Exhibit 99.1

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

��
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Searchable text section of graphics shown above
[LOGO]
Investor Presentation
Forward-Looking Statements
Statements in this presentation that are not historical facts are “forward-looking statements” that involve risks and uncertainties. Forward-looking statements can be identified by the use of words such as “opportunities,” “trends,” “potential,” “estimates,” “may,” “will,” “should,” “anticipates,” “expects” or comparable terminology or by discussions of strategy. Such forward looking statements include statements as to the potential uses, activity, safety and/or effectiveness of TPI 287, the initiation of clinical trials, and patient enrollment in those clinical trials. Such statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance or achievements to be materially different from those expressed in or implied by such forward-looking statements. Such risks, uncertainties and other factors include risks that additional testing of TPI 287 may reveal deficiencies that would delay or preclude clinical trials; that we are unable to manufacture TPI 287 according to GMP standards or in sufficient quantities; that clinical trials show TPI to be unsafe and/or ineffective in treating cancer in humans or to be no more effective than competitive treatments; and that the start of clinical trials may be delayed because the FDA requires us to conduct additional preclinical testing or to submit additional data and because of delays in obtaining institutional approvals and in recruiting patients. General implementation risks associated with development of any of our products include those that we are blocked or limited in the development of our product candidates because of the intellectual property rights of third parties; that we are limited in our ability to obtain, maintain and enforce our own intellectual property; that development of our product candidates is delayed or terminated because the costs of further development exceed the value of such candidates; and that the Company’s resources are insufficient to continue development. Additional risks, uncertainties and other factors are identified under the captions “Risk Factors,” “Special Note Regarding Forward-Looking Statements” or “Cautionary Note Regarding Forward-Looking Statements” in the Company’s documents filed from time to time with the SEC, including the Company’s Current Report on Form 8-K/A, dated February 11, 2004, Annual Report on Form 10-K/A for the year ended December 31, 2003 filed with the SEC on May 5, 2004, and Quarterly Report on Form 10-Q for the quarter ended September 29, 2004. The Company disclaims any intention or obligation to update publicly or revise any forward-looking statements, whether as a result of new or additional information, future events or otherwise.
For further information, please contact L. Robert Cohen, Vice President, Investor Relations of Tapestry Phamaceuticals, Inc., 212 218 8715.
[LOGO]
Focused on proprietary therapeutics for the treatment of cancer
History of the Company
• 1991 – Company established
• 1994 – Initial Public Offering
• 2002 – Company’s generic paclitaxel approved in US & 25 other countries
• Dec 2003 – Company’s worldwide Paclitaxel business sold to Mayne Pharma for approximately $70 million
• Dec 2004 – Filed 1st proprietary IND – TPI 287
Development Pipeline
Oncology Clinical Candidate | ||
| ||
• | TPI 287 (IND Filed) | Potential indications: Breast, Colon, Lung, Prostate |
|
|
|
Oncology Preclinical Programs | ||
| ||
• | Quassinoid Programs | Novel class of compounds |
|
|
|
• | Peptide Linked Programs | Novel class of compounds |
|
|
|
Genomic Preclinical Program | ||
| ||
• | Oligo Therapy | Proprietary oligonucleotide for the treatment of Huntington’s Disease |
TPI 287
A Third Generation Taxane
Taxanes are highly active chemotherapeutic agents in the treatment of advanced common malignancies such as NSCLC and cancers of the breast, ovary, prostate and head and neck.
However, many of these neoplasms ultimately become resistant to the effects of currently available taxanes. A taxane that could overcome resistance would be a valuable addition to the oncologic therapeutic armamentarium.
TPI 287 Tubulin Binding Model
[GRAPHIC]
Design of TPI 287
• The Company leveraged its extensive paclitaxel chemistry experience in order to develop this novel taxane.
• The molecular construct was optimized for desired attributes such as MDR independence and mutant tubulin binding.
• Potential exists to treat both chemoresistant and chemonaïve patients.
TPI 287: A Third Generation Taxane
• Preclinical data shows more activity than paclitaxel or docetaxelin a variety of taxane resistant human tumor cell lines
• The compound:
• May circumvent MDR-1 seen in breast, colon, lung and prostate cancers
• May be active in tumor cells that express mutant tubulin
In Vitro IC50 Studies for TPI 287
Tumor Cell Line |
| Origin |
| TPI 287 |
| Comparator |
| Differential Factor |
|
MCF-7/NCI-ADR (mdr1+) |
| Breast |
| 0.0100 |
| PTX: 2.0 |
| 200 |
|
MCF-7 (mdr1-) |
| Breast |
| 0.0006 |
| PTX: 0.0003 |
| 0.5 |
|
HCT-15 (mdr1+++, mrp+) |
| Colon |
| 0.0095 |
| SN-38: 0.05 |
| 5.3 |
|
HCT-116 (mdr1+) |
| Colon |
| 0.0035 |
| SN-38: 0.0045 |
| 1.3 |
|
H69 (mrp-, myc+) |
| Lung |
| 0.0046 |
| Docetaxel: 0.0072 |
| 1.6 |
|
MIA PaCa-2 (bcl-2 inducible) |
| Pancreas |
| 0.0763 |
| GEM: 0.124 |
| 1.6 |
|
HepG2 (mdr1+, mrp+) |
| Hepatocellular |
| 0.1000 |
| Oxaliplatin: 5.5 |
| 55 |
|
DU-145 (mdr1+) |
| Prostate |
| 0.0050 |
| Cisplatin: 1.4 |
| 280 |
|
MESSA/Dx5 (mdr1+) |
| Uterine |
| 0.0100 |
| Doxorubicin: 0.8 |
| 80 |
|
MESSA (mdr1-) |
| Uterine |
| 0.0053 |
| Doxorubicin: 0.2 |
| 38 |
|
SK-N-FI (mdr1+) |
| Neuroblastoma |
| 0.0034 |
| Vincristine: 134 |
| 3900 |
|
SK-N-AS (mdr1-, TGF-β 1+) |
| Neuroblastoma |
| 0.0049 |
| Vincristine: 17.7 |
| 3600 |
|
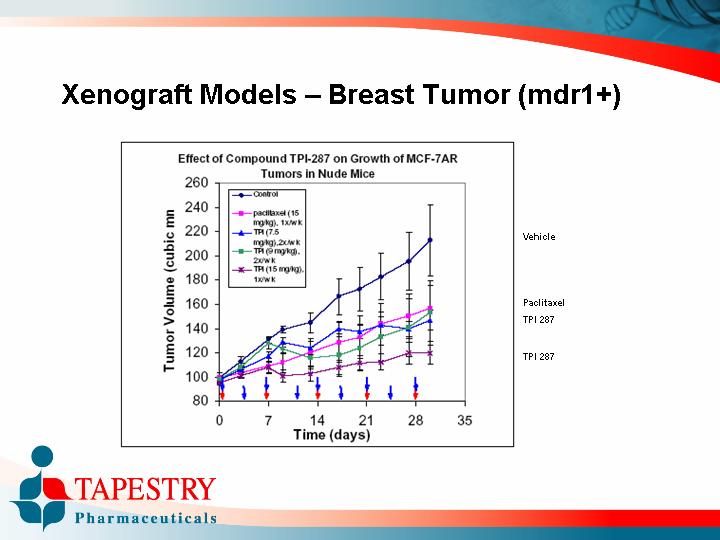
Xenograft Models – Breast Tumor (mdr1+)
Effect of Compound TPI-287 on Growth of MCF-7AR Tumors in Nude Mice
[CHART]
Xenograft Models – Breast Tumor (mdr1-)
Effect of Compound TPI-287 on Growth of MCF-7 tumors in Nude Mice
[CHART]
Xenograft Models – Colon Tumor (mdr1+)
Tumor Growth of TPI-287 vs.
HCT-116 Human Colon Tumor Xenograft Model
[CHART]
Xenograft Models – Lung Tumor (chemoresistant)
Tumor Growth of TPI-287 vs.
MV 522 Human Lung Tumor Xenograft Model
[CHART]
Xenograft Models – Prostate Tumor (mdr+)
Tumor Growth of TPI-287 vs.
PC-3 Human Prostate Tumor Xenograft Model
[CHART]
Toxicology: Single Dose Studies
• Intravenous or Intraperitoneal dose tolerance and MTD
• Maximum Tolerated Dose
• Mouse – 80 mg/kg
• Rat – 48 mg/kg
• Dog – 12.5 mg/kg
• Notable side effects
• Bloody diarrhea, vomiting
• Emaciation and weight loss
• Gastric paresis with distended stomachs
• Ataxia, increased salivation
Note: These effects are routinely seen with other cytotoxics.
Toxicology: Repeat Dose Studies
• Maximum Tolerated Dose
• Mouse – 12 mg/kg or 36 mg/m2
• Rat – 14 mg/kg
• Dog – 4 mg/kg
• Notable side-effects
• Weight loss in testes, ovaries, thymus, and adrenal glands
• Bloody diarrhea, abnormal feces, vomiting
• Emaciation and weight loss
• Abnormal positioning
• Gastric paresis, distended stomach, changes in bowel histology
• Decrease in WBC and neutrophils
• Slight decrease in HCT and platelets
• Increase in liver function tests
Note: These effects are routinely seen with other cytotoxics.
Preclinical Conclusions
• In Vitro Studies
• Evidence of activity in various cell lines including: breast, colon, lung, pancreas, prostate, and others
• Tumor Xenograft Studies
• Evidence of activity in breast, colon, lung, and prostate tumors models
• Toxicity
• Generally well-tolerated
• Key side-effects are GI in nature
• MTD based on mouse repeat study is 12 mg/kg or 36 mg/m2
• Starting human dose
• Based on 1/10 of MTD in mice
• Will be 3 mg/m2
Phase I Clinical Program
TPI 287
Phase I Objectives
• Primary
• To determine the maximum tolerated dose (MTD) of TPI 287
• Secondary
• To determine the safety profile
• To determine the PK profile
• To report any responses based on RECIST criteria
• To determine the time to treatment failure
• To determine the duration of response
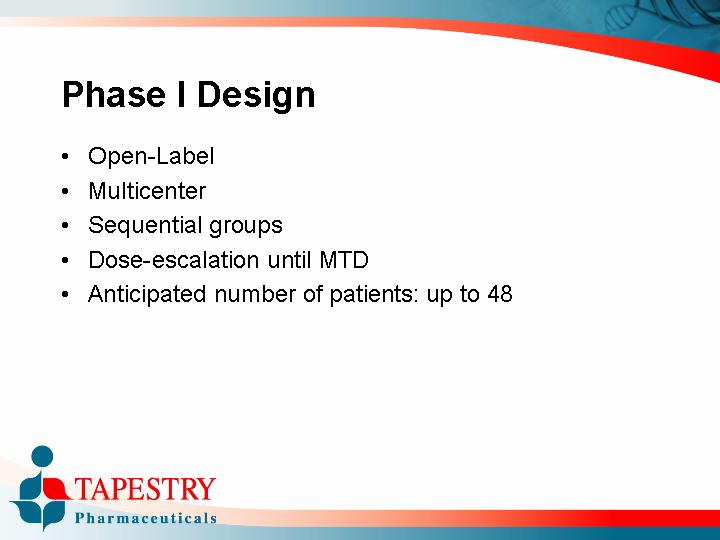
Phase I Design
• Open-Label
• Multicenter
• Sequential groups
• Dose-escalation until MTD
• Anticipated number of patients: up to 48
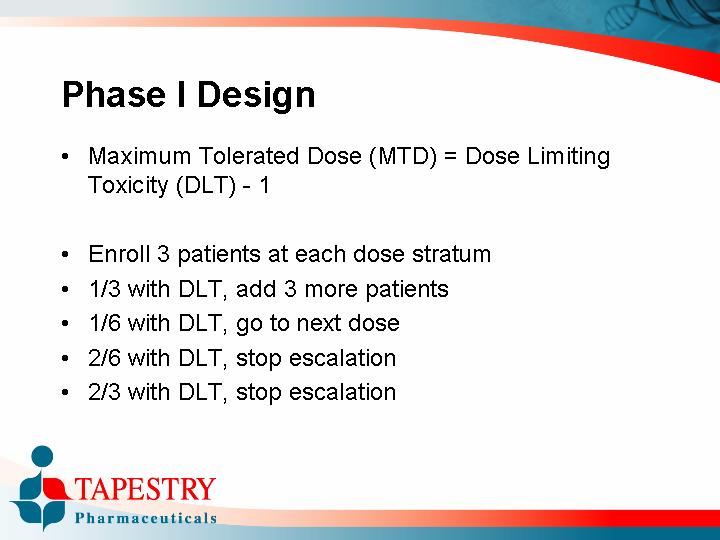
• Maximum Tolerated Dose (MTD) = Dose Limiting Toxicity (DLT) -1
• Enroll 3 patients at each dose stratum
• 1/3 with DLT, add 3 more patients
• 1/6 with DLT, go to next dose
• 2/6 with DLT, stop escalation
• 2/3 with DLT, stop escalation
Definition of Dose Limiting Toxicity
• Grade 4 neutropenia or thrombocytopenia that persits for 7 days
• Febrile neutropenia
• Grade 3 thrombocytopenia with bleeding
• Grade 3 elevation of transaminases that persists for 7 days
• Any other Grade 3 non-hematological toxicity, excluding inadequately treated nausea or vomiting
• Inability to initiate the next cycle of treatment for 3 weeks due to unresolved toxicity
Key Inclusion Criteria
• Documented recurrent or refractory cancer
• Histologically demonstrated cancer
• Patients must have advanced solid tumors that have recurred or progressed following standard therapy or who are not candidates for standard therapy
• Patient was treated for three or less chemotherapeutic regimens which were administered for progress disease
• Patient is ambulatory with an ECOG score of 0 or 1, and an estimated life expectancy of > 3 months
• Patient is older than 18
Key Exclusion Criteria
• Patient has a history of cancer other than primary cancer (excluding resected basal cell or squamous cell carcinoma of the skin). Patient may be included if disease free of other cancer for 5 years
• Patient has had prior local radiation therapy within the last 4 weeks or chemotherapy within the last 4 weeks
• Patient has another active medical condition(s) or organ disease(s):
• WBC < 3,000/µL
• Absolute neutrophil count < 1,500/µL
• Platelets < 100,000/µL
• Total bilirubin > 3 x upper limit of normal
• ALT or AST > 3 x upper limit of normal if no liver metastases or > 5 upper limit of normal if the patient has liver metastases
• Serum Creatine > 3 x upper limit of normal
• INR > 2.0
Key Exclusion Criteria (continued)
• Patient has clinically significant cardiac co-morbidities
• Patient has been treated with any investigational drug, investigational biologic, or investigational therapeutic device within 30 days of initiating study treatment
• Prior or concurrent CNS disease including stroke (Brain metastasis are acceptable as long as the patient has had prior radiation therapy, has no CNS symptoms, and hs been on a stable dose of steriods for at least one month)
• Grade II peripheral neuropathy
Dose Escalation
• If a cohort exhibits no patients Dose Limiting Toxicity, the next cohort will be enrolled at twice the dose
• If a cohort exhibits one patient with Dose Limiting Toxicity, the next cohort will be enrolled at 1.25 X the previous dose
• If a cohort exhibits two or more patients with Dose Limiting Toxicity, there will be no further dose escalation
Study Duration
• Patients will be treated until the following:
• Dose Limiting Toxicity
• Patient or investigator decision
• Progressive disease
• Patient develops significant other illness
• If they are not discontinued for above reasons, they can stay on trial until:
• 6 cycles or
• If they demonstrate a partial response, they can stay on trial until progression
Timelines
• IND Submission: December, 2004
• First patient enrolled: Q2, 2005
Overview
STRENGTHS
• Potent compound
• Overcomes Paclitaxel resistance in vitro
• Extensive taxane experience
• Extensive chemistry work completed
• MTD established in mice
OPPORTUNITIES
• Large taxane resistant market
• Newer, targeted therapies will probably still be given with cytotoxics
• May have efficacy in tumors that are taxane resistant
WEAKNESSES
• Potent compound
• Difficult synthesis
• Expensive synthesis
• No clinical results
• May be perceived as “me-too” drug to paclitaxel
THREATS
• Generic taxanes
• Reformulated taxanes
• Rising regulatory bar for follow-on therapies
Oncology Advisory Board
• Paul A. Bunn, Jr., M.D. (Chair)
Director of the University of Colorado Cancer Center, past president of American Society of Clinical Oncology (2002-2003), and former chairman of the Oncologic Drugs Advisory Committee (ODAC) for FDA
• S. Gail Eckhardt, M.D.
Professor of Medicine and Director of the Developmental Therapeutics and GI Malignancies Programs at the University of Colorado Health Sciences Center in Denver
• Eric K. Rowinsky, M.D.
Former Director of the Institute for Drug Development, Cancer Therapy and Research Center and Clinical Professor of Medicine at the University of Medicine at the University of Texas Health Science Center at San Antonio
• Daniel D. Von Hoff, M.D.
Professor of Medicine, Pathology, Molecular and Cellular Biology and the Director of the Arizona Health Sciences Center’s Cancer Therapeutics Program
Board of Directors
• Leonard P. Shaykin
Chairman of the Board, Chief Executive Officer
• Stephen K. Carter, M.D.
Former Senior Vice President, Worldwide Clinical Research and Development, Bristol-Myers Squibb and Deputy Director, Division of Cancer Treatment, National Cancer Institute
• Edward L. Erickson
Chairman of the Board, President, and Chief Executive Officer of Immunicon Corporation
• George M. Gould, Esq.
Attorney, Of Counsel, Gibbons, Del Deo, Dolan, Giffinger & Vecchione; formerly Vice President and Chief Patent Counsel of Hoffman-La Roche
• Arthur Hull Hayes, Jr., M.D.
President, MediScience Associates a pharmaceutical consulting company; Former FDA Commissioner
• Elliot M. Maza, J.D., C.P.A.
Chief Financial Officer of Emisphere Technologies, Inc.
• The Honorable Richard N. Perle
Former U.S. Assistant Secretary of Defense; Former Director of Hollinger International, Inc.; Autonomy, PLC
• Patricia Pilia, Ph.D.
Executive VP, Secretary, Co-founder of Company
• Robert E. Pollack, Ph.D.
Professor of Biological Sciences and Director of the Center for the Study of Science and Religion at Columbia University
Investment Opportunity
• IND filed for TPI 287 in December, 2004 – Phase I studies to proceed pending regulatory & institutional approvals
• ~ $42 million cash resources available to advance development programs as of September 29, 2004
• Experienced drug development team
• Experienced business development team
• Extensive intellectual property position
• Distinguished scientific and clinical advisors
[LOGO]
Strategies for LifeTM