AstraZeneca PLC
Legal & Secretary’s Department
2 Kingdom Street
London
W2 6BD
For the attention of Adrian Kemp
By fax 020 7604 8151 & by post
28 March 2012
Dear Ladies and Gentlemen
BUREAU VERITAS STATEMENT OF ASSURANCE FOR ANNUAL REPORT AND FORM 20-F INFORMATION 2011
In connection with the anticipated filing by AstraZeneca PLC (“AstraZeneca”) of certain registration statements with the US Securities and Exchange Commission, Bureau Veritas hereby authorizes AstraZeneca to refer to Bureau Veritas’s external assurance on corporate responsibility related information as stated on pages 48 and 112 and highlighted as identified on the pages of the Annual Report and Form 20-F Information for the fiscal year ended December 31, 2011 (the “Annual Report”) annexed as Exhibit A, which is incorporated by reference in the registration statements No. 33-83774 for AstraZeneca and Zeneca Wilmington Inc. and No. 333-145848, No. 333-114165 and No. 333-171306 for AstraZeneca, each on Form F-3, and in the registration statements No. 333-09060, No. 333-09062, No. 33-65362, No, 33-65366, No. 333-12310, No. 333-12426, No. 333-12428, No. 333-13328, No. 333-13918, No. 333-124689, No. 333-152767 and No. 333-170381 on Form S-8 for AstraZeneca.
Our authorization is subject to AstraZeneca’s acknowledgement and agreement that:
| 1) | Bureau Veritas has undertaken an independent review of the corporate responsibility information disclosed in the Annual Report and provided an opinion as to the accuracy and reliability of the information subject to the scope, objectives and limitations defined in the full assurance statement posted on AstraZeneca’s responsibility website; |
| 2) | AstraZeneca acknowledges and agrees that Bureau Veritas shall not be deemed an “Expert” in respect of AstraZeneca’s securities filings, and AstraZeneca agrees that it shall not characterize Bureau Veritas as such; and |
| 3) | AstraZeneca accepts full responsibility for the disclosure of all information and data, including that relating to Bureau Veritas, set forth in the Annual Report as filed with the SEC and agrees to indemnify Bureau Veritas from any third party claims that may arise therefrom. |
Please indicate your agreement to the foregoing by signing in the space indicated below. Our authorization will not become effective until accepted and agreed by AstraZeneca.
Very truly yours,
/s/ Tracy Oates
Tracy Oates, Practice Manager (Sustainability Services)
For and on behalf of Bureau Veritas UK Ltd
ACCEPTED AND AGREED
this 28 day of March 2012
AstraZeneca PLC
/s/ Adrian Kemp
Name: Adrian Kemp
Title: Company Secretary
Exhibit A
Responsible Business
We are committed to acting
responsibly and to the sustainabledevelopment of our business

In this section we describe how we are working to deliver business success responsibly, including summary information about our commitment and performance in certain key areas. Further information about these areas and others is available on our website, astrazeneca.com/responsibility.
Introduction
At AstraZeneca, we are dedicated to the research, development, manufacture and marketing of medicines that make a difference in healthcare. For us, this is at the core of our responsibility to our stakeholders and to society. Successful pharmaceutical innovation, delivered responsibly, brings benefits for patients, creates sustainable value for shareholders and contributes to the economic development of the communities we serve.
Previous sections have described our strategic business priorities and how we are enhancing our R&D, expanding our footprint in Emerging Markets, continuing our efforts to source innovation from outside AstraZeneca and increasingly working in partnerships that broaden the base for success in improving healthcare. At the same time, we continue to drive efficiency and effectiveness across the organisation, including increased outsourcing to a diverse range of strategic suppliers.
All of these efforts are underpinned by our continued commitment to the sustainable development of our business which delivers value for our stakeholders and for us. To that end, our responsible business objectives must be closely aligned to, and support delivery of, our business strategy. Our new Responsible Business Plan, published in April 2011, provides our framework for delivering business success responsibly. It puts at the top of our agenda those areas most impacted by our strategic priorities and which are therefore key enablers of our business strategy.
This means a specific focus on:
| > | Clinical trials and animal research – underpinning our drive for innovation with sound ethical R&D practice worldwide |
| > | Sales and marketing practices – driving consistently high ethical standards to promote our medicines responsibly worldwide |
| > | Access to healthcare – exploring ways of increasing access to healthcare for underserved patient populations in a sustainable way |
| > | Human rights – making sure that we continue to develop and drive a consistent approach across all our activities |
| > | Diversity and inclusion – ensuring that diversity, in its broadest sense, is appropriately represented in our leadership, our workforce and our thinking. See the People section from page 40 for more information |
| > | Suppliers – working only with organisations who embrace ethical standards that are consistent with our own. |

| | |
| AstraZeneca Annual Report and Form 20-F Information 2011 | | Delivering our strategy Responsible Business47 |
Delivering our strategy
As well as managing specific responsible business challenges associated with the changes to our strategy, we are maintaining focus on other aspects of our responsibility:
| > | Employee safety, health and wellbeing |
A summary of these areas of focus is provided in this section. The full Responsible Business Plan is available on our website, astrazeneca.com/responsibility.
Accountabilities and responsibilities
The Board is responsible for our Responsible Business framework and Non-Executive Director, Dame Nancy Rothwell, oversees implementation and reporting to the Board.
The SET and senior managers throughout the Group are accountable for operating responsibly within their areas taking into account national, functional and site issues and priorities. Line managers are accountable for ensuring that their teams understand the requirements and that people are clear about what is expected of them as they work to achieve AstraZeneca’s business goals. Individually, everyone has a responsibility to integrate sustainability considerations into their day-to-day decision making, actions and behaviours.
Our dedicated Global Corporate Responsibility Team (CR Team) works together with the SET areas across the business to ensure that responsible business risks and opportunities are identified and managed appropriately, in line with our strategic business objectives.
Responsible business governance
During 2011, we established the Responsible Business Council (the Council) – a cross functional team of senior leaders, chaired by our EVP, HR and Corporate Affairs. The Council will meet twice a year and their agenda is focused on driving long-term value creation by agreeing, among other things:
| > | Responsible Business priorities for the Group in line with strategic business objectives |
| > | Strategy and overseeing performance as measured by short- and long-term objectives, targets and key performance indicators recorded in the Responsible Business Plan |
| > | Appropriate policy positions to support AstraZeneca’s business objectives and reputation management. |
The Council is supported by a newly established Responsible Business Working Group (the Working Group) of SET area representatives and our CR Team, chaired by the Head of Corporate Affairs Strategy, Brand and CR. Among other things, the Working Group continuously reviews external issues with the potential to impact AstraZeneca and, as appropriate, prepares management and measurement proposals for the Council’s consideration. The Working Group will meet four times a year.
External engagement and benchmarking
Stakeholder dialogue was critical in the development of our Responsible Business Plan and we continue to engage with our stakeholders to ensure that our strategy development and risk management take account of their feedback.
During 2011, we developed a global framework for multi-stakeholder engagement to provide a consistent, best practice-based approach across AstraZeneca and to improve how we capture feedback from around the world.
We held a number of multi-stakeholder events throughout the year. These included an event specifically for key Socially Responsible Investor (SRI) contacts. The agenda reflected areas of interest expressed by the SRI community and focused on growth in Emerging Markets, sustaining innovation in R&D and managing environmental impact. Feedback following the event was generally positive and the opportunity to interact with senior AstraZeneca leaders was particularly welcomed by the participants. Discussion centred on how we are managing the potential challenges to sustainability as we expand our business and drive R&D productivity. The SRIs also highlighted that they wanted more information on our access to healthcare strategy.
We also hosted a discussion on global product security during the year. This brought together representatives from diverse organisations, geographies and perspectives to gain insights into what our stakeholders expect from us in the area of product security, and to gain new perspectives on how we can reduce the threat that counterfeiting and illegal trade pose to global health. Attendees included NGOs, supply chain partners, academics and enforcement professionals from both the developed and developing world. The discussion focused mainly on the critical need for collaboration between all the key players in this area, including the role that AstraZeneca can play, working with other manufacturers and influencing broader stakeholders regarding policy and regulation, enforcement and activities to influence patient understanding and behaviour. Product security is an inherent part of our commitment to patient safety, which continues to be a fundamental consideration.
In addition, we use the insights we gain from external surveys to develop our approach in line with global best practice. A member of the Dow Jones Sustainability Index since 2001, AstraZeneca achieved its highest ever placing in the 2011 World Index. We also retained our listing on the DJSI STOXX – European index (the top 20% of the 600 largest European companies) for the fourth year running (one of only four pharmaceutical companies to do so out of 14 assessed). We achieved a total score of 85% (2010: 81%) compared with a sector best score of 87% (2010: 87%). We increased individual scores for 14 out of 23 criteria for 2011 (compared to nine out of 23 criteria in 2010) including corporate governance, R&D, environmental policy and supplier standards. While these scores are encouraging, we lost ground in some areas including marketing practices and health outcomes contribution. To better understand these lower scores, we have commissioned an in-depth external benchmark survey and the analysis will be used to inform our improvement planning. The survey is expected to report in the first quarter of 2012.
External assurance
Bureau Veritas has provided external assurance on the responsible business information contained within this Responsible Business section of this Annual Report and of the detailed content of the Responsibility section of our website. Bureau Veritas has found the responsible business information provided within this Annual Report to be accurate and reliable (based on the evidence provided and subject to the scope, objectives and limitations defined in the full assurance statement). The full assurance statement which contains detailed scope, methodology, overall opinion and recommendations can be found on our website, astrazeneca.com; web page content assured by Bureau Veritas is marked at the bottom of each page. Bureau Veritas is an independent professional services company that specialises in quality, health, safety, social and environmental management with a long history of providing independent assurance services.
| | |
| 48Delivering our strategy Responsible Business | | AstraZeneca Annual Report and Form 20-F Information 2011 |
Delivering our strategy
R&D ethics
We want to be recognised for our high quality science and for the impact we can make on serious diseases, and to be trusted for the way we work. Our standards of R&D ethics are global and apply to all AstraZeneca research activity, in all locations, whether conducted by us or on our behalf by external contract research organisations (CROs). We continue to work to ensure that these standards are applied, particularly as we expand our activity in countries such as China and Russia.
Clinical trials
We conduct clinical trials at multiple sites in several different countries. A broad geographic span helps us to ensure that those taking part in our studies reflect the diversity of patients around the world for whom the new medicine is intended. This approach also helps to identify the types of people for whom the treatment may be most beneficial.
Our global governance process for determining where we place clinical trials provides the framework for ensuring a consistent approach worldwide. We take several factors into account, including the availability of experienced and independent ethics committees and a robust regulatory regime, as well as sufficient numbers of trained healthcare professionals and patients willing to participate in a trial.
Before a trial begins, we work to make sure that those taking part understand the nature and purpose of the research and that proper procedures for gaining informed consent are followed (including managing any special circumstances, such as different levels of literacy). Protecting participants throughout the trial process is a core priority and we have strict procedures in place to ensure that they are not exposed to any unnecessary risks.
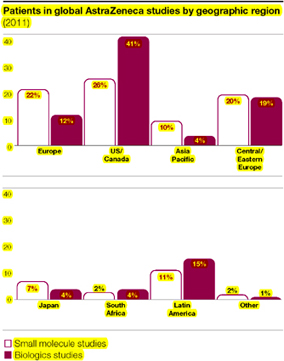
We recognise that situations may exist where continued provision of a non-approved clinical study drug to patients is both appropriate and necessary following the completion of a clinical study. During 2011, we introduced a new standard to provide global guidance in this area. Factors we take into account include the severity of the disease, the availability of alternative treatments, the individual patient response to the medicine, and the overall benefit/risk profile of the medicine based on completed and ongoing studies. If we continue to provide a clinical study drug after the original study is completed, we ensure that appropriate oversight measures are in place, such as dispensing treatment in the context of a clinical study or a compassionate use programme.
All our clinical studies are conceptually designed and finally interpreted in-house but a percentage of them are run for us by CROs. In 2011, around 39% of patients in our small molecule studies and around 66% of patients in our biologics studies were monitored by CROs on our behalf. We contractually require CROs to work to our global standards and we conduct risk-based audits to monitor compliance.
We publish information about the registration and results of all our clinical trials, whether favourable or unfavourable to AstraZeneca, on a range of public websites including our own dedicated site, astrazenecaclinicaltrials.com. By the end of 2011, we had registered over 1,370 trials and published the results of more than 1,150.
Animal research
Animal studies continue to play a vital role in the search for new medicines. They provide essential information, not available through other methods, about the effects of a potential new therapy on disease and the body. Regulatory authorities around the world also require safety data from preclinical testing in animals before a new medicine can be tested in humans.
As we work to improve our R&D productivity, we remain committed to minimising our use of animals without compromising the quality of the research data. All research using animals is carefully considered and justified, not only to confirm the scientific need for a study, but also to make sure that it has been designed so that the minimum number of animals is used and that they are exposed to as little pain and distress as possible.
Wherever possible, we use non-animal methods, such as computer modelling, that eliminate the need to use animals early in drug development or reduce the number required. We also work to refine our existing methods. This replacement, reduction and refinement of animal studies is known as ‘the 3Rs’ and to support our drive for continuous improvement, we work both within AstraZeneca and the wider scientific community to share 3Rs knowledge and learning.
The number of animals we use will continue to vary because it depends on a number of factors, including the amount of preclinical research we are doing, the complexity of the diseases under investigation and regulatory requirements. We believe that, without our active commitment to the 3Rs, our animal use would be much greater. In 2011, we used approximately 381,400 animals in-house (2010: 408,000). In addition, approximately 16,600 animals were used by external CROs on our behalf (2010: 21,000).
The welfare of the animals we use continues to be a top priority and our standards apply worldwide. In addition to mandatory inspections by government authorities, we have a formal programme of regular peer reviews of our internal animal research facilities conducted by our own qualified staff. External CROs that conduct animal studies on our behalf are required to comply with our global standards and we undertake audits to ensure ourexpectations are being met.
| | |
| 50Delivering our strategy Responsible Business | | AstraZeneca Annual Report and Form 20-F Information 2011 |
Protests
AstraZeneca acknowledges the right of every individual to express their views on the use of animals in research but we condemn the use of violence and other illegal acts. We firmly reject any harassment, intimidation or harming of our employees and their families, our suppliers and our other stakeholders as totally unacceptable.
Sales and marketing ethics
Delivering consistently high standards of sales and marketing practice continues to be one of our top priorities and is at the core of our commitment to driving commercial success responsibly. Our activities centre on ensuring that the appropriate information is provided to those who need it to support the safe and effective use of our medicines and enhance patient care.
We have always had a range of sales and marketing policies and standards in place but, following a review in 2010, we further strengthened the requirements and consolidated the range to form a single new Global Policy on External Interactions (the Policy). Launched in April 2011, the new Policy provides a single common, principle-based approach to all our interactions worldwide. Everyone in AstraZeneca, wherever they are located, is required to work to our global standards of ethical sales and marketing practice. We believe this is especially important as we grow our business in Emerging Markets, such as China and Russia, alongside our continued efforts in Established Markets, including the US and Japan.
The diversity of business cultures around the world means that putting a global approach into practice at a local level is a challenge. Nevertheless, we are committed to making it work. During 2011, we continued to provide targeted training for our people to ensure expectations and accountabilities were clear and understood as well as where to obtain further advice and support if needed. We are also talking to our customers and other stakeholders to explain the changes they are seeing to the way we are working with them.
We have comprehensive processes in place for monitoring compliance with our Code of Conduct and global policies, including dedicated compliance professionals who support our line managers locally in monitoring their staff activities. We also have a nominated signatory network that works to ensure that our promotional materials meet all applicable requirements.
Instances of potential non-compliance are collected through our compliance incident management processes and reviewed by senior management in local and/or regional compliance committees. Serious breaches are reviewed by the Audit Committee and, if appropriate, the Board. More information about our compliance and risk assurance processes is contained in the Managing risk section from page 129.
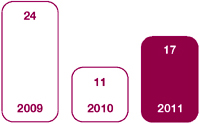
We take all breaches very seriously and act to prevent repeat occurrences. In 2011, we identified a total of 17 confirmed breaches of external sales and marketing regulations or codes globally (2010: 11; 2009: 24). Excluding the confirmed external breaches, there were 1,275 instances of failure to comply with our Code of Conduct and global policies in our Commercial organisation, including contract staff. In relation to all these breaches we removed 214 people from their role, formally warned 570 people, and provided further guidance or coaching on our policies for 971 people. It is important to note that a single breach can involve more than one employee failing to meet the standards required.
We believe that the increase in identified breaches is due in part to our enhanced management oversight of compliance and heightened awareness of policy requirements through targeted training, alongside improved data capture mechanisms. However, we acknowledge that our numbers are likely to continue to vary as we reshape our business and geographic footprint. We will continue to focus our complianceefforts appropriately.
Global KPI: Breaches of external sales and marketing codes and regulations ruled by external bodies
Disciplinary actions: Breaches of Code of Conduct by our Commercial organisation including contract staff
| | | | | | | | |
| | | | Number of people | |
Action taken | | | 2011 | | | | 2010 | |
Removed from role* | | | 214 | | | | 117 | |
Formal warning | | | 570 | | | | 740 | |
Guidance and coaching | | | 971 | | | | 768 | |
Total | | | 1,755 | | | | 1,625 | |
| * | In the majority of cases, this means dismissal from the Company/contract termination, but it can include resignations and demotions. |
US Corporate Integrity Agreement reporting
In April 2010, AstraZeneca signed an agreement with the US Department of Justice to settle an investigation relating to the sales and marketing of Seroquel IR. The requirements of the associated Corporate Integrity Agreement between AstraZeneca and the Office of the Inspector General of the US Department of Health and Human Services (OIG) include a number of active monitoring and self-reporting obligations that differ from self-reporting required by authorities in the rest of the world. To meet these obligations, AstraZeneca provides notices to the OIG describing the outcomes of particular investigations potentially relating to violations of certain laws, as well as a separate annual report to the OIG summarising monitoring and investigation outcomes relevant to Corporate Integrity Agreement requirements.
Access to healthcare
Providing sustainable access to healthcare for all those who need it is a significant global challenge. The complexities surrounding the issue mean that there is no ‘one size fits all’ solution. Factors affecting access range from the affordability of medicines to the availability of healthcare systems and the resources to make them effective. We believe it will take a combined global effort involving all related stakeholders to drive sustainable progress in increasing access to healthcare worldwide and we know that, as a global biopharmaceutical company, we can make a meaningful contribution to that effort.
Our strategy takes account of the different barriers to healthcare in different parts of the world and, because access to healthcare can also vary within a country, our approach is tailored locally to meet the needs of different patient populations. We are pursuing a range of different initiatives across these populations to understand what works best and in what context. We believe we will be able to make the biggest contribution to improving health where we are able to adopt a commercial approach. Our goal is always to improve health for patients and add value for our stakeholders and our business.
| > | Our mainstream business will continue to focus on those people for whom healthcare is readily available and who can afford our medicines. The selling of these medicines in our Established Markets helps enable us to generate the revenue we need to provide our shareholders with a return, invest in continued innovation and pursue other opportunities to increase theavailability of our medicines. |

| | |
| AstraZeneca Annual Report and Form 20-F Information 2011 | | Delivering our strategy Responsible Business51 |
Delivering our strategy
| > | As we expand our business in new geographies, we are exploring broad market strategies to reach new patients, in particular the emerging middle income populations who are increasingly able to access healthcare systems and for whom our medicines are becoming affordable. You can read more about our efforts to broaden access to our medicines in the Sales and Marketing section from page 36. |
| > | The availability of medicines is not always the primary challenge. Access to healthcare also depends on having a functioning healthcare system and the right allocation of resources to make sure that medicines are used appropriately as part of overall health management. For people in communities with limited healthcare infrastructure we partner with others to help strengthen healthcare frameworks and capabilities. |
We have defined some common criteria to guide our commitment and ensure that all our partnerships centre on delivering meaningful and enduring benefit. The key principles are that our partnerships:
| > | lead to positive, measurable outcomes in underserved communities |
| > | can be scaled up and potentially replicated to improve outcomes for a greater number of people |
| > | deliver a sustainable framework that can ultimately be owned and managed locally, without the need for our support. |
Such partnerships can also contribute to our business development, by enabling us to understand better the health needs of, and build important relationships in, markets of the future. An example is our Phakamisa initiative in South Africa (see page 55 for further information).
We also partner with NGOs who are experienced at tackling disease at a community level. For example, we have been supporting the British Red Cross since 2002 in their work to tackle TB and TB/HIV in Kyrgyzstan, Turkmenistan and Kazakhstan and, more recently, in South Africa and Lesotho. To date, over 16,000 people have been directly supported in completing their TB treatment across all our partnership countries and TB mortality and morbidity rates continue to fall in our partnership countries in central Asia. Our partnership with the African Medical and Research Foundation (AMREF), created in 2007, centres on strengthening healthcare systems and integrating the management of malaria, HIV/AIDS and TB (MAT) programmes in Uganda, where there is a high burden of all three diseases. Progress to date includes six laboratories upgraded to Ministry of Health standards to support improved diagnosis and over one million patient visits recorded in the Health Management Information System as having received MAT diagnostic, treatment and other services.
Our most recent community investment, the AstraZeneca Young Health Programme (YHP) is designed to help young people in need around the world deal with the health issues they face so they can improve their chances of living a better life. We are working with expert partners, Plan International and Johns Hopkins Bloomberg School of Public Health, to identify the needs in our local communities and to help address these needs with a combination of work on the ground, research and advocacy. Adolescent health remains an underserved part of the healthcare agenda and this global investment initiative aims to make a measurable and sustainable difference. YHP initiatives are now in place in nine countries and our target is 15 by the end of 2012. By 2015, YHP will reach 500,000 young people between the ages of 10 and 24 directly and will touch an additional 500,000 lives indirectly.
| > | On a broader basis, we collaborate at a global level to increase understanding of fast-emerging and existing health threats in the developing world, and to lend our skills and resources to addressing these. For example: |
| > | In partnership with the IFPMA, AstraZeneca is undertaking policy research to understand practical steps to overcome the barriers to treatment and care for non-communicable diseases (NCDs) which are fast overtaking communicable diseases as a developing world health threat. The challenges that NCDs present are not new to us. We have many decades of experience in NCD treatment, with a strong product portfolio and pipeline of new medicines targeting these areas. The majority of our research investment continues to centre on NCDs. |
| > | Alongside the rising challenge of NCDs, the battle against TB and neglected tropical diseases (NTDs) is far from over. Scientists at our dedicated research facility in Bangalore, India are focused on finding a new treatment for TB. For further information about this, see the Infection section from page 64. As outlined in the Research and Development section from page 30, during 2011, we joined the World Intellectual Property Organization’s (WIPO) Re:Search initiative. This unprecedented collaboration between the private sector and public partners will make publicly available a searchable database of available IP assets and resources for use in NTD research to speed the discovery and development of new potential treatments. |
More information about our Access to healthcare strategy and the associated initiatives is available on our website, astrazeneca.com/responsibility.
Human rights
As we reshape our organisation, grow our business and increase our outsourcing, we are working to make sure that human rights continue to be appropriately integrated into our policies and processes.
AstraZeneca is a signatory to the United Nations Global Compact (UNGC), a strategic public-private initiative for organisations committed to social and environmental sustainability. This means that we have committed to uphold 10 internationally recognised principles in the areas of human rights, labour standards, environmental sustainability and anti-corruption. These are long-standing principles for AstraZeneca (as described in our Code of Conduct and global policies) but being part of the UNGC reinforces how seriously we take these principles. It also gives us the framework for further developing our approach.
In recent years, we participated in a project led by the Danish Institute for Human Rights (DIHR), working with the pharmaceutical industry to develop a human rights assessment tool for pharmaceutical companies, based on the DIHR’s existing Human Rights Compliance Tool. The first pharmaceutical industry version of the tool was launched in November 2010 and we used it to conduct a labour review in 11 of our marketing companies, including some countries where national labour standards are inconsistent with global best practice. The review focused on International Labour Organization core areas (freedom of association and collective bargaining, forced and bonded labour, child labour, discrimination and working time and wages).
Building on the experience of this review, we adapted and simplified the employment section of the assessment tool and, during the remainder of 2011, used it to conduct a labour review in every country where AstraZeneca has employees.
The results of all our 2011 reviews are currently being collated and analysed to identify what we are doing well and where we may need to improve. Any areas identified for improvement will be included in our local people strategies in the relevant countries.
| | |
| 52Delivering our strategy Responsible Business | | AstraZeneca Annual Report and Form 20-F Information 2011 |
Also in 2011, the DIHR in collaboration with AstraZeneca, GSK, Novartis and Merck established the Human Rights Assessment Tool for Pharmaceuticals Companies Forum (the Forum). The Forum is a means of sharing information, experience and best practice, helping members to better understand what it means to integrate human rights into daily business practice and to further clarify human rights responsibilities for pharmaceutical companies.
Working with suppliers
Our ongoing drive to support increased efficiency through our procurement activity continues to be underpinned by our work to make sure that our purchasing is directed only to those organisations which embrace ethical standards consistent with our own.
Our Global Responsible Procurement Standard (the Standard) defines one of the key business processes for integrating our ethical standards into our procurement activity and decision making worldwide. It includes detailed expectations of suppliers. In addition, responsible procurement clauses that include audit requirements are incorporated in supplier contracts. We continue to review the Standard to ensure it appropriately reflects our commitment and during 2011, revised it to strengthen the anti-bribery and anti-corruption (ABAC) requirements in line with our ABAC policy and the requirements of the UK Bribery Act.
The process outlined in our Standard applies to suppliers of goods and services globally and is focused on ensuring that our responsible business expectations are being met. Specific expectations of suppliers such as healthcare professionals or CROs are managed within the relevant functions using specific assessment and monitoring processes.
Our Responsible Procurement process is based on an escalating set of risk-based due diligence activities, applied in a pragmatic way. The same initial assessment process is used for all suppliers and more detailed, focused assessments are then made, relevant to the service provided. Full details of the process are available on our website, astrazeneca.com/responsibility.
By the end of 2011, we had completed 3,342 responsible procurement risk assessments accounting for 71% of our third party spend.
We categorise suppliers as high, medium or low risk. We focus our auditing efforts on high and medium risk rated suppliers but we also audit some suppliers that we consider to be lower risk, to confirm our performance expectations across all suppliers we do business with. In 2011, we worked with our suppliers to substantially increase our audit activity. 727 suppliers across 55 countries have participated in 751 audits undertaken this year (48 audits and 42 suppliers in 2010).
Forty five percent of supplier sites audited demonstrated standards that met our expectations with a further 51% implementing improvements to address non-compliances. We monitor progress across all corrective actions and 4% of suppliers audited this year will require significant follow up to confirm they will make the improvements we require. We will not use suppliers who are unable or unwilling to meet our expectations in a timely way.
Our audits are conducted through a combination of internal resources and external independent auditors. Our assessment programmes reflect best practice from other industry sectors as well as the principles of the Pharmaceutical Supply Chain Initiative.
Patient safety
The safety of the patients who take our medicines will always be a fundamental consideration for us. All drugs have potential side effects and we aim to minimise the risks and maximise the benefits of each of our medicines, beginning with the discovery of a potential new medicine and continuing throughout its development, launch and marketing.
We have an experienced, in-house team of clinical patient safety professionals working around the world who are dedicated to the task of ensuring that we meet our commitment to patient safety. At a global level, every medicine in development and on the market is allocated a Global Safety Physician and a team of patient safety scientists. In each of our markets we also have dedicated safety managers with responsibility for patient safety at a local level.
Our two Chief Medical Officers (one for small molecule products and one for biologics) have overall accountability for the benefit/risk profiles of the products we have in development and those on the market. They provide medical oversight and ensure that appropriate risk assessment processes are in place to enable informed decisions to be made about safety as quickly as possible.
We use an external provider, Tata Consultancy Services (TCS), to manage the data entry process for individual case safety reports relating to our products. As experts in their field, TCS continues to drive improvements in the efficiency and consistency of data entry across AstraZeneca and using TCS for this work means our patient safety teams can focus primarily on case prioritisation, the medical aspects of patient safety and continuing to improve our safety science. TCS is contractually required to comply with our patient safety standards and is closely monitored through audits against detailed quality and compliance performance indicators.
Environmental sustainability
Managing our environmental impact is a core commitment. In January 2011, we implemented our new SHE strategy and associated objectives and targets for 2011-2015 which are closely aligned with our business objectives and provide the framework for driving our environmental sustainability going forward. This section includes summary information about certain key areas of the framework. Full details of our strategy, objectives and targets are available on our website, astrazeneca.com/responsibility.
We aim to minimise our environmental impact by reducing the carbon footprint and natural resource demands of our business activities, and improve the environmental profile of our products. We believe we are on track to deliver our 2015 targets.
We work to reduce our CO2 emissions by, among other things, improving our energy efficiency and pursuing lower-carbon alternatives to fossil fuels at our sites, and making sure that our travel and transport activities are as efficient as possible. Our carbon footprint is also affected by some of our respiratory therapies, specifically our

| | |
| AstraZeneca Annual Report and Form 20-F Information 2011 | | Delivering our strategy Responsible Business53 |
Delivering our strategy
pressurised metered-dose inhalers that rely on hydrofluoroalkane (HFA) propellants to deliver the medicine to a patient’s airways. While HFAs have no ozone depletion potential and a third or less of the global warming potential than the chlorofluorocarbons (CFCs) they replace, they are still greenhouse gases. Our target is to reduce our operational greenhouse gas footprint (excluding emissions from patient use of our inhaler therapies) by 20% by 2015. In 2011, our greenhouse gas emissions (from all sources) totalled 1.17 million tonnes (35 tonnes/$m indexed to Group revenue).
The management of waste is another key aspect of our commitment and we have a 2015 target of a 15% reduction in hazardous and non-hazardous waste. Our primary focus is waste prevention, but where this is not practical, we concentrate on waste minimisation and appropriate treatment or disposal to maximise the reuse and recycling of materials and minimise disposal to landfill. In 2011, our total waste was 45.9 thousand tonnes (excluding our biologics capabilities) with a tonnes/$m index of 1.41.
We recognise the need to use water responsibly and where possible to minimise the use of water in our facilities. To support the delivery of our target to reduce water use by 25% by 2015, we now have water conservation plans at our largest sites. In 2011, our water use was 4.4 million m3 with a m3/$m index of 130.
Alongside these efforts, we are also working to ensure that we measure and report the impact of our external manufacturing activity on the environment, and that our suppliers have appropriate environmental improvement targets.
Our continued commitment to product stewardship is underpinned by our ongoing work to integrate environmental considerations into a medicine’s complete life-cycle, from discovery and development, through manufacturing, marketing, use and, ultimately, disposal. Further information is available on our website, astrazeneca.com/ responsibility, including environmental risk assessment data for our medicines.
Employee safety, health and wellbeing
Providing a safe workplace and promoting the health and wellbeing of all our people remains a core consideration. We provide a wide range of health and wellbeing improvement programmes across AstraZeneca, designed to help people understand their personal health risks and support them in proactively managing these risks.
In January 2011, we implemented our new SHE strategy and a complementary Health and Wellbeing strategy, with associated objectives and targets for 2011-2015. The new targets reflect our determination to stay focused on continuous improvement as we grow and reshape our business.
Driver safety remains our highest priority for improvement and our focus is on promoting driver safety among our sales forces, collectively the single largest group of employees who drive on company business. Our long-standing ‘Road Scholars’ scheme in the US continues to be a valuable channel for building awareness and improving driver skills. Outside the US, our ‘Drive Success’ programme takes into account the different driving environments in the various countries in which we operate and provides a high-level framework of common standards and measures to be applied by each country. Driver safety targets are included in regional and local scorecards. Performance is monitored centrally to assess progress and identify areas for improvement.
We regret that during 2011 one of our employees was killed in a road traffic accident while driving on AstraZeneca business. A detailed investigation was carried out, including an audit of the implementation of the Drive Success programme. An action plan was drawn up to respond to the findings of the investigation which include further enhancements to the Drive Success programme and increased communications to drivers. These actions are being tracked and learning will be shared widely across the business.
In 2011, we achieved a 23% improvement in the lost time injury/illness rate compared to the baseline year (2010), exceeding our annual improvement target. This puts us well on track to achieve our 2015 target of a 25% reduction in the lost time injury/illness rate.
Work-related stress remains our greatest single category of occupational illness with high workloads, interpersonal issues and organisational change identified as significant factors. As part of our ongoing efforts in this area, we are adopting an increasingly proactive, risk-based approach, using wellbeing risk assessment tools to identify high-risk areas and target interventions more effectively.
Community investment
Wherever AstraZeneca operates worldwide, we aim to make a positive contribution to our local communities through partnerships, charitable donations and other initiatives that help to make a sustainable difference. Our investment is focused on improving health and promoting science skills.
In 2011, we spent a total of $1.27 billion (2010: $1.41 billion) on community sponsorships, partnerships and charitable donations worldwide, including our product donation and patient assistance programmes which make our medicines available free of charge or at reduced prices. Through our three patient assistance programmes in the US we donated products valued at an average wholesale price of over $938 million (2010: $1.38 billion). We also donated products worth $8.2 million, valued at average wholesale price, to the charitable organisations: Americares and Direct Relief International.
Disaster relief
During 2011, we made a number of contributions to disaster relief efforts, including donations from our Charities Aid Foundation (CAF) account. We also developed an enhanced protocol for working with the British Red Cross, our global disaster relief partner, to improve our internal coordination and enable us to respond in a timely, consistent and effective way to emergencies as and when they arise. This protocol was used to inform the following contributions to disaster relief efforts during the year.
| > | In February 2011, we donated£10,000 (approximately $16,000) from our CAF account to the British Red Cross New Zealand Earthquake Appeal. In March 2011, we donated £100,000 (approximately $162,000) from our CAF account to the British Red Cross Libya and Region Appeal to help support those who had fled to neighbouring countries to escape the violence in Libya. |
| > | Following the earthquake in Japan, we donated 51 million Yen (approximately $640,000) as part of an overall pledge of 100 million Yen (approximately $1.3 million) to Ashinaga Ikueikai in support of their ongoing relief and rebuilding effort. In addition, employee donations from AstraZeneca in Japan totalling 25.5 million Yen (approximately $320,000) were matched by the Company. We donated $25,000 to AMREF to support their local networks in North East Kenya, where the Horn of Africa drought was having a devastating impact. |
| | |
| 54Delivering our strategy Responsible Business | | AstraZeneca Annual Report and Form 20-F Information 2011 |
Corporate Governance Report
Political donations
Neither the Company nor its subsidiaries made any EU political donations or incurred any EU political expenditure in 2011 and they do not intend to do so in the future in respect of which shareholder authority is required, or for which disclosure in this Annual Report is required, under the Companies Act 2006. However, to enable the Company and its subsidiaries to continue to support interest groups or lobbying organisations concerned with the review of government policy or law reform without inadvertently breaching the Companies Act 2006, which defines political donations and other political expenditure in broad terms, a resolution will be put to shareholders at the 2012 AGM, similar to that passed at the 2011 AGM, to authorise the Company and its subsidiaries to:
| > | make donations to political parties or independent election candidates |
| > | make donations to political organisations other than political parties |
| > | incur political expenditure, up to an aggregate limit of $250,000. |
Corporate political contributions in the US are permitted in defined circumstances under the First Amendment of the US Constitution and are subject to both federal and state laws and regulations. In 2011, the Group’s US legal entities made contributions amounting in aggregate to $1,099,450 (2010: $1,999,150) to national political organisations, state-level political party committees and to campaign committees of various state candidates. No corporate donations were made at the federal level and all contributions were made only where allowed by US federal and state law. We publicly disclose details of our corporate US political contributions, which can be found at astrazeneca-us.com/ responsibility/transparency/. The annual corporate contributions budget is reviewed and approved by the US General Counsel, the US Vice-President, Corporate Affairs and the President of our US business to ensure robust governance and oversight. US citizens or individuals holding valid green cards exercised decision making over the contributions and the funds were not provided or reimbursed by any non-US legal entity. Such contributions do not constitute political donations or political expenditure for the purposes of the Companies Act 2006 and were made without any involvement of persons or entities outside the US.
Significant agreements
There are no significant agreements to which the Company is a party that take effect, alter or terminate on a change of control of the Company following a takeover bid. There are no persons with whom we have contractual or other arrangements, who are deemed by the Directors to be essential to our business.
Use of financial instruments
Notes 15 and 23 to the Financial Statements, from pages 161 and 171 respectively, include further information on our use of financial instruments.
Creditor payment policy
It is not our policy formally to comply with the Confederation of British Industry’s code of practice on the prompt payment of suppliers. It is, however, our policy to agree appropriate payment terms with all suppliers when agreeing to the terms of each transaction, to ensure that those suppliers are made aware of the terms of payment and, subject to their compliance, to abide by the terms of payment. The total amount of money owed by the Company’s subsidiaries to trade creditors at the balance sheet date was equivalent to 50 days’ average purchases (2010: 62 days). A considerable part of the trade creditors’ balance continues to relate to the Merck account in the US, which has particularly long contractual payment terms. By removing this balance and other items not directly related to trade purchases in the US, a more accurate average of 43 days is obtained (2010: 57 days).
The Company has no external trade creditors.
Annual General Meeting
The Company’s AGM will be held on 26 April 2012. The meeting place will be in London, UK. A Notice of AGM will be sent to all registered holders of Ordinary Shares and, where requested, to the beneficial holders of shares.
External auditor
A resolution will be proposed at the AGM on 26 April 2012 for the re-appointment of KPMG Audit Plc as auditor of the Company. The external auditor has undertaken various non-audit work for us during 2011. More information about this work and the audit and non-audit fees that we have paid are set out in Note 27 to the Financial Statements on page 190. The external auditor is not engaged by us to carry out any non-audit work in respect of which it might, in the future, be required to express an audit opinion. As explained more fully in the Audit Committee section from page 107, the Audit Committee has established pre-approval policies and procedures for audit and non-audit work permitted to be carried out by the external auditor and has carefully monitored the objectivity and independence of the external auditor throughout 2011.
Bureau Veritas
Bureau Veritas is an independent professional services company that specialises in quality, health, safety, social and environmental management with a long history of providing independent assurance services.
Bureau Veritas has provided external assurance on corporate responsibility related information within this Annual Report and of the detailed content of the ‘Responsibility’ section of our website. Bureau Veritas has found the information provided within this Annual Report to be accurate and reliable (based on the evidence provided and subject to the scope, objectives and limitations defined in the full assurance statement). The full assurance statement which contains detailed scope, methodology, overall opinion and recommendations can be found on our website, astrazeneca.com. The web page content assured by Bureau Veritas is marked at the bottom of each page.
Directors’ Report
The Directors’ Report, which has been prepared in accordance with the requirements of the Companies Act 2006, comprises the following sections:
| > | Strategy and Performance |
and has been signed on behalf of the Board.
A C N Kemp
Company Secretary
2 February 2012
| | |
| 112Corporate Governance Report | | AstraZeneca Annual Report and Form 20-F Information 2011 |



