Exhibit 99.2

FOCUSED ON NEW THERAPIES FOR ONCOLOGY
Annual Meeting of Stockholders
September 2006
Forward Looking Statements
This presentation includes forward-looking statements that reflect Somanta’s current views with respect to future events and financial performance. The words “believe,” “expect,” “intend”, “plan”, “will”, “plan”, “may”, “anticipate,” and similar expressions identify forward-looking statements. Forward-looking statements are subject to a variety of risks, uncertainties, and other factors that could cause actual results to differ materially from those expressed in any such forward-looking statements. These factors include, but are not limited to: (1) the ability to successfully complete development and commercialization of products, including the cost, scope and results of preclinical and clinical testing; (2) the ability to successfully complete product research and further development, including preclinical and clinical studies; (3) the time, cost and uncertainty of obtaining regulatory approvals; (4) the ability to obtain substantial additional funding; (5) the ability to develop and commercialize products before competitors; and (6) other factors detailed from time to time in filings with the Securities and Exchange Commission and in particular Somanta’s most recently file 10-KSB.
Formation of Somanta
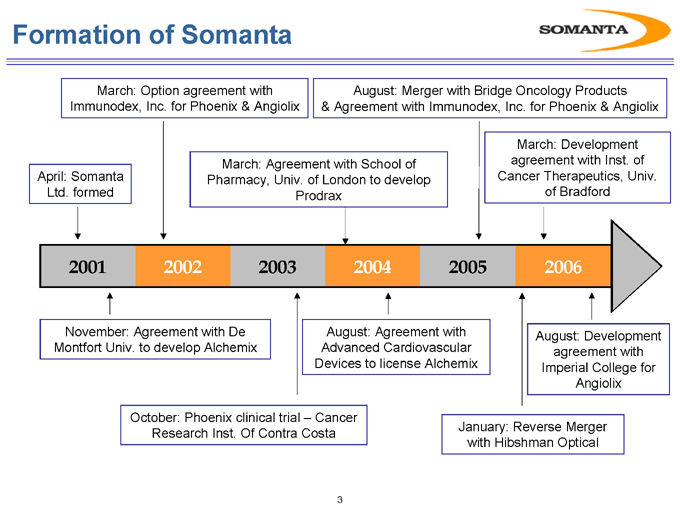
March: Option agreement with Immunodex, Inc. for Phoenix & Angiolix
August: Merger with Bridge Oncology Products & Agreement with Immunodex, Inc. for Phoenix & Angiolix
April: Somanta Ltd. formed
March: Agreement with School of Pharmacy, Univ. of London to develop Prodrax
March: Development agreement with Inst. of Cancer Therapeutics, Univ. of Bradford
2001 2002 2003 2004 2005 2006
November: Agreement with De Montfort Univ. to develop Alchemix
August: Agreement with Advanced Cardiovascular Devices to license Alchemix
August: Development agreement with Imperial College for Angiolix
October: Phoenix clinical trial – Cancer Research Inst. Of Contra Costa
January: Reverse Merger with Hibshman Optical

Results of Annual Meeting
Re-election of Board
Michael Ashton – retired CEO of SkyePharma, Chair of Nominating and Corporate Governance Committee, member of Audit and Compensation Committees
Terrance Bruggeman – Executive Chairman and CFO
Jeffrey Davis – President of SCO Financial Group, Chair of Compensation Committee and member of Audit Committee
Agamemnon Epenetos MD PhD- President and Chief Executive Officer
John Gibson MD – Senior VP, Global Development of Allergan. Member of Compensation Committee and Nominating Committee
Kathleen Van Sleen – Principal REventures and former CFO of Diversa. Chair of Audit Committee and member of Nominating Committee
Ratification of Stonefield Josephson as independent registered public accountants for fiscal year ended April 30, 2007
Experienced Management
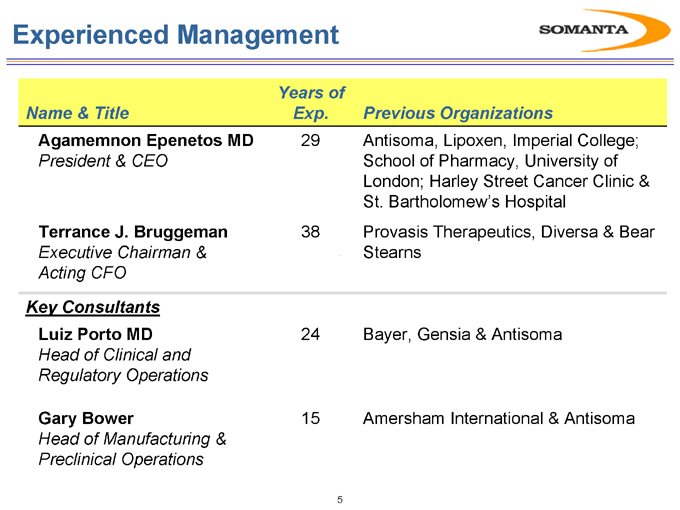
Name & Title
Agamemnon Epenetos MD
President & CEO
Terrance J. Bruggeman
Executive Chairman & Acting CFO
Key Consultants
Luiz Porto MD
Head of Clinical and Regulatory Operations
Gary Bower
Head of Manufacturing & Preclinical Operations
Years of Exp.
29 38 24 15
Previous Organizations
Antisoma, Lipoxen, Imperial College; School of Pharmacy, University of London; Harley Street Cancer Clinic & St. Bartholomew’s Hospital Provasis Therapeutics, Diversa & Bear Stearns
Bayer, Gensia & Antisoma
Amersham International & Antisoma
Scientific Advisory Board
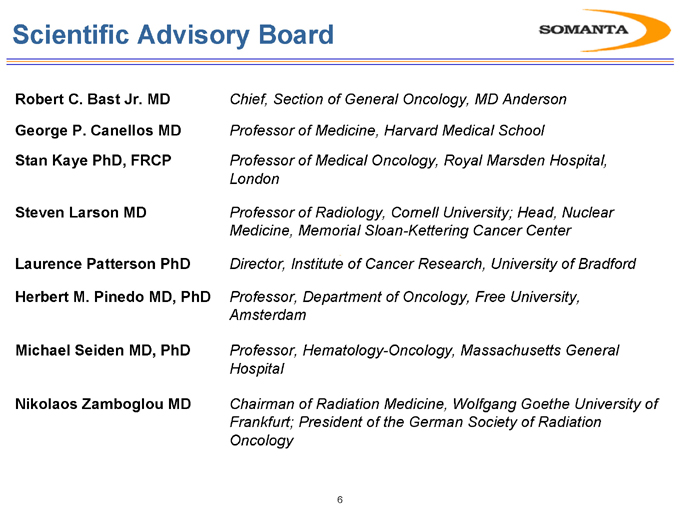
Robert C. Bast Jr. MD
George P. Canellos MD Stan Kaye PhD, FRCP
Steven Larson MD
Laurence Patterson PhD
Herbert M. Pinedo MD, PhD
Michael Seiden MD, PhD
Nikolaos Zamboglou MD
Chief, Section of General Oncology, MD Anderson
Professor of Medicine, Harvard Medical School
Professor of Medical Oncology, Royal Marsden Hospital, London
Professor of Radiology, Cornell University; Head, Nuclear Medicine, Memorial Sloan-Kettering Cancer Center
Director, Institute of Cancer Research, University of Bradford
Professor, Department of Oncology, Free University, Amsterdam
Professor, Hematology-Oncology, Massachusetts General Hospital
Chairman of Radiation Medicine, Wolfgang Goethe University of Frankfurt; President of the German Society of Radiation Oncology
Cancer Therapeutic Market
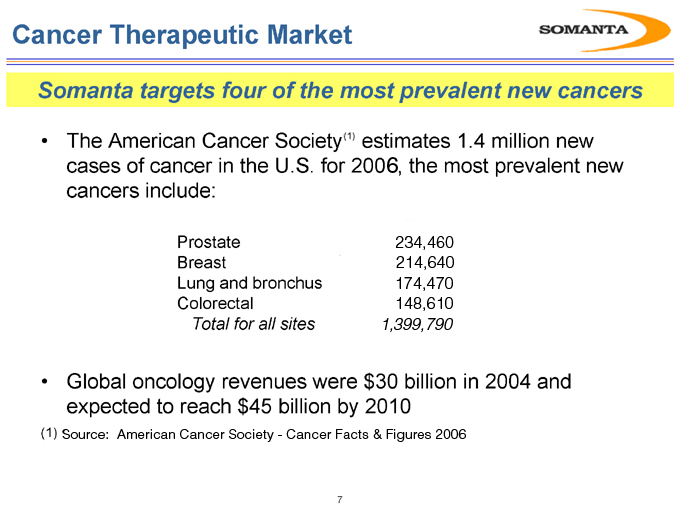
Somanta targets four of the most prevalent new cancers
The American Cancer Society(1) estimates 1.4 million new cases of cancer in the U.S. for 2006, the most prevalent new cancers include:
Prostate 234,460
Breast 214,640
Lung and bronchus 174,470
Colorectal 148,610
Total for all sites 1,399,790
Global oncology revenues were $30 billion in 2004 and expected to reach $45 billion by 2010
(1) | | Source: American Cancer Society—Cancer Facts & Figures 2006 |
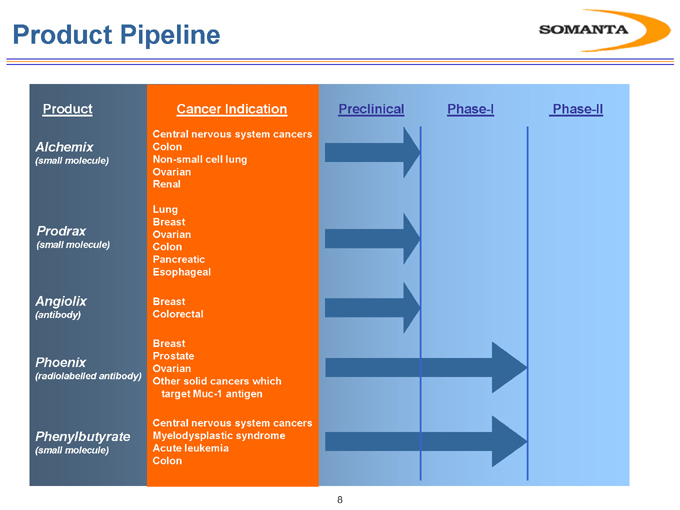
Product Pipeline
Product
Alchemix
(small molecule)
Prodrax
(small molecule)
Angiolix
(antibody)
Phoenix
(radiolabelled antibody)
Phenylbutyrate
(small molecule)
Cancer Indication
Central nervous system cancers Colon Non-small cell lung Ovarian Renal
Lung Breast Ovarian Colon Pancreatic Esophageal
Breast Colorectal
Breast Prostate Ovarian
Other solid cancers which target Muc-1 antigen
Central nervous system cancers Myelodysplastic syndrome Acute leukemia Colon
Preclinical Phase-I Phase-II
Alchemix Overview
Overcoming drug resistance in chemotherapy
Novel small molecule highly toxic to cancer cells
Interrupts cancer cell growth cycle by inhibition of DNA processing enzymes (Topoisomerase-II) that are required for cancer cells to multiply
Combine anti-cancer mechanisms of known cytotoxics such as cisplatin & doxorubicin
Excellent anti-tumor activity in doxorubicin and cisplatin resistant cell lines shown during in vitro and in vivo testing
Well tolerated in animals – similar to other approved chemotherapeutic drugs
Plan to optimize molecule, develop through Phase-II and then partner
9
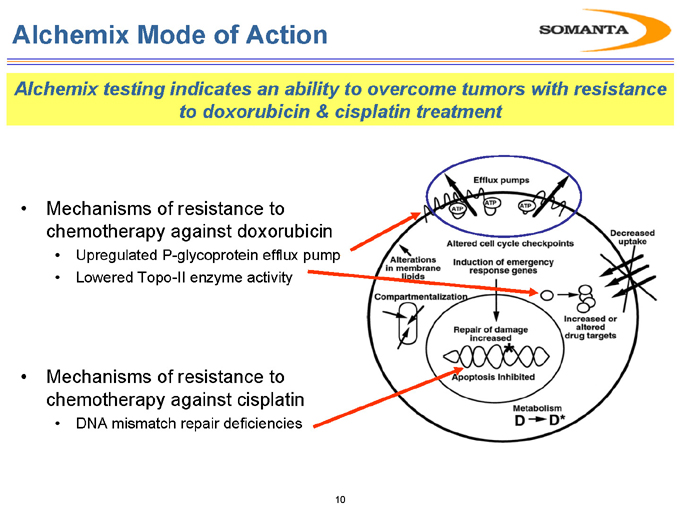
Alchemix Mode of Action
Alchemix testing indicates an ability to overcome tumors with resistance to doxorubicin & cisplatin treatment
Mechanisms of resistance to chemotherapy against doxorubicin
Upregulated P-glycoprotein efflux pump
Lowered Topo-II enzyme activity
Mechanisms of resistance to chemotherapy against cisplatin
DNA mismatch repair deficiencies
10

Prodrax Overview
Taking cancer’s strength and turning it into its weakness
A family of small molecule “prodrugs” which are inactive in normally oxygenated cells but convert to highly toxic DNA binding agents in hypoxic tumors
Estimated that over 50% of all solid tumors exhibit clinically significant hypoxic areas
Radiation is markedly less effective in hypoxic areas and conventional chemotherapy has more limited penetration
Plan to optimize molecule, develop through Phase-II and then partner
11
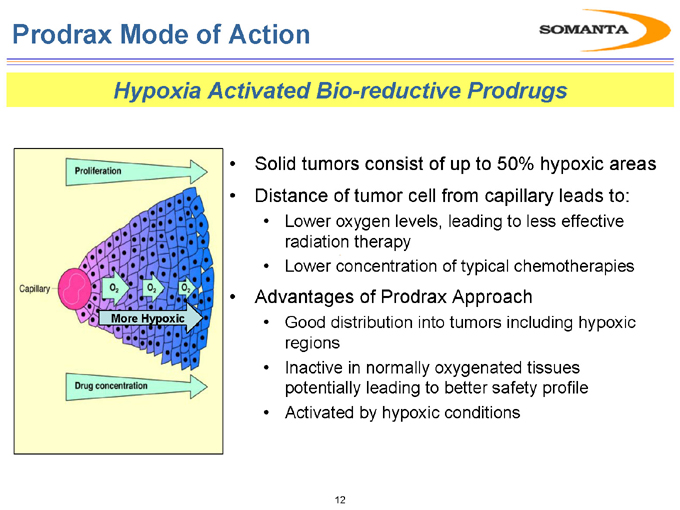
Prodrax Mode of Action
Hypoxia Activated Bio-reductive Prodrugs
Solid tumors consist of up to 50% hypoxic areas
Distance of tumor cell from capillary leads to:
Lower oxygen levels, leading to less effective radiation therapy
Lower concentration of typical chemotherapies
Advantages of Prodrax Approach
Good distribution into tumors including hypoxic regions
Inactive in normally oxygenated tissues potentially leading to better safety profile
Activated by hypoxic conditions
12
Angiolix Overview
Targeting Angiogenesis
A humanized monoclonal antibody (huMc3) that targets Lactadherin
Lactadherin is a protein expressed in and around blood vessels of tumors and has a critical role in promoting the growth of new blood vessels to support tumor growth
Angiolix binds to Lactadherin and inhibits formation of new blood vessels, blocking blood supply to the tumor and causing the tumor to die
Angiolix has similar effects to Avastin® which targets VEGF instead of Lactadherin
No other product against Lactadherin for the treatment of cancer can be developed without a sub-license from Somanta
Plan to develop through Phase-II and then partner
13
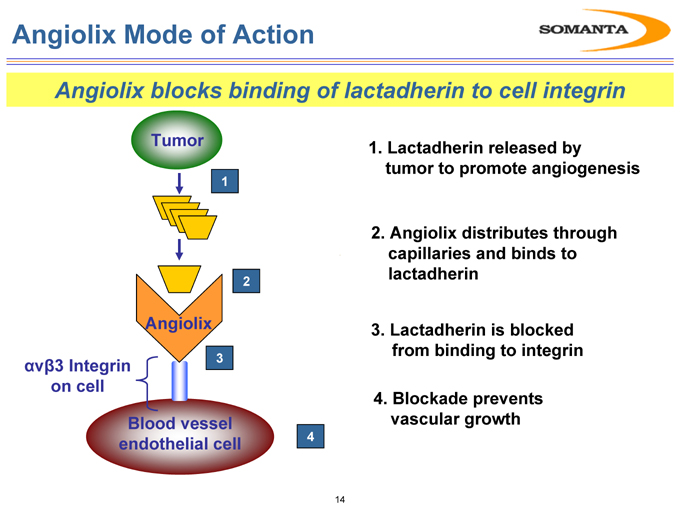
Angiolix Mode of Action
Angiolix blocks binding of lactadherin to cell integrin
Tumor
Angiolix
?v?3 Integrin on cell
Blood vessel endothelial cell
1. Lactadherin released by tumor to promote angiogenesis
2. Angiolix distributes through capillaries and binds to lactadherin
3. Lactadherin is blocked from binding to integrin
4. Blockade prevents vascular growth
14
Phoenix Overview
Targeting cancer related antigen
Phoenix (huBrE-3 tiuxetan)—a patented radiolabeled humanized monoclonal antibody delivering high-energy radiation to cancer cells by targeting PEM
PEM – polymorphic epithelial mucin – an antigen over expressed in tumor cells; encoded by MUC-1 gene
Phoenix binds to PEM on the surface of cancer cells; when radiolabelled can deliver a localized dose of high-energy beta radiation (Yttrium-90) directly to tumors
Clinical studies have shown antibody can be localized and tumor shrinkage was evident
Seeking partner for further clinical development
15
Phoenix Clinical Study
25% response rate in Stage IV Breast Cancer patients in Phase-I Study
Phase-I study at University Hospital, Denver, Colorado
17 patients
Advanced (stage IV) breast cancer
Single dose
Radiation myelotoxicity as expected
4/17 PI-defined responses (~25% response rate)
16
Sodium Phenylbutyrate
A new use for an approved drug
Well-tolerated: many years of clinical use for hyperuremia (a rare genetic disorder in infants)
Renewed interest as an anti-cancer drug
Phase-I dose established (IV and oral)
Indications of activity in Phase-II
Novel mechanism of action for cancer therapy as non-cytotoxic HDAC inhibitor
Sodium phenylbutyrate works in synergy with radiation and chemotherapy
17
Sodium Phenylbutyrate
Ongoing trials and development plans
Our licensor, Virium Pharmaceuticals, to initiate Phase-I/II in recurrent glioblastoma in combination with radiation
Somanta to evaluate Virium data and then initiate Phase-IIb trials for glioblastoma + other cancers in Europe and elsewhere
Opportunity for development of improved formulation
18
Corporate Objectives
Create and develop a diversified pipeline of cancer therapeutics
Advance clinical programs of three most promising products through Phase-II clinical trials
Seek high value corporate partnerships for two products currently in Phase-II
Continue to seek additional in-licensing opportunities
19
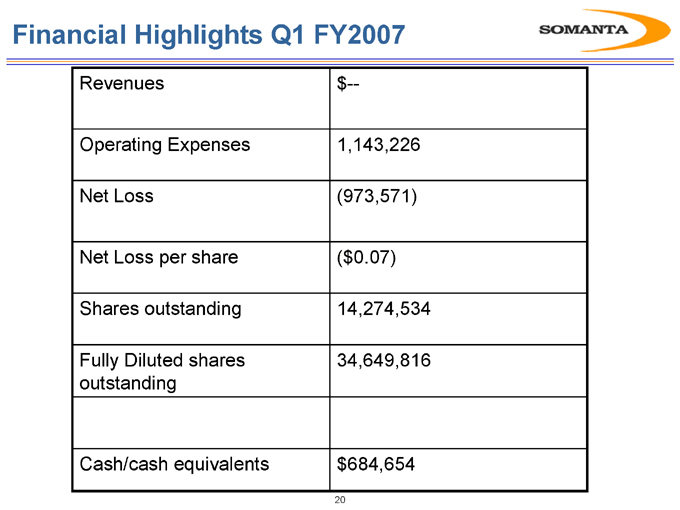
Financial Highlights Q1 FY2007
Revenues $—
Operating Expenses 1,143,226
Net Loss (973,571)
Net Loss per share ($0.07)
Shares outstanding 14,274,534
Fully Diluted shares 34,649,816
outstanding
Cash/cash equivalents $684,654
20

Investment Highlights
Focused on oncology therapies targeting 11 tumor types
Management team with proven oncology drug development expertise and commercialization background
In-licensing driven business model mitigates R&D risk
Diversified oncology portfolio with 3 small molecules and 2 antibodies in development
All products are novel approaches with defined Modes of Action
Strong IP position for all products
Low-cost “virtual” operation: 5 employees & key service providers
Bulk of investor dollars going to product development
21
APPENDIX

ALCHEMIX
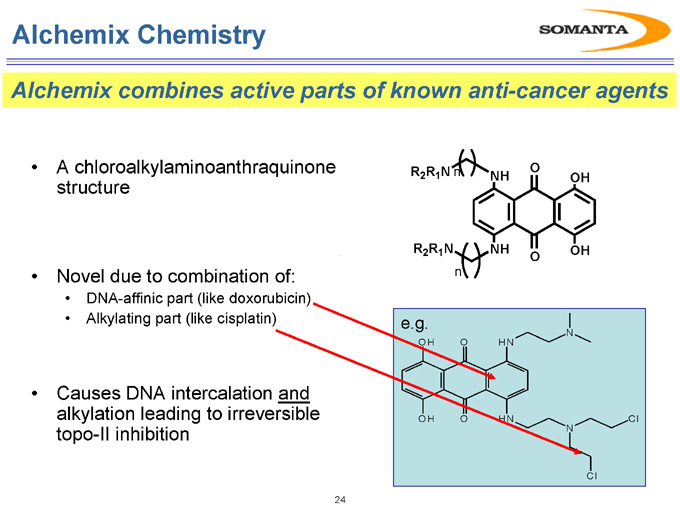
Alchemix Chemistry
Alchemix combines active parts of known anti-cancer agents
A chloroalkylaminoanthraquinone structure
Novel due to combination of:
DNA-affinic part (like doxorubicin)
Alkylating part (like cisplatin)
Causes DNA intercalation and alkylation leading to irreversible topo-II inhibition
R2R1Nn NH O OH
O n
e.g.
N O H O H N
O H O H N C l N
C l
24
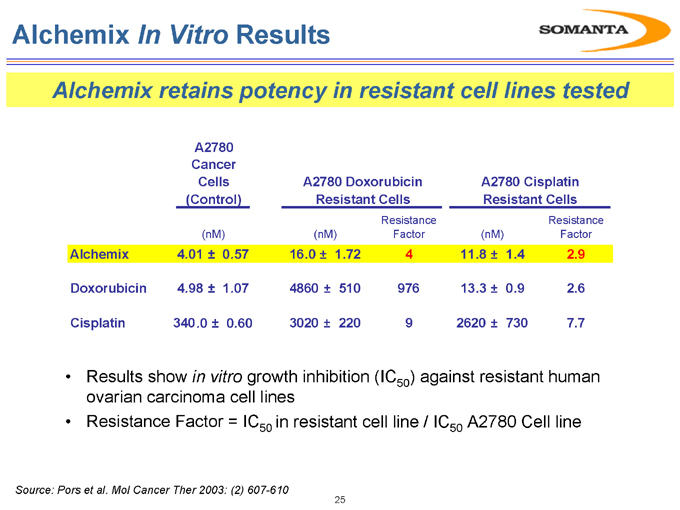
Alchemix In Vitro Results
Alchemix retains potency in resistant cell lines tested
A2780 Cancer
Cells A2780 Doxorubicin A2780 Cisplatin (Control) Resistant Cells Resistant Cells
Alchemix Doxorubicin Cisplatin
(nM)
4 .01 ± 0.57
4.98 ± 1.07 340.0 ± 0.60
(nM)
16.0 ± 1.72 4860 ± 510 3020 ± 220
Resistance Factor
(nM)
11.8 ± 1.4 13.3 ± 0.9 2620 ± 730
Resistance Factor
2.9 2.6 7.7
Results show in vitro growth inhibition (IC50) against resistant human
ovarian carcinoma cell lines
Resistance Factor = IC50 in resistant cell line / IC50 A2780 Cell line
Source: Pors et al. Mol Cancer Ther 2003: (2) 607-610
25
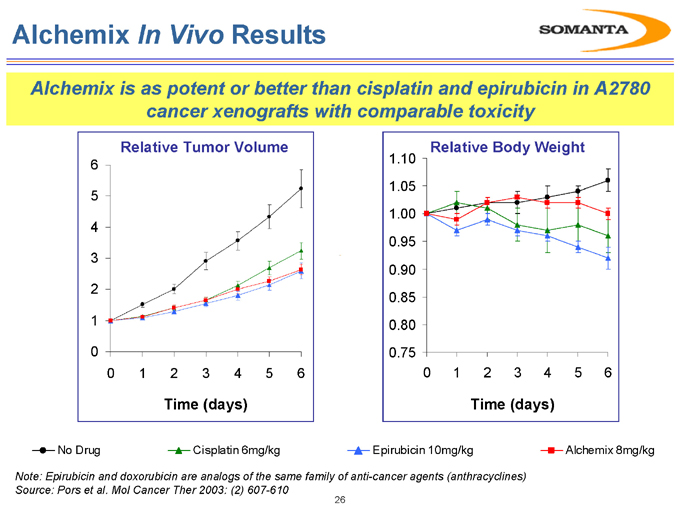
Alchemix In Vivo Results
Alchemix is as potent or better than cisplatin and epirubicin in A2780 cancer xenografts with comparable toxicity
Relative Tumor Volume
0 1 2 3 4 5 6
Time (days)
Relative Body Weight
1.10 1.05 1.00 0.95
0.90 0.85 0.80 0.75
0 1 2 3 4 5 6
Time (days)
No Drug Cisplatin 6mg/kg Epirubicin 10mg/kg Alchemix 8mg/kg
Note: Epirubicin and doxorubicin are analogs of the same family of anti-cancer agents (anthracyclines) Source: Pors et al. Mol Cancer Ther 2003: (2) 607-610
26
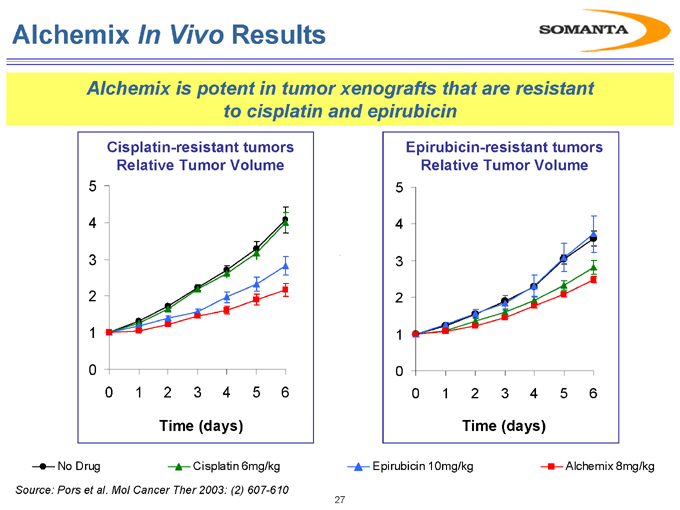
Alchemix In Vivo Results
Alchemix is potent in tumor xenografts that are resistant to cisplatin and epirubicin
Cisplatin-resistant tumors Relative Tumor Volume
0 1 2 3 4 5 6
Time (days)
Epirubicin-resistant tumors Relative Tumor Volume
0 1 2 3 4 5 6
Time (days)
No Drug Cisplatin 6mg/kg Epirubicin 10mg/kg Alchemix 8mg/kg
Source: Pors et al. Mol Cancer Ther 2003: (2) 607-610
27

PRODRAX
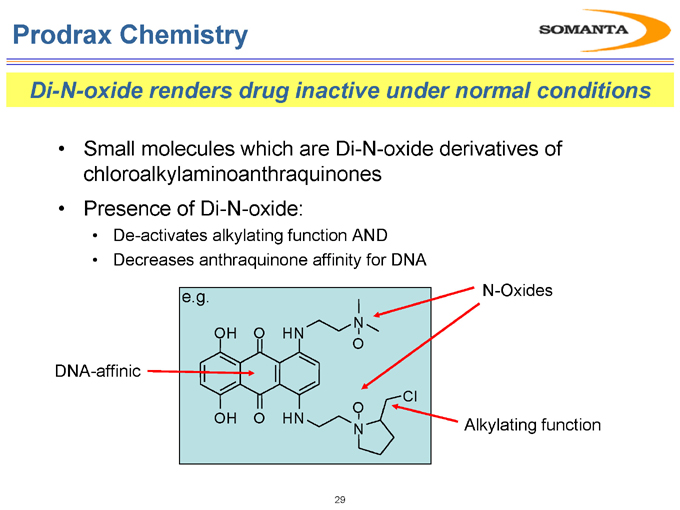
Prodrax Chemistry
Di-N-oxide renders drug inactive under normal conditions
Small molecules which are Di-N-oxide derivatives of chloroalkylaminoanthraquinones
Presence of Di-N-oxide:
De-activates alkylating function AND
Decreases anthraquinone affinity for DNA
DNA-affinic
N-Oxides
Alkylating function
e.g.
N OH O HN
O
Cl O
OH O HN
N
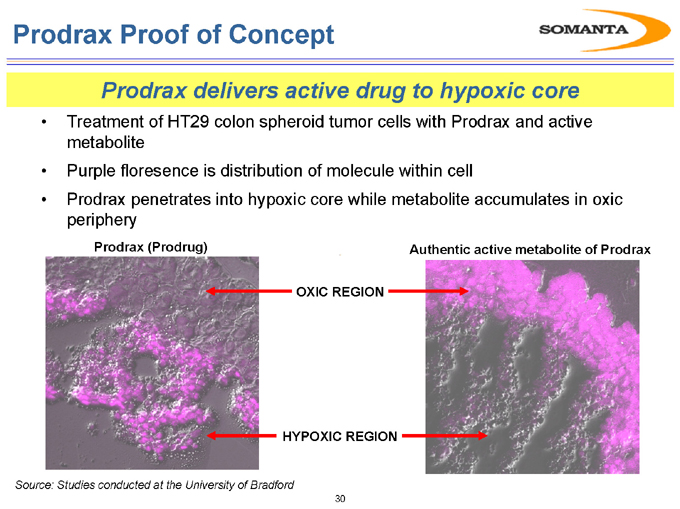
Prodrax Proof of Concept
Prodrax delivers active drug to hypoxic core
Treatment of HT29 colon spheroid tumor cells with Prodrax and active metabolite
Purple floresence is distribution of molecule within cell
Prodrax penetrates into hypoxic core while metabolite accumulates in oxic periphery
Prodrax (Prodrug)
Authentic active metabolite of Prodrax
OXIC REGION
HYPOXIC REGION
Source: Studies conducted at the University of Bradford
30
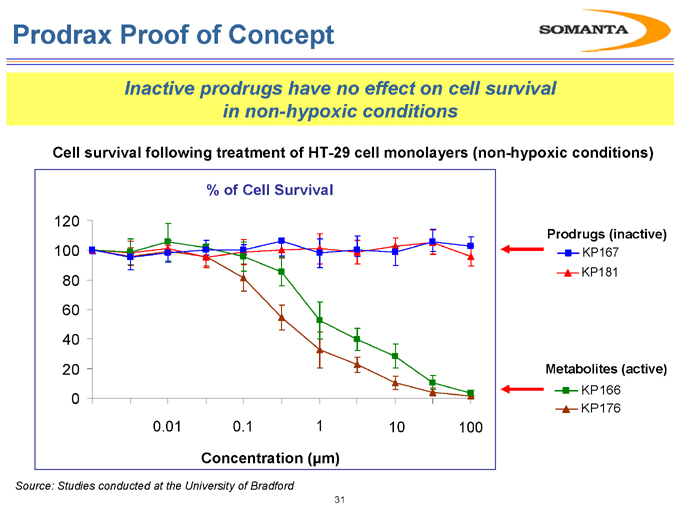
Prodrax Proof of Concept
Inactive prodrugs have no effect on cell survival in non-hypoxic conditions
Cell survival following treatment of HT-29 cell monolayers (non-hypoxic conditions)
% of Cell Survival
Prodrugs (inactive)
KP167 KP181
Metabolites (active)
KP166 KP176
120 100 80 60 40 20 0
0.01 0.1 1 10 100
Concentration (µm)
Source: Studies conducted at the University of Bradford
31
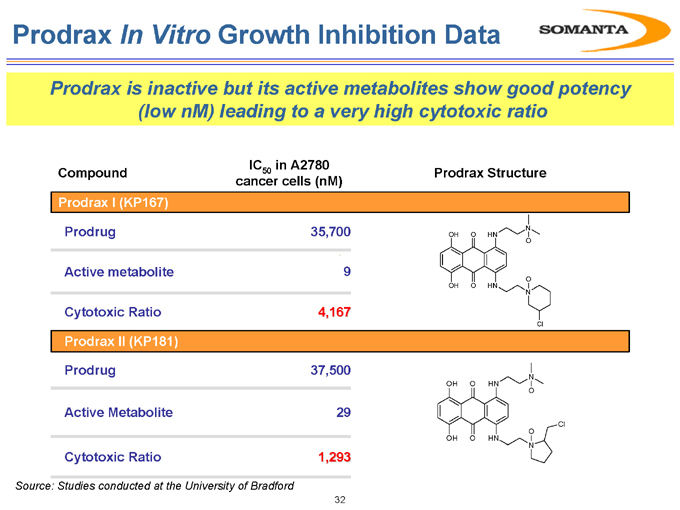
Prodrax In Vitro Growth Inhibition Data
Prodrax is inactive but its active metabolites show good potency (low nM) leading to a very high cytotoxic ratio
Compound IC50 in A2780 Prodrax Structure cancer cells (nM)
Prodrax I (KP167)
Prodrug
Active metabolite
Cytotoxic Ratio Prodrax II (KP181) Prodrug
Active Metabolite
Cytotoxic Ratio
35,700 9 4,167
37,500 29 1,293
N OH O HN
O
O OH O HN
N
Cl
N OH O HN
O
Cl O
OH O HN
N
Source: Studies conducted at the University of Bradford
32
ANGIOLIX
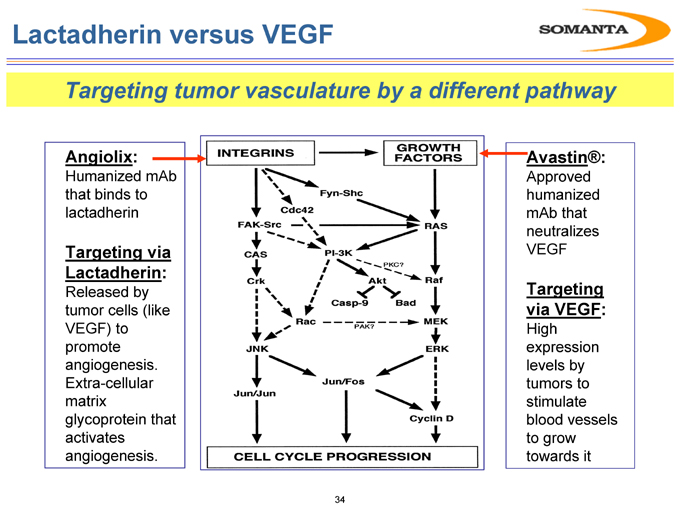
Lactadherin versus VEGF
Targeting tumor vasculature by a different pathway
Angiolix:
Humanized mAb that binds to lactadherin
Targeting via Lactadherin:
Released by tumor cells (like VEGF) to promote angiogenesis. Extra-cellular matrix glycoprotein that activates angiogenesis.
Avastin®:
Approved humanized mAb that neutralizes VEGF
Targeting via VEGF:
High expression levels by tumors to stimulate blood vessels to grow towards it
34
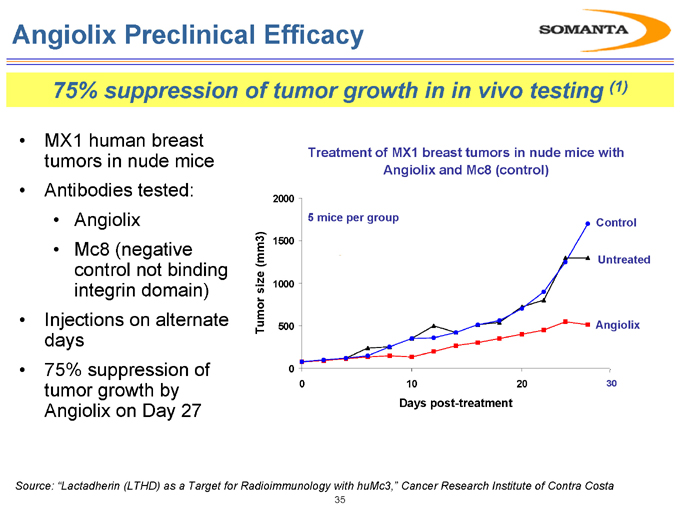
Angiolix Preclinical Efficacy
75% suppression of tumor growth in in vivo testing (1)
• MX1 human breast tumors in nude mice
• Antibodies tested:
• Angiolix
• Mc8 (negative control not binding integrin domain)
• Injections on alternate days
• 75% suppression of tumor growth by Angiolix on Day 27
Treatment of MX1 breast tumors in nude mice with Angiolix and Mc8 (control)
Control
Untreated
Angiolix
2000 1500 1000 500 0
0 10 20 30
Days post-treatment
Tumor size (mm3)
Source: “Lactadherin (LTHD) as a Target for Radioimmunology with huMc3,” Cancer Research Institute of Contra Costa
35

PHOENIX
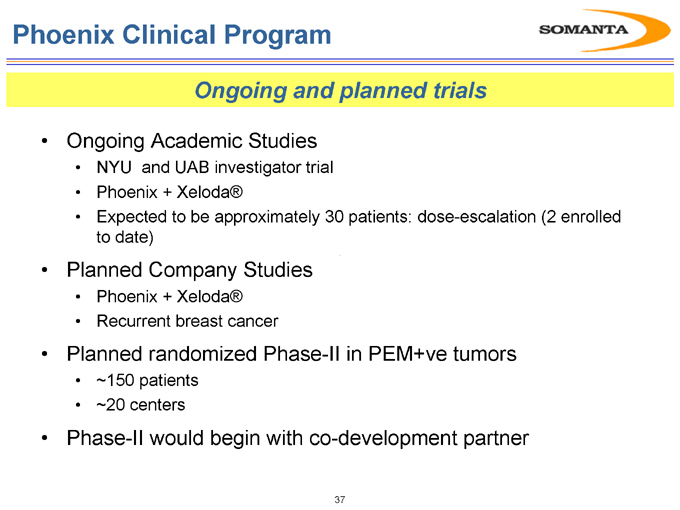
Phoenix Clinical Program
Ongoing and planned trials
Ongoing Academic Studies
NYU and UAB investigator trial
Phoenix + Xeloda®
Expected to be approximately 30 patients: dose-escalation (2 enrolled to date)
Planned Company Studies
Phoenix + Xeloda®
Recurrent breast cancer
Planned randomized Phase-II in PEM+ve tumors
~150 patients
~20 centers
Phase-II would begin with co-development partner
37

SODIUM PHENYLBUTYRATE
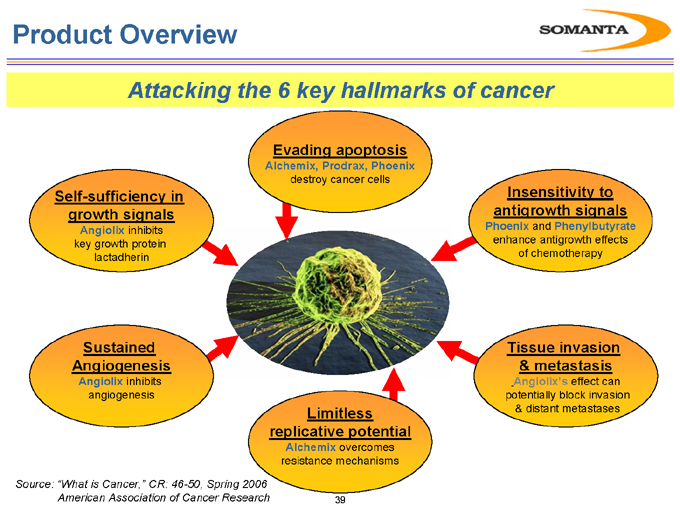
Product Overview
Attacking the 6 key hallmarks of cancer
Self-sufficiency in growth signals
Angiolix inhibits key growth protein lactadherin
Sustained Angiogenesis
Angiolix inhibits angiogenesis
Limitless replicative potential
Alchemix overcomes resistance mechanisms
Tissue invasion & metastasis
Angiolix’s effect can potentially block invasion & distant metastases
Insensitivity to antigrowth signals
Phoenix and Phenylbutyrate enhance antigrowth effects of chemotherapy
Evading apoptosis
Alchemix, Prodrax, Phoenix destroy cancer cells
Source: “What is Cancer,” CR: 46-50, Spring 2006
American Association of Cancer Research 39

FINANCIAL OVERVIEW
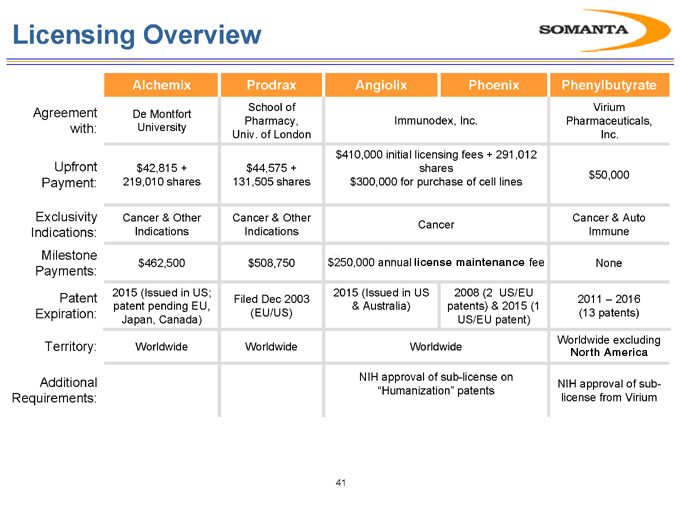
Licensing Overview
Agreement with:
Upfront Payment:
Exclusivity Indications: Milestone Payments:
Patent Expiration:
Territory:
Additional Requirements:
Alchemix
De Montfort University
$42,815 + 219,010 shares
Cancer & Other Indications
$462,500
2015 (Issued in US; patent pending EU, Japan, Canada)
Worldwide
Prodrax
School of Pharmacy, Univ. of London
$44,575 + 131,505 shares
Cancer & Other Indications
$508,750
Filed Dec 2003 (EU/US)
Worldwide
Angiolix Phoenix
Immunodex, Inc.
$410,000 initial licensing fees + 291,012 shares $300,000 for purchase of cell lines
Cancer
$250,000 annual license maintenance fee
2015 (Issued in US
& Australia)
2008 (2 US/EU patents) & 2015 (1 US/EU patent)
Worldwide
NIH approval of sub-license on “Humanization” patents
Phenylbutyrate
Virium Pharmaceuticals, Inc.
$50,000
Cancer & Auto Immune
None
2011 – 2016 (13 patents)
Worldwide excluding North America
NIH approval of sub-license from Virium
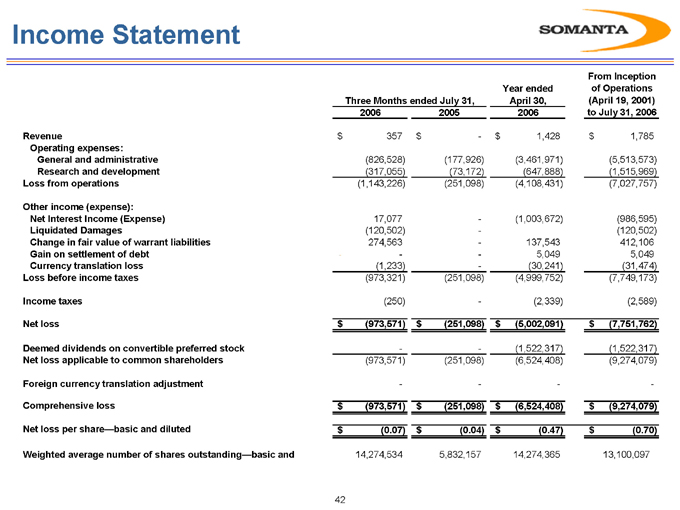
Income Statement
Three Months ended July 31, Year ended April 30, 2006 From Inception of Operations (April 19, 2001) to July 31, 2006
2006 2005
Revenue $357 $- $1,428 $1,785
Operating expenses:
General and administrative (826,528) (177,926) (3,461,971) (5,513,573)
Research and development (317,055) (73,172) (647,888) (1,515,969)
Loss from operations (1,143,226) (251,098) (4,108,431) (7,027,757)
Other income (expense):
Net Interest Income (Expense) 17,077 — (1,003,672) (986,595)
Liquidated Damages (120,502) — (120,502)
Change in fair value of warrant liabilities 274,563 — 137,543 412,106
Gain on settlement of debt —— 5,049 5,049
Currency translation loss (1,233) — (30,241) (31,474)
Loss before income taxes (973,321) (251,098) (4,999,752) (7,749,173)
Income taxes (250) — (2,339) (2,589)
Net loss $(973,571) $(251,098) $(5,002,091) $(7,751,762)
Deemed dividends on convertible preferred stock —— (1,522,317) (1,522,317)
Net loss applicable to common shareholders (973,571) (251,098) (6,524,408) (9,274,079)
Foreign currency translation adjustment ——— -
Comprehensive loss $(973,571) $(251,098) $(6,524,408) $(9,274,079)
Net loss per share—basic and diluted $(0.07) $(0.04) $(0.47) $(0.70)
Weighted average number of shares outstanding—basic and 14,274,534 5,832,157 14,274,365 13,100,097
42
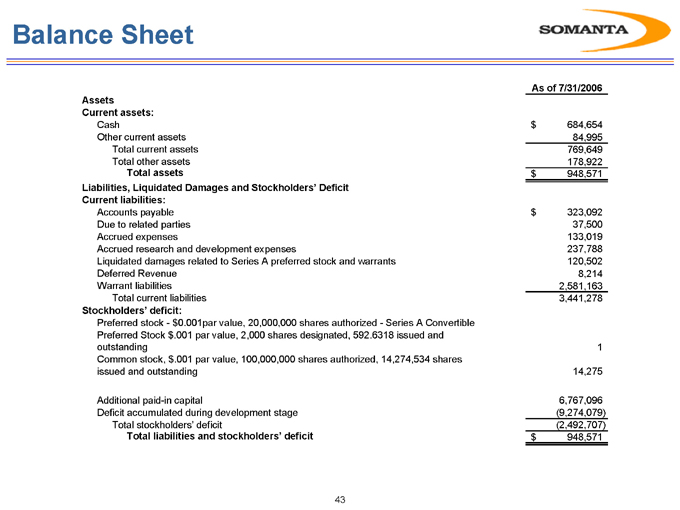
Balance Sheet
As of 7/31/2006
Assets
Current assets:
Cash $684,654
Other current assets 84,995
Total current assets 769,649
Total other assets 178,922
Total assets $948,571
Liabilities, Liquidated Damages and Stockholders’ Deficit
Current liabilities:
Accounts payable $323,092
Due to related parties 37,500
Accrued expenses 133,019
Accrued research and development expenses 237,788
Liquidated damages related to Series A preferred stock and warrants 120,502
Deferred Revenue 8,214
Warrant liabilities 2,581,163
Total current liabilities 3,441,278
Stockholders’ deficit:
Preferred stock—$0.001par value, 20,000,000 shares authorized—Series A Convertible
Preferred Stock $.001 par value, 2,000 shares designated, 592.6318 issued and
outstanding 1
Common stock, $.001 par value, 100,000,000 shares authorized, 14,274,534 shares
issued and outstanding 14,275
Additional paid-in capital 6,767,096
Deficit accumulated during development stage (9,274,079)
Total stockholders’ deficit (2,492,707)
Total liabilities and stockholders’ deficit $948,571
43

Capitalization Table
As of 7/31/2006
Cash $684,654
Capitalization
Debt
Warrant liabilities 2,581,163 (1)
Shareholder’s Deficit
Preferred stock 1
Common stock 14,275
Additional paid-in capital 6,767,096
Deficit accumulated during development stage (9,274,079)
Total stockholders’ deficit (2,492,707)
Total Capitalization $88,456
(1) | | Net cash settlement value of warrants if company fails to issue and deliver common stop upon exercise |
44
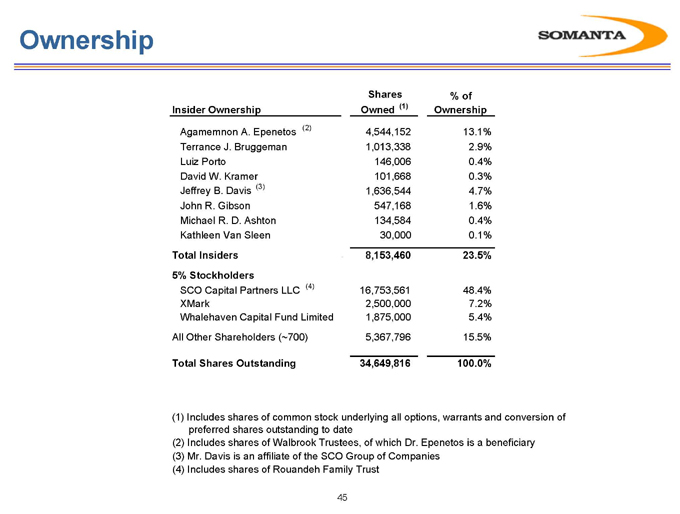
Ownership
Insider Ownership Shares Owned (1) % of Ownership
Agamemnon A. Epenetos (2) 4,544,152 13.1%
Terrance J. Bruggeman 1,013,338 2.9%
Luiz Porto 146,006 0.4%
David W. Kramer 101,668 0.3%
Jeffrey B. Davis (3) 1,636,544 4.7%
John R. Gibson 547,168 1.6%
Michael R. D. Ashton 134,584 0.4%
Kathleen Van Sleen 30,000 0.1%
Total Insiders 8,153,460 23.5%
5% Stockholders
SCO Capital Partners LLC (4) 16,753,561 48.4%
XMark 2,500,000 7.2%
Whalehaven Capital Fund Limited 1,875,000 5.4%
All Other Shareholders (
700) 5,367,796 15.5%
Total Shares Outstanding 34,649,816 100.0%
(1) | | Includes shares of common stock underlying all options, warrants and conversion of preferred shares outstanding to date |
(2) Includes shares of Walbrook Trustees, of which Dr. Epenetos is a beneficiary (3) Mr. Davis is an affiliate of the SCO Group of Companies (4) Includes shares of Rouandeh Family Trust
45

FOCUSED ON NEW THERAPIES FOR ONCOLOGY













































