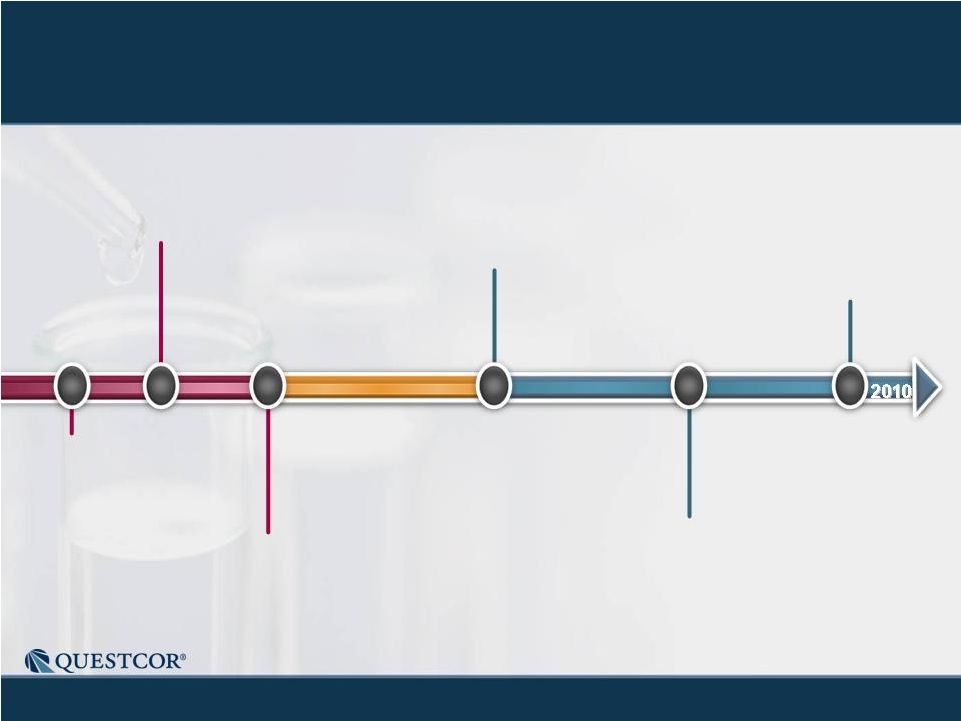
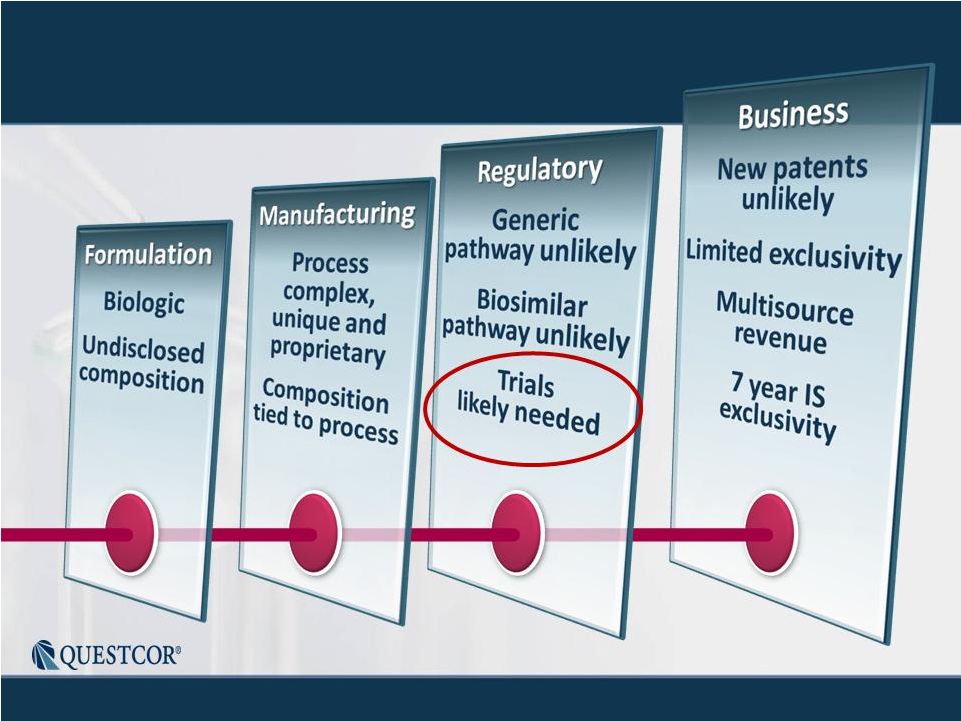
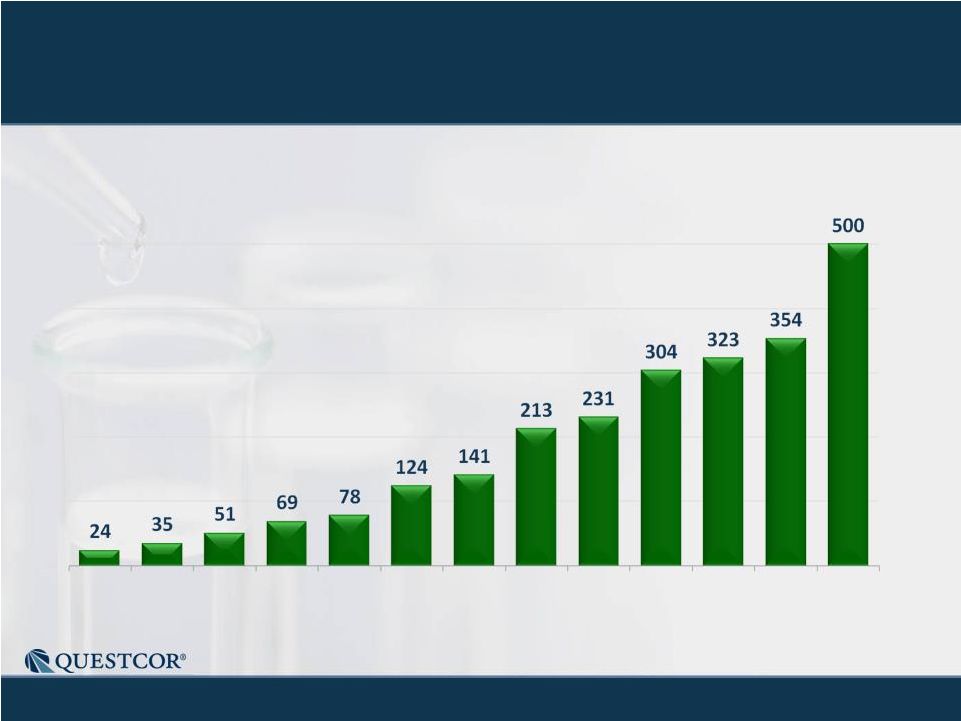
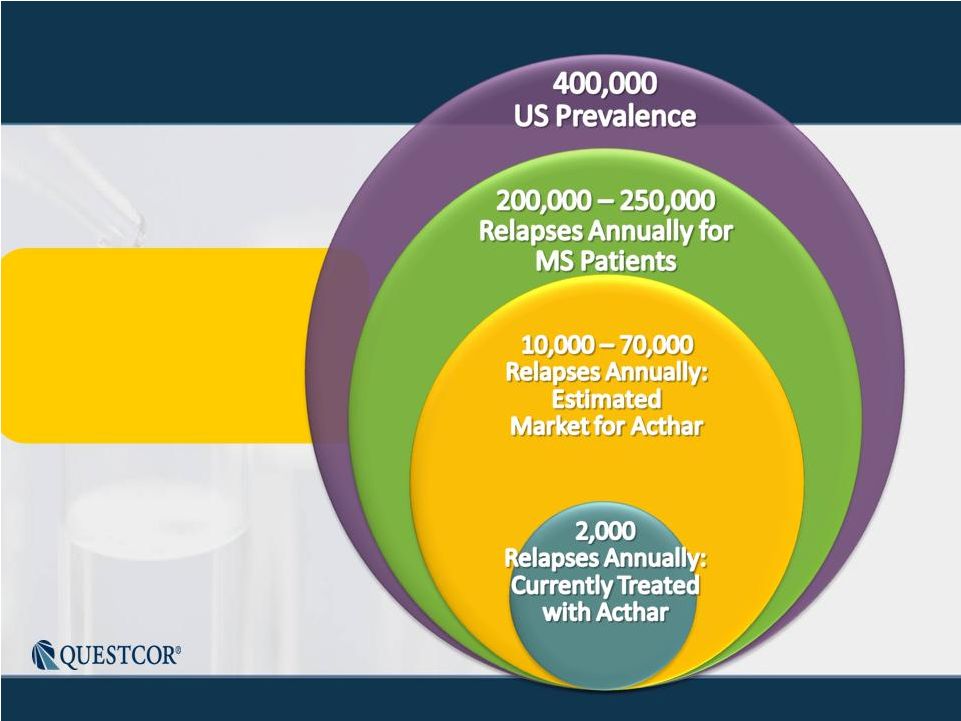
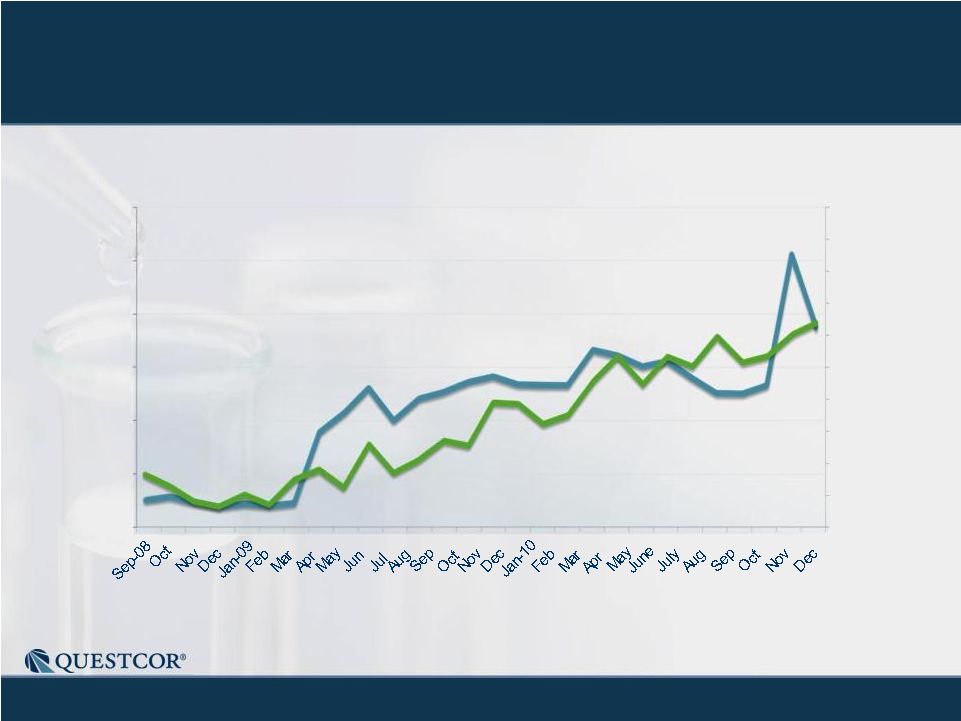
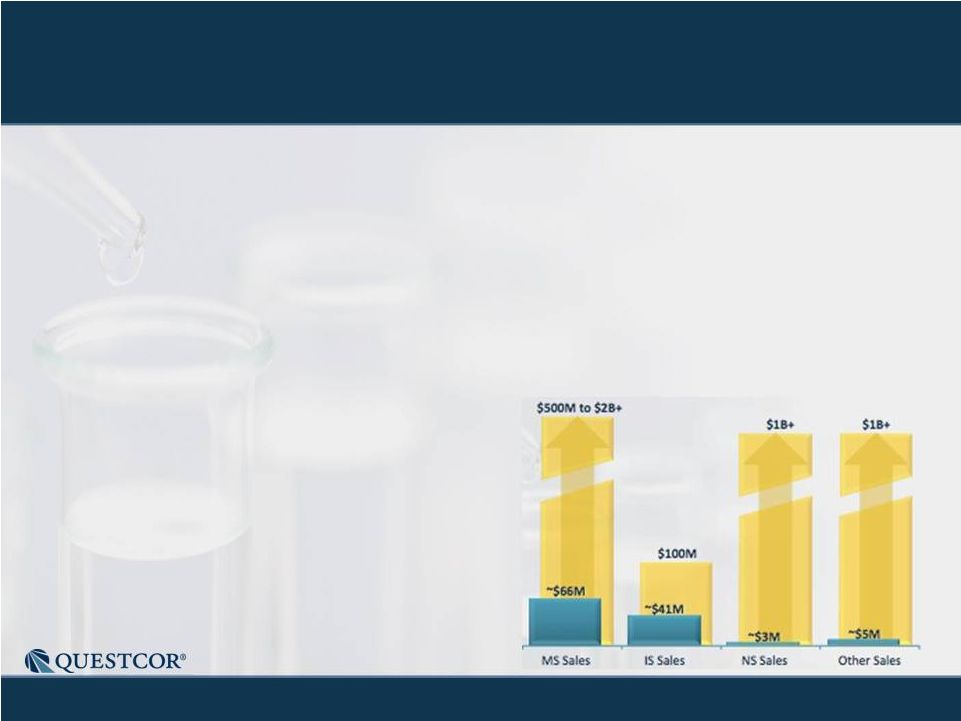
3 Safe Harbor Statement Note: Except for the historical information contained herein, this press release contains forward-looking statements that have been made pursuant to the Private Securities Litigation Reform Act of 1995. These statements relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “believes,” “continue,” “could,” “estimates,” “expects,” “growth,” “may,” “plans,” “potential,” “should,” “substantial” or “will” or the negative of such terms and other comparable terminology. These statements are only predictions. Actual events or results may differ materially. Factors that could cause or contribute to such differences include, but are not limited to, the following: Our reliance on Acthar for substantially all of our net sales and profits; The complex nature of our manufacturing process and the potential for supply disruptions or other business disruptions; The lack of patent protection for Acthar; and the possible FDA approval and market introduction of competitive products; Our ability to generate revenue from sales of Acthar to treat on-label indications associated with NS, and our ability to develop other therapeutic uses for Acthar; Research and development risks, including risks associated with Questcor's preliminary work in the area of nephrotic syndrome and our reliance on third-parties to conduct research and development and the ability of research and development to generate successful results; Regulatory changes or other policy actions by governmental authorities and other third parties as recently adopted U.S. healthcare reform legislation is implemented; Our ability to receive high reimbursement levels from third party payers; An increase in the proportion of our Acthar unit sales comprised of Medicaid-eligible patients and government entities; Our ability to estimate reserves required for Acthar used by government entities and Medicaid-eligible patients and the impact that unforeseen invoicing of historical Medicaid sales may have upon our results; Our ability to operate within an industry that is highly regulated at both the Federal and state level; Our ability to effectively manage our growth and our reliance on key personnel; The impact to our business caused by economic conditions; Our ability to protect our proprietary rights; Our ability to maintain effective controls over financial reporting; The risk of product liability lawsuits; Unforeseen business interruptions; Volatility in Questcor's monthly and quarterly Acthar shipments and end-user demand, as well as volatility in our stock price; and Other risks discussed in Questcor's annual report on Form 10-K for the year ended December 31, 2010 and other documents filed with the Securities and Exchange Commission. The risk factors and other information contained in these documents should be considered in evaluating Questcor's prospects and future financial performance. Note: Except for the historical information contained herein, this press release contains forward-looking statements that have been made pursuant to the Private Securities Litigation Reform Act of 1995. These statements relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “believes,” “continue,” “could,” “estimates,” “expects,” “growth,” “may,” “plans,” “potential,” “should,” “substantial” or “will” or the negative of such terms and other comparable terminology. These statements are only predictions. Actual events or results may differ materially. Factors that could cause or contribute to such differences include, but are not limited to, the following: Our reliance on Acthar for substantially all of our net sales and profits; The complex nature of our manufacturing process and the potential for supply disruptions or other business disruptions; The lack of patent protection for Acthar; and the possible FDA approval and market introduction of competitive products; Our ability to generate revenue from sales of Acthar to treat on-label indications associated with NS, and our ability to develop other therapeutic uses for Acthar; Research and development risks, including risks associated with Questcor's preliminary work in the area of nephrotic syndrome and our reliance on third-parties to conduct research and development and the ability of research and development to generate successful results; Regulatory changes or other policy actions by governmental authorities and other third parties as recently adopted U.S. healthcare reform legislation is implemented; Our ability to receive high reimbursement levels from third party payers; An increase in the proportion of our Acthar unit sales comprised of Medicaid-eligible patients and government entities; Our ability to estimate reserves required for Acthar used by government entities and Medicaid-eligible patients and the impact that unforeseen invoicing of historical Medicaid sales may have upon our results; Our ability to operate within an industry that is highly regulated at both the Federal and state level; Our ability to effectively manage our growth and our reliance on key personnel; The impact to our business caused by economic conditions; Our ability to protect our proprietary rights; Our ability to maintain effective controls over financial reporting; The risk of product liability lawsuits; Unforeseen business interruptions; Volatility in Questcor's monthly and quarterly Acthar shipments and end-user demand, as well as volatility in our stock price; and Other risks discussed in Questcor's annual report on Form 10-K for the year ended December 31, 2010 and other documents filed with the Securities and Exchange Commission. The risk factors and other information contained in these documents should be considered in evaluating Questcor's prospects and future financial performance. |