
Exhibit 99.2 st 41 Annual J.P. Morgan Healthcare Conference Karim Mikhail President & CEO January 23

Forward Looking Statements & Disclaimer This presentation contains forward-looking statements, such as those relating to the commercial potential of VASCEPA® (VAZKEPA® in Europe), clinical and regulatory efforts and timelines, potential regulatory and pricing approvals, patent litigation, generic product launch, intellectual property, cash flow, research and development, and other statements that are forward-looking in nature and depend upon or refer to future events or conditions, including financial guidance and milestones. These statements involve known and unknown risks, uncertainties and other factors that can cause actual results to differ materially. Investors should not place undue reliance on forward-looking statements, which speak only as of the presentation date of this presentation. Please refer to the “Risk Factors” section in Amarin’s most recent Forms 10-K and 10-Q filed with the SEC and cautionary statements outlined in recent press releases for more complete AMARIN, VASCEPA, VAZKEPA and REDUCE-IT are trademarks descriptions of risks in an investment in Amarin. of Amarin Pharmaceuticals Ireland Limited. VAZKEPA is a registered trademark in Europe and other countries and regions and is pending registration in the United States. THIS PRESENTATION IS INTENDED FOR COMMUNICATION WITH INVESTORS AND NOT FOR DRUG PROMOTION.

Summary 01 The Amarin Journey Changing the Paradigm in Cardiovascular Care 02 Progress In 2022 Solid Execution Against Ambitious Objectives 03 The Next Chapter Becoming a Diversified, Global Cardiometabolic Player AMARIN, VASCEPA, VAZKEPA and REDUCE-IT are trademarks of Amarin Pharmaceuticals Ireland Limited. VAZKEPA is a registered trademark in Europe and other countries and regions and is pending 04 registration in the United States. 2023 Key Priorities Positioned for Successful Execution

The Amarin Journey Changing the Paradigm in Cardiovascular Care
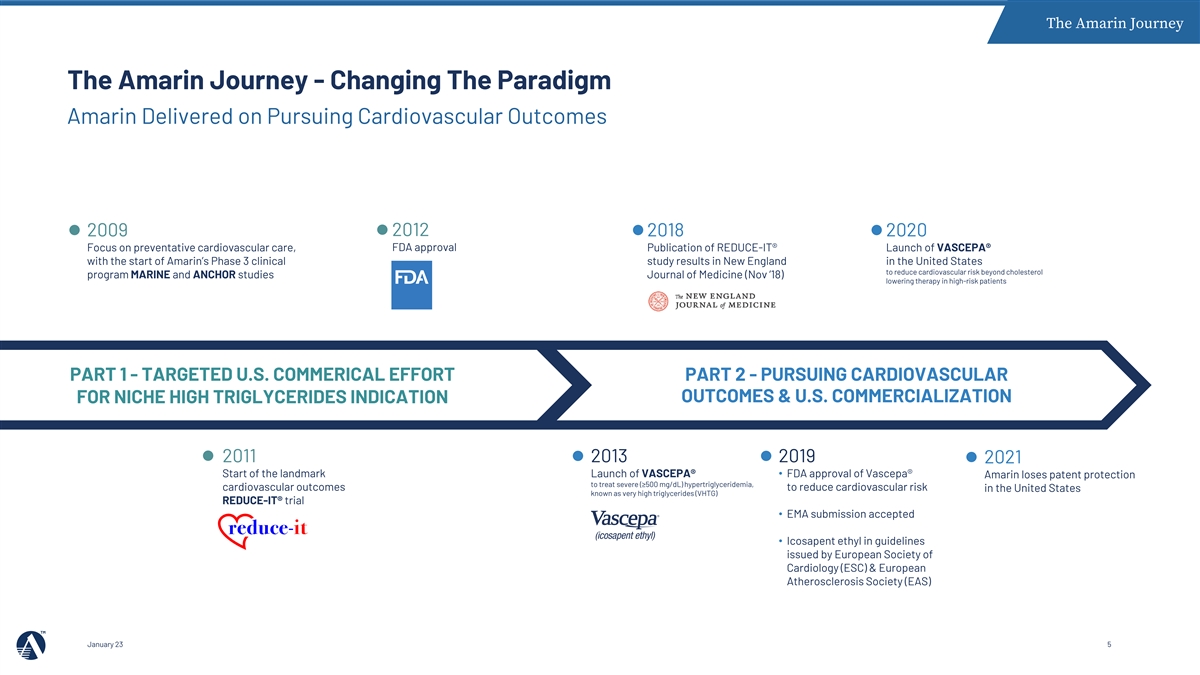
The Amarin Journey The Amarin Journey - Changing The Paradigm Amarin Delivered on Pursuing Cardiovascular Outcomes 2012 2009 2018 2020 FDA approval Focus on preventative cardiovascular care, Publication of REDUCE-IT® Launch of VASCEPA® with the start of Amarin’s Phase 3 clinical study results in New England in the United States to reduce cardiovascular risk beyond cholesterol program MARINE and ANCHOR studies Journal of Medicine (Nov ’18) lowering therapy in high-risk patients PART 1 - TARGETED U.S. COMMERICAL EFFORT PART 2 - PURSUING CARDIOVASCULAR OUTCOMES & U.S. COMMERCIALIZATION FOR NICHE HIGH TRIGLYCERIDES INDICATION 2011 2013 2019 2021 Start of the landmark Launch of VASCEPA® • FDA approval of Vascepa® Amarin loses patent protection to treat severe (≥500 mg/dL) hypertriglyceridemia, cardiovascular outcomes to reduce cardiovascular risk in the United States known as very high triglycerides (VHTG) REDUCE-IT® trial • EMA submission accepted • Icosapent ethyl in guidelines issued by European Society of Cardiology (ESC) & European Atherosclerosis Society (EAS) January 23 5
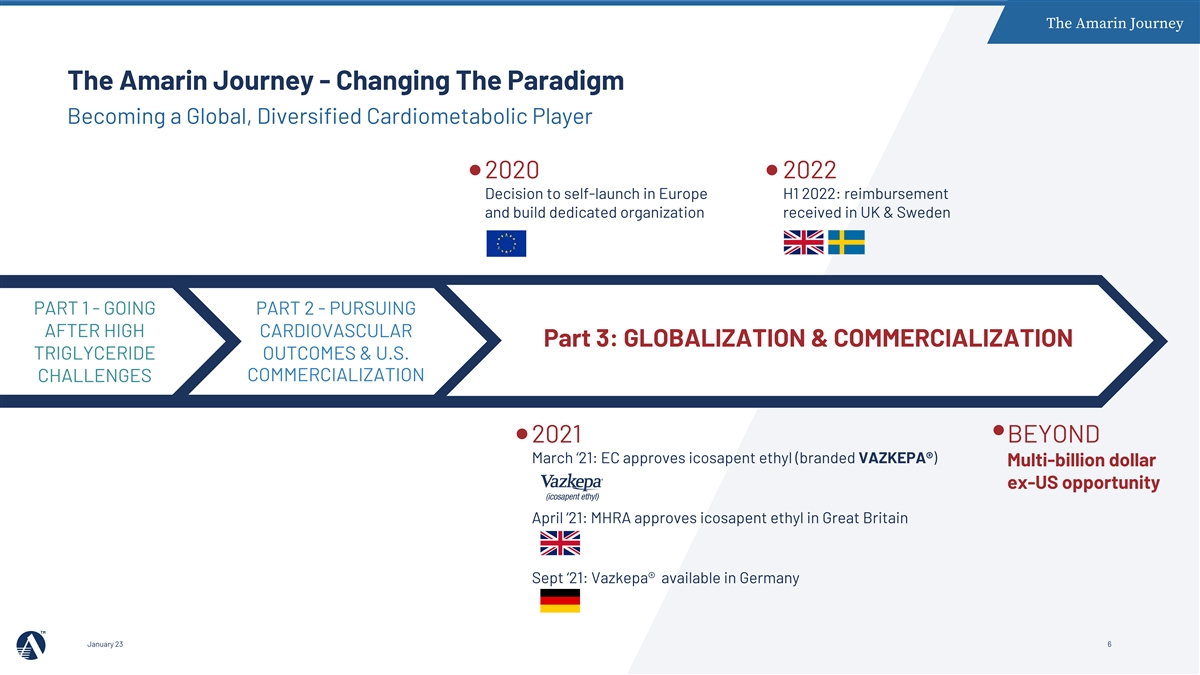
The Amarin Journey The Amarin Journey - Changing The Paradigm Becoming a Global, Diversified Cardiometabolic Player 2020 2022 Decision to self-launch in Europe H1 2022: reimbursement and build dedicated organization received in UK & Sweden PART 1 - GOING PART 2 - PURSUING AFTER HIGH CARDIOVASCULAR Part 3: GLOBALIZATION & COMMERCIALIZATION TRIGLYCERIDE OUTCOMES & U.S. COMMERCIALIZATION CHALLENGES 2021 BEYOND March ‘21: EC approves icosapent ethyl (branded VAZKEPA®) Multi-billion dollar ex-US opportunity April ‘21: MHRA approves icosapent ethyl in Great Britain Sept ‘21: Vazkepa® available in Germany January 23 6

2022 Progress Solid Execution Against Ambitious Objectives
2022 Solid Execution Against Ambitious Objectives We Made Progress Against All Three Strategic Pillars January 23 8 8 8
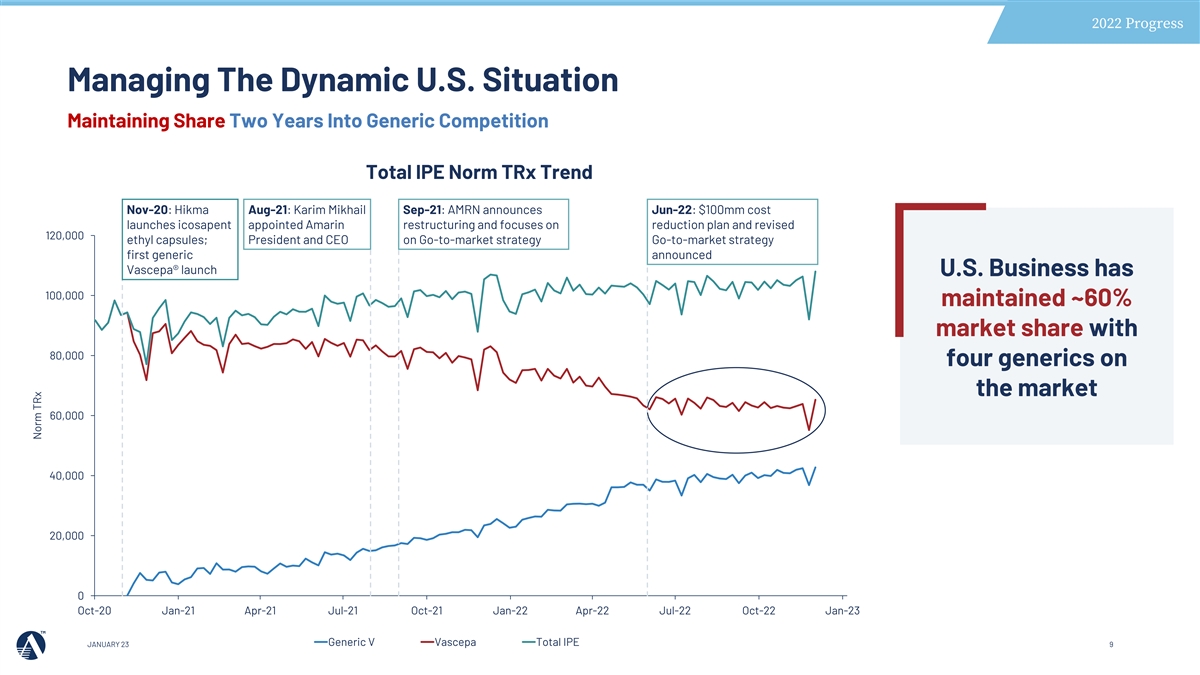
2022 Progress Managing The Dynamic U.S. Situation Maintaining Share Two Years Into Generic Competition Total IPE Norm TRx Trend Nov-20: Hikma Aug-21: Karim Mikhail Sep-21: AMRN announces Jun-22: $100mm cost launches icosapent appointed Amarin restructuring and focuses on reduction plan and revised 120,000 ethyl capsules; President and CEO on Go-to-market strategy Go-to-market strategy first generic announced Vascepa® launch U.S. Business has 100,000 maintained ~60% market share with 80,000 four generics on the market 60,000 40,000 20,000 0 Oct-20 Jan-21 Apr-21 Jul-21 Oct-21 Jan-22 Apr-22 Jul-22 Oct-22 Jan-23 Generic V Vascepa Total IPE JANUARY 23 9 Norm TRx
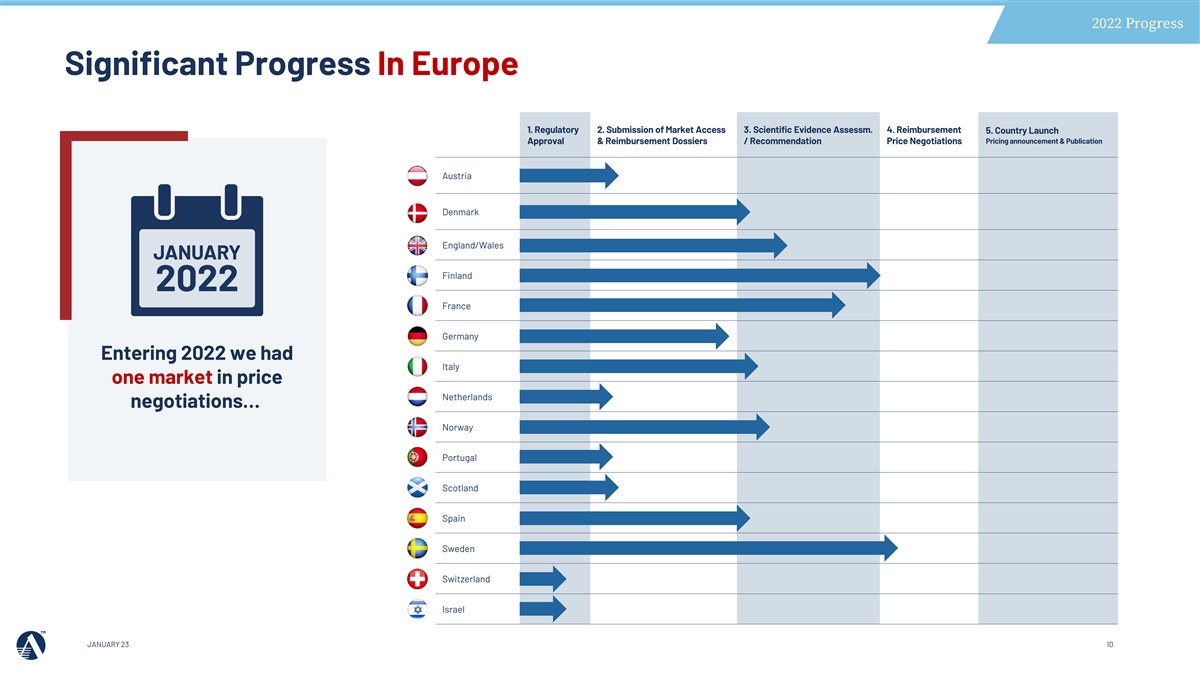
2022 Progress Significant Progress In Europe 1. Regulatory 2. Submission of Market Access 3. Scientific Evidence Assessm. 4. Reimbursement 5. Country Launch Approval & Reimbursement Dossiers / Recommendation Price Negotiations Pricing announcement & Publication Austria Denmark England/Wales Finland France Germany Entering 2022 we had Italy one market in price Netherlands negotiations… Norway Portugal Scotland Spain Sweden Switzerland Israel JANUARY 23 10

2022 Progress Significant Progress In Europe 1. Regulatory 2. Submission of Market Access 3. Scientific Evidence Assessm. 4. Reimbursement 5. Country Launch Approval & Reimbursement Dossiers / Recommendation Price Negotiations Pricing announcement & Publication Launched Austria Individual reimbursement > national reimbursement expansion 2023 (Individual Reimbursement) Launched Denmark Individual reimbursement > national reimbursement expansion 2023 (Individual Reimbursement) England/Wales National reimbursement Launched Finland National reimbursement Launched CEPS negotiations ongoing France Final Arbitration Decision Germany Amarin suspended VAZKEPA® supply and German business operations November 2022 At year end we had Positive CTS, CPR negotiations started Italy VAZKEPA® available in Netherlands Positive ZIN recommendation, MoH pricing negotiations ongoing 5 markets, and in the Norway Pending NOMA decision pricing negotiation stage in another 5 markets INFARMED submission ongoing Portugal SMC submission ongoing Scotland Spain Positive IPT recommendation, Pending MoH decision National reimbursement Sweden Launched Switzerland BAG submission ongoing Israel JANUARY 23 11
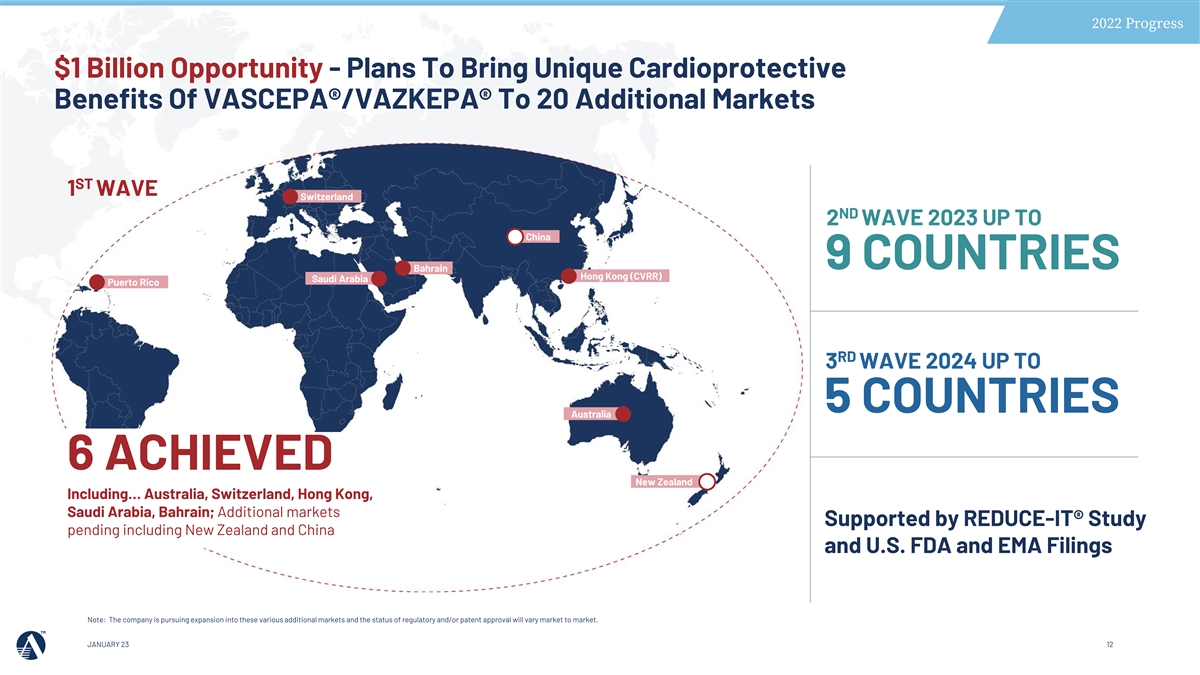
2022 Progress $1 Billion Opportunity - Plans To Bring Unique Cardioprotective Benefits Of VASCEPA®/VAZKEPA® To 20 Additional Markets ST 1 WAVE Switzerland ND 2 WAVE 2023 UP TO China 9 COUNTRIES Bahrain Hong Kong (CVRR) Saudi Arabia Puerto Rico RD 3 WAVE 2024 UP TO 5 COUNTRIES Australia 6 ACHIEVED New Zealand Including… Australia, Switzerland, Hong Kong, Saudi Arabia, Bahrain; Additional markets Supported by REDUCE-IT® Study pending including New Zealand and China and U.S. FDA and EMA Filings Note: The company is pursuing expansion into these various additional markets and the status of regulatory and/or patent approval will vary market to market. JANUARY 23 12

2022 Progress 2022 Solid Execution Against Ambitious Objectives Transformed Amarin Through Focus on Operational Excellence SIGNIFICANT PROGRESS ON CASH TRANSFORMED LEADERSHIP PRESERVATION INITIATIVES TEAM AND BOARD • Added global, pharma, leadership • Cost savings plans to achieve $100 million and financial expertise in annual savings by mid-2023 on target • Built a leading European leadership team • Renegotiations of supply agreements to best execute on significant continue to progress resulting in commercial opportunity reduction of inventory purchases • 70% of executive leadership team new in 2022 JANUARY 23 13

2022 Progress Full Year 2022 Preliminary Results Support Solid Foundation For Future Growth Q1 Q2 Q3 Q4 160 Stabilization Of 140 U.S. Revenue Drives 120 Strong Performance Est • Fourth quarter 2022 $88-90 100 million revenues expected between $88 and $90 million 80 • Despite ongoing generic competition, experienced 60 stable revenue trends 40 throughout 2022 for VASCEPA® in the U.S. 20 0 2021 2022 January 23 14 Total Net Revenues $M
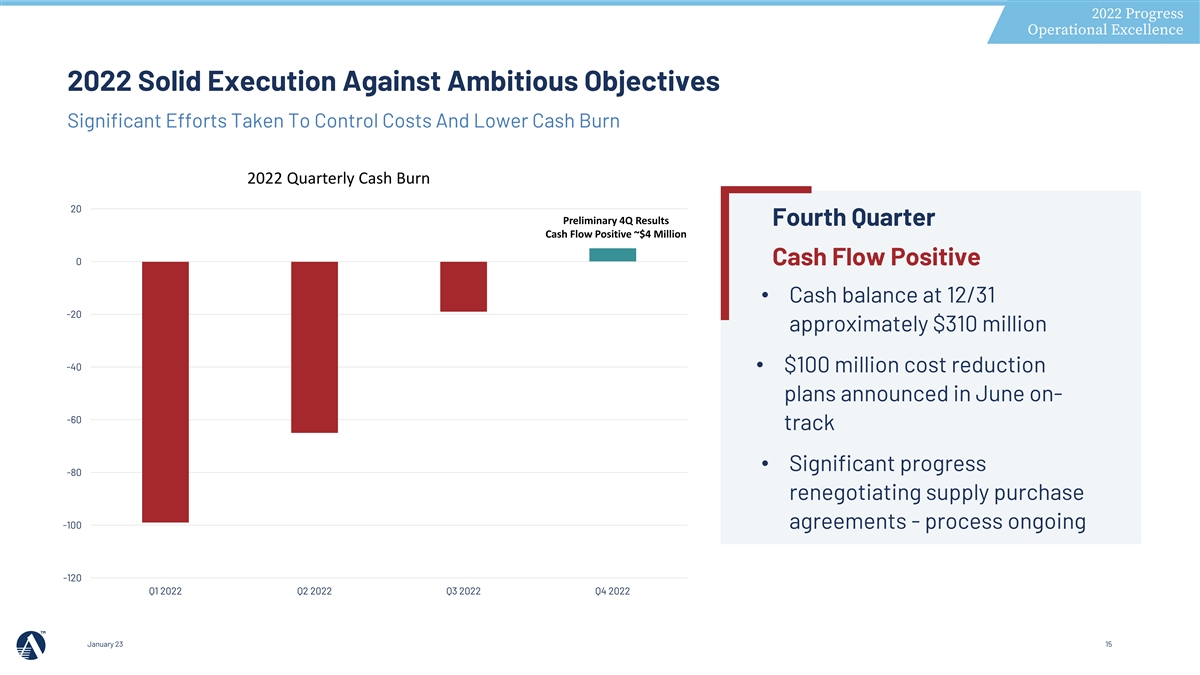
2022 Progress Operational Excellence 2022 Solid Execution Against Ambitious Objectives Significant Efforts Taken To Control Costs And Lower Cash Burn 2022 Quarterly Cash Burn 20 Preliminary 4Q Results Fourth Quarter Cash Flow Positive ~$4 Million 0 Cash Flow Positive • Cash balance at 12/31 -20 approximately $310 million -40 • $100 million cost reduction plans announced in June on- -60 track • Significant progress -80 renegotiating supply purchase -100 agreements - process ongoing -120 Q1 2022 Q2 2022 Q3 2022 Q4 2022 January 23 15

2022 Progress Transformed And Strengthened Leadership Team 25+ 45+ 100+ CV & RELATED DIFFERENT YEARS PRODUCTS LAUNCHED MARKETS IN CV Caroline Maraval Laurent Abuaf Dr. Nabil Abadir Tom Reilly Lisa DeFrancesco David Keenan Jordan Zwick Aaron Berg Steven Ketchum, PhD Donna Pasek VP, Chief of Staff SVP, President SVP, Chief SVP, Chief SVP, Corporate SVP, Technical SVP, Corporate EVP, President-U.S. EVP, Chief Scientific SVP, Human March 2022 of Europe Medical Officer Financial Officer Affairs and Operations Business November 2012 Officer Resources August 2021 April 2022 June 2022 Investor Relations May 2022 Development February 2012 May 2012 January 2022 May 2022 JANUARY 23 16

2022 Progress Established Best-in-Class European Leadership Team 30+ 40+ 125+ CV & RELATED DIFFERENT YEARS PRODUCTS LAUNCHED MARKETS IN CV Laurent Abuaf Scott Curley Luca Ruffini Henrik Salvador Lopez Hakima Laurent Josse, PhD Tamara Barghout David Jakouloff, MD Christos SVP, President UK – General Italy – General Asmussen Iberia – General Hannachi France – General Head of Head of Market Papadopoulos of Europe Manager Manager Mid Europe & Manager Head of Medical Manager Marketing & Access CCE – General Nordics – Affairs Europe Launch Lead Manager General Manager JANUARY 23 17
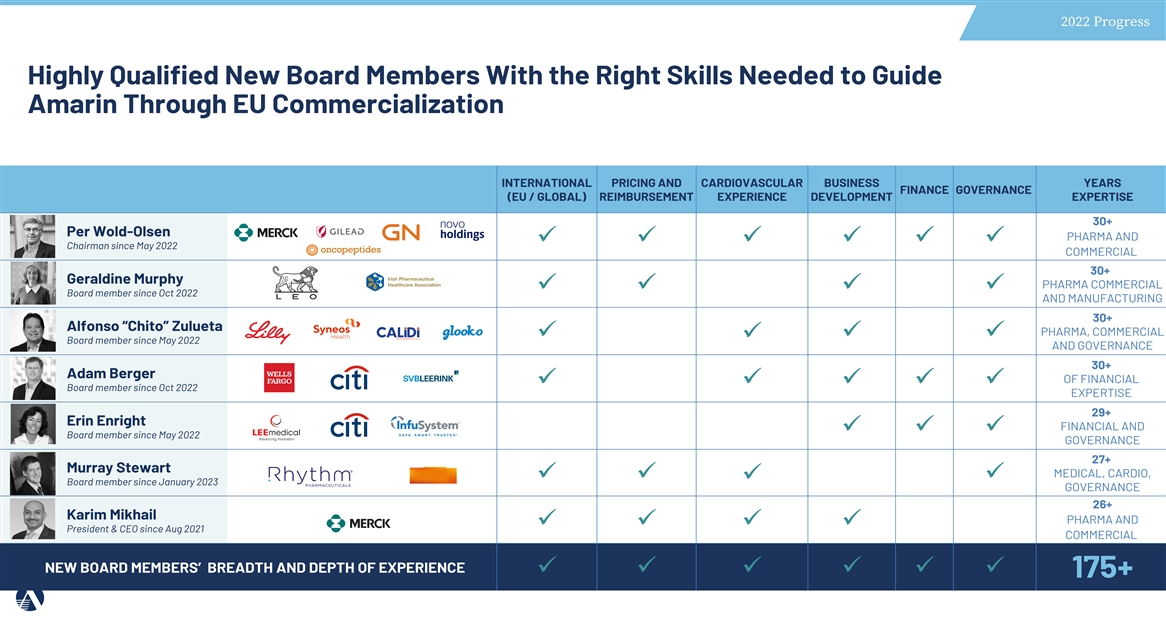
2022 Progress Highly Qualified New Board Members With the Right Skills Needed to Guide Amarin Through EU Commercialization INTERNATIONAL PRICING AND CARDIOVASCULAR BUSINESS YEARS FINANCE GOVERNANCE (EU / GLOBAL) REIMBURSEMENT EXPERIENCE DEVELOPMENT EXPERTISE 30+ Per Wold-Olsen PHARMA AND üüüüüü Chairman since May 2022 COMMERCIAL 30+ Geraldine Murphy PHARMA COMMERCIAL üüüü Board member since Oct 2022 AND MANUFACTURING 30+ Alfonso “Chito” Zulueta PHARMA, COMMERCIAL üüü ü Board member since May 2022 AND GOVERNANCE 30+ Adam Berger OF FINANCIAL üüüüü Board member since Oct 2022 EXPERTISE 29+ Erin Enright FINANCIAL AND üüü Board member since May 2022 GOVERNANCE 27+ Murray Stewart MEDICAL, CARDIO, üüü ü Board member since January 2023 GOVERNANCE 26+ Karim Mikhail PHARMA AND üüüü President & CEO since Aug 2021 COMMERCIAL NEW BOARD MEMBERS’ BREADTH AND DEPTH OF EXPERIENCE üüüüüü 175+ JANUARY 23 18

The Next Chapter Becoming a Diversified, Global Cardiometabolic Player

The Next Chapter Becoming a Diversified, Global Cardiometabolic Player OPERATIONAL PORTFOLIO EVOLUTION DIVERSIFICATION GEOGRAPHIC EXPANSION Solid foundation entering growth and expansion period JANUARY 23 20
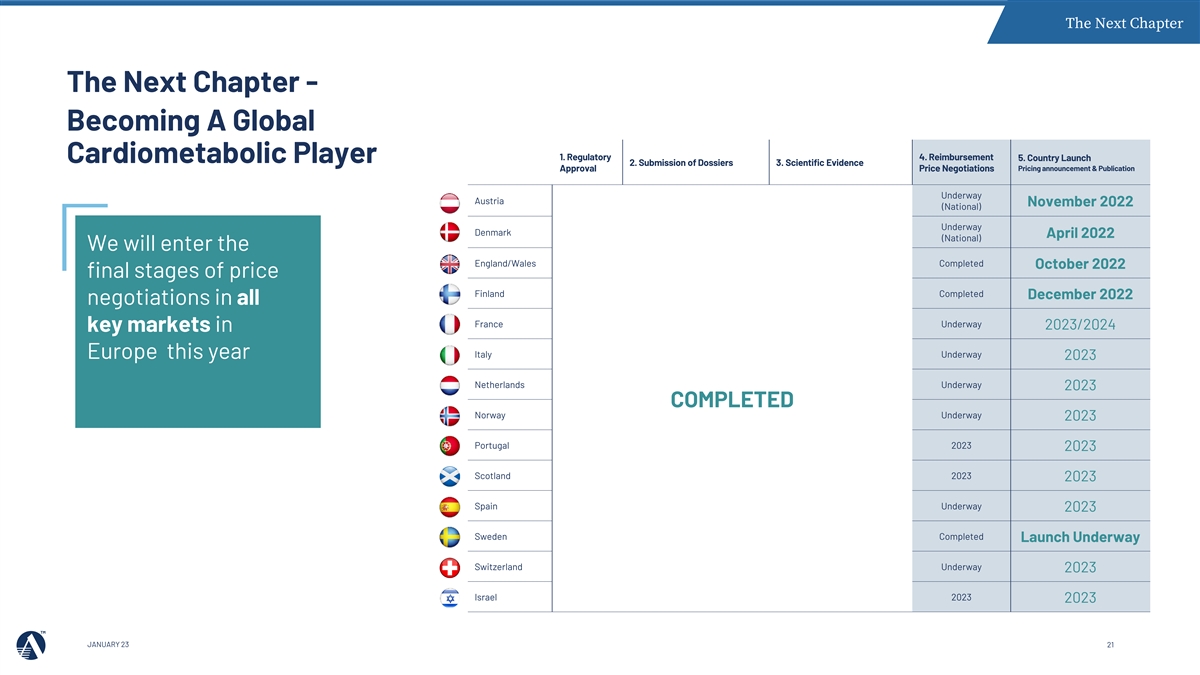
The Next Chapter The Next Chapter - Becoming A Global 1. Regulatory 4. Reimbursement 5. Country Launch Cardiometabolic Player 2. Submission of Dossiers 3. Scientific Evidence Pricing announcement & Publication Approval Price Negotiations Underway Austria November 2022 (National) Underway Denmark April 2022 (National) We will enter the England/Wales Completed October 2022 final stages of price Finland Completed December 2022 negotiations in all France Underway 2023/2024 key markets in Italy Underway Europe this year 2023 Netherlands Underway 2023 COMPLETED Norway Underway 2023 Portugal 2023 2023 Scotland 2023 2023 Spain Underway 2023 Sweden Completed Launch Underway Switzerland Underway 2023 Israel 2023 2023 JAN JANU UAR ARY 2 Y 23 3 21

The Next Chapter International Expansion A Significant Focus – 20 Markets By 2024 Switzerland China NEXT WAVE UP TO Bahrain Hong Kong (CVRR) Saudi Arabia Puerto Rico 14 BY YEAR END 2024 Additional markets pending including China, New Zealand Australia ST Negotiate Partnership Opportunities 1 WAVE New Zealand to Drive Access While Maintaining Flexibility 6 COMPLETE Supported by REDUCE-IT® Study and U.S. FDA and EMA Filings JANUARY 23 22

The Next Chapter We Will Continue To Focus On Operational Excellence As Part Of Our Evolution FOCUS ON PROFITABILITY IN THE US Continue to anticipate and manage effectively to market dynamics MAINTAIN FINANCIAL DISCIPLINE Identify additional cost savings opportunities SEQUENCED INVESTMENTS IN GROWTH Staged investment approach to launch activities in Europe JANUARY 23 23

The Next Chapter The Next Chapter – Becoming A Diversified Cardiometabolic Player Explore Strategic Opportunities to Leveraging our Strengths for Future Success Scientific R&D Commercial Strong Expertise Capabilities Cardiovascular Network REDUCE-IT®, US Commercial Cardiovascular Capabilities and Building Global Medical Affairs Development and EU Commercial Team and Advocacy Global Regulatory JANUARY 23 24
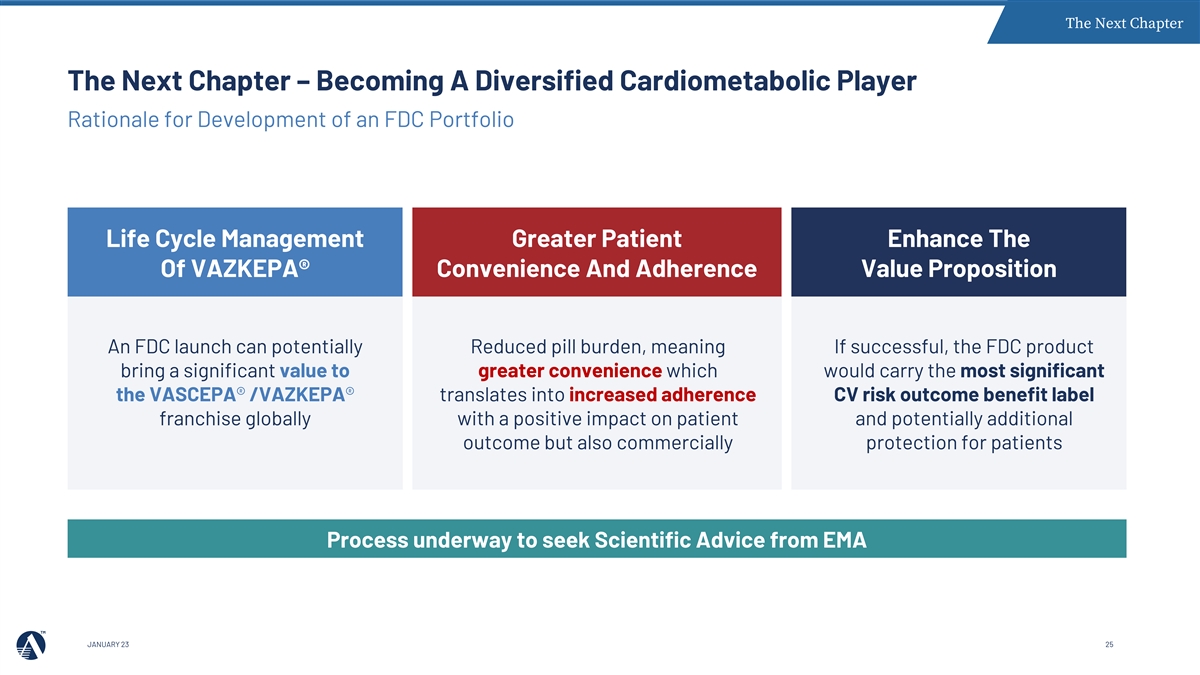
The Next Chapter The Next Chapter – Becoming A Diversified Cardiometabolic Player Rationale for Development of an FDC Portfolio Life Cycle Management Greater Patient Enhance The Of VAZKEPA® Convenience And Adherence Value Proposition An FDC launch can potentially Reduced pill burden, meaning If successful, the FDC product bring a significant value to greater convenience which would carry the most significant the VASCEPA® /VAZKEPA® translates into increased adherence CV risk outcome benefit label franchise globally with a positive impact on patient and potentially additional outcome but also commercially protection for patients Process underway to seek Scientific Advice from EMA JANUARY 23 25

2023 Priorities Positioned for Successful Execution
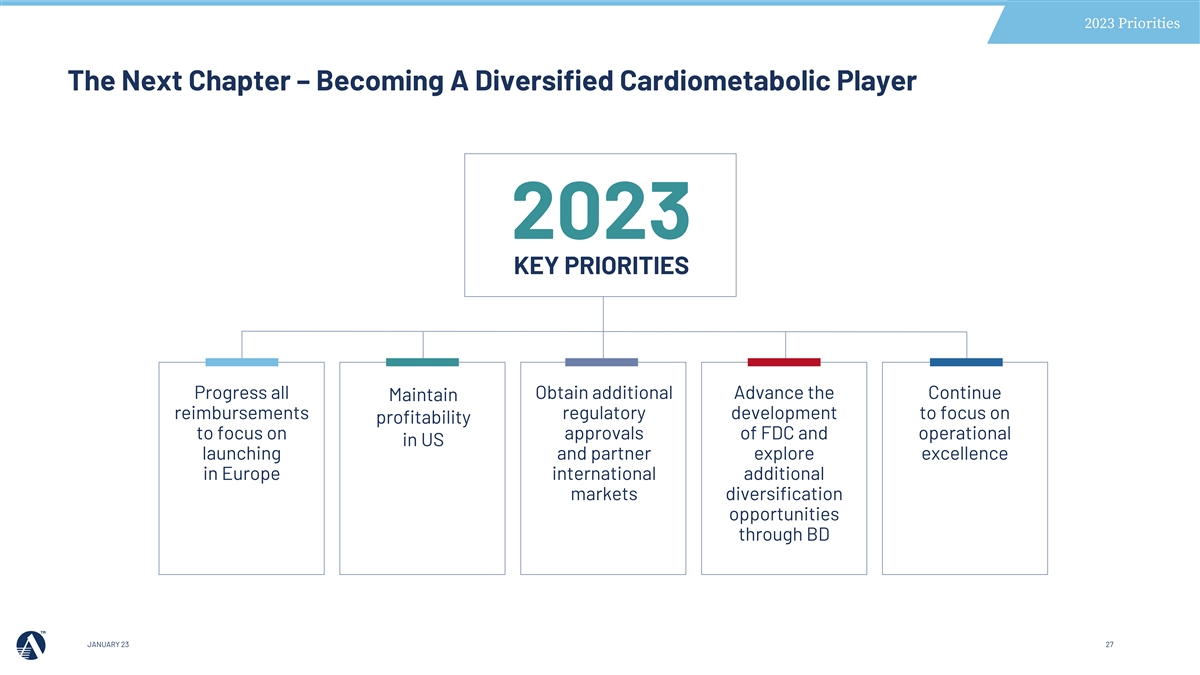
2023 Priorities The Next Chapter – Becoming A Diversified Cardiometabolic Player 2023 KEY PRIORITIES Progress all Obtain additional Advance the Continue Maintain reimbursements regulatory development to focus on profitability to focus on approvals of FDC and operational in US launching and partner explore excellence in Europe international additional markets diversification opportunities through BD JANUARY 23 27

Leading a new paradigm in preventive cardiovascular care and growing our impact for patients globally AMBITIONS for

THANK YOU JANUARY 23




























