
Myriad Genetics Fiscal Second-Quarter 2020 Earnings Call February 6, 2020 Exhibit 99.2
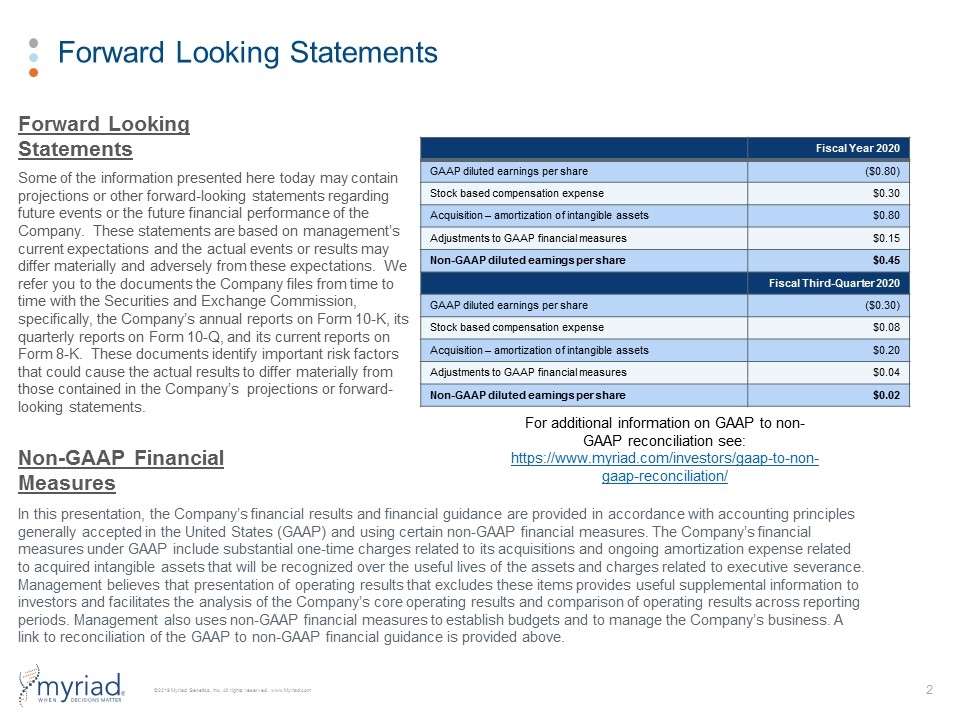
Forward Looking Statements Some of the information presented here today may contain projections or other forward-looking statements regarding future events or the future financial performance of the Company. These statements are based on management’s current expectations and the actual events or results may differ materially and adversely from these expectations. We refer you to the documents the Company files from time to time with the Securities and Exchange Commission, specifically, the Company’s annual reports on Form 10-K, its quarterly reports on Form 10-Q, and its current reports on Form 8-K. These documents identify important risk factors that could cause the actual results to differ materially from those contained in the Company’s projections or forward-looking statements. In this presentation, the Company’s financial results and financial guidance are provided in accordance with accounting principles generally accepted in the United States (GAAP) and using certain non-GAAP financial measures. The Company’s financial measures under GAAP include substantial one-time charges related to its acquisitions and ongoing amortization expense related to acquired intangible assets that will be recognized over the useful lives of the assets and charges related to executive severance. Management believes that presentation of operating results that excludes these items provides useful supplemental information to investors and facilitates the analysis of the Company’s core operating results and comparison of operating results across reporting periods. Management also uses non-GAAP financial measures to establish budgets and to manage the Company’s business. A link to reconciliation of the GAAP to non-GAAP financial guidance is provided above. Forward Looking Statements Non-GAAP Financial Measures For additional information on GAAP to non-GAAP reconciliation see: https://www.myriad.com/investors/gaap-to-non-gaap-reconciliation/ Fiscal Year 2020 GAAP diluted earnings per share ($0.80) Stock based compensation expense $0.30 Acquisition – amortization of intangible assets $0.80 Adjustments to GAAP financial measures $0.15 Non-GAAP diluted earnings per share $0.45 Fiscal Third-Quarter 2020 GAAP diluted earnings per share ($0.30) Stock based compensation expense $0.08 Acquisition – amortization of intangible assets $0.20 Adjustments to GAAP financial measures $0.04 Non-GAAP diluted earnings per share $0.02

Fiscal Second-Quarter 2020 Financial Results 2Q20 Actual Results 2Q19 Actual Results Revenue (in mil.) $195.1 $216.8 GAAP EPS ($0.11) $0.03 Adjusted EPS $0.23 $0.38

Financial Overview
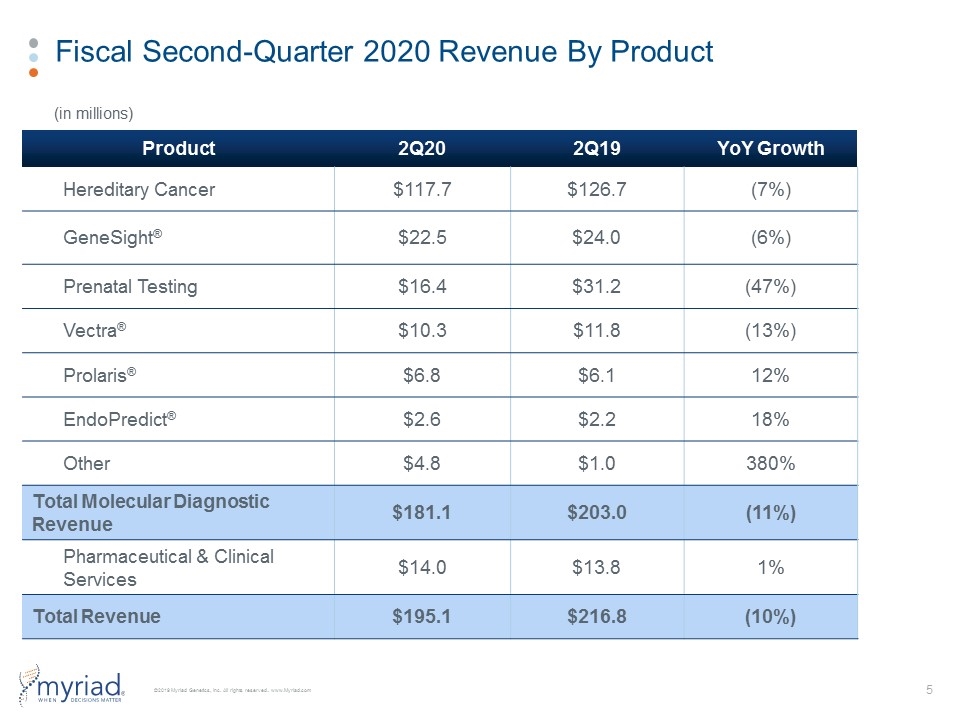
Fiscal Second-Quarter 2020 Revenue By Product Product 2Q20 2Q19 YoY Growth Hereditary Cancer $117.7 $126.7 (7%) GeneSight® $22.5 $24.0 (6%) Prenatal Testing $16.4 $31.2 (47%) Vectra® $10.3 $11.8 (13%) Prolaris® $6.8 $6.1 12% EndoPredict® $2.6 $2.2 18% Other $4.8 $1.0 380% Total Molecular Diagnostic Revenue $181.1 $203.0 (11%) Pharmaceutical & Clinical Services $14.0 $13.8 1% Total Revenue $195.1 $216.8 (10%) (in millions)
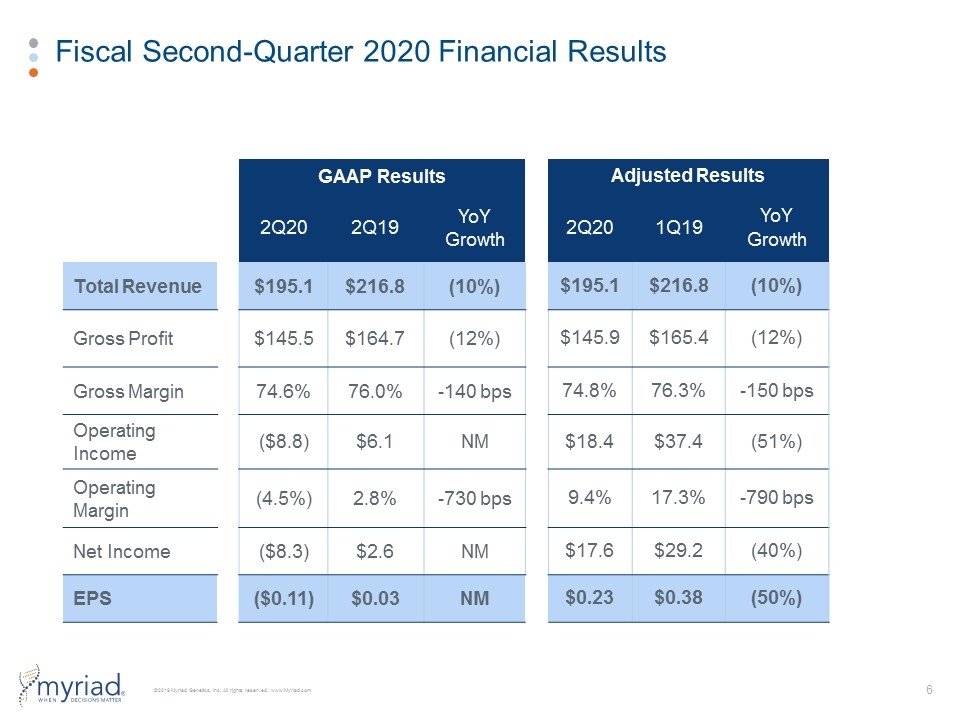
Fiscal Second-Quarter 2020 Financial Results GAAP Results Adjusted Results 2Q20 2Q19 YoY Growth 2Q20 1Q19 YoY Growth Total Revenue $195.1 $216.8 (10%) $195.1 $216.8 (10%) Gross Profit $145.5 $164.7 (12%) $145.9 $165.4 (12%) Gross Margin 74.6% 76.0% -140 bps 74.8% 76.3% -150 bps Operating Income ($8.8) $6.1 NM $18.4 $37.4 (51%) Operating Margin (4.5%) 2.8% -730 bps 9.4% 17.3% -790 bps Net Income ($8.3) $2.6 NM $17.6 $29.2 (40%) EPS ($0.11) $0.03 NM $0.23 $0.38 (50%)
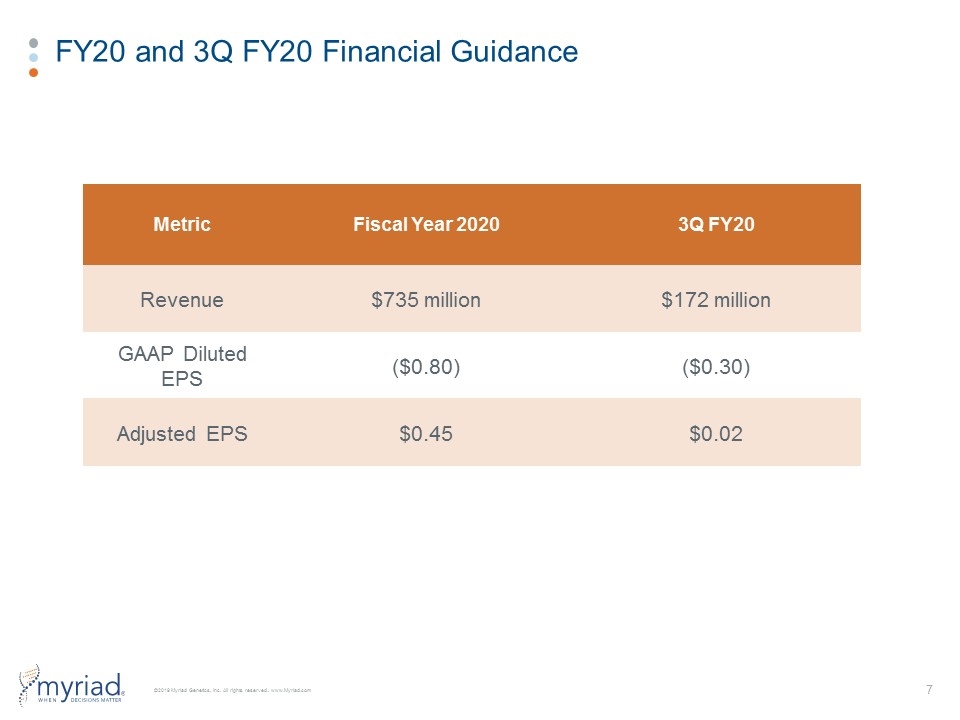
FY20 and 3Q FY20 Financial Guidance Metric Fiscal Year 2020 3Q FY20 Revenue $735 million $172 million GAAP Diluted EPS ($0.80) ($0.30) Adjusted EPS $0.45 $0.02
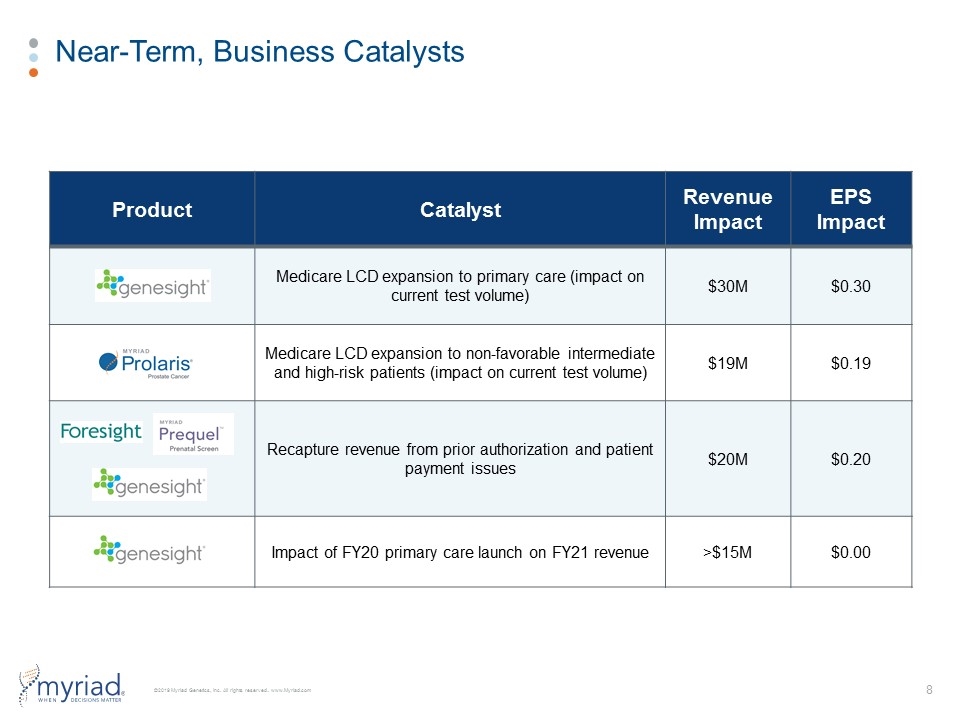
Near-Term, Business Catalysts Product Catalyst Revenue Impact EPS Impact Medicare LCD expansion to primary care (impact on current test volume) $30M $0.30 Medicare LCD expansion to non-favorable intermediate and high-risk patients (impact on current test volume) $19M $0.19 Recapture revenue from prior authorization and patient payment issues $20M $0.20 Impact of FY20 primary care launch on FY21 revenue >$15M $0.00
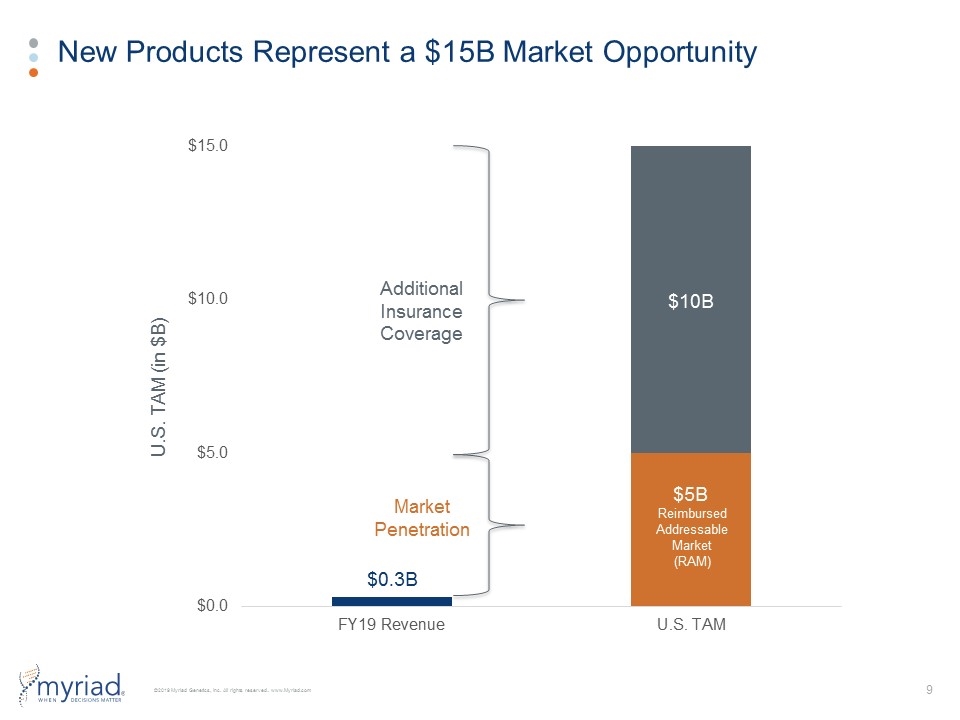
New Products Represent a $15B Market Opportunity $5B Reimbursed Addressable Market (RAM) $10B Additional Insurance Coverage Market Penetration U.S. TAM (in $B) $0.3B
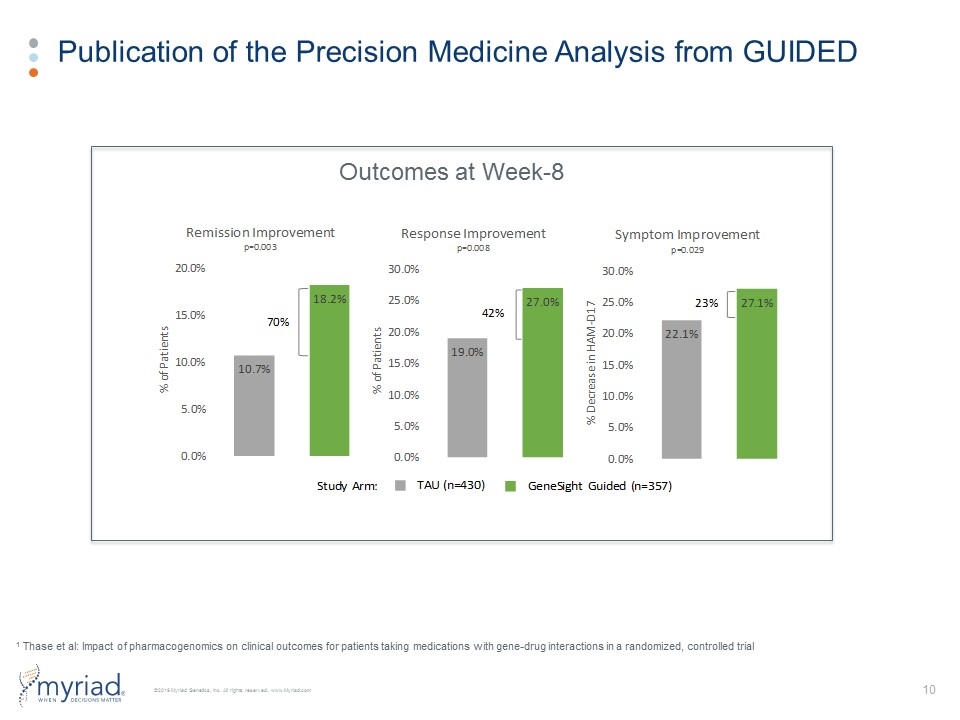
Publication of the Precision Medicine Analysis from GUIDED Outcomes at Week-8 1 Thase et al: Impact of pharmacogenomics on clinical outcomes for patients taking medications with gene-drug interactions in a randomized, controlled trial 70% 42% 23% Study Arm: TAU (n=430) GeneSight Guided (n=357)
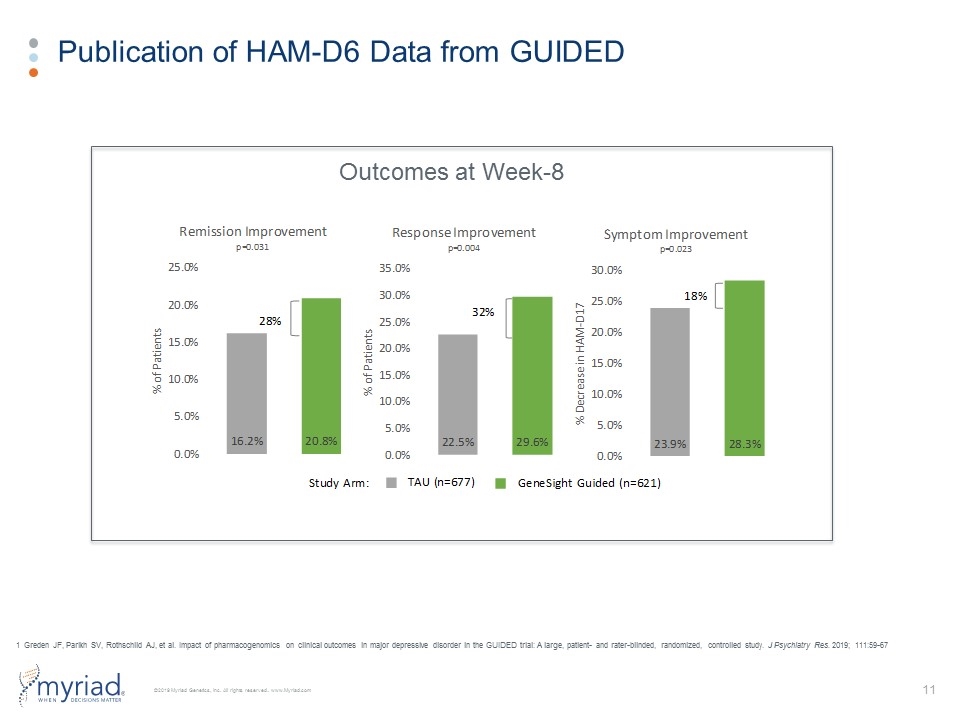
Publication of HAM-D6 Data from GUIDED Outcomes at Week-8 1 Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatry Res. 2019; 111:59-67
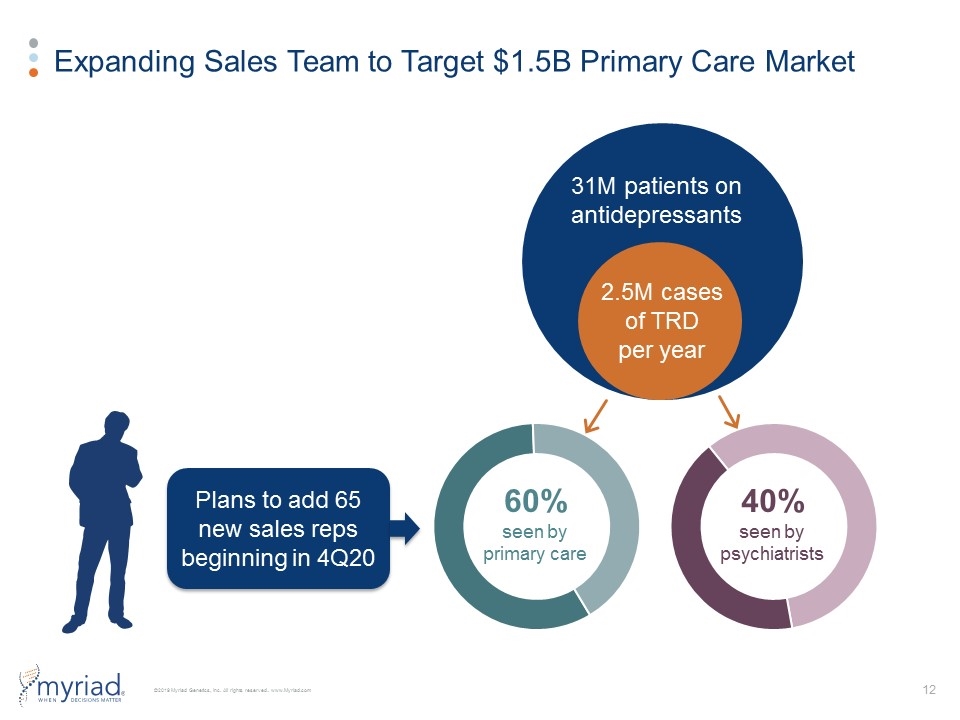
Expanding Sales Team to Target $1.5B Primary Care Market 31M patients on antidepressants 2.5M cases of TRD per year 60% seen by primary care 40% seen by psychiatrists Plans to add 65 new sales reps beginning in 4Q20
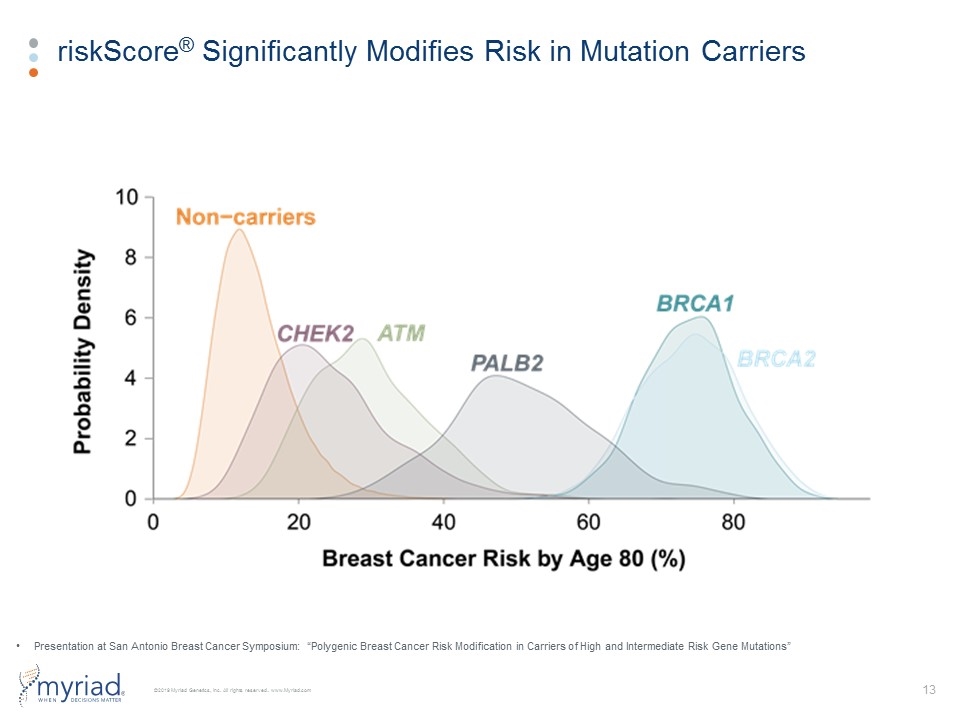
riskScore® Significantly Modifies Risk in Mutation Carriers Presentation at San Antonio Breast Cancer Symposium: “Polygenic Breast Cancer Risk Modification in Carriers of High and Intermediate Risk Gene Mutations”
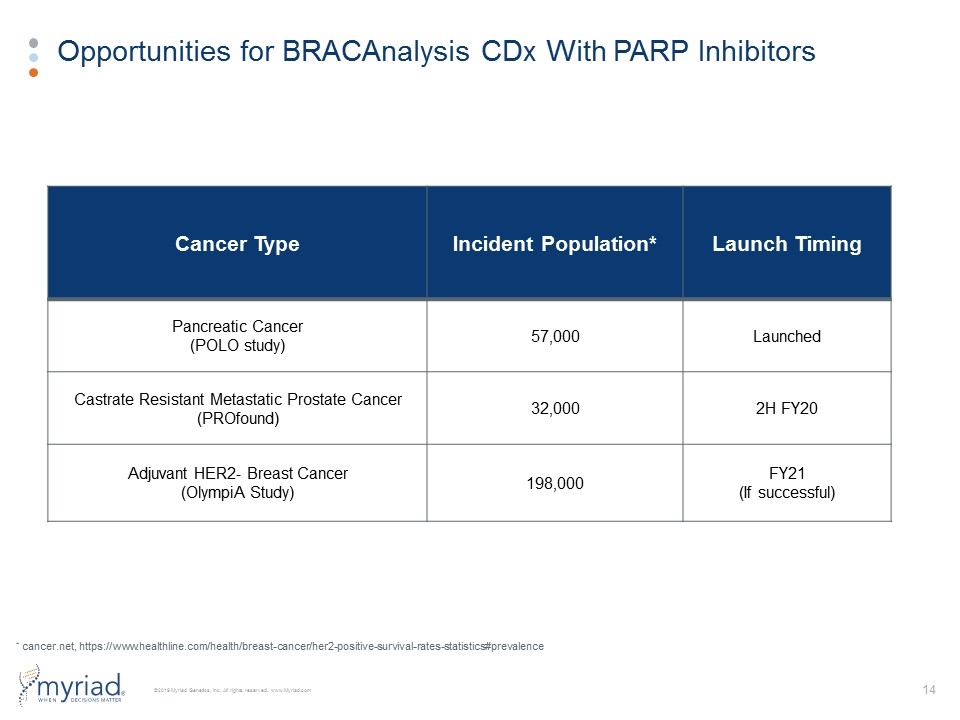
Opportunities for BRACAnalysis CDx With PARP Inhibitors Cancer Type Incident Population* Launch Timing Pancreatic Cancer (POLO study) 57,000 Launched Castrate Resistant Metastatic Prostate Cancer (PROfound) 32,000 2H FY20 Adjuvant HER2- Breast Cancer (OlympiA Study) 198,000 FY21 (If successful) * cancer.net, https://www.healthline.com/health/breast-cancer/her2-positive-survival-rates-statistics#prevalence
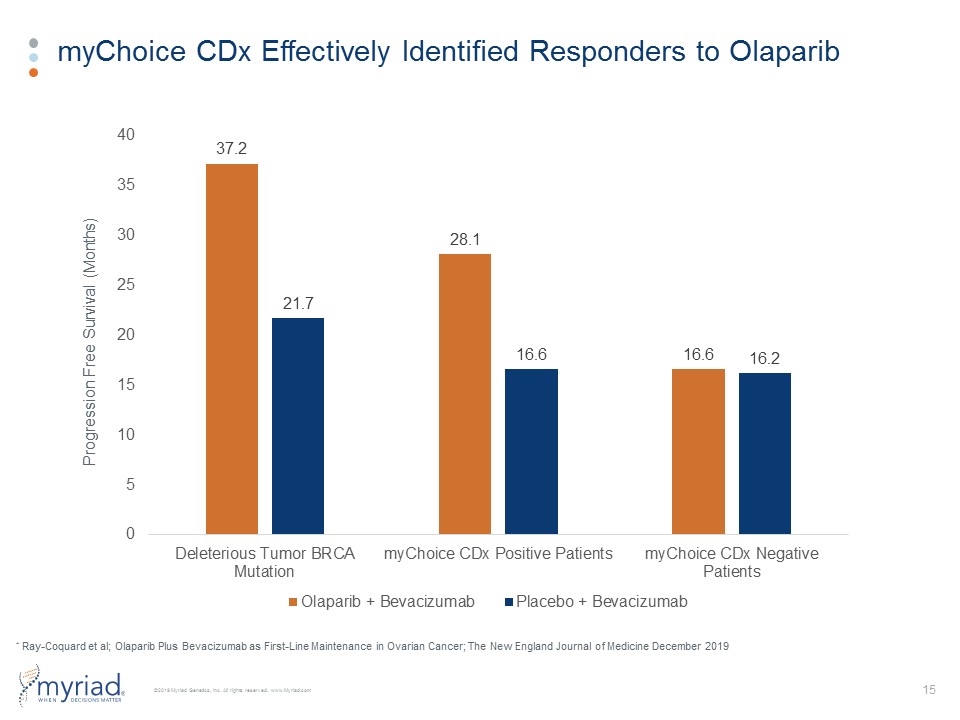
myChoice CDx Effectively Identified Responders to Olaparib * Ray-Coquard et al; Olaparib Plus Bevacizumab as First-Line Maintenance in Ovarian Cancer; The New England Journal of Medicine December 2019 Progression Free Survival (Months)
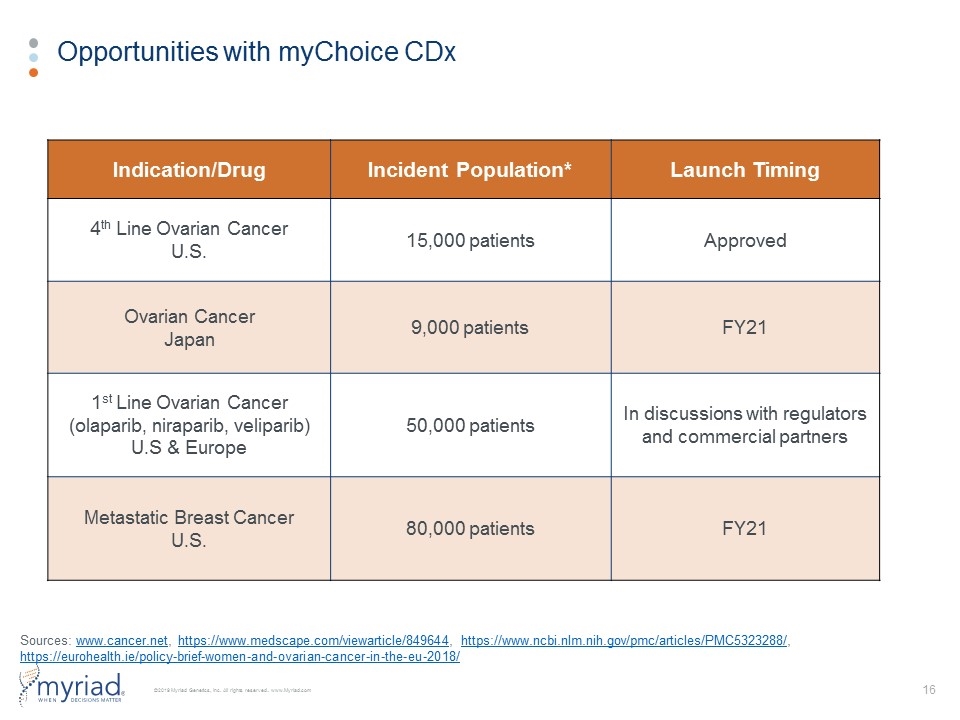
Opportunities with myChoice CDx Indication/Drug Incident Population* Launch Timing 4th Line Ovarian Cancer U.S. 15,000 patients Approved Ovarian Cancer Japan 9,000 patients FY21 1st Line Ovarian Cancer (olaparib, niraparib, veliparib) U.S & Europe 50,000 patients In discussions with regulators and commercial partners Metastatic Breast Cancer U.S. 80,000 patients FY21 Sources: www.cancer.net, https://www.medscape.com/viewarticle/849644, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5323288/, https://eurohealth.ie/policy-brief-women-and-ovarian-cancer-in-the-eu-2018/

Prequel® is the Most Accurate NIPS Test in Women With High BMI Source: Muzzey et al: Noninvasive prenatal screening for patients with high body mass index: Evaluating the impact of a customized whole genome sequencing workflow on sensitivity and residual risk No call rate of up to 24% in high BMI women using SNP arrays with 4% fetal fraction cutoff Prequel has demonstrated high diagnostic accuracy in women below a 4% fetal fraction Prequel maintained high analytical sensitivity in women with high BMIs 50% of pregnant women are overweight or obese No call rate for Prequel is 1 in 1,000
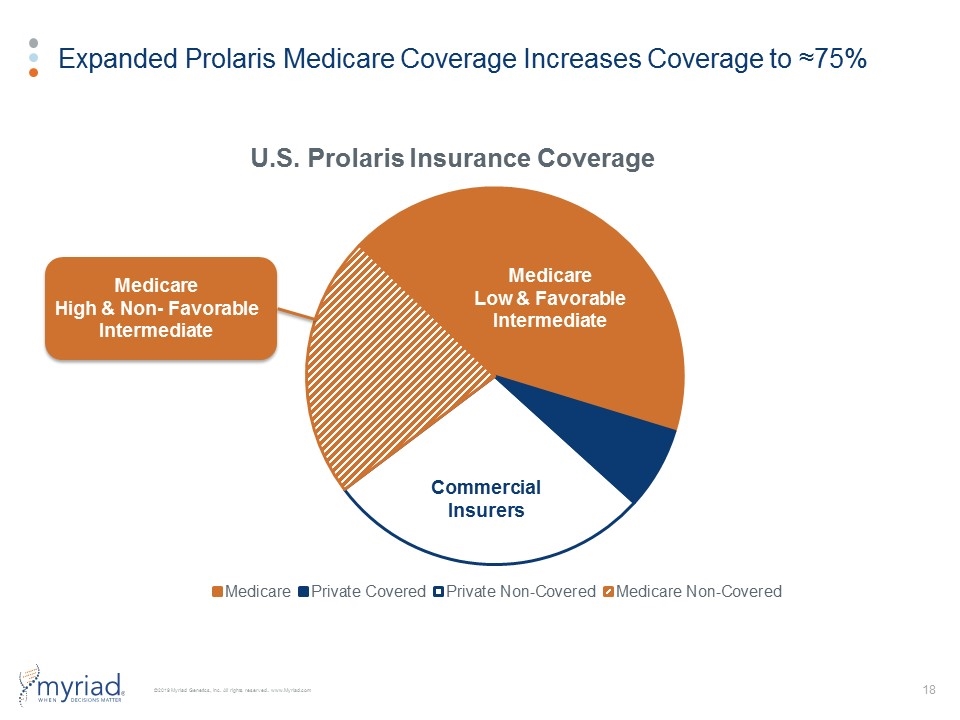
Expanded Prolaris Medicare Coverage Increases Coverage to ≈75% 225 Sales Reps 80 Sales Reps Commercial Insurers Medicare Low & Favorable Intermediate Medicare High & Non- Favorable Intermediate

















