 CORPORATE PRESENTATION September 2014 Exhibit 99.1 |
 Forward Looking Statement This presentation includes forward-looking statements within the meaning of the Safe Harbor provisions of the Private Securities Litigation Reform Act of 1995. Such statements are subject to a number of risks and uncertainties, the outcome of which could materially and/or adversely affect actual future results and the trading price of CTI's securities. Such statements include, but are not limited to, statements regarding expectations with respect to the following: the development of CTI and its product and product candidate portfolio, including expectations with respect to existing or potential partnerships; expected receipt and timing of milestone progress payments; market size/potential for our compounds; timing of PIXUVRI reimbursement and pricing decisions; potential advantages of pacritinib and expansion of pacritinib market opportunity; timing of our Phase 3 trial for pacritinib and projected availability of trial data; timing of opening of E.U. sites for PIXUVRI post-marketing commitment study; potential growth of PIXUVRI sales; benefits and results from tosedostat and our ability to manage expenses and generate non-equity based operating capital; liquidity projections; and our 2014 target milestones. Risks that contribute to the uncertain nature of the forward-looking statements include, among others, risks associated with the biopharmaceutical industry in general and with CTI and its product and product candidate portfolio in particular including, among others, risks associated with the following: that CTI cannot predict or guarantee the pace or geography of enrollment of its clinical trials, that CTI cannot predict or guarantee the outcome of preclinical and clinical studies, that CTI may not obtain reimbursement for PIXUVRI in certain markets in the E.U. as planned, that the conditional marketing authorization for PIXUVRI may not be renewed, that the second Phase 3 clinical trial of pacritinib will not occur as planned, that CTI may not obtain favorable determinations by other regulatory, patent and administrative governmental authorities, that CTI may experience delays in the commencement of preclinical and clinical studies, risks related to the costs of developing, producing and selling PIXUVRI, pacritinib, tosedostat and CTI's other product candidates, and other risks, including, without limitation, competitive factors, technological developments, costs of developing, producing and selling PIXUVRI, that CTI's operating expenses continue to exceed its net revenues, that CTI may not be able to sustain its current cost controls or further reduce its operating expenses, that CTI's average net operating burn rate may increase, that CTI will continue to need to raise capital to fund its operating expenses, but may not be able to raise sufficient amounts to fund its continued operation, as well as other risks listed or described from time to time in CTI's most recent filings with the Securities and Exchange Commission on Forms 10-K, 10-Q and 8-K. Except as required by law, CTI does not intend to update any of the statements in this presentation upon further developments. 2 |
 CTI BioPharma Opportunity • 1 st approved therapy in the E.U. for relapsed aggressive B-cell NHL beyond 2 nd line • Partnership goal: expand market potential, upfront & cost share; retain U.S./E.U. rights PIXUVRI ® : Marketed in 10 E.U. Countries Pacritinib: JAK2/FLT3 Inhibitor Completing Phase 3 • Fast Track designated drug; Registration trial conducted under FDA - SPA • 1 st Phase 3 trial enrollment completed; Top-line data 1Q-2015 • 2 nd Phase 3 trial enrollment completion estimate: 1H-2015 • $360mm collaboration with BAXTER International Cash-flow Focus • Targeting net positive product margin contribution from PIXUVRI sales 4Q-2014 • Generating non-equity based revenue/operating capital through product partnerships • Current cash balance, plus BAXTER upfront/progress payments, potential PIXUVRI collaboration upfront and projected PIXUVRI European sales expected to provide adequate capital into 3Q-2015 Marketed Oncology Product & Late Stage Pipeline 3 |
 Therapeutic Focus: Novel Targeted Therapies Blood-Related Cancers Lymphoma Leukemia MPNs Aggressive NHL AML and MDS Myelofibrosis 4 |
 |
 • 1 st and only approved mono-therapy in the E.U. for aggressive B-cell NHL 3 rd /4 th line • Novel MOA: DNA alkylation/mitotic instability • Works in anthracycline-resistant aggressive B-cell NHL • No label warning for cardiotoxicity or cumulative dose restrictions • Predictable and manageable toxicity (neutropenia) - No severe nausea, vomiting or hair loss PIXUVRI ® : Overview One of the most active agents in R/R aggressive B-cell NHL where there is no standard therapy 6 |
 Relapsed Aggressive NHL: High Unmet Need ~37,000 new cases in the E.U. each year 1,2 1 Harris NL, et al. Ann Oncol. 1999;10(12):1419-32. 2 European Cancer Observatory, Cancer Fact Sheets, 2008. 3 Swain S, Whaley FS, Ewer MS. Cancer 2003;97:2869-79. 4 Mordente A, Meucci E, Silvestrini A et al. Curr Med Chem 2009; 16:1656-72. 5 Hagemeister FB. Cancer Chemother Pharmacol 2002;49(suppl 1):S13-20. 7 • Typically Anthracycline-based treatment • Anthracyclines limited mostly to 1 st line use due to association with irreversible, cumulative dose related heart damage 3,4 • Intensive non-anthracycline based toxic salvage therapy (R- DHAP), +/-autologous stem cell transplantation • 95% of patients will relapse after 2 nd -line therapy 5 • Prior options for patients were palliative care or clinical trials 1 st Line 2 nd Line 3 rd Line |
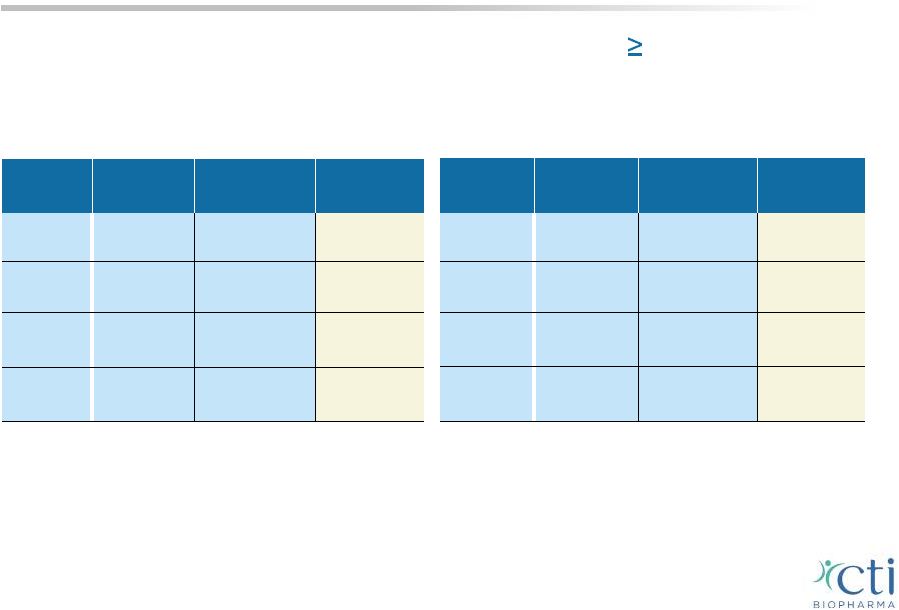 PIXUVRI Highlights 3 rd /4 th line B-cell Results Only randomized controlled Phase 3 trial in 3 rd -line aggressive NHL (n=140 patients) PIXUVRI (n=50) Comparator (n=49) p value CR/CRu 28% 4.0% 0.002 ORR 48% 12.2% <0.001 PFS 5.8 mos. 2.8 mos. 0.002 (HR=0.50) Median OS 13.9 mos. 7.8 mos. 0.275 (HR=0.76) PIXUVRI (n=70) Comparator (n=70) p value CR/CRu 24% 7.1% 0.009 ORR 40% 14.3% 0.001 PFS 5.3 mos. 2.6 mos. 0.005 (HR=0.60) OS 10.2 mos. 7.6 mos. 0.251 (HR=0.79) ITT Population EXTEND (PIX301) study published in Lancet Oncology, May 2012 8 |
 PIXUVRI: E.U. Commercial Update Attractive Market Opportunity Potential >8,000 patients on product at peak in E.U.** Label expansion study underway (PIX306) Reimbursement in Key Major Market Countries Reimbursement/market access in U.K., Germany, France, Italy NICE: cost effective for NHS in England and Wales Standard of care per treatment guidelines in key E.U. countries Weighted average pricing: €16,500 or $20,000/patient/year *Autologous Stem Cell Transplant **Market penetration projections provided by Kantar Research. 9 2 nd salvage (3 rd line) = 2 nd chance at potentially curative ASCT* 13,500** addressable market |
 PIXUVRI: PIX306 Study Eligibility Criteria Relapse after CHOP-R therapy or an equivalent regimen and are ineligible for stem cell transplant 1:1 Randomization n = ~220 Primary Endpoint PFS Secondary Endpoints CR ORR OS Gemcitabine* Rituximab Pixantrone* Rituximab Post-marketing commitment Phase 3 study • Sites currently open in U.S. and E.U. • Target enrollment completion 4Q-2015; data in 2016 • If positive, should extend label to 2 nd line combination therapy - Expected to increase E.U. addressable market by 12,000* patients - Potential to provide basis for U.S. NDA 10 *Friedberg J 2011 American Society of Hematology. |
 Pacritinib |
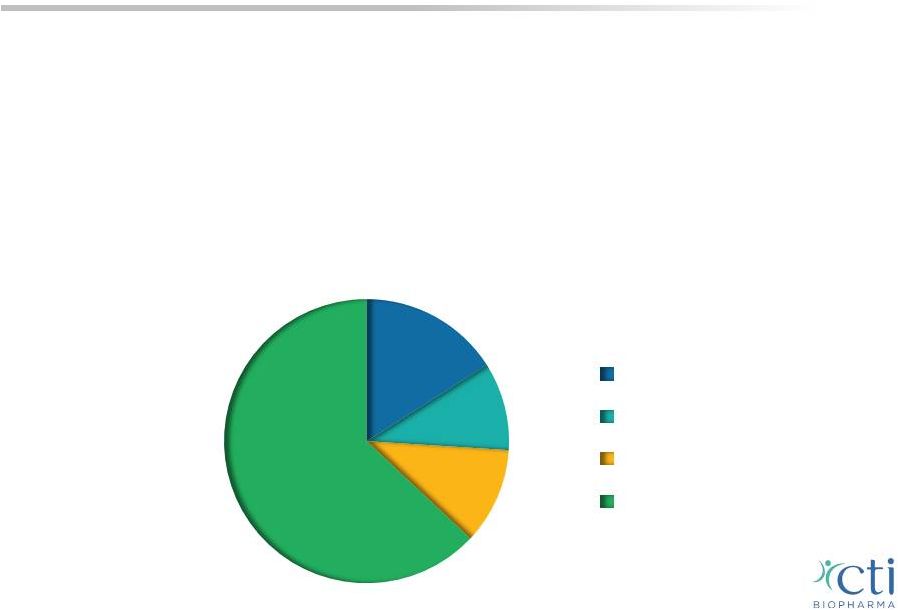 12 Myelofibrosis: A Chronic Disease • Malignant bone marrow disorder triggers an inflammatory response, scarring the bone marrow and limiting its ability to produce blood cells prompting the spleen and liver to compensate • Symptoms include: enlargement of the spleen, anemia, thrombocytopenia, extreme fatigue, pain, severe itching and GI side effects • Estimated prevalence ~18,000 – 30,000 patients in the U.S., 1 ~37% of whom are thrombocytopenic with platelet counts below 150,000/µL 2 1 Based on Mesa R, ASH 2012 poster 2 Visani et al. 1990 Br J Haematol; Caramazza et al. 2011 Leukemia; Tam et al. 2009 JCO <50,000/µL >50,000/µL but <100,000/µL >100,000/µL but <150,000/µL >150,000/µL 10% 11% 16% 63% |
 Clinical trials of JAK inhibitors before PERSIST excluded patients with platelet counts <100,000/µL or <50,000/µL Treatment emergent thrombocytopenia is a limitation of most JAK1/JAK2 inhibitors (ruxolitinib) Spleen Volume Total Symptom Score 1 Harrison C, et al. JAK Inhibition with Ruxolitinib vs Best Available Therapy for Myelofibrosis. N Eng J Med 2012;366;787-98. 2 Verstovsek S, European Focus on MPNs & MDS 2014, Tips on using rux in everyday practice. 3 Fonseca E, ASH 2013 Abstract 2833 Ruxolitinib Discontinuation in Patients with Myelofibrosis: An Analysis from Clinical Practice. Thrombocytopenia in Myelofibrosis 13 • 92% patients in COMFORT II (ruxolitinib) had normal platelet counts 1 n=101 n=103 • Low dose ( 10mg bid) provides suboptimal disease/symptom control 2 • Discontinuation rates for ruxolitinib (60%) within the first 3 months 3 |
 Baseline Platelets 100,000/µL (n=28) Baseline Platelets 100,000/µL (n=37) All (n=65) Platelet Count (x10 3 /µl) Median (IQR) 59 (34-70) 227 (163-315) 121 (60-238) Range 15-97 104-859 15-859 Duration on Treatment Median Weeks (range)* 46.9 (5.7-86.4) 40.9 (1.3-87.4) 44.1 (1.3-87.4) Komrokji, R; Versovsek, S, et al. ASH 2013 Oral Presentation of Phase 2 Trial *In the 003 study drug was not provided beyond 12 months Pacritinib Phase 2: Treatment Duration Independent of Platelet Counts 14 |
 Endpoint Time Period Baseline Platelets 100,000/µL (n=28) Baseline Platelets 100,000/µL (n=37) All (n=65) 35% Reduction in spleen volume by MRI Up to 24 weeks 7 / 23 (30%) 6 / 26 (23%) 13 / 49 (27%) Up to last visit on treatment 10 / 23 (44%) 8 / 26 (31%) 18 / 49 (37%) Pacritinib Phase 2: Spleen Response Independent of Platelet Counts 15 Komrokji, R; Versovsek, S, et al. ASH 2013 Oral Presentation of Phase 2 Trial |
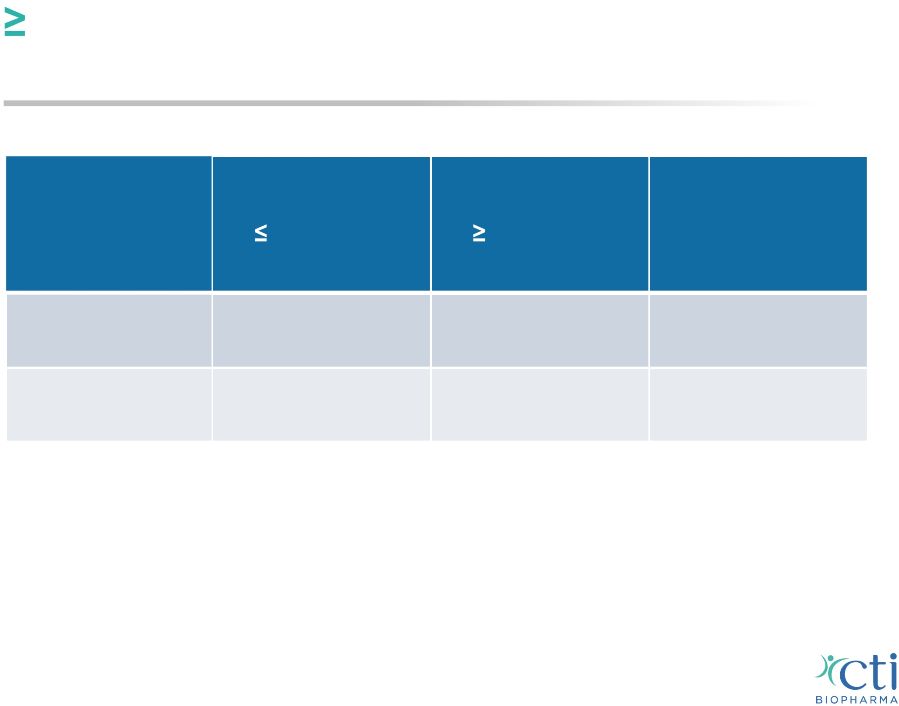 50% Reduction In Patient-Reported Symptom Score Independent of Platelet Counts* 16 Baseline Platelets 100,000/µL (n=28)** Baseline Platelets 100,000/µL (n=37)** All (n=65) Up to 24 weeks 11 / 28 (39%) 16 / 34 (47%) 27 / 62 (44%) Up to last visit on treatment 13 / 28 (46%) 17 / 34 (50%) 30 / 62 (48%) * The symptom score is the sum of the individual scores for worst fatigue, early satiety, abdominal pain or discomfort, night sweats, itching and bone pain reported on the MFSAF (Mesa et al, Leuk Res 2009, 33(9)1199). ** No significant differences were observed between the two groups Komrokji, R; Versovsek, S, et al. ASH 2013 Oral Presentation of Phase 2 Trial |
 Pacritinib: Addressing Unmet Need in MF Fast Track Designation; Phase 3 Program Underway PERSIST-1 Topline results expected early 2015 INT-1, 2 High risk, no restrictions on platelet counts PERSIST-2 Conducted under Special Protocol Assessment (SPA) Enrollment completion expected 1H-2015 17 • Most inclusive randomized trial of MF patient population representative of actual clinical practice • - Designed to demonstrate impact of full dose pacritinib over titrated dose ruxolitinib or BAT in patients with disease or drug related thrombocytopenia ( 100,000/µL) • • • |
 Sites: Europe, Australia, New Zealand, Russia and U.S. Patient Accrual: Completed Anticipated Topline Results: 1Q-2015 Principal Investigators: *Cross-over from BAT allowed after progression or assessment of the primary endpoint. Eligibility Criteria No exclusion for platelet levels, stratified for platelet counts of 100,000/µL and 50,000/µL No prior treatment with JAK2 inhibitors 2:1 Randomization* n = ~320 Primary Endpoint % of patients achieving 35% reduction in spleen size from baseline to Week 24 Pacritinib Pacritinib: PERSIST-1 Phase 3 18 Best Available Therapy (BAT) excluding ruxolitinib Ruben Mesa, M.D., Mayo Clinic Cancer Center, Arizona Claire Harrison, M.D., Guy’s Hospital, London |
 Sites: Predominantly North America, Europe, Australia, and New Zealand Anticipated Patient Accrual: Principal Investigator: * Cross-over from BAT allowed after progression or assessment of the primary endpoint. ** BAT may include ruxolitinib at the approved dose per its label. Pacritinib: PERSIST-2 Phase 3 19 Co-Primary Endpoints % of patients achieving 35% reduction in spleen volume from baseline to Week 24 Srdan (Serge) Verstovsek, M.D. MD Anderson Cancer Center, Texas 1H-2015 Eligibility Criteria Patients with platelet counts 100,000/µL, prior/current JAK2 therapy allowed 1:1:1 Randomization n = ~300 Best Available Therapy (BAT) ** Pacritinib 400 mg QD Pacritinib 200 mg BID Patients achieving 50% reduction in total symptom score (TSS) from baseline to Week 24 * |
 • Announced November 2013 • CTI and BAXTER: Joint commercialization, profit split in U.S. • BAXTER: Exclusive rights to commercialize in all indications outside U.S., BAXTER generally responsible for 75% of global development program • Total value: $362mm; potential milestones of up to $127mm through regulatory submission - $60mm upfront payment, includes $30mm equity investment in CTI - $67mm potential milestone progress payments expected through 2015 ($20mm received in conjunction with completion of PERSIST-1 enrollment) • CTI to receive tiered high single-digit to mid-teen percentage royalties based on net sales for myelofibrosis; higher double-digit royalties for all other indications Summary of Financial Terms Pacritinib: Collaboration with Baxter 20 |
 • Oral JAK2/FLT3 inhibitor that lacks treatment emergent myelosuppression associated with marketed JAK1/JAK2 agent Potential to demonstrate superior disease and symptom control over titrated doses of ruxolitinib May be used in combination regimens w/o overlapping myelosuppression Potential for longer duration of therapy than observed with JAK1/JAK2 inhibitors • Durable improvement in splenomegaly (24+ months) and MF-related symptoms • Unique kinome profile - potential application in spectrum of blood-related cancers (AML - MDS) Pacritinib: Key Takeaways 21 - - - |
 Tosedostat |
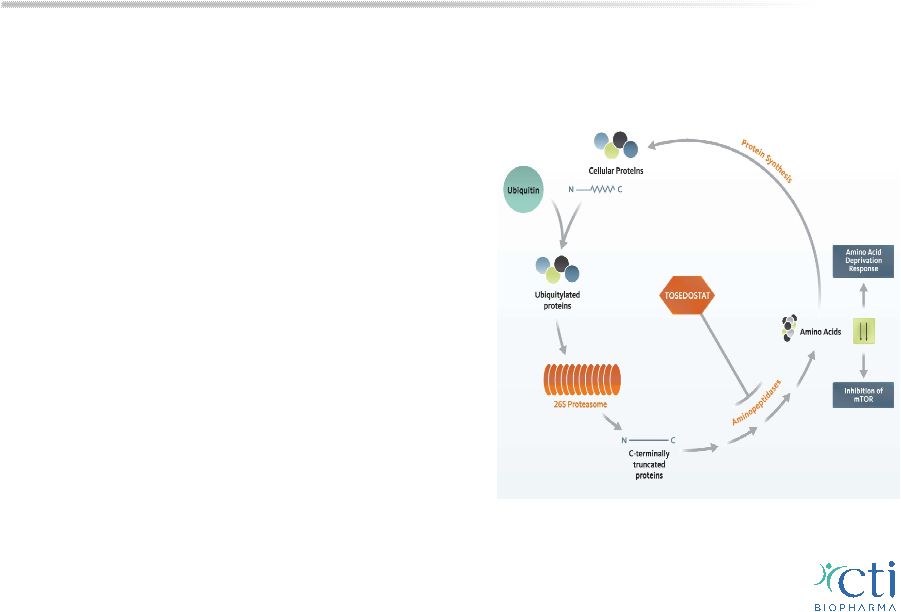 Tosedostat Novel Treatment for Blood-Related Cancers: MDS & AML • Oral, once-daily, aminopeptidase inhibitor interferes with protein recycling, prevents breakdown of peptides into amino acids necessary for tumor cell survival • Synergy with targeted agents (HMA, proteosome inhibitors) or chemotherapy • Encouraging CR rates (54%) and median OS (12 months) in combination with HMA or LDAC in 1 st line elderly high risk AML/MDS • Several ISTs in AML or MDS underway 23 |
 Financial Overview Capital Structure and Financial Statistics Exchanges NASDAQ and MTA: CTIC Market Capitalization* ~$390mm Shares Outstanding ~149.9mm Pro Forma Cash as of June 30, 2014** $53.2mm Debt (unsecured) $15mm *As of September 12, 2014 stock price of $2.61 per share 24 **In August 2014, CTI received a $20mm development milestone payment related to the PERSIST- 1 trial under the agreement with Baxter |
 2014 Key Business Priorities Initiate PERSIST-2 (2 nd Phase 3 trial of pacritinib in MF) Earn $20mm milestone from BAXTER after completion of enrollment in PERSIST-1 Be in position to report PERSIST-1 top-line results in 1Q-2015 • Secure ROW partner (ex-U.S.) to expand commercial potential for PIXUVRI in countries where CTI doesn’t have a commercial presence • Advance PERSIST-2 to complete enrollment in 1H-2015 • Generate PIXUVRI E.U. sales to achieve a net positive contribution margin by year-end 2014 25 |
 Why Invest in CTI BioPharma Biopharmaceutical company with a marketed oncology drug in the E.U. Strategic partner provides validation, financial resources and commercial support for high-potential JAK2/FLT3 inhibitor Phase 3 program Building value through development of late-stage pipeline, with emphasis in areas that address unmet medical needs of patients with blood-related cancers Commitment to manage expenses within guidance Stock with good liquidity and potential near-term catalysts 26 |
 NASDAQ & MTA: CTIC THANK YOU! |