Exhibit 99.1
A PHASE 1 STUDY OF TPI 287; A THIRD GENERATION TAXANE ADMINSTERED WEEKLY IN PATIENTS WITH ADVANCED CANCER
Jimmy Hwang MD*, John Marshall MD*, Tauseef Ahmed MD†, Hoo G. Chun MD†, Michele Basche MD‡, Allen Cohn MD‡, Michael Kurman MD**, Gilles Tapolsky PhD**, Sandra Silberman MD PhD+, Ernest Allen**
* Georgetown University Medical Center, Washington, DC
† New York Medical College, Valhalla, NY
‡ Rocky Mountain Cancer Centers, Denver, CO
** Tapestry Pharmaceuticals, Boulder, CO
+ Tapestry Pharmaceuticals, Roseland, NJ
1
INTRODUCTION
Taxanes have become key components of the chemotherapeutic armamentarium for a variety of cancers including tumors of the breast, lung, prostate, ovary, stomach, and head and neck.
Unfortunately, resistance to the antineoplastic effects of taxanes often develops, limiting their efficacy. Two putative mechanisms for resistance have been proposed:
· Taxanes are substrates for the efflux protein encoded by the mdr1 gene
· Alteration of the tubulin structure (genetic or epigenetic changes) may interfere with the mechanism of action of taxanes, rendering them unable to produce microtubule stabilization
2
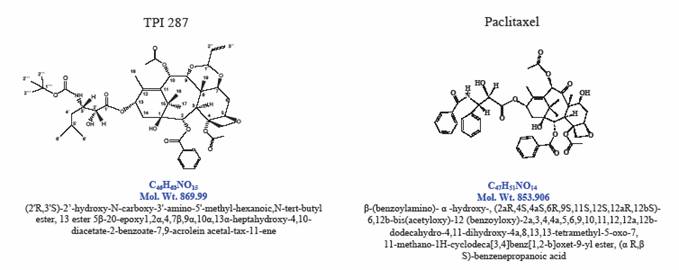
Tapestry Pharmaceuticals, Inc. has developed a novel taxane analog, TPI 287, which is a semi-synthetic compound manufactured from yew starting material. TPI 287 synthesis involves modifications of the taxane side chain, rendering the molecule more lipophilic. In addition, modifications of the baccatin ring structure were designed to enhance the affinity of ß-tubulin binding, and to circumvent multi-drug resistance.
3
Figure 1: TPI 287 and Paclitaxel Structures
4
Preclinical evaluation of TPI 287 in vitro demonstrated significant anti-tumor activity in both MDR- and MDR+ cell lines. TPI 287 was active in tumor xenograft models in mice (breast cancers MCF-7 and MCF-7AR, colon cancers HCT-15 and HCT-116, and prostate cancer PC3). Toxicology and safety studies were performed in rodents and dogs. The LD10 value for multiple-dose TPI 287 was found to be 4 mg/kg/day IV (71 mg/m2/day) in dogs, the most sensitive species. Allometric scaling of 1/10 of the dog LD10 determined an initial human starting dose of 7 mg/m2.
5
Study Objectives
Primary Objective:
To determine the Maximum Tolerated Dose (MTD) of TPI 287 administered once weekly for three weeks followed by one week of no treatment, in order to determine a safe, well tolerated dose.
Secondary Objectives:
To determine the safety of TPI 287
To determine the pharmacokinetic profile of TPI 287
This is a multi-center, dose-escalation, sequential cohort Phase I study in patients with recurrent or refractory solid tumors or Non-Hodgkin’s lymphoma. Patients were either no longer candidates for standard therapy, had no standard therapy available, or chose not to pursue standard therapy.
6
TPI 287 was administered intravenously over 60 minutes, starting with a dose of 7 mg/m2 in the first cohort. Up to four patients could be enrolled at each dose level and dose escalation was permitted after at least three patients had received the first cycle of therapy. Escalation to the next dose occurred if no dose-limiting toxicity (DLT) was observed in any patient in that first cycle. If one patient experienced a DLT, up to three additional patients were enrolled at this dose, with a minimum of six patients treated to determine the MTD. If two or more patients in a cohort experienced a DLT, dose escalation was stopped. Dose refinement was then initiated, which was a way to define the MTD more accurately, by enrolling three patients at a dose approximately half way between the dose level where two or more patients experienced a DLT at the preceding dose. If no patient or one patient experienced a DLT at this intermediate dose, the cohort was expanded by an additional three patients, and if none or one patient in the expanded cohort experienced a DLT, this intermediate dose was considered to be the MTD. However, if more than one patient in the expanded cohort experienced a DLT, the
7
algorithm was repeated, i.e. three more patients were enrolled at an intermediate, lower dose. Once the MTD is defined, additional patients will be treated at that dose, to bring the total number treated at the MTD to nine.
DLT was defined as a Grade 4 hematological toxicity lasting seven days, febrile neutropenia, Grade 3 or 4 thrombocytopenia with bleeding, Grade 3 or 4 elevation of transaminases lasting seven days, Grade 3 neuropathy or any other Grade 3 or Grade 4 drug-related non-hematologic toxicity other than inadequately treated nausea or vomiting.
After at least three patients in each cohort had been enrolled, the investigators, medical monitor and the sponsor reviewed the safety data in order to decide whether to recommend further dose escalation. Escalation or de-escalation at smaller dosage increments than stipulated in the protocol, and enrollment of additional patients was recommended in some instances, as outlined above.
8
Patients whose tumors did not progress, or who did not discontinue TPI 287 for other medical or administrative reasons, were treated with a maximum of six cycles of therapy (approximately six months) on this protocol, and were eligible to participate in a separate extension study in order to continue to receive study drug.
9
Demographics
|
| Cohort 1 |
| Cohort 2 |
| Cohort 3 |
| Cohort 4 |
| Cohort 5 |
| Cohort 6 |
| Cohort 7 |
|
|
|
|
| 7 mg/m2 |
| 14 mg/m2 |
| 28 mg/m2 |
| 56 mg/m2 |
| 85 mg/m2 |
| 127.5 mg/m2 |
| 185 mg/m2 |
| Total |
|
|
| N = 4 |
| N = 4 |
| N = 4 |
| N = 4 |
| N = 4 |
| N = 5 |
| N = 5 |
| N = 30 |
|
Age (years) |
| 51.8 |
| 51.8 |
| 58.3 |
| 61.3 |
| 66.5 |
| 64.2 |
| 66.8 |
| 60.4 |
|
Mean |
| 52.5 |
| 54.0 |
| 58.5 |
| 60.0 |
| 67.0 |
| 60.0 |
| 71.0 |
| 60.5 |
|
Median |
| 37, 65 |
| 24, 75 |
| 47, 69 |
| 54, 71 |
| 53, 79 |
| 49, 86 |
| 42, 82 |
| 24, 86 |
|
Min, Max |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Male |
| 3 |
| 1 |
| 3 |
| 2 |
| 4 |
| 2 |
| 2 |
| 17 |
|
Female |
| 1 |
| 3 |
| 1 |
| 2 |
| 0 |
| 3 |
| 3 |
| 13 |
|
Race |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Asian |
| 0 |
| 0 |
| 0 |
| 0 |
| 1 |
| 0 |
| 0 |
| 1 |
|
Black |
| 1 |
| 0 |
| 0 |
| 0 |
| 1 |
| 1 |
| 1 |
| 4 |
|
Caucasian |
| 3 |
| 3 |
| 4 |
| 4 |
| 2 |
| 4 |
| 4 |
| 24 |
|
Hispanic |
| 0 |
| 1 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 1 |
|
An additional group (Cohort 8) of patients treated with 150mg/m2 is currently being enrolled
10
Thirty patients have been enrolled, 17 males and 13 females, ranging in age from 24 to 86. The majority (24) were Caucasian, four patients were Black, one was Asian, and one Hispanic.
The patients’ tumor types were as follows:
Colon (7), Prostate (3), Pancreatic (3), Lung (3), Ovarian (2), Breast (1), Hepatocellular (1), Kidney (1), Osteosarcoma (1), Cervical (2), Endometrial (1), Basal cell (1), Melanoma (1), Brain (1), Gastric (1), Squamous of buccal mucosa (1).
Twenty-nine (93%) patients had metastatic disease at the time of enrollment. Prior cancer therapy included surgery (23 patients, 77%), radiotherapy (18 patients, 60%) chemotherapy (29 patients, 97%), and hormonal, immunological, biological or other therapy (14 patients, 47%). One patient was enrolled but was then found to be ineligible, and is not included in this analysis (i.e. 29/30 patients are included in the analysis).
11
Safety
Most Frequent Treatment-Emergent Adverse Events (Any Grade) Occurring in ≥ 15% of Patients
Adverse Event |
| Total |
|
|
|
Any Adverse Event |
| 28 (97%) |
|
|
|
Fatigue |
| 15 (52%) |
|
|
|
Constipation |
| 10 (34%) |
|
|
|
Dyspnea |
| 9 (31%) |
|
|
|
Nausea |
| 9 (31%) |
|
|
|
Abdominal Pain |
| 7 (24%) |
|
|
|
Vomiting |
| 8 (24%) |
|
|
|
Urinary Tract Infection |
| 7 (24%) |
|
|
|
Cough |
| 7 (24%) |
|
|
|
Pyrexia |
| 6 (21%) |
12
Back Pain |
| 6 (21%) |
|
|
|
Anorexia |
| 6 (21%) |
|
|
|
Anemia |
| 5 (17%) |
|
|
|
Diarrhea |
| 5 (17%) |
|
|
|
Neuropathy |
| 5 (17%) |
|
|
|
Chest Pain |
| 5 (17%) |
13
Safety
Treatment-Emergent Grade 3 or 4 Adverse Events occurring in ≥ 5% of All Patients
|
| Cohort 1 - 3 |
| Cohort 4 |
| Cohort 5 |
| Cohort 6 |
| Cohort 7 |
|
|
|
|
| ||||||||||
|
| 7 (N = 4), 14 |
| 56 mg/m2 |
| 85 mg/m2 |
| 127.5 mg/m2 |
| 185 mg/m2 |
| Total |
| ||||||||||||
Grade |
| 3 |
| 4 |
| 3 |
| 4 |
| 3 |
| 4 |
| 3 |
| 4 |
| 3 |
| 4 |
| 3 |
| 4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dyspnea |
| 3 (25%) |
| 0 |
| 2 (50%) |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 5 (17%) |
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Neuropathy* |
| 1 (8%) |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 1 (20%) |
| 0 |
| 1 (20%) |
| 0 |
| 3 (10%) |
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chest pain |
| 2 (17%) |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 2 (7%) |
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pneumonia |
| 0 |
| 0 |
| 0 |
| 0 |
| 1 (25%) |
| 0 |
| 1 (25%) |
| 0 |
| 0 |
| 0 |
| 2 (7%) |
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INR increased |
| 0 |
| 0 |
| 1 (25%) |
| 0 |
| 0 |
| 0 |
| 1 (20%) |
| 0 |
| 0 |
| 0 |
| 2 (7%) |
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hypoxia |
| 1 (8%) |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 1 (20%) |
| 0 |
| 0 |
| 0 |
| 2 (7%) |
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fatigue |
| 0 |
| 0 |
| 0 |
| 0 |
| 1 (25%) |
| 0 |
| 0 |
| 0 |
| 0 |
| 1 (25%) |
| 1 (3%) |
| 1 (3%) |
|
14
Liver Function Test Elevated† |
| 1 (8%) |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 0 |
| 1 (3%) |
|
Dose-limiting Toxicity, 2/3 patients had baseline Grade 1 neuropathy
† alkaline phosphatase elevated
15
Safety
Summary of Safety
· In this ongoing study, the maximum dose of TPI 287, administered weekly was 185 mg/m2. Treatment with 150 mg/m2 is currently ongoing.
· Overall, 28/29 patients experienced at least one adverse event, which were typically grade 1 and 2 in severity, with the most frequently cited being fatigue (52% of patients), constipation (34% of patients), dyspnea (31% of patients) and nausea (31% of patients). There was a trend toward more patients experiencing fatigue with higher dose (80% of patients at 185 mg/m2). The most frequent adverse events considered probably or definitely related to TPI 287 were anemia, nausea, diarrhea and neuropathy.
· In the 29 patients analyzed, overall, 17 (57%) patients experienced at least one adverse event of Grade 3 severity and 3 (10%) patients experienced at least one adverse event which was Grade 4. The most frequent grade 3 or 4 adverse events experienced by ≥ 5% of patients were dyspnea, neuropathy, chest pain, pneumonia, increased INR, hypoxia and fatigue. Three patients receiving 14 mg/m2, 127.5 mg/m2 and 185 mg/m2 respectively had Grade 3 neuropathy which proved to be the dose-limiting toxicity in this study. Two of these patients had baseline neuropathy of Grade 1 due to prior therapy and co-morbid conditions. One patient receiving 7 mg/m2 had Grade 3 elevated alkaline phosphatase. There were no Grade 3 or 4 myelosuppressive events.
· Twelve patients experienced serious adverse events (SAE), resulting in cessation of treatment in three patients and interruption of treatment in four patients. In two patients the SAEs were considered probably related (anemia) or definitely related (hypersensitivity reaction with dyspnea, dizziness/back pain/ flushing) to TPI 287 treatment.
16
Pharmacokinetics of TPI 287
Blood samples for the measurement of pharmacokinetic parameters for TPI 287 were taken from 31 patients before the first dose, and 1-2 minutes, 5, 15 and 30 minutes, and 1, 2, 3, 4, 5 , 6 and 24 hours after the end of the infusion. The levels of TPI 287 in plasma were measured using a validated LC-MS method. PK parameters were determined using Win Nonlin software applying non-compartmental analysis and 2-compartment modeling.
17
Non-compartmental PK Analysis
| Dose (mg/m2) |
| |||||||||||||
|
| 7 |
| 14 |
| 28 |
| 56 |
| 84 |
| 127.5 |
| 185 |
|
Number of Patients |
| 4 |
| 4 |
| 3 |
| 4 |
| 4 |
| 6 |
| 6 |
|
Cmax (ng/mL) |
| 542.5 |
| 159.6 |
| 235.2 |
| 896 |
| 2234.4 |
| 2575.5 |
| 7825.5 |
|
Mean (SD) |
| (483.7 | ) | (77.1 | ) | (137.2 | ) | (208.9 | ) | (1083.6 | ) | (947.3 | ) | (2701 | ) |
AUClast (ng*h/mL) |
| 329 |
| 196 |
| 369.6 |
| 1876 |
| 5905.2 |
| 4347.7 |
| 11174 |
|
Mean (SD) |
| (363 | ) | (99.4 | ) | (86.8 | ) | (1075.2 | ) | (3855.6 | ) | (3060 | ) | (2109 | ) |
CL (L/hr/m2) |
| — |
| 29.1 |
| 49.6 |
| 28.6 |
| 16.6 |
| 30.2 |
| 15.8 |
|
Mean (SD) |
|
|
| (19.2 | ) | (5.0 | ) | (13.2 | ) | (9.3 | ) | (11.9 | ) | (2.7 | ) |
T1/2 (h) |
| — |
| 8.5 |
| 6.1 |
| 7.2 |
| 8.9 |
| 8.5 |
| 8.0 |
|
Mean (SD) |
|
|
| (4.1 | ) | (1.6 | ) | (2.3 | ) | (2.1 | ) | (1.3 | ) | (0.7 | ) |
The AUC and Cmax were generally dose-proportional, the half-life of TPI 287 in blood was approximately 8 hours and clearance at 185 mg/m2 was 15.8 L/hr/m2.
18
Efficacy
One 62-year-old female patient with pancreatic cancer had stable disease for four cycles of TPI 287 administered at 185 mg/m2.
Another 59-year-old male patient with non-small cell lung cancer had a 57% reduction in one lesion as assessed by RECIST (Figure 2) after treatment with TPI 287 at 85 mg/m2; however this patient discontinued treatment due to progression of an unrelated condition.
19
Figure 2: Pre- and Post- Treatment CT Scans in Patient with Non-Small Cell Lung Cancer

20
Conclusions
· In this ongoing Phase 1 study, the maximum tolerated dose of TPI 287 to date, administered as an IV infusion over 60 minutes weekly for three weeks followed by one week rest, is still being explored.
· Dose-limiting toxicity of Grade 3 neuropathy was seen at 185 mg/m2 in three patients, two of whom had baseline neuropathy of Grade 1.
· No clinically significant myelosuppression was seen.
· Pharmacokinetics show dose-proportionality and TPI 287 had a half life of approximately 8 hours.
· Two patients enrolled at the highest dose levels had clinical benefit as documented by disease regression (Non-small cell lung cancer) or stabilization of disease (pancreatic cancer).
21