Exhibit 10.2
NOTE: CERTAIN CONFIDENTIAL INFORMATION HAS BEEN OMITTED FROM
THIS DOCUMENT AND REPLACED BY "[*]". A COMPLETE COPY OF THIS
DOCUMENT INCLUDING THE CONFIDENTIAL INFORMATION HAS BEEN FILED
SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
EXCLUSIVE PATENT LICENSE AGREEMENT
BETWEEN
GLAXOSMITHKLINE LLC
AND
NPS PHARMACEUTICALS, INC.
EXCLUSIVE PATENT LICENSE AGREEMENT
THIS EXCLUSIVE PATENTLICENSE AGREEMENT ("Agreement"),is entered into as of July 29, 2011 (the"Effective Date")byand between GlaxoSmithKline LLC, a Delaware limited liability company with an address at One Franklin Plaza, Philadelphia, Pennsylvania 19106 (formerly known as SmithKline Beecham Corporation) ("GSK"),and NPS Pharmaceuticals, Inc., a Delaware corporation with an address at 550 Hills Dr., 3rd Floor, Bedminster, New Jersey 07921 ("NPS").GSKand NPS are referred to in this Agreement each individually as a"Party"and collectively as the"Parties".
WHEREAS, GSK and NPS entered into that certain Collaborative Research and License Agreement effective November 1, 1993, as amended from time to time (collectively, the "Original Agreement"), pursuant to which GSK and NPS conducted Research for the purpose of discovering compounds that interact with Calcium Receptors to determine their potential for use in the diagnosis, treatment or prophylaxis of certain diseases and disorders;
WHEREAS, in the Original Agreement, GSK had the exclusive right to make, use and sell certain compounds arising from the Research in the Original Field;
WHEREAS, GSK desires to further develop the compound known as Ronacaleret in the GSK Field, and NPS is willing to grant such rights and licenses to GSK in accordance with the terms and conditions of this Agreement; and
WHEREAS, NPS desires to further develop Existing Compounds and Transferred Compounds and to receive from GSK certain IND filings, preclinical data, patents, and other information relating thereto, and GSK is willing to transfer such materials to NPS in accordance with the terms and conditions of this Agreement.
NOW THEREFORE, in consideration of the foregoing premises, the mutual covenants and obligations hereinafter contained, and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the Parties agree as follows:
1. DEFINITIONS. As used in this Agreement, each of the following capitalized terms shall have the meaning ascribed to such term in thisSection 1. All capitalized terms not defined in thisSection 1 shall have the meaning ascribed to such terms in the body of this Agreement. Defined terms may be used in the singular and the plural.
1.1 "`003 Patent" means U.S. Patent No. [*].
1.2 "`557 Compound" means the Compound designated SB 423557 with the chemical name [*].
1.3 "`562 Compound" means the Compound designated SB 423562 with the chemical name [*].
1.4 "`684 Patent" means U.S. Patent No. [*].
2
1.5 "ADHH" means Autosomal Dominant Hypocalcemia with Hypercalciuria.
1.6 "Affiliate"means: (a) any entity that owns directly or indirectly, a Controlling Interest in a Party, by stock ownership or otherwise; (b) any entity in which a Party owns a Controlling Interest, by stock ownership or otherwise; or (c) any entity under common Control with a Party, directly or indirectly. As used in this definition,"Control"or"Controlling Interest"means the ownership, directly or indirectly, of more than fifty percent (50%) (or such lesser percentage which is the maximum allowed to be owned by an entityin a particular jurisdiction) of voting stock permitted to vote for the election of the board of directors or any other arrangement resulting in control of or the right to control the management and the affairs of the entity or Party in question.
1.7 "Alliance Manager" has the meaning set forth in Section 4.1.
1.8 "Applicable Laws" means all laws, statutes, rules, regulations, orders, judgments, or ordinances having the effect of law of any federal, national, multinational, state, provincial, county, city or other political subdivision.
1.9 "Breaching Party" has the meaning set forth in Section 8.2.
1.10 "Brigham Agreement" means that certain Patent Agreement, effective February 19, 1993, by and between The Brigham and Women's Hospital, Inc. and NPS, as amended.
1.11 "Business Day" means a day on which banking institutions in New York, New York, United States, and London, England are open for business, excluding any Saturday or Sunday and the nine (9) consecutive calendar days beginning onDecember24th and continuing throughJanuary1st of each calendar year during theTerm.
1.12 "Calcium Receptor"means any protein(s) located on the cell surface (plasma membrane) that detects, as their primary physiological ligand, extracellular Ca2+, and such detection enables certain cells in the body to respond to changes in the concentration of extracellular Ca2+.
1.13 "Commercially Reasonable Efforts" means, with respect to a Party, such efforts that are consistent with the efforts and resources normally used by such Party in the exercise of its reasonable business discretion relating to the research, development and commercialization of a pharmaceutical product owned by it or to which it has exclusive rights, with similar product characteristics, which is of similar market potential at a similar stage in its development or product life, taking into account issues of patent coverage, safety and efficacy, product profile, the competitiveness of the marketplace, the proprietary position of the compound or product, the regulatory structure involved, the potential or actual profitability of the applicable products (including pricing and reimbursement status achieved or to be achieved), and other relevant factors, including technical, legal, scientific and/or medical factors.Forpurposes of clarity,Commercially Reasonable Effortswould be determined on a market-by-market and indication-by-indication basisfora particular product and it is anticipated that the level of effort may be differentfordifferent markets and may change over time, reflecting changes in the status of the product and the market(s) involved.
3
1.14 "Compound(s)" means compositions of matter that interact with Calcium Receptors on parathyroid cells, osteoclast cells, C-cell, kidney cells, gastrointestinal cells and other cells involved in regulation of bone metabolism (i.e. resorption, formation and remodeling), and whose Principal Mode of Action is indicative of utility in the Original Field. For the avoidance of doubt, Compounds do not include calcimimetic compounds.
1.15 "Control"means, with respect to any information or intellectual property right, possession by a Party of the ability to grant the right to access or use, or to grant a license or a sublicense to, such information or intellectual property right as provided for herein without violating the terms of any agreement or other arrangement with any Third Party.
1.16 "Cost of Goods Sold" means all direct costs and all indirect costs incurred to manufacture, test and store Ronacaleret in finished dosage form, including indirect labor, supplies, returns, breakage and other incidental expenses of manufacturing, such as: indirect labor, employee fringe benefits, recruiting and relocations, project related travel, facilities expenses, telephone and communication, printing and duplicating, electronic data processing equipment rent, maintenance and services, vehicle expenses, equipment depreciation and other general equipment expenses, consulting, outside services, indirect materials and supplies, postage and freight, dues and subscriptions, meetings, miscellaneous taxes and fees, general and administrative expenses allocated to manufacturing. GSK's methods for allocating direct and indirect costs shall be consistent with IFRS.
1.17 "Country Termination Notice" has the meaning set forth in Section 8.3.
1.18 "Data" means any and all results, data, information, compositions of matter (other than compositions of matter relating to Existing Compounds, Transferred Compounds or Ronacaleret) and expertise, including without limitation, all chemical, pharmacological, toxicological, clinical, assay, chemistry, manufacturing and control data, and any other information and reagents.
1.19 "Delay Period" has the meaning set forth in Section 8.3.
1.20 "Delivery Period" has the meaning set forth in Section 2.2(e).
1.21 "Development" means all activities relating to non-clinical, preclinical and clinical trials, including without limitation all testing related to toxicology, pharmacokinetics, pathology, chemistry, modification, optimization, efficacy and drug metabolism, statistical analysis, publication and presentation of study results and reporting, preparation and submission of filings and applications related to the foregoing activities to Regulatory Authorities and obtaining and maintaining Regulatory Approvals. For the avoidance of doubt, "Development" shall include any work undertaken to inform future development plans or as deemed necessary or useful to obtain Regulatory Approvals.
1.22 "Disclosing Party" has the meaning set forth in Section 10.1.
1.23 "Existing Compound Know-How" means all Data solely relating to Existing Compounds that is useful or required for the Development or commercialization of Existing Compounds which was (a) developed by or became known to NPS or GSK during the term of
4
the Original Agreement, (b) made legally available to NPS or GSK in accordance with agreements entered into underSection 2.02 of the Original Agreement, or (c) in existence as of the Effective Date of the Original Agreement.
1.24 "Existing Compounds" means the Compounds that are disclosed in the Patents listed onSchedule 1.24, excluding Ronacaleret and the Transferred Compounds.
1.25 "FDA" means the United States Food and Drug Administration or any successor entity thereto.
1.26 "First Commercial Sale" means (i) with respect to each Product, the first sale for which revenue has been recognized by GSK or its Affiliates or sublicensees for use or consumption by the general public of such Product in any country in the Territory for which all Regulatory Approvals that are legally required in order to sell such Product in such country have been granted, and (ii) with respect to each NPS Compound Product or [*] Product, as the case may be, the first sale for which revenue has been recognized by NPS or its Affiliates or sublicensees for use or consumption by the general public of such NPS Compound Product or [*] Product in any country in the Territory for which all Regulatory Approvals and pricing or reimbursement approvals that are legally required in order to sell such NPS Compound Product or [*] Product in such country have been granted.
1.27 "GAAP"means generally accepted accounting principles, consistently applied.
1.28 "GSK Field" means the diagnosis and/or treatment and/or prophylaxis and/or palliation of [*] disorders, such as, but not limited to[*], such as, but not limited to [*]. "GSK Field" specifically excludes HPT and ADHH. For clarity, for purposes of payment for milestone achievement set forth in Section 5.1, the same application of the Product in connection with [*], or the approval of a Product as a first line therapy after being approved as a second line therapy for treatment of the same disease or condition shall not be deemed to be separate indications.
1.29 "GSK Inventions" has the meaning set forth in Section 11.2(a).
1.30 "GSK Licensed Ronacaleret Patents" has the meaning set forth in Section 3.2.
1.31 "GSK Royalty Term" has the meaning set forth in Section 5.2(a).
1.32 "HPT" means hyperparathyroidism, including, without limitation, renal osteodystrophy secondary to hyperparathyroidism, provided that HPT shall specifically exclude the diagnosis and/or treatment and/or prophylaxis and/or palliation of osteoporosis and bone metabolism disorders, such as, but not limited to, rheumatoid arthritis and osteoarthritis.
1.33 "IFRS"means International Financial Reporting Standards, consistently applied.
1.34 "IND" means mean an Investigational New Drug Application for a Transferred Compound filed by or on behalf of GSK with the FDA or equivalent application(s) filed with the appropriate regulatory authorities in any other country or territory of the world.
1.35 "Joint Inventions" has the meaning set forth in Section 11.1(b).
5
1.36 "Liabilities" means all debts, duties, liabilities and obligations, whether accrued or fixed, absolute or contingent, mature or unmatured or determined or determinable, including those arising under any Applicable Laws, those arising under any contract, agreement, commitment, instrument, permit, license, franchise or undertaking and those arising as a result of any act or omission.
1.37 "NDA" means a New Drug Application and all amendments and supplements thereto filed with the FDA.
1.38 "Negotiation Period" has the meaning set forth in Section 9.5(c).
1.39 "Net Sales"means with respect to any Product, the gross amounts invoiced by GSK, its Affiliates and sublicensees (each, a "Selling Party") to Third Party customers for sales of such Product, less the following deductions actually incurred, allowed, paid, accrued or specifically allocated in its financial statements in accordance with (as applicable to the Selling Party) GAAP or IFRS, for:
- customary and reasonable trade, quantity, and cash discounts and wholesaler allowances;
- customary and reasonable credits, rebates and chargebacks (including those to managed-care entities and government agencies), and allowances or credits to customers on account of rejection or returns (including, but not limited to, wholesaler and retailer returns) or on account of retroactive price reductions affecting such Product;
- freight, postage and duties, and transportation charges relating to such Product, including handling and insurance thereto; and
- sales (such as VAT or its equivalent) and excise taxes, other consumption taxes, customs duties and compulsory payments to governmental authorities and any other governmental charges imposed upon the importation, use or sale of such Product to Third Parties (excluding any taxes paid on the income from such sales) to the extent the Selling Party is not otherwise entitled to a credit or a refund for such taxes, duties or payments made; and
- the lesser of (1) two percent (2%) of the gross amount invoiced on sales of the Product in a particular country or (2) the actual amount of any write-offs for bad debt relating to such sales during the period in which a Selling Party has the obligation to pay a royalty; provided, that if any such written-off amounts are subsequently collected, such collected amounts shall be included in Net Sales in the period in which they are subsequently collected.
If non-monetary consideration is received for any Product, Net Sales will be calculated based on the average price charged for such Product during the preceding royalty period, or in the absence of such sales, the fair market value of the Product, as determined by GSK. Notwithstanding the foregoing, Net Sales shall not be imputed to transfers of Product for use in clinical trials, non-clinical Development activities or other Development activities, for bona fide charitable purposes or for compassionate use if no monetary consideration is received for such transfers. Sales between or among GSK and its Affiliates or sublicensees shall be excluded from
6
the computation of Net Sales except where such Affiliates or sublicensees are end users, but Net Sales shall include the subsequent final sales to Third Parties by such Affiliates or sublicensees.
If a Product is sold as part of a Combination Product (as defined below), Net Sales will be the product of (i) Net Sales of the Combination Product calculated as above (i.e., calculated as for a non-Combination Product) and (ii) the fraction (A/(A+B)), where:
"A" is the average wholesale acquisition cost of the Product comprising Ronacaleret as the sole therapeutically active ingredient during the four (4) most recently completed Calendar Quarters during which such non-Combination Products were sold in such country; and
"B" is the average wholesale acquisition cost in such country of the other therapeutically active ingredients contained in the Combination Product when sold separately during the four (4) most recently completed Calendar Quarters during which such products were sold in such country.
If "A" or "B" cannot be determined by reference to non-Combination Product sales as described above, then Net Sales for purposes of determining royalty payments will be calculated as above, but the average wholesale acquisition cost in the above equation shall be determined by GSK prior to the end of the accounting period in question based on an equitable method of determining same that takes into account, in the applicable country, variations in dosage units and the relative fair market value of each therapeutically active ingredient in the Combination Product.
As used in this Section 1.30, "Combination Product" means a Product that contains one or more additional active ingredients (whether co-formulated or co-packaged) that are neither Ronacaleret nor generic or other non-proprietary compositions-of-matter. Pharmaceutical dosage form vehicles, adjuvants and excipients shall be deemed not to be "active ingredients".
To the extent the Net Sales definition is used herein with respect to an NPS Compound Product or [*] Product, Net Sales shall have the meaning set forth above, with all references to "GSK" replaced by "NPS", all references to "Product" replaced with "NPS Compound Product" or "[*] Product", as applicable.
1.40 "Non-Breaching Party" has the meaning set forth in Section 8.2.
1.41 "NPS Compound Product" has the meaning set forth in Section 5.7.
1.42 "NPS Field" means any and all human therapeutic, prophylactic, palliative and diagnostic indications other than the GSK Field.
1.43 "NPS Inventions" has the meaning set forth in Section 11.2(b).
1.44 "NPS Licensed Ronacaleret Patents" has the meaning set forth in Section 3.1.
1.45 "NPSP558" has the meaning set forth in Section 7.1(d).
1.46 "[*]Product" has the meaning set forth in Section 9.5(b).
7
1.47 "NPS Compound Product Royalty Term" has the meaning set forth in Section 5.7.
1.48 "[*]Product Royalty Term" has the meaning set forth in Section 9.5(b).
1.49 "Original Field" means the diagnosis and/or treatment and/or prophylaxis and/or palliation of [*] disorders, such as, but not limited to, [*], but specifically excluding HPT.
1.50 "Other Know-How" means any and all Data obtained from the Research or that otherwise arose in the performance of activities under the Original Agreement, other than Ronacaleret Know-How, Transferred Compound Know-How or Existing Compound Know-How.
1.51 "Patents" means all patents and patent applications filed in any country of the world, including any continuation, continuation-in-part, division, provisional or any substitute applications, any patent issued with respect to any such patent applications, any reissue, reexamination, renewal or extension (including any supplementary protection certificate) of any such patent, any confirmation patent or registration patent or patent of addition based on any such patent, all SPCs and all foreign counterparts of any of the foregoing.
1.52 "Principle Mode of Action" means the predominate mechanism by which a composition of matter causes a biological and/or physiological effect.
1.53 "Proceeding" has the meaning set forth in Section 8.4(c).
1.54 "Product" means any pharmaceutical product comprising Ronacaleret, whether or not as the sole active ingredient and in any dosage form or formulation.
1.55 "Proof of Concept ("POC") Criteria" means clinical and non-clinical criteria (including without limitation safety, efficacy, chemistry, manufacturing and controls, and patentability criteria) established by GSK solely, to determine if Ronacaleret demonstrates a clinically meaningful benefit-to-risk profile in treating the applicable indication within the GSK Field. This shall include a target population that is reasonably representative of the proposed patient population; appropriate and validated endpoint(s) that may be used in further phases of development; doses in a final commercializable formulation that show efficacy with a reasonable safety and tolerability profile and efficacy responses that are consistent with those delineated in a target product profile and any other reasonable parameters as established by GSK. For the avoidance of doubt, the PoC Criteria may be different for each indication within the GSK Field.
1.56 "Proof of Concept ("POC") Trial" means a clinical trial that meets the requirements of 21 C.F.R. Section 312.21(b) and is intended to demonstrate the POC Criteria.
1.57 "Receiving Party" has the meaning set forth in Section 10.1.
1.58 "Regulatory Approval" means the approval, license or authorization of the applicable Regulatory Authority necessary for the marketing and sale of a product for a particular indication in a country in the Territory, including separate pricing or reimbursement approvals that may be required.
8
1.59 "Regulatory Authority" means the FDA in the U.S. or any health regulatory authority in another country in the Territory that is a counterpart to the FDA and holds responsibility for granting Regulatory Approval for a product in such country.
1.60 "Research" means the collaborative research program conducted by NPS and GSK under the Original Agreement, which was directed towards discovering Compounds. The term of the Research expired on May 31, 2003.
1.61 "Responsible Persons" has the meaning set forth in Section 10.2.
1.62 "Ronacaleret" means (a) the Compound known as Ronacaleret, as described inSchedule 1.62 and also known as 3-{3,4-Difluoro-5-[(R)-2-hydroxy-3-(2-indan-2-yl-1,1-dimethyl-ethylamino)-propoxy]-phenyl}-propionic acid hydrochloride and (b) free bases or acids, zwitterions, esters or other salts of the foregoing.
1.63 "Ronacaleret Know-How" means all Data, inventions and discoveries relating to Ronacaleret that are useful or required for the Development or commercialization of Ronacaleret in the GSK Field which was (a) developed by or became known to NPS or GSK during the term of the Original Agreement, (b) made legally available to NPS or GSK in accordance with agreements entered into underSection 2.02 of the Original Agreement, (c) in existence as of the Effective Date of the Original Agreement, or (d) with respect to stem cell indications, owned or Controlled by NPS as of the Effective Date of this Agreement, whether such Data was made or generated in the course of performing under the Original Agreement or otherwise.
1.64 "Ronacaleret Patents" means all Patents that, as of the Effective Date or during the Term of this Agreement, generically or specifically claim Ronacaleret, a process for manufacturing Ronacaleret, an intermediate used in such process, a method to formulate or deliver Ronacaleret, or a use of Ronacaleret, that are owned or Controlled by either Party or jointly by both Parties during the Term of this Agreement. For the avoidance of doubt, "Ronacaleret Patents" may disclose, describe or claim either Existing Compounds or Transferred Compounds in addition to Ronacaleret. The Ronacaleret Patents in existence as of the Effective Date are set forth onSchedule 1.64. For the avoidance of doubt, and by way of example only, if as of the Effective Date, a Patent discloses, but does not claim Ronacaleret generically or specifically, then such Patent is not a Ronacaleret Patent; provided, that if during the Term of this Agreement, such Patent issues or is otherwise modified in a manner that results in the claiming of Ronacaleret, then such Patent thereafter shall be deemed a Ronacaleret Patent for all purposes of this Agreement.
1.65 "Sale Date" has the meaning set forth in Section 9.5(b).
1.66 [*]
1.67 "SPCs" means a right which is not a Patent but which is based upon a Patent to exclude others from making, using or selling Ronacaleret, Existing Compounds or Transferred Compounds, as the case may be, such as a Supplementary Protection Certificate.
1.68 "Term" has the meaning set forth in Section 8.1.
9
1.69 "Territory" means all countries and territories of the world.
1.70 "Third Party" means any person or entity other than NPS or GSK that is not an Affiliate of NPS or of GSK.
1.71 "Transferred Assets" has the meaning set forth in Section 2.2.
1.72 "Transferred Compounds" means the `557 Compound and the `562 Compound.
1.73 "Transferred Compound Know-How" means all Data, inventions and discoveries solely relating to Transferred Compounds that are useful or required for the Development or commercialization of Transferred Compounds which was (a) developed by or became known to NPS or GSK during the term of the Original Agreement, (b) made legally available to NPS or GSK in accordance with agreements entered into underSection 2.02 of the Original Agreement, or (c) in existence as of the Effective Date of the Original Agreement.
1.74 "Transferred Compound Patents" means all Patents, which generically or specifically claim any Transferred Compound and/or Existing Compound, a process for manufacturing any Transferred Compound and/or Existing Compound, an intermediate used in such process, a method to formulate or deliver any Transferred Compound and/or Existing Compound, or a use of any Transferred Compound and/or Existing Compound, that are owned or Controlled by GSK or jointly by GSK and NPS as of the Effective Date. For the avoidance of doubt, Transferred Compound Patents do not include Patents that, as of the Effective Date or during the Term of this Agreement, claim Ronacaleret generically or specifically, and therefore, Transferred Compound Patents do not include Ronacaleret Patents. The Transferred Compound Patents are set forth onSchedule 1.74. For the avoidance of doubt and by way of example only, if as of the Effective Date, a Patent discloses, but does not claim, Ronacaleret generically or specifically, and claims a Transferred Compound and/or Existing Compound, then such Patent is a Transferred Compound Patent; provided, that if during the Term of this Agreement, such Patent issues or is otherwise modified in a manner that results in the claiming of Ronacaleret, then such Patent thereafter shall be deemed a Ronacaleret Patent for all purposes of this Agreement.
1.75 "Transferred Compound Regulatory Information" has the meaning set forth in Section 2.2(a)(iv).
2. TERMINATION OF THE ORIGINAL AGREEMENT.
2.1 Termination. The Parties agree that the Original Agreement is terminated effective as of the Effective Date, except (i) as expressly referenced in this Agreement, or (ii) with respect to the indemnity obligations under Sections 19.06, 19.07, 19.08 and 19.09 of the Original Agreement, which provisions shall continue to survive in accordance with the terms of the Original Agreement. Neither Party shall have any further liability nor obligation under the Original Agreement, including without limitation, any provisions which survive termination of the Original Agreement as provided for under Article 13 of the Original Agreement.
2.2 Transfer of Transferred Compounds.
10
- GSK hereby assigns, grants, transfers, and delivers to NPS and NPS shall accept from GSK, free and clear of all Liens, all of GSK's rights, title and interest in and to the assets listed below, which are referred to herein collectively as the "Transferred Assets", as more fully set forth onSchedule 2.2:
- samples and inventory of the Transferred Compounds (whether packaged, in bulk form, or work-in-process);
- the Transferred Compound Patents (and all file wrappers related thereto);
- the Transferred Compound Know-How;
- the INDs relating to the Transferred Compounds, all regulatory documentation generated solely in connection with the Transferred Compounds, including all correspondence and other documentation related to communications to or from the FDA or other Regulatory Authorities ("Transferred Compound Regulatory Information"); and
- all rights and claims or causes of action against Third Parties relating to any of the Transferred Assets arising from or based on events or circumstances occurring or existing or omissions to act occurring prior to the Effective Date.
All properties, assets, and rights of GSK or GSK's Affiliates not specifically listed and identified in this Section 2.2(a) or onSchedule 2.2are not part of the transfer contemplated hereunder, are excluded from the Transferred Assets, shall be retained by GSK, and shall remain the property of GSK after the Effective Date. Notwithstanding the foregoing, (1) NPS shall permit GSK to access the Transferred Assets solely to the extent GSK transferred the original forms of such Transferred Assets and access to such originals are required by a Regulatory Authority, and (2) GSK shall permit NPS to access records supporting the underlying methodology of preclinical studies of the Transferred Compounds, such as, for example, records relating to personnel training or study room environmental conditions, solely to the extent required by a Regulatory Authority.
No Transfer of Existing Compound Know-How. GSK shall not be required to transfer any Existing Compound Know-How to NPS, or otherwise enable NPS to conduct any research, Development or commercialization of Existing Compounds other than the assignment of Transferred Compound Patents as described in this Section 2.2.No Transfer of Liabilities. As between NPS and GSK, GSK will be solely responsible for and pay, perform and/or otherwise discharge when due those Liabilities (including any Liabilities arising in respect of taxes) arising out of or in connection with or related to the Transferred Assets or the use thereof, arising on or prior to the Effective Date, including any claims filed after the Effective Date that relate to periods prior to the Effective Date. As between NPS and GSK, NPS will be solely responsible for Liabilities (including any Liabilities in respect of taxes) arising out of or in connection with or related to the Transferred Assets or the use, marketing or sale thereof, arising after the Effective Date (except to the extent such Liabilities relate to activities or events first arising prior to the Effective Date).11
- Assignment Agreement. GSK shall execute and deliver an Assignment Agreement in the form attached hereto as Exhibit A to further effectuate such assignment. The Parties shall use reasonable efforts to cooperate as needed to complete and execute the Assignment Agreement within forty-five (45) days after the Effective Date.
- Delivery of Tangible Transferred Assets. As soon as practicable (and in any case within one hundred eighty (180) days) after the Effective Date (the "Delivery Period"), GSK shall have delivered all tangible assets included in the Transferred Assets at NPS's risk and expense. All such tangible assets included in the Transferred Assets shall be shipped EXW GSK's facilities (as defined in INCOTERMS, 2010 edition, published by the International Chamber of Commerce). From and after making the tangible assets included in the Transferred Assets available for pickup at GSK's facilities, NPS shall bear all risk of loss for such items and shall be solely responsible for all tangible assets included in the Transferred Assets against any such loss.
- EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, THE TRANSFERRED ASSETS ARE BEING TRANSFERRED "AS IS, WHERE IS, WITH ALL FAULTS," AND GSK MAKES NO REPRESENTATION OR WARRANTY, EXPRESS OR IMPLIED, ORAL OR WRITTEN, AT LAW OR IN EQUITY, IN RESPECT OF THE CONDITION, VALUE OR QUALITY OF THE TRANSFERRED ASSETS, AND THE PROSPECTS (FINANCIAL OR OTHERWISE), RISKS AND OTHER INCIDENTS OF THE TRANSFERRED ASSETS, INCLUDING WITH RESPECT TO ANY WARRANTY OF MERCHANTABILITY, NON-INFRINGEMENT, VALIDITY, SCOPE OR ENFORCEABILITY, SUITABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE, OR ANY OTHER MATTER WITH RESPECT TO THE TRANSACTIONS CONTEMPLATED HEREBY, AND ANY SUCH OTHER REPRESENTATIONS OR WARRANTIES ARE HEREBY EXPRESSLY DISCLAIMED.
3. LICENSE GRANTS.
3.1 By NPS.Subject to the terms and conditions of this Agreement, NPS hereby grants to GSK an exclusive license, with the right to grant sublicenses, under NPS' interests and rights in the Ronacaleret Patents set forth onSchedule 3.1 (the "NPS Licensed Ronacaleret Patents") and Ronacaleret Know-How owned or Controlled by NPS, to make, have made, use, offer for sale, sell and import Ronacaleret in the GSK Field in the Territory. NPS retains its rights in the NPS Licensed Ronacaleret Patents and Ronacaleret Know-How for all other purposes, subject to Section 7.2(d).
3.2 By GSK. Subject to the terms and conditions of this Agreement, GSK hereby grants to NPS an exclusive license, with the right to grant sublicenses, under GSK's interests and rights in the Ronacaleret Patents set forth onSchedule 3.2 (the "GSK Licensed Ronacaleret Patents") and Ronacaleret Know-How owned or Controlled by GSK, to make, have made, use, offer for sale, sell and import Transferred Compounds and/or Existing Compounds in the NPS Field in the Territory.GSK retains its rights in the GSK Licensed Ronacaleret Patents and Ronacaleret Know-How to make, have made, use, offer for sale, sell and import Ronacaleret in the GSK Field.
12
3.3 GSK Option for Additional Indications. If, during the Term, GSK identifies additional indications for Ronacaleret outside of the GSK Field, then the parties agree to negotiate in good faith a mutually acceptable agreement to allow GSK to develop, manufacture and commercialize Ronacaleret in such additional indications.
3.1 Other Know-How; No Other Rights. Each Party retains all of its rights to Other Know-How owned or Controlled by it to the extent of its individual or joint interest therein, as applicable. Each Party will be entitled to practice and sublicense Other Know-How without restriction or consent of the other or an obligation to account to the other Party, whether such Other Know-How is owned or Controlled solely by such Party or jointly by the Parties. Nothing under this Agreement is deemed to grant to a Party any right to or interest in the Other Know-How owned or Controlled by the other Party. Except as expressly stated herein, neither Party shall have any other right to use, or interest in, any intellectual property owned or Controlled by the other Party. Neither Party makes any grant of intellectual property rights by implication.
4. GOVERNANCE.
4.1 Alliance Managers.Promptly after theEffective Date, eachPartyshall appoint an individual to act as alliance managerforsuchParty(each, an "Alliance Manager"). TheAlliance Managersshall be the primary point of contactforthePartiesregarding the activities contemplated by thisAgreementand the provision of reports as set forth in Section 4.2. The name and contact informationforsuchAlliance Managers, as well as any replacement(s) chosen by NPS orGSK, in their sole discretion, from time to time, shall be promptly provided to the otherPartyin accordance with Section 14.6 of thisAgreement; provided, that GSK has provided name and contact information for its Alliance Manager to NPS prior to the Effective Date.
4.2 Reporting. GSK will submit reasonably detailed reports to NPS on an annual basis, regarding Development activities with respect to Ronacaleret and Products, commencing on the first anniversary of the Effective Date. All such reports will be considered Confidential Information of GSK. Such reports and any other related communications shall be handled via the Alliance Managers.
5. FINANCIAL TERMS.
5.1 Milestone Payments.
- In partial consideration for the rights granted to GSK hereunder, GSK shall make the non-refundable, non-creditable milestone payments to NPS set forth below upon the first achievement by GSK, or its respective Affiliates or sublicensees of the corresponding milestone events with respect to Ronacaleret in the GSK Field (other than indications in the Original Field):
Milestone | Payment |
The decision by GSK to continue Development in the first indication following the [*] | U.S. $[*] |
12
First dosing of the fifth subject in the first Phase III clinical trial for each indication |
U.S. $[*]
|
First Commercial Sale for the first indication |
U.S. $[*]
|
First Commercial Sale for [*] |
U.S. $[*]
|
- As additional consideration for the rights granted to GSK hereunder, GSK shall make the non-refundable, non-creditable milestone payment to NPS set forth below upon the first achievement by GSK, or its respective Affiliates or sublicensees of the corresponding milestone event with respect to Ronacaleret in the Original Field:
Milestone | Payment |
Acceptance of NDA filing for review by FDA |
U.S. $[*]
|
- For the avoidance of doubt, the milestones set forth in Section 5.1(a) for "The decision by GSK to continue Development in the first indication following the [*]" and "First Commercial Sale for the first indication" are payable only one time each. In addition, notwithstanding anything in the definition of "Development" to the contrary, the milestone set forth in Section 5.1(a) entitled "The decision by GSK to continue Development in the first indication following the [*]" shall be deemed to have been met upon the earlier to occur of either GSK's determination that the [*] have been achieved following such [*], or the dosing of the first patient in a [*] for the first indication if GSK determines that the [*] have not been achieved following the [*]. Upon achievement by or on behalf of GSK, its Affiliates or sublicensees of a milestone event, GSK shall promptly (but in no event more than ten (10) Business Days after achievement thereof) notify NPS of such achievement, and GSK shall pay NPS the corresponding milestone payment within sixty (60) after receipt of an invoice from NPS for the milestone payment. Invoices shall be sent in PDF format to GSK's Alliance Manager and Mark.R.Hancock@gsk.com with a copy to Robin.B.Markovitz@gsk.com (or such other email address(es) as may be notified to NPS by GSK.
5.2 Royalty Payments by GSK.
- As additional consideration for the licenses granted to GSK underSection 3.1 of this Agreement, GSK shall pay NPS incremental royalties on worldwide annual Net Sales of the Product, at the royalty rates set forth in the table below:
Worldwide Annual Net Sales | Royalty Rate |
Net Sales up to and including [*] |
[*]%
|
Net Sales in excess of [*] | [*]% |
14
For illustrative purposes only, if worldwide annual Net Sales of a Product were $[*], the royalties payable with respect to such annual Net Sales, subject to adjustment as set forth in this Section 5.2 below and Section 6.2, would be [*]. GSK's royalty obligations to NPS under thisSection 5.2(a) in each country shall commence on a country-by-country and Product-by-Product basis on the date of First Commercial Sale of the Product by GSK, its Affiliates or sublicensees to a Third Party in the relevant country, and shall expire on a country-by-country and Product-by-Product basis upon the later of the following: (i) the expiration or invalidation of the last remaining NPS Licensed Ronacaleret Patent in such country which claims the composition of matter or method of using Ronacaleret or the Product or (ii) the tenth (10th) anniversary of the First Commercial Sale of such Product in such country by GSK, its Affiliates or sublicensees (the "GSK Royalty Term").
- The foregoing provisions of Section 5.2(a) notwithstanding, the royalties payable with respect to Net Sales of a Product shall be reduced, on a country-by-country basis, to [*] of Net Sales during any portion of the GSK Royalty Term when (i) all NPS Licensed Ronacaleret Patents in such country that claim the composition of matter or method of using Ronacaleret or the Product have lapsed, been abandoned or held invalid or unenforceable by a decision of a court or tribunal of competent jurisdiction from which no appeal is or can be taken; and (b) substantial competition exists in such country. "Substantial competition", as used in thisSection 5.2(b), means a composition of matter that either is the same chemical entity or has the same mechanism of action as Ronacaleret, and the period of substantial competition shall be applied retroactively to the date of first commercial sale of such substantial competition in such country, and shall continue only for so long as such commercialization continues.
- Royalties on Net Sales of the Product for veterinary use, diagnostic use or any other use other than a human ethical pharmaceutical use shall be calculated separately at [*] of the royalty rates set forth inSections 5.2(a) or5.2(b), as applicable.
5.3 Records. GSK shall keep, and require its Affiliates and sublicensees to keep, complete and accurate records of all sales of Products for a period of three (3) years after the relevant payment is owed to NPS pursuant toSection 5.2(a) or(b). Upon ninety (90) days' prior written notice, NPS shall have the right to examine such records during regular business hours using a certified public accountant or like person reasonably acceptable to GSK. Such examination shall not be performed more frequently than once in any twelve (12)-month period, and shall be conducted under appropriate confidentiality provisions, for the sole purpose of verifying the accuracy and completeness of all financial, accounting and numerical information and calculations provided under this Agreement with respect to royalties paid under Section 5.2 and reports provided under Section 5.4. The certified public accountant shall have the right to make copies of relevant portions of GSK's books and records; provided, that any such copies shall be the Confidential Information of GSK, shall be protected by appropriate confidentiality obligations and shall not be shared with NPS or any Third Party. The sole deliverable to NPS shall be a copy of the accounting firm's final report, which also shall be provided to GSK at the same time. Such examination is to be made at the expense of NPS, except if the results of the
15
audit reveal an underpayment of royalties under this Agreement of five percent (5%) or more in any calendar year, in which case reasonable audit fees for such examination shall be paid by GSK. Any underpayment determined by such audit shall be paid promptly to NPS.
5.4 Reports. Until the expiration of all applicable Royalty Terms in all countries, GSK shall make written reports to NPS within [*] days afterthe end of eachcalendar quarter, setting forthNet Sales on a country-by-country and Product-by-Product basis and the royalties due to NPSby GSK during the preceding calendar quarter. Concurrent with the delivery of each such report,GSK shall make the royalty payment due toNPSas set forth in the report.
5.5 Payment Terms.
- All royalties due under this Agreement shall be payable in U.S. dollars. If governmental regulations prevent remittances from a country to any other country with respect to sales made in that country, the obligation of GSK to pay royalties on sales in that country shall be suspended until such remittances are possible, and once they are possible, GSK shall pay NPS any back royalties which may be owed. Alternatively, NPS shall have the right, upon giving written notice to GSK, to receive payment in that country in local currency.
- With respect to sales of the Product invoiced in Dollars, the Net Sales and the amounts due hereunder will be expressed in Dollars. With respect to Net Sales invoiced in a currency other than Dollars, the Net Sales and amounts due hereunder will be reported in Dollars, calculated using the standard methodologies employed by GSK for consolidation purposes. As of the Effective Date, the method utilized by GSK's group reporting system uses spot exchange rates sourced from Reuters/Bloomberg.
5.6 Taxes. Any tax paid or required to be withheld by GSK on account of royalties payable to NPS under this Agreement shall be deducted from the amount of royalties otherwise due. GSK shall secure and send to NPS written proof of any such taxes withheld and paid by GSK, its Affiliates or its sublicensees for the benefit of NPS in a form sufficient to satisfy the United States Internal Revenue Service.
5.6 Royalty Payments by NPS.As consideration for the assignment of Transferred Compound Patents related to Existing Compounds to NPS under Section 2.2 of this Agreement, NPS shall pay GSK a royalty rate of [*] on worldwide annual Net Sales of any pharmaceutical product comprising an Existing Compound, whether or not as the sole active ingredient and in any dosage form or formulation ("NPS Compound Product"). NPS' royalty obligations under thisSection 5.7 shall commence on a country-by-country and NPS Compound Product-by-NPS Compound Product basis on the date of First Commercial Sale of the NPS Compound Product by NPS, its Affiliates or sublicensees to a Third Party in the relevant country, and shall expire on a country-by-country and NPS Compound Product-by-NPS Compound Product basis upon the later of the following: (i) the expiration or invalidation of the last remaining Transferred Compound Patent in such country which claims the composition of matter or method of using the Existing Compound or the NPS Compound Product or (ii) the tenth (10th) anniversary of the First Commercial Sale of such NPS Compound Product in such country by NPS, its Affiliates or sublicensees (the "NPS Compound Product Royalty Term"). The terms of Sections 5.3, 5.4, 5.5 and 5.6 would applymutatis mutandis to the payment of royalties to GSK under this Section 5.7.
16
6. COMPULSORY LICENSES AND THIRD PARTY LICENSES.
6.1 Compulsory Licenses. In the event that a governmental agency in any country or territory grants or compels NPS to grant a license to the NPS Licensed Ronacaleret Patents to any Third Party to make, have made, use or sell Ronacaleret, GSK shall have the benefit in such country or territory of the terms granted to such Third Party to the extent that such terms are more favorable to the Third Party than those of this Agreement.
6.1 Third Party Licenses. If, during the Term, GSK's Average Gross Margin on Net Sales of the Product during a particular calendar year falls below [*] in a particular country as a result of royalty payments made by GSK to Third Parties on such Net Sales for license rights to Patents or Data owned or Controlled by such Third Parties that are necessary for the manufacture, use, offer for sale, sale or importation of such Product in such country, then GSK's royalty obligations underSection 5.2(a) shall be reduced by the amount required to raise GSK's Average Gross Margin to [*]; provided that the reduction shall not be greater than (x) [*] of the total royalty payable by GSK to such Third Parties or (y) [*] of the royalty payable by GSK to NPS on such Net Sales in such country underSection 5.2(a). "Average Gross Margin" means Net Sales during a calendar year minus Cost of Goods Sold attributable to the Product sold, minus all royalties payable by GSK to NPS and all Third Parties on such Net Sales.
7. DEVELOPMENT AND COMMERCIALIZATION.
7.1 Development and Commercialization of Ronacaleret by GSK.
- GSK shall have full control, authority and responsibility for the Development, manufacture and commercialization of Ronacaleret within the GSK Field in its sole discretion, and shall use Commercially Reasonable Efforts with respect thereto; provided, that GSK shall inform NPS promptly after any decision to discontinue all Development and/or commercialization of Ronacaleret has been made by the applicable management committee within GSK responsible for making such decisions, and the material rationale for such discontinuance. All such Development, manufacture and commercialization of Ronacaleret as described above shall be undertaken at GSK's expense.
- GSK shall keep NPS reasonably informed of the progress of GSK's efforts relating to the Development of Ronacaleret in the GSK Field as set forth in Section 4.2.
- NPS shall provide to GSK, at GSK's request and expense, reasonable technical assistance within its area of expertise concerning Development, registration, production and commercialization of Ronacaleret.
- GSK understands that NPS currently is developing recombinant full-length parathyroid hormone ("NPSP558") for the prevention, diagnosis and treatment of hypoparathyroidism. GSK shall not, and shall cause its Affiliates to not, directly or indirectly through any Third Party, make, have made, use, sell or otherwise develop or commercialize Ronacaleret for the prevention, diagnosis or treatment of hypoparathyroidism if NPS, its
17
Affiliates or sublicensees launches a pharmaceutical product containing NPSP558 on or before June 1, 2017. The foregoing covenant shall not prevent, limit or restrict GSK or any of its Affiliates or licensees from making, having made, using, selling or otherwise developing or commercializing any compound or product other than Ronacaleret for the prevention, diagnosis or treatment of hypoparathyroidism. In addition, nothing in the foregoing covenant shall prohibit GSK from selling Ronacaleret in the GSK Field, even if the particular indication within the GSK Field involves modulation of parathyroid hormone.
- GSK shall not, and shall cause its Affiliates to not, directly or indirectly through any Third Party, make, have made, use, sell or otherwise Develop or commercialize Transferred Compounds or Existing Compounds.
7.2 Development and Commercialization of Transferred Compounds and Existing Compounds by NPS.
- NPS shall have full control, authority and responsibility for the Development, manufacture and commercialization of Transferred Compounds and Existing Compounds within the NPS Field, in its sole discretion, and shall use Commercially Reasonable Efforts with respect thereto. All such activities as described above shall be undertaken at NPS' expense.
- For a period of three (3) months following the expiration of the Delivery Period, GSK shall provide to NPS, at NPS' request and expense, reasonable technical assistance within its area of expertise concerning Development, registration, production and commercialization of Transferred Compounds and Existing Compounds.
- NPS shall not, and shall cause its Affiliates to not, directly or indirectly through any Third Party, make, have made, use, sell or otherwise Develop or commercialize the Transferred Compounds and Existing Compounds in the GSK Field. The Parties understand and agree that the foregoing covenant shall not prevent, limit or restrict NPS, its Affiliates or any of its licensees from making, having made, using, selling or otherwise Developing or commercializing any compound or product other than the Transferred Compounds and Existing Compounds in any field of use or indication, including, without limitation, ADHH.
- NPS shall not, and shall cause its Affiliates to not, directly or indirectly through any Third Party, make, have made, use, sell or otherwise Develop or commercialize Ronacaleret.
8. TERM AND TERMINATION.
8.1 Term. This Agreement shall become effective on the Effective Date and, unless earlier terminated pursuant to this Article 8, shall remain in effect until it expires (the "Term") as follows:
- On a Product-by-Product and country-by-country basis, this Agreement shall expire on the date of the expiration of all applicable GSK Royalty Terms with respect to such Product in such country; and
18
- This Agreement shall expire in its entirety upon the expiration of all applicable GSK Royalty Terms under this Agreement with respect to all Products in all countries in the Territory.
- Upon expiration of the Term, GSK shall have a fully paid-up, non-exclusive, perpetual license to use the applicable NPS Licensed Ronacaleret Patents and Ronacaleret Know-How to make, have made, use, sell, offer for sale and import Ronacaleret and Products in the GSK Field. To the extent that any [*] Product Royalty Term or NPS Compound Product Royalty Term survives expiration of the Term, NPS shall be obligated to continue to pay royalties to GSK for the remainder of the applicable [*] Product Royalty Term or NPS Compound Product Royalty Term.
8.2 Termination for Default.Either Party (the "Non- Breaching Party") may, without prejudice to any other remedies available to it under Applicable Law or in equity, terminate this Agreement if the other Party (the "Breaching Party") shall have materially breached or defaulted in the performance of its obligations hereunder, and such default shall have continued for sixty (60) days after written notice thereof was provided to the Breaching Party by the Non-Breaching Party, such notice describing the alleged breach. Any such termination of this Agreement shall become effective at the end of such sixty (60) day cure period, unless:
- the Breaching Party has cured such breach or default prior to the expiration of such cure period; or
- such breach is not susceptible to cure within such cure period even with the use of Commercially Reasonable Efforts, in which event the Non-Breaching Party's right to termination shall be suspended only if and for so long as (i) the Breaching Party has provided to the Non-Breaching Party a written plan that is reasonably calculated to effect a cure, (ii) such plan is reasonably acceptable to the Non-Breaching Party, and (iii) the Breaching Party commits to and does carry out such plan.Where theNon-breachingPartyhas accepted any such plan in accordance with the preceding sentence, theNon-breachingPartymay terminate thisAgreementimmediately upon written notice to theBreachingPartyif theBreachingPartysubsequently fails to carry out such plan.
8.3 Country Specific Termination. GSK may terminate this Agreement with respect to any country or all countries by giving NPS at least thirty (30) days written notice thereof (the "Country Termination Notice") based on a reasonable determination by GSK, using the same standards GSK would use in assessing whether or not to continue Development or commercialization of a product solely and exclusively owned by it, that the patent, medical/scientific, technical, regulatory or commercial profile of Ronacaleret does not justify continued Development or commercialization of Ronacaleret in such country or countries; provided, that NPS may delay the effective date of such termination for up to six (6) months (the "Delay Period") in any country in which GSK, its Affiliates or sublicensees are commercializing Ronacaleret on the date on which GSK provides the Country Termination Notice if, within sixty (60) days of NPS' receipt of the Country Termination Notice, NPS (a) gives written notice to GSK that NPS intends to continue the commercialization of Ronacaleret on its own or through Third Party sublicensees, in accordance with a reasonable marketing plan provided to GSK with
19
such notice and (b) during the Delay Period, NPS uses Commercially Reasonable Efforts to implement such plan. If NPS uses Commercially Reasonable Efforts to implement such marketing plan during the Delay Period, then GSK hereby grants to NPS an exclusive license to Ronacaleret Patents and Ronacaleret Know-How owned or Controlled by GSK to make, have made, use, offer to sell, sell and import Ronacaleret in the GSK Field in such terminated countries as described in thisSection 8.3.Termination of this Agreement with respect to any country under thisSection 8.3 shall terminate all licenses granted to GSK in such country underSection 3.1 with full reversion to NPS of all NPS' interest and rights in NPS Licensed Ronacaleret Patents and Ronacaleret Know-How in such country. Termination by GSK of this Agreement with respect to any countries or all countries under this Section 8.3 shall not affect the license granted to NPS by GSK in accordance with Section 3.2 which shall remain in effect after any such termination, subject to all applicable terms and conditions of this Agreement.
8.4 Termination for Bankruptcy.
- Either Party may terminate this Agreement if, at any time, the other Party shall file in any court or agency pursuant to any statute or regulation of any state or country, a petition in bankruptcy or insolvency or for reorganization or for an arrangement or for the appointment of a receiver or trustee of the Party or of its assets, or if such Party proposes a written agreement of composition or extension of its debts, or is served with an involuntary petition against it, filed in any insolvency proceeding, and such petition is not dismissed within sixty (60) days after the filing thereof, or if such Party shall propose or be a party to any dissolution or liquidation, or if such Party shall make an assignment for the benefit of creditors.
- Notwithstanding the bankruptcy of NPS, or the impairment of performance by NPS of its obligations under this Agreement as a result of bankruptcy or insolvency of NPS, GSK shall be entitled to retain the licenses granted herein, subject to NPS' rights to terminate this Agreement for reasons other than bankruptcy or insolvency as expressly provided in this Agreement, and subject to performance by GSK of its preexisting obligations under this Agreement. Notwithstanding the bankruptcy of GSK, or the impairment of performance by GSK of its obligations under this Agreement as a result of bankruptcy or insolvency of GSK, NPS shall be entitled to retain the licenses granted herein, subject to GSK's rights to terminate this Agreement for reasons other than bankruptcy or insolvency as expressly provided in this Agreement, and subject to performance by NPS of its preexisting obligations under this Agreement.
- All rights and licenses granted under or pursuant to this Agreement by NPS to GSK, and by GSK to NPS, are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of rights to "intellectual property" as defined under Section 101(35A) of the U.S. Bankruptcy Code. The Parties agree that each Party, as a licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code, or any other provisions of Applicable Law outside the United States that provide similar protection for intellectual property rights, subject to performance by the licensee of its preexisting obligations under this Agreement. The Parties further agree that, in the event that any proceeding shall be instituted by or against licensor under the U.S. Bankruptcy Code seeking to adjudicate it bankrupt or insolvent, or seeking liquidation, winding up, reorganization, arrangement, adjustment, protection, relief or composition of it or its
20
debts under any law relating to bankruptcy, insolvency or reorganization or relief of debtors, or seeking an entry of an order for relief or the appointment of a receiver, trustee or other similar official for it or any substantial part of its property or it shall take any action to authorize any of the foregoing actions (each a "Proceeding"), the licensee shall have the right to retain and enforce its rights under this Agreement, including but not limited to the following rights:
- The right to continue to use the relevant license materials and all versions and derivatives thereof, and all documentation and other supporting material related thereto, in accordance with the terms and conditions of this Agreement; and
- The right to a complete duplicate of (or complete access to, as appropriate) all relevant license materials, intellectual property, and all embodiments of the foregoing, and the same, if not already in licensee's possession. Such materials shall be promptly delivered to licensee (1) upon any such commencement of a Proceeding upon written request therefor by licensee, unless licensor elects to continue to perform all of its obligations under this Agreement; or (2) if not delivered under (1) above, upon the rejection of this Agreement by or on behalf of licensor upon written request therefor by licensee;
- The right to obtain from licensor all documentation and other supporting materials related to the relevant license materials and intellectual property, and all versions and derivatives thereof.
8.5 Termination for Non-Development of Ronacaleret.NPS may terminate this Agreement upon ninety (90) days prior written notice to GSK if Ronacaleret is not in Development or under commercialization by GSK, its Affiliates or sublicensees for a period of [*]. Upon receipt of such written notice, GSK will have forty-five (45) days from the receipt of such notice to verify to NPS that it either (i) is [*] Ronacaleret, (ii) has Ronacaleret [*] or (iii) intends to have Ronacaleret enter [*]. If GSK reasonably demonstrates such intent to the reasonable satisfaction of NPS, there shall be no such termination.
9. RIGHTS AND DUTIES UPON TERMINATION.
9.1 Payments. Upon termination of this Agreement, each Party shall have the right to retain any sums already paid to it hereunder, and each Party shall pay all sums accrued hereunder which are then due.
9.2 Effect of Termination for Default.
- In the event that GSK terminates this Agreement pursuant toSection 8.2 for a default by NPS of Section 7.2(c) or (d), all rights and obligations hereunder shall terminate; provided, however, that GSK shall retain all licenses granted to it by NPS under this Agreement and have the right to continue to exercise its rights thereunder, subject to the payment to NPS of [*] set forth in Section 5.2.
- In the event that NPS terminates this Agreement pursuant toSection 8.2 for a default by GSK, all rights and obligations hereunder shall terminate; provided, however, that NPS shall retain all licenses granted to it by GSK under this Agreement and have the right to continue to exercise its rights thereunder.
21
9.3 Right to Sell Off Inventory. Termination of this Agreement with respect to all countries underSection 8.3 shall terminate GSK's obligation to make any remaining payments required bySection 5 for periods after the effective date of termination.Upon termination of this Agreement in its entirety or with respect to any country underSection 8.3, GSK shall notify NPS of the amount of Product that GSK, its Affiliates, sublicensees and distributors then have on hand, the sale of which would, but for the termination, be subject to royalty, and GSK, its Affiliates, sublicensees and distributors shall thereupon be permitted to sell that amount of Ronacaleret provided that GSK pays the royalty thereon at the time herein provided for.
9.4 Effect of Termination for Bankruptcy.
- If, as a result of the happening of any event described inSection 8.4(a), any tangible or intangible asset owned by NPS relating to Ronacaleret (e.g., intellectual property) is offered for sale or license, in addition to other remedies in law or equity which may be available to GSK, GSK shall have a right of first refusal with respect to the asset; provided, that to the extent the asset also relates to subject matter other than Ronacaleret, GSK's right of first refusal shall extend only to so much of the asset as relates to Ronacaleret.
- If, as a result of the happening of any event described inSection 8.4(a), any tangible or intangible asset owned by GSK relating to the Transferred Compounds or Existing Compounds (e.g., intellectual property) is offered for sale or license, in addition to other remedies in law or equity which may be available to NPS, NPS shall have a right of first refusal with respect to the asset; provided, that to the extent the asset also relates to subject matter other than the Transferred Compounds or Existing Compounds, NPS' right of first refusal shall extend only to so much of the asset as relates to the Transferred Compounds or Existing Compounds.
9.5 [*].
- If this Agreement is terminated by [*], then the following shall apply:
- All rights and licenses granted by[*]in this Agreement will automatically terminate;
- [*]will promptly assign to[*] all regulatory filings and regulatory approvals and all data, materials and information supporting such regulatory approvals, owned by[*]or, if such assignment is not legally permissible, grant[*] the right to access, use and cross-reference such filings, approvals and data;
- [*] will grant to[*] the non-exclusive right and license to use[*] Patents owned by[*], necessary to develop, manufacture or commercialize[*]; and
- [*] will assume complete responsibility for the preparation, filing, prosecution and maintenance of all[*]Patents, and all costs associated therewith in a prompt manner.[*] may request that[*] provide such reasonable assistance as is necessary to[*] in such efforts and[*] shall be entitled to reimbursement for any and all costs incurred by[*] in such efforts.
22
- Upon termination of this Agreement in accordance with [*], and in consideration of the transfer of rights to [*] under thisSection 9.5, if [*] subsequently commercializes [*] will pay to [*] a royalty of [*] of worldwide Net Sales of [*] (for purposes of thisSection 9.5, the "[*]Product") billed or invoiced by [*] or its Affiliates or licensees, and in such event, the provisions of Sections 5.3, 5.4, 5.5 and 5.6 would applymutatis mutandis to the payment of royalties [*] under this Section 9.5(b). Royalties shall be payable on a country-by-country basis, commencing upon the First Commercial Sale of the [*] Product in such country by [*], its Affiliates or a licensee (or, if [*], its Affiliates or licensees are continuing [*] commercialization of [*], then the date upon which [*], its Affiliates or licensees assume responsibility for the selling and booking of sales of [*]) (the foregoing collectively referred to as the "Sale Date"), until the later of either (a) ten (10) years from the Sale Date in such country, or (b) the expiration or invalidation of the last remaining [*] Patent in such country which claims the composition of matter or method of using [*] the Product (the "[*]Product Royalty Term").
- If after the termination of the Agreement pursuant to [*], and the subsequent transfer of rights to [*] as set forth in Section 9.5, [*] desires to sell or offer for sale [*] in the Territory, then [*] will give [*] written notice of such desire, and [*] will have an exclusive right of first negotiation to obtain such rights on an exclusive basis. Such written notification will include all relevant data and information required to enable [*] to make an informed decision. Within ninety (90) days of [*] receipt of [*] written notice, [*] shall notify [*] whether it desires to pursue an exclusive arrangement for such rights. If [*] so notifies [*], then [*] shall have, for a period of sixty (60) days thereafter (the "Negotiation Period") the exclusive right to negotiate the terms of such exclusive arrangement. If no agreement is reached within the Negotiation Period, then [*] shall not, within the three (3) month period thereafter, offer to, or accept from any Third Party, any terms that are less favorable on the whole to [*] than the last terms offered by [*] without first offering such terms to [*]. Thereafter, [*] may pursue discussions with Third Parties with respect to such commercialization rights.
9.6 Remedies. All rights to terminate, and rights upon termination, provided for either Party in this Agreement are in addition to other remedies in law or equity which may be available to either Party.
9.7 Survival. The following Articles and Sections shall survive any expiration or termination of this Agreement: Article 1, Article 2, Section 3.4, Sections 5.2 through and including 5.7 with respect to amounts due and owing, audit rights and obligations to maintain records, Section 6.2 with respect to amounts due and owing, Section 8.4(c), Articles 9 and 10, Sections 11.1(a) and 11.1(b), and Articles 12, 13 and 14, as well as any other provisions which are expressed to survive termination or expiration or which are required to give effect to such termination or expiration.
10. CONFIDENTIALITY.
10.1 Definition of Confidential Information. "Confidential Information" means any and all proprietary information furnished or made available to one Party (the "Receiving Party") by the other Party (the "Disclosing Party") relating to the Disclosing Party's current or future business, or any other proprietary information of the Disclosing Party whether in written, oral, electronic or other form. For purposes of clarification, all data, information and know-how
23
contained in any Transferred Assets shall be deemed to be Confidential Information of NPS, and all Confidential Information exchanged during the term of the Original Agreement shall remain subject to the terms and conditions of this Article 10. In addition, the terms and conditions of this Agreement are hereby deemed to be Confidential Information of both Parties.
10.2 Obligations of Nonuse and Nondisclosure. The Receiving Party (a) shall hold the Disclosing Party's Confidential Information in strict confidence, (b) shall not disclose such Confidential Information to any Third Party and shall employ reasonable practices and procedures necessary to prevent such disclosure, which steps shall include at least those taken by the Receiving Party to protect its own confidential information of like kind, and (c) shall not use such Confidential Information unless the Disclosing Party has agreed otherwise in writing. Notwithstanding the foregoing, the Receiving Party may disclose the Disclosing Party's Confidential Information to the Receiving Party's Affiliates, employees, licensees, agents, advisors, financing sources, potential acquirors, sublicensees or distributors (collectively "Responsible Persons") who have abona fide need to know, but only to the extent reasonably necessary to perform its obligations under this Agreement or to perform due diligence on the Receiving Party. The Receiving Party (i) will inform all Responsible Persons in writing that such Confidential Information is confidential and is not to be disclosed to Third Parties, (ii) will obligate all Responsible Persons in writing to abide by the nondisclosure and nonuse obligations of this Agreement, and (iii) will be responsible for any noncompliance by Responsible Persons. Notwithstanding the foregoing, but subject toSection 10.3, these obligations of nonuse and nondisclosure shall not be breached by disclosures that are required for purposes of investigating, developing, manufacturing or marketing an Existing Compound, Transferred Compound or Ronacaleret, or for securing essential or desirable authorizations, privileges or rights from governmental agencies with respect to Existing Compounds, Transferred Compounds or Ronacaleret, or that are necessary to file or prosecute patent applications concerning Existing Compounds, Transferred Compounds or Ronacaleret, or to carry out litigation concerning Existing Compounds, Transferred Compounds or Ronacaleret. Further, nothing in thisSection 10.2 shall be construed as preventing or in any way inhibiting GSK or NPS from complying with statutory and regulatory requirements governing the Development, manufacture, use and sale or other distribution of an Existing Compound, Transferred Compound or Ronacaleret in any manner which it reasonably deems appropriate.
10.3 Exclusions from Confidential Information. The obligations of nondisclosure and nonuse inSection 10.2 shall not apply to any part of the Confidential Information which the Receiving Party can show by clear and convincing evidence using written documents (a) was already known to the Receiving Party before receiving such information from the Disclosing Party, (b) is or becomes known to the public or generally available to the public through no fault of the Receiving Party, (c) is rightfully furnished to the Receiving Party by a Third Party who has not received such Confidential Information, directly or indirectly, from the Disclosing Party or any other party under an obligation of nondisclosure or nonuse, or (d) is independently developed by or for the Receiving Party without use of Confidential Information received from the Disclosing Party.
10.4 Judicial Order. The obligation of nondisclosure in this Agreement shall not be breached by disclosure required in a judicial proceeding or governmental investigation, provided
24
the Receiving Party gives the Disclosing Party prior written notice of such requirement and affords the Disclosing Party an opportunity to oppose such disclosure or seek a protective order.
10.5 Public Announcement. No public announcement or other disclosure to Third Parties concerning the existence of or terms of this Agreement shall be made, either directly or indirectly, by any Party to this Agreement, except as may be legally required or as may be required for recording purposes, without first obtaining the approval of the other Party and agreement upon the nature and text of such announcement or disclosure. The Party desiring to make any such public announcement or other disclosure shall inform the other Party of the proposed announcement or disclosure in reasonably sufficient time prior to public release, and shall provide the other Party with a written copy thereof, in order to allow such other Party to comment upon such announcement or disclosure. Each Party agrees that it shall cooperate fully with the other with respect to all disclosures regarding this Agreement to the Securities Exchange Commission and any other governmental or regulatory agencies, including requests for confidential treatment of proprietary information of either Party included in any such disclosure.
10.6 Clinical Trial Register.GSKshall have the right to publish the results or summaries of results of all clinical trials (including meta-analysis or observational studies) conducted by or on behalf ofGSKwith respect toRonacaleretorProductsin any clinical trial register maintained byGSKor itsAffiliatesand the protocols of clinical trials relating toRonacaleretorProductson www.ClinicalTrials.gov (and/or in each case publish the results, summaries and/or protocols of clinical trials on such other websites and/or repositories as required by Applicable Laws orGSK's or itsAffiliates' policies). Each such publication made in accordance with this Section10.6shall not be a breach of the confidentiality obligations provided in this Article 10. It is understood and agreed that NPS shall not have the right to publish any information regarding the Development of Ronacaleret, whether such Development was conducted during the term of the Original Agreement or under this Agreement, without the prior written consent of GSK, which consent GSK may withhold in its discretion; provided, that if GSK consents to such publication, then GSK shall have the right to review, comment on and approve such publication which shall be made in accordance with all relevant GSK policies and procedures, including all policies regarding scientific engagement.
10.7 Return of Confidential Information. Except as otherwise provided in Article 9 of this Agreement, upon termination of this Agreement, each Party hereto and its Affiliates shall use Commercially Reasonable Efforts to return all Confidential Information of the other Party in its possession to the other Party; provided, that each Party may retain: (a) a single archival copy of the Confidential Information of the other Party; (b) any portion of the Confidential Information of the other Party which is contained in laboratory notebooks or other electronic systems, the deletion from which would not be practicable; in either case,solely for the purpose of determining the extent of disclosure of Confidential Information hereunder, assuring compliance with the surviving provisions of this Agreement, relevant document retention policies of the Party and Applicable Laws.
11. INVENTIONS, PATENTS AND PATENT PROSECUTION.
11.1 Ownership of Inventions.
25
- Except as otherwise set forth in this Agreement, each Party shall have and retain sole and exclusive title to all inventions, discoveries and Data that are made, conceived, reduced to practice or generated solely by its employees or agents in the course of or as a result of activities performed in furtherance of this Agreement or as a result of activities performed in furtherance of the Original Agreement.
- Except as otherwise set forth in this Agreement, each Party shall own a fifty percent (50%) undivided interest in all inventions, discoveries and Data that are made, conceived, reduced to practice or generated jointly by employees or agents of both Parties in the course of or as a result of activities performed in furtherance of this Agreement or as a result of activities performed in furtherance of the Original Agreement ("Joint Inventions"). Each Party shall own, and may fully exploit, all of its rights and interests in such inventions, discoveries and Data, without any encumbrances to each other or any Third Party, except as otherwise specifically set forth in this Agreement.
11.2 Patent Prosecution, Maintenance, Defense and Enforcement.
- Subject to Section 11.4, with respect to all (i) inventions and discoveries that are made, conceived, reduced to practice or generated solely by GSK's employees or agents as described in Section 11.1(a) ("GSK Inventions"), and (ii) Ronacaleret Patents (including NPS Licensed Ronacaleret Patents), GSK shall have the sole right, using in-house or outside legal counsel selected at GSK's sole discretion, to prepare, file, prosecute, maintain and extend Patents concerning all such GSK Inventions and Ronacaleret Patents in countries of GSK's choice throughout the world, for which GSK shall bear the costs relating to such activities. Notwithstanding the foregoing, and subject to Section 11.4, NPS shall have the right to prosecute, maintain and extend Ronacaleret Patents (including NPS Licensed Ronacaleret Patents) solely to the extent that, as of the Effective Date or during the Term of this Agreement, such Patents generically or specifically claim [*], including the `003 Patent and the `684 Patent. If GSK elects not to file, prosecute or maintain Patents for which it has responsibility pursuant to this Section 11.2(a) in any country of the Territory, GSK shall give NPS notice thereof within a reasonable period prior to allowing such Patents to lapse or become abandoned or unenforceable, and NPS shall thereafter have the right, at its sole expense, to prepare, file, prosecute and maintain such Patents in such country.
- Subject to Section 11.4, with respect to all (i) inventions and discoveries that are made, conceived, reduced to practice or generated solely by NPS' employees or agents as described in Section 11.1(a) ("NPS Inventions"), and (ii) all Transferred Compound Patents, NPS shall have the sole right, using in-house or outside legal counsel selected at NPS' sole discretion, to prepare, file, prosecute, maintain and extend Patents concerning all such NPS Inventions and Transferred Compound Patents in countries of NPS's choice throughout the world, for which NPS shall bear the costs relating to such activities. If NPS elects not to file, prosecute or maintain Patents for which it has responsibility pursuant to this Section 11.2(b) in any country of the Territory, NPS shall give GSK notice thereof within a reasonable period prior to allowing such Patents to lapse or become abandoned or unenforceable, and GSK shall thereafter have the right, at its sole expense, to prepare, file, prosecute and maintain such Patents in such country.
26
- GSK shall have the sole right but not the obligation to defend any suit brought by a Third Party for patent infringement involving the manufacture, use or sale of Ronacaleret anywhere in the Territory at its own expense. GSK shall have the sole right (except as provided below) but not the obligation to bring, at its own expense, an infringement action or file any other appropriate action or claim directly related to infringement of any Ronacaleret Patent (including NPS Licensed Ronacaleret Patents), wherein such infringement relates to Ronacaleret, against any Third Party. GSK shall have full control over the conduct of such action, at its sole expense, including settlement thereof; provided, that if such infringement relates to a Ronacaleret Patent (including NPS Licensed Ronacaleret Patents) that, at the time of such infringement action, generically or specifically claims [*], then NPS shall control such action, at its sole expense, subject to Section 11.4. NPS shall have the sole right but not the obligation to bring, at its own expense, an infringement action or file any other appropriate action or claim directly related to infringement of the `003 Patent and/or the `684 Patent, wherein such infringement does not relate to Ronacaleret, against any Third Party. NPS shall have full control over the conduct of such action, at its sole expense, including settlement thereof.
- NPS shall have the sole right but not the obligation to defend any suit brought by a Third Party for patent infringement involving the manufacture, use or sale of any Transferred Compound and/or Existing Compound anywhere in the Territory at its own expense. NPS shall have the sole right (except as provided below) but not the obligation to bring, at its own expense, an infringement action or file any other appropriate action or claim directly related to infringement of any Transferred Compound Patent against any Third Party. NPS shall have full control over the conduct of such action, at its sole expense, including settlement thereof.
11.3 Assistance. Notwithstanding the provisions ofSection 11.1, each Party shall, at its own expense, provide reasonable assistance to the other Party as required (i) to facilitate filing of all patent applications covering inventions referred to inSection 11.1 and shall execute all documents deemed necessary or desirable therefor, and (ii) in connection with any litigation regarding Patents as described in Sections 11.2(c) and 11.2(d).
11.4 Consultation; Cooperation. With respect to any Transferred Compound Patents that disclose or describe Ronacaleret, and any Ronacaleret Patents that disclose, describe or claim Transferred Compounds, respectively, the Parties will use reasonable efforts to keep each other informed of issues in the prosecution, enforcement or defense of Transferred Compound Patents that are likely to have a material effect on Ronacaleret, and Ronacaleret Patents that are likely to have a material effect on Transferred Compounds, respectively. Each Party will allow the other Party the right to review and comment on any such material issues, and each Party will consider the input of the other in good faith. The Parties shall be responsible for formulating a strategy for the protection of Transferred Compound Patents that disclose or describe Ronacaleret, and Ronacaleret Patents that disclose, describe or claim Transferred Compounds to ensure consistency between the Parties with respect to the prosecution, enforcement and defense of all such Patents. The foregoing principles shall also apply to the extent that NPS is controlling any prosecution, maintenance, enforcement or defense of Ronacaleret Patents that generically or specifically claim [*] as provided in this Article 11, including the `003 Patent and the `684 Patent, and which prosecution, maintenance, enforcement or defense is likely to have a material effect on Ronacaleret.
27
12. REPRESENTATIONS, WARRANTIES AND COVENANTS.
12.1 Representations and Warranties of BothParties. EachPartyhereby represents, warrants and covenants to the otherParty, as of theEffective Date, that:
- suchPartyis duly organized, validly existing and in good standing under Applicable Laws of the jurisdiction of its incorporation and has full corporate power and authority to enter into thisAgreementand to carry out the provisions hereof.
- suchPartyhas taken all necessary action on its part to authorize the execution and delivery of thisAgreementand the performance of its obligations hereunder.
- thisAgreementhas been duly executed and delivered on behalf of suchParty, and constitutes a legal, valid, binding obligation, enforceable against it in accordance with the terms hereof, except as enforcement may be affected by bankruptcy, insolvency or other similar laws and by general principles of equity.
- the execution, delivery and performance of thisAgreementby suchPartydoes not conflict with anymaterial agreement, instrument or understanding, oral or written, to which it is a party or by which it is bound, nor violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over suchParty.
- no government authorization, consent, approval, license, exemption of or filing or registration with any court or governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, under any Applicable Laws currently in effect, is necessaryfor the transaction contemplated by thisAgreementor any otheragreementor instrument executed in connection herewith, orforthe performance by it of its obligations under thisAgreement.
- SuchPartyshall perform its activities pursuant to thisAgreementin material compliance with Applicable Laws, including any and all Applicable Laws related to anti-bribery and anti-corruption.
- EachPartyshall notify the otherPartyin writing promptly in the event that it has actual knowledge of the material breach of any covenant under Section 12.1 or the material breach of any representation or warranty provided by eitherPartyunder Sections12.1, 12.2 or 12.3.
12.2 By NPS. NPS hereby represents and warrants to GSK, as of the Effective Date, that:
- To its knowledge it has not breached any provision of the Original Agreement.
- To the best of its knowledge, it has the right to grant the licenses outlined inSection 3.1 with respect to NPS Licensed Ronacaleret Patents and Ronacaleret Know-How, free and clear of all liens and encumbrances, and has the right to enter into this Agreement.
28
- Other than the Original Agreement and the Brigham Agreement, there are no Third Party agreements NPS has entered into as of the Effective Date, which will limit NPS' ability to perform all of the obligations undertaken by NPS hereunder, or which grants any ownership interest to any Third Party to Ronacaleret, Ronacaleret Patents or Ronacaleret Know-How.
- To its knowledge,Schedule 3.1 contains a complete and accurate list of the NPS Licensed Ronacaleret Patents owned or Controlled by NPS, in whole or in part.
12.3 By GSK.GSK hereby represents and warrants to NPS, as of the Effective Date, that:
- To its knowledge it has not breached any provision of the Original Agreement.
- To its knowledge, it owns the entire right and title to the extent of its ownership interest (which may be joint ownership with NPS) in the Transferred Assets and has the right to convey its interest in the Transferred Assets to NPS, free and clear of all Liens.
- Other than the Original Agreement, there are no Third Party agreements GSK has entered into as of the Effective Date, which will limit GSK's ability to perform all of the obligations undertaken by GSK hereunder, or which grants any ownership interest to any Third Party to any Transferred Assets.
- To its knowledge,Schedule 1.67 contains a complete and accurate list of the Transferred Compound Patents andSchedule 3.2 contains a complete and accurate list of the GSK Licensed Ronacaleret Patents, in each case, owned or Controlled by GSK, in whole or in part.
12.4 Mutual Covenant.If, after the Effective Date, either (a) GSK becomes aware of any additional Existing Compounds not previously disclosed to NPS, or (b) NPS becomes aware of any additional Ronacaleret Know-How not previously disclosed to GSK, then such Party shall use reasonable efforts to promptly take all appropriate action to make such Existing Compounds or Ronacaleret Know-How, as the case may be, known to the other Party.
12.5 EXCEPT AS EXPRESSLY SET FORTH IN THISSECTION 12, NEITHER PARTY MAKES ANY REPRESENTATIONS OR WARRANTIES CONCERNING THE RONACALERET PATENTS, RONACALERET KNOW-HOW OR TRANSFERRED ASSETS, OR OTHERWISE IN CONNECTION WITH THIS AGREEMENT, AND EACH PARTY HEREBY SPECIFICALLY DISCLAIMS ALL WARRANTIES, EXPRESS, IMPLIED, STATUTORY OR OTHERWISE, INCLUDING WITHOUT LIMITATION ANY WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, TITLE, NONINFRINGMENT, VALIDITY, SCOPE OR ENFORCEABILITY OF ANY RONACALERET PATENTS, RONACALERET KNOW-HOW OR TRANSFERRED ASSETS.
29
13. INDEMNIFICATION.
13.1 By NPS.
- NPS shall indemnify, defend and hold harmless GSK and its officers, directors, shareholders, employees, successors and assigns from and against any and all damages, claims, costs, losses, liabilities or expenses (including reasonable attorneys' fees) from any Third Party claims arising out of, or resulting from or in connection with NPS' activities under this Agreement, including, but not limited to any breach of a representation or warranty or covenant made to GSK by NPS under this Agreement, provided:
- NPS shall not be obligated under thisSection 13.1(a) if it is shown by evidence acceptable in a court of law having jurisdiction over the subject matter and meeting the appropriate degree of proof for such action, that the injury was the result of the negligence or willful misconduct of any employee or agent of GSK;
- NPS shall have no obligation under thisSection 13.1(a) unless GSK (1) gives NPS prompt written notice of any claim or lawsuit or other action for which it seeks to be indemnified under this Agreement, (2) NPS is granted full authority and control over the defense, including settlement, against such claim or lawsuit or other action, and (3) GSK cooperates fully with NPS and its agents in defense of the claims or lawsuit or other action; and
- GSK shall have the right to participate at its cost in the defense of any such claim, complaint, suit, proceeding or cause of action referred to in thisSection 13.1(a) utilizing attorneys of its choice, provided, however, that NPS shall have full authority and control to handle any such claim, complaint, suit, proceeding or cause of action, including any settlement or other disposition thereof, for which GSK seeks indemnification under thisSection 13.1(a).
NPS shall indemnify, defend and hold harmless GSK, its officers, directors, shareholders, employees, successors and assigns from any loss, damage, or liability, including reasonable attorney's fees, resulting from any claim, complaint, suit, proceeding or cause of action against any of them from any Third Party claims alleging physical or other injury, including death, brought by or on behalf of an injured party, loss of service or consortium or a similar such claim, complaint, suit, proceeding or cause of action brought by a friend, spouse, relative or companion of an injured Third Party due to such physical injury or death and rising out of the administration, utilization and/or ingestion of an Existing Compound or Transferred Compound manufactured, sold or otherwise provided to the injured Third Party by NPS (or its permitted sublicensee), except to the extent such damages, claims, costs, losses, liabilities or expenses are directly and proximately caused by GSK's negligent or wrongful actions and provided:- NPS shall not be obligated under thisSection 13.1(b) if it is shown by evidence acceptable in a court of law having jurisdiction over the subject matter and meeting the appropriate degree of proof for such action, that the injury was the result of the negligence or willful misconduct of any employee or agent of GSK;
30
- NPS shall have no obligation under thisSection 13.1(b) unless GSK (1) gives NPS prompt written notice of any claim or lawsuit or other action for which it seeks to be indemnified under this Agreement, (2) NPS is granted full authority and control over the defense, including settlement, against such claim or lawsuit or other action, and (3) GSK cooperates fully with NPS and its agents in defense of the claims or lawsuit or other action; and
- GSK shall have the right to participate at its cost in the defense of any such claim, complaint, suit, proceeding or cause of action referred to in thisSection 13.1(b) utilizing attorneys of its choice, provided, however, that NPS shall have full authority and control to handle any such claim, complaint, suit, proceeding or cause of action, including any settlement or other disposition thereof, for which GSK seeks indemnification under thisSection 13.1(b).
13.2 By GSK.
- GSK shall defend, indemnify and hold harmless NPS and its officers, directors, shareholders, employees, successors and assigns from and against any and all damages, claims, costs, losses liabilities or expenses (including reasonable attorney's fees) from any Third Party claims arising out of, or resulting from or in connection with GSK's activities under this Agreement, including, but not limited to GSK's activities related to Development, or any breach of a representation or warranty or covenant made to NPS by GSK under this Agreement, provided:
- GSK shall not be obligated under thisSection 13.2(a) if it is shown by evidence acceptable in a court of law having jurisdiction over the subject matter and meeting the appropriate degree of proof for such action, that the injury was the result of the negligence or willful misconduct of any employee or agent of NPS;
- GSK shall have no obligation under thisSection 13.2(a) unless NPS (1) gives GSK prompt written notice of any claim or lawsuit or other action for which it seeks to be indemnified under this Agreement, (2) GSK is granted full authority andcontrol over the defense, including settlement, against such claim or lawsuit or other action, and (3) NPS cooperates fully with GSK and its agents in defense of the claims or lawsuit or other action; and
- NPS shall have the right to participate at its cost in the defense of any such claim, complaint, suit, proceeding or cause of action referred to in thisSection 13.2(a) utilizing attorneys of its choice, provided, however, that GSK shall have full authority and control to handle any such claim, complaint, suit, proceeding or cause of action, including any settlement or other disposition thereof, for which NPS seeks indemnification under thisSection 13.2(a).
GSK shall indemnify, defend and hold harmless NPS, its officers, directors, shareholders, employees, successors and assigns from any loss, damage, or liability, including reasonable attorney's fees, resulting from any claim, complaint, suit, proceeding or cause of action against any of them from any Third Party claims alleging physical or other injury, including death, brought by or on behalf of an injured party; loss of service or consortium or a similar such claim, complaint, suit, proceeding or cause of action brought by a friend,31
spouse, relative or companion of an injured party due to such physical injury or death and rising out of the administration, utilization and/or ingestion of Ronacaleret manufactured, sold or otherwise provided to the injured party by GSK (or its permitted sublicensee), provided:
- GSK shall not be obligated under thisSection (b) if it is shown by evidence acceptable in a court of law having jurisdiction over the subject matter and meeting the appropriate degree of proof for such action, that the injury was the result of the negligence or willful misconduct of any employee or agent of NPS;
- GSK shall have no obligation under thisSection (b) unless NPS (1) gives GSK prompt written notice of any claim or lawsuit or other action for which it seeks to be indemnified under this Agreement, (2) GSK is granted full authority and control over the defense, including settlement, against such claim or lawsuit or other action, and (3) NPS cooperates fully with GSK and its agents in defense of the claims or lawsuit or other action; and
- NPS shall have the right to participate at its cost in the defense of any such claim, complaint, suit, proceeding or cause of action referred to in thisSection (b) utilizing attorneys of its choice, provided, however, that GSK shall have full authority and control to handle any such claim, complaint, suit, proceeding or cause of action, including any settlement or other disposition thereof, for which NPS seeks indemnification under thisSection 13.2(b).
14. MISCELLANEOUS.
14.1 Force Majeure. If the performance of any part of this Agreement (other than the payment of any amounts due hereunder) by either Party, is prevented, restricted, interfered with or delayed by reason of any cause beyond the reasonable control of the Party liable to perform, unless conclusive evidence to the contrary is provided, the Party so affected shall, upon giving written notice to the other Party, be excused from such performance to the extent of such prevention, restriction, interference or delay, provided that the affected Party shall use its reasonable best efforts to avoid or remove such causes of non-performance and shall continue performance with the utmost dispatch whenever such causes are removed. When such circumstances arise, the parties shall discuss what, if any, modification of the terms of this Agreement may be required in order to arrive at an equitable solution.
14.2 Governing Law. This Agreement shall be deemed to have been made in the State of Delaware and its form, execution, validity, construction and effect shall be determined in accordance with the laws of the State of Delaware without reference to conflicts of laws principles; provided, that with respect to matters involving the enforcement of intellectual property rights, the Applicable Laws of the applicable country shall apply.
14.3 Dispute Resolution. Any dispute, controversy or claim arising out of or relating to this Agreement (hereinafter collectively referred to as "Dispute") shall be attempted to be settled by the parties, in good faith, by submitting each such Dispute to appropriate senior management representatives of each Party in an effort to affect a mutually acceptable resolution thereof. In the event no mutually acceptable resolution is achieved, then each Party shall be
32
entitled to seek relief for such Dispute by using any appropriate mechanism which may be available, such as, but not limited to, judicial relief.
14.4 Severability.In the event any portion of this Agreement shall be held illegal, void or ineffective, the remaining portions shall remain in full force and effect. If any of the terms or provisions of this Agreement are in conflict with any applicable statute or rule of law, then such terms or provisions shall be deemed inoperative to the extent that they may conflict therewith and shall be deemed to be modified to conform with such statute or rule of law. In the event that the terms and conditions of this Agreement are materially altered as a result of thisSection 14.4, the Parties will renegotiate such terms and conditions of this Agreement to resolve any inequities.
14.5 Entire Agreement. This Agreement, entered into as of the Effective Date, constitutes the entire agreement between the Parties relating to the subject matter hereof and supersedes all previous writings and understandings, including the Original Agreement and the Amendments. No terms or provisions of this Agreement shall be varied or modified by any prior or subsequent statement, conduct or act of either of the Parties, except that the Parties may amend this Agreement by written instruments specifically referring to and executed in the same manner as this Agreement.
14.6 Notices.
- Any notice required or permitted under this Agreement shall be sent by air mail, postage pre-paid, to the following addresses of the parties:
NPS
NPS Pharmaceuticals, Inc.
550 Hills Dr., 3rd Floor
Bedminster, New Jersey 07921
Attention: General Counsel
copy to:
Barton W. Giddings
Stoel Rives LLP
201 S. Main St., 11th Floor
Salt Lake City, Utah 84111
GSK
GlaxoSmithKline
709 Swedeland Road
P.O. Box 1539
King of Prussia, PA 19406-0939
Attention: Senior Vice President, Worldwide Business Development
Facsimile: +1-610-270-5880
30
With a copy (which shall not constitute notice) to:
GlaxoSmithKline
2301 Renaissance Boulevard
Mailcode RN0220
King of Prussia, PA 19406-2772
Attention: Vice President and Associate General Counsel, Business Development Transactions
Facsimile: +1-610-787-7084
- Any notice required or permitted to be given concerning this Agreement shall be effective upon receipt by the Party to whom it is addressed.
14.7 Assignment. This Agreement and the licenses herein granted shall be binding upon and inure to the benefit of the successors in interest of the respective Parties. Neither this Agreement nor any interest hereunder shall be assignable by either Party without the written consent of the other Party provided, however, that either Party may assign this Agreement or any Patent owned by it to any Affiliate, or to any corporation with which it may merge or consolidate or to which it may transfer all or substantially all of its assets to which this Agreement relates, without obtaining the consent of the other Party.
14.8 Recording. Either Party shall have the right, at any time, to record, register, or otherwise notify this Agreement in appropriate governmental or regulatory offices anywhere, and each Party shall provide reasonable assistance to the other Party in effecting such recording, registering or notifying.
14.9 Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument.
34
IN WITNESS WHEREOF the parties have caused this Agreement to be executed by their duly authorized officers as of the Effective Date.
NPS PHARMACEUTICALS, INC.
By:/s/ Luke Beshar______________________________________
Name:Luke Beshar___________________________________
Title:SVP & CFO____________________________________
GlaxoSmithKline LLC
By:/s/ William J. Mosher_____________________________________
Name:William J. Mosher___________________________________
Title:Vice President & Secretary____________________________________
35
EXHIBIT A
ASSIGNMENT AGREEMENT
This ASSIGNMENT AGREEMENT(this "Assignment Agreement") is made as of this ___ day of ___________, 2011 (the "Assignment Effective Date"), by and between GlaxoSmithKline LLC, a Delaware limited liability company with an address at One Franklin Plaza, Philadelphia, Pennsylvania 19106 (formerly known as SmithKline Beecham Corporation) ("GSK"),and NPS Pharmaceuticals, Inc., a Delaware corporation with an address at 550 Hills Dr., 3rd Floor, Bedminster, New Jersey 07921 ("NPS"). Each of GSK and NPS are sometimes referred to herein, individually, as a "Party" and, collectively, as the "Parties."
WHEREAS, GSK and NPS have entered into that certain Exclusive Patent License Agreement, dated as of July 28, 2011 (the "License Agreement"); and
WHEREAS, pursuant to the License Agreement, GSK has agreed to assign ownership of the Transferred Assets to NPS, and NPS has agreed to accept such assignment of the Transferred Assets from GSK.
NOW THEREFORE, in consideration of the mutual covenants and agreements set forth in this Assignment Agreement and the License Agreement, and for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and intending to be legally bound hereby, the Parties hereby agree as follows:
- Definitions. Unless otherwise defined herein, all capitalized terms used in this Assignment Agreement shall have the meanings set forth in the License Agreement.
- Assignment and Acceptance. In accordance with the provisions of the License Agreement, GSK hereby sells, assigns, grants, transfers and delivers to NPS the entire right, title and interest of GSK in and to the Transferred Assets, and NPS hereby accepts such assignment, grant, transfer and delivery, in each case free and clear of all Liens.
- Delivery of Tangible Transferred Assets. As soon as practicable (and in any case within one hundred eighty (180) days) after the Effective Date, GSK shall have delivered all tangible assets included in the Transferred Assets at NPS's risk and expense. All such tangible assets included in the Transferred Assets shall be shipped EXW GSK's facilities (as defined in INCOTERMS, 2010 edition, published by the International Chamber of Commerce). From and after making the tangible assets included in the Transferred Assets available for pickup at GSK's facilities, NPS shall bear all risk of loss for such items and shall be solely responsible for all tangible assets included in the Transferred Assets against any such loss.
- No Transfer of Liabilities. As between NPS and GSK, GSK will be solely responsible for and pay, perform and/or otherwise discharge when due those Liabilities (including any Liabilities arising in respect of taxes) arising out of or in connection with or
36
related to the Transferred Assets or the use thereof, arising on or prior to the Effective Date, including any claims filed after the Effective Date that relate to periods prior to the Effective Date. As between NPS and GSK, NPS will be solely responsible for Liabilities (including any Liabilities in respect of taxes) arising out of or in connection with or related to the Transferred Assets or the use, marketing or sale thereof, arising after the Effective Date (except to the extent such Liabilities relate to activities or events first arising prior to the Effective Date.
- License Agreement Controls. Notwithstanding any other provisions of this Assignment Agreement to the contrary, nothing contained herein shall in any way supersede, modify, replace, amend, change, rescind, waive, exceed, expand, enlarge or in any way affect the provisions, including warranties, covenants, agreements, conditions, representations or, in general any of rights and remedies, and any of the obligations of GSK or NPS set forth in the License Agreement. This Assignment Agreement is subject to and governed entirely in accordance with the terms and conditions of the License Agreement.
- Further Acts. GSK hereby agrees to take such further action and to execute such additional documents as NPS may reasonably request that are required to carry out and fulfill the purposes and intent of this Assignment Agreement.
- Miscellaneous.
- Governing Law. This Assignment Agreement shall be deemed to have been made in the State of Delaware and its form, execution, validity, construction and effect shall be determined in accordance with the laws of the State of Delaware without reference to conflicts of laws principles; provided, that with respect to matters involving the enforcement of intellectual property rights, the Applicable Laws of the applicable country shall apply.
- Assignment. This Assignment Agreement shall be binding upon and inure to the benefit of the successors in interest of the respective Parties. Neither this Assignment Agreement nor any interest hereunder shall be assignable by either Party without the written consent of the other Party provided, however, that either Party may assign this Assignment Agreement or any Patent owned by it to any Affiliate, or to any corporation with which it may merge or consolidate or to which it may transfer all or substantially all of its assets to which this Agreement relates, without obtaining the consent of the other Party.
- Severability. In the event any portion of this Assignment Agreement shall be held illegal, void or ineffective, the remaining portions shall remain in full force and effect. If any of the terms or provisions of this Assignment Agreement are in conflict with any applicable statute or rule of law, then such terms or provisions shall be deemed inoperative to the extent that they may conflict therewith and shall be deemed to be modified to conform with such statute or rule of law. In the event that the terms and conditions of this Assignment Agreement are materially altered as a result of thisSection 7(c), the Parties will renegotiate such terms and conditions of this Agreement to resolve any inequities.
- Entire Agreement. This Assignment Agreement and the License Agreement constitute the entire agreement between the Parties relating to the subject matter hereof and supersede all previous writings and understandings, including the Original Agreement and the Amendments. No terms or provisions of this Agreement shall be varied or
37
modified by any prior or subsequent statement, conduct or act of either of the Parties, except that the Parties may amend this Assignment Agreement by written instruments specifically referring to and executed in the same manner as this Assignment Agreement.
- This Assignment Agreement may be executed in any number of counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument.
IN WITNESS WHEREOF the Parties have caused this Agreement to be executed by their duly authorized officers as of the Effective Date.
NPS Pharmaceuticals, Inc.
By: ______________________________________
Name: ___________________________________
Title: ____________________________________
GlaxoSmithKline LLC
By: _____________________________________
Name: ___________________________________
Title: ____________________________________
38
SCHEDULE 1.24
EXISTING COMPOUNDS
[*]
39
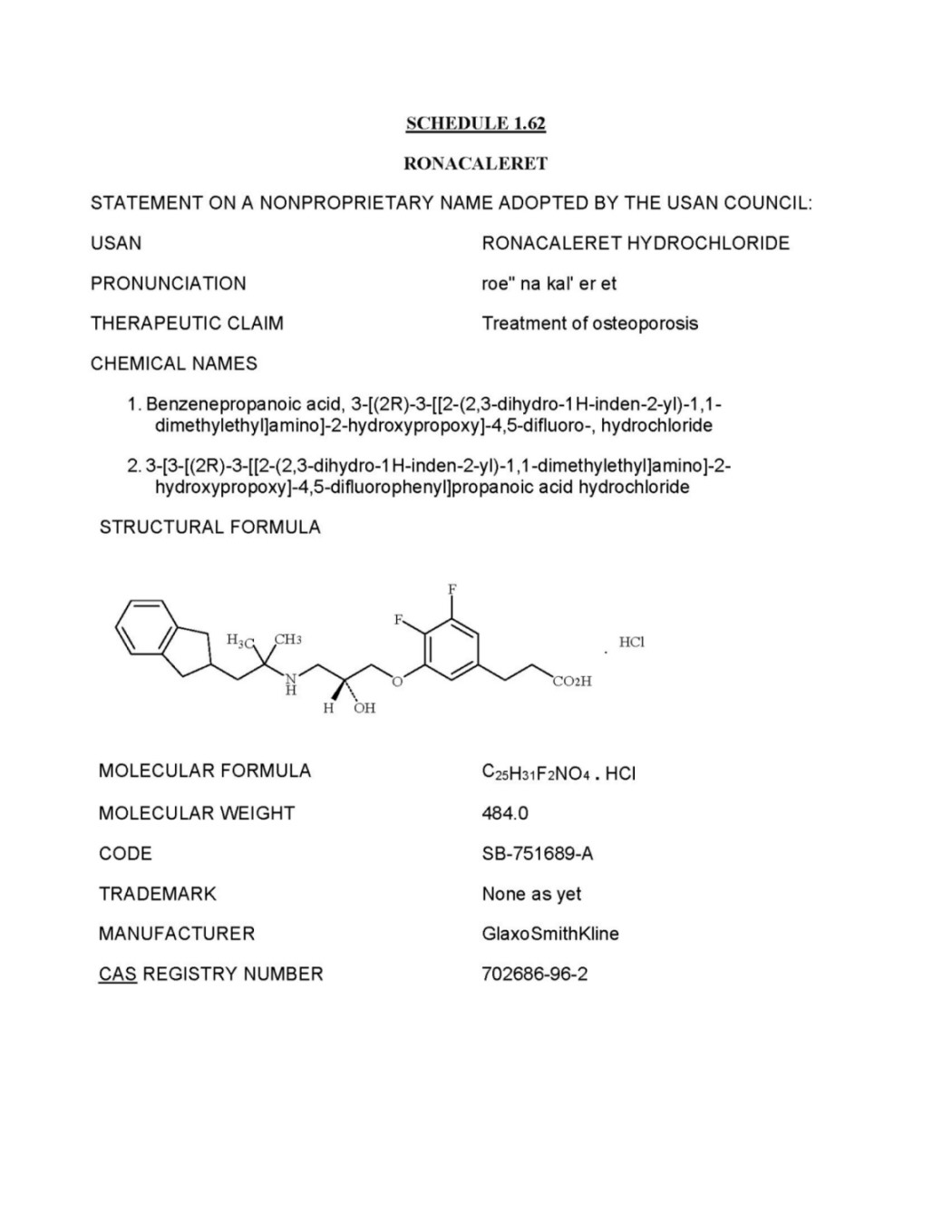
40
SCHEDULE 1.64
RONACALERET PATENTS
The Parties will use reasonable efforts to complete this schedule within forty-five (45) days after the Effective Date.
41
SCHEDULE 1.74
TRANSFERRED COMPOUND PATENTS
The Parties will use reasonable efforts to complete this schedule within forty-five (45) days after the Effective Date.
42
SCHEDULE 2.2
TRANSFERRED ASSETS
- Regulatory files in GSK's possession, including material communications with FDA.
- Final preclinical study reports.
- Safety assessment reports.
- Any study files and specimens in GSK's possession relating to preclinical GLP studies (i.e., wet tissues, paraffin blocks, slides). If preclinical GLP studies were conducted by a third party such as a contract research organization and study files and specimens are in such third party's possession, GSK will inform such third parties as soon as reasonably practicable after the Effective Date that ownership of the applicable study files and any archived specimens has been transferred to NPS as of the Effective Date of the Agreement.
With respect to electronic information, one electronic copy will be transferred to NPS and one electronic copy may be retained by GSK for archival purposes. In the case of physical records, the original document(s) will be transferred to NPS and one copy may be retained by GSK for archival purposes.
43
SCHEDULE 3.1
NPS LICENSED RONACALERET PATENTS
The Parties will use reasonable efforts to complete this schedule within forty-five (45) days after the Effective Date.
44
SCHEDULE 3.2
GSK LICENSED RONACALERET PATENTS
The Parties will use reasonable efforts to complete this schedule within forty-five (45) days after the Effective Date.
45
