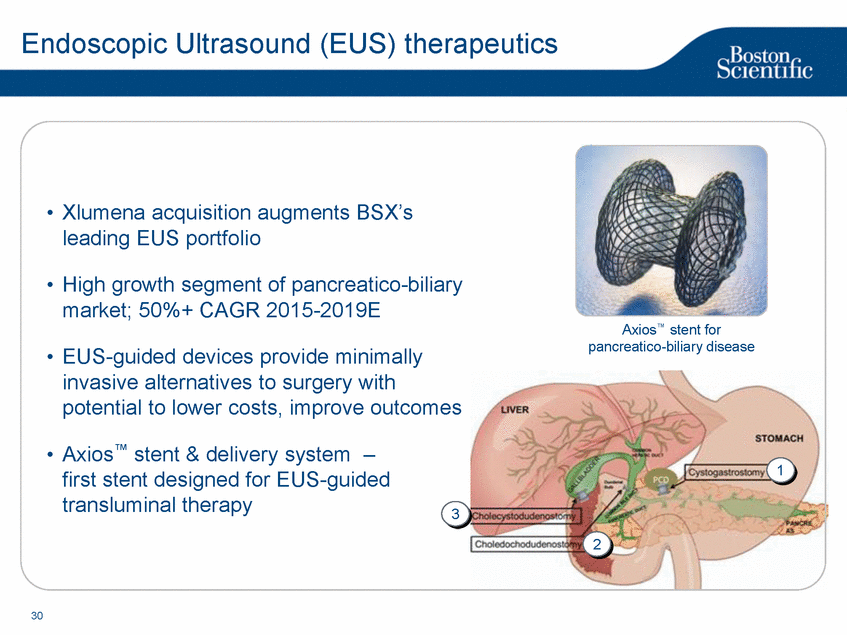
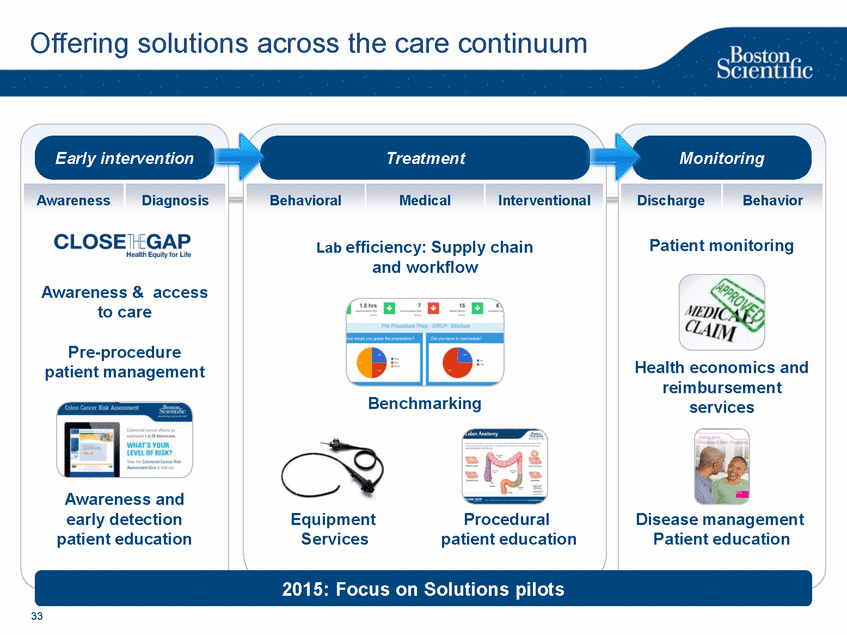
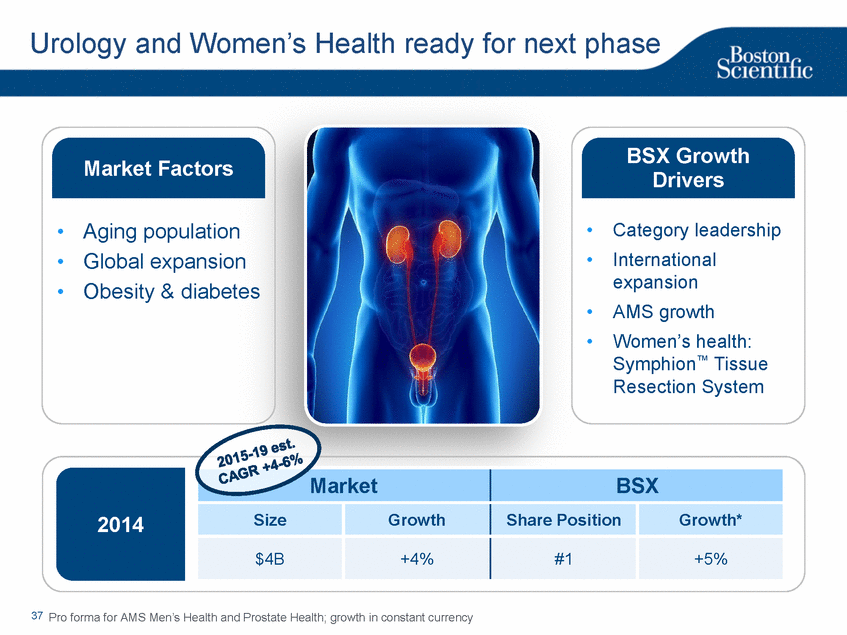
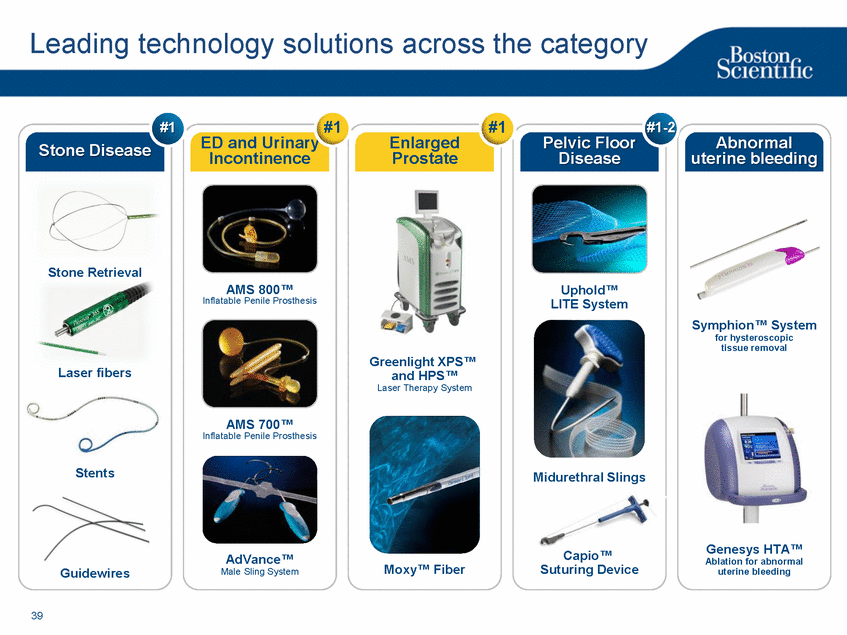
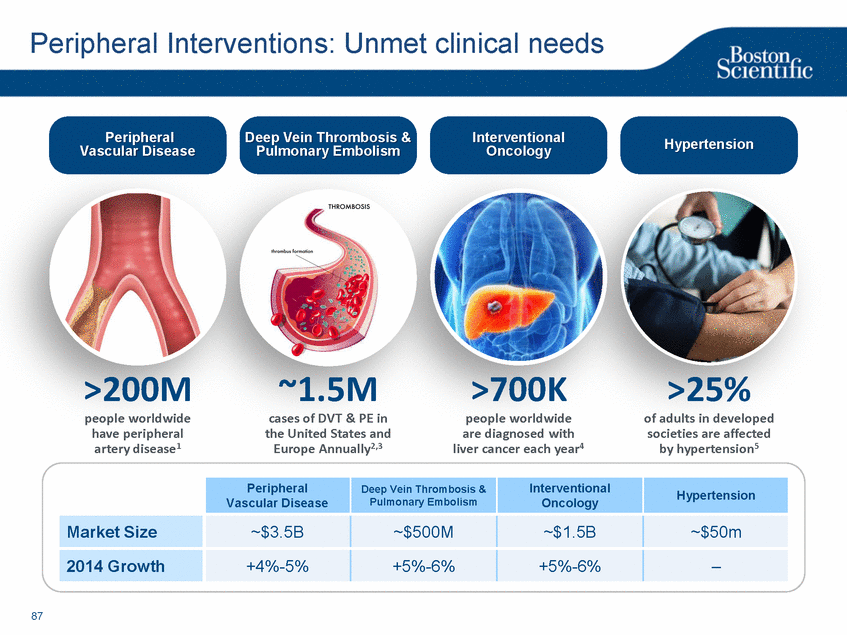
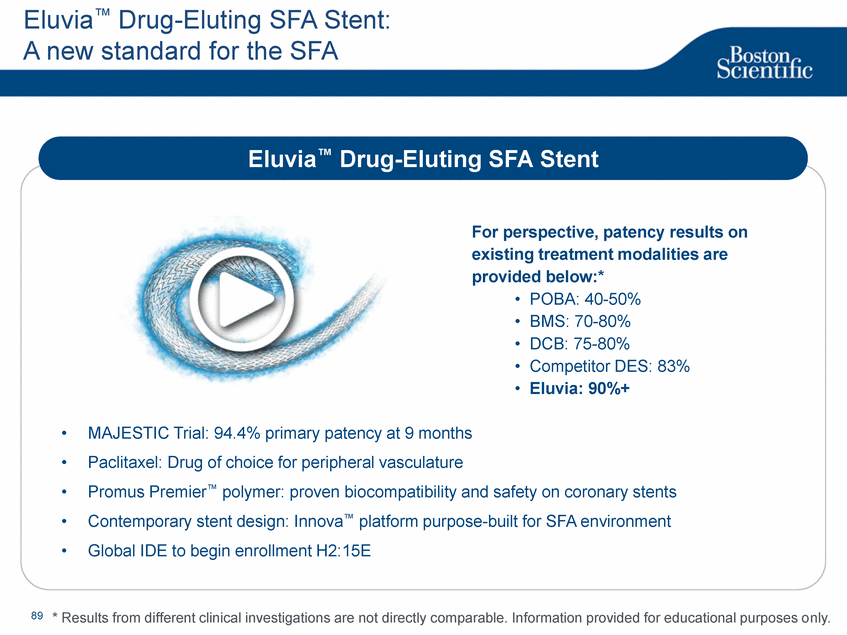
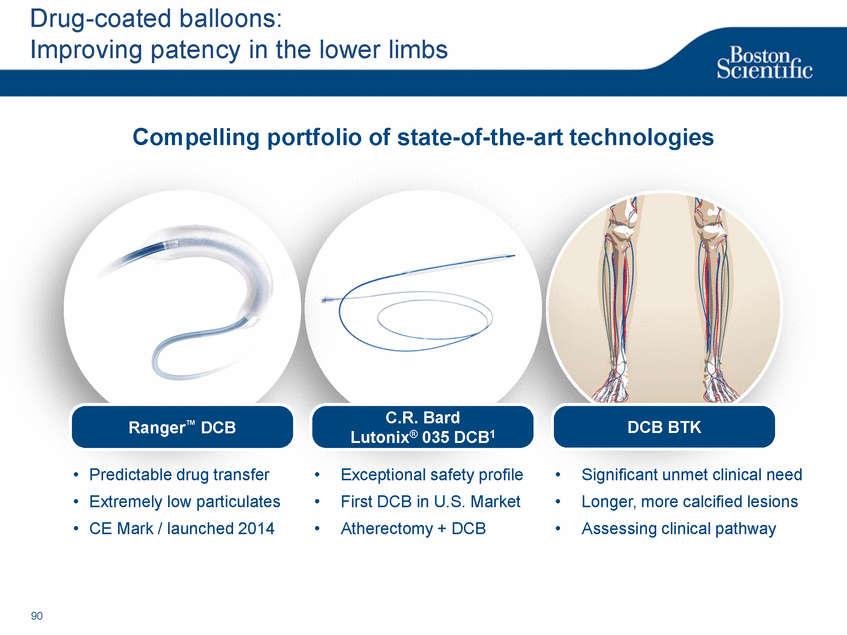
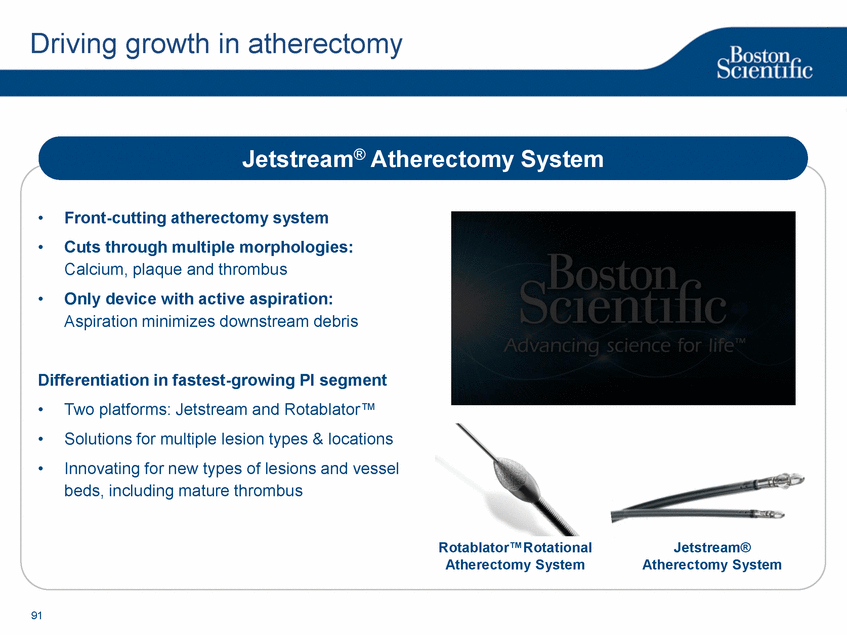
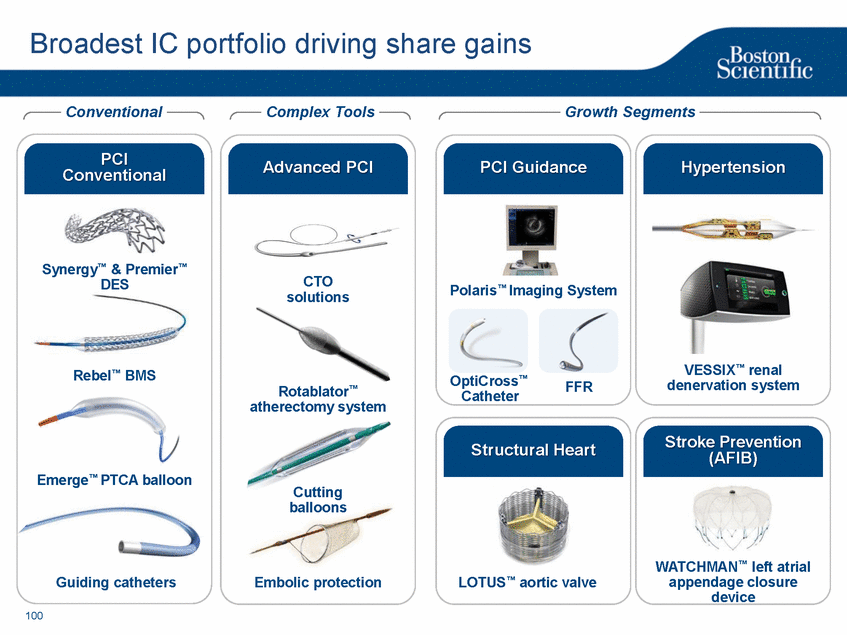
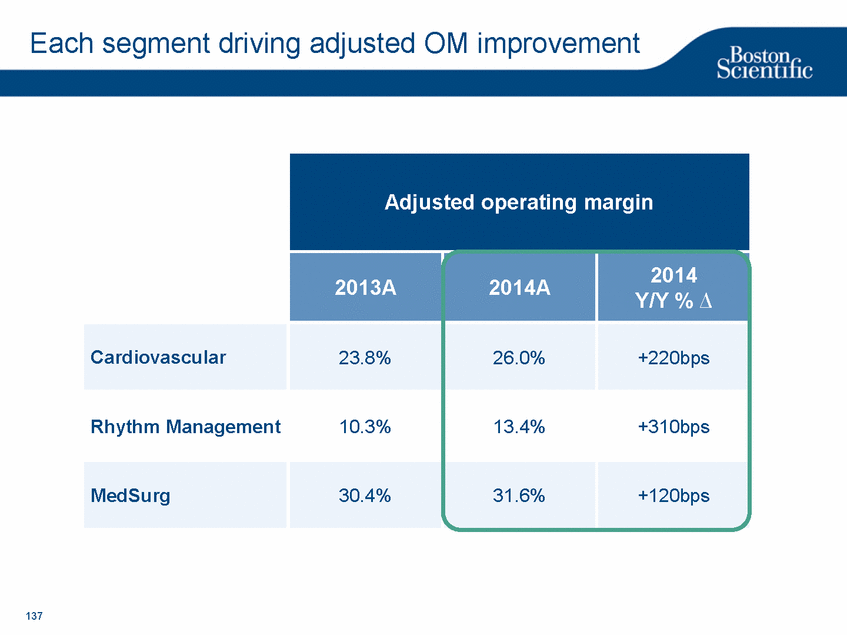
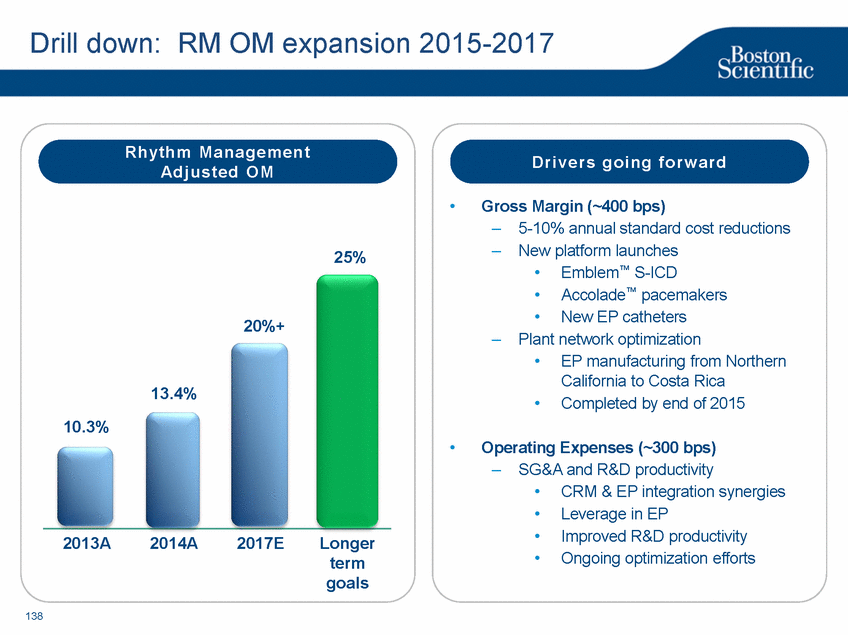
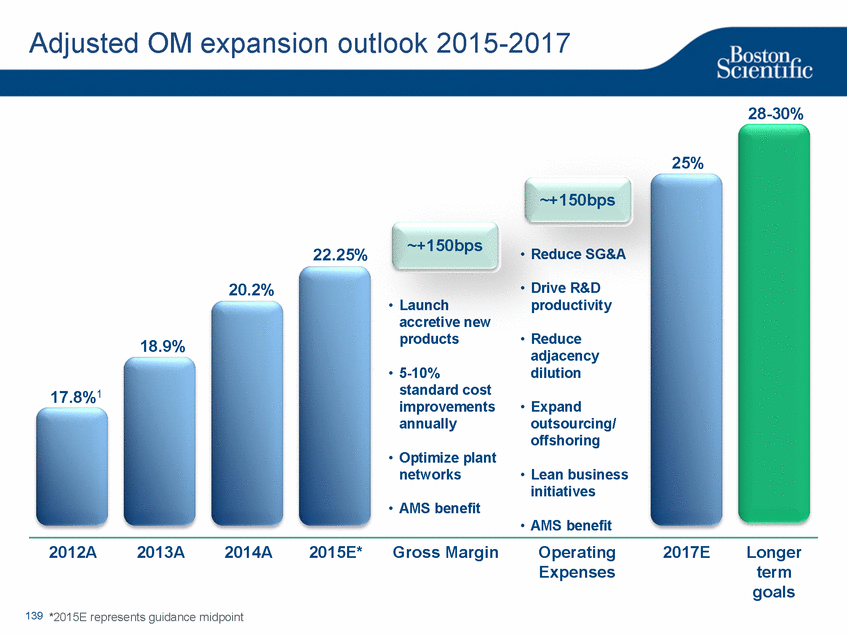
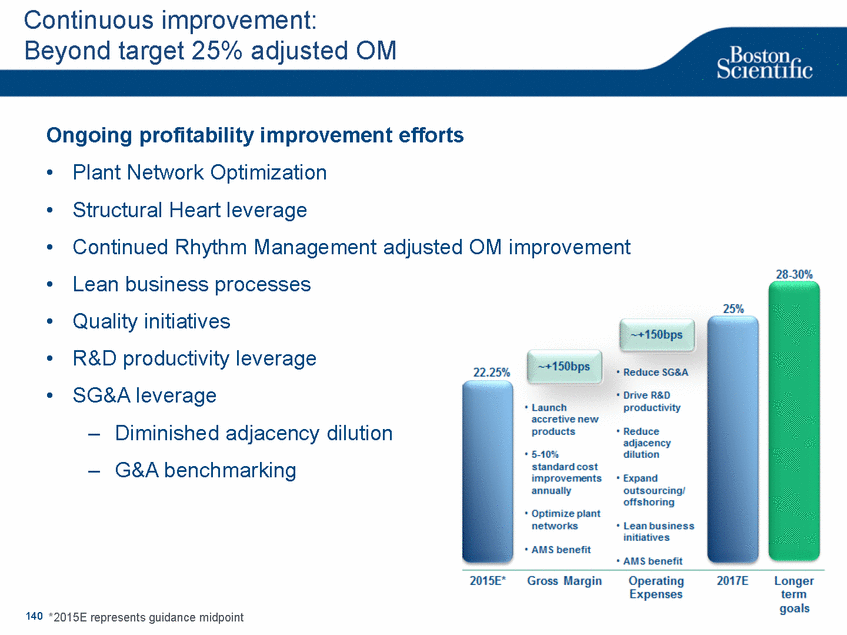
| Footnotes Slide 2: 1.Growth in constant currency. For reconciliations of non-GAAP financial measures to the most directly comparable GAAP figures, please refer to the Investor Relations section of our website at www.bostonscientific.com Slide 3: 1.BSC estimates 2.Yancy, C., Circulation AHA 4/7/08. (online before print) Slide 6: 1.Ellis C, Markus T, Dickerman D, Orton J, Hassan S, Good E, Okabe T, Greenspon A. Ampere Hour as a Predictor of CRT ICD Pulse Generator Longevity: A Multi-Center Study. Presented at HFSA 2014. http://www.onlinejcf.com/article/S1071-9164(14)00337-6/fulltext. Ampere Hour (Ah) as a Predictor of CRT ICD Pulse Generator Battery Longevity Study. The five major institutions performing the study include, at Vanderbilt University, Eastside Cardiovascular Medicine, University of Michigan, Thomas Jefferson University, Robert Wood Johnson University Hospital, Cooper Health System and North Ohio Research. Boston Scientific = 173 patients, Medtronic = 587 patients, St. Jude Medical = 153 patients. Survival rate calculated using device replacements for battery depletion as indicated by ERI. 2.J. Williams, R. Stevenson. Contemporary cardiac resynchronization implantable cardioverter defibrillator battery longevity in a community hospital heart failure cohort. Presented at HFSA 2014. http://www.onlinejcf.com/article/S1071-9164(14)00389-3/fulltext. Boston Scientific = 53 patients, Medtronic = 28 patients, St. Jude Medical = 10 patients. Survival rate calculated using device replacements for battery depletion as indicated by ERI. 3.Haarbo J, Hjortshoj S, Johansen J, Jorgensen O, Nielsen J, Petersen H. Device Longevity in Cardiac Resynchronization Therapy Implantable Cardioverter Defibrillators Differs Between Manufacturers: Data from the Danish ICD Registry. Presented at HRS 2014. http://ondemand.hrsonline.org/common/presentation-detail.aspx/15/35/1241/9000. Boston Scientific = 136 patients, Medtronic = 651 patients, St. Jude Medical = 1,587 patients, Bitronik = 369 patients. Time to exchange of the device because of battery depletion or device failure recorded in the Danish ICD Registry was the endpoint. 4.Alam M, Munir B, Rattan R, Flanigan S, Adelstein E, Jan S, Saba S. Battery Longevity in Cardiac Resynchronization Therapy Defibrillators. 2013; Europace (2013) doi: 10.1093/europace/eut301. First published online: October 6, 2013. Kaplan Meier curves depicting survival of CRT devices free from battery depletion by device manufacturer. Battery Longevity in Cardiac Medtronic = 416 patients, Boston Scientific = 173 patients, St. Jude Medical = 57 patients. Survival rate calculated using device replacements for battery depletion as indicated by ERI. 5.Boston Scientific CRM Product Performance Report, Data on file. Medtronic CRM Product Performance Report, C154DWK Concerto CRT-D DR, 2014 CDRM Product Performance eSource, data August 3, 2014. Medtronic CRM Product Performance Report, D224TRK Consulta CRT-D, 2014 CDRM Product Performance eSource, data August 3, 2014. St. Jude Medical Product Performance Report, Promote RF CRT-D, 60061673 REV_A, page 38, Last updated May 5, 2014. 6.Not intended to replace longevity estimates found in labeling. Analysis of LATITUDE Patient Management system data (data on file): From oldest 1000 VR, DR and 999 CRT-Ds as of October 2013. This distributions may be different than later groups. Data on file. Individual symptoms, situations, circumstances and results vary. This information is not intended to be used for medical diagnosis or treatment or as a substitute for medical advice. Device programming was determined by physicians. Accordingly, the aggregate average represents a mean value that is based upon real-world programming. The data reflect projected longevities based upon parameter settings, rather than observed performance. This information is a defined data set and could change in the future. The low variability may be the result of the devices still being quite young. As the devices continue to age, patient differences in pacing and other factors may cause greater variability in the Approximate Time to Explant. The LATITUDE data are assumed to be representative of the general patient population. The distribution is non-normal; therefore the standard deviation must be interpreted with care. It may not necessarily be true that ~ 95% of the data lie within standard deviations of the mean, ~99.7% within three, etc. Slide 8: 1.Burke M., et al., Safety and efficacy of a totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE Study and EFFORTLESS Registry. JACC 2015. Online April 2015. Slide 10: 1.Data on file with BSC. 74 |