Exhibit 99.1

New York City Microbiome Clinical Program Seminar December 10, 2015

Introduction Jeff Riley, President & Chief Executive Officer

Forward - Looking Statements This presentation includes forward - looking statements on Synthetic Biologics’ current expectations and projections about future events . In some cases forward - looking statements can be identified by terminology such as "may," "should," "potential," "continue," "expects," "anticipates," "intends," "plans," "believes,“ "estimates,” “indicates,” and similar expressions . These statements are based upon current beliefs, expectations and assumptions and are subject to a number of risks and uncertainties, many of which are difficult to predict and include statements regarding our clinical trials, our establishment of collaborations and our execution of our growth strategy . The forward - looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those set forth or implied by any forward - looking statements . Important factors that could cause actual results to differ materially from those reflected in Synthetic Biologics’ forward - looking statements include, among others, a failure of our product candidates to be demonstrably safe and effec tive , or results that are consistent with prior results a failure to initiate clinical trials and if initiated, a failure to achieve the desired results, a failure to obtain regulatory approval for our product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to our ability to promote or commercialize our product candidates for the specific indications, a lack of acceptance of our product candidates in the marketplace, a failure of us to become or remain profitable, a failure to establish collaborations, our inability to obtain or maintain the capital or grants necessary to fund our research and development activities, a loss of any of our key scientists or management personnel, and other factors described in Synthetic Biologics’ annual report on Form 10 - K for the year ended December 31 , 2014 , subsequent quarterly reports on Form 10 - Qs and any other filings we make with the SEC . The information in this presentation is provided only as of the date presented, and Synthetic Biologics undertakes no obligation to update any forward - looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law .

Management Team • Jeffrey Riley, CEO Pfizer, Nichols Institute (Quest), SmithKline Beecham, QIC • Steven Shallcross , CFO Vanda Pharmaceuticals, Inc., Empire Petroleum Partners, LLC, Innocoll AG (formerly privately held Innocoll Holdings, Inc .) • John Monahan, PhD, EVP R&D Avigen, Somatix, Triton Biosciences, Hoffman - LaRoche • Joseph Sliman, MD, MPH, SVP Clinical/Regulatory Vanda Pharmaceuticals, Inc., MedImmune , Inc., DynPort Vaccine • Raymond Stapleton, PhD, SVP, Manufacturing Merck & Co., Inc. • Klaus Gottlieb, MD, FACG, VP Clinical/Regulatory Quintiles, U.S. Food & Drug Administration • Maureen Early, MBA, VP Commercial Rhone Poulenc Rorer/Aventis , Upside Endeavors

Investment Considerations NYSE MKT: SYN • Clinical - stage therapeutics to protect the gut microbiome while targeting pathogen - specific diseases • Addressing multi - billion dollar market opportunities addressing significant unmet medical needs • Multiple near - term and long - term clinical milestones • Experienced management team with extensive clinical and commercial track record

Microbiome Product Pipeline C - Cedars - Sinai Medical Center collaboration Completed Planned – 2015 Therapeutic Area Product Candidate Discovery Preclinical Phase 1 Phase 2 Phase 3 C. difficile infection/ Antibiotic - associated diarrhea (AAD) SYN - 004 Irritable bowel syndrome with constipation (IBS - C) SYN - 010 C

Milestones: Achieved & Upcoming Therapeutic Area/ Product Candidate Timeline C. difficile /AAD – SYN - 004: Phase 1a/1b 1Q 2015 – Positive topline Phase 1b results 1Q 2015 – Positive Phase 1a/1b PK data Phase 2a open - label (1 st ileostomy study; ceftriaxone) 1Q 2015 – Initiated Phase 2a 3Q 2015 – Supportive Phase 2a data from initial 4 of 12 expected participants 4Q 2015 – Report Phase 2a topline data Phase 2a open label (2 nd ileostomy study; ceftriaxone + PPI) 2Q 2015 – Initiated Phase 2a 1H 2016 – Report Phase 2a topline data Phase 2b proof - of - concept ( double - blind, placebo - controlled) 3Q 2015 – Initiated Phase 2b trial 1H 2016 – Interim analysis of blinded data by independent monitor committee Pivotal Phase 3 trial(s) 2016 – Initiate Phase 3 trial(s) IBS - C – SYN - 010: 1Q 2015 – SYN - 010 modified - release formulation of lovastatin Phase 2 (1 st study; acute, placebo - controlled) 2Q 2015 – Initiated Phase 2 4Q 2015 – Report Phase 2 topline data Phase 2 (2 nd study; extension, SYN - 010 only) 2H 2015 – Initiate Phase 2 1H 2016 – Report Phase 2 topline data Pivotal Phase 3 trial(s) 2016 – Initiate Phase 3 trial(s)
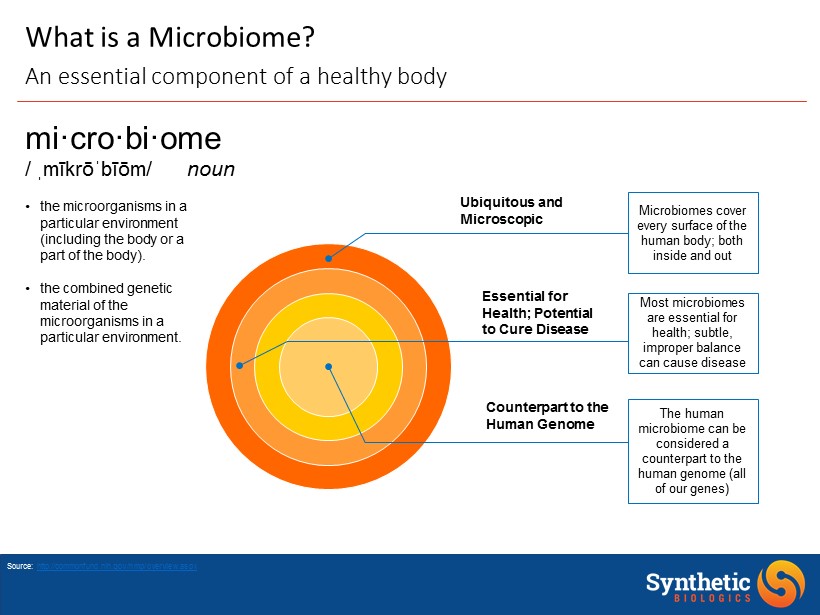
Most microbiomes are essential for health; subtle, improper balance can cause disease Ubiquitous and Microscopic Essential for Health; Potential to Cure Disease The human microbiome can be considered a counterpart to the human genome (all of our genes ) Microbiomes cover every surface of the human body; both inside and out Source: http://commonfund.nih.gov/hmp/overview.aspx mi·cro·bi·ome / ˌ mīkrōˈbīōm / noun • the microorganisms in a particular environment (including the body or a part of the body). • the combined genetic material of the microorganisms in a particular environment. Counterpart to the Human Genome What is a Microbiome? An essential component of a healthy body
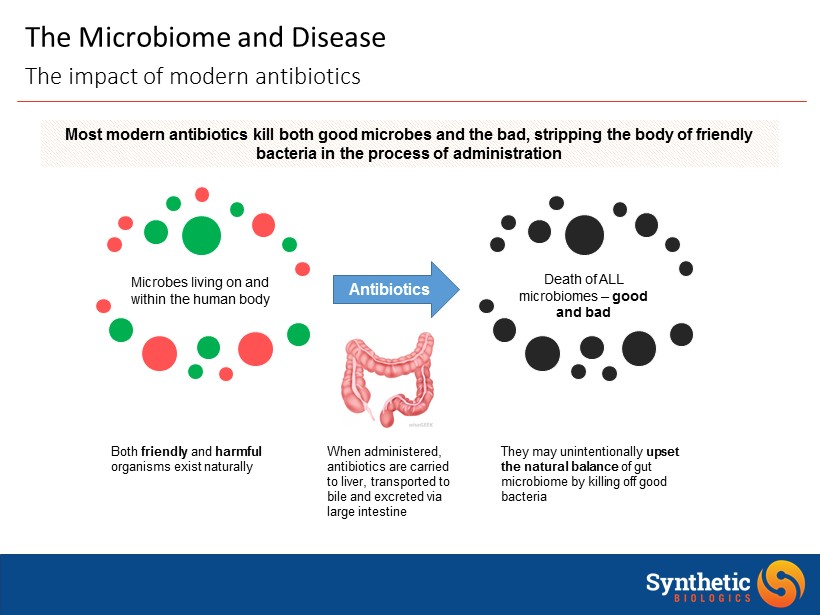
The Microbiome and Disease The impact of modern antibiotics Microbes living on and within the human body Most modern antibiotics kill both good microbes and the bad, stripping the body of friendly bacteria in the process of administration Death of ALL microbiomes – good and bad Antibiotics They may unintentionally upset the natural balance of gut microbiome by killing off good bacteria When administered, antibiotics are carried to liver, transported to bile and excreted via large intestine Both friendly and harmful organisms exist naturally

The Microbiome and Disease Disrupting the microbiome can have disastrous consequences http://learn.genetics.utah.edu/content/microbiome/disease/ A microbial imbalance in the gut microbiome provides an opportunity for overgrowth of harmful pathogenic organisms Interactions between microbes and the cells that line the intestine appear to be important in inflammatory bowel disease This overgrowth / imbalance may cause severe diarrhea , damage to the colon and in some cases, even death In autoimmune diseases , the immune system becomes confused and starts attacking the body's own tissues. Microbes in and around the large intestine are a big part of protecting us from this scenario S everal studies have shown that children with autism have different microbes living in their intestines than those without the disorder ALSO LINKED TO: Gut - brain hypothesis : depression; Asthma / atopy; Peripheral vascular disease; Obesity / metabolic syndrome; Altered xenobiotic / drug metabolism; Biliary disease; Colon cancer; Hypertension / ischemic heart disease

Source: US National Library of Medicine. Image source: Ottman N, et al. (2012) The function of our microbiota : who is out there and what do they do? Front. Cell. Inf. Microbio . 2:104. Human Microbiome Over Time Response to environmental conditions and life stages

Joe Sliman, MD, MPH Senior Vice President, Clinical & Regulatory Affairs • Senior Medical Director and Head of Patient Safety and Pharmacovigilance at Vanda Pharmaceuticals Inc . • Responsible for a New Drug Application for HETLIOZ ( tasimelteon ), which is indicated for the treatment of Non - 24 Hour Disorder in totally blind adults • Medical Director in Vaccines and Infectious Diseases at MedImmune , Inc . • Associate Medical Director at Dynport Vaccine Company • United States Navy • Led the U. S. Pacific Fleet disease surveillance programs, including influenza surveillance, preparedness, and prevention, as well as communicable disease and injury surveillance and prevention and health policy development

Klaus Gottlieb, MD, FACG Vice President, Clinical & Regulatory Affairs • Quintiles • Gastroenterology Therapeutic Area Lead • USFDA • Senior Clinical Reviewer, Division of Gastroenterology & Inborn Errors • Dr . Gottlieb’s responsibilities include: • Lead the Clinical Study teams through End - of - Phase 2 • Assist in development of strategy and execution of Phase 3

Prevention of C. difficile Infection and Antibiotic - Associated Diarrhea / SYN - 004 Joseph Sliman, M.D., MPH, SVP, Clinical & Regulatory Affairs

Professor Mark H. Wilcox, M.D., FRCPath C. difficile Keynote Speaker • Consultant / Head of Microbiology, Leeds Teaching Hospitals NHS Trust • Professor of Medical Microbiology, University of Leeds • Lead on C. difficile Infection, Public Health England • Chairman of Synthetic Biologics’ C. difficile Clinical Advisory Board

Professor Eric Sicard, MD C. difficile Keynote Speaker • Principal Investigator, Algorithme Pharma, Inc. • Family doctor and biologist with a strong interest for research and preventative medicine • Medical Director, Gamma Dynacare • Board Member, Tactio Medical Advisory Board • Principal Investigator for SYN - 004 Phase 2a clinical trials

SYN - 004 Clinical Development Mark Wilcox, MD. FRCPath , Leeds Teaching Hospitals NHS Trust

SYN - 004 • Completed Phase 1a (40 participants) and 1b (24 participants) trials • PK data supports SYN - 004 should have no effect on the antibiotic in the bloodstream • No clinically significant safety events were observed; well tolerated by participants • Completed first Phase 2a trial (10 evaluable participants) • Demonstrated the ability of SYN - 004 to degrade ceftriaxone in the chyme of healthy participants with functioning ileostomies without affecting ceftriaxone in the bloodstream (Dr. Eric Sicard) • Second Phase 2a trial ongoing • Characterize SYN - 004 activity on ceftriaxone in the small intestine in the presence of esomeprazole, an approved, OTC proton pump inhibitor Clinical trial development to date

• Initiated Phase 2b Proof - of - Concept trial in September 2015 • Evaluate the ability of SYN - 004 to prevent C. difficile infection (CDI) and antibiotic - associated diarrhea in patients admitted for LRTI receiving at least 5 days of IV ceftriaxone • Screening and enrollment in the U.S. and Canada has begun • Site initiation in Eastern Europe has begun • 6 countries with antibiotic treatment protocols similar to those used in U.S. and Canada • Interim Analysis scheduled for late 1Q 2016 • First 120 patients • Evaluate baseline rate of CDI in placebo group SYN - 004 Clinical trial development to date

20 ~75 Global Clinical Sites 370 patients SYN - 004 + Ceftriaxone Placebo + Ceftriaxone Primary Endpoint: • Prevention of CDI and C. difficile associated diarrhea (CDAD) Secondary Endpoints: • Prevention of AAD • Limiting disruption of gut microbiome diversity 1:1 SYN - 004 Clinical trial development to date

SYN - 004 First Phase 2a Clinical Trial Results Eric Sicard, MD, Algorithme Pharma, Principal Investigator

SYN - 004 Study SB - 004 - 01 - 003 • Study SB - 004 - 01 - 003 study was conducted in healthy volunteers with functioning ileostomy • Provided in vivo data that complements in vitro and nonclinical data to support the mechanism of action (MOA) of SYN - 004 • Following oral administration, SYN - 004 acts locally in the intestinal tract to inactivate residual ceftriaxone thus, potentially, reducing or preventing antibiotic induced changes to the gut microbiome • SYN - 004, formulated as a delayed release capsule, does not impact the systemic PK profile of ceftriaxone Unique approach to support MOA

SYN - 004 Study SB - 004 - 01 - 003 • Primary : Evaluate the effect of two strengths of oral SYN - 004 (75 mg or 150 mg) administered twice in six hours on the chyme concentrations of ceftriaxone administered as an intravenous (IV) infusion • Secondary : • Compare ceftriaxone PK parameters in plasma following administration with SYN - 004 vs. administration of ceftriaxone alone • Determine the concentration of SYN - 004 in chyme . • Determine the systemic absorption, if any, of SYN - 004 in plasma of ileostomy subjects • Determine the safety and tolerability of SYN - 004 in healthy adult subjects with a functioning ileostomy co - treated with ceftriaxone Objectives

SYN - 004 Study SB - 004 - 01 - 003 • Randomized, multi - center, open - label study • Healthy subjects with functioning ileostomies between the ages of 18 and 80 years • Treatment Period 1: all subjects received an IV infusion of 1 gram (g) ceftriaxone. • Treatment Period 2: all subjects received an IV infusion of 1 g ceftriaxone and 2 doses of SYN - 004 (either 75 mg or 150 mg, as randomized), 30 minutes before and 5.5 hours after the ceftriaxone infusion Study design

SYN - 004 Study SB - 004 - 01 - 003 • Subjects enrolled: 11* • 8 males/Ages 23 - 74 • 3 females/Ages 52 - 62 • Subjects dosed with SYN - 004: 10 • Subjects completing study: 10 Enrollment * One subject withdrew from the study during period 1 ceftriaxone infusion due to an adverse reaction to the infusion. Subject did not receive SYN - 004

Ceftriaxone Chyme Concentrations - Period 1 and Period 2 Without 2 outlier subjects (n=8) IV Ceftriaxone Infusion Oral SYN - 004 Dosage

Chyme Ceftriaxone Concentrations for Two Outlier Subjects

Chyme SYN - 004 Concentrations A ll subjects
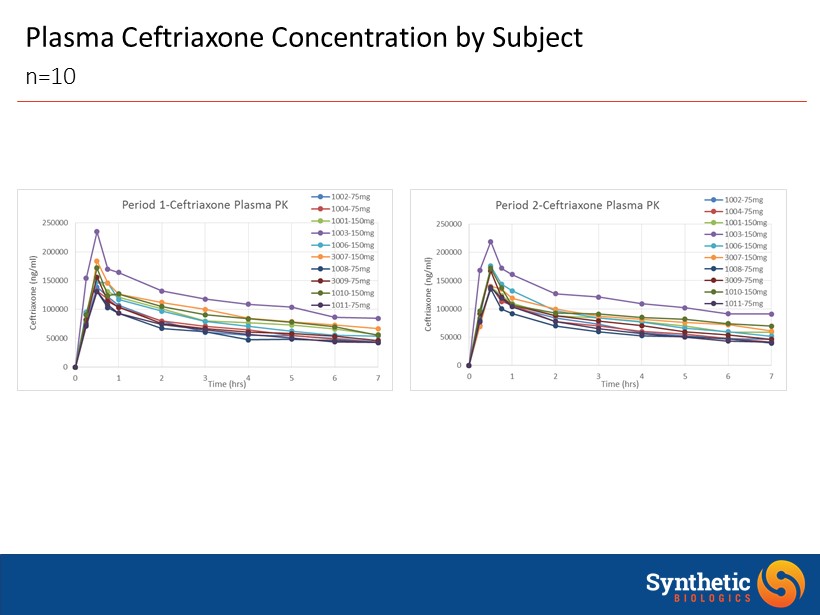
Plasma Ceftriaxone Concentration by Subject n=10

SYN - 004 Plasma Concentration • No SYN - 004 was detected in any plasma sample • Max dose 300 mg given as 150 mg twice 6 hours apart • LLOQ of the Assay is 0.8 ng/mL • Consistent with results from SAD and MAD studies n=10

Incidence of Treatment - Emergent Adverse Events (TEAEs ) Considered Related To Study Drug by MedDRA Coded Terms In Period 2 -- Safety Population Treatment AB: Ceftriaxone + 75 mg SYN - 004 (N=6) Treatment AC: Ceftriaxone + 150 mg SYN - 004 (N=5) Total (N=11) Total # of Related TEAEs 2 1 3 Subjects With at Least One Related TEAE 2 1 3 GASTROINTESTINAL DISORDERS 1 1 Abdominal pain 1 1 NERVOUS SYSTEM DISORDERS 1 1 Somnolence 1 1 RENAL AND URINARY DISORDERS 1 1 Pollakiuria 1 1 • All AEs were grade 1 • No SAEs were reported during the study

SYN - 004 Study SB - 004 - 01 - 003 • SYN - 004 reduced the ceftriaxone concentration in the intestinal chyme of subjects dosed with IV ceftriaxone and oral SYN - 004 • Ceftriaxone concentrations observed in Period 2 were reduced to near below or below detection limits in the chyme when both substances were present • Ceftriaxone plasma concentrations in each subject after oral administration of SYN - 004 was similar to that observed without SYN - 004 • SYN - 004 was not detectable in the plasma of the ileostomy subjects • SYN - 004 was safe and well tolerated when co - administered with IV ceftriaxone in subjects with functioning ileostomies • No discernible differences were noted between 75 mg and 150 mg doses of SYN - 004 Outcomes

Prevention of CDI and AAD / SYN - 004 Q&A Session

Irritable Bowel Syndrome with Constipation (IBS - C) Joseph Sliman, M.D., MPH, SVP, Clinical & Regulatory Affairs

Mark Pimentel, MD, FRCP(C) IBS - C Keynote Speaker • Cedars - Sinai Medical Center • Director, GI Motility Program • Professor of Medicine • Serves as a principal investigator or co - investigator for numerous basic science, translational and clinical studies in IBS, and the relationship between gut flora composition and human health • Chairman of Synthetic Biologics’ IBS - C Clinical Advisory Board

SYN - 010 First Phase 2 Results Mark Pimentel, MD, FRCP(C), Cedars - Sinai Medical Center

SYN - 010 Safety and Efficacy in IBS - C Patients • Randomized, double - blind, parallel - group, placebo - controlled study in breath methane positive IBS - C patients • Patients dosed at 13 centers in the United States • Compared SYN - 010 21 mg and 42 mg with placebo • 28 days of single, daily dosing • Primary objective to evaluate changes from baseline in breath methane after 7 and 28 days of SYN - 010 treatment • Secondary objective to evaluate changes from baseline in the number and consistency of bowel movements, and in abdominal pain and bloating SB - 2 - 010 - 001 Phase 2 clinical trial
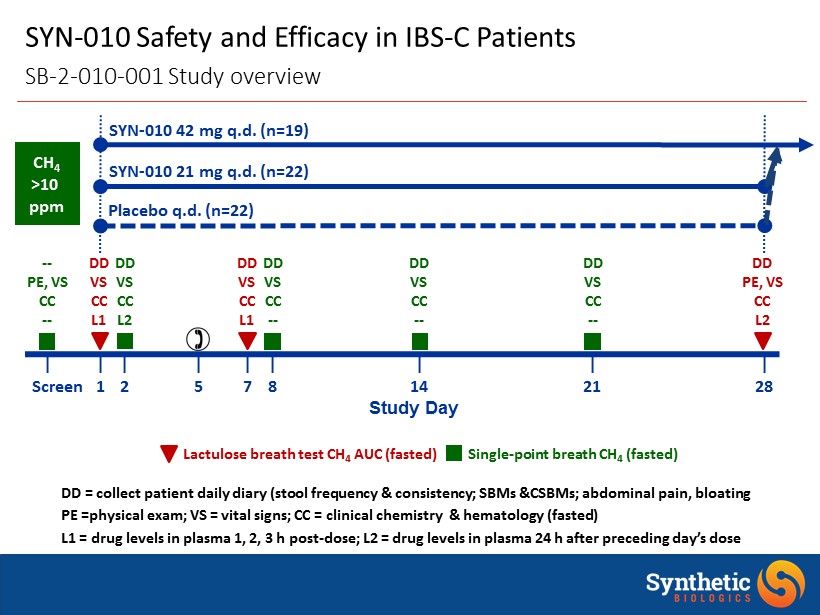
SYN - 010 Safety and Efficacy in IBS - C Patients SB - 2 - 010 - 001 Study overview Lactulose breath test CH 4 AUC (fasted) Single - point breath CH 4 (fasted) SYN - 010 21 mg q.d . (n=22) Placebo q.d . (n=22) SYN - 010 42 mg q.d . (n=19) | | | | | | | | | Screen 1 2 5 7 8 14 21 28 Study Day DD VS CC L2 DD VS CC L1 -- PE, VS CC -- DD VS CC L1 DD PE, VS CC L2 DD VS CC -- DD VS CC -- DD VS CC -- CH 4 >10 ppm DD = collect patient daily diary ( stool frequency & consistency; SBMs &CSBMs; abdominal pain, bloating PE =physical exam; VS = vital signs; CC = clinical chemistry & hematology (fasted) L1 = drug levels in plasma 1, 2, 3 h post - dose; L2 = drug levels in plasma 24 h after preceding day’s dose
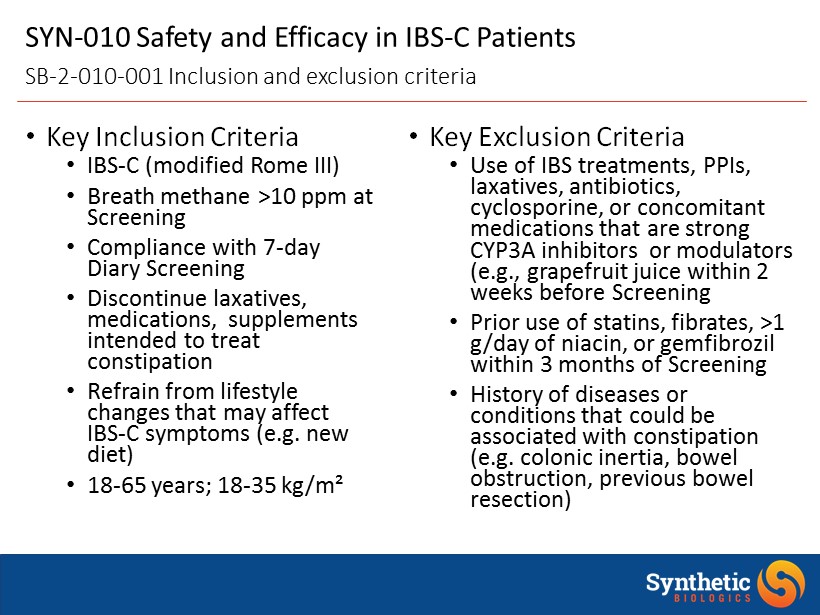
• Key Inclusion Criteria • IBS - C (modified Rome III) • Breath methane >10 ppm at Screening • Compliance with 7 - day Diary Screening • Discontinue laxatives, medications, supplements intended to treat constipation • Refrain from lifestyle changes that may affect IBS - C symptoms (e.g. new diet) • 18 - 65 years; 18 - 35 kg/m² • Key Exclusion Criteria • Use of IBS treatments, PPIs, laxatives, antibiotics, cyclosporine, or concomitant medications that are strong CYP3A inhibitors or modulators (e.g., grapefruit juice within 2 weeks before Screening • Prior use of statins, fibrates , >1 g/day of niacin, or gemfibrozil within 3 months of Screening • History of diseases or conditions that could be associated with constipation (e.g. colonic inertia, bowel obstruction, previous bowel resection) SYN - 010 Safety and Efficacy in IBS - C Patients SB - 2 - 010 - 001 Inclusion and exclusion criteria

SYN - 010 in IBS - C Patients SB - 2 - 010 - 001 Subject demographics Placebo SYN - 010 21 mg SYN - 010 42 mg Female, n/total (%) 17/22 (77.3%) 19/22 (86.4%) 14/19 (73.7%) Age , years* 46.4 ± 10.3 42.6 ± 6.0 44.7 ± 9.5 Breath CH 4 , ppm*† 38 ± 20 39 ± 30 42 ± 28 Race, n (%) White 16 (72.7%) 21 (95.5%) 15 (78.9%) Black/African American 4 (18.2%) 1 (4.5)% 4 (21.1%) Other 2 ( 9.1%) -- -- Ethnicity, n (%) Hispanic/Latino 21 (95.5%) 20 (90.9%) 17 (89.5%) Weight , kg* 79.5 ± 12.6 69.3 ± 12.0 71.7 ± 10.7 BMI, mg/kg²* 29.4 ± 3.3 26.2 ± 3.1 26.4 ± 3.2 * Mean ± SD . †Value at Screening

0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 45.0 0 20 40 60 80 100 120 140 160 180 200 Breath Methane (ppm) Time (min) Subject A Subject B • Standardized measurement of methane production • Day 1, Day 7 and Day 28 prior to SYN - 010 dosing • Patient fasts overnight then provides a breath sample (Time 0) • Patient then consumes a solution of lactulose • Patient provides breath samples every 20 min after lactulose consumption up to 180 min • Methane area under the concentration vs. time curve (AUC; ppm*h) estimates overall methane production SYN - 010 Safety and Efficacy in IBS - C Patients Measuring methane using the lactulose b reath test (LBT)

- 10.6 - 31.0 11.1 - 22.6 - 34.6 - 34.3 - 120 - 100 - 80 - 60 - 40 - 20 0 20 40 Change in Breath Methane AUC (ppm*h) Placebo SYN - 010 21 mg SYN - 010 42 mg SYN - 010 Reduced Breath Methane in IBS - C Patients Decrease from baseline in methane AUC ( mITT Population) p=0.64 p=0.88 p=0.02* p=0.15 p=0.03* p=0.01* *Statistically different from baseline AUC by paired t - test; data normalized (square root) prior to analysis Day 7 Day 28

16% 41% 22% 0% 10% 20% 30% 40% 50% 60% 70% 80% % Weeks Patients Had a Stool Response Placebo SYN - 010 21 mg SYN - 010 42 mg SYN - 010 Improved Weekly Stool Frequency Response Percentage of weeks subjects had a response ( mITT Population) *Statistically different from Placebo based an a Wilcoxon Mann - Whitney test P=0.02* P=0.54 A Weekly Stool Frequency Responder is a subject who experiences a stool frequency increase of 1 or more complete spontaneous bowel movements per week compared with baseline.

11% 15% 29% 0% 10% 20% 30% 40% 50% 60% 70% 80% % Weeks Patients Had a Pain Response Placebo SYN - 010 21 mg SYN - 010 42 mg SYN - 010 Improved Weekly Abdominal Pain Response Percentage of weeks subjects has a response ( mITT Population) P=0.08† P=0.26† †P - value based an a Wilcoxon Mann - Whitney test for the comparison of the SYN - 010 treatment to Placebo A Weekly Pain Responder is a subject who experiences a decrease in weekly average score for worst abdominal pain in the past 24 hours of at least 30% compared with baseline

1% 7% 9% 0% 5% 10% 15% 20% 25% 30% % Weeks Patients Had a Weekly Response Placebo SYN - 010 21 mg SYN - 010 42 mg SYN - 010 Effects on Combined Weekly Response Trend to dose - dependent improvement ( mITT Population) †P - value based an a Wilcoxon Mann - Whitney test for the comparison of the SYN - 010 treatment to Placebo P=0.13† P=0.10† A Weekly Responder is a subject who experiences a decrease in weekly average score for worst abdominal pain in the past 24 hours of at least 30% compared with baseline AND a stool frequency increase of 1 or more complete spontaneous bowel movements per week compared with baseline.

82% 14% 59% 41% 68% 32% 27% 73% 58% 42% 47% 53% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Abdominal Pain No Response (0% Weeks) Abdominal Pain Any Response (25 - 100% Weeks) Stool Frequency No Response (0% Weeks) Stool Frequency Any Response (25 - 100% Weeks) % of Patients with Response Placebo SYN - 010 21 mg SYN - 010 42 mg SYN - 010 Dose - Response in IBS - C Patients Total no. responses increased at higher dose ( mITT Population) Increased Response Reduced Non - Response
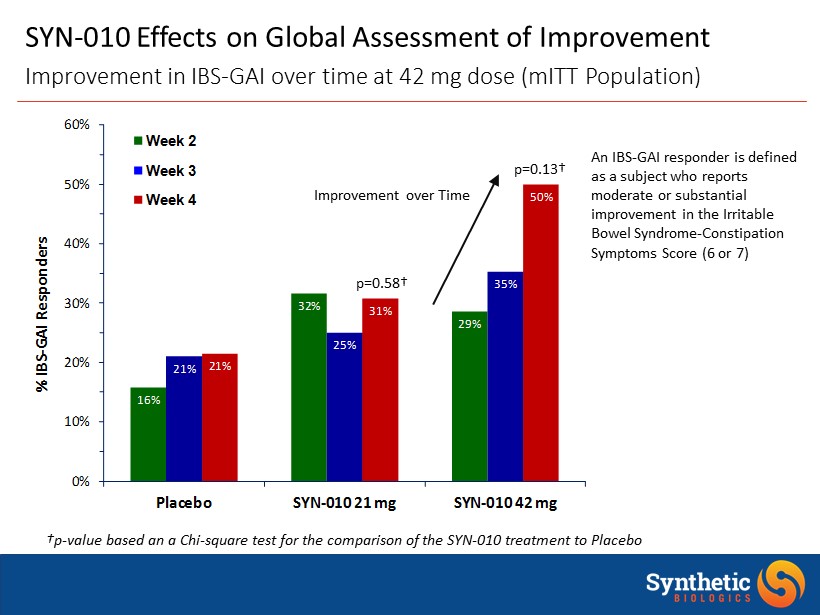
16% 32% 29% 21% 25% 35% 21% 31% 50% 0% 10% 20% 30% 40% 50% 60% Placebo SYN - 010 21 mg SYN - 010 42 mg % IBS - GAI Responders Week 2 Week 3 Week 4 SYN - 010 Effects on Global Assessment of Improvement Improvement in IBS - GAI over time at 42 mg dose ( mITT Population) †p - value based an a Chi - square test for the comparison of the SYN - 010 treatment to Placebo An IBS - GAI responder is defined as a subject who reports moderate or substantial improvement in the Irritable Bowel Syndrome - Constipation Symptoms Score (6 or 7) p=0.13† p=0.58† Improvement over Time
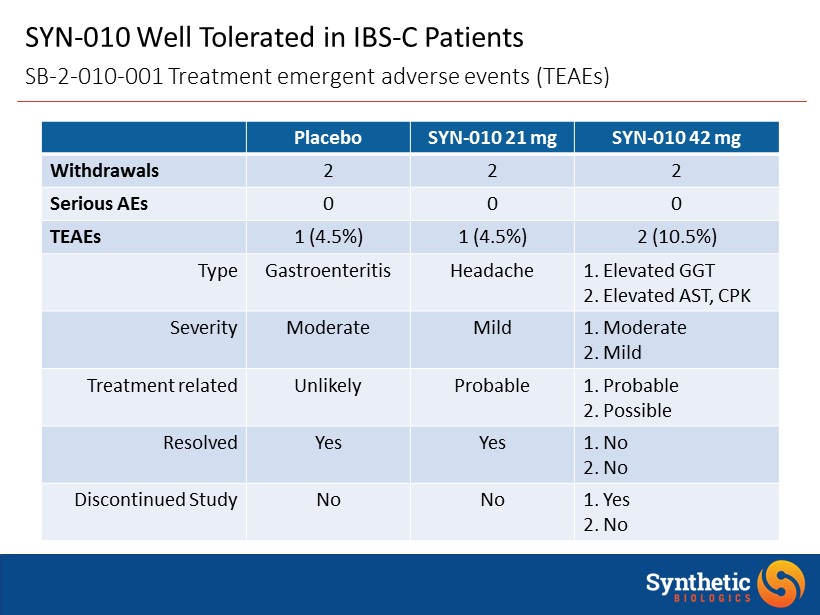
SYN - 010 Well Tolerated in IBS - C Patients SB - 2 - 010 - 001 Treatment emergent adverse events (TEAEs) Placebo SYN - 010 21 mg SYN - 010 42 mg Withdrawals 2 2 2 Serious AEs 0 0 0 TEAEs 1 (4.5%) 1 (4.5%) 2 (10.5%) Type Gastroenteritis Headache 1. Elevated GGT 2. Elevated AST, CPK Severity Moderate Mild 1. Moderate 2. Mild Treatment related Unlikely Probable 1. Probable 2. Possible Resolved Yes Yes 1. No 2. No Discontinued Study No No 1. Yes 2. No

SYN - 010 Summary of Clinical Outcomes to Date • SYN - 010 was well tolerated in breath methane positive IBS - C patients treated with single, daily, oral doses of 21 mg or 42 mg for 28 days • Only 3 TEAEs (7.3%) observed in SYN - 010 treated patients • SYN - 010 reduced breath methane in breath methane positive IBS - C patients • Dose - response apparent despite high variability • SYN - 010 provided an improvement in IBS - C symptoms • Weekly stool frequency response; weekly pain response SB - 2 - 010 - 001 Top - line data

SYN - 010 Development in IBS - C • SB - 2 - 010 - 001 complete data set to be reviewed • Data release Q1 2016 • 8 week extension study nearing completion • 54 patients from SB - 2 - 010 - 001 continued into an open - label 8 week extension study administering single, daily, doses of SYN - 010 42 mg. • SB - 2 - 010 - 002 designed to evaluate the sustainability of methane reduction and trends in IBS - C symptoms • SYN - 010 clinical pharmacokinetics to be evaluated • Advise dose(s) for advanced clinical testing Pending data and on - going studies

IBS - C / SYN - 010 Q&A Session

Closing Jeff Riley, President & Chief Executive Officer

New York City Microbiome Clinical Program Seminar December 10, 2015 SYN Microbiome Clin Prog Seminar (SYN Slides) - FINAL