
RARE INSPIRATION. CHANGING LIVES. Confidential – For internal use only

IN THE ROOM - B L U E B E L L 2 | RARE INSPIRATION. CHANGING LIVES. John Orloff, MD Gianluca Pirozzi Camilo Garcia EVP SVP VP G l o b a l Hematology & R & D H R ROLE H e a d o f Nephrology L e a d R&D R&D TA Head T I M E W I T H 2 Y e a r s 2 Y e a r s ALEXION 3 W e e k s Confidential – For internal use only

IN THE ROOM – N E W H A V E N 3 | RARE INSPIRATION. CHANGING LIVES. Aradhana Sarin, MD Sharon Barr Rachael Alford EVP VP VP H e a d o f Chief Strategy G l o b a l and Business H e a d o f ROLE P r o d u c t O f f i c e r R e s e a r c h Development T I M E W I T H 2 Y e a r s 1 5 Y e a r s ALEXION 6 y e a r s Confidential – For internal use only

ALEXION TO ACQUIRE ACHILLION 4 | RARE INSPIRATION. CHANGING LIVES. Strategic Rationale ✓ Opportunity to diversify into additional complement-mediated diseases using oral therapies • Adds clinical-stage portfolio of oral small molecule Factor D inhibitors to Alexion pipeline ✓ Potential to enhance treatment for PNH patients • Opportunity to treat the small portion of PNH patients experiencing clinical extravascular hemolysis (EVH) ✓ Potential first-in-class treatment for C3 glomerulopathy • Severe kidney disease with no approved treatment ✓ Promising development platform • Significant opportunity for Factor D inhibition in other alternative pathway complement-mediated rare diseases • Small molecule chemistry expertise and library ✓ Aligned with Alexion BD strategy Confidential – For internal use only

ALEXION BY THE NUMBERS 5 | RARE INSPIRATION. CHANGING LIVES. 3000+ TALENTED COLLEAGUES AROUND THE GLOBE PRIX3 GALIEN AWARDS 20+ YEARS OF LEADERSHIP IN COMPLEMENT BIOLOGY APPROVED $4.75 4 DEVASTATING6 THERAPIES RARE DISEASES -4.8b SERVING PATIENTS IN Projected Revenues in 2019 50 (Per 2Q19 Earnings release COUNTRIES MADDOX July 24, 2019) LIVING WITH HPP Confidential – For internal use only

OUR 25+ YEAR JOURNEY 6 | RARE INSPIRATION. CHANGING LIVES. 1992 2015 2017 2018 Alexion founded in New Haven, CT STRENSIQ® (asfotase alfa) first approved Partnered with Halozyme Therapeutics and ULTOMIRIS® (ravulizumab-cwvz) first ® for the treatment of patients with HPP established license agreement for ENHANZE approved for the treatment of adult patients 1996 drug-delivery technology with PNH Alexion goes public with ticker ALXN 2015 2018 ® (sebelipase alfa) first approved 2019 KANUMA Opened global headquarters in Boston 2007 for the treatment of patients with LAL-D Established partnerships with Caelum Biosciences, Zealand Pharma and Affibody SOLIRIS® (eculizumab) first approved for the treatment of patients with PNH 2018 2016 Acquired Wilson Therapeutics, the company Opened Center of Excellence in New Haven 2019 that developed ALXN1840 (WTX101) for the SOLIRIS first approved for the treatment of 2011 treatment of Wilson Disease adult patients with NMOSD SOLIRIS first approved for the 2017 treatment of patients with aHUS SOLIRIS first approved for the treatment of patients with gMG 2019 2018 Announced Japanese license agreement with 2012 Acquired Syntimmune, the company that BridgeBio to develop and commercialize Eidos’ Acquired asfostase alfa, a potential 2017 began development of ALXN1830 (SYNT001) AG10 for transthyretin amyloidosis (ATTR) treatment for patients living with HPP for the treatment of rare IgG-mediated Partnered with Halozyme Therapeutics and diseases established license agreement for ENHANZE® 2015 drug-delivery technology 2019 ULTOMIRIS first approved for the treatment of Acquired Synageva and added to its 2018 adults and pediatric patients one month of age pipeline KANUMA, a potential treatment Established partnerships with Complement and older with aHUS to inhibit complement- for patients living with LAL-D Pharma and Dicerna mediated thrombotic microangiopathy (TMA) APPROVALS ACQUISITIONS PARTNERSHIPS Confidential – For internal use only

MANAGEMENT TEAM 7 | RARE INSPIRATION. CHANGING LIVES. Ludwig Hantson, PhD Ellen Chiniara, JD Paul Clancy Indrani Franchini, JD Brian Goff Chief Executive Officer EVP, General Counsel EVP, Chief Financial Officer EVP, Chief Compliance Officer EVP, Chief Commercial Officer Anne-Marie Law John Orloff, MD Aradhana Sarin, MD Rana Strellis EVP, Chief Patient & Employee EVP, Global Head of Research EVP, Chief Strategy and SVP, Global Communications Experience Officer & Development Business Officer & Culture Confidential – For internal use only
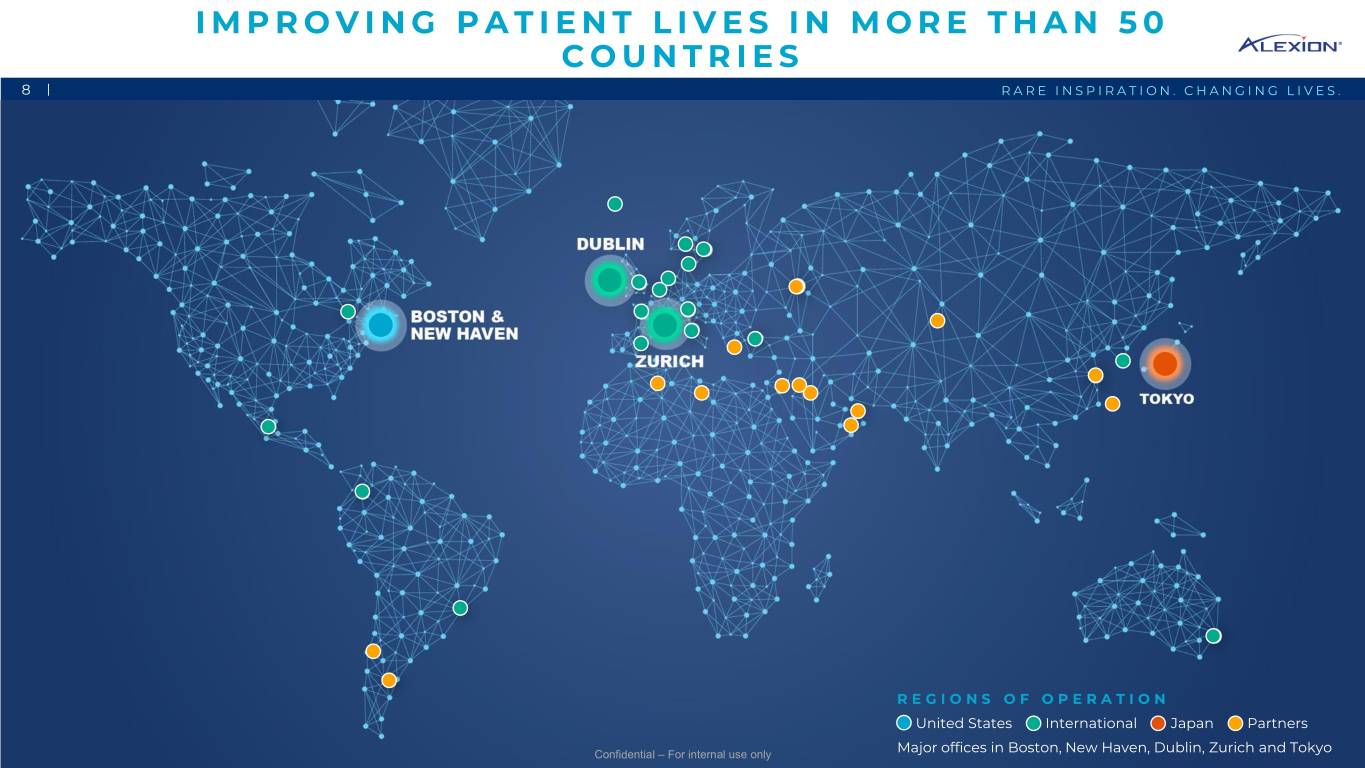
IMPROVING PATIENT LIVES IN MORE THAN 50 COUNTRIES 8 | RARE INSPIRATION. CHANGING LIVES. REGIONS OF OPERATION United States International Japan Partners Confidential – For internal use only Major offices in Boston, New Haven, Dublin, Zurich and Tokyo

O U R V A L U E S 9 | RARE INSPIRATION. CHANGING LIVES. Our culture is rooted in integrity, inclusiveness, Serve Act with and our dedication to Patients Integrity joining and supporting the communities in which Empower Innovate for we live and work. People Solutions Confidential – For internal use only

THE MOST REWARDING COMPANY TO WORK FOR 10 | RARE INSPIRATION. CHANGING LIVES. WORLD CLASS LEADERSHIP AND INNOVATION CAPABILITY Leaders cultivate the climate for innovation and connect us to the experience of those we serve Alexion delivers world-class innovation to patients and caregivers to help them fully live their best lives PATIENT & EMPLOYEE EXPERIENCE We invest in and value By cultivating a deep understanding of the experience of both people who believe in the patients and employees, Alexion creates meaningful and importance of our purpose and fulfilling work, and a feeling of value, belonging and reward, understand what it takes for all employees to deliver on it. Confidential – For internal use only

SERVING THE COMMUNITIES WHERE WE LIVE AND WORK 11 | RARE INSPIRATION. CHANGING LIVES. 85+ PROJECTS 20+ COUNTRIES Our Global Day of Service is an annual opportunity for 8,500+ us to make a positive impact VOLUNTEER HOURS on the lives of the people in our local communities and each 1,700+ other EMPLOYEES Confidential – For internal use only
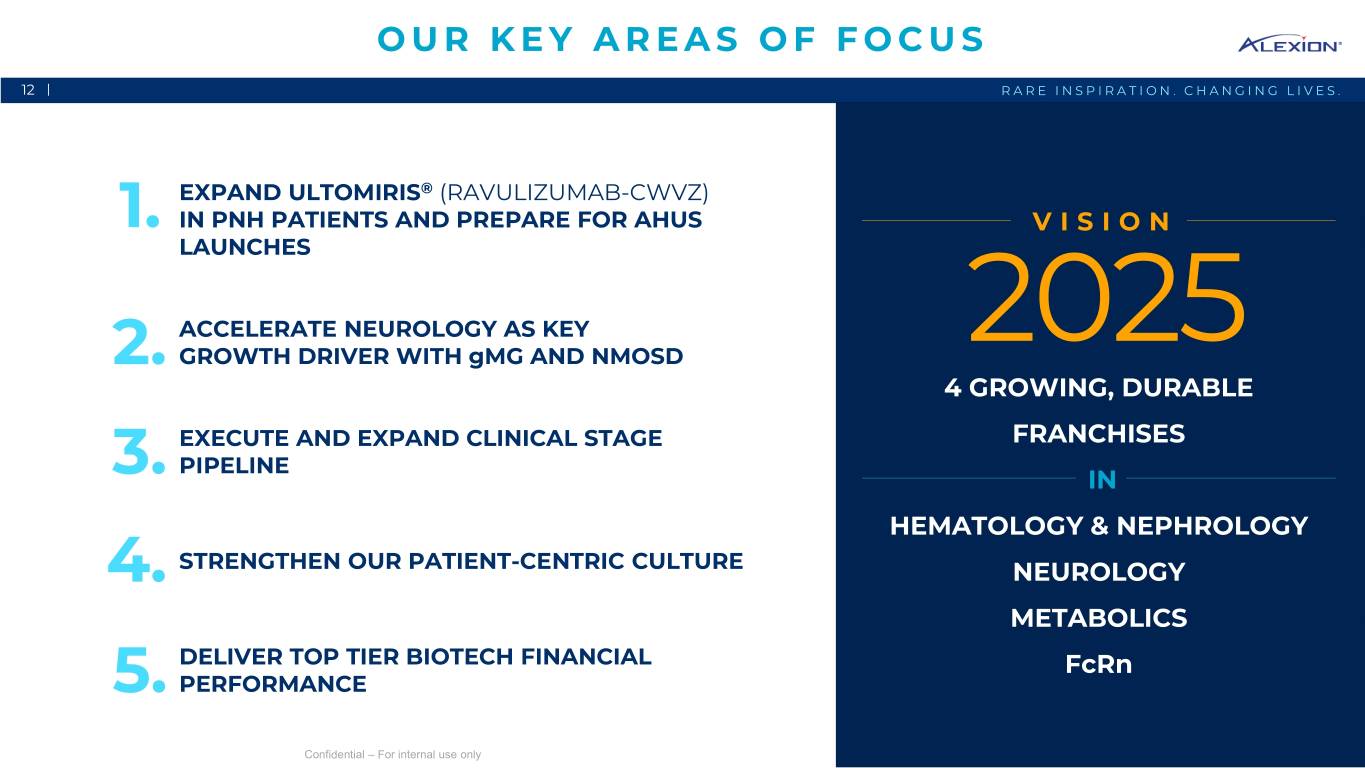
OUR KEY AREAS OF FOCUS 12 | RARE INSPIRATION. CHANGING LIVES. EXPAND ULTOMIRIS® (RAVULIZUMAB-CWVZ) 1. IN PNH PATIENTS AND PREPARE FOR AHUS VISION LAUNCHES ACCELERATE NEUROLOGY AS KEY 2. GROWTH DRIVER WITH gMG AND NMOSD 2025 4 GROWING, DURABLE EXECUTE AND EXPAND CLINICAL STAGE FRANCHISES PIPELINE 3. IN HEMATOLOGY & NEPHROLOGY 4. STRENGTHEN OUR PATIENT-CENTRIC CULTURE NEUROLOGY METABOLICS DELIVER TOP TIER BIOTECH FINANCIAL FcRn 5. PERFORMANCE Confidential – For internal use only

OUR MEDICINES 13 | RARE INSPIRATION. CHANGING LIVES. ULTOMIRIS® SOLIRIS® STRENSIQ® KANUMA® (RAVULIZUMAB-CWVZ) (ECULIZUMAB) (ASFOTASE ALFA) (SEBELIPASE ALFA) FOR FOR FOR FOR PNH PNH HPP LAL-D aHUS aHUS gMG NMOSD Confidential – For internal use only

2017 PIPELINE 14 | RARE INSPIRATION. CHANGING LIVES. 5 Development Preclinical Early Clinical Advanced Clinical Registration Programs Internal Complement ULTOMIRIS QW SC ALXN1210 IV PNH eculizumab gMG Programs Other Research ALXN1210 IV aHUS eculizumab NMOSD Hematology & Nephrology Metabolics Neurology FcRn TBD / Other Confidential – For internal use only
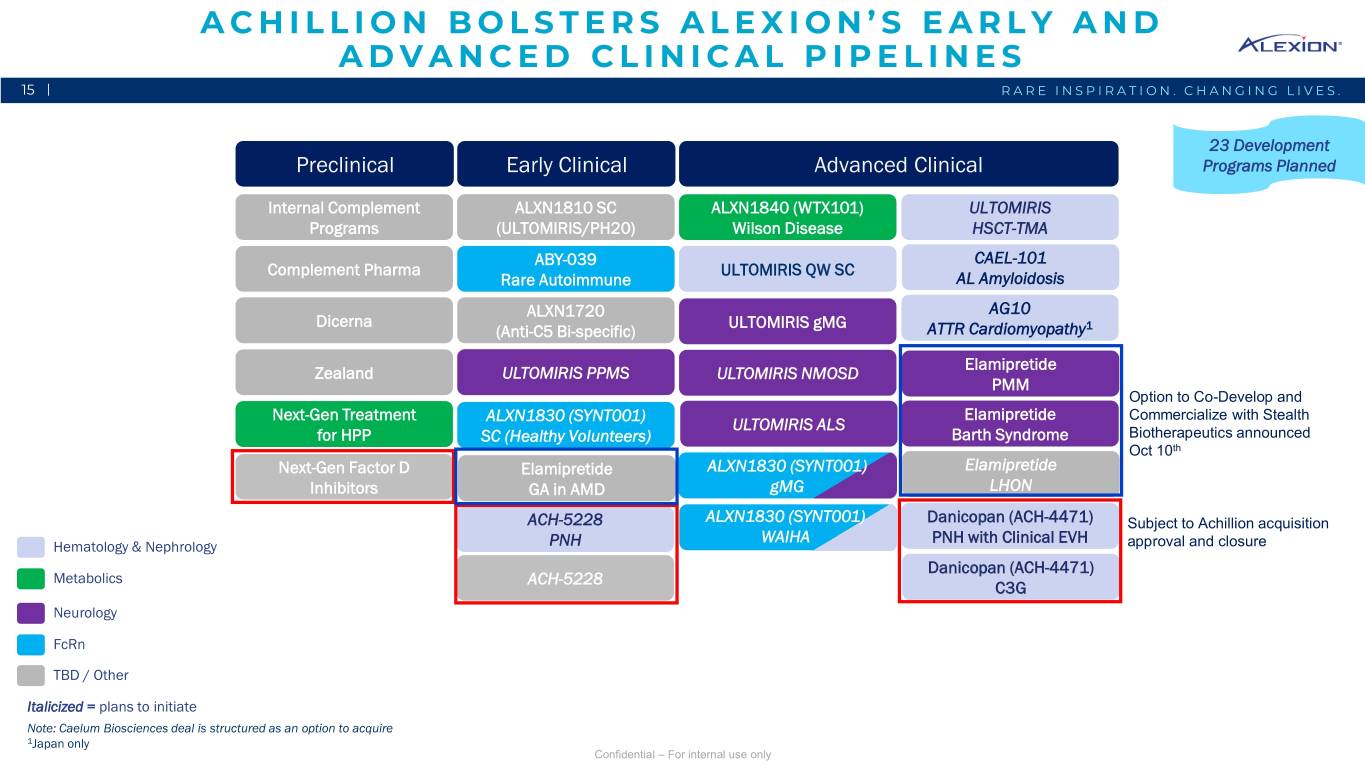
ACHILLION BOLSTERS ALEXION’S EARLY AND ADVANCED CLINICAL PIPELINES 15 | RARE INSPIRATION. CHANGING LIVES. 23 Development Preclinical Early Clinical Advanced Clinical Programs Planned Internal Complement ALXN1810 SC ALXN1840 (WTX101) ULTOMIRIS ALXN1210 QW SubQ Programs (ULTOMIRIS/PH20) Wilson Disease HSCT-TMA ABY-039 CAEL-101 Complement Pharma ULTOMIRISALXN1210 IVQW PNH SC Rare Autoimmune AL Amyloidosis ALXN1720 AG10 Dicerna ULTOMIRIS gMG (Anti-C5 Bi-specific) ATTR Cardiomyopathy1 Elamipretide Zealand ULTOMIRIS PPMS ULTOMIRIS NMOSD PMM Option to Co-Develop and Next-Gen Treatment ALXN1830 (SYNT001) Elamipretide Commercialize with Stealth ULTOMIRIS ALS for HPP SC (Healthy Volunteers) Barth Syndrome Biotherapeutics announced Oct 10th Next-Gen Factor D Elamipretide ALXN1830 (SYNT001) Elamipretide Inhibitors GA in AMD gMG LHON ACH-5228 ALXN1830 (SYNT001) Danicopan (ACH-4471) Subject to Achillion acquisition WAIHA PNH with Clinical EVH Hematology & Nephrology PNH approval and closure Danicopan (ACH-4471) Metabolics ACH-5228 C3G Neurology FcRn TBD / Other Italicized = plans to initiate Note: Caelum Biosciences deal is structured as an option to acquire 1Japan only Confidential – For internal use only

R&D HIRING TO DELIVER THE PORTFOLIO 16 | RARE INSPIRATION. CHANGING LIVES. +293 ~1200 R&D COLLEAGUES ADDED OR POSITIONS OFFERED YTD 2019 R&D COLLEAGUES AROUND THE GLOBE BY THE END OF 2020 PLAN TO HIRE UP TO …. +~175 from 2019 365 Yearend Headcount THIS YEAR IN TOTAL (pending budget approval) (+72 ADDITIONAL BY YEAREND) Confidential – For internal use only

ACQUISITION NEXT STEPS 17 | RARE INSPIRATION. CHANGING LIVES. • Alexion’s acquisition of Achillion is subject to: • The approval of Achillion shareholders • Satisfaction of other customary closing conditions • Approval from relevant regulatory agencies, including clearance under the Hart-Scott Rodino Antitrust Improvements Act • Pending these approvals, the transaction is expected to close in the first half of 2020 • Until this time, we will continue to operate as two independent companies; Achillion will continue to make all decisions for all aspects of its business until the closing • Details for integration planning will be worked out over the coming months; as such, we are not able to provide answers on all the questions that you have today • We are committed to have a Day 1 kickoff to share details with employees once the deal closes • We are focused on ensuring that continuity is maintained in the clinical trials that are ongoing • We are committed to the KOLs and patients that are involved in the danicopan and ACH-5228 trials • We welcome having a presence in Pennsylvania to support these programs Confidential – For internal use only

18 | RARE INSPIRATION. CHANGING LIVES. QUESTIONS? Confidential – For internal use only

ADDITIONAL INFORMATION ABOUT THE PROPOSED TRANSACTION AND WHERE TO FIND IT 19 | RARE INSPIRATION. CHANGING LIVES. • This document does not constitute a solicitation of any vote or approval. In connection with the proposed acquisition of Achillion by Alexion, Achillion intends to file with the SEC a proxy statement, as well as other relevant documents concerning the proposed transaction. INVESTORS AND SECURITY HOLDERS OF ACHILLION ARE URGED TO READ THE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION WHEN IT BECOMES AVAILABLE AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THOSE DOCUMENTS, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. You may obtain these documents (when they become available) free of charge through the website maintained by the SEC at http://www.sec.gov. Copies of the documents filed with the SEC by Alexion will be available free of charge on Alexion’s internet website at http://www.alexion.com under the tab, “Investors” and under the heading “SEC Filings” or by contacting Alexion’s Investor Relations Department at investorrelations@alexion.com. Copies of the documents filed with the SEC by Achillion will be available free of charge on Achillion’s internet website at http://www.achillion.com under the tab “Investors and News” and under the heading “SEC Filings” or by contacting Achillion’s Investor Relations Department through http://ir.achillion.com/contact-us • Alexion, Achillion and their respective directors and executive officers may be considered participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Alexion is set forth in its Annual Report on Form 10-K for the year ended December 31, 2018, which was filed with the SEC on February 6, 2019, and its proxy statement for its May 14, 2019 annual meeting of stockholders, which was filed with the SEC on March 26, 2019. Information about the directors and executive officers of Achillion is set forth in its Annual Report on Form 10-K for the year ended December 31, 2018, which was filed with the SEC on March 7, 2019, and its proxy statement for its May 30, 2019 annual meeting of stockholders, which was filed with the SEC on April 15, 2019. Other information regarding the participants in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the proxy statement and other relevant materials to be filed with the SEC regarding the proposed transaction when they become available Confidential – For internal use only


















