EXHIBIT 99.1

Caraco Pharmaceutical
Laboratories, Ltd.
Safe Harbor: This news release contains forward-looking statements made pursuant to the
safe-harbor provisions of the Private Securities Litigation Reform Act of 1995. Such
statements are based on management's current expectations and are subject to risks and
uncertainties that could cause actual results to differ materially from those described in the
forward-looking statements. These risks and uncertainties are contained in the
Corporation’s filings with the Securities and Exchange Commission and include:
information of a preliminary nature that may be subject to adjustment, potentially not
obtaining or delay in obtaining FDA approval for new products, governmental restrictions
on the sale of certain products, dependence on key personnel, development by
competitors of new or superior products or cheaper products or new technology for the
production of products, the entry into the market of new competitors, market and customer
acceptance and demand for new pharmaceutical products, availability of raw materials,
timing and success of product development and launches, integrity and reliability of the
Corporation’s data, compliance with regard to regulatory and cGMP managing our recent
rapid growth and anticipated future growth, occasional credits to certain customers
reflecting price reductions on products previously sold to them and still available as a
shelf-stock adjustment, possibility of an incorrect estimate of charge-backs and the impact
of such an incorrect estimate on net sales, gross profit and net income, dependence on
few products generating majority of sales, product liability claims for which the Company
may be inadequately insured, subjectivity in judgment of management in applying certain
significant accounting policies derived based on historical experience, terms of contracts,
accounts receivable allowances including chargebacks, rebates, income taxes, values of
assets and inventories, litigation involving claims of patent infringement, litigation involving
claims for royalties, and other risks identified in this report and identified from time to time
in our reports and registration statements filed with the Securities and Exchange
Commission. These forward-looking statements represent our judgment as of the date of
this report. We disclaim, however, any intent or obligation to update our forward-looking statements.
Caraco Overview
What sets us apart from the pack?
Size of our Product Portfolio vs. the Size of
Company
Experienced Management Team
Vertically Integrated via Sun API’s
Disciplined Management
Ability to Service our Customers’ Immediate Needs
Capable of Creating Markets that Previously did not
Exist
Caraco Overview
What sets us apart from the pack?
Low Cost Production
Size Plays to our Advantage
Ability to Penetrate the Market
Building to fit the Current Environment
Improved Customer Mix
Removal of Barriers, Execution
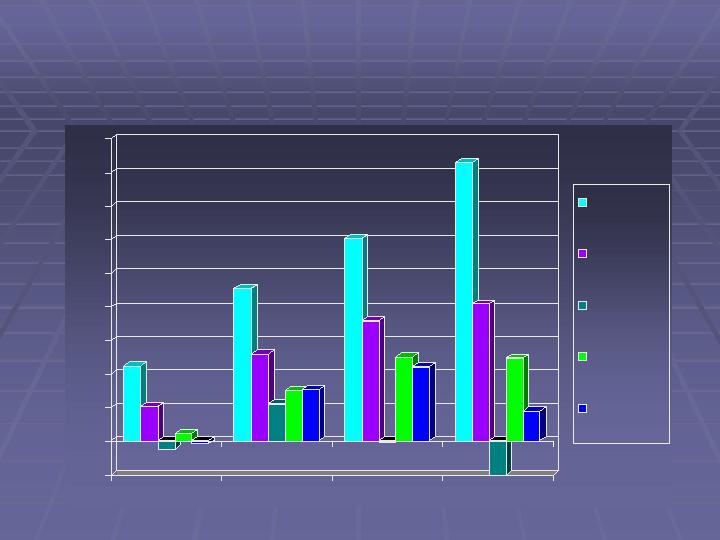
Caraco Pharmaceutical Laboratories, LTD
Annual Historical Results (in US $ millions)
22
10
(2)
2
(1)
46
26
11
15
16
60
36
(0)
25
22
83
41
(10)
25
9
($10)
$0
$10
$20
$30
$40
$50
$60
$70
$80
$90
2002
2003
2004
F2006*
Net Sales
Gross Profit
Net Income
Net Cash
Income
Cash Flow from
operations
*Change in fiscal year

Caraco Pharmaceutical Laboratories, LTD
Quarterly Analysis (in US $ millions)
20
10
(5)
6
(1)
21
10
(1)
7
5
25
13
(6)
8
4
25
13
5
10
7
28
14
2
10
8
($6)
($1)
$4
$9
$14
$19
$24
$29
Q2-F2006
Q3-F2006
Q4-F2006
Q1-F2007
Q2-F2007
Net Sales
Gross Profit
Net Income
Net Cash
Income
Cash Flow
From
Operations
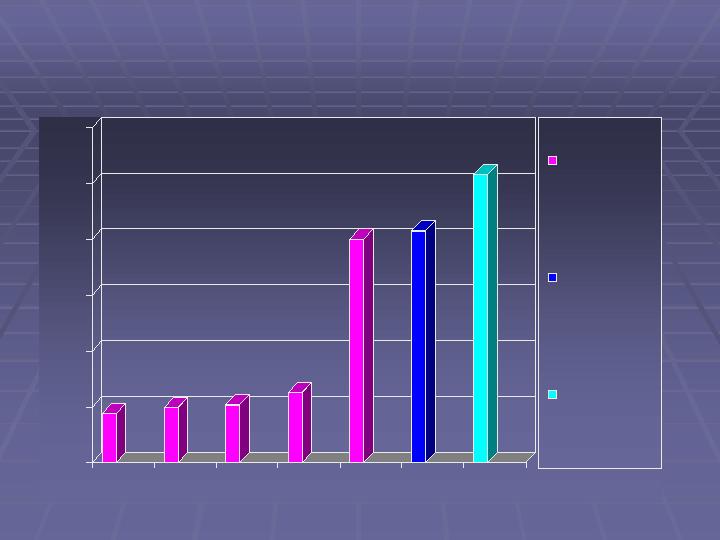
Caraco Pharmaceutical Laboratories, LTD
SALES for Fiscal 2006
(in US $ millions)
18
20
21
25
80
83
103
$0
$20
$40
$60
$80
$100
$120
Actual Q1
F2006
Actual Q2
F2006
Actual Q3
F2006
Actual Q4
F2006
Forecast
F2006
Actual
F2006*
Forecast
F2007
Net Sales
Represents 40%
growth over
Calendar 2004
Represents 25%
growth over
Fiscal 2006
* Change in fiscal year
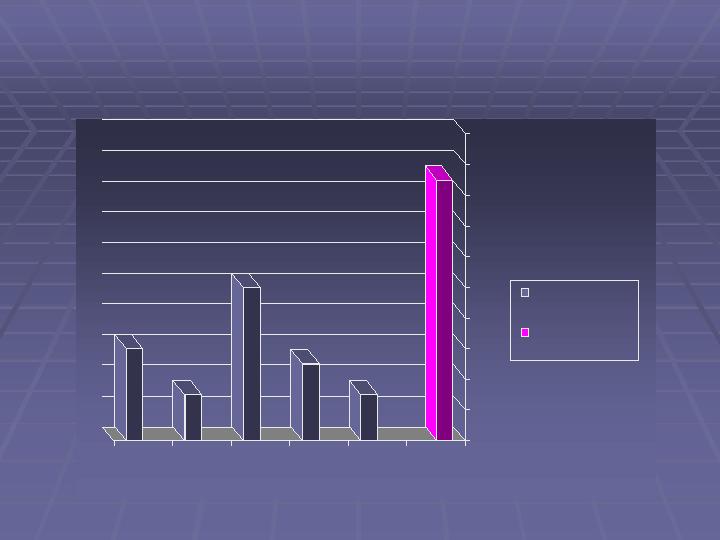
Caraco Pharmaceutical Laboratories, LTD
Product Development Quarterly Filings (ANDAS)
17
3
5
10
3
6
0
2
4
6
8
10
12
14
16
18
20
Y 2004
QT 2005
Fiscal 2006
YTD Fiscal
2007
2Q-F2007
Number of ANDA
Filings
ANDAs Awaiting
Approval*
*Includes two tentative FDA approvals

Caraco Pharmaceutical Laboratories, LTD
Product Market Share Trends
Caraco is Currently Marketing 26 Generic
Pharmaceuticals and One Brand Product
Three of the 26 Products were Launched in the
Second Quarter of Fiscal 2007
Eight of the 26 Products are in the Top Three of
Generic Market Share
Tramadol with Acetaminophen (generic Ultracet®)
was the Fastest Ramp up in Market Share in
Caraco’s History Representing Approximately 18.5%
in Three Months
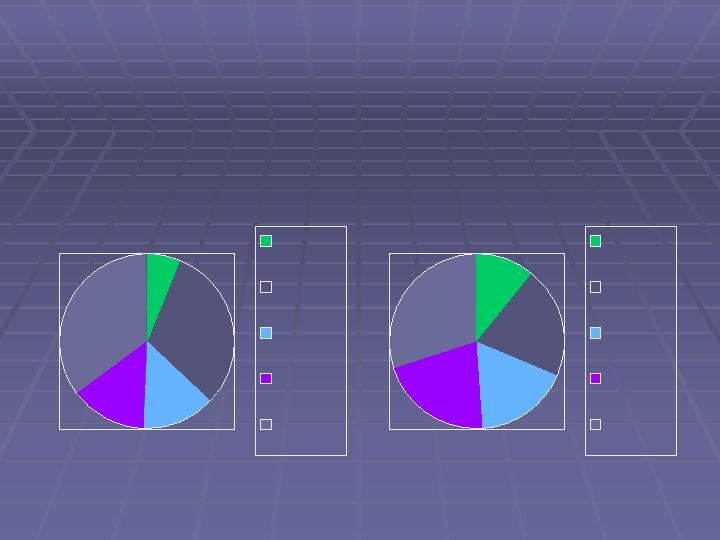
Caraco Pharmaceutical Laboratories, LTD
Net Sales by Trade Class
July 2005 - September 2005
Q2-F2006
Chain -
1.27MM
Dist -
6.05MM
Whole -
2.68MM
Mg Care -
2.82MM
Govt -
6.95MM
July 2006 - September 2006
Q2-F2007
Chain -
3.06MM
Dist -
5.81MM
Whole -
4.97MM
Mg Care -
5.91
Govt -
8.48MM

Caraco Pharmaceutical Laboratories, LTD
Net Sales by Trade Class
0
1
2
3
4
5
6
7
8
9
$ in millions
July-Sept 2005
(2Q-F2006)
July-Sept 2006
(2Q-F2007)
Chain
Dist
Whole
Mg Care
Govt
Growth Percentages
Chain : 140.9%
Dist : - 4.0%
Whole : 85.4%
Mg Care : 109.6%
Govt : 22.0%

Second Quarter Fiscal 2007
Highlights
Recognized by One of the Big Three
Wholesalers in US as Best Trade
Representative for Companies Under $100
Million in Sales with that Wholesaler
Acquired Packaging Facility
Signed Definitive Agreement with New Product
Development Partner
Second Quarter Fiscal 2007
Highlights
Launched Three Products - Baclofen, Meloxicam and
Glipizide
Filed Three Products with FDA Bringing Total
Products Awaiting Approval to 17 Including Two
Tentative Approvals
Continued Profitability (with stock transfer for
two products versus 1 last quarter)
Record Production 298 Million Tablets During
October

Second Quarter Fiscal 2007
Highlights
Second Quarter Net Sales Growth of 43%
Year-over-Year (YTD 42%)
Gross Profit 50%
Successive Quarterly Growth of 14%
Expansion
Current facility is 80,000
sq ft and lease another
62,000 sq ft for total
footprint of 142,000 sq ft
Need to build 80,000 -
100,000 sq ft facility or
move to 250,000 sq. ft
cGMP facility for
complete company move
Fiscal 2007 forecast is for
an average of 263 million
tablets per month. Need to
average 400 million tablets
per month to support
growth
6 acre parcel approved by
city to acquire
Ability to expand in
increments as cash flow
dictates
Can expand up to 450,000
sq ft at current sites

Accomplishments Fiscal 2006
Increased Training in cGMP
Award of Top 500 Fastest Growing
Technology Companies Ranked 96th by
Deloitte & Touche
Award of Future 50 Representing the top 50
Growth Companies in Metro Detroit Based on
Sales and Employment
Accomplishments Fiscal 2006
Filed 10 Products with the FDA in Fiscal 2006
Surpassed our Net Sales Forecast for 2006 by
Almost $3 Million
Increased Manufacturing Capacity by 35%
Maintained Gross Profit of 49% which is Above the
Generic Industry Average
Developed Clozapine Registry Web site for
Electronic Patient Monitoring Information

Caraco Pharmaceutical Laboratories, LTD
Clozapine Sales Update & Web Registry
Development
Caraco successfully launched
www.caracoclozapine.com on March
20th 2006
New confirmed customer
commitments
Distributor Class of Trade
Large distributor buying group
Large managed care (HMO) player
Two of Big Three Wholesale Source
Programs
Large mass merchandiser
New customers in process
Mid size chain
Small to midsize buying groups
Product Litigation
Lexapro – Teva Lost Infringement Case and is Set to
Appeal; Possible Upside if Caraco Prevails
Prandin – First to File Position
Provigil – Determine Next Steps to Monetize
Ultracet – Ortho Filed Suit Based on New Patent
Effective August 1st which we Certified we do not
Infringe; Original Summary Judgment is Under
Appeal

Drivers Fiscal 2007
Approvals - Baclofen, Meloxicam, Glipizide,
Full-Year Tramadol with Acetaminophen
Approvals Pending
Increase / Maintain Share
Increase and Close Alternate Development
Projects

Drivers Fiscal 2007
Expand Current Development Streams and
Pursue New Opportunities
Expansion of Current Facilities
Improve Packaging
Continue Staff Improvements and Succession
Planning

CARACO
Thank You