Table of Contents
Filed Pursuant to 424(B)(3)
Registration No. 333-118821
The information in this preliminary prospectus supplement and the accompanying prospectus is not complete and may be changed. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS SUPPLEMENT | Subject to Completion | January 24, 2005 |
(To Prospectus dated December 6, 2004)
7,000,000 Shares

Common Stock
We are offering all of the 7,000,000 shares of our common stock offered by this prospectus supplement. We will receive all of the net proceeds from the sale of such common stock.
Our common stock is quoted on The Nasdaq National Market under the symbol “NUVO.” The last reported sale price for our common stock on January 20, 2005 was $9.19 per share.
Investing in our common stock involves a high degree of risk. Before buying any shares you should carefully read the discussion of material risks of investing in our common stock under the heading “Risk factors” beginning on page S-9 of this prospectus supplement and on page 1 of the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus are truthful or complete. Any representation to the contrary is a criminal offense.
| Per share | Total | |||
Public offering price | $ | $ | ||
Underwriting discounts and commissions | $ | $ | ||
Proceeds, before expenses, to us | $ | $ |
The underwriters may also purchase up to an additional 1,050,000 shares of common stock from us at the public offering price, less underwriting discounts and commissions payable by us to cover over-allotments, if any, within 30 days from the date of this prospectus supplement. If the underwriters exercise the option in full, the total underwriting discounts and commissions will be , and the total proceeds, before expenses, to us will be $ .
The underwriters are offering the shares of common stock as set forth under “Underwriting.” Delivery of the shares will be made on or about February , 2005.
| Sole Book-Running Manager | ||
| UBS Investment Bank | Deutsche Bank Securities |
| CIBC World Markets | Needham & Company, Inc. |
Table of Contents
You should rely only on the information contained or incorporated by reference in this prospectus supplement and the accompanying prospectus. We have not, and the underwriters have not, authorized anyone to provide you with additional or different information. We are not making an offer of these securities in any jurisdiction where the offer is not permitted. You should assume that the information in this prospectus supplement is accurate only as of the date on the front of the document and that any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus supplement and the accompanying prospectus or of any sale of our common stock. Unless the context otherwise requires, references to “we,” or the “company” in this prospectus supplement and the accompanying prospectus mean Nuvelo, Inc. and its subsidiaries.
| Prospectus Supplement | Page | |
| S-1 | ||
| S-9 | ||
| S-32 | ||
| S-32 | ||
| S-33 | ||
| S-33 | ||
| S-34 | ||
| S-36 | ||
| S-37 | ||
| S-40 | ||
| S-43 | ||
| S-43 | ||
| S-43 | ||
| S-43 | ||
| Prospectus | Page | |
| 1 | ||
| 1 | ||
| 1 | ||
| 2 | ||
| 2 | ||
| 2 | ||
| 3 | ||
| 12 | ||
| 14 | ||
| 16 | ||
| 18 | ||
| 20 | ||
| 20 | ||
| 20 | ||
We own or have rights to use trademarks or trade names that we use in conjunction with the operation of our business. Nuvelo is a registered trade and service mark of ours. All other trademarks, service marks and trade names referred to in this prospectus supplement or the accompanying prospectus are the property of their respective owners.
Table of Contents
This summary highlights information contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. This summary does not contain all of the information that you should consider before investing in our common stock. You should carefully read the entire prospectus supplement and the accompanying prospectus, including the “Risk factors” section, as well as the financial statements and the other information incorporated by reference herein before making an investment decision.
BUSINESS OVERVIEW
We are a biopharmaceutical company strategically focused on the discovery, development and commercialization of therapeutics for the treatment of acute cardiovascular indications and cancer.
We currently have three drug candidates in clinical trials. Our lead drug candidate, alfimeprase, is a thrombolytic agent, or blood clot dissolver. In 2004, we completed two separate Phase 2 clinical trials for alfimeprase for the treatment of acute peripheral arterial occlusion, or PAO, and catheter occlusion. We anticipate initiating a Phase 3 trial for alfimeprase in acute PAO in the first half of 2005 and a Phase 3 trial for alfimeprase in patients with occluded central venous catheters in the second half of 2005.
Our second drug candidate, recombinant nematode anticoagulant protein c2, or rNAPc2, is a recombinant version of a naturally occurring protein that has anticoagulant properties. These properties arise from its ability to block the factor VIIa/tissue factor protease complex, which is responsible for the initiation of the process leading to blood clot formation. rNAPc2 is currently undergoing a Phase 2a double-blind, placebo-controlled clinical trial for use in treating acute coronary syndromes, or ACS, including unstable angina, or UA, and non-ST segment elevation myocardial infarction, or NSTEMI. We expect to complete enrollment of this trial in the first half of 2005.
Our third drug candidate is ARC183, a novel thrombin inhibitor which is currently in a Phase 1 clinical development program for use as an anticoagulant in coronary artery bypass graft, or CABG, surgery. We entered into a 50/50 cost/profit sharing collaboration agreement with Archemix Corporation in January 2004 for the development and commercialization of ARC183. We anticipate completing enrollment of the Phase 1 clinical program for ARC183 in the first half of 2005.
We have exclusive worldwide rights to develop and commercialize alfimeprase and, for the indications we are currently pursuing, rNAPc2, and we share worldwide commercialization rights to ARC183 with Archemix.
In addition to our clinical and development stage drug candidates, we have an on-going discovery effort that is focused on therapeutic secreted proteins and antibody targets. Our secreted protein program includes our collaboration with the pharmaceutical division of Kirin Brewery Company, Ltd. and our internal discovery program. Our antibody program is focused on screening our proprietary gene sequence collection to identify proteins located on the surface of tumor cells that could be targeted by therapeutic monoclonal antibodies. We recently initiated pre-clinical studies on our first internally-discovered drug candidate, NU206. We expect to leverage discoveries in our research programs to extend and expand our drug pipeline and to create revenue-generating licensing and partnering arrangements.
S-1
Table of Contents
OUR LEAD DRUG CANDIDATES
ALFIMEPRASE
Alfimeprase, our lead development candidate, recently completed Phase 2 clinical trials in two distinct indications, acute PAO and catheter occlusion. Alfimeprase is a thrombolytic agent, or blood clot dissolver, with a novel mechanism of action. It is a modified and recombinant version of fibrolase, a naturally occurring enzyme that directly degrades fibrin, the protein that provides the structural scaffold of blood clots. Thrombolytics currently on the market such as urokinase (Abbokinase) or alteplase (Activase), are plasminogen activators that work by activating plasminogen to form plasmin which, in turn, degrades fibrin. In contrast, alfimeprase directly degrades fibrin, creating the potential for more rapid clot dissolution or lysis. Alfimeprase is locally delivered at the site of the blood clot and is inactivated quickly by a naturally occurring protein in the bloodstream. We believe this clearance mechanism limits the amount of drug in systemic circulation and implies that patients may experience fewer associated side effects. Phase 2 clinical data suggest that alfimeprase has the potential to rapidly lyse clots while also reducing the bleeding complications resulting from currently available agents.
Alfimeprase was identified through a research program at Amgen Inc. In January 2002 we entered into a 50/50 cost/profit sharing arrangement with Amgen for the development and commercialization of alfimeprase. In October 2004, Amgen exercised its rights pursuant to the terms of this collaboration agreement to terminate its collaboration with us and enter instead into an exclusive license whereby we are granted the worldwide rights to develop and commercialize alfimeprase in exchange for the payment to Amgen of previously negotiated milestone payments and royalties. Under the terms of our license agreement with Amgen, Amgen will transfer the technology necessary for the manufacture of alfimeprase to us or to a manufacturer acceptable to Amgen. Amgen is required to continue to supply alfimeprase to us during the transition period. On January 21, 2005, we entered into an Interim Agreement with Avecia Limited for the manufacture of alfimeprase, and we are currently in negotiations with Avecia for a definitive agreement. In connection with the termination of the collaboration agreement with Amgen, we also entered into an opt-out, termination, settlement and release agreement with Amgen in October 2004, whereby we made a payment of $8.5 million to Amgen, of which $8.3 million was related to the remaining reimbursement of its manufacturing costs incurred under the collaboration agreement.
Alfimeprase in Acute Peripheral Arterial Occlusion (PAO)
Our lead medical indication for alfimeprase is acute PAO. Acute PAO is a significant cause of morbidity in the United States with over 100,000 cases reported annually. Acute PAO occurs when arterial blood flow is blocked to a distant part of the body, usually the leg, by a blood clot. Traditionally, bypass surgery and angioplasty have been used to treat acute PAO. However, thrombolytic agents such as urokinase (Abbokinase) or alteplase (Activase) have been increasingly used as a less-invasive alternative, even though they have not received regulatory approval to treat acute PAO. Studies have shown that patients receiving current thrombolytic therapies experience intracerebral hemorrhage at rates of between one to two percent. We believe alfimeprase has the potential to be a more effective agent than existing agents for use in treating acute PAO by reducing the treatment time and potential bleeding side effects.
We completed our Phase 2 alfimeprase trial in patients with acute PAO in the second quarter of 2004. This trial was a multi-center, open label, dose-escalation study to evaluate the safety and activity of alfimeprase, and involved 113 patients across centers in the United States, Western Europe, Hungary, Russia and South Africa. The Phase 2 results indicate that alfimeprase has the potential to offer significant advances in the rapid resolution of a clot while minimizing potentially fatal side effects such as hemorrhagic stroke and other bleeding complications. In analysis of the Phase 2 results, alfimeprase showed potential to break up blood clots within four hours of initiation of dosing with rates of up to 76 percent, and partial or complete clot lysis and restoration of arterial flow with rates of up to 60 percent. Up to 69 percent of study patients were able to avoid open vascular surgical intervention in
S-2
Table of Contents
the 30 days following treatment with alfimeprase. Among the 113 patients enrolled, there were no intracerebral hemorrhages or deaths at 30 days. There were 7 major bleeding events reported.
Of these, only one was categorized by the investigator as possibly related to alfimeprase. Incidents of transient hypotension were also reported and were dose related.
We expect to initiate a multi-center, multi-national, randomized, double-blind, placebo-controlled Phase 3 program to determine the efficacy and safety of alfimeprase for the treatment of patients with acute PAO in the first half of 2005. This Phase 3 program, also known as NAPA-2, or Novel Arterial Perfusion with Alfimprase-2, will be led by Dr. Kenneth Ouriel, chairman of the division of surgery at the Cleveland Clinic and Dr. Gunnar Tepe, associate professor of radiology in the department of diagnostic radiology at the University of Tubingen, Germany. This program is expected to consist of two overlapping trials that will include a total of approximately 700 patients, who will be randomized to receive either 0.3 mg/kg of alfimeprase or placebo. The primary endpoint will be avoidance of open vascular surgery within 30 days. Secondary endpoints will include restoration of arterial blood flow and increase in ankle brachial index, which is a measure of ankle blood pressure. We have obtained orphan drug status for alfimeprase in the United States for the treatment of acute PAO, which may provide us with seven years of market exclusivity in the United States.
Alfimeprase in catheter occlusion
Our second medical indication for alfimeprase is catheter occlusion. Catheter occlusion is the obstruction of blood flow through a central venous catheter by a blood clot. It is estimated that about five million catheters are implanted in patients each year in the United States, and approximately 25% become occluded. Current treatment for catheter occlusion includes invasive surgery to remove and replace the catheter, or treatment with alteplase (Cathflo Activase). Based on clinical trial evidence of alfimeprase’s activity, we believe alfimeprase has the potential to restore flow to these occluded catheters more rapidly than Cathflo Activase.
In the third quarter of 2004 we announced that we had closed patient enrollment in a Phase 2 multi-center, double-blind, randomized study in patients with occluded central venous catheters comparing three doses (0.3 mg, 1.0 mg and 3.0 mg) of alfimeprase against the approved dose of Cathflo Activase (2.0 mg). We treated 55 patients in this U.S. trial. The alfimeprase 3.0 mg dose produced cumulative flow rates of 50% at 15 minutes after the first dose, 60% at 120 minutes after the first dose, and 80% at 120 minutes after the second dose. This is compared to CathfloActivase (2.0 mg) which produced flow rates of 0% at 15 minutes after the first dose, 46% at 120 minutes after the first dose, and 62% at 120 minutes after the second dose. No major hemorrhagic events were reported in any treated patients and only one patient had a catheter-related infection. Results from this Phase 2 study support further evaluation of alfimeprase in fixed doses ranging from 1.0 mg to 3.0 mg for the treatment of occluded catheters. We expect to initiate a Phase 3 pivotal trial of alfimeprase in catheter occlusion in the second half of 2005.
rNAPc2
Our second drug candidate, rNAPc2, is a recombinant version of a naturally occurring protein that has anticoagulant properties. Specifically, rNAPc2 has been shown to block the factor VIIa/tissue factor protease complex, which is responsible for the initiation of the process leading to blood clot formation. Compared to other commercially available anticoagulants, which all exert their effects at later stages of the blood coagulation cascade, rNAPc2 is designed to block the first step in the clotting cascade. By blocking the coagulation cascade before amplification of the coagulation process, rNAPc2 could prove to be more effective in treating patients with conditions such as acute coronary syndrome or as a prophylactic against clot formation in conditions such as deep venous thrombosis.
ACS occurs when an atherosclerotic plaque ruptures in a coronary artery which triggers the coagulation cascade and results in the formation of a blood clot. The clot blocks the flow of blood to the heart
S-3
Table of Contents
muscle, depriving it of oxygen and causing chest pain and, if severe, permanent heart muscle death. In the United States, ACS accounts for approximately 1.4 million hospital admissions annually. Patients with ACS are traditionally given aspirin and heparin, among other agents, to stabilize their medical condition. Recent guidelines also recommend the addition of the antiplatelet agent clopidogrel (Plavix) to the standard of care. However, based upon the significant number of patients with ACS who continue to experience poor outcomes such as recurrent angina, myocardial infarction or death, we believe there is a clear need for better antithrombotic therapy.
rNAPc2, given alone or with standard therapy, may reduce the risk of subsequent heart attack or death in patients suffering from ACS. Unlike aspirin and heparin, or current antithrombotic agents, which all exert their effects at later stages of the blood coagulation cascade, rNAPc2 blocks the first step in the clotting cascade. A medical regimen that includes rNAPc2 could, therefore, enable a multi-pronged attack at several points along the blood coagulation process. Alternatively, by stopping coagulation at the outset, rNAPc2 could also prove effective as a stand-alone therapy.
We licensed the worldwide rights for all indications of rNAPc2 and all of the rNAPc molecules owned by Dendreon Corporation in February 2004. The United States government may claim a non-exclusive right to use rNAPc2 with respect to the treatment of hemorrhagic fever. We don’t currently contemplate development of rNAPc2 to treat hemorrhagic fever. To date, rNAPc2 has been shown to be well-tolerated in over 500 patients and healthy volunteers in several Phase 1 and 2 studies.
In May of 2004 we reinitiated a Phase 2a double-blind, placebo controlled clinical trial to determine a safe and effective dose of rNAPc2 in moderate to high-risk patients with ACS. The study is being conducted in three parts, each of which is investigating rNAPc2 in combination with current anticoagulant and antiplatelet therapies. Currently, the study is being conducted with the TIMI Study Group led by Dr. Eugene Braunwald of Brigham and Women’s Hospital and Harvard Medical School. We plan to complete patient enrollment of the Phase 2a study in the first half of 2005.
ARC183
Our third drug candidate, ARC183, is a DNA aptamer, a single-stranded nucleic acid that binds to thrombin with high affinity and specificity. The key advantage of ARC183 compared to other thrombin inhibitors is its rapid onset of action and short half-life, giving it the potential to be highly effective for medical procedures that require rapid reversal of anticoagulation shortly after the procedure is completed.
In January of 2004 we announced a collaboration agreement with Archemix, a privately held biotechnology company, located in Cambridge, Massachusetts, for the development and commercialization of ARC183. Our lead indication for ARC183 is as a thrombin inhibitor for use in CABG surgery.
According to the American Heart Association, more than 500,000 CABG procedures are performed in the United States annually. Currently, heparin is used to limit blood clotting in this indication, but it is difficult to dose and can cause side effects such as bleeding and heparin-induced thrombocytopenia, or HIT. Moreover, the effect of heparin must be reversed with the use of an antidote called protamine. Protamine is not approved by the FDA for reversal of heparin in CABG surgery and is associated with significant complications including hypotension, platelet dysfunction, complement activation and thrombus formation. We believe that there is a significant unmet medical need for a safe, fast-acting anticoagulant for use in CABG surgery that is easier to administer, does not require a reversal agent and limits adverse side effects such as bleeding and HIT.
S-4
Table of Contents
ARC183 has shown potential in pre-clinical studies to be equally effective, with fewer side effects, than heparin and protamine in combination. Due to its very short half-life, we believe ARC183 has the potential for more predictable dosing as well as reduced incidence of bleeding side effects compared to heparin.
In August 2004 we and our partner, Archemix, initiated a Phase 1 clinical program for ARC183 for use in CABG surgery. These studies are evaluating the safety, tolerability, anticoagulation activity and titratability of ARC183. We expect to complete enrollment of the Phase 1 clinical program of ARC183 in the first half of 2005.
RESEARCH AND DEVELOPMENT PROGRAMS
In addition to our clinical and development stage drug candidates, we have an ongoing discovery program focused on the identification of novel human genes that encode proteins with therapeutic potential. Over the long-term, we intend to develop additional product opportunities from our ongoing discovery efforts focused on secreted proteins and antibody targets.
In the second half of 2004, we initiated pre-clinical studies for our first internally-generated drug candidate, NU206. In addition to the development of internal therapeutic candidates, we intend to leverage these discoveries to create revenue-generating licensing and partnering arrangements.
The secreted protein program includes our collaboration with Kirin and our internal discovery program. We have already advanced several secreted protein candidates to more extensive studies to better define their therapeutic utility based upon early findings in initial mouse models. Within our internal secreted protein discovery program, we have developed a fast and efficient method of expressing human secreted proteins in mice. This program could significantly bolster our ability to identify which secreted proteins within our patent estate have the greatest potential for therapeutic use.
The antibody program is focused on screening our proprietary gene sequence collection to identify proteins located on the surface of tumor cells that could be targeted by therapeutic monoclonal antibodies.
PRODUCT PIPELINE
The following table summarizes key information about our current product pipeline:
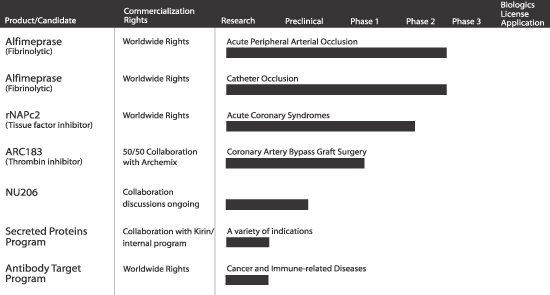
S-5
Table of Contents
OUR STRATEGY
We are focused on building a successful biopharmaceutical business and committed to creating a valuable product-focused company that leverages our drug discovery and development expertise. Key elements of our strategy are to:
Successfully develop and commercialize our lead drug candidate, alfimeprase
We are seeking to successfully develop and commercialize our lead drug candidate, alfimeprase, for the treatment of acute PAO and catheter occlusion. We recently completed Phase 2 clinical trials in these two indications, and we expect to initiate pivotal, Phase 3 trials in both indications in 2005. We have acquired worldwide, exclusive rights to this compound and are currently exploring potential partnering opportunities that would enable us to participate in its commercialization, particularly in the United States.
Leverage our expertise in cardiovascular disease to advance our clinical development program
We are primarily focused on the development of acute, hospital-based, cardiovascular drug candidates. We believe this portfolio leverages our expertise in cardiovascular drug development, provides synergy with alfimeprase during both development and commercialization and enables us to pursue a more rapid path toward drug development.
Build a diversified pipeline of product candidates
We are pursuing several drug development candidates in various stages of clinical and pre-clinical development. We believe this strategy reduces our exposure to the impact of any single product failure and increases our flexibility to eliminate programs we deem less promising. By broadening our product portfolio, we intend to increase the probability of clinical and commercial success. In addition, we focus on molecules that we believe have a greater chance of success due to the predictability of pre-clinical models used in their development.
Opportunistically seek to license or acquire complementary products and technologies
We intend to supplement our internal drug discovery efforts through the acquisition of products and technologies that complement our development strategy. We continue to identify, evaluate and pursue the acquisition or licensing of strategically valuable product opportunities.
CORPORATE INFORMATION
We were incorporated as “Hyseq, Inc.” in Illinois in 1992 and reincorporated in Nevada in 1993. On January 31, 2003, we merged with Variagenics, Inc., a publicly traded Delaware corporation based in Massachusetts, and, in connection with the merger, changed our name to “Nuvelo, Inc.” On March 25, 2004, we reincorporated from Nevada to the State of Delaware. Our principal executive offices are located at 675 Almanor Avenue, Sunnyvale, California 94085 and our telephone number is (408) 215-4000. Our World Wide Web address is http://www.nuvelo.com. We have not incorporated by reference into this prospectus supplement or the accompanying prospectus the information contained on our website and you should not consider it to be part of this prospectus supplement or the accompanying prospectus.
S-6
Table of Contents
The offering
Common stock we are offering | 7,000,000 shares | |
Common stock to be outstanding after this offering | 39,228,732 shares | |
Use of proceeds | We estimate the net proceeds to us from this offering will be approximately $59.9 million, after payment of underwriting discounts and commissions and estimated expenses of this offering, or approximately $68.9 million if the underwriters exercise their over-allotment option in full. We intend to use the net proceeds to us from this offering for general corporate purposes, including capital expenditures, working capital needs, current and future clinical trials of our lead drug candidate, alfimeprase, as well as other research and drug development activities. See “Use of proceeds.” | |
Nasdaq National Market Symbol | NUVO | |
Risk factors | See “Risk factors” beginning on page S-9 for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. | |
The number of shares of our common stock to be outstanding immediately after the closing of this offering is based on 32,228,732 shares of our common stock outstanding as of December 31, 2004, but excludes:
| Ø | an aggregate of 3,871,594 shares of our common stock issuable upon exercise of stock options outstanding as of December 31, 2004, granted under our 2004 Equity Incentive Plan, 2002 Equity Incentive Plan, 1995 Stock Option Plan, Non-Employee Director Stock Option Plan, Scientific Advisory Board/Consultants Stock Option Plan and the Variagenics, Inc. Amended 1997 Employee, Director and Consultant Stock Option Plan, and as of December 31, 2004, an aggregate of 895,075 shares of common stock issuable upon the exercise of stock options granted outside of any of our stock option plans, with exercise prices of all outstanding options ranging from $0.03 to $304.31 per share and a weighted average exercise price of $18.77 per share; |
| Ø | an aggregate of 3,760,298 shares of common stock reserved for issuance pursuant to future option grants under the 2004 Equity Incentive Plan, based on options outstanding as of December 31, 2004; |
| Ø | an aggregate of 56,736 shares of common stock issuable under our Employee Stock Purchase Plan as of December 31, 2004; |
| Ø | an aggregate of 1,516,792 shares of our common stock issuable upon the exercise of warrants, with exercise prices ranging from $4.05 to $25.53 per share, and a weighted average exercise price of $20.88 per share, outstanding as of December 31, 2004; and |
| Ø | 542,235 shares of common stock issuable at our option to repay our note held by Affymetrix and 907,113 shares of common stock issuable upon mutual agreement to convert the promissory note under the Rathmann line of credit, both as of December 31, 2004. |
Unless otherwise stated, all information contained in this prospectus supplement assumes that the underwriters do not exercise their over-allotment option to purchase up to an additional 1,050,000 shares of common stock and all currency amounts are in United States dollars.
S-7
Table of Contents
Summary consolidated financial data
The tables below present summary consolidated statement of operations and balance sheet data. The summary financial data for the years ended December 31, 2001 through December 31, 2003 are derived from our audited consolidated financial statements for those periods. The summary data for the nine month period ended September 30, 2004, is derived from our unaudited condensed consolidated financial statements for that period. This information is only a summary and should be read in conjunction with our historical consolidated financial statements and related notes contained in our annual reports, quarterly reports and recent current reports on file with the SEC incorporated by reference in this prospectus supplement and the accompanying prospectus. For more details on how you can obtain our SEC filings, you should read the section of this prospectus supplement entitled “Incorporation by reference” beginning on page S-43. Our consolidated statement of operations data includes the results of operations of Variagenics, Inc. from February 1, 2003. The as adjusted consolidated balance sheet data gives effect to the sale by us of 7,000,000 shares of our common stock in this offering, assuming a public offering price of $9.19 per share and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
| Year ended December 31, | Nine months ended September 30, | |||||||||||||||||||
| Consolidated statement of operations data: | 2001 | 2002 | 2003 | 2003 | 2004 | |||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||
Revenue | $ | 24,590 | $ | 26,433 | $ | 2,290 | $ | 1,940 | $ | 2,219 | ||||||||||
Research and development expenses | 46,506 | 50,157 | 33,084 | 26,421 | 34,845 | |||||||||||||||
General and administrative expenses | 13,452 | 18,108 | 17,223 | 14,384 | 6,667 | |||||||||||||||
Total operating expenses | 60,783 | 70,368 | 51,532 | 42,017 | 41,487 | |||||||||||||||
Interest income (expense), net | (572 | ) | (1,155 | ) | (945 | ) | (720 | ) | (239 | ) | ||||||||||
Net loss | (36,472 | ) | (44,978 | ) | (50,187 | ) | (40,797 | ) | (39,507 | ) | ||||||||||
Net loss per common share, basic and diluted | $ | (6.78 | ) | $ | (6.24 | ) | $ | (2.37 | ) | $ | (2.08 | ) | $ | (1.30 | ) | |||||
Shares used in computation of basic and diluted net loss per share | 5,386 | 7,220 | 21,054 | 19,656 | 30,427 | |||||||||||||||
| September 30, 2004 | ||||||||
| Consolidated balance sheet data: | Actual | As adjusted | ||||||
| (unaudited) | ||||||||
Cash, cash equivalents and short-term investments | $ | 70,671 | $ | 130,521 | ||||
Working capital | 53,663 | 113,513 | ||||||
Total assets | 100,339 | 160,189 | ||||||
Current portion of capital lease, note and line of credit obligations | 6,698 | 6,698 | ||||||
Non-current portion of capital lease, note and line of credit obligations | 9,886 | 9,886 | ||||||
Accumulated deficit | (243,066 | ) | (243,066 | ) | ||||
Total stockholders’ equity | 58,516 | 118,366 | ||||||
S-8
Table of Contents
Investing in our common stock involves a high degree of risk. You should consider carefully the risk factors described below, in the accompanying prospectus and all other information contained in or incorporated by reference in this prospectus supplement and the accompanying prospectus before deciding to invest in our common stock. If any of the following risks actually occur, they may materially harm our business, financial condition, operating results and cash flow. As a result, the market price of our common stock could decline, and you could lose all or part of your investment. Additional risks and uncertainties that are not yet identified or that we think are immaterial may also materially harm our business, operating results and financial condition and could result in a complete loss of your investment.
RISKS RELATED TO OUR BUSINESS
Development of our products will take years, and our products require regulatory approval before they can be sold.
We have three clinical stage drug candidates. All of our other potential products currently are in research or pre-clinical development and revenues from the sales of any products resulting from this research and development may not occur for several years, if at all. We cannot be certain that any of our products will be demonstrated to be safe and effective or that we will obtain regulatory approvals. We cannot predict whether we will be able to develop and commercialize any of our drug candidates successfully. If we are unable to obtain regulatory approval and successfully commercialize our potential products, our business, results of operations and financial condition will be affected in a materially adverse manner.
We do not yet have products in the commercial markets. We must demonstrate that our product candidates satisfy rigorous standards of safety and efficacy before the FDA and comparable agencies in foreign markets. We cannot apply for regulatory approval of our potential products until we have performed significant additional research and development and testing. We cannot be certain that we, or our strategic partners, will be permitted to undertake clinical testing of our potential products or continue clinical testing of alfimeprase, rNAPc2, or ARC183. If we are successful in initiating clinical trials, we may experience delays in conducting them. Our clinical trials may not demonstrate the safety and efficacy of our potential products, and we may encounter unacceptable side effects or other problems in the clinical trials that may prevent or limit the use of our products. Should this occur, we may have to delay or discontinue development of the potential product that causes the problem. After a successful clinical trial, we cannot market products in the United States until we receive regulatory approval. Even if we are able to gain regulatory approval of our products after successful clinical trials and then commercialize and sell those products, we may be unable to manufacture enough products to maintain our business, which could have a negative impact on our financial condition.
Our clinical trials may not yield results that will enable us to obtain regulatory approval for our products.
We will only receive regulatory approval for a drug candidate if we can demonstrate in carefully designed and conducted clinical trials that the drug candidate is safe and effective. We do not know whether our pending or any future clinical trials will demonstrate sufficient safety and efficacy to obtain the requisite regulatory approvals or will result in marketable products. Clinical trials are lengthy, complex, and expensive processes with uncertain results. It will take us several years to complete our testing, and failure can occur at any stage of testing. Results attained in pre-clinical testing and early clinical studies, or trials, may not be predictive of results that are obtained in later studies. We may suffer significant setbacks in advanced clinical trials, even after promising results in earlier studies. Based on results at any stage of clinical trials, we may decide to repeat or redesign a trial or discontinue development of one or more of our drug candidates. If we fail to adequately demonstrate the safety and efficacy of our products
S-9
Table of Contents
Risk factors
under development, we will not be able to obtain the required regulatory approvals to commercialize our drug candidates, and our business, results of operations and financial condition will be materially adversely affected.
Clinical trials are subject to continuing oversight by governmental regulatory authorities and institutional review boards, or IRBs, and must meet the requirements of these authorities in the United States, including those for informed consent and good clinical practices. We may not be able to comply with these requirements and the FDA, an IRB or we may suspend or terminate clinical trials at any time.
Administering our drug candidates to humans may produce undesirable side effects. These side effects could interrupt, delay or halt clinical trials of our drug candidates and could result in the FDA or other regulatory authorities denying approval of our drug candidates for any or all targeted indications.
We rely on third parties, including contract research organizations and outside consultants, to assist us in managing and monitoring clinical trials. Our reliance on these third parties may result in delays in completing, or in failing to complete, these trials if they fail to perform with the speed and competency we expect.
If clinical trials for a drug candidate are unsuccessful, we will be unable to commercialize the drug candidate. If one or more of our clinical trials are delayed, we will be unable to meet our anticipated development or commercialization timelines. Either circumstance could cause the price of our shares to decline.
If we encounter difficulties enrolling patients in our clinical trials, our trials could be delayed or otherwise adversely affected.
Clinical trials for our drug candidates require that we identify and enroll a large number of patients with the disorder under investigation. We may not be able to enroll a sufficient number of patients to complete our clinical trials in a timely manner.
Patient enrollment is affected by factors including:
| Ø | design of the protocol; |
| Ø | the size of the patient population; |
| Ø | eligibility criteria for the study in question; |
| Ø | perceived risks and benefits of the drug under study; |
| Ø | availability of competing therapies; |
| Ø | efforts to facilitate timely enrollment in clinical trials; |
| Ø | patient referral practices of physicians; and |
| Ø | availability of clinical trial sites. |
If we have difficulty enrolling a sufficient number of patients to conduct our clinical trials as planned, we may need to delay or terminate ongoing or planned clinical trials, either of which would have a negative effect on our business. Delays in enrolling patients in our clinical trials would also adversely affect our ability to generate product and royalty revenues and could impose significant additional costs on us or our collaborators. In addition, we have never conducted Phase 3 clinical trials, and we may be unable to successfully conduct multiple Phase 3 clinical trials involving the numbers of clinical sites and the numbers of patients planned for our alfimeprase Phase 3 clinical trials.
S-10
Table of Contents
Risk factors
We face heavy government regulation, and FDA regulatory approval of our products is uncertain.
The research, testing, manufacturing and marketing of drug products such as those proposed to be developed by us or our collaboration partners are subject to extensive regulation by federal, state and local governmental authorities, including the FDA, and comparable agencies in other countries. To obtain regulatory approval of a drug product, we or our collaboration partners must demonstrate to the satisfaction of the applicable regulatory agency, among other things, that the product is safe and effective for its intended uses. In addition, we must show that the manufacturing facilities used to produce the products are in compliance with current Good Manufacturing Practices, or cGMP, requirements.
The process of obtaining FDA and other required regulatory approvals and clearances typically takes several years and will require us to expend substantial capital and resources. Despite the time and expense expended, regulatory approval is never guaranteed. The number of pre-clinical and clinical tests that will be required for FDA approval varies depending on the drug candidate, the disease or condition that the drug candidate is in development for, and the regulations applicable to that particular drug candidate. The FDA or comparable international regulatory authorities can delay, limit or deny approval of a drug candidate for many reasons, including:
| Ø | a drug candidate may not be safe or effective; |
| Ø | FDA or comparable international regulatory authorities may interpret data from pre-clinical and clinical testing in different ways than we and our collaboration partners interpret them; |
| Ø | the FDA or comparable international regulatory authorities may not approve our manufacturing processes or facilities or the processes or facilities of our collaboration partners; or |
| Ø | the FDA or comparable international regulatory officials may change their approval polices or adopt new regulations. |
Moreover, if and when our products do obtain such approval or clearances, the marketing, distribution and manufacture of such products would remain subject to extensive ongoing regulatory requirements. Failure to comply with applicable regulatory requirements could result in:
| Ø | warning letters; |
| Ø | fines; |
| Ø | civil penalties; |
| Ø | injunctions; |
| Ø | recall or seizure of products; |
| Ø | total or partial suspension of production; |
| Ø | refusal of the government to grant approvals; or |
| Ø | withdrawal of approvals and criminal prosecution. |
Any delay or failure by us or our collaboration partners to obtain regulatory approvals for our product candidates:
| Ø | would adversely affect our ability to generate product and royalty revenues; |
| Ø | could impose significant additional costs on us or our collaboration partners; |
| Ø | could diminish competitive advantages that we may attain; |
| Ø | would adversely affect the marketing of our products; and |
| Ø | could cause the prices of our shares to decline. |
S-11
Table of Contents
Risk factors
Even if we do receive regulatory approval for our drug candidates, the FDA or international regulatory authorities may impose limitations on the indicated uses for which our products may be marketed, subsequently withdraw approval or take other actions against us or our products that are adverse to our business. The FDA and comparable international regulatory authorities generally approve products for particular indications. An approval for a limited indication reduces the size of the potential market for the product. Product approvals, once granted, may be withdrawn if problems occur after initial marketing.
We also are subject to numerous federal, state and local laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices, the experimental use of animals, the environment and the use and disposal of hazardous substances used in connection with our discovery, research and development work, including radioactive compounds and infectious disease agents. In addition, we cannot predict the extent of government regulations or the impact of new governmental regulations that might significantly harm the discovery, development, production and marketing of our products. We may be required to incur significant costs to comply with current or future laws or regulations, and we may be adversely affected by the cost of such compliance.
If we fail to maintain existing third-party arrangements and collaborative agreements or fail to develop new collaborative arrangements, our business will be harmed.
The success of our business is dependent, in significant part, upon our ability to enter into multiple collaboration agreements and to manage effectively the numerous issues that arise from such arrangements. Management of our relationships with these third parties has required and will require:
| Ø | a significant amount of our management team’s time and effort; |
| Ø | effective allocation of our and third-party resources to multiple projects; |
| Ø | agreements with third parties as to ownership of proprietary rights and development plans, including clinical trials or regulatory approval strategy; and |
| Ø | an ability to obtain and retain management, scientific and other personnel. |
In October 2004, Amgen Inc., exercised its rights under the collaboration agreement entered into by us and Amgen in January 2002, to convert the relationship from a collaboration into a licensing arrangement in accordance with terms agreed upon by us and Amgen. In November 2004, we and Amgen entered into a license agreement granting us worldwide rights to develop and commercialize alfimeprase in exchange for payment of previously negotiated development milestones and royalties. Under the terms of the license agreement, Amgen will transfer the technology necessary for the manufacture of alfimeprase to us or a manufacturer acceptable to Amgen. Amgen is required to continue to supply alfimeprase to us during the transition period. On January 21, 2005, we entered into an Interim Agreement with Avecia Limited for the manufacture of alfimeprase. Either party may terminate this agreement at any time. While we currently believe we have enough supplies of alfimeprase for phase 3 trials for the treatment of PAO and catheter occlusion, additional supplies may be necessary, and we do not yet have a definitive agreement for the manufacture of additional supplies of alfimeprase. We cannot be certain that we will be able to reach a definitive agreement with Avecia or any other manufacturer, upon commercially reasonable terms for alfimeprase’s manufacture or that Avecia or any other manufacturer will be able to produce alfimeprase in the quantities and with the quality we need for our clinical trials. If we are unable to find a manufacturer, or manufacturers, to produce alfimeprase in the quantities and with the quality we need, at a commercially reasonable price, we may incur significant, additional expenses and our efforts to complete our clinical trials and obtain FDA approval to market alfimeprase could be significantly delayed.
S-12
Table of Contents
Risk factors
In our collaboration with Archemix for the development and commercialization of ARC183, we have been sharing all research and developments costs equally since the third quarter of 2004 when we completed funding of the first $4.0 million in research and development costs, and will share any revenue from this collaboration. We will make milestone payments of $10.0 million upon commencement of a Phase 2 trial and $1.0 million upon the designation of any backup compound selected by both Nuvelo and Archemix for pre-clinical studies. We are obligated to make the Phase 2 milestone payment to Archemix even if Archemix terminates the collaboration or Archemix does not meet its obligations under the agreement and we terminate the collaboration for Archemix’s default. We have the option to lead commercialization in which both parties may participate if we establish commercialization capabilities; however, if we do not establish such commercialization capabilities, Archemix, or a third party selected by the parties’ joint steering committee, will have the option to lead commercialization. We do not currently have established commercialization experience or an internal trained sales force and we may not successfully develop such capabilities without incurring additional expenses. If we cannot develop an internal sales force, we will not be able to lead commercialization activities on our own. If we do not lead the commercialization efforts, we are dependent on Archemix or a third party’s experience in commercialization and ability to perform and we may also incur additional expenses for a third party to undertake commercialization efforts.
We are subject to a number of additional risks associated with our collaboration with Archemix for ARC183, including the right of Archemix to terminate its collaboration with us on limited notice and for reasons outside our control, and to the loss of significant rights if the collaboration is terminated because we fail to meet our obligations under it. In particular, if Archemix terminates the collaboration for our breach, all of our rights to ARC183 and other collaboration products will become the property of Archemix, and we may not practice certain activities related to anti-thrombin compounds in the field of modifying blood-clotting times in therapeutic applications through the use of aptamers such as ARC183, including research and development, manufacturing and commercialization activities.
Pursuant to our licensing arrangement with Dendreon relating to rNAPc2, we are obligated to make milestone payments ranging from $2.0 million to $6.0 million each upon the first dosing of the first patient in a Phase 3 clinical trial, upon submission of a new drug application, or NDA, and upon commercialization for the first and second indications. If all milestones are achieved, total milestone payments to Dendreon can reach as much as $23.5 million.
Our efforts to manage simultaneously a number of collaboration arrangements may not be successful, and our failure to manage effectively such collaborations would significantly harm our business, financial condition and results of operations.
Due to these factors and other possible disagreements with Amgen, Archemix, Dendreon and Kirin, we may be delayed or prevented from developing or commercializing alfimeprase, ARC183 and rNAPc2 or our pre-clinical product candidates or we may become involved in litigation or arbitration, which would be time-consuming or expensive and could have a material adverse effect on our stock price.
In addition to our existing collaborations, we will focus on effecting new collaborative arrangements where we would share costs of identifying, developing and marketing drug candidates. We cannot assure you that we will be able to negotiate new collaboration arrangements of this type on acceptable terms, or at all.
We are currently dependent on third parties for a variety of functions and may enter into future collaborations for the manufacture and sale of our products. Our arrangements with these third parties may not provide us with the benefits we expect.
We currently rely upon third parties to perform administrative functions and functions related to the research, development, pre-clinical testing and clinical trials of our drug candidates. In addition, because
S-13
Table of Contents
Risk factors
we do not have the resources, facilities or experience to manufacture our drug candidates on our own, we currently rely, and will continue to rely, on third parties to manufacture our drug candidates for clinical trials, and, if our products are approved, in quantities for commercial sales. We currently rely on a number of sole-source service providers and suppliers and do not have long-term supply agreements with our third-party manufacturers.
We do not currently have significant manufacturing facilities for clinical or commercial production of our drug candidates and depend on contract research and manufacturing organizations. We may not be able to finalize contractual arrangements, transfer technology or maintain relationships with such organizations in order to file an investigational new drug application, or IND, with the FDA, and proceed with clinical trials for any of our drug candidates. We currently rely on Amgen to manufacture our clinical drug product, alfimeprase. We have entered into an Interim Agreement with Avecia and are in the process of transitioning manufacture of alfimeprase from Amgen to Avecia, but do not yet have a definitive agreement with Avecia. If our efforts are unsuccessful, we may not have adequate supplies of alfimeprase to complete our clinical trials or to commercialize alfimeprase on our anticipated schedule.
We are dependent on third-party contract research organizations to conduct certain research, including good laboratory practices toxicology studies, in order to gather the data necessary to file INDs with the FDA for any of our drug candidates. Our drug candidates have never been manufactured on a commercial scale. Third-party manufacturers may not be able to manufacture these drug candidates at a cost or in quantities necessary to make them commercially viable. In addition, if and when any of our other drug candidates enter the clinical trial phase, we will initially depend on third-party contract manufacturers to produce the volume of current good manufacturing practices materials needed to complete such trials. We will need to enter into contractual relationships with these or other organizations in order to (1) complete the Good Laboratory Practices, or GLP, toxicology and other studies necessary to file an IND with the FDA, and (2) produce a sufficient volume of current cGMP grade material in order to conduct clinical trials of ARC183 and our other drug candidates. We cannot be certain that we will be able to do so on a timely basis or that we will be able to obtain sufficient quantities of material on commercially reasonable terms. In addition, the failure of any of these relationships with third-party contract organizations may delay our filing for an IND or impede our progress through the clinical trial phase. Any significant delay or interruption would have a material adverse effect on our ability to file an IND with the FDA and/or proceed with the clinical trial phase for any of our drug candidates.
Moreover, contract manufacturers that we may use must continually adhere to current cGMP regulations enforced by the FDA through a facilities inspection program. If one of our contract manufacturers fails to maintain compliance, the production of our product candidates could be interrupted, resulting in delays, additional costs and potentially lost revenues. In addition, if the facilities of such manufacturers do not pass a pre-approval plant inspection, the FDA will not grant pre-market approval of our products.
Our reliance on these relationships poses a number of risks, including:
| Ø | disagreements with third parties that could disrupt our operation or delay or terminate the research, development or manufacturing of drug candidates, or result in litigation or arbitration; |
| Ø | our inability to effectively control the resources devoted by our partners to our programs or products; |
| Ø | inadequate contractual protection or difficulty in enforcing the contracts if one of our partners fails to perform; |
| Ø | failure of these third parties to comply with regulatory requirements; |
| Ø | conflicts of interest between third parties’ work for us and their work for another entity, and the resulting loss of their services; |
S-14
Table of Contents
Risk factors
| Ø | failure to identify acceptable manufacturers or other suppliers or enter into favorable long-term agreements with them; |
| Ø | inability of third parties to manufacture our drug candidates in a cost-effective or timely manner or in quantities needed for clinical trials or commercial sales; |
| Ø | delays in, or failures to achieve, scale-up to commercial quantities, or changes to current raw material suppliers or product manufacturers (whether the change is attributable to us or the supplier or manufacturer), resulting in delayed clinical studies, regulatory submissions and commercialization of our drug candidates; and |
| Ø | lack of all necessary intellectual property rights to manufacture and sell our drug candidates. |
Given these risks, our current and future collaborative efforts with third parties may not be successful. If these efforts fail, we would be required to devote additional internal resources to the activities currently performed, or to be performed, by third parties, to seek alternative third-party collaborators, or to delay our product development or commercialization.
We may not achieve our projected development goals in the time frames we announce and expect.
We set goals for and make public statements regarding the timing of certain accomplishments, such as the commencement and completion of clinical trials, anticipated regulatory approval dates and time of product launch, which we sometimes refer to as milestones. The actual timing of these events can vary dramatically due to a number of factors such as delays or failures in our clinical trials, the uncertainties inherent in the regulatory approval process and delays in achieving manufacturing or marketing arrangements sufficient to commercialize our products. There can be no assurance that our clinical trials will be completed, that we will make regulatory submissions or receive regulatory approvals as planned or that we will be able to adhere to our current schedule for the launch of any of our products. If we fail to achieve one or more of these milestones as planned, our business will be materially adversely affected and the price of our shares could decline.
The success of our potential products in pre-clinical studies does not guarantee that these results will be replicated in humans.
Although our clinical development-stage drug candidates have shown results in pre-clinical studies, these results may not be replicated in our clinical trials with humans. Consequently, there is no assurance that the results in our pre-clinical studies are predictive of the results that we will see in our clinical trials with humans or that they are predictive of whether the resulting products will be safe and effective in humans.
We are dependent on key personnel and we must attract and retain qualified employees, collaborators and consultants.
The success of our business is highly dependent on the principal members of our scientific and management staff, including our senior management team. The loss of the services of any such individual might seriously harm our product development and commercialization efforts. In addition, we will require additional skilled personnel in areas such as clinical development. Retaining and training personnel with the requisite skills is challenging, and, if general economic conditions improve, is likely to become extremely competitive, particularly in Northern California where we are located.
Our success will depend on our ability to attract and retain qualified employees to help develop our potential products and execute our research and development strategy. We have programs in place to retain personnel, including programs to create a positive work environment and competitive compensation packages. Because competition for employees in our field is intense, however, we may be unable to retain our existing personnel or attract additional qualified employees. Our success also
S-15
Table of Contents
Risk factors
depends on the continued availability of outside scientific collaborators, including collaborators at research institutions, to perform research and develop processes to advance and augment our internal research efforts. Competition for collaborators is intense. We also rely on services provided by outside consultants. Attracting and retaining qualified outside consultants is competitive, and, generally, outside consultants can terminate their relationship with us at will. If we do not attract and retain qualified personnel, outside consultants and scientific collaborators, or if we experience turnover or difficulties recruiting new employees or outside consultants, our research and development programs could be delayed and we could experience difficulties in generating sufficient revenue to maintain our business.
In addition, we do not currently have a marketing and sales organization. As the potential commercialization of our products approaches, we intend to hire marketing and sales personnel to enable us to participate in the commercialization of our products in the United States. If we are unsuccessful in hiring and retaining sales and marketing personnel with appropriate qualifications and talent, our ability to generate product revenues would be adversely affected.
Because we have not yet commercialized any of our drug candidates, our ability to develop and subsequently commercialize products is unproven.
We have not yet commercialized any of our in-licensed therapeutic product candidates. Moreover, we have not developed any therapeutic products using proteins produced by the genes we have discovered in our internal research programs. Before we make any products available to the public from our internal research and development programs, we or our collaboration partners will need to conduct further research and development and complete laboratory testing and animal and human studies. We or our collaboration partners will need to obtain regulatory approval before releasing any drug products. We have spent, and expect to continue to spend, significant amounts of time and money in our internal research programs in determining the function of genes and the proteins they produce, using our own capabilities and those of our collaboration partners. Such a determination process constitutes the first step in developing commercial products from our internal research programs. We also have spent and will continue to spend significant amounts of time and money in developing processes for manufacturing of our recombinant proteins under pre-clinical development, yet we may not be able to produce sufficient proteins for pre-clinical studies. A commercially viable product may never be developed from our gene discoveries.
Our commercialization of products is subject to several risks, including but not limited to:
| Ø | the possibility that a product is toxic, ineffective or unreliable; |
| Ø | failure to obtain regulatory approval for the product; |
| Ø | difficulties in manufacturing the product on a large scale, or inability to market in an economically feasible manner; |
| Ø | competition from superior products; or |
| Ø | third-party patents that preclude us from marketing a product. |
Our internally developed drug development programs are currently in the research stage or in pre-clinical development. None of our potential therapeutic protein candidates from our own portfolio has advanced to Phase 1 clinical trials. Our programs may not move beyond their current stages of development. Even if our internal research does advance, we will need to engage in certain additional pre-clinical development efforts to determine whether a product is sufficiently safe and effective to enter clinical trials. We have little experience with these activities and may not be successful in developing or commercializing products.
S-16
Table of Contents
Risk factors
Under our Kirin collaboration arrangement, Kirin has primary responsibility for clinical development in its territory and we have primary responsibility in our territory. Under our collaboration with Archemix, Archemix leads development until Phase 2 clinical trials are reached, and thereafter, a joint steering committee will designate one party to lead development until commercialization. With respect to these arrangements, we run the risk that Kirin or Archemix may not pursue clinical development in a timely or effective manner.
Any regulatory approvals that we or our collaboration partners receive for our product candidates may be subject to limitations on the intended uses for which the product candidates may be marketed or contain requirements for potentially costly post-marketing follow-up studies. In addition, if the FDA approved of our or our collaboration partners’ product candidates, the labeling, packaging, adverse event reporting, storage, advertising, promotion and record-keeping for the products will be subject to extensive regulatory requirements.
We, our collaborators and our suppliers may also not be able to produce any products in commercial quantities at a reasonable cost or may not be able to successfully market such products. If we do not develop a commercially viable product, then we will suffer significant harm to our business, financial condition and operating results.
We lack marketing and commercialization experience for biopharmaceutical products and we may have to rely on third parties for these capabilities.
We currently have no sales, marketing or distribution capability. As the potential commercialization of our products approaches, we intend to hire marketing and sales personnel to enable us to participate in the commercialization of our products in the United States. If we are unsuccessful in hiring and retaining sales and marketing personnel with appropriate technical and sales expertise or in developing an adequate distribution capability to support them, our ability to generate product revenues would be adversely affected. To the extent we cannot or choose not to use internal resources for the marketing, sales or distribution of any potential products in the United States or elsewhere, we intend to rely on collaboration partners or licensees. We may not be able to establish or maintain such relationships. To the extent that we depend on collaboration partners or other third parties for marketing and distribution, any revenues we receive will depend upon their efforts. Such efforts may not be successful, and we will not be able to control the amount and timing of resources that collaboration partners or other third parties devote to our products.
Our products may not be accepted in the marketplace, and we may not be able to generate significant revenue, if any.
Even if they are approved for marketing, our products, if any, may never achieve market acceptance among physicians, patients and the medical community. Our products, if successfully developed, will compete with a number of traditional drugs and therapies manufactured and marketed by major pharmaceutical and other biotechnology companies. Our products will also compete with new products currently under development by such companies and others. The degree of market acceptance of any products developed by us, alone, or in conjunction with our collaboration partners, will depend on a number of factors, including:
| Ø | the establishment and demonstration of the clinical efficacy and safety of the products; |
| Ø | convenience and ease of administration; |
| Ø | cost-effectiveness; |
| Ø | our products’ potential advantages over alternative treatment methods; |
S-17
Table of Contents
Risk factors
| Ø | marketing, sales and distribution support of our products; and |
| Ø | reimbursement policies of government and third-party payers. |
Physicians, patients or the medical community in general may not accept and utilize any of the products that we alone, or in conjunction with our collaboration partners, develop. In practice, competitors may be more effective in marketing their drugs. The lack of such market acceptance would significantly harm our business, financial condition and results of operations.
We face intense competition.
The biopharmaceutical industry is intensely competitive and is accentuated by the rapid pace of technological development. We expect to face increased competition in the future as new companies enter our markets. Research and discoveries by others may result in breakthroughs that render our potential products obsolete even before they begin to generate any revenue. Our competitors include major pharmaceutical and biotechnology firms, many of which have substantially greater research and product development capabilities and financial, scientific, marketing and human resources than we have. Our lead product candidate alfimeprase, if approved, will face competition in the catheter occlusion indication from alteplase, an approved Genentech, Inc. product, and will potentially face competition in the peripheral arterial occlusion, or PAO, indication from product candidates being developed and/or marketed by Abbot Laboratories, Centocor, Inc. and Genentech.
Our competitors may obtain patents and regulatory approvals for their competing products more rapidly than we or our collaboration partners, or develop products that are more effective than those developed by us or our collaboration partners. Any potential products based on genes we identify ultimately will face competition from other companies developing gene-based products as well as from companies developing other forms of treatment for diseases which may be caused by, or related to, the genes we identify. Similarly, our products will face competition from other companies developing similar products as well as from companies developing other forms of treatment for the same conditions.
Many of the companies developing competing products have significantly greater financial resources than we have. Many such companies also have greater expertise than we or our collaboration partners have in discovery, research and development, manufacturing, pre-clinical and clinical testing, obtaining regulatory approvals and marketing. Other smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies and institutions compete with us in recruiting and retaining qualified scientific and management personnel as well as in acquiring technologies complementary to our programs. We will face competition with respect to:
| Ø | product efficacy and safety; |
| Ø | the timing and scope of regulatory approvals; |
| Ø | availability of resources; |
| Ø | reimbursement coverage; and |
| Ø | price and patent position, including the potentially dominant patent positions of others. |
There can be no assurance that research and development by others will not render the products that we may develop obsolete or uneconomical, or result in treatments or cures superior to any therapy developed by us or that any therapy we develop will be preferred to any existing or newly developed alternative products.
S-18
Table of Contents
Risk factors
We face uncertainty with respect to coverage, pricing, third-party reimbursements and health care reform.
Our ability to collect significant royalties from our products may depend on our ability, and the ability of our collaboration partners or customers, to obtain adequate levels of coverage for our products and reimbursement from third-party payers such as:
| Ø | government health administration authorities; |
| Ø | private health insurers; |
| Ø | health maintenance organizations; |
| Ø | pharmacy benefit management companies; and |
| Ø | other health care related organizations. |
Third-party payers may deny coverage or offer inadequate levels of reimbursement if they determine that a prescribed product or device has not received appropriate clearances from the FDA or other government regulators, is not used in accordance with cost-effective treatment methods as determined by the third-party payer, or is experimental, unnecessary or inappropriate. If third-party payers deny coverage or offer inadequate levels of reimbursement, we may not be able to market our products effectively. We also face the risk that we will have to offer our products at prices lower than anticipated as a result of the current trend in the United States towards managed health care through health maintenance organizations. Currently, third-party payers are increasingly challenging the prices charged for medical products and services. Prices could be driven down by health maintenance organizations that control or significantly influence purchases of health care services and products. Existing U.S. laws, such as the Medicare Prescription Drug and Modernization Act of 2003, or future legislation to reform health care or reduce government insurance programs could also adversely affect prices of our approved products, if any. The cost containment measures that health care providers are instituting and the results of potential health care reforms may prevent us from maintaining prices for our products that are sufficient for us to realize profits and may otherwise significantly harm our business, financial condition and operating results. In addition, to the extent that our products are marketed outside of the United States, foreign government pricing controls and other regulations may prevent us from maintaining prices for our products that are sufficient for us to realize profits and may otherwise significantly harm our business, financial condition and operating results.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud.
Effective internal controls are necessary for us to provide reliable financial reports and effectively prevent fraud. Any inability to provide reliable financial reports or prevent fraud could harm our business. We are in the process of evaluating our internal procedures to satisfy the requirements of the Sarbanes-Oxley Act of 2002, which require management and our auditors to evaluate and assess the effectiveness of our internal controls. We are continuing to evaluate and, where appropriate, enhance our policies, procedures and internal controls. If we fail to maintain the adequacy of our internal controls, as such standards are modified, supplemented or amended from time to time, we could be subject to regulatory scrutiny, civil or criminal penalties or shareholder litigation. In addition, failure to maintain adequate internal controls could result in financial statements that do not accurately reflect our financial condition. We might not be able to complete the work necessary to fully comply with the requirements of the Sarbanes-Oxley Act. Our auditors might not complete their review and assessment of our internal controls in a timely manner. Finally, our management and our auditors might not conclude that our internal controls are effective.
S-19
Table of Contents
Risk factors
We may merge with or acquire other companies and our failure to receive the anticipated benefits in these transactions could harm our business.
In January 2003, we merged with Variagenics, and we may merge with or acquire other companies in the future. The success of any merger or acquisition depends, in part, on our ability to realize the anticipated synergies, cost savings and growth opportunities from integrating the business of the merged or acquired company with our business. The integration of two independent companies is a complex, costly and time-consuming process. The difficulties of combining the operations of the companies and/or our subsidiary include, among others:
| Ø | consolidating research and development operations; |
| Ø | retaining key employees; |
| Ø | consolidating corporate and administrative infrastructures; |
| Ø | preserving the research and development and other important relationships of the companies; |
| Ø | integrating and managing the technology of two companies; |
| Ø | using the merged or acquired company’s liquid capital and other assets efficiently to develop the business of the combined company; |
| Ø | minimizing the diversion of management’s attention from ongoing business concerns; and |
| Ø | coordinating geographically separate organizations. |
Moreover, we have assumed the costs of defending against litigation claims asserted against Variagenics, and anytime we or our subsidiary merge with or acquire another company, we will be exposed to similar costs. In addition, we may be exposed to a number of other risks in connection with future transactions, including:
| Ø | we may experience unbudgeted expenses in attempting to complete the transaction and integration process and exposure to unknown liabilities of the merged or acquired business; and |
| Ø | our stock price may suffer if the former stockholders of the merged or acquired entity dispose of significant numbers of shares of our common stock that they receive in the transaction within a short period of time. |
We cannot assure you that we will receive all of the anticipated benefits of any mergers or acquisitions, or that any of the risks described above will not occur. Our failure to receive anticipated benefits of, and our exposure to inherent risks in, any such merger or acquisition transaction could significantly harm our business, financial condition and operating results.
We may not receive any benefits from and we may have lost potential income as a result of the sale of our equity holdings in our former Callida subsidiary.
On December 3, 2004, we entered into and consummated a Stock Purchase Agreement with SBH Genomics, Inc., Radoje Drmanac, Snezana Drmanac and Affymetrix, Inc., pursuant to which we sold all of the stock we held in our subsidiary, Callida Genomics, Inc., or Callida, to SBH Genomics, Inc., a privately held Delaware corporation. Prior to the sale, we owned approximately 90% of Callida’s issued and outstanding capital stock. Affymetrix, a minority stockholder in Callida, also sold its Callida shares to SBH Genomics as part of the same negotiated transaction. We and Affymetrix sold our stock in Callida in exchange for convertible promissory notes in the principal amount of $1 million and potential additional payments to us from SBH Genomics based on future revenues. The notes are convertible into preferred shares of SBH Genomics under certain circumstances. The notes may prove uncollectible, and we cannot assure you that we will receive the anticipated benefits, if any, of our sale of Callida stock,
S-20
Table of Contents
Risk factors
and through this transaction we may have lost certain benefits that we would have otherwise received from the continued ownership of our Callida holdings.
We are subject to the risk of natural disasters.
Our facilities are located in Northern California. If a fire or other natural disaster (such as an earthquake) disrupts our research or development efforts, our business, financial condition and operating results could be materially adversely affected. Some of our landlords maintain earthquake coverage for our facilities. Although we maintain personal property and business interruption coverage, we do not maintain earthquake coverage for personal property or resulting business interruption.
RISKS RELATED TO OUR CAPITAL STRUCTURE AND FINANCIAL RESULTS
We have not been profitable, anticipate continuing losses and may never become profitable.
For the year ended December 31, 2001, we had a net loss of $36.5 million. For the year ended December 31, 2002, we had a net loss of $45.0 million. For the year ended December 31, 2003, we had a net loss of $50.2 million. For the nine months ended September 30, 2004, we had a net loss of $39.5 million. As of September 30, 2004, we had an accumulated deficit of $243.1 million.
All of our product candidates are in various stages of product development, and some are still in research or in early development. None of them are approved for sale. The process of developing our drug products will require significant additional research and development, pre-clinical testing, clinical trials and regulatory approvals.
These activities, together with general administrative and other expenses, are expected to result in operating losses for the foreseeable future. To date, we have not generated any revenues from product sales. We do not expect to achieve significant product sales or royalty revenue for several years, and we may never do so. We expect to incur additional operating losses in the future, and these losses may increase significantly as we continue pre-clinical research and clinical trials, apply for regulatory approvals, develop our drug candidates, expand our operations and develop systems that support commercialization of our potential products. These losses, among other things, have caused and may cause our stockholders’ equity and working capital to decrease. We may not be successful in developing our drug candidates, obtaining regulatory approvals and commercializing our products, and our operations may not be profitable even if any of our drug candidates are commercialized. We may never generate profits and, as a result, the trading price of our common stock could decline.
Moreover, utilization of our net operating loss carryforwards and credits may be subject to an annual limitation due to the “change in ownership” provisions of the Internal Revenue Code of 1986 and similar state law provisions. It is possible that certain transactions that we have entered into, including our merger with Variagenics that occurred in January 2003, when considered in connection with other transactions, may result in a “change in ownership” for purposes of these provisions.
In January 2005, we entered into a lease agreement for approximately 55,000 square feet of industrial space in San Carlos, California. In connection with our lease of this new facility, we are examining the potential to sublease or otherwise exit our existing facility at 985 Almanor Avenue in Sunnyvale, California, which is currently primarily being used for storage and for which we have a lease through May 30, 2011. In accordance with Statement of Financial Accounting Standards No. 146, “Accounting for Costs Associated with Exit or Disposal Activities,” if we sublease or otherwise exit this facility, we could incur a potentially significant charge to our earnings based on the remaining lease rental expense of $36.1 million for our existing facility reduced by the estimated income from sublease rental, if any. Our remaining lease obligations with respect to the facility at 985 Almanor Avenue total approximately $46.2
S-21
Table of Contents
Risk factors
million, excluding deferred rent credits of $10.1 million. We are obligated to pay the full amount of such remaining lease rental obligation, net of any sublease payments we may receive, from time to time as it comes due under the terms of the lease for this facility.
We will need to raise additional capital, and such capital may be unavailable to us when we need it or not available on acceptable terms.
Even though we intend to raise capital through this offering, we will need to raise significant additional capital to finance the research and clinical development of our drug products. If future securities offerings are successful, they could dilute our current shareholders’ equity interests and reduce the market price of our common stock. Financing may be unavailable when we need it or may not be available on acceptable terms. The unavailability of financing may require us to delay, scale back or eliminate expenditures for our research, development and marketing activities necessary to commercialize our potential biopharmaceutical products. We may also be required to raise capital by granting rights to third parties to develop and market drug candidates that we would prefer to develop and market on our own, potentially reducing the ultimate value that we could realize from these drug candidates.
If we are unable to obtain additional financing when we need it, the capital markets may perceive that we are not able to raise the amount of financing we desire, or on the terms that we desire. This perception, if it occurs, may negatively affect the trading price of our common stock. If sufficient capital is not available, we may be forced to delay, reduce the scope of, eliminate or divest one or more of our research or development programs. Any such action could significantly harm our business, financial condition and results of operations.
Our future capital requirements and the adequacy of our currently available funds will depend on many factors, including, among others, the following:
| Ø | our ability to maintain, and the financial commitments involved in, our existing collaborative and licensing arrangements; |
| Ø | our ability to establish new collaborative relationships with other companies to share costs and expertise of identifying and developing drug candidates; |
| Ø | the magnitude and scope of our research and development programs, including development of product candidates; |
| Ø | continued scientific progress in our research and development programs, including progress in our research and pre-clinical studies; |
| Ø | the cost involved in any facilities expansion to support research and development of our product candidates; |
| Ø | the cost of manufacturing our material for pre-clinical, clinical and commercial purposes; |
| Ø | progress in clinical studies of our products, including alfimeprase, rNAPc2 and ARC 183; |
| Ø | the cost of prosecuting and enforcing our intellectual property rights; |
| Ø | the time and cost involved in obtaining regulatory approvals; |
| Ø | our need to develop, acquire or license new technologies or products; |
| Ø | competing technological and market developments; |
| Ø | our ability to use our common stock to repay the outstanding note to Affymetrix and our line of credit from our Chairman, Dr. George B. Rathmann; |
S-22
Table of Contents
Risk factors
| Ø | future funding commitments to our collaborators; |
| Ø | general conditions in the financial markets and in the biotech sector; |
| Ø | the uncertain condition of the capital markets and in the biotech sector; and |
| Ø | other factors not within our control. |
We may face fluctuations in operating results.
Our operating results may rise or fall significantly as a result of many factors, including:
| Ø | the amount of research and development we engage in; |
| Ø | the number of product candidates we have and their progress in research and pre-clinical studies; |
| Ø | our ability to expand our facilities to support our operations; |
| Ø | our ability to maintain existing and enter into new strategic relationships; |
| Ø | the scope, duration and effectiveness of our collaborative arrangements; |
| Ø | the costs involved in prosecuting, maintaining and enforcing patent claims; |
| Ø | the possibility that others may have or obtain patent rights that are superior to ours; |
| Ø | changes in government regulation; and |
| Ø | release of successful products into the market by our competitors. |
Excluding our three clinical stage drug candidates, our potential products currently are in research or pre-clinical development, and revenues from the sales of any of our potential products may not occur for several years, if at all. A high percentage of our expenses are fixed costs such as lease obligations. As a result, we may experience fluctuations in our operating results from quarter to quarter and continue to generate losses. Quarterly comparisons of our financial results may not necessarily be meaningful, and investors should not rely upon such results as an indication of our future performance. In addition, investors may react adversely if our reported operating results are less favorable than in a prior period or are less favorable than those anticipated by investors or the financial community, which may result in a drop of our stock price.
We have implemented anti-takeover provisions that could discourage, prevent or delay a takeover, even if the acquisition would be beneficial to our stockholders.
The existence of our stockholder rights plan and provisions of our certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions:
| Ø | establish a classified board of directors so that not all members of our board may be elected at one time; |
| Ø | authorize the issuance of up to 5,000,000 shares of preferred stock that could be issued by our board of directors to increase the number of outstanding shares and hinder a takeover attempt; |
| Ø | limit who may call a special meeting of stockholders; |
| Ø | prohibit stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; and |
| Ø | establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon at a stockholder meeting. |
S-23
Table of Contents
Risk factors
Specifically, our certificate of incorporation provides that all stockholder action must be effected at a duly called meeting and not by a consent in writing. The by-laws provide, however, that our stockholders may call a special meeting of stockholders only upon a request of stockholders owning at least 50% of our common stock. These provisions of our certificate of incorporation and our by-laws could discourage potential acquisition proposals and could delay or prevent a change in control. We designed these provisions to reduce our vulnerability to unsolicited acquisition proposals and to discourage certain tactics that may be used in proxy fights. These provisions, however, could also have the effect of discouraging others from making tender offers for our shares. As a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. Such provisions also may have the effect of preventing changes in our management.
We are permitted to issue shares of our preferred stock without stockholder approval upon such terms as our board of directors determines. Therefore, the rights of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of our preferred stock that may be issued in the future. In addition, the issuance of preferred stock could have a dilutive effect on the holdings of our current stockholders.
On June 5, 1998, our board of directors adopted a rights plan and declared a dividend with respect to each share of our common stock then outstanding. This dividend took the form of a right, which entitles the holders to purchase one one-thousandth of a share of our Series A junior participating preferred stock at a purchase price that is subject to adjustment from time to time. These rights have also been issued in connection with each share of our common stock issued after June 15, 1998. The rights are exercisable only if a person or entity or affiliated group of persons or entities acquires, or has announced its intention to acquire, 15% (27.5% in the case of certain approved stockholders) or more of our outstanding common stock. The adoption of the rights plan makes it more difficult for a third party to acquire control of us without the approval of our board of directors. This rights agreement was amended on March 19, 2004, to ensure that it would remain in full force and effect after our reincorporation under Delaware law.
We are subject to the Delaware anti-takeover laws regulating corporate takeovers. These anti-takeover laws prevent a Delaware corporation from engaging in a merger or sale of more than 10 percent of its assets with any stockholder, including all affiliates and associates of the stockholder, who owns 15 percent or more of the corporation’s outstanding voting stock, for three years following the date that the stockholder acquired 15 percent or more of the corporation’s stock unless:
| Ø | the board of directors approved the transaction where the stockholder acquired 15 percent or more of the corporation’s stock; |
| Ø | after the transaction in which the stockholder acquired 15 percent or more of the corporation’s stock, the stockholder owned at least 85 percent of the corporation’s outstanding voting stock, excluding shares owned by directors, officers and employee stock plans in which employee participants do not have the right to determine confidentially whether shares held under the plan will be tendered in a tender or exchange offer; or |
| Ø | on or after this date, the merger or sale is approved by the board of directors and the holders of at least two-thirds of the outstanding voting stock that is not owned by the stockholder. |
S-24
Table of Contents
Risk factors
The provisions of our governing documents, stockholder rights plan and current Delaware law may, collectively:
| Ø | lengthen the time required for a person or entity to acquire control of us through a proxy contest for the election of a majority of our board of directors; |
| Ø | discourage bids for our common stock at a premium over market price; and |
| Ø | generally deter efforts to obtain control of us. |
We have adopted an Executive Change in Control and Severance Benefit Plan that could discourage, prevent or delay a takeover, even if the acquisition would be beneficial to our stockholders.
On December 14, 2004, our Board of Directors approved an “Executive Change in Control and Severance Benefit Plan” for our executive officers. The purpose of the plan is to provide for the payment of severance benefits and/or change in control benefits to certain of our eligible employees, and the plan supersedes and replaces any change in control and/or severance plans adopted by us previously. All of our executive employees at the level of Vice President or above are eligible to participate in the plan and our Board of Directors may designate certain other individuals as eligible to participate. The plan provides that, upon a change in control of the company as defined under the plan, all Nuvelo stock options and stock awards held by a plan participant will become fully vested. Such shares held by a plan participant will also become fully vested if the participant is terminated without cause or constructively terminated within one month preceding our change in control. If a participant is terminated without cause or constructively terminated one month before or one year after our change in control, he or she will also be entitled to certain cash severance and continued medical benefits and shall be credited with an additional year of vesting with respect to Nuvelo stock options and stock awards held by such participant. The change in control and severance benefits for certain of our employees provided for under this plan could make it more difficult and expensive, or less desirable, for a third party to acquire us, even if doing so would benefit our stockholders.
RISKS RELATED TO INTELLECTUAL PROPERTY AND OTHER LEGAL MATTERS
The commercial success of our products will be dependent upon our ability to protect the intellectual property rights associated with our products and drug candidates.
Our competitive success will depend in part on our ability to obtain and maintain patent protection for our inventions, technologies and discoveries, including intellectual property that we license. The patent positions of biotechnology companies involve complex legal and factual questions, and we cannot assure you that our patents and licenses will successfully preclude others from using our technology. We could incur substantial costs in seeking enforcement of our proprietary rights against infringement. In addition, to obtain a patent on a novel gene or the protein it encodes, we need to identify a utility for the novel gene or the encoded protein we seek to protect under patent law. Identifying a utility may require significant research and development with respect to which we may incur a substantial expense and invest a significant amount of time.
We currently have, or have in-licensed, issued patents and pending patent applications that cover portions of our in-licensed clinical products. ARC183 is covered both by a U.S. patent specifically claiming ARC183 and by U.S. patents covering aptamers generically. However, there are no equivalent international applications pending specifically claiming ARC183. International patent applications generically covering aptamers are pending but we cannot assure you that such patents will issue. We licensed the worldwide rights for all indications of rNAPc2 and all of the rNAPc molecules owned by Dendreon in February 2004. The United States government may claim a non-exclusive right to use rNAPc2 with respect to the treatment of hemorrhagic fever. We also currently have patents that cover some of our technological discoveries and patent applications that we expect to protect some of our gene, protein and technological discoveries. We have approximately 37 issued patents relating to our gene and protein discoveries. We also currently have or have rights to 27
S-25
Table of Contents
Risk factors
issued patents which cover or describe single nucleotide polymorpohisms and their application to pharmacogenetic studies, genotyping and haplotyping methods, and allele specific inhibitors. In addition, we have rights to 21 issued U.S. patents relating to the in-licensed clinical products. We will continue to apply for patents for our discoveries. We cannot assure you that any of our applications will issue as patents, or that any patent issued or licensed to us will not be challenged, invalidated, circumvented or held unenforceable by way of an interference proceeding or litigation.
The timing of the grant of a patent cannot be predicted. Patent applications describing and seeking patent protection of methods, compositions, or processes relating to proprietary inventions involving human therapeutics could require us to generate data, which may involve substantial costs. Our pending patent applications may lack priority over others’ applications or may not result in the issuance of patents. Even if issued, our patents may not be sufficiently broad to provide protection against competitors with similar technologies and may be challenged, invalidated or circumvented.
In addition to patents, we rely on a combination of trade secrets, copyright and trademark laws, nondisclosure agreements, licenses and other contractual provisions and technical measures to maintain and develop our competitive position with respect to intellectual property. Nevertheless, these measures may not be adequate to safeguard the technology underlying our products. For example, employees, consultants and others who participate in the development of our products may breach their agreements with us regarding our intellectual property and we may not have adequate remedies for the breach. Our trade secrets could become known through other unforeseen means. We depend on our collaborators and other third parties that license intellectual property to us to protect our licensed intellectual property. These collaborators and other third parties could fail to take a necessary step to protect our licensed intellectual property, which could seriously harm our intellectual property position.
We also may not be able to effectively protect our intellectual property rights in some foreign countries, as many countries do not offer the same level of legal protection for intellectual property as the United States. Furthermore, certain of the patent applications describing our proprietary methods are filed only in the United States. Even where we have filed our patent applications internationally, for some cases and in certain countries, we have chosen not to maintain foreign patent protection by opting not to enter national phase or opting not to pay maintenance annuities.
Notwithstanding our efforts to protect our intellectual property, our competitors may independently develop similar or alternative technologies or products that are equal or superior to our technology. Our competitors may also develop similar products without infringing on any of our intellectual property rights or design around our proprietary technologies.
If our products infringe on the intellectual property rights of others, we could face costly litigation, which could cause us to pay substantial damages and limit our ability to sell some or all of our products.
Extensive litigation regarding patents and other intellectual property rights has been common in the biopharmaceutical industry. Litigation may be necessary to assert infringement claims, enforce patent rights, protect trade secrets or know-how and determine the enforceability, scope and validity of certain proprietary rights. The defense and prosecution of intellectual property lawsuits, United States Patent and Trademark Office interference proceedings, and related legal and administrative proceedings in the United States and internationally involve complex legal and factual questions. As a result, such proceedings are costly and time-consuming to pursue and their outcome is uncertain.
Regardless of merit or outcome, our involvement in any litigation, interference or other administrative proceedings could cause us to incur substantial expense and could significantly divert the efforts of our technical and management personnel. An adverse determination may subject us to the loss of our
S-26
Table of Contents
Risk factors
proprietary position or to significant liabilities, or require us to seek licenses that may include substantial cost and ongoing royalties. Licenses may not be available from third parties, or may not be obtainable on satisfactory terms. An adverse determination or a failure to obtain necessary licenses may restrict or prevent us from manufacturing and selling our products, if any. These outcomes could materially harm our business, financial condition and results of operations.
Our market success depends in part on us neither infringing valid, enforceable patents or proprietary rights of third parties, nor breaching any licenses that may relate to our technologies and products. We are aware of third-party patents that may relate to our technology. We may be required to obtain licenses to patents or other proprietary rights of others in order to conduct research, development, or commercialization of some or all of our programs. We plan to seek licenses, as we deem appropriate, but it is possible that we may infringe upon these patents or proprietary rights of third parties. If we do not obtain these licenses, we may encounter delays in product market introductions, incur substantial costs while we attempt to design around existing patents or not be able to develop, manufacture or sell products. In response, third parties may assert infringement or other intellectual property claims against us. We may consequently be subjected to substantial damages for past infringement or be required to modify our products if it is ultimately determined that our products infringe a third party’s proprietary rights. Further, we may be prohibited from selling our products before we obtain a license, which, if available at all, may require us to pay substantial royalties, which could adversely impact our product costs and have an impact on our business. Further, if we do obtain these licenses, the agreed terms may necessitate reevaluation of the potential commercialization of any one of our programs. Failing to obtain a license could result in litigation. Even if these claims are without merit, defending a lawsuit takes significant time, may be expensive and may divert management attention from other business concerns. Any public announcements related to litigation or interference proceedings initiated or threatened against us could cause our stock price to decline.
We face product liability exposure and potential unavailability of insurance.
We risk financial exposure to product liability claims in the event that the use of products developed by us or our collaboration partners, if any, result in personal injury.
We may experience losses due to product liability claims in the future. We have obtained limited product liability insurance coverage. Such coverage, however, may not be adequate or may not continue to be available to us in sufficient amounts or at an acceptable cost, or at all. We may not be able to obtain commercially reasonable product liability insurance for any product approved for marketing. A product liability claim or other claim, product recalls, as well as any claims for uninsured liabilities or in excess of insured liabilities, may significantly harm our business, financial condition and results of operations.
We use hazardous materials, chemicals and patient samples in our business and any disputes relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development and production activities involve the controlled use of hazardous or radioactive materials, chemicals, including oxidizing and reducing reagents, patient tissue and blood samples. We, our collaborators and service providers are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and certain waste products. We could be liable for accidental contamination or discharge or any resultant injury from hazardous materials, and conveyance, processing, and storage of and data on patient samples. If we, our collaborators or service providers fail to comply with applicable laws or regulations, we could be required to pay penalties or be held liable for any damages that result and this liability could exceed our financial resources. Further, future changes to environmental health and safety laws could cause us to incur additional expense or restrict our operations.
S-27
Table of Contents
Risk factors
In addition, our collaborators and service providers may be working with these types of hazardous materials, including viruses and hazardous chemicals, in connection with our collaborations. In the event of a lawsuit or investigation, we could be held responsible for any injury caused to persons or property by exposure to, or release of, patient samples that may contain viruses and hazardous materials. The cost of this liability could exceed our resources.
Variagenics has been named as a defendant in a class action suit and defending this litigation could hurt our business.
Variagenics has been named as a defendant in a securities class action lawsuit alleging the failure to disclose additional and excessive commissions purportedly solicited by and paid to underwriters who are also named defendants in the lawsuit. Plaintiffs in the suit allege that underwriters took these commissions and in exchange allocated shares of Variagenics’ stock to their preferred customers through alleged agreements with these preferred customers that tied the allocation of initial public offering shares to agreements by the customers to make additional aftermarket purchases at pre-determined prices. As a result of our merger with Variagenics, we are obligated to continue to defend against this litigation. Currently we are in the process of approving a settlement by and between the issuers that are defendants in the lawsuit, the insurers of those issuers, and the plaintiffs. We believe that any loss or settlement amount will not be material to our financial position or results of operation, and that any settlement payment and attorneys’ fees accrued with respect to the suit will be paid by our insurance provider. However, we cannot assure you that this will be the case until a final settlement is executed. Failure to finalize a settlement could require us to pay substantial damages.
RISKS RELATED TO THIS OFFERING
Our involvement in a January 2005 magazine article about Nuvelo could be held to be in violation of the Securities Act of 1933. You should rely only on statements made in this prospectus in making your investment decision.
Information about Nuvelo has been published in an article appearing in the January 31, 2005 issue of Forbes entitled “Leg Attack.” The text of the article contains information derived from an interview with our Chief Executive Officer, Dr. Ted Love, conducted in November 2004, prior to this offering. The article attributed certain information to Dr. Love and presented certain statements about our company in isolation and did not disclose many of the related risks and uncertainties described in this prospectus supplement. In making your investment decision, you should rely only on the information contained in this prospectus supplement or the documents incorporated by reference in this prospectus supplement. In addition, you should be aware of the following clarifications with respect to content contained in the Forbes article:
The Forbes article indicated that the market for alfimeprase could be worth $500 million in annual sales. We believe that any such projections are based upon a number of estimates and assumptions and are inherently subject to significant uncertainties and contingencies, including successfully completing our clinical trials, demonstrating the safety and efficacy of alfimeprase, obtaining necessary regulatory approvals, contracting for the manufacture of alfimeprase, the timing of commercializing alfimeprase and competing against other drugs and procedures. Projections are necessarily speculative in nature, and one or more of the estimates on which the projections were based may not materialize or may vary significantly from actual results.
In addition, the Forbes article suggests that alfimeprase could be used to treat an estimated 11 million Americans with peripheral arterial disease; however, our clinical trials have not focused on treating all indications of peripheral arterial disease. As stated in this prospectus, our development of alfimeprase has focused solely on two distinct indications, (i) acute PAO, with over 100,000 cases reported annually in
S-28
Table of Contents
Risk factors
the United States and (ii) catheter occlusion, which is not a form of peripheral arterial disease and which is estimated to occur in approximately 25% of the estimated five million catheters implanted annually in the United States. Although alfimeprase may in the future prove effective in treating types of peripheral arterial disease other than acute PAO, we expect to continue to focus our initial commercialization efforts on the two indications discussed in this prospectus.
We have received, and may continue to receive, media coverage, including coverage that is not directly attributable to statements made by our officers and employees. Neither we nor any of the underwriters in this offering have confirmed, endorsed or adopted any statements that were not made by us for utilization by, or distribution to, prospective purchasers in this offering. To the extent any statements are inconsistent with, or conflict with, the information contained in this prospectus, or relate to information not contained in this prospectus, they are disclaimed by us and the underwriters and you should not rely on them in making your investment decision.
We do not believe that our involvement in the Forbes article constitutes a violation of the Securities Act of 1933. However, if our involvement were held by a court to be in violation of the Securities Act of 1933, we could be required to repurchase the shares sold to purchasers in this offering at the original purchase price, plus statutory interest from the date of purchase, for a period of one year following the date of the violation. We would contest vigorously any claim that a violation of the Securities Act occurred.
Our stock price has historically been and is likely to remain highly volatile, and an investment in our stock could suffer a decline in value.
Stock prices and trading volumes for many biopharmaceutical companies fluctuate widely for a number of reasons, including factors which may be unrelated to their businesses or results of operations such as media coverage, legislative and regulatory measures and the activities of various interest groups or organizations. This market volatility, as well as general domestic or international economic, market and political conditions, could materially and adversely affect the market price of our common stock and the return on your investment.
Historically, our stock price has been extremely volatile. Between January 1, 2004 and December 31, 2004, the price ranged between a high of $16.50 per share and a low of $6.77 per share. The significant market price fluctuations of our common stock are due to a variety of factors, including:
| Ø | the depth of the market for the common stock; |
| Ø | the experimental nature of our potential products; |
| Ø | actual or anticipated fluctuations in our operating results; |
| Ø | sales of our common stock by existing holders, or sales of shares issuable upon exercise of outstanding options and warrants, upon repayment of our outstanding note to Affymetrix, or upon repayment of our line of credit with Dr. Rathmann; |
| Ø | market conditions relating to the biopharmaceutical and pharmaceutical industries; |
| Ø | any announcements of technological innovations, new commercial products, or clinical progress or lack thereof by us, our collaborative partners or our competitors; |
| Ø | announcements concerning regulatory developments, developments with respect to proprietary rights and our collaborations; |
| Ø | changes in or our failure to meet market or, to the extent securities analysts follow our common stock, securities analysts’ expectations; |
S-29
Table of Contents
Risk factors
| Ø | loss of key personnel; |
| Ø | changes in accounting principles; |
| Ø | general market conditions; or |
| Ø | public concern with respect to our products. |
In addition, the stock market in general, and the market for biotechnology and other life science stocks in particular, has historically been subject to extreme price and volume fluctuations. This volatility has had a significant effect on the market prices of securities issued by many companies for reasons unrelated to the operating performance of these companies. In the past, following periods of volatility in the market price of a company’s securities, class action securities litigation has often been instituted against such a company. Any such litigation instigated against us could result in substantial costs and a diversion of management’s attention and resources, which could significantly harm our business, financial condition and operating results.
Future sales of our common stock may depress the market price of our common stock.
Sales in the public market of substantial amounts of our common stock could depress prevailing market prices of our common stock. As of December 31, 2004, we had 32,228,732 shares of our common stock outstanding. All of these shares are freely transferable without restriction or further registration under the Securities Act of 1933, as amended, or the Securities Act, except for shares held by our affiliates and unregistered shares held by non-affiliates. As of December 31, 2004, our directors, officers and greater than five percent stockholders held 4,923,431 shares of our common stock, which are transferable pursuant to Rule 144 or in some cases Rule 145, each as promulgated under the Securities Act, or pursuant to effective registration statements. Although we do not believe that our directors, officers and greater than five percent stockholders have any present intentions to dispose of any shares of common stock owned by them, there can be no assurance that such intentions will not change in the future. The sale of these additional shares could depress the market price of our common stock.
We have granted registration rights in connection with the issuance of our securities to a number of our stockholders and warrant holders. In the aggregate, as of December 31, 2004, these registration rights covered approximately 1,516,792 shares of our common stock which were then outstanding or which may become outstanding upon the exercise of warrants that were then outstanding. If these registration rights, or similar registration rights that may apply to securities we may issue in the future, are exercised, it could result in additional sales of our common stock in the market, which may have an adverse effect on our stock price.
In addition, under registration statements on Form S-8 under the Securities Act, we have registered approximately 4,766,669 shares of our common stock for sale upon the exercise of outstanding options under our 2004 Equity Incentive Plan, 2002 Equity Incentive Plan, 1995 Stock Option Plan, Non-Employee Director Stock Option Plan, Scientific Advisory Board/Consultants Stock Option Plan, the Variagenics, Inc. Amended 1997 Employee Director and Consultant Stock Option Plan and stock option agreements entered into outside of any of our stock option plans. Included in this 4,766,669 shares, options to exercise 3,871,594 shares of our common stock were outstanding under the specified plans, and shares of our common stock acquired pursuant to these plans and agreements are available for sale in the open market. Additionally, included in the 4,766,669 shares, we have reserved approximately 895,075 shares of our common stock for issuance upon the exercise of outstanding options under stock option agreements entered into outside of any of our stock option plans. As of December 31, 2004, 3,591,344 shares of the total 6,283,461 shares of these warrants and options were exercisable. In addition, as of December 31, 2004, we had 3,760,298 shares of our common stock remaining for future option grants under our 2004 Equity Incentive Plan. Under our Employee Stock Purchase Plan, we have
S-30
Table of Contents
Risk factors
approximately 56,736 shares of our common stock reserved for future issuance as of December 31, 2004. The existence of the currently outstanding warrants and options to purchase our common stock may negatively affect our ability to complete future equity financings at acceptable prices and on acceptable terms. The exercise of those options or warrants, and the prompt resale of shares of our common stock received, may result in downward pressure on the price of our common stock.
As of December 31, 2004, 542,235 shares of our common stock were issuable, at our option, to repay a note in the principal amount of $4,000,000 held by Affymetrix. Affymetrix has the ability to declare all outstanding principal and interest under the note immediately due and payable in the event that our market capitalization is under $50 million and Affymetrix reasonably determines that the loan evidenced by the note is impaired, and we have an obligation to prepay amounts owing under the note to the extent that the amounts outstanding exceed 10% of our market capitalization. Moreover, we have registered for resale a portion of these shares on a registration statement that has been declared effective by the Securities and Exchange Commission. If we decide to repay this note with our common stock, whether pursuant to acceleration of the note or otherwise, the resale of shares of our common stock by Affymetrix may also result in significant downward pressure on the price of our common. In addition, as of December 31, 2004, 907,113 shares of common stock were issuable, upon mutual agreement, to convert the promissory note that we have issued under a line of credit with George Rathmann. If we agree to repay this note with our common stock, whether pursuant to acceleration of the note or otherwise, the resale of shares of our common stock received by George Rathmann may also result in significant downward pressure on the price of our common stock.
Upon completion of this offering, based on information as of December 31, 2004 we will have outstanding an aggregate of 39,228,732 shares of common stock, assuming no exercise of outstanding options or warrants, no issuance of shares under our Employee Stock Purchase Plan, and excluding the shares of our common stock issuable, at our option, to repay our note held by Affymetrix, and shares of common stock issuable, upon mutual agreement, to convert the promissory note under the Rathmann line of credit. All of the shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act unless these shares are purchased by affiliates.
We will need to raise significant additional capital to finance the research and clinical development of our drug products. If future securities offerings are successful, they could dilute our current shareholders’ equity interests and reduce the market price of our common stock.
Our management will have broad discretion with respect to the use of proceeds of this offering, and may not apply the proceeds to uses that will benefit stockholders.
Our management will have broad discretion as to how to use the proceeds of this offering. You will be relying on the judgment of our management regarding the application of the proceeds of this offering. The results and effectiveness of the use of proceeds are uncertain.
We do not intend to pay cash dividends on our common stock in the foreseeable future.
We do not anticipate paying cash dividends on our common stock in the foreseeable future. Any payment of cash dividends will depend upon our financial condition, results of operations, capital requirements and other factors and will be at the discretion of the Board of Directors. Under our August 31, 2004 Loan and Security Agreement with Silicon Valley Bank, we cannot pay dividends without Silicon Valley Bank’s prior written consent, except for dividends paid in shares of our capital stock. Furthermore, we may incur additional indebtedness that may severely restrict or prohibit the payment of dividends.
S-31
Table of Contents
All statements included or incorporated by reference in this prospectus supplement and the accompanying prospectus, other than statements of historical facts, that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements are typically characterized by terminology such as “believe,” “anticipate,” “should,” “intend,” “plan,” “will,” “expect,” “estimate,” “project,” “positioned,” “strategy,” and similar expressions. These statements are based on assumptions and assessments made by our management in light of its experience and its perception of historical trends, current conditions, expected future developments and other factors our management believes to be appropriate. These forward-looking statements are subject to a number of risks and uncertainties, including those risks described or incorporated by reference in this prospectus supplement and the accompanying prospectus under “Risk factors”, as well as other factors that our management has not yet identified. Any such forward-looking statements are not guarantees of future performance and actual results, developments and business decisions may differ from those contemplated by such forward-looking statements. We disclaim any duty to update any forward-looking statements.
We encourage you to read this prospectus supplement and the accompanying prospectus, as well as the information that is incorporated by reference in this prospectus supplement and the accompanying prospectus, in their entireties. You should carefully consider the facts set forth under “Risk factors” beginning on page S-9 in this prospectus supplement and in the other reports incorporated by reference herein before making an investment decision to purchase shares of our common stock.
We estimate the net proceeds to us from this offering will be approximately $59.9 million, assuming a public offering price of $9.19 per share and after payment of underwriting discounts and commissions and estimated expenses of this offering, or approximately $68.9 million if the underwriters exercise their over-allotment option in full.
We intend to use the net proceeds to us from this offering for general corporate purposes, including capital expenditures, working capital needs, current and future clinical trials of our lead drug candidate, alfimeprase, as well as other research and drug development activities. The amounts and timing of the expenditures will depend on numerous factors, such as the timing and progress of our clinical trials and research and development efforts, technological advances and the competitive environment for our drug candidates. We expect from time to time to evaluate the acquisition of businesses, products and technologies for which a portion of the net proceeds may be used, although we currently are not planning or negotiating any such transactions. As of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from the offering. Accordingly, we will retain broad discretion over the use of these proceeds.
Pending such uses, we may invest the net proceeds in investment-grade interest-bearing securities.
S-32
Table of Contents
Our common stock is quoted on The Nasdaq National Market under the symbol “NUVO.” The following table sets forth, for the periods indicated, the high and low reported sales prices of our common stock as reported on The Nasdaq National Market, after giving effect to the one-for-three reverse split of our common stock on February 23, 2004:
| Stock Price | ||||||
| High | Low | |||||
Year ended December 31, 2002 | ||||||
First quarter | $ | 27.00 | $ | 15.96 | ||
Second quarter | 15.99 | 5.10 | ||||
Third quarter | 8.52 | 4.05 | ||||
Fourth quarter | 5.70 | 2.55 | ||||
Year ended December 31, 2003 | ||||||
First quarter | $ | 4.47 | $ | 1.92 | ||
Second quarter | 8.16 | 2.58 | ||||
Third quarter | 12.03 | 5.28 | ||||
Fourth quarter | 12.66 | 7.47 | ||||
Year ended December 31, 2004 | ||||||
First quarter | $ | 16.50 | $ | 10.36 | ||
Second quarter | 13.20 | 7.57 | ||||
Third quarter | 10.44 | 6.77 | ||||
Fourth quarter | 11.23 | 8.36 | ||||
As of December 31, 2004, we estimate there were approximately 267 holders of record of our common stock. On January 20, 2005, the last sale price reported on The Nasdaq National Market for our common stock was $9.19 per share.
The holders of our common stock are entitled to dividends in such amounts and at such times, if any, as may be declared by our board of directors out of legally available funds. We have not paid any dividends on our common stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. Under our August 31, 2004 Loan and Security Agreement with Silicon Valley Bank, we cannot pay dividends without Silicon Valley Bank’s prior written consent, except for dividends paid in shares of our capital stock.
S-33
Table of Contents
The following table shows our cash, cash equivalents and investments and capitalization as of September 30, 2004:
| Ø | on an actual basis; and |
| Ø | on an as adjusted basis to give effect to our sale of 7,000,000 shares of common stock offered by us, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
This table should be read with “Management’s discussion and analysis of financial condition and results of operations” and our financial statements and the related notes incorporated by reference in this prospectus supplement and the accompanying prospectus.
| As of September 30, 2004 | ||||||||
| Actual | As adjusted | |||||||
(in thousands, except share and per share data) | ||||||||
Cash, cash equivalents and short-term investments(1) | $ | 70,671 | $ | 130,521 | ||||
Current portion of capital lease, note and line of credit obligations | 6,698 | 6,698 | ||||||
Non-current portion of capital lease, note and line of credit obligations | 9,886 | 9,886 | ||||||
Stockholders’ equity: | ||||||||
Preferred stock, $0.001 par value; 5,000,000 shares authorized; no shares issued or outstanding, actual and as adjusted | — | — | ||||||
Common stock $0.001 par value; 100,000,000 shares authorized 32,199,571 shares issued and outstanding, actual; 39,199,571 shares issued and outstanding, as adjusted | 32 | 39 | ||||||
Additional paid-in capital | 301,629 | 361,472 | ||||||
Accumulated other comprehensive loss | (79 | ) | (79 | ) | ||||
Accumulated deficit | (243,066 | ) | (243,066 | ) | ||||
Total stockholders’ equity | 58,516 | 118,366 | ||||||
Total capitalization | $ | 75,100 | $ | 134,950 | ||||
| (1) | As of December 31, 2004, cash, cash equivalents and short-term investments totaled approximately $50,625,000. |
The number of shares of common stock outstanding is based on the actual number of shares outstanding as of September 30, 2004, but excludes:
| Ø | an aggregate of 3,461,882 shares of our common stock issuable upon exercise of stock options outstanding as of September 30, 2004, granted under our 2004 Equity Incentive Plan, 2002 Equity Incentive Plan, 1995 Stock Option Plan, Non-Employee Director Stock Option Plan, Scientific Advisory Board/Consultants Stock Option Plan and the Variagenics, Inc. Amended 1997 Employee, Director and Consultant Stock Option Plan, and as of September 30, 2004, an aggregate of 895,075 shares of common stock issuable upon the exercise of stock options granted outside of any of our stock option plans, with exercise prices of all outstanding options ranging from $0.03 to $304.31 per share and a weighted average exercise price of $19.63 per share; |
| Ø | an aggregate of 4,188,424 shares of common stock reserved for issuance pursuant to future option grants under the 2004 Equity Incentive Plan, based on options outstanding as of September 30, 2004; |
S-34
Table of Contents
Capitalization
| Ø | an aggregate of 64,997 shares of common stock issuable under our Employee Stock Purchase Plan as of September 30, 2004; |
| Ø | an aggregate of 1,516,792 shares of our common stock issuable upon the exercise of warrants, with exercise prices ranging from $4.05 to $25.53 per share, and a weighted average exercise price of $20.88 per share, outstanding as of September 30, 2004; and |
| Ø | 583,149 shares of common stock issuable at our option to repay our note held by Affymetrix and 1,053,321 shares of common stock issuable upon mutual agreement to convert the promissory note under the Rathmann line of credit, both as of September 30, 2004. |
S-35
Table of Contents
If you invest in our common stock, your interest will be diluted to the extent of the difference between the public offering price per share you pay in this offering and the net tangible book value per share of our common stock immediately after this offering. Our net tangible book value as of September 30, 2004 was approximately $52.9 million, or $1.64 per share of common stock. Net tangible book value per share is equal to our total tangible assets minus total liabilities, all divided by the number of shares of common stock outstanding as of September 30, 2004. After giving effect to the sale by us of the 7,000,000 shares of common stock we are offering, assuming a public offering price of $9.19 per share and after deducting underwriting discounts and commissions and our estimated offering expenses, our as adjusted net tangible book value would have been approximately $112.8 million, or $2.88 per share of common stock. This represents an immediate increase in net tangible book value of $1.24 per share to our existing stockholders and an immediate dilution of $6.31 per share to new investors. The following table illustrates this calculation on a per share basis:
Public offering price per share | $ | 9.19 | ||||
Net tangible book value per share as of September 30, 2004 | $ | 1.64 | ||||
Increase per share attributable to the offering | 1.24 | |||||
As adjusted net tangible book value per share after this offering | 2.88 | |||||
Dilution per share to new investors | $ | 6.31 | ||||
If the underwriters exercise their over-allotment option in full, the as adjusted net tangible book value as of September 30, 2004 would have been $3.03 per share, representing an increase to existing stockholders of $1.39 per share, and there will be an immediate dilution of $6.16 per share to new investors.
The foregoing table does not take into effect further dilution to new investors that could occur upon the exercise of outstanding options and warrants having a per share exercise price less than the offering price per share in this offering. As of September 30, 2004, there were:
| Ø | an aggregate of 3,461,882 shares of our common stock issuable upon exercise of stock options outstanding as of September 30, 2004, granted under our 2004 Equity Incentive Plan, 2002 Equity Incentive Plan, 1995 Stock Option Plan, Non-Employee Director Stock Option Plan, Scientific Advisory Board/Consultants Stock Option Plan and the Variagenics, Inc. Amended 1997 Employee, Director and Consultant Stock Option Plan, and as of September 30, 2004, an aggregate of 895,075 shares of common stock issuable upon the exercise of stock options granted outside of any of our stock option plans, with exercise prices of all outstanding options ranging from $0.03 to $304.31 per share and a weighted average exercise price of $19.63 per share; |
| Ø | an aggregate of 4,188,424 shares of common stock reserved for issuance pursuant to future option grants under the 2004 Equity Incentive Plan, based on options outstanding as of September 30, 2004; |
| Ø | an aggregate of 64,997 shares of common stock issuable under our Employee Stock Purchase Plan as of September 30, 2004; |
| Ø | an aggregate of 1,516,792 shares of our common stock issuable upon the exercise of warrants, with exercise prices ranging from $4.05 to $25.53 per share, and a weighted average exercise price of $20.88 per share, outstanding as of September 30, 2004; and |
| Ø | 583,149 shares of common stock issuable at our option to repay our note held by Affymetrix and 1,053,321 shares of common stock issuable upon mutual agreement to convert the promissory note under the Rathmann line of credit, both as of September 30, 2004. |
S-36
Table of Contents
Set forth below is information regarding each of our executive officers and directors as of December 31, 2004.
| Name | Age | Position | ||
George B. Rathmann, Ph.D.(1) | 77 | Chairman of the Board of Directors | ||
Ted W. Love, M.D. | 45 | President, Chief Executive Officer and Director | ||
Lee Bendekgey, J.D. | 47 | Senior Vice President, Chief Financial Officer, and General Counsel | ||
Linda A. Fitzpatrick | 48 | Senior Vice President of Human Resources | ||
Michael D. Levy, M.D., F.F.P.M. | 43 | Senior Vice President of Research and Development | ||
Gary S. Titus, C.P.A. | 44 | Vice President of Finance and Chief Accounting Officer | ||
Burton E. Sobel, M.D. | 68 | Director | ||
Mary K. Pendergast, J.D., L.L.M.(1) | 54 | Director | ||
Mark L. Perry, J.D.(2),(3) | 49 | Director | ||
Martin A. Vogelbaum(1),(3) | 41 | Director | ||
Barry L. Zubrow, J.D., M.B.A.(2),(3) | 51 | Director |
| (1) | Member of the Nominating and Governance Committee of the Board of Directors. |
| (2) | Member of the Compensation Committee of the Board of Directors. |
| (3) | Member of the Audit Committee of the Board of Directors. |
George B. Rathmann, Ph.D. has served as chairman and a member of our board of directors since February 2000. Dr. Rathmann served as our chief executive officer from May 2000 to March 2001 and also served as our president from May 2000 to January 2001. Dr. Rathmann was a founder of ICOS Corporation, a publicly held biopharmaceutical company, in 1990 and served as its chairman until January 2000. While at ICOS, he also served as chief executive officer and president from September 1991 until June 1999. In 1980, he co-founded Amgen Inc., a publicly held biotechnology company. He was a director of Amgen until 1993 and at various times also served as its chairman of the board, president and chief executive officer. Dr. Rathmann was also associated with Abbott Laboratories, Inc., a healthcare products manufacturer, where from 1975 to 1977 he was director of research and development and from 1977 to 1980 he was divisional vice president. Dr. Rathmann received his Ph.D. in physical chemistry from Princeton University.
Ted W. Love, M.D. has served as our president since January 2001, our chief executive officer since March 2001 and as a director since February 2001. Dr. Love served as our president and chief operating officer from January 2001 until March 2001. Prior to joining us, Dr. Love served as senior vice president of development at Theravance Inc. (formerly Advanced Medicine, Inc.) from 1998 to 2001 and as a research physician and vice president of product development at Genentech from 1992 to 1998. Dr. Love holds a B.A. in molecular biology from Haverford College and a M.D. from Yale Medical School.
Lee Bendekgey, J.D. joined us in July 2004 as our senior vice president and general counsel. On July 23, 2004, Mr. Bendekgey also became our chief financial officer. Mr. Bendekgey brings to Nuvelo over 20 years of legal experience. Prior to joining us, Mr. Bendekgey spent approximately six years at Incyte Corporation where he held several executive positions, including executive vice president, general counsel, acting chief financial officer and acting general manager of information business. From 1993 to 1997, Mr. Bendekgey worked for Silicon Graphics, Inc. in Mountain View, California where he held a variety of positions in their legal group including director of legal services, products and technology and director of strategic relations, helping the company negotiate strategic relationships and providing legal support to
S-37
Table of Contents
Management
all product divisions. Prior to his time at Silicon Graphics, he served as a partner at Graham & James (now Squire Sanders & Dempsey) where he specialized in intellectual property, corporate and commercial law and founded the firm’s Palo Alto office. Mr. Bendekgey graduated magna cum laude with a bachelor of arts degree from Kalamazoo College and received his J.D. from Stanford University.
Linda A. Fitzpatrick joined us in April 2001 as our senior vice president of human resources. Prior to joining us, Ms. Fitzpatrick served as senior advisor at Theravance, Inc. (formerly Advanced Medicine, Inc.) from 1998 to 2000 and vice president, human resources, corporate communications and operations at Gilead Sciences, Inc. from 1992 to 1998. Prior to her tenure at Gilead Sciences, Ms. Fitzpatrick served eight years at Genentech, Inc. where her positions included director, investor relations and director, compensation, benefits and systems. Ms. Fitzpatrick graduated with honors with a Bachelor of Science degree in sociology and psychology from San Francisco State University.
Michael D. Levy, M.D., F.F.P.M. joined us in November 2004, as our senior vice president, research and development. Dr. Levy comes to Nuvelo from Tularik Inc. (recently acquired by Amgen Inc.) where he served as vice president of development and chief medical officer. While at Tularik, Dr. Levy created and built a new development organization and directed the clinical development of seven novel drugs across three therapeutic areas: oncology, metabolic disease and inflammation/immunology. Prior to joining Tularik, Dr. Levy spent 12 years at Glaxo SmithKline Inc., or GSK, in Canada, where he held several positions including his final role as senior vice president of R&D and chief medical officer. While at GSK, he had dual responsibilities for supporting global drug development and the Canadian commercial business. Dr. Levy earned his B.A., M.A. and M.D. at Cambridge University, U.K. He undertook his surgical residency at the Mayo Clinic and received the distinction of being elected a Fellow of the Faculty of Pharmaceutical Medicine of the Royal College of Physicians.
Gary S. Titus, C.P.A.joined us in January 2003 as senior director of finance and was promoted to the position of Vice President Finance and Chief Accounting Officer in July 2004. Mr. Titus brings 18 years of financial management experience to Nuvelo. Prior to Nuvelo, he served as a senior director of finance at emerging life science companies including Metabolex, Inc. from January 2002 to January 2003 and IntraBiotics Pharmaceuticals, Inc. from January 2000 to January 2002. In addition, he held a variety of financial management positions at Johnson & Johnson from August 1997 to January 2000. Mr. Titus is a certified public accountant and has a bachelor’s degree in finance from the University of Florida and a bachelor’s degree in accounting from the University of South Florida.
Burton E. Sobel, M.D.joined our board of directors in September 2004. Dr. Sobel is currently at the University of Vermont and Fletcher Allen Health Care where he has served as the E.L. Amidon professor and chair of the department of medicine and professor of biochemistry since 1994, as well as the director of the Cardiovascular Center since 2002. Prior to joining our board, Dr. Sobel served as senior counsel to the executive dean of the University of Vermont College of Medicine and to the executive vice president of Fletcher Allen Health Care from 1996 to 1998. From 1994 to 1996, Dr. Sobel served as adjunct professor of medicine at Washington University in St. Louis, Missouri. Dr. Sobel received his M.D. from the Harvard Medical School, magna cum laude, and his A.B. from Cornell University.
Mary K. Pendergast, J.D., L.L.M. has served as a member of our board of directors since May 2002. Ms. Pendergast has served as executive vice president, government affairs for Elan Corporation since January 1998. Ms. Pendergast was deputy commissioner/senior advisor to the Food and Drug Administration, Department of Health and Human Services from November 1990 to January 1998. Ms. Pendergast received her L.L.M. from Yale Law School in 1977, her J.D. from the University of Iowa College of Law in 1976 and her B.A. from Northwestern University in 1972.
S-38
Table of Contents
Management
Mark L. Perry, J.D. has served as a member of our board of directors since October 2003. Mr. Perry currently serves as the executive vice president of operations for Gilead Sciences, Inc., a biopharmaceutical company located in Foster City, California. Mr. Perry joined Gilead in 1994 as vice president, general counsel and secretary, later assumed the additional responsibilities of chief financial officer in 1996, and was promoted to his current position in 2000. At Gilead, Mr. Perry is responsible for worldwide commercial operations and the legal, intellectual property, manufacturing and facilities functions. Prior to joining Gilead, Mr. Perry served as a partner at the law firm Cooley Godward LLP, based in San Francisco and Palo Alto, California. Mr. Perry also sits on the board of directors of IntraBiotics Pharmaceuticals, Inc. and DNA Sciences, Inc. Mr. Perry received his J.D. from the University of California, Davis and is a member of the California bar.
Martin A. Vogelbaum has served as a member of our board of directors since our merger with Variagenics in January 2003. Mr. Vogelbaum served as a member of the board of directors of Variagenics from 1997 until 2003. Since June 2000, Mr. Vogelbaum has been a general partner of Apple Tree Partners. Previously, Mr. Vogelbaum was a general partner of Oxford Bioscience Partners. Prior to joining Oxford in 1993, he held senior executive positions at the public and investor relations firms of Burns McClellan, Inc. and Hill & Knowlton, where he implemented marketing and investor initiatives for biotechnology and pharmaceutical companies. Previously, he was a research associate in the Bone Marrow Transplant Unit at Memorial Sloan-Kettering Cancer Center. Mr. Vogelbaum received his A.B. in biology and history from Columbia University.
Barry L. Zubrow, J.D., M.B.A. has served as a member of our board of directors since February 3, 2004. Mr. Zubrow brings over 26 years of corporate finance experience to our board of directors. From 1977 to 2003, Mr. Zubrow held a variety of positions at The Goldman Sachs Group including chief administrative officer and head of the operations and the administration division. As the firm’s first chief administrative officer, he was responsible for overseeing a 4,500 plus person group of departments in charge of financial reporting, credit and risk functions, operations and trade processing, facilities, security and corporate services. Prior to that, Mr. Zubrow was elected the firm’s first chief credit officer, with responsibility for overseeing the entire firm’s global credit exposures. He received his J.D. and M.B.A. for the University of Chicago and is an active board member for a number of non-profit organizations.
S-39
Table of Contents
We are offering the shares of our common stock described in this prospectus supplement through the underwriters named below. UBS Securities LLC, Deutsche Bank Securities Inc., CIBC World Markets Corp. and Needham & Company, Inc. are the representatives of the underwriters. UBS Securities LLC is the sole book-running manager of this offering and Deutsche Bank Securities Inc. is acting as the co-lead manager.
We have entered into an underwriting agreement with the representatives. Subject to the terms and conditions of the underwriting agreement, each of the underwriters has severally agreed to purchase the number of shares of common stock listed next to its name in the following table.
| Underwriters | Number of shares | |
UBS Securities LLC | ||
Deutsche Bank Securities Inc. | ||
CIBC World Markets Corp. | ||
Needham & Company, Inc. | ||
Total | 7,000,000 | |
The underwriting agreement provides that the underwriters buy all of the shares if they buy any of them. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
Our common stock is offered subject to a number of conditions, including:
| Ø | receipt and acceptance of our common stock by the underwriters; and |
| Ø | the underwriters’ right to reject orders in whole or in part. |
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectus supplements and prospectuses electronically.
OVER-ALLOTMENT OPTION
We have granted the underwriters an option to buy up to an aggregate of 1,050,000 additional shares of our common stock. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. The underwriters have 30 days from the date of this prospectus supplement to exercise this option. If the underwriters exercise this option, they will each purchase additional shares approximately in proportion to the amounts specified in the table above.
COMMISSIONS AND DISCOUNTS
Shares sold by the underwriters to the public will initially be offered at the public offering price set forth on the cover of this prospectus supplement. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the public offering price. Any of these securities dealers may resell any shares purchased from the underwriters to other brokers or dealers at a discount of up to $ per share from the public offering price. If all the shares are not sold at the public offering price, the representatives may change the offering price and the other selling terms.
S-40
Table of Contents
Underwriting
The following table shows the per share and total underwriting discounts and commissions we will pay to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase up to an additional 1,050,000 shares.
| Paid by the Company | No exercise | Full exercise | ||||
Per share | $ | $ | ||||
Total | $ | $ | ||||
We estimate that the total expenses of this offering payable by us, not including underwriting discounts and commissions, will be approximately $620,000.
NO SALES OF SIMILAR SECURITIES
We and our executive officers and directors have entered into lock-up agreements with the underwriters. Under these agreements, we and each of these persons generally may not, without the prior written approval of UBS Securities LLC, subject to certain permitted exceptions, offer, sell, contract to sell or otherwise dispose of or hedge our common stock or securities convertible into or exercisable or exchangeable for our common stock. These restrictions will be in effect for a period of 90 days after the date of this prospectus supplement. The 90-day lock up period may be extended for up to 37 days under certain circumstances where we announce or pre-announce earnings or material news or a material event within approximately 18 days prior to, or approximately 16 days after, the termination of the 90-day period. At any time and without public notice UBS Securities LLC may in its sole discretion release all or some of the securities from these lock-up agreements.
INDEMNIFICATION AND CONTRIBUTION
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we have agreed to contribute to payments the underwriters may be required to make in respect of those liabilities.
NASDAQ NATIONAL MARKET QUOTATION
Our common stock is quoted on The Nasdaq National Market under the symbol “NUVO.”
PRICE STABILIZATION, SHORT POSITIONS, PASSIVE MARKET MAKING
In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our common stock, including:
| Ø | stabilizing transactions; |
| Ø | short sales; |
| Ø | purchases to cover positions created by short sales; |
| Ø | imposition of penalty bids; |
| Ø | syndicate covering transactions; and |
| Ø | passive market making. |
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of our common stock while this offering is in progress. These transactions may also include making short sales of our common stock, which involve the sale by the underwriters of
S-41
Table of Contents
Underwriting
a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered short sales,” which are short positions in an amount not greater than the underwriters’ over-allotment option referred to above, or may be “naked short sales,” which are short positions in excess of that amount.
The underwriters may close out any covered short position by either exercising their over-allotment option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchased in this offering.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of that underwriter in stabilizing or short covering transactions.
As a result of these activities, the price of our common stock may be higher than the price that otherwise might exist in the open market. If the activities are commenced, they may be discontinued by the underwriters at any time. The underwriters may carry out these transactions on The Nasdaq National Market, in the over-the-counter market or otherwise.
In addition, in connection with this offering, certain of the underwriters (and selling group members) may engage in passive market making transactions in our common stock on The Nasdaq National Market prior to the pricing and completion of this offering. Passive market making consists of displaying bids on The Nasdaq National Market no higher than the bid prices of independent market makers and making purchases at prices no higher than these independent bids and effected in response to order flow. Net purchases by a passive market maker on each day are limited to a specified percentage of the passive market maker’s average daily trading volume in the common stock during a specified period and must be discontinued when such limit is reached. Passive market making may cause the price of our common stock to be higher than the price that otherwise would exist in the open market in the absence of these transactions. If passive market making is commenced, it may be discontinued at any time.
AFFILIATIONS
UBS Securities LLC, CIBC World Markets Corp., and Needham & Company, Inc. have in the past provided, and certain of the underwriters may in the future provide, financial advisory services to us. For these services, we have paid, or will pay, them customary compensation. The underwriters and their affiliates may from time to time in the future engage in transactions with us and perform services for us in the ordinary course of their business.
S-42
Table of Contents
The validity of the securities being offered by this prospectus supplement will be passed upon for us by Cooley Godward LLP of Palo Alto, California. Davis Polk & Wardwell, Menlo Park, California, is counsel for the underwriters in connection with this offering.
The consolidated financial statements of Nuvelo, Inc. as of December 31, 2003 and 2002 and for each of the years in the three year period ended December 31, 2003 have been incorporated by reference herein and in the registration statement in reliance upon the report of KPMG LLP, independent accountants, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
The consolidated financial statements of Variagenics, Inc. as of December 31, 2002 and 2001, and for each of the three years in the period ended December 31, 2002 incorporated in this prospectus supplement by reference to the Current Report on Form 8-K/A, dated July 3, 2003, of Nuvelo, Inc., have been so incorporated in reliance on the reports of PricewaterhouseCoopers LLP, independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
Where you can find more information
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission, or the SEC. You may read and copy these reports, proxy statements and other information at the SEC’s public reference rooms at 450 Fifth Street, N.W., Washington, D.C., 20549. You can request copies of these documents by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the public reference rooms. In addition, you can read and copy our SEC filings at the office of the National Association of Securities Dealers, Inc. at 1735 K Street, Washington, D.C. 20006. Our SEC filings are also available at the SEC’s web site at www.sec.gov and our website at www.nuvelo.com. We have not incorporated by reference into this prospectus the information contained on our website and you should not consider it to be part of this prospectus.
The SEC allows us to “incorporate by reference” information that we file with them, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is an important part of this prospectus supplement, and information that we file later with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below and any future filings we will make with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, after the date of this prospectus supplement but before the end of any offering made under this prospectus supplement and accompanying prospectus (other than current reports or portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K):
| Ø | our current report on Form 8-K, filed with the SEC on February 4, 2003, as amended on Form 8-K/A filed with the SEC on February 14, 2003, and as further amended on Form 8-K/A filed with the SEC on July 3, 2003; |
S-43
Table of Contents
Incorporation by reference
| Ø | our annual report on Form 10-K for the fiscal year ended December 31, 2003, filed with the SEC on March 12, 2004; |
| Ø | our proxy statement for our stockholders’ meeting on May 6, 2004, filed on April 12, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on March 26, 2004; |
| Ø | our quarterly report on Form 10-Q for the quarter ended March 31, 2004, filed with the SEC on May 10, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on May 10, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on July 29, 2004; |
| Ø | our quarterly report on Form 10-Q for the quarter ended June 30, 2004, filed with the SEC on August 9, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on September 8, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on September 15, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on September 16, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on September 20, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on November 4, 2004; |
| Ø | our quarterly report on Form 10-Q for the quarter ended September 30, 2004, filed with the SEC on November 9, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on December 9, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on December 20, 2004; |
| Ø | our current report on Form 8-K/A, filed with the SEC on December 23, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on January 14, 2005; and |
| Ø | our current report on Form 8-K, filed with the SEC on January 24, 2005. |
We will provide to you at no cost a copy of any and all of the information incorporated by reference into this prospectus supplement and the accompanying prospectus. You may make a request for copies of this information in writing or by telephone. Requests should be directed to:
Nuvelo, Inc.
Attention: Lee Bendekgey
675 Almanor Avenue
Sunnyvale, CA 94085
(408) 215-4000
S-44
Table of Contents
PROSPECTUS
$100,000,000

Debt Securities
Preferred Stock
Common Stock
We may sell from time to time in one or more offerings up to $100,000,000 in the aggregate of:
| Ø | our secured or unsecured debt securities, in one or more series, which may be either senior, senior subordinated or subordinated debt securities; |
| Ø | shares of our preferred stock in one or more series; |
| Ø | shares of our common stock; and |
| Ø | any combination of the foregoing. |
We will provide the specific terms of these securities in supplements to this prospectus. You should read this prospectus and any prospectus supplement carefully before you invest.This prospectus may not be used to offer or sell any securities unless accompanied by a prospectus supplement.
Investing in our securities involves risks. see “Risk Factors“ beginning on page 1.
Our common stock is quoted on The Nasdaq National Market under the symbol “NUVO.” On November 30, 2004, the last reported sale price for our common stock on The Nasdaq National Market was $9.75 per share. The applicable prospectus supplement will contain information, where applicable, as to any other listing on The Nasdaq national market or any securities exchange of the securities covered by the prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
December 6, 2004
Table of Contents
TABLE OF CONTENTS
| Page | ||
| 1 | ||
| 1 | ||
| 1 | ||
| 2 | ||
| 2 | ||
| 2 | ||
| 3 |
| Page | ||
| 12 | ||
| 14 | ||
| 16 | ||
| 18 | ||
| 20 | ||
| 20 | ||
| 20 |
This prospectus is part of a registration statement we filed with the Securities and Exchange Commission. You should rely only on the information we have provided or incorporated by reference in this prospectus or any prospectus supplement. We have not authorized anyone to provide you with additional or different information. We are not making an offer of these securities in any jurisdiction where the offer is not permitted. You should assume that the information in this prospectus or any prospectus supplement is accurate only as of the date on the front of the document and that any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference.
We own or have rights to use trademarks or trade names that we use in conjunction with the operation of our business. Nuvelo is a registered trade and service mark of ours. All other trademarks, service marks and trade names referred to in this prospectus are the property of their respective owners.
Table of Contents
This prospectus is part of a Registration Statement on Form S-3 that we filed with the Securities and Exchange Commission utilizing a “shelf” registration process. Under this shelf process, we may offer any combination of securities described in this prospectus in one or more offerings. This prospectus provides you with a general description of the securities we may offer. Each time we use this prospectus to offer securities, we will provide a prospectus supplement that will contain specific information about the terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus. You should read both this prospectus and any prospectus supplement together with additional information described below under the heading “Where You Can Find More Information.” THIS PROSPECTUS MAY NOT BE USED TO CONSUMMATE A SALE OF SECURITIES UNLESS IT IS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
An investment in our debt securities, preferred stock or common stock involves a high degree of risk. You should consider carefully the risk factors contained in our most recent Annual Report on Form 10-K, and in our most recent Quarterly Report on Form 10-Q, both as filed with the Securities and Exchange Commission, or SEC, and both of which are incorporated herein by reference. You should also consider all other information contained in and incorporated by reference in this prospectus before making an investment decision. Additional risks and uncertainties that are not yet identified or that we think are immaterial may also materially harm our business, operating results and financial condition and could result in a complete loss of your investment.
We are strategically focused on the discovery, development and commercialization of life improving therapeutics for the treatment of acute cardiovascular indications and cancer. As part of this plan, we intend to dedicate our resources to advancing our most promising biopharmaceutical discovery and development programs, including our lead product candidate alfimeprase, which recently completed two Phase 2 trials in acute peripheral arterial occlusion, or PAO, and catheter occlusion, rNAPc2, an anticoagulant which is currently in a Phase 2a clinical trial in acute coronary syndromes, or ACS, and ARC183, a thrombin inhibitor that began a Phase 1 trial in August 2004.
We were incorporated as “Hyseq, Inc.” in Illinois in 1992 and reincorporated in Nevada in 1993. On January 31, 2003, we merged with Variagenics, Inc., a publicly traded Delaware corporation based in Massachusetts, and, in connection with the merger, changed our name to “Nuvelo, Inc.” On March 25, 2004, we reincorporated in the State of Delaware. Our principal executive offices are located at 675 Almanor Avenue, Sunnyvale, California 94085 and our telephone number is (408) 215-4000. Our World Wide Web address is http://www.nuvelo.com. We have not incorporated by reference into this prospectus the information contained on our website and you should not consider it to be part of this prospectus.
1
Table of Contents
Cautionary note regarding forward looking information
All statements included or incorporated by reference in this prospectus, other than statements of historical facts, that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future are forward looking statements. Such statements are typically characterized by terminology such as “believe,” “anticipate,” “should,” “intend,” “plan,” “will,” “expect,” “estimate,” “project,” “positioned,” “strategy,” and similar expressions. These statements are based on assumptions and assessments made by our management in light of its experience and its perception of historical trends, current conditions, expected future developments and other factors our management believes to be appropriate. These forward looking statements are subject to a number of risks and uncertainties, including those risks described or incorporated by reference in this prospectus under “Risk Factors” above, as well as other factors that our management has not yet identified. Any such forward looking statements are not guarantees of future performance and actual results, developments and business decisions may differ from those contemplated by such forward looking statements. We disclaim any duty to update any forward looking statements.
Unless otherwise indicated in the prospectus supplement, the net proceeds from the sale of securities offered by this prospectus will be used for general corporate purposes, including capital expenditures and to meet working capital needs. We expect from time to time to evaluate the acquisition of businesses, products and technologies for which a portion of the net proceeds may be used, although we currently are not planning or negotiating any such transactions.
Pending such uses, we may invest the net proceeds in interest bearing securities.
Ratio of earnings to fixed charges
Our earnings were insufficient to cover fixed charges in each of the years in the five-year period ended December 31, 2003 and in the nine-month period ended September 30, 2004. Earnings consist of loss from continuing operations before income taxes, extraordinary items, cumulative effect of accounting changes, equity in net losses of affiliates and fixed charges, adjusted for capitalized interest. Fixed charges consist of interest expense, including the interest component of rent expense, and capitalized and amortized premiums, discounts and capitalized expenses related to indebtedness. The extent to which earnings were insufficient to cover fixed charges is as follows:
| Fiscal year Ended December 31, | Nine Months ended September 30, 2004 | |||||||||||||||||||||||
| 1999 | 2000 | 2001 | 2002 | 2003 | ||||||||||||||||||||
Ratio of earnings to fixed charges(1) | — | — | — | — | — | — | ||||||||||||||||||
Coverage deficiency(2) | $ | (18,547 | ) | $ | (22,253 | ) | $ | (36,472 | ) | $ | (44,978 | ) | $ | (50,187 | ) | $ | (39,507 | ) | ||||||
| (1) | We have not included a ratio of earnings to combined fixed charges and preferred stock dividends because we do not have any preferred stock outstanding. |
| (2) | Defined as adjusted earnings less fixed charges, as these are defined above. |
2
Table of Contents
Description of debt securities
The following description, together with the additional information we include in any applicable prospectus supplements, summarizes the material terms and provisions of the debt securities that we may offer under this prospectus. While the terms we have summarized below will apply generally to any future debt securities we may offer under this prospectus, we will describe the particular terms of any debt securities that we may offer in more detail in the applicable prospectus supplement. The terms of any debt securities we offer under a prospectus supplement may differ from the terms we describe below. However, no prospectus supplement shall fundamentally change the terms that are set forth in this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness. In this description of the debt securities, the words “Nuvelo,” “we,” “us” or “our” refer only to Nuvelo and not to any of our subsidiaries. As of the date of this prospectus, Nuvelo has no outstanding issuer debt.
We will issue the senior debt securities under the senior indenture that we will enter into with the trustee named in the senior indenture. We will issue the subordinated debt securities under the subordinated indenture that we will enter into with the trustee named in the subordinated indenture. We have filed forms of these documents as exhibits to the registration statement which includes this prospectus. We use the term “indentures” in this prospectus to refer to both the senior indenture and the subordinated indenture.
The indentures will be qualified under the Trust Indenture Act of 1939. We use the term “trustee” to refer to either the senior trustee or the subordinated trustee, as applicable.
The following summaries of material provisions of the senior debt securities, the subordinated debt securities and the indentures are subject to, and qualified in their entirety by reference to, all the provisions of the indenture applicable to a particular series of debt securities. Except as we may otherwise indicate, the terms of the senior indenture and the subordinated indenture are identical.
GENERAL
Debt securities may be issued in separate series without limitation as to aggregate principal amount. We may specify a maximum aggregate principal amount for the debt securities of any series.
We are not limited as to the amount of debt securities we may issue under the indentures. The prospectus supplement will set forth:
| Ø | whether the debt securities will be senior or subordinated; |
| Ø | the offering price; |
| Ø | the title; |
| Ø | any limit on the aggregate principal amount; |
| Ø | the person who shall be entitled to receive interest, if other than the record holder on the record date; |
| Ø | the date the principal will be payable; |
| Ø | the interest rate, if any, the date interest will accrue, the interest payment dates and the regular record dates; |
| Ø | the place where payments may be made; |
3
Table of Contents
Description of debt securities
| Ø | any mandatory or optional redemption provisions; |
| Ø | if applicable, the method for determining how the principal, premium, if any, or interest will be calculated by reference to an index or formula; |
| Ø | if other than U.S. currency, the currency or currency units in which principal, premium, if any, or interest will be payable and whether we or the holder may elect payment to be made in a different currency; |
| Ø | the portion of the principal amount that will be payable upon acceleration of stated maturity, if other than the entire principal amount; |
| Ø | if the principal amount payable at stated maturity will not be determinable as of any date prior to stated maturity, the amount which will be deemed to be the principal amount; |
| Ø | any defeasance provisions if different from those described below under “Satisfaction and Discharge; Defeasance;” |
| Ø | any conversion or exchange provisions; |
| Ø | any obligation to redeem or purchase the debt securities pursuant to a sinking fund; |
| Ø | whether the debt securities will be issuable in the form of a global security; |
| Ø | any subordination provisions, if different from those described below under “Subordinated Debt Securities;” |
| Ø | any deletions of, or changes or additions to, the events of default or covenants; and |
| Ø | any other specific terms of such debt securities. |
Unless otherwise specified in the prospectus supplement:
| Ø | the debt securities will be registered debt securities; and |
| Ø | registered debt securities denominated in U.S. dollars will be issued in denominations of $1,000 or an integral multiple of $1,000. |
Debt securities may be sold at a substantial discount below their stated principal amount, bearing no interest or interest at a rate which at the time of issuance is below market rates.
EXCHANGE AND TRANSFER
Debt securities may be transferred or exchanged at the office of the security registrar or at the office of any transfer agent designated by us.
We will not impose a service charge for any transfer or exchange, but we may require holders to pay any tax or other governmental charges associated with any transfer or exchange.
In the event of any potential redemption of debt securities of any series, we will not be required to:
| Ø | issue, register the transfer of, or exchange, any debt security of that series during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption and ending at the close of business on the day of the mailing; or |
| Ø | register the transfer of or exchange any debt security of that series selected for redemption, in whole or in part, except the unredeemed portion being redeemed in part. |
4
Table of Contents
Description of debt securities
We may initially appoint the trustee as the security registrar. Any transfer agent, in addition to the security registrar, initially designated by us will be named in the prospectus supplement. We may designate additional transfer agents or change transfer agents or change the office of the transfer agent. However, we will be required to maintain a transfer agent in each place of payment for the debt securities of each series.
GLOBAL SECURITIES
The debt securities of any series may be represented, in whole or in part, by one or more global securities. Each global security will:
| Ø | be registered in the name of a depositary that we will identify in a prospectus supplement; |
| Ø | be deposited with the depositary or nominee or custodian; and |
| Ø | bear any required legends. |
No global security may be exchanged in whole or in part for debt securities registered in the name of any person other than the depositary or any nominee unless:
| Ø | the depositary has notified us that it is unwilling or unable to continue as depositary or has ceased to be qualified to act as depositary; |
| Ø | an event of default is continuing; or |
| Ø | any other circumstances described in a prospectus supplement. |
As long as the depositary, or its nominee, is the registered owner of a global security, the depositary or nominee will be considered the sole owner and holder of the debt securities represented by the global security for all purposes under the indenture. Except in the above limited circumstances, owners of beneficial interests in a global security:
| Ø | will not be entitled to have the debt securities registered in their names, |
| Ø | will not be entitled to physical delivery of certificated debt securities, and |
| Ø | will not be considered to be holders of those debt securities under the indentures. |
Payments on a global security will be made to the depositary or its nominee as the holder of the global security. Some jurisdictions have laws that require that certain purchasers of securities take physical delivery of such securities in definitive form. These laws may impair the ability to transfer beneficial interests in a global security.
Institutions that have accounts with the depositary or its nominee are referred to as “participants.” Ownership of beneficial interests in a global security will be limited to participants and to persons that may hold beneficial interests through participants. The depositary will credit, on its book-entry registration and transfer system, the respective principal amounts of debt securities represented by the global security to the accounts of its participants.
Ownership of beneficial interests in a global security will be shown on and effected through records maintained by the depositary, with respect to participants’ interests, or any participant, with respect to interests of persons held by participants on their behalf.
Payments, transfers and exchanges relating to beneficial interests in a global security will be subject to policies and procedures of the depositary.
5
Table of Contents
Description of debt securities
The depositary policies and procedures may change from time to time. Neither we nor the trustee will have any responsibility or liability for the depositary’s or any participant’s records with respect to beneficial interests in a global security.
PAYMENT AND PAYING AGENT
The provisions of this paragraph will apply to the debt securities unless otherwise indicated in the prospectus supplement. Payment of interest on a debt security on any interest payment date will be made to the person in whose name the debt security is registered at the close of business on the regular record date. Payment on debt securities of a particular series will be payable at the office of a paying agent or paying agents designated by us. However, at our option, we may pay interest by mailing a check to the record holder. The corporate trust office will be designated as our sole paying agent.
We may also name any other paying agents in the prospectus supplement. We may designate additional paying agents, change paying agents or change the office of any paying agent. However, we will be required to maintain a paying agent in each place of payment for the debt securities of a particular series.
All moneys paid by us to a paying agent for payment on any debt security which remain unclaimed at the end of two years after such payment was due will be repaid to us. Thereafter, the holder may look only to us for such payment.
CONSOLIDATION, MERGER AND SALE OF ASSETS
We may not consolidate with or merge into any other person in a transaction in which we are not the surviving corporation or convey, transfer or lease our properties and assets substantially as an entirety to any person, unless:
| Ø | the successor, if any, is a U.S. corporation, limited liability company, partnership, trust or other entity; |
| Ø | the successor assumes our obligations on the debt securities and under the indenture; |
| Ø | immediately after giving effect to the transaction, no default or event of default shall have occurred and be continuing; and |
| Ø | certain other conditions are met. |
If the debt securities are convertible for our other securities or securities of other entities, the person with whom we consolidate or merge or to whom we sell all of our property must make provisions for the conversion of the debt securities into securities which the holders of the debt securities would have received if they had converted the debt securities before the consolidation, merger or sale.
EVENTS OF DEFAULT
Unless we inform you otherwise in the prospectus supplement, the indenture will define an event of default with respect to any series of debt securities as one or more of the following events:
| (1) | failure to pay principal of or any premium on any debt security of that series when due; |
| (2) | failure to pay any interest on any debt security of that series for 90 days when due; |
| (3) | failure to deposit any sinking fund payment when due; |
| (4) | failure to perform any other covenant in the indenture continued for 90 days after being given the notice required in the indenture; |
6
Table of Contents
Description of debt securities
| (5) | our bankruptcy, insolvency or reorganization; and |
| (6) | any other event of default specified in the prospectus supplement. |
An event of default of one series of debt securities is not necessarily an event of default for any other series of debt securities.
If an event of default, other than an event of default described in clause (5) above, shall occur and be continuing, either the trustee or the holders of at least 25% in aggregate principal amount of the outstanding securities of that series may declare the principal amount of the debt securities of that series to be due and payable immediately.
If an event of default described in clause (5) above shall occur, the principal amount of all the debt securities of that series will automatically become immediately due and payable. Any payment by us on the subordinated debt securities following any such acceleration will be subject to the subordination provisions described below under “Subordinated Debt Securities.”
After acceleration the holders of a majority in aggregate principal amount of the outstanding securities of that series may, under certain circumstances, rescind and annul such acceleration if all events of default, other than the non-payment of accelerated principal or other specified amount, have been cured or waived.
Other than the duty to act with the required care during an event of default, the trustee will not be obligated to exercise any of its rights or powers at the request of the holders unless the holders shall have offered to the trustee reasonable indemnity. Generally, the holders of a majority in aggregate principal amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the trustee or exercising any trust or power conferred on the trustee.
A holder will not have any right to institute any proceeding under the indentures, or for the appointment of a receiver or a trustee, or for any other remedy under the indentures, unless:
| (1) | the holder has previously given to the trustee written notice of a continuing event of default with respect to the debt securities of that series; |
| (2) | the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made a written request and have offered reasonable indemnity to the trustee to institute the proceeding; and |
| (3) | the trustee has failed to institute the proceeding and has not received direction inconsistent with the original request from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series within 90 days after the original request. |
Holders may, however, sue to enforce the payment of principal, premium or interest on any debt security on or after the due date or to enforce the right, if any, to convert any debt security without following the procedures listed in (1) through (3) above.
We will furnish the trustee an annual statement by our officers as to whether or not we are in default in the performance of the indenture and, if so, specifying all known defaults.
7
Table of Contents
Description of debt securities
MODIFICATION AND WAIVER
Nuvelo and the trustee may make modifications and amendments to the indentures with the consent of the holders of a majority in aggregate principal amount of the outstanding securities of each series affected by the modification or amendment.
However, neither we nor the trustee may make any modification or amendment without the consent of the holder of each outstanding security of that series affected by the modification or amendment if such modification or amendment would:
| Ø | change the stated maturity of any debt security; |
| Ø | reduce the principal, premium, if any, or interest on any debt security; |
| Ø | reduce the principal of an original issue discount security or any other debt security payable on acceleration of maturity; |
| Ø | reduce the rate of interest on any debt security; |
| Ø | change the currency in which any debt security is payable; |
| Ø | impair the right to enforce any payment after the stated maturity or redemption date; |
| Ø | waive any default or event of default in payment of the principal of, premium or interest on any debt security; |
| Ø | waive a redemption payment or modify any of the redemption provisions of any debt security; |
| Ø | adversely affect the right to convert any debt security in any material respect; or |
| Ø | change the provisions in the indenture that relate to modifying or amending the indenture. |
SATISFACTION AND DISCHARGE; DEFEASANCE
We may be discharged from our obligations on the debt securities of any series that have matured or will mature or be redeemed within one year if we deposit with the trustee enough cash to pay all the principal, interest and any premium due to the stated maturity date or redemption date of the debt securities.
Each indenture contains a provision that permits us to elect:
| Ø | to be discharged from all of our obligations, subject to limited exceptions, with respect to any series of debt securities then outstanding; and/or |
| Ø | to be released from our obligations under the following covenants and from the consequences of an event of default resulting from a breach of these covenants: |
| (1) | the subordination provisions under the subordinated indenture; and |
| (2) | covenants as to payment of taxes and maintenance of corporate existence. |
To make either of the above elections, we must deposit in trust with the trustee enough money to pay in full the principal, interest and premium on the debt securities. This amount may be made in cash and/or U.S. government obligations. As a condition to either of the above elections, we must deliver to the trustee an opinion of counsel that the holders of the debt securities will not recognize income, gain or loss for Federal income tax purposes as a result of the action.
8
Table of Contents
Description of debt securities
If any of the above events occurs, the holders of the debt securities of the series will not be entitled to the benefits of the indenture, except for the rights of holders to receive payments on debt securities or the registration of transfer and exchange of debt securities and replacement of lost, stolen or mutilated debt securities.
NOTICES
Notices to holders will be given by mail to the addresses of the holders in the security register.
GOVERNING LAW
The indentures and the debt securities will be governed by, and construed under, the law of the State of New York.
REGARDING THE TRUSTEE
The indenture limits the right of the trustee, should it become a creditor of us, to obtain payment of claims or secure its claims.
The trustee is permitted to engage in certain other transactions. However, if the trustee acquires any conflicting interest, and there is a default under the debt securities of any series for which it is trustee, the trustee must eliminate the conflict or resign.
SUBORDINATED DEBT SECURITIES
Payment on the subordinated debt securities will, to the extent provided in the indenture, be subordinated in right of payment to the prior payment in full of all of our senior indebtedness. The subordinated debt securities also are effectively subordinated to all debt and other liabilities, including trade payables and lease obligations, if any, of our subsidiaries.
Upon any distribution of our assets or upon any dissolution, winding up, liquidation or reorganization, the payment of the principal of and interest on the subordinated debt securities will be subordinated in right of payment to the prior payment in full in cash or other payment satisfactory to the holders of senior indebtedness of all senior indebtedness. In the event of any acceleration of the subordinated debt securities because of an event of default, the holders of any senior indebtedness would be entitled to payment in full in cash or other payment satisfactory to such holders of all senior indebtedness before the holders of the subordinated debt securities are entitled to receive any payment or distribution. The indenture requires us or the trustee to promptly notify holders of designated senior indebtedness if payment of the subordinated debt securities is accelerated because of an event of default.
We may not make any payment on the subordinated debt securities, including upon redemption at the option of the holder of any subordinated debt securities or at our option, if:
| Ø | a “payment default” takes place, whereby a default in the payment of the principal, premium, if any, interest, rent or other obligations in respect of designated senior indebtedness occurs and is continuing beyond any applicable period of grace; or |
| Ø | a “non-payment default” takes place, whereby a default other than a payment default on any designated senior indebtedness occurs and is continuing that permits holders of designated senior indebtedness to accelerate its maturity, and the trustee receives notice of such default, which is called a “payment blockage notice from us or any other person permitted to give such notice under the indenture. |
9
Table of Contents
Description of debt securities
We may resume payments and distributions on the subordinated debt securities:
| Ø | in the case of a payment default, upon the date on which such default is cured or waived or ceases to exist; and |
| Ø | in the case of a non-payment default, the earlier of the date on which such nonpayment default is cured or waived or ceases to exist and 179 days after the date on which the payment blockage notice is received by the trustee, if the maturity of the designated senior indebtedness has not been accelerated. |
No new period of payment blockage may be commenced pursuant to a payment blockage notice unless 365 days have elapsed since the initial effectiveness of the immediately prior payment blockage notice and all scheduled payments of principal, premium and interest, including any liquidated damages, on the notes that have come due have been paid in full in cash. No non-payment default that existed or was continuing on the date of delivery of any payment blockage notice shall be the basis for any later payment blockage notice unless the non-payment default is based upon facts or events arising after the date of delivery of such payment blockage notice.
If the trustee or any holder of the notes receives any payment or distribution of our assets in contravention of the subordination provisions on the subordinated debt securities before all senior indebtedness is paid in full in cash, property or securities, including by way of set-off or other payment satisfactory to holders of senior indebtedness, then such payment or distribution will be held in trust for the benefit of holders of senior indebtedness or their representatives to the extent necessary to make payment in full in cash or payment satisfactory to the holders of senior indebtedness of all unpaid senior indebtedness.
In the event of our bankruptcy, dissolution or reorganization, holders of senior indebtedness may receive more, ratably, and holders of the subordinated debt securities may receive less, ratably, than our other creditors (including our trade creditors). This subordination will not prevent the occurrence of any event of default under the indenture.
As of October 31, 2004, $18.4 million in senior indebtedness was outstanding. We are not prohibited from incurring debt, including senior indebtedness, under the indenture. We may from time to time incur additional debt, including senior indebtedness.
We are obligated to pay reasonable compensation to the trustee and to indemnify the trustee against certain losses, liabilities or expenses incurred by the trustee in connection with its duties relating to the subordinated debt securities. The trustee’s claims for these payments will generally be senior to those of noteholders in respect of all funds collected or held by the trustee.
CERTAIN DEFINITIONS
“indebtedness” means:
| (1) | all indebtedness, obligations and other liabilities for borrowed money, including overdrafts, foreign exchange contracts, currency exchange agreements, interest rate protection agreements, and any loans or advances from banks, or evidenced by bonds, debentures, notes or similar instruments, other than any account payable or other accrued current liability or obligation incurred in the ordinary course of business in connection with the obtaining of materials or services; |
| (2) | all reimbursement obligations and other liabilities with respect to letters of credit, bank guarantees or bankers’ acceptances; |
10
Table of Contents
Description of debt securities
| (3) | all obligations and liabilities in respect of leases required in conformity with generally accepted accounting principles to be accounted for as capitalized lease obligations on our balance sheet; |
| (4) | all obligations and liabilities, contingent or otherwise, as lessee under leases for facility equipment (and related assets leased together with such equipment) and under any lease or related document (including a purchase agreement, conditional sale or other title retention or synthetic lease agreement) in connection with the lease of real property or improvement thereon (or any personal property included as part of any such lease) which provides that such Person is contractually obligated to purchase or cause a third party to purchase the leased property or pay an agreed upon residual value of the leased property, including the obligations under such lease or related document to purchase or cause a third party to purchase such leased property (whether or not such lease transaction is characterized as an operating lease or a capitalized lease in accordance with GAAP) or pay an agreed upon residual value of the leased property to the lessor; |
| (5) | all obligations with respect to an interest rate or other swap, cap or collar agreement or other similar instrument or agreement or foreign currency hedge, exchange, purchase agreement or other similar instrument or agreement; |
| (6) | all direct or indirect guaranties or similar agreements in respect of, and our obligations or liabilities to purchase, acquire or otherwise assure a creditor against loss in respect of, indebtedness, obligations or liabilities of others of the type described in (1) through (5) above; |
| (7) | any indebtedness or other obligations described in (1) through (6) above secured by any mortgage, pledge, lien or other encumbrance existing on property which is owned or held by us; and |
| (8) | any and all refinancings, replacements, deferrals, renewals, extensions and refundings of, or amendments, modifications or supplements to, any indebtedness, obligation or liability of the kind described in clauses (1) through (7) above. |
“senior indebtedness” means the principal, premium, if any, interest, including any interest accruing after bankruptcy, and rent or termination payment on or other amounts due on our current or future indebtedness, whether created, incurred, assumed, guaranteed or in effect guaranteed by us, including any deferrals, renewals, extensions, refundings, amendments, modifications or supplements to the above. However, senior indebtedness does not include:
| Ø | indebtedness that expressly provides that it shall not be senior in right of payment to the subordinated debt securities or expressly provides that it is on the same basis or junior to the subordinated debt securities; |
| Ø | our indebtedness to any of our majority-owned subsidiaries; and |
| Ø | the subordinated debt securities. |
11
Table of Contents
Description of preferred stock
We currently have authorized 5,000,000 shares of preferred stock, of which 100,000 shares have been designated Series A Junior Participating Preferred Stock. As of November 30, 2004, we do not have any shares of preferred stock outstanding.
GENERAL
Prior to issuance of shares of each series of our undesignated preferred stock, our Board of Directors is required by the Delaware General Corporate Law, or DGCL, and our Amended and Restated Certificate of Incorporation, or certificate of incorporation, to adopt resolutions and file a Certificate of Designation with the Secretary of State of the State of Delaware, fixing for each such series the designations, powers, preferences, rights, qualifications, limitations and restrictions of the shares of such series. Our Board of Directors could authorize the issuance of shares of preferred stock with terms and conditions which could have the effect of discouraging a takeover or other transaction which holders of some, or a majority, of such shares might believe to be in their best interests or in which holders of some, or a majority, of such shares might receive a premium for their shares over the then-market price of such shares.
Subject to limitations prescribed by the DGCL, our certificate of incorporation and our Amended and Restated Bylaws, or bylaws, our Board of Directors is authorized to fix the number of shares constituting each series of preferred stock and the designations, powers, preferences, rights, qualifications, limitations and restrictions of the shares of such series, including such provisions as may be desired concerning voting, redemption, dividends, dissolution or the distribution of assets, conversion or exchange, and such other subjects or matters as may be fixed by resolution of the Board of Directors. Each series of preferred stock that we offer under this prospectus will, when issued, be fully paid and nonassessable and will not have, or be subject to, any preemptive or similar rights.
The applicable prospectus supplement(s) will describe the following terms of the series of preferred stock in respect of which this prospectus is being delivered:
| Ø | the title and stated value of the preferred stock; |
| Ø | the number of shares of the preferred stock offered, the liquidation preference per share and the purchase price of the preferred stock; |
| Ø | the dividend rate(s), period(s) and/or payment date(s) or the method(s) of calculation for dividends; |
| Ø | whether dividends shall be cumulative or non-cumulative and, if cumulative, the date from which dividends on the preferred stock shall accumulate; |
| Ø | the procedures for any auction and remarketing, if any, for the preferred stock; |
| Ø | the provisions for a sinking fund, if any, for the preferred stock; |
| Ø | the provisions for redemption, if applicable, of the preferred stock; |
| Ø | any listing of the preferred stock on any securities exchange or market; |
| Ø | the terms and conditions, if applicable, upon which the preferred stock will be convertible into common stock or another series of our preferred stock, including the conversion price (or its manner of calculation) and conversion period; |
| Ø | the terms and conditions, if applicable, upon which preferred stock will be exchangeable into our debt securities, including the exchange price, or its manner of calculation, and exchange period; |
12
Table of Contents
Description of preferred stock
| Ø | voting rights, if any, of the preferred stock; |
| Ø | a discussion of any material and/or special United States federal income tax considerations applicable to the preferred stock; |
| Ø | whether interests in the preferred stock will be represented by depositary shares; |
| Ø | the relative ranking and preferences of the preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of our affairs; |
| Ø | any limitations on issuance of any series of preferred stock ranking senior to or on a parity with the preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of our affairs; and |
| Ø | any other specific terms, preferences, rights, limitations or restrictions on the preferred stock. |
Unless otherwise specified in the prospectus supplement, the preferred stock will, with respect to dividend rights and rights upon liquidation, dissolution or winding up of Nuvelo rank:
| Ø | senior to all classes or series of our common stock, and to all equity securities issued by us the terms of which specifically provide that such equity securities rank junior to the preferred stock with respect to dividend rights or rights upon the liquidation, dissolution or winding up of us; |
| Ø | on a parity with all equity securities issued by us that do not rank senior or junior to the preferred stock with respect to dividend rights or rights upon the liquidation, dissolution or winding up of us; and |
| Ø | junior to all equity securities issued by us the terms of which do not specifically provide that such equity securities rank on a parity with or junior to the preferred stock with respect to dividend rights or rights upon the liquidation, dissolution or winding up of us (including any entity with which we may be merged or consolidated or to which all or substantially all of our assets may be transferred or which transfers all or substantially all of our assets). |
As used for these purposes, the term “equity securities” does not include convertible debt securities.
TRANSFER AGENT AND REGISTRAR
The transfer agent and registrar for any series of preferred stock will be set forth in the applicable prospectus supplement.
13
Table of Contents
The following is only a summary of the material terms of our common stock and our stockholder rights agreement. Because it is only a summary, it does not contain all the information that may be important to you. Accordingly, you should read carefully the more detailed provisions of our amended and restated certificate of incorporation, bylaws and rights agreement, each of which has been filed with the SEC, as well as applicable Delaware law.
We currently have authorized 100,000,000 shares of common stock, par value $0.001, and, as of October 29, 2004, we had 32,214,676 shares of common stock outstanding. As of October 29, 2004, we had an aggregate of 3,508,264 shares of common stock reserved for issuance upon exercise of outstanding stock options granted under our 2004 Equity Incentive Plan, 2002 Equity Incentive Plan, 1997 Stock Option Plan, Stock Option Plan, Non-Employee Director Stock Option Plan, Scientific Advisory Board/ Consultants Stock Option Plan, and the Variagenics, Inc. Amended 1997 Employee Director and Consultant Stock Option Plan, and an aggregate of 4,129,424 shares of common stock reserved for issuance pursuant to future grants under these plans. As of October 29, 2004, we also had 64,997 shares of common stock reserved for issuance under our Employee Stock Purchase Plan. As of October 29, 2004, we had an aggregate of 895,075 shares of common stock reserved for issuance upon the exercise of stock options granted outside of any of our stock option plans. As of October 29, 2004, we had warrants to purchase an aggregate of 1,516,792 shares of our common stock outstanding, with exercise prices ranging from $4.05 to $25.53 per share, and a weighted average exercise price of $20.88 per share.
COMMON STOCK
Holders of our common stock are entitled to one vote per share for the election of directors and all other matters submitted for stockholder vote, except matters submitted to the vote of another class or series of shares. Holders of common stock are not entitled to cumulative voting rights. The approval of 66 2/3% of the voting rights of the common stock is required to make certain amendments to our certificate of incorporation, amend our by-laws, and to remove a director from our board of directors. The affirmative vote of the holders of a majority of the outstanding shares of our common stock is required to approve the sale by us of U.S. Patent 5,202,231, or the exclusive license or assignment by us of U.S. Patent 5,202,231 to a single person or entity having the same effect as a sale of all rights, title and interest in it.
The holders of common stock are entitled to dividends in such amounts and at such times, if any, as may be declared by our board of directors out of legally available funds. We have not paid any dividends on our common stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. Upon liquidation, dissolution or winding up of us, the holders of our common stock are entitled to share ratably in all net assets available for distribution to stockholders after payments to creditors and holders of senior securities. The common stock is not redeemable and has no preemptive, conversion or sinking fund rights. The rights of the holders of our common stock are subject to the rights of the holders of any preferred stock which may, in the future, be issued. All outstanding shares of our common stock are, and any shares of common stock issued pursuant to this prospectus when issued will be, duly authorized, validly issued, fully paid and non-assessable.
As of October 29, 2004, we had 32,214,676 shares of common stock issued and outstanding.
14
Table of Contents
Description of common stock
TRANSFER AGENT
The transfer agent and registrar for our common stock is U.S. Stock Transfer Corporation. Its offices are located at 1745 Gardena Ave., Glendale, California 91204, and its telephone number is (818) 502-1404.
STOCKHOLDER RIGHTS AGREEMENT
On June 5, 1998, our board of directors adopted a stockholder rights agreement, or rights agreement, which was subsequently amended on November 9, 2002 and March 19, 2004. Pursuant to the rights agreement, one whole right attaches to each outstanding share of our common stock. Each right entitles the registered holder to purchase from us one one-thousandth (1/1000) of a share of our Series A Junior Participating Preferred Stock at an initial purchase price of $175.00, subject to customary antidilution adjustments. The rights do not become exercisable until the earlier to occur of:
| Ø | 10 business days following a public announcement that a person or group has acquired beneficial ownership of 15% (or 27.5% in the of an approved stockholder) or more of our outstanding common stock (any such person or group is referred to as an acquiring case person); or |
| Ø | 10 business days (or a later date as determined by our board of directors) following the commencement or announcement of an intention to make a tender offer or exchange offer, that would result in a person or entity becoming an acquiring person. |
The rights will expire on June 5, 2008, unless they are redeemed or exchanged by us before that time. Until a right is exercised, the rights do not convey the right to vote, receive dividends or otherwise provide the holder with any rights as a stockholder.
When a person or group becomes an acquiring person (or at such later time as determined by independent directors of our board of directors) then each registered holder of a right, except for such person or group, will be entitled to purchase, for the purchase price, shares of our common stock having a then current market value equal to two times the purchase price of the right. Subject to specified exemptions, in the event that we are involved in a merger, or we sell more than 50% of our assets or earning power to an acquiring company, each right will entitle the holder, other than an acquiring person, to purchase, upon exercise, a number of shares of common stock of the acquiring company having a then current market value of two times the purchase price of the right.
We may, at our option, at any time prior to the close of business on the tenth day following the day a person or group becomes an acquiring person, redeem all of the then-outstanding rights at a redemption price of $0.001 per right, subject to certain adjustments. At any time after a person or group becomes an acquiring person and prior to the acquisition by that person or group of 50% or more of the outstanding shares of our common stock, our board of directors may cause us to acquire the rights (other than rights owned by the acquiring person), in whole or in part, in exchange for one share of common stock per right.
While the rights are redeemable, we may supplement or amend any provision of the rights agreement in any respect without the approval of any holders of rights or share of common stock. When the rights are no longer redeemable, we may supplement or amend the rights agreement without the approval of any holders of rights certificates as long as the supplement or amendment does not adversely affect the interests of the holders of rights (other than an acquiring person). Any supplement or amendment to the rights agreement shall require the affirmative vote of a majority of our independent directors. Any extension of the final expiration date of the rights shall require the affirmative vote of three-quarters of the independent directors.
15
Table of Contents
Additional information concerning our capital stock
ANTI-TAKEOVER EFFECTS OF OUR CERTIFICATE OF INCORPORATION AND BYLAWS
Our certificate of incorporation and by-laws include a number of provisions that may have the effect of encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions:
| Ø | establish a classified board of directors so that not all members of our board may be elected at one time; |
| Ø | establish that the holders of 66 2/3% of the voting rights of all classes of stock entitled to vote are required to remove our directors or to amend the by-laws and certain provisions of our certificate of incorporation; |
| Ø | authorize the issuance of up to 5,000,000 shares of preferred stock that could be issued by our board of directors to increase the number of outstanding shares and hinder a takeover attempt; |
| Ø | limit who may call a special meeting of stockholders; |
| Ø | prohibit stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; and |
| Ø | establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon at a stockholder meeting. |
These provisions could discourage, delay or prevent certain types of transactions involving an actual or potential change in control of us, including transactions in which stockholders might otherwise receive a premium for their shares over current market prices.
BUSINESS COMBINATION STATUTE UNDER DELAWARE LAW
We are also subject to provisions of Delaware law that could discourage, delay or prevent an actual or potential change in control of us. These anti-takeover laws prevent a Delaware corporation from engaging in a merger or sale of more than 10 percent of its assets with any stockholder, including all affiliates and associates of the stockholder, who owns 15 percent or more of the corporation’s outstanding voting stock, for three years following the date that the stockholder acquired 15 percent or more of the corporation’s stock unless:
| Ø | the board of directors approved the transaction where the stockholder acquired 15 percent or more of the corporation’s stock; |
| Ø | after the transaction in which the stockholder acquired 15 percent or more of the corporation’s stock, the stockholder owned at least 85 percent of the corporation’s outstanding voting stock, excluding shares owned by directors, officers and employee stock plans in which employee participants do not have the right to determine confidentially whether shares held under the plan will be tendered in a tender or exchange offer; or |
| Ø | on or after this date, the merger or sale is approved by the board of directors and the holders of at least two-thirds of the outstanding voting stock that is not owned by the stockholder. |
16
Table of Contents
Additional information concerning our capital stock
LIMITATION OF LIABILITY AND INDEMNIFICATION
To the fullest extent permitted by the Delaware law, our certificate of incorporation provides that directors shall not be personally liable to us or any of our stockholders for monetary damages for breach of fiduciary duty as a director. However, this provision does not eliminate the duty of care, and in appropriate circumstances, equitable remedies such as injunctive or other forms of nonmonetary relief that will remain available under Delaware law. In addition, each director will continue to be subject to liability for (i) breach of the directors duty of loyalty to us or our stockholders, (ii) acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) violating Section 174 of the Delaware General Corporation Law, or (iv) any transaction from which the director derived an improper personal benefit. The provision also does not affect a director’s responsibilities under any other law, such as the federal securities laws or state or federal environmental laws.
In accordance with Delaware law, our by-laws provide that we shall indemnify any person who was or is a party or is threatened to be made a party to, or otherwise becomes involved in, any proceeding (other than an action by or in the right of Nuvelo) by reason of the fact that he is an officer, director or agent of Nuvelo against losses actually and reasonably incurred by that person if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to our best interests. Losses are the total amount that the officer, director or agent becomes legally obligated to pay, including judgments, fines, amounts paid in settlement, attorneys’ fees, expenses of establishing a right to indemnification and other expenses. If the proceeding is a criminal proceeding, the person to be indemnified must have had no reasonable cause to believe his or her conduct was unlawful.
Our bylaws provide for similar indemnification for expenses resulting from an action by or in the right of Nuvelo, except that no indemnification will be made if the person is adjudged by a court of competent jurisdiction after exhaustion of all appeals to be liable to us or for amounts paid in settlement to us unless the court determines that the person is fairly and reasonably entitled to indemnity for expenses. Expenses of officers, directors and agents include attorneys’ fees, any expenses of establishing a right to indemnification and amounts paid in settlement. Our bylaws also provide for advancement of expenses.
We also maintain liability insurance for our officers and directors and have entered into indemnification agreements with them.
17
Table of Contents
We may sell the securities separately or together:
| Ø | through one or more underwriters or dealers in a public offering and sale by them; |
| Ø | directly to investors; or |
| Ø | through agents. |
We may sell the securities from time to time in one or more transactions at a fixed price or prices, which may be changed from time to time:
| Ø | at market prices prevailing at the times of sale; |
| Ø | at prices related to such prevailing market prices; or |
| Ø | at negotiated prices. |
We will describe the method of distribution of the securities in the prospectus supplement.
Underwriters, dealers or agents may receive compensation in the form of discounts, concessions or commissions from us or our purchasers (as their agents in connection with the sale of securities). These underwriters, dealers or agents may be considered to be underwriters under the Securities Act. As a result, discounts, commissions, or profits on resale received by the underwriters, dealers or agents may be treated as underwriting discounts and commissions. The prospectus supplement will identify any such underwriter, dealer or agent, and describe any compensation received by them from us. Only underwriters named in the prospectus supplement are underwriters of the securities offered by the prospectus supplement. Any initial public offering price and any discounts or concessions allowed or reallowed or paid to dealers may be changed from time to time.
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
Underwriters, dealers and agents may be entitled to indemnification by us against certain civil liabilities, including liabilities under the Securities Act, or to contribution with respect to payments made by the underwriters, dealers or agents, under agreements between us and the underwriters, dealers and agents.
We may grant underwriters who participate in the distribution of securities an option to purchase additional securities to cover over-allotments, if any, in connection with the distribution. Any underwriter may engage in overallotment, stabilizing transactions, short covering transactions and penalty bids in accordance with Regulation M under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Overallotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing
18
Table of Contents
Plan of distribution
bids do not exceed a specified maximum. Short covering transactions involve purchases of the common stock in the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the common stock originally sold by the dealer is purchased in a covering transaction to cover short positions. Those activities may cause the price of the common stock to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any underwriters who are qualified market makers on The Nasdaq National Market may engage in passive market making transactions in the common stock on The Nasdaq National Market in accordance with Rule 103 of Regulation M, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the common stock. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded.
In compliance with guidelines of the National Association of Securities Dealers, or NASD, the maximum consideration or discount to be received by any NASD member or independent broker dealer may not exceed 8% of the aggregate amount of the securities offered pursuant to this prospectus and any applicable prospectus supplement.
All securities we offer, other than common stock, will be new issues of securities with no established trading market. Underwriters involved in the public offering and sale of these securities may make a market in these securities. However, they are not obligated to make a market and may discontinue market making activity at any time. No assurance can be given as to the liquidity of the trading market for any of these securities.
Underwriters or agents and their associates may be customers of, engage in transactions with or perform services for us in the ordinary course of business.
19
Table of Contents
The validity of the securities being offered by this prospectus will be passed upon for us by Cooley Godward LLP of Palo Alto, California.
The consolidated financial statements of Nuvelo, Inc. as of December 31, 2003 and 2002, and for each of the years in the three-year period ended December 31, 2003, have been incorporated by reference herein and in the registration statement in reliance upon the report of KPMG LLP, independent auditors, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
The consolidated financial statements of Variagenics, Inc. as of December 31, 2002 and 2001, and for each of the three years in the period ended December 31, 2002 incorporated in this prospectus by reference to the Current Report on Form 8-K/A, dated July 3, 2003, of Nuvelo, Inc., have been so incorporated in reliance on the reports of PricewaterhouseCoopers LLP, independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
Where you can find more information
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange Commission, or the SEC. You may read and copy these reports, proxy statements and other information at the SEC’s public reference rooms at 450 Fifth Street, N.W., Washington, D.C., 20549. You can request copies of these documents by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the public reference rooms. Our SEC filings are also available at the SEC’s web site at www.sec.gov and our website at www.nuvelo.com. We have not incorporated by reference into this prospectus the information contained on our website and you should not consider it to be part of this prospectus. In addition, you can read and copy our SEC filings at the office of the National Association of Securities Dealers, Inc. at 1735 K Street, Washington, D.C. 20006.
The SEC allows us to “incorporate by reference” information that we file with them, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is an important part of this prospectus, and information that we file later with the SEC will automatically update and supersede this information. Further, all filings we make under the Securities Exchange Act after the date of the initial registration statement and prior to effectiveness of the registration statement shall be deemed to be incorporated by reference into this prospectus. We incorporate by reference the documents listed below and any future filings we will make with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934:
| Ø | our current report on Form 8-K, filed with the SEC on February 4, 2003, as amended on Form 8-K/A filed with the SEC on February 14, 2003, and as further amended on Form 8-K/A filed with the SEC on July 3, 2003; |
| Ø | our annual report on Form 10-K for the fiscal year ended December 31, 2003, filed with the SEC on March 12, 2004; |
20
Table of Contents
Where you can find more information
| Ø | our proxy statement for our stockholders’ meeting on May 6, 2004, filed on April 12, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on March 26, 2004; |
| Ø | our quarterly report on Form 10-Q for the quarter ended March 31, 2004, filed with the SEC on May 10, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on May 10, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on July 29, 2004; |
| Ø | our quarterly report on Form 10-Q for the quarter ended June 30, 2004, filed with the SEC on August 9, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on September 8, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on September 15, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on September 16, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on September 20, 2004; |
| Ø | our current report on Form 8-K, filed with the SEC on November 4, 2004; and |
| Ø | our quarterly report on Form 10-Q for the quarter ended September 30, 2004, filed with the SEC on November 9, 2004. |
We will provide to you at no cost a copy of any and all of the information incorporated by reference into the registration statement of which this prospectus is a part. You may make a request for copies of this information in writing or by telephone. Requests should be directed to:
Nuvelo, Inc.
Attention: Lee Bendekgey
675 Almanor Avenue
Sunnyvale, CA 94085
(408) 215-4000
21
Table of Contents
