | | | | | | | | | |
| Net revenues | | $ | 83,725 | | | $ | 82,454 | | | $ | 81,287 | | | $ | 79,387 | |
| | | | | | | | | | | | | |
| Net income | | $ | (4,417 | ) | | $ | 1,835 | | | $ | (5,762 | )(1) | | $ | (60,451 | )(2) |
| | | | | | | | | | | | | |
| Net income per share: | | | | | | | | | | | | | | | | |
| Basic | | $ | (0.12 | ) | | $ | 0.05 | | | $ | 0.15 | | | $ | (1.61 | ) |
| Diluted | | $ | (0.12 | ) | | $ | 0.05 | | | $ | 0.15 | | | $ | (1.61 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted average shares outstanding: | | | | | | | | | | | | | | | | |
| Basic | | | 37,544 | | | | 37,544 | | | | 37,554 | | | | 37,556 | |
| Diluted | | | 37,544 | | | | 37,553 | | | | 37,554 | | | | 37,556 | |
| | | | | | | | | | | | | |
| | |
| (1) | | Includes $5,700 recorded in Cost of legal proceedings and settlements, net, related to the settlement of all claims related to the DOJ investigation. |
| |
| (2) | | Includes $82,233 for a goodwill impairment charge offset by $18,470 of deferred tax benefits primarily related to the reversal of deferred tax liabilities associated with indefinite life intangible assets. |
| |
| (3) | | Includes $5,750 recorded in Cost of legal proceedings and settlements, net, related to the settlement of all claims related to the Anthurium patent litigation settlement. |
| |
| (4) | | Includes $500 recorded in Cost of legal proceedings and settlements, net, related to the settlement of all claims related to the Reseller arbitration settlement. |
F-32
MedQuist Inc. and Subsidiaries
Schedule II — Valuation and Qualifying Accounts
(In thousands)
Allowance for Doubtful Accounts and Returns
| | | | | | | | | | | | | | | |
| | | Balance at | | Charges to | | Doubtful | | Balance at |
| | | Beginning | | Revenues and | | Accounts | | End of |
| | | of Period | | and Expenses | | Written Off | | Period |
| December 31, 2007 | | $ | 4,494 | | | | 4,967 | | | (5,102) | | $ | 4,359 | |
| December 31, 2008 | | $ | 4,359 | | | | 3,073 | | | (2,630) | | $ | 4,802 | |
| December 31, 2009 | | $ | 4,802 | | | | 2,306 | | | (3,949) | | $ | 3,159 | |
Includes amounts written off to costs and expenses for bad debts of $197, $627 and $(17) for the years ended December 31, 2009, 2008 and 2007, respectively, and amounts charged to revenues for customer credits of $2,109, $2,446 and $4,984 for the years ended December 31, 2009, 2008 and 2007, respectively.
F-33
EXHIBIT INDEX
| | | |
| No. | | Description |
| 10.12.7 | | Amendment No. 7 to Licensing Agreement, dated as of November 10, 2009, between MedQuist Inc. and Nuance Communications, Inc. as the successor-in-interest to Philips Speech Recognition Systems GmbH |
| | | |
| 10.13# | | Third Amended and Restated OEM Supply Agreement dated November 10, 2009, between MedQuist Inc. and Nuance Communications, Inc. as the successor-in-interest to Philips Speech Recognition Systems GmbH |
| | | |
| 10.29# | | Licensing Agreement by and between Nuance Communications, Inc. and MedQuist Inc. dated November 10, 2009 |
| | | |
| 23 | | Consent of KPMG LLP |
| | | |
| 24 | | Power of Attorney (included on the signature page hereto) |
| | | |
| 31.1 | | Certification of Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 31.2 | | Certification of Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 32.1 | | Certification of Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 32.2 | | Certification of Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | |
| # | | Portions of this Exhibit were omitted and filed separately with the Secretary of the SEC pursuant to a request for confidential treatment that has been filed with the SEC. |

