Exhibit 99.1
 | Shire and NPS Pharma Leadership in Rare Diseases GRAPHIC OMMITTED GRAPHIC OMMITTED GRAPHIC OMMITTED |
 | THIS COMMUNICATION IS FOR INFORMATIONAL PURPOSES ONLY AND DOES NOT CONSTITUTE AN OFFER TO PURCHASE OR A SOLICITATION OF AN OFFER TO SELL NPS PHARMA COMMON STOCK. THE OFFER TO BUY NPS PHARMA COMMON STOCK WILL ONLY BE MADE PURSUANT TO A TENDER OFFER STATEMENT (INCLUDING THE OFFER TO PURCHASE, LETTER OF TRANSMITTAL AND OTHER RELATED TENDER OFFER MATERIALS) . INVESTORS AND SECURITY HOLDERS ARE URGED TO READ BOTH THE TENDER OFFER STATEMENT (WHICH WILL BE FILED BY SHIRE AND A SUBSIDIARY OF SHIRE WITH THE SECURITIES AND EXCHANGE COMMISSION (SEC)) AND THE SOLICITATION/RECOMMENDATION STATEMENT ON SCHEDULE 14D-9 WITH RESPECT TO THE TENDER OFFER (WHICH WILL BE FILED BY NPS PHARMA WITH THE SEC) WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION, INCLUDING THE TERMS AND CONDITIONS OF THE OFFER. INVESTORS AND SECURITY HOLDERS MAY OBTAIN A FREE COPY OF THESE MATERIALS (WHEN AVAILABLE) AND OTHER DOCUMENTS FILED BY SHIRE AND NPS PHARMA WITH THE SEC AT THE WEBSITE MAINTAINED BY THE SEC AT WWW.SEC.GOV. THE TENDER OFFER STATEMENT AND RELATED MATERIALS, AND THE SOLICITATION/RECOMMENDATION STATEMENT, MAY ALSO BE OBTAINED (WHEN AVAILABLE) FOR FREE BY CONTACTING SHIRE INVESTOR RELATIONS AT +1 484 595 2220 or +44 1256 894157. COPIES OF THESE MATERIALS AND ANY DOCUMENTATION RELATING TO THE TENDER OFFER ARE NOT BEING, AND MUST NOT BE, DIRECTLY OR INDIRECTLY, MAILED OR OTHERWISE FORWARDED, DISTRIBUTED OR SENT IN, INTO OR FROM ANY JURISDICTION WHERE TO DO SO WOULD BE UNLAWFUL. GRAPHIC OMMITTED 1 |
 | Forward Looking Statements Statements included in this communication that are not historical facts are forward -looking statements. Such forward -looking statements involve a number of risks and uncertainties and are subject to change at any time. In the event such risks or uncertainties materialize, Shire's results could be materially adversely affected. The risks and uncertainties include, but are not limited to, that: [] Shire's products may not be a commercial success; [] revenues from ADDERALL XR and INTUNIV are subject to generic erosion; [] the failure to obtain and maintain reimbursement, or an adequate level of reimbursement, by third-party payors in a timely manner for Shire's products may impact future revenues, financial condition and results of operations; [] Shire conducts its own manufacturing operations for certain of its products and is reliant on third party contract manufacturers to manufacture other products and to provide goods and services. Some of Shire's products or ingredients are only available from a single approved source for manufacture. Any disruption to the supply chain for any of Shire's products may result in Shire being unable to continue marketing or developing a product or may result in Shire being unable to do so on a commercially viable basis for some period of time; [] the development, approval and manufacturing of Shire's products is subject to extensive oversight by various regulatory agencies. Submission of an application for regulatory approval of any of our product candidates, such as our planned submission of a New Drug Application to the FDA for Lifitegrast, may be delayed for any number of reasons and, once submitted, may be subjected to lengthy review and ultimately rejected. Moreover, regulatory approvals or interventions associated with changes to manufacturing sites, ingredients or manufacturing processes could lead to significant delays, increase in operating costs, lost product sales, an interruption of research activities or the delay of new product launches; [] the actions of certain customers could affect Shire 's ability to sell or market products profitably. Fluctuations in buying or distribution patterns by such customers can adversely impact Shire's revenues, financial condition or results of operations; [] investigations or enforcement action by regulatory authorities or law enforcement agencies relating to Shire's activities in the highly regulated markets in which it operates may result in significant legal costs and the payment of substantial compensation or fines; [] adverse outcomes in legal matters and other disputes, including Shire's ability to enforce and defend patents and other intellectual property rights required for its business, could have a material adverse effect on Shire's revenues, financial condition or results of operations; [] Shire faces intense competition for highly qualified personnel from other companies, academic institutions, government entities and other organizations. Shire is undergoing a corporate reorganization and the consequent uncertainty could adversely impact Shire's ability to attract and/or retain the highly skilled personnel needed for Shire to meet its strategic objectives; To be as brave as the people we help. 2 |
 | Forward Looking Statements [] failure to achieve Shire's strategic objectives with respect to the acquisition of ViroPharma Incorporated may adversely affect Shire's financial condition and results of operations; [] Shire's proposed acquisition of NPS Pharma may not be consummated due to the occurrence of an event, change or other circumstances that gives rise to the termination of the merger agreement; [] a governmental or regulatory approval required for the proposed acquisition of NPS Pharma may not obtained, or may be obtained subject to conditions that are not anticipated, or another condition to the closing of the proposed acquisition may not be satisfied; [] NPS Pharma may be unable to retain and hire key personnel and/or maintain its relationships with customers, suppliers and other business partners pending the consummation of the proposed acquisition by Shire, or NPS Pharma's business may be disrupted by the proposed acquisition, including increased costs and diversion of management time and resources; [] difficulties in integrating NPS Pharma into Shire may lead to the combined company not being able to realize the expected operating efficiencies, cost savings, revenue enhancements, synergies or other benefits at the time anticipated or at all; [] and other risks and uncertainties detailed from time to time in Shire's or NPS Pharma's filings with the U.S. Securities and Exchange Commission, including their respective most recent Annual Reports on Form 10-K. THIS COMMUNICATION IS FOR INFORMATIONAL PURPOSES ONLY AND DOES NOT CONSTITUTE AN OFFER TO PURCHASE OR A SOLICITATION OF AN OFFER TO SELL NPS PHARMA COMMON STOCK. THE OFFER TO BUY NPS PHARMA COMMON STOCK WILL ONLY BE MADE PURSUANT TO A TENDER OFFER STATEMENT (INCLUDING THE OFFER TO PURCHASE, LETTER OF TRANSMITTAL AND OTHER RELATED TENDER OFFER MATERIALS) . INVESTORS AND SECURITY HOLDERS ARE URGED TO READ BOTH THE TENDER OFFER STATEMENT (WHICH WILL BE FILED BY SHIRE AND A SUBSIDIARY OF SHIRE WITH THE SECURITIES AND EXCHANGE COMMISSION (SEC)) AND THE SOLICITATION/RECOMMENDATION STATEMENT ON SCHEDULE 14D-9 WITH RESPECT TO THE TENDER OFFER (WHICH WILL BE FILED BY NPS PHARMA WITH THE SEC) WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION, INCLUDING THE TERMS AND CONDITIONS OF THE OFFER. INVESTORS AND SECURITY HOLDERS MAY OBTAIN A FREE COPY OF THESE MATERIALS (WHEN AVAILABLE) AND OTHER DOCUMENTS FILED BY Shire AND NPS PHARMA WITH THE SEC AT THE WEBSITE MAINTAINED BY THE SEC AT WWW.SEC.GOV. THE TENDER OFFER STATEMENT AND RELATED MATERIALS, AND THE SOLICITATION/RECOMMENDATION STATEMENT, MAY ALSO BE OBTAINED (WHEN AVAILABLE) FOR FREE BY CONTACTING SHIRE INVESTOR RELATIONS AT +1 484 595 2220 IN THE US AND +44 1256 894157 IN THE UK. COPIES OF THESE MATERIALS AND ANY DOCUMENTATION RELATING TO THE TENDER OFFER ARE NOT BEING, AND MUST NOT BE, DIRECTLY OR INDIRECTLY, MAILED OR OTHERWISE FORWARDED, DISTRIBUTED OR SENT IN, INTO OR FROM ANY To be as brave as the people we help. JURISDICTION WHERE TO DO SO WOULD BE UNLAWFUL. 3 |
 | Shire patient video, "My Name Is[]" GRAPHIC OMMITTED 4 |
 |
At Shire we have delivered hope to millions of patients []
We've served millions of patients across our therapeutic areas
GRAPHIC OMMITTED
Our patient support services covered 2.6K patients, including 100K+ calls
and 1.3K+ donated vials
GRAPHIC OMMITTED
We work with regulators to alleviate shortages when they occur
GRAPHIC OMMITTED
We support 50+ ADHD patients to attend college each year
Mike Yasick ADHD Scholarship
GRAPHIC OMMITTED
We partner with countless societies to raise awareness of rare and orphan
diseases
GRAPHIC OMMITTED
We work closely with patient groups to support them and their families
|
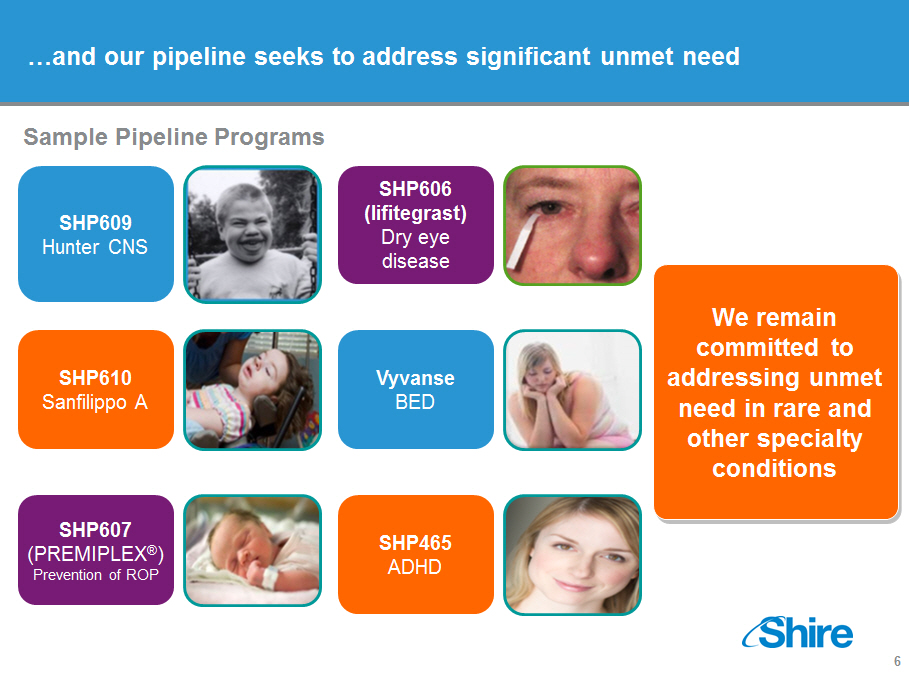 |
[]and our pipeline seeks to address significant unmet need
Sample Pipeline Programs
SHP609
Hunter CNS
GRAPHIC OMMITTED
SHP606 (lifitegrast)
Dry eye disease
GRAPHIC OMMITTED
SHP610
Sanfilippo A
GRAPHIC OMMITTED
Vyvanse
BED
GRAPHIC OMMITTED
SHP607
(PREMIPLEX ([R]))
Prevention of ROP
GRAPHIC OMMITTED
SHP465
ADHD
GRAPHIC OMMITTED
We remain committed to addressing unmet need in rare and other
specialty conditions
6
|
 |
Key Shire facts
[] Founded in 1986 in the United
Kingdom
[] Growth through original research,
creative acquisitions, and innovative
licensing agreements
[] Listed on both the London Stock
Exchange and NASDAQ
[] Market capitalization: $40 billion
[] Hubs in both Zug, Switzerland and
Lexington, Massachusetts
[] Over 5,000 employees globally,
offices in 31 countries
GRAPHIC OMMITTED
DRAFT
7
|
 |
Shire's culture
We have a clear and strong patient-focused culture where we all strive to be
BRAVE :
[] Bold: We have the courage to lead the way
[] Resilient: We are agile and adaptable to meet
the changing needs of our stakeholders
[] Accountable: We deliver on our promises to all
of our stakeholders
[] Visionary: We fearlessly innovate to address
unmet patient need
[] Ethical: We do the right thing, in the right way
Our culture comes to life through our employees who together form One Shire. We
value and invest in our employees to ensure they have the capabilities and
support to implement our strategy, achieve our vision and deliver value to our
patients, payors T be andas brave shareholders. as the people we help.
GRAPHIC OMMITTED
8
|
 | NPS Pharma and Shire have similar values GRAPHIC OMMITTED * Integrity* Respect* Excellence * Personal Ownership* Teamwork * Entrepreneurial Spirit * Fun GRAPHIC OMMITTED * Bold* Resilient* Accountable * Visionary* Ethical 9 |
 |
Strategy executed via our In-Line and Pipeline Teams
In-Line
Drive optimum performance from our currently marketed products
Pipeline
Build our future assets through both RandD and Business Development
[] Increase sales for rare diseases treatments through geographic expansion,
patient identification and increase awareness through medical education
[] Execute on market expansion initiatives
[] Optimize the sales performance of all our BUs
[] Continuously assess to leverage strengths
[] Develop promising pipeline
[] MandA to expand pipeline and on-market presence (e. g. , NPS and ViroPharma)
GRAPHIC OMMITTED
|
 |
Shire's global organization
4 Business Units
Rare Diseases BU
Neuroscience BU
Gastrointestinal and Internal Medicine BU
Ophthalmics BU
1 geographic organization
International Commercial
9 global functions
RandD Technical Ops*
Finance Corporate
Development**
Compliance and HR
Risk management
Commercial Legal
Excellence***
Corporate Comms
and Public Affairs
*Includes IT, Procurement and Real Estate
**Includes Business Development, Strategic Planning, and Program Management ***
Includes Market Access and Global Commercial Operations
|
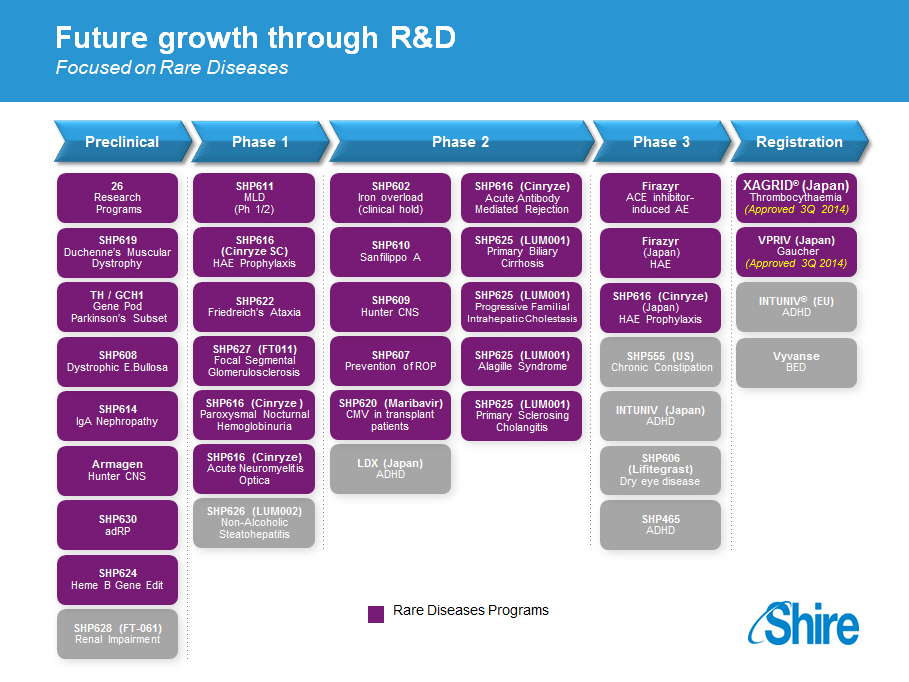 |
Future growth through RandD
Focused on Rare Diseases
Preclinical Phase 1 Phase 2 Phase 3 Registration
26 SHP611 SHP602 SHP616 (Cinryze) Firazyr XAGRID([R]) (Japan)
Research MLD Iron overload Acute Antibody ACE inhibitor- Thrombocythaemia
Programs (Ph 1/2) (clinical hold) Mediated Rejection induced AE (Approved 3Q 2014)
SHP619 SHP616 SHP610 SHP625 (LUM001) Firazyr VPRIV (Japan)
Duchenne's Muscular (Cinryze SC) Sanfilippo A Primary Biliary (Japan) Gaucher
Dystrophy HAE Prophylaxis Cirrhosis HAE (Approved 3Q 2014)
TH / GCH1 SHP622 SHP609 SHP625 (LUM001) SHP616 (Cinryze) INTUNIV([R]) (EU)
Gene Pod Friedreich's Ataxia Hunter CNS Progressive Familial (Japan) ADHD
Parkinson's Subset Intrahepatic Cholestasis HAE Prophylaxis
SHP627 (FT011)
SHP608 Focal Segmental SHP607 SHP625 (LUM001) SHP555 (US) Vyvanse
Dystrophic E.Bullosa Glomerulosclerosis Prevention of ROP Alagille Syndrome Chronic Constipation BED
SHP616 (Cinryze ) SHP620 (Maribavir) SHP625 (LUM001)
SHP614 Paroxysmal Nocturnal CMV in transplant Primary Sclerosing INTUNIV (Japan)
IgA Nephropathy Hemoglobinuria patients Cholangitis ADHD
SHP616 (Cinryze) SHP606
Armagen Acute Neuromyelitis LDX (Japan) (Lifitegrast)
Hunter CNS Optica ADHD Dry eye disease
SHP626 (LUM002)
SHP630 Non-Alcoholic SHP465
adRP Steatohepatitis ADHD
SHP624
Heme B Gene Edit
Rare Diseases Programs
SHP628 (FT-061)
Renal Impairment
|
 | We look forward to combining the best of Shire and NPS Pharma on behalf of patients* Shire [] Broad international commercial footprint with products marketed in over 50 countries [] Innovative services to identify, support and manage patients [] Best-in-class market access and launch capabilities [] #2 GI sales force as ranked by U.S. GI's(1) [] Deep development portfolio, including SHP625 for rare GI / hepatic diseases GRAPHIC OMMITTED NPS Pharma [] Rare disease expertise [] Proven successes in product development and commercialization [] First-in / best-in rare disease pipeline portfolio [] Patient -centric organization [] Emerging global footprint (1) IMS Attributable Ranking Study 2013 *Subject to regulatory and other customary closing conditions 13 |
 |
What's Next? Integration Planning
[] Integration Planning Team will be established -- staff from NPS (selected by NPS) will be
members of the Integration Team
[] Team will be focused on the development of critical plans for all transition and integration activities of
the NPS business post-close
[] NPS must continue to run its business independently and make its own decisions regarding its
business
[] During this pre-close period -- we can plan for integration but cannot execute on any element
of integration
[] Transparent communication is critical to success -- we will communicate key information as
decisions are made
[] During the period before close, communication to NPS employees will continue to be the
responsibility of your management. All communications must flow through Integration Teams
-- no direct communications unless approved by Legal.
[] Ultimate goal -- ensure that patient needs are met and that there is no interruption to their
treatment
[] Key next steps -- business meetings (scheduled via integration leads only)
[] Manager-to-manager meetings to review and gain understanding of ongoing activities, staff
responsibilities and other key elements of the business
[] Critical business meetings with key internal/external stakeholders (e.g. CMOs)
[] These meetings will drive planning related to critical and/or priority integration events
14
|
 | This acquisition will allow NPS Pharma's products to transform the lives of even more patients GRAPHIC OMMITTED 15 |
 | Questions? 16 |
 | GRAPHIC OMMITTED |