Product Litigation
Lexapro - Forest has two U.S. patents listed with the FDA that they claim cover Lexapro, the ‘712 patent and the ‘941 patent. We filed an ANDA in which we certified to the noninfringement and/or invalidity of both patents. On July 10, 2006, Forest sued us on the ‘712 patent in federal court in Detroit, Michigan. On February 23, 2007, the court partially stayed discovery pending a decision in the U.S. Court of Appeals for the Federal Circuit in a related case in federal court in Delaware. The Delaware case involved Forest’s assertion of the ‘712 patent against Ivax Pharmaceuticals. On December 3, 2007, after the appellate court affirmed the Delaware district court’s finding that the ‘712 patent was valid and infringed by Ivax, the Detroit judge issued a ruling allowing discovery to proceed in our case. Accordingly, discovery is now ongoing.
We also filed a separate case challenging the ‘941 patent on the grounds of noninfringement. On April 23, 2007, Forest granted us a covenant not to sue on the ‘941 patent and, based upon that covenant, the Detroit court dismissed the case for lack of a controversy. For strategic reasons, we appealed. On April 1, 2008, the Federal Circuit reversed, finding that the covenant not to sue did not moot the controversy between the parties and that we could therefore proceed with our lawsuit. Forest subsequently filed a motion for en banc review of the decision, which was denied on June 24, 2008. The case is in the process of being returned to the district court.
Prandin – Novo Nordisk filed suit against us in Michigan for patent infringement. FDA granted tentative approval on August 13, 2007. The parties are in discovery. No trial date has been set.
Ultracet - Ortho originally filed suit in Michigan for patent infringement. We certified to noninfringement and obtained summary judgment of noninfringement. This decision was recently affirmed by the Federal Circuit. Our generic version of Ultracet is presently on the market. Ortho subsequently filed for a reissue patent, and again sued for patent infringement in New Jersey. We certified to invalidity of the reissue patent and filed another motion for summary judgment. On April 18, 2008, the United States District Court of New Jersey granted our motion for summary judgment that the claims of the reissue patent are invalid. Ortho has moved for entry of final judgment so that it can appeal the district court’s decision.
Clarinex - Schering filed suit in both New Jersey and Michigan for patent infringement against us and Sun. Sun certified to noninfringement and invalidity. We filed a motion to dismiss for lack of subject matter jurisdiction because Sun is the ANDA filer, not us. Schering also filed suit against several other ANDA filers. Each of these proceedings has been consolidated in New Jersey for pretrial proceedings. Fact discovery is ongoing. No trial date has been set.

Capacity
At the start of 2007, facilities included 114,000 sq ft owned and 67,000 sq ft
leased for total footprint of approximately 180,000 sq ft
2008 additions
Acquired and opened 135,000 sq ft distribution warehouse
Expansion of 140,000 sq ft to be occupied approximately November 1,
2008 using redevelopment incentives from the City of Detroit and State of
MI
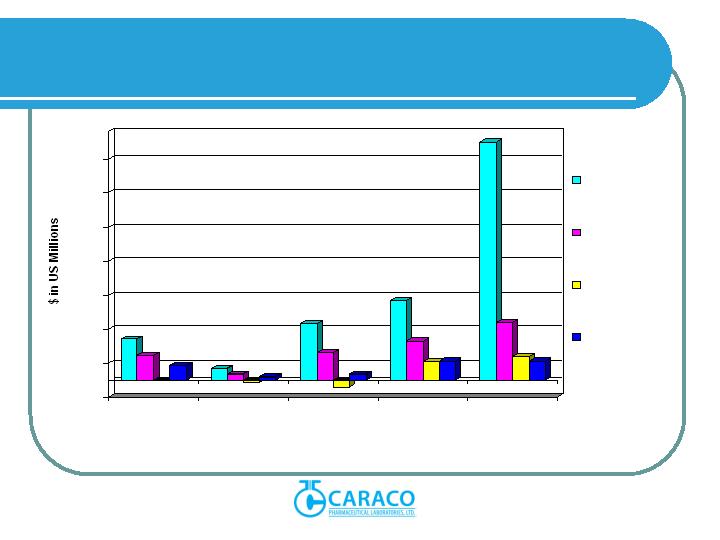
Fiscal 2008 production was approximately 300 million tablets per month, up
from Fiscal 2007 production of approximately 270 million tablets per month.
Currently running two shifts, five days a week and partial shifts on weekends
Manual equipment being replaced by automatic equipment as we grow for better
throughput

Contents
I.
Industry Overview
II.
Caraco Overview
III.
Fiscal 2008 in Review
IV.
Fiscal 2009 Initiatives & Drivers
V.
Summary
Initiatives Fiscal 2009
Quality
Continue to focus and improve on FDA compliance.
Increase cGMP training to accommodate growing staff
and compliance.
Management
Increase focus on succession planning.
Increase management training and development.
Initiatives Fiscal 2009
Research & Development
Increase research and development activities, with a view
to increase the number of ANDA filings.
Research possible development of brands for existing
stream of products where such potential exists.
Sales
Increased market share for certain existing products and
recently introduced products.
Enhanced customer reach and satisfaction.
Initiatives Fiscal 2009
Capacity and Efficiency
Continue to invest in equipment and facilities to
expand capacity to meet requirements of projected
short and long-term growth while improving quality.
Build or lease new facilities to meet the increased
demand for production and warehousing in short and
long term.
Achieving further operational efficiencies by attaining
economies of scale and cost reduction per unit.
Initiatives Fiscal 2009
Business Opportunities
Increase revenue and cash by marketing ANDAs
owned by Sun Pharma and other third parties.
Expand our relationships with financial institutions to
fortify our credit position and borrowings if necessary.
Look for potential acquisitions that either complement
or are synergistic to our current business model.
Research alternate product development sources and
product licenses

Drivers Fiscal 2009
FDA product approvals
Maximize market share on current portfolio
Improve operational execution
Streamline processes and reduce operational costs
Contents
I.
Industry Overview
II.
Caraco Overview
III.
Fiscal 2008 in Review
IV.
Fiscal 2009 Initiatives & Drivers
V.
Summary

Summary
We are Built to Fit the Current Competitive Environment
Size and management structure conducive to success
Large basket of products awaiting FDA approval
Vertical integration with Sun allows us to enjoy longer lifecycles and value
on products that we market
Increasing capacity
Focus on Quality