Exhibit 99.2
Exhibit 99.2
REVENUE DRIVERS
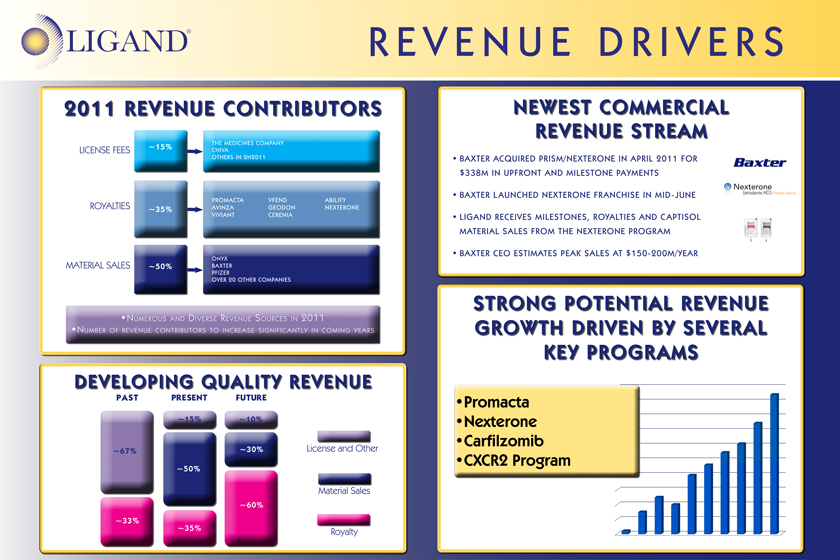
2011 REVENUE CONTRIBUTORS
LICENSE FEES ~15% THE MEDICINES COMPANY CHIVA OTHERS IN 2H2011
ROYALTIES ~ 35% PROMACTA AVINZA VIVIANT
VFEND GEODON CERENIA
ABILIFY NEXTERONE
MATERIAL SALES ~50% ONYX BAXTER PFIZER OVER 20 OTHER COMPANIES
NUMEROUS AND DIVERSE REVENUE SOURCES IN 2011
NUMBERS OF REVENUE CONTRIBUTORS TO INCREASE SIGNIFICANTLY IN COMING YEARS
DEVELOPING QUALITY REVENUE
PAST ~67% ~33%
PRESENT ~15% ~50% ~35%
FUTURE ~10% ~30% ~60%
License and Other
Material Sales
Royalty
NEWEST COMMERCIAL REVENUE STREAM
BAXTER ACQUIRED PRISM / NEXTERONE IN APRIL 2011 FOR $338m IN UPFRONT AND MILESTONE PAYMENTS
BAXTER LAUNCHED NEXTERONE FRANCHISE IN MID-JUNE
LIGAND RECEIVES MILESTONES, ROYALTIES AND CAPTISOL MATERIAL SALES FROM THE NEXTERONE PROGRAM
BAXTER CEO ESTIMATES PEAK SALES AT $150-200M/YEAR
STRONG POTENTIAL REVENUE GROWTH DRIVEN BY SEVERAL KEY PROGRAMS
Promacta
Nexterone
Carfilzomib
CXCR2 Program
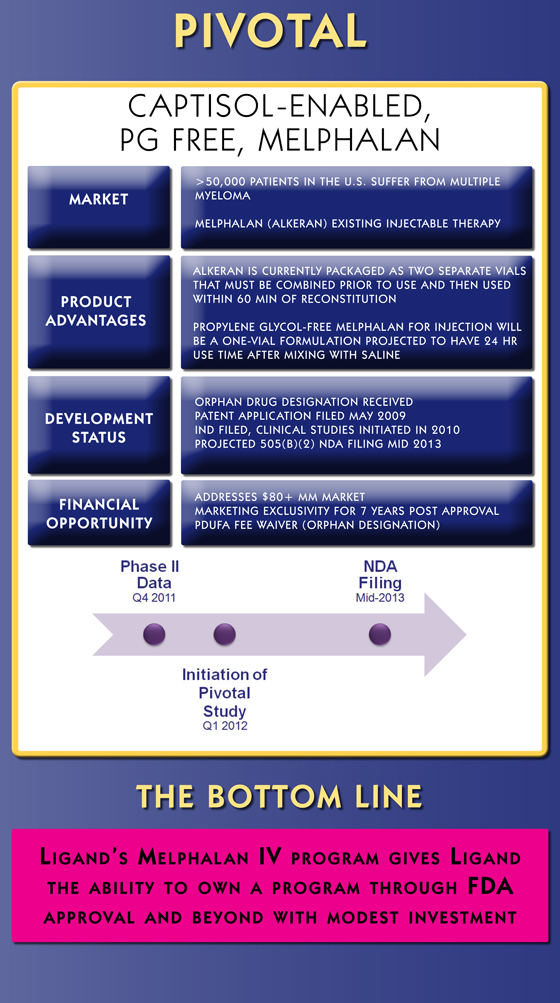
Pivotal
Captisol-EnablEd, pG FrEE, MElphal an
market
>50,000 patients in the U.s. sUffer from mUltiple myeloma
melphal an (alkeran) existing injectable therapy
Product advantages
alkeran is cUrrently packaged as two separate vials that mUst be combined prior to Use and then Used within 60 min of reconstitUtion
propylene glycol-free melphal an for injection will be a one-vial formUl ation projected to have 24 hr Use time after mixing with saline
develoPment status
orphan drUg designation received patent applic ation filed may 2009 ind filed, clinic al stUdies initiated in 2010 projected 505(b)(2) nda filing mid 2013
Financial oPPortunity
addresses $80+ mm market marketing exclUsivity for 7 years post approval pdUfa fee waiver (orphan designation)
the Bottom line
L igand ‘ s M eLphaL an iV prograM giVes L igand
the abiLity to own a prograM through Fda
approVaL and beyond with Modest investment

Phase ii ready
sarM
lgd-4033
sarm
preclinical
Supportive animal toxicity data
Unique AR binding & selective activity Muscle and bone building activity in animal models
clinical
Most potent oral SARM
Ph. I efficacy trends
Well tolerated with improved safety over anabolic steroids
commercial
Potential to address large unmet
medical needs in both specialty
& long-term muscle wasting patient
Populations
lean mass (kg) change from Baseline up to day 28
average leg Press Force (newton) change from Baseline up to day 28
the Bottom line
Ligand’s sarM prograM oFFers opportunities For new reVenue through potentiaL Licensing
PRECLINICAL
TOPICAL JAK3
target ProFile
Small-molecule inhibitors of Janus kinase 3 (JAK3) for the topical or ocular treatment or prevention of skin and eye diseases
Specific inhibition of JAK3, which is selectively expressed in immune cells, should provide a
lower potential for dose-limiting toxicity than currently available immunosuppressive drugs
Jak3 Program status
Ligand retains rights to certain JAK3 compounds developed during an alliance with Wyeth/Pfizer for use in the treatment or prevention of skin and eye diseases Multiple compounds that are potent JAK3 inhibitors (IC50 range: 0.1 – 8 nM)
Many with >10-fold selectivity vs JAK2 and other related kinases
Active in cell-based functional assays
Compounds identified that are effective systemically or topically in mouse models of JAK3
inhibition
No safety issues observed in preliminary studies (i genotoxicity, CYP inhibition, hERG
photocytotoxicity, skin irritation)
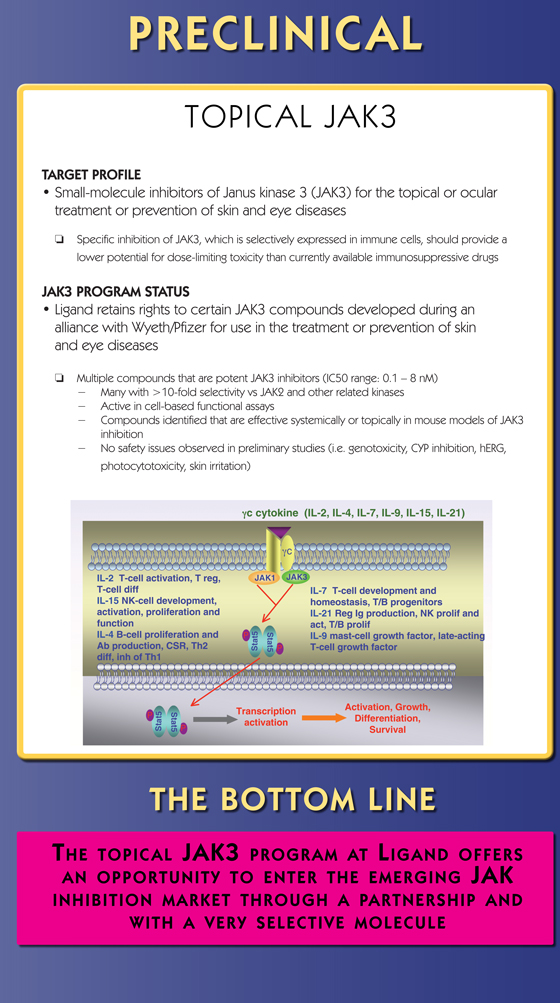
c cytokine
(IL-2, IL-4, IL-7, IL-9, IL-15, IL-21)
c
IL-2 T-cell activation, T reg,
JAK1
JAK3
T-cell diff
IL-7 T-cell development and
IL-15 NK-cell development,
homeostasis, T/B progenitors
activation, proliferation and
IL-21 Reg Ig production, NK prolif andfunction act, T/B prolif IL-4 B-cell proliferation and P
IL-9 mast-cell growth factor, late-acting
Ab production, CSR, Th2
Stat5
Stat5 P
T-cell growth factor
diff, inh of Th1
Transcription
Activation, Growth,
P Stat5
Stat5
activation
Differentiation,
P
Survival
the Bottom line
the topic aL JaK3 prograM at Ligand oFFers an opportunity to enter the eMerging JaK inhibition MarKet through a partnership and with a Very seLectiVe MoLecuLe

Discovery
Pii
Fructose BisPhosPha-tase (FBP) inhiBitor
Novel dIABeteS MeChANISM of ACtIoN ClINICAl PoC dAtA IN hANd
Preclinical
glucagon recePtor antagonist
Novel dIABeteS MeChANISM of ACtIoN heP-dIReCt dRIveN lIveR tARgetINg minimizes side-effects
discovery
dgat inhiBitor
Novel dIABeteS MeChANISM of ACtIoN PoteNtIAl foR dUAl dIABeteS/oBeSIty activity
discovery
glucokinase (gk) activator
Novel dIABeteS MeChANISM of ACtIoN lIgANd tISSUe tARgetINg teChNology
the Bottom line
Ligand s portFoLio
oF diabetes
assets
giVes
us
the
opportunity
to
engage
in
a
broad
M
etaboLic
d isease
c oLL aboration