Exhibit 99.1

DX - 2930 Phase 1b Data Review MARCH 31, 2015

This presentation contains forward - looking statements, including statements regarding the prospects for therapeutic benefits and treatment advantages of an investigational product, DX - 2930, being developed by Dyax for hereditary angioedema (HAE). Statements that are not historical facts are based on our current expectations, beliefs, assumptions, estimates, forecasts an d projections about the industry and markets in which Dyax competes. The statements contained in this presentation are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results to differ materia lly from what is expressed in such forward - looking statements. We caution investors not to place undue reliance on such forward - looking statements. There are many factors that could cause actual results to differ materially from those in these forward - looking statements. Thes e factors include the following: • The results from our Phase 1b study may not be predictive of the results or success of future clinical trials that will be re qui red to permit application for regulatory approval of DX - 2930; • even if DX - 2930 progresses through clinical trials and gains regulatory approval, it may not gain market acceptance; • others may develop technologies or products superior to DX - 2930 or that reach the market before DX - 2930; • Dyax is dependent on the expertise, effort, priorities and contractual obligations of third parties in the manufacture, quali ty control, storage and clinical development of DX - 2930; • t he costs of prosecuting, maintaining, defending and enforcing our patents and other intellectual property rights; • t he overall condition of the financial markets; and • a variety of other risks common to our industry. Additional factors that could cause actual results to differ materially from those projected or suggested in any forward - looking statements are contained in our recent annual and quarterly reports filed with the Securities and Exchange Commission, includ ing those factors discussed under the caption "Risk Factors" in such filings, which are incorporated in this presentation by this reference. Forward - looking statements speak only as of the date of this presentation, and we undertake no obligation to update o r revise them, except as may be required by law. Forward Looking Statements 2

DX - 2930 Phase 1b Data Review

DX - 2930 phase 1b data review Presenters Burt Adelman, Executive Vice President of R&D and Chief Medical Officer Ryan Iarrobino, Senior Director Clinical Development Dan Sexton, Distinguished Scientist Yung Chyung, Vice President Medical Research 4

5 DX - 2930 phase 1b study Key analyses: Safety & tolerability Pharmacokinetic profile Pharmacodynamic bioactivity Efficacy in preventing HAE attacks M ulti - center , randomized, placebo - controlled, multiple ascending dose trial in subjects with HAE

6 DX - 2930 phase 1b study design 3 dosing cohorts originally planned: 30 mg, 100 mg, 300 mg Subjects randomized to active drug vs placebo in 2:1 ratio (each subject receives 2 doses separated by 14 days ) Follow - up visits occur through 15 weeks following second dose Protocol allows for expansion of additional subjects and additional dose levels

7 Expansion and data analysis 16 additional subjects randomized to 400 mg DX - 2930 or placebo (overall total of 37 treated subjects) Data analysis was conducted after all subjects in the 400 mg dose group completed Day 50 follow - up visit Analyses include safety, pharmacokinetics, pharmacodynamics, and efficacy Study is ongoing until all subjects in 400 mg dose group reach Day 120

8 Subject disposition and demographics Disposition • One subject in the 400 mg dose group received only one dose and was replaced. • No subjects discontinued due to adverse events . DX - 2930 and Placebo were balanced in terms of age, race , ethnicity, and BMI. • Slightly more females in DX - 2930 (67%) vs placebo (54 %) Dose (mg) Number of subjects 30 4 100 4 300 5 400 11 Placebo 13
Phase 1b Safety
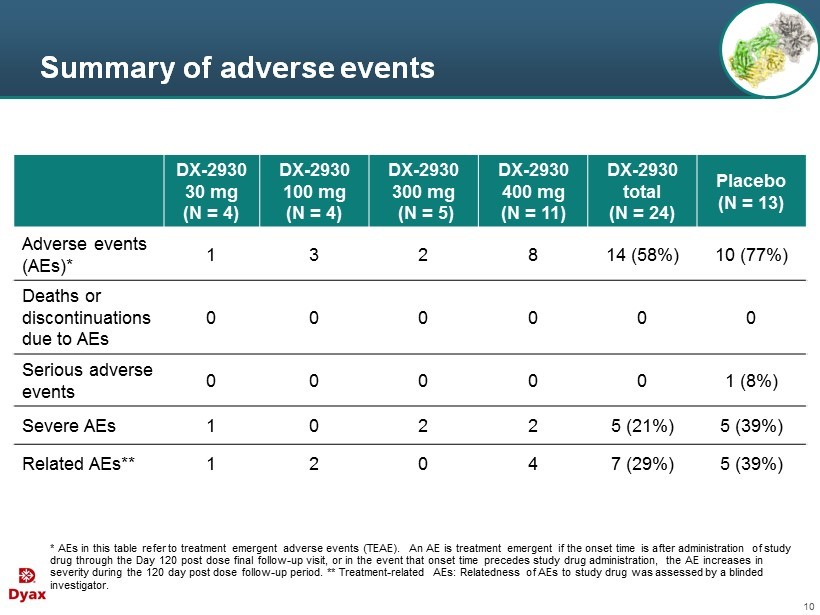
10 Summary of adverse events DX - 2930 30 mg (N = 4) DX - 2930 100 mg (N = 4) DX - 2930 300 mg (N = 5) DX - 2930 400 mg (N = 11) DX - 2930 total (N = 24) Placebo (N = 13) Adverse events (AEs)* 1 3 2 8 14 (58%) 10 (77%) Deaths or discontinuations due to AEs 0 0 0 0 0 0 Serious adverse events 0 0 0 0 0 1 (8%) Severe AEs 1 0 2 2 5 (21%) 5 (39%) Related AEs** 1 2 0 4 7 (29%) 5 (39%) * AEs in this table refer to treatment emergent adverse events (TEAE). An AE is treatment emergent if the onset time is after administration of study drug through the Day 120 post dose final follow - up visit, or in the event that onset time precedes study drug administration, th e AE increases in severity during the 120 day post dose follow - up period . ** Treatment - related AEs: Relatedness of AEs to study drug was assessed by a blinded investigator.

11 Adverse events Most common AEs were HAE attacks, injection site pain, and headache; r ates not appreciably higher in DX - 2930 2 subjects with 3 related severe TEAEs • One DX - 2930 subject (30 mg) with injection site pain lasting 1 minute • One DX - 2930 subject (400 mg) with worsening headache lasting 1 minute and night sweats

12 Summary of safety assessments No safety signals identified for ● Vital signs ● P hysical examinations ● Clinical laboratory tests ● Electrocardiogram (ECG)

13 Summary of safety R esults suggest DX - 2930 was well tolerated in this study N o evidence of dose - limiting toxicity at doses up to 400 mg

Phase 1b Pharmacokinetics and Pharmacodynamics

15 Mean DX - 2930 concentration following SC administration to HAE subjects Pharmacokinetics Note: 400 mg PK analysis excludes one patient who received only 1 dose of DX - 2930. C max ( ng /ml) T max (day) t ½ (day) 30 mg 4063.2 20 14.3 100 mg 7728.1 21 14.7 300 mg 31275.7 19.6 13.8 400 mg pending pending pending 0 10000 20000 30000 40000 50000 0 20 40 60 80 100 120 DX - 2930 ( ng /mL ) Days 30 mg (n=4) 100 mg (n=4) 300 mg (n=5) 400 mg (n=10)

16 Levels of HMWK cleavage in citrated plasma 300 and 400 mg DX - 2930 reduce biomarker activity in HAE subjects to levels seen in healthy subjects One - Way Analysis of Variance. %2 - chain in each dose group at day 8 and 22 compared to all subject predose %2 - chain. error bars = SEM. HV = healthy volunteer. 0.0 10.0 20.0 30.0 40.0 50.0 60.0 70.0 Pre - dose Day 8 Day 22 P=0.041 P<0.001 P<0.001 P=0.004 HV (8.3%) % 2 - Chain Predose (N=35) 30 mg (N=4) 100 mg (N=4) 300 mg (N=5) 400 mg (N=9)

Phase 1b Efficacy

18 Primary efficacy assessment period 300 mg DX - 2930 400 mg DX - 2930 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day Notable drug exposure from day 8 to day 50

19 Prospective primary efficacy analysis Included subjects with a minimum baseline history of at least 2 attacks in the 3 months (0.15 attacks/week) prior to study ● 11 of 13 placebo subjects ● 15 of 16 subjects treated with 300 or 400 mg DX - 2930 Baseline HAE attack rates (attacks/week) ● Placebo = 0.39 ● 300 mg = 0.33 ● 400 mg = 0.55 ● 300 and 400 mg combined = 0.49 Intent - to - treat (ITT) analysis ● Repeated measurements with ANOVA employing baseline attack rates as a covariate

20 Primary efficacy analysis (Day 8 to 50) DX - 2930 300 mg (N = 4) DX - 2930 400 mg (N = 11) DX - 2930 Combined 300 and 400 mg (N = 15) % Reduction vs placebo 100 88 91 P - value vs placebo * <0.0001 0.005 0.0012 Note: Only subjects who have a baseline attack rate of at least 2 attacks in the last 3 months are included. * Mixed Model Repeated Measurements with Analysis of Variance (baseline attack frequency as covariate) and assuming Poisson distribution.

21 Observed attack rates (over placebo) Note: Baseline is defined as historical HAE attacks over last 3 months prior to dosing. Only includes patients with a baseline rate of ≥ 2 attacks in the last 3 months. Day 8 to 50 a ttack rates are adjusted for baseline rates. Percent reduction in HAE attack rate over placebo and p - value calculated based upon Mixed Model Repeated Measurements with Analysis of Variance (baseline attack rate as covariate) and assuming Poiss on distribution. 0 0.1 0.2 0.3 0.4 0.5 0.6 Baseline Day 8 to 50 HAE Attack Rate Per Week 300 mg (n = 4) 400 mg (n = 11) Combined (n = 15) Placebo (n = 13) 88% P=0.005 100% P<0.0001 91% P=0.0012
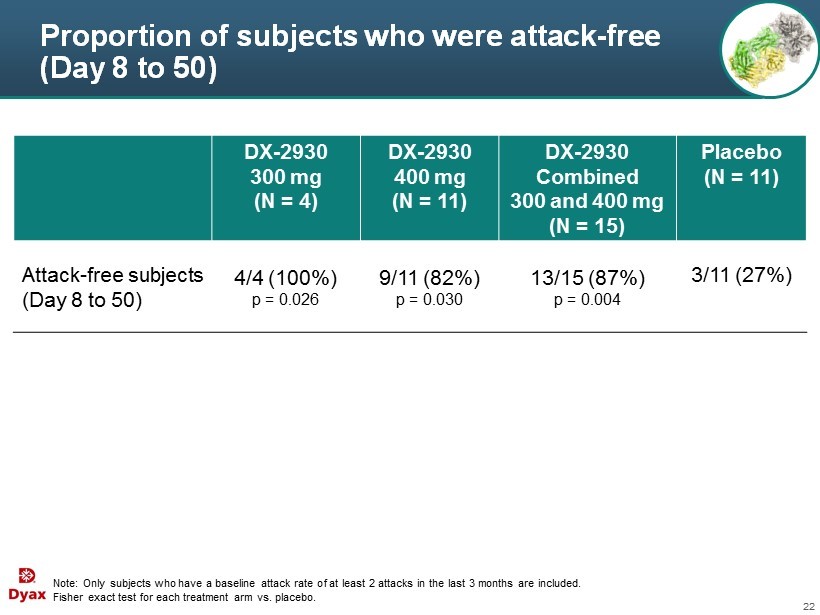
22 Proportion of subjects who were attack - free (Day 8 to 50) Note: Only subjects who have a baseline attack rate of at least 2 attacks in the last 3 months are included . Fisher exact test for each treatment arm vs. placebo. DX - 2930 300 mg (N = 4) DX - 2930 400 mg (N = 11) DX - 2930 Combined 300 and 400 mg (N = 15) Placebo (N = 11) Attack - free subjects (Day 8 to 50) 4/4 (100%) p = 0.026 9/11 (82%) p = 0.030 13/15 (87%) p = 0.004 3/11 (27%)

23 Characteristics of HAE attacks (Day 8 to 50) DX - 2930 300 mg (N = 4) DX - 2930 400 mg (N = 11) Placebo (N = 11) Attacks 0 3 24 Primary Attack Location Peripheral 0 3 10 Abdominal 0 0 13 Laryngeal 0 0 1 Attack Severity Mild 0 1 8 Moderate 0 1 6 Severe 0 1 10 Acute attacks requiring treatment 0 2 22

24 Modified intent - to - treat ( M ITT) analysis Goal: Evaluate clinical effects in the context of HAE patients receiving the dose regimen as specified Post - hoc analysis Same as ITT population except 2 subjects excluded ● 1 subject received only 1 administration of DX - 2930 (subject discontinued for reasons unrelated to treatment) – Subject had 2 peripheral attacks within day 8 to 50 ● 1 subject did not have HAE type I or II – No attacks during day 8 to 50 Note: Only subjects who have a baseline attack rate of at least 2 attacks in the last 3 months are included .

25 Primary efficacy analysis (Day 8 to 50) (modified intent - to - treat analysis) Note: Only subjects who have a baseline attack rate of at least 2 attacks in the last 3 months are included . M ITT population excludes 2 subjects, one subject without type I or II HAE and one subject who received only 1 administration of DX - 2930. * Mixed Model Repeated Measurements with Analysis of Variance (baseline attack frequency as covariate) and assuming Poisson distribution. DX - 2930 300 mg (N = 4) DX - 2930 400 mg (N = 9) DX - 2930 Combined 300 and 400 mg (N = 13) % Reduction vs placebo 100 95 97 P - value vs placebo * <0.0001 0.0022 0.0007

26 Attack incidence plotted against mean PK curves Drug Concentration Time Attacks o bserved Few or n o attacks Attacks r e - emerge Hypothesis: Higher DX - 2930 drug levels should be associated with few or no attacks Note: Hypothetical data for illustrative purposes.
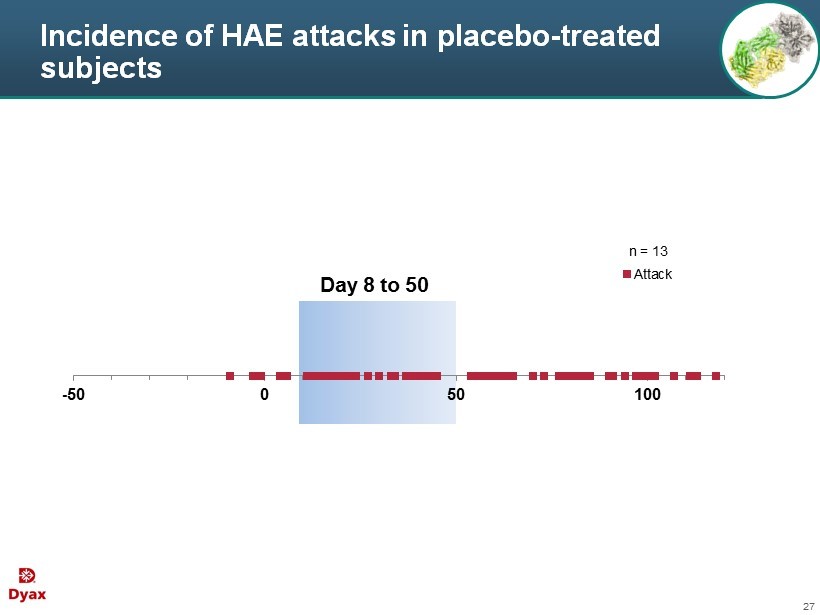
27 Incidence of HAE attacks in placebo - treated subjects -50 0 50 100 Attack Day 8 to 50 n = 13

28 Mean PK plots and HAE attack incidence following 30 and 100 mg DX - 2930 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 30 mg (n = 4) Attack 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 100 mg (n = 4) Attack Day 8 to 50 Day 8 to 50

29 Mean PK plots and HAE attack incidence following 300 mg DX - 2930 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 300 mg (n = 5) Attack Day 8 to 50

30 Mean PK plots and HAE attack incidence following 400 mg DX - 2930 Note: Excluding patient who received only one dose (this was necessary to derive the mean pharmacokinetic curve). 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 -50 0 50 100 DX - 2930 (ng/mL) Study Day 400 mg (n = 10) Attack Day 8 to 50

31 DX - 2930 concentration and HAE attacks in patients with historical attack rate ≥ 9 attacks in past 3 months -45 5 55 105 DX - 2930 (ng/mL) Study Day Placebo Historically: 35 attacks in last 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 300 mg Historically: 9 attacks in last 3 months attack DX - 2930

32 DX - 2930 concentration and HAE attacks in patients with historical attack rate ≥ 9 attacks in past 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 400 mg Historically: 9 attacks in last 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 400 mg Historically: 36 attacks in last 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 400 mg Historically: 9 attacks in last 3 months 0 10,000 20,000 30,000 40,000 50,000 -45 5 55 105 DX - 2930 (ng/mL) Study Day 400 mg Historically: 9 attacks in last 3 months attack DX - 2930

33 Summary of phase 1b No apparent safety signals have emerged to date for DX - 2930 PK profile is consistent with that of a monoclonal antibody, supporting dosing once every 2 weeks (or less frequently) Kininogen biomarker assays demonstrate DX - 2930 normalizes instability of HAE plasma A highly statistically significant result of 100% and 88% reduction in HAE attacks over placebo in the 300 and 400 mg DX - 2930 treatment groups, respectively

Regulatory Update 34

Regulatory update • US Status • Orphan Drug designation • Fast Track designation • US Next Steps • Phase 1b data to inform the design and discussion of the Phase 3 pivotal trial design with the FDA • EU Next Steps • Meet with EU regulators for scientific advice regarding evidence required for approval 35

DX - 2930 Phase 1b Data Review MARCH 31, 2015 For additional information, please contact: Jennifer Robinson 617 - 250 - 5741 jrobinson@dyax.com



































