Included in the above total are contracts related to certain product purchase and licence agreements with deferred consideration obligations, the amounts of which are variable depending upon particular ‘milestone’ achievements. Sales of the products to which these ‘milestones’ relate could give rise to additional payments, contingent upon the sales levels achieved. Guarantees and contingencies arising in the ordinary course of business, for which no security has been given, are not expected to result in any material financial loss.
In 1998, Astra and Merck & Co., Inc restructured their joint venture (the “restructuring”) which had been established some years earlier for the purpose of selling and marketing certain Astra products in the US.
Under the terms of the 1998 restructuring, the merger between Astra and Zeneca in 1999 triggered two one-time payments from AstraZeneca to Merck:
Back to Contents
| 102 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Financial Statements | |
Notes to the Financial Statements continued
34 Assets pledged, commitments and contingent liabilities (continued)
In addition, in 2008 there will be a true up of the Advance Payment. The calculation of this will be based on a multiple of the previous three years’ contingent payments in respect of all the agreement products with the exception of Prilosec and Nexium, plus other defined amounts, which are then reduced by the Appraised Value (whether paid or not), the Partial Redemption and the Advance Payment. This could result in a further payment by AstraZeneca to Merck or a payment by Merck to AstraZeneca.
The precise amount of settlements with Merck under the Partial Redemption and the First Option cannot be determined at this time, as some of the payments are based on calculations based on sales between 2005 and 2007, and another is contingent upon Merck exercising the First Option. However, if Merck does exercise this option, the combined effect will involve a minimum amount payable to Merck in 2008 of approximately $4.7bn. If AstraZeneca exercises this option in 2010, the combined effect will involve a minimum aggregate payable to Merck in 2008 and 2010 of approximately $4.7bn.
Finally, in 2008 Merck will repay to AstraZeneca a loan in the amount of $1.4bn made at the time of the restructuring.
Second Option
A Second Option exists whereby AstraZeneca has the option to re-purchase Merck’s interests in Prilosec and Nexium in the US. This option is exercisable by AstraZeneca two years after the exercise of the First Option in either 2008 or 2010. Exercise of the Second Option by AstraZeneca at a later date is also provided for in 2017 or if combined annual sales of the two products fall below a minimum amount provided, in each case only so long as the First Option has been exercised. The exercise price for the Second Option is the fair value of these product rights as determined at the time of exercise. If the Second Option is exercised, Merck will have no further rights to contingent payments from AstraZeneca.
Environmental costs and liabilities
The Group’s expenditure on environmental protection, including both capital and revenue items, relates to costs which are necessary for meeting current good practice standards and regulatory requirements for processes and products.
They are an integral part of normal ongoing expenditure for maintaining the Group’s manufacturing capacity and product ranges and are not separated from overall operating and development costs. There are no known changes in environmental, regulatory or other requirements resulting in material changes to the levels of expenditure for 2000, 2001 or 2002.
In addition to expenditure for meeting current and foreseen environmental protection requirements, the Group incurs substantial costs in investigating and cleaning up land and groundwater contamination. In particular, AstraZeneca has environmental liabilities at some currently or formerly owned, leased and third party sites in the US and Europe. AstraZeneca, or its indemnitees, have been named under US legislation (the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended) as potentially responsible parties (PRP) in respect of 32 sites (although AstraZeneca expects to be indemnified against liabilities associated with nine of these sites by the seller or owner of the businesses associated with such sites) and, where appropriate, actively participates in or monitors the clean-up activities at sites in respect of which it is a PRP. Stauffer Management Company, a subsidiary of AstraZeneca established in 1987 to own and manage certain assets of Stauffer Chemical Company which was acquired that year, has identified 28 sites (including 18 for which an AstraZeneca indemnitee has been named a PRP) for which it may have responsibility that will, in aggregate, require significant expenditure on clean-up and monitoring.
Liabilities are generally more likely to crystallise where a contaminated site is to be sold, its use changed or where a regulatory authority imposes a particular remedial measure. Costs of these liabilities may be offset by amounts recovered from third parties, such as previous owners of the sites in question or through insurance.
The future level of investigation and clean up costs will depend on a number of factors, including the nature and extent of any contamination that may ultimately be found to exist, the need for and type of any remedial work to be undertaken and the standards required by applicable current and future environmental laws and regulations and the number and financial viability of other PRPs. The relative importance of these factors varies significantly from site to site. Many sites are at different stages in the regulatory process or at different stages in the process of evaluating environmental damage or alternative remediation methods. It is therefore difficult to form meaningful ranges of estimates for such costs.
AstraZeneca had provisions at 31 December 2002 in respect of such costs in accordance with the accounting policies on page 64. Although there can be no assurance, management believes that, taking account of these provisions, the costs of addressing currently identified environmental obligations, as AstraZeneca currently views those obligations, is unlikely to impair materially AstraZeneca’s financial position.
Such contingent costs, to the extent that they exceed applicable provisions, could have a material adverse effect on AstraZeneca’s results of operations for the relevant period.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Financial Statements | 103 |
Legal proceedings
Losec/Prilosec (omeprazole)
In June 1997, the German Federal Patent Court declared invalid a previously granted supplementary protection certificate which extended protection for omeprazole, the active ingredient contained in Losec, from 1999 to 2003. The decision was appealed and on 1 February 2000, at AstraZeneca’s request, the German Supreme Court decided to refer the case to the European Court of Justice for a preliminary ruling. The court heard the case on 8 November 2001 and its decision is pending. The case does not involve any financial claims.
In March 2000, the German Federal Patent Court declared that AstraZeneca’s formulation patent for omeprazole was invalid. The decision has been appealed to the German Supreme Court. As a consequence, all pending infringement actions in Germany have been stayed awaiting the outcome of the appeal. There is one interlocutory injunction in force against ratiopharm GmbH based on the formulation patent. If the final decision on the validity of the formulation patent goes against AstraZeneca, ratiopharm may claim damages for lost sales due to the interlocutory injunction.
In 1998, Astra filed suits in the US against Andrx Pharmaceuticals, Inc. and Genpharm, Inc. This followed the filing of abbreviated new drug applications by Andrx and Genpharm with the US Food and Drug Administration (FDA) concerning the two companies’ intention to market generic omeprazole products in the US. During 1999, Astra also filed suits against Kremers Urban Development Company and Schwarz Pharma, Inc., and against Cheminor Drugs Ltd., Reddy-Cheminor Inc. and Schein Pharmaceuticals, Inc. During 2000, AstraZeneca filed further suits against Lek Pharmaceutical and Chemical Company d.d, Impax Laboratories Inc., Eon Labs Manufacturing Inc. and Mylan Pharmaceuticals Inc. During 2001, AstraZeneca filed further suits against Torpharm, Inc. and Zenith Goldline Pharmaceuticals, Inc. (Ivax). The basis for the proceedings is that the actions of all the companies infringe several patents relating to omeprazole (Prilosec in the US). The cases are proceeding under the US Hatch-Waxman legislation. AstraZeneca filed additional patent infringement suits during 2001 against Andrx and Genpharm in respect of one other omeprazole patent outside the Hatch-Waxman legislation. The trial against Andrx, Genpharm, Kremers Urban Development Company and Cheminor started in December 2001 and ended in July 2002.
In October 2002, the US District Court for the Southern District of New York ruled that two AstraZeneca patents (‘230 and ‘505) relating to the formulation of omeprazole are valid until 2007, that Andrx, Genpharm and Cheminor all infringed both patents but that Kremers Urban Development Company did not infringe either patent. The court did not rule on the ‘281 patent relating to a manufacturing process for omeprazole formulations in respect of which AstraZeneca has sued Andrx only. AstraZeneca has appealed the judgement with regard to non-infringement and Kremers Urban Development Company. Andrx, Genpharm and Cheminor have appealed the decision with regard to infringement and validity of the patents.
In April 2001, Andrx filed a case in the US District Court for the Southern District of New York against AstraZeneca, Merck & Co., Inc. and the FDA alleging that the listing of certain patents in the FDA’s Orange Book was improper and constituted violations of certain provisions of the Sherman Act, the US federal anti-trust legislation, and a state statute analogous to the federal anti-trust laws. Andrx seeks injunctive relief compelling the parties to delist omeprazole-related patents it claims were improperly listed in the Orange Book and prohibiting the defendants from using patents to delay the effective date of the FDA’s approval of Andrx’s ANDA for omeprazole. AstraZeneca and Merck have filed motions to dismiss the case, which are pending.
AstraZeneca and Merck & Co., Inc. were named as defendants in three class actions; two in the US District Court for the Southern District of New York and one in the US District Court for the District of New Jersey. The plaintiffs are consumers and third party payers who have alleged that they and others who are similarly situated have been forced to pay higher prices for omeprazole as a result of agreements that AstraZeneca and Merck entered into that resulted in ‘unreasonable restraints of trade and competition’. Furthermore, the plaintiffs have alleged that AstraZeneca and Merck engaged in conduct designed to extend their monopoly power ‘beyond the lawful boundaries of their patents’. The plaintiffs are seeking declarative, equitable and injunctive relief enjoining AstraZeneca and Merck from continuing their alleged illegal activities, costs of suit, reasonable attorney’s fees and expenses and any other relief determined by the court. AstraZeneca filed a motion in March 2002 to dismiss the two class actions before the US District Court for the Southern District of New York, which was granted in June 2002. The plaintiffs did not appeal. The plaintiffs voluntarily dismissed the New Jersey case also in June 2002.
In October 2000, the Federal Court of Australia (Full Court) handed down a patent ruling pertaining to omeprazole in connection with a dispute between AstraZeneca and the generic company, Alphapharm Pty Ltd. The court declared that AstraZeneca’s formulation patent was invalid. In November 2001, AstraZeneca applied for special leave to appeal the decision to the High Court of Australia and this application was granted in December 2001. The appeal was heard by the High Court in May 2002 and in December 2002 the High Court reversed the judgement of the lower court. The High Court ruled that AstraZeneca’s formulation patent is valid and that the case should be returned to the lower court for determination of the remaining issues.
During 2000, AstraZeneca was granted interlocutory injunctions based on certain of AstraZeneca’s omeprazole patents and supplementary protection certificates against the generic company, Scandinavian Pharmaceuticals-Generics AB (Scand Pharm), in Sweden, Denmark and Norway. In October 2000, the District Court of Stockholm ruled that Scand Pharm had infringed one of AstraZeneca’s supplementary protection certificates for omeprazole. Scand Pharm has appealed this decision. In October 2001, Oslo City Court in Norway found that Scand Pharm had infringed AstraZeneca’s formulation patent for omeprazole. At the same time, the court declared AstraZeneca’s formulation patent valid. As a result of the Norwegian case, Scand Pharm cannot sell its omeprazole product in Norway, nor can it do so in Sweden or Denmark pending the outcome of the main actions in the cases in these countries. If the final decisions in these cases are against AstraZeneca, Scand Pharm may claim damages for lost sales due to the interlocutory injunctions.
Back to Contents
| 104 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Financial Statements | |
Notes to the Financial Statements continued
34 Assets pledged, commitments and contingent liabilities (continued)
In March 2002, the Patents Court in the UK handed down a ruling invalidating certain of AstraZeneca’s formulation patents for omeprazole. AstraZeneca applied for leave to appeal the decision to the Court of Appeal and this application was granted. The appeal was heard by the Court of Appeal in October 2002 and the court affirmed the original decision of the Patents Court invalidating the formulation patents.
In the Netherlands, Pharmachemie BV has filed a claim against two AstraZeneca companies alleging that AstraZeneca has misused its exclusive rights in the Netherlands in relation to the expiration date for AstraZeneca’s supplementary protection certificate for omeprazole. AstraZeneca denies the allegations and is defending the case.
Other court cases relating to omeprazole patents are pending worldwide. However, the financial impact if AstraZeneca loses is not considered to be material.
In February 2000, the European Commission commenced an investigation relating to certain omeprazole intellectual property rights, and associated regulatory and patent infringement litigation. The investigation is pursuant to Article 82 of the EC Treaty, which prohibits an abuse of a dominant position. The investigation was precipitated by a complaint by a party to a number of patent and other proceedings involving AstraZeneca and relates to a limited number of European countries. AstraZeneca has, in accordance with its corporate policy, co-operated with the Commission. AstraZeneca remains of the view that the complaint is unfounded and that it has complied with all relevant competition laws. In particular, it considers that the matters raised by the complaint are more properly dealt with by the courts in the context of the litigation in which the complainant is involved. The Commission has recently requested certain factual patent and regulatory information from AstraZeneca and AstraZeneca will continue to co-operate with the Commission.
Zoladex (goserelin acetate implant) investigation
The US Department of Justice has been conducting a civil and criminal investigation into the sale and marketing of Zoladex (goserelin acetate implant). The investigation was prompted by the filing of a qui tam complaint by a private party in 1997 and involves allegations of improper submissions of claims to the Medicare and Medicaid programmes. The Company and federal and state authorities are in the process of negotiating a potential settlement of the civil and criminal claims at issue in the investigation. As a result, although no final agreement has been concluded, the Company believes it appropriate to accrue $350m to cover estimated settlement costs.
Plendil (felodipine)
In August 2000, AstraZeneca LP received a letter from Mutual Pharmaceutical Co., Inc. informing AstraZeneca of Mutual’s intention to market a generic version of AstraZeneca’s felodipine extended release tablets (Plendil) prior to the expiration of AstraZeneca’s patent covering the extended release formulation. AstraZeneca filed a patent infringement action against Mutual in the US District Court for the Eastern District of Pennsylvania. Mutual responded and filed counterclaims alleging non-infringement and invalidity. Expert discovery is due to close in March 2003. A trial date has not yet been set.
In May 2001, AstraZeneca Pharmaceuticals LP received a similar letter from Zenith Goldline Pharmaceuticals, Inc. and in July 2001, AstraZeneca filed a patent infringement action against Zenith in the US District Court for the District of New Jersey. Zenith responded and filed counterclaims alleging non-infringement. Fact discovery is due to close in May 2003. A trial date has not yet been set.
Nolvadex (tamoxifen)
AstraZeneca is a co-defendant with Barr Laboratories, Inc. in numerous purported class actions filed in federal and state courts throughout the US. All of the state court actions were removed to federal court and have been consolidated, along with all of the cases originally filed in federal court, in a federal multi-district litigation proceeding pending in the US District Court for the Eastern District of New York. Some of the cases were filed by plaintiffs representing a putative class of consumers who purchased tamoxifen. The other cases were filed on behalf of a putative class of ‘third party payers’ (including health maintenance organisations, insurers and other managed care providers and health plans) that have reimbursed or otherwise paid for prescriptions of tamoxifen. The plaintiffs allege that they paid ‘supra-competitive and monopolistic prices’ for tamoxifen as a result of the settlement of patent litigation between Zeneca and Barr in 1993. The plaintiffs seek injunctive relief, treble damages under the anti-trust laws, disgorgement and restitution. In April 2002, AstraZeneca filed a motion to dismiss the cases for failure to state a cause of action. The court’s decision is awaited.
In August 2002, AstraZeneca’s US distribution agreement with Barr Laboratories, Inc. for non-branded tamoxifen expired, as did AstraZeneca’s patent for Nolvadex (tamoxifen). At the same time, a six month period of market exclusivity, awarded by the US Food and Drug Administration in connection with the successful completion of certain paediatric testing with the product, commenced. Barr thereafter commenced litigation against the FDA in the US District Court for the District of Columbia, challenging the FDA’s refusal to grant Barr final approval for its own generic tamoxifen prior to expiration of AstraZeneca’s exclusivity period. Barr also declined AstraZeneca’s offer to extend the distribution agreement through the end of the exclusivity period. Therefore, in October 2002, AstraZeneca began shipping its own non-branded tamoxifen to customers to ensure an uninterrupted supply to patients. In December 2002, the Court held that Barr could not obtain final FDA approval for its own generic tamoxifen prior to the expiration of AstraZeneca’s paediatric exclusivity for Nolvadex. In January 2003, Barr made a claim that AstraZeneca improperly thwarted Barr’s entry into the tamoxifen market and caused Barr monetary damages. AstraZeneca disputes the claim.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Financial Statements | 105 |
Zestril (lisinopril)
In 1986, AstraZeneca’s predecessor company and Merck & Co., Inc. entered into licence agreements under which AstraZeneca was granted the right to make, use and sell lisinopril (Zestril), in return for which AstraZeneca agreed to pay royalties to Merck. In April 2002, AstraZeneca commenced arbitration proceedings against Merck under one of the licence agreements. In the arbitration, AstraZeneca is seeking repayment of approximately $38m of prior royalty amounts and a prospective reduction in the royalty rate going forward, based on a provision of the licence agreement which reduces the royalty rate if sales of lisinopril by third parties exceed a certain level. The case is currently progressing under the arbitration rules of the International Chamber of Commerce.
Retail pharmacies’/drug purchasers’ actions
Since October 1993, several thousand retail pharmacies and certain retail drug purchasers have commenced purported class actions and individual actions in various federal and state courts throughout the US alleging that, with respect to brand name prescription drugs, manufacturers and wholesalers engaged in discriminatory pricing practices, discriminatory discounting and rebate practices, and/or conspired with one another to fix prices and artificially maintain high prices to the plaintiffs in restraint of trade and commerce. More than 20 brand name prescription drug manufacturers and eight wholesalers have been named defendants in some or all of these suits.
AstraZeneca entered into a settlement agreement with the retail class plaintiffs whose anti-trust claims were consolidated in a federal multi-district litigation proceeding pending in the US District Court for the Northern District of Illinois. AstraZeneca also reached settlements with numerous independent and chain pharmacies that opted out of the federal class action, although there are still actions brought by certain chain and independent pharmacies pending in federal court. AstraZeneca has settled or been dismissed from all of the state cases except for a consumer case pending in state court in Alabama. AstraZeneca has consistently denied liability and continues to believe it has meritorious defences to all of these claims. However, it believes that entering into these settlements is the prudent course of action given the inherent risks and costs of litigation and to avoid further business disruption.
Average wholesale price class action litigation
In January 2002, AstraZeneca was named as a defendant along with 24 other pharmaceutical manufacturers in a class action suit, in Massachusetts, brought on behalf of a putative class of plaintiffs alleged to have overpaid for prescription drugs as a result of inflated wholesale list prices. The suit seeks to recover unspecified damages. AstraZeneca has also been named as a co-defendant with various other pharmaceutical manufacturers in similar class action suits filed in five other states. Most of these suits have been consolidated with the Massachusetts action for pre-trial purposes pursuant to federal multi-district litigation procedures. AstraZeneca believes that it has meritorious defences to all of these claims.
Additional government investigations into drug marketing practices
As is true for most, if not all, major prescription pharmaceutical companies operating in the US, AstraZeneca is currently involved in multiple additional US federal and state criminal and civil investigations into drug marketing and pricing practices. AstraZeneca has received subpoenas from the US Attorney’s Office in Boston requesting production of documents relating to the sale and promotion of Prilosec to the New England Medical Center in Boston. A separate subpoena from the same office requests documents relating to Prilosec purchasing and services agreements with AdvancePCS, the pharmacy benefits management company. AstraZeneca has also received a subpoena from the Massachusetts Attorney General’s Office seeking documents relating to the sale and promotion of five products (Prilosec, Seroquel, Rhinocort Aqua, Toprol-XL and Zestril) within Massachusetts. AstraZeneca has received an investigative demand from the Missouri Attorney General’s Office seeking documents and information relating to agreements with drug retailers doing business within Missouri. Most recently, AstraZeneca has received a Civil Investigative Demand from the US Federal Trade Commission for certain information concerning AstraZeneca’s advertising and marketing of Nexium. AstraZeneca is cooperating with these investigations. It is not possible to predict the outcome of any of these investigations, which could include the payment of damages and the imposition of fines, penalties and administrative remedies.
General
AstraZeneca is also involved in various other legal proceedings considered typical to its businesses, including some remaining US retail pharmacy anti-trust class and individual actions outside the scope of the settlements described above and litigation relating to employment, product liability, commercial disputes, infringement of intellectual property rights and the validity of certain patents. Although there can be no assurance regarding the outcome of any of the legal proceedings or investigations referred to in this Note 34 to the Financial Statements, AstraZeneca does not expect them to have a materially adverse effect on AstraZeneca’s financial position or profitability.
Back to Contents
| 106 | AstraZeneca Annual Report and Form 20-F 2002 www.astrazeneca.com | Financial Statements | |
Notes to the Financial Statements continued
35 Leases
Total rentals under operating leases charged to profit and loss account were as follows:
| | 2002
$m | | 2001
$m | | 2000
$m | |
|
|
|
|
|
| |
| Hire of plant and machinery | 23 | | 25 | | 15 | |
|
|
|
|
|
| |
| Other | 96 | | 76 | | 74 | |
|
|
|
|
|
| |
| | 119 | | 101 | | 89 | |
|
|
|
|
|
| |
Commitments under operating leases to pay rentals during the year following the year of these Financial Statements analysed according to the period in which each lease expires were as follows:
| | Land and buildings
| | Other assets
| |
| | 2002
$m | | 2001
$m | | 2002
$m | | 2001
$m | |
|
|
|
|
|
|
|
| |
| Expiring within one year | 5 | | 5 | | 11 | | 12 | |
|
|
|
|
|
|
|
| |
| Expiring in years two to five | 25 | | 37 | | 15 | | 13 | |
|
|
|
|
|
|
|
| |
| Expiring thereafter | 32 | | 25 | | 2 | | 2 | |
|
|
|
|
|
|
|
| |
| | 62 | | 67 | | 28 | | 27 | |
|
|
|
|
|
|
|
| |
The future minimum lease payments under operating leases that have initial or remaining terms in excess of one year at 31 December 2002 were as follows:
| | Operating leases
| |
| | 2002
$m | | 2001
$m | |
|
|
|
| |
Obligations under leases comprise | | | | |
| Rentals due within one year | 90 | | 94 | |
|
|
|
| |
| Rentals due after more than one year | | | | |
| After five years from balance sheet date | 94 | | 97 | |
|
|
|
| |
| From four to five years | 21 | | 20 | |
|
|
|
| |
| From three to four years | 27 | | 21 | |
|
|
|
| |
| From two to three years | 38 | | 25 | |
|
|
|
| |
| From one to two years | 47 | | 35 | |
|
|
|
| |
| | 227 | | 198 | |
|
|
|
| |
| | 317 | | 292 | |
|
|
|
| |
The Group had no commitments (2001 $nil) under finance leases at the balance sheet date which were due to commence thereafter.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002 www.astrazeneca.com | Financial Statements | 107 |
36 Statutory and other information
| | | | 2002
$m | | 2001
$m | | 2000
$m | |
|
|
|
|
|
|
|
| |
| Statutory audit fees | | | | | | | | |
| KPMG Audit Plc | | | 3.5 | | 2.5 | | 3.2 | |
|
|
|
|
|
|
|
| |
| Others | | | 0.1 | | 0.1 | | – | |
|
|
|
|
|
|
|
| |
| | | | 3.6 | | 2.6 | | 3.2 | |
|
|
|
|
|
|
|
| |
| | | | | | | | | |
| Fees for other services | | | | | | | | |
| KPMG Audit Plc and associates | – UK | | 0.4 | | 3.2 | | 8.9 | |
|
|
|
|
|
|
|
| |
| | – Worldwide | | 3.1 | | 2.0 | | 5.0 | |
|
|
|
|
|
|
|
| |
| | | | 3.5 | | 5.2 | | 13.9 | |
|
|
|
|
|
|
|
| |
Non statutory audit fees paid to KPMG Audit Plc and its associates were in relation to other assurance services $1.5m (2001 $1.8m); taxation $1.8m (2001 $2.1m); and other non audit services $0.2m (2001 $1.3m).
In addition to the above, in 2000 KPMG Audit Plc and its associates charged fees for other services of $8.0m that were borne by Syngenta AG in relation to its demerger from AstraZeneca.
The charge for the statutory audit of the Company, AstraZeneca PLC, was $1,600 (2001 $1,600, 2000 $1,600). KPMG Audit Plc were sole auditors to AstraZeneca in 2002 and 2001.
The bulk of fees for other services charged by KPMG Audit Plc and its associates (aside from the Zeneca Agrochemicals demerger and associated restructuring work) were incurred in the early months of 2000, completing 1999 integration projects.
Related party transactions
The Group had no material related party transactions which might reasonably be expected to influence decisions made by the users of these Financial Statements.
Subsequent events
No significant change has occurred since the date of the annual Financial Statements.
Back to Contents
| 108 | AstraZeneca Annual Report and Form 20-F 2002 www.astrazeneca.com | Financial Statements | |
Notes to the Financial Statements continued
37 Company information
Company Balance Sheet
| At 31 December | Notes | | 2002
$m | | 2001
$m | |
|
|
|
|
|
| |
| Fixed assets | | | | | | |
| Fixed asset investments | 37 | | 7,236 | | 6,736 | |
|
|
|
|
|
| |
| | | | 7,236 | | 6,736 | |
|
|
|
|
|
| |
| | | | | | | |
| Current assets | | | | | | |
| Debtors – amounts owed by subsidiaries | | | 27,104 | | 27,998 | |
|
|
|
|
|
| |
| Total assets | | | 34,340 | | 34,734 | |
|
|
|
|
|
| |
| | | | | | | |
| Creditors due within one year | | | | | | |
| Non-trade creditors | 37 | | (2,961 | ) | (835 | ) |
|
|
|
|
|
| |
| | | | (2,961 | ) | (835 | ) |
|
|
|
|
|
| |
| Net current assets | | | 24,143 | | 27,163 | |
|
|
|
|
|
| |
| Total assets less current liabilities | | | 31,379 | | 33,899 | |
|
|
|
|
|
| |
| | | | | | | |
| Creditors due after more than one year | | | | | | |
| Loans – owed to subsidiaries | 37 | | (295 | ) | (590 | ) |
|
|
|
|
|
| |
| Net assets | | | 31,084 | | 33,309 | |
|
|
|
|
|
| |
| | | | | | | |
| Capital and reserves | | | | | | |
| Called-up share capital | 38 | | 429 | | 436 | |
|
|
|
|
|
| |
| Share premium account | 37 | | 403 | | 334 | |
|
|
|
|
|
| |
| Capital redemption reserve | 37 | | 16 | | 9 | |
|
|
|
|
|
| |
| Other reserves | 37 | | 1,841 | | 2,239 | |
|
|
|
|
|
| |
| Profit and loss account | 37 | | 28,395 | | 30,291 | |
|
|
|
|
|
| |
| Shareholders’ funds – equity interests | | | 31,084 | | 33,309 | |
|
|
|
|
|
| |
The financial statements on pages 58 to 122 were approved by the Board of Directors on 30 January 2003 and were signed on its behalf by:
| Sir Tom McKillop | Jonathan Symonds |
| Director | Director |
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Financial Statements | 109 |
37 Company information (continued)
Deferred taxationThe parent company had no deferred tax assets or liabilities (actual or potential) at 31 December 2002.
| | Investments in subsidiaries | |
| |
| |
| Fixed asset investments | Shares
$m | | Loans
$m | | Total
$m | |
|
|
|
|
|
| |
| Cost at beginning of year | 6,145 | | 591 | | 6,736 | |
|
|
|
|
|
| |
| Additions | 500 | | – | | 500 | |
|
|
|
|
|
| |
| Net book value at 31 December 2002 | 6,645 | | 591 | | 7,236 | |
|
|
|
|
|
| |
| Net book value at 31 December 2001 | 6,145 | | 591 | | 6,736 | |
|
|
|
|
|
| |
| | | | | | | |
| Non-trade creditors | | | 2002
$m | | 2001
$m | |
|
|
|
|
|
| |
| Amounts due within one year | | | | | | |
| Short term borrowings (unsecured) | | | 3 | | 3 | |
|
|
|
|
|
| |
| Other creditors | | | 50 | | 4 | |
|
|
|
|
|
| |
| Amounts owed to subsidiaries | | | 2,100 | | 8 | |
|
|
|
|
|
| |
| Dividends to Shareholders | | | 808 | | 820 | |
|
|
|
|
|
| |
| | | | 2,961 | | 835 | |
|
|
|
|
|
| |
| | | | | | | |
| Loans – owed to subsidiaries | Repayment
Dates | | 2002
$m | | 2001
$m | |
|
|
|
|
|
| |
| Loans (unsecured) | | | | | | |
| US dollars | | | | | | |
| 6.58% loan | 2003 | | 295 | | 295 | |
|
|
|
|
|
| |
| 7.2% loan | 2023 | | 295 | | 295 | |
|
|
|
|
|
| |
| Total loans | | | 590 | | 590 | |
|
|
|
|
|
| |
| | | | | | | |
| Loans or instalments thereof are repayable | | | | | | |
| After five years from balance sheet date | | | 295 | | 295 | |
|
|
|
|
|
| |
| From two to five years | | | – | | – | |
|
|
|
|
|
| |
| From one to two years | | | – | | 295 | |
|
|
|
|
|
| |
| Total unsecured | | | 295 | | 590 | |
|
|
|
|
|
| |
| Total due within one year | | | 295 | | – | |
|
|
|
|
|
| |
| Total loans | | | 590 | | 590 | |
|
|
|
|
|
| |
Back to Contents
| 110 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Financial Statements | |
Notes to the Financial Statements continued
37 Company information (continued)Reserves
| Share
premium
account
$m | | Capital
redemption
reserve
$m | | Other
reserves
$m | | Profit
and loss
account
$m | | 2002
Total
$m | | 2001
Total
$m | |
|
|
|
|
|
|
|
|
|
|
|
| |
| At beginning of year | 334 | | 9 | | 2,239 | | 30,291 | | 32,873 | | 32,759 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Net profit for the year | – | | – | | – | | 102 | | 102 | | 2,314 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Dividends | – | | – | | (398 | ) | (808 | ) | (1,206 | ) | (1,225 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Share re-purchase | – | | 7 | | – | | (1,190 | ) | (1,183 | ) | (1,074 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Share premiums | 69 | | – | | – | | – | | 69 | | 99 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| At end of year | 403 | | 16 | | 1,841 | | 28,395 | | 30,655 | | 32,873 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Distributable reserves at end of year | – | | – | | 443 | | 1,614 | | 2,057 | | 1,623 | |
|
|
|
|
|
|
|
|
|
|
|
| |
As permitted by section 230 of the Companies Act 1985, the Company has not presented its profit and loss account.
At 31 December 2002 $26,781m (31 December 2001 $29,440m) of the profit and loss account reserve was not available for distribution. The majority of this non-distributable amount relates to profit arising on the sale of Astra AB to a subsidiary in 1999, which becomes distributable as the underlying receivable is settled in cash. During 2002, $2,659m of the profit was realised by repayment. Subsequent to the year end a further $825m was repaid on 23 January 2003 resulting in additional distributable reserves not included in the figures above. Included in other reserves is a special reserve of $157m, arising on the redenomination of share capital in 1999.
| Reconciliation of movement in shareholders’ funds | 2002
$m | | 2001
$m | |
|
|
|
| |
| Shareholders’ funds at beginning of year | 33,309 | | 33,201 | |
|
|
|
| |
| Net profit for the financial year | 102 | | 2,314 | |
|
|
|
| |
| Dividends | (1,206 | ) | (1,225 | ) |
|
|
|
| |
| Issues of AstraZeneca PLC Ordinary Shares | 69 | | 99 | |
|
|
|
| |
| Re-purchase of AstraZeneca PLC Ordinary Shares | (1,190 | ) | (1,080 | ) |
|
|
|
| |
| Net (reduction in)/addition to shareholders’ funds | (2,225 | ) | 108 | |
|
|
|
| |
| Shareholders’ funds at end of year | 31,084 | | 33,309 | |
|
|
|
| |
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Financial Statements | 111 |
38 Called-up share capital of parent company
| | Authorised | | Allotted, called-up
and fully paid | |
| |
| |
| |
| | 2002
$m | | 2002
$m | | 2001
$m | |
|
|
|
|
|
| |
| Ordinary Shares ($0.25 each) | 429 | | 429 | | 436 | |
|
|
|
|
|
| |
| Unissued Ordinary Shares ($0.25 each) | 171 | | – | | – | |
|
|
|
|
|
| |
| Redeemable Preference Shares (£50,000) | – | | – | | – | |
|
|
|
|
|
| |
| | 600 | | 429 | | 436 | |
|
|
|
|
|
| |
The Redeemable Preference Shares carry limited class voting rights and no dividend rights. This class of shares is capable of redemption at par at the option of the Company on the giving of seven days’ written notice to the registered holder of the shares.
The movements in share capital during the year can be summarised as follows:
| | No. of shares
(million) | | $m | |
|
|
|
| |
| At beginning of year | 1,745 | | 436 | |
|
|
|
| |
| Issues of shares | 2 | | – | |
|
|
|
| |
| Re-purchase of shares | (28 | ) | (7 | ) |
|
|
|
| |
| At 31 December 2002 | 1,719 | | 429 | |
|
|
|
| |
Share buy-back
During the year the Company purchased, and subsequently cancelled, 28,386,560 Ordinary Shares at an average price of 2785 pence per share for a consideration, including expenses, of $1,190m. The excess of the consideration over the nominal value has been charged against the profit and loss account reserve.
Share schemes
A total of 1,737,401 shares were issued during the year in respect of share schemes. Details of movements in the number of shares under option are shown in Note 33; details of options granted to Directors are shown in the Directors’ Remuneration Report.
Back to Contents
| 112 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Financial Statements | |
Principal Subsidiaries, Joint Ventures and Associates
| | | Percentage of voting | | |
| At 31 December 2002 | Country | share capital held | | Principal activity |
|
| UK | | | | |
| AstraZeneca UK Limited | England | 100 | # | Research, production, marketing |
|
| AstraZeneca Insurance Company Limited | England | 100 | | Insurance and reinsurance underwriting |
|
| AstraZeneca Treasury Limited | England | 100 | | Treasury |
|
| | | | | |
| Continental Europe | | | | |
| NV AstraZeneca SA | Belgium | 100 | | Marketing |
|
| ASP SA | France | 100 | | Production |
|
| AstraZeneca Pharma SA | France | 100 | | Research, production, marketing |
|
| AstraZeneca GmbH | Germany | 100 | | Development, production, marketing |
|
| AstraZeneca Holding GmbH | Germany | 100 | | Production, marketing |
|
| AstraZeneca SpA | Italy | 100 | | Production, marketing |
|
| AstraZeneca Farmaceutica Spain SA | Spain | 100 | | Production, marketing |
|
| AstraZeneca AB | Sweden | 100 | | Research and development, |
| | | | | production, marketing |
|
| Astra Tech AB | Sweden | 100 | | Research and development, |
| | | | | production, marketing |
|
| AstraZeneca BV | The Netherlands | 100 | | Marketing |
|
| | | | | |
| The Americas | | | | |
| AstraZeneca do Brasil Ltda. | Brazil | 100 | | Production, marketing |
|
| AstraZeneca Canada Inc. | Canada | 100 | | Research, production, marketing |
|
| IPR Pharmaceuticals Inc. | Puerto Rico | 100 | | Development, production, marketing |
|
| AstraZeneca LP | US | 99 | | Development, production, marketing |
|
| AstraZeneca Pharmaceuticals LP | US | 100 | | Development, production, marketing |
|
| Salick Health Care, Inc. | US | 100 | | Provision of disease-specific |
| | | | | healthcare services |
|
| Zeneca Holdings Inc. | US | 100 | | Production, marketing |
|
| | | | | |
| Asia, Africa & Australasia | | | | |
| AstraZeneca Pty Limited | Australia | 100 | | Research, production, marketing |
|
| AstraZeneca Pharmaceutical Co., Limited | China | 100 | | Production, marketing |
|
| AstraZeneca Hong Kong Limited | Hong Kong | 100 | | Production |
|
| AstraZeneca KK | Japan | 80 | | Production, marketing |
|
| # shares held directly |
The companies and other entities listed above are those whose results or financial position principally affected the figures shown in the Group’s annual financial statements. A full list of subsidiaries, joint ventures and associates will be annexed to the Company’s next annual return filed with the Registrar of Companies. The country of registration or incorporation is stated alongside each company. The accounting dates of principal subsidiaries and associates are 31 December, except for Salick Health Care, Inc. which is 30 November. AstraZeneca operates through 235 subsidiary companies worldwide. Products are manufactured in some 20 countries worldwide and are sold in over 100 countries.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | 113 |
Additional Information for US Investors
Differences between UK and US accounting principles
The accompanying consolidated financial statements included in this Annual Report are prepared in accordance with UK GAAP. Certain significant differences between UK GAAP and US GAAP which affect AstraZeneca’s net income and shareholders’ equity are set out below.
Purchase accounting adjustments
Under UK GAAP the merger of Astra and Zeneca was accounted for as a ‘merger of equals’ (pooling-of-interests). Under US GAAP the merger was accounted for as the acquisition of Astra by Zeneca using ‘purchase accounting’. Under purchase accounting, the cost of the investment is calculated at the market value of the shares issued together with other incidental costs and the assets and liabilities of the acquired entity are recorded at fair value. As a result of the fair value exercise, increases in the values of Astra’s tangible fixed assets and inventory were recognised and values attributed to their in-process research and development, existing products and assembled work force, together with appropriate deferred taxation effects. The difference between the cost of investment and the fair value of the assets and liabilities of Astra was recorded as goodwill. The amount allocated to in-process research and development was, as required by US GAAP, expensed immediately in the first reporting period after the business combination. Fair value adjustments to the recorded amount of inventory were expensed in the period the inventory was utilised. Additional amortisation and depreciation have also been recorded in respect of the fair value adjustments to tangible and intangible assets and the resulting goodwill.
In the consolidated financial statements prepared under UK GAAP, goodwill arising on acquisitions made prior to 1 January 1998 accounted for under the purchase method has been eliminated against shareholders’equity. Under the requirements of UK Financial Reporting Standard 10 ‘Goodwill and Intangible Assets’, goodwill on acquisitions made after 1 January 1998 is capitalised and amortised over its estimated useful life which is generally presumed not to exceed 20 years. UK GAAP requires that on subsequent disposal or termination of a previously acquired business, any goodwill previously taken directly to shareholders’equity is then charged in the income statement against the profit or loss on disposal or termination. Up until 1 January
2002, under US GAAP, goodwill was required to be capitalised and amortised. Now, instead of being amortised, goodwill is tested annually for impairment. Amortisation charged under UK GAAP is added back in the reconciliation of net income. The intangible recognised as assembled workforce has been reclassified as goodwill.
Identifiable intangible assets, which principally include patents, ‘know-how’ and product registrations, are amortised over their estimated useful lives which vary between 5 years and 20 years with a weighted average life of approximately 13 years.
At 31 December 2002 and 2001, shareholders’ equity includes capitalised goodwill of $13,600m and $12,169m respectively (net of amortisation and impairment of $2,383m and $2,180m) and capitalised identifiable intangible assets of $9,433m and $9,789m respectively (net of amortisation and impairment of $4,566m and $3,475m). Goodwill on businesses disposed of is charged to the gain or loss on disposal.
On disposal of a business, the gain or loss under US GAAP may differ from that under UK GAAP due principally to goodwill capitalised and amortised, together with the appropriate share of other differences between UK and US accounting principles recognised previously.
Capitalisation of interest
AstraZeneca does not capitalise interest in its financial statements. US GAAP requires interest incurred as part of the cost of constructing fixed assets to be capitalised and amortised over the life of the asset.
Dividends
Under UK GAAP Ordinary Share dividends proposed are provided for in the year in respect of which they are recommended by the Board of Directors for approval by the shareholders. Under US GAAP such dividends are not provided for until declared by the Board.
Deferred taxation
Deferred taxation is provided on a full liability basis under US GAAP, which permits deferred tax assets to be recognised if their realisation is considered to be more likely than not. Under current UK GAAP, full provision is also made although there are a number of different bases on which this calculation is made, eg rolled over capital gains.
Pension and post-retirement benefits
There are four main differences between current UK GAAP and US GAAP in accounting for pension costs:
| (i) | US GAAP requires measurements of plan assets and obligations to be made as at the date of the financial statements or a date not more than three months prior to that date. Under UK GAAP, calculations may be based on the results of the latest actuarial valuation; |
| | |
| (ii) | US GAAP mandates a particular actuarial method – the projected unit credit method – and requires that each significant assumption necessary to determine annual pension cost reflects best estimates solely with regard to that individual assumption. UK GAAP does not mandate a particular method, but requires that the method and assumptions taken as a whole should be compatible and lead to the actuary’s best estimate of the cost of providing the benefits promised; |
| | |
| (iii) | under US GAAP, a negative pension cost may arise where a significant unrecognised net asset or gain exists at the time of implementation. This is required to be amortised on a straight-line basis over the average remaining service period of employees. Under UK GAAP, AstraZeneca’s policy is not to recognise pension credits in its financial statements unless a refund of, or reduction in, contributions is likely; and |
| | |
| (iv) | under US GAAP, a minimum pension liability is recognised through other comprehensive income in certain circumstances when there is a deficit of plan assets relative to the projected benefits obligation. Under UK GAAP, there is no such requirement. |
Back to Contents
| 114 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | |
Additional Information for US Investors continued
Differences between UK and US accounting principles (continued)
Restructuring costs
Under UK GAAP, provisions are made for restructuring costs once a detailed formal plan is in place and valid expectations have been raised in those affected that the restructuring will be carried out. US GAAP requires a number of specific criteria to be met before such costs can be recognised as an expense. Among these are the requirements that the costs incurred are incremental to other costs incurred by the company, or represent amounts to be incurred under contractual obligations which are not associated with or do not benefit activities that will be continued. Also, all significant actions arising from a restructuring and their completion dates must be identified by the balance sheet date. To the extent that restructuring costs are related to the activities of the acquired company, US GAAP allows them to be recognised as a liability upon acquisition.
Software costs
Under UK GAAP, AstraZeneca capitalises certain defined software costs. Under US GAAP software costs are generally capitalised and amortised over three to five years.
Foreign exchange
Under UK GAAP, unrealised gains and losses on foreign currency transactions to hedge anticipated, but not firmly committed, foreign currency transactions may be deferred and accounted for at the same time as the anticipated transactions. Under US GAAP such deferral is not permitted except in certain defined circumstances.
Derivative instruments and hedging activities
Under US GAAP, all derivative instruments should be recognised as assets or liabilities in the balance sheet at fair value. Gains and losses are recognised in net income unless they are regarded as hedges. Under UK GAAP, these instruments are measured at cost and gains or losses deferred until the underlying transactions occur.
Deferred income
Under UK GAAP, profits or losses from the sale of product related intangible assets are classified in other operating income and are stated after taking account of product disposal costs and costs of minor outstanding obligations. Under US GAAP, such profits are deferred and recognised in
the income statement in subsequent periods until all disposal obligations and commitments have been completed.
Current assets and liabilities
In the Group’s financial statements prepared under UK GAAP, no cost is accrued for the share options awarded to employees under the Zeneca 1994 Executive Share Option Scheme, the AstraZeneca Share Option Plan, and the AstraZeneca Savings-Related Share Option Scheme as the exercise price is equivalent to the market value at the date of grant. Under US GAAP the cost is calculated as the difference between the option price and the market price at the date of grant or, for variable plans, at the end of the reporting period (until measurement date). Under the requirements of APB Opinion No. 25 any compensation cost would be amortised over the period from the date the options are granted to the date they are first exercisable. Under US GAAP in the net income reconciliation, the Group has adjusted for stock compensation costs and calculated under APB Opinion No. 25.
Statement of cash flows: Basis of preparation
AstraZeneca’s Statement of Group Cash Flow is prepared in accordance with United Kingdom Financial Reporting Standard 1 (Revised 1996) (‘FRS 1’), whose objective and principles are similar to those set out in SFAS No. 95, ‘Statement of Cash Flows’. The principal differences between the standards relate to classification. Under FRS 1, the Company presents its cash flows for (a) operating activities; (b) dividends received from joint ventures and associates; (c) returns on investments and servicing of finance; (d) tax paid; (e) capital expenditure and financial investment; (f) acquisitions and disposals; (g) dividends paid to shareholders; (h) management of liquid resources; and (i) financing. SFAS No. 95 requires only three categories of cash flow activity being (a) operating; (b) investing; and (c) financing.
Cash flows from taxation, returns on investments and servicing of finance and dividends received from joint ventures and associates under FRS 1 would be included as operating activities under SFAS No. 95; capital expenditure and financial investment and acquisitions and disposals would be included as investing activities; and distributions would be included as a financing activity under SFAS No. 95. Under FRS 1 cash comprises cash in hand and deposits repayable on demand, less overdrafts repayable on demand; and liquid resources
comprise current asset investments held as readily disposable stores of value. Under SFAS No. 95 cash equivalents, comprising short term highly liquid investments, generally with original maturities of three months or less, are grouped together with cash; short term borrowings repayable on demand would not be included within cash and cash equivalents and movements on those borrowings would be included in financing activities.
New accounting standards adopted
Statement of Financial Accounting Standards SFAS No. 141 ‘Business Combinations’ and SFAS No. 142 ‘Goodwill and Other Intangible Assets’ were issued in July 2001 and are effective for accounting periods commencing on or after 15 December 2001. Under SFAS No. 141, all business combinations initiated after 30 June 2001 must be accounted for using the purchase method. The pooling of interest method is no longer permitted. Intangible assets arising on acquisitions are required to be amortised to residual values over their estimated useful lives unless they are regarded as having indefinite useful lives, in which case they are tested annually for impairment. Goodwill, arising on a combination of business, is tested for impairment annually in lieu of amortisation. SFAS No. 142 requires that goodwill and intangible assets acquired prior to 1 July 2001 should continue to be amortised and tested for impairment until the adoption of the standard. Upon adoption of SFAS No. 142 an impairment test must be carried out on all intangible assets with indefinite useful lives and goodwill. Any impairment loss identified on the date of adoption of SFAS No. 142 should be accounted for as a cumulative effect of a change in accounting principle. At the same time, the estimated useful lives of amortised intangible assets must be reviewed.
Adoption of these new accounting standards has resulted in an estimated increase in net income of $755m (including amortisation charged under UK GAAP of $55m). Initial adoption of SFAS No. 142 did not result in an impairment charge, nor was there any impairment at the subsequent annual test. Had goodwill not been amortised in 2001, net income would have increased from $1,397m to $2,125m (2000 $865m to $1,716m) with a corresponding increase in basic and diluted earnings per share from $0.77 to $1.21 (2000 $0.49 to $0.97). No changes were made to estimated useful lives of intangible assets.
SFAS No. 144 ‘Accounting for the Impairment or Disposal of Long-Lived Assets’
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | 115 |
addresses financial accounting and reporting for the impairment or disposal of long-lived assets and supersedes SFAS No. 121, ‘Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed of’ and the accounting and reporting provisions of APB Opinion No. 30, ‘Reporting the Results of Operations – Reporting the Effects of Disposal of a Segment of a Business, and Extraordinary, Unusual and Infrequently Occurring Events and Transactions’, for the disposal of a segment of a business. It is effective for accounting periods beginning on or after 15 December 2001. The adoption of SFAS No. 144 did not have a material effect.
New accounting standards not yet adopted
SFAS No. 143 ‘Accounting for Asset Retirement Obligation’ addresses the accounting and reporting for obligations associated with the retirement of long-lived assets and the associated asset retirement costs. It is effective for accounting periods beginning on or after 15 June 2002. The adoption of SFAS No. 143 is not expected to have a material effect.
SFAS No.146 ‘Accounting for Costs Associated with Exit or Disposal Activities’, issued on 30 July 2002 requires costs associated with exit or disposal activities to be recognised when the costs are incurred rather than at the date of commitment to an exit or disposal plan. The provisions are effective for disposals initialised after 31 December 2002 and restatement of prior periods is not required. As SFAS No. 146 may apply to future activities which are not currently envisaged it is not possible to assess the impact of SFAS No. 146.
SFAS No. 148 ‘Accounting for Stock Based Compensation – Transition and Disclosure – an amendment of FASB Statement No. 123’ permits two additional transition methods for entities that adopt the fair value based method of accounting for stock-based employee compensation. The Statement also requires new disclosures about the ramp-up effect of stock-based employee compensation on reported results and that those effects be disclosed more prominently by specifying the form, content and location of those disclosures. The transition guidance and annual disclosure provisions of SFAS No. 148 are effective for fiscal years ending after 15 December 2002. AstraZeneca has not yet determined whether it will adopt the transition provisions of SFAS No. 148.
Back to Contents
| 116 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | |
Additional Information for US Investors continued
Differences between UK and US accounting principles (continued)Introduction
As a result of the significant difference between the UK GAAP and US GAAP treatment of the combination of Astra and Zeneca in the year of acquisition, and in the results of preceding periods, condensed statements of operations and cash flow under US GAAP have been prepared for the benefit of US investors.
The following is a summary of the material adjustments to net income and shareholders’ equity which would have been required if US GAAP had been applied instead of UK GAAP. As noted on page 62, 2001 and 2000 net income and shareholders’ equity under UK GAAP have been restated under FRS19 – Deferred Tax. On this basis the deferred tax adjustment below as been restated for those years.
| Net income | 2002
$m | | 2001
$m | | 2000
$m | |
|
|
|
|
|
| |
| Net income, as shown in the consolidated statements | | | | | | |
| of income before exceptional items (restated) | 3,186 | | 3,044 | | 2,858 | |
|
|
|
|
|
| |
| Exceptional items after tax | (350 | ) | (138 | ) | (581 | ) |
|
|
|
|
|
| |
| Net income for the period under UK GAAP (restated) | 2,836 | | 2,906 | | 2,277 | |
|
|
|
|
|
| |
| | | | | | | |
| Adjustments to conform to US GAAP | | | | | | |
Purchase accounting adjustments (including goodwill and intangibles)
Deemed acquisition of Astra
Amortisation and other acquisition adjustments | (864 | ) | (1,514 | ) | (1,756 | ) |
|
|
|
|
|
| |
| Others | 55 | | – | | (20 | ) |
|
|
|
|
|
| |
| Capitalisation, less disposals and amortisation of interest | 46 | | 57 | | 45 | |
|
|
|
|
|
| |
| Deferred taxation | | | | | | |
| On fair values of Astra | 239 | | 249 | | 284 | |
|
|
|
|
|
| |
| Others (restated) | (99 | ) | (198 | ) | 115 | |
|
|
|
|
|
| |
| Pension expense | (50 | ) | (33 | ) | (50 | ) |
|
|
|
|
|
| |
| Post-retirement benefits/plan amendment | 4 | | 4 | | 4 | |
|
|
|
|
|
| |
| Software costs | (46 | ) | (10 | ) | 98 | |
|
|
|
|
|
| |
| Restructuring costs | – | | (22 | ) | (97 | ) |
|
|
|
|
|
| |
| Share based compensation | 33 | | (7 | ) | (33 | ) |
|
|
|
|
|
| |
| Fair value of derivative financial instruments | 93 | | 18 | | – | |
|
|
|
|
|
| |
| Deferred income recognition | 61 | | (75 | ) | – | |
|
|
|
|
|
| |
| Unrealised losses on foreign exchange and others | (1 | ) | (10 | ) | (2 | ) |
|
|
|
|
|
| |
| Net income before cumulative effect of change in accounting policy | 2,307 | | 1,365 | | 865 | |
|
|
|
|
|
| |
| Cumulative effect of change in accounting policy, net of tax, on adoption of SFAS No 133 | – | | 32 | | – | |
|
|
|
|
|
| |
| Net income in accordance with US GAAP | 2,307 | | 1,397 | | 865 | |
|
|
|
|
|
| |
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | 117 |
Differences between UK and US accounting principles (continued)US GAAP Condensed Consolidated Statement of Operations
| For the years ended 31 December | | 2002
$m | | 2001
$m | | 2000
$m | |
|
|
|
|
|
|
| |
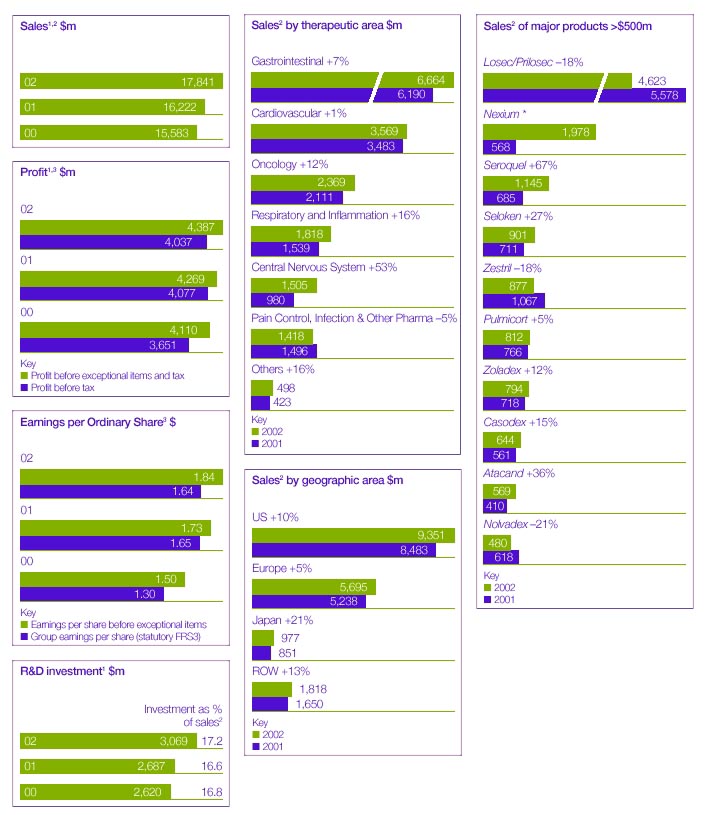
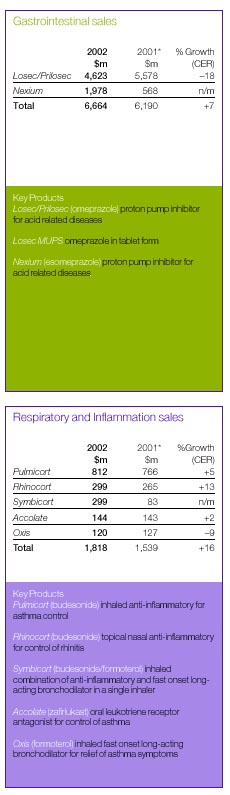
| Sales | | 17,841 | | 16,222 | | 15,583 | |
|
|
|
|
|
|
| |
| Cost of sales | | (4,520 | ) | (4,198 | ) | (3,960 | ) |
|
|
|
|
|
|
| |
| Distribution costs | | (141 | ) | (122 | ) | (210 | ) |
|
|
|
|
|
|
| |
| Research and development | | (3,069 | ) | (2,687 | ) | (2,620 | ) |
|
|
|
|
|
|
| |
| Selling, general and administrative expenses | | (6,165 | ) | (5,219 | ) | (4,861 | ) |
|
|
|
|
|
|
| |
| Acquisition related costs | | – | | (224 | ) | (419 | ) |
|
|
|
|
|
|
| |
| Amortisation of intangibles and goodwill | | (1,052 | ) | (1,769 | ) | (2,043 | ) |
|
|
|
|
|
|
| |
| Other income | | 308 | | 283 | | 223 | |
|
|
|
|
|
|
| |
| Operating income | | 3,202 | | 2,286 | | 1,693 | |
|
|
|
|
|
|
| |
| Net interest income | | 140 | | 188 | | 183 | |
|
|
|
|
|
|
| |
| Income from continuing operations before taxation | | 3,342 | | 2,474 | | 1,876 | |
|
|
|
|
|
|
| |
| Taxes on income from continuing operations | | (1,035 | ) | (1,109 | ) | (969 | ) |
|
|
|
|
|
|
| |
| Net income from continuing operations | | 2,307 | | 1,365 | | 907 | |
|
|
|
|
|
|
| |
Discontinued operations:
Net income from discontinued operations | | – | | – | | (42 | ) |
|
|
|
|
|
|
| |
| Net income before cumulative effect of change in accounting policy | | 2,307 | | 1,365 | | 865 | |
|
|
|
|
|
|
| |
| Cumulative effect of change in accounting policy on adoption of SFAS No 133 | | – | | 32 | | – | |
|
|
|
|
|
|
| |
| Net income for the year | | 2,307 | | 1,397 | | 865 | |
|
|
|
|
|
|
| |
| | | | | | | | |
Weighted average number of $0.25 Ordinary Shares
in issue (millions of shares) | | 1,733 | | 1,758 | | 1,768 | |
|
|
|
|
|
|
| |
| Dilutive impact of share options outstanding (millions of shares) | | 2 | | 3 | | 2 | |
|
|
|
|
|
|
| |
Diluted weighted average number of $0.25 Ordinary Shares
in accordance with US GAAP (millions of shares) | | 1,735 | | 1,761 | | 1,770 | |
|
|
|
|
|
|
| |
Net income per $0.25 Ordinary Share and ADS before change
in accounting policy in accordance with US GAAP – basic and diluted ($) | | $1.33 | | $0.77 | | $0.49 | |
|
|
|
|
|
|
| |
Net income per $0.25 Ordinary Share and ADS after change in
accounting policy in accordance with US GAAP – basic and diluted ($) | | $1.33 | | $0.79 | | $0.49 | |
|
|
|
|
|
|
| |
| | | | | | | | |
| | | | | | | | |
| | | 2002 | | 2001 | | 2000 | |
|
|
|
|
|
|
| |
Net income from continuing operations per $0.25 Ordinary Share and ADS
in accordance with US GAAP – basic and diluted ($) | | $1.33 | | $0.79 | | $0.51 | |
|
|
|
|
|
|
| |
Net loss from discontinued operations per $0.25 Ordinary Share and ADS
in accordance with US GAAP – basic and diluted ($) | | – | | – | | ($0.02 | ) |
|
|
|
|
|
|
| |
The dividend in specie in 2000 in respect of the demerger of Zeneca Agrochemicals under US GAAP amounted to $836m, after realised exchange gains on the translation of foreign currency financial statements of $297m.
As noted on page 62, cash settlement discounts have been reclassified from cost of sales to sales. Comparative information for 2001 and 2000 has also been reclassified for consistency of presentation.
Back to Contents
| 118 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information or US Investors | |
Additional Information for US Investors continued
Differences between UK and US accounting principles (continued)| | | | | | | |
| US GAAP Statement of Comprehensive Income | | | | | | |
| For the years ended 31 December | 2002
$m | | 2001
$m | | 2000
$m | |
| |
| Net income for the year | 2,307 | | 1,397 | | 865 | |
| |
| Exchange gains/(losses) net of tax | 2,919 | | (1,473 | ) | (2,184 | ) |
| |
| Exchange realised on demerger of Zeneca Agrochemicals | – | | – | | (297 | ) |
| |
| Other movements | (73 | ) | – | | (2 | ) |
| |
| Total Comprehensive Income | 5,153 | | (76 | ) | (1,618 | ) |
| |
Other movements in 2002 include the recognition of a minimum liability under SFAS 87 of $45m.
The cumulative exchange gains and losses (net of tax) on the translation of foreign currency financial statements under US GAAP are set out in the following note:
| For the years ended 31 December | 2002
$m | | 2001
$m | | 2000
$m | |
| |
| Balance at 1 January | (4,318 | ) | (2,845 | ) | (364 | ) |
| |
| Movement in year | 2,919 | | (1,473 | ) | (2,481 | ) |
| |
| Balance at 31 December | (1,399 | ) | (4,318 | ) | (2,845 | ) |
| |
Stock compensation
In the Group’s financial statements prepared under UK GAAP, no cost is accrued for the share options awarded to employees under the Zeneca 1994 Executive Share Option Scheme, the AstraZeneca Share Option Plan, and the AstraZeneca Savings-Related Share Option Scheme as the exercise price is equivalent to the market value at the date of grant. Under US GAAP the cost is calculated as the difference between the option price and the market price at the date of grant or, for variable plans, at the end of the reporting period (until measurement date). Under the requirements of APB Opinion No. 25 any compensation cost would be amortised over the period from the date the options are granted to the date they are first exercisable. Under US GAAP in the net income reconciliation, the Group has adjusted for stock compensation costs as calculated under APB Opinion No 25, SFAS No.123 sets out an alternative methodology for recognising the compensation cost based on the fair value at grant date. Had the Group adopted this methodology, the incremental effect on net income under US GAAP is shown below:
| | 2002 | | 2001 | | 2000 | |
| $m | $m | $m |
| |
| Net income under US GAAP as reported | 2,307 | | 1,397 | | 865 | |
| |
| Compensation cost (after adjusting for APB 25 credit of $33m) | (155 | ) | (76 | ) | (46 | ) |
| |
| Pro forma net income | 2,152 | | 1,321 | | 819 | |
| |
| Pro forma net income per $0.25 Ordinary Share and ADS in accordance with US GAAP (basic and diluted): | | | | | | |
| |
| As reported ($) | $1.33 | | $0.79 | | $0.49 | |
| |
| Pro forma ($) | $1.24 | | $0.75 | | $0.46 | |
| |
The fair value of options granted is estimated, based on the stock price at the grant date, using the Black-Scholes option pricing model with the following assumptions:
| | 2002 | | 2001 | | 2000 | |
| |
| Dividend yield | 1.6% | | 1.5% | | 2.0% | |
| |
| Expected volatility | 30.0% | | 20.0% | | 20.0% | |
| |
| Risk-free interest rate | 5.2% | | 4.2% | | 5.9% | |
| |
| Expected lives: 1994 Scheme | – | | – | | 6.0 years | |
| |
| Expected lives: AstraZeneca Share Option Plan | 6.0 years | | 6.0 years | | 6.0 years | |
| |
| Expected lives: SAYE Scheme | 4.3 years | | 4.3 years | | 4.6 years | |
| |
In the initial phase-in period, the effects of applying SFAS No.123 for disclosing compensation cost may not be representative of the effects on pro forma net income and earnings per share for future years.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | 119 |
Differences between UK and US accounting principles (continued)Pension and post-retirement benefits
For the purposes of US GAAP, the pension costs of the major UK retirement plan and of the retirement plans of the major non-UK subsidiaries have been restated in the following tables in accordance with the requirements of SFAS No. 132. These plans comprise a substantial portion of the actuarial liabilities of all AstraZeneca retirement plans. The changes in projected benefit obligations, plan assets and details of the funded status of these retirement plans, together with the changes in the accumulated other post-retirement benefit obligations, under SFAS No. 132 are as follows:
| | Pension benefits | | Other post-retirement benefits | |
| |
| |
| |
| Change in projected benefit obligation | 2002 | | 2001 | | 2002 | | 2001 | |
| | $m | | $m | | $m | | $m | |
| |
| Benefit obligation at beginning of year | 4,337 | | 4,188 | | 205 | | 197 | |
| |
| Service cost | 114 | | 102 | | 8 | | 7 | |
| |
| Interest cost | 263 | | 243 | | 14 | | 14 | |
| |
| Participant contributions | 18 | | 17 | | – | | – | |
| |
| Plan amendments | – | | (11 | ) | – | | – | |
| |
| Actuarial (gain)/loss | 80 | | 75 | | 23 | | (1 | ) |
| |
| Special termination benefits | 12 | | 19 | | – | | – | |
| |
| Settlement and curtailment | – | | – | | (24 | ) | – | |
| |
| Benefits paid | (206 | ) | (198 | ) | (19 | ) | (14 | ) |
| |
| Exchange | 408 | | (98 | ) | 3 | | 2 | |
| |
| Benefit obligation at end of year | 5,026 | | 4,337 | | 210 | | 205 | |
| |
| | Pension benefits | | Other post-retirement benefits | |
| |
| |
| |
| Change in plan assets | 2002 | | 2001 | | 2002 | | 2001 | |
| | $m | | $m | | $m | | $m | |
| |
| Fair value at 1 January | 3,753 | | 3,803 | | – | | – | |
| |
| Actual return on plan assets | (142 | ) | 45 | | (16 | ) | – | |
| |
| Group contribution | 284 | | 170 | | 161 | | – | |
| |
| Participant contributions | 18 | | 17 | | – | | – | |
| |
| Settlement and curtailment | – | | – | | – | | – | |
| |
| Benefits paid | (205 | ) | (198 | ) | (12 | ) | – | |
| |
| Exchange | 330 | | (84 | ) | – | | – | |
| |
| Fair value of plan assets at end of year | 4,038 | | 3,753 | | 133 | | – | |
| |
| Funded status of plans | (988 | ) | (584 | ) | (77 | ) | (205 | ) |
| |
| Unrecognised net loss/(profit) | 938 | | 396 | | – | | – | |
| |
| Prior service cost not recognised | 29 | | 35 | | – | | – | |
| |
| Unrecognised net obligation on implementation | 3 | | 6 | | – | | – | |
| |
| | (18 | ) | (147 | ) | (77 | ) | (205 | ) |
| |
| Adjustments to recognise minimum liability | | | | | | | | |
| Intangible assets | (45 | ) | – | | – | | – | |
| |
| Accumulated other comprehensive income | (45 | ) | – | | – | | – | |
| |
| Accrued benefit liability | (108 | ) | (147 | ) | (77 | ) | (205 | ) |
| |
Back to Contents
| 120 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | |
Additional Information for US Investors continued
Differences between UK and US accounting principles (continued)At 31 December 2002, the projected benefit obligation, accumulated benefit obligation and fair value of the plan assets in respect of the retirement plans above with accumulated benefit obligations in excess of plan assets were $4,249m, $3,557m and $3,296m, (2001 $97m, $73m and $nil) respectively.
Assumed discount rates and rates of increase in remuneration used in calculating the projected benefit obligations together with long term rates of return on plan assets vary according to the economic conditions of the country in which the retirement plans are situated. The weighted average rates used for calculation of year end benefit obligations and forecast benefits cost in the main retirement plans and other benefit obligations for SFAS No. 132 purposes were as follows:
| | Pension benefits | | Other post-retirement benefits | |
| |
| |
| |
| | 2002
% | | 2001
% | | 2000
% | | 2002
% | | 2001
% | | 2000
% | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Discount rate | 5.8 | | 6.0 | | 5.6 | | 6.6 | | 7.1 | | 7.1 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Long term rate of increase in remuneration | 4.1 | | 4.4 | | 4.4 | | 4.8 | | n/a | | n/a | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Expected long term return on assets | 6.4 | | 6.5 | | 6.2 | | 7.8 | | n/a | | n/a | |
|
|
|
|
|
|
|
|
|
|
|
| |
| |
The Group has assumed a long term rate of increase in healthcare costs of 11.0%, reducing to 5.0%.
| | Pension benefits | | Other post-retirement benefits | |
| |
| |
| |
| | 2002
$m | | 2001
$m | | 2000
$m | | 2002
$m | | 2001
$m | | 2000
$m | |
|
|
|
|
|
| |
|
|
|
|
| |
| Net periodic cost | | | | | | | | | | | | |
| Service cost – present value of benefits | | | | | | | | | | | | |
| accruing during the year | 114 | | 102 | | 152 | | 8 | | 7 | | 10 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Interest cost on projected benefit obligations | 263 | | 243 | | 301 | | 14 | | 14 | | 17 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Expected return on assets | (263 | ) | (242 | ) | (322 | ) | – | | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
| |
| Net amortisation and deferral | 28 | | 39 | | 46 | | (1 | ) | (2 | ) | (1 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |
| Net periodic cost for the year | 142 | | 142 | | 177 | | 21 | | 19 | | 26 | |
|
|
|
|
|
|
|
|
|
|
|
| |
| |
It is estimated that a 1 percentage point change in the weighted average healthcare costs trend would have the following effects on the accumulated benefit obligation and net periodic cost at 31 December 2002:
| | | | 1 percentage point | |
| | | |
| |
| | | | increase
$m | | decrease
$m | |
| |
| Accumulated benefit obligation | | | 10 | | (9 | ) |
| |
| Net periodic cost | | | 2 | | (1 | ) |
| |
| | | | | | | |
| Taxation | | | | | | |
| Years ended 31 December | 2002
$m | | 2001
$m | | 2000
$m | |
|
|
|
|
|
| |
| Taxes on income from continuing operations | | | | | | |
| UK taxation | | | | | | |
| Corporation tax | 165 | | 147 | | 79 | |
|
|
|
|
|
| |
| Double taxation relief | (7 | ) | (4 | ) | (42 | ) |
|
|
|
|
|
| |
| Deferred | 40 | | 10 | | (27 | ) |
|
|
|
|
|
| |
| Overseas taxation | | | | | | |
| Overseas taxes | 921 | | 831 | | 956 | |
|
|
|
|
|
| |
| Deferred taxation | (84 | ) | 125 | | – | |
|
|
|
|
|
| |
| Share of taxation of joint ventures and associates | – | | – | | 3 | |
|
|
|
|
|
| |
| Taxes on income from continuing operations | 1,035 | | 1,109 | | 969 | |
|
|
|
|
|
| |
| |
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | 121 |
Differences between UK and US accounting principles (continued)
The table below reconciles the UK statutory tax charge to the Group’s actual charge on income from continuing operations.
| Years ended 31 December | 2002
$m | | 2001
$m | | 2000
$m | |
| |
| Income on continuing operations | 3,342 | | 2,506 | | 1,876 | |
|
|
|
|
|
| |
| Taxation charge at UK corporation | | | | | | |
| tax rate of 30% for 2002 (30% for 2001, 30% for 2000) | 1,002 | | 751 | | 563 | |
|
|
|
|
|
| |
| Acquisition related items | – | | 4 | | 29 | |
|
|
|
|
|
| |
| Goodwill, Advanta, and Salick Health Care impairment | – | | 190 | | 576 | |
|
|
|
|
|
| |
| Net effect of different rates and eligible costs in other jurisdictions | (21 | ) | (43 | ) | (86 | ) |
|
|
|
|
|
| |
| Exceptional items | 105 | | 4 | | 19 | |
|
|
|
|
|
| |
| Other | (51 | ) | 203 | | (132 | ) |
|
|
|
|
|
| |
| Tax on income from continuing operations | 1,035 | | 1,109 | | 969 | |
| |
| |
In 2002, claims amounting to $43m (2001 $109m) for tax relief arising as a result of a restructuring of the AMI joint venture in 1998 were made. Under US GAAP, these reliefs are adjusted against the goodwill arising on the restructuring and included in other adjustments.
| Shareholders’ equity | 2002
$m | | 2001
$m | |
| |
| Total shareholders’ equity under UK GAAP (restated) | 11,172 | | 9,586 | |
|
|
|
| |
| | | | | |
| Adjustments to conform to US GAAP | | | | |
| Purchase accounting adjustments (including goodwill and intangibles) | | | | |
| Deemed acquisition of Astra | | | | |
| Goodwill | 12,692 | | 11,062 | |
|
|
|
| |
| Tangible and intangible fixed assets | 7,707 | | 8,139 | |
|
|
|
| |
| Others | 86 | | 31 | |
|
|
|
| |
| Capitalisation, less disposals and amortisation of interest | 238 | | 192 | |
|
|
|
| |
| Deferred taxation | | | | |
| On fair value of Astra | (2,305 | ) | (2,313 | ) |
|
|
|
| |
| Others (restated) | (159 | ) | (68 | ) |
|
|
|
| |
| Dividend | 808 | | 820 | |
|
|
|
| |
| Pension expense | (271 | ) | (162 | ) |
|
|
|
| |
| Post-retirement benefits/plan amendment | (24 | ) | (28 | ) |
|
|
|
| |
| Software costs capitalised | 64 | | 110 | |
|
|
|
| |
| Fair value of derivative financial instruments | 99 | | 50 | |
|
|
|
| |
| Deferred income recognition | (14 | ) | (75 | ) |
|
|
|
| |
| Others | 90 | | 58 | |
|
|
|
| |
| Shareholders’ equity in accordance with US GAAP | 30,183 | | 27,402 | |
|
|
|
| |
| |
Back to Contents
| 122 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | |
Additional Information for US Investors continued
Differences between UK and US accounting principles (continued)US GAAP Condensed Consolidated Statement of Cash Flows
| For the years ended 31 December | 2002
$m | | 2001
$m | | 2000
$m | |
|
|
|
|
|
| |
| Cash flows from operating activities | 4,833 | | 3,126 | | 3,554 | |
|
|
|
|
|
| |
| Cash flows from investing activities | | | | | | |
| Movement in short term investments and fixed deposits | (806 | ) | 260 | | (608 | ) |
|
|
|
|
|
| |
| New fixed asset investments | (1 | ) | (5 | ) | (3 | ) |
|
|
|
|
|
| |
| Disposal of fixed assets | 66 | | 44 | | 37 | |
|
|
|
|
|
| |
| Acquisitions and disposals | – | | (44 | ) | 740 | |
|
|
|
|
|
| |
| Capital expenditure | (1,608 | ) | (1,582 | ) | (1,460 | ) |
|
|
|
|
|
| |
| Net cash outflows from investing activities | (2,349 | ) | (1,327 | ) | (1,294 | ) |
|
|
|
|
|
| |
| Net cash flow before financing | 2,484 | | 1,799 | | 2,260 | |
|
|
|
|
|
| |
| Cash flows from financing activities | | | | | | |
| Equity dividends paid | (1,234 | ) | (1,236 | ) | (1,220 | ) |
|
|
|
|
|
| |
| Repurchase of AstraZeneca PLC Ordinary Shares | (1,154 | ) | (994 | ) | (334 | ) |
|
|
|
|
|
| |
| Net (decrease)/increase in short term borrowings | (13 | ) | 7 | | (67 | ) |
|
|
|
|
|
| |
| Loans repaid/new loans | (105 | ) | 28 | | 3 | |
|
|
|
|
|
| |
| Repayment of lease finance | – | | – | | (2 | ) |
|
|
|
|
|
| |
| Net cash outflows from financing activities | (2,506 | ) | (2,195 | ) | (1,620 | ) |
|
|
|
|
|
| |
| (Decrease)/increase in cash | (22 | ) | (396 | ) | 640 | |
|
|
|
|
|
| |
| Cash: | | | | | | |
| At 1 January | 510 | | 908 | | 262 | |
|
|
|
|
|
| |
| (Decrease)/increase in cash | (22 | ) | (396 | ) | 640 | |
|
|
|
|
|
| |
| Exchange movements | 36 | | (2 | ) | 6 | |
|
|
|
|
|
| |
| At 31 December | 524 | | 510 | | 908 | |
|
|
|
|
|
| |
| (1) | Interest paid was $96m in 2002, $84m in 2001, $145m in 2000. Interest received was $142m in 2002, $232m in 2001, $180m in 2000. |
| (2) | Tax paid was $795m in 2002, $792m in 2001, $648m in 2000. |
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information for US Investors | 123 |
Group Financial Record – UK GAAP
| For the years ended 31 December | 1995
(restated)
$m | | 1996
(restated)
$m | | 1997
(restated)
$m | | 1998
(restated)
$m | | 1999
(restated)
$m | | 2000
(restated)
$m | | 2001
(restated)
m | | 2002
$m | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Turnover and profits | | | | | | | | | | | | | | | | |
| Group turnover | 12,007 | | 13,106 | | 13,055 | | 15,260 | | 18,257 | | 17,882 | | 16,222 | | 17,841 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Cost of sales | (4,018 | ) | (4,225 | ) | (3,952 | ) | (4,819 | ) | (5,849 | ) | (5,270 | ) | (4,232 | ) | (4,520 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Distribution costs | (374 | ) | (385 | ) | (364 | ) | (367 | ) | (343 | ) | (286 | ) | (122 | ) | (141 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Research and development | (1,671 | ) | (1,961 | ) | (2,170 | ) | (2,473 | ) | (2,923 | ) | (2,893 | ) | (2,773 | ) | (3,069 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Selling, general and administrative expenses | (3,566 | ) | (3,751 | ) | (3,838 | ) | (4,812 | ) | (6,585 | ) | (5,691 | ) | (5,509 | ) | (6,348 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Other income | 189 | | 193 | | 126 | | 353 | | 189 | | 266 | | 368 | | 243 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Group operating profit | 2,567 | | 2,977 | | 2,857 | | 3,142 | | 2,746 | | 4,008 | | 3,954 | | 4,006 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Group operating profit
before exceptional items | 2,670 | | 2,977 | | 2,857 | | 3,051 | | 3,908 | | 4,330 | | 4,156 | | 4,356 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Exceptional items charged to
operating profit | (103 | ) | – | | – | | 91 | | (1,162 | ) | (322 | ) | (202 | ) | (350 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Share of operating profit of
joint ventures and associates | 354 | | 504 | | 722 | | 539 | | (7 | ) | (149 | ) | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Exceptional items | (306 | ) | (56 | ) | – | | (29 | ) | (776 | ) | (150 | ) | – | | – | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Profits on sale of fixed assets | – | | – | | – | | – | | – | | – | | 10 | | – | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Dividend income | – | | – | | – | | – | | – | | 3 | | 8 | | 1 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Net interest | 75 | | 118 | | 81 | | 47 | | (4 | ) | 135 | | 105 | | 30 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Profit on ordinary activities before taxation | 2,690 | | 3,543 | | 3,660 | | 3,699 | | 1,959 | | 3,847 | | 4,077 | | 4,037 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Taxation | (802 | ) | (1,087 | ) | (1,081 | ) | (1,118 | ) | (661 | ) | (1,560 | ) | (1,160 | ) | (1,177 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Profit on ordinary activities after taxation | 1,888 | | 2,456 | | 2,579 | | 2,581 | | 1,298 | | 2,287 | | 2,917 | | 2,860 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Attributable to minorities | (25 | ) | (19 | ) | (9 | ) | (2 | ) | (1 | ) | (10 | ) | (11 | ) | (24 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Net profit for the financial year | 1,863 | | 2,437 | | 2,570 | | 2,579 | | 1,297 | | 2,277 | | 2,906 | | 2,836 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Return on sales | | | | | | | | | | | | | | | | |
Group operating profit before exceptional
items as a percentage of sales | 22.2 | % | 22.7 | % | 21.9 | % | 20.0 | % | 21.4 | % | 24.2 | % | 25.6 | % | 24.9 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Ratio of earnings to fixed charges
(UK GAAP)
| 18.3 | | 28.3 | | 28.1 | | 26.1 | | 10.1 | | 25.2 | | 42.8 | | 45.6 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | | | | | | | | | | | | | | | | |
| At 31 December | 1995
(restated)
$m | | 1996
(restated)
$m | | 1997
(restated)
$m | | 1998
(restated)
$m | | 1999
(restated)
$m | | 2000
(restated)
$m | | 2001
(restated)
$m | | 2002
$m | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Balance sheets | | | | | | | | | | | | | | | | |
Fixed assets (tangible and intangible)
and goodwill | 5,251 | | 5,661 | | 5,894 | | 8,721 | | 9,717 | | 7,908 | | 8,109 | | 9,404 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Fixed asset investments | 834 | | 1,005 | | 1,027 | | 353 | | 185 | | 11 | | 23 | | 46 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Current assets | 8,343 | | 9,387 | | 9,355 | | 9,630 | | 10,393 | | 10,938 | | 10,364 | | 12,126 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Total assets | 14,428 | | 16,053 | | 16,276 | | 18,704 | | 20,295 | | 18,857 | | 18,496 | | 21,576 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Creditors due within one year | (4,540 | ) | (4,599 | ) | (4,459 | ) | (5,650 | ) | (7,019 | ) | (6,897 | ) | (6,480 | ) | (8,215 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Total assets less current liabilities | 9,888 | | 11,454 | | 11,817 | | 13,054 | | 13,276 | | 11,960 | | 12,016 | | 13,361 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Creditors due after more than one year | (917 | ) | (912 | ) | (902 | ) | (801 | ) | (1,202 | ) | (927 | ) | (787 | ) | (362 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Provisions for liabilities and charges | (1,452 | ) | (1,511 | ) | (1,478 | ) | (1,472 | ) | (1,765 | ) | (1,617 | ) | (1,600 | ) | (1,773 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Minority equity interests | 169 | | 184 | | 60 | | 59 | | 46 | | 27 | | 43 | | 54 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Shareholders’ funds – equity interests | 7,350 | | 8,847 | | 9,377 | | 10,722 | | 10,263 | | 9,389 | | 9,586 | | 11,172 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Shareholders’ funds and minority interests | 7,519 | | 9,031 | | 9,437 | | 10,781 | | 10,309 | | 9,416 | | 9,629 | | 11,226 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net profit and shareholders’funds have been restated under FRS19 – Deferred Tax for the years 1995 to 2001. In addition, the information under sales and costs of sales has been reclassified for cash settlement discounts which are now deducted from sales as opposed to being included in cost of sales.
Back to Contents
| 124 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Group Financial Record – UK GAAP | |
Group Financial Record – UK GAAP continued
For the years ended 31 December
| 1995
$m | | 1996
$m | | 1997
$m | | 1998
$m | | 1999
$m | | 2000
$m | 2001
$m | | 2002
$m | |
| |
| Cash flow | | | | | | | | | | | | | | | | |
| Net cash inflow from operating activities | 3,005 | | 3,198 | | 3,355 | | 3,832 | | 3,113 | | 4,183 | | 3,762 | | 5,593 | |
| |
| Dividends received from | | | | | | | | | | | | | | | | |
| joint ventures and associates | 243 | | 328 | | 369 | | 262 | | 3 | | – | | – | | – | |
| |
| Returns on investments and servicing of finance | 65 | | 98 | | (31 | ) | 103 | | 29 | | 19 | | 156 | | 35 | |
| |
| Tax paid | (788 | ) | (719 | ) | (750 | ) | (775 | ) | (1,020 | ) | (648 | ) | (792 | ) | (795 | ) |
| |
| Capital expenditure and financial investment | (918 | ) | (1,182 | ) | (1,292 | ) | (1,369 | ) | (2,731 | ) | (1,426 | ) | (1,543 | ) | (1,543 | ) |
| |
| Acquisitions and disposals | (531 | ) | 227 | | (321 | ) | (2,013 | ) | 1,978 | | 740 | | (44 | ) | – | |
| |
| Equity dividends paid to Shareholders | (628 | ) | (750 | ) | (882 | ) | (995 | ) | (1,216 | ) | (1,220 | ) | (1,236 | ) | (1,234 | ) |
| |
| Net cash flow before management of liquid resources and financing | 448 | | 1,200 | | 448 | | (955 | ) | 156 | | 1,648 | | 303 | | 2,056 | |
| |
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Group Financial Record – US GAAP | 125 |
Group Financial Record – US GAAP
Group Financial Record – US GAAP
The selected financial data set out below for each of the years in the five year period ended 31 December 2002, has been extracted or derived from audited Financial Statements.
The selected financial data should be read in conjunction with, and are qualified in their entirety by reference to, the Financial Statements of AstraZeneca and the notes thereto, which are included elsewhere in this document.
Consolidated income statement data
For the years ended 31 December | 1998 | | 1999 | | 2000 | | 2001 | | 2002 | |
| |
| Net income/(loss) from operations ($ million) | 1,036 | | (3,539 | ) | 865 | | 1,397 | | 2,307 | |
| |
| Net income/(loss) from operations per Ordinary Share | $1.09 | | ($2.26 | ) | $0.49 | | $0.79 | | $1.33 | |
| |
| Diluted income/(loss) from operations per Ordinary Share | $1.09 | | ($2.26 | ) | $0.49 | | $0.79 | | $1.33 | |
| |
| | | | | | | | | | | |
| Net income/(loss) from operations had SFAS No 141 been adopted | 1,129 | | (2,833 | ) | 1,716 | | 2,125 | | | |
| |
Net and diluted income/(loss) per Ordinary Share from operations
had SFAS No 141 been adopted | $1.19 | | ($1.81 | ) | $0.97 | | $1.21 | | | |
| |
Ratio of earnings to fixed charges
For the Group with estimated material
adjustments to accord with US GAAP | 11.7 | | (19.3 | ) | 15.5 | | 25.0 | | 36.7 | |
| |
| | | | | | | | | | | |
Consolidated balance sheet data
At 31 December | 1998
$m | | 1999
$m | | 2000
$m | | 2001
$m | | 2002
$m | |
| |
| Total assets | 10,675 | | 46,640 | | 41,500 | | 38,081 | | 42,578 | |
| |
| Shareholders’ equity | 5,558 | | 33,735 | | 29,707 | | 27,402 | | 30,183 | |
| |
Merger accounting
For the purpose of US GAAP, the merger has been regarded as a purchase accounting acquisition of Astra by Zeneca.
Accordingly the US GAAP results above for 1998 are not restated for the merger with Astra and represent the previously reported results of Zeneca Group PLC.
Ratio of earnings to fixed charges (UK and US GAAP)
For the purpose of computing these ratios, earnings consist of the income from continuing ordinary activities before taxation of Group companies and income received from companies owned 50% or less, plus fixed charges (excluding capitalised interest). Fixed charges consist of interest (including capitalised interest) on all indebtedness, amortisation of debt discount and expense and that portion of rental expense representative of the interest factor. The comparative figures have been restated from those previously disclosed to reflect the reclassification of the operations of Specialties and Agrochemicals as discontinued.
Back to Contents
| 126 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Shareholder Information | |
Shareholder Information
| AstraZeneca | 1999 | * | 2000 | | 2001 | | 2002 | |
|
|
|
|
|
|
|
| |
| Ordinary Shares in issue – millions | | | | | | | | |
| At year end | 1,775 | | 1,766 | | 1,745 | | 1,719 | |
|
|
|
|
|
|
|
| |
| Weighted average for year | 1,776 | | 1,768 | | 1,758 | | 1,733 | |
|
|
|
|
|
|
|
| |
| Stock Market price – per $0.25 Ordinary Share | | | | | | | | |
| Highest (pence) | 2946 | | 3600 | | 3555 | | 3625 | |
|
|
|
|
|
|
|
| |
| Lowest (pence) | 2208 | | 1926 | | 2880 | | 1799 | |
|
|
|
|
|
|
|
| |
| At year end (pence) | 2568 | | 3375 | | 3098 | | 2220 | |
|
|
|
|
|
|
|
| |
| Earnings per $0.25 Ordinary Share before exceptional items1 | $1.63 | | $1.62 | | $1.73 | | $1.84 | |
|
|
|
|
|
|
|
| |
| Earnings per $0.25 Ordinary Share (basic)1 | $0.73 | | $1.30 | | $1.65 | | $1.64 | |
|
|
|
|
|
|
|
| |
| Earnings per $0.25 Ordinary Share (diluted)1 | $0.73 | | $1.30 | | $1.65 | | $1.64 | |
|
|
|
|
|
|
|
| |
| Dividends | $0.70 | | $0.70 | † | $0.70 | | $0.70 | |
|
|
|
|
|
|
|
| |
| * | For the period 1 January 1999 to 31 December 1999 (except for Stock Market prices which are for the period from 6 April 1999 to 31 December 1999). |
| † | In addition, shareholders received a distribution of shares in Syngenta AG as a dividend in specie in respect of the demerger of Zeneca Agrochemicals. |
| 1 | Earnings per share have been restated for the effect of the adoption of FRS19 – Deferred Tax |
| | | | | | | | | |
| Zeneca | 1999 | * | | | | | | |
|
| | | | | | | |
| Ordinary Shares in issue – millions | | | | | | | | |
| At period end | 953 | | | | | | | |
|
| | | | | | | |
| Weighted average for period | 951 | | | | | | | |
|
| | | | | | | |
| Stock Market price – per 25 pence Ordinary Share | | | | | | | | |
| Highest (pence) | 3037 | | | | | | | |
|
| | | | | | | |
| Lowest (pence) | 2406 | | | | | | | |
|
| | | | | | | |
| At period end (pence) | 3037 | | | | | | | |
|
| | | | | | | |
| * | For the period from 1 January 1999 to 6 April 1999 | | | | | | | | |
| | | | | | | | | |
| Astra | 1999 | * | | | | | | |
|
| | | | | | | |
| Ordinary Shares in issue – millions | | | | | | | | |
| At period end | 1,643 | | | | | | | |
|
| | | | | | | |
| Weighted average for period | 1,643 | | | | | | | |
|
| | | | | | | |
| Stock Market price – per Astra A Share | | | | | | | | |
| Highest (SEK) | 190 | | | | | | | |
|
| | | | | | | |
| Lowest (SEK) | 154 | | | | | | | |
|
| | | | | | | |
| At period end (SEK) | 190 | | | | | | | |
|
| | | | | | | |
| Stock Market price – per Astra B Share | | | | | | | | |
| Highest (SEK) | 190 | | | | | | | |
|
| | | | | | | |
| Lowest (SEK) | 154 | | | | | | | |
|
| | | | | | | |
| At period end (SEK) | 190 | | | | | | | |
|
| | | | | | | |
| * | For the period from 1 January 1999 to 6 April 1999 | | | | | | | | |
| | | | | | | | | | |
Percentage analysis at 31 December 2002 of issued share capital
By size of account
No. of shares
| 2002
% | |
|
| |
| 1 – 250 | 0.6 | |
|
| |
| 251 – 500 | 0.8 | |
|
| |
| 501 – 1,000 | 1.1 | |
|
| |
| 1,001 – 5,000 | 1.7 | |
|
| |
| 5,001 – 10,000 | 0.3 | |
|
| |
| 10,001 – 50,000 | 1.4 | |
|
| |
| 50,001 – 1,000,000 | 12.2 | |
|
| |
| over 1,000,000† | 81.9 | |
|
| |
| Issued share capital | 100.0 | |
|
| |
| † | includes VPC and ADR holdings | | |
At 31 December 2002, AstraZeneca PLC had 177,573 registered holders of 1,718,666,329 Ordinary Shares of $0.25 each. In addition there were approximately 46,000 holders of American Depositary Receipts (ADRs) representing 5.31% of the issued share capital and 156,000 holders of shares held under the VPC Services Agreement representing 23.56% of the issued share capital. The ADRs, each of which is equivalent to one Ordinary Share, are issued by JPMorgan Chase Bank.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Shareholder Information | 127 |
AstraZeneca PLC
Since April 1999, following the AstraZeneca merger, the principal markets for trading in the shares of AstraZeneca PLC are the London, Stockholm and New York Stock Exchanges. The table below sets forth, for the four quarters of 2001 and for the first two quarters and last six months of 2002 the reported high and low share prices of AstraZeneca PLC, on the following bases:
| > | for shares listed on the London Stock Exchange (‘LSE’) the reported high and low middle market closing quotations are derived from The Daily Official List; |
| | |
| > | for shares listed on the Stockholm Stock Exchange (‘SSE’) the high and low closing sales prices are as stated in the Official List; |
| | |
| > | for American Depositary Shares (‘ADS’) listed on the New York Stock Exchange the reported high and low sales prices are as reported by Dow Jones (ADR quotations). |
| | |
| | | AstraZeneca | |
| | |
| |
| | | Ordinary LSE | | ADS | | Ordinary SSE | * |
| | |
| |
| | | High
(pence) | | Low
(pence) | | High
(US$) | | Low
(US$)> | | High
(SEK) | | Low
(SEK) | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| 2001 | – Quarter 1 | 3385 | | 2880 | | 50.88 | | 42.70 | | 501 | | 400 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – Quarter 2 | 3555 | | 3149 | | 50.40 | | 45.68 | | 540 | | 460.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – Quarter 3 | 3512 | | 2913 | | 51.11 | | 42.60 | | 534 | | 431 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – Quarter 4 | 3285 | | 3012 | | 48.14 | | 44.01 | | 507 | | 458.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| 2002 | – Quarter 1 | 3625 | | 3051 | | 52.00 | | 43.72 | | 541 | | 455.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – Quarter 2 | 3574 | | 2634 | | 52.04 | | 39.12 | | 536 | | 366 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – July | 2680 | | 2002 | | 41.30 | | 29.90 | | 392 | | 286 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – August | 2406 | | 1822 | | 38.00 | | 28.30 | | 360.5 | | 264 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – September | 2067 | | 1799 | | 32.15 | | 28.00 | | 305 | | 255 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – October | 2400 | | 1947 | | 38.15 | | 30.16 | | 351.5 | | 279 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – November | 2540 | | 2259 | | 40.48 | | 34.19 | | 365 | | 316.5 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| | – December | 2430 | | 2194 | | 38.47 | | 34.82 | | 350 | | 304 | |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| * | Principally held in bearer form |
During 2002 AstraZeneca’s share re-purchase programme which was introduced in 1999 continued with the re-purchase and subsequent cancellation of 28.4 million shares at a total cost of $1,190m, representing 1.6 per cent of the total issued share capital of the Company. The average price paid per share in 2002 was 2818 pence. Between 1999 and 2001 a total of 37.1 million Ordinary Shares were re-purchased, and subsequently cancelled, at an average price of 2910 pence per share for a consideration, including expenses, of $1,615m. The excess of the consideration over the nominal value was charged against the profit and loss account reserve. Shares issued in respect of share schemes totalled 1.7 million.
In 1999 in connection with the merger, AstraZeneca’s share capital was redenominated into US dollars. On 6 April 1999, Zeneca shares were cancelled and US dollar shares issued, credited as fully paid on the basis of one dollar share for each Zeneca share then held. This was achieved by a reduction of capital under section 135 of the Companies Act 1985. Upon the reduction of capital becoming effective, all issued and unissued Zeneca shares were cancelled and the sum arising as a result thereof credited to a special reserve which was converted into US dollars at the rate of exchange prevailing on the record date. This US dollar reserve was then applied in paying up at par newly created US dollar shares.
At the same time as the US dollar shares were issued, the Company issued £50,000 Redeemable Preference Shares for cash at par. The Redeemable Preference Shares carry limited class voting rights and no dividend rights. This class of shares is also capable of redemption at par at the option of the Company on the giving of seven days’ written notice to the registered holder of the shares.
A total of 826 million AstraZeneca shares were issued to Astra shareholders who accepted the merger offer before the final closing date, 21 May 1999. AstraZeneca received acceptances from Astra shareholders representing 99.6 per cent of Astra’s shares and the remaining 0.4 per cent was acquired in 2000 for cash.
Back to Contents
| 128 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Shareholder Information | |
Shareholder Information continued
Major shareholdings
On 29 January 2003 (not more than one month prior to the date of the Notice of Annual General Meeting) the following had disclosed an interest in the issued Ordinary Share capital of the Company in accordance with the requirements of Sections 198-208 of the Companies Act 1985:
| Shareholder | Number of shares | | Date of disclosure
to Company* | Percentage of issued
share capital | |
|
|
|
|
| |
| The Capital Group Companies, Inc. | 204,812,653 | | 14 Jan 2003 | 11.92% | |
|
|
|
|
| |
| Investor AB | 91,545,308 | | 16 Apr 1999 | 5.33% | |
|
|
|
|
| |
| Putnam Investment Management, LLC and The Putnam Advisory Company, LLC | 52,643,485 | | 8 Feb 2002 | 3.06% | |
|
|
|
|
| |
| Legal & General Investment Management Limited | 52,518,020 | | 13 Jun 2002 | 3.06% | |
|
|
|
|
| |
No other person held a notifiable interest in shares, comprising 3% or more of the issued Ordinary Share capital of the Company, appearing in the register of interests in shares maintained under the provisions of Section 211 of the Companies Act 1985.
Significant changes in the percentage ownership held by major shareholders during the past three years are set out below. Major shareholders do not have different voting rights.
*Since the date of disclosure to the Company, the interest of any person listed above in the Ordinary Shares of the Company may have increased or decreased. No requirement to notify the Company of any increase or decrease would have arisen unless the holding moved up or down through a whole number percentage level.
| | Percentage of issued share capital | | | |
Shareholder | 29 Jan 2003In AstraZeneca | | 17 Feb 2002In AstraZeneca | | 9 Feb 2001In AstraZeneca | | 14 Mar 2000In AstraZeneca | |
|
|
|
|
|
|
|
| |
| The Capital Group Companies, Inc. | 11.92% | | 11.09% | | 10.02% | | 7.80% | |
|
|
|
|
|
|
|
| |
| Investor AB | 5.33% | | 5.25% | | 5.18% | | 5.20% | |
|
|
|
|
|
|
|
| |
| Putnam Investment Management, LLC and The Putnam Advisory Company, LLC | 3.06% | | 3.02% | | <3.00% | | <3.00% | |
|
|
|
|
|
|
|
| |
| Legal & General Investment Management Limited | 3.06% | | <3.00% | | <3.00% | | <3.00% | |
|
|
|
|
|
|
|
| |
AstraZeneca PLC American Depositary Shares (each representing one Ordinary Share) evidenced by American Depositary Receipts issued by JPMorgan Chase Bank, as depositary, are listed on the New York Stock Exchange. As of 29 January 2003, the proportion of Ordinary Shares represented by American Depositary Shares was 5.32% of the Ordinary Shares outstanding.
| Number of registered holders of Ordinary Shares as of 29 January 2003: | | |
| – In the US | 804 | |
| – Total | 176,842 | |
| | | |
| Number of record holders of American Depositary Receipts as of 29 January 2003: | | |
| – In the US | 3,133 | |
| – Total | 3,167 | |
So far as the Company is aware, it is neither directly nor indirectly owned nor controlled by one or more corporations or by any government.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Shareholder Information | 129 |
As of 29 January 2003 the total amount of the Company’s voting securities owned by Directors and Officers of the Company was:
| Title of class | Amount owned | Per cent of class |
| | ($0.25 shares) | |
|
| Ordinary Shares | 508,201 | 0.03% |
|
|
The Company does not know of any arrangements the operation of which might result in a change in the control of the Company.
Related party transactions
During the period 1 January 2003 to 29 January 2003 there were no transactions, loans, or proposed transactions between the Company and any related parties which were material to either the Company or the related party, or which were unusual in their nature or conditions. (See also Note 36 Statutory and other information).
Options to purchase securities from registrant or subsidiaries
| (a) | As of 29 January 2003, options outstanding to subscribe for Ordinary Shares of $0.25 of the Company were: |
Number of shares | | Subscription price | | Normal expiry date |
|
|
|
|
|
| 34,608,810 | | 630p-3487p | | 2003-2012 |
|
|
|
|
|
The weighted average subscription price of options outstanding at 29 January 2003 was 3000p. All options were granted under Company employee schemes.
| (b) | Included in paragraph (a) are options granted to Directors and Officers of AstraZeneca as follows: |
Number of shares | | Subscription price | | Normal expiry date |
|
|
|
|
|
| 1,562,652 | | 748p-3487p | | 2004-2012 |
|
|
|
|
|
| (c) | Included in paragraph (b) are options granted to individually named Directors. Details of these option holdings as at 31 December 2002 are shown in the Directors’ Remuneration Report. |
| | |
| | During the period 1 January 2003 to 29 January 2003 no Director exercised any options. |
Dividend payments
The record date for the second interim dividend for 2002 payable on 7 April 2003 (in the UK, US and Sweden) is 21 February 2003. Shares trade ex-dividend on the London and Stockholm Stock Exchanges from 19 February 2003 and ADRs trade ex-dividend on the New York Stock Exchange from the same date. Future dividends will normally be paid as follows:
The record date for the first interim dividend for 2003 payable on 6 October 2003 (in the UK, US and Sweden) is 22 August 2003.
| First interim: | Announced end of July and paid in October |
| Second interim: | Announced end of January and paid in April |
Registrar and Transfer Office The AstraZeneca Registrar Lloyds TSB Registrars The Causeway Worthing West Sussex BN99 6DA Telephone 0870 600 3956
Shareview
AstraZeneca’s shareholders with internet access may visit www.shareview.co.uk and register their details to create a portfolio. Shareview is a free and secure on-line service from Lloyds TSB Registrars that gives access to shareholdings including balance movements, indicative share prices and information about recent dividends.
Results
Unaudited trading results of AstraZeneca in respect of the first three months of 2003 will be published on 30 April 2003 and results in respect of the first six months of 2003 will be published on 24 July 2003.
Documents on display
The Memorandum and Articles of Association of the Company and other documents concerning the Company which are referred to in this document may be inspected at the Company’s registered office at 15 Stanhope Gate, London W1K 1LN.
Back to Contents
| 130 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Shareholder Information | |
Shareholder Information continued
Taxation for US residents
The following summary of the principal UK and certain US tax consequences of ownership of Ordinary Shares or ADRs held as capital assets by US resident shareholders is based on current UK and US federal income tax law and practice and in part on representations of JPMorgan Chase Bank as Depositary for ADRs and assumes that each obligation in the deposit agreement among the Company, the Depositary and the holders from time to time of ADRs and any related agreement will be performed in accordance with its terms. The US Treasury has expressed concerns that parties to whom ADRs are pre-released may be taking actions that are inconsistent with the claiming, by US holders of ADRs, of foreign tax credits for US federal income tax purposes. Accordingly, the analysis of the creditability of UK taxes described below could be affected by future actions that may be taken by the US Treasury.
UK and US income taxes and tax treaties affecting remittance of dividends
Under the current Double Taxation (Income) Convention (the ‘Convention’) between the UK and the US, US resident individuals who are the beneficial owners of dividends on Ordinary Shares, or ADRs representing Ordinary Shares, in UK corporations are generally entitled to a tax credit payment in respect of dividends equal to one-ninth (1/9th) of the dividend paid (the ‘Tax Credit Amount’). This tax credit payment is reduced by a UK withholding (the ‘UK withholding’) of up to 15% of the gross dividend paid. Therefore, a US holder will not actually receive any payment of this credit.
US resident corporate shareholders are generally treated in the same way as individuals provided that either alone, or together with associated corporations, they do not control directly or indirectly 10% or more of the voting shares of the Company and do not constitute investment or holding companies, 25% or more of the capital of which is owned, directly or indirectly, by persons that are not individuals resident in, and are not nationals of, the US.
The UK and the US have signed a new double taxation convention (the ‘New Convention’), which must be ratified by the UK Parliament and the US Senate before its provisions enter into force. No assurance can be provided as to when the New Convention will enter into force. When the Convention ceases to apply, US resident shareholders will no longer be entitled to the Tax Credit Amount because the New Convention does not provide for that entitlement.
For US federal income tax purposes, the dividend paid and, if a US resident shareholder elects under the Convention to claim a foreign tax credit with respect to the UK withholding, the associated Tax Credit Amount are includible in gross income by US resident shareholders and, for foreign tax credit limitation purposes, are foreign source income, treated separately, together with other items of ‘passive income’ (or, in the case of certain holders, ‘financial services income’). The UK withholding is treated as a foreign income tax which may, subject to certain limitations and restrictions, be eligible for credit against a US resident shareholder’s US federal income tax liability (or deductible by such shareholders in computing their taxable income) for a US resident shareholder who elects to include the associated Tax Credit Amount in income.
The election described in the preceding paragraph will not be available under the New Convention and, accordingly, no foreign tax credit for the related UK withholding will be available under the New Convention with respect to dividends paid to US resident shareholders.
Shareholders whose holdings are effectively connected with a permanent establishment or fixed base in the UK, or who are corporations also resident in the UK for the purpose of the Convention, are not entitled to payment of the Tax Credit Amount nor are they subject to any deductions from the dividend.
Taxation on capital gains
Under the Convention (and the New Convention) each contracting state may in general tax capital gains in accordance with the provisions of its domestic law. Under present UK law, individuals who are neither resident nor ordinarily resident in the UK, and companies which are not resident in the UK will not be liable to UK tax on capital gains made on the disposal of their Ordinary Shares or ADRs, unless such Ordinary Shares or ADRs are held in connection with a trade, profession or vocation carried on in the UK through a branch or agency. A US resident shareholder will recognise capital gain or loss for US federal income tax purposes on the sale or exchange of the Ordinary Shares or ADRs in the same manner as such holder would on the sale or exchange of any other shares held as capital assets. As a result, a US resident shareholder will generally recognise capital gain or loss for US federal income tax purposes equal to the difference between the amount realised and such holder’s adjusted basis in the Ordinary
Shares or ADRs. The gain or loss will generally be US source income or loss. US resident shareholders should consult their own tax advisors about the treatment of capital gains, which may be taxed at lower rates than ordinary income for non-corporate taxpayers and capital losses, the deductibility of which may be limited.
UK inheritance tax
Under the current Double Taxation (Estates) Convention (the ‘Estate Tax Convention’) between the US and the UK, Ordinary Shares or ADRs held by an individual shareholder who is domiciled for the purposes of the Estate Tax Convention in the US, and is not for the purposes of the Estate Tax Convention a national of the UK, will generally not be subject to the UK inheritance tax on the individual’s death or on a chargeable gift of the Ordinary Shares or ADRs during the individual’s lifetime provided that any applicable US federal gift or estate tax liability is paid, unless the Ordinary Shares or ADRs are part of the business property of a permanent establishment of the individual in the UK or, in the case of a shareholder who performs independent personal services, pertain to a fixed base situated in the UK. Where the ADRs or Ordinary Shares have been placed in trust by a settlor who, at the time of settlement, was a US resident shareholder, the ADRs or Ordinary Shares will generally not be subject to UK inheritance tax unless the settlor, at the time of settlement, was not domiciled in the US and was a UK national. In the exceptional case where the Ordinary Shares or ADRs are subject both to UK inheritance tax and to US federal gift or estate tax, the Estate Tax Convention generally provides for double taxation to be relieved by means of credit relief.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Shareholder Information | 131 |
Taxation for US residents (continued)Exchange controls and other limitations affecting security holders
| (a) | There are no governmental laws, decrees or regulations in the UK restricting the import or export of capital or affecting the remittance of dividends, interest or other payments to non-resident holders of Ordinary Shares or ADRs. However, a 1.5% stamp duty reserve tax is payable upon the deposit of Ordinary Shares in connection with the creation of but not subsequent dealing in ADRs. This is in lieu of the normal 0.5% stamp duty on all purchases of Ordinary Shares. |
| | |
| (b) | There are no limitations under English law or the Company’s Memorandum and Articles of Association on the right of non-resident or foreign owners to be the registered holders of and to vote Ordinary Shares or to be registered holders of notes or debentures of Zeneca Wilmington Inc. |
Exchange rates
For the periods up to April 1999, Astra accounted for and reported its results in Swedish kronor, whereas Zeneca accounted for and reported its results in sterling. Consistent with AstraZeneca’s decision to publish its Financial Statements in US dollars, the financial information in this document has been translated from kronor and sterling into US dollars at the following applicable exchange rates:
| | SEK/USD | | USD/GBP |
| Average rates (profit and loss account, cash flow) | | | |
| 1995 | 7.1100 | | 1.5796 |
|
|
|
|
| 1996 | 6.7000 | | 1.5525 |
|
|
|
|
| 1997 | 7.6225 | | 1.6386 |
|
|
|
|
| 1998 | 7.9384 | | 1.6603 |
|
|
|
|
| 1999 | 8.2189 | | 1.6247 |
|
|
|
|
| | | | |
| End of year spot rates (balance sheet) | | | |
| 1995 | 6.6500 | | 1.5500 |
|
|
|
|
| 1996 | 6.8400 | | 1.6900 |
|
|
|
|
| 1997 | 7.8500 | | 1.6600 |
|
|
|
|
| 1998 | 8.0400 | | 1.6600 |
|
|
|
|
| 1999 | 8.5130 | | 1.6185 |
|
|
|
|
The following information relating to average and spot exchange rates used by AstraZeneca is provided for convenience:
| | SEK/USD | | USD/GBP |
| Average rates (profit and loss account, cash flow) | | | |
| 2000 | 8.9103 | | 1.5341 |
|
|
|
|
| 2001 | 10.3235 | | 1.4447 |
|
|
|
|
| 2002 | 9.8558 | | 1.4817 |
|
|
|
|
| | | | |
| End of year spot rates (balance sheet) | | | |
| 2000 | 9.5390 | | 1.4925 |
|
|
|
|
| 2001 | 10.5420 | | 1.4501 |
|
|
|
|
| 2002 | 8.7700 | | 1.6093 |
|
|
|
|
Back to Contents
| 132 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Shareholder Information |
Shareholder Information continued
Definitions
In this Annual Report and Form 20-F the following words and expressions shall, unless the context otherwise requires, have the following meanings:
| ADR | American Depositary Receipt evidencing title to an ADS |
|
| ADS | American Depositary Share representing one underlying Ordinary Share |
|
| Depositary | JPMorgan Chase Bank, as depositary under the deposit agreement pursuant to which the ADRs are issued |
|
| Directors | The Directors of the Company |
|
| Company | AstraZeneca PLC |
|
| AstraZeneca, AstraZeneca Group or the Group | The Company and its subsidiaries |
|
| Ordinary Shares | Ordinary Shares of $0.25 each in the capital of the Company |
|
| LSE | London Stock Exchange Limited |
|
| NYSE | New York Stock Exchange, Inc. |
|
| SSE | Stockholm Stock Exchange |
|
| Pound sterling, £, GBP, pence or p | References to UK currency |
|
| SEK, kronor | References to Swedish currency |
|
| UK | United Kingdom of Great Britain and Northern Ireland |
|
| US dollar, US$, USD or $ | References to US currency |
|
| US | United States of America |
|
| FDA | Food and Drug Administration of the US |
|
Figures in parentheses in tables and financial statements are used to represent negative numbers.
Except where otherwise indicated, figures included in this report relating to pharmaceutical product market sizes and market shares are obtained from syndicated industry sources, primarily IMS Health (IMS), a market research firm internationally recognised by the pharmaceutical industry. The 2002 market share figures included in this report are based primarily on data obtained from an online IMS database.
IMS data may differ from that compiled by the Group with respect to its own products. Of particular significance in this regard are the following: (1) AstraZeneca publishes its financial results on a financial year and quarterly interim basis, whereas IMS issues its data on a monthly and quarterly basis; (2) the online IMS database is updated quarterly and uses the average exchange rates for the relevant quarter; (3) IMS data from the US is not adjusted for Medicaid and similar state rebates; and (4) IMS sales data is compiled using actual wholesaler data and data from statistically representative panels of retail and hospital pharmacies, which data are then projected by IMS to give figures for national markets.
References to prevalence of disease have been derived from a variety of sources and are not intended to be indicative of the current market or any potential market for AstraZeneca’s pharmaceutical products since, among other things, there may be no correlation between the prevalence of a disease and the number of individuals who are treated for such disease.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Shareholder Information | 133 |
| | |
| | |
|
|
| Terms used in the Annual Report and Form 20-F | US equivalent or brief description |
| Accruals | Accrued expenses |
|
| Allotted | Issued |
|
| Bank borrowings | Payable to banks |
|
| Called-up share capital | Issued share capital |
|
| Capital allowances | Tax term equivalent to US tax depreciation allowances |
|
| Creditors | Liabilities/payables |
|
| Current instalments of loans | Long term debt due within one year |
|
| Debtors | Receivables and prepaid expenses |
|
| Earnings | Net income |
|
| Finance lease | Capital lease |
|
| Fixed asset investments | Non-current investments |
|
| Freehold | Ownership with absolute rights in perpetuity |
|
| Interest receivable | Interest income |
|
| Interest payable | Interest expense |
|
| Loans | Long term debt |
|
| Prepayments | Prepaid expenses |
|
| Profit | Income |
|
| Profit and loss account | Income statement/consolidated statement of income |
|
| Reserves | Retained earnings |
|
| Short term investments | Redeemable securities and short term deposits |
|
| Share premium account | Premiums paid in excess of par value of Ordinary Shares |
|
| Statement of Total Recognised Gains and Losses | Statement of Comprehensive Income |
|
| Stocks | Inventories |
|
| Tangible fixed assets | Property, plant and equipment |
|
| Turnover | Sales/revenues |
|
|
Back to Contents
| 134 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Risk Factors | |
Risk Factors
Risk of loss or expiration of patents, marketing exclusivity or trade marks
Scientific development and technological innovation is crucial if AstraZeneca is to deliver long term market success. In the pharmaceutical market, a drug, diagnostic or medical device is normally only subject to competition from alternative products, in the same therapy area, during the period of patent protection or other types of marketing exclusivity, but once patent protection or other types of marketing exclusivity has expired the product is generally open to competition from generic copy products. Products under patent protection or other types of marketing exclusivity usually generate significantly higher revenues than those not protected by patents or other types of marketing exclusivity. We believe that we have patent protection for many of our most important products.
For example, lisinopril, the active ingredient in Zestril lost protection in the US in June 2002 and in Japan, the UK and most other major markets during 2002. As anticipated, a major erosion of our sales of lisinopril products occurred during the second half of 2002.
Also, Nolvadex patent protection in the US expired in August 2002, although the FDA requested and we submitted paediatric data for Nolvadex which resulted in Nolvadex being granted six months’ marketing exclusivity until February 2003.
Increasingly, manufacturers of generic pharmaceutical products whether based in developing countries, such as those in Asia, or elsewhere in the world seek to challenge our patents or other types of marketing exclusivity in order to allow access to the market for their own generic products.
For example, AstraZeneca was involved in litigation in the US during 2002 relating to omeprazole, the active ingredient in Losec/Prilosec, concerning the infringement of certain patents, including formulation patents, by four generic manufacturers. The US Court for the Southern District of New York upheld the validity of two of these patents, ruled that three generic companies infringed the patents, but that one did not. The infringement and non-infringement decisions are all under appeal but, in the meantime, the fourth generic company launched its generic omeprazole in the US market in December 2002. Other patent litigation relating to omeprazole is proceeding or pending in several countries.
Trade mark protection for our products is also an important element of our overall product marketing programmes. Combined with patent protection or other types of marketing exclusivity, products protected by a valid trade mark usually generate significantly higher revenues than those not protected by a trade mark. We believe that we have trade mark protection for many of our most important products. However, trade mark protection may expire or be challenged by third parties.
The expiration or loss of certain patents, marketing exclusivity or trade marks could have an adverse effect on pricing and sales with respect to these products and, consequently, could result in a material adverse effect on AstraZeneca’s financial condition and results of operations.
Impact of fluctuations in exchange rates
The results of AstraZeneca’s operations are accounted for in US dollars. Approximately 57% of our 2002 sales were in the Americas (comprised of the US, Canada and Latin America) with a significant proportion of that figure being in respect of US sales. The US is, and is expected to remain, our largest and potentially fastest growing major market. Sales in certain other countries are also in US dollars, or in currencies whose exchange rates are linked to the US dollar. Major components of our cost base are, however, located in Europe, where an aggregate of approximately 60% of our employees are based. Movements in the exchange rates used to translate foreign currencies into US dollars may therefore have a material adverse effect on AstraZeneca’s financial condition and results of operations.
Certain subsidiaries of AstraZeneca import and export goods and services in currencies other than their own functional currency, although we minimise this practice. The results of such subsidiaries could, therefore, be affected by currency fluctuations arising between the transaction dates and the settlement dates for those transactions. We hedge these exposures through financial instruments in the form of forward contracts and currency swaps. The notional principal amount of financial instruments used to hedge these exposures, principally forward foreign exchange contracts and purchased currency options, at 31 December 2002 was $47 million. We have policies that seek to mitigate the effect of exchange rate fluctuations on the value of foreign currency cash flows and in turn their effects on the results of the various subsidiaries, but do not
seek to remove all such risks. In general, a unilateral strengthening of the US dollar adversely affects our results whereas a weakening of the US dollar is generally favourable. We cannot ensure that exchange rate fluctuations will not have a material adverse effect on AstraZeneca’s financial condition and results of operations in the future.
Risk that R&D will not yield new products that achieve commercial success
As a result of the complexities and uncertainties associated with pharmaceutical research, it cannot be ensured that compounds currently under development will achieve success in laboratory, animal or clinical trials and ultimately be granted the regulatory approvals needed to market such products successfully. For example, in 2002 our anti-cancer drug, Iressa, unexpectedly failed in clinical trials to show any benefit when used in combination with the most common chemotherapy treatments and we discontinued our development of AZD7545, a potential anti-diabetic, due to failure to meet our target profile. Development of a number of other drugs was also discontinued during 2002 for the same reason: these included ZD9331 (a direct acting anti-folate for potential treatment of cancers), D5522 (an intranasal steroid for the potential treatment of rhinitis) and NAD-299 (a potential treatment for anxiety and depression). There can be no absolute assurances regarding the development and commercial success of any of the products in our current pipeline. The commercial success of pipeline products is of particular importance to us in view of the recent and anticipated expiry of patent protection in major markets for a number of our key current products in the 2002-2003 period.
Competition, price controls and price reductions
The principal markets for our pharmaceutical products are the Americas, the countries of the European Union and Japan. These markets are highly competitive. We compete in all of them, and elsewhere in the world, against major prescription pharmaceutical companies which, in many cases, are able to match or exceed the resources which we have available to us, particularly in the areas of R&D and marketing investment. Recent industry consolidation has resulted in the formation of a small number of very large companies with which we compete as well. Some of our most important products for future growth, such as Crestor, will compete directly with similar products marketed by
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Risk Factors | 135 |
some of these companies. Increasingly, we also compete directly with biotechnology companies and companies which manufacture generic versions of our products following the expiry or loss of patent or other marketing exclusivity.
In most of the principal markets in which we sell our products, there is continued economic, regulatory and political pressure to limit the cost of pharmaceutical products. Certain groups have been involved in exerting price pressure on pharmaceutical companies to ensure medicines are affordable to those who need them.
Currently there is no direct government control of prices for non-government sales in the US. In 1990, however, federal legislation was enacted which required drug manufacturers to agree to substantial rebates in order for the manufacturer’s drugs to be reimbursed by state Medicaid programmes, and an additional rebate if manufacturer price increases after 1990 exceed the increase in inflation. In addition, certain states have taken action to require further manufacturer rebates on Medicaid drug utilisation and for other state pharmaceutical assistance programmes. Congress also has enacted statutes that place a ceiling on the price manufacturers may charge US government agencies, thereby causing a substantial discount, as well as establishing a minimum discount (comparable to the Medicaid rebate) on manufacturers’ sales to certain clinics and hospitals that serve the poor and other populations with special needs. These government initiatives together with competitive market pressures have contributed to restraints on realised prices.
Pending legislation in the US may also affect the pricing of and access to pharmaceutical products. If drug importation into the US market from other countries with lower prices becomes a reality, parallel import activity will affect realised prices. On the other hand, outpatient prescription drug coverage could improve access to pharmaceutical products for senior citizens, albeit at potentially lower realised prices.
In addition, realised prices are being depressed by pressure from managed care and institutional purchasers who use cost considerations to restrict the sale of preferred drugs that their physicians may prescribe as well as other competitive activity. Such limited lists or formularies may force manufacturers either to reduce prices or be excluded from the list, thereby losing all the sales revenue
from patients covered by that formulary. The use of strict formularies by institutional customers is increasing rapidly in response to the current cost containment environment, resulting in lower margins on such sales.
Some governments in Europe, notably Italy and Spain, set price controls having regard to the medical, economic and social impact of the product. In other European countries, primarily Germany, the UK, the Netherlands and, more recently, France, governments are exerting a strong downward pressure on prices by incentives and sanctions to encourage doctors to prescribe cost-effectively. Efforts by the European Commission to harmonise the disparate national systems have met with little immediate success, leaving the industry exposed to ad hoc national cost containment measures on prices and the consequent parallel trading of products from markets with prices depressed by governments into those where higher prices prevail.
There is formal central government control of prices in Japan. New product prices are determined primarily by comparison with existing products for the same medical condition. All existing products are subject to a price review at least every two years. Regulations introduced in 2000 included provisions allowing a drug’s price to be set according to the average price of the product in four major countries (the US, the UK, Germany and France).
Taxation
The UK is party to various double tax treaties with foreign jurisdictions which enable AstraZeneca’s revenues and capital gains to escape a double tax charge to both UK and foreign jurisdiction tax. If any of these double tax treaties should be withdrawn or amended, or should any member of the AstraZeneca Group become involved in taxation disputes with any tax authority, such withdrawal, amendment or a negative outcome of such disputes could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
Risk of substantial product liability claims
Given the widespread impact ethical prescription drugs may have on the health of large patient populations, pharmaceutical and medical device companies have, historically, been subject to large product liability damages claims, settlements and awards for injuries allegedly caused by the use of their products. Substantial product liability claims that are not covered by
insurance could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
Risk of reliance on third parties for supplies of materials and services
Like most, if not all, major prescription pharmaceutical companies, in some of its key business operations, such as the manufacture, formulation and packaging of products, AstraZeneca relies on third parties for the timely supply of specified raw materials, equipment, contract manufacturing, formulation or packaging services and maintenance services. Although we actively manage these third party relationships to ensure continuity of supplies on time and to our required specifications, some events beyond our control could result in the complete or partial failure of supplies or in supplies not being delivered on time. Any such failure could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
Risk of delay to new product launches
AstraZeneca’s continued success depends on the development and successful launch of innovative new drugs. The anticipated launch dates of major new products have a significant impact on a number of areas of our business including investment in large clinical trials, the manufacture of pre-launch stocks of the products and the timing of anticipated future revenue streams from commercial sales of the products. Any delay to the anticipated launch dates may therefore impact AstraZeneca’s business and operations in a number of ways. For example, we had expected our new statin for the treatment of lipid disorders, Crestor, to be launched in the US in the second half of 2002. However, the approval of products in this class has been subject to additional regulatory scrutiny partly as a result of the previous withdrawal from the market of cerivastatin. Although Crestor received marketing approval in the Netherlands in November 2002, launch in the US is now expected in the latter part of 2003 subject to the FDA being satisfied by additional trial data to be submitted by AstraZeneca in Q1 of 2003. Significant delay to the anticipated launch dates of new products could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
Difficulties of obtaining government regulatory approvals for new products
AstraZeneca is subject to strict controls on the manufacture, labelling, distribution and marketing of pharmaceutical products. The
Back to Contents
| 136 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Risk Factors |
Risk Factors continued
requirement to obtain regulatory approval based on safety, efficacy and quality before such products may be marketed in a particular country and to maintain and to comply with licences and other regulations relating to their manufacture are particularly important. The submission of an application to a regulatory authority does not guarantee that approval to market the products will be granted. The countries that constitute material markets for our pharmaceutical products include the US, the countries of the European Union and Japan. Approval of such products is required by the relevant regulatory authority in each country, although in Europe, single marketing authorisation can govern the approval of products throughout the European Union through a centralised procedure. In addition, each jurisdiction has very high standards of regulatory approval and, consequently, in most cases, a lengthy approval process. Furthermore, each regulatory authority may impose its own requirements and may refuse to grant, or may require additional data before granting, an approval even though the relevant product has been approved in another country.
Risk of failure to observe ongoing regulatory oversight
AstraZeneca’s products are only licenced following exhaustive regulatory approval processes. Once a product is licenced it is subject to ongoing control and regulation such as the manner of its manufacture distribution and marketing. Regulatory authorities have wide-ranging administrative powers to deal with any failure to comply with their ongoing regulatory oversight. These powers include withdrawal of a licence approval previously granted, product recalls, seizure of products and other sanctions for non-compliance. Regulatory sanction following a failure to comply with such ongoing regulatory oversight could have a material adverse effect on AstraZeneca’s financial condition and results of operations.
Environmental liabilities
AstraZeneca has environmental liabilities at some currently or formerly owned, leased and third party sites in the US as described in more detail on page 102. There is no reason for us to believe that current and expected expenditure and risks occasioned by these circumstances are likely to have a material adverse effect on AstraZeneca’s financial position and results of operations although they could, to the extent that they exceed applicable provisions, have a material adverse effect on AstraZeneca’s financial position and results of operations for the relevant period. In
addition, a change in circumstances (including a change in applicable laws or regulations) may result in such a material adverse effect. Although we take great care to ensure that we operate our business at all of our sites within all applicable environmental laws, regulations, licences and permits, a significant environmental incident for which we were responsible could result in AstraZeneca being liable to pay compensation, fines or remediation costs. In some circumstances, such liability could have a material adverse effect on AstraZeneca’s financial position and results of operations.
Risks associated with forward-looking statements
This report contains certain forward-looking statements about AstraZeneca. Although we believe our expectations are based on reasonable assumptions, any forward-looking statements may be influenced by factors that could cause actual outcomes and results to be materially different from those predicted. Forward-looking statements are identified in this report, by using the words ‘anticipates’, ‘believes’, ‘expects’, ‘intends’ and similar expressions in such statements. These forward-looking statements are subject to numerous risks and uncertainties. Important factors that could cause actual results to differ materially from those in forward-looking statements, certain of which are beyond our control, include, among other things: the loss or expiration of patents, marketing exclusivity or trade marks; exchange rate fluctuations; the risk that R&D will not yield new products that achieve commercial success; the impact of competition, price controls and price reductions; taxation risks; the risk of substantial product liability claims; the impact of any failure by third parties to supply materials or services; the risk of delay to new product launches, the difficulties of obtaining and maintaining governmental approvals for products; and the risk of environmental liabilities.
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | AstraZeneca Code of Conduct | 137 |
AstraZeneca Code of Conduct
Introductory Message from the Chief Executive
The reputation of the AstraZeneca Group and the trust and confidence of those with whom it deals are of great importance to its business. It is essential that AstraZeneca maintains high ethical standards in its dealings with all those with whom it is involved. All employees are required to comply with the letter and spirit of the Code of Conduct and with the detailed standards issued in support of it. Taken together, the policy and standards comprise the Group’s Code of Conduct which was approved by the Board of Directors in April 2000.
Tom McKillop
Policy
The Group requires its companies, and their employees, to observe high standards of integrity and honesty and act with due skill, care, diligence and fairness in the conduct of business. To this end all Group companies, and their employees, are required to comply with the laws of all countries in which they operate and with the high ethical standards detailed by the Group in support of this policy.
Compliance
It is the responsibility of management to ensure that the Group Code of Conduct and standards are communicated, understood and acted upon. They must positively promote them by personal example and are not entitled to permit exceptions to the required behaviour.
All employees should familiarise themselves with the Code of Conduct and must comply with it. Failure to act in compliance with the Code is likely to result in disciplinary action against both the employee committing the breach and others who condone it.
The Standards set out in the Code are general and do not address each and every situation that may confront employees in markets around the world. Guidance on the application of the Code to particular situations should be sought from management. In addition, Legal Department and Group Internal Audit are available on a confidential basis as independent sources of advice. Where confidential phone lines are available, these should be used to raise issues of concern. So long as this is done in good faith,
the Group assures individual employees who raise issues that they will be protected from any adverse impact on their employment as a result.
Standards of Conduct
Business practices
Group companies, and their employees, should comply with the laws of all countries in which they operate, with appropriate international and national industry codes of practice and with the high ethical standards specified by the Group.
It is the responsibility of all employees to ensure, by taking advice where appropriate, that they are fully aware of all relevant laws, practices and codes of practice. While this standard applies without exception, particular areas where compliance must be ensured are laws concerned with competition, employment, new product research and development, manufacturing, marketing and selling and safety, health and the environment.
Employees should ensure that, within their sphere of business activity, Group companies carry out their contractual obligations in a proper and timely manner and are not in breach of contract.
Business practice, and what amounts to improper conduct, varies from country to country and from industry to industry. All employees will comply with (a) the high ethical standards specified by the Group (b) any overall AstraZeneca Code published relating to business practices and (c) any international and national codes of practice applicable to the conduct of business in each environment.
Gifts, entertainment and personal favours may only be offered to a third party if they are consistent with customary business practice and not in contravention of any applicable law or code of practice.
No employee should seek or accept a gift, entertainment or personal favour which might reasonably be believed to have a significant influence on business transactions. An offer of entertainment must not be accepted unless the offer is within the bounds of accepted business hospitality. Gifts which do not meet the above criteria should be reported to management who shall determine how they shall be dealt with.
Group funds should not be used in payments, direct or indirect, to government officials,
people participating in government bodies, employees of state organisations or representatives of political parties, for unlawful or improper purposes.
Equal opportunities
All employees should be treated with equal respect and dignity and should be provided with equality of opportunity to develop themselves and their careers.
AstraZeneca values the individuality, diversity and creative potential that every employee brings to its business and supports the continuous development of their skills and abilities.
Judgements about people for the purpose of recruitment, development or promotion should be made solely on the basis of a person’s ability and potential in relation to the needs of the job and should take no account of any matter not relevant to the performance of that job. Overall, success and advancement within the Group should depend solely on personal ability and work performance.
In some countries these principles may be modified by national legal requirements for positive discrimination.
Personal harassment
Conduct involving the harassment of any employee of the Group, its suppliers or customers is unacceptable. In particular, sexual harassment will not be tolerated.
Any person who believes they have been personally harassed should report the incident and circumstances to their immediate manager or personnel manager or other senior manager who will arrange for it to be investigated impartially and confidentially.
AstraZeneca is fully supportive of the principles set forth in the UN Declaration of Human Rights. These include freedom from torture and arbitrary arrest, the right to a fair trial and equality before the law.
Political contributions
Any political contributions by Group companies must be lawful and approved under procedures laid down by the board or governing body of the company concerned.
Approval should not be given to any political contributions which, by their scale or affiliation, might embarrass the Group. The Group’s accounting procedures require any
Back to Contents
| 138 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | AstraZeneca Code of Conduct |
AstraZeneca Code of Conduct continued
political contributions to be reported to Group headquarters as part of the annual consolidation of results.
Conflicts of interest
Employees dealing with the Group’s business must act in the best interests of the Group and must disregard any personal preference or advantage.
Employees should avoid entering into situations in which their personal, family or financial interests may conflict with those of the AstraZeneca Group. Where any potential conflict of interest may arise, the employee should declare that interest and seek advice from senior management.
Examples of conflict that must be declared and resolved include:
| > | having a family interest in a transaction with AstraZeneca or one of its subsidiaries (the Company) or any supplier or customer; |
| | |
| > | hiring of a family member in any capacity; |
| | |
| > | having an interest in a competitor, supplier or customer of the Company; |
| | |
| > | having an interest in an organisation that has, or seeks to do business with the Company; |
| | |
| > | acquiring an interest in property (such as real estate, patent rights or securities) where the Company has, or might have, an interest. |
These examples do not extend to normal financial investments in publicly quoted companies.
Insider information
Employees must not use confidential information obtained through their employment for personal gain.
It is AstraZeneca policy, and in certain countries a legal requirement carrying criminal sanctions, that employees in possession of confidential ‘price sensitive’ information (in relation to securities) must not make use of such information to deal in securities of AstraZeneca or provide such information to third parties for that purpose. The same considerations apply in relation to confidential ‘price sensitive’ information relating to other companies and dealing in their securities.
Group property and resources
Group resources should be kept securely and should only be applied for the proper advancement of its business and not for personal gain.
Individuals expending Group resources should recognise that they owe a duty of care to the shareholders of the Group, who are its ultimate owners. Commitments and expenditure should only be such as could be justified to shareholders if the facts were known.
Group resources include not only tangible assets such as materials, equipment and cash, but also intangible assets such as computer systems, trade secrets and confidential information. Employees must observe Group and local guidelines concerning the classifying and handling of documents and electronic data. The storage of personal data in an electronic medium may be governed by laws with which relevant employees should familiarise themselves.
Information generated within the Group, including research and development and manufacturing data, costs, prices, sales, profits, markets, customers and methods of doing business, is the property of the Group and should not, unless legally required, be disclosed outside the Group without proper authority.
Group policies, delegated authorities and reserved powers
Group employees are expected to make themselves aware of and comply with the letter and spirit of all Group policies and with the reserved powers and delegated authorities established by the Board from time to time. Copies of these are available on the Company’s intranet site.
The freedoms which individuals have to carry out their jobs should be exercised within both the letter and spirit of Group policies and procedures, reserved powers and delegated authorities. These are designed to empower people to carry out their responsibilities within a necessary framework of corporate control and legal responsibility but are not so voluminous as to prescribe appropriate action in every circumstance. In the exercise of their authorities individuals must bear Group and legal entity requirements in mind and must surface problems, and consult on issues, which have significant Group implications. When considering whether an issue does
require reference to another authority, the overall substance of the issue must be considered and when sharing an issue with another authority all facts relevant to a decision must be fully and fairly presented.
June 2000
Back to Contents
| | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Additional Information | 139 |
Additional Information
History and development of the Company
AstraZeneca PLC was incorporated in England and Wales on 17 June 1992 under the Companies Act 1985. It is a public limited company domiciled in the UK. The Company’s registered number is 2723534 and its registered office is at 15 Stanhope Gate, London W1K 1LN (telephone + 44 (0)20 7304 5000). From February 1993 until April 1999, the Company was called Zeneca Group PLC. On 6 April 1999, the Company changed its name to AstraZeneca PLC.
The Company was formed when the pharmaceutical, agrochemical and specialty chemical businesses of Imperial Chemical Industries PLC were demerged in 1993. In 1999, the Company sold the specialty chemical business. Also in 1999, the Company merged with Astra AB of Sweden. In 2000, it demerged the agrochemical business and merged it with the similar agribusiness of Novartis AG to form a new company called Syngenta AG.
The Company owns and operates numerous R&D, production and marketing facilities worldwide. Its corporate headquarters are at 15 Stanhope Gate, London, W1K 1LN and its R&D headquarters are at SE-151 85 Södertälje, Sweden.
Memorandum and Articles of Association
Objects
As is typical of companies registered in England and Wales, the Company’s objects, which are detailed in the Memorandum of Association, are broad and wide-ranging and include manufacturing, distributing and trading pharmaceutical products.
Directors
Subject to certain exceptions, Directors do not have power to vote at Board Meetings on matters in which they have a material interest.
The quorum for meetings of the Board of Directors is a majority of the full Board, of whom at least four must be Non-Executive Directors. In the absence of a quorum, the Directors do not have power to determine compensation arrangements for themselves or any member of the Board.
The Board of Directors may exercise all the powers of the Company to borrow money. Variation of these borrowing powers would require the passing of a special resolution of the Company’s shareholders.
Directors are not required to retire at a particular age.
Directors are required to beneficially own Ordinary Shares in the Company of an aggregate nominal amount of $125. At present, this means they must own at least 500 shares.
Rights, preferences and restrictions attaching to shares
The share capital of the Company is divided into 2,400,000,000 Ordinary Shares with a nominal value of $0.25 each and 50,000 Redeemable Preference Shares with a nominal value of £1.00 each. The rights and restrictions attaching to the Redeemable Preference Shares differ from those attaching to Ordinary Shares as follows:
| > | the Redeemable Preference Shares carry no rights to receive dividends; |
| | |
| > | the holders of Redeemable Preference Shares have no rights to receive notices of, attend or vote at general meetings except in certain limited circumstances; they have one vote for every 50,000 Redeemable Preference Shares held; |
| | |
| > | on a distribution of assets of the Company, on a winding-up or other return of capital (subject to certain exceptions), the holders of Redeemable Preference Shares have priority over the holders of Ordinary Shares to receive the capital paid up on those shares; and |
| | |
| > | subject to the provisions of theCompanies Act 1985, the Company has the right to redeem the Redeemable Preference Shares at any time on giving not less than seven days’written notice. |
Action necessary to change the rights of shareholders
In order to vary the rights attached to any class of shares, the consent in writing of the holders of three quarters in nominal value of the issued shares of that class or the sanction of an extraordinary resolution passed at a general meeting of such holders is required.
Annual general meetings and extraordinary general meetings
Annual general meetings and extraordinary general meetings where a special resolution is to be passed or a Director is to be appointed require 21 clear days’ notice to shareholders. All other extraordinary general meetings require 14 clear days’ notice.
For all general meetings, a quorum of two shareholders present in person or by proxy is required.
Shareholders and their duly appointed proxies and corporate representatives are entitled to be admitted to general meetings.
Limitations on the rights to own shares
There are no limitations on the rights to own shares.
ShareGift
AstraZeneca welcomes and values all its shareholders, no matter how many or how few shares they own. However, shareholders who have only a small number of shares whose value makes it uneconomic to sell them, either now or at some stage in the future, may wish to consider donating them to charity through ShareGift, an independent charity share donation scheme. One of the advantages of the scheme is that there is no gain or loss for capital gains tax purposes on gifts of shares through ShareGift and it may now also be possible to obtain income tax relief on the donation. Further information about ShareGift can be found on its website, www.sharegift.org, or by contacting ShareGift on 020 7337 0501 or at 46 Grosvenor Street, London W1K 3HN. More information about the tax position on gifts of shares to ShareGift can be obtained from the Inland Revenue whose website address is www.inlandrevenue.gov.uk. The share transfer form needed to make a donation may be obtained from the AstraZeneca Registrar, Lloyds TSB Registrars whose address can be found on page 129. ShareGift is administered by The Orr Mackintosh Foundation, registered charity number 1052686.
The Unclaimed Assets Register
AstraZeneca supplies unclaimed dividend data to the Unclaimed Assets Register (UAR) which provides investors who have lost track of shareholdings with an opportunity to search the UAR’s database of unclaimed financial assets on payment of a small, fixed fee.The UAR donates part of the search fee to charity. The UAR can be contacted at Leconfield House, Curzon Street, London W1J 5JA and at www.uar.co.uk.
Back to Contents
| 140 | AstraZeneca Annual Report and Form 20-F 2002
www.astrazeneca.com | Cross Reference to Form 20-F |
Cross Reference to Form 20-F
The information in this document that is referenced on this page is included in the Annual Report on Form 20-F for 2002 (2002 Form 20-F) and is filed with the Securities and Exchange Commission (SEC). The 2002 Form 20-F is the only document intended to be incorporated by reference into any filings by AstraZeneca under the Securities Act of 1933, as amended. References to major headings include all information under such major headings, including subheadings. References to subheadings include only the information contained under such subheadings. Graphs are not included unless specifically identified opposite. The 2002 Form 20-F has not been approved or disapproved by the SEC nor has the SEC passed comment upon the accuracy or adequacy of the 2002 Form 20-F. The 2002 Form 20-F filed with the SEC may contain modified information and may be updated from time to time.
| Item | | | Page |
|
|
|
|
| 3 | Key Information | |
|
|
|
|
| | A. | Selected financial data | |
| | | Financial Highlights | 2 |
| | | Group Financial Record | 123 |
| | | Shareholder Information | 126 |
| | D. | Risk factors | 134 |
| 4 | Information on the Company | |
|
|
|
|
| | A. | History and development of the Company | 139 |
| | | Note 11 – Tangible fixed assets | 78 |
| | | Note 26 – Acquisitions of subsidiaries and purchases of minority interests | 90 |
| | | Note 28 – Disposals | 91 |
| | B. | Business overview | |
| | | Operational Review | 8 |
| | C. | Organisational structure | |
| | | Directors’ Report – Principal activities | 44 |
| | | Note 1 – Composition of the Group | 65 |
| | | Principal Subsidiaries, Joint Ventures and Associates | 112 |
| | D. | Property, plants and equipment | |
| | | Operational Review – Main Facilities | 26 |
| 5 | Operating and Financial Review and Prospects | |
|
|
|
|
| | A-D. | Financial Review | 30 |
| 6 | Directors, Senior Management and Employees | |
|
|
|
|
| | A. | Directors and senior management | |
| | | Board of Directors and Officers of the Company | 6 |
| | B. | Compensation | |
| | | Directors’ Remuneration Report | 49 |
| | C. | Board practices | |
| | | Board of Directors and Officers of the Company | 6 |
| | | Directors’ Remuneration Report | 49 |
| | | Directors’ Report | 44 |
| | D. | Employees | |
| | | Note 10 – Segment information, employees | 76 |
| | | Directors’ Report – Employees | 47 |
| | E. | Share ownership | |
| | | Directors’ Remuneration Report – Directors’ Interests in Shares | 53 |
| 7 | Major Shareholders and Related Party Transactions | |
|
|
|
|
| | A. | Major shareholders | |
| | | Shareholder Information – Major shareholdings | 128 |
| | B. | Related party transactions | |
| | | Shareholder Information – Related party transactions | 129 |
| | | Note 36 – Statutory and other information | 107 |
| 8 | Financial Information | |
|
|
|
|
| | A. | Consolidated statements and other financial information | |
| | | Financial Statements (excluding Directors’ responsibilities on page 56 and Auditor’ s opinion on page 57) | 58 |
| | B. | Significant changes | n/a |
| 9 | The Offer and Listing | |
|
|
|
|
| | A4. | Price history of stock listed | |
| | | Shareholder Information | 126 |
| | C. | Markets | |
| | | Shareholder Information | 127 |
| 10 | Additional Information | |
|
|
|
|
| | B. | Memorandum and Articles of Association | 139 |
| | C. | Material contracts | n/a |
| | D. | Exchange controls | 131 |
| | E. | Taxation | 130 |
| | H. | Documents on display | 129 |
| | I. | Subsidiary information | 112 |
| 11 | Quantitative and Qualitative Disclosures about Market Risk | |
|
|
|
|
| | Financial Policies – Treasury | 35 |
| 13 | Defaults, Dividend Arrearages and Delinquencies | n/a |
|
|
|
|
| 14 | Material Modifications to the Rights of Security Holders and Use of Proceeds | n/a |
|
|
|
|
| 15 | Controls and Procedures | |
|
|
|
|
| | Directors’ Report – Audit Committee, Internal Controls and Management of Risk | 46 |
| 18 | Financial Statements | |
|
|
|
|
| | Financial Statements (excluding Directors’ responsibilities on page 56 and Auditor’s opinion on page 57) | 58 |
|
|
|
|
Back to Contents
Back to Contents
www.astrazeneca.com
Registered office and corporate headquarters:
AstraZeneca PLC
15 Stanhope Gate
London W1K 1LN
UK
Tel: +44 (0)20 7304 5000
Fax: +44 (0)20 7304 5183
R&D headquarters address:
AstraZeneca R&D Södertälje
SE-151 85 Södertälje
Sweden
Tel: +46 (0)8 553 260 00
Fax: +46 (0)8 553 290 00
Investor relations contacts:
UK and Sweden: As above or e-mail:
investor-relations@astrazeneca.com
US:
Investor Relations
AstraZeneca LP
1800 Concord Pike
PO Box 15438
Wilmington
DE 19850-5438
US
Tel: +1 (302) 886 3000
Fax: +1 (302) 886 2972
Registrar and transfer office:
Lloyds TSB Registrars
The Causeway
Worthing
West Sussex
BN99 6DA
UK
Tel (in the UK): 0870 600 3956
Tel (outside the UK):
+44 (0)121 433 8000
Swedish securities registration centre:
VPC AB
PO Box 7822
SE-103 97 Stockholm
Sweden
Tel: +46 (0)8 402 9000
US depositary:
JPMorgan Chase Bank
PO Box 43013
Providence
RI 02940-3013
US
Tel (toll free in the US): 888 697 8018
Tel: +1 (781) 575 4328
-->