
Myriad Genetics myChoice HRD® Update 06/30/2016 Exhibit 99.1

Forward Looking Statements Some of the information presented here today may contain projections or other forward-looking statements regarding future events or the future financial performance of the Company. These statements are based on management’s current expectations and the actual events or results may differ materially and adversely from these expectations. We refer you to the documents the Company files from time to time with the Securities and Exchange Commission, specifically, the Company’s annual reports on Form 10-K, its quarterly reports on Form 10-Q, and its current reports on Form 8-K. These documents identify important risk factors that could cause the actual results to differ materially from those contained in the Company’s projections or forward-looking statements.

A Pioneering Discovery: myChoice HRD
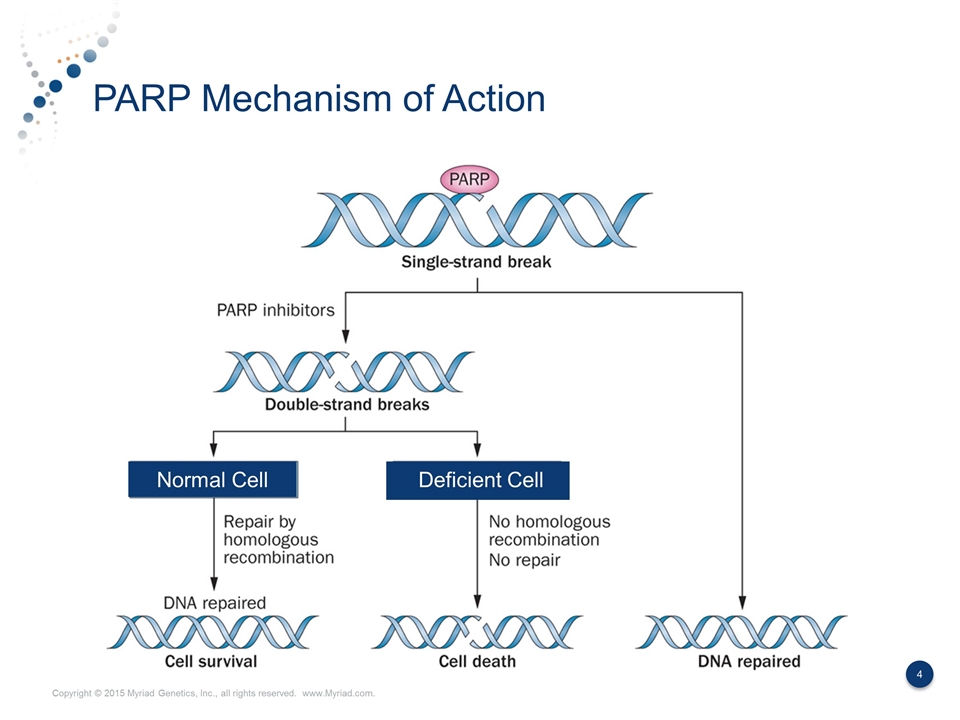
PARP Mechanism of Action Normal Cell Deficient Cell
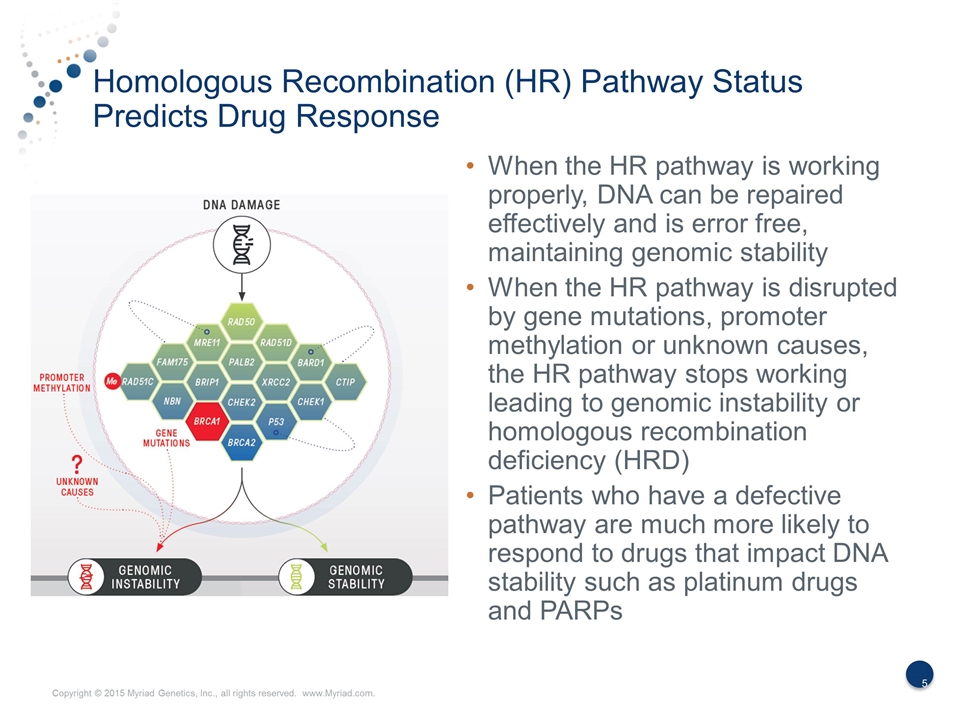
Homologous Recombination (HR) Pathway Status Predicts Drug Response When the HR pathway is working properly, DNA can be repaired effectively and is error free, maintaining genomic stability When the HR pathway is disrupted by gene mutations, promoter methylation or unknown causes, the HR pathway stops working leading to genomic instability or homologous recombination deficiency (HRD) Patients who have a defective pathway are much more likely to respond to drugs that impact DNA stability such as platinum drugs and PARPs
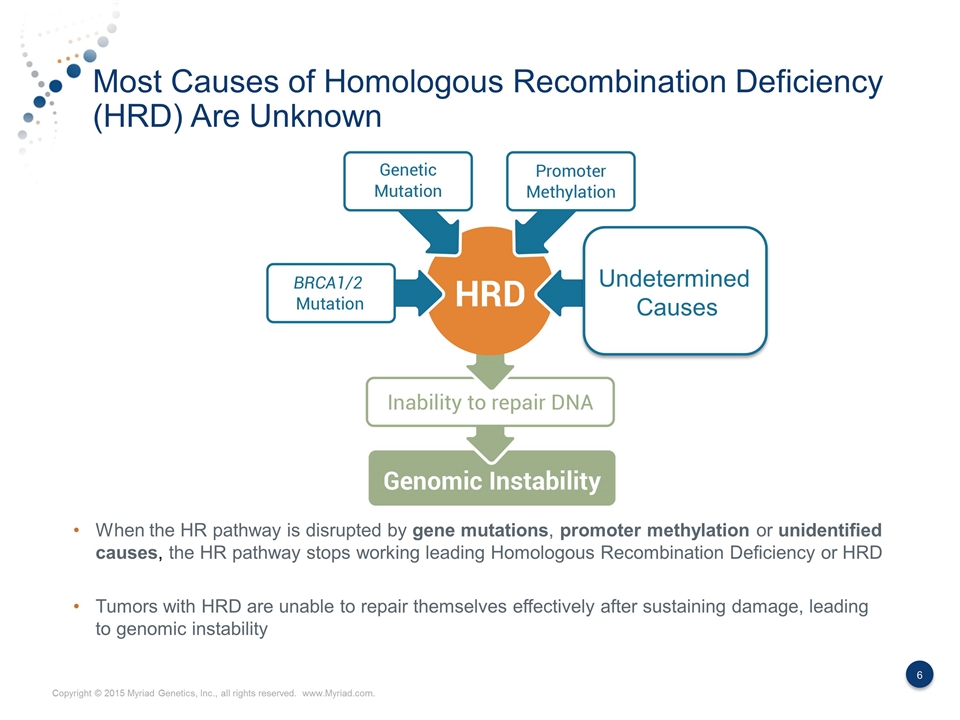
Most Causes of Homologous Recombination Deficiency (HRD) Are Unknown When the HR pathway is disrupted by gene mutations, promoter methylation or unidentified causes, the HR pathway stops working leading Homologous Recombination Deficiency or HRD Tumors with HRD are unable to repair themselves effectively after sustaining damage, leading to genomic instability Undetermined Causes

The Cause? Lane closure? Traffic light failure? Overturned truck? Fender bender? Presidential motorcade? Who cares…. Pioneering Discovery: Measure the Effects vs. the Causes The EFFECT…..
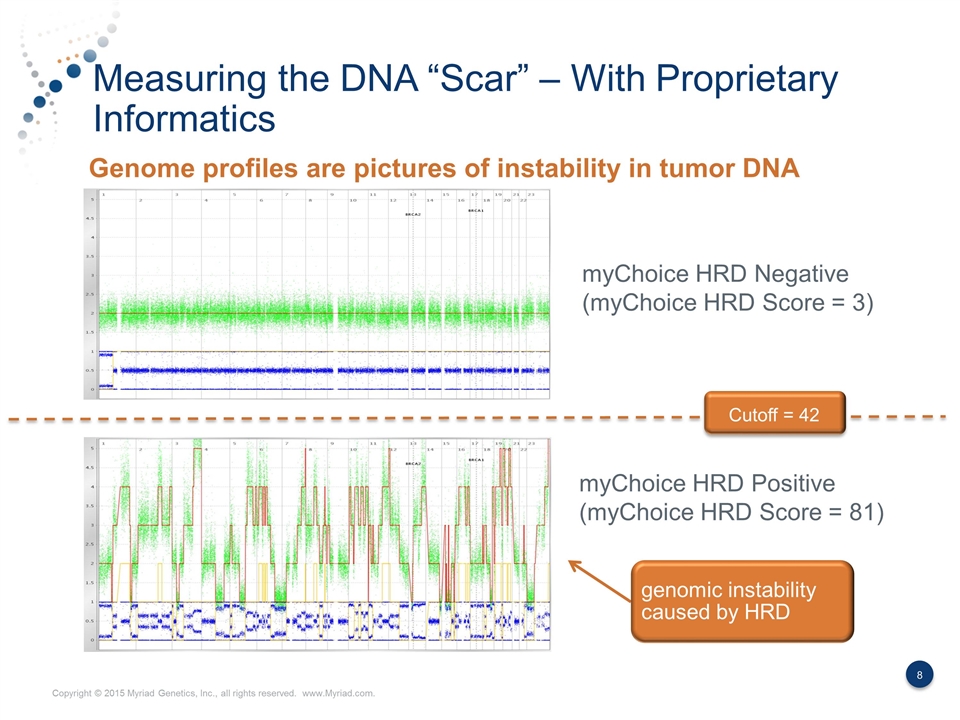
Measuring the DNA “Scar” – With Proprietary Informatics myChoice HRD Negative (myChoice HRD Score = 3) myChoice HRD Positive (myChoice HRD Score = 81) Genome profiles are pictures of instability in tumor DNA Cutoff = 42 genomic instability caused by HRD
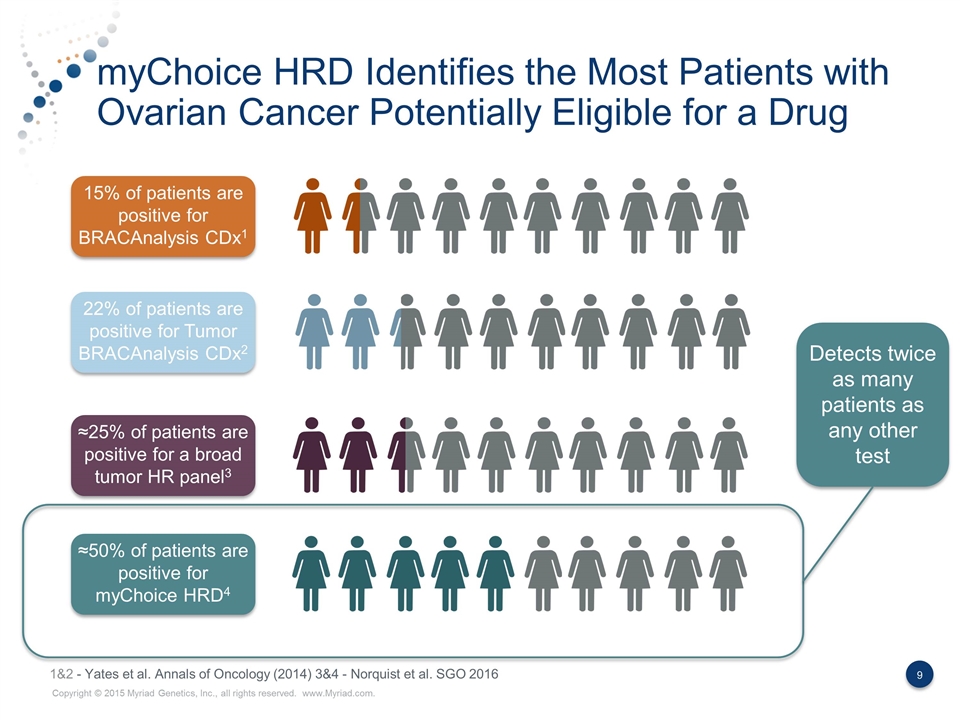
myChoice HRD Identifies the Most Patients with Ovarian Cancer Potentially Eligible for a Drug 15% of patients are positive for BRACAnalysis CDx1 22% of patients are positive for Tumor BRACAnalysis CDx2 ≈25% of patients are positive for a broad tumor HR panel3 ≈50% of patients are positive for myChoice HRD4 Detects twice as many patients as any other test 1&2 - Yates et al. Annals of Oncology (2014) 3&4 - Norquist et al. SGO 2016
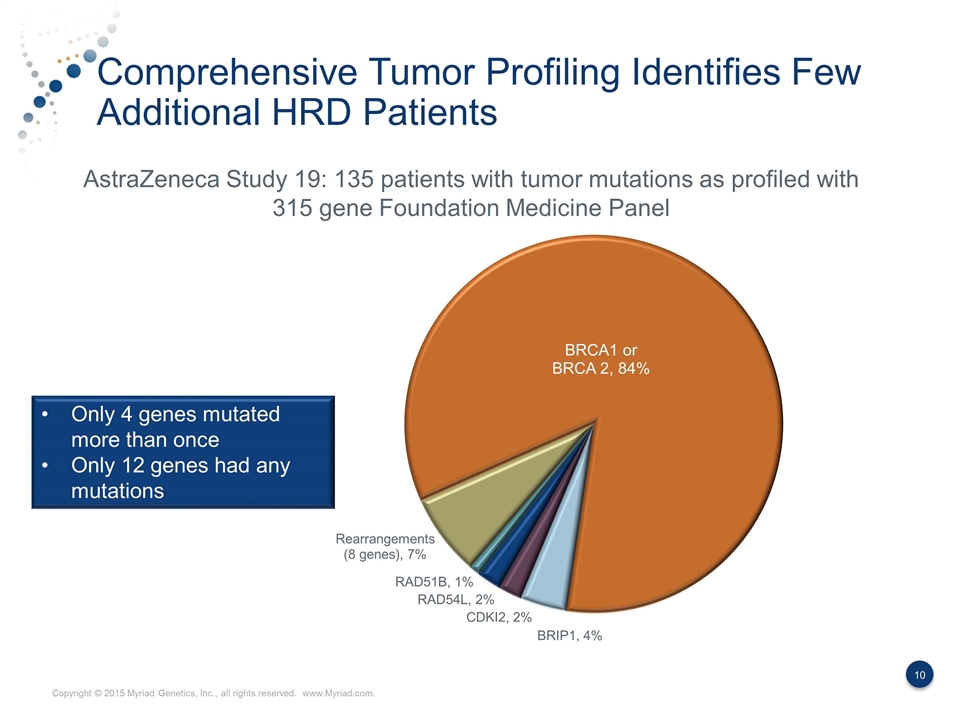
Comprehensive Tumor Profiling Identifies Few Additional HRD Patients AstraZeneca Study 19: 135 patients with tumor mutations as profiled with 315 gene Foundation Medicine Panel Only 4 genes mutated more than once Only 12 genes had any mutations
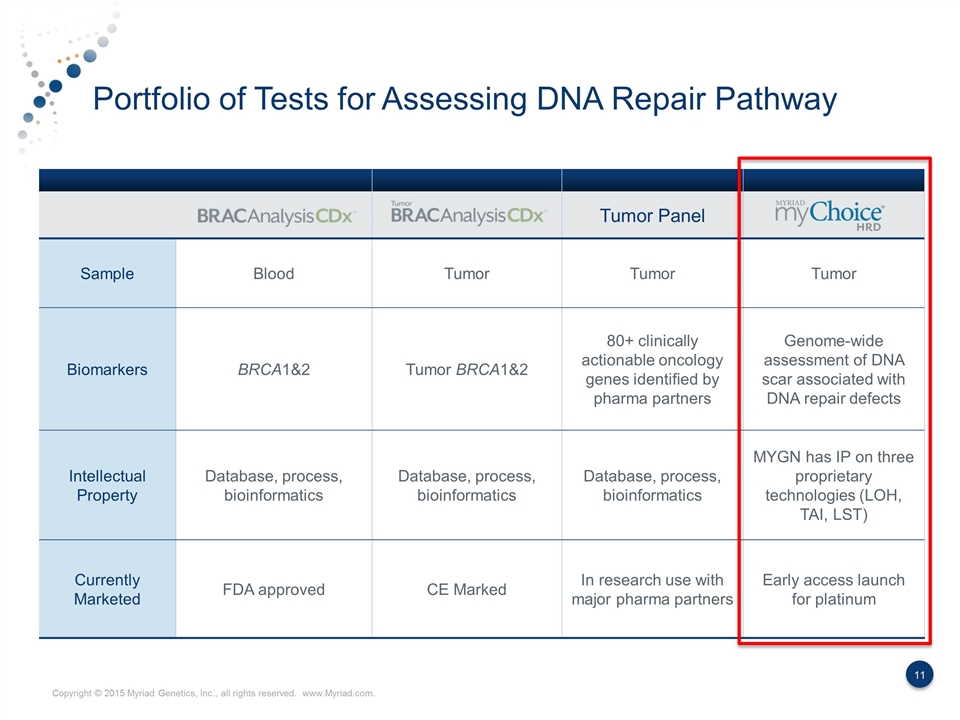
Portfolio of Tests for Assessing DNA Repair Pathway Tumor Panel Sample Blood Tumor Tumor Tumor Biomarkers BRCA1&2 Tumor BRCA1&2 80+ clinically actionable oncology genes identified by pharma partners Genome-wide assessment of DNA scar associated with DNA repair defects Intellectual Property Database, process, bioinformatics Database, process, bioinformatics Database, process, bioinformatics MYGN has IP on three proprietary technologies (LOH, TAI, LST) Currently Marketed FDA approved CE Marked In research use with major pharma partners Early access launch for platinum
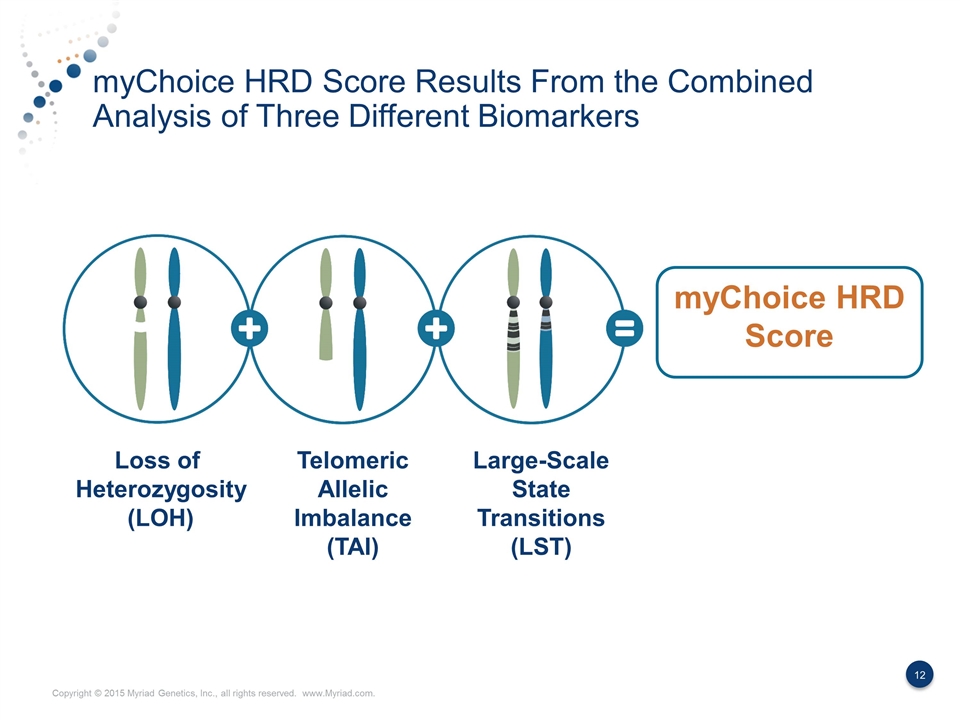
myChoice HRD Score Results From the Combined Analysis of Three Different Biomarkers myChoice HRD Score Loss of Heterozygosity (LOH) Telomeric Allelic Imbalance (TAI) Large-Scale State Transitions (LST)
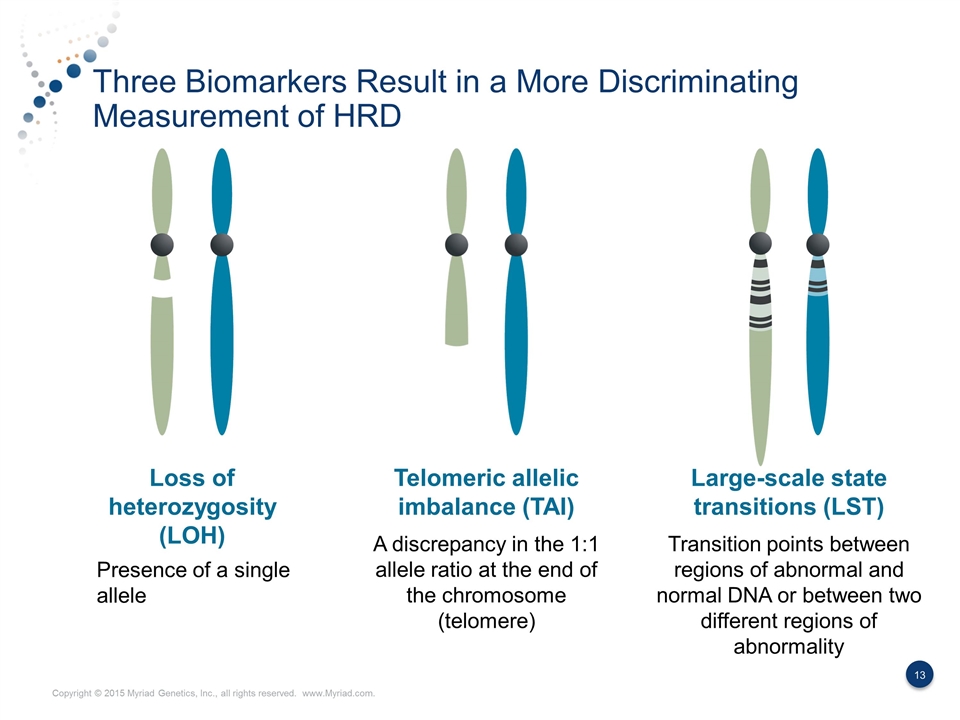
Three Biomarkers Result in a More Discriminating Measurement of HRD Loss of heterozygosity (LOH) Telomeric allelic imbalance (TAI) Large-scale state transitions (LST) Presence of a single allele A discrepancy in the 1:1 allele ratio at the end of the chromosome (telomere) Transition points between regions of abnormal and normal DNA or between two different regions of abnormality
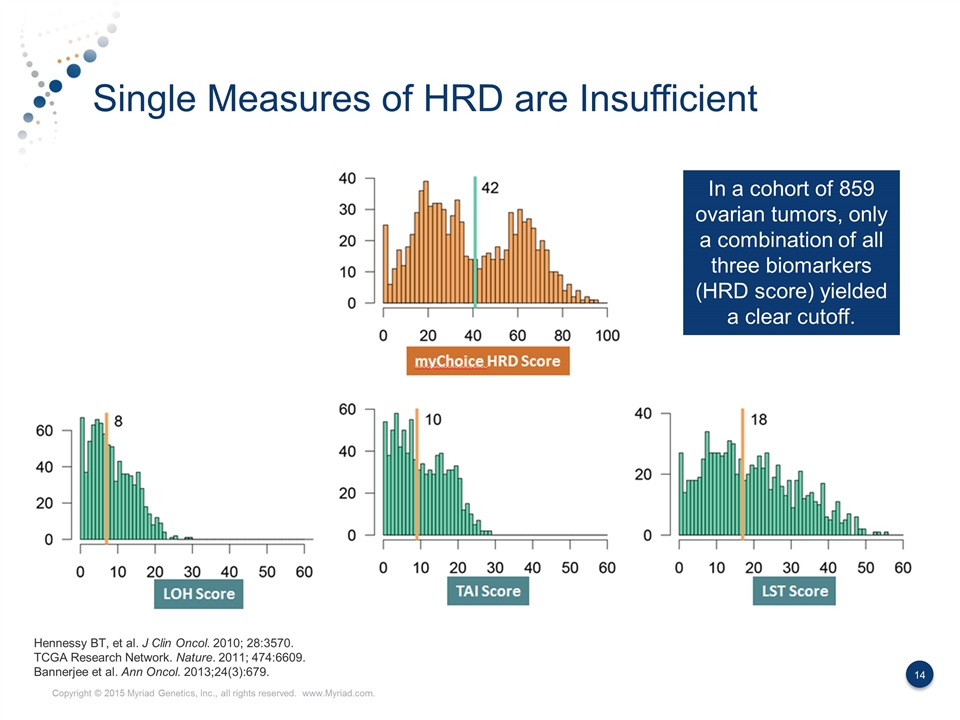
Single Measures of HRD are Insufficient Hennessy BT, et al. J Clin Oncol. 2010; 28:3570. TCGA Research Network. Nature. 2011; 474:6609. Bannerjee et al. Ann Oncol. 2013;24(3):679. In a cohort of 859 ovarian tumors, only a combination of all three biomarkers (HRD score) yielded a clear cutoff.

NOVA Study and Data
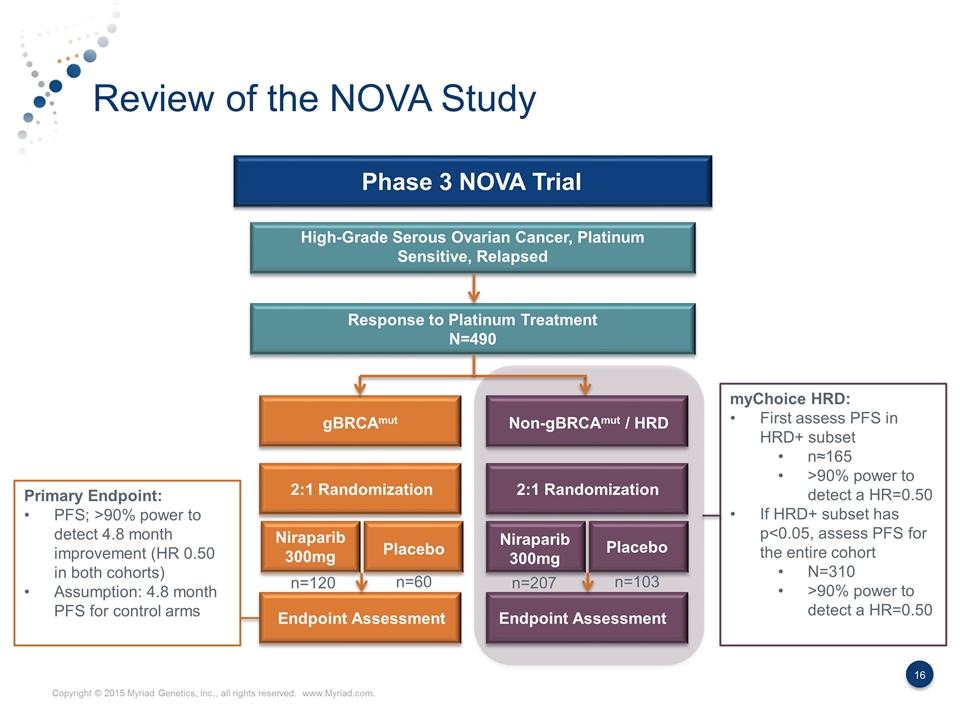
Review of the NOVA Study Phase 3 NOVA Trial High-Grade Serous Ovarian Cancer, Platinum Sensitive, Relapsed Response to Platinum Treatment N=490 gBRCAmut Non-gBRCAmut / HRD 2:1 Randomization 2:1 Randomization Niraparib 300mg Niraparib 300mg Placebo Placebo Endpoint Assessment Endpoint Assessment myChoice HRD: First assess PFS in HRD+ subset n≈165 >90% power to detect a HR=0.50 If HRD+ subset has p<0.05, assess PFS for the entire cohort N=310 >90% power to detect a HR=0.50 n=120 n=60 n=207 n=103 Primary Endpoint: PFS; >90% power to detect 4.8 month improvement (HR 0.50 in both cohorts) Assumption: 4.8 month PFS for control arms
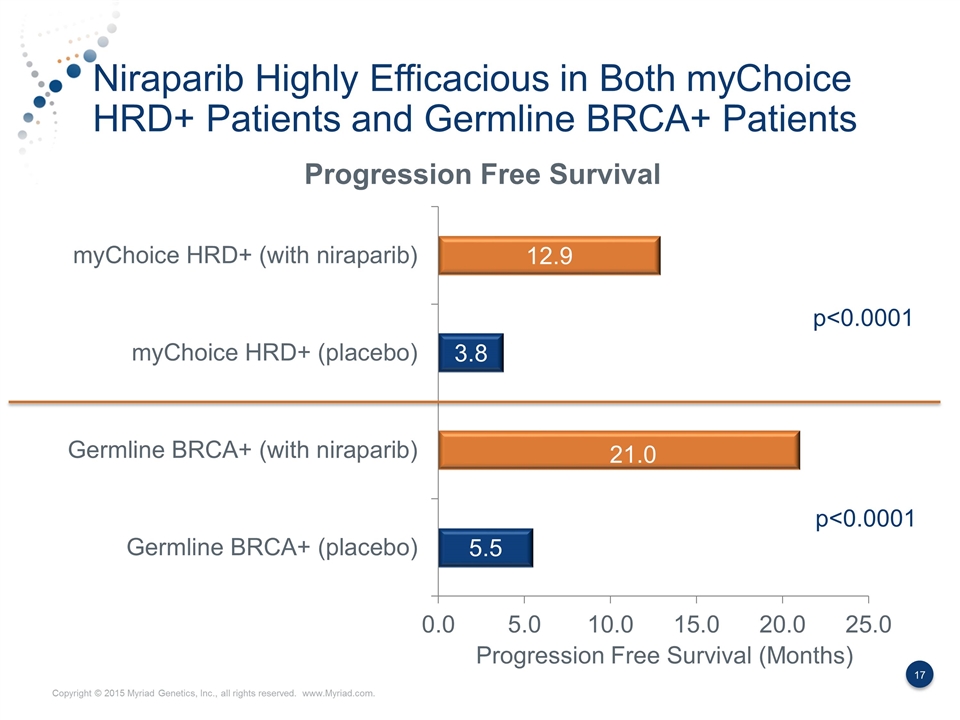
Niraparib Highly Efficacious in Both myChoice HRD+ Patients and Germline BRCA+ Patients p<0.0001 ? 21.0 Progression Free Survival (Months) p<0.0001
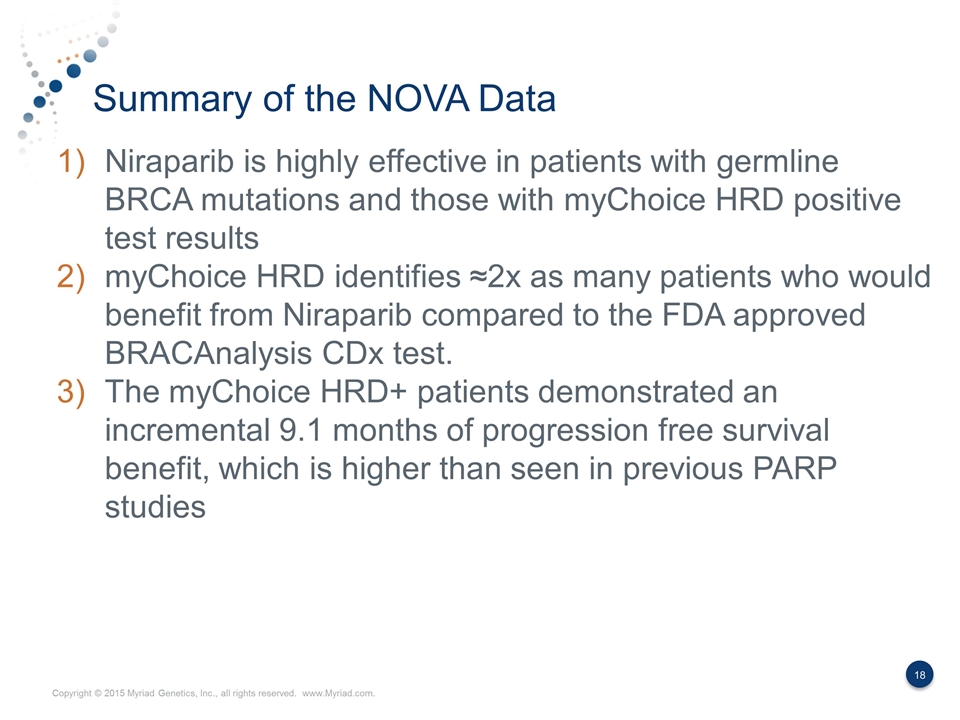
Summary of the NOVA Data Niraparib is highly effective in patients with germline BRCA mutations and those with myChoice HRD positive test results myChoice HRD identifies ≈2x as many patients who would benefit from Niraparib compared to the FDA approved BRACAnalysis CDx test. The myChoice HRD+ patients demonstrated an incremental 9.1 months of progression free survival benefit, which is higher than seen in previous PARP studies

Business Impact
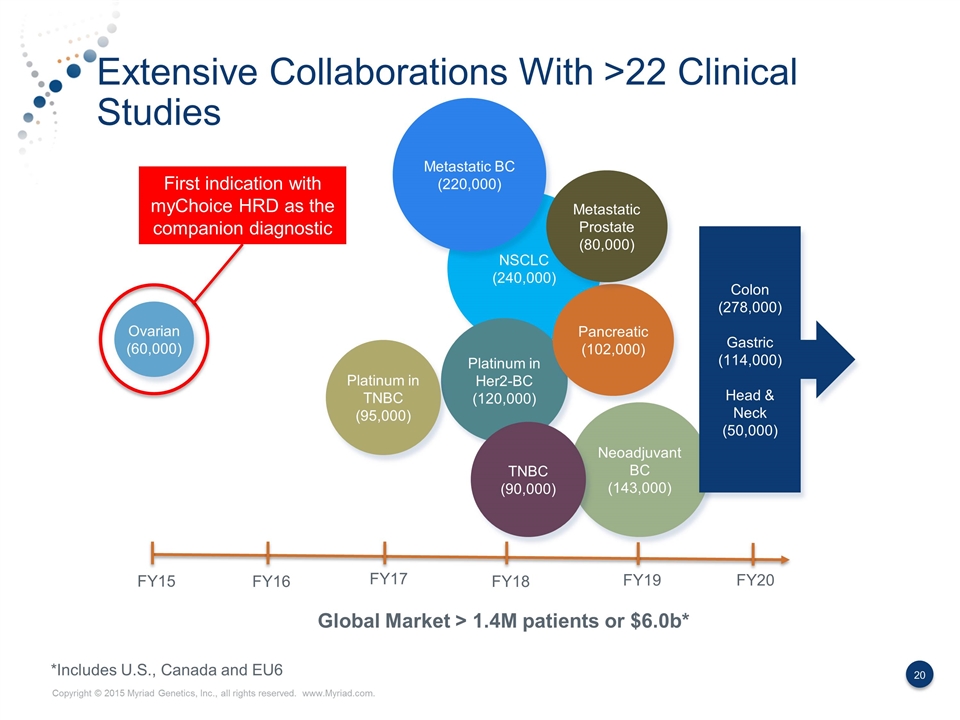
Extensive Collaborations With >22 Clinical Studies NSCLC (240,000) Global Market > 1.4M patients or $6.0b* Neoadjuvant BC (143,000) Ovarian (60,000) Platinum in Her2-BC (120,000) FY15 FY16 FY17 FY18 FY19 FY20 *Includes U.S., Canada and EU6 Platinum in TNBC (95,000) Pancreatic (102,000) Metastatic Prostate (80,000) Colon (278,000) Gastric (114,000) Head & Neck (50,000) Metastatic BC (220,000) TNBC (90,000) First indication with myChoice HRD as the companion diagnostic
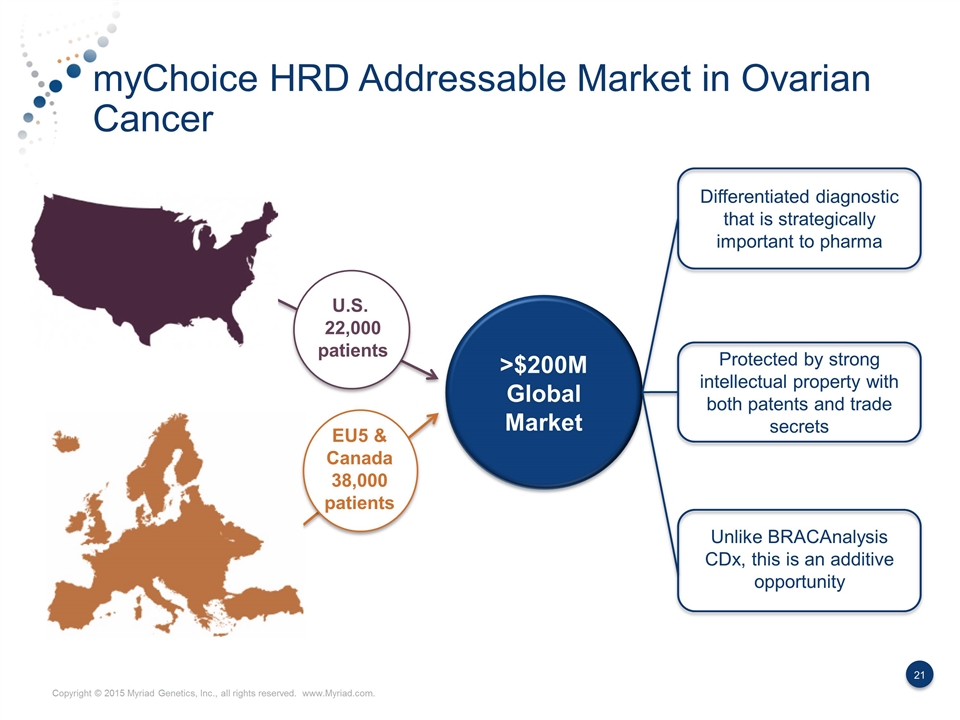
myChoice HRD Addressable Market in Ovarian Cancer U.S. 22,000 patients EU5 & Canada 38,000 patients Differentiated diagnostic that is strategically important to pharma Protected by strong intellectual property with both patents and trade secrets Unlike BRACAnalysis CDx, this is an additive opportunity >$200M Global Market
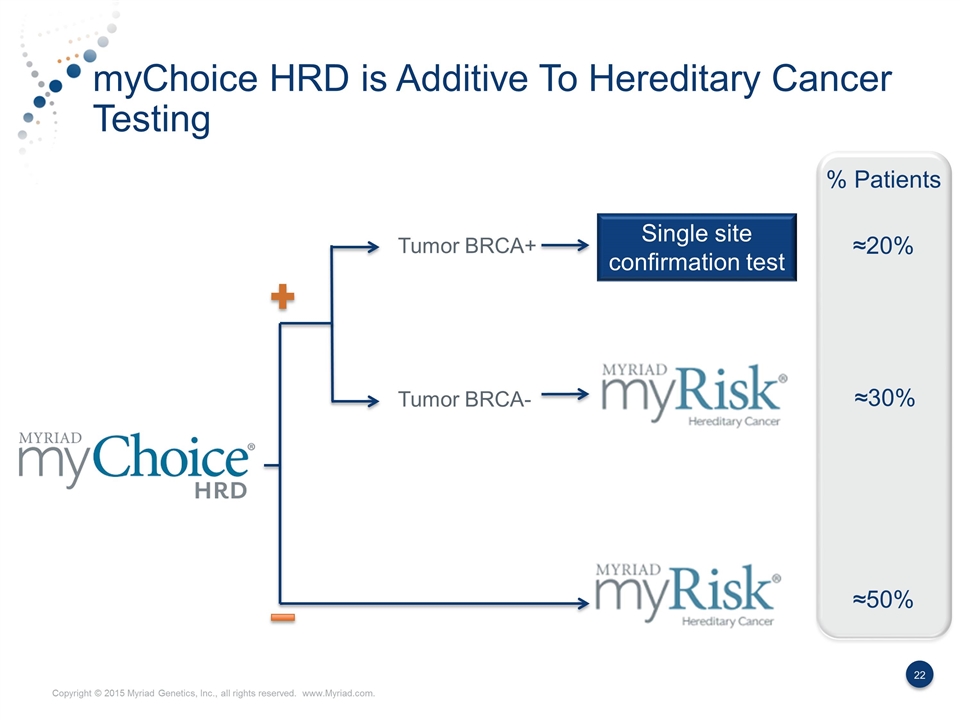
myChoice HRD is Additive To Hereditary Cancer Testing Tumor BRCA+ Single site confirmation test Tumor BRCA- % Patients ≈20% ≈30% ≈50%
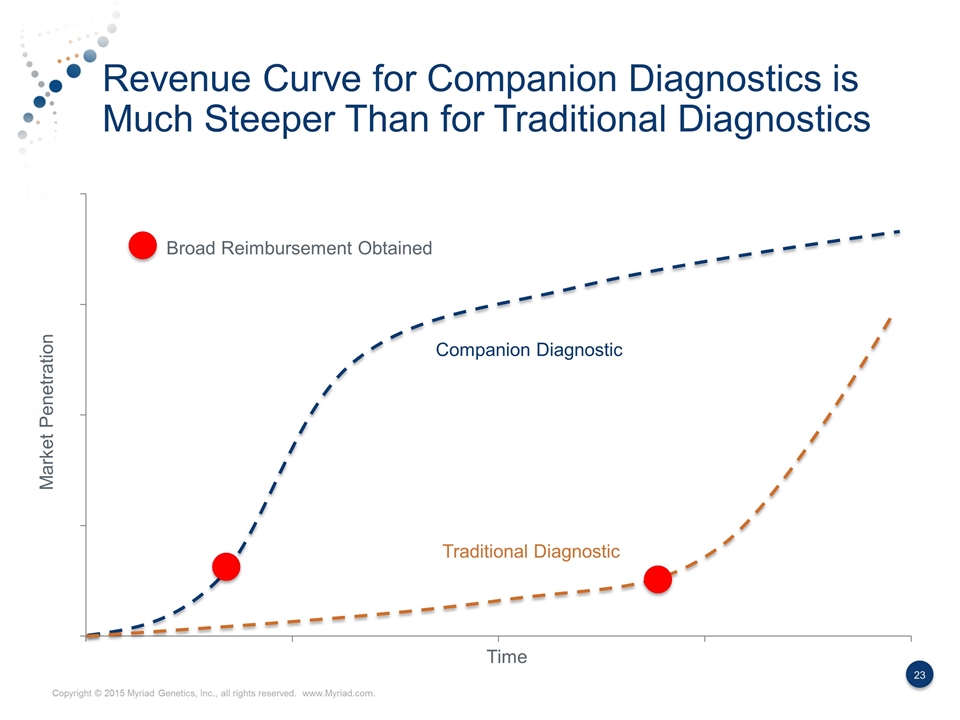
Revenue Curve for Companion Diagnostics is Much Steeper Than for Traditional Diagnostics Market Penetration Time Broad Reimbursement Obtained Companion Diagnostic Traditional Diagnostic

Other Strategic Considerations First prospective data and major validation of myChoice HRD – reduces risk for pharma partners and likely drives additional collaborations Growing core competency in FDA approved, high-value companion diagnostics Intellectual property around myChoice HRD is significant and provides global differentiation























