
ARCA biopharma Pharmacogenetic Precision Medicine for Cardiovascular Diseases February 2020 Nasdaq: ABIO Exhibit 99.2

Safe Harbor Statement This presentation contains "forward-looking statements" for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, potential future development plans for Gencaro, ARCA’s ability to complete any clinical trials, the likelihood for PRECISION-AF results to satisfy the requirements of the U.S. FDA Special Protocol Assessment (SPA) agreement, ARCA's ability to launch any clinical trials, or announce any results therefrom, on or before any particular date, ARCA’s ability to raise sufficient capital to fund the PRECSION-AF trial and its other operations, the expected features and characteristics of Gencaro, including the potential for genetic variations to predict individual patient response to Gencaro, Gencaro’s potential to treat AF and/or HF, future treatment options for patients with AF and/or HF, the potential for Gencaro to be the first genetically-targeted AF prevention treatment, and the potential market opportunity for Gencaro, should it receive regulatory approval. Such statements are based on management's current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, the risks and uncertainties associated with: ARCA’s financial resources and whether they will be sufficient to meet its business objectives and operational requirements; ARCA may not be able to raise sufficient capital on acceptable terms, or at all, to continue development of Gencaro or to otherwise continue operations in the future; an FDA SPA agreement does not guarantee approval of Gencaro or any other particular outcome from regulatory review; results of earlier clinical trials may not be confirmed in future trials; the protection and market exclusivity provided by ARCA’s intellectual property; risks related to the drug discovery and the regulatory approval process; and, the impact of competitive products and technological changes. These and other factors are identified and described in more detail in ARCA’s filings with the Securities and Exchange Commission, including without limitation ARCA’s annual report on Form 10-K for the year ended December 31, 2019, and subsequent filings. ARCA disclaims any intent or obligation to update these forward-looking statements.
Investment Highlights Late stage, genetically-targeted cardiovascular company PRECISION-AF: single pivotal Phase 3 trial for AF in HF│FDA SPA agreement Anticipated P3 trial timeline - initiation: 4Q2020│interim analysis: 1H2022│topline data: 1H2023 GencaroTM FDA Fast Track Designation for AF in Genotype-Defined HF Population Large and growing market opportunity for Gencaro Potentially first genetically-targeted cardiovascular therapeutic AF in HF is significant unmet medical need with no FDA approved treatments Estimated $12.5 billion global atrial fibrillation market by 2020 Gencaro in AF/HF potential annual sales of $400M ‒ $900M in US; significant markets in EU & Asia Pharmacogenetic platform with additional cardiovascular programs AB171 – development for HF, PAD │ IND anticipated 2021 Potential third asset – development for pediatric HF (Orphan Indication) │ In-licensing in progress

Experienced Leadership Michael R. Bristow, MD, PhD: President /CEO Thomas Keuer: Chief Operating Officer Chris Ozeroff: General Counsel Brian Selby: VP, Finance & Chief Accounting Officer Debra Marshall, MD, FACC: SVP, Medical Affairs Chris Dufton, PhD: VP, Clinical Development Sharon Perry: Sr Director, Regulatory Affairs & Quality Board of Directors Michael R. Bristow, MD, PhD Robert E. Conway (Chairman) Linda Grais, MD, JD Anders Hove, MD Dan Mitchell Raymond L. Woosley, MD, PhD
Pharmacogenetics: Precision Medicine Applied to Drug Therapy Our Platform Approach Pharmacogenetic drug development by targeting genetic variation in candidate drug targets, using cardiovascular tissue and cells from an extensive and exclusive human heart tissue bank Identify functionally different and important genetic variants with allele frequency ≥ 10% Screen compounds for variant-specific selective favorable action using human in vitro CV systems, including tissue from the world’s largest human heart biobank1,2 Generate Phase 1B ® Phase 2 data for pharmacogenetic POC and IP Potential benefits Maximizes potential efficacy and therapeutic index Avoids exposing patients unlikely to benefit Enables smaller clinical development programs Intellectual property (IP) generated later in development process 1Liggett et al, PNAS 103:11288-93, 2006; 2Sucharov et al, JACC 73:1173-84, 2019
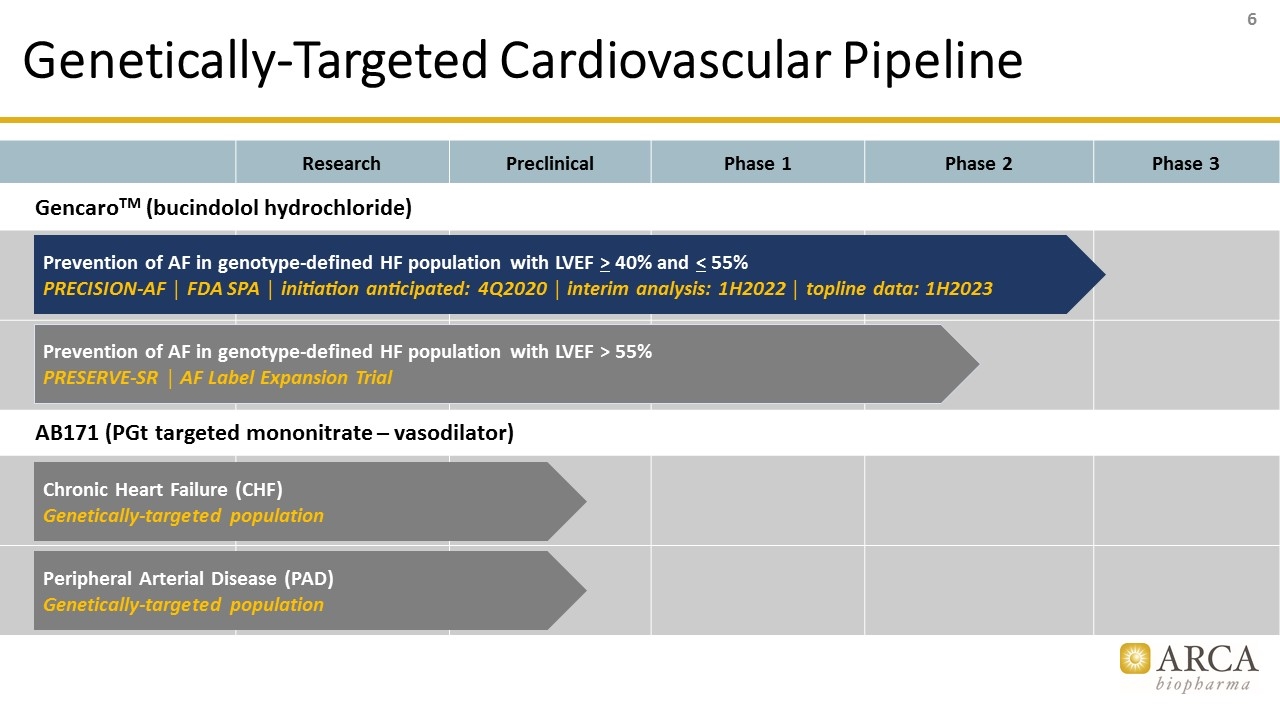
Genetically-Targeted Cardiovascular Pipeline Research Preclinical Phase 1 Phase 2 Phase 3 GencaroTM (bucindolol hydrochloride) AB171 (PGt targeted mononitrate – vasodilator) Prevention of AF in genotype-defined HF population with LVEF > 40% and < 55% PRECISION-AF │ FDA SPA │ initiation anticipated: 4Q2020 │ interim analysis: 1H2022 │ topline data: 1H2023 Peripheral Arterial Disease (PAD) Genetically-targeted population Chronic Heart Failure (CHF) Genetically-targeted population Prevention of AF in genotype-defined HF population with LVEF > 55% PRESERVE-SR │ AF Label Expansion Trial
Atrial Fibrillation – A Significant Market Opportunity AF is the most common sustained cardiac arrhythmia Affects ~5.2 million (2015) Americans1 2015 worldwide prevalence of AF was estimated at 33 million2 AF is considered an epidemic cardiovascular disease Based on the rate of increase in incidence in the U.S. and industrialized countries3 Estimated global atrial fibrillation market4 $7.2B in 2015 growing to $12.5B in 2020 ARCA estimate of Gencaro opportunity $400M to $900M peak revenue in US; similar in EU5 1- American Journal of Cardiology 2013: 112: 1142-1147 “Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population; AHA – “Cardiovascular Disease: A Costly Burden for America” (Jan 2017), page 7 2- AHA Statistical Update – Heart and Stroke Stats 2017, p306 3- Journal of the American Medical Association. 2001; 285(18):237 0-2375 4- DelveInsight – “Atrial Fibrillation – Market Insights & Drug Sales Forecast - 2020”, May 2016
Atrial Fibrillation in Heart Failure – An Unmet Medical Need No FDA approved drug treatments for this indication Approved antiarrhythmic agents are associated with significant side effects Most are contraindicated or have warnings for HF patients New onset AF markedly worsens HF morbidity & increases mortality β-blockers approved for HF but used off-label for AF Demonstrated only limited efficacy for AF prevention No agents approved for AF or HF have genetically influenced clinical response
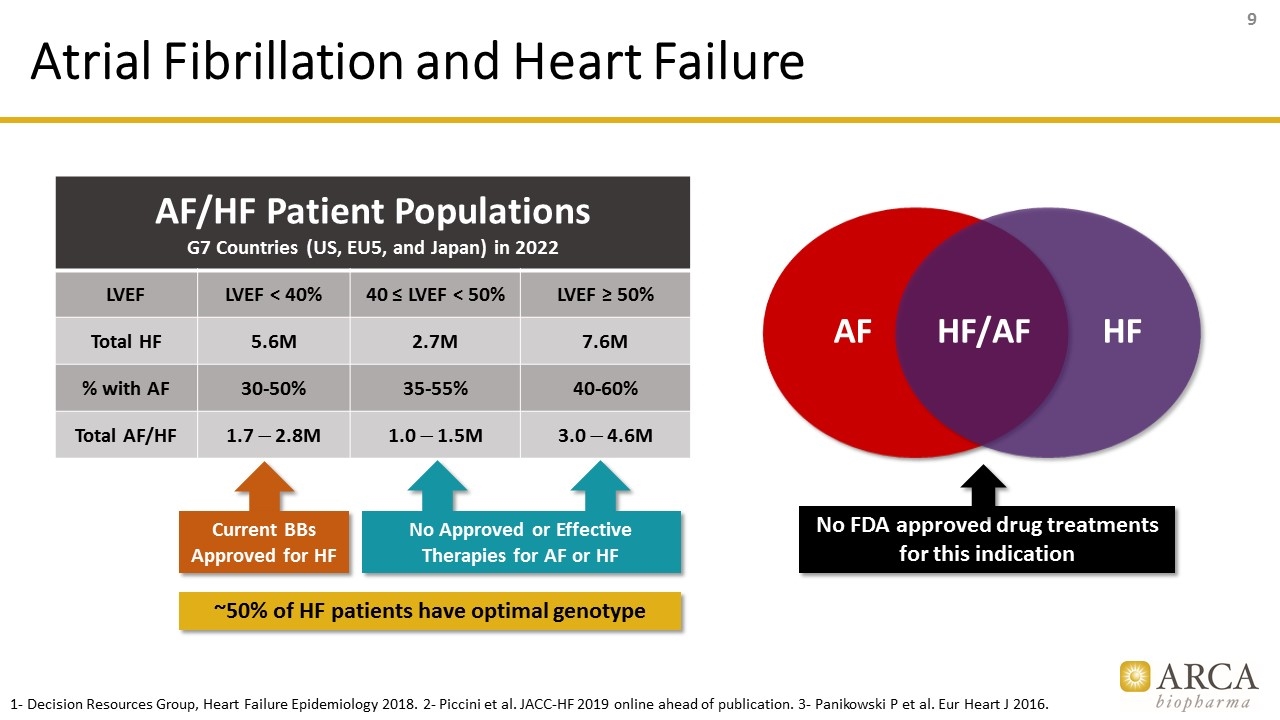
Atrial Fibrillation and Heart Failure AF/HF Patient Populations G7 Countries (US, EU5, and Japan) in 2022 LVEF LVEF < 40% 40 ≤ LVEF < 50% LVEF ≥ 50% Total HF 5.6M 2.7M 7.6M % with AF 30-50% 35-55% 40-60% Total AF/HF 1.7 - 2.8M 1.0 - 1.5M 3.0 - 4.6M ~50% of HF patients have optimal genotype No Approved or Effective Therapies for AF or HF AF HF No FDA approved drug treatments for this indication HF/AF 1- Decision Resources Group, Heart Failure Epidemiology 2018. 2- Piccini et al. JACC-HF 2019 online ahead of publication. 3- Panikowski P et al. Eur Heart J 2016. Current BBs Approved for HF
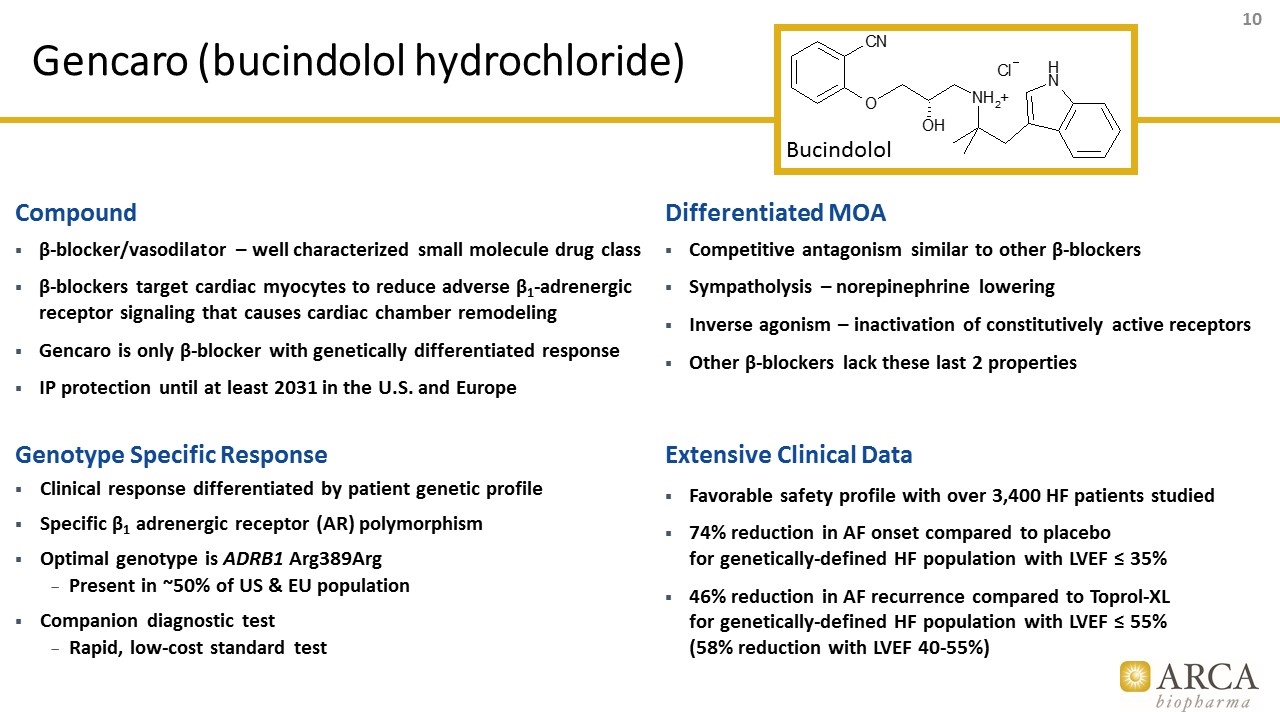
Gencaro (bucindolol hydrochloride) Compound β-blocker/vasodilator – well characterized small molecule drug class β-blockers target cardiac myocytes to reduce adverse β1-adrenergic receptor signaling that causes cardiac chamber remodeling Gencaro is only β-blocker with genetically differentiated response IP protection until at least 2031 in the U.S. and Europe Genotype Specific Response Clinical response differentiated by patient genetic profile Specific β1 adrenergic receptor (AR) polymorphism Optimal genotype is ADRB1 Arg389Arg Present in ~50% of US & EU population Companion diagnostic test Rapid, low-cost standard test Differentiated MOA Competitive antagonism similar to other β-blockers Sympatholysis – norepinephrine lowering Inverse agonism – inactivation of constitutively active receptors Other β-blockers lack these last 2 properties Extensive Clinical Data Favorable safety profile with over 3,400 HF patients studied 74% reduction in AF onset compared to placebo for genetically-defined HF population with LVEF ≤ 35% 46% reduction in AF recurrence compared to Toprol-XL for genetically-defined HF population with LVEF ≤ 55% (58% reduction with LVEF 40-55%) Bucindolol

Differentiated AF Response by Genotype Seen in BEST DNA Substudy1 b1389 Arg/Arg (n = 441; 36 events) Risk reduction 74% Hazard Ratio = 1.01 (0.56 – 1.84) P-value = 0.969 b1389 Gly carriers (n = 484; 44 events) No risk reduction Hazard Ratio = 0.26 (0.12 – 0.57) P-value = 0.0003 b-blocker class 27% average risk reduction in AF onset in ~12,000 patient meta-analysis of Phase 3 HF trials.2 Metoprolol and Carvedilol are not influenced by ADRB1 Arg389Gly3,4 1- Aleong RG et al, JACC-HF 1:338-344, 2013. 2- Abi Nasr I et al, EHJ 28: 457–462, 2007. 3- Sehnert AJ, et al. JACC 52:644-651, 2008; 4- Data on file at ARCA. Gencaro Gencaro Placebo Placebo Data from the BEST DNA pharmacogenetic substudy for patients with NYHA Class III/IV, LVEF ≤ 35% who were not in AF at randomization.
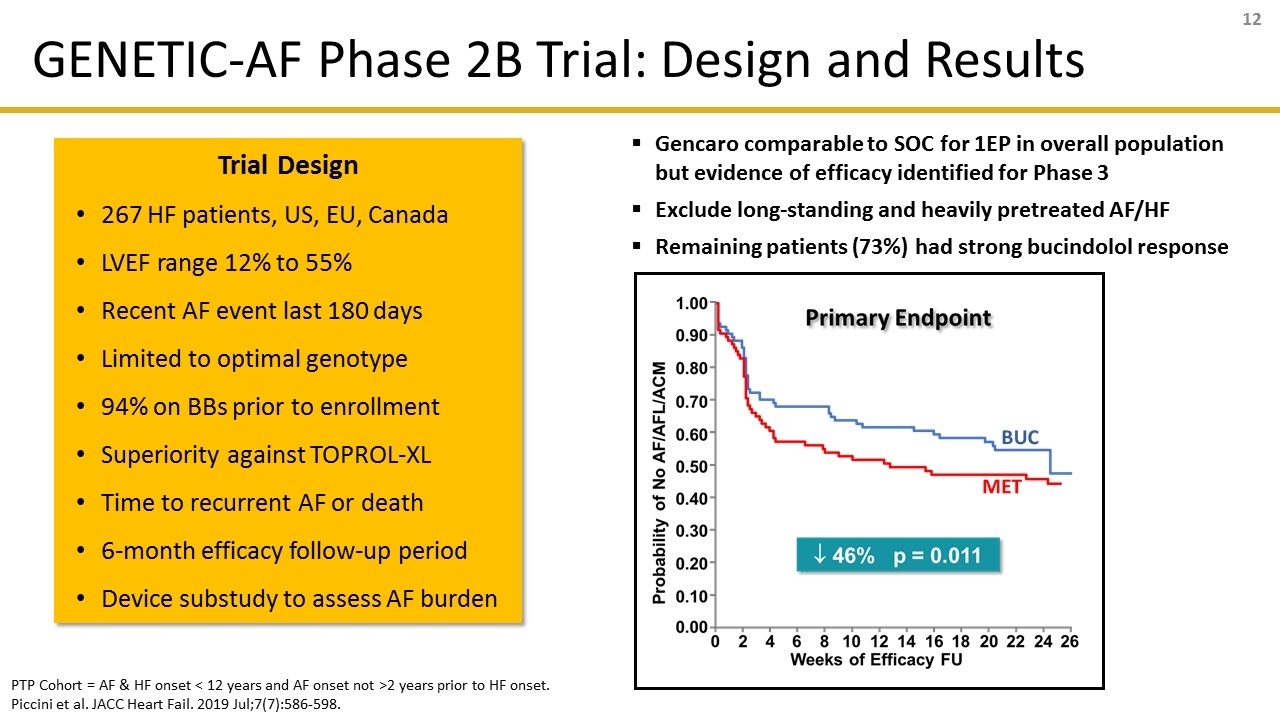
Trial Design 267 HF patients, US, EU, Canada LVEF range 12% to 55% Recent AF event last 180 days Limited to optimal genotype 94% on BBs prior to enrollment Superiority against TOPROL-XL Time to recurrent AF or death 6-month efficacy follow-up period Device substudy to assess AF burden Gencaro comparable to SOC for 1EP in overall population but evidence of efficacy identified for Phase 3 Exclude long-standing and heavily pretreated AF/HF Remaining patients (73%) had strong bucindolol response PTP Cohort = AF & HF onset < 12 years and AF onset not >2 years prior to HF onset. Piccini et al. JACC Heart Fail. 2019 Jul;7(7):586-598. GENETIC-AF Phase 2B Trial: Design and Results
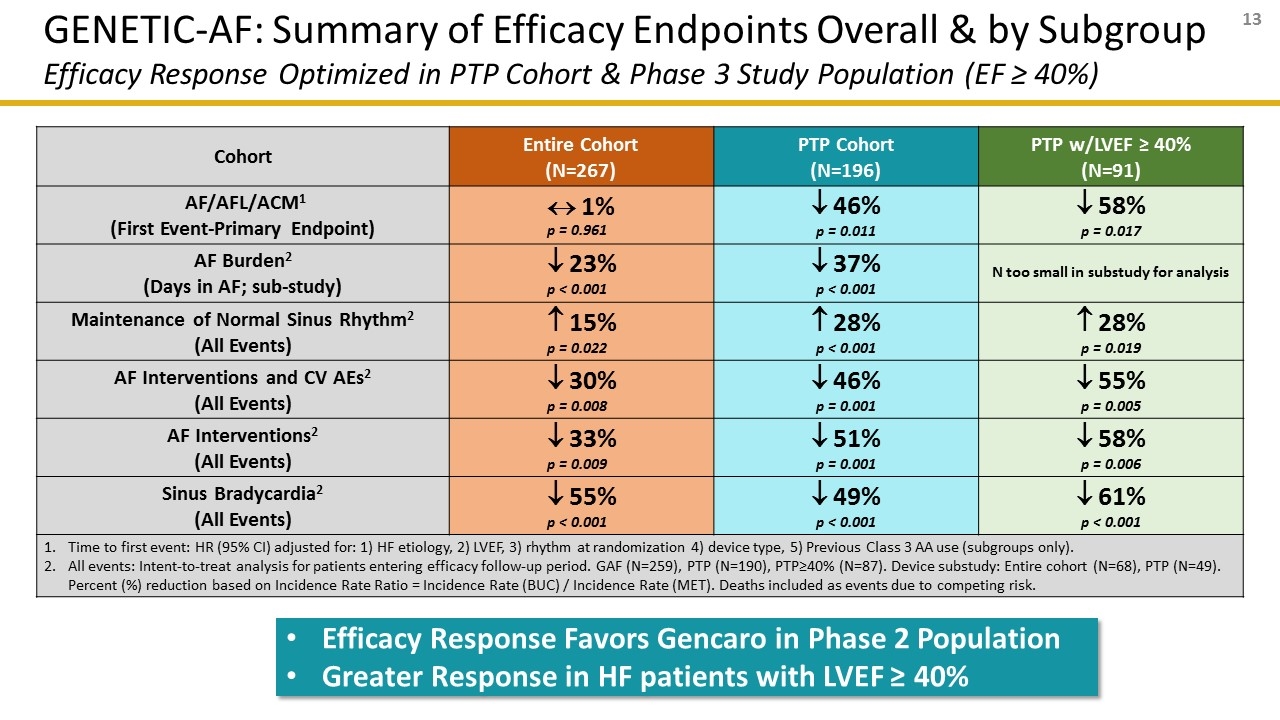
Cohort Entire Cohort (N=267) PTP Cohort (N=196) PTP w/LVEF ≥ 40% (N=91) AF/AFL/ACM1 (First Event-Primary Endpoint) « 1% p = 0.961 ¯ 46% p = 0.011 ¯ 58% p = 0.017 AF Burden2 (Days in AF; sub-study) ¯ 23% p < 0.001 ¯ 37% p < 0.001 N too small in substudy for analysis Maintenance of Normal Sinus Rhythm2 (All Events) 15% p = 0.022 28% p < 0.001 28% p = 0.019 AF Interventions and CV AEs2 (All Events) ¯ 30% p = 0.008 ¯ 46% p = 0.001 ¯ 55% p = 0.005 AF Interventions2 (All Events) ¯ 33% p = 0.009 ¯ 51% p = 0.001 ¯ 58% p = 0.006 Sinus Bradycardia2 (All Events) ¯ 55% p < 0.001 ¯ 49% p < 0.001 ¯ 61% p < 0.001 Time to first event: HR (95% CI) adjusted for: 1) HF etiology, 2) LVEF, 3) rhythm at randomization 4) device type, 5) Previous Class 3 AA use (subgroups only). All events: Intent-to-treat analysis for patients entering efficacy follow-up period. GAF (N=259), PTP (N=190), PTP≥40% (N=87). Device substudy: Entire cohort (N=68), PTP (N=49). Percent (%) reduction based on Incidence Rate Ratio = Incidence Rate (BUC) / Incidence Rate (MET). Deaths included as events due to competing risk. GENETIC-AF: Summary of Efficacy Endpoints Overall & by Subgroup Efficacy Response Optimized in PTP Cohort & Phase 3 Study Population (EF ≥ 40%) Efficacy Response Favors Gencaro in Phase 2 Population Greater Response in HF patients with LVEF ≥ 40%
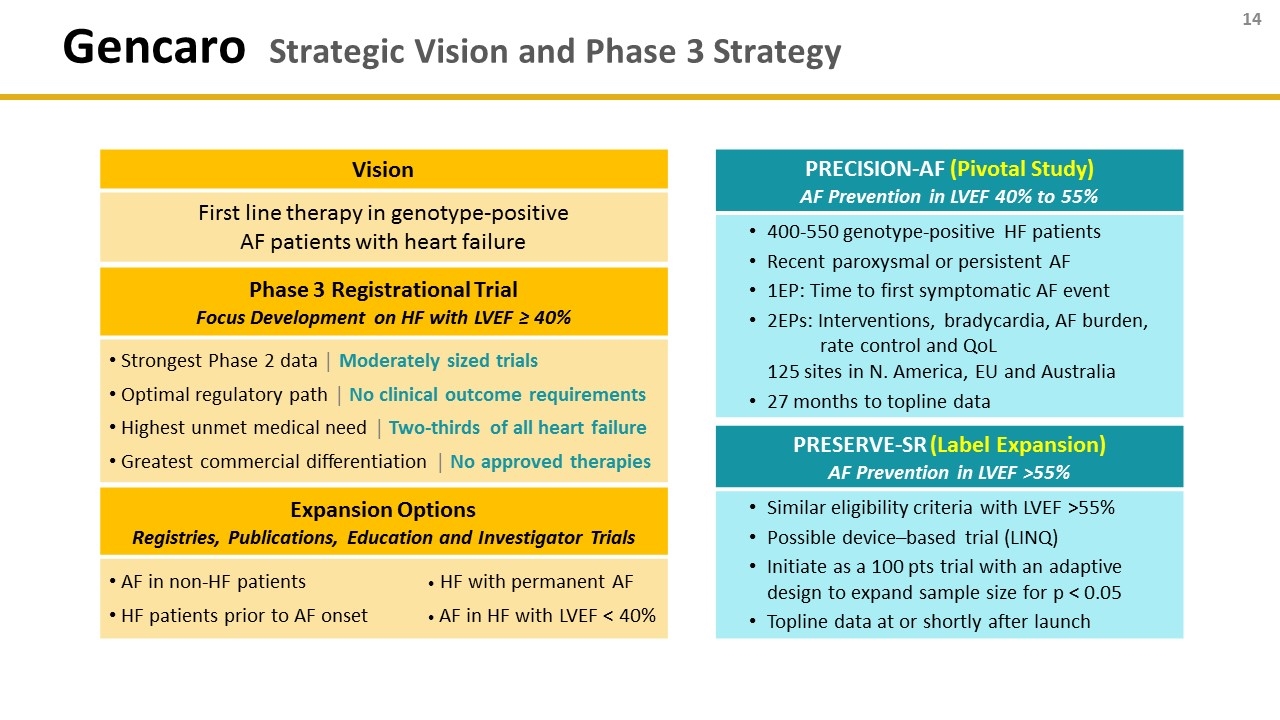
Gencaro Strategic Vision and Phase 3 Strategy Vision First line therapy in genotype-positive AF patients with heart failure Phase 3 Registrational Trial Focus Development on HF with LVEF ≥ 40% Strongest Phase 2 data │ Moderately sized trials Optimal regulatory path │ No clinical outcome requirements Highest unmet medical need │ Two-thirds of all heart failure Greatest commercial differentiation │ No approved therapies Expansion Options Registries, Publications, Education and Investigator Trials AF in non-HF patients · HF with permanent AF HF patients prior to AF onset · AF in HF with LVEF < 40% PRECISION-AF (Pivotal Study) AF Prevention in LVEF 40% to 55% 400-550 genotype-positive HF patients Recent paroxysmal or persistent AF 1EP: Time to first symptomatic AF event 2EPs: Interventions, bradycardia, AF burden, rate control and QoL 125 sites in N. America, EU and Australia 27 months to topline data PRESERVE-SR (Label Expansion) AF Prevention in LVEF >55% Similar eligibility criteria with LVEF >55% Possible device–based trial (LINQ) Initiate as a 100 pts trial with an adaptive design to expand sample size for p < 0.05 Topline data at or shortly after launch
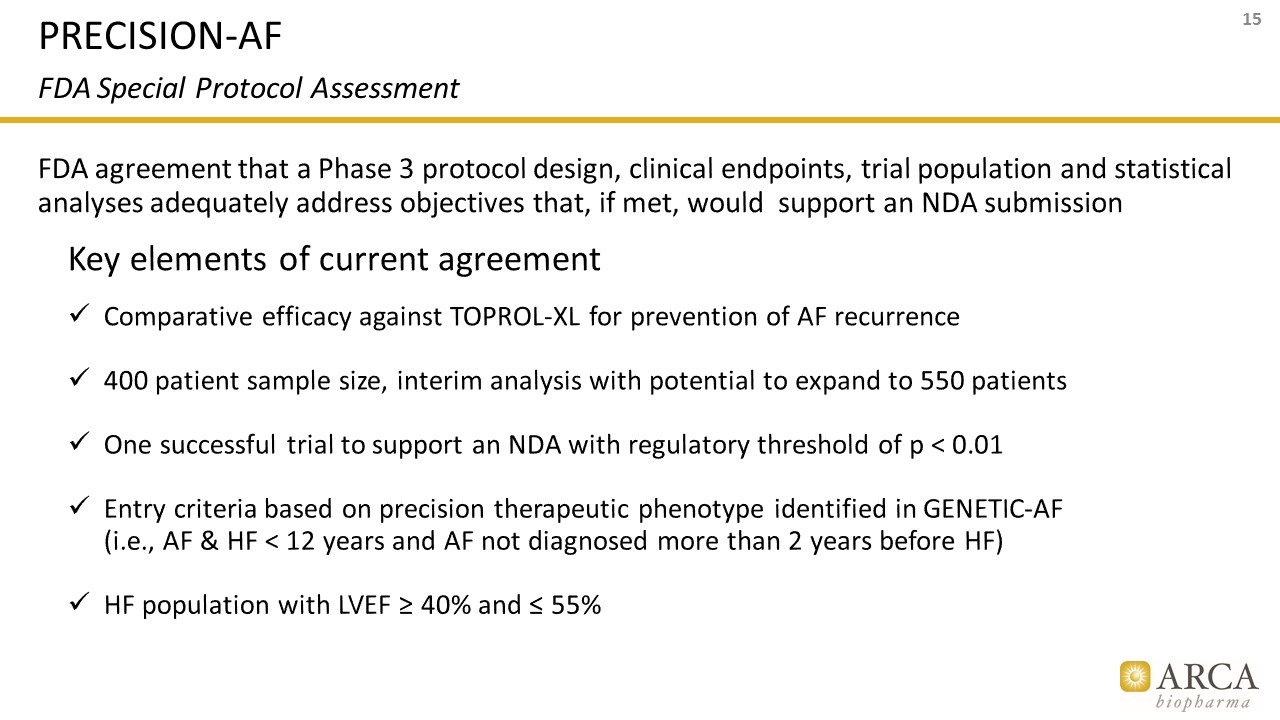
FDA agreement that a Phase 3 protocol design, clinical endpoints, trial population and statistical analyses adequately address objectives that, if met, would support an NDA submission Key elements of current agreement Comparative efficacy against TOPROL-XL for prevention of AF recurrence 400 patient sample size, interim analysis with potential to expand to 550 patients One successful trial to support an NDA with regulatory threshold of p < 0.01 Entry criteria based on precision therapeutic phenotype identified in GENETIC-AF (i.e., AF & HF < 12 years and AF not diagnosed more than 2 years before HF) HF population with LVEF ≥ 40% and ≤ 55% PRECISION-AF FDA Special Protocol Assessment
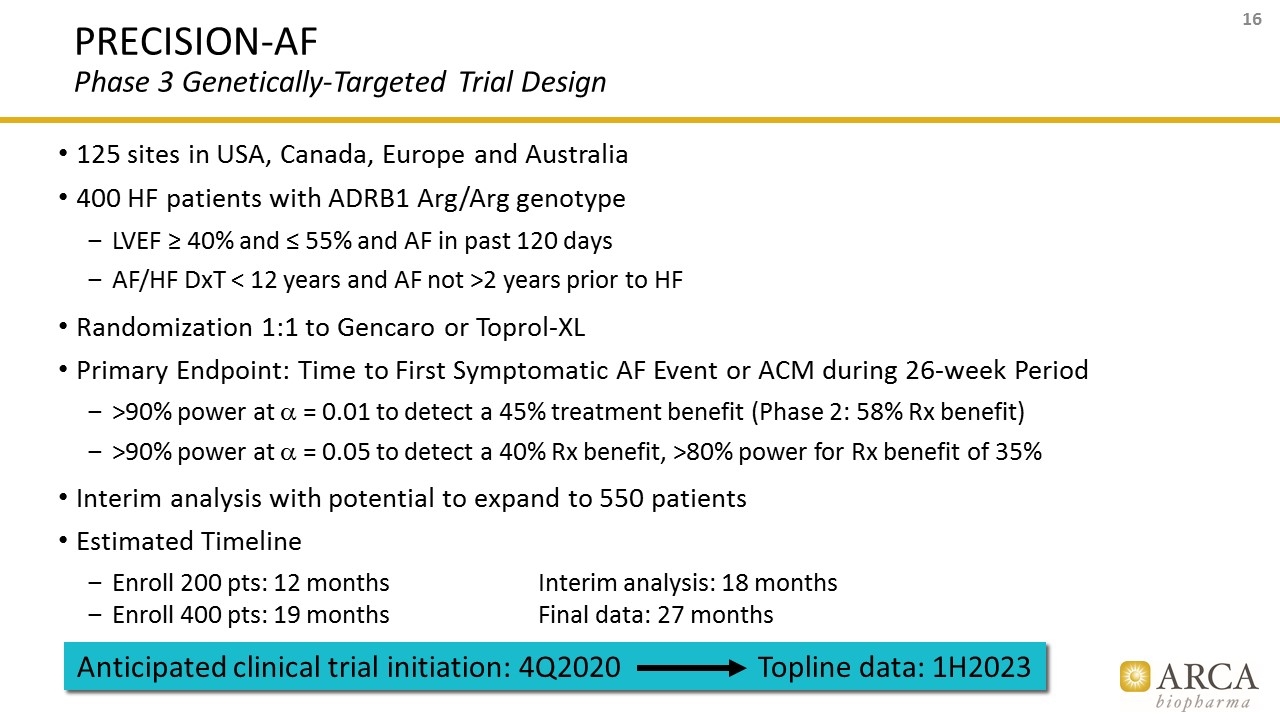
PRECISION-AF Phase 3 Genetically-Targeted Trial Design 125 sites in USA, Canada, Europe and Australia 400 HF patients with ADRB1 Arg/Arg genotype LVEF ≥ 40% and ≤ 55% and AF in past 120 days AF/HF DxT < 12 years and AF not >2 years prior to HF Randomization 1:1 to Gencaro or Toprol-XL Primary Endpoint: Time to First Symptomatic AF Event or ACM during 26-week Period >90% power at a = 0.01 to detect a 45% treatment benefit (Phase 2: 58% Rx benefit) >90% power at a = 0.05 to detect a 40% Rx benefit, >80% power for Rx benefit of 35% Interim analysis with potential to expand to 550 patients Estimated Timeline Enroll 200 pts: 12 monthsInterim analysis: 18 months Enroll 400 pts: 19 monthsFinal data: 27 months Anticipated clinical trial initiation: 4Q2020 Topline data: 1H2023
AF in mid-range and preserved heart failure More than two-thirds of all HF patients have EF ≥ 40% | Current BBs not indicated for this population 35-50% of HF patients have AF co-morbidity | Worsens HF prognosis including mortality 50% have optimal Gencaro genotype | High frequency biomarker No efficacious and safe drug therapies for AF/HF in EF ≥ 40% | Major unmet cardiovascular need Significant commercial opportunity $400M to $900M peak revenue in U.S. for first indication | Substantial market Options for label expansion that more than double the initial indication| Broad clinical application Significant markets in EU and Asia| Global partnering opportunity GENCARO Medical Need and Commercial Opportunity Potentially first precision medicine-based cardiovascular therapeutic
Gencaro Intellectual Property Patents + New Chemical Entity Provide Robust Protection Issued Patents + NCE status expected to provide minimum 8-10 years exclusivity in major markets Newly-filed IP based on Phase 2 discoveries would extend to 2039 U.S. Exclusivity U.S. patents provide longest exclusivity Main patent to mid-2031; 2034 with isomer development New IP from Phase 2 findings would extend to 2039 NCE exclusivity overlaps— 5+ to 7.5 years from approval EU & Japan EMA NCE exclusivity: 10-11 years from approval EU Patents extend into 2030, new IP would take to 2039 Japan: current patent into 2030, new IP would take to 2039 Japan NCE PMS period: 8 years from approval
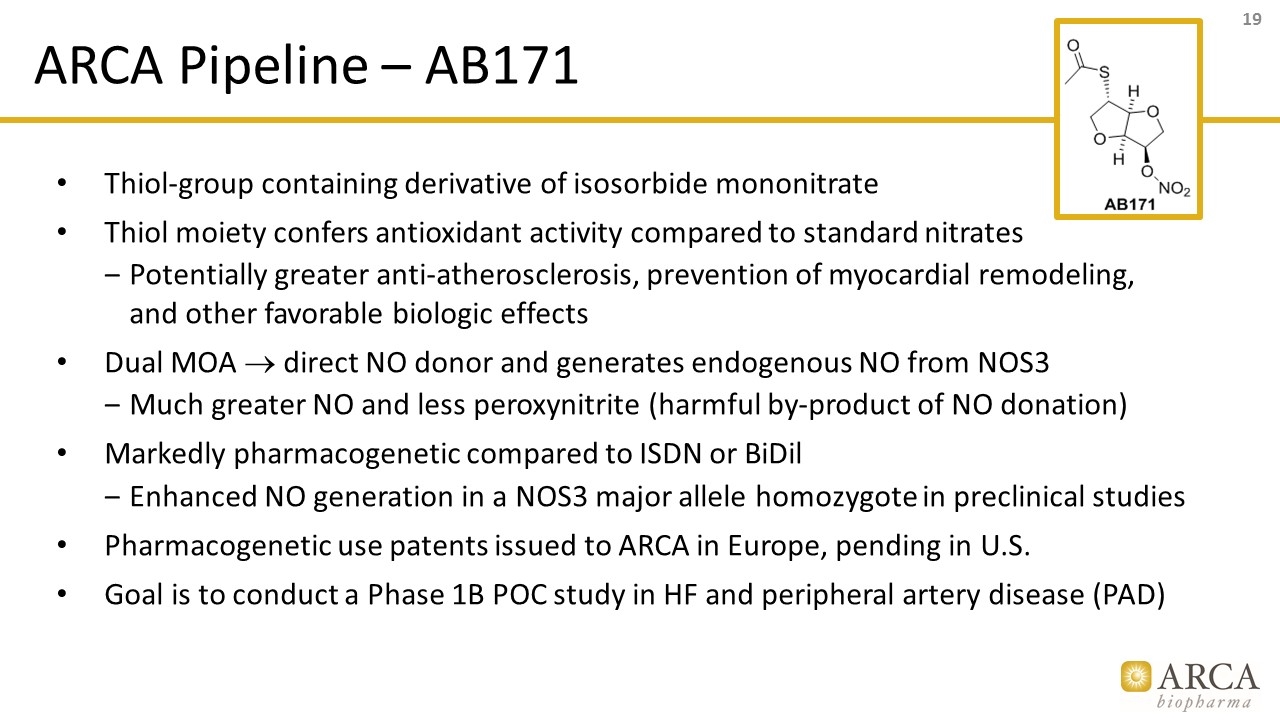
ARCA Pipeline – AB171 Thiol-group containing derivative of isosorbide mononitrate Thiol moiety confers antioxidant activity compared to standard nitrates Potentially greater anti-atherosclerosis, prevention of myocardial remodeling, and other favorable biologic effects Dual MOA ® direct NO donor and generates endogenous NO from NOS3 Much greater NO and less peroxynitrite (harmful by-product of NO donation) Markedly pharmacogenetic compared to ISDN or BiDil Enhanced NO generation in a NOS3 major allele homozygote in preclinical studies Pharmacogenetic use patents issued to ARCA in Europe, pending in U.S. Goal is to conduct a Phase 1B POC study in HF and peripheral artery disease (PAD)

Capital Structure Shares outstanding (12/31/19)1.59 million Potentially dilutive securities 0.17 million Fully diluted1.76 million Cash and cash equivalents (as of 12/31/19)$8.4 million No debt Estimated cash runway through end of third quarter 2020

ARCA biopharma Pharmacogenetic Precision Medicine for Cardiovascular Diseases Nasdaq: ABIO




















