
Developing clinical stage small molecule therapeutics to treat hormonal and reproductive system disorders

Repros Disclaimer Any statements made by the Company that are not historical facts contained in this release are forward - looking statements that involve risks and uncertainties, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of its Androxal ® and Proellex ® programs, have success in the clinical development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks which are identified in the Company's most recent Annual Report on Form 10 - K and in any subsequent quarterly reports on Form 10 - Q. These documents are available on request from Repros Therapeutics or at www.sec.gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise.
Investment Highlights • Focused strategy: small molecule therapeutics for reproductive disorders • Two late stage clinical programs each with +$1B sales potential • Androxal ® : PHASE 3 (SPA) oral treatment for Low Testosterone with pending patent/ patent life to the mid 2020’s(growing +$2B market) – Restoration of testicular function and testosterone levels in treatment of 2 º hypogonadism (most common cause of low T) • Proellex : PHASE 2 treatment for uterine fibroids and endometriosis with pending patent/ patent life to the mid 2020’s (+$5B market) – Chronic relief of uterine fibroid symptoms – Fibroid de - bulking – Chronic relief of the symptoms associated with endometriosis – Potential breast cancer intervention • Key late stage clinical & regulatory events driven news flow in 2013
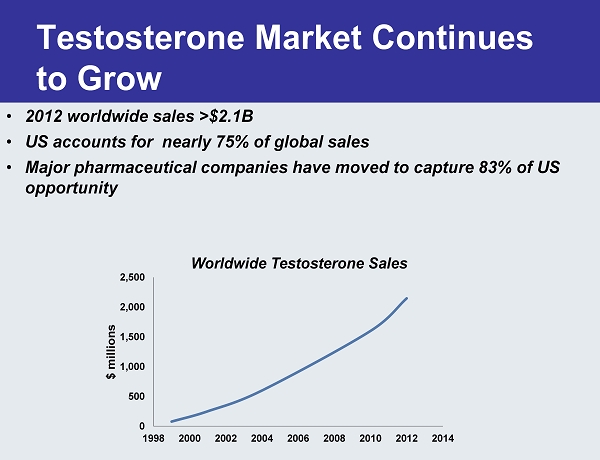
Testosterone Market Continues to Grow 0 500 1,000 1,500 2,000 2,500 1998 2000 2002 2004 2006 2008 2010 2012 2014 $ millions • 2012 worldwide sales >$2.1B • US accounts for nearly 75% of global sales • Major pharmaceutical companies have moved to capture 83% of US opportunity Worldwide Testosterone Sales
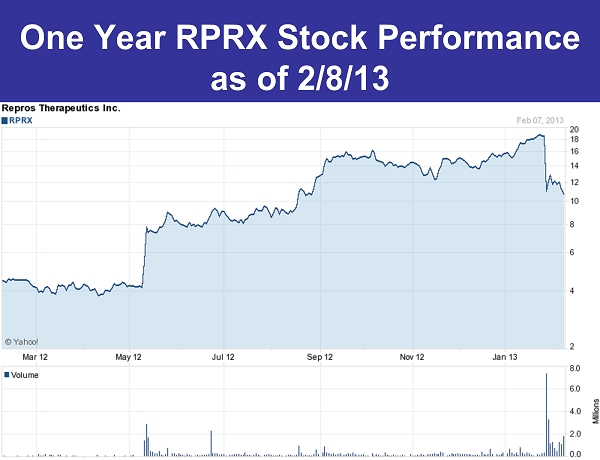
One Year RPRX Stock Performance as of 2/8/13

Recent Events • Company gives guidance that expected top - line results from first pivotal anticipated in Q3 - ’13 • Early January release suggested results available in April • Reason for change – Company advised FDA high enrolling site in both pivotal studies with apparent different population of subjects than other sites – Company suggested enrolling additional subjects into both studies to allow for subset analysis • First study fully enrolled in 2012 • Adding subjects will result in a delay beyond the original April date – Awaiting FDA guidance

Excerpt: FDA SPA Minutes 1) Proportion of subjects with average serum concentration ( Cavg ) for T in the normal range (i.e. serum T of 300 ng / dL – 1040 ng / dL ). 2) Proportion of subjects with a 50% or greater decrease in sperm concentration from baseline to endpoint. To demonstrate efficacy with regards to the first endpoint, at least 75% of subjects in the Androxal group should achieve a Cavg for T in the normal range with the lower bound of the 95% confidence interval not below 67%. At least 100 Androxal subjects would be required to demonstrate a point estimate of 75% or better. For the second endpoint, Androxal should be non - inferior to placebo with respect to the difference in responder rates We have found a 20% non - inferiority margin to be acceptable in prior similar trials. Values for serum T and sperm concentration at baseline and endpoint should be based on at least two assessments. Semen sampling at each time point (baseline and endpoint) should be separated by at least 48 hours. Cmax is an important safety issue. The percentage of patients with Cmax above the following three pre - determined limits (listed below) should be a secondary endpoint: • Cmax >1500 ng / dL • Cmax >1800 ng / dL and <2499 ng / dL • Cmax >2500 ng / dL

ZA - 301 • Mean Age – Site 9 (n=40): 45.9 – Other 16 sites: 46.6 • Mean BMI – Site 9: 29.6 – Other 16 Sites: 31.9 • Baseline Sperm Concentration – Site 9: 17.6 x 10 6 sperm/ mL (StDev:1.7million) – Other 16 Sites: 80.7 x 10 6 sperm/ mL ( StDev : 74 million) • Baseline T – Site 9: 202 ng / dL – Other 16 Sites: 167 ng / dL
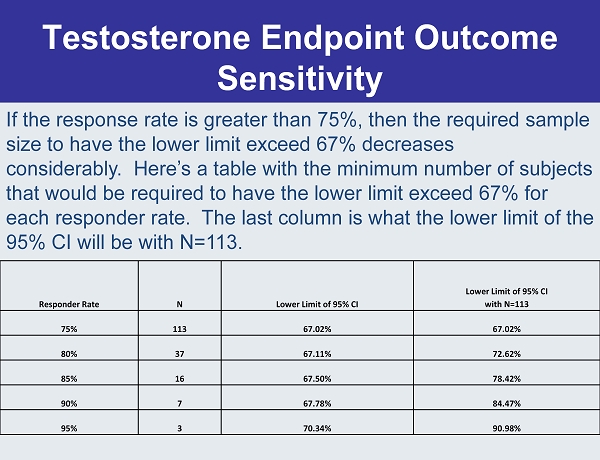
Testosterone Endpoint Outcome Sensitivity Responder Rate N Lower Limit of 95% CI Lower Limit of 95% CI with N=113 75% 113 67.02% 67.02% 80% 37 67.11% 72.62% 85% 16 67.50% 78.42% 90% 7 67.78% 84.47% 95% 3 70.34% 90.98% If the response rate is greater than 75%, then the required sample size to have the lower limit exceed 67% decreases considerably. Here’s a table with the minimum number of subjects that would be required to have the lower limit exceed 67% for each responder rate. The last column is what the lower limit of the 95% CI will be with N=113.
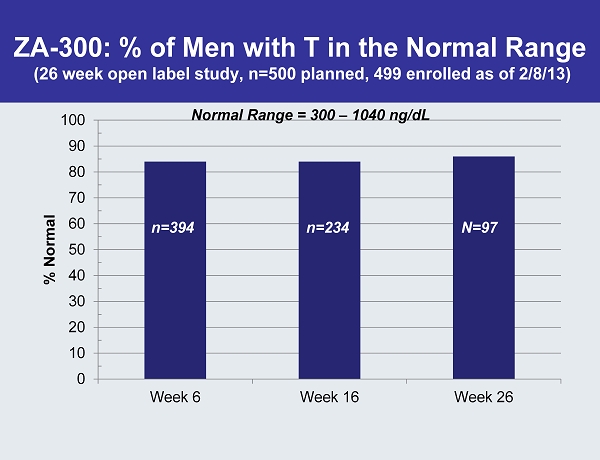
ZA - 300: % of Men with T in the Normal Range (26 week open label study, n=500 planned, 499 enrolled as of 2/8/13) 0 10 20 30 40 50 60 70 80 90 100 Week 6 Week 16 Week 26 % Normal n=394 n=234 N=97 Normal Range = 300 – 1040 ng / dL
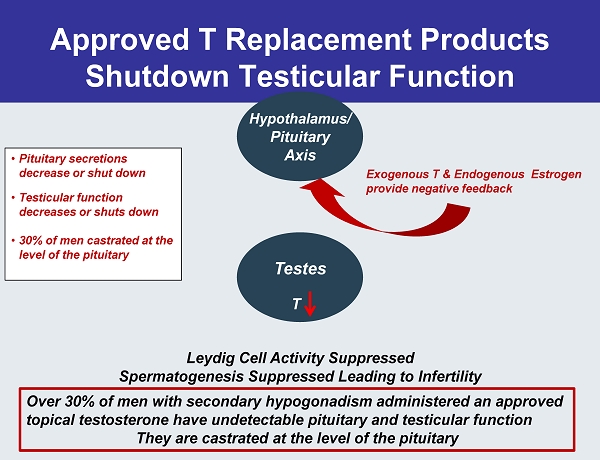
Approved T Replacement Products Shutdown Testicular Function Testes Hypothalamus / Pituitary Axis • Pituitary secretions decrease or shut down • Testicular function decreases or shuts down • 30% of men castrated at the level of the pituitary Leydig Cell Activity Suppressed Spermatogenesis Suppressed Leading to Infertility T Over 30% of men with secondary hypogonadism administered an approved topical testosterone have undetectable pituitary and testicular function They are castrated at the level of the pituitary Exogenous T & Endogenous Estrogen provide negative feedback

Sperm Endpoint Outcome Sensitivity Assuming that 1% of the placebo group and 10% of the Androxal group experience a 50% or greater decrease in sperm concentration with a 3:1 randomization the study requires 114 Androxal /38 placebo/152 total provides for a 0.05 significance level and assuming a non - inferiority limit of 20%. Translating the statistical jargon into English, if no subjects in the placebo arm drop below 50% of their baseline counts up to 14 subjects in the Androxal arm can drop below 50% and the results meet the non - inferiority outcome.
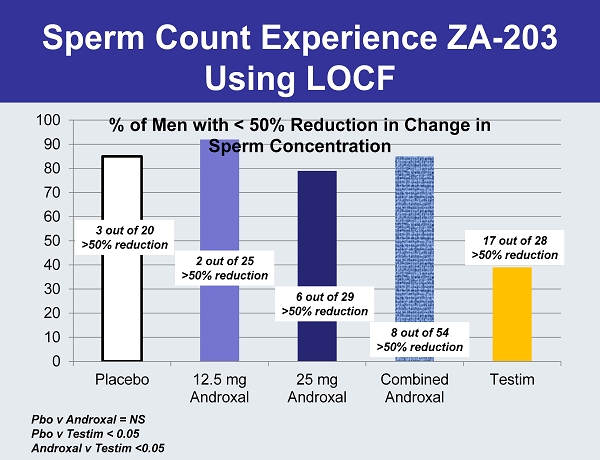
Sperm Count Experience ZA - 203 Using LOCF 0 10 20 30 40 50 60 70 80 90 100 Placebo 12.5 mg Androxal 25 mg Androxal Combined Androxal Testim % of Men with < 50% Reduction in Change in Sperm Concentration 3 out of 20 >50% reduction 2 out of 25 >50% reduction 6 out of 29 >50% reduction 8 out of 54 >50% reduction 17 out of 28 >50% reduction Pbo v Androxal = NS Pbo v Testim < 0.05 Androxal v Testim <0.05
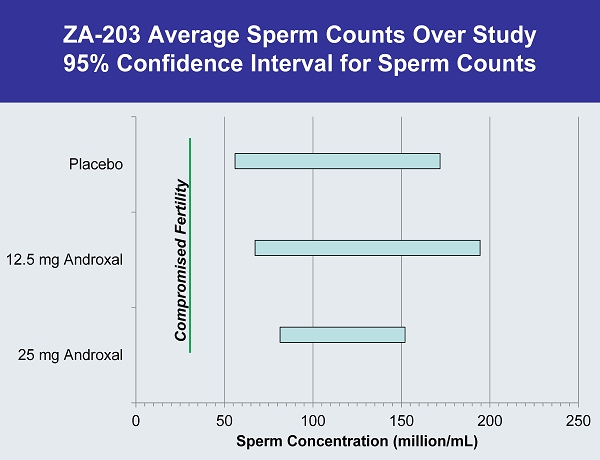
ZA - 203 Average Sperm Counts Over Study 95% Confidence Interval for Sperm Counts 0 50 100 150 200 250 25 mg Androxal 12.5 mg Androxal Placebo Sperm Concentration (million/ mL ) Compromised Fertility
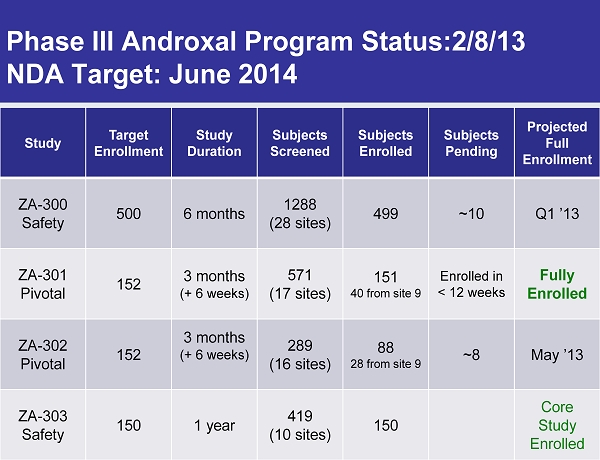
Phase III Androxal Program Status:2/8/13 NDA Target: June 2014 Study Target Enrollment Study Duration Subjects Screened Subjects Enrolled Subjects Pending Projected Full Enrollment ZA - 300 Safety 500 6 months 1288 (28 sites) 499 ~10 Q1 ’13 ZA - 301 Pivotal 152 3 months (+ 6 weeks) 571 (17 sites) 151 40 from site 9 Enrolled in < 12 weeks Fully Enrolled ZA - 302 Pivotal 152 3 months (+ 6 weeks) 289 (16 sites) 88 28 from site 9 ~8 May ’13 ZA - 303 Safety 150 1 year 419 (10 sites) 150 Core Study Enrolled
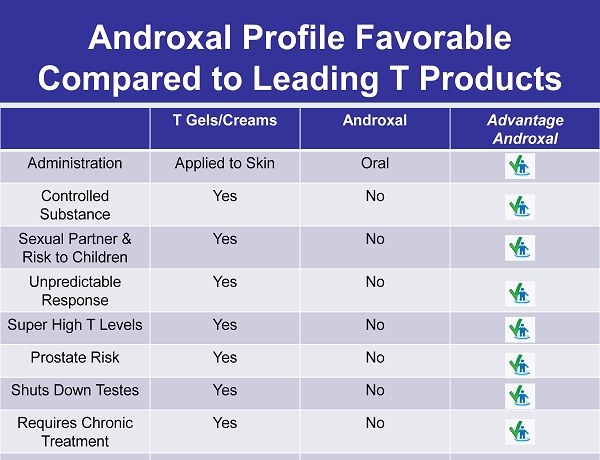
Androxal Profile Favorable Compared to Leading T Products T Gels/Creams Androxal Advantage Androxal Administration Applied to Skin Oral Controlled Substance Yes No Sexual Partner & Risk to Children Yes No Unpredictable Response Yes No Super High T Levels Yes No Prostate Risk Yes No Shuts Down Testes Yes No Requires Chronic Treatment Yes No
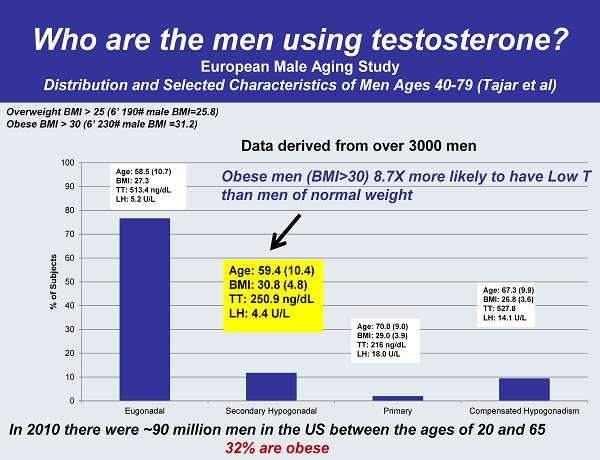
Who are the men using testosterone? European Male Aging Study Distribution and Selected Characteristics of Men Ages 40 - 79 ( Tajar et al) 0 10 20 30 40 50 60 70 80 90 100 Eugonadal Secondary Hypogonadal Primary Compensated Hypogonadism % of Subjects Age: 58.5 (10.7) BMI: 27.3 TT: 513.4 ng / dL LH: 5.2 U/L Age: 59.4 (10.4) BMI: 30.8 (4.8) TT: 250.9 ng / dL LH: 4.4 U/L Age: 70.0 (9.0) BMI: 29.0 (3.9) TT: 216 ng / dL LH: 18.0 U/L Age: 67.3 (9.9) BMI: 26.8 (3.6) TT: 527.8 LH: 14.1 U/L Data derived from over 3000 men Obese men (BMI>30) 8.7X more likely to have Low T than men of normal weight In 2010 there were ~90 million men in the US between the ages of 20 and 65 32% are obese Overweight BMI > 25 (6’ 190# male BMI=25.8) Obese BMI > 30 (6’ 230# male BMI =31.2)

Androxal Preserves Testicular Function Approved Topical Products Suppress the Hypothalamic - Pituitary - Testes Axis
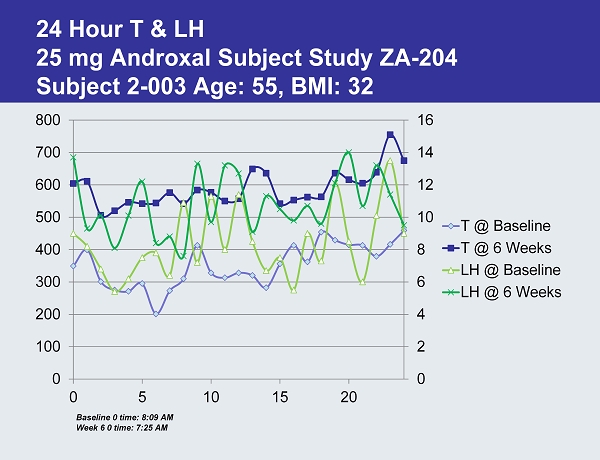
24 Hour T & LH 25 mg Androxal Subject Study ZA - 204 Subject 2 - 003 Age: 55, BMI: 32 0 2 4 6 8 10 12 14 16 0 100 200 300 400 500 600 700 800 0 5 10 15 20 T @ Baseline T @ 6 Weeks LH @ Baseline LH @ 6 Weeks Baseline 0 time: 8:09 AM Week 6 0 time: 7:25 AM
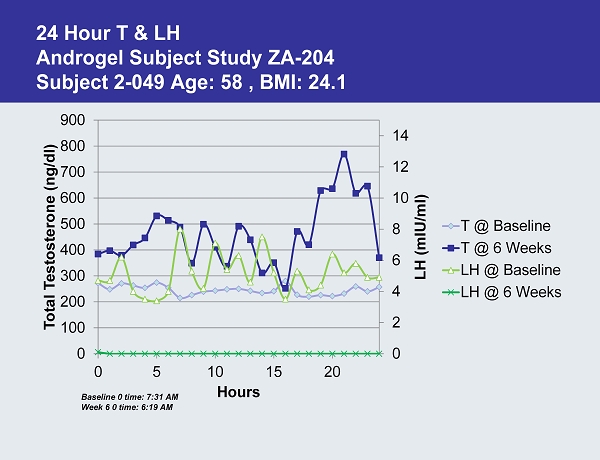
24 Hour T & LH Androgel Subject Study ZA - 204 Subject 2 - 049 Age: 58 , BMI: 24.1 0 2 4 6 8 10 12 14 0 100 200 300 400 500 600 700 800 900 0 5 10 15 20 LH ( mIU /ml) Total Testosterone ( ng /dl) Hours T @ Baseline T @ 6 Weeks LH @ Baseline LH @ 6 Weeks Baseline 0 time: 7:31 AM Week 6 0 time: 6:19 AM
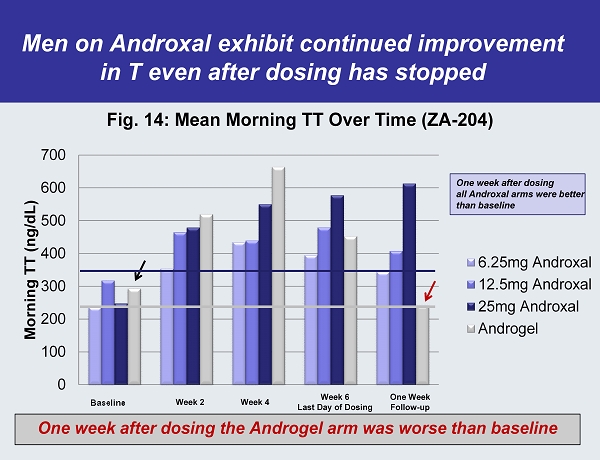
0 100 200 300 400 500 600 700 Morning TT (ng/dL) Fig. 14: Mean Morning TT Over Time (ZA - 204) 6.25mg Androxal 12.5mg Androxal 25mg Androxal Androgel Week 2 Week 4 Week 6 Last Day of Dosing One Week Follow - up One week after dosing all Androxal arms were better than baseline One week after dosing the Androgel arm was worse than baseline Men on Androxal exhibit continued improvement in T even after dosing has stopped
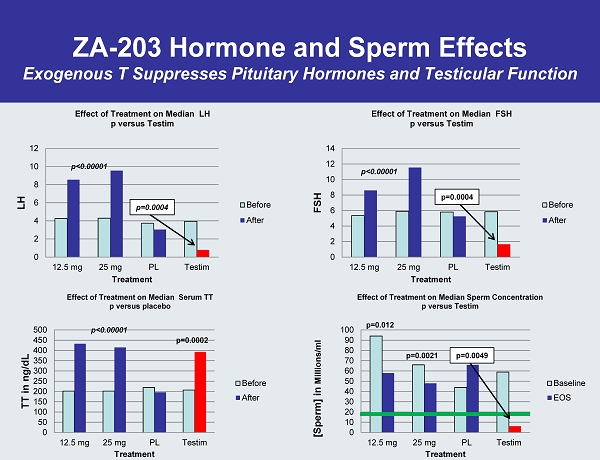
ZA - 203 Hormone and Sperm Effects Exogenous T Suppresses Pituitary Hormones and Testicular Function 0 2 4 6 8 10 12 14 12.5 mg 25 mg PL Testim FSH Treatment Effect of Treatment on Median FSH p versus Testim Before After p<0.00001 p=0.0004 0 10 20 30 40 50 60 70 80 90 100 12.5 mg 25 mg PL Testim [Sperm] in Millions/ml Treatment Effect of Treatment on Median Sperm Concentration p versus Testim Baseline EOS p=0.012 p=0.0021 p=0.0049 0 2 4 6 8 10 12 12.5 mg 25 mg PL Testim LH Treatment Effect of Treatment on Median LH p versus Testim Before After p=0.0004 0 50 100 150 200 250 300 350 400 450 500 12.5 mg 25 mg PL Testim TT in ng/dL Treatment Effect of Treatment on Median Serum TT p versus placebo Before After p<0.00001 p=0.0002 p<0.00001
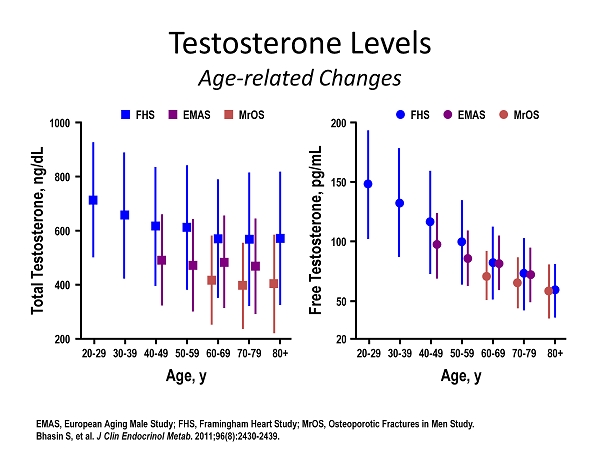
Testosterone Levels Age - related Changes EMAS, European Aging Male Study; FHS, Framingham Heart Study; MrOS, Osteoporotic Fractures in Men Study. Bhasin S, et al. J Clin Endocrinol Metab . 2011;96(8):2430 - 2439. Total Testosterone, ng/dL 20 - 29 30 - 39 40 - 49 50 - 59 60 - 69 70 - 79 1000 800 200 600 400 80+ Age, y FHS EMAS MrOS Free Testosterone, pg/mL 20 - 29 30 - 39 40 - 49 50 - 59 60 - 69 70 - 79 200 150 20 100 50 80+ Age, y FHS EMAS MrOS

Third Party Study Indicates Favorable Reimbursement Potential for Androxal Majority of payers believe Androxal’s oral administration and non - chronic use may offer overall cost savings – Third party assessment of payers indicates vast majority (>90%) would add Androxal to formularies • Cost will be key for tier placement • 50% of plans indicated they would require a PA(Prior Authorization) to show proper diagnosis • 62 % of respondents expect Androxal to be priced at parity to Androgel • Anticipated Androxal pricing of $170 - 350/month would be competitive with Androgel

Androxal Take Home Message • Because of Obesity, 30% of American Males are at Risk of Secondary Hypogonadism – Co - morbidities include diabetes and cardiovascular disease • Approved T Products Worsen the Underlying Condition • We believe only Androxal + Diet + Exercise can reverse this disorder

Proellex for the Treatment of Uterine Fibroids and Endometriosis Over 30 million women of reproductive age in the US afflicted with symptomatic uterine fibroids or endometriosis Over 300,000 hysterectomies performed every year in the US to treat these two disorders No acceptable chronic therapeutic options available today

An Effective Dose of Proellex Stops Menstruation in Majority of Women Proellex induced amenorrhea
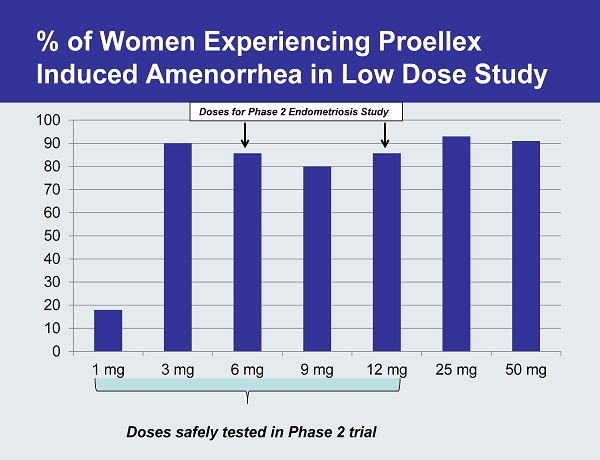
% of Women Experiencing Proellex Induced Amenorrhea in Low Dose Study 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg Doses for Phase 2 Endometriosis Study Doses safely tested in Phase 2 trial
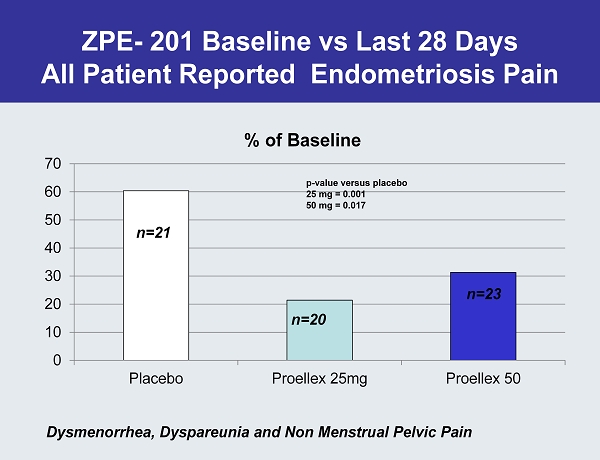
ZPE - 201 Baseline vs Last 28 Days All Patient Reported Endometriosis Pain 0 10 20 30 40 50 60 70 Placebo Proellex 25mg Proellex 50 % of Baseline n=23 p - value versus placebo 25 mg = 0.001 50 mg = 0.017 n=20 n=21 Dysmenorrhea , Dyspareunia and Non Menstrual Pelvic Pain
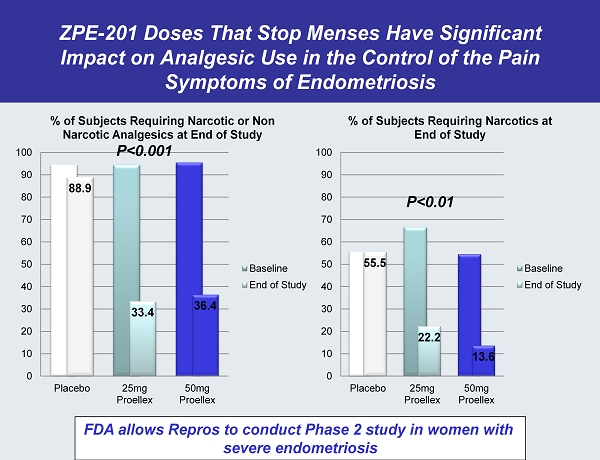
ZPE - 201 Doses That Stop Menses Have Significant Impact on Analgesic Use in the Control of the Pain Symptoms of Endometriosis 88.9 33.4 36.4 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotic or Non Narcotic Analgesics at End of Study Baseline End of Study 55.5 22.2 13.6 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotics at End of Study Baseline End of Study FDA allows Repros to conduct Phase 2 study in women with severe endometriosis P<0.01 P<0.001
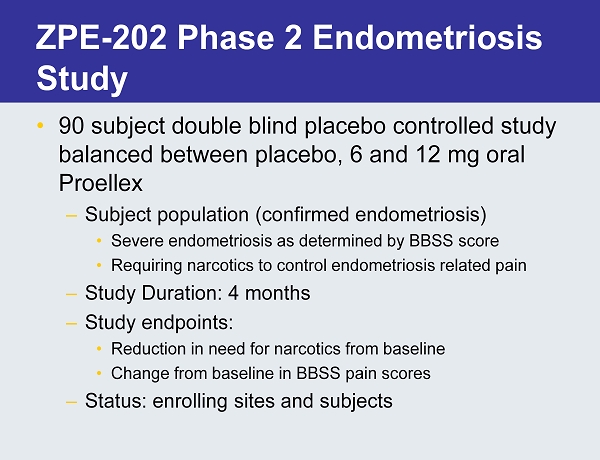
ZPE - 202 Phase 2 Endometriosis Study • 90 subject double blind placebo controlled study balanced between placebo, 6 and 12 mg oral Proellex – Subject population (confirmed endometriosis) • Severe endometriosis as determined by BBSS score • Requiring narcotics to control endometriosis related pain – Study Duration: 4 months – Study endpoints: • Reduction in need for narcotics from baseline • Change from baseline in BBSS pain scores – Status: enrolling sites and subjects
Vaginal Proellex to Eliminate the Need for Hysterectomy in Most Situations – Initial Phase 2 study to test four doses of vaginal administration in the treatment of uterine fibroids completed • Assess reduction of fibroid size and elimination of symptoms • Top line data reported – End of Phase 2 meeting request with FDA for end of April 2013 – Propose 90 subject 1 st Phase 3 study and….. • 2 Phase 3 studies • 200 subjects for +1 year • 300 - 600 subjects for +6 months – Separate IND from low dose
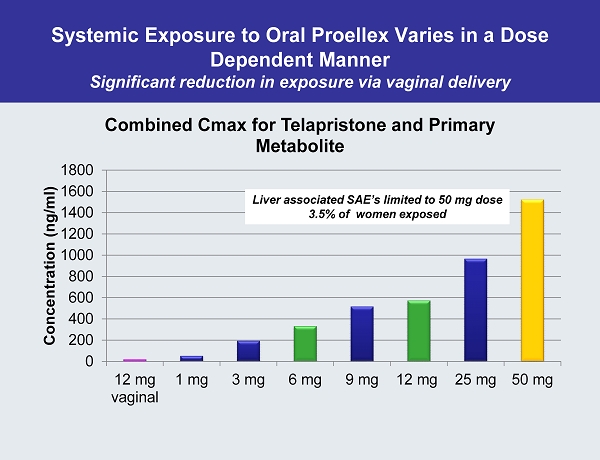
Systemic Exposure to Oral Proellex Varies in a Dose Dependent Manner Significant reduction in exposure via vaginal delivery 0 200 400 600 800 1000 1200 1400 1600 1800 12 mg vaginal 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg Concentration ( ng /ml) Combined Cmax for Telapristone and Primary Metabolite Liver associated SAE’s limited to 50 mg dose 3.5% of women exposed
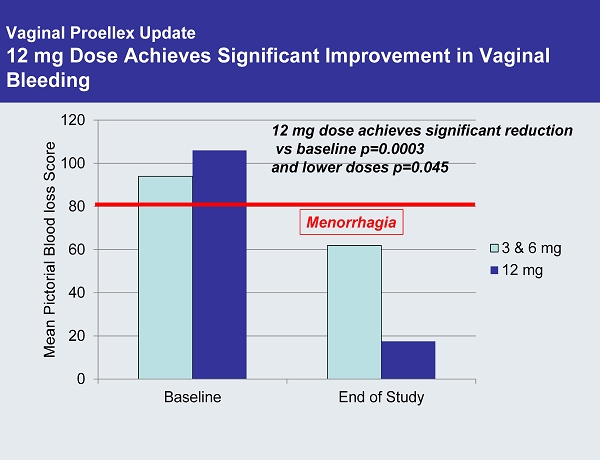
Vaginal Proellex Update 12 mg Dose Achieves Significant Improvement in Vaginal Bleeding 0 20 40 60 80 100 120 Baseline End of Study Mean Pictorial Blood loss Score 3 & 6 mg 12 mg 12 mg dose achieves significant reduction vs baseline p=0.0003 and lower doses p=0.045 Menorrhagia
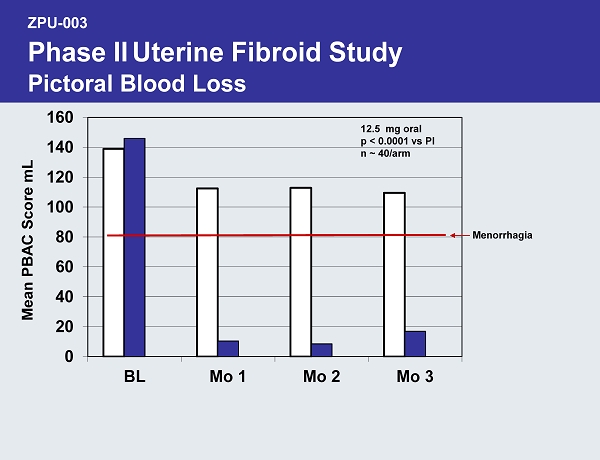
ZPU - 003 Phase II Uterine Fibroid Study Pictoral Blood Loss 0 20 40 60 80 100 120 140 160 BL Mo 1 Mo 2 Mo 3 Mean PBAC Score mL 12.5 mg oral p < 0.0001 vs Pl n ~ 40/arm Menorrhagia
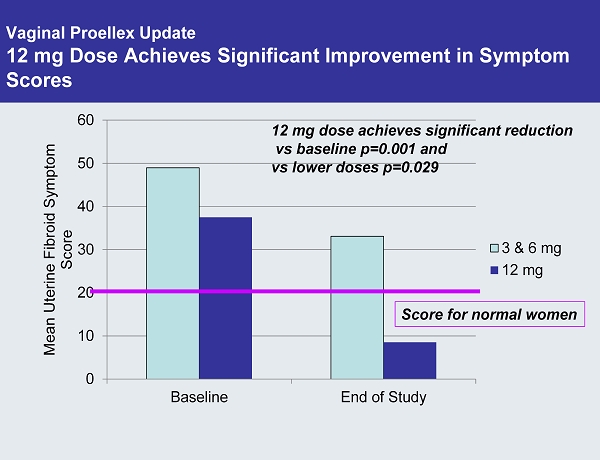
Vaginal Proellex Update 12 mg Dose Achieves Significant Improvement in Symptom Scores 0 10 20 30 40 50 60 Baseline End of Study Mean Uterine Fibroid Symptom Score 3 & 6 mg 12 mg 12 mg dose achieves significant reduction vs baseline p=0.001 and vs lower doses p=0.029 Score for normal women
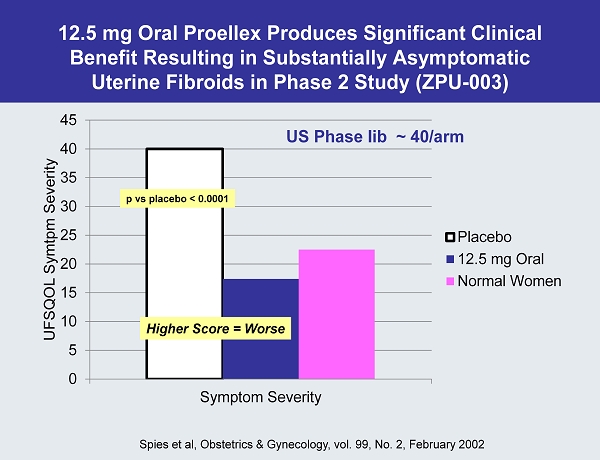
12.5 mg Oral Proellex Produces Significant Clinical Benefit Resulting in Substantially Asymptomatic Uterine Fibroids in Phase 2 Study (ZPU - 003) 0 5 10 15 20 25 30 35 40 45 Symptom Severity UFSQOL Symtpm Severity Placebo 12.5 mg Oral Normal Women US Phase Iib ~ 40/arm Spies et al, Obstetrics & Gynecology, vol. 99, No. 2, February 2002 p vs placebo < 0.0001 Higher Score = Worse
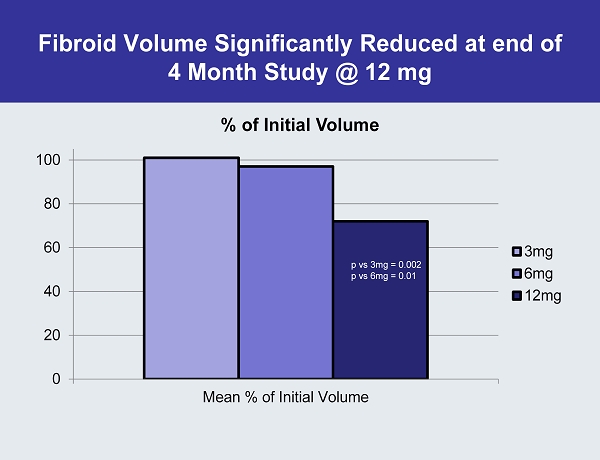
Fibroid Volume Significantly Reduced at end of 4 Month Study @ 12 mg 0 20 40 60 80 100 Mean % of Initial Volume % of Initial Volume 3mg 6mg 12mg p vs 3mg = 0.002 p vs 6mg = 0.01

Financial Summary • Cash and equivalents (as of 1/2/13, unaudited) ~ $24.2M • Cash burn = 2012 = ~$14 M • Cash runway = Mid 2014 • Current shares outstanding – 18.6 M shares – This includes approximately 1.5 million shares resulting from the cashless exercise of 872,133 Series A Warrants and 713,741 Series B Warrants on Jan. 29, 2013. – Warrants Outstanding – Series A – 877,137 (purchased in unit deal @ $2.45); Series B – 855,680 @ $2.49 exercise price.

2013 Milestones • Report results for Phase 2 Vaginal Proellex Study Q1 - 13 • Fully Enroll 1 year Dexa Study Q1 - 13 • Fully Enroll 500 subject 6 mos. Androxal Study Q1 - 13 • Report Results for 1 st Pivotal Androxal Study Q3 - 13 • End of Phase 2 Meeting with FDA for Vaginal Proellex Q2 - 13 • Commence Phase 3 Vaginal Proellex Study Q3 - 13 • Complete 500 subject 6 mos. Androxal Study Q3 - 13 • Report Phase 2 low dose Oral Proellex Study Q4 - 13 • Report 2 nd Pivotal Androxal Study Q4 - 13 • Request Androxal Pre - NDA Meeting with FDA for Q1’14 Q4 - 13 • Submit Androxal NDA Mid - 2014







































