
GENETIC-AF Phase II Trial of Pharmacogenetic Guided Beta-Blocker Therapy with Bucindolol vs. Metoprolol for the Prevention of Atrial Fibrillation/Flutter in Heart Failure William T. Abraham, MD Professor of Medicine, Physiology and Cell Biology and Director, Division of Cardiovascular Medicine at the Ohio State University Exhibit 99.2
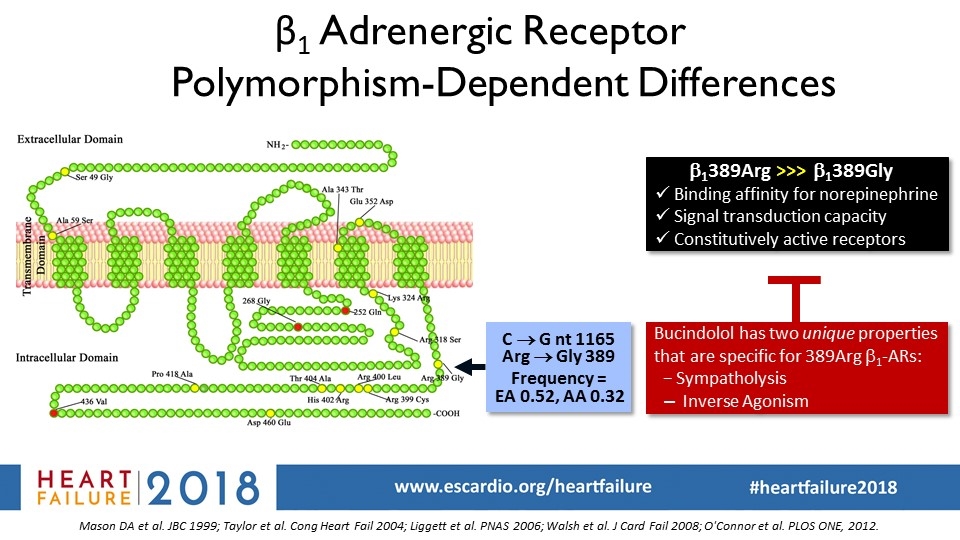
β1 Adrenergic Receptor Polymorphism-Dependent Differences Mason DA et al. JBC 1999; Taylor et al. Cong Heart Fail 2004; Liggett et al. PNAS 2006; Walsh et al. J Card Fail 2008; O'Connor et al. PLOS ONE, 2012. b1389Arg >>> b1389Gly Binding affinity for norepinephrine Signal transduction capacity Constitutively active receptors C ® G nt 1165 Arg ® Gly 389 Frequency = EA 0.52, AA 0.32 Bucindolol has two unique properties that are specific for 389Arg b1-ARs: − Sympatholysis Inverse Agonism
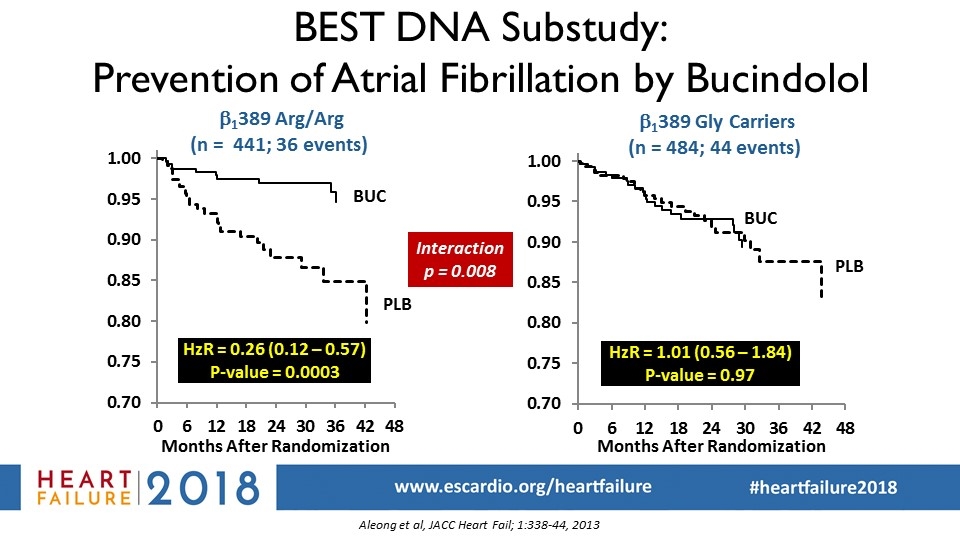
Interaction p = 0.008 Kaplan-Meier curves for prevention of atrial fibrillation in BEST: DNA substudy (Aleong et al, JACC Heart Fail; 1:338-44, 2013) BEST DNA Substudy: Prevention of Atrial Fibrillation by Bucindolol b1389 Arg/Arg (n = 441; 36 events) HzR = 0.26 (0.12 – 0.57) P-value = 0.0003 BUC PLB HzR = 1.01 (0.56 – 1.84) P-value = 0.97 b1389 Gly Carriers (n = 484; 44 events) BUC PLB Aleong et al, JACC Heart Fail; 1:338-44, 2013
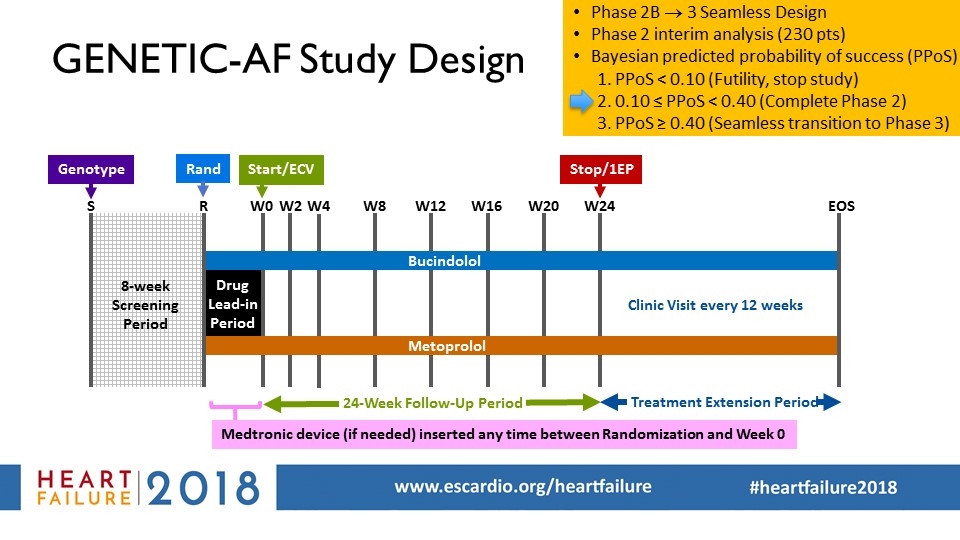
W8 W4 S W12 W0 W20 24-Week Follow-Up Period Start/ECV R Drug Lead-in Period Treatment Extension Period Clinic Visit every 12 weeks EOS W16 W24 Stop/1EP Bucindolol Metoprolol W2 8-week Screening Period Medtronic device (if needed) inserted any time between Randomization and Week 0 Genotype GENETIC-AF Study Design Phase 2B ® 3 Seamless Design Phase 2 interim analysis (230 pts) Bayesian predicted probability of success (PPoS) PPoS < 0.10 (Futility, stop study) 0.10 ≤ PPoS < 0.40 (Complete Phase 2) PPoS ≥ 0.40 (Seamless transition to Phase 3) Rand
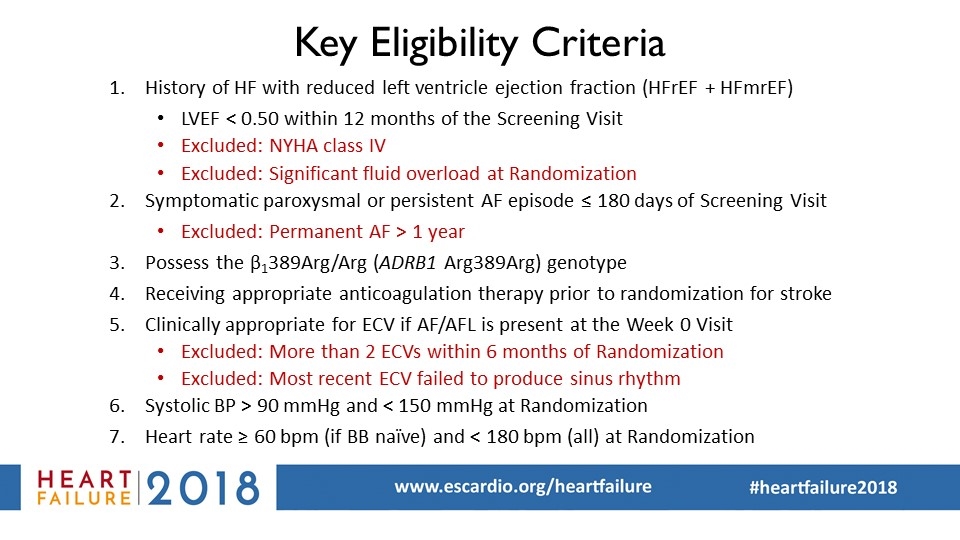
Key Eligibility Criteria History of HF with reduced left ventricle ejection fraction (HFrEF + HFmrEF) LVEF < 0.50 within 12 months of the Screening Visit Excluded: NYHA class IV Excluded: Significant fluid overload at Randomization Symptomatic paroxysmal or persistent AF episode ≤ 180 days of Screening Visit Excluded: Permanent AF > 1 year Possess the β1389Arg/Arg (ADRB1 Arg389Arg) genotype Receiving appropriate anticoagulation therapy prior to randomization for stroke Clinically appropriate for ECV if AF/AFL is present at the Week 0 Visit Excluded: More than 2 ECVs within 6 months of Randomization Excluded: Most recent ECV failed to produce sinus rhythm Systolic BP > 90 mmHg and < 150 mmHg at Randomization Heart rate ≥ 60 bpm (if BB naïve) and < 180 bpm (all) at Randomization
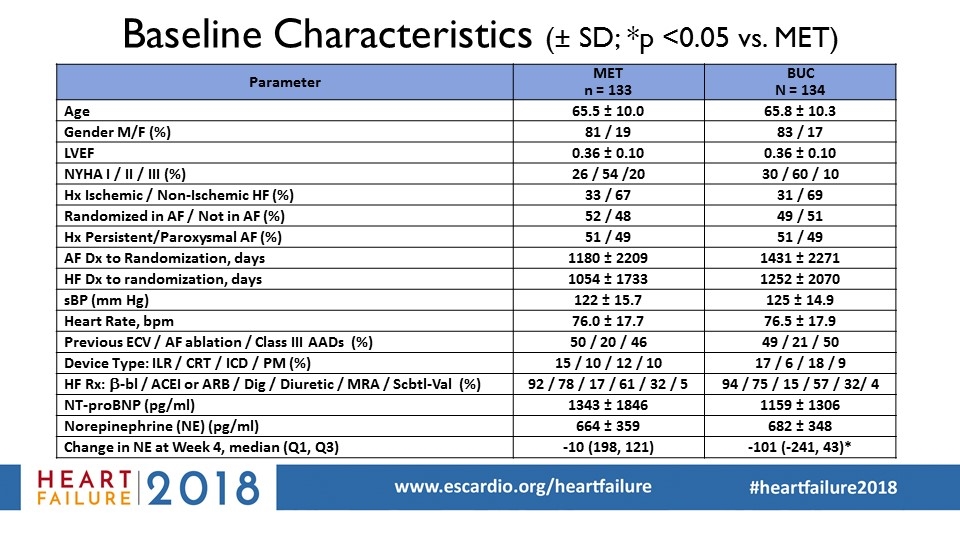
Baseline Characteristics (± SD; *p <0.05 vs. MET) Parameter MET n = 133 BUC N = 134 Age 65.5 ± 10.0 65.8 ± 10.3 Gender M/F (%) 81 / 19 83 / 17 LVEF 0.36 ± 0.10 0.36 ± 0.10 NYHA I / II / III (%) 26 / 54 /20 30 / 60 / 10 Hx Ischemic / Non-Ischemic HF (%) 33 / 67 31 / 69 Randomized in AF / Not in AF (%) 52 / 48 49 / 51 Hx Persistent/Paroxysmal AF (%) 51 / 49 51 / 49 AF Dx to Randomization, days 1180 ± 2209 1431 ± 2271 HF Dx to randomization, days 1054 ± 1733 1252 ± 2070 sBP (mm Hg) 122 ± 15.7 125 ± 14.9 Heart Rate, bpm 76.0 ± 17.7 76.5 ± 17.9 Previous ECV / AF ablation / Class III AADs (%) 50 / 20 / 46 49 / 21 / 50 Device Type: ILR / CRT / ICD / PM (%) 15 / 10 / 12 / 10 17 / 6 / 18 / 9 HF Rx: b-bl / ACEI or ARB / Dig / Diuretic / MRA / Scbtl-Val (%) 92 / 78 / 17 / 61 / 32 / 5 94 / 75 / 15 / 57 / 32/ 4 NT-proBNP (pg/ml) 1343 ± 1846 1159 ± 1306 Norepinephrine (NE) (pg/ml) 664 ± 359 682 ± 348 Change in NE at Week 4, median (Q1, Q3) -10 (198, 121) -101 (-241, 43)*
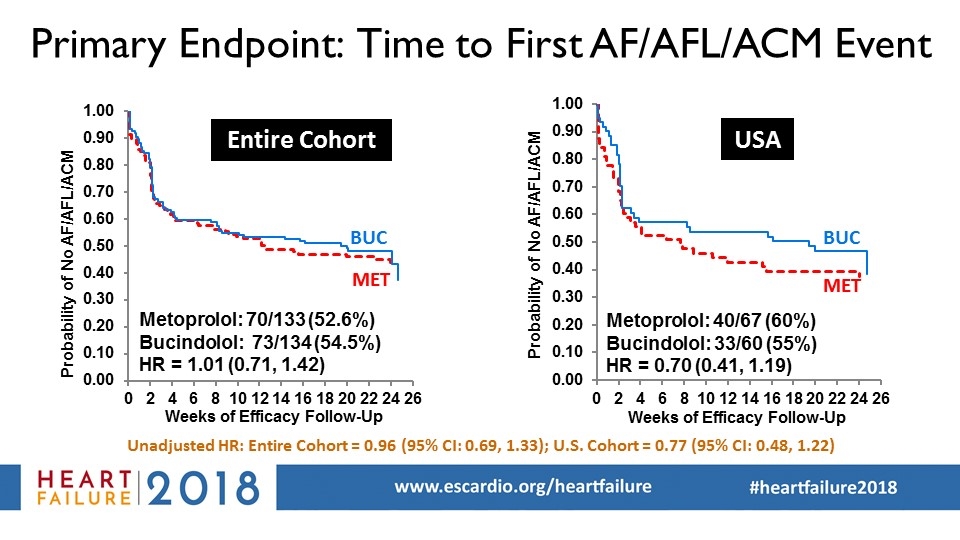
Primary Endpoint: Time to First AF/AFL/ACM Event Unadjusted HR: Entire Cohort = 0.96 (95% CI: 0.69, 1.33); U.S. Cohort = 0.77 (95% CI: 0.48, 1.22) BUC MET Entire Cohort USA
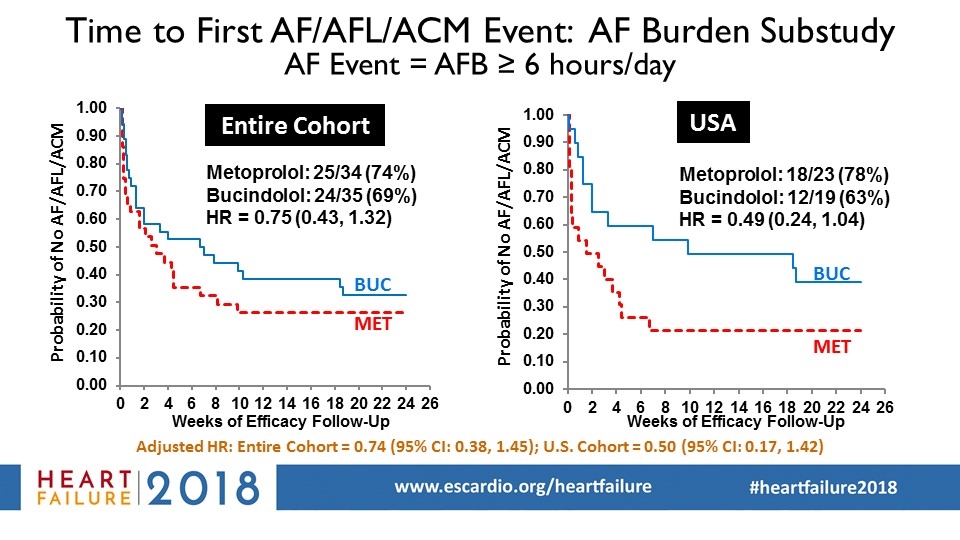
Time to First AF/AFL/ACM Event: AF Burden Substudy AF Event = AFB ≥ 6 hours/day Probability of No AF/AFL/ACM Entire Cohort USA Adjusted HR: Entire Cohort = 0.74 (95% CI: 0.38, 1.45); U.S. Cohort = 0.50 (95% CI: 0.17, 1.42)
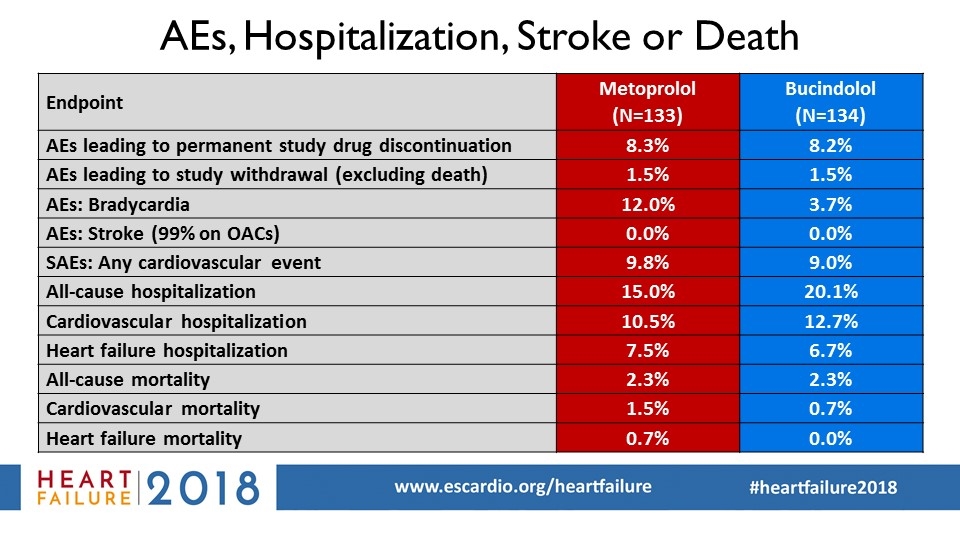
AEs, Hospitalization, Stroke or Death Endpoint Metoprolol (N=133) Bucindolol (N=134) AEs leading to permanent study drug discontinuation 8.3% 8.2% AEs leading to study withdrawal (excluding death) 1.5% 1.5% AEs: Bradycardia 12.0% 3.7% AEs: Stroke (99% on OACs) 0.0% 0.0% SAEs: Any cardiovascular event 9.8% 9.0% All-cause hospitalization 15.0% 20.1% Cardiovascular hospitalization 10.5% 12.7% Heart failure hospitalization 7.5% 6.7% All-cause mortality 2.3% 2.3% Cardiovascular mortality 1.5% 0.7% Heart failure mortality 0.7% 0.0%
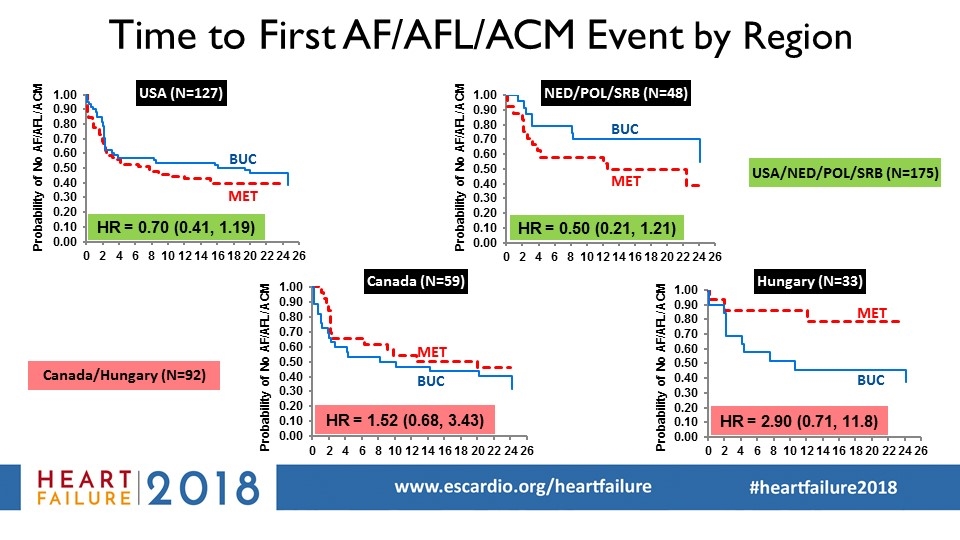
USA (N=127) Canada (N=59) Hungary (N=33) Time to First AF/AFL/ACM Event by Region BUC MET BUC MET BUC MET BUC MET NED/POL/SRB (N=48) Probability of No AF/AFL/ACM Probability of No AF/AFL/ACM Probability of No AF/AFL/ACM Probability of No AF/AFL/ACM USA/NED/POL/SRB (N=175) Canada/Hungary (N=92)

Time to First AF/AFL/ACM Event LVEF < 0.39 (median) or LVEF 0.39-0.49 with HF Dx to Rand - AF Dx to Rand (DTRI) >-30 days Unadjusted HR: Entire Cohort = 0.84 (95% CI: 0.58, 1.22) Country Included (%) All 77% USA 85% Canada 78% Hungary 39% Poland 78% Serbia 81% Netherlands 75%
GENETIC-AF Conclusions Pharmacogenetic guided bucindolol did not reduce AF/AFL/ACM recurrence compared to the active comparator metoprolol in the overall population Trends for bucindolol benefit were observed in several large subpopulations Bucindolol appears to have a similar safety profile compared to metoprolol These Phase 2 results merit further investigation in a redefined population HFmrEF (LVEF ≥0.40 and <0.50) if DTRI > -30 days HFrEF (LVEF < 0.40) Symptomatic paroxysmal/persistent AF ≤ 180 days of randomization β1389Arg/Arg genotype











