Filed by Trimeris, Inc.
Pursuant to Rule 425 under the
Securities Act of 1933 (the “Securities Act”) and
deemed filed pursuant to Rule 14a-12 under the
Securities Exchange Act of 1934 (the “Exchange Act”)
Securities Act File Number: 333-175512
Subject Company: Trimeris, Inc.
Exchange Act File Number: 000-23155
This filing relates to the proposed merger involving Trimeris, Inc. (“Trimeris”) and Synageva BioPharma Corp. (“Synageva”), pursuant to the terms of an Agreement and Plan of Merger and Reorganization, dated as of June 13, 2011, by and among Trimeris, Tesla Merger Sub, Inc., a wholly owned subsidiary of Trimeris, and Synageva. Beginning on September 7, 2011, the following PowerPoint slides are being used for presentations by Synageva’s management to certain investors:
|
© Copyright 2011 Synageva BioPharma™ Company Presentation September 2011 |
|
2 © Copyright 2011 Synageva BioPharma™ Forward-Looking Statements These materials and oral statements made from time to time by Synageva representatives in respect of the same subject matter may contain “forward-looking statements.” These statements can be identified by introductory words such as “expects,” “plans,” “intends,” “believes,” “will,” “estimates,” “forecasts,” “projects,” or words of similar meaning, and by the fact that they do not relate strictly to historical or current facts. Forward-looking statements frequently are used in discussing potential product applications, potential collaborations, product development activities, clinical studies, regulatory submissions and approvals, and similar operating matters. Many factors may cause actual results to differ from forward-looking statements, including inaccurate assumptions and a broad variety of risks and uncertainties, some of which are known and others of which are not. No forward-looking statement is a guarantee of future results or events, and one should avoid placing undue reliance on such statements. Synageva undertakes no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Synageva cannot be sure when or if it will be permitted by regulatory agencies to undertake additional clinical trials or to commence any particular phase of clinical trials or how quickly patent enrollment in clinical trials will occur. Because of this, statements regarding the expected timing of clinical trials or ultimate regulatory approval cannot be regarded as actual predictions of when Synageva will obtain regulatory approval for any “phase” of clinical trials or when it will obtain ultimate regulatory approval by a particular regulatory agency. |
|
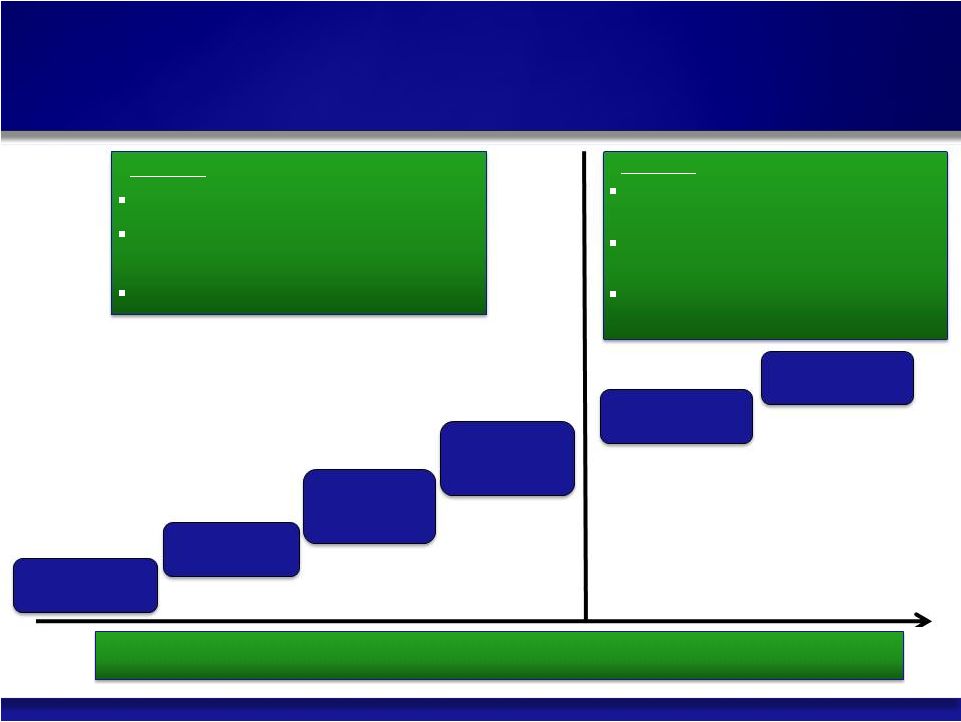
Synageva Strategy & Direction 3 © Copyright 2011 Synageva BioPharma™ Vehicle Focus Count-Down |
|
Deal Synageva and Trimeris merge in all-stock transaction 3:1 exchange ratio Transaction has been unanimously approved by both boards Rationale Provides Synageva financial resources to aggressively pursue corporate strategy Gives Synageva access to public markets Allows Synageva to maintain focus 4 © Copyright 2011 Synageva BioPharma™ Synageva Transaction |
|
Synageva Investment Highlights 5 © Copyright 2011 Synageva BioPharma™ Team Rare Disease Lead Product Status Commercial Manufacturing Rare Disease Experience Unmet Medical Need ERT for LSD – Model is Predictive Patient Dosing Commenced Pull-through Expertise Robust, Efficient and Scalable |
|
Chief Executive Officer Product Development Chief Financial Officer 6 © Copyright 2011 Synageva BioPharma™ Sanj K. Patel Carsten Boess Mark Goldberg Eric Grinstead Anthony Quinn Tara O’Meara Joe DeCourcey Commercial Operations Chief Medical Officer Clinical Operations Manufacturing Proven Management Team |
|
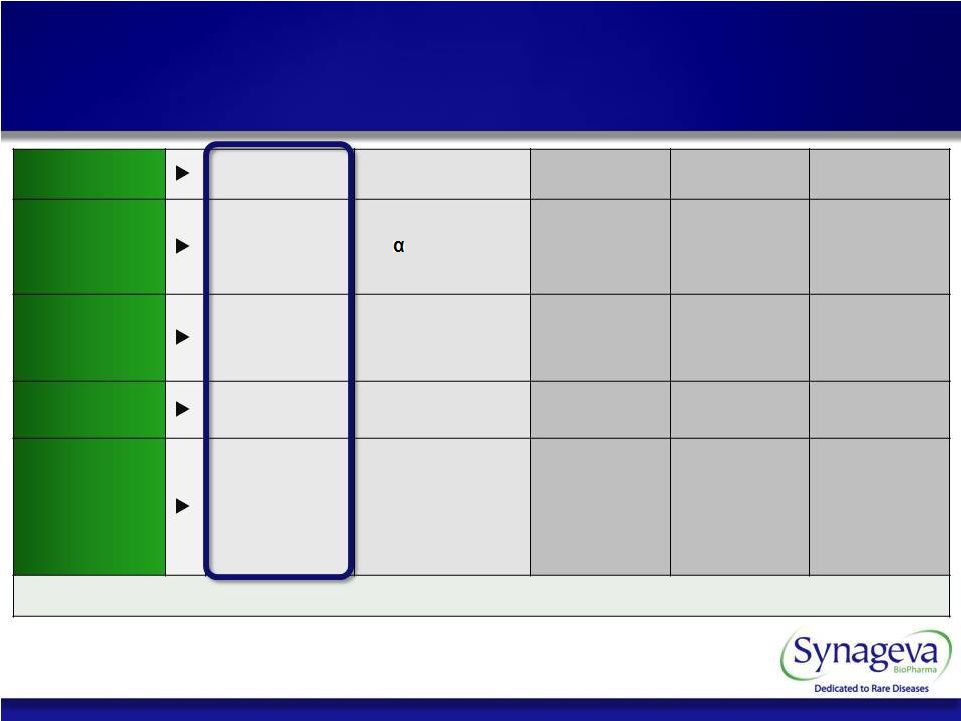
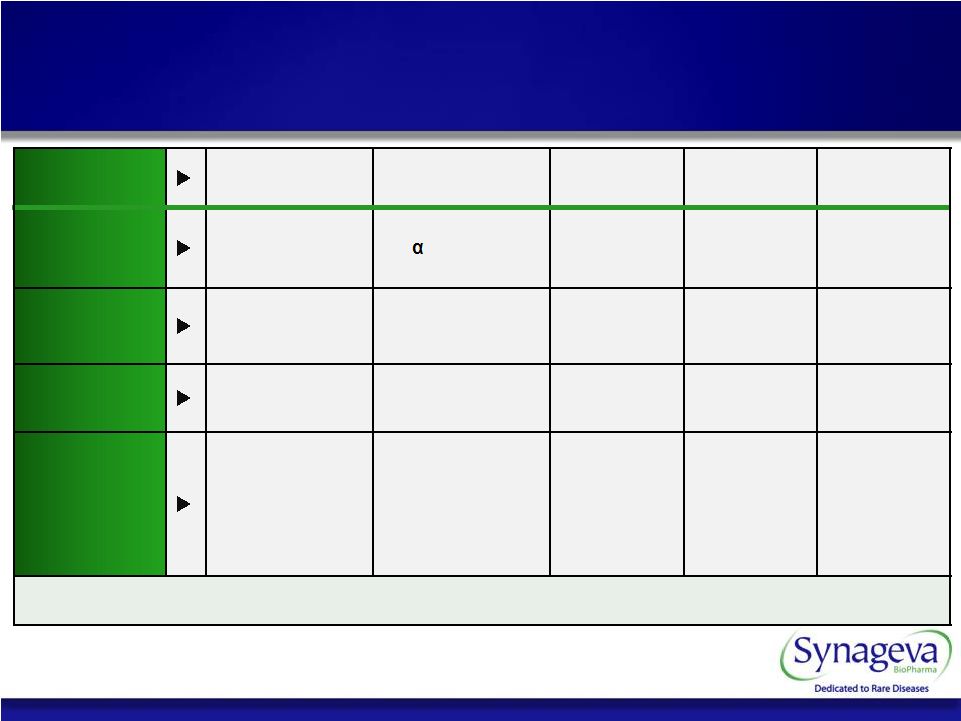
7 © Copyright 2011 Synageva BioPharma™ Program SBC-102 (rhLAL) SBC-103 (rhNAGLU) SBC-104 SBC-105 SBC-106 Therapeutic Recombinant Lysosomal Acid Lipase Recombinant -N-acetyl- glucosaminidase Extra Cellular Protein Enzyme Replacement Therapy Enzyme Replacement Therapy Disease LAL Deficiency (LSD) MPS IIIB/ Sanfilippo B (LSD) Severe Genetic Condition Severe Metabolic Disorder Severe Genetic Condition Development Status Clinical Preclinical Preclinical Preclinical Preclinical Regulatory Opportunity Orphan Designation • Granted US • Granted EU Fast Track Designation Potential for Orphan & Fast Track Designation Potential for Orphan & Fast Track Designation Potential for Orphan & Fast Track Designation Potential for Orphan & Fast Track Designation Significant unmet medical need Synageva Pipeline |
|
Lead Program 8 © Copyright 2011 Synageva BioPharma™ ERT for Lysosomal Acid Lipase Deficiency (beselipase alfa)* *Name under review by INN |
|
LDL LDL receptors coated vesicle Cholesterol esters & TG FFA Free Cholesterol Free cholesterol and fatty acids are key regulators of lipid synthesis lysosome ENDOCYTOSIS Liver lipid content in model of LAL Deficiency Cholesteryl Ester Triglyceride © Copyright 2011 Synageva BioPharma™ LAL Deficient LAL Deficient Normal Normal LAL Deficient Normal 9 |
|
Shortened lifespan and morbidity Prominent hepatic manifestations – Fatty liver – Elevated transaminases – Fibrosis – Liver failure Other manifestations: splenomegaly, type II hyperlipidemia Rare disease in a common phenotype Abdominal distention due to liver failure Liver biopsy showing cirrhosis in CESD 10 © Copyright 2011 Synageva BioPharma™ SBC-102 Late Onset LAL Deficiency (CESD) |
|
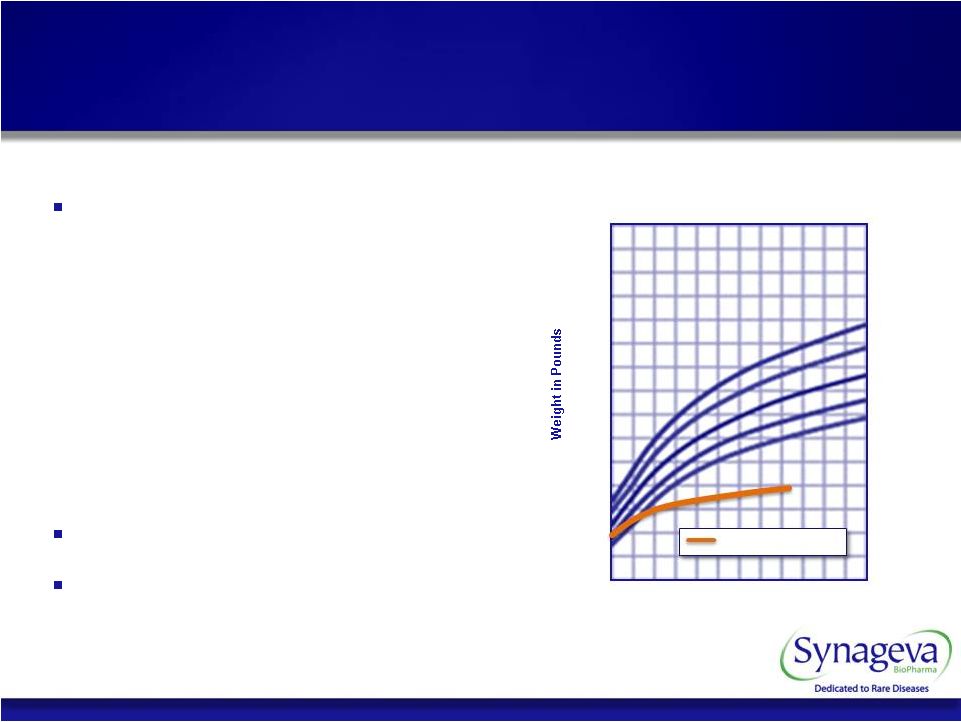
Prominent hepatic and GI manifestations – Hepatomegaly and liver failure – Splenomegaly – Persistent vomiting – Abdominal distension – Profound growth failure Adrenal calcification Rapidly progressive and fatal 4.4 6.6 8.8 11 13.2 15.4 17.6 19.8 22.1 24.3 26.5 28.7 30.9 33.1 3rd 15th 50th 85th 97th 1 2 3 4 5 6 7 8 9 10 11 12 LAL Deficient Age (months) Weight-for-age percentiles: Boys, birth to 12 months © Copyright 2011 Synageva BioPharma™ 11 SBC-102 Early Onset LAL Deficiency (Wolman) |
|
Terminal mannose/GlcNac and mannose-6-phosphate (M6P) for targeted delivery Uptake into key cells, including macrophages Lysosomal localization demonstrated Corrects enzyme deficiency Lysotracker red Overlap images Oregon green- labeled SBC-102 12 © Copyright 2011 Synageva BioPharma™ * * * *Data in macrophages 0 1 2 3 4 5 6 0 0.16 0.5 1.6 5 SBC-102 (ug/ml) Normal Human Fibroblasts LD Fibroblasts 0 0.16 0.5 1.6 5.0 SBC-102 Targeting and Activity |
|
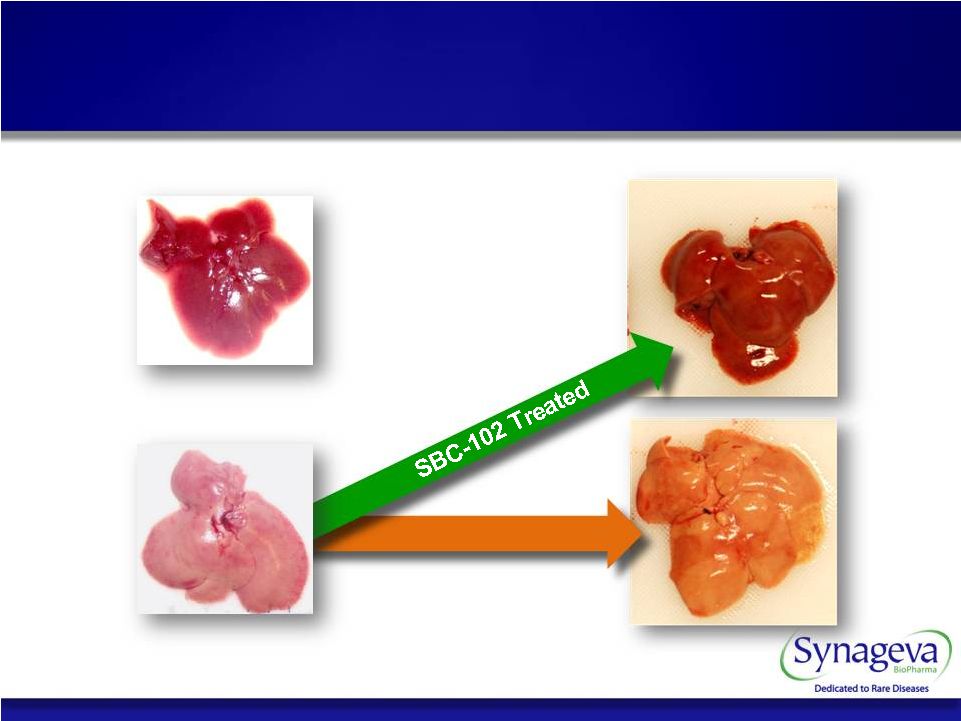
13 Normal 4 weeks 8 weeks Normalizes liver Untreated LAL Deficient © Copyright 2011 Synageva BioPharma™ SBC-102 Corrects Key Disease Related Abnormalities |
|
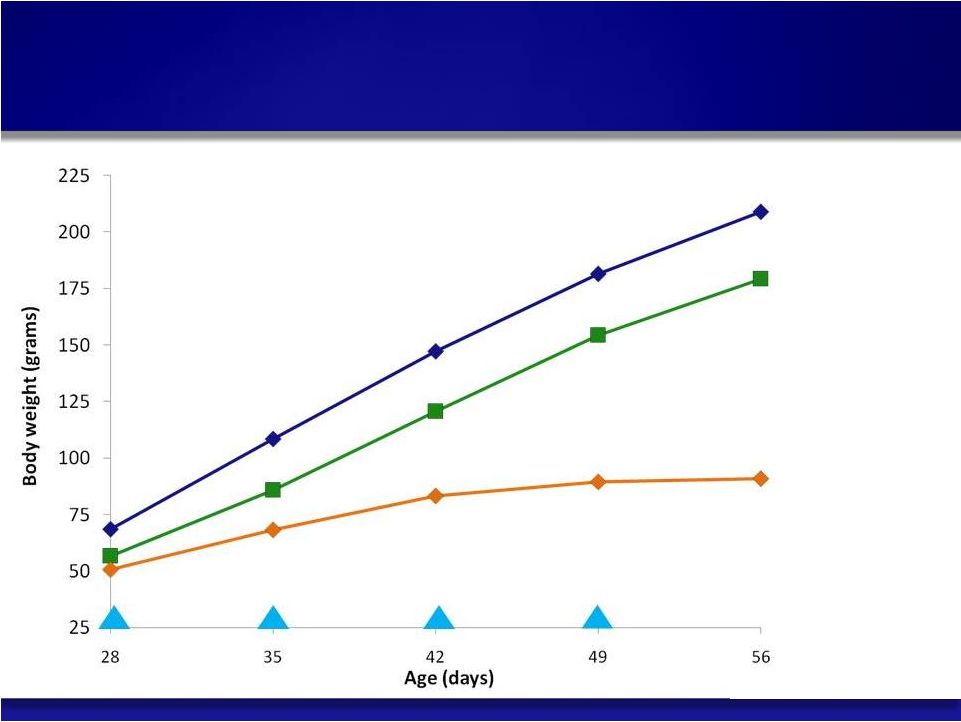
14 Normal LAL Deficient SBC-102 Treated, qw © Copyright 2011 Synageva BioPharma™ SBC-102 Rapid Correction of Growth Failure in LAL Model |
|
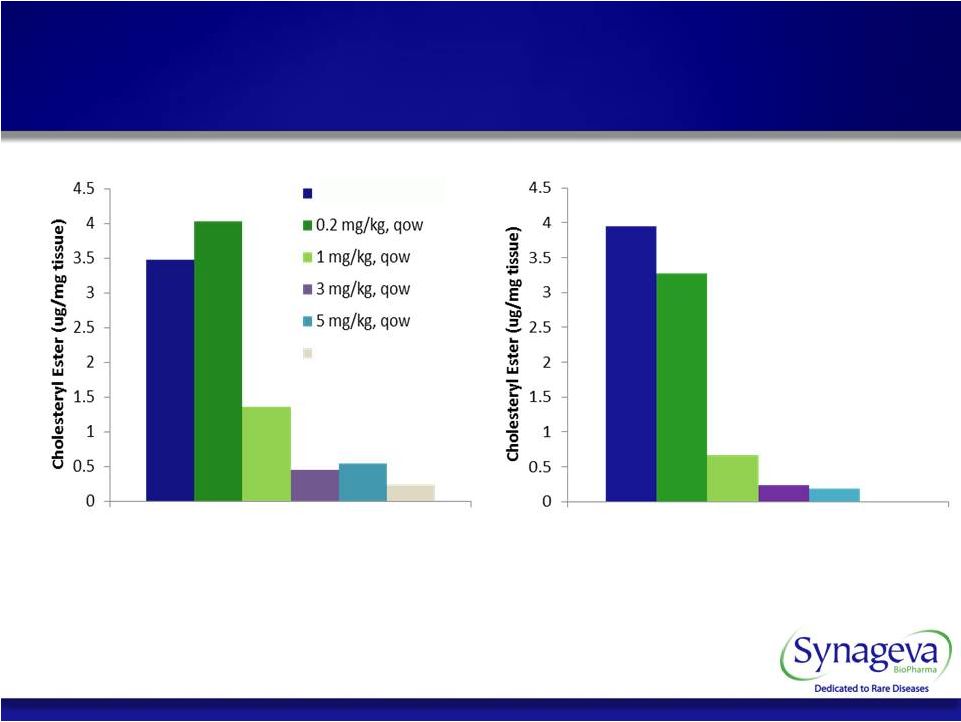
Liver Spleen Marked Reduction In Liver Cholesteryl Ester Content Across A Broad Range Of Doses © Copyright 2011 Synageva BioPharma™ 15 SBC-102 Efficacy With Every Other Week Dosing LAL Deficient Normal |
|
Frequency mg/kg 16 © Copyright 2011 Synageva BioPharma™ Therapy Pre-Clinical Effective Dose Approved Dose Disease Model Key Results Aldurazyme 0.5-2mg/kg once weekly (canine) QW 0.58 Lysosomal targeting & substrate reduction Fabrazyme 0.3-3mg/kg twice monthly (rodent) QOW 1 Naglazyme 1-2mg/kg once weekly (feline) QW 1 Myozyme 10-100mg/kg once weekly or twice monthly (rodent) QOW 20 SBC-102 0.35-3mg/kg once weekly or twice monthly (rodent) Under investigation Lysosomal targeting & substrate reduction Endpoints that translate to humans SBC-102 ERT Models Predict Efficacy and Dose |
|
Proof of concept established in disease model Small patient numbers accelerate development timelines (~5 years vs 5-10 years for common diseases) Patient dosing initiated within 3 months of IND and CTA clearance Conventional Development Programs 2020 2020 2011 2011 PIVOTAL PHASE I/II Late Onset Early Onset 17 © Copyright 2011 Synageva BioPharma™ SBC-102 Clinical Development COMMERCIALIZATION |
|
18 Adult patients with liver dysfunction due to LAL Deficiency Open label dose escalation 4 week dosing 3 dose levels Multicenter N = 9 Patient dosing underway Followed by extension protocol Phase I/II Open-Label Phase I/II Open-Label Primary endpoint: Safety Secondary endpoint: Pharmacokinetics Trial Measurements: Pharmacodynamic markers Liver Function Tests Endpoints: Safety, Efficacy and Pharmacokinetics Patients with liver dysfunction due to LAL Deficiency Randomized, placebo-controlled study 6 months dosing 3 arm study Multicenter N = ~24 Randomized, Double-Blind, Randomized, Double-Blind, Placebo-controlled Trial Placebo-controlled Trial Late Onset Natural History Study On-going © Copyright 2011 Synageva BioPharma™ SBC-102 Clinical Development Late Onset (CESD) |
|
19 Growth failure in children due to LAL Deficiency Open label dose escalation 4 month dosing Multicenter N= 8 Active Phase I/II Open-Label Phase I/II Open-Label Secondary endpoints: Efficacy (including growth, weight gain) Pharmacokinetics Pharmacodynamics Primary endpoint: Long term efficacy and safety (including survival) Secondary endpoint: Efficacy (including growth, weight gain, organ function) Pharmacokinetics Patients from Phase I/II study Continuation with dose regimen from Phase I/II Dosing for > 6 months Multicenter N= ~8 Open-Label Long-term Extension Open-Label Long-term Extension Early Onset Natural History Study On-going © Copyright 2011 Synageva BioPharma™ SBC-102 Clinical Development Early Onset (Wolman) Primary endpoint: Safety and tolerability |
|
IND filed on schedule and cleared within 30 days EU CTAs filed 3 months ahead of schedule US & EU orphan drug designations granted 2010 Fast Track designation granted by the FDA Clinical production for studies on schedule, internal manufacturing Initiated multiple US and EU clinical trial sites Patient dosing commenced 20 © Copyright 2011 Synageva BioPharma™ SBC-102 Clinical and Regulatory Milestones |
|
An infant with LAL Deficiency has received treatment with SBC-102 on a compassionate use basis – Presented with anemia, increasingly abnormal liver function tests, and growth failure prior to treatment – Child has received weekly infusions since April 2011 with no complications and is demonstrating substantial improvements in: • Growth rate • Liver function tests (reduction in serum transaminases) • Other disease-associated abnormalities consistent with Synageva’s preclinical data for SBC-102 Infant continues to receive treatment with SBC-102 © Copyright 2011 Synageva BioPharma™ 21 SBC-102 Compassionate Use: Patient Data |
|
22 © Copyright 2011 Synageva BioPharma™ ERT for MPS IIIB / Sanfilippo B SBC-103 (rhNAGLU) |
|
Heparan sulfate accumulation in CNS, liver, spleen, skin and joints May affect as many as 8 lives per million Progressive mental impairment with sleep disturbances and behavioral manifestations – Severe phenotype - wheelchair dependent and death within first two decades – Attenuated phenotype – early onset intellectual disability with delayed progression Strategy – Builds on ERT experience with SBC-102 – Slow CNS onset provides Rx window – Leverages progress in intrathecal delivery © Copyright 2011 Synageva BioPharma™ 23 From curekirby.org Age 9 Age 18 SBC-103 MPS IIIB / Sanfilippo B |
|
24 Historical attempts to produce NAGLU ERT for MPS IIIB have failed due to lack of cell uptake © Copyright 2011 Synageva BioPharma™ Yogalingham G et al, 2000 NAG (nmol/h per mg) Normal fibroblasts 25.12 ± 2.23 MPS-IIIB fibroblasts 0.101 ± 0.025 MPS-IIIB fibroblasts + MPS-IIIB/LNCNAG 0.388 ± 0.048 conditioned medium Markedly increased cellular enzyme levels Properties substantially better than cell culture produced material in previous publications SBC-103 NAGLU Production for ERT Demonstrated good uptake into enzyme-deficient human fibroblasts Synageva |
|
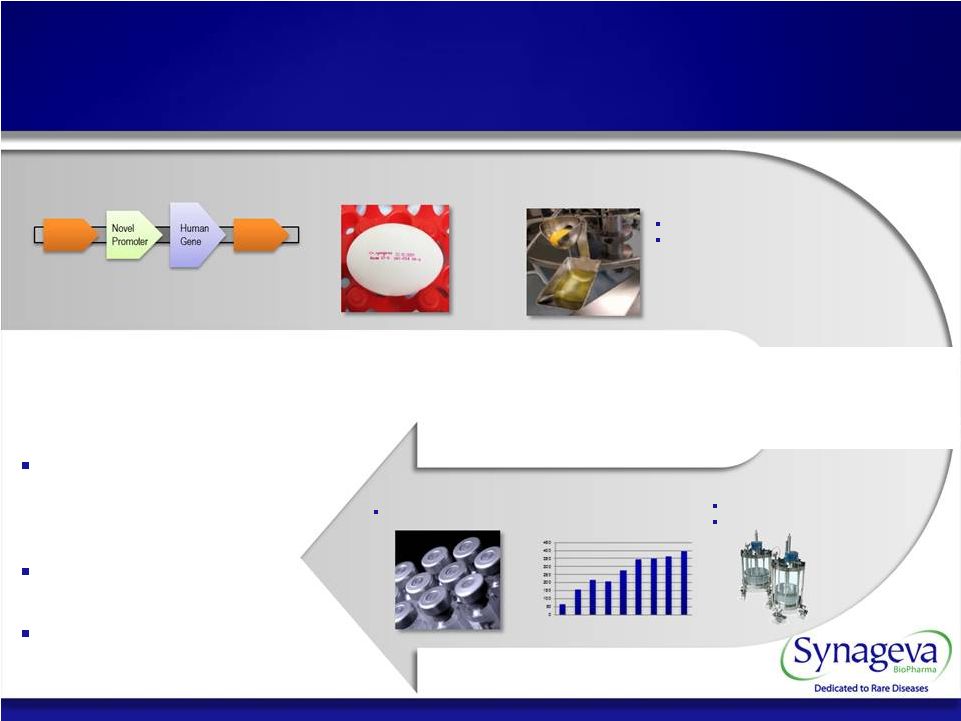
Proprietary Vector System Human Biopharmaceutical Expression in Egg White FDA-accepted Aseptic Protein Harvest GMP compliant facilities Automated commercial scale infrastructure Finished Product Consistent and Reliable Scale-up Purification Established industry standard IND approved process Consistent Scalable Efficient Clinically validated – Studies included over 250 patients, including 189 pt Ph II GCSF trial Multiple cleared INDs – 4 INDs Pipeline generator 25 © Copyright 2011 Synageva BioPharma™ Stable commercial supply Synageva Expression Platform |
|
Drug SBC-102 Cerezyme Fabrazyme Myozyme Soliris SBC-103 Indication LAL Deficiency Type I Gaucher Disease Fabry Disease Pompe Disease PNH MPS IIIB/ Sanfilippo B Estimated Prevalence 1 in 59,000 to 86,000 2 1 in 40,000 to 476,000 2 1 in 40,000 to 146,000 2 1 in ~77,000 3 1 in 125,000 4 to 211,000 5 1. Muntoni, et al 2007. Prevalence of Cholesteryl Ester Storage Disease, Arteriosclerosis, Thrombosis, and Vascular Biology, 27, p.1866 2. Genzyme, AMCP Dossier for Cerezyme 3. Alexion, AMCP Formulary Dossier 4. Héron B, et al. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am J Med Genet A. 2011 Jan;155A(1):58-68. 5. Meikle, P.J., Hopwood, J.J., Clague, A.E. & Carey, W.F., 1999. Prevalence of lysosomal storage disorders. JAMA, 281(3), p.249. 26 © Copyright 2011 Synageva BioPharma™ SBC-102 Commercial: Ultra-Orphan Opportunity 1 in 40,000 1 |
|
~17,000 homozygotes with LAL mutation Enzyme DNA 1 1 Estimated distribution for US, EU and BR Estimated distribution for US, EU and BR © Copyright 2011 Synageva BioPharma™ 27 Simple blood test Enzyme Assay DNA Sequencing Lab Prototypes 1. Baylor College of Medicine 2. Willink Lab, St Mary’s Manchester, UK Simple blood test Enzyme Assay DNA Sequencing Lab Prototypes 1. Baylor College of Medicine 2. Willink Lab, St Mary’s Manchester, UK Right Right Right Test Geneticists Suspected Patients Hepatologists Liver Lipidologists Lipid SBC-102 Commercial: Finding Patients Practice Patient Phenotype Phenotype 1 |
|
30-60M Americans have NAFLD 6-10M Americans have NASH 6K Americans are predicted to have CESD 1 http://www.digestive.niddk.nih.gov/ddiseases/pubs/NASH/ 2 Muntoni, et al; “Prevalence of Cholesteryl Ester Storage Disease”, Arteriosclerosis, Thrombosis, and Vascular Biology, July 19,2010 28 © Copyright 2011 Synageva BioPharma™ 1 2 SBC-102 Commercial: Rare & Devastating Disease in a 1 Common Phenotype ..…CESD should more often be considered as a differential diagnosis in liver diseases unknown (nonalcoholic steatohepatitis or NASH) or known (alcoholic steatohepatitis) origin and in dyslipidemic patients with combined hyperlipidemia and low HDL-cholesterol (Familial Combined Hyperlipidemia). –Muntoni of |
|
Investing in the future Valued as a clinical stage biotech First-ever ERT for LAL in humans Proof of Concept Established Human Data on SBC-102 Pipeline products entering clinic Filing and Approval for SBC-102 Commercial-stage biotech company Potential Five product introductions expected Valued on revenue and margin development Fast Revenue Ramp Attractive Margin Profile Stage 2 Stage 1 Multiple Value Drivers 29 © Copyright 2011 Synageva BioPharma™ Synageva Value Drivers in Two Distinct Stages company |
|
Trimeris cash ending Q2 2011 was approx $52 million Synageva cash ending Q2 2011 was approx $18 million May 25th, 2011 Trimeris signed new agreement with Roche: Trimeris received $4.9 million upfront fee Trimeris to receive a 16% royalty on global sales of Fuzeon, an HIV drug marketed globally by Roche (55 countries) 30 © Copyright 2011 Synageva BioPharma™ Pro forma Synageva would have approximately $70 million in cash ending Q2 2011 Financials Cash balance will allow Synageva to reach multiple value creating events - - Summary |
|
Program SBC-102 (rhLAL) SBC-103 (rhNAGLU) SBC-104 SBC-105 SBC-106 Therapeutic Recombinant Lysosomal Acid Lipase Recombinant -N-accetyl- glucosaminidase Extra Cellular Protein Enzyme Replacement Therapy Enzyme Replacement Therapy Disease LAL Deficiency (LSD) MPS IIIB/ Sanfilippo B (LSD) Severe Genetic Condition Severe Metabolic Disorder Severe Genetic Condition Development Status Clinical Preclinical Preclinical Preclinical Preclinical Regulatory Opportunity Orphan Designation • Granted US • Granted EU Fast Track Designation Potential for Orphan & Fast Track Designation Potential for Orphan & Fast Track Designation Potential for Orphan & Fast Track Designation Potential for Orphan & Fast Track Designation MULTIPLE VALUE DRIVING EVENTS 31 © Copyright 2011 Synageva BioPharma™ Synageva Pipeline of Opportunity |
|
© Copyright 2011 Synageva BioPharma™ |
About Synageva BioPharma Corp.
Synageva is a clinical stage biopharmaceutical company focused on the discovery, development, and commercialization of therapeutic products for patients with life-threatening rare diseases and unmet medical need. SBC-102 has been granted orphan designations by the U.S. Food and Drug Administration (“FDA”) and the European Medicines Agency (“EMA”), andfast track designation by the FDA. Synageva has several protein therapeutics in its pipeline, including two enzyme replacement therapies for lysosomal storage disorders and two programs for life-threatening genetic conditions for which there are currently no approved treatments. The Company has assembled a team with a proven record of bringing orphan therapies to patients.
On June 13, 2011, Synageva announced that they had entered into a definitive agreement under which Synageva will merge with Trimeris, Inc. (NASDAQ: TRMS) in an all-stock transaction. Upon closing, the combined company will be named Synageva BioPharma Corp., and will create a publicly-traded company focused on the development of novel therapeutics for patients with rare diseases and unmet medical need.
Further information regarding Synageva BioPharma Corp. is available at http://www.synageva.com.
About Trimeris, Inc.
Trimeris, Inc. (NASDAQ: TRMS) pioneered the development of a class of antiviral drug treatments called fusion inhibitors. Trimeris’ currently marketed product is FUZEON, an anti-HIV fusion inhibitor which was developed by Trimeris in collaboration with Roche. Substantially all of Trimeris’ revenues are derived from its collaboration with Roche relating to FUZEON. For more information about Trimeris, please visit the company’s website at http://www.trimeris.com.
Important Merger Information and Additional Information and Where to Find It
This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities of Trimeris or Synageva or the solicitation of any vote or approval. In connection with the proposed merger, Trimeris filed a Registration Statement on Form S-4, filed with the SEC on July 13, 2011 and amended on August 23, 2011 (the “Registration Statement”), which includes a preliminary joint proxy statement of Trimeris and Synageva and constitutes a preliminary prospectus of Trimeris. These materials are not yet final and will be further amended. The joint proxy statement/prospectus of Trimeris and Synageva will be mailed to the stockholders of Trimeris and Synageva once it is final.Investors are strongly urged to read the definitive joint proxy statement/prospectus when it becomes available and other documents filed with the SEC by Trimeris, because they will contain important information about Trimeris, Synageva and the proposed merger.
Investors and security holders of Trimeris and Synageva may obtain free copies of the joint proxy statement/prospectus for the proposed merger and other documents filed with the SEC by Trimeris through the website maintained by the SEC at www.sec.gov. In addition, investors and security holders of Trimeris will be able to obtain free copies of the joint proxy statement/prospectus for the proposed merger by contacting Trimeris, Inc., Attn: James Thomas, Chief Financial Officer. Investors and security holders of Synageva will be able to obtain free copies of the joint proxy statement/prospectus for the merger by contacting Synageva BioPharma Corp., Attn: Secretary, 128 Spring Street, Suite 520, Lexington, MA 02421.
Trimeris and Synageva, and their respective directors and certain of their executive officers, may be deemed to be participants in the solicitation of proxies in respect of the transactions contemplated by the agreement between Trimeris and Synageva. Information regarding Trimeris’ directors and executive officers is contained in Trimeris’ Annual Report on Form 10-K for the fiscal year ended December 31, 2010, which was filed with the SEC on March 14, 2011, and in its proxy statement prepared in connection with its 2010 Annual Meeting of Stockholders, which was filed with the SEC on March 16, 2010. Information regarding Synageva’s directors and officers and a more complete description of the interests of Trimeris’ and Synageva’s respective directors and officers in the proposed transaction is available in the Registration Statement.