Exhibit 99.1

| Zonagen Annual Meeting September 29, 2004 |

| This presentation contains forward-looking statements and information regarding the future performance and actions of Zonagen that involve risks and uncertainties that could cause actual results and actions to differ materially from those set forth in the presentation, including, but not limited to, failure to begin and complete the clinical trials associated with Progenta and Androxal as set forth in this presentation and Zonagen's ability to raise additional capital on acceptable terms or at all. We refer you to the documents that Zonagen files from time to time with the Securities and Exchange Commission, including Zonagen's annual report on Form 10-K for the fiscal year ended December 31, 2003 and the Forms 10-Q and Forms 8-K filed during 2004, which contain additional factors that could cause actual results to differ from Zonagen's current expectations and from the forward-looking statements made in this presentation. |

| INVESTMENT HIGHLIGHTS Focused on large, underserved markets Uterine fibroids affect 8mm women in the US - no current drug cure Endometriosis affects 5mm women in the US Testosterone market is greater than $375mm in US alone Progenta offers significant potential Multiple indications Compelling preclinical data Competing compounds cause significant loss of bone mineral density Androxal has demonstrated safety and superior efficacy Only oral non-testosterone therapy Strong IP Attractive valuation for company with multiple compounds in the clinic Experienced management Multiple milestones to drive value |

| COMPANY OVERVIEW POST DUTCH TENDER OFFER Four employees President, CFO, Chief Scientist, Office Manager Company operates in near virtual fashion Lower overhead cost Use of CROs New focused board Currently seven members Experience includes FDA, big pharma and biotech |
| KEY FINANCIAL DATA Cash @ 6/30/04 = $7.62 million Projected 2004 cash burn = $5.2 million Approx. 5 million shares outstanding |
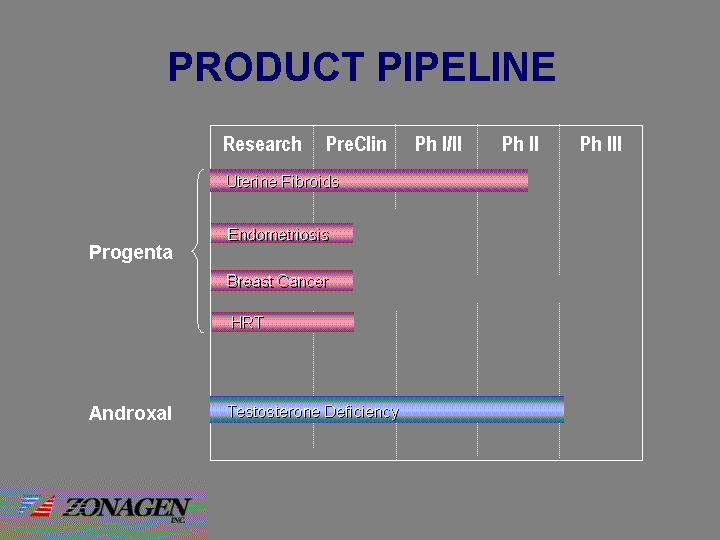
| PRODUCT PIPELINE Research PreClin Progenta Androxal Research PreClin Ph I/II Ph II Ph III Uterine Fibroids Uterine Fibroids Endometriosis Endometriosis Breast Cancer Breast Cancer Testosterone Deficiency Testosterone Deficiency HRT HRT Research PreClin Progenta Androxal Research PreClin Ph I/II Ph II Ph III Uterine Fibroids Uterine Fibroids Endometriosis Endometriosis Breast Cancer Breast Cancer Testosterone Deficiency Testosterone Deficiency HRT HRT |
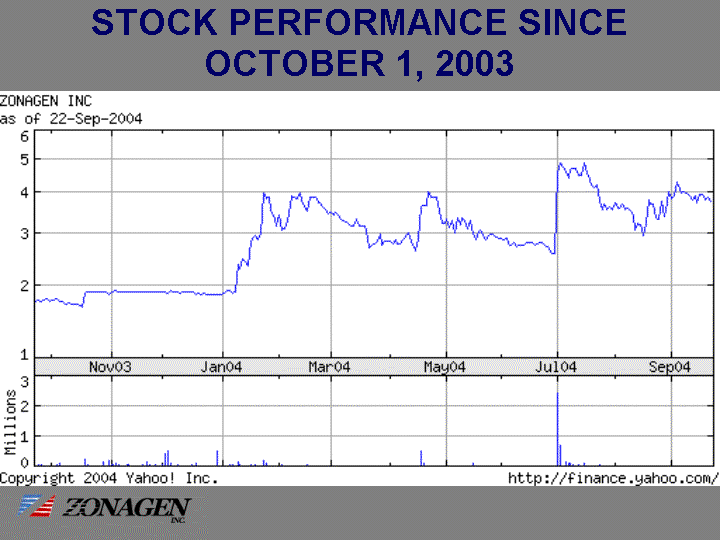
| STOCK PERFORMANCE SINCE OCTOBER 1, 2003 |

| PROGENTA OVERVIEW New class of [ANTIPROGESTIN] progesterone receptor modulator Potential indications: uterine fibroids, endometriosis, breast cancer and HRT Potential significant advantages over existing therapies No loss of bone mineral density in clinical trials No other drugs available for fibroids Composition of matter patents Development status: Phase I/II in Europe ongoing Pre-IND meeting in US planned for 2H04 |

| UTERINE FIBROIDS 30% of women > Age 30 8,000,000 women in US experience abnormal bleeding due to fibroids Most frequent indication for hysterectomy $1-$1.5 Billion Spent Annually in US No current drug cure |
| PHASE I/II UTERINE FIBROID TRIAL Design: N=30 Women with signs and symptoms of uterine fibroids Doses of 0, 12.5, 25, 50 mg Progenta and Lupron Treated for 1 month with potential for 2 month extension Endpoints: Safety, PK and efficacy trial Reduction in fibroid size Positive change in hematocrit Bone loss markers |
| ENDOMETRIOSIS 5 million women in US affected 10-15% of Women Ages 25-44 No acceptable chronic therapy Chronic GnRH agonist therapy results in bone loss and severe hot flashes Laparoscopic surgery and pregnancy provide relief |

| ANDROXAL OVERVIEW Orally administered, centrally acting antagonist that induces production of endogenous testosterone Indication: Restoration of normal testosterone levels Advantages over existing therapies Superior efficacy data in clinical trials Only oral non-testosterone therapy (oral testosterone has major side effects) Capable of replicating normal circadian testosterone levels Significantly lower systemic DHT levels than dermal preparations Drug composition patents filed Recently reported positive Phase I/II results |
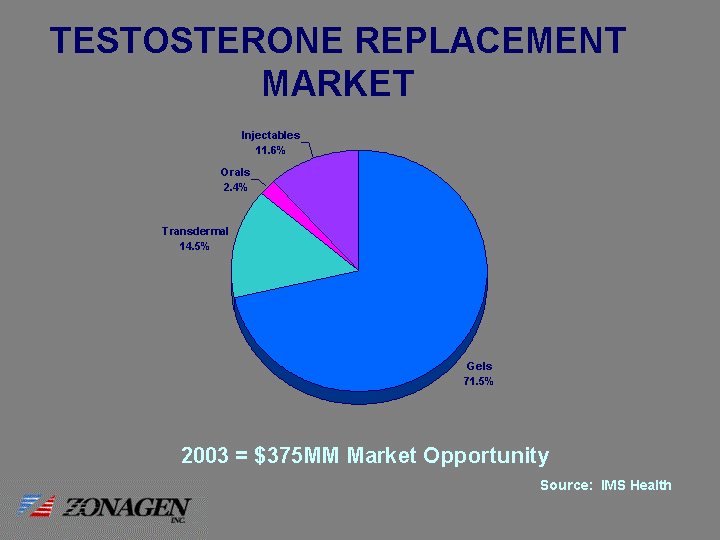
| TESTOSTERONE REPLACEMENT MARKET Gels Transdermal Orals Injectables 71.5 14.5 2.4 11.6 2003 = $375MM Market Opportunity Source: IMS Health |
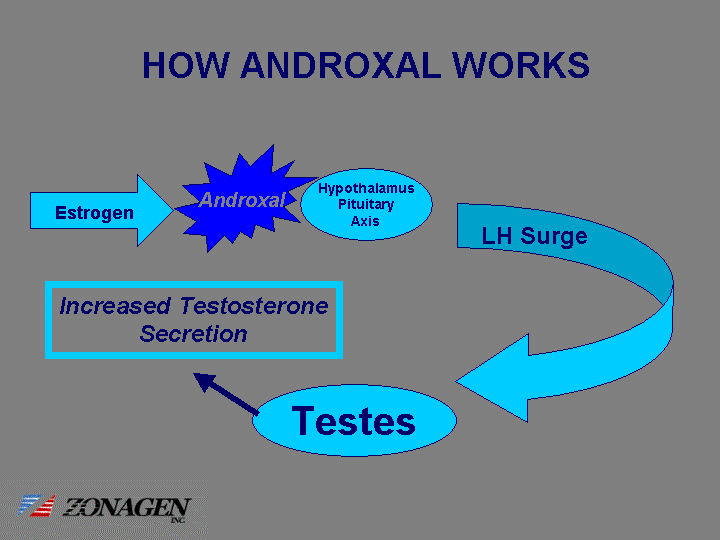
| HOW ANDROXAL WORKS Hypothalamus Pituitary Axis Testes Estrogen Androxal LH Surge Increased Testosterone Secretion |
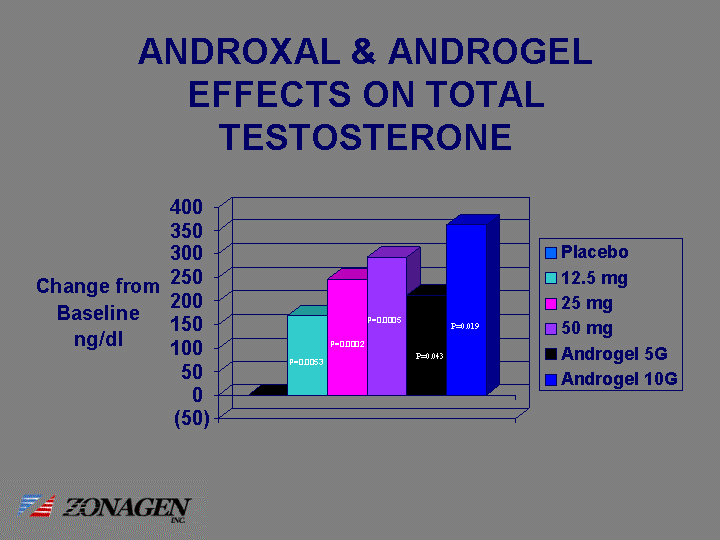
| ANDROXAL & ANDROGEL EFFECTS ON TOTAL TESTOSTERONE P=0.0053 P=0.0002 P=0.0005 P=0.043 P=0.019 |
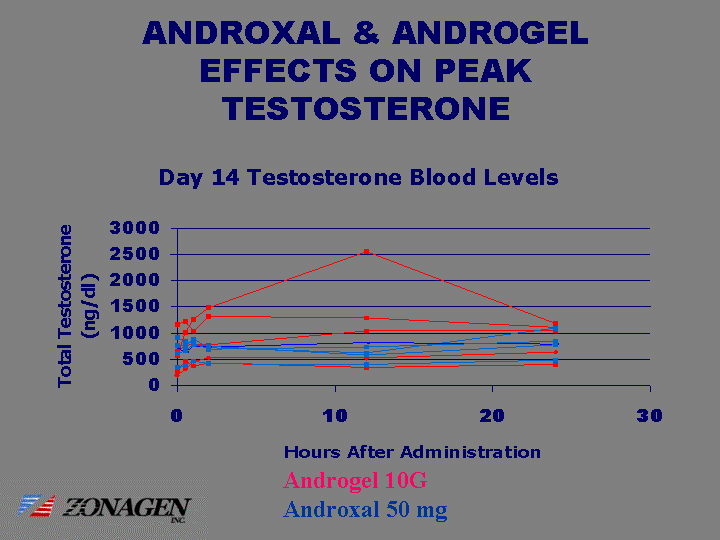
| ANDROXAL & ANDROGEL EFFECTS ON PEAK TESTOSTERONE 0 0.5 1 2 12 24 Androgel 192 325 457 523 523 635 Androgel 270 470 373 435 351 401 Androgel 651 761 798 776 1036 1036 Androgel 1163 1221 1046 1321 1293 1113 Androgel 568 1016 1268 1493 2552 1183 50 mg 347 389 470 419 402 482 50 mg 768 770 883 720 626 1104 50 mg 709 634 747 742 819 793 50 mg 607 663 775 750 586 779 50mg 920 851 805 701 739 848 Androgel 10G Androxal 50 mg |

| ANDROXAL INTELLECTUAL PROPERTY Drug composition patent application filed Currently involved in IP dispute Other party holds patent on treatment of androgen deficiency using selective Clomid and its isomers The PTO has reexamined the other patent and has rejected all claims Other party has until Nov. 9th 2004 to respond |

| 2004 MAJOR MILESTONES First Half Commenced European Phase I/II Uterine Fibroid Study Reported US Androxal Phase I/II Results Second Half Report Progenta Phase I/II Results for Fibroids Publish Results of Primate Endometriosis Study Progenta Pre-IND Meeting Androxal End-of-Study Meeting |

| 2005 MAJOR MILESTONES First Half Commence US Phase II/III Uterine Fibroid Study Commence US Phase II Endometriosis Study US Phase II/III Androxal Study Second Half Report Progenta Phase II/III Results for Fibroids Report Progenta Phase II Results for Endometriosis Report Androxal Phase II/III Results |