- AZN Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
SC TO-C Filing
AstraZeneca (AZN) SC TO-CInformation about tender offer
Filed: 15 May 06, 12:00am

| This presentation does not constitute an offer to sell or an invitation to purchase any securities or the solicitation of an offer for or buy any securities, pursuant to the Offer or otherwise. The Offer will be made solely by the Offer Document, the Form of Acceptance and Letter of Transmittal accompanying the Offer Document, which will contain the full terms and conditions of the Offer, including details of how the Offer may be accepted and any restrictions on the extent to which the Offer is capable of acceptance in certain jurisdictions. Goldman Sachs International, which is authorised and regulated by the Financial Services Authority is acting exclusively for AstraZeneca UK Limited ("AstraZeneca") and no one else in connection with the Offer and will not be responsible to anyone other than AstraZeneca for providing the protections afforded to clients of Goldman Sachs International or for providing advice in relation to the Offer or any other matters referred to herein. Morgan Stanley is acting exclusively for Cambridge Antibody Technology Group plc ("CAT") in connection with the Offer and no one else and will not be responsible to anyone other than CAT for providing the protections afforded to clients of Morgan Stanley or for providing advice in relation to the Offer or any other matters referred to herein. Copies of this presentation and any documentation relating to the Offer are not being, and must not be, directly or indirectly, mailed or otherwise forwarded, distributed or sent in or into or from Canada or Japan or any other jurisdiction where to do so would be unlawful. In the United States, AstraZeneca will file a Tender Offer Statement containing the Offer Document and other related documentation with the US Securities and Exchange Commission (the "SEC") on Schedule TO and CAT will file a Solicitation/Recommendation Statement with the SEC on Schedule 14D-9 on or around the date an Offer Document is mailed to CAT shareholders, including holders of CAT American Depositary Shares (collectively, "CAT Shareholders"). Free copies of the Schedule TO, the Schedule 14D-9 and the other related documents to be filed by AstraZeneca or CAT in connection with this Offer will be available from the date an Offer Document is mailed to CAT Shareholders on the SEC's website at http://www.sec.gov. The Offer Document, the Form of Acceptance and the Letter of Transmittal accompanying the Offer Document will be made available to all CAT Shareholders at no charge to them. CAT Shareholders are advised to read the Offer Document and the accompanying Form of Acceptance and Letter of Transmittal when they are sent to them because they will contain important information. CAT Shareholders in the United States are also advised to read the Tender Offer Statement and the Solicitation/Recommendation Statement because they will contain important information. This presentation may contain certain forward-looking statements about AstraZeneca and CAT which are subject to risks and uncertainties and may be influenced by factors that could cause actual outcomes and results to be materially different from those predicted. We have identified the forward-looking statements by using the words 'anticipates', 'believes', 'expects', 'intends' and similar expressions in such statements. Important factors that could cause actual results to differ materially from those contained in forward-looking statements, certain of which are beyond our control, include, among other things: difficulties in integrating CAT, unexpected and greater costs arising out of the offer for CAT or the integration of AstraZeneca and CAT; regulatory conditions being imposed on the acquisition of CAT; the loss or expiration of patents, marketing exclusivity or trade marks by either AstraZeneca or CAT; exchange rate fluctuations; the risk that R&D will not yield new products that achieve commercial success; the impact of competition, price controls and price reductions; taxation risks; the risk of substantial product liability claims; the impact of any failure by third parties to supply materials or services; the risk of delay to new product launches; the difficulties of obtaining and maintaining governmental approvals for products; the risk of failure to observe ongoing regulatory oversight; the risk that new products do not perform as we expect; and the risk of environmental liabilities. For further discussion of factors that may could cause AstraZeneca's or CAT's actual results to differ from expectations, you should read AstraZeneca's and CAT's most recent respective UK Annual Report and filings and submissions to the SEC, including the Annual Reports on Form 20-F. You are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this presentation. Except as required by law or the UK Listing Rules, AstraZeneca does not undertake any obligation to publicly release any update or revisions to these forward-looking statements. Disclaimers: Exhibit 99.(a)(5) |

| Peter Chambre |

| Dr John Patterson Executive Director Development, AstraZeneca |

| Offer of £13.20 per share Unanimously recommended by CAT Board Irrevocable undertakings to accept from each CAT Director Offer document to be posted shortly Closing expected around the end of June 2006 Recommended Offer |

| AstraZeneca's Approach We turn good ideas into effective medicines designed to fight disease in important areas of healthcare and make a difference for patients worldwide. We are one of the world's leading pharmaceutical companies, with extensive research, development, manufacturing and marketing capabilities. We are committed to sustainable development of our business and the delivery of a flow of new medicines that bring benefit for patients and add value for wider society. |

| Strategic Context Strengthening the pipeline is AstraZeneca's top priority Biological therapeutics offer excellent prospects for growth This offer will transform AstraZeneca's approach to biological therapeutics |

| World Class People World class people and proprietary technology Phage and ribosome display platforms producing: Now: Optimised fully human antibodies Future: Optimised peptides, proteins and protein fragments Providing a foundation for AstraZeneca's biological therapeutics strategy |
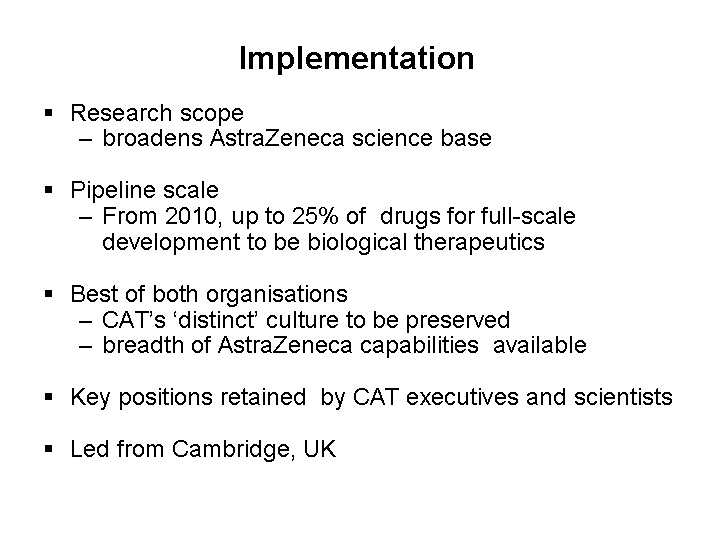
| Implementation Research scope broadens AstraZeneca science base Pipeline scale From 2010, up to 25% of drugs for full-scale development to be biological therapeutics Best of both organisations CAT's 'distinct' culture to be preserved breadth of AstraZeneca capabilities available Key positions retained by CAT executives and scientists Led from Cambridge, UK |
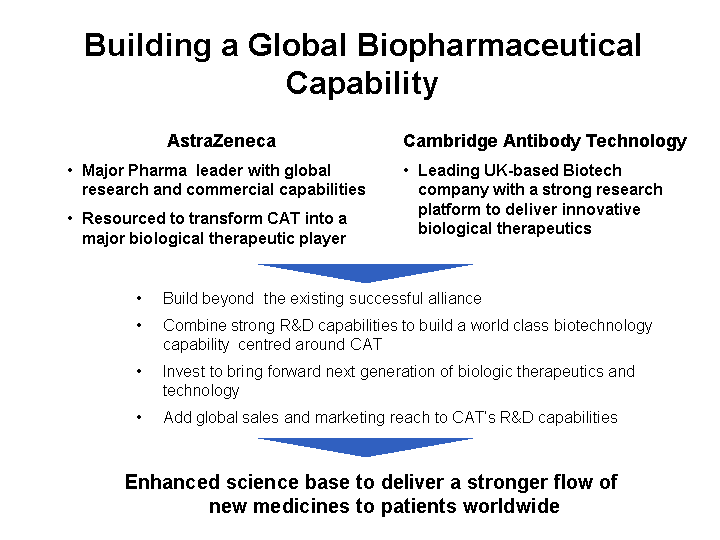
| Building a Global Biopharmaceutical Capability AstraZeneca Major Pharma leader with global research and commercial capabilities Resourced to transform CAT into a major biological therapeutic player Build beyond the existing successful alliance Combine strong R&D capabilities to build a world class biotechnology capability centred around CAT Invest to bring forward next generation of biologic therapeutics and technology Add global sales and marketing reach to CAT's R&D capabilities Enhanced science base to deliver a stronger flow of new medicines to patients worldwide Cambridge Antibody Technology Leading UK-based Biotech company with a strong research platform to deliver innovative biological therapeutics |
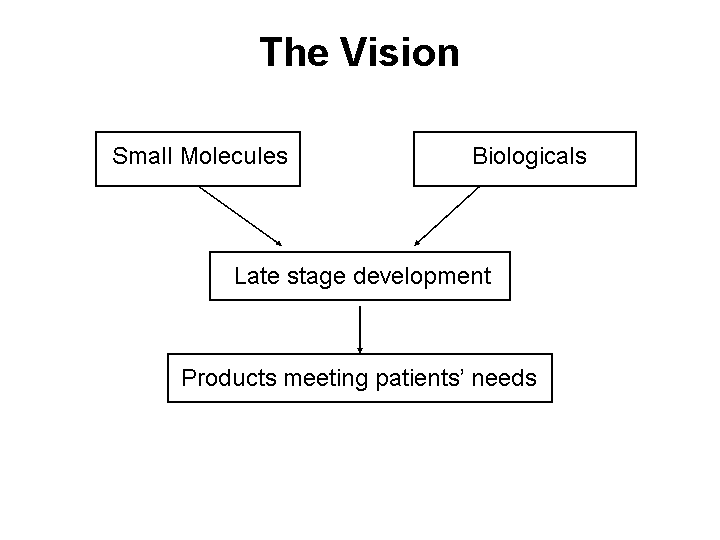
| The Vision Small Molecules Biologicals Late stage development Products meeting patients' needs |

| Hamish Cameron |

| Paul Nicholson |

| Peter Chambre |