Filed Pursuant to Rule 424(b)(3)
Registration Statement No. 333-180562
February 12, 2013
PROSPECTUS SUPPLEMENT NO. 15
SYNTHETIC BIOLOGICS, INC.
112,573 Shares of Common Stock
This prospectus supplement amends and supplements our prospectus, dated July 26, 2012 relating to the resale, from time to time, of up to 112,573 shares of common stock of Synthetic Biologics, Inc. upon the exercise of warrants issued in July 2011 at an exercise price of $1.00 per share and warrants sold in our July 2010 offering at an exercise price of $1.32 per share. We will receive proceeds if the warrants are exercised for cash; to the extent we receive such proceeds, they will be used for working capital purposes.
Our common stock became eligible for trading on the NYSE MKT October 16, 2008. Our common stock is eligible for quotation on the NYSE MKT under the symbol “SYN”. The closing price of our stock on February 11, 2013 was $1.93.
This prospectus supplement is being filed to include the information set forth in the Current Report on Form 8-K filed on February 11, 2013, which is set forth below. This prospectus supplement should be read in conjunction with the prospectus dated July 26, 2012, supplement no. 1 dated August 9, 2012, prospectus supplement no. 2 dated August 15, 2012, prospectus supplement no. 3 dated August 15, 2012, prospectus supplement no. 4 dated September 12, 2012, prospectus supplement no. 5 dated October 9, 2012, prospectus supplement no. 6 dated October 17, 2012, prospectus supplement no. 7 dated November 1, 2012, prospectus supplement no. 8 dated November 14, 2012; prospectus supplement no. 9 dated November 15, 2012; prospectus supplement no. 10 dated November 15, 2012; prospectus supplement no. 11 dated December 3, 2012; prospectus supplement no. 12 dated December 6, 2012; prospectus supplement no. 13 dated December 21, 2012, and prospectus supplement no. 14 dated January 8, 2013, which are to be delivered with this prospectus supplement.
Investing in our securities involves a high degree of risk. See “Risk Factors ” beginning on page 4 of the original prospectus for more information.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus or the prospectus to which it relates is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement No. 15 is February 12, 2013.
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): February 11, 2013
SYNTHETIC BIOLOGICS, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 1-12584 | 13-3808303 | ||
| (State or other jurisdiction of incorporation) | (Commission File No.) | (IRS Employer Identification No.) |
617 Detroit Street, Suite 100, Ann Arbor, Michigan, 48104
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (734) 332-7800
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |||
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |||
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |||
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) | |||
Item 7.01. Regulation FD Disclosure
Beginning on February 11, 2013, Jeffrey Riley, Chief Executive Officer and President of Synthetic Biologics, Inc. (the “Company”), will be making several investor presentations during the next few weeks, including a presentation on February 12, 2013 at the 2013 BIO CEO & Investor Conference. In connection with the presentations, Mr. Riley intends to discuss the slide presentation furnished as Exhibit 99.1 hereto, which is incorporated herein by reference.
The slide presentation attached as Exhibit 99.1 to this Report includes “safe harbor” language pursuant to the Private Securities Litigation Reform Act of 1995, as amended, indicating that certain statements contained in the slide presentation or in the press release are “forward-looking” rather than historical.
The information included in this Item 7.01 and in Exhibit 99.1 shall not be deemed filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section or incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing. The Company undertakes no duty or obligation to update or revise information included in this Report or any of the Exhibits.
Item 9.01. Financial Statements and Exhibits
(d) Exhibits
The following exhibit is being filed as part of this Report.
Exhibit Number |
Description |
| 99.1 | Presentation materials to be provided at Synthetic Biologics, Inc.’s investor presentations |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SYNTHETIC BIOLOGICS, INC. | |
| Date: February 11, 2013 | By: /s/ C. Evan Ballantyne |
| Name: C. Evan Ballantyne | |
| Title: Chief Financial Officer |
EXHIBIT INDEX
Exhibit Number |
Description |
| 99.1 | Presentation materials to be provided at Synthetic Biologics, Inc.’s investor presentations |

NYSE MKT: SYN February 11, 2013

Forward - Looking Statements This presentation includes forward - looking statements on Synthetic Biologics’ current expectations and projections about future events . In some cases forward - looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes,“ "estimates,” “indicates,” and similar expressions . These statements are based upon current beliefs, expectations and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and include statements regarding our clinical trials, our establishment of collaborations and our execution of our growth strategy . The forward - looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those set forth or implied by any forward - looking statements . Important factors that could cause actual results to differ materially from those reflected in Synthetic Biologics’ forward - looking statements include, among others, a failure of our product candidates to be demonstrably safe and effective, a failure to initiate clinical trials and if initiated, a failure to achieve the desired results, a failure to obtain regulatory approval for our product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to our ability to promote or commercialize our product candidates for the specific indications, a lack of acceptance of our product candidates in the marketplace, a failure of us to become or remain profitable, a failure to establish collaborations, our inability to obtain or maintain the capital or grants necessary to fund our research and development activities, a loss of any of our key scientists or management personnel, and other factors described in Synthetic Biologics’ report on Form 10 - K/A for the year ended December 31 , 2011 , subsequent Form 10 - Qs and any other filings with the SEC . The information in this presentation is provided only as of the date presented, and Synthetic Biologics undertakes no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law . 2

Corporate Snapshot • Current Price : $2.00 (as of 2/8/13) • 52 Week Range: $1.46 - $ 2.95 • Average Volume (3 months): 63,337 • Shares Outstanding: ~44.3 million (as of 12/7/12 ) • Warrants Outstanding: ~2.0 million * • Vested Options Outstanding: ~2.6 million ** • Market Capitalization: ~$88.6 million • Cap Structure: Common, no debt, no preferred • Cash: ~$14.2 million ( pro forma 10/31/12) • Offices in Rockville, Maryland 3 * As of October 31, 2012; weighted average exercise price is $2.72 ** As of October 31, 2012; weighted average exercise price is $1.76; total options outstanding: ~4.3 million NYSE MKT: SYN

Corporate Overview • Clinical - stage programs ̶ Relapsing - remitting multiple sclerosis (MS) – Phase II ̶ Cognitive dysfunction in MS – Phase II ̶ Prevention of Clostridium difficile ( C. diff ) – Phase II* • Discovery/preclinical stage programs ̶ Pertussis (whooping cough) ̶ Prevention of C. diff infections ̶ Acinetobacter baumannii infections ̶ Pulmonary arterial hypertension (PAH) • Two strategic collaborations with Intrexon Corporation − Private biotechnology company led by CEO, RJ Kirk 4 NYSE MKT: SYN * Suite of oral enzyme candidates; first generation candidate (formerly known as P1A) is in Phase II

Management Team 5 • Jeffrey Riley, CEO Pfizer, Nichols Institute (Quest), SmithKline Beecham, QIC • C. Evan Ballantyne , CFO Clinical Data, Inc., Avedro , ZymeQuest , ACNielsen, IMS • John Monahan, Ph.D., EVP R&D Avigen , Somatix , Triton Biosciences, Hoffman - LaRoche • Carol Reed, M.D., SVP Clinical/Regulatory Clinical Data, Inc., Genaissance Pharmaceuticals, Inc., Bayer Pharmaceuticals, Inc. • Michael Kaleko , M.D., Ph.D ., SVP R&D Genetic Therapy, Inc. (Novartis)
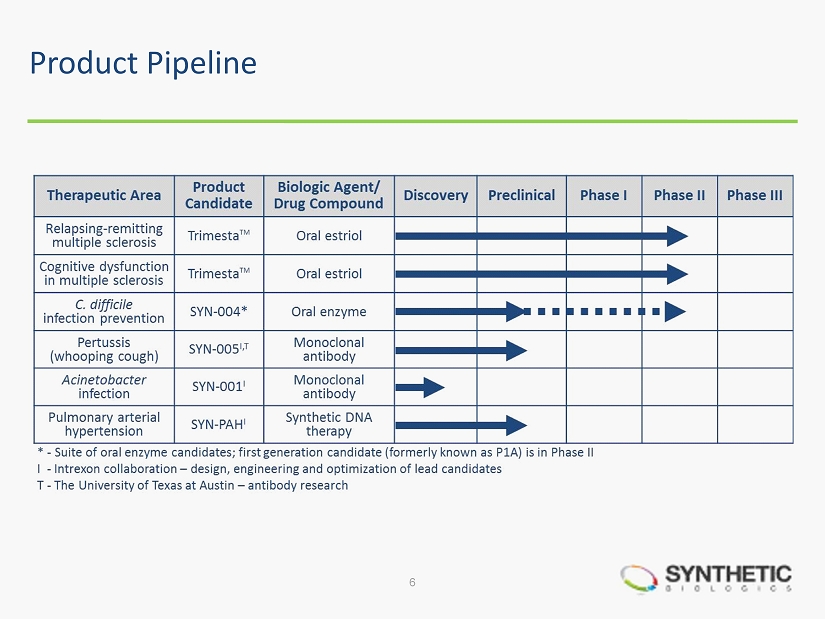
Product Pipeline 6
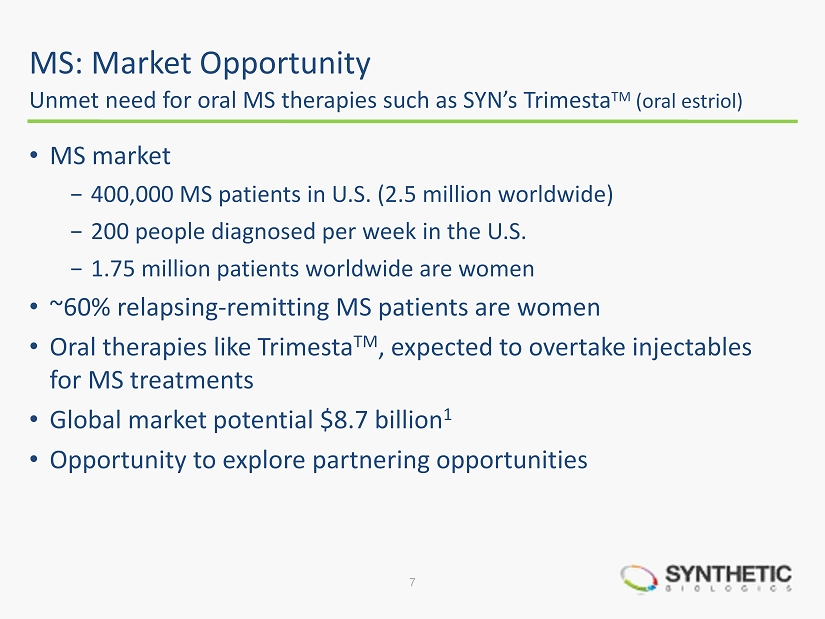
MS: Market Opportunity • MS market − 400,000 MS patients in U.S. (2.5 million worldwide) − 200 people diagnosed per week in the U.S. − 1.75 million patients worldwide are women • ~60% relapsing - remitting MS patients are women • Oral therapies like Trimesta TM , expected to overtake injectables for MS treatments • Global market potential $8.7 billion 1 • Opportunity to explore partnering opportunities Unmet need for oral MS therapies such as SYN’s Trimesta TM (oral estriol) 7

MS: Historical Efficacy of Estriol “Pregnancy Hormone” – Linked to decreased MS relapse rates • Estriol − Hormone produced by placenta during pregnancy − Solid safety profile (approved in Europe/Asia for 40+ years) • Landmark study published in New England Journal of Medicine 2 − Study of 254 women diagnosed with MS prior to pregnancy − Relapse rates were significantly reduced (p<0.001) in 3 rd trimester of pregnancy compared to pre - pregnancy levels − Relapse rates were significantly increased (p<0.001) during three months post - partum compared to pre - pregnancy levels − Decrease in relapse rate during pregnancy was more prominent than any therapeutic effect reported to date 8
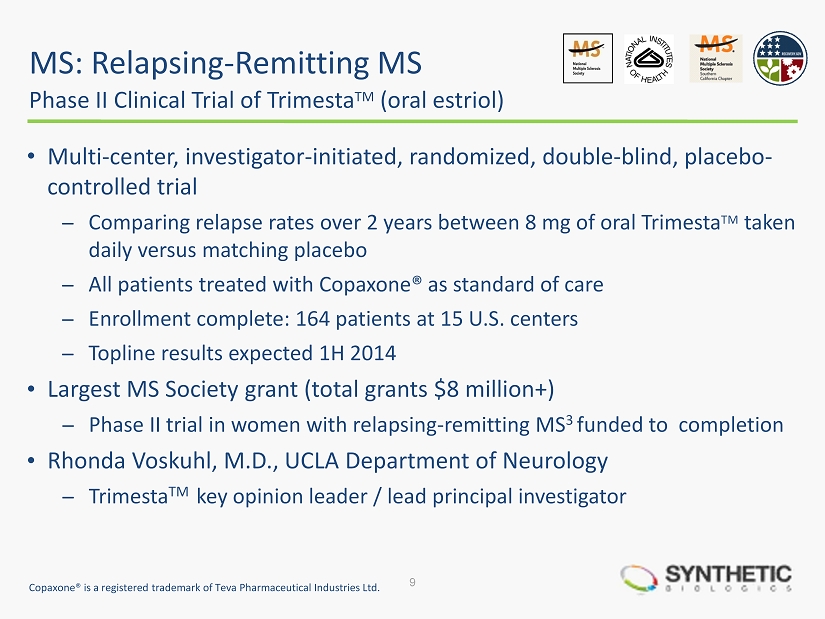
MS: Relapsing - Remitting MS Phase II Clinical Trial of Trimesta TM (oral estriol) • Multi - center, investigator - initiated, randomized, double - blind, placebo - controlled trial ̶ Comparing relapse rates over 2 years between 8 mg of oral Trimesta TM taken daily versus matching placebo ̶ All patients treated with Copaxone ® as standard of care ̶ Enrollment complete: 164 patients at 15 U.S. centers ̶ Topline results expected 1H 2014 • Largest MS Society grant (total grants $8 million+) ̶ Phase II trial in women with relapsing - remitting MS 3 funded to completion • Rhonda Voskuhl , M.D., UCLA Department of Neurology ̶ Trimesta TM key opinion leader / lead principal investigator 9 Copaxone ® is a registered trademark of Teva Pharmaceutical Industries Ltd.

Emerging Worldwide Crisis 10 Multidrug - resistant infectious diseases “Infections were the second leading cause of death worldwide in 2002, killing nearly 15 million people – almost 1 in 3 deaths across the globe ” “Multidrug - resistant (MDR) microbes infect more than 2 million Americans each year, killing over 100,000 people ” Brad Spellberg, M.D., author of the book, Rising Plague 4
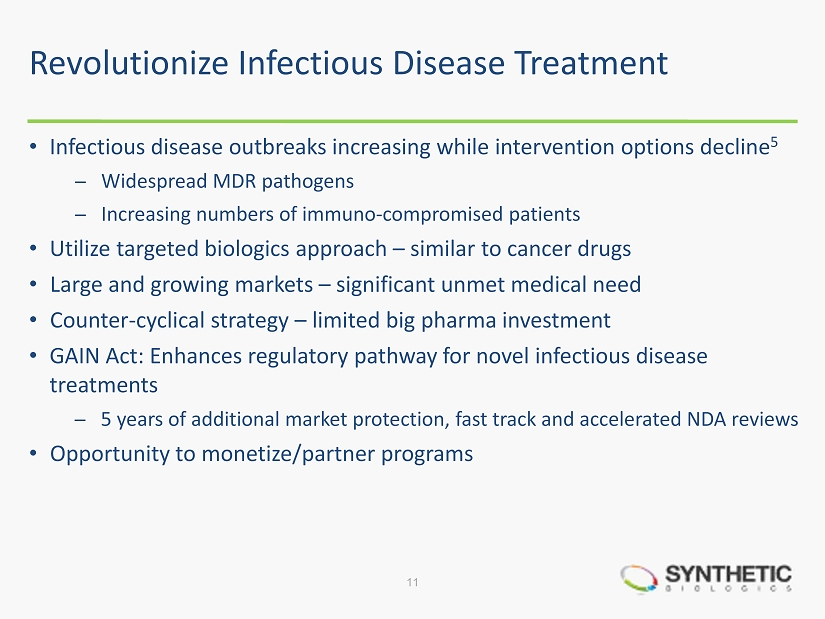
Revolutionize Infectious Disease Treatment • Infectious disease outbreaks increasing while intervention options decline 5 ̶ Widespread MDR pathogens ̶ Increasing numbers of immuno - compromised patients • Utilize targeted biologics approach – similar to cancer drugs • Large and growing markets – significant unmet medical need • Counter - cyclical strategy – limited big pharma investment • GAIN Act: Enhances regulatory pathway for novel infectious disease treatments ̶ 5 years of additional market protection, fast track and accelerated NDA reviews • Opportunity to monetize/partner programs 11

12 • Infections caused by C. diff in the U.S. (2009) resulted in: ̶ 337,000+ hospitalizations 6 ̶ Cost per patient $24,000 ̶ 30,000 deaths 6 ̶ $8.2 billion in costs associated with C. diff - related stays in the hospital 7 ̶ Increasing incidence and often multidrug - resistant • C . diff toxins cause serious diarrhea and in some cases death • Leading cause of hospital acquired infections (HAIs) C. difficile : Market Overview Impact on patients and growing economic burden There are currently no approved products for the prevention of C. diff infections
13 Infectious Disease: Clostridium difficile (C. diff) Acquired clinical - stage program November 2012 • Prophylactic treatment for prevention of C. diff infection • First generation candidate (formerly known as P1A), a proprietary oral enzyme co - administered with certain penicillins , eliminated the antibiotics from the GI tract and protected the native microflora ̶ Phase I and II studies demonstrated safety, tolerability and efficacy ▪ Maintained natural GI microflora balance ▪ Remained in the GI tract – did not cross over into blood stream • Develop SYN - 004, a next generation product designed to protect the GI tract microflora from penicillins and, in addition, most cephalosporins (the antibiotics most commonly associated with C. diff infection) ‒ ~ 8.7 million Americans administered IV β - lactam antibiotics in 2011 8
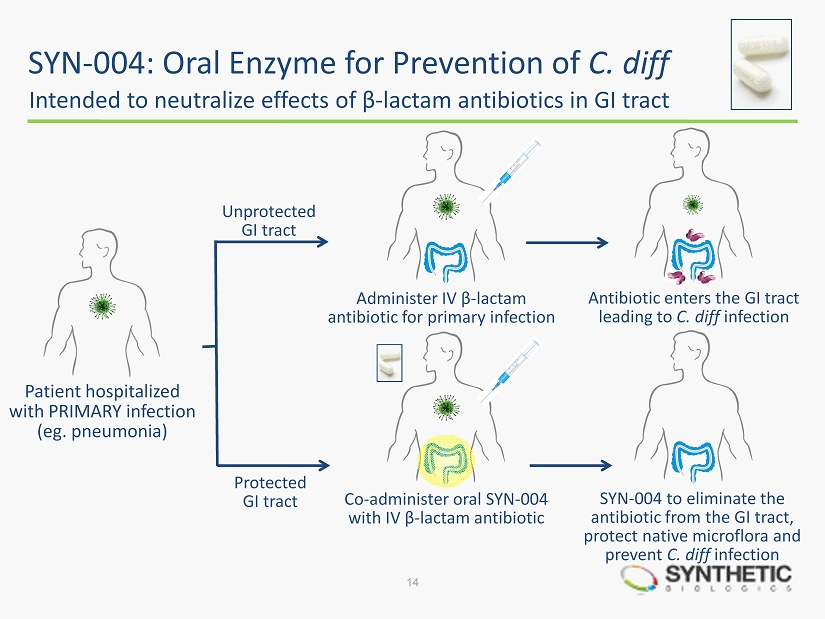
SYN - 004: Oral Enzyme for Prevention of C. diff Intended to neutralize effects of β - lactam antibiotics in GI tract 14 Patient hospitalized with PRIMARY infection ( eg . pneumonia) Administer IV β - lactam antibiotic for primary infection SYN - 004 to eliminate the antibiotic from the GI tract, protect native microflora and prevent C. diff infection Co - administer oral SYN - 004 with IV β - lactam antibiotic Antibiotic enters the GI tract leading to C. diff infection Unprotected GI tract Protected GI tract
Pertussis : Market Overview Serious threat from steady increase in whooping cough outbreaks • 294,000 deaths worldwide 9 ̶ Primarily young, unvaccinated children • Increased incidence associated with ̶ Infants not yet fully vaccinated ̶ Unvaccinated individuals ̶ Individuals with diminished immunity ̶ Individual carriers with bacteria in lungs ▪ May or may not have active disease • Whooping cough cases increasing to a 60 - year high in U.S. 10,11 15
Pertussis: Monoclonal Antibody Approach 16 mAb development • I nfectious disease collaboration with Intrexon • Licensed rights related to certain pertussis antibodies from The University of Texas at Austin ̶ Antibody research conducted by Jennifer A. Maynard, Ph.D. ̶ Pending patents • Develop SYN - 005 – mAb designed to neutralize pertussis toxin and reverse the course of disease ̶ Reduce the mortality rate in infants ̶ Shorten the chronic cough in adults • Antibiotics can only eliminate B. pertussis bacteria from respiratory tract – cannot neutralize pertussis toxin

Acinetobacter Infection : Market Overview Deadly gram - negative bacteria • M ortality rates as high as 43% reported 12 • Multi - billion dollar market opportunity 13 • Multidrug - resistant due to pathogen’s ability to rapidly mutate 14 • Survives on dry surfaces for up to 36 days ̶ Survives twice as long as non - biofilm - forming pathogens 15 • Acinetobacter disease profile ̶ 2.6% of HAIs 16 ̶ 1.3% of bloodstream infections 16 ̶ 7% of ICU respiratory tract infections in U.S. 16 ̶ Pneumonia , bacteremia, endocarditis, skin and soft tissue infections, urinary tract infections and meningitis • Increasing trauma center risk for wounded military personnel and natural disaster victims 17 17
Acinetobacter : Monoclonal Antibody Approach 18 Intrexon collaboration for mAb development Intrexon’s proprietary technologies/processes produce mAbs to detect and destroy viruses, bacteria and toxins • mAbLogix ™ – fully human antibody discovery platform • LEAP ™ – rapid identification and selection of targeted cells of interest • Protein engineering – improved or novel protein functions mAbLogix ™ and LEAP™ are registered trademarks of Intrexon Corporation.
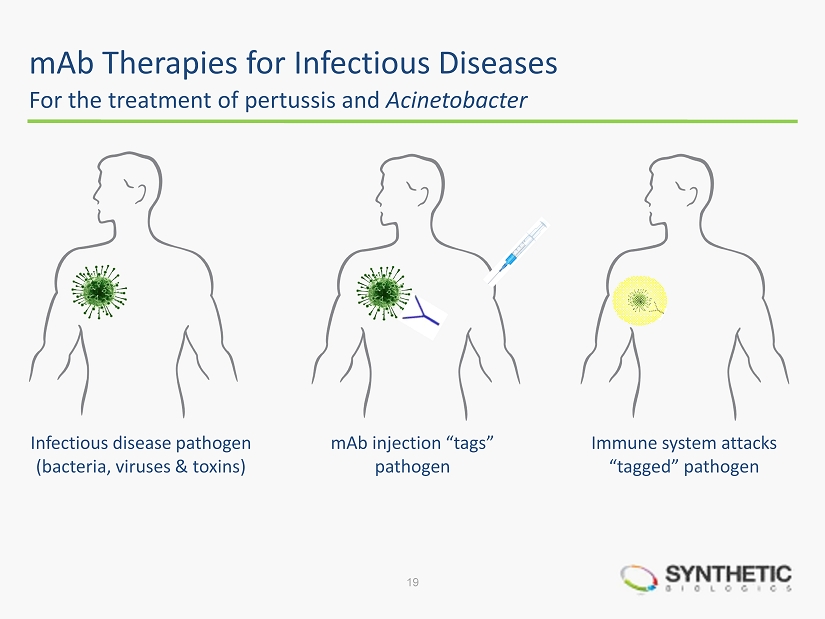
mAb Therapies for Infectious Diseases Infectious disease pathogen (bacteria, viruses & toxins) mAb injection “tags” pathogen Immune system attacks “tagged” pathogen For the treatment of pertussis and Acinetobacter 19

20 Anticipated Milestones • MS (oral estriol ) ̶ Continued patient enrollment in Phase II cognitive dysfunction in MS trial (ongoing) ̶ Topline results from Phase II relapsing - remitting MS trial (1H 2014) ̶ Results from cognitive dysfunction trial (2H 2014) • C. diff (oral enzyme) ‒ Generate master cell bank and initiate manufacturing process for C. diff program (2Q 2013) ‒ File IND to conduct clinical trial for prevention of C. diff (2014) • Pertussis ( mAb ) ‒ Verify efficacy of optimized pertussis mAb in animal models (1H 2013) • Acinetobacter ( mAb ) ̶ Generate panel of recombinant antibodies ( 1H 2013) ̶ Identify optimal candidate and confirm in vivo efficacy (2013) ̶ Initiate preclinical studies (2013) • PAH (synthetic DNA - based therapy) ‒ Transduction and vector optimization of therapy for PAH (underway)

NYSE MKT: SYN February 11, 2013 Feb 2013 – FINAL – 2.11.2013

References Slide 7 : 1 Current estimated sales of injectable disease - modifying MS therapies. Research and Markets: Multiple Sclerosis Therapeutics – Pi peline Assessment and Market Forecasts to 2019. Novartis 2011 Financial Report. Slide 8 : 2 Pr egnancy I n M ultiple S clerosis (PRIMS) Study; Confavreux , C., Hutchinson, M., Hours, M.M., Cortinvis - Tourniaire , P., Moreau, T., and the Pregnancy in MS Group (1998). Rate of Pregnancy - Related Relapse in Multiple Sclerosis. New England Journal of Medicine , 339, 285 - 291. Slide 9 : 3 www.clinicaltrials.gov/ct2/show/NCT00451204 Slide 10 : 4 Spellberg, B. Rising Plague: The Global Threat from Deadly Bacteria and Our Dwindling Arsenal to Fight Them. Copyright 2009. Slide 11 : 5 Saylor, C, Dadachova , E, Casadevall , A, Monoclonal antibody - based therapies for microbial diseases. Vaccine. 2009 December 30; 27 ( Suppl 6): G38 - G46. Slide 12 : 6 U.S. Department of Health & Human Services. Agency for Healthcare Research and Quality. January 25, 2012. Available at http://www.ahrq.gov/news/nn/nn012512.htm . Accessed November 5, 2012. 7 Agency for Healthcare Research and Quality. Healthcare and Cost Utilization Project. Statistical Brief #124. Clostridium difficile Infections (CDI) in Hospital Stays, 2009. January 2012. Available at http://www.hcup - us.ahrq.gov/reports/statbriefs/sb124.pdf . Slide 13 : 8 GlobalData . Beta - lactam Antibiotics Sales - United States of America, 2011. Prepared for Synthetic Biologics, Inc. November 2012. 22
