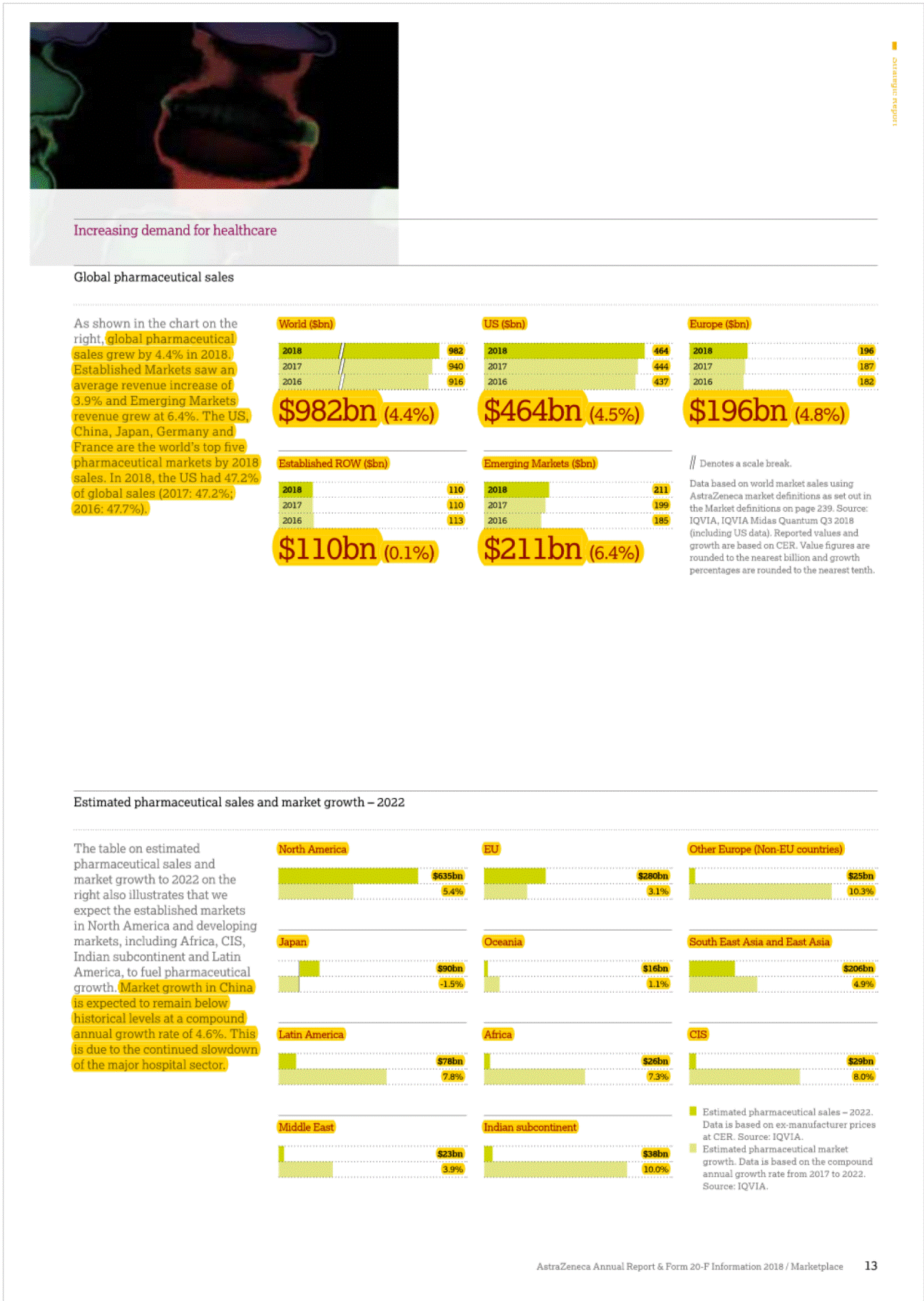
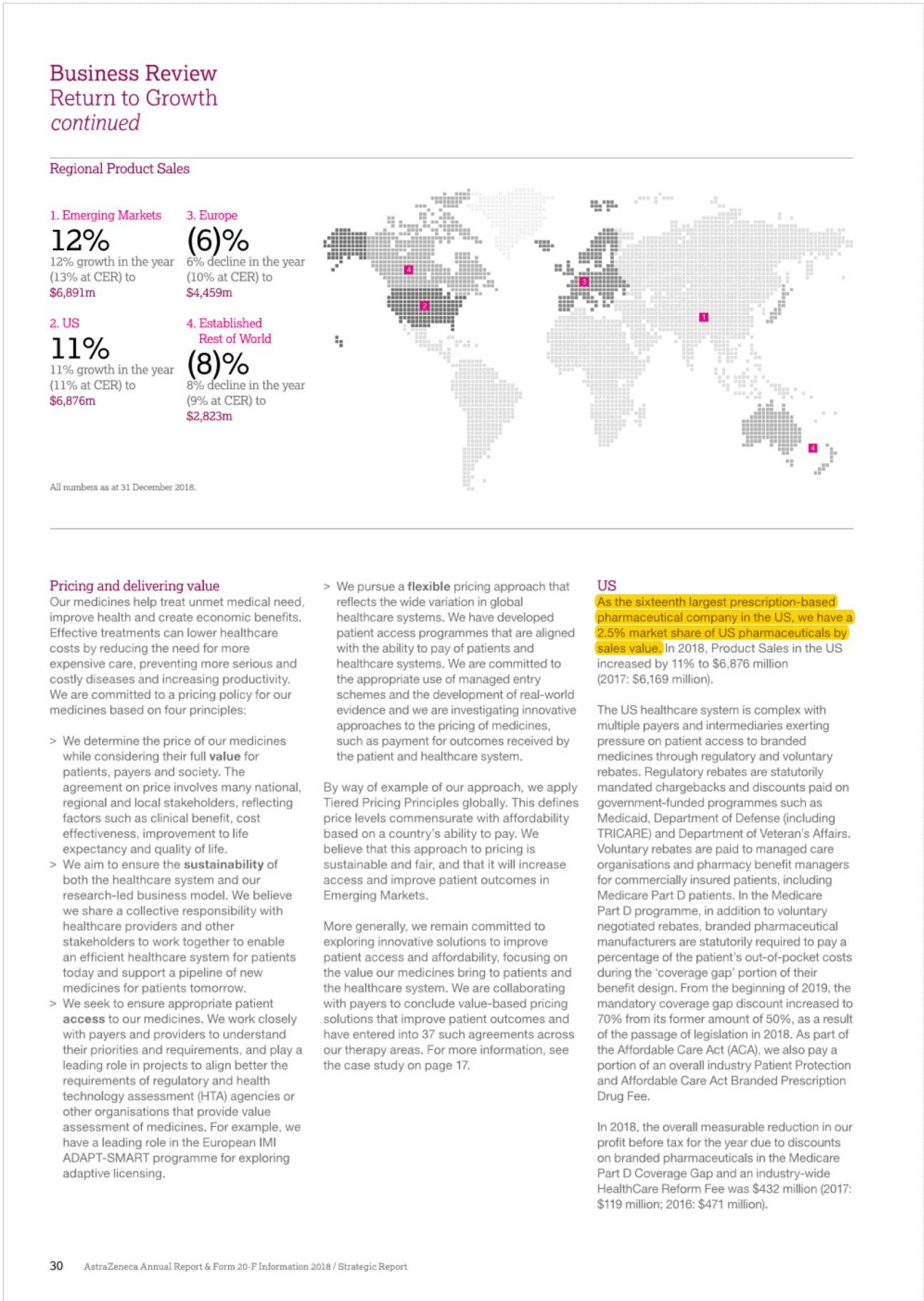
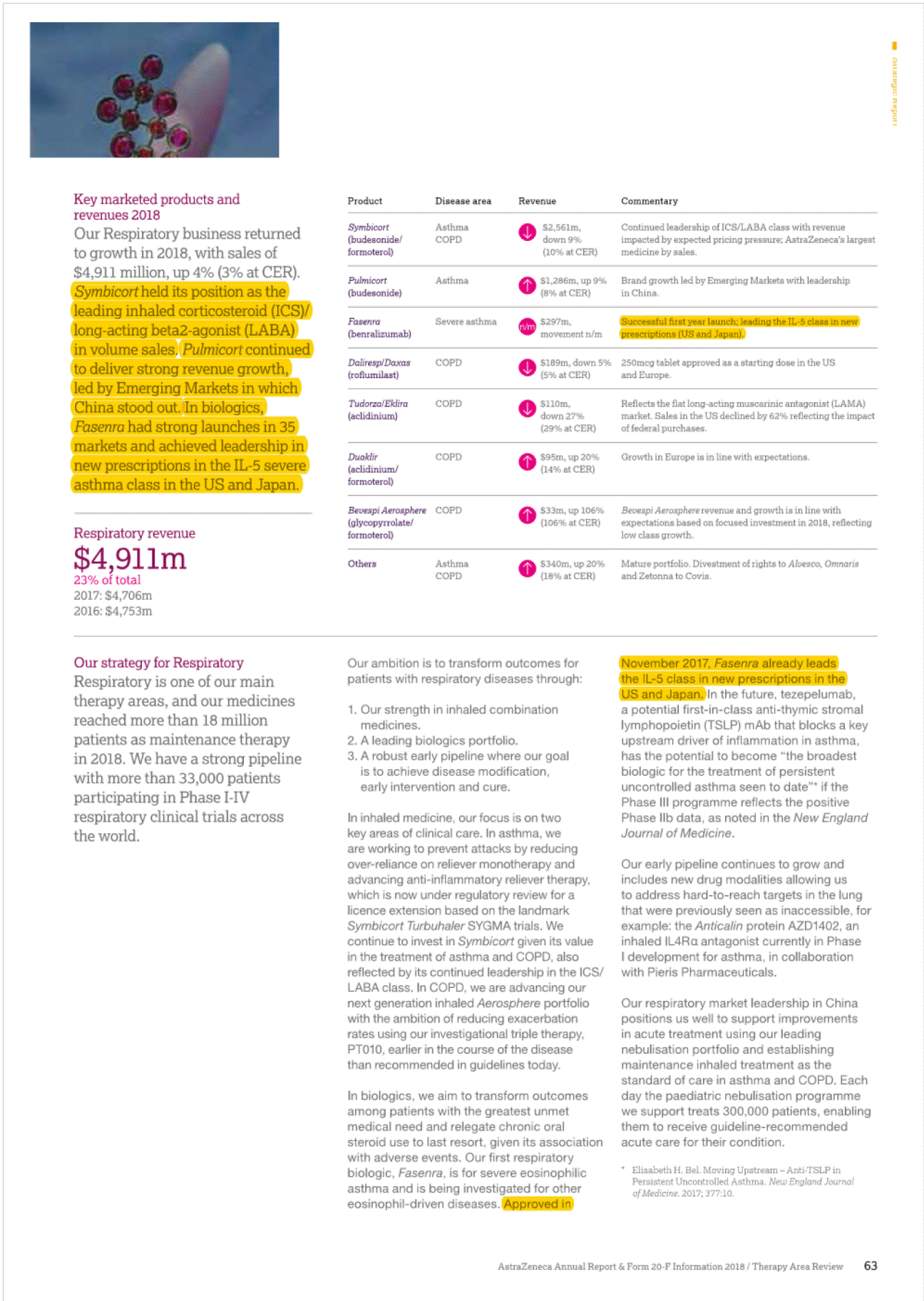
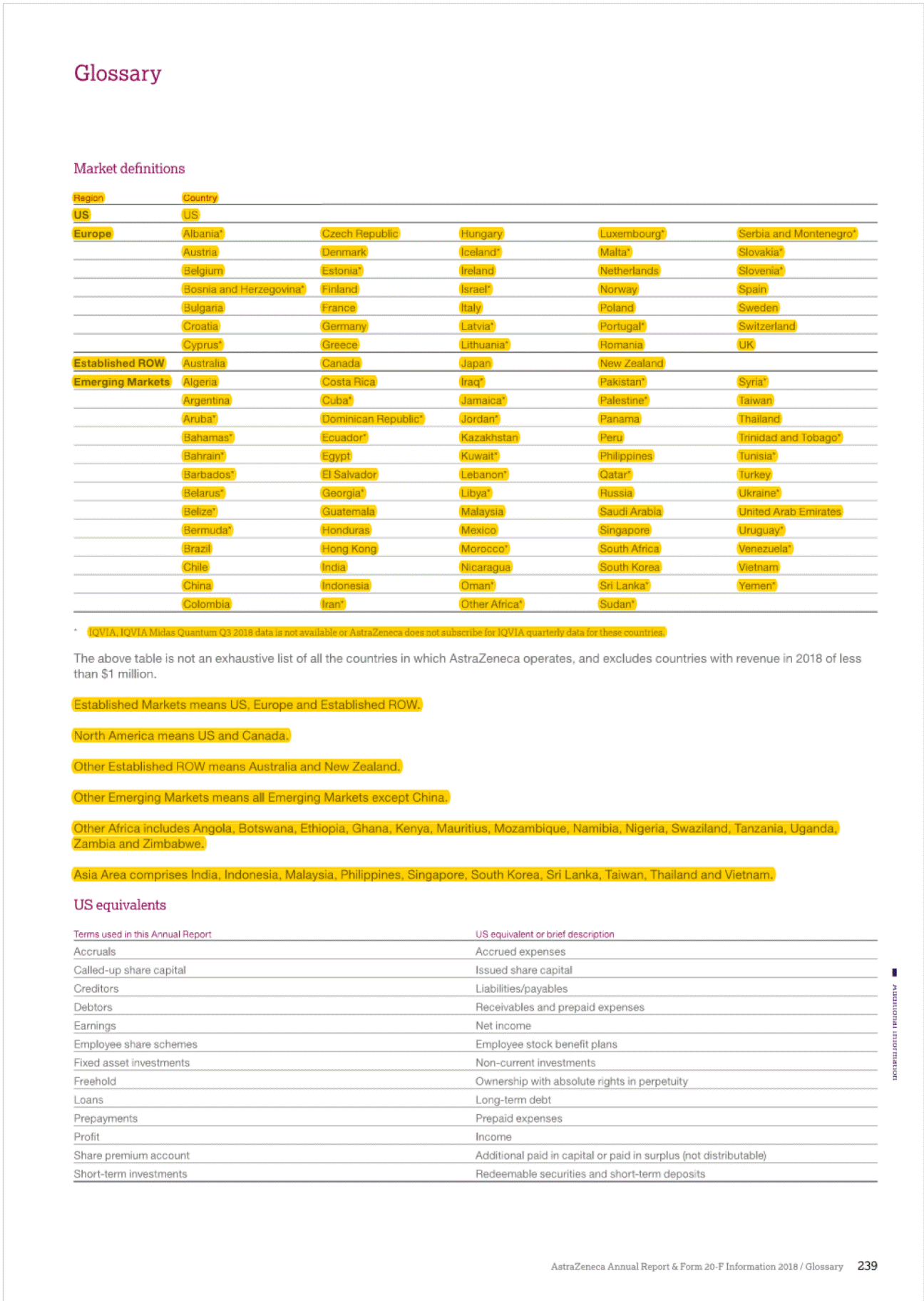
I 2• "'' ", g• Established Rest of World (ROW)' In 2018,Product Sales in Japan decreased by 9% at actual rate of exchange (11% at CER) to $2,004 million (2017: $2,208 mlilion), as a result of the biennialgovernment price cuts and increased intervent on from the government to rapidly increase the volume share of generic products.Qii.SOe tejjj6e("201(jjere£i® uthorised generic entered the market an ·n cember 2017 we saw more tha g n ric c rumies enter thpw;;e;s:;:e:;s"t '"t"i"j"jl market with generic rosuvastatin which ha strongJyJmpacted Crestor Product Sale with a decrease of 60%.Leaving aside these generic restraints, a(:!an is presenting stron growth from the brands in our Growl (Piat!Qijjj§)and Nexium. In addition,there were particularly strong performances from Tagrisso,Fasenra, tmfinzi, Lynparza and the Diabetes franchise. e now old twel (ijOSitionin the ranking of pharmaceutic 9mpanies by sales of medicines in,)Man at::>an remains an attraclil! e market foq In the US,there is signif cant pricing pressure driven by payer consolidation, restrictive reimbursement policies and cost controltools. such as exclusionary formularies and price protection clauses.Many formularies,which specify particular medicines that are approved to be prescribed in a healthcare system,or under a healthinsurance poilcy,employ 'generic first' strategies and/or require physicians to obtainprior approval for the use of a branded medicine where a generic alternative exists. These mechanisms canbe used by intermediaries to limthe use of branded products andput pressureonmanufacturers to reduce net prices.In 2018 84.8% o We understand that our medicines will not benefit patientsif they are unable to afford them and that is why we offer a number of resources and programmes that can help increase patients' access to medication and reduce their out-of-pocket costs. We focus our formulary access on affordability for patients through rebate payments as well as savings cards for eligible patients when the out-of-pocket costs are not affordable. AstraZeneca has one of the longest-standing patient assistance programmes in the industry, AZ&Me,which provides eligible patients with AstraZeneca medicines at no cost. AstraZeneca has provided prescription savings to four million patients across the US and Puerto Rico over the past 10 years. rescriptions dispensedin the, US we@Jleneric with 84.9% in 201 In add ion, patients are seeing changes in the design of their health planbenefits andmay experience variation, ncluding increases,in both premiums and out-of-pocket payments for their branded medications. The pat ent out-of-pocket spend is generally in the form of a co-payment or co-insurance,but there is a growing trend towards high-deductible healthplans which require patients to pay the fulllist price untilthey meet certain out-of-pocket thresholds. [!] Formore informahon, see Community mveatment on page48. Europe The totalEuropean pharmace uticalmark et; o h $196 billionin 2018. We are the · ee Oargest_prescription-basedpharmaceuti mpanyin Europe (see Market definiot innovative harmaceuticals Canada has a mixed public/private payer system for medicines that is funded by the provinces, insurers and individual patients. It has also now become common for public payers to negotiate lower non-transparent prices after they have gone through a review by the Canadian Agency for Drugs and Technology in Health, a health technology assessment body.Most private insurers pay full price,although there is increasing pressure to achieve lower pricing. Overall,the split for AstraZeneca's portfolio is 62% funded by private payers and 38% by public plans. am_page 239) ith a 2.0% market shar harmaceutical sales bval ue. o Ongoing scrutiny of the US pharmaceut cal industry, focused largely on pricing,has been the basis of multiple policy proposals in the US.In May 2018, the Trump Administrat on issued its 'Blueprint to Lower Drug Pr ces and Reduce Out-of-Pocket Costs', which included a wide range of policy proposals that would impact the US pharmaceuticalindustryif implemented. Proposed changes under consideration include,but are notlimited to, fundamentally changing the role of rebates in the pharmaceutical supply chain,reforms to the 3408 Drug Pricing Program,and policies to increase competit on in the Medicare programme and encourage generic drug use. The Trump Administration has already taken action on several of the policies discussed in the Blueprint, and more policy actions are pending.In addition, lawmakers at both the federal and state level have sought increased drug pricing transparency and have proposed and implemented poilcies thatinclude measures relating to the submission of proprietary manufacturer data,establishment of price parameters that are indexed to certain federalprogrammes. and reporting of changes in pricing beyond certain thresholds. In 2018, our Product Sales in Europe decreased by 6% at actualrate of exchange (10% at CER) to S4,459 million (2017:$4,753 million).Key drivers of the decline,leaving aside theimpact of divestments such as Seloken, Atacand,Nexium and Zomig, were cont nued competition from Symbicort analogues, loss of exclusivity for Crestor, and the cont nued impact of early generic entry in certain markets for Faslodex, which we expect to continue in 2019. The continued macroeconomic environment, pricing pressure from payers and parallel trade across markets also affected sales. Our sales in Australia and New Zealand declined by 16% at actual rate of exchange (14% at CER) in 2018.This was primar ly due to the continued erosion of Nexium and Seroque/ by generic medicines, further price reduct ons on established brands and entry of an analogue for Symbicort in Australia, which had an impact onboth price and volume. Consequently, sales in 2018 declined at a greater rate compared to that seen in 2017.However, the pace of generic erosion has moderated notably with Crestor and Atacand, while the sales growth from new products such as Brilinta, Lynparza and the Diabetes portfolio has continued. Brilinta, Lynparza and the Diabetes portfolio grew by 4% at actualrate of exchange (6% at CER), 41% at actual rate of exchange (43% at CER) and 4% at actual rate of exchange (6% at CER),respectively. Despite these condit ons, we continued to launch innovative medicines across Europe and saw encouraging performance for certain products across our Growth Platforms, in particular with Forxiga, Brilinta, Fasenra, Lynparza and Tagrisso. Oncology sales in Europe grew by 19% {14% at CER},partly driven by the approval of Tagrisso for the treatment of patients in the 1st line EGFRm setting in June 2018. Lynparza sales grew by 46% {41% at CER), partly benefiting from the approval in May 2018 for its use as a tablet based treatment for platinum-sensiti ve ovarian cancer,regardless of BRCA status.Brilique sales growth of 18% (13% at CER) was accompanied by Forxiga sales growth of 30% (24% at CER).Fasenra was successfully launched in several European countries, with a strong initial uptake. Though widespread adoption of a broad national price control scheme in the near future is unlikely,we continue to comply with new state-levelregulations in this area and we recognise the sustained potential for substantial changes to laws andregulations regarding drug pricing that could have a significant impact on the pharmaceutical industry. EstablishedROW comprises Australia and New Zealand. Canada and Japan. 31 AstraZeneca Annual Report & Fonn 20-F Information 2018/ Business Revtew