Exhibit 99.1

Enclomiphene ( EnCyzix ™) A Mechanistically Consistent Approach to the Treatment of Secondary Hypogonadism

Repros Disclaimer Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to various risks, uncertainties and other factors that could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by such forward - looking statements . These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “believe,” “plan,” “seek,” “could,” “can,” “should” or similar expressions . These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current conditions, expected future developments and other factors the Company believes are appropriate in these circumstances . Forward - looking statements include, but are not limited to, those relating to anticipated milestones for Enclomiphene and Proellex®, the conduct of planned clinical studies and the timing and nature of the results thereof, the markets for the Company’s products and the potential success of the Company in penetrating those markets and that the Company’s need for and use of financial resources . Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factors that may cause actual events to be materially different from those expressed or implied by such forward - looking statements, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of its Enclomiphene and Proellex® programs, the ability to have success in the clinical development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks as are identified in the Company's most recent Annual Report on Form 10 - K and the subsequent quarterly report on Form 10 - Q and in the prospectus supplement and the accompanying prospectus included in the registration statement mentioned below . These documents are available on request from Repros or at www . sec . gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise . In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry . We obtained this information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates . Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information . While we believe that each of these studies and publications is reliable, we have not independently verified such data, and we make no any representation as to the accuracy of such information . Similarly, we believe our internal research is reliable, but it has not been verified by any independent sources .

CM - 3 Age - Related Hypogonadism In recent years, use of testosterone therapies has increased markedly among middle - aged and elderly men for a controversial condition that the FDA calls “ age - related hypogonadism , ” also referred to as “ late onset hypogonadism , ” a condition which is typically diagnosed in men who, for no discernable reason other than older age , have serum testosterone concentrations below the normal range for healthy young men, as well as signs and symptoms that may or may not be caused by low testosterone concentrations . (Nguyen NEJM 2015)
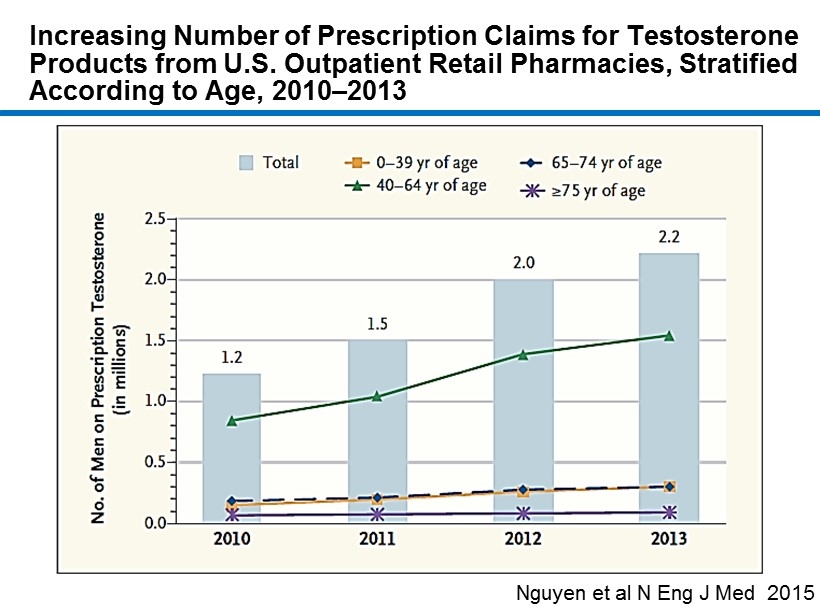
CM - 4 Increasing Number of Prescription Claims for Testosterone Products from U.S. Outpatient Retail Pharmacies, Stratified According to Age, 2010 – 2013 Nguyen et al N Eng J Med 2015
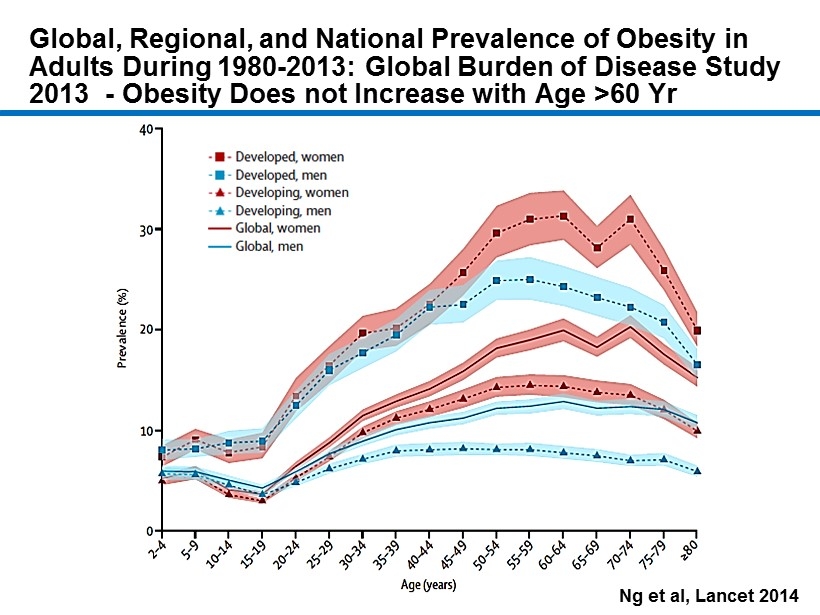
CM - 5 Global, Regional, and National Prevalence of Obesity in Adults During 1980 - 2013: Global Burden of Disease Study 2013 - Obesity Does not Increase with Age >60 Yr Ng et al, Lancet 2014
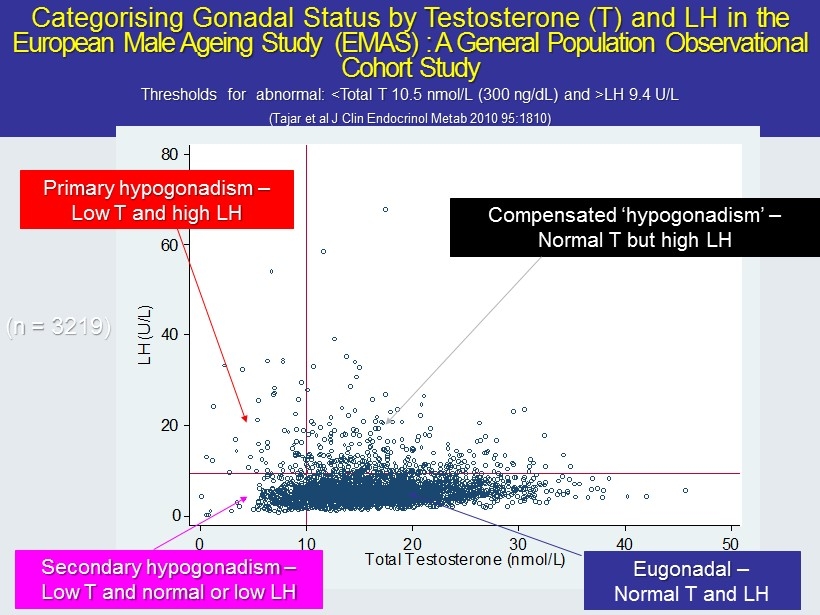
Categorising Gonadal Status by Testosterone (T) and LH in the European Male Ageing Study (EMAS) : A General Population Observational Cohort Study Thresholds for abnormal: <Total T 10.5 nmol /L (300 ng/ dL ) and >LH 9.4 U/L (Tajar et al J Clin Endocrinol Metab 2010 95:1810) 0 20 40 60 80 LH (U/L) 0 10 20 30 40 50 Total Testosterone (nmol/L) Secondary hypogonadism – Low T and normal or low LH Eugonadal – Normal T and LH Primary hypogonadism – Low T and high LH Compensated ‘ hypogonadism ’ – Normal T but high LH (n = 3219)
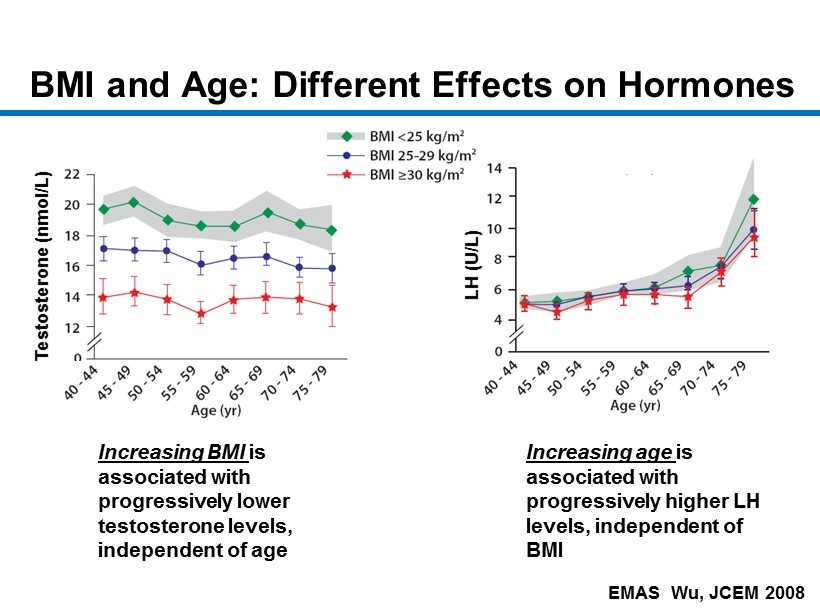
CM - 7 EMAS Wu, JCEM 2008 BMI and Age: Different Effects on Hormones Increasing BMI is associated with progressively lower testosterone levels, independent of age Increasing age is associated with progressively higher LH levels, independent of BMI Testosterone ( nmol /L) LH (U/L)
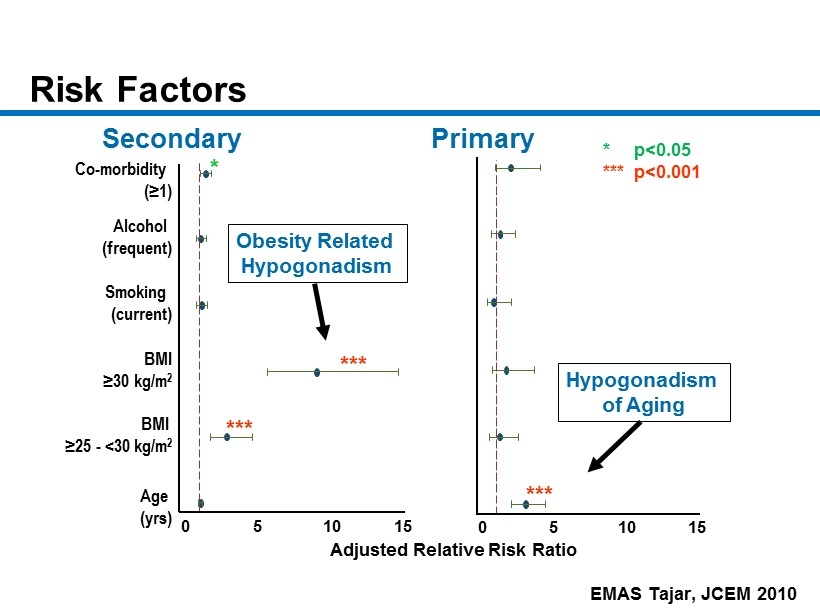
CM - 8 Risk Factors Age ( yrs ) BMI ≥ 25 - <30 kg/m 2 BMI ≥ 30 kg/m 2 Smoking ( current) Alcohol ( frequent) Co - morbidity ( ≥ 1) 0 5 10 15 *** *** * Adjusted Relative Risk Ratio Secondary Obesity Related Hypogonadism EMAS Tajar, JCEM 2010 0 5 10 15 *** Hypogonadism of Aging * p<0.05 *** p<0.001 Primary
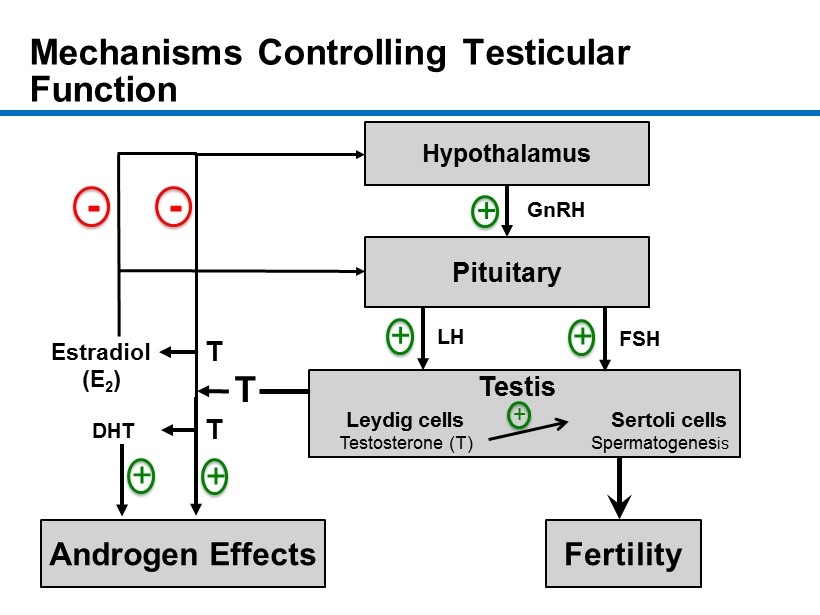
CM - 9 Mechanisms Controlling Testicular Function 9 Hypothalamus Pituitary Androgen Effects Fertility Estradiol (E 2 ) DHT GnRH LH FSH + + + + - T + Testis + - Leydig cells Sertoli cells Testosterone ( T) Spermatogenes is T T
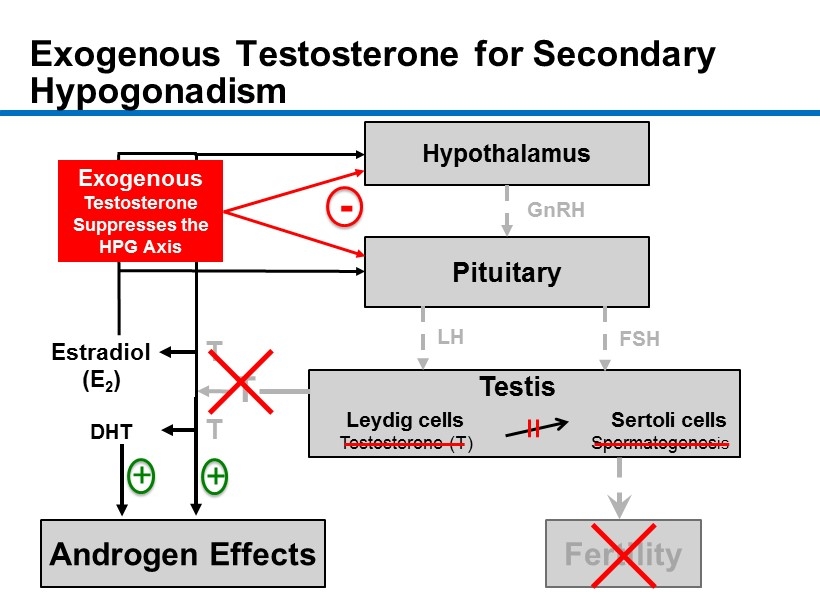
CM - 10 Exogenous Testosterone for Secondary Hypogonadism 10 Hypothalamus Pituitary Androgen Effects Fertility Estradiol (E 2 ) DHT GnRH LH FSH + - T + Testis Leydig cells Sertoli cells Testosterone ( T) Spermatogenes is T T Exogenous Testosterone Suppresses the HPG Axis

CM - 11 So What Do We Know About the Current Group of Men Seeking Treatment for Hypogonadism? The majority are secondary hypogonadal and are fertile Confirmatory diagnosis not practiced in the US No studies conducted by hormone replacement sponsors to assess impact on spermatogenesis “But these men don’t care about fertility” – True for some – Not true for all Modest increase in “off label” scripts for Clomid being written by physicians
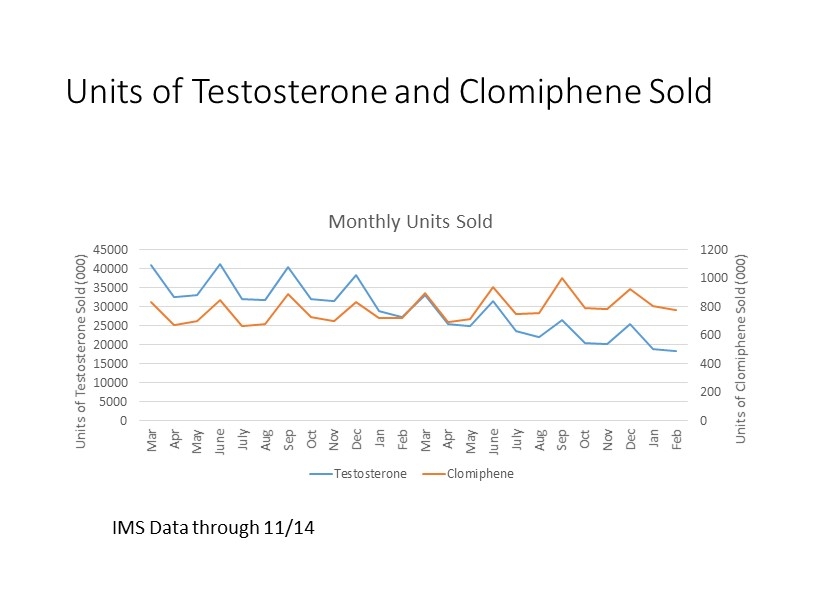
Units of Testosterone and Clomiphene Sold 0 200 400 600 800 1000 1200 0 5000 10000 15000 20000 25000 30000 35000 40000 45000 Mar Apr May June July Aug Sep Oct Nov Dec Jan Feb Mar Apr May June July Aug Sep Oct Nov Dec Jan Feb Units of Clomiphene Sold (000) Units of Testosterone Sold (000) Monthly Units Sold Testosterone Clomiphene IMS Data through 11/14
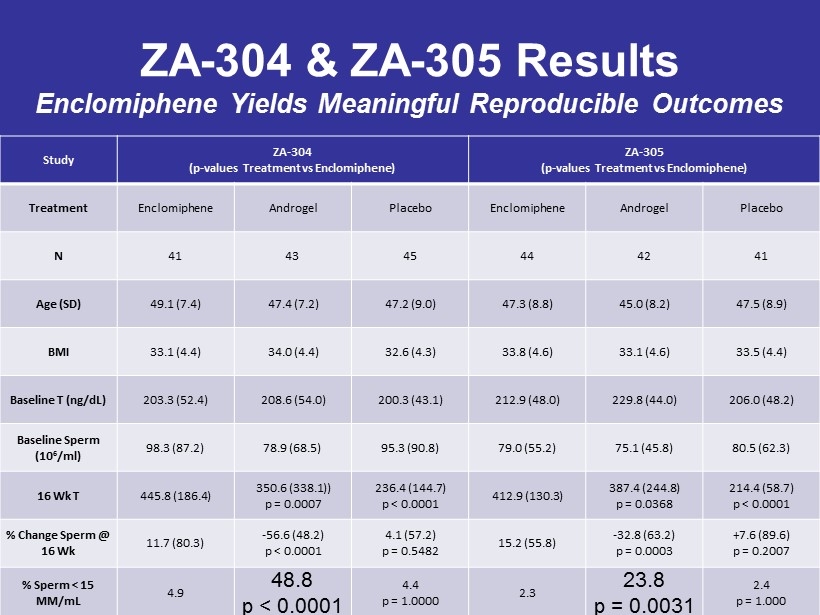
ZA - 304 & ZA - 305 Results Enclomiphene Yields Meaningful Reproducible Outcomes Study ZA - 304 (p - values Treatment vs Enclomiphene) ZA - 305 (p - values Treatment vs Enclomiphene) Treatment Enclomiphene Androgel Placebo Enclomiphene Androgel Placebo N 41 43 45 44 42 41 Age (SD) 49.1 (7.4) 47.4 (7.2) 47.2 (9.0) 47.3 (8.8) 45.0 (8.2) 47.5 (8.9) BMI 33.1 (4.4) 34.0 (4.4) 32.6 (4.3) 33.8 (4.6) 33.1 (4.6) 33.5 (4.4) Baseline T (ng/dL) 203.3 (52.4) 208.6 (54.0) 200.3 (43.1) 212.9 (48.0) 229.8 (44.0) 206.0 (48.2) Baseline Sperm (10 6 /ml) 98.3 (87.2) 78.9 (68.5) 95.3 (90.8) 79.0 (55.2) 75.1 (45.8) 80.5 (62.3) 16 Wk T 445.8 (186.4) 350.6 (338.1)) p = 0.0007 236.4 (144.7) p < 0.0001 412.9 (130.3) 387.4 (244.8) p = 0.0368 214.4 (58.7) p < 0.0001 % Change Sperm @ 16 Wk 11.7 (80.3) - 56.6 (48.2) p < 0.0001 4.1 (57.2) p = 0.5482 15.2 (55.8) - 32.8 (63.2) p = 0.0003 + 7.6 (89.6) p = 0.2007 % Sperm < 15 MM/mL 4.9 48.8 p < 0.0001 4.4 p = 1.0000 2.3 23.8 p = 0.0031 2.4 p = 1.000
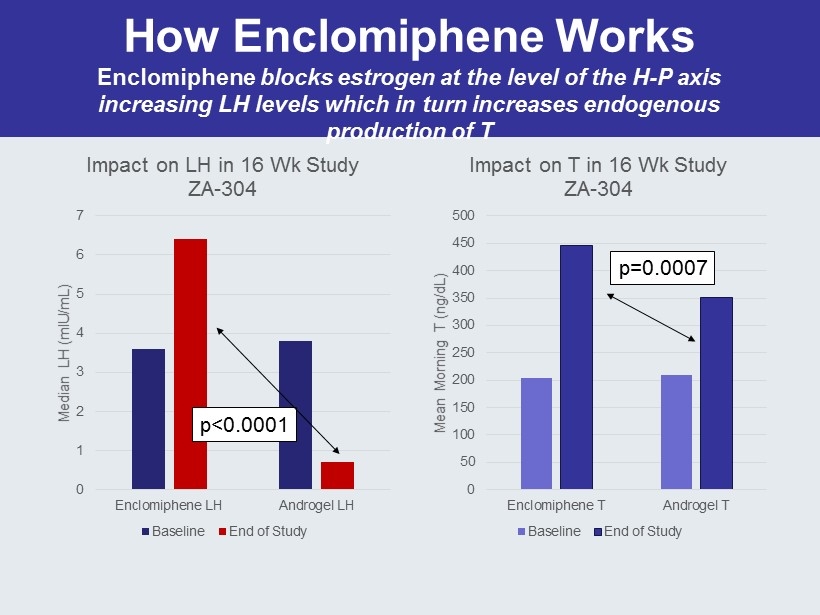
How Enclomiphene Works Enclomiphene blocks estrogen at the level of the H - P axis increasing LH levels which in turn increases endogenous production of T 0 1 2 3 4 5 6 7 Enclomiphene LH Androgel LH Median LH ( mIU /mL) Impact on LH in 16 Wk Study ZA - 304 Baseline End of Study 0 50 100 150 200 250 300 350 400 450 500 Enclomiphene T Androgel T Mean Morning T (ng/ dL ) Impact on T in 16 Wk Study ZA - 304 Baseline End of Study p<0.0001 p=0.0007
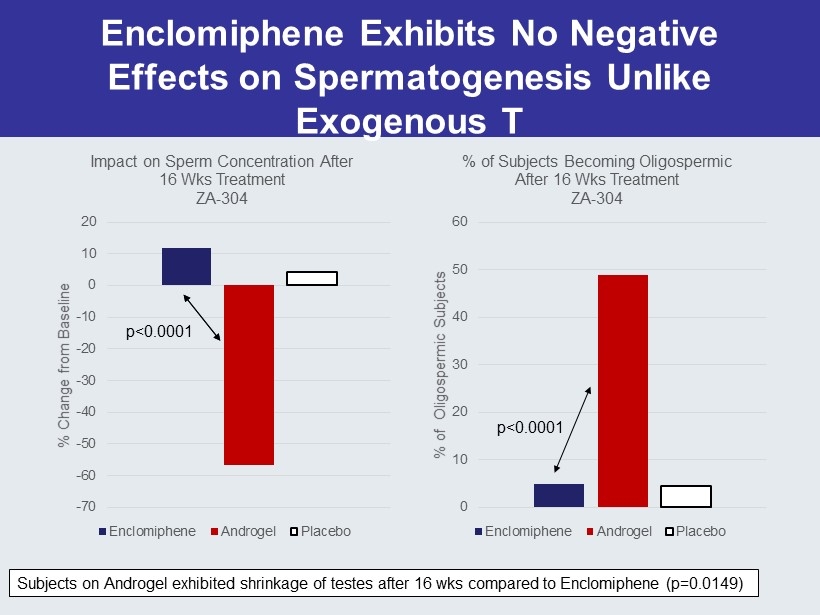
Enclomiphene Exhibits No Negative Effects on Spermatogenesis Unlike Exogenous T -70 -60 -50 -40 -30 -20 -10 0 10 20 % Change from Baseline Impact on Sperm Concentration After 16 Wks Treatment ZA - 304 Enclomiphene Androgel Placebo 0 10 20 30 40 50 60 % of Oligospermic Subjects % of Subjects Becoming Oligospermic After 16 Wks Treatment ZA - 304 Enclomiphene Androgel Placebo Subjects on Androgel exhibited shrinkage of testes after 16 wks compared to Enclomiphene (p=0.0149) p<0.0001 p<0.0001
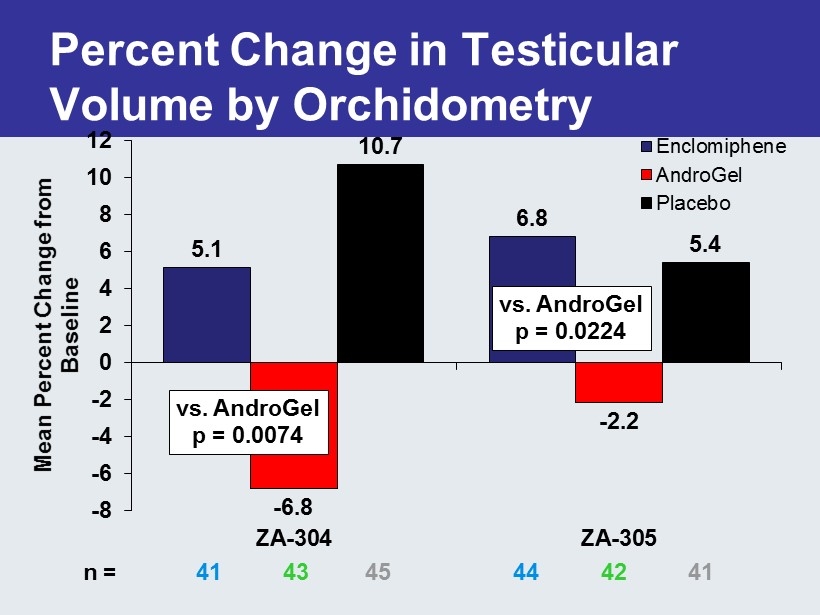
5.1 6.8 - 6.8 - 2.2 10.7 5.4 -8 -6 -4 -2 0 2 4 6 8 10 12 ZA-304 ZA-305 Mean Percent Change from Baseline Enclomiphene AndroGel Placebo Percent Change in Testicular Volume by Orchidometry vs. AndroGel p = 0.0074 vs. AndroGel p = 0.0224 n = 41 43 45 44 42 41
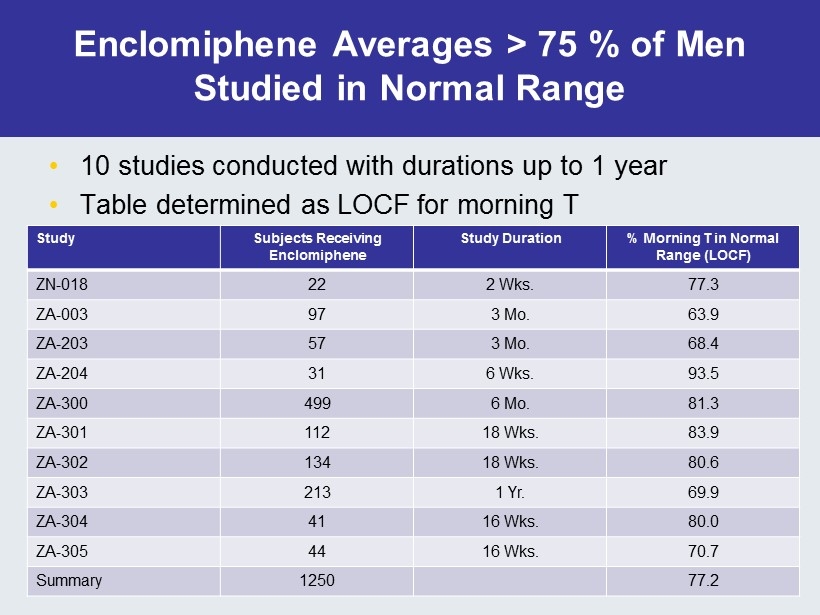
Enclomiphene Averages > 75 % of Men Studied in Normal Range • 10 studies conducted with durations up to 1 year • Table determined as LOCF for morning T Study Subjects Receiving Enclomiphene Study Duration % Morning T in Normal Range (LOCF) ZN - 018 22 2 Wks. 77.3 ZA - 003 97 3 Mo. 63.9 ZA - 203 57 3 Mo. 68.4 ZA - 204 31 6 Wks. 93.5 ZA - 300 499 6 Mo. 81.3 ZA - 301 112 18 Wks. 83.9 ZA - 302 134 18 Wks. 80.6 ZA - 303 213 1 Yr. 69.9 ZA - 304 41 16 Wks. 80.0 ZA - 305 44 16 Wks. 70.7 Summary 1250 77.2

CM - 18 “So is there anything else to worry about if I take T?” “I don’t want to have any more kids” You have a reversible disorder if you are willing to make a lifestyle change If you start taking testosterone you may need to take it for the rest of your life
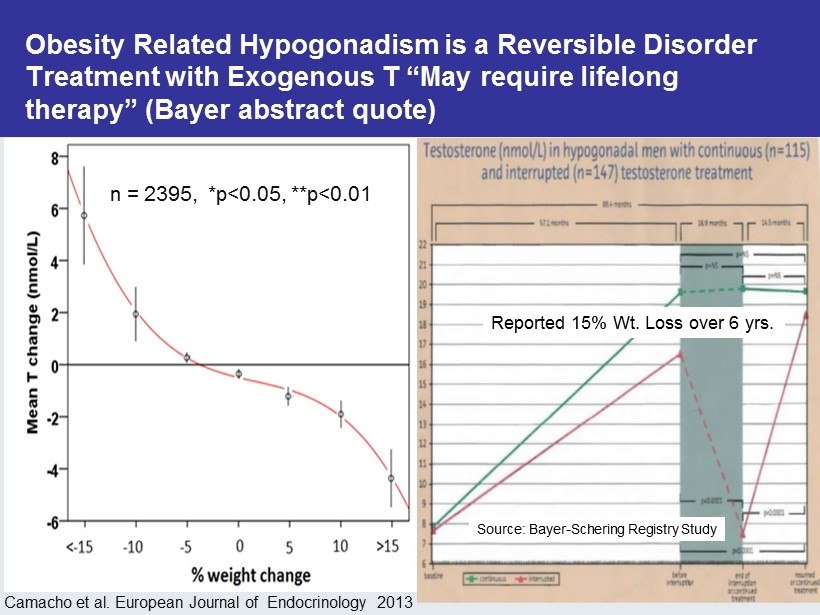
Obesity Related Hypogonadism is a Reversible Disorder Treatment with Exogenous T “May require lifelong therapy” (Bayer abstract quote) Camacho et al. European Journal of Endocrinology 2013 n = 2395, *p<0.05, **p<0.01 Reported 15% Wt. Loss over 6 yrs. Source: Bayer - Schering Registry Study
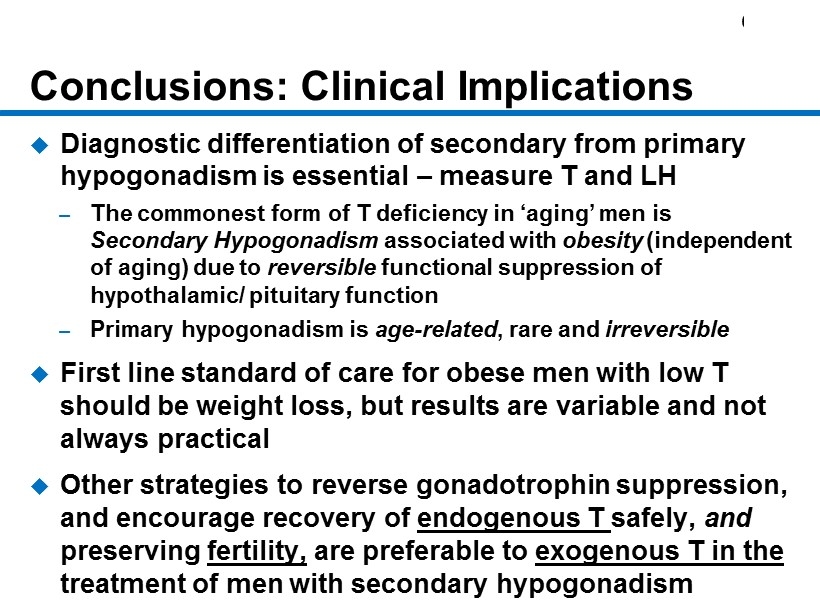
CM - 20 Conclusions: Clinical Implications Diagnostic differentiation of secondary from primary hypogonadism is essential – measure T and LH – The commonest form of T deficiency in ‘aging’ men is Secondary Hypogonadism associated with obesity (independent of aging) due to reversible functional suppression of hypothalamic/ pituitary function – Primary hypogonadism is age - related , rare and irreversible First line standard of care for obese men with low T should be weight loss, but results are variable and not always practical Other strategies to reverse gonadotrophin suppression, and encourage recovery of endogenous T safely, and preserving fertility, are preferable to exogenous T in the treatment of men with secondary hypogonadism
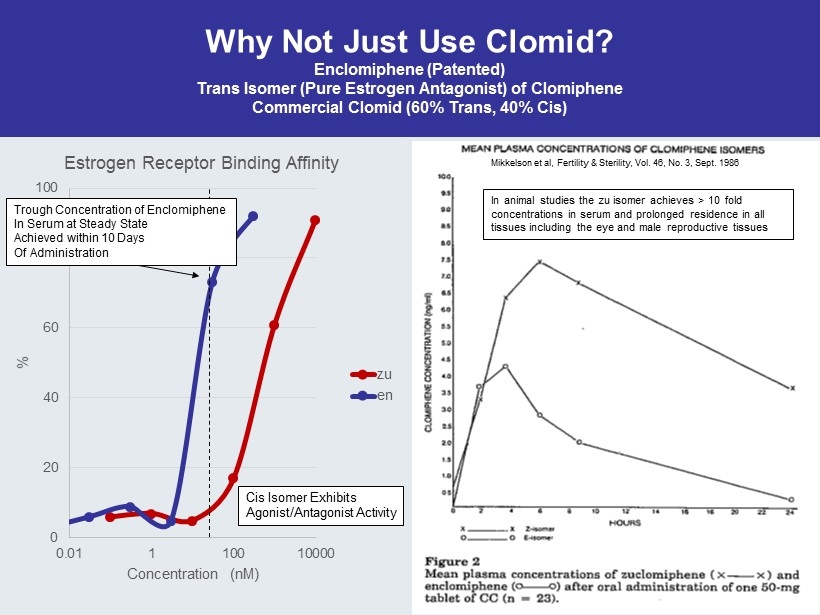
Why Not Just Use Clomid ? Enclomiphene (Patented) Trans Isomer (Pure Estrogen Antagonist) of Clomiphene Commercial Clomid (60% Trans, 40% Cis) 0 20 40 60 80 100 0.01 1 100 10000 % Concentration ( nM ) Estrogen Receptor Binding Affinity zu en Trough Concentration of Enclomiphene In Serum at Steady State Achieved within 10 Days Of Administration Cis Isomer Exhibits Agonist/Antagonist Activity Mikkelson et al, Fertility & Sterility, Vol. 46, No. 3, Sept. 1986 In animal studies the zu isomer achieves > 10 fold concentrations in serum and prolonged residence in all tissues including the eye and male reproductive tissues
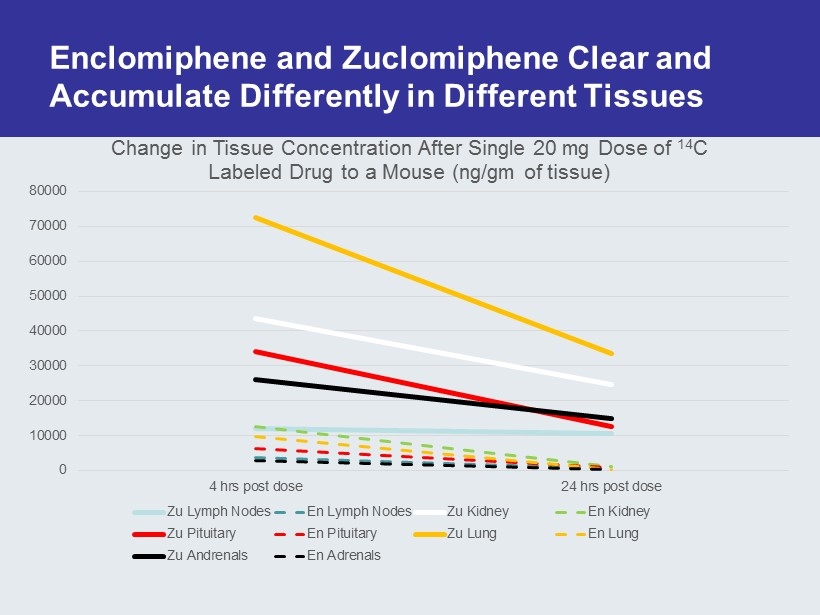
Enclomiphene and Zuclomiphene Clear and Accumulate Differently in Different Tissues 0 10000 20000 30000 40000 50000 60000 70000 80000 4 hrs post dose 24 hrs post dose Change in Tissue Concentration After Single 20 mg Dose of 14 C Labeled Drug to a Mouse (ng/gm of tissue) Zu Lymph Nodes En Lymph Nodes Zu Kidney En Kidney Zu Pituitary En Pituitary Zu Lung En Lung Zu Andrenals En Adrenals
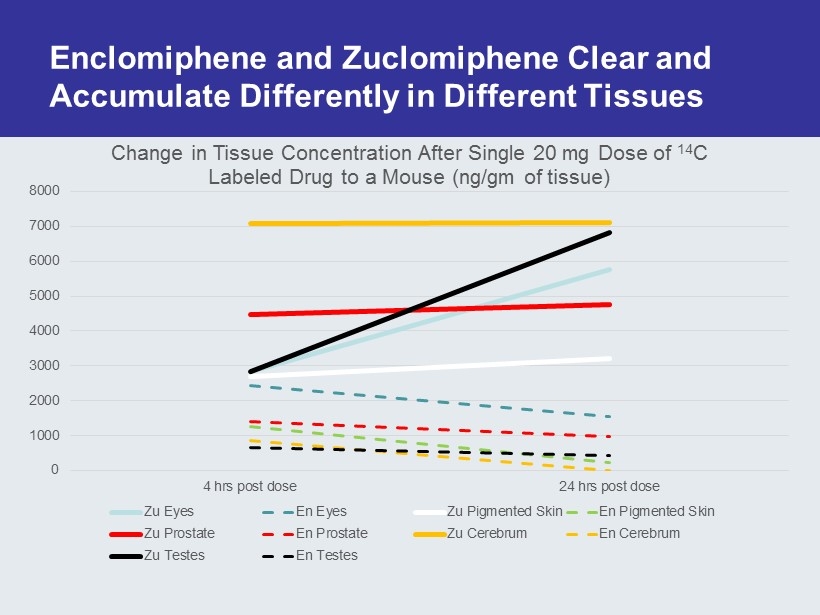
Enclomiphene and Zuclomiphene Clear and Accumulate Differently in Different Tissues 0 1000 2000 3000 4000 5000 6000 7000 8000 4 hrs post dose 24 hrs post dose Change in Tissue Concentration After Single 20 mg Dose of 14 C Labeled Drug to a Mouse (ng/gm of tissue) Zu Eyes En Eyes Zu Pigmented Skin En Pigmented Skin Zu Prostate En Prostate Zu Cerebrum En Cerebrum Zu Testes En Testes
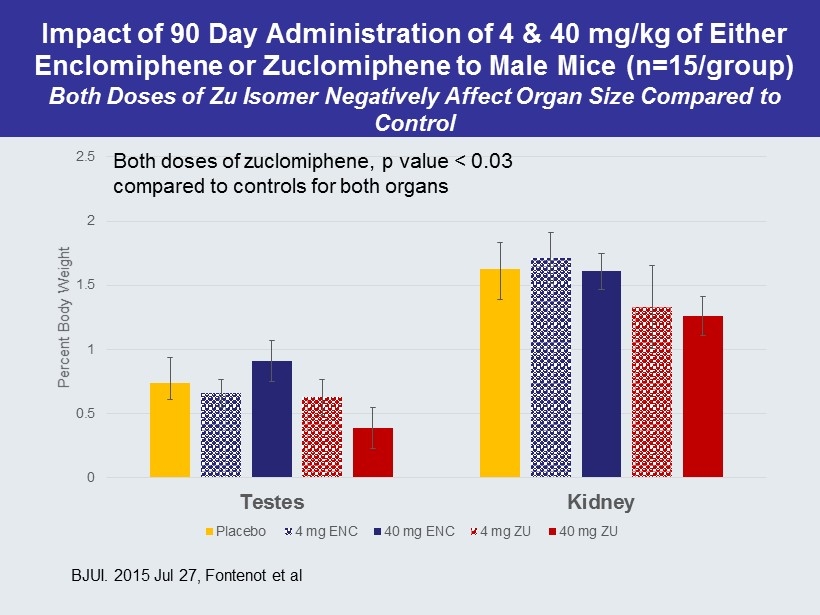
0 0.5 1 1.5 2 2.5 Testes Kidney Percent Body Weight Placebo 4 mg ENC 40 mg ENC 4 mg ZU 40 mg ZU Both doses of zuclomiphene , p value < 0.03 compared to controls for both organs Impact of 90 Day Administration of 4 & 40 mg/kg of Either Enclomiphene or Zuclomiphene to Male Mice (n=15/group) Both Doses of Zu Isomer Negatively Affect Organ Size Compared to Control BJUI. 2015 Jul 27, Fontenot et al
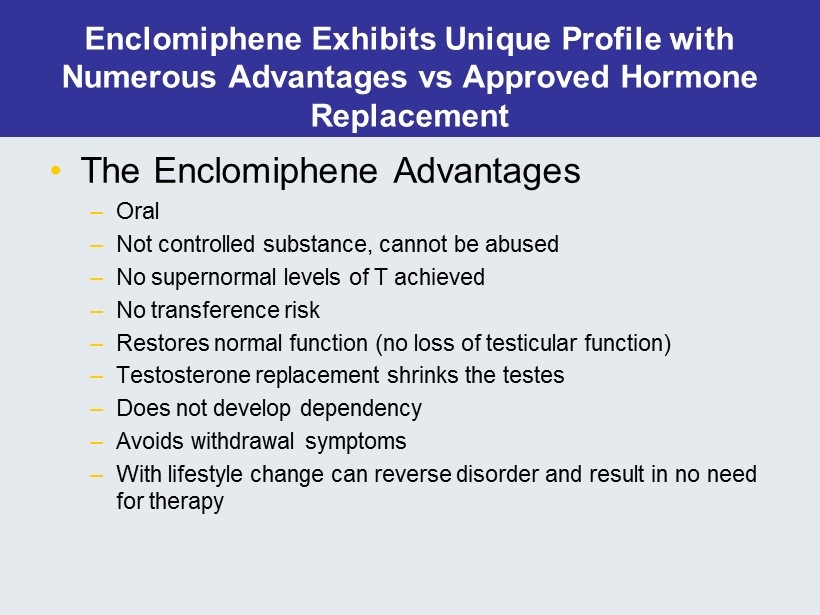
Enclomiphene Exhibits Unique Profile with Numerous Advantages vs Approved Hormone Replacement • The Enclomiphene Advantages – Oral – Not controlled substance, cannot be abused – No supernormal levels of T achieved – No transference risk – Restores normal function (no loss of testicular function ) – Testosterone replacement shrinks the testes – Does not develop dependency – Avoids withdrawal symptoms – With lifestyle change can reverse disorder and result in no need for therapy
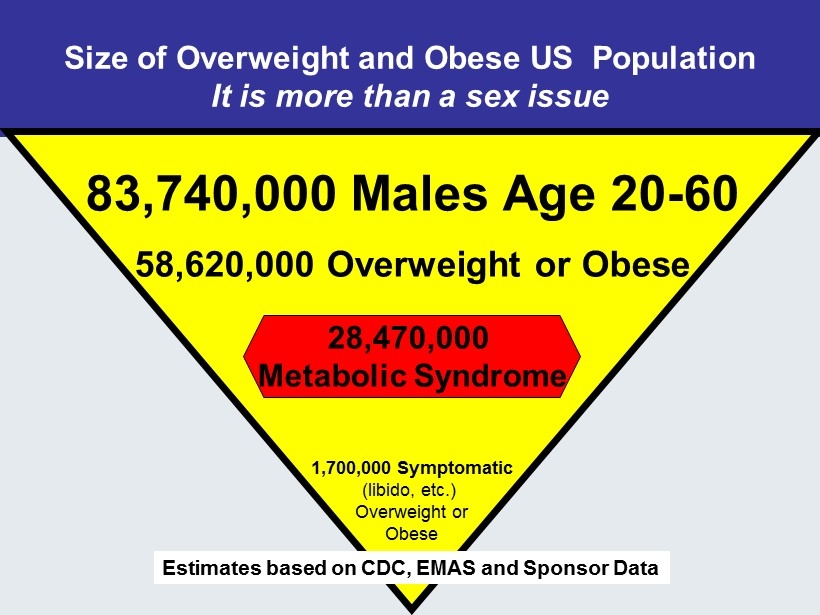
Size of Overweight and Obese US Population It is more than a sex issue 83,740,000 Males Age 20 - 60 58,620,000 Overweight or Obese 28,470,000 Metabolic Syndrome 1,700,000 Symptomatic (libido, etc.) Overweight or Obese Estimates based on CDC, EMAS and Sponsor Data
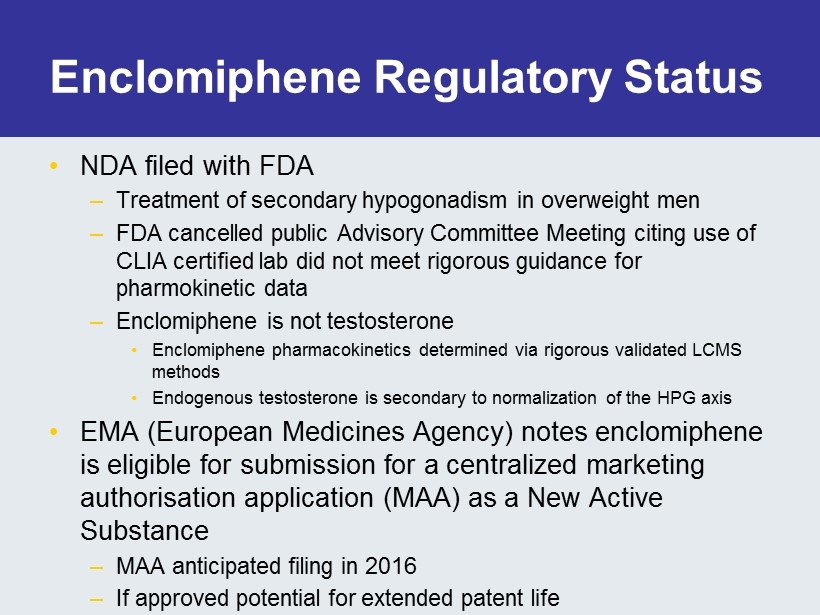
Enclomiphene Regulatory Status • NDA filed with FDA – Treatment of secondary hypogonadism in overweight men – FDA cancelled public Advisory Committee Meeting citing use of CLIA certified lab did not meet rigorous guidance for pharmokinetic data – Enclomiphene is not testosterone • Enclomiphene pharmacokinetics determined via rigorous validated LCMS methods • Endogenous testosterone is secondary to normalization of the HPG axis • EMA (European Medicines Agency) notes enclomiphene is eligible for submission for a centralized marketing authorisation application (MAA) as a New Active Substance – MAA anticipated filing in 2016 – If approved potential for extended patent life
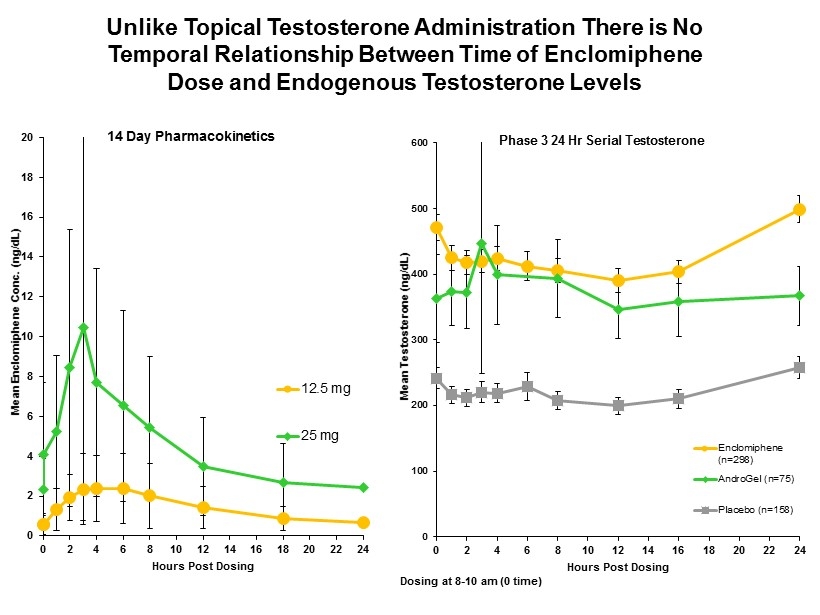
OT - 28 0 100 200 300 400 500 600 0 2 4 6 8 10 12 14 16 18 20 22 24 Mean Testosterone (ng/ dL ) Hours Post Dosing Phase 3 24 Hr Serial Testosterone Enclomiphene (n=298) AndroGel (n=75) Placebo (n=158) Unlike Topical Testosterone Administration There is No Temporal Relationship Between Time of Enclomiphene Dose and Endogenous Testosterone Levels Dosing at 8 - 10 am (0 time) 0 2 4 6 8 10 12 14 16 18 20 0 2 4 6 8 10 12 14 16 18 20 22 24 Mean Enclomiphene Conc. (ng/ dL ) Hours Post Dosing 14 Day Pharmacokinetics 12.5 mg 25 mg
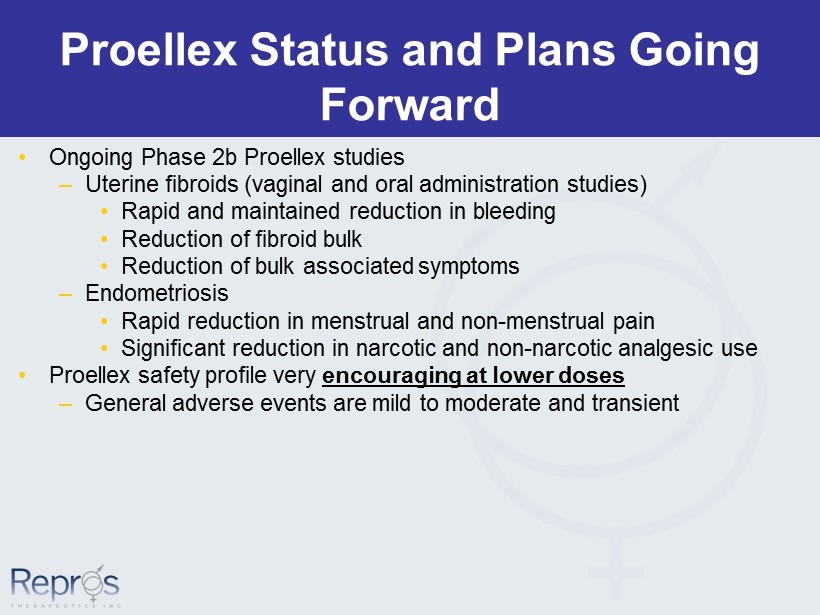
Proellex Status and Plans Going Forward • Ongoing Phase 2b Proellex studies – Uterine fibroids (vaginal and oral administration studies) • Rapid and maintained reduction in bleeding • Reduction of fibroid bulk • Reduction of bulk associated symptoms – Endometriosis • Rapid reduction in menstrual and non - menstrual pain • Significant reduction in narcotic and non - narcotic analgesic use • Proellex safety profile very encouraging at lower doses – General adverse events are mild to moderate and transient
Proellex for the Treatment of Uterine Fibroids and Endometriosis • Over 30 million women of reproductive age in the US afflicted with symptomatic uterine fibroids or endometriosis • Over 300,000 hysterectomies performed every year in the US to treat these two disorders • No acceptable chronic therapeutic options available today
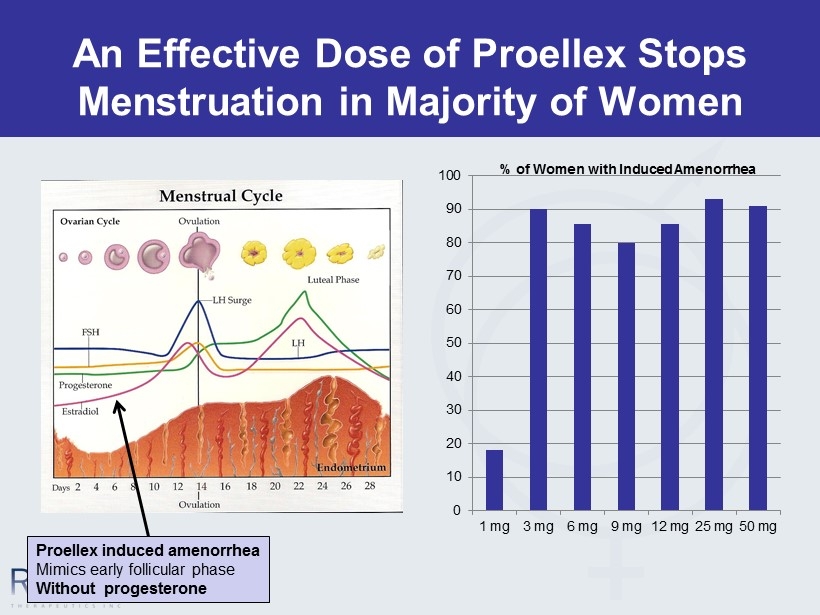
An Effective Dose of Proellex Stops Menstruation in Majority of Women 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg % of Women with Induced Amenorrhea Proellex induced amenorrhea Mimics early follicular phase Without progesterone
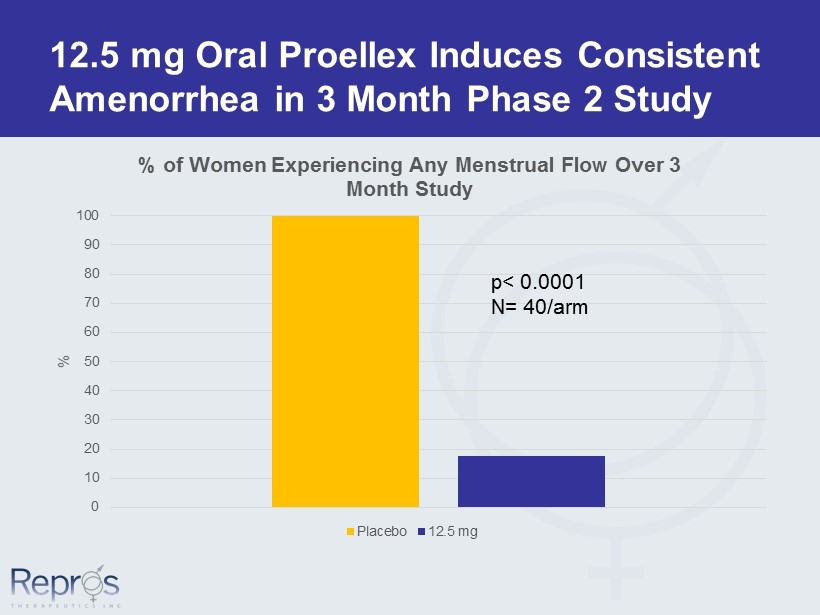
12.5 mg Oral Proellex Induces Consistent Amenorrhea in 3 Month Phase 2 Study 0 10 20 30 40 50 60 70 80 90 100 % % of Women Experiencing Any Menstrual Flow Over 3 Month Study Placebo 12.5 mg p< 0.0001 N= 40/arm
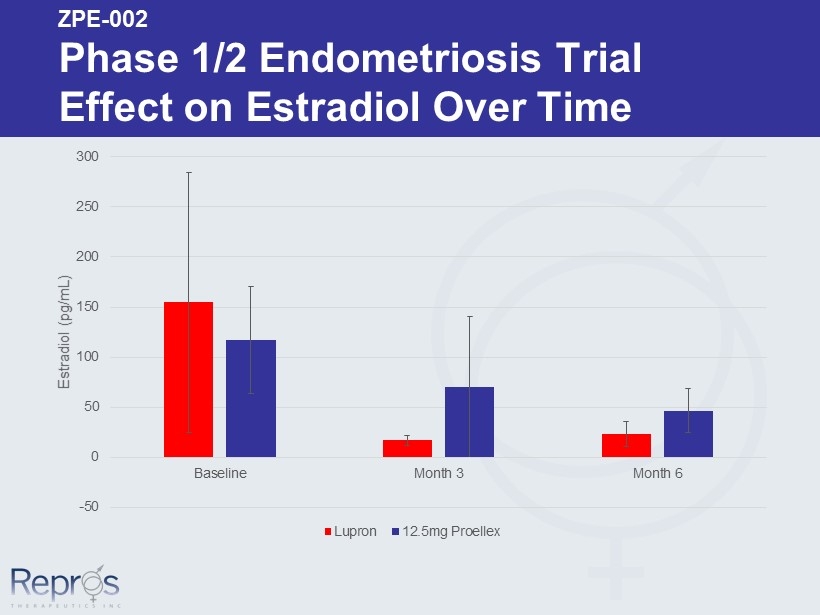
-50 0 50 100 150 200 250 300 Baseline Month 3 Month 6 Estradiol ( pg /mL) Lupron 12.5mg Proellex ZPE - 002 Phase 1/2 Endometriosis Trial Effect on Estradiol Over Time
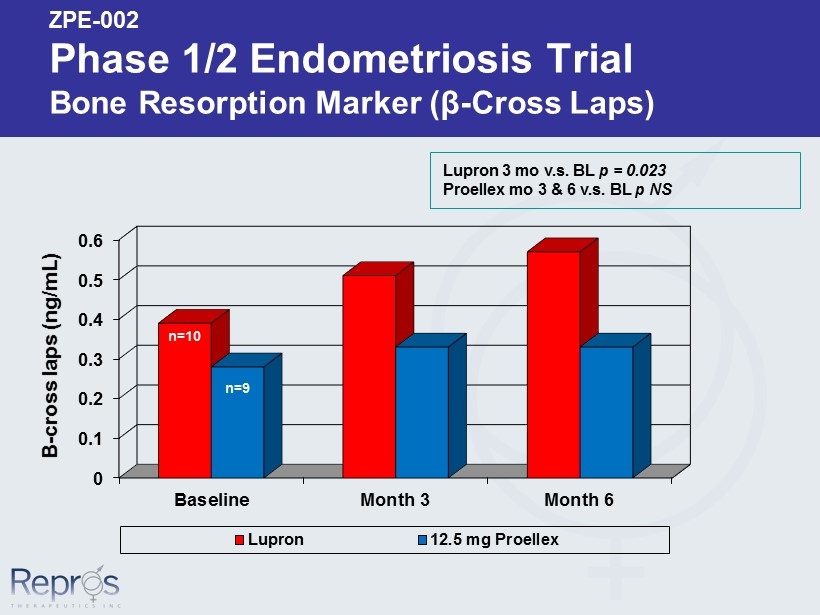
ZPE - 002 Phase 1/2 Endometriosis Trial Bone Resorption Marker ( β - Cross Laps) 0 0.1 0.2 0.3 0.4 0.5 0.6 Baseline Month 3 Month 6 Lupron 12.5 mg Proellex Lupron 3 mo v.s . BL p = 0.023 Proellex mo 3 & 6 v.s . BL p NS n=10 Β - cross laps (ng/mL) n=9
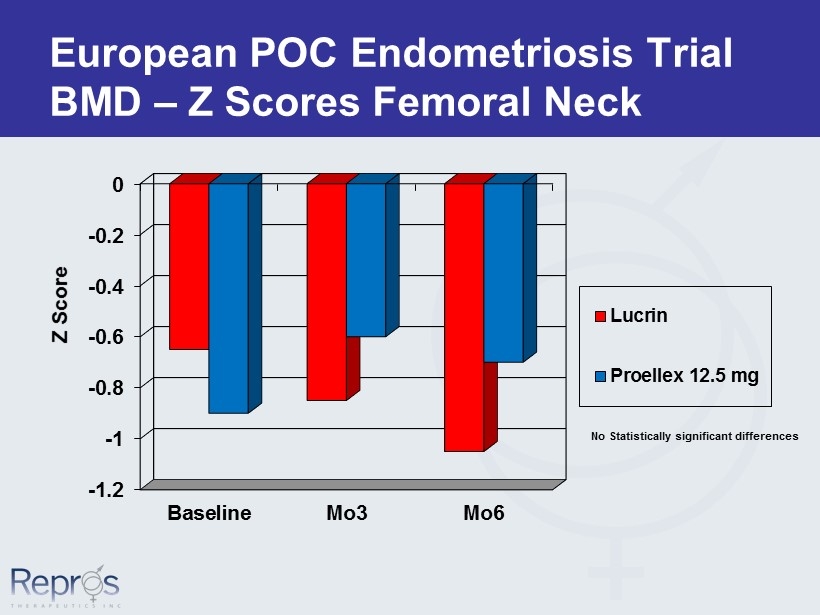
European POC Endometriosis Trial BMD – Z Scores Femoral Neck -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 Baseline Mo3 Mo6 Z Score Lucrin Proellex 12.5 mg No Statistically significant differences

Vaginal Proellex to Eliminate the Need for Hysterectomy in Most Situations • Initial Phase 2 study tested four doses of vaginal administration in the treatment of uterine fibroids completed – Assess reduction of fibroid size and elimination of symptoms – Top line data reported • FDA required additional Phase 2b study before proceeding to Phase 3 to insure proper dose selection – 45 subject Phase 2b study (placebo, 6 and 12 mg) – Separate IND from low dose oral
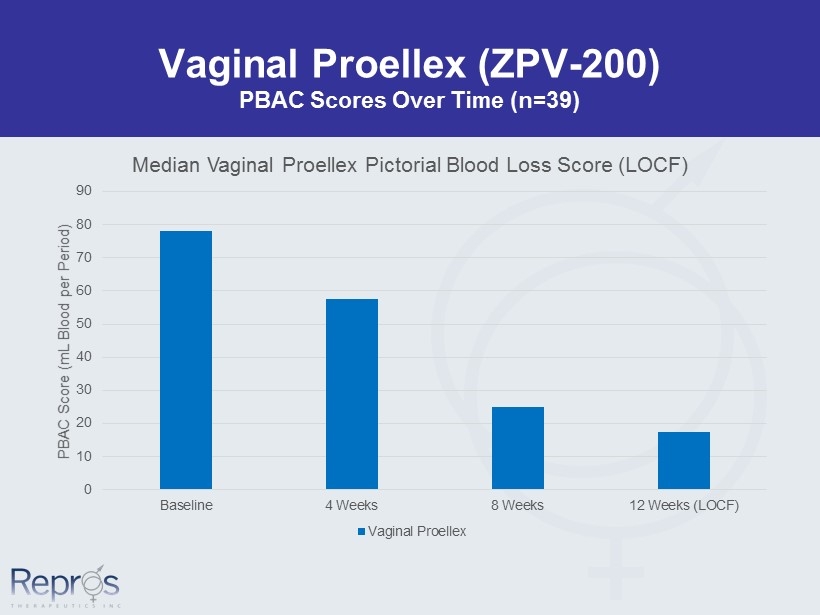
Vaginal Proellex (ZPV - 200) PBAC Scores Over Time (n=39) 0 10 20 30 40 50 60 70 80 90 Baseline 4 Weeks 8 Weeks 12 Weeks (LOCF) PBAC Score (mL Blood per Period) Median Vaginal Proellex Pictorial Blood Loss Score (LOCF) Vaginal Proellex
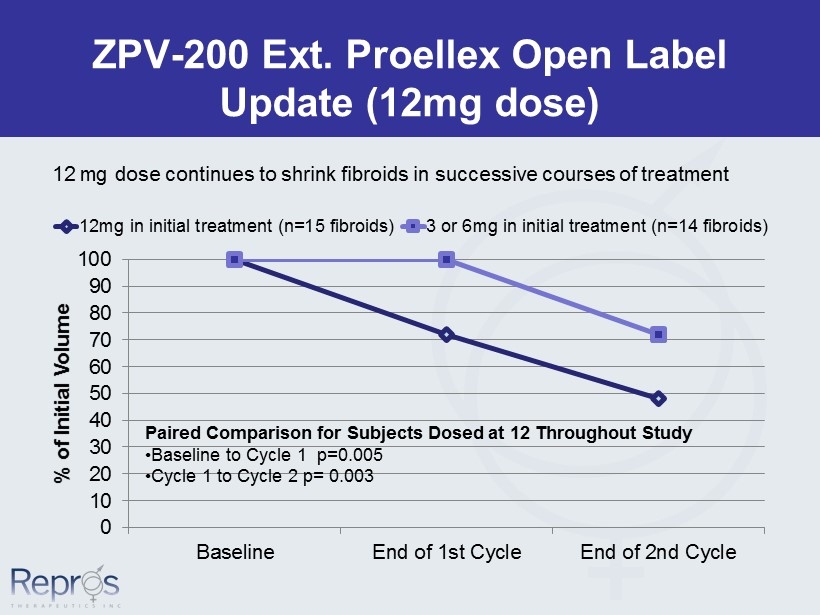
ZPV - 200 Ext. Proellex Open Label Update (12mg dose) 0 10 20 30 40 50 60 70 80 90 100 Baseline End of 1st Cycle End of 2nd Cycle % of Initial Volume 12mg in initial treatment (n=15 fibroids) 3 or 6mg in initial treatment (n=14 fibroids) Paired Comparison for Subjects Dosed at 12 Throughout Study • Baseline to Cycle 1 p=0.005 • Cycle 1 to Cycle 2 p= 0.003 12 mg dose continues to shrink fibroids in successive courses of treatment
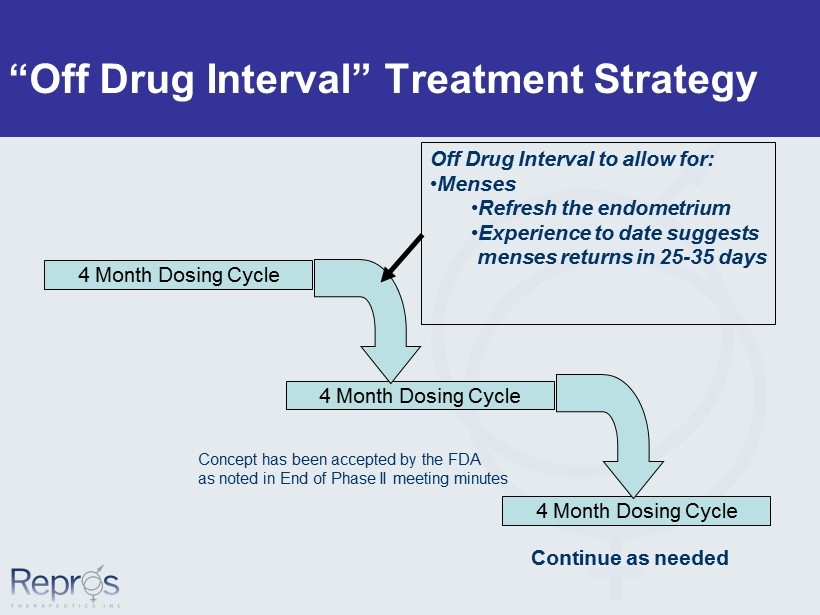
“Off Drug Interval” Treatment Strategy 4 Month Dosing Cycle 4 Month Dosing Cycle 4 Month Dosing Cycle Off Drug Interval to allow for: • Menses • Refresh the endometrium • Experience to date suggests menses returns in 25 - 35 days Continue as needed Concept has been accepted by the FDA as noted in End of Phase II meeting minutes
Financial Summary • Cash and equivalents: (unaudited end of Q3 2015) $26.2 M • Cash used in 2015 (through September 30, 2015) : $20.4 M • Cash runway: YE 2016 • Current shares outstanding: 24.3 M shares – Warrants Outstanding – Series A – 2,502 (purchased in unit deal @ $2.45); Series B – 429,704 @ $2.49 exercise price.







































