United States Securities and Exchange Commission
Washington, D.C. 20549
FORM 10‑K
[X] | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| FOR THE FISCAL YEAR ENDED DECEMBER 31, 2015 | |
| OR | |
[ ] | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
FOR THE TRANSITION PERIOD FROM TO
COMMISSION FILE NUMBER 1‑11846

AptarGroup, Inc.
475 WEST TERRA COTTA AVENUE, SUITE E, CRYSTAL LAKE, ILLINOIS 60014
815‑477‑0424
Securities Registered Pursuant to Section 12(b) of the Act:
Title of each class | | Name of each exchange on which registered |
Common Stock $.01 par value | | New York Stock Exchange |
Securities Registered Pursuant to Section 12 (g) of the Act:
NONE
Indicate by check mark if the registrant is a well‑known seasoned issuer, as defined in Rule 405 of the Securities Act.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15 (d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S‑T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S‑K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10‑K or any amendment to this Form 10‑K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non‑accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b‑2 of the Exchange Act.
Large accelerated filer ☒ | Accelerated filer ☐ | Non‑accelerated filer ☐ | Smaller reporting company ☐ |
| | (Do not check if a smaller reporting company) | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b‑2 of the Act).
The aggregate market value of the common stock held by non‑affiliates as of June 30, 2015 was $3,908,285,956.
The number of shares outstanding of common stock, as of February 23, 2016, was 62,842,639 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive Proxy Statement to be delivered to stockholders in connection with the Annual Meeting of Stockholders to be held May 4, 2016 are incorporated by reference into Part III of this report.
AptarGroup, Inc.
FORM 10‑K
For the Year Ended December 31, 2015
INDEX
PART I
ITEM 1. BUSINESS
BUSINESS OF APTARGROUP
We are a leading global provider of a broad range of innovative packaging, dispensing and sealing solutions, primarily for the beauty, personal care, home care, prescription drug, consumer health care, injectables, food and beverage markets. Our creative packaging solutions enhance the convenience, safety and security of consumers around the globe and allow our customers to differentiate their products in the market.
Our business was started in the late 1940’s, manufacturing and selling aerosol valves in the United States, and has grown primarily through the acquisition of relatively small companies and internal expansion. We were incorporated in Delaware in 1992. In this report, we may refer to AptarGroup, Inc. and its subsidiaries as “AptarGroup”, “Aptar” or the “Company”.
We have manufacturing facilities located throughout the world including North America, Europe, Asia and South America. We have approximately 5,000 customers with no single customer or group of affiliated customers accounting for greater than 8% of our 2015 net sales.
Sales of our dispensing systems have traditionally grown at a faster rate than the overall packaging industry as consumers’ preference for convenience has increased and product differentiation through packaging design has become more important to our customers. Also, consumer product marketers have converted many of their packages from non-dispensing formats to those with dispensing systems that offer the benefit of enhanced shelf appeal, convenience, cleanliness or accuracy of dosage. We expect these trends to continue.
While we offer a wide variety of dispensing and sealing solutions, our primary products are dispensing pumps, closures, aerosol valves and elastomeric primary packaging components.
Dispensing pumps are finger‑actuated dispensing systems that dispense a spray or lotion from non‑pressurized containers. The style of pump used depends largely on the nature of the product being dispensed, from small, fine mist pumps used with perfume and pharmaceutical products to lotion pumps for more viscous formulas.
Closures are primarily dispensing closures but to a lesser degree can include non‑dispensing closures. Dispensing closures are plastic caps which allow a product to be dispensed without removing the cap.
Aerosol valves dispense product from pressurized containers. The majority of the aerosol valves that we sell are continuous spray valves, with the balance being metered dose inhaler valves.
We also manufacture and sell elastomeric primary packaging components. These components are used in the injectables market. Products include stoppers for infusion, antibiotic, lyophilization and diagnostic vials. Our elastomeric components also include pre‑filled syringe components, such as plungers, needle shields, tip caps and cartridges, as well as dropper bulbs and syringe plungers.
On January 25, 2016, the Company signed an agreement to acquire Megaplast GmbH, Megaplast France S.a.r.l. and Mega Pumps L.P. (“Mega Airless”), a leading provider of innovative all-plastic airless dispensing systems for the beauty, personal care and pharmaceutical markets. The transaction is subject to customary regulatory approvals and has not closed as of the date of this report but is expected to close prior to the end of the first quarter of 2016.
AVAILABLE INFORMATION
Our periodic and current reports, and any amendments to those reports, are available, free of charge, through a link on the Investors page of our website (www.aptar.com), as soon as reasonably practicable after the material is electronically filed with, or furnished to, the Securities and Exchange Commission (“SEC”). These filing are also available to the public over the Internet at the SEC’s website (http://www.sec.gov). You may also read and copy any document we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. Please call the SEC at 1-800-SEC-0330 for further information on the reference room.
Also posted on our website are the charters for our Audit, Compensation, Governance and Executive Committees, our Governance Principles, our Code of Business Conduct & Ethics, our Director Independence Standards and our Conflict Minerals Statement. Within the time period required by the SEC and the New York Stock Exchange (“NYSE”), we will post on our website any amendment or waiver to the Code of Business Conduct & Ethics applicable to any executive officer or director. The information provided on our website is not part of this report and is therefore not incorporated herein by reference.
OUR STRATEGY
We seek to enhance our position as a leading global provider of innovative packaging dispensing and sealing solutions and deliver increased value to our customers and shareholders through strategic focus and execution in the following areas:
| (i) | | Market Focused: We are committed to develop market focused packaging solutions and convert less convenient or non-dispensing applications to convenient dispensing systems or replacing current dispensing applications with more value-added dispensing products. We see opportunities to convert to more convenient and value-added solutions across all of our segments and markets. |
| (ii) | | Diversification: We aim to continue to expand, through internal growth or through acquisition, our geographic footprint and portfolio of solutions in order to serve global consumer and pharmaceutical companies across a number of end uses. Our ability to diversify our business globally and across a number of end uses is a key strategic priority which improves our competitive position and reduces an over reliance on any one particular region or market. |
| (iii) | | Niche Provider: Our customers look to us to develop products that provide them market differentiation related to convenience of use, product security and regulatory compliance. We invest in consumer research, product design and manufacturing technologies to be a leader in packaging delivery solutions. |
| (iv) | | Innovation: We use our market research and insight into consumer behaviors to develop and license innovative new products. Additionally, we actively adapt and transfer innovative product technologies and concepts across business segments and geographic regions. |
| (v) | | Operationally Efficient: We compete with global and local companies. We are focused on executing programs to improve our operational efficiency, including actions around local sourcing, production efficiency and process redesign. We understand the importance of continuous improvement to protect our ability to compete in the market. |
| (vi) | | Talented Workforce: Execution of our strategy requires a talented and highly motivated team. We have a focused talent acquisition and development strategy to ensure our team has the right skills to execute our strategy. |
Facilitating the execution of our strategy are our core values, which dictate how we interact internally and with our customers, suppliers and all stakeholders.
DESCRIPTION OF APTARGROUP’S REPORTING SEGMENTS
INFORMATION ABOUT SEGMENTS
AptarGroup’s organizational structure consists of three market‑focused business segments which are Beauty + Home, Pharma and Food + Beverage. This is a strategic structure which allows us to be more closely aligned with our customers and the markets in which they operate. We primarily sell our products through our own sales force to the largest beauty, personal care, pharmaceutical, home care, food and beverage marketers in the world. To a limited extent, we use independent representatives and distributors to reach smaller customers and export markets.
Operations that sell dispensing systems primarily to the beauty, personal care and home care markets form the Beauty + Home segment. Operations that sell dispensing systems and sealing solutions to the prescription drug, consumer health care and injectables markets form the Pharma segment. Operations that sell dispensing systems and sealing solutions to the food and beverage markets form the Food + Beverage segment. Each of these three business segments is described more fully below. A summary of sales, segment income and total assets based upon this reporting structure for each of the last three years is shown in Note 17 to the Consolidated Financial Statements in Item 8 (which is incorporated by reference herein).
BEAUTY + HOME
The Beauty + Home segment is our largest segment in terms of net sales and total assets representing 55% and 45% of AptarGroup’s Net Sales and Total Assets, respectively. The Beauty + Home segment primarily sells pumps, closures, aerosol valves and accessories to the personal care and home care markets and pumps and decorative components to the beauty market. We believe we are a leading supplier for the majority of the products we sell primarily to the beauty, personal care and home care markets.
Beauty. Sales to the beauty market accounted for approximately 46% of the segment’s total net sales in 2015. The beauty market requires a broad range of spray and lotion pumps and sampling dispensing systems to meet functional as well as aesthetic requirements. A considerable amount of research, time and coordination with our customers is required to qualify a pump for use with their products. Within the market, we expect the use of pumps to continue to increase, particularly in the cosmetics and sampling sectors. In the cosmetic sector, packaging for certain products such as natural and organic cosmetics and anti‑aging lotions continue to provide us with growth opportunities. We are a leading provider of packaging solutions for prestige and mass market fragrance products. Our cosmetic lotion pumps, airless dispensing systems, lotion sampling devices and decorative capabilities will also provide growth opportunities. We see continued growth opportunities in Latin America and significant opportunities for growth in the sale of our products for cosmetic applications in Asia.
Personal Care. Sales to the personal care market accounted for approximately 45% of the segment’s total net sales in 2015 and primarily included sales of fine mist spray pumps, lotion pumps, closures and continuous spray aerosol valves. Personal care spray pump applications include hair care, body care and sun care products. Typical lotion pump applications include skin moisturizers, hand sanitizers and soap. Personal care closures applications include shampoos and conditioners. Personal care continuous spray aerosol valve applications include hair care products, deodorants, shaving creams and sun care products. Our research and development teams continue to design unique accessories that increase the value of our continuous spray aerosol valve offerings.
Home Care. Sales to the home care market accounted for approximately 8% of the segment’s total net sales in 2015 and primarily included sales of continuous or metered dose spray aerosol valves, closures and to a lesser degree spray and lotion pumps. Applications for continuous spray valves include disinfectants, spray paints, insecticides and automotive products. Metered dose valves are used for air fresheners. Closure applications include liquid detergents and household cleansers. Spray and lotion pump applications primarily include household and industrial cleaners. There is a trend towards concentrated cleaners and laundry care products that could provide opportunities for incorporating our dosing dispensing technologies.
PHARMA
The Pharma segment is our second largest segment in terms of net sales and total assets, accounting for 31% and 24% of AptarGroup’s Net Sales and Total Assets, respectively, and is our most profitable segment. We believe we are a leading supplier of pumps and metered dose inhaler valves (“MDI’s”) to the pharmaceutical market worldwide and we are a supplier of elastomer for injectables primary packaging components worldwide. Characteristics of this market include (i) governmental regulation of our pharmaceutical customers, (ii) contaminant‑controlled manufacturing environments and (iii) a significant amount of time and research from initially working with pharmaceutical companies at the molecular development stage of a medication through the eventual distribution to the market. We have clean‑room manufacturing facilities in Argentina, China, France, Germany, India, Switzerland and the United States. We believe that providing an alternative to traditional medication forms such as pills with value‑added, convenient dispensing systems will continue to offer opportunities for our business. In addition, with the trend towards health care reform we believe there are opportunities for growth in the over the counter and generic pharmaceutical categories.
Prescription Drug. Sales to the prescription drug market accounted for approximately 56% of the segment’s total net sales in 2015. Pumps sold to the prescription drug market deliver medications nasally, orally or topically. Currently the majority of our pumps sold are for nasal allergy treatments. Recently, there is a trend of nasal allergy products moving from prescription‑only to being sold over the counter without a prescription. This trend could provide us with growth opportunities as this movement could allow consumers easier access to these types of treatments. Our nasal pumps and unit dose devices are also used to deliver pain management products. Potential opportunities for providing alternatives to traditional pill and injectable dosage forms of medication include pump dispensing systems for vaccines, cold and flu treatments, central nervous systems applications and hormone replacement therapies.
MDI’s are used for dispensing precise amounts of aerosolized medication. This technology allows medication to be broken up into very fine particles, which enables the drug to be delivered typically via the pulmonary system. Currently the majority of our MDI’s sold are used for respiratory ailments such as asthma and COPD (chronic obstructive pulmonary disease).
We continue to develop new dispensing systems and accessories in this segment. For example, we provide single dose delivery devices suitable for central nervous system applications. While we expect that these types of new products will come to market in the future, it is difficult to estimate when, as the rigors of pharmaceutical regulations affect the timing of product introductions by our pharmaceutical customers which use our dispensing systems.
Consumer Health Care. Sales to the consumer health care market accounted for approximately 25% of the segment’s total net sales in 2015. Applications for this market are similar to the prescription market; however, these applications are sold over the counter without a prescription. Typical consumer health care spray pump applications include nasal decongestants, nasal salines and cough and cold applications. Typical consumer health care valve applications include nasal saline using our bag‑on valve technology. We have developed a multi dose ophthalmic dispensing device suitable for unpreserved medicinal formulations. This technology is successfully marketed in Europe and is under development for other markets. Other products sold to this market include airless pump systems for dermal applications. We have recently seen a trend to more child resistant and senior‑friendly packaging solutions and have developed products to meet these market needs.
Injectables. Sales to the injectables market accounted for approximately 19% of the segment’s total net sales in 2015. Injectables are elastomeric primary packaging components for injectable drug delivery. Injectable products offered include stoppers for vials, pre‑filled syringe components, such as plungers, needle shields, tip caps and components for cartridges, as well as syringe plungers. Our recent investment in this business will allow us to market coated stoppers which better preserve the contents of the vial and adds value to our customers and the consumer. Pharmaceutical applications for this market include vaccines, anti‑thrombotic, small molecules and biologics.
FOOD + BEVERAGE
The Food + Beverage segment is our smallest segment in terms of net sales and total assets representing 14% and 10% of AptarGroup’s Net Sales and Total Assets, respectively, but has been experiencing strong product growth over recent years. We primarily sell dispensing closures and, to a lesser degree, non‑dispensing closures, spray pumps and aerosol valves.
Sales of dispensing closures have grown as consumers worldwide have demonstrated a preference for a package utilizing the convenience of a dispensing closure. At the same time, consumer marketers are trying to differentiate their products by incorporating performance enhancing features such as bonded aluminum liners to plastic, flow‑control and no‑drip dispensing, inverted packaging and directional flow to make packages simpler to use, cleaner and more appealing to consumers. We also have a number of product solutions that address the increased use of flexible packaging formats.
Food. Sales to the food market accounted for approximately 56% of the segment’s total net sales in 2015 and primarily include sales of dispensing closures and elastomeric flow‑control components. To a lesser degree we also sell non‑dispensing closures, continuous spray aerosol valves and spray pumps to this market. Applications for dispensing closures include sauces, condiments and food products. Applications for non‑dispensing closures include granular and powder food products along with baby food closures. Applications for continuous spray aerosol valves include cooking sprays. Spray pump applications primarily include butter or salad dressing sprays.
Beverage. Sales to the beverage market accounted for approximately 42% of the segment’s total net sales in 2015 and primarily include sales of dispensing closures and elastomeric flow‑control components. Sales of dispensing closures to the beverage market have increased significantly over the last several years as we continue to see an increase of interest from marketers using dispensing closures for their products. Examples of beverage products currently utilizing dispensing closures include bottled water, sport and energy drinks, juices and concentrated water flavorings.
GENERAL BUSINESS INFORMATION
RESEARCH AND DEVELOPMENT
Our commitment to innovation, one of our competitive strengths, has resulted in an emphasis on research and development directed toward developing affordable, new, innovative packaging delivery solutions and adapting existing products for new markets or customer requirements. In certain cases, our customers share in the research and development expenses of customer initiated projects. Occasionally, we acquire or license from third parties technologies or products that are in various stages of development. Expenditures for research and development activities, net of certain research and development credits, were $67.1 million, $76.2 million and $71.8 million in 2015, 2014 and 2013, respectively.
PATENTS AND TRADEMARKS
We customarily seek patent and trademark protection for our products and brands. We own and currently have numerous applications pending for patents and trademarks in many regions of the world. In addition, certain of our products are produced under patent licenses granted by third parties. We believe that we possess certain technical capabilities in making our products that make it difficult for a competitor to duplicate. While valuable to our overall product portfolio, sales of any one individually patented product are not considered material to any specific segment or to the Company’s consolidated results.
TECHNOLOGY
We have technical expertise regarding injection molding, robotics, clean-room facilities and high‑speed assembly. We also have expertise regarding the formulation and finishing of elastomer and silicone components. In addition, we offer a variety of sterilization options for elastomeric components for the pharmaceutical industry. Pumps and aerosol valves require the assembly of several different plastic, metal and rubber components using high‑speed equipment. When molding dispensing closures, or plastic components to be used in pump or aerosol valve products, we use advanced plastic injection molding technology, including large cavitation plastic injection molds. We are able to mold within tolerances as small as one one‑thousandth of an inch and we assemble products in a high‑speed, cost‑effective manner. We are experts in molding liquid silicone that is used in certain dispensing closures as well as rubber gasket formulation and production primarily for the prescription drug and consumer health care markets.
MANUFACTURING AND SOURCING
The majority of our worldwide production is located outside of the United States. In order to augment capacity and to maximize internal capacity utilization (particularly for plastic injection molding), we use subcontractors to supply certain plastic, metal and rubber components. Certain suppliers of these components have unique technical abilities that make us dependent on them, particularly for aerosol valve and pump production. The principal raw materials used in our production are plastic resins, rubber and certain metal products. We believe an adequate supply of such raw materials is available from existing and alternative sources. We attempt to offset cost increases through improving productivity and increasing selling prices over time, as allowed by market conditions or contractual commitments. Our pharmaceutical products often use plastic resin and rubber components specifically approved by our customers. Significant delays in receiving these components or discontinuance of an approved raw material would require us to seek alternative sources, which could result in higher costs as well as impact our ability to supply products in the short-term.
BACKLOG
Our sales are primarily made pursuant to standard purchase orders for delivery of products. While most orders placed with us are ready for delivery within 120 days, we continue to experience a trend towards shorter lead times requested by our customers. Some customers place blanket orders, which extend beyond this delivery period. However, deliveries against purchase orders are subject to change, and only a small portion of the order backlog is noncancelable. The dollar amount associated with the noncancelable portion is not material. Therefore, we do not believe that backlog as of any particular date is an accurate indicator of future results.
CUSTOMERS
We have approximately 5,000 customers with no single customer or group of affiliated customers accounting for greater than 8% of 2015 net sales. A consolidation of our customer base has occurred and this trend is expected to continue. A concentration of customers presents opportunities for increasing sales due to the breadth of our product line, our international presence and our long‑term relationships with certain customers. However, consolidation of our customers could lead to pricing pressures, concentration of credit risk and fewer opportunities to introduce new products to the market.
INTERNATIONAL BUSINESS
We are geographically diverse with manufacturing and sales operations in Asia, Europe, Latin America (including Mexico) and North America. Europe is our largest region where sales for the years ended December 31, 2015, 2014 and 2013 were approximately 56%, 58% and 58%, respectively. Asia and Latin America when aggregated represented approximately 17%, 17% and 17% of our consolidated sales for the years ended December 31, 2015, 2014 and 2013, respectively. Export sales from the United States were $151.2 million, $161.4 million and $143.9 million in 2015, 2014 and 2013, respectively. For additional financial information about geographic areas, please refer to Note 17 in the Notes to the Consolidated Financial Statements in Item 8 (which is incorporated by reference herein).
FOREIGN CURRENCY
Because of our international presence, movements in exchange rates have a significant impact on the translation of the financial statements of our foreign subsidiaries. Our primary foreign exchange exposure is to the Euro, but we have foreign exchange exposure to the Chinese Yuan, Brazilian Real, Mexican Peso, Swiss Franc and other Asian, European and South American currencies. A strengthening U.S. dollar relative to foreign currencies has a dilutive translation effect on our financial statements. Conversely, a weakening U.S. dollar has an additive effect. We manage our exposures to foreign exchange principally with forward exchange contracts to economically hedge recorded transactions and firm purchase and sales commitments denominated in foreign currencies.
WORKING CAPITAL PRACTICES
Collection and payment periods tend to be longer for our operations located outside the United States due to local business practices. We have also seen an increasing trend in pressure from certain customers to lengthen their payment terms. As the majority of our products are made to order, we have not needed to keep significant amounts of finished goods inventory to meet customer requirements.
To the extent our financial position allows and there is a clear financial benefit, we from time-to-time benefit from early payment discounts with some suppliers.
EMPLOYEE AND LABOR RELATIONS
AptarGroup has approximately 13,000 full‑time employees. Of the full‑time employees, approximately 7,600 are located in Europe, 3,300 are located in Asia and South America and the remaining 2,100 are located in North America. The majority of our European and Latin American employees are covered by collective bargaining arrangements made at either the local or national level in their respective countries and approximately 160 of the North American employees are covered by a collective bargaining agreement. Termination of employees at certain of our international operations could be costly due to local regulations regarding severance benefits. There were no material work stoppages in 2015 and management considers our employee relations to be satisfactory.
COMPETITION
All of the markets in which we operate are highly competitive and we continue to experience price competition in all product lines and markets. Competitors include privately and publicly held entities that range from regional to international companies. We expect the market for our products to remain competitive. We believe our competitive advantages are consistent high levels of innovation, quality and service, geographic diversity and breadth of products. Our manufacturing strength lies in the ability to mold complex plastic components and formulate and finish elastomer and silicone components in a cost‑effective manner and to assemble products at high speeds. Our business is somewhat capital intensive and it is becoming more important to our customers for Aptar to have global manufacturing capabilities. Both of these serve as barriers to entry for new competitors wanting to enter our business.
While we have experienced some competition in Europe, Latin America and the United States from low cost Asian suppliers, particularly in the low‑end beauty and personal care market, this has not been significant. Although using low cost Asian supplies may have a cost advantage, some customers prefer local suppliers citing better quality, better customer service and shorter lead times.
ENVIRONMENT
Our manufacturing operations primarily involve plastic injection molding, automated assembly processes, elastomer and silicone formulation and finishing and, to a limited degree, metal anodization and vacuum metallization of plastic components. Historically, the environmental impact of these processes has been minimal, and we believe we meet current environmental standards in all material respects. To date, our manufacturing operations have not been significantly affected by environmental laws and regulations relating to the environment.
Recently there is increased interest and awareness from consumers, and from our customers, in environmentally sustainable products, especially through the sourcing of alternate materials. We are focused on reducing our environmental impacts through product life cycle assessments, alternate material trials, operational eco-efficiency initiatives and renewable energy sourcing. We are designing for sustainability by providing products that improve recyclability and use less material. Future regulations on environmental matters regarding recycling or material inputs could impact our business.
GOVERNMENT REGULATION
Certain of our products are indirectly affected by government regulation. Demand for aerosol and pump packaging is affected by government regulations regarding the release of volatile organic compounds (“VOCs”) into the atmosphere. Europe and the United States have regulations that require the reduction in the amount of VOCs that can be released into the atmosphere and the potential exists for this type of regulation to expand worldwide. These regulations required certain of our customers to reformulate certain aerosol and pump products, which may have affected the demand for such products. We own patents and have developed systems to function with alternative propellant and product formulations.
Future government regulations could include medical cost containment policies. For example, reviews by various governments to determine the number of drugs, or prices thereof, that will be paid by their insurance systems could affect future sales to the pharmaceutical industry. Such regulation could adversely affect prices of and demand for our pharmaceutical products. We believe that the focus on the cost effectiveness of the use of medications as compared to surgery and hospitalization provides us with an opportunity to expand sales to the pharmaceutical market.
EXECUTIVE OFFICERS
Our executive officers as of February 25, 2016 are as follows:
Name | Age | Position with the Company |
| | |
Stephen Hagge | 64 | President and Chief Executive Officer |
Mr. Hagge has been President and Chief Executive Officer since January 2012. Prior to this, Mr. Hagge was Chief Operating Officer from 2008 to 2011, Executive Vice President from 1993 to 2011 and Secretary from 1993 to June 2011. |
| | |
Robert Kuhn | 53 | Executive Vice President, Chief Financial Officer and Secretary |
Mr. Kuhn has been Executive Vice President and Chief Financial Officer since September 2008. Mr. Kuhn has been Secretary since June 2011. |
| | |
Eldon Schaffer | 50 | President, Aptar Beauty + Home |
Mr. Schaffer has been President of Aptar Beauty + Home since January 2016. Prior to this, Mr. Schaffer was President of Aptar Food + Beverage from 2012 to 2015 and President of Aptar Beauty + Home North America from 2010 to 2011. |
| | |
Gael Touya | 46 | President, Aptar Food + Beverage |
Mr. Touya has been President of Aptar Food + Beverage since January 2016. Prior to this, Mr. Touya was President of Aptar Food + Beverage Europe from 2012 to 2015 and Business Development Vice President Skin Care and Color Cosmetics from 2010 to 2011. |
| | |
Salim Haffar | 42 | President, Aptar Pharma |
Mr. Haffar has been President of Aptar Pharma since January 2014. From 2012 to 2013 Mr. Haffar worked with Capsugel, a leading pharmaceutical supplier of gelatin capsules for the oral drug delivery industry. From 2010 to 2012, he was President of Aptar Pharma’s Prescription division. |
| | |
Ursula Saint‑Léger | 52 | Vice President of Human Resources |
Ms. Saint‑Léger has been Vice President of Human Resources since October 2010. |
There were no arrangements or understandings between any of the executive officers and any other person(s) pursuant to which such officers were elected.
ITEM 1A. RISK FACTORS
Set forth below and elsewhere in this report and in other documents we file with the Securities and Exchange Commission are risks and uncertainties that could cause our actual results to materially differ from the results contemplated by the forward‑looking statements contained in this report and in other documents we file with the Securities and Exchange Commission. Additional risks and uncertainties not presently known to us, or that we currently deem immaterial, may also impair our business operations. You should carefully consider the following factors in addition to other information contained in this report on Form 10‑K before purchasing any shares of our common stock.
FACTORS AFFECTING OPERATIONS OR OPERATING RESULTS
Geopolitical conditions, including direct or indirect acts of war or terrorism, could have a material adverse effect on our operations and financial results. Our operations could be disrupted by geopolitical conditions such as international boycotts and sanctions, acts of war, terrorist activity or other similar events. Such events could make it difficult or impossible to manufacture or deliver products to our customers, receive production materials from our suppliers, or perform critical functions, which could adversely affect our business globally or in certain regions. While we maintain similar manufacturing capacities at different locations and coordinate multi‑source supplier programs on many of our materials which would better enable us to respond to these types of events, we cannot be sure that our plans will fully protect us from all such disruptions. In addition, our customers may export their finished products using our dispensing devices that were sold in other regions and an adverse geopolitical event, such as recent conflicts in Eastern Europe, may impact the sales of our customers’ products and thus indirectly negatively impact the demand for our dispensing solutions.
If there is deterioration in economic conditions in a particular region or market, our business and operating results could be materially adversely impacted. Due to our strong balance sheet, diverse product offerings, various end‑markets served, and our broad geographic presence, we believe we are well positioned to withstand slowness in any one particular region or market. However, economic uncertainties affect businesses such as ours in a number of ways, making it difficult to accurately forecast and plan our future business activities. A tightening of credit in financial markets or other factors may lead consumers and businesses to postpone spending, which may cause our customers to cancel, decrease or delay their existing and future orders with us. In addition, financial difficulties experienced by our suppliers, customers or distributors
could result in product delays, increased accounts receivable defaults, inventory or supply challenges and pricing pressures. An interruption in supply may also impact our ability to meet customer demands. Consumer demand for our customers’ products and shifting consumer preferences are unpredictable and could have a negative impact on our customers and our customers’ demand for our products.
We have foreign currency translation and transaction risks that may materially adversely affect our operating results. A majority of our operations are located outside of the United States. Because of this, movements in exchange rates may have a significant impact on the translation of the financial statements of our foreign entities. Our primary foreign exchange exposure is to the Euro, but we have foreign exchange exposure to the Chinese Yuan, Brazilian Real, Mexican Peso, Swiss Franc, and other Asian, European and South American currencies. A strengthening U.S. dollar relative to foreign currencies has a dilutive translation effect on our financial statements. Conversely, a weakening U.S. dollar has an additive translation effect. In some cases, we sell products denominated in a currency different from the currency in which the related costs are incurred. We manage our exposures to foreign exchange principally with forward exchange contracts to economically hedge certain transactions and firm purchase and sales commitments denominated in foreign currencies. The volatility of currency exchange rates may materially affect our operating results.
Government regulation on environmental matters regarding recycling or environmental sustainability policies could impact our business. Future government regulations mandating the use or limitations of certain materials could impact our manufacturing processes or the technologies we use forcing us to reinvest in alternative materials or assets used in the production of our products.
Consolidation of customer base could impact of business. We believe mergers and acquisitions within our customer base create opportunities for increasing sales due to the breadth of our product line, our international presence and our long‑term relationships with certain customers. However, consolidation of our customers could lead to pricing pressures, concentration of credit risk and fewer opportunities to introduce new products to the market.
We face strong global competition and our market share could decline. All of the markets in which we operate are highly competitive and we continue to experience price competition in all product lines and segments. Competitors include privately and publicly held entities. Our competitors mainly range from regional to international companies. Some fragrance marketers are sourcing their manufacturing requirements including filling of their product in Asia and importing the finished product back into the United States or Europe. If we are unable to compete successfully, our market share may decline, which could materially adversely affect our results of operations and financial condition.
If our expansion initiatives are unsuccessful, our operating results and reputation may suffer. We are expanding our operations in a number of markets, including facilities expansions in France, North America and Asia, while also planning the integration of Mega Airless. Expansion of our operations and the integration of Mega Airless will continue to require a significant amount of time and attention from our senior management and/or capital investment. These activities present considerable challenges and risks, including the general economic and political conditions in the markets that we enter, attracting, training and retaining qualified and talented employees, infrastructure disruptions, fluctuations in currency exchange rates, the imposition of restrictions by governmental authorities, compliance with current, new and changing governmental laws and regulations and the cost of such compliance activities. If any of our expansion or integration efforts are unsuccessful, our operating results and reputation may suffer.
The success or failure of our customers’ products, particularly in the pharmaceutical market, may materially affect our operating results and financial condition. In the pharmaceutical market, the proprietary nature of our customers’ products and the success or failure of their products in the market using our dispensing systems may have a material impact on our operating results and financial condition. We may potentially work for years on modifying our dispensing device to work in conjunction with a customer’s drug formulation. If the customer’s pharmaceutical product is not approved by regulatory bodies or it is not successful on the market, the associated costs may not be recovered.
Higher raw material costs and other inputs and an inability to increase our selling prices may materially adversely affect our operating results and financial condition. The cost of raw materials and other inputs (particularly plastic resin, rubber, metal, anodization costs and transportation and energy costs) are volatile and susceptible to rapid and substantial changes due to factors beyond our control, such as changing economic conditions, currency fluctuations, weather conditions, political unrest and instability in energy‑producing nations, and supply and demand pressures. Raw material costs may increase in the coming years and, although we have generally been able to increase selling prices to cover increased costs, future market conditions may prevent us from passing these increased costs on to our customers through timely price increases. In addition, we may not be able to improve productivity or realize savings from our cost reduction programs sufficiently enough to offset the impact of increased raw material costs. As a result, higher raw material costs could result in declining margins and operating results.
In difficult market conditions, our fixed costs structure combined with potentially lower revenues may negatively impact our results. Our business is characterized by relatively high fixed costs and, notwithstanding our utilization of third‑party manufacturing capacity, most of our production requirements are met by our own manufacturing facilities. In difficult environments, we are generally faced with a decline in the utilization rates of our manufacturing facilities due to decreases in product demand. During such periods, our plants do not operate at full capacity and the costs associated with
this excess capacity are charged directly to cost of sales. Difficult market conditions in the future may adversely affect our utilization rates and consequently our future gross margins, and this, in turn, could have a material negative impact on our business, financial condition and results of operations.
If our unionized employees were to engage in a strike or other work stoppage, our business, operating results and financial position could be materially adversely affected. The majority of our European and Latin American employees are covered by collective bargaining arrangements made either at the local or national level in their respective countries and approximately 160 of our North American employees are covered by a collective bargaining agreement. Although we believe that our relations with our employees are satisfactory, no assurance can be given that this will continue. If disputes with our unions arise, or if our unionized workers engage in a strike or other work stoppage, we could incur higher labor costs or experience a significant disruption of operations, which could have a material adverse effect on our business, operating results and financial position.
Single sourced materials and manufacturing sites could adversely impact our ability to deliver product. The Company sources certain materials, especially some resins and rubber components for our pharmaceutical segment, from a single source. Any disruption in the supply of these materials could adversely impact our ability to deliver product to our customers. Similarly, we have certain components and / or products that are manufactured at a single location or from a single machine or mold. Any disruption to the manufacturing process could also adversely impact our ability to deliver product to our customers.
If we were to incur a significant product liability claim above our current insurance coverage, our business, operating results and financial condition could be materially adversely affected. Approximately 31% of our net sales are made to customers in the pharmaceutical industry. If our devices fail to operate as intended, medication prescribed for patients may be under administered, or may be over administered. The failure of our devices to operate as intended may result in a product liability claim against us. We believe we maintain adequate levels of product liability insurance coverage. A product liability claim or claims in our Pharma segment or our other segments in excess of our insurance coverage or not covered by existing insurance may materially adversely affect our business, operating results and financial condition.
Increased cybersecurity threats could pose a risk to our operations. Increased global information security threats and more sophisticated, targeted computer crime pose a risk to the confidentiality, availability and integrity of our data, operations and infrastructure. While we do not believe we have experienced a specific cyber incident such as unauthorized access to our systems or disruption of functionality, we attempt to mitigate these types of risks by employing a number of measures, including employee training, comprehensive monitoring of our networks and systems, and maintenance of backup and protective systems. We also periodically test our systems for vulnerabilities and have on occasion used a third party to conduct such tests. Even with these mitigations, our information systems remain potentially vulnerable to sophisticated cybersecurity threats. Depending on their nature and scope, such threats could potentially lead to the compromise of confidential information, improper use of our systems and networks, manipulation and destruction of data, production downtimes and operational disruptions, which in turn could adversely affect our reputation, competitiveness and results of operations.
We have approximately $310 million in recorded goodwill at December 31, 2015, and changes in future business conditions could cause this asset to become impaired, requiring write‑downs that would reduce our operating income. We evaluate the recoverability of goodwill amounts annually, or more frequently when evidence of potential impairment exists. The impairment test is based on several factors requiring judgment. A decrease in expected reporting unit cash flows or changes in market conditions may indicate potential impairment of recorded goodwill and, as a result, our operating results could be materially adversely affected. See “Critical Accounting Estimates” in Part II, Item 7 for additional information.
FACTOR AFFECTING APTARGROUP STOCK
Ownership by Certain Significant Shareholders. Currently, Aptar has six institutional shareholders who each own between 5% and 11% of our outstanding common stock. None of these shareholders have direct representation on our Board of Directors. If one of these shareholders decides to sell significant volumes of our stock, this could put downward pressure on the price of the stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS
The Company has no unresolved comments from the SEC.
ITEM 2. PROPERTIES
We lease or own our principal offices and manufacturing facilities. None of the owned principal properties is subject to a lien or other encumbrance material to our operations. We believe that existing operating leases will be renegotiated as they expire, will be acquired through purchase options or that suitable alternative properties will be leased on acceptable terms. We consider the condition and extent of utilization of our manufacturing facilities and other properties to be generally good, and the capacity of our plants to be adequate for the needs of our business. The locations of our principal manufacturing facilities, by country, are set forth below:
ARGENTINA | GERMANY | SPAIN |
Florencio Varela (1 & 2) | Böhringen (1 & 2) | Madrid (1) |
Tortuguitas (1 & 3) | Dortmund (1) | Torello (1 & 3) |
| Eigeltingen (2) | |
BRAZIL | Freyung (1 & 3) | SWITZERLAND |
Cajamar (1) | Menden (1) | Mezzovico (2) |
Maringá Paraná (1 & 3) | | |
Jundiai (1) | INDIA | THAILAND |
| Himachal Pradesh (1) | Chonburi (1) |
CHINA | Hyderabad (1 & 3) | |
Suzhou (1, 2 & 3) | Mumbai (2) | UNITED KINGDOM |
| | Leeds, England (1 & 3) |
COLOMBIA | INDONESIA | |
Cali (1) | Cikarang, Bekasi (1) | UNITED STATES |
| | Cary, Illinois (1, 2 & 3) |
CZECH REPUBLIC | IRELAND | Congers, New York (2) |
Ckyne (1 & 3) | Ballinasloe, County Galway (1) | Libertyville, Illinois (1 & 3) |
| | Lincolnton, North Carolina (3) |
FRANCE | ITALY | McHenry, Illinois (1 & 2) |
Annecy (1 & 2) | Manoppello (1) | Midland, Michigan (1 & 3) |
Brecey (2) | San Giovanni Teatino (Chieti) (1 & 3) | Mukwonago, Wisconsin (1, 2 & 3) |
Charleval (1 & 2) | | Stratford, Connecticut (1) |
Granville (2) | MEXICO | Torrington, Connecticut (1) |
Le Neubourg (1) | Queretaro (1 & 3) | Watertown, Connecticut (1) |
Le Vaudreuil (2) | | |
Oyonnax (1) | RUSSIA | |
Poincy (1 & 3) | Vladimir (1 & 3) | |
Verneuil Sur Avre (1) | | |
| (1) | | Locations of facilities manufacturing for the Beauty + Home segment. |
| (2) | | Locations of facilities manufacturing for the Pharma segment. |
| (3) | | Locations of facilities manufacturing for the Food + Beverage segment. |
We also have sales personnel in countries other than those listed above. Our corporate office is located in Crystal Lake, Illinois.
ITEM 3. LEGAL PROCEEDINGS
The Company, in the normal course of business, is subject to a number of lawsuits and claims both actual and potential in nature. While management believes the resolution of these claims and lawsuits will not have a material adverse effect on the Company’s financial position or results of operations or cash flows, claims and legal proceedings are subject to inherent uncertainties, and unfavorable outcomes could occur that could include amounts in excess of any accruals which management has established. Were such unfavorable final outcomes to occur, it is possible that they could have a material adverse effect on our financial position, results of operations and cash flows.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
MARKET FOR REGISTRANT’S COMMON EQUITY
Information regarding market prices of our Common Stock and dividends declared may be found in Note 21 to the Consolidated Financial Statements in Item 8 (which is incorporated by reference herein). Our Common Stock is traded on the New York Stock Exchange under the symbol ATR. As of February 19, 2016, there were approximately 250 holders of record of our Common Stock. A substantially greater number of holders of our Common Stock are “street name” or beneficial holders, whose shares of record are held by banks, brokers, and other financial institutions.
RECENT SALES OF UNREGISTERED SECURITIES
The employees of Aptargroup UK Holdings Limited (French Branch) and Aptar France S.A.S., our subsidiaries, are eligible to participate in the FCP Aptar Savings Plan (the “Plan”). All eligible participants are located outside of the United States. An independent agent purchases shares of Common Stock available under the Plan for cash on the open market and we do not issue shares. We do not receive any proceeds from the purchase of Common Stock under the Plan. The agent under the Plan is Banque Nationale de Paris Paribas Fund Services. No underwriters are used under the Plan. All shares are sold in reliance upon the exemption from registration under the Securities Act of 1933 provided by Regulation S promulgated under that Act. During the quarter ended December 31, 2015, the Plan purchased no shares of our common stock on behalf of the participants, and sold 1,600 shares of our Common Stock on behalf of the participants at an average price of $70.61 per share, for an aggregate amount of $113 thousand. At December 31, 2015, the Plan owned 50,600 shares of our Common Stock.
ISSUER PURCHASES OF EQUITY SECURITIES
On October 30, 2014, the Company announced a share repurchase authorization of up to $350 million of Common Stock. This authorization replaces previous authorizations and has no expiration date. AptarGroup may repurchase shares through the open market, privately negotiated transactions or other programs, subject to market conditions.
On December 16, 2014, the Company entered into an agreement to repurchase approximately $250 million of its Common Stock under an accelerated share repurchase program (the “ASR program”). The ASR program was part of the Company’s $350 million share repurchase authorization. On December 17, 2014, the Company paid $250 million to Wells Fargo Bank N.A. (“Wells Fargo”) in exchange for approximately 3.1 million shares. On September 25, 2015, the Company settled the ASR program with Wells Fargo and received approximately 719 thousand additional shares. The total number of shares repurchased under the ASR program was approximately 3.8 million shares.
After the ASR settlement, the Company spent $13.9 million to repurchase approximately 0.2 million additional shares during the fourth quarter of 2015.
The following table summarizes the Company’s purchases of its securities for the quarter ended December 31, 2015:
| | | | | | | | | | | | |
| | | | | | | | | Dollar Value Of | |
| | | | | | | Total Number Of Shares | | Shares that May Yet be | |
| | | Total Number | | | | Purchased as Part Of | | Purchased Under The | |
| | | Of Shares | | Average Price | | Publicly Announced | | Plans or Programs | |
Period | | | Purchased | | Paid Per Share | | Plans Or Programs | | (in millions) | |
| | | | | | | | | | | | |
10/1 – 10/31/15 | | | — | | $ | — | | — | | $ | 100.0 | |
11/1 – 11/30/15 | | | 190,350 | | | 72.98 | | 190,350 | | | 86.1 | |
12/1 – 12/31/15 | | | — | | | — | | — | | | 86.1 | |
Total | | | 190,350 | | $ | — | | 190,350 | | $ | 86.1 | |
SHARE PERFORMANCE
The following graph shows a five year comparison of the cumulative total stockholder return on AptarGroup’s Common Stock as compared to the cumulative total return of the Standard & Poor’s 500 Composite Stock Price Index and to an index of peer group companies we selected. The companies included in the peer group are: A. Schulman, Inc., AEP Industries Inc., Bemis Company, Inc., Berry Plastics Group, Inc., Crown Holdings, Inc., Graphic Packaging Holding Company, Greif Inc., H.B. Fuller Company, International Flavors and Fragrances, Inc., Owen’s‑Illinois, Inc., Packaging Corporation of America, PH Glatfelter Co., Sealed Air Corporation, Silgan Holdings, Inc., Sonoco Products Company, TriMas Corporation and West Pharmaceutical Services Inc.
Comparison of 5 Year Cumulative Stockholder Returns
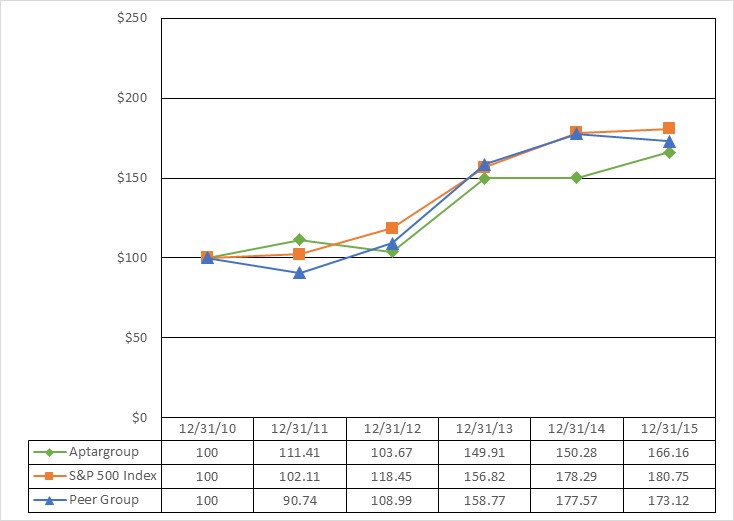
The graph and other information furnished in the section titled “Share Performance” under this Part II, Item 5 of this Form 10‑K shall not be deemed to be “soliciting” material or to be “filed” with the Securities and Exchange Commission or subject to Regulation 14A or 14C, or to the liabilities of Section 18 of the Securities Exchange Act of 1934, as amended.
ITEM 6. SELECTED FINANCIAL DATA
FIVE YEAR SUMMARY OF SELECTED FINANCIAL DATA
| | | | | | | | | | | | | | | | | | | | |
Dollars in millions, except per share data | | | | | | | | | | | | | | | | | | | | |
Years Ended December 31, | | | 2015 | | | | 2014 | | | | 2013 | | | | 2012 | | | | 2011 | |
| | | | | | | | | | | | | | | | | | | | |
Statement of Income Data: | | | | | | | | | | | | | | | | | | | | |
Net Sales | | $ | 2,317.1 | | | $ | 2,597.8 | | | $ | 2,520.0 | | | $ | 2,331.0 | | | $ | 2,337.2 | |
Cost of sales (exclusive of depreciation and amortization shown below) (1) | | | 1,502.7 | | | | 1,755.3 | | | | 1,708.9 | | | | 1,590.4 | | | | 1,568.3 | |
% of Net Sales | | | 64.8 | % | | | 67.6 | % | | | 67.8 | % | | | 68.2 | % | | | 67.1 | % |
Selling, research & development and administrative | | | 351.5 | | | | 383.9 | | | | 364.7 | | | | 341.6 | | | | 347.6 | |
% of Net Sales | | | 15.2 | % | | | 14.8 | % | | | 14.4 | % | | | 14.7 | % | | | 14.9 | % |
Depreciation and amortization (2) | | | 138.9 | | | | 152.2 | | | | 150.0 | | | | 137.0 | | | | 134.2 | |
% of Net Sales | | | 6.0 | % | | | 5.8 | % | | | 6.0 | % | | | 5.9 | % | | | 5.7 | % |
Restructuring initiatives | | | — | | | | — | | | | 11.8 | | | | 3.1 | | | | (0.1) | |
% of Net Sales | | | — | | | | — | | | | 0.5 | % | | | 0.1 | % | | | — | |
Operating Income | | | 324.1 | | | | 306.4 | | | | 284.6 | | | | 258.9 | | | | 287.1 | |
% of Net Sales | | | 14.0 | % | | | 11.8 | % | | | 11.3 | % | | | 11.1 | % | | | 12.3 | % |
Net Income | | | 199.3 | | | | 191.6 | | | | 171.9 | | | | 162.4 | | | | 183.6 | |
% of Net Sales | | | 8.6 | % | | | 7.4 | % | | | 6.8 | % | | | 7.0 | % | | | 7.9 | % |
Net Income Attributable to AptarGroup, Inc. | | | 199.3 | | | | 191.7 | | | | 172.0 | | | | 162.6 | | | | 183.7 | |
% of Net Sales | | | 8.6 | % | | | 7.4 | % | | | 6.8 | % | | | 7.0 | % | | | 7.9 | % |
Net Income Attributable to AptarGroup, Inc. per Common Share: | | | | | | | | | | | | | | | | | | | | |
Basic | | | 3.19 | | | | 2.95 | | | | 2.60 | | | | 2.45 | | | | 2.76 | |
Diluted | | | 3.09 | | | | 2.85 | | | | 2.52 | | | | 2.38 | | | | 2.65 | |
Balance Sheet and Other Data: | | | | | | | | | | | | | | | | | | | | |
Capital Expenditures | | $ | 149.3 | | | $ | 161.9 | | | $ | 151.5 | | | $ | 174.1 | | | $ | 179.7 | |
Total Assets | | | 2,438.7 | | | | 2,437.2 | | | | 2,497.8 | | | | 2,324.4 | | | | 2,159.3 | |
Long-Term Obligations | | | 762.5 | | | | 588.9 | | | | 354.8 | | | | 352.9 | | | | 254.9 | |
Net Debt (3) | | | 299.8 | | | | 441.1 | | | | 184.7 | | | | 197.8 | | | | 61.0 | |
AptarGroup, Inc. Stockholders’ Equity | | | 1,149.4 | | | | 1,103.4 | | | | 1,479.8 | | | | 1,379.9 | | | | 1,289.8 | |
Capital Expenditures % of Net Sales | | | 6.4 | % | | | 6.2 | % | | | 6.0 | % | | | 7.5 | % | | | 7.7 | % |
Interest Bearing Debt to Total Capitalization (4) | | | 41.6 | % | | | 43.2 | % | | | 25.1 | % | | | 23.7 | % | | | 25.4 | % |
Net Debt to Net Capitalization (5) | | | 20.7 | % | | | 28.6 | % | | | 11.1 | % | | | 12.5 | % | | | 4.5 | % |
Cash Dividends Declared per Common Share | | | 1.14 | | | | 1.09 | | | | 1.00 | | | | 0.88 | | | | 0.80 | |
| (1) | | Cost of sales includes $7.4 million reduction in expense for 2015 due to a change in accounting method relating to our inventory accounting methods. |
| (2) | | Depreciation and amortization includes $2.7 million and $1.6 million of accelerated depreciation related to the European restructuring plan for the year ended December 31, 2013 and 2012, respectively. |
| (3) | | Net Debt is interest bearing debt less cash and cash equivalents. |
| (4) | | Total Capitalization is AptarGroup, Inc. Stockholders’ Equity plus Interest Bearing Debt. |
| (5) | | Net Capitalization is AptarGroup, Inc. Stockholders’ Equity plus Net Debt. |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
(In thousands, expect per share amounts or otherwise indicated)
The objective of the following Management’s Discussion and Analysis of Consolidated Results of Operations and Financial Condition (“MD&A”) is to help the reader understand the financial performance of AptarGroup, Inc. MD&A is presented in eight sections: Overview, Results of Operations, Liquidity and Capital Resources, Off‑Balance Sheet Arrangements, Overview of Contractual Obligations, Recently Issued Accounting Pronouncements, Critical Accounting Estimates, Operations Outlook and Forward‑Looking Statements. MD&A should be read in conjunction with our Consolidated Financial Statements and accompanying Notes to Consolidated Financial Statements contained elsewhere in this Annual Report on Form 10‑K.
In MD&A, “we,” “our,” “us,” “AptarGroup,” “AptarGroup, Inc.”, “Aptar” and the “Company” refer to AptarGroup, Inc. and its consolidated subsidiaries.
OVERVIEW
GENERAL
We are a leading global provider of a broad range of innovative packaging dispensing and sealing solutions primarily for the beauty, personal care, home care, prescription drug, consumer health care, injectables, food and beverage markets. Our creative packaging solutions enhance the convenience, safety and security of consumers around the globe and allow our customers to differentiate their products in the market.
We define core sales as net sales excluding acquisitions and changes in foreign currency rates. Core sales is a non‑GAAP financial measure. We present this measure as supplemental information to help our investors better understand the trends in our business results over time. Our management uses core sales to evaluate our business on a consistent basis. A reconciliation of core sales growth to net sales growth, the most comparable GAAP measure, can be found on page 15.
2015 was a challenging year with sluggish macroeconomic conditions, foreign currency translation headwinds and specific softness in several key markets. In spite of these issues, we grew the top line on a core sales basis, adapted to the softer market conditions with a Company-wide focus on cost containment and benefitted from lower input costs. As a result, we were able to improve margins and grow operating income by 6%. We also executed our balanced capital allocation strategy by deploying capital to fund internal growth projects and increasing our dividend. Just after year-end, we also announced our agreement to acquire a leading provider of all-plastic airless dispensing systems for the beauty, personal care and pharmaceutical markets.
2015 HIGHLIGHTS
| · | | We achieved record annual net income and earnings per share. |
| · | | Core sales increased 1%, while reported sales decreased 11% largely due to changes in foreign currency translation rates. |
| · | | Performance of our Pharma and Food + Beverage segments offset certain softness in our Beauty + Home segment. |
| · | | Lower material costs, cost savings initiatives and productivity improvements drove margin improvements across all three segments. |
| · | | We paid increased annual dividends for the 22nd consecutive year (current annualized dividend is $1.20 per share). |
RESULTS OF OPERATIONS
The following table sets forth the consolidated statements of income and the related percentages of net sales for the periods indicated:
| | | | | | | | | | | | | | | | | | |
| | 2015 | | 2014 | | 2013 | |
Years Ended December 31, | | Amount in | | % of | | | Amount in | | % of | | | Amount in | | % of | |
| | $ Thousands | | Net Sales | | | $ Thousands | | Net Sales | | | $ Thousands | | Net Sales | |
| | | | | | | | | | | | | | | | | | |
Net sales | | $ | 2,317,149 | | 100.0 | % | | $ | 2,597,809 | | 100.0 | % | | $ | 2,520,013 | | 100.0 | % |
Cost of sales (exclusive of depreciation and amortization shown below) | | | 1,502,650 | | 64.8 | | | | 1,755,266 | | 67.6 | | | | 1,708,936 | | 67.8 | |
Selling, research & development and administrative | | | 351,461 | | 15.2 | | | | 383,909 | | 14.8 | | | | 364,747 | | 14.4 | |
Depreciation and amortization | | | 138,893 | | 6.0 | | | | 152,218 | | 5.8 | | | | 149,956 | | 6.0 | |
Restructuring initiatives | | | — | | — | | | | — | | — | | | | 11,800 | | 0.5 | |
Operating income | | | 324,145 | | 14.0 | | | | 306,416 | | 11.8 | | | | 284,574 | | 11.3 | |
Other expense | | | (29,574) | | (1.3) | | | | (20,115) | | (0.8) | | | | (20,191) | | (0.8) | |
Income before income taxes | | | 294,571 | | 12.7 | | | | 286,301 | | 11.0 | | | | 264,383 | | 10.5 | |
Net Income | | | 199,295 | | 8.6 | | | | 191,624 | | 7.4 | | | | 171,926 | | 6.8 | |
Effective tax rate | | | 32.3 | % | | | | | 33.1 | % | | | | | 35.0 | % | | |
NET SALES
We reported net sales of $2.3 billion for 2015, 11% below 2014 reported net sales of $2.6 billion. The average U.S. dollar exchange rate strengthened significantly relative to the Euro and also strengthened relative to other currencies impacting our business, resulting in a negative currency translation impact of 12%. Therefore, sales excluding changes in foreign currency rates (“core sales”) increased by 1% in 2015 compared to 2014. Core sales growth in the Pharma and Food + Beverage segments was substantially offset by a decline in core sales in the Beauty + Home segment.
In 2014, we reported net sales of $2.6 billion, 3% above 2013 reported net sales of $2.5 billion. The negative translation effect of 2% mainly relates to the strength of the U.S. Dollar compared to Latin American and Asian currencies as the Euro rates remained relatively stable when compared to the U.S. Dollar for the full year. All three operating segments saw core sales increases in 2014, but the consolidated 5% core sales growth was mainly driven by the strong results of our Food + Beverage and Pharma segments.
| | | | | |
Net Sales Change versus Prior Year | | 2015 | | 2014 | |
| | | | | |
Core Sales Growth | | 1 | % | 5 | % |
Foreign Currency Effects | | (12) | % | (2) | % |
Total Reported Net Sales Growth | | (11) | % | 3 | % |
Foreign currency effects are approximations of the adjustment necessary to state the prior year net sales using current period exchange rates. For further discussion on net sales by reporting segment, please refer to the segment analysis of net sales and operating income on the following pages.
The following table sets forth, for the periods indicated, net sales by geographic location:
| | | | | | | | | | | | | | | | |
Years Ended December 31, | | | 2015 | | % of Total | | | 2014 | | % of Total | | | 2013 | | % of Total | |
| | | | | | | | | | | | | | | | |
Domestic | | $ | 633,522 | | 27 | % | $ | 642,060 | | 25 | % | $ | 634,418 | | 25 | % |
Europe | | | 1,287,309 | | 56 | % | | 1,506,992 | | 58 | % | | 1,452,041 | | 58 | % |
Other Foreign | | | 396,318 | | 17 | % | | 448,757 | | 17 | % | | 433,554 | | 17 | % |
COST OF SALES (EXCLUSIVE OF DEPRECIATION AND AMORTIZATION SHOWN BELOW)
Our cost of sales as a percent of net sales decreased to 64.8% in 2015 compared to 67.6% in 2014. The decrease is partly due to a one-time favorable $7.4 million change in our inventory accounting principle for the inventory valuation method of certain operating entities in our North American business to the first-in first-out (FIFO) method from the last-in first-out (LIFO) method which was recorded in the second quarter of 2015. Operationally, we benefitted from our mix of products sold. Our Pharma segment sales represented a higher percentage of our overall sales in 2015 compared to 2014. This positively impacts our cost of sales percentage as margins on our pharmaceutical products typically are higher than the overall Company average. We also benefitted from lower material costs, cost savings initiatives and productivity improvements along with a positive impact from the timing delay of resin pass-throughs of approximately $8.9 million as resin prices declined significantly during 2015.
In 2014, our cost of sales as a percentage of net sales decreased to 67.6% compared to 67.8% in 2013. Positively impacting the cost of sales percentage in 2014 were the mix of products sold as the Pharma segment sales represented a higher percentage of our overall sales in 2014 compared to 2013, along with incremental cost savings from a restructuring plan to optimize certain production capacities in Europe which was fully realized in 2014. These savings were partially offset by underutilized U.S. overhead as soft demand in certain product lines negatively impacted our North American cost of sales, mainly in our Beauty + Home segment. We also incurred additional costs in Latin America as we recognized approximately $3.0 million in one-time costs related to the start-up of our Colombian operations and incurred $1.3 million of expenses related to a fire that occurred in one of our Brazilian facilities.
SELLING, RESEARCH & DEVELOPMENT AND ADMINISTRATIVE
Our Selling, Research & Development and Administrative expenses (“SG&A”) decreased approximately 8% or $32.4 million in 2015 compared to the same period a year ago. Excluding changes in foreign currency rates, SG&A increased by approximately $11.6 million compared to the same period a year ago. Cost savings initiatives were offset by higher professional fees related to our ongoing information systems implementations and approximately $1.9 million of transaction costs related to an agreement to acquire Mega Airless which is expected to close in the first quarter of 2016. We also realized higher pension costs related to lower discount rates along with other normal inflationary increases. For 2015, SG&A as a percentage of net sales increased to 15.2% compared to 14.8% of net sales in the same period of the prior year.
In 2014, our SG&A expenses increased approximately 5% or $19.2 million compared to the same period a year ago. Excluding changes in foreign currency rates, SG&A increased approximately $24.2 million compared to the same period a year earlier. The increase was due to several factors, including higher research and development costs mainly related to our bonded aluminum to plastic technology. We also realized higher information technology costs associated with our ongoing enterprise resource planning system roll-ins along with higher stock compensation expense. For 2014, SG&A as a percentage of net sales increased to 14.8% compared to 14.4% of net sales in the same period of the prior year.
DEPRECIATION AND AMORTIZATION
Depreciation and amortization expense decreased approximately 9% or $13.3 million in 2015. Excluding changes in foreign currency rates, depreciation and amortization increased by approximately $4.4 million compared to the same period a year ago. This increase is mainly due to our investments in new products and our global enterprise resource planning system. Depreciation and amortization as a percentage of net sales increased to 6.0% compared to 5.8% for the same period a year ago.
In 2014, depreciation and amortization expense increased approximately 2% or $2.3 million. Excluding changes in foreign currency rates, depreciation and amortization increased by approximately $3.6 million compared to the same period a year earlier. The investments in our new products and the continued roll-out of our global enterprise resource planning system mentioned above exceeded exceptional charges incurred in 2013. Depreciation and amortization as a percentage of net sales decreased slightly to 5.8% compared to 6.0% in 2013.
RESTRUCTURING INITIATIVES
On November 1, 2012, the Company announced a plan to optimize certain capacity in Europe. Due to increased production efficiencies and to better position the Company for future growth in Europe, AptarGroup transferred and consolidated production capacity involving twelve facilities. Under the plan, two facilities closed which impacted approximately 170 employees. During 2013, we recognized $11.8 million of restructuring expenses along with $2.7 million accelerated depreciation of assets. The plan was substantially completed at the end of 2013 with total costs of approximately $19.5 million.
OPERATING INCOME
Operating income increased approximately $17.7 million or 6% to $324.1 million in 2015. Excluding changes in currency rates, operating income increased by approximately $57.6 million in 2015. We benefitted from the $7.4 million change in inventory accounting principle along with the strong results of our Pharma and Food + Beverage segments. We also benefitted from a positive impact from the timing delay in passing through resin cost decreases and certain cost saving initiatives driven by the segments, which were slightly offset by transaction costs related to an agreement to acquire Mega Airless. Reported operating income as a percentage of net sales increased to 14.0% in 2015 compared to 11.8% for the same period in the prior year due to the same factors mentioned above.
In 2014, operating income increased approximately $21.8 million or 8% to $306.4 million. Excluding changes in currency rates, operating income increased by approximately $22.7 million in 2014. 2013 results included the negative impact of the European restructuring plan charges of $11.8 million. The remaining $10.9 million increase in operating income over the prior year is due to additional operating income from our sales growth, primarily from the Pharma and Food + Beverage segments. Reported operating income as a percentage of net sales increased to 11.8% in 2014 compared to 11.3% for the same period in the prior year due to the costs associated with our European restructuring plan in 2013.
NET OTHER EXPENSES
Net other expenses in 2015 increased to $29.6 million compared to $20.1 million in 2014. During 2015, we incurred $13.6 million of higher interest expense related to our additional $475 million senior notes established late 2014 and early 2015 along with increased costs of our forward exchange contracts. These increases were offset by $2.9 million of gain due to an insurance recovery on the involuntary conversion of fixed assets related to a fire in one of our Brazilian facilities. In addition, 2014 net other expenses included a $1.5 million write-down on a non-controlling investment.
In 2014, net other expenses decreased slightly to $20.1 million compared to $20.2 million in 2013. Higher interest income and lower hedging costs were mostly offset by the recognition of a $1.5 million write-down on a non-controlling investment taken during the first quarter of 2014 to align with the current fair value.
EFFECTIVE TAX RATE
The reported effective tax rate on net income for 2015 and 2014 was 32.3% and 33.1%, respectively. The lower tax rate for 2015 is attributable to the reduction of valuation allowances related to U.S. state tax credits and tax benefits associated with exceptional depreciation provisions enacted in France during 2015.
The reported effective tax rate on net income for 2014 and 2013 was 33.1% and 35.0%, respectively. The Company did not repatriate any earnings from foreign jurisdictions to the U.S. in 2014 and this reduced our reported effective tax rate compared to 2013.
NET INCOME ATTRIBUTABLE TO APTARGROUP, INC.
We reported net income of $199.3 million compared to $191.7 million reported in 2014 and $172.0 million reported in 2013.
BEAUTY + HOME SEGMENT
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | % Change | | % Change | |
Years Ended December 31, | | | 2015 | | | | 2014 | | | | 2013 | | 2015 vs. 2014 | | 2014 vs. 2013 | |
| | | | | | | | | | | | | | | | |
Net Sales | | $ | 1,272,946 | | | $ | 1,498,297 | | | $ | 1,488,145 | | (15.0) | % | 0.7 | % |
Segment Income (1) | | | 98,707 | | | | 98,368 | | | | 109,272 | | 0.3 | | (10.0) | |
Segment Income as a percentage of Net Sales | | | 7.8 | % | | | 6.6 | % | | | 7.3 | % | | | | |
| (1) | | Segment income is defined as earnings before net interest expense, certain corporate expenses, restructuring initiatives and income taxes. The Company evaluates performance of its business units and allocates resources based upon segment income. For a reconciliation of segment income to income before income taxes, see Note 17 to the Consolidated Financial Statements in Item 8. |
Net sales decreased approximately 15% in 2015 to $1.27 billion compared to $1.50 billion in 2014. Changes in foreign currency negatively impacted reported sales for 2015 by 13%. Core sales, which exclude changes in exchange rates, decreased 2% in 2015 compared to the prior year. Core sales to the personal care, beauty and home care markets decreased by 5%, 1% and 2%, respectively, in 2015 compared to 2014. We experienced global market softness in all regions except Asia leading to a sales decline on a constant currency basis compared to the prior year. Decreases in selling prices due to contractual resin cost pass-throughs to our customers negatively impacted sales by $9.1 million. Customer tooling sales, excluding foreign currency changes, increased $8.0 million in 2015 to $36.9 million compared to $28.9 million in the prior year.
In 2014, net sales increased approximately 1% to $1.50 billion compared to $1.49 billion in 2013. Changes in foreign currency negatively impacted reported sales for 2014 by 2%. Core sales, which exclude changes in exchange rates, increased 3% in 2014 compared to the prior year. Core sales to the beauty market increased approximately 4% while core sales to the personal care and home care markets increased approximately 3% and 4%, respectively, in 2014 compared to 2013. The increase in beauty sales was primarily due to growth of our prestige fragrance and skin care products. Personal care increased over the prior year due to stronger sales to our personal cleansing and sun care customers while sales to the home care market increased mainly due to improved laundry care sales. Geographically, all four regions reported increases in net sales with strong core sales growth in our emerging markets along with moderate growth in Europe and the U.S. Customer tooling sales, excluding foreign currency changes, decreased in 2014 to $33.9 million compared to $37.7 million in the prior year.
In spite of the decrease in net sales, segment income for 2015 increased slightly to $98.7 million from $98.4 million reported in 2014. The negative impacts of foreign currency rate changes and lower product sales were more than offset by lower material costs, cost savings initiatives and improved productivity, mainly in North America. We recognized a $2.4 million negative transaction effect related to the importing of components into Latin America from different regions due to the continued devaluation of certain Latin American currencies. However, this was offset by a $5.1 million favorable impact from the timing of resin pass-throughs in 2015 primarily in North America.
In 2014, segment income decreased approximately 10% to $98.4 million from $109.3 million reported in 2013. Soft demand in certain product lines negatively impacted our North American region. We also recognized approximately $3.0 million in one-time costs related to the start-up of our Colombian operations. Due to the significant devaluation of certain Latin American currencies, we recognized approximately $3.3 million of negative transaction effects related to the importing of components from different regions. In addition, we incurred $1.3 million of expense related to a fire that occurred in one of our Brazilian facilities.
PHARMA SEGMENT
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | % Change | | % Change | |
Years Ended December 31, | | | 2015 | | | | 2014 | | | | 2013 | | 2015 vs. 2014 | | 2014 vs. 2013 | |
| | | | | | | | | | | | | | | | |
Net Sales | | $ | 712,220 | | | $ | 751,226 | | | $ | 708,774 | | (5.2) | % | 6.0 | % |
Segment Income | | | 210,509 | | | | 204,698 | | | | 189,689 | | 2.8 | | 7.9 | |
Segment Income as a percentage of Net Sales | | | 29.6 | % | | | 27.2 | % | | | 26.8 | % | | | | |
Net sales decreased approximately 5% in 2015 to $712.2 million compared to $751.2 million in 2014. Foreign currency changes negatively impacted total segment sales by 13%. Core sales, which exclude changes in exchange rates, increased 8% in 2015 compared to the prior year. Core sales to the prescription and injectables markets increased 13% and 7%, respectively, in 2015 compared to the same period in the prior year. Prescription growth was led by strong demand for allergy products in both over the counter and prescription versions in the U.S. market as well as generics in Europe. We also experienced strong demand for our Asthma/COPD metered dose inhaler aerosol valves from key branded and generics customers worldwide. Injectables grew on increased stopper and syringe component sales with strong demand in Europe, U.S., India and Latin America. Core sales to the consumer health care market were down slightly to the prior year as growth in our non-prescription nasal decongestant business was offset by the softness in Eastern Europe and customer inventory reduction plans. Customer tooling sales, excluding foreign currency changes, increased $6.0 million in 2015 to $20.3 million compared to $14.3 million in the prior year.
In 2014, net sales increased approximately 6% to $751.2 million compared to $708.8 million in 2013. Foreign currency changes negatively impacted total segment sales by 1%. Core sales, which exclude changes in exchange rates, increased 7% in 2014 compared to the prior year. Core sales of our products increased in each end market and geographic region we serve. Core sales to the prescription drug market increased 6% on strong demand for our metered dose inhaler valves for various asthma and COPD treatments, including new generic launches in developing regions. We continued to see strong demand for our consumer health care products, which increased 12% partially due to preservative free eye care launches in Europe using our innovative ophthalmic squeeze dispenser along with strong sales of our products used for nasal decongestant. Excluding foreign currency rate changes, sales to the injectables market increased 1%. Customer tooling sales, excluding foreign currency changes, decreased in 2014 to $16.9 million compared to $21.1 million in the prior year.
Segment income increased approximately 3% to $210.5 million in 2015 compared to $204.7 million in 2014. This increase is mainly attributed to the higher sales volumes and improved product mix within the segment as well as cost containment initiatives. Prior year results also included a $1.5 million write-down on a non-controlling investment to align with the current fair value.
In 2014, segment income increased approximately 8% to $204.7 million compared to $189.7 million in 2013. This increase is mainly attributed to higher sales for the prescription and consumer health care markets discussed above. The Pharma segment also recognized a $1.5 million expense in the first quarter of 2014 related to the write-down of a non-controlling investment to fair value.
FOOD + BEVERAGE SEGMENT
| | | | | | | | | | | | | | |
| | | | | | | | | | | % Change | | % Change | |
Years Ended December 31, | | 2015 | | | 2014 | | | | 2013 | | 2015 vs. 2014 | | 2014 vs. 2013 | |
| | | | | | | | | | | | | | |
Net Sales | | 331,983 | | $ | 348,286 | | | $ | 323,094 | | (4.7) | % | 7.8 | % |
Segment Income | | 42,731 | | | 37,728 | | | | 35,186 | | 13.3 | | 7.2 | |
Segment Income as a percentage of Net Sales | | 12.9 | % | | 10.8 | % | | | 10.9 | % | | | | |
Net sales decreased by approximately 5% in 2015 to $332.0 million compared to $348.3 million in 2014. Excluding changes in foreign currency rates, sales increased 2%. Decreases in prices due to resin pass-throughs to our customers negatively impacted sales by $6.6 million while customer tooling sales, excluding foreign currency changes, decreased $11.5 million in 2015 to $15.6 million compared to $27.1 million in the prior year. Sales of our products, excluding foreign currency changes, to the food market increased 3% and core sales of our products to the beverage market increased approximately 1% in 2015 compared to the prior year. Broad based growth in the food market was partially offset by lower tooling sales of $10.9 million in 2015 compared to 2014. Beverage sales increased slightly as higher bottled water sales offset weakness in the functional drink and concentrated beverage flavorings applications.
In 2014, net sales increased by approximately 8% to $348.3 million compared to $323.1 million in 2013. Excluding changes in foreign currency rates, sales increased 9%. Sales of our products, excluding foreign currency changes, to the food market increased 7% and core sales of our products to the beverage market increased approximately 11% in 2014 compared to the prior year. The food sales increase was mainly due to improved sales of our products used to dispense sauces/condiments and dairy creamers. The beverage sales increase was driven by the continued success of our products used on functional beverages in the Asian region as well as growth in the concentrate beverage flavoring market in Europe. Customer tooling sales, excluding foreign currency changes, also increased in 2014 to $28.5 million compared to $27.2 million in the prior year.
Segment income increased approximately 13% to $42.7 million in 2015 compared to $37.7 million in 2014. This increase is mainly attributed to higher product sales volumes, improved operational performance and a $3.8 million favorable impact due to the timing of resin pass-throughs.
In 2014, segment income increased approximately 7% to $37.7 million compared to $35.2 million in 2013. This increase is mainly driven by the increase in product sales to both the food and beverage markets.
CORPORATE & OTHER
Certain costs that are not allocated to our three operating business segments are classified as “Corporate & Other,” which is presented separately in Note 17 of the Notes to the Consolidated Financial Statements. Corporate & Other primarily includes certain corporate compensation, professional fees, certain information system costs and LIFO inventory adjustments (prior to our accounting change in the second quarter of 2015).
Corporate & Other expense in 2015 decreased to $28.4 million compared to $38.3 million in 2014. This decrease is primarily due to the favorable impact of a $7.4 million change in accounting principle related to our inventory valuation method during the second quarter, $2.9 million of gain due to an insurance recovery on the involuntary conversion of fixed assets related to a fire in one of our Brazilian facilities along with the positive effect of changes in exchange rates on the translation of foreign based costs. These decreases were partially offset by $1.9 million of transaction costs related to an agreement to acquire Mega Airless.
In 2014, Corporate & Other expense increased slightly to $38.3 million compared to $38.0 million in 2013. The increase was mainly due to increases in professional fees associated with our legal entity reorganization project in 2014. The 2013 Corporate & Other expense included a $1.5 million adjustment for accelerated depreciation recognized on certain corporate assets in 2013.
LIQUIDITY AND CAPITAL RESOURCES
Our primary sources of liquidity are cash flows provided by our operations and our revolving credit facility. In 2015, our operations provided cash flows that totaled $324.5 million compared to $316.3 million in 2014 and $286.1 million in 2013. The increase in cash flow from operations in 2015 is mainly due to profit growth resulting from operational improvements, cost containment efforts and lower input costs. Comparing 2014 to 2013, the increase is mainly due to lower cash used for working capital compared to the prior year.
We used $176.2 million in cash for investing activities during 2015, compared to $159.2 million during 2014 and $157.1 million during 2013. The increase in cash used for investing activities is due to the purchase of $32.8 million in short term investments. Short term investments reflect funds invested in a time deposit instrument with a two-year maturity. However, during the life of the investment the funds can be redeemed at any time with a 35-90 day notice. There are no penalties for early redemption. This increase in cash used for short term investments was partially offset by a decrease in capital expenditures of $12.6 million. Comparing 2014 to 2013, the increase in cash used for investing activities is primarily related to the increase in capital expenditures of approximately $10.4 million during the year. We estimate that we will spend approximately $160 million (assuming current exchange rates) on capital expenditures in 2016.
Our net cash used for financing activities in 2015 was $33.1 million compared to $20.7 million in 2014 and $67.8 million in 2013. During the fourth quarter 2015, we repurchased $13.9 million shares for retirement. Comparing 2014 to 2013, the decrease in cash used for financing activities was primarily due to an increase in our proceeds from long-term debt which was primarily used to fund share repurchases through our accelerated share repurchase program.
Cash and equivalents increased $90.1 million to $489.9 million at the end of 2015 from $399.8 million at the end of 2014. Total short and long-term interest bearing debt decreased to $819.5 million at the end of 2015 from $840.9 million at the end of 2014. The ratio of our Net Debt (interest bearing debt less cash and cash equivalents) to Net Capital (Stockholders’ Equity plus Net Debt) decreased to 20.7% at the end of 2015 compared to 28.6% as of December 31, 2014.
The Company maintains a revolving credit facility that provides for unsecured financing of up to $300 million. Each borrowing under this credit facility will bear interest at rates based on LIBOR, prime rates or other similar rates, in each case plus an applicable margin. A facility fee on the total amount of the facility is also payable quarterly, regardless of usage. The applicable margins for borrowings under the credit facility and the facility fee percentage may change from time to time depending on changes in AptarGroup's consolidated leverage ratio. The facility matures on December 16, 2019. At December 31, 2015, there was no outstanding balance under the credit facility. The outstanding balance under the credit facility was $230 million at December 31, 2014 and is reported as notes payable in the current liabilities section of the Condensed Consolidated Balance Sheets. We incurred approximately $0.9 million and $2.7 million in interest and fees related to this credit facility during 2015 and 2014, respectively.
Our revolving credit facility and certain long-term obligations require us to satisfy certain financial and other covenants including:
| | | | |
| | Requirement | | Level at December 31, 2015 |
Consolidated Leverage Ratio (a) | | Maximum of 3.50 to 1.00 | | 0.83 to 1.00 |
Consolidated Interest Coverage Ratio (a) | | Minimum of 3.00 to 1.00 | | 14.11 to 1.00 |
| (a) | | Definitions of ratios are included as part of the revolving credit facility agreement. |
Based upon the above consolidated leverage ratio covenant, we would have the ability to borrow approximately an additional $1.7 billion before the 3.50 to 1.00 ratio requirement would be exceeded.
Our foreign operations have historically met cash requirements with the use of internally generated cash or borrowings. These foreign subsidiaries have financing arrangements with several foreign banks to fund operations located outside the U.S., but the majority of these arrangements are uncommitted. Cash generated by foreign operations has generally been reinvested locally. The majority of our $489.9 million in cash and equivalents is located outside of the U.S. We manage our global cash requirements considering (i) available funds among the many subsidiaries through which we conduct business, (ii) the geographic location of our liquidity needs, and (iii) the cost to access international cash balances. The repatriation of non‑U.S. cash balances from certain subsidiaries could have adverse tax consequences as we may be required to pay income tax on those funds. Historically, the tax consequences associated with repatriating current year earnings to the U.S. has been between 10% and 14% of the repatriated amount. We do not expect future impacts to be materially different.
We believe we are in a strong financial position and have the financial resources to meet our business requirements in the foreseeable future. We have historically used cash flow from operations as our primary source of liquidity. Our primary uses of liquidity are to invest in equipment and facilities that are necessary to support our growth and to make acquisitions that will contribute to the achievement of our strategic objectives. We currently anticipate funding the Mega Airless acquisition using available cash on hand in Europe and an existing revolving line of credit in the U.S. Other uses of liquidity include paying dividends to shareholders and repurchasing shares of our common stock. In the event that customer demand would decrease significantly for a prolonged period of time and negatively impact cash flow from operations, we would have the ability to restrict and significantly reduce capital expenditure levels, as well as evaluate our acquisition strategy and dividend and share repurchase programs. A prolonged and significant reduction in capital expenditure levels could increase future repairs and maintenance costs as well as have a negative impact on operating margins if we were unable to invest in new innovative products.
OFF‑BALANCE SHEET ARRANGEMENTS
We lease certain warehouse, plant and office facilities as well as certain equipment under noncancelable operating leases expiring at various dates through the year 2027. Most of the operating leases contain renewal options and certain equipment leases include options to purchase during or at the end of the lease term. Other than operating lease obligations, we do not have any off‑balance sheet arrangements. See the following section “Overview of Contractual Obligations” for future payments relating to operating leases.
OVERVIEW OF CONTRACTUAL OBLIGATIONS
Below is a table of our outstanding contractual obligations and future payments as of December 31, 2015:
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | 2021 and | |
Payment Due by Period | | | Total | | | 2016 | | 2017-2018 | | 2019-2020 | | After | |
| | | | | | | | | | | | | | | | |
Long-term debt (1) | | $ | 812,785 | | $ | 51,368 | | $ | 76,102 | | $ | 84,371 | | $ | 600,944 | |
Capital lease obligations (1) | | | 1,628 | | | 522 | | | 1,067 | | | 39 | | | — | |
Operating leases | | | 65,943 | | | 15,418 | | | 21,683 | | | 12,790 | | | 16,052 | |
Notes payable (2) | | | 5,083 | | | 5,083 | | | — | | | — | | | — | |
Purchase obligations (3) | | | 85,866 | | | 38,941 | | | 34,937 | | | 11,988 | | | — | |
Interest obligations (4) | | | 222,941 | | | 30,782 | | | 55,624 | | | 48,135 | | | 88,400 | |
Required minimum pension contribution (5) | | | — | | | — | | | — | | | — | | | — | |
Other liabilities reflected on the balance sheet under GAAP (6) | | | — | | | — | | | — | | | — | | | — | |
Total Contractual Obligations | | $ | 1,194,246 | | $ | 142,114 | | $ | 189,413 | | $ | 157,323 | | $ | 705,396 | |
| (1) | | The future payments listed above for capital lease obligations and long‑term debt repayments reflect only principal payments. |
| (2) | | Notes payable mainly includes local, non-U.S. short-term borrowings. The future payments listed above assume that no additional amounts will be drawn under this credit facility. |
| (3) | | Purchase obligations are agreements to purchase goods or services that are enforceable and legally binding on the Company that specify all significant terms, including: fixed or minimum quantities to be purchased; fixed, minimum, or variable price provisions; and the approximate timing of the transactions. |
| (4) | | Approximately 0.2% of our total interest bearing long‑term debt has variable interest rates. Using our long‑term variable rate debt outstanding as of December 31, 2015 of approximately $1.2 million at an average rate of approximately 7.6%, we included approximately $0.1 million of variable interest rate obligations in 2015. No variable interest rate obligations were included in subsequent years. |
| (5) | | This line represents the required minimum pension contribution obligation for the Company’s U.S. plans. At this time, the Company is not required to make a contribution. The Company also makes contributions to its foreign pension plans but amounts are expected to be discretionary in 2016 and future years. Therefore amounts related to these plans are not included in the preceding table. |
| (6) | | This line represents the current portion of the liability for uncertain tax positions. Aside from deferred income taxes, we have approximately $94.1 million of other deferred long‑term liabilities on the balance sheet, which consist primarily of retirement plan obligations as described in Note 8 to the Consolidated Financial Statements. The Company is not able to reasonably estimate the timing of the long‑term payments or the amount by which the liability will increase or decrease over time. Therefore, the long‑term portion of the liability is excluded from the preceding table. |
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS
We have reviewed the recently issued accounting standards updates to the Financial Accounting Standards Board’s (“FASB’s”) Accounting Standards Codification that have future effective dates. Standards which are effective for 2015 are discussed in Note 1 of the Notes to Consolidated Financial Statements.
In May 2014, the FASB amended the guidance for recognition of revenue from customer contracts. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in the amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In August 2015, the FASB decided to defer the effective date by one year to December 15, 2017 for annual reporting periods beginning after that date. The FASB also decided to allow early adoption of the standard, but not before the original effective date of December 15, 2016. We are currently evaluating the impact the adoption of this standard will have on our consolidated financial statements.
In April 2015, the FASB issued an Accounting Standards Update (“ASU”) intended to simplify GAAP by changing the presentation of debt issuance costs. Under the new standard, debt issuance costs will be presented as a reduction of the carrying amount of the related liability, rather than as an asset. The new treatment is consistent with debt discounts. In August 2015, the FASB issued an ASU clarifying that debt issuance costs related to line of credit arrangements can be classified as an asset and amortized ratably over the term of the line of credit arrangement. These standards are effective for annual reporting periods beginning after December 15, 2015, but early adoption is permitted. The Company does not believe that this new guidance will have a material impact on its consolidated financial statements.
In April 2015, the FASB issued new guidance on a customer's accounting for fees paid in a cloud computing arrangement (CCA). Previously, there was no specific GAAP guidance on accounting for such fees from the customer's perspective. Under the new standard, customers will apply the same criteria as vendors to determine whether a CCA contains a software license or is solely a service contract. This standard is effective for annual reporting periods beginning after December 15, 2015, but early adoption is permitted. The Company does not believe that this new guidance will have a material impact on its consolidated financial statements.
In July 2015, the FASB issued new guidance for simplifying the measurement of inventory. The core principle of the guidance is that an entity should measure inventory at the lower of cost and net realizable value. This standard is effective for annual reporting periods beginning after December 15, 2016. The Company does not believe that this new guidance will have a material impact on its consolidated financial statements.
In November 2015, the FASB issued guidance simplifies the balance sheet classification of deferred taxes. The new guidance requires that deferred tax liabilities and assets be presented as non-current in a classified statement of financial position. This standard is effective for annual reporting periods beginning after December 15, 2016. Other than presentation, the Company does not believe that this new guidance will have a material impact on its consolidated financial statements.
Other accounting standards that have been issued by the FASB or other standards‑setting bodies that do not require adoption until a future date are not expected to have a material impact on our consolidated financial statements upon adoption.
CRITICAL ACCOUNTING ESTIMATES
The preparation of the financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We continually evaluate our estimates, including those related to bad debts, inventories, intangible assets, income taxes, pensions and contingencies. We base our estimates on historical experience and on a variety of other assumptions believed to be reasonable in order to make judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our more significant judgments and estimates used in preparation of our Consolidated Financial Statements. Management has discussed the development and selection of these critical accounting estimates with the audit committee of our Board of Directors and the audit committee has reviewed our disclosure relating to it in this Management’s Discussion and Analysis of Consolidated Results of Operations and Financial Condition.
IMPAIRMENT OF GOODWILL
In accordance with current accounting standards, goodwill has an indefinite life and is not amortized. We evaluate our goodwill for impairment at the reporting unit level on an annual basis, or whenever indicators of impairment exist. We have determined that our business segments represent our reporting units except for Injectables which, based on our review, qualifies as a separate reporting unit for goodwill impairment testing. As of December 31, 2015, we have $310.2 million of goodwill, which is allocated as follows: $33.7 million is allocated to the Pharma reporting unit, $95.6 million is allocated to the Injectables reporting unit, $164.6 million is allocated to the Beauty + Home reporting unit and $16.3 million is allocated to the Food + Beverage reporting unit.
We believe that the accounting estimate related to determining the fair value of our reporting units is a critical accounting estimate because: (1) it is highly susceptible to change from period to period because it requires Company management to make assumptions about the future cash flows for each reporting unit over several years in the future, and (2) the impact that recognizing an impairment would have on the assets reported on our balance sheet as well as our results of operations could be material. Management’s assumptions about future cash flows for the reporting units require significant judgment and actual cash flows in the future may differ significantly from those forecasted today. The estimate for future cash flows and its impact on the impairment testing of goodwill is a critical accounting estimate for all the segments of our business.
For our goodwill impairment assessment we have adopted a standard that provides an entity the option to first assess qualitative factors to determine whether the existence of events or circumstances leads to a determination that it is more likely than not (greater than 50 percent chance) that the fair value of a reporting unit is less than its carrying amount. Such qualitative factors may include the following: macroeconomic conditions; industry and market considerations; cost factors; overall financial performance; and other relevant entity‑specific events. In the absence of sufficient qualitative factors, goodwill impairment is determined utilizing a two‑step process. If it is determined that the fair value of a reporting unit is below its carrying amount, where necessary, goodwill will be impaired at that time.
We performed our annual goodwill impairment assessment as of December 31, 2015 for each of our reporting units. Based on our qualitative assessment of macroeconomic, industry, and market events and circumstances as well as the overall financial performance of the reporting units, we determined it was more likely than not that the fair value of goodwill attributed to these reporting units was greater than its carrying amount. As such, the annual two‑step impairment test was deemed not necessary to be performed for our reporting units for the year ended December 31, 2015.
ALLOWANCE FOR DOUBTFUL ACCOUNTS
We record an allowance for doubtful accounts as an estimate of the inability of our customers to make their required payments. We determine the amount of our allowance for doubtful accounts by looking at a variety of factors. First, we examine an aging report of the accounts receivable in each entity within the Company. The aging report lists past due amounts according to invoice terms. In addition, we consider historical experience with the customers, the current economic environment, the credit rating of the customers and general overall market conditions. In some countries we maintain credit insurance, which can be used in certain cases of non‑payment.
We believe that the accounting estimate related to the allowance for doubtful accounts is a critical accounting estimate because: (1) it requires management to make assumptions about the ability to collect amounts owed from customers in the future, and (2) changes to these assumptions or estimates could have a material impact on our results of operations. The estimate for the allowance for doubtful accounts is a critical accounting estimate for all of our segments.
When we determine that a customer is unlikely to pay, we record a charge to bad debt expense in the income statement and an increase to the allowance for doubtful accounts. When it becomes certain the customer cannot pay (typically driven by the customer filing for bankruptcy) we write off the receivable by removing the accounts receivable amount and reducing the allowance for doubtful accounts accordingly. In 2015, we reduced the allowance for doubtful accounts by approximately $813 thousand and we wrote off doubtful accounts of $728 thousand. Please refer to Schedule II—Valuation and Qualifying Accounts for activity in the allowance for doubtful accounts over the past three years.
We had approximately $391.6 million in net accounts receivable at December 31, 2015. At December 31, 2015, we had approximately $2.7 million recorded in the allowance for doubtful accounts to cover potential future customer non‑payments net of any credit insurance reimbursement we would potentially recover. We believe our allowance for doubtful accounts is adequate to cover future non‑payments of our customers. However, if economic conditions deteriorate significantly or one of our large customers was to declare bankruptcy, a larger allowance for doubtful accounts might be necessary. It is extremely difficult to estimate how much of an additional reserve would be necessary, but we expect the largest potential customer balance at any one time would not exceed $18.2 million. An additional loss of $18.2 million would reduce our Total Assets as of December 31, 2015 by approximately 0.7% and would have reduced Income Before Income Taxes by approximately 6.2%.
If we had been required to recognize an additional $18.2 million in bad debt expense, it would likely not have significantly affected our liquidity and capital resources because, in spite of any such additional expense, we would have been within the terms of our debt covenants.
VALUATION OF PENSION BENEFITS
The benefit obligations and net periodic pension cost associated with our domestic and foreign noncontributory pension plans are determined using actuarial assumptions. Such assumptions include discount rates to reflect the time value of money, rate of employee compensation increases, demographic assumptions to determine the probability and timing of benefit payments, and the long‑term rate of return on plan assets. The actuarial assumptions are based upon management’s best estimates, after consulting with outside investment advisors and actuaries. Because assumptions and estimates are used, actual results could differ from expected results.
The discount rate is utilized principally in calculating our pension obligations, which are represented by the Accumulated Benefit Obligation (ABO) and the Projected Benefit Obligation (PBO), and in calculating net periodic benefit cost. In establishing the discount rate for our foreign plans, we review a number of relevant interest rates including Aa corporate bond yields. In establishing the discount rate for our domestic plans, we match the hypothetical duration of our plans, using a weighted average duration that is based upon projected cash payments, to a simulated bond portfolio (Citigroup Pension Index Curve). At December 31, 2015, the discount rates for our domestic and foreign plans were 4.24% and 2.10%, respectively.
We believe that the accounting estimates related to determining the valuation of pension benefits are critical accounting estimates because: (1) changes in them can materially affect net income, and (2) we are required to establish the discount rate and the expected return on fund assets, which are highly uncertain and require judgment. The estimates for the valuation of pension benefits are critical accounting estimates for all of our segments.
To the extent the discount rates increase (or decrease), our PBO and net periodic benefit cost will decrease (or increase) accordingly. The estimated effect of a 1% decrease in each discount rate would be a $49.3 million increase in the PBO ($35.6 million for the domestic plans and $13.7 million for the foreign plans) and a $7.2 million increase in net periodic benefit cost ($6.1 million for the domestic plans and $1.1 million for the foreign plans). To the extent the PBO increases, the after‑tax effect of such increase could reduce Other Comprehensive Income and Stockholders’ Equity. The estimated effect of a 1% increase in each discount rate would be a $38.2 million decrease in the PBO ($27.3 million for the domestic plans and $10.9 million for the foreign plans) and a $5.6 million decrease in net periodic benefit cost ($4.7 million for the domestic plans and $0.9 million for the foreign plans). A decrease of this magnitude in the PBO would eliminate the current year reduction recognized in Other Comprehensive Income and Stockholders’ Equity as related to pension assumptions.
The assumed expected long‑term rate of return on assets is the average rate of earnings expected on the funds invested to provide for the benefits included in the PBO. Of domestic plan assets, approximately 47% was invested in equities, 30% was invested in fixed income securities, 1% was invested in money market funds, 14% was invested in hedge funds and 8% was invested in infrastructure securities at December 31, 2015. Of foreign plan assets, approximately 89% was invested in investment funds, 3% was invested in corporate securities, 1% was invested in fixed income securities, 4% was invested in equity securities and 3% was invested in cash at December 31, 2015.
The expected long‑term rate of return assumptions are determined based on our investment policy combined with expected risk premiums of equities and fixed income securities over the underlying risk‑free rate. This rate is utilized principally in calculating the expected return on the plan assets component of the net periodic benefit cost. To the extent the actual rate of return on assets realized over the course of a year is greater or less than the assumed rate, that year’s net periodic benefit cost is not affected. Rather, this gain (or loss) reduces (or increases) future net periodic benefit cost over a period of approximately 15 to 20 years. To the extent the expected long‑term rate of return on assets increases (or decreases), our net periodic benefit cost will decrease (or increase) accordingly. The estimated effect of a 1% decrease (or increase) in each expected long‑term rate of return on assets would be a $1.6 million increase (or decrease) in net periodic benefit cost.
The average rate of compensation increase is utilized principally in calculating the PBO and the net periodic benefit cost. The estimated effect of a 0.5% decrease in each rate of expected compensation increase would be a $5.8 million decrease in the PBO ($1.5 million for the domestic plans and $4.3 million for the foreign plans) and a $1.1 million decrease to the net periodic benefit cost. The estimated effect of a 0.5% increase in each rate of expected compensation increase would be a $6.2 million increase in the PBO ($1.6 million for the domestic plans and $4.6 million for the foreign plans) and a $1.2 million increase to the net periodic benefit cost.
Our primary pension related assumptions as of December 31, 2015 and 2014 were as follows:
| | | | | | | |
Actuarial Assumptions as of December 31, | | 2015 | | 2014 | | 2013 | |
| | | | | | | |
Discount rate: | | | | | | | |
Domestic plans | | 4.24 | % | 3.83 | % | 4.75 | % |
Foreign plans | | 2.10 | % | 1.90 | % | 3.24 | % |
Expected long‑term rate of return on plan assets: | | | | | | | |
Domestic plans | | 7.00 | % | 7.00 | % | 7.00 | % |
Foreign plans | | 3.66 | % | 3.54 | % | 3.79 | % |
Rate of compensation increase: | | | | | | | |
Domestic plans | | 4.00 | % | 4.00 | % | 4.00 | % |
Foreign plans | | 3.00 | % | 3.00 | % | 3.00 | % |
In order to determine the 2016 net periodic benefit cost, the Company expects to use the discount rates, expected long‑term rates of return on plan assets and rates of compensation increase assumptions as of December 31, 2015. The estimated impact of the changes to the assumptions as noted in the table above on our 2016 net periodic benefit cost is expected to be a decrease of approximately $2.8 million.
SHARE‑BASED COMPENSATION
The Company uses the Black‑Scholes option‑valuation model to value stock options, which requires the input of subjective assumptions. These assumptions include the length of time employees will retain their vested stock options before exercising them (“expected term”), the estimated volatility of the Company’s stock price, risk‑free interest rate, the expected dividend yield and stock price. The expected term of the options is based on historical experience of similar awards, giving consideration to the contractual terms, vesting schedules and expectations of future employee behavior. The expected term determines the period for which the risk‑free interest rate and volatility must be applied. The risk‑free interest rate is based on the expected U.S. Treasury rate over the expected term. Expected stock price volatility is based on historical volatility of the Company’s stock price. Dividend yield is management’s long‑term estimate of annual dividends to be paid as a percentage of share price.
For 2015, expense related to share‑based compensation for employee stock option plans was $17.9 million. Future changes in the subjective assumptions used in the Black‑Scholes option‑valuation model or estimates associated with forfeitures could impact our share‑based compensation expense. For example, a one year reduction in the expected term of the options would decrease the Black‑Scholes valuation and reduce share‑based compensation by approximately $0.6 million. In comparison, a one year increase in the expected term of the option would increase the Black‑Scholes valuation and increase share‑based compensation by approximately $0.5 million. In addition, changes in the share price at the date of the grant would impact our share‑based compensation expense. For example, a $5 decrease in the stock price would decrease the Black‑Scholes valuation and reduce share‑based compensation by approximately $0.8 million. In comparison, a $5 increase in the share price would increase the Black‑Scholes valuation and increase share‑based compensation by approximately $0.8 million.
OPERATIONS OUTLOOK
We don’t anticipate significant changes in the various macro challenges that we are facing in the first quarter of 2016. The foreign currency exchange environment is expected to continue to be volatile and will impact our reported results. We will remain flexible to adapt to changing market conditions with a continued focus on containing costs while we invest in innovation and new solutions that will help our customers grow their businesses.
AptarGroup expects earnings per share for the first quarter, excluding any impacts from costs associated with the Mega Airless acquisition, to be in the range of $0.69 to $0.74 per share compared to $0.70 per share reported in the prior year. Assuming a comparable foreign currency exchange rate environment, comparable earnings per share for the prior year would have been approximately $0.68 per share.
FORWARD‑LOOKING STATEMENTS
Certain statements in Management’s Discussion and Analysis and other sections of this Form 10‑K are forward‑looking and involve a number of risks and uncertainties, including certain statements set forth in the Liquidity and Capital Resources, Off‑Balance Sheet Arrangements, and Operations Outlook sections of this Form 10‑K. Words such as “expects,” “anticipates,” “believes,” “estimates,” “future” and other similar expressions or future or conditional verbs such as “will,” “should,” “would” and “could” are intended to identify such forward‑looking statements. Forward‑looking statements are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on our beliefs as well as assumptions made by and information currently available to us. Accordingly, our actual results may differ materially from those expressed or implied in such forward‑looking statements due to known or unknown risks and uncertainties that exist in our operations and business environment, including but not limited to:
| · | | the ability to complete the Mega Airless acquisition; |
| · | | economic conditions worldwide, including potential deflationary conditions in regions we rely on for growth; |
| · | | political conditions worldwide, including current conflicts in Eastern Europe; |
| · | | significant fluctuations in foreign currency exchange rates; |
| · | | changes in customer and/or consumer spending levels; |
| · | | financial conditions of customers and suppliers; |
| · | | consolidations within our customer or supplier base; |
| · | | fluctuations in the cost of raw materials, components and other input costs (particularly resin, metal, anodization costs and transportation and energy costs); |
| · | | the availability of raw materials and components (particularly from sole sourced suppliers) as well as the financial viability of these suppliers; |
| · | | our ability to increase prices, contain costs and improve productivity; |
| · | | our ability to successfully implement facility expansions and new facility projects; |
| · | | our ability to increase prices, contain costs and improve productivity; |
| · | | changes in capital availability or cost, including interest rate fluctuations; |
| · | | volatility of global credit markets; |
| · | | the timing and magnitude of capital expenditures; |
| · | | our ability to identify potential new acquisitions and to successfully acquire and integrate such operations or products; |
| · | | cybersecurity threats that could impact our networks and reporting systems; |
| · | | the impact of natural disasters and other weather‑related occurrences; |
| · | | fiscal and monetary policies and other regulations, including changes in worldwide tax rates; |
| · | | direct or indirect consequences of acts of war or terrorism; |
| · | | changes or difficulties in complying with government regulation; |
| · | | changing regulations or market conditions regarding environmental sustainability; |
| · | | work stoppages due to labor disputes; |
| · | | competition, including technological advances; |
| · | | our ability to protect and defend our intellectual property rights, as well as litigation involving intellectual property rights; |
| · | | the outcome of any legal proceeding that has been or may be instituted against us and others; |
| · | | our ability to meet future cash flow estimates to support our goodwill impairment testing; |
| · | | the demand for existing and new products; |
| · | | our ability to manage worldwide customer launches of complex technical products, in particular in developing markets; |
| · | | the success of our customers’ products, particularly in the pharmaceutical industry; |
| · | | difficulties in product development and uncertainties related to the timing or outcome of product development; |
| · | | significant product liability claims; and |
| · | | other risks associated with our operations. |
Although we believe that our forward‑looking statements are based on reasonable assumptions, there can be no assurance that actual results, performance or achievements will not differ materially from any future results, performance or achievements expressed or implied by such forward‑looking statements. Readers are cautioned not to place undue reliance on forward‑looking statements. We undertake no obligation to update publicly any forward‑looking statements, whether as a result of new information, future events or otherwise, except as required by law. Please refer to Item 1A (“Risk Factors”) of Part I included in this Form 10‑K for additional risk factors affecting the Company.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
MARKET RISKS
A majority of our operations are located outside of the United States. Because of this, movements in exchange rates may have a significant impact on the translation of the financial condition and results of operations of our subsidiaries. Our primary foreign exchange exposure is to the Euro, but we also have foreign exchange exposure to the Chinese Yuan, Brazilian Real, Mexican Peso, Swiss Franc and other Asian, European and South American currencies. A strengthening U.S. dollar relative to foreign currencies has a dilutive translation effect on our financial condition and results of operations. Conversely, a weakening U.S. dollar has an additive effect.
Additionally, in some cases, we sell products denominated in a currency different from the currency in which the related costs are incurred. Any changes in exchange rates on such inter‑country sales may impact our results of operations.
We manage our exposures to foreign exchange principally with forward exchange contracts to hedge certain firm purchase and sales commitments and intercompany cash transactions denominated in foreign currencies.
The table below provides information, as of December 31, 2015, about our forward currency exchange contracts. The majority of the contracts expire before the end of the first quarter of 2016.
| | | | | | | | |
In thousands | | | | | | | | |
Year Ended December 31, 2015 | | | | | Average | | Min / Max | |
| | | | | Contractual | | Notional | |
Buy/Sell | | | Contract Amount | | Exchange Rate | | Volumes | |
| | | | | | | | |
Euro/Brazilian Real | | $ | 19,895 | | 4.1571 | | 14,721-23,710 | |
Euro/Indian Rupee | | | 10,324 | | 76.1946 | | 9,208-11,156 | |
Euro/U.S. Dollar | | | 9,643 | | 1.0903 | | 8,190-17,777 | |
Brazilian Real/Euro | | | 6,610 | | 0.2353 | | 0-6,610 | |
Euro/Colombian Peso | | | 5,504 | | 3,163.0388 | | 5,504-6,386 | |
U.S. Dollar/Brazilian Real | | | 4,420 | | 4.0169 | | 1,430-4,650 | |
U.S. Dollar/Chinese Yuan | | | 4,390 | | 6.4822 | | 470-6,810 | |
U.S. Dollar/Euro | | | 3,650 | | 0.9207 | | 914-5,586 | |
Swiss Franc/Euro | | | 3,586 | | 0.9258 | | 3,586-73,521 | |
Czech Koruna/Euro | | | 2,932 | | 0.0371 | | 2,526-8,739 | |
British Pound/Euro | | | 2,721 | | 1.3902 | | 2,180-10,425 | |
Euro/Mexican Peso | | | 2,441 | | 19.8143 | | 2,441-6,547 | |
Euro/Thai Baht | | | 2,034 | | 37.7826 | | 1,680-2,093 | |
Euro/Indonesian Rupiah | | | 1,762 | | 18,425.0000 | | 1,762-1,918 | |
Other | | | 1,609 | | | | | |
Total | | $ | 81,521 | | | | | |
As of December 31, 2015, the Company has recorded the fair value of foreign currency forward exchange contracts of $1.9 million in prepayments and other, $0.1 million in miscellaneous other assets, $1.2 million in accounts payable and accrued liabilities and $45 thousand in deferred and other non-current liabilities in the Consolidated Balance Sheets.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
AptarGroup, Inc.
CONSOLIDATED STATEMENTS OF INCOME
| | | | | | | | | | |
In thousands, except per share amounts | | | | | | | | | | |
| | | | | | | | | | |
Years Ended December 31, | | 2015 | | 2014 | | 2013 | |
Net Sales | | $ | 2,317,149 | | $ | 2,597,809 | | $ | 2,520,013 | |
Operating Expenses: | | | | | | | | | | |
Cost of sales (exclusive of depreciation and amortization shown below) | | | 1,502,650 | | | 1,755,266 | | | 1,708,936 | |
Selling, research & development and administrative | | | 351,461 | | | 383,909 | | | 364,747 | |
Depreciation and amortization | | | 138,893 | | | 152,218 | | | 149,956 | |
Restructuring initiatives | | | — | | | — | | | 11,800 | |
| | | 1,993,004 | | | 2,291,393 | | | 2,235,439 | |
Operating Income | | | 324,145 | | | 306,416 | | | 284,574 | |
Other (Expense) Income: | | | | | | | | | | |
Interest expense | | | (34,615) | | | (21,029) | | | (20,514) | |
Interest income | | | 5,596 | | | 4,797 | | | 3,233 | |
Equity in results of affiliates | | | (718) | | | (1,917) | | | (883) | |
Miscellaneous, net | | | 163 | | | (1,966) | | | (2,027) | |
| | | (29,574) | | | (20,115) | | | (20,191) | |
Income before Income Taxes | | | 294,571 | | | 286,301 | | | 264,383 | |
Provision for Income Taxes | | | 95,276 | | | 94,677 | | | 92,457 | |
Net Income | | $ | 199,295 | | $ | 191,624 | | $ | 171,926 | |
Net Loss Attributable to Noncontrolling Interests | | | 53 | | | 34 | | | 68 | |
Net Income Attributable to AptarGroup, Inc. | | $ | 199,348 | | $ | 191,658 | | $ | 171,994 | |
Net Income Attributable to AptarGroup, Inc. per Common Share: | | | | | | | | | | |
Basic | | $ | 3.19 | | $ | 2.95 | | $ | 2.60 | |
Diluted | | $ | 3.09 | | $ | 2.85 | | $ | 2.52 | |
See accompanying notes to consolidated financial statements.
AptarGroup, Inc.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
| | | | | | | | | | |
In thousands | | | | | | | | | | |
| | | | | | | | | | |
Years Ended December 31, | | 2015 | | 2014 | | 2013 | |
Net Income | | $ | 199,295 | | $ | 191,624 | | $ | 171,926 | |
Other Comprehensive (Loss) Income: | | | | | | | | | | |
Foreign currency translation adjustments | | | (163,887) | | | (192,824) | | | 29,879 | |
Changes in treasury locks, net of tax | | | 25 | | | 24 | | | 45 | |
Net loss on derivatives, net of tax | | | — | | | — | | | — | |
Defined benefit pension plan, net of tax | | | | | | | | | | |
Actuarial gain / (loss), net of tax | | | 7,253 | | | (29,842) | | | 14,791 | |
Prior service cost, net of tax | | | (538) | | | — | | | — | |
Amortization of prior service cost included in net income, net of tax | | | 168 | | | 206 | | | 234 | |
Amortization of net loss included in net income, net of tax | | | 4,664 | | | 2,632 | | | 4,130 | |
Total defined benefit pension plan, net of tax | | | 11,547 | | | (27,004) | | | 19,155 | |
Total other comprehensive (loss) income | | | (152,315) | | | (219,804) | | | 49,079 | |
Comprehensive Income (Loss) | | | 46,980 | | | (28,180) | | | 221,005 | |
Comprehensive Loss Attributable to Noncontrolling Interests | | | 66 | | | 42 | | | 57 | |
Comprehensive Income (Loss) Attributable to AptarGroup, Inc. | | $ | 47,046 | | $ | (28,138) | | $ | 221,062 | |
See accompanying notes to consolidated financial statements.
AptarGroup, Inc.
CONSOLIDATED BALANCE SHEETS
| | | | | | | |
In thousands | | | | | | | |
| | | | | | | |
December 31, | | 2015 | | 2014 | |
Assets | | | | | | | |
Current Assets: | | | | | | | |
Cash and equivalents | | $ | 489,901 | | $ | 399,762 | |
Short-term investments | | | 29,816 | | | — | |
| | | 519,717 | | | 399,762 | |
Accounts and notes receivable, less allowance for doubtful accounts of $2,710 in 2015 and $4,251 in 2014 | | | 391,571 | | | 406,976 | |
Inventories | | | 294,912 | | | 311,072 | |
Prepayments and other | | | 88,794 | | | 96,128 | |
| | | 1,294,994 | | | 1,213,938 | |
Property, Plant and Equipment: | | | | | | | |
Buildings and improvements | | | 343,698 | | | 353,683 | |
Machinery and equipment | | | 1,866,627 | | | 1,919,507 | |
| | | 2,210,325 | | | 2,273,190 | |
Less: Accumulated depreciation | | | (1,465,873) | | | (1,484,546) | |
| | | 744,452 | | | 788,644 | |
Land | | | 20,931 | | | 23,011 | |
| | | 765,383 | | | 811,655 | |
Other Assets: | | | | | | | |
Investments in affiliates | | | 4,590 | | | 5,760 | |
Goodwill | | | 310,240 | | | 329,741 | |
Intangible assets | | | 33,210 | | | 40,045 | |
Miscellaneous | | | 30,309 | | | 36,051 | |
| | | 378,349 | | | 411,597 | |
Total Assets | | $ | 2,438,726 | | $ | 2,437,190 | |
See accompanying notes to consolidated financial statements.
AptarGroup, Inc.
CONSOLIDATED BALANCE SHEETS
| | | | | | | |
In thousands, except share and per share amounts | | | | | | | |
| | | | | | | |
December 31, | | 2015 | | 2014 | |
Liabilities and Stockholders’ Equity | | | | | | | |
Current Liabilities: | | | | | | | |
Notes payable | | $ | 5,083 | | $ | 233,284 | |
Current maturities of long-term obligations | | | 51,889 | | | 18,692 | |
Accounts payable and accrued liabilities | | | 354,928 | | | 352,762 | |
| | | 411,900 | | | 604,738 | |
Long-Term Obligations | | | 762,524 | | | 588,892 | |
Deferred Liabilities and Other: | | | | | | | |
Deferred income taxes | | | 20,486 | | | 25,521 | |
Retirement and deferred compensation plans | | | 87,763 | | | 109,517 | |
Deferred and other non-current liabilities | | | 6,347 | | | 4,606 | |
Commitments and contingencies | | | — | | | — | |
| | | 114,596 | | | 139,644 | |
Stockholders’ Equity: | | | | | | | |
AptarGroup, Inc. stockholders’ equity | | | | | | | |
Common stock, $.01 par value, 199 million shares authorized, and 66.7 and 86.3 million shares issued at 2015 and 2014, respectively | | | 667 | | | 862 | |
Capital in excess of par value | | | 495,462 | | | 498,702 | |
Retained earnings | | | 1,185,681 | | | 1,740,005 | |
Accumulated other comprehensive (loss) | | | (262,347) | | | (110,045) | |
Less: Treasury stock at cost, 4.2 million and 24.3 million shares in 2015 and 2014, respectively | | | (270,052) | | | (1,026,117) | |
Total AptarGroup, Inc. Stockholders’ Equity | | | 1,149,411 | | | 1,103,407 | |
Noncontrolling interests in subsidiaries | | | 295 | | | 509 | |
Total Stockholders’ Equity | | | 1,149,706 | | | 1,103,916 | |
Total Liabilities and Stockholders’ Equity | | $ | 2,438,726 | | $ | 2,437,190 | |
See accompanying notes to consolidated financial statements.
AptarGroup, Inc.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | |
In thousands, brackets denote cash outflows | | | | | | | | | | |
| | | | | | | | | | |
Years Ended December 31, | | 2015 | | 2014 | | 2013 | |
Cash Flows from Operating Activities: | | | | | | | | | | |
Net income | | $ | 199,295 | | $ | 191,624 | | $ | 171,926 | |
Adjustments to reconcile net income to net cash provided by operations: | | | | | | | | | | |
Depreciation | | | 134,647 | | | 146,893 | | | 144,923 | |
Amortization | | | 4,246 | | | 5,325 | | | 5,033 | |
Stock based compensation | | | 20,612 | | | 19,749 | | | 14,392 | |
(Recovery of) provision for doubtful accounts | | | (813) | | | 741 | | | (385) | |
Deferred income taxes | | | (7,141) | | | (18,973) | | | 6,844 | |
Defined benefit plan expense | | | 20,685 | | | 16,699 | | | 19,408 | |
Equity in results of affiliates in excess of cash distributions received | | | 718 | | | 1,917 | | | 883 | |
Changes in balance sheet items, excluding effects from foreign currency adjustments: | | | | | | | | | | |
Accounts and other receivables | | | (27,759) | | | (16,322) | | | (32,806) | |
Inventories | | | (18,925) | | | 5,205 | | | (29,918) | |
Prepaid and other current assets | | | (6,982) | | | (6,496) | | | (6,394) | |
Accounts payable and accrued liabilities | | | 39,330 | | | (24,319) | | | 1,144 | |
Income taxes payable | | | 3,397 | | | (10,949) | | | 16,712 | |
Retirement and deferred compensation plan liabilities | | | (29,576) | | | (370) | | | (19,441) | |
Other changes, net | | | (7,219) | | | 5,605 | | | (6,221) | |
Net cash provided by operations | | | 324,515 | | | 316,329 | | | 286,100 | |
Cash Flows from Investing Activities: | | | | | | | | | | |
Capital expenditures | | | (149,323) | | | (161,940) | | | (151,510) | |
Proceeds from sale of property and equipment | | | 827 | | | 5,106 | | | 436 | |
Insurance proceeds on property claim | | | 3,739 | | | — | | | — | |
Purchase of short-term investments | | | (32,769) | | | — | | | — | |
Intangible assets | | | — | | | (9) | | | (725) | |
Investment in unconsolidated affiliate | | | — | | | — | | | (5,256) | |
Notes receivable, net | | | 1,296 | | | (2,357) | | | (65) | |
Net cash used by investing activities | | | (176,230) | | | (159,200) | | | (157,120) | |
Cash Flows from Financing Activities: | | | | | | | | | | |
Proceeds from notes payable | | | — | | | 95,816 | | | 94,184 | |
Repayments of notes payable | | | (227,362) | | | — | | | — | |
Proceeds from long-term obligations | | | 209,236 | | | 253,520 | | | 2,994 | |
Repayments of long-term obligations | | | (981) | | | (778) | | | (28,320) | |
Dividends paid | | | (71,247) | | | (71,072) | | | (66,133) | |
Credit facility costs | | | (1,216) | | | (720) | | | (498) | |
Proceeds from stock option exercises | | | 64,003 | | | 36,015 | | | 42,684 | |
Purchase of treasury stock | | | — | | | (340,517) | | | (118,813) | |
Common stock repurchased and retired | | | (13,887) | | | — | | | — | |
Excess tax benefit from exercise of stock options | | | 8,388 | | | 6,998 | | | 6,104 | |
Net cash used by financing activities | | | (33,066) | | | (20,738) | | | (67,798) | |
Effect of Exchange Rate Changes on Cash | | | (25,080) | | | (46,490) | | | 18,924 | |
Net increase in Cash and Equivalents | | | 90,139 | | | 89,901 | | | 80,106 | |
Cash and Equivalents at Beginning of Period | | | 399,762 | | | 309,861 | | | 229,755 | |
Cash and Equivalents at End of Period | | $ | 489,901 | | $ | 399,762 | | $ | 309,861 | |
Supplemental Cash Flow Disclosure: | | | | | | | | | | |
Interest paid | | $ | 31,664 | | $ | 20,352 | | $ | 20,679 | |
Income taxes paid | | | 79,502 | | | 94,578 | | | 47,445 | |
See accompanying notes to consolidated financial statements.
AptarGroup, Inc.
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
Years Ended December 31, 2015, 2014 and 2013
| | | | | | | | | | | | | | | | | | | | | |
In thousands | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | AptarGroup, Inc. Stockholders’ Equity | | | | | | | | | |
| | | | | Accumulated | | | | | | | | | | | | | | | |
| | | | | Other | | Common | | | | | Capital in | | Non- | | | |
| | Retained | | Comprehensive | | Stock | | Treasury | | Excess of | | Controlling | | Total |
| | Earnings | | (Loss) Income | | Par Value | | Stock | | Par Value | | Interest | | Equity |
Balance - December 31, 2012 | | $ | 1,513,558 | | $ | 60,683 | | $ | 840 | | $ | (622,075) | | $ | 426,884 | | $ | 608 | | $ | 1,380,498 |
Net income | | | 171,994 | | | | | | | | | | | | | | | (68) | | | 171,926 |
Foreign currency translation adjustments | | | | | | 29,868 | | | | | | | | | | | | 11 | | | 29,879 |
Changes in unrecognized pension gains/losses and related amortization, net of tax | | | | | | 19,155 | | | | | | | | | | | | | | | 19,155 |
Changes in treasury locks, net of tax | | | | | | 45 | | | | | | | | | | | | | | | 45 |
Stock option exercises & restricted stock vestings | | | | | | | | | 13 | | | 2,330 | | | 61,408 | | | | | | 63,751 |
Cash dividends declared on common stock | | | (66,133) | | | | | | | | | | | | | | | | | | (66,133) |
Treasury stock purchased | | | | | | | | | | | | (118,813) | | | | | | | | | (118,813) |
Balance - December 31, 2013 | | $ | 1,619,419 | | $ | 109,751 | | $ | 853 | | $ | (738,558) | | $ | 488,292 | | $ | 551 | | $ | 1,480,308 |
Net income | | | 191,658 | | | | | | | | | | | | | | | (34) | | | 191,624 |
Foreign currency translation adjustments | | | | | | (192,816) | | | | | | | | | | | | (8) | | | (192,824) |
Changes in unrecognized pension gains/losses and related amortization, net of tax | | | | | | (27,004) | | | | | | | | | | | | | | | (27,004) |
Changes in treasury locks, net of tax | | | | | | 24 | | | | | | | | | | | | | | | 24 |
Stock option exercises & restricted stock vestings | | | | | | | | | 9 | | | 2,958 | | | 60,410 | | | | | | 63,377 |
Cash dividends declared on common stock | | | (71,072) | | | | | | | | | | | | | | | | | | (71,072) |
Treasury stock purchased | | | | | | | | | | | | (290,517) | | | (50,000) | | | | | | (340,517) |
Balance - December 31, 2014 | | $ | 1,740,005 | | $ | (110,045) | | $ | 862 | | $ | (1,026,117) | | $ | 498,702 | | $ | 509 | | $ | 1,103,916 |
Net income | | | 199,348 | | | | | | | | | | | | | | | (53) | | | 199,295 |
Foreign currency translation adjustments | | | | | | (163,874) | | | | | | | | | | | | (13) | | | (163,887) |
Changes in unrecognized pension gains/losses and related amortization, net of tax | | | | | | 11,547 | | | | | | | | | | | | | | | 11,547 |
Changes in treasury locks, net of tax | | | | | | 25 | | | | | | | | | | | | | | | 25 |
Stock option exercises & restricted stock vestings | | | | | | | | | 14 | | | 5,447 | | | 79,107 | | | | | | 84,568 |
Cash dividends declared on common stock | | | (71,247) | | | | | | | | | | | | | | | | | | (71,247) |
Treasury stock purchased | | | | | | | | | | | | (50,000) | | | 50,000 | | | | | | — |
Treasury stock retired | | | (669,801) | | | | | | (207) | | | 800,618 | | | (130,610) | | | | | | — |
Common stock repurchased and retired | | | (12,624) | | | | | | (2) | | | | | | (1,261) | | | | | | (13,887) |
Non controlling interest repurchased | | | | | | | | | | | | | | | (476) | | | (148) | | | (624) |
Balance - December 31, 2015 | | $ | 1,185,681 | | $ | (262,347) | | $ | 667 | | $ | (270,052) | | $ | 495,462 | | $ | 295 | | $ | 1,149,706 |
See accompanying notes to consolidated financial statements.
AptarGroup, Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in Thousands, Except Share and per Share Amounts, or as Otherwise Indicated)
NOTE 1 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION
The accompanying consolidated financial statements include the accounts of AptarGroup, Inc. and our subsidiaries. The terms “AptarGroup” or “Company” as used herein refer to AptarGroup, Inc. and our subsidiaries. All significant intercompany accounts and transactions have been eliminated. Certain previously reported amounts have been reclassified to conform to the current period presentation.
AptarGroup’s organizational structure consists of three market‑focused lines of business which are Beauty + Home, Pharma and Food + Beverage. This is a strategic structure which allows us to be more closely aligned with our customers and the markets in which they operate.
On November 1, 2012, the Company initiated our European restructuring plan (see Note 20 Restructuring Initiatives for further details). During 2013, the Company recognized approximately $14.6 million of expense related to the plan, of which $2.7 million was accelerated depreciation. For presentation purposes, the accelerated depreciation related to this plan is reported in Depreciation and Amortization within the Consolidated Statements of Income.
CHANGE IN ACCOUNTING PRINCIPLE
During the second quarter of 2015, the Company changed its inventory valuation method for certain operating entities in its North American business to the first-in first-out (FIFO) method from the last-in first-out (LIFO) method. Prior to the change, the Company utilized two methods of inventory costing: LIFO for inventories in these operating entities and FIFO for inventories in other operating entities. The Company believes that the FIFO method is preferable as it better reflects the current value of inventory on the Company's Consolidated Balance Sheet, provides better matching of revenues and expenses, results in uniformity across the Company's global operations with respect to the method of inventory accounting and improves comparability with the Company's peers. The cumulative pre-tax effect of this change is a gain of approximately $7.4 million and was recognized as a decrease to Cost of sales (exclusive of depreciation and amortization). The effect of the change on Net Income Attributable to AptarGroup was approximately $4.8 million, representing approximately $0.08 per diluted share. We have determined that this change is not material to the Company's previously issued financial statements and that the cumulative effect of the change is not material to current operations or to the trend of reported results of operations. Therefore, we conclude it was appropriate to recognize the cumulative effect of the change as an operating item in the current period's Consolidated Statement of Income and not to adopt the change by retrospective application.
ACCOUNTING ESTIMATES
The financial statements are prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). This process requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
CASH MANAGEMENT
The Company considers all investments which are readily convertible to known amounts of cash with an original maturity of three months or less when purchased to be cash equivalents. Short-term investments reflect funds invested in a time deposit instrument with a two-year maturity. However, during the life of the investment the funds can be redeemed at any time with a 35-90 day notice. There are no penalties for early redemption. We do not consider this investment a marketable security as there is no active market for this type of product.
INVENTORIES
Inventories are stated at lower of cost or market. Costs included in inventories are raw materials, direct labor and manufacturing overhead. As discussed above, the Company changed its inventory valuation method for certain operating entities in its North American business to the FIFO method from the LIFO method during the second quarter of 2015 resulting in all entities utilizing the FIFO method at the end of 2015.
INVESTMENTS IN AFFILIATED COMPANIES
The Company accounts for its investments in 20% to 50% owned affiliated companies using the equity method. There were no dividends received from affiliated companies in 2015, 2014 and 2013. The Company accounts for its investments less than 20% on the cost method.
PROPERTY AND DEPRECIATION
Properties are stated at cost. Depreciation is determined on a straight‑line basis over the estimated useful lives for financial reporting purposes and accelerated methods for income tax reporting. Generally, the estimated useful lives are 25 to 40 years for buildings and improvements and 3 to 10 years for machinery and equipment.
FINITE‑LIVED INTANGIBLE ASSETS
Finite‑lived intangibles, consisting of patents, non‑compete agreements and license agreements acquired in purchase transactions, are capitalized and amortized over their useful lives which range from 3 to 20 years.
GOODWILL
Management believes the excess purchase price over the fair value of the net assets acquired (“Goodwill”) in purchase transactions has continuing value. Goodwill is not amortized and must be tested annually, or more frequently as circumstances dictate, for impairment. The annual goodwill impairment test may first consider qualitative factors to determine whether it is more likely than not (i.e. greater than 50 percent chance) that the fair value of a reporting unit is less than its book value. This is sometimes referred to as the “step zero” approach and is an optional step in the annual goodwill impairment analysis. Management has performed this qualitative assessment as of December 31, 2015 for all four of our reporting units. Based on our review of macroeconomic, industry, and market events and circumstances as well as the overall financial performance of the reporting units, we determined it was more likely than not that the fair value of goodwill attributed to all four of our reporting units was greater than its carrying amount. Therefore, no impairment of goodwill has been recorded.
IMPAIRMENT OF LONG‑LIVED ASSETS
Long‑lived assets, such as property, plant and equipment and finite‑lived intangibles, are evaluated for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. An impairment loss is recognized when estimated undiscounted future cash flows expected to result from the use of the asset plus net proceeds expected from disposition of the asset (if any) are less than the carrying value of the asset. During 2013, we recognized a $1.5 million adjustment for accelerated depreciation on certain corporate assets to reduce the carrying amount to the fair value.
DERIVATIVES INSTRUMENTS AND HEDGING ACTIVITIES
Derivative financial instruments are recorded in the consolidated balance sheets at fair value as either assets or liabilities. Changes in the fair value of derivatives are recorded in each period in earnings or accumulated other comprehensive income, depending on whether a derivative is designated and effective as part of a hedge transaction.
RETIREMENT OF TREASURY SHARES
During the third quarter of 2015, the Company retired 20 million shares of treasury stock. Common stock was reduced by the number of shares retired at $0.01 par value while treasury stock was reduced by the purchase price of the shares retired. The excess of purchase price over par or stated value may either be charged entirely to retained earnings or allocated between additional paid-in capital and retained earnings. The Company has elected to allocate the excess purchase price over par value between additional paid-in capital and retained earnings.
RESEARCH & DEVELOPMENT EXPENSES
Research and development costs, net of any customer funded research and development or government research and development credits, are expensed as incurred. These costs amounted to $67.1 million, $76.2 million and $71.8 million in 2015, 2014 and 2013, respectively.
INCOME TAXES
The Company computes taxes on income in accordance with the tax rules and regulations of the many taxing authorities where the income is earned. The income tax rates imposed by these taxing authorities may vary substantially. Taxable income may differ from pretax income for financial accounting purposes. To the extent that these differences create timing differences between the tax basis of an asset or liability and its reported amount in the financial statements, an appropriate provision for deferred income taxes is made.
In its determination of which foreign earnings are permanently reinvested in foreign operations, the Company considers numerous factors, including the financial requirements of the U.S. parent company and those of its foreign subsidiaries, the U.S. funding needs for dividend payments and stock repurchases, and the tax consequences of remitting earnings to the U.S. From this analysis, current year repatriation decisions are made in an attempt to provide a proper mix of debt and shareholder capital both within the U.S. and for non‑U.S. operations. The Company’s policy is to permanently reinvest its accumulated foreign earnings and will only make a distribution to the U.S. out of current year earnings in order to meet the cash needs at the parent company. As such, the Company does not provide taxes on earnings that are deemed to be permanently reinvested.
The Company provides a liability for the amount of tax benefits realized from uncertain tax positions. This liability is provided whenever the Company determines that a tax benefit will not meet a more-likely-than-not threshold for recognition. See Note 5 for more information.
TRANSLATION OF FOREIGN CURRENCIES
The functional currencies of the majority of the Company's foreign operations are the local currencies. Assets and liabilities of the Company’s foreign operations are translated into U.S. dollars at the rates of exchange on the balance sheet date. Sales and expenses are translated at the average rates of exchange prevailing during the year. The related translation adjustments are accumulated in a separate section of Stockholders’ Equity. Realized and unrealized foreign currency transaction gains and losses are reflected in income, as a component of miscellaneous income and expense, and represented a loss of $3.9 million in 2015, a loss of $37 thousand in 2014 and a loss of $6.3 million in 2013.
STOCK BASED COMPENSATION
Accounting standards require the application of the non‑substantive vesting approach which means that an award is fully vested when the employee’s retention of the award is no longer contingent on providing subsequent service. Under this approach, compensation costs are recognized over the requisite service period of the award instead of ratably over the vesting period stated in the grant. As such, costs are recognized immediately if the employee is retirement eligible on the date of grant or over the period from the date of grant until retirement eligibility if retirement eligibility is reached before the end of the vesting period stated in the grant. See Note 15 for more information.
REVENUE RECOGNITION
Product Sales. The Company’s policy is to recognize revenue from product sales when price is fixed and determinable, when the title and risk of loss has transferred to the customer, when the Company has no remaining obligations regarding the transaction and when collection is reasonably assured. The majority of the Company’s products shipped from the U.S. transfers title and risk of loss when the goods leave the Company’s shipping location. The majority of the Company’s products shipped from non‑U.S. operations transfer title and risk of loss when the goods reach their destination. Tooling revenue is also recognized when the title and risk of loss transfers to the customer.
Services and Other. The Company occasionally invoices customers for certain services. The Company also receives revenue from other sources such as license or royalty agreements. Revenue is recognized when services are rendered or rights to use assets can be reliably measured and when collection is reasonably assured. License and royalty revenue is typically recognized when the contractual terms of each agreement are met. Service and other revenue is not material to the Company’s results of operations for any of the years presented.
ADOPTION OF RECENT ACCOUNTING PRONOUNCEMENTS
Changes to GAAP are established by the Financial Accounting Standards Board (“FASB”) in the form of accounting standards updates to the FASB’s Accounting Standards Codification.
In May 2014, the FASB amended the guidance for recognition of revenue from customer contracts. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in the amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In August 2015, the FASB decided to defer the effective date by one year to December 15, 2017 for annual reporting periods beginning after that date. The FASB also decided to allow early adoption of the standard, but not before the original effective date of December 15, 2016. We are currently evaluating the impact the adoption of this standard will have on our consolidated financial statements.
In July 2013, the FASB issued authoritative guidance on the presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists. This standard requires an entity to present an unrecognized tax benefit, or a portion of an unrecognized tax benefit, in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, a similar tax loss, or a tax credit carryforward. The guidance is effective for the Company’s fiscal years beginning after December 15, 2013. This standard did not impact our current year financial statements as this was already the Company’s existing reporting treatment.
In March 2013, the FASB issued authoritative guidance which permits an entity to release cumulative translation adjustments into net income when a reporting entity (parent) ceases to have a controlling financial interest in a subsidiary or group of assets that is a business within a foreign entity. Accordingly, the cumulative translation adjustment should be released into net income only if the sale or transfer results in the complete or substantially complete liquidation of the foreign entity in which the subsidiary or group of assets had resided, or if a controlling financial interest is no longer held. The guidance is effective for the Company’s fiscal years beginning after December 15, 2013. This standard did not have a material impact on our current year financial statements.
In February 2013, the FASB issued authoritative guidance that amends the presentation of accumulated other comprehensive income and clarifies how to report the effect of significant reclassifications out of accumulated other comprehensive income. The guidance requires footnote disclosures regarding the changes in accumulated other comprehensive income by component and the line items affected in the statements of earnings. The adoption of this standard had no impact on the Consolidated Financial Statements other than disclosure. Additional information can be found in Note 9 of the Notes to the Consolidated Financial Statements.
In January 2013, the FASB issued authoritative guidance requiring new asset and liability offsetting disclosures for derivatives, repurchase agreements and security lending transactions to the extent that they are offset in the financial statements or are subject to an enforceable master netting arrangement or similar agreement. We do not have any repurchase agreements and do not participate in securities lending transactions. Our derivative instruments are not offset in the financial statements. Accordingly, the adoption of this standard had no impact on the Consolidated Financial Statements other than disclosure. Additional information can be found in Note 10 of the Notes to the Consolidated Financial Statements.
Other accounting standards that have been issued by the FASB or other standards-setting bodies did not have a material impact on our Consolidated Financial Statements.
REVISION OF PRIOR PERIOD FINANCIAL STATEMENTS
During the third quarter of 2015, the Company determined that it had incorrectly accounted for the reissuance of treasury shares in connection with certain employee stock option exercises. The Company's policy is to reissue treasury shares at cost on a first-in, first-out (FIFO) basis. However, beginning in 2007 shares were reissued at a cost other than FIFO. The effect of correcting this error results in a credit adjustment to the treasury stock at cost with a corresponding debit adjustment to the capital in excess of par value. As this adjustment represents a reclassification between two accounts within Stockholders' Equity, the Condensed Consolidated Balance Sheets and the Condensed Consolidated Statements of Changes in Equity are impacted by this change. The revisions, which the Company determined are not material, had no impact on consolidated results of operations or cash flows. Following is a summary of the previously issued financial statement line items impacted by this revision for all periods and statements included in this report:
| | | | | | | | | | |
| | Year Ended December 31, 2014 | |
| | As Previously | | | | | | | |
| | Reported | | Adjustment | | As Revised | |
Revised Condensed Consolidated Balance Sheets | | | | | | | | | | |
Year Ended December 31, 2014 | | | | | | | | | | |
Capital in excess of par value | | $ | 507,313 | | $ | (8,611) | | $ | 498,702 | |
Less treasury stock at cost | | | (1,034,728) | | | 8,611 | | | (1,026,117) | |
Total Stockholders’ Equity | | | 1,103,916 | | | — | | | 1,103,916 | |
| | | | | | | | | | |
| | As Previously | | | | | | | |
| | Reported | | Adjustment | | As Revised | |
Revised Condensed Consolidated Statements of Changes in Equity | | | | | | | | | | |
Balance – December 31, 2012 | | | | | | | | | | |
Capital in excess of par value | | $ | 430,210 | | $ | (3,326) | | $ | 426,884 | |
Treasury Stock | | | (625,401) | | | 3,326 | | | (622,075) | |
Total Equity | | | 1,380,498 | | | — | | | 1,380,498 | |
Stock option exercises & restricted stock vestings | | | | | | | | | | |
Capital in excess of par value | | $ | 63,737 | | $ | (2,329) | | $ | 61,408 | |
Treasury Stock | | | 1 | | | 2,329 | | | 2,330 | |
Total Equity | | | 63,751 | | | — | | | 63,751 | |
Balance – December 31, 2013 | | | | | | | | | | |
Capital in excess of par value | | $ | 493,947 | | $ | (5,655) | | $ | 488,292 | |
Treasury Stock | | | (744,213) | | | 5,655 | | | (738,558) | |
Total Equity | | | 1,480,308 | | | — | | | 1,480,308 | |
Stock option exercises & restricted stock vestings | | | | | | | | | | |
Capital in excess of par value | | $ | 63,366 | | $ | (2,956) | | $ | 60,410 | |
Treasury Stock | | | 2 | | | 2,956 | | | 2,958 | |
Total Equity | | | 63,377 | | | — | | | 63,377 | |
Balance – December 31, 2014 | | | | | | | | | | |
Capital in excess of par value | | $ | 507,313 | | $ | (8,611) | | $ | 498,702 | |
Treasury Stock | | | (1,034,728) | | | 8,611 | | | (1,026,117) | |
Total Equity | | | 1,103,916 | | | — | | | 1,103,916 | |
NOTE 2 INVENTORIES
Inventories, by component, consisted of:
| | | | | | | |
| | 2015 | | 2014 | |
Raw materials | | $ | 91,214 | | $ | 108,618 | |
Work in process | | | 90,625 | | | 94,414 | |
Finished goods | | | 113,073 | | | 115,809 | |
Total | | | 294,912 | | | 318,841 | |
Less LIFO reserve | | | — | | | (7,769) | |
Total | | $ | 294,912 | | $ | 311,072 | |
At December 31, 2014, approximately 19% of the total inventories were accounted for by the LIFO method. As discussed in Note 1 above, the Company changed its inventory valuation method for certain operating entities in its North American business to the first-in first-out (FIFO) method from the last-in first-out (LIFO) method during the second quarter of 2015. Had this change not been implemented, the Company would have reported a LIFO reserve of $4,974 at December 31, 2015, as compared to $7,769 at December 31, 2014.
NOTE 3 GOODWILL AND OTHER INTANGIBLE ASSETS
The changes in the carrying amount of goodwill for the year ended December 31, 2015 are as follows by reporting segment:
| | | | | | | | | | | | | | | | |
| | Beauty + | | | | | Food + | | Corporate | | | | |
| | Home | | Pharma | | Beverage | | & Other | | Total | |
Balance as of December 31, 2013 | | | | | | | | | | | | | | | | |
Goodwill | | $ | 181,002 | | $ | 159,949 | | $ | 17,914 | | $ | 1,615 | | $ | 360,480 | |
Accumulated impairment losses | | | — | | | — | | | — | | | (1,615) | | | (1,615) | |
| | $ | 181,002 | | $ | 159,949 | | $ | 17,914 | | $ | — | | $ | 358,865 | |
Foreign currency exchange effects | | | (9,853) | | | (18,357) | | | (914) | | | — | | | (29,124) | |
Balance as of December 31, 2014 | | | | | | | | | | | | | | | | |
Goodwill | | $ | 171,149 | | $ | 141,592 | | $ | 17,000 | | $ | 1,615 | | $ | 331,356 | |
Accumulated impairment losses | | | — | | | — | | | — | | | (1,615) | | | (1,615) | |
| | $ | 171,149 | | $ | 141,592 | | $ | 17,000 | | $ | — | | $ | 329,741 | |
Foreign currency exchange effects | | | (6,559) | | | (12,232) | | | (710) | | | — | | | (19,501) | |
Balance as of December 31, 2015 | | | | | | | | | | | | | | | | |
Goodwill | | $ | 164,590 | | $ | 129,360 | | $ | 16,290 | | $ | 1,615 | | $ | 311,855 | |
Accumulated impairment losses | | | — | | | — | | | — | | | (1,615) | | | (1,615) | |
| | $ | 164,590 | | $ | 129,360 | | $ | 16,290 | | $ | — | | $ | 310,240 | |
The Company has also completed the annual impairment analysis of its reporting units as of December 31, 2015 using a qualitative analysis of goodwill commonly referred to as the “step zero” approach. Based on our review of macroeconomic, industry, and market events and circumstances as well as the overall financial performance of the reporting units, we determined it was more likely than not that the fair value of goodwill attributed to all four of our reporting units was greater than its carrying amount. Therefore, no impairment of goodwill has been recorded.
The table below shows a summary of intangible assets for the years ended December 31, 2015 and 2014.
| | | | | | | | | | | | | | | | | | | | | |
| | | | 2015 | | 2014 | |
Weighted Average | | Gross | | | | | | | | Gross | | | | | | | |
Amortization Period | | Carrying | | Accumulated | | Net | | Carrying | | Accumulated | | Net | |
| | (Years) | | Amount | | Amortization | | Value | | Amount | | Amortization | | Value | |
Amortization intangible assets: | | | | | | | | | | | | | | | | | | | | | |
Patents | | 0.1 | | $ | 15,358 | | $ | (15,330) | | $ | 28 | | $ | 17,001 | | $ | (16,852) | | $ | 149 | |
Acquired Technology | | 15.0 | | | 32,030 | | | (8,543) | | | 23,487 | | | 35,701 | | | (5,950) | | | 29,751 | |
License agreements and other | | 5.3 | | | 30,082 | | | (20,387) | | | 9,695 | | | 32,804 | | | (22,659) | | | 10,145 | |
Total intangible assets | | 8.3 | | $ | 77,470 | | $ | (44,260) | | $ | 33,210 | | $ | 85,506 | | $ | (45,461) | | $ | 40,045 | |
Aggregate amortization expense for the intangible assets above for the years ended December 31, 2015, 2014 and 2013 was $4,246, $5,325 and $5,033, respectively.
Estimated amortization expense for the years ending December 31 is as follows:
| | | |
2016 | | $ | 3,654 |
2017 | | | 3,243 |
2018 | | | 3,241 |
2019 | | | 3,027 |
2020 and thereafter | | | 20,045 |
Future amortization expense may fluctuate depending on changes in foreign currency rates. The estimates for amortization expense noted above are based upon foreign exchange rates as of December 31, 2015.
NOTE 4 ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
At December 31, 2015 and 2014, accounts payable and accrued liabilities consisted of the following:
| | | | | | | |
| | 2015 | | 2014 | |
Accounts payable, principally trade | | $ | 115,061 | | $ | 116,374 | |
Accrued employee compensation costs | | | 125,724 | | | 125,227 | |
Customer deposits and other unearned income | | | 33,253 | | | 42,641 | |
Other accrued liabilities | | | 80,890 | | | 68,520 | |
Total | | $ | 354,928 | | $ | 352,762 | |
NOTE 5 INCOME TAXES
Income before income taxes consists of:
| | | | | | | | | | |
Years Ended December 31, | | 2015 | | 2014 | | 2013 | |
United States | | $ | 73,492 | | $ | 31,681 | | $ | 28,968 | |
International | | | 221,079 | | | 254,620 | | | 235,415 | |
Total | | $ | 294,571 | | $ | 286,301 | | $ | 264,383 | |
The provision (benefit) for income taxes is comprised of:
| | | | | | | | | | |
Years Ended December 31, | | 2015 | | 2014 | | 2013 | |
Current: | | | | | | | | | | |
U.S. Federal | | $ | 19,749 | | $ | 14,023 | | $ | 7,174 | |
State/Local | | | 82 | | | 42 | | | (631) | |
International | | | 82,586 | | | 99,585 | | | 79,070 | |
| | $ | 102,417 | | $ | 113,650 | | $ | 85,613 | |
Deferred: | | | | | | | | | | |
U.S. Federal/State | | $ | 1,605 | | $ | (10,130) | | $ | 9,575 | |
International | | | (8,746) | | | (8,843) | | | (2,731) | |
| | $ | (7,141) | | $ | (18,973) | | $ | 6,844 | |
Total | | $ | 95,276 | | $ | 94,677 | | $ | 92,457 | |
The difference between the actual income tax provision and the tax provision computed by applying the statutory federal income tax rate of 35.0% in 2015, 2014 and 2013 to income before income taxes is as follows:
| | | | | | | | | | |
Years Ended December 31, | | 2015 | | 2014 | | 2013 | |
Income tax at statutory rate | | $ | 103,100 | | $ | 100,205 | | $ | 92,534 | |
State income (benefits) taxes, net of federal (tax) benefit | | | (1,254) | | | 770 | | | (610) | |
Provision for distribution of current foreign earnings | | | 2,031 | | | 695 | | | 11,388 | |
Rate differential on earnings of foreign operations | | | (6,395) | | | (5,478) | | | (10,167) | |
Other items, net | | | (2,206) | | | (1,515) | | | (688) | |
Actual income tax provision | | $ | 95,276 | | $ | 94,677 | | $ | 92,457 | |
Effective income tax rate | | | 32.3 | % | | 33.1 | % | | 35.0 | % |
The tax provision for 2015 reflects a benefit of $1.6 million related to the reduction of valuation allowances mostly associated with U.S. state tax credits. Additional benefits of $3.1 million associated with the exceptional depreciation allowances enacted in France during 2015 were partially offset by a $2.4 million charge for expected income tax assessments in France for a transfer pricing issue. While the Company expects to seek compensating offsets for this amount, no receivable has been recorded at this time. The $2.0 million charge pertaining to the distribution of earnings reflects $1.6 million of tax incurred in 2015 for income recognized under the U.S. deemed dividend provisions and $0.4 million for planned cash movements in Europe during 2016.
In 2014, we did not repatriate foreign earnings to the U.S. Therefore, the 2014 tax provision is lower than the prior year as 2013 included $10.1 million of net additional tax expense due to repatriations.
The tax provision for 2013 reflects an increase of $6.7 million due to tax law changes in France. These changes were enacted on December 31, 2013 but retroactive to January 1, 2013. An additional $2.3 million of tax was incurred as a result of new French distribution taxes effective for distributions after August 17, 2012. The increases were partially offset
by a benefit of $3.6 million from the expected use of net operating losses in Brazil and a benefit of $1.4 million from a tax law change in Italy.
Significant deferred tax assets and liabilities as of December 31, 2015 and 2014 are comprised of the following temporary differences:
| | | | | | | |
| | 2015 | | 2014 | |
Deferred Tax Assets: | | | | | | | |
Pension liabilities | | $ | 27,155 | | $ | 35,582 | |
Stock compensation | | | 20,170 | | | 15,672 | |
Foreign tax credit carryforward | | | 7,471 | | | 10,348 | |
Net operating loss carryforwards | | | 5,053 | | | 7,896 | |
U.S. state tax credits | | | 8,554 | | | 7,641 | |
Vacation | | | 4,167 | | | 5,892 | |
Workers compensation | | | 4,693 | | | 4,526 | |
Other | | | 6,503 | | | 9,584 | |
Total gross deferred tax assets | | | 83,766 | | | 97,141 | |
Less valuation allowance | | | (6,125) | | | (7,734) | |
Net deferred tax assets | | | 77,641 | | | 89,407 | |
Deferred Tax Liabilities: | | | | | | | |
Depreciation, amortization and leases | | | 47,154 | | | 53,121 | |
Acquisition related intangibles | | | 15,720 | | | 18,058 | |
Total gross deferred tax liabilities | | | 62,874 | | | 71,179 | |
Net deferred tax assets | | $ | 14,767 | | $ | 18,228 | |
The foreign tax credit carryforward will expire in the years 2023 and 2024. There is no expiration date on $4.3 million of the tax‑effected net operating loss carry forwards and $0.7 million (tax effected) will expire in the years 2016 to 2034. The U.S. state tax credit carryforwards of $8.6 million (tax effected) will expire in the years 2016 to 2030. No state tax credit carryforwards are expected to expire unused in 2016.
The Company evaluates the deferred tax assets and records a valuation allowance when it is believed it is more likely than not that the benefit will not be realized. The Company has established a valuation allowance of $2.1 million of the $5.1 million of tax effected net operating loss carry forwards. These losses are generally in start‑up jurisdictions or locations that have not produced an operating profit to date. A valuation allowance of $3.8 million has been established against the $8.6 million of U.S. state tax credit carry forwards. A valuation allowance of $0.2 million has been established related to other future tax deductions in non‑U.S. jurisdictions, the benefit of which management believes will not be realized.
As of December 31, 2015, the Company had $1.4 billion of undistributed earnings from non‑U.S. subsidiaries which have been designated as permanently reinvested. The Company has not made a provision for U.S. or additional foreign taxes on this amount. However, we estimate the amount of additional tax that might be payable on these earnings to be in the range of $75 million to $100 million. These earnings will continue to be reinvested indefinitely and could become subject to the additional tax if they were remitted as dividends or lent to a U.S. affiliate, or if the Company should sell its stock in the subsidiaries.
The Company has not provided for taxes on certain tax‑deferred income of a foreign operation. The income arose predominately from government grants. Taxes of approximately $1.7 million would become payable in the event the terms of the grant are not fulfilled.
INCOME TAX UNCERTAINTIES
The Company provides a liability for the amount of tax benefits realized from uncertain tax positions. A reconciliation of the beginning and ending amount of income tax uncertainties is as follows:
| | | | | | | | | | |
| | 2015 | | 2014 | | 2013 | |
Balance at January 1 | | $ | 6,408 | | $ | 7,988 | | $ | 8,464 | |
Increases based on tax positions for the current year | | | 255 | | | 113 | | | 110 | |
Increases based on tax positions of prior years | | | 2,684 | | | 228 | | | 381 | |
Decreases based on tax positions of prior years | | | (518) | | | (1,073) | | | (92) | |
Settlements | | | (207) | | | (407) | | | (515) | |
Lapse of statute of limitations | | | (688) | | | (441) | | | (360) | |
Balance at December 31 | | $ | 7,934 | | $ | 6,408 | | $ | 7,988 | |
The amount of income tax uncertainties that, if recognized, would impact the effective tax rate is close to $7.9 million. The Company estimates that it is reasonably possible that the liability for uncertain tax positions will decrease no more than $7.0 million in the next twelve months from the resolution of various uncertain positions as a result of the completion of tax audits, litigation and the expiration of the statute of limitations in various jurisdictions.
The Company recognizes interest and penalties accrued related to unrecognized tax benefits as a component of income taxes. As of December 31, 2015, 2014 and 2013, the Company had approximately $1.1 million, $0.7 million and $0.9 million, respectively, accrued for the payment of interest and penalties, of which approximately $0.4 million, ($0.2) million and ($0.4) million was recognized in income tax expense in the years ended December 31, 2015, 2014 and 2013, respectively.
The Company or its subsidiaries file income tax returns in the U.S. Federal jurisdiction and various state and foreign jurisdictions. The major tax jurisdictions the Company files in, with the years still subject to income tax examinations, are listed below:
| | | |
| | Tax Years | |
Major Tax | | Subject to | |
Jurisdiction | | Examination | |
United States — Federal | | 2013-2015 | |
United States — State | | 2009-2015 | |
France | | 2012-2015 | |
Germany | | 2011-2015 | |
Italy | | 2011-2015 | |
China | | 2006-2015 | |
NOTE 6 DEBT
The Company maintains certain short-term notes payable, including a revolving credit facility. These short-term notes payable are reported as notes payable in the current liabilities section of the Consolidated Balance Sheets. Average borrowings under these short-term notes payable were $42.7 million and $198.3 million for 2015 and 2014, respectively. The average annual interest rate on short‑term notes payable was approximately 6.8% for 2015 and 1.5% for 2014. The higher average annual interest rate in 2015 is due to our remaining short-term notes payable primarily consisting of borrowings in emerging markets after we paid off our revolving credit facility in February 2015 and replaced those borrowings with long-term debt. There are no compensating balance requirements associated with short‑term borrowings. Each borrowing under this credit facility will bear interest at rates based on LIBOR, prime rates or other similar rates, in each case plus an applicable margin. A facility fee on the total amount of the facility is also payable quarterly, regardless of usage. The applicable margins for borrowings under the credit facility and the facility fee percentage may change from time to time depending on changes in AptarGroup’s consolidated leverage ratio. The outstanding balance under the credit facility was $0 and $230 million at December 31, 2015 and 2014, respectively. We incurred approximately $0.9 million and $2.7 million in interest and fees related to this credit facility during 2015 and 2014, respectively. The revolving credit and the senior unsecured debt agreements contain covenants, with which the Company is in compliance, that include certain financial tests.
The Company also maintains long-term notes obligations, including private placement facilities. In December 2014, we executed a $475 million private placement to take advantage of low long-term interest rates. At that time, we closed on $250 million of the private placement to fund our accelerated share repurchase ("ASR") program (see Note 13). This closing consisted of two maturity tranches, with $125 million of 9 year notes at an interest rate of 3.49% and $125
million of 11 year notes at an interest rate of 3.61%. We closed on the remaining $225 million of the private placement in February 2015, consisting of $100 million of 9 year notes at an interest rate of 3.49% and $125 million of 11 year notes at an interest rate of 3.61%. The proceeds from this closing were used to pay down the existing revolving line of credit.
At December 31, the Company’s long‑term obligations consisted of the following:
| | | | | | | |
| | 2015 | | 2014 | |
Notes payable 0.61% – 16.00%, due in monthly and annual installments through 2027 | | $ | 3,785 | | $ | 5,160 | |
Senior unsecured notes 2.3%, due in 2015 | | | — | | | 16,000 | |
Senior unsecured notes 6.0%, due in 2016 | | | 50,000 | | | 50,000 | |
Senior unsecured notes 6.0%, due in 2018 | | | 75,000 | | | 75,000 | |
Senior unsecured notes 3.8%, due in 2020 | | | 84,000 | | | 84,000 | |
Senior unsecured notes 3.2%, due in 2022 | | | 75,000 | | | 75,000 | |
Senior unsecured notes 3.5%, due in 2023 | | | 125,000 | | | 125,000 | |
Senior unsecured notes 3.4%, due in 2024 | | | 50,000 | | | 50,000 | |
Senior unsecured notes 3.5%, due in 2024 | | | 100,000 | | | — | |
Senior unsecured notes 3.6%, due in 2025 | | | 125,000 | | | 125,000 | |
Senior unsecured notes 3.6%, due in 2026 | | | 125,000 | | | — | |
Capital lease obligations | | | 1,628 | | | 2,424 | |
| | | 814,413 | | | 607,584 | |
Current maturities of long-term obligations | | | (51,889) | | | (18,692) | |
Total long-term obligations | | $ | 762,524 | | $ | 588,892 | |
Aggregate long‑term maturities, excluding capital lease obligations, which is discussed in Note 7, due annually for the five years beginning in 2016 are $51,368, $919, $75,183, $185, $84,186 and $600,944 thereafter.
NOTE 7 LEASE COMMITMENTS
The Company leases certain warehouse, plant, and office facilities as well as certain equipment under noncancelable operating and capital leases expiring at various dates through the year 2027. Most of the operating leases contain renewal options and certain leases include options to purchase during or at the end of the lease term.
Amortization expense related to capital leases is included in depreciation expense. Rent expense under operating leases (including taxes, insurance and maintenance when included in the rent) amounted to $26,583, $30,813 and $30,720 in 2015, 2014 and 2013, respectively.
Assets recorded under capital leases consist of:
| | | | | | | |
| | 2015 | | 2014 | |
Buildings | | $ | 2,184 | | $ | 2,434 | |
Machinery and equipment | | | 831 | | | 1,076 | |
| | $ | 3,015 | | $ | 3,510 | |
Accumulated depreciation | | | (663) | | | (758) | |
| | $ | 2,352 | | $ | 2,752 | |
Future minimum payments, by year and in the aggregate, under the capital leases and noncancelable operating leases with initial or remaining terms of one year or more consisted of the following at December 31, 2015:
| | | | | | | |
| | Capital | | Operating | |
| | Leases | | Leases | |
2016 | | $ | 569 | | $ | 15,418 | |
2017 | | | 633 | | | 12,385 | |
2018 | | | 521 | | | 9,298 | |
2019 | | | 43 | | | 8,006 | |
2020 | | | — | | | 4,784 | |
Subsequent to 2020 | | | — | | | 16,052 | |
Total minimum lease payments | | | 1,766 | | $ | 65,943 | |
Amounts representing interest | | | (138) | | | | |
Present value of future minimum lease payments | | | 1,628 | | | | |
Lease amount due in one year | | | (521) | | | | |
Total | | $ | 1,107 | | | | |
NOTE 8 RETIREMENT AND DEFERRED COMPENSATION PLANS
The Company has various noncontributory retirement plans covering certain of its domestic and foreign employees. Benefits under the Company’s retirement plans are based on participants’ years of service and annual compensation as defined by each plan. Annual cash contributions to fund pension costs accrued under the Company’s domestic plans are generally at least equal to the minimum funding amounts required by the Employee Retirement Income Security Act of 1974, as amended (ERISA). Certain pension commitments under its foreign plans are also funded according to local requirements or at the Company’s discretion.
The following table presents the changes in the benefit obligations and plan assets for the most recent two years for the Company’s domestic and foreign plans.
| | | | | | | | | | | | | |
| | Domestic Plans | | Foreign Plans | |
| | 2015 | | 2014 | | 2015 | | 2014 | |
Change in benefit obligation: | | | | | | | | | | | | | |
Benefit obligation at beginning of year | | $ | 171,501 | | $ | 129,448 | | $ | 91,160 | | $ | 84,660 | |
Service cost | | | 10,016 | | | 8,042 | | | 4,570 | | | 4,186 | |
Interest cost | | | 6,355 | | | 5,928 | | | 1,654 | | | 2,711 | |
Curtailment/Settlement | | | — | | | — | | | (227) | | | — | |
Prior service cost | | | — | | | — | | | 821 | | | — | |
Actuarial (gain) loss | | | (19,568) | | | 33,058 | | | (1,486) | | | 14,765 | |
Benefits paid | | | (7,309) | | | (4,975) | | | (2,265) | | | (3,111) | |
Foreign currency translation adjustment | | | — | | | — | | | (9,420) | | | (12,051) | |
Benefit obligation at end of year | | $ | 160,995 | | $ | 171,501 | | $ | 84,807 | | $ | 91,160 | |
| | | | | | | | | | | | | |
| | Domestic Plans | | Foreign Plans | |
| | 2015 | | 2014 | | 2015 | | 2014 | |
Change in plan assets: | | | | | | | | | | | | | |
Fair value of plan assets at beginning of year | | $ | 111,094 | | $ | 100,567 | | $ | 55,394 | | $ | 54,075 | |
Actual return on plan assets | | | (930) | | | 5,431 | | | 927 | | | 2,875 | |
Employer contribution | | | 10,079 | | | 10,071 | | | 10,157 | | | 8,016 | |
Benefits paid | | | (7,309) | | | (4,975) | | | (2,265) | | | (3,111) | |
Foreign currency translation adjustment | | | — | | | — | | | (5,696) | | | (6,461) | |
Fair value of plan assets at end of year | | $ | 112,934 | | $ | 111,094 | | $ | 58,517 | | $ | 55,394 | |
Funded status at end of year | | $ | (48,060) | | $ | (60,407) | | $ | (26,288) | | $ | (35,766) | |
The following table presents the funded status amounts recognized in the Company’s Consolidated Balance Sheets as of December 31, 2015 and 2014.
| | | | | | | | | | | | | |
| | Domestic Plans | | Foreign Plans | |
| | 2015 | | 2014 | | 2015 | | 2014 | |
Current liabilities | | $ | (173) | | $ | (471) | | $ | — | | $ | — | |
Non-current liabilities | | | (47,887) | | | (59,936) | | | (26,288) | | | (35,766) | |
| | $ | (48,060) | | $ | (60,407) | | $ | (26,288) | | $ | (35,766) | |
The following table presents the amounts not recognized as components of periodic benefit cost that are recognized in accumulated other comprehensive loss as of December 31, 2015 and 2014.
| | | | | | | | | | | | | |
| | Domestic Plans | | Foreign Plans | |
| | 2015 | | 2014 | | 2015 | | 2014 | |
Net actuarial loss | | $ | 47,820 | | $ | 64,272 | | $ | 33,839 | | $ | 36,219 | |
Net prior service cost | | | — | | | — | | | 4,036 | | | 3,471 | |
Tax effects | | | (17,933) | | | (24,102) | | | (12,211) | | | (12,762) | |
| | $ | 29,887 | | $ | 40,170 | | $ | 25,664 | | $ | 26,928 | |
Changes in benefit obligations and plan assets recognized in other comprehensive income in 2015, 2014 and 2013 are as follows:
| | | | | | | | | | |
| | Domestic Plans | |
| | 2015 | | 2014 | | 2013 | |
Current year actuarial gain (loss) | | $ | 11,048 | | $ | (34,211) | | $ | 20,548 | |
Amortization of net loss | | | 5,404 | | | 2,869 | | | 5,103 | |
Amortization of prior service cost | | | — | | | — | | | 3 | |
| | $ | 16,452 | | $ | (31,342) | | $ | 25,654 | |
| | | | | | | | | | |
| | Foreign Plans | |
| | 2015 | | 2014 | | 2013 | |
Current year actuarial gain (loss) | | $ | 568 | | $ | (12,589) | | $ | 3,005 | |
Current year prior service cost | | | (821) | | | — | | | — | |
Amortization of net loss | | | 1,812 | | | 1,214 | | | 1,416 | |
Amortization of prior service cost | | | 256 | | | 313 | | | 373 | |
| | $ | 1,815 | | $ | (11,062) | | $ | 4,794 | |
The following table presents the amounts in accumulated other comprehensive loss as of December 31, 2015 expected to be recognized as components of periodic benefit cost in 2016.
| | | | | | | |
| | Domestic Plans | | Foreign Plans | |
Amortization of net loss | | $ | 2,988 | | $ | 1,510 | |
Amortization of prior service cost | | | — | | | 343 | |
| | $ | 2,988 | | $ | 1,853 | |
Components of net periodic benefit cost:
| | | | | | | | | | |
| | Domestic Plans | |
| | 2015 | | 2014 | | 2013 | |
Service cost | | $ | 10,016 | | $ | 8,042 | | $ | 8,539 | |
Interest cost | | | 6,355 | | | 5,928 | | | 4,992 | |
Expected return on plan assets | | | (7,590) | | | (6,585) | | | (5,775) | |
Amortization of net loss | | | 5,404 | | | 2,869 | | | 5,103 | |
Amortization of prior service cost | | | — | | | — | | | 3 | |
Net periodic benefit cost | | $ | 14,185 | | $ | 10,254 | | $ | 12,862 | |
| | | | | | | | | | |
| | Foreign Plans | |
| | 2015 | | 2014 | | 2013 | |
Service cost | | $ | 4,570 | | $ | 4,186 | | $ | 3,901 | |
Interest cost | | | 1,654 | | | 2,711 | | | 2,676 | |
Expected return on plan assets | | | (1,792) | | | (1,979) | | | (1,821) | |
Amortization of net loss | | | 1,676 | | | 1,214 | | | 1,416 | |
Amortization of prior service cost | | | 256 | | | 313 | | | 373 | |
Net periodic benefit cost | | $ | 6,364 | | $ | 6,445 | | $ | 6,545 | |
Settlement | | | 136 | | | — | | | — | |
Curtailment | | | — | | | — | | | 1 | |
Total Net periodic benefit cost | | $ | 6,500 | | $ | 6,445 | | $ | 6,546 | |
The accumulated benefit obligation (“ABO”) for the Company’s domestic defined benefit pension plans was $145.5 million and $153.8 million at December 31, 2015 and 2014, respectively. The accumulated benefit obligation for the Company’s foreign defined benefit pension plans was $65.5 million and $70.7 million at December 31, 2015 and 2014, respectively.
The following table provides the projected benefit obligation (“PBO”), ABO, and fair value of plan assets for all pension plans with an ABO in excess of plan assets as of December 31, 2015 and 2014.
| | | | | | | | | | | | | |
| | Domestic Plans | | Foreign Plans | |
| | 2015 | | 2014 | | 2015 | | 2014 | |
Projected benefit obligation | | $ | 160,995 | | $ | 171,501 | | $ | 81,343 | | $ | 87,759 | |
Accumulated benefit obligation | | | 145,482 | | | 153,778 | | | 61,990 | | | 67,317 | |
Fair value of plan assets | | | 112,934 | | | 111,094 | | | 55,054 | | | 51,993 | |
The following table provides the PBO, ABO, and fair value of plan assets for all pension plans with a PBO in excess of plan assets as of December 31, 2015 and 2014.
| | | | | | | | | | | | | |
| | Domestic Plans | | Foreign Plans | |
| | 2015 | | 2014 | | 2015 | | 2014 | |
Projected benefit obligation | | $ | 160,995 | | $ | 171,501 | | $ | 81,343 | | $ | 87,759 | |
Accumulated benefit obligation | | | 145,482 | | | 153,778 | | | 61,990 | | | 67,317 | |
Fair value of plan assets | | | 112,934 | | | 111,094 | | | 55,054 | | | 51,993 | |
Assumptions:
| | | | | | | | | | | | | |
| | Domestic Plans | | Foreign Plans | |
| | 2015 | | 2014 | | 2013 | | 2015 | | 2014 | | 2013 | |
Weighted-average assumptions used to determine benefit obligations at December 31: | | | | | | | | | | | | | |
Discount rate | | 4.24 | % | 3.83 | % | 4.75 | % | 2.10 | % | 1.90 | % | 3.24 | % |
Rate of compensation increase | | 4.00 | % | 4.00 | % | 4.00 | % | 3.00 | % | 3.00 | % | 3.00 | % |
Weighted-average assumptions used to determine net periodic benefit cost for years ended December 31: | | | | | | | | | | | | | |
Discount rate | | 3.83 | % | 4.75 | % | 3.80 | % | 1.90 | % | 3.24 | % | 3.19 | % |
Expected long-term return on plan assets | | 7.00 | % | 7.00 | % | 7.00 | % | 3.54 | % | 3.79 | % | 3.79 | % |
Rate of compensation increase | | 4.00 | % | 4.00 | % | 4.00 | % | 3.00 | % | 3.00 | % | 3.00 | % |
The Company develops the expected long‑term rate of return assumptions based on historical experience and by evaluating input from the plans’ asset managers, including the managers’ review of asset class return expectations and benchmarks, economic indicators and long‑term inflation assumptions.
In order to determine the 2016 net periodic benefit cost, the Company expects to use the December 31, 2015 discount rates, December 31, 2015 rates of compensation increase assumptions and the same assumed long‑term returns on domestic and foreign plan assets used for the 2015 net periodic benefit cost.
The Company’s domestic and foreign pension plan weighted‑average asset allocations at December 31, 2015 and 2014 by asset category are as follows:
Plan Assets:
| | | | | | | | | |
| | Domestic Plans Assets | | Foreign Plans Assets | |
| | at December 31, | | at December 31, | |
| | 2015 | | 2014 | | 2015 | | 2014 | |
Equity securities | | 47 | % | 47 | % | 4 | % | 5 | % |
Fixed income securities | | 30 | % | 27 | % | 1 | % | 1 | % |
Corporate debt securities | | — | | — | | 3 | % | 12 | % |
Infrastructure | | 8 | % | 8 | % | — | | — | |
Hedge funds | | 14 | % | 15 | % | — | | — | |
Money market | | 1 | % | 3 | % | 3 | % | 2 | % |
Investment Funds | | — | | — | | 89 | % | 80 | % |
Total | | 100 | % | 100 | % | 100 | % | 100 | % |
The Company’s investment strategy for its domestic and foreign pension plans is to maximize the long‑term rate of return on plan assets within an acceptable level of risk. The investment policy strives to have assets sufficiently diversified so that adverse or unexpected results from one security type will not have an unduly detrimental impact on the entire portfolio and accordingly, establishes a target allocation for each asset category within the portfolio. The domestic plan asset allocation is reviewed on a quarterly basis and the foreign plan asset allocation is reviewed annually. Rebalancing occurs as needed to comply with the investment strategy. The domestic plan target allocation for 2016 is 60% equity securities and 40% fixed income securities and infrastructure. The foreign plan target allocation for 2016 is 100% investment funds.
Authoritative guidelines require the categorization of assets and liabilities into three levels based upon the assumptions (inputs) used to price the assets or liabilities. Level 1 provides the most reliable measure of fair value, whereas Level 3 generally requires significant management judgment. The three levels are defined as follows:
| · | | Level 1: Unadjusted quoted prices in active markets for identical assets and liabilities. |
| · | | Level 2: Observable inputs other than those included in Level 1. For example, quoted prices for similar assets or liabilities in active markets or quoted prices for identical assets or liabilities in inactive markets. |
| · | | Level 3: Unobservable inputs reflecting management’s own assumptions about the inputs used in pricing the asset or liability. |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Domestic Fair Value Measurement | | Foreign Fair Value Measurement | |
| | at December 31, 2015 | | at December 31, 2015 | |
(In Thousands $) | | Total | | (Level 1) | | (Level 2) | | (Level 3) | | Total | | (Level 1) | | (Level 2) | | (Level 3) | |
Cash and Short-term Securities (a) | | $ | 1,189 | | $ | 1,189 | | $ | — | | $ | — | | $ | 1,907 | | $ | 1,907 | | $ | — | | $ | — | |
USD | | | — | | | 1,189 | | | — | | | — | | | — | | | — | | | — | | | — | |
EUR | | | — | | | — | | | — | | | — | | | — | | | 1,907 | | | — | | | — | |
Equity Securities (a) | | $ | 53,985 | | $ | 47,725 | | $ | 6,260 | | | — | | $ | 2,258 | | $ | 2,258 | | | — | | | — | |
US Large Cap Equities | | | — | | | 28,797 | | | — | | | — | | | — | | | — | | | — | | | — | |
US Small Cap Equities | | | — | | | 5,349 | | | — | | | — | | | — | | | 2,258 | | | — | | | — | |
Global Equities | | | — | | | — | | | 6,260 | | | — | | | — | | | — | | | — | | | — | |
International Equities | | | — | | | 13,579 | | | — | | | — | | | — | | | — | | | — | | | — | |
Fixed Income (a&b) | | $ | 33,291 | | $ | 21,918 | | $ | 11,373 | | | — | | $ | 559 | | $ | 559 | | | — | | | — | |
Corporate debts securities | | | — | | | — | | | — | | | — | | $ | 1,542 | | $ | 1,542 | | | — | | | — | |
Euro Corporate Bonds (a) | | | — | | | — | | | — | | | — | | | — | | | 1,542 | | | — | | | — | |
Hedge Fund (b) | | $ | 15,509 | | | — | | $ | 15,509 | | | — | | | — | | | — | | | — | | | — | |
Investment Funds | | | — | | | — | | | — | | | — | | $ | 52,251 | | $ | 19,446 | | $ | 32,805 | | | — | |
Mutual Funds in Equities (a) | | | — | | | — | | | — | | | — | | | — | | | 3,392 | | | — | | | — | |
Mutual Funds in Bonds (a) | | | — | | | — | | | — | | | — | | | — | | | 15,501 | | | — | | | — | |
Mutual Funds Diversified (a&b) | | | — | | | — | | | — | | | — | | | — | | | 553 | | | 32,805 | | | — | |
Infrastructure (c) | | $ | 8,960 | | | — | | | — | | $ | 8,960 | | | — | | | — | | | — | | | — | |
Total Investments | | $ | 112,934 | | $ | 70,832 | | $ | 33,142 | | $ | 8,960 | | $ | 58,517 | | $ | 25,712 | | $ | 32,805 | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Domestic Fair Value Measurement | | Foreign Fair Value Measurement | |
| | at December 31, 2014 | | at December 31, 2014 | |
(In Thousands $) | | Total | | (Level 1) | | (Level 2) | | (Level 3) | | Total | | (Level 1) | | (Level 2) | | (Level 3) | |
Cash and Short-term Securities (a) | | $ | 3,190 | | $ | 3,190 | | $ | — | | $ | — | | $ | 1,289 | | $ | 1,289 | | $ | — | | $ | — | |
USD | | | — | | | 3,190 | | | — | | | — | | | — | | | — | | | — | | | — | |
EUR | | | — | | | — | | | — | | | — | | | — | | | 1,289 | | | — | | | — | |
Equity Securities (a) | | $ | 52,840 | | $ | 52,840 | | | — | | | — | | $ | 2,625 | | $ | 2,625 | | | — | | | — | |
US Large Cap Equities | | | — | | | 29,381 | | | — | | | — | | | — | | | — | | | — | | | — | |
US Small Cap Equities | | | — | | | 8,493 | | | — | | | — | | | — | | | — | | | — | | | — | |
International Equities | | | — | | | 14,966 | | | — | | | — | | | — | | | 2,625 | | | — | | | — | |
Fixed Income (a&b) | | $ | 29,762 | | $ | 19,713 | | $ | 10,049 | | | — | | $ | 574 | | $ | 574 | | | — | | | — | |
Corporate debts securities | | | — | | | — | | | — | | | — | | $ | 6,758 | | $ | 6,758 | | | — | | | — | |
Euro Corporate Bonds (a) | | | — | | | — | | | — | | | — | | | — | | | 6,758 | | | — | | | — | |
Hedge Fund (b&c) | | $ | 16,371 | | | — | | $ | 16,099 | | $ | 272 | | | — | | | — | | | — | | | — | |
Investment Funds | | | — | | | — | | | — | | | — | | $ | 44,148 | | $ | 14,643 | | $ | 29,505 | | | — | |
Mutual Funds in Equities (a) | | | — | | | — | | | — | | | — | | | — | | | 2,687 | | | — | | | — | |
Mutual Funds in Bonds (a) | | | — | | | — | | | — | | | — | | | — | | | 11,386 | | | — | | | — | |
Mutual Funds Diversified (a&b) | | | — | | | — | | | — | | | — | | | — | | | 570 | | | 29,505 | | | — | |
Infrastructure (c) | | $ | 8,931 | | | — | | | — | | $ | 8,931 | | | — | | | — | | | — | | | — | |
Total Investments | | $ | 111,094 | | $ | 75,743 | | $ | 26,148 | | $ | 9,203 | | $ | 55,394 | | $ | 25,889 | | $ | 29,505 | | $ | — | |
| (a) | | Based on third party quotation from financial institution. |
| (b) | | Based on observable market transactions. |
| (c) | | Based on a quarterly statement prepared by the fund manager that reflects contributions, distributions and realized/unrealized gains and losses. |
The following table sets forth a summary of changes in fair value of the pension plan investments classified as Level 3 for the year ended December 31, 2015.
| | | | | | | |
| | Infrastructure | | | | |
| | Fund | | Hedge Fund | |
Balance, 12/31/13 | | $ | 8,587 | | $ | 9,731 | |
Purchases, sales and settlements, net | | | — | | | (9,387) | |
Return on assets held | | | 476 | | | (72) | |
Admin fees and other | | | (132) | | | — | |
Balance, 12/31/14 | | $ | 8,931 | | $ | 272 | |
Purchases, sales and settlements, net | | | — | | | (273) | |
Return on assets held | | | 147 | | | 1 | |
Admin fees and other | | | (118) | | | — | |
Balance, 12/31/15 | | $ | 8,960 | | $ | — | |
CONTRIBUTIONS
Annual cash contributions to fund pension costs accrued under the Company’s domestic plans are generally at least equal to the minimum funding amounts required by ERISA. The Company contributed $10.1 million to its domestic defined benefit plans in 2015 and although the Company has no minimum funding requirement, we plan to contribute approximately $10.0 million in 2016. Contributions to fund pension costs accrued under the Company’s foreign plans are made in accordance with local laws or at the Company’s discretion. The Company contributed approximately $10.2 million to its foreign defined benefit plan in 2015 and expects to contribute approximately $5.0 million in 2016.
ESTIMATED FUTURE BENEFIT PAYMENTS
As of December 31, 2015, the Company expects the plans to make the following estimated benefit payments relating to its defined benefit plans over the next ten years:
| | | | | | | |
| | Domestic Plans | | Foreign Plans | |
2016 | | $ | 7,489 | | $ | 1,831 | |
2017 | | | 7,861 | | | 1,751 | |
2018 | | | 8,393 | | | 2,355 | |
2019 | | | 8,622 | | | 2,705 | |
2020 | | | 9,302 | | | 3,996 | |
2021 - 2025 | | | 58,818 | | | 21,751 | |
OTHER PLANS
The Company has a non‑qualified supplemental pension plan for domestic employees which provides for pension amounts that would have been payable from the Company’s principal domestic pension plan if it were not for limitations imposed by income tax regulations. The liability for this plan, which is not funded, was $8.4 million and $8.5 million at December 31, 2015 and 2014, respectively. This amount is included in the liability for domestic plans shown above.
The Company has a defined contribution 401(k) employee savings plan available to substantially all domestic employees. Company matching contributions are made in cash up to a maximum of 3% of the participating employee’s salary subject to income tax regulations. For each of the years ended December 31, 2015, 2014 and 2013, total contributions made by the Company to these plans were approximately $3.0 million, $2.8 million and $2.6 million, respectively.
The Company has several foreign defined contribution plans, which require the Company to contribute a percentage of the participating employee’s salary according to local regulations. For each of the years ended December 31, 2015, 2014 and 2013, total contributions made by the Company to these plans were approximately $2.1 million, $2.3 million and $2.1 million, respectively.
The Company has no additional postretirement or postemployment benefit plans.
NOTE 9 ACCUMULATED OTHER COMPREHENSIVE INCOME/(LOSS)
Changes in Accumulated Other Comprehensive Income by Component:
| | | | | | | | | | | | | |
| | Foreign | | Defined Benefit | | | | | | | |
| | Currency | | Pension Plans | | Other | | Total | |
Balance - December 31, 2013 | | $ | 149,965 | | $ | (40,093) | | $ | (121) | | $ | 109,751 | |
Other comprehensive income before reclassifications | | | (192,476) | | | (29,842) | | | — | | | (222,318) | |
Amounts reclassified from accumulated other comprehensive income | | | (340) | | | 2,838 | | | 24 | | | 2,522 | |
Net current-period other comprehensive income | | | (192,816) | | | (27,004) | | | 24 | | | (219,796) | |
Balance - December 31, 2014 | | $ | (42,851) | | $ | (67,097) | | $ | (97) | | $ | (110,045) | |
Other comprehensive income before reclassifications | | | (163,874) | | | 6,715 | | | — | | | (157,159) | |
Amounts reclassified from accumulated other comprehensive income | | | — | | | 4,832 | | | 25 | | | 4,857 | |
Net current-period other comprehensive income | | | (163,874) | | | 11,547 | | | 25 | | | (152,302) | |
Balance - December 31, 2015 | | $ | (206,725) | | $ | (55,550) | | $ | (72) | | $ | (262,347) | |
Reclassifications Out of Accumulated Other Comprehensive Income:
| | | | | | | | | | |
| | Amount Reclassified from | | | |
Details about Accumulated Other | | Accumulated Other | | Affected Line in the Statement | |
Comprehensive Income Components | | Comprehensive Income | | Where Net Income is Presented | |
Years Ended December 31, | | 2015 | | 2014 | | | | |
Defined Benefit Pension Plans | | | | | | | | | | |
Amortization of net loss | | $ | 7,216 | | $ | 4,083 | | | (a) | |
Amortization of prior service cost | | | 256 | | | 313 | | | (a) | |
| | | 7,472 | | | 4,396 | | | Total before tax | |
| | | (2,640) | | | (1,558) | | | Tax benefit | |
| | $ | 4,832 | | $ | 2,838 | | | Net of tax | |
Foreign Currency | | | | | | | | | | |
Foreign currency gain | | $ | — | | $ | (340) | | | Miscellaneous, net | |
| | | — | | | (340) | | | Total before tax | |
| | | — | | | — | | | Tax benefit | |
| | $ | — | | $ | (340) | | | Net of tax | |
Other | | | | | | | | | | |
Changes in treasury locks | | $ | 39 | | $ | 38 | | | Interest Expense | |
| | | 39 | | | 38 | | | Total before tax | |
| | | (14) | | | (14) | | | Tax benefit | |
| | $ | 25 | | $ | 24 | | | Net of tax | |
Total reclassifications for the period | | $ | 4,857 | | $ | 2,522 | | | | |
| (a) | | These accumulated other comprehensive income components are included in the computation of total net periodic benefit costs, net of tax (see Note 8 - Retirement and Deferred Compensation Plans for additional details). |
NOTE 10 DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
The Company maintains a foreign exchange risk management policy designed to establish a framework to protect the value of the Company’s non‑functional denominated transactions from adverse changes in exchange rates. Sales of the Company’s products can be denominated in a currency different from the currency in which the related costs to produce the product are denominated. Changes in exchange rates on such inter‑country sales or intercompany loans can impact the Company’s results of operations. The Company’s policy is not to engage in speculative foreign currency hedging activities, but to minimize its net foreign currency transaction exposure defined as firm commitments and transactions recorded and denominated in currencies other than the functional currency. The Company may use foreign currency forward exchange contracts, options and cross currency swaps to economically hedge these risks.
For derivative instruments designated as hedges, the Company formally documents the nature and relationships between the hedging instruments and the hedged items, as well as the risk management objectives, strategies for undertaking the various hedge transactions, and the method of assessing hedge effectiveness. Additionally, in order to designate any derivative instrument as a hedge of an anticipated transaction, the significant characteristics and expected terms of any anticipated transaction must be specifically identified, and it must be probable that the anticipated transaction will occur.
HEDGE OF NET INVESTMENTS IN FOREIGN OPERATIONS
A significant number of the Company’s operations are located outside of the United States. Because of this, movements in exchange rates may have a significant impact on the translation of the financial condition and results of operations of the Company’s foreign entities. A strengthening U.S. dollar relative to foreign currencies has a dilutive translation effect on the Company’s financial condition and results of operations. Conversely, a weakening U.S. dollar has an additive effect. The Company in some cases maintains debt in these subsidiaries to offset the net asset exposure. The Company does not otherwise actively manage this risk using derivative financial instruments. In the event the Company plans on a full or partial liquidation of any of its foreign subsidiaries where the Company’s net investment is likely to be monetized, the Company will consider hedging the currency exposure associated with such a transaction.
OTHER
As of December 31, 2015, the Company has recorded the fair value of foreign currency forward exchange contracts of $1.9 million in prepayments and other, $0.1 million in miscellaneous other assets, $1.2 million in accounts payable and accrued liabilities and $45 thousand in deferred and other non-current liabilities in the balance sheet. All forward exchange contracts outstanding as of December 31, 2015 had an aggregate contract amount of $81.5 million.
Fair Value of Derivative Instruments in the Consolidated Balance Sheets as of December 31, 2015 and December 31, 2014
| | | | | | | | | |
Derivative Contracts Not Designated | | | | December 31, | | December 31, | |
as Hedging Instruments | | Balance Sheet Location | | 2015 | | 2014 | |
Derivative Assets | | | | | | | | | |
Foreign Exchange Contracts | | Prepayments and other | | $ | 1,924 | | $ | 1,037 | |
Foreign Exchange Contracts | | Miscellaneous other assets | | | 112 | | | 7 | |
| | | | $ | 2,036 | | $ | 1,044 | |
Derivative Liabilities | | | | | | | | | |
Foreign Exchange Contracts | | Accounts payable and accrued liabilities | | $ | 1,152 | | $ | 2,378 | |
Foreign Exchange Contracts | | Deferred and other non-current liabilities | | | 45 | | | 115 | |
| | | | $ | 1,197 | | $ | 2,493 | |
The Effect of Derivative Instruments on the Consolidated Statements of Income
for the Fiscal Years Ended December 31, 2015 and December 31, 2014
| | | | | | | | | |
| | | | Amount of Gain (Loss) | |
Derivatives Not Designated | | Location of Gain (Loss) Recognized | | Recognized in Income | |
as Hedging Instruments | | in Income on Derivatives | | on Derivatives | |
| | | | 2015 | | 2014 | |
Foreign Exchange Contracts | | Other Income (Expense) | | $ | 1,704 | | $ | (2,368) | |
| | Miscellaneous, net | | | | | | | |
| | | | $ | 1,704 | | $ | (2,368) | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | Gross Amounts not Offset | | | | |
| | | | | | | Net Amounts | | in the Statement of | | | | |
| | | | | Gross Amounts | | Presented in | | Financial Position | | | | |
| | Gross | | Offset in the | | the Statement of | | Financial | | Cash Collateral | | Net | |
| | Amount | | Financial Position | | Financial Position | | Instruments | | Received | | Amount | |
Description | | | | | | | | | | | | | | | | |
December 31, 2015 | | | | | | | | | | | | | | | | |
Derivative Assets | | $ | 2,036 | | — | | $ | 2,036 | | — | | — | | $ | 2,036 | |
Total Assets | | $ | 2,036 | | — | | $ | 2,036 | | — | | — | | $ | 2,036 | |
| | | | | | | | | | | | | | | | |
Derivative Liabilities | | $ | 1,197 | | — | | $ | 1,197 | | — | | — | | $ | 1,197 | |
Total Liabilities | | $ | 1,197 | | — | | $ | 1,197 | | — | | — | | $ | 1,197 | |
| | | | | | | | | | | | | | | | |
December 31, 2014 | | | | | | | | | | | | | | | | |
Derivative Assets | | $ | 1,044 | | — | | $ | 1,044 | | — | | — | | $ | 1,044 | |
Total Assets | | $ | 1,044 | | — | | $ | 1,044 | | — | | — | | $ | 1,044 | |
| | | | | | | | | | | | | | | | |
Derivative Liabilities | | $ | 2,493 | | — | | $ | 2,493 | | — | | — | | $ | 2,493 | |
Total Liabilities | | $ | 2,493 | | — | | $ | 2,493 | | — | | — | | $ | 2,493 | |
NOTE 11 FAIR VALUE
Authoritative guidelines require the categorization of assets and liabilities into three levels based upon the assumptions (inputs) used to price the assets or liabilities. Level 1 provides the most reliable measure of fair value, whereas Level 3 generally requires significant management judgment. The three levels are defined as follows:
| · | | Level 1: Unadjusted quoted prices in active markets for identical assets and liabilities. |
| · | | Level 2: Observable inputs other than those included in Level 1. For example, quoted prices for similar assets or liabilities in active markets or quoted prices for identical assets or liabilities in inactive markets. |
| · | | Level 3: Unobservable inputs reflecting management’s own assumptions about the inputs used in pricing the asset or liability. |
As of December 31, 2015, the fair values of our financial assets and liabilities were categorized as follows:
| | | | | | | | | | | | | |
| | Total | | Level 1 | | Level 2 | | Level 3 | |
Assets | | | | | | | | | | | | | |
Forward exchange contracts (1) | | $ | 2,036 | | $ | — | | $ | 2,036 | | $ | — | |
Total assets at fair value | | $ | 2,036 | | $ | — | | $ | 2,036 | | $ | — | |
Liabilities | | | | | | | | | | | | | |
Forward exchange contracts (1) | | $ | 1,197 | | $ | — | | $ | 1,197 | | $ | — | |
Total liabilities at fair value | | $ | 1,197 | | $ | — | | $ | 1,197 | | $ | — | |
As of December 31, 2014, the fair values of our financial assets and liabilities were categorized as follows:
| | | | | | | | | | | | | |
| | Total | | Level 1 | | Level 2 | | Level 3 | |
Assets | | | | | | | | | | | | | |
Forward exchange contracts (1) | | $ | 1,044 | | $ | — | | $ | 1,044 | | $ | — | |
Total assets at fair value | | $ | 1,044 | | $ | — | | $ | 1,044 | | $ | — | |
Liabilities | | | | | | | | | | | | | |
Forward exchange contracts (1) | | $ | 2,493 | | $ | — | | $ | 2,493 | | $ | — | |
Total liabilities at fair value | | $ | 2,493 | | $ | — | | $ | 2,493 | | $ | — | |
| (1) | | Market approach valuation technique based on observable market transactions of spot and forward rates. |
The carrying amounts of the Company’s other current financial instruments such as cash and equivalents, notes payable and current maturities of long‑term obligations approximate fair value due to the short‑term maturity of the instrument. The Company considers its long‑term obligations a Level 2 liability and utilizes the market approach valuation technique based on interest rates that are currently available to the Company for issuance of debt with similar terms and maturities. The estimated fair value of the Company’s long‑term obligations was $760 million as of December 31, 2015 and $606 million as of December 31, 2014.
NOTE 12 COMMITMENTS AND CONTINGENCIES
The Company, in the normal course of business, is subject to a number of lawsuits and claims both actual and potential in nature. While management believes the resolution of these claims and lawsuits will not have a material adverse effect on the Company’s financial position or results of operations or cash flows, claims and legal proceedings are subject to inherent uncertainties, and unfavorable outcomes could occur that could include amounts in excess of any accruals which management has established. Were such unfavorable final outcomes to occur, it is possible that they could have a material adverse effect on our financial position, results of operations and cash flows.
Under our Certificate of Incorporation, the Company has agreed to indemnify our officers and directors for certain events or occurrences while the officer or director is, or was, serving at our request in such capacity. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited; however, the Company has a directors and officers liability insurance policy that covers a portion of our exposure. As a result of our insurance policy coverage, the Company believes the estimated fair value of these indemnification agreements is minimal. The Company has no liabilities recorded for these agreements as of December 31, 2015.
NOTE 13 STOCK REPURCHASE PROGRAM
On October 30, 2014, the Company announced a share repurchase authorization of up to $350 million of common stock. This authorization replaced previous authorizations and has no expiration date. AptarGroup may repurchase shares through the open market, privately negotiated transactions or other programs, subject to market conditions.
On December 16, 2014, the Company entered into an agreement to repurchase approximately $250 million of its common stock under an accelerated share repurchase program (the “ASR program”). The ASR program is part of the Company’s $350 million share repurchase authorization. On December 17, 2014, the Company paid $250 million to Wells Fargo Bank N.A. ("Wells Fargo") in exchange for approximately 3.1 million shares. On September 25, 2015, the Company settled the ASR program with Wells Fargo and received approximately 719 thousand additional shares. The total number of shares repurchased under the ASR program was approximately 3.8 million shares.
Subsequent to the completion of the ASR program, the Company repurchased approximately 190 thousand shares in the open market in November 2015 at a total cost of $13.9 million. Prior to November 2015, shares repurchased were returned to Treasury Stock. Beginning with the shares purchased in November 2015 and with all subsequent repurchases, such shares are immediately retired. In 2014, the Company repurchased approximately 4.5 million shares of its outstanding common stock at a total cost of $340.5 million, which included the $250 million payment related to the ASR program.
NOTE 14 CAPITAL STOCK
We have 199 million authorized shares of common stock. The number of shares of common stock and treasury stock and the share activity were as follows:
| | | | | | | | | |
| | Common Shares | | Treasury Shares | |
| | 2015 | | 2014 | | 2015 | | 2014 | |
Balance at the beginning of the year | | 86,267,467 | | 85,364,303 | | 24,336,367 | | 19,979,833 | |
Employee option exercises | | 1,248,629 | | 851,396 | | (167,878) | | (177,280) | |
Director option exercises | | 65,167 | | 41,844 | | — | | — | |
Restricted stock vestings | | 13,331 | | 9,924 | | — | | — | |
Common stock repurchases | | — | | — | | 718,866 | | 4,533,814 | |
Common stock repurchased and retired | | (190,350) | | — | | — | | — | |
Stock retirements | | (20,718,866) | | — | | (20,718,866) | | — | |
Balance at the end of the year | | 66,685,378 | | 86,267,467 | | 4,168,489 | | 24,336,367 | |
The cash dividends paid on the common stock for the years ended December 31, 2015, 2014 and 2013 aggregated $71.2 million, $71.1 million and $66.1 million, respectively.
NOTE 15 STOCK‑BASED COMPENSATION
The Company issues stock options and restricted stock units to employees under Stock Awards Plans approved by shareholders. Stock options and restricted stock units are issued to non‑employee directors under Director Stock Option Plans and the Director Restricted Stock Unit Plan approved by shareholders. Options are awarded with the exercise price equal to the market price on the date of grant and generally become exercisable over three years and expire 10 years after grant. Restricted stock units granted to employees generally vest over three years.
Compensation expense recorded attributable to stock options for the year ended December 31, 2015 was approximately $17.9 million ($11.6 million after tax). The income tax benefit related to this compensation expense was approximately $6.3 million. Approximately $15.7 million of the compensation expense was recorded in selling, research & development and administrative expenses and the balance was recorded in cost of sales. Compensation expense recorded attributable to stock options for the year ended December 31, 2014 was approximately $18.0 million ($11.7 million after tax). The income tax benefit related to this compensation expense was approximately $6.3 million. Approximately $16.0 million of the compensation expense was recorded in selling, research & development and administrative expenses and the balance was recorded in cost of sales. Compensation expense recorded attributable to stock options for the year ended December 31, 2013 was approximately $13.7 million ($9.2 million after tax). The income tax benefit related to this compensation expense was approximately $4.5 million. Approximately $12.1 million of the compensation expense was recorded in selling, research & development and administrative expenses and the balance was recorded in cost of sales.
The Company uses historical data to estimate expected life and volatility. The weighted‑average fair value of stock options granted under the Stock Awards Plans was $12.83, $14.82 and $10.16 per share in 2015, 2014 and 2013, respectively. These values were estimated on the respective dates of grant using the Black‑Scholes option‑pricing model with the following weighted‑average assumptions:
| | | | | | | |
Stock Awards Plans: | | 2015 | | 2014 | | 2013 | |
Years ended December 31, | | | | | | | |
Dividend Yield | | 1.7 | % | 1.7 | % | 1.8 | % |
Expected Stock Price Volatility | | 21.9 | % | 22.2 | % | 22.7 | % |
Risk-free Interest Rate | | 1.6 | % | 2.3 | % | 1.3 | % |
Expected Life of Option (years) | | 6.9 | | 6.9 | | 6.9 | |
There were no grants under the Director Stock Option Plan during 2015 as this plan was cancelled and replaced by the Director Restricted Stock Unit Plan. The fair value of stock options granted under the Director Stock Option Plan was $14.07 and $10.89 per share in 2014 and 2013, respectively. These values were estimated on the respective date of the grant using the Black‑Scholes option‑pricing model with the following weighted‑average assumptions:
| | | | | |
Director Stock Option Plans: | | | | | |
Years ended December 31, | | 2014 | | 2013 | |
Dividend Yield | | 1.8 | % | 1.9 | % |
Expected Stock Price Volatility | | 22.2 | % | 23.0 | % |
Risk-free Interest Rate | | 2.2 | % | 1.3 | % |
Expected Life of Option (years) | | 6.9 | | 6.9 | |
A summary of option activity under the Company’s stock option plans as of December 31, 2015, and changes during the period then ended is presented below:
| | | | | | | | | | | | |
| | Stock Awards Plans | | Director Stock Option Plans | |
| | | | | Weighted Average | | | | Weighted Average | |
| | | Options | | Exercise Price | | Options | | Exercise Price | |
Outstanding, January 1, 2015 | | | 8,107,806 | | $ | 46.74 | | 368,668 | | $ | 53.52 | |
Granted | | | 1,391,355 | | | 64.60 | | — | | | — | |
Exercised | | | (1,416,507) | | | 37.15 | | (65,167) | | | 41.93 | |
Forfeited or expired | | | (50,624) | | | 60.84 | | — | | | — | |
Outstanding at December 31, 2015 | | | 8,032,030 | | $ | 51.44 | | 303,501 | | $ | 56.00 | |
Exercisable at December 31, 2015 | | | 5,324,545 | | $ | 45.23 | | 221,159 | | $ | 53.22 | |
Weighted-Average Remaining Contractual Term (Years): | | | | | | | | | | | | |
Outstanding at December 31, 2015 | | | 6.1 | | | | | 6.8 | | | | |
Exercisable at December 31, 2015 | | | 4.9 | | | | | 6.4 | | | | |
Aggregate Intrinsic Value: | | | | | | | | | | | | |
Outstanding at December 31, 2015 | | $ | 170,340 | | | | $ | 5,052 | | | | |
Exercisable at December 31, 2015 | | $ | 145,978 | | | | $ | 4,297 | | | | |
Intrinsic Value of Options Exercised During the Years Ended: | | | | | | | | | | | | |
December 31, 2015 | | $ | 43,041 | | | | $ | 1,478 | | | | |
December 31, 2014 | | $ | 33,059 | | | | $ | 741 | | | | |
December 31, 2013 | | $ | 37,822 | | | | $ | 732 | | | | |
The grant date fair value of options vested during the years ended December 31, 2015, 2014 and 2013 was $16.1 million, $14.1 million and $13.0 million, respectively. Cash received from option exercises was approximately $64.0 million and the actual tax benefit realized for the tax deduction from option exercises was approximately $12.5 million in the year ended December 31, 2015. As of December 31, 2015, the remaining valuation of stock option awards to be expensed in future periods was $12.8 million and the related weighted‑average period over which it is expected to be recognized is 1.3 years.
The fair value of restricted stock unit grants is the market price of the underlying shares on the grant date. A summary of restricted stock unit activity as of December 31, 2015, and changes during the period then ended is presented below:
| | | | | | | | | | | |
| | | | | | | Director Restricted | |
| | Stock Awards Plans | | Stock Unit Plan | |
| | | | Weighted Average | | | | Weighted Average | |
| | RSUs | | Grant‑Date Fair Value | | RSUs | | Grant-Date Fair Value | |
| | | | | | | | | | | |
Nonvested at January 1, 2015 | | 61,750 | | $ | 64.09 | | — | | $ | — | |
Granted | | 17,957 | | | 67.96 | | 18,857 | | | 63.10 | |
Vested | | (13,331) | | | 56.75 | | — | | | — | |
Nonvested at December 31, 2015 | | 66,376 | | $ | 66.61 | | 18,857 | | $ | 63.10 | |
Compensation expense recorded attributable to restricted stock unit grants for the years ended December 31, 2015, 2014 and 2013 was approximately $2.7 million, $1.8 million and $664 thousand, respectively. The fair value of units vested during the years ended December 31, 2015, 2014 and 2013 was $757 thousand, $614 thousand and $571 thousand, respectively. The intrinsic value of units vested during the years ended December 31, 2015, 2014 and 2013 was $877 thousand, $761 thousand and $661 thousand, respectively. As of December 31, 2015, there was $1.6 million of total unrecognized compensation cost relating to restricted stock unit awards which is expected to be recognized over a weighted average period of 1.3 years.
During the first quarter of 2014, the Company approved a new long-term incentive program for certain employees. Each award is based on the cumulative total stockholder return of our common stock during a three year performance period. Total expense related to this program is expected to be approximately $2.7 million over the performance period, of which $1.2 million and $530 thousand was recognized for the year ended December 31, 2015 and 2014, respectively.
NOTE 16 EARNINGS PER SHARE
The reconciliation of basic and diluted earnings per share for the years ended December 31, 2015, 2014 and 2013 are as follows:
| | | | | | | | | |
| | Income | | Shares | | Per Share | |
| | (Numerator) | | (Denominator) | | Amount | |
For the Year Ended December 31, 2015 | | | | | | | | | |
Basic EPS | | | | | | | | | |
Income available to common stockholders | | $ | 199,348 | | 62,585 | | $ | 3.19 | |
Effect of Dilutive Securities | | | | | | | | | |
Stock options | | | | | 1,864 | | | | |
Restricted stock | | | — | | 43 | | | | |
Diluted EPS | | | | | | | | | |
Income available to common stockholders | | $ | 199,348 | | 64,492 | | $ | 3.09 | |
For the Year Ended December 31, 2014 | | | | | | | | | |
Basic EPS | | | | | | | | | |
Income available to common stockholders | | $ | 191,658 | | 65,009 | | $ | 2.95 | |
Effect of Dilutive Securities | | | | | | | | | |
Stock options | | | | | 2,248 | | | | |
Restricted stock | | | — | | 35 | | | | |
Diluted EPS | | | | | | | | | |
Income available to common stockholders | | $ | 191,658 | | 67,292 | | $ | 2.85 | |
For the Year Ended December 31, 2013 | | | | | | | | | |
Basic EPS | | | | | | | | | |
Income available to common stockholders | | $ | 171,994 | | 66,090 | | $ | 2.60 | |
Effect of Dilutive Securities | | | | | | | | | |
Stock options | | | | | 2,106 | | | | |
Restricted stock | | | — | | 12 | | | | |
Diluted EPS | | | | | | | | | |
Income available to common stockholders | | $ | 171,994 | | 68,208 | | $ | 2.52 | |
NOTE 17 SEGMENT INFORMATION
The Company is organized into three reporting segments. Operations that sell dispensing solutions primarily to the personal care, beauty and home care markets form the Beauty + Home segment. Operations that sell dispensing solutions to the prescription drug, consumer health care and injectables markets form the Pharma segment. Operations that sell dispensing solutions primarily to the food and beverage markets form the Food + Beverage segment.
The accounting policies of the segments are the same as those described in Note 1, Summary of Significant Accounting Policies. The Company evaluates performance of its business segments and allocates resources based upon segment income. Segment income is defined as earnings before net interest expense, certain corporate expenses, restructuring initiatives and income taxes.
Financial information regarding the Company’s reportable segments is shown below:
| | | | | | | | | | |
Years Ended December 31, | | 2015 | | 2014 | | 2013 | |
Total Sales: | | | | | | | | | | |
Beauty + Home | | $ | 1,291,545 | | $ | 1,522,444 | | $ | 1,501,611 | |
Pharma | | | 712,220 | | | 751,226 | | | 709,058 | |
Food + Beverage | | | 335,365 | | | 349,297 | | | 323,469 | |
Total Sales | | $ | 2,339,130 | | $ | 2,622,967 | | $ | 2,534,138 | |
Less: Intersegment Sales: | | | | | | | | | | |
Beauty + Home | | $ | 18,599 | | $ | 24,147 | | $ | 13,466 | |
Pharma | | | — | | | — | | | 284 | |
Food + Beverage | | | 3,382 | | | 1,011 | | | 375 | |
Total Intersegment Sales | | $ | 21,981 | | $ | 25,158 | | $ | 14,125 | |
Net Sales: | | | | | | | | | | |
Beauty + Home | | $ | 1,272,946 | | $ | 1,498,297 | | $ | 1,488,145 | |
Pharma | | | 712,220 | | | 751,226 | | | 708,774 | |
Food + Beverage | | | 331,983 | | | 348,286 | | | 323,094 | |
Net Sales | | $ | 2,317,149 | | $ | 2,597,809 | | $ | 2,520,013 | |
Segment Income: | | | | | | | | | | |
Beauty + Home | | $ | 98,707 | | $ | 98,368 | | $ | 109,272 | |
Pharma | | | 210,509 | | | 204,698 | | | 189,689 | |
Food + Beverage | | | 42,731 | | | 37,728 | | | 35,186 | |
Restructuring Initiatives and Related Depreciation (1) | | | — | | | — | | | (14,525) | |
Corporate & Other | | | (28,357) | | | (38,261) | | | (37,958) | |
Income before interest and taxes | | $ | 323,590 | | $ | 302,533 | | $ | 281,664 | |
Interest expense, net | | | (29,019) | | | (16,232) | | | (17,281) | |
Income before income taxes | | $ | 294,571 | | $ | 286,301 | | $ | 264,383 | |
Depreciation and Amortization (1): | | | | | | | | | | |
Beauty + Home | | $ | 75,289 | | $ | 85,469 | | $ | 83,328 | |
Pharma | | | 36,008 | | | 41,690 | | | 39,812 | |
Food + Beverage | | | 21,347 | | | 20,179 | | | 18,871 | |
Restructuring Initiatives | | | — | | | — | | | 2,725 | |
Corporate & Other | | | 6,249 | | | 4,880 | | | 5,220 | |
Depreciation and Amortization | | $ | 138,893 | | $ | 152,218 | | $ | 149,956 | |
Capital Expenditures: | | | | | | | | | | |
Beauty + Home | | $ | 61,156 | | $ | 76,544 | | $ | 81,247 | |
Pharma | | | 48,133 | | | 37,455 | | | 32,643 | |
Food + Beverage | | | 25,337 | | | 32,148 | | | 19,339 | |
Corporate & Other | | | 14,697 | | | 15,793 | | | 18,281 | |
Capital Expenditures | | $ | 149,323 | | $ | 161,940 | | $ | 151,510 | |
Total Assets: | | | | | | | | | | |
Beauty + Home | | $ | 1,086,482 | | $ | 1,193,370 | | $ | 1,318,933 | |
Pharma | | | 591,500 | | | 619,304 | | | 676,420 | |
Food + Beverage | | | 250,369 | | | 263,742 | | | 253,774 | |
Corporate & Other | | | 510,375 | | | 360,774 | | | 248,635 | |
Total Assets | | $ | 2,438,726 | | $ | 2,437,190 | | $ | 2,497,762 | |
| (1) | | Restructuring Initiatives and related Depreciation includes the following income/(expense) items for the twelve months ended December 31, 2015, 2014 and 2013 as follows: |
| | | | | | | | | | |
Years Ended December 31, | | 2015 | | 2014 | | 2013 | |
European Restructuring Plan | | | | | | | | | | |
Depreciation | | $ | — | | $ | — | | $ | 2,725 | |
Employee Severance and Other Costs | | | — | | | — | | | 11,844 | |
Prior Year Initiatives | | | — | | | — | | | (44) | |
Total Restructuring Initiatives and Related Depreciation Expense | | $ | — | | $ | — | | $ | 14,525 | |
Restructuring Initiatives and Related Depreciation Expense by Segment | | | | | | | | | | |
Beauty + Home | | $ | — | | $ | — | | $ | 14,548 | |
Pharma | | | — | | | — | | | — | |
Food + Beverage | | | — | | | — | | | (23) | |
Total Restructuring Initiatives and Related Depreciation Expense | | $ | — | | $ | — | | $ | 14,525 | |
GEOGRAPHIC INFORMATION
The following are net sales and long‑lived asset information by geographic area and product information for the years ended December 31, 2015, 2014 and 2013:
| | | | | | | | | | |
| | 2015 | | 2014 | | 2013 | |
Net Sales to Unaffiliated Customers (1): | | | | | | | | | | |
United States | | $ | 633,522 | | $ | 642,060 | | $ | 634,418 | |
Europe: | | | | | | | | | | |
France | | | 712,470 | | | 850,817 | | | 810,528 | |
Germany | | | 326,782 | | | 363,898 | | | 339,858 | |
Italy | | | 125,511 | | | 141,808 | | | 136,617 | |
Other Europe | | | 122,546 | | | 150,469 | | | 165,038 | |
Total Europe | | | 1,287,309 | | | 1,506,992 | | | 1,452,041 | |
Other Foreign Countries | | | 396,318 | | | 448,757 | | | 433,554 | |
Total | | $ | 2,317,149 | | $ | 2,597,809 | | $ | 2,520,013 | |
Plant, Property & Equipment: | | | | | | | | | | |
United States | | $ | 175,367 | | $ | 192,265 | | $ | 226,917 | |
Europe: | | | | | | | | | | |
France | | | 216,966 | | | 228,049 | | | 263,913 | |
Germany | | | 125,186 | | | 136,491 | | | 156,970 | |
Italy | | | 43,631 | | | 48,940 | | | 57,729 | |
Other Europe | | | 54,483 | | | 57,946 | | | 72,297 | |
Total Europe | | | 440,266 | | | 471,426 | | | 550,909 | |
Other Foreign Countries | | | 149,750 | | | 147,964 | | | 86,836 | |
Total | | $ | 765,383 | | $ | 811,655 | | $ | 864,662 | |
Product Net Sales Information: | | | | | | | | | | |
Pumps | | $ | 991,583 | | $ | 1,111,536 | | $ | 1,093,514 | |
Closures | | | 558,212 | | | 611,144 | | | 594,135 | |
Valves | | | 306,367 | | | 350,106 | | | 327,635 | |
Injectables | | | 130,503 | | | 143,631 | | | 142,116 | |
Other | | | 330,484 | | | 381,392 | | | 362,613 | |
Total | | $ | 2,317,149 | | $ | 2,597,809 | | $ | 2,520,013 | |
| (1) | | Sales are attributed to countries based upon where the sales invoice to unaffiliated customers is generated. |
No single customer represents 10% or more of the Company’s net sales in 2015, 2014 or 2013.
NOTE 18 INSURANCE SETTLEMENT RECEIVABLE
A fire caused damage to the roof and production area of one of the Company’s facilities in Brazil during September 2014. There were no injuries. The facility is primarily an internal supplier of anodized aluminum components for certain dispensing systems sold to the regional beauty and personal care markets. Repairs of the facility were essentially completed in the fourth quarter 2015. AptarGroup is insured for the damages caused by the fire, including business interruption insurance. While the Company is still in the process of reviewing claims with our insurance carriers, we have currently recognized a $4.9 million receivable related to costs incurred but not yet reimbursed, which is included in Prepayments and Other in the Consolidated Balance Sheet. During 2015, we recognized $2.9 million of gain due to the insurance recovery on the involuntary conversion of fixed assets related to this fire, which is included in Other (Expense) Income on the Consolidated Statements of Income. Otherwise, this incident did not have a material impact on our financial results during 2015 and we expect to reach a final insurance settlement during the first half of 2016.
NOTE 19 ACQUISITIONS
On January 25, 2016, the Company announced it has signed an agreement to acquire Mega Airless. Further information about this transaction can be found in Note 22 – Subsequent Events.
In December 2013, AptarGroup acquired a 20% non-controlling investment in Bapco Closures Holding Limited (Bapco) for approximately $5.2 million. In addition to this equity stake, the Company secured an exclusive global license related to innovative closures sealing technology that provides package integrity and tamper evidence. This investment is being accounted for under the equity method of accounting from the date of acquisition and, since it does not have a material impact on the results of operations in 2015, 2014 or 2013, pro forma information is not presented.
NOTE 20 RESTRUCTURING INITIATIVES
In November 2012, the Company announced a plan to optimize certain capacity in Europe. Due to increased production efficiencies and to better position the Company for future growth in Europe, AptarGroup transferred and consolidated production capacity involving twelve facilities. Two facilities have closed impacting approximately 170 employees. The locations involved in the plan are facilities serving the beauty, personal care, food, beverage, and consumer health care markets. During 2013, the Company recognized approximately $14.6 million of expense related to the plan, of which $2.7 million was accelerated depreciation. For presentation purposes, the accelerated depreciation related to this plan is reported in Depreciation and amortization within the Consolidated Statements of Income. As of December 31, 2013, the plan was substantially complete. The cumulative expense incurred was $19.5 million.
NOTE 21 QUARTERLY DATA (UNAUDITED)
Quarterly results of operations and per share information for the years ended December 31, 2015 and 2014 are as follows:
| | | | | | | | | | | | | | | | |
| | Quarter | | Total | |
| | First | | Second | | Third | | Fourth | | for Year | |
Year Ended December 31, 2015: | | | | | | | | | | | | | | | | |
Net sales | | $ | 589,811 | | $ | 594,275 | | $ | 586,290 | | $ | 546,773 | | $ | 2,317,149 | |
Gross profit (1) | | | 170,853 | | | 185,917 | | | 170,498 | | | 152,584 | | | 679,852 | |
Net Income | | | 45,099 | | | 57,541 | | | 53,262 | | | 43,393 | | | 199,295 | |
Net Income Attributable to AptarGroup, Inc. | | | 45,171 | | | 57,539 | | | 53,247 | | | 43,391 | | | 199,348 | |
Per Common Share — 2015: | | | | | | | | | | | | | | | | |
Net Income Attributable to AptarGroup, Inc. | | | | | | | | | | | | | | | | |
Basic | | $ | .73 | | $ | .92 | | $ | .85 | | $ | .69 | | $ | 3.19 | |
Diluted | | | .70 | | | .90 | | | .83 | | | .68 | | | 3.09 | |
Dividends declared | | | .28 | | | .28 | | | .28 | | | .30 | | | 1.14 | |
Stock price high (2) | | | 67.68 | | | 66.31 | | | 70.34 | | | 75.96 | | | 75.96 | |
Stock price low (2) | | | 61.39 | | | 61.98 | | | 60.73 | | | 65.79 | | | 60.73 | |
Average number of shares outstanding: | | | | | | | | | | | | | | | | |
Basic | | | 62,292 | | | 62,697 | | | 62,886 | | | 62,461 | | | 62,585 | |
Diluted | | | 64,494 | | | 64,276 | | | 64,454 | | | 64,266 | | | 64,492 | |
Year Ended December 31, 2014: | | | | | | | | | | | | | | | | |
Net sales | | $ | 676,051 | | $ | 670,631 | | $ | 651,942 | | $ | 599,185 | | $ | 2,597,809 | |
Gross profit (1) | | | 186,791 | | | 182,483 | | | 171,547 | | | 154,829 | | | 695,650 | |
Net Income | | | 48,408 | | | 53,084 | | | 48,620 | | | 41,512 | | | 191,624 | |
Net Income Attributable to AptarGroup, Inc. | | | 48,389 | | | 53,076 | | | 48,595 | | | 41,598 | | | 191,658 | |
Per Common Share — 2014: | | | | | | | | | | | | | | | | |
Net Income Attributable to AptarGroup, Inc. | | | | | | | | | | | | | | | | |
Basic | | $ | .74 | | $ | .81 | | $ | .75 | | $ | .65 | | $ | 2.95 | |
Diluted | | | .71 | | | .79 | | | .73 | | | .63 | | | 2.85 | |
Dividends declared | | | .25 | | | .28 | | | .28 | | | .28 | | | 1.09 | |
Stock price high (2) | | | 68.78 | | | 67.84 | | | 68.00 | | | 68.67 | | | 68.78 | |
Stock price low (2) | | | 61.18 | | | 64.54 | | | 60.52 | | | 55.59 | | | 55.59 | |
Average number of shares outstanding: | | | | | | | | | | | | | | | | |
Basic | | | 65,468 | | | 65,328 | | | 64,886 | | | 64,368 | | | 65,009 | |
Diluted | | | 68,232 | | | 67,438 | | | 66,845 | | | 66,121 | | | 67,292 | |
| (1) | | Gross profit is defined as net sales less cost of sales and depreciation. |
| (2) | | The stock price high and low amounts are based upon intra‑day New York Stock Exchange composite price history. |
NOTE 22 SUBSEQUENT EVENTS
On January 25, 2016, the Company announced it signed an agreement to acquire 100% of Mega Airless. Mega Airless is a leading provider of innovative all-plastic airless dispensing systems for the beauty, personal care and pharmaceutical markets and operates two manufacturing facilities in Germany and one in the United States. The transaction is subject to customary regulatory approvals and had not closed as of the date of this report but is expected to close prior to the end of the first quarter of 2016.
Under the terms of the agreement, the Company will acquire Mega Airless for an enterprise value of approximately €200 million ($218 million) in cash. For the year ended December 31, 2015, we recognized $1.9 million in transaction costs related to the agreement. These costs are reflected in the selling, research & development and administrative section of the Condensed Consolidated Statements of Income.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of AptarGroup, Inc.:
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of AptarGroup, Inc. and its subsidiaries at December 31, 2015 and December 31, 2014, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2015 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in “Management’s Report on Internal Control over Financial Reporting”, under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Chicago, Illinois
February 25, 2016
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
DISCLOSURE CONTROLS AND PROCEDURES
The Company’s management has evaluated, with the participation of the chief executive officer and chief financial officer of the Company, the effectiveness of the Company’s disclosure controls and procedures (as such term is defined in Rule 13a‑15(e) under the Securities Exchange Act of 1934) as of December 31, 2015. Based on that evaluation, the chief executive officer and chief financial officer have concluded that these controls and procedures were effective as of such date.
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a‑15(f) under the Securities Exchange Act of 1934. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. The Company’s management has evaluated, with the participation of the chief executive officer and chief financial officer of the Company, the effectiveness of our internal control over financial reporting as of December 31, 2015 based on the framework in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation under the framework in Internal Control—Integrated Framework, management has concluded that our internal control over financial reporting was effective as of December 31, 2015.
PricewaterhouseCoopers LLP, independent registered public accounting firm, has issued an attestation report on the effectiveness of our internal control over financial reporting. This report appears on page 61.
CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
No changes in the Company’s internal control over financial reporting (as such term is defined in Rule 13a‑15(f) under the Securities Exchange Act of 1934) occurred during the Company’s fiscal quarter ended December 31, 2015 that materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information with respect to directors may be found under the caption “Proposal 1—Election of Directors” in the Company’s Proxy Statement for the Annual Meeting of Stockholders to be held on May 4, 2016 (the “2016 Proxy Statement”) and is incorporated herein by reference.
Information with respect to executive officers may be found under the caption “Executive Officers” in Part I of this report and is incorporated herein by reference.
Information with respect to audit committee members and audit committee financial experts may be found under the caption “Corporate Governance—Audit Committee” in the 2016 Proxy Statement and is incorporated herein by reference.
Information with respect to the Company’s Code of Business Conduct and Ethics may be found under the caption “Corporate Governance—Code of Business Conduct and Ethics” in the 2016 Proxy Statement and is incorporated herein by reference. Our Code of Business Conduct and Ethics is available through the Corporate Governance link on the Investor Relations page of our website (www.aptar.com).
The information set forth under the heading “Section 16(a) Beneficial Ownership Reporting Compliance” in the 2016 Proxy Statement is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information set forth under the headings “Board Compensation”, “Executive Officer Compensation” and “Compensation Committee Report” in the 2016 Proxy Statement is incorporated herein by reference. The information included under the heading “Compensation Committee Report” in the 2016 Proxy Statement shall not be deemed to be “soliciting” material or to be “filed” with the Securities and Exchange Commission or subject to Regulation 14A or 14C, or to the liabilities of Section 18 of the Securities Exchange Act of 1934, as amended.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information set forth under the heading “Security Ownership of Certain Beneficial Owners, Directors and Management” and “Equity Compensation Plan Information” in the 2016 Proxy Statement is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information set forth under the heading “Transactions with Related Persons” and “Corporate Governance—Independence of Directors” in the 2016 Proxy Statement is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Information with respect to the independent registered public accounting firm fees and services may be found under the caption “Proposal [4]—Ratification of the Appointment of PricewaterhouseCoopers LLP as the Independent Registered Public Accounting Firm for 2016” in the 2016 Proxy Statement. Such information is incorporated herein by reference.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| (a) | | The following documents are filed as a part of this report: |
| (b) | | Exhibits required by Item 601 of Regulation S‑K are incorporated by reference to the Exhibit Index on pages 66-69 of this report. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | |
| | | | AptarGroup, Inc. | |
| | | | (Registrant) | |
| | | | | |
Date: February 25, 2016 | | By | | /s/ Robert W. Kuhn | |
| | | | Robert W. Kuhn | |
| | | | Executive Vice President, | |
| | | | Chief Financial Officer and Secretary | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant in the capacities and on the date indicated.
| | |
Signature | Title | Date |
| | |
/s/ King Harris King Harris | Chairman of the Board and Director | February 25, 2016 |
| | |
/s/ Stephen J. Hagge Stephen J. Hagge | President and Chief Executive Officer and Director (Principal Executive Officer) | February 25, 2016 |
| | |
/s/ Robert W. Kuhn Robert W. Kuhn | Executive Vice President, Chief Financial Officer and Secretary (Principal Accounting and Financial Officer) | February 25, 2016 |
| | |
/s/ Alain Chevassus Alain Chevassus | Director | February 25, 2016 |
| | |
/s/ George L. Fotiades George L. Fotiades | Director | February 25, 2016 |
| | |
/s/ Maritza Gomez Montiel Maritza Gomez Montiel | Director | February 25, 2016 |
| | |
/s/ Giovanna Kampouri-Monnas Giovanna Kampouri-Monnas | Director | February 25, 2016 |
| | |
/s/ Andreas Kramvis Andreas Kramvis | Director | February 25, 2016 |
| | |
/s/ Peter Pfeiffer Peter Pfeiffer | Director | February 25, 2016 |
| | |
/s/ Dr. Joanne C. Smith Dr. Joanne C. Smith | Director | February 25, 2016 |
| | |
/s/ Ralf Wunderlich Ralf Wunderlich | Director | February 25, 2016 |
AptarGroup, Inc.
SCHEDULE II – VALUATION AND QUALIFYING ACCOUNTS
For the years ended December 31, 2015, 2014 and 2013
| | | | | | | | | | | | | |
Dollars in thousands | | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | Balance at | | Charged to | | Deductions | | Balance | |
| | Beginning | | Costs and | | from | | at End of | |
| | Of Period | | Expenses | | Reserve (a) | | Period | |
2015 | | | | | | | | | | | | | |
Allowance for doubtful accounts | | $ | 4,251 | | $ | (813) | | $ | (728) | | $ | 2,710 | |
Deferred tax valuation allowance | | | 7,734 | | | 564 | | | (2,173) | | | 6,125 | |
| | | | | | | | | | | | | |
2014 | | | | | | | | | | | | | |
Allowance for doubtful accounts | | $ | 4,416 | | $ | 741 | | $ | (906) | | $ | 4,251 | |
Deferred tax valuation allowance | | | 4,840 | | | 3,396 | | | (502) | | | 7,734 | |
| | | | | | | | | | | | | |
2013 | | | | | | | | | | | | | |
Allowance for doubtful accounts | | $ | 6,751 | | $ | (381) | | $ | (1,954) | | $ | 4,416 | |
Deferred tax valuation allowance | | | 7,033 | | | 2,708 | | | (4,901) | | | 4,840 | |
| (a) | | Write-off accounts considered uncollectible, net of recoveries and foreign currency impact adjustments. |
INDEX TO EXHIBITS
| |
Exhibit
Number | Description |
2.1* | Share Purchase Agreement, dated as of January 25, 2016, between shareholders of MEGAPLAST GmbH, Megaplast France S.a.r.l. and MEGA Pumps L.P., and Aptargroup Holding SAS. |
3(i) | Amended and Restated Certificate of Incorporation of AptarGroup, Inc., as amended, filed as Exhibit 4(a) to AptarGroup Inc.’s Registration Statement on Form S‑8, Registration Number 333‑152525, filed on July 25, 2008, is hereby incorporated by reference. |
3(ii) | Amended and Restated By‑Laws of the Company, filed as Exhibit 3(ii) to the Company’s Annual Report on Form 10‑K for the year ended December 31, 2002 (File No. 1‑11846), is hereby incorporated by reference. |
| The Company hereby agrees to provide the Commission, upon request, copies of instruments defining the rights of holders of long‑term debt of the Registrant and its subsidiaries as are specified by item 601(b)(4)(iii)(A) of Regulation S‑K. |
4.1 | Note Purchase Agreement dated as of July 31, 2006, among AptarGroup, Inc. and the purchasers listed on Schedule A thereto, filed as Exhibit 4.2 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2006 (File No. 1‑11846), is hereby incorporated by reference. |
4.2 | Form of AptarGroup, Inc. 6.04% Series 2006‑A Senior Notes Due July 31, 2016, filed as Exhibit 4.3 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2006 (File No. 1‑11846), is hereby incorporated by reference. |
4.3 | Note Purchase Agreement dated as of July 31, 2008, among AptarGroup, Inc. and the purchasers listed on Schedule A thereto, filed as Exhibit 4.1 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2008 (File No. 1‑11846), is hereby incorporated by reference. |
4.4 | Form of AptarGroup, Inc. 6.03% Series 2008‑A‑2 Senior Notes Due July 31, 2018, filed as Exhibit 4.2 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2008 (File No. 1‑11846), is hereby incorporated by reference. |
4.5 | First Amendment to 2006 Note Purchase Agreement, dated as of November 30, 2010, among the Company and each of the institutions listed as signatories thereto, filed as Exhibit 4.1 to the Company’s Current Report on Form 8‑K filed on December 1, 2010 (File No. 1‑11846), is hereby incorporated by reference. |
4.6 | First Amendment to 2008 Note Purchase Agreement, dated as of November 30, 2010, among the Company and each of the institutions listed as signatories thereto, filed as Exhibit 4.2 to the Company’s Current Report on Form 8‑K filed on December 1, 2010 (File No. 1‑11846), is hereby incorporated by reference. |
4.7 | Supplemental Note Purchase Agreement, dated as of November 30, 2010, among the Company and each of the purchasers listed in Exhibit A thereto, filed as Exhibit 4.3 to the Company’s Current Report on Form 8‑K filed on December 1, 2010 (File No. 1‑11846), is hereby incorporated by reference. |
4.8 | Form of AptarGroup, Inc. 2.33% Series 2008‑B‑1 Senior Notes due November 30, 2015, filed as Exhibit 4.4 to the Company’s Current Report on Form 8‑K filed on December 1, 2010 (File No. 1‑11846), is hereby incorporated by reference. |
4.9 | Form of AptarGroup, Inc. 3.78% Series 2008‑B‑2 Senior Notes due November 30, 2020, filed as Exhibit 4.5 to the Company’s Current Report on Form 8‑K filed on December 1, 2010 (File No. 1‑11846), is hereby incorporated by reference. |
4.10 | Second Supplemental Note Purchase Agreement, dated as of September 5, 2012, among the Company and each of the purchasers listed in Exhibit A thereto, filed as Exhibit 4.1 to the Company’s Current Report on Form 8‑K filed on September 5, 2012 (File No. 1‑11846), is hereby incorporated by reference. |
4.11 | Form of AptarGroup, Inc. 3.25% Series 2008‑C‑1 Senior Notes Due September 5, 2022, filed as Exhibit 4.2 to the Company’s Current Report on Form 8‑K filed on September 5, 2012 (File No. 1‑11846), is hereby incorporated by reference. |
4.12 | Form of AptarGroup, Inc. 3.40% Series 2008‑C‑2 Senior Notes Due September 5, 2024, filed as Exhibit 4.3 to the Company’s Current Report on Form 8‑K filed on September 5, 2012 (File No. 1‑11846), is hereby incorporated by reference. |
4.13 | Note Purchase Agreement, dated as of December 16, 2014, among the Company and each of the purchasers listed in Schedule B thereto, filed as Exhibit 4.1 to the Company’s Current Report on Form 8‑K filed on December 17, 2014 (File No. 1‑11846), is hereby incorporated by reference. |
4.14 | Form of AptarGroup, Inc. 3.49% Series 2014‑A‑1 Senior Notes due December 16, 2023 (included as a part of Exhibit 4.13), filed as Exhibit 4.2 to the Company’s Current Report on Form 8‑K filed on December 17, 2014 (File No. 1‑11846), is hereby incorporated by reference. |
| |
Exhibit
Number | Description |
4.15 | Form of AptarGroup, Inc. 3.49% Series 2014‑A‑2 Senior Notes due February 26, 2024 (included as a part of Exhibit 4.13), filed as Exhibit 4.3 to the Company’s Current Report on Form 8‑K filed on December 17, 2014 (File No. 1‑11846), is hereby incorporated by reference. |
4.16 | Form of AptarGroup, Inc. 3.61% Series 2014‑A‑3 Senior Notes due December 16, 2025 (included as a part of Exhibit 4.13), filed as Exhibit 4.4 to the Company’s Current Report on Form 8‑K filed on December 17, 2014 (File No. 1‑11846), is hereby incorporated by reference. |
4.17 | Form of AptarGroup, Inc. 3.61% Series 2014‑A‑4 Senior Notes due February 26, 2026 (included as a part of Exhibit 4.13), filed as Exhibit 4.5 to the Company’s Current Report on Form 8‑K filed on December 17, 2014 (File No. 1‑11846), is hereby incorporated by reference. |
4.18 | Second Amendment to 2006 Note Purchase Agreement, dated as of December 16, 2014, among the Company and each of the noteholders listed on the signature pages thereto, filed as Exhibit 4.6 to the Company’s Current Report on Form 8‑K filed on December 17, 2014 (File No. 1‑11846), is hereby incorporated by reference. |
4.19 | Second Amendment to 2008 Note Purchase Agreement, dated as of December 16, 2014, among the Company and each of the noteholders listed on the signature pages thereto, filed as Exhibit 4.7 to the Company’s Current Report on Form 8‑K filed on December 17, 2014 (File No. 1‑11846), is hereby incorporated by reference. |
10.1 | AptarGroup, Inc. 2000 Stock Awards Plan, filed as Appendix A to the Company’s Proxy Statement, dated April 6, 2000 (File No. 1‑11846), is hereby incorporated by reference.** |
10.2 | AptarGroup, Inc. 2004 Stock Awards Plan, filed as Appendix A to the Company’s Proxy Statement, dated March 26, 2004 (File No. 1‑11846), is hereby incorporated by reference.** |
10.3 | AptarGroup, Inc. 2004 Director Stock Option Plan, filed as Appendix B to the Company’s Proxy Statement, dated March 26, 2004 (File No. 1‑11846), is hereby incorporated by reference.** |
10.4 | AptarGroup, Inc., Stock Option Agreement for Employees pursuant to the AptarGroup, Inc. 2004 Stock Awards Plan, filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10‑Q for the quarter ended September 30, 2004 (File No. 1‑11846), is hereby incorporated by reference.** |
10.5 | AptarGroup, Inc. Stock Option Agreement for Non‑Employee Directors pursuant to the AptarGroup, Inc. 2004 Director Option Plan, filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10‑Q for the quarter ended September 30, 2004 (File No. 1‑11846), is hereby incorporated by reference.** |
10.6 | AptarGroup, Inc. Stock Option Agreement for Employees pursuant to the AptarGroup, Inc. 2000 Stock Awards Plan, filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10‑Q for the quarter ended September 30, 2004 (File No. 1‑11846), is hereby incorporated by reference.** |
10.7 | AptarGroup, Inc. Restricted Stock Unit Award Agreement pursuant to the AptarGroup, Inc. 2000 Stock Awards Plan, filed as Exhibit 10.4 to the Company’s Quarterly Report on Form 10‑Q for the quarter ended September 30, 2004 (File No. 1‑11846), is hereby incorporated by reference.** |
10.8 | Form of AptarGroup, Inc. Stock Option Agreement for Employees pursuant to the AptarGroup, Inc. 2014 Stock Awards Plan, filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10‑Q for the quarter ended June 30, 2014 (File No. 1‑11846), is hereby incorporated by reference.** |
10.9 | Form of AptarGroup, Inc. Restricted Stock Unit Award Agreement pursuant to the AptarGroup, Inc. 2014 Stock Awards Plan, filed as Exhibit 10.3 to the Company’s Quarterly Report on Form 10‑Q for the quarter ended June 30, 2014 (File No. 1‑11846), is hereby incorporated by reference.** |
10.10 | Supplementary Pension Plan—France dated August 24, 2001, filed as Exhibit 10.2 to the Company’s Quarterly Report on Form 10‑Q for the quarter ended March 31, 2004 (File No. 1‑11846), is hereby incorporated by reference.** |
10.11 | AptarGroup, Inc. Supplemental Retirement Plan dated October 6, 2008, filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10‑Q for the quarter ended September 30, 2008 (File No. 1‑11846), is hereby incorporated by reference.** |
10.12 | Indemnification Agreement dated January 1, 1996 of King Harris, filed as Exhibit 10.25 to the Company’s quarterly report on Form 10‑Q for the quarter ended March 31, 2001 (File No. 1‑11846), is hereby incorporated by reference.** |
10.13 | Employment Agreement effective January 1, 2012 of Stephen J. Hagge, filed as Exhibit 10.1 to the Company’s quarterly report on Form 10‑Q for the quarter ended September 30, 2012 (File No. 1‑11846), is hereby incorporated by reference.** |
10.14 | Employment Agreement effective January 1, 2012 of Robert W. Kuhn, filed as Exhibit 10.2 to the Company’s quarterly report on Form 10‑Q for the quarter ended September 30, 2012 (File No. 1‑11846), is hereby incorporated by reference.** |
| |
Exhibit
Number | Description |
10.15 | Employment Agreement effective January 1, 2012 of Patrick F. Doherty, filed as Exhibit 10.3 to the Company’s quarterly report on Form 10‑Q for the quarter ended September 30, 2012 (File No. 1‑11846), is hereby incorporated by reference.** |
10.16 | Employment Agreement effective January 1, 2012 of Eldon W. Schaffer, filed as Exhibit 10.4 to the Company’s quarterly report on Form 10‑Q for the quarter ended September 30, 2012 (File No. 1‑11846), is hereby incorporated by reference.** |
10.17* | Employment Agreement dated February 10, 2016 of Gael Touya.** |
10.18 | Employment Agreement dated October 1, 2010 of Ursula Saint Léger, filed as Exhibit 10.21 to the Company’s annual report on Form 10‑K for the fiscal year ended December 31, 2010 (File No. 1‑11846), is hereby incorporated by reference. ** |
10.19 | Employment Agreement effective August 1, 2014 of Salim Haffar, filed as Exhibit 10.4 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2014 (File No. 1‑11846), is hereby incorporated by reference. ** |
10.20 | AptarGroup, Inc. 2008 Stock Option Plan, filed as Exhibit 10.3 to AptarGroup, Inc.’s Current Report on Form 8‑K filed on May 1, 2008, is hereby incorporated by reference.** |
10.21 | AptarGroup, Inc. 2008 Director Stock Option Plan, filed as Exhibit 10.1 to AptarGroup, Inc.’s Current Report on Form 8‑K filed on May 1, 2008, is hereby incorporated by reference.** |
10.22 | Form of AptarGroup, Inc. Stock Option Agreement for Employees pursuant to the AptarGroup, Inc. 2008 Stock Option Plan, filed as Exhibit 10.4 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2008 (File No. 1‑11846), is hereby incorporated by reference.** |
10.23 | Form of AptarGroup, Inc. Stock Option Agreement for Non‑Employee Directors pursuant to the AptarGroup, Inc. 2008 Director Stock Option Plan, filed as Exhibit 10.5 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2008 (File No. 1‑11846), is hereby incorporated by reference.** |
10.24 | Form of AptarGroup, Inc. Restricted Stock Unit Award Agreement pursuant to the AptarGroup, Inc. 2004 Stock Awards Plan, filed as Exhibit 10.6 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2008 (File No. 1‑11846), and amended as of January 1, 2010.** |
10.25 | Form of AptarGroup, Inc. Stock Option Agreement for Employees pursuant to the AptarGroup, Inc. 2011 Stock Awards Plan, filed as Exhibit 10.2 to the Company’s quarterly report on Form 10‑Q for the quarter ended September 30, 2011 (File No. 1‑11846), is hereby incorporated by reference.** |
10.26 | Form of AptarGroup, Inc. Restricted Stock Unit Award Agreement pursuant to the AptarGroup, Inc. 2011 Stock Awards Plan, filed as Exhibit 10.3 to the Company’s quarterly report on Form 10‑Q for the quarter ended September 30, 2011 (File No. 1‑11846), is hereby incorporated by reference.** |
10.27 | AptarGroup, Inc. 2011 Stock Awards Plan, filed as Exhibit 10.1 to the Company’s Current Report on Form 8‑K filed on May 10, 2011 (File No. 1‑11846), is hereby incorporated by reference.** |
10.28 | AptarGroup, Inc. 2014 Stock Awards Plan, filed as Exhibit 10.1 to the Company’s Current Report on Form 8‑K filed on May 12, 2014 (File No. 1‑11846), is hereby incorporated by reference.** |
10.29 | Amendment to Stock Option Award Agreements, filed as Exhibit 10.1 to the Company’s quarterly report on Form 10‑Q for the quarter ended September 30, 2015 (File No. 1‑11846), is hereby incorporated by reference.** |
10.30 | AptarGroup Performance Incentive Plan, filed as Exhibit 10.1 to AptarGroup, Inc.’s Current Report on Form 8‑K filed on May 13, 2013 (File No. 1‑11846), is hereby incorporated by reference.** |
10.31 | AptarGroup, Inc. 2014 Long‑Term Incentive Program (as Amended and Restated) under the AptarGroup, Inc. Performance Incentive Plan, filed as Exhibit 10.28 to the Company’s annual report on Form 10‑K for the fiscal year ended December 31, 2013 (File No. 1‑11846), is hereby incorporated by reference.** |
10.32 | AptarGroup, Inc. 2014 Long‑Term Incentive Program (as Amended and Restated) under the AptarGroup, Inc. Performance Incentive Plan filed as Exhibit 10.31 to the Company’s annual report on Form 10‑K for the fiscal year ended December 31, 2015 (File No. 1‑11846), is hereby incorporated by reference.** |
10.33 | AptarGroup, Inc. 2015 Director Restricted Stock Unit Plan, filed as Exhibit 4(c) to AptarGroup, Inc.’s Registration Statement on Form S-8, Registration Number 333-203905, filed on May 6, 2015, is hereby incorporated by reference.** |
10.34 | Form of AptarGroup, Inc. 2015 Restricted Stock Unit Award Agreement for Directors pursuant to the AptarGroup, Inc. 2015 Director Restricted Stock Unit Plan, filed as Exhibit 10.2 to the Company’s quarterly report on Form 10‑Q for the quarter ended June 30, 2015 (File No. 1‑11846), is hereby incorporated by reference.** |
| |
Exhibit
Number | Description |
10.35 | Credit Agreement dated as of January 31, 2012 among AptarGroup, Inc. and the financial institutions party thereto as Lenders, Wells Fargo Bank, National Association, as administrative agent, Bank of America, N.A. and JPMorgan Chase Bank, N.A., as co‑syndication agents, HSBC Bank USA, N.A. and Union Bank, N.A., as co‑documentation agents, and Wells Fargo Securities, LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC, as joint lead arrangers and joint bookrunners, filed as Exhibit 4.1 to the Company’s Current Report on Form 8‑K filed on February 3, 2012 (File No. 1‑11846), is hereby incorporated by reference (replaced herein by Exhibit 10.36). |
10.36 | Amendment No. 1 to Credit Agreement dated as of January 31, 2013 by and among AptarGroup, Inc., and the financial institutions party thereto as Lenders and Wells Fargo Bank, National Association, as administrative agent and swingline lender, filed as Exhibit 4.4 to the Company’s Annual Report on Form 10‑K filed on February 28, 2013 (File No. 1‑11846), is hereby incorporated by reference. |
10.37 | Amendment No. 2 to Credit Agreement dated as of January 31, 2014 by and among AptarGroup, Inc., and the financial institutions party thereto as Lenders and Wells Fargo Bank, National Association, as administrative agent and swingline lender, filed as Exhibit 10.31 to the Company’s annual report on Form 10‑K for the fiscal year ended December 31, 2013 (File No. 1‑11846), is hereby incorporated by reference. |
10.38 | Amendment No. 3 to Credit Agreement, dated as of December 16, 2014, among the Company, the financial institutions party thereto as Lenders and Wells Fargo Bank, National Association, as administrative agent and swingline lender, filed as Exhibit 10.1 to the Company’s Current Report on Form 8‑K filed on December 17, 2014 (File No. 1‑11846), is hereby incorporated by reference. |
21* | List of Subsidiaries. |
23* | Consent of Independent Registered Public Accounting Firm. |
31.1* | Certification Pursuant to Section 302 of the Sarbanes‑Oxley Act of 2002. |
31.2* | Certification Pursuant to Section 302 of the Sarbanes‑Oxley Act of 2002. |
32.1* | Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes‑Oxley Act of 2002. |
32.2* | Certification Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes‑Oxley Act of 2002. |
101* | The following financial information from AptarGroup, Inc.’s Annual Report on Form 10‑K for the fiscal year ended December 31, 2015, filed with the SEC on February 25, 2016, formatted in Extensible Business Reporting Language (XBRL): (1) the consolidated statements of income for the years ended December 31, 2015, 2014 and 2013, (2) the consolidated statements of comprehensive income for the years ended December 31, 2015, 2014 and 2013, (3) the consolidated balance sheets as of December 31, 2015 and 2014, (4) the consolidated statements of cash flows for the years ended December 31, 2015, 2014 and 2013, (5) the consolidated statements of changes in equity for the years ended December 31, 2015, 2014 and 2013 and (6) notes to the consolidated financial statements, tagged as blocks of text. |
*Filed herewith.
**Management contract or compensatory plan or arrangement.

