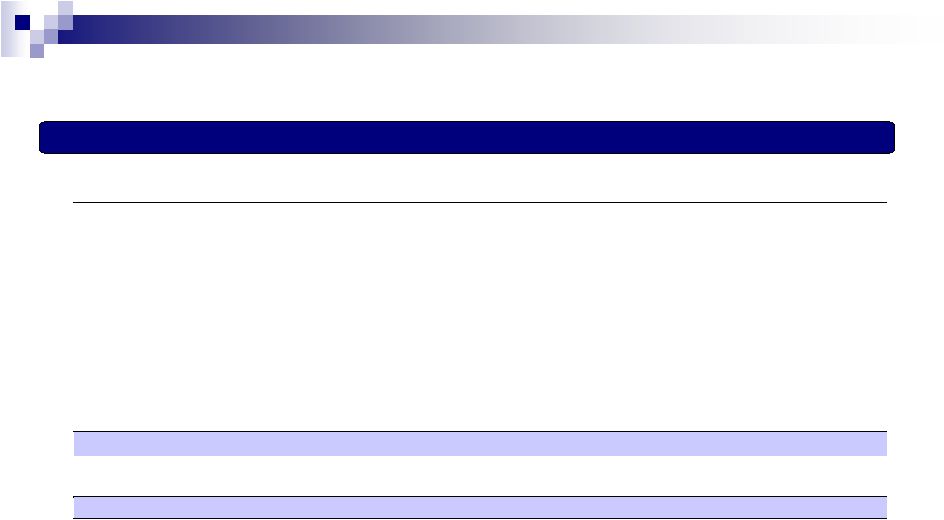
3 (a) Net Interest expense excludes amortization and write-offs of debt discounts and debt issuance costs. (b) Includes impairments of finite-lived intangible assets and amortization and write-off of debt discounts and debt issuance costs. (c) Non-cash and other charges include amortization of inventory step up and alliance product assets and PP&E step up, contingent consideration fair value adjustments, and other one-time items. Also includes a net gain of $323.9mm related to the divestiture of the filler and toxin assets in Q3 of 2014. (d) Gives pro forma effect for the historical results of acquisitions and divestitures consummated by the Company as if they occurred on January 1, 2014, including anticipated synergies the Company expects to realize within 12 months of the date of the applicable acquisition. (e) Gives pro forma effect for recent acquisition of products from Marathon Pharmaceuticals, LLC and Dendreon as if they had occurred on January 1, 2014, including the anticipated synergies of approximately $130mm related to Dendreon that the Company expects to realize within 12 months of the date of the transaction. LTM Pro Forma Adjusted EBITDA (As of December 31,2014) Valeant Adjusted EBITDA Reconciliation LTM EBITDA ($ in millions) LTM 12/31/14 Net income $912 Adjustments: Interest expense, net (a) 896 Provision (benefit) for income taxes 180 Depreciation and amortization (b) 1,808 Restructuring, integration and other costs 382 Acquisition-related costs 6 In-process research and development impairment and other charges 41 Share-based compensation expense 78 Loss on extinguishment of debt 130 Non-cash and other charges (gains) (c) (76) Adjusted EBITDA $4,357 + EBITDA and synergies from prior acquisitions / divestitures (d) 15 + EBITDA and synergies from subsequent transactions (e) 262 Pro Forma Adjusted EBITDA $4,634 |