Exhibit (a)(1)(N)

Merck Announces Acquisition of Cubist
December 23, 2014
(Updated)
MERCK
Be well

Forward-Looking Statement
This communication includes “forward-looking statements” within the meaning of the safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995. Forward looking statements include statements regarding the timing and closing of the tender offer and the merger transactions, the ability of Merck to complete the transactions considering the various closing conditions, and any assumptions underlying any of the foregoing. These statements are based upon the current beliefs and expectations of Merck’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline products that the products will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Merck’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of Merck’s patents and other protections for innovative products; the exposure to litigation, including patent litigation, and/or regulatory actions; timing of the tender offer and merger; uncertainties as to how many Cubist stockholders will tender shares in the tender offer; the possibility that competing offer may be made; the possibility that various closing conditions to transactions may not be satisfied or waived, including that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of the transactions; or that a material adverse effect occurs with respect to Cubist.
Merck undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in Merck’s 2013 Annual Report on Form 10-K and the company’s other filings with the SEC available at the SEC’s Internet site (www.sec.gov).
MERCK
Be well
2

Important Information about the Tender Offer
This presentation is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares. On December 19, 2014, Merck filed a tender offer statement on Schedule TO with the SEC, and Cubist filed a solicitation/recommendation statement on Schedule 14D-9 with respect to the tender offer. The tender offer materials (including an offer to purchase, a related letter of transmittal and other tender offer documents) and the solicitation/ recommendation statement, as each may be amended or supplemented from time to time, contain important information that holders of Cubist common stock shares are urged to read carefully before making any decision regarding tendering their shares. All of these materials are available at no charge on the SEC’s website at www.sec.gov. In addition, copies of the tender offer materials may be obtained at no charge by contacting Merck at 2000 Galloping Hill Road, Kenilworth, N.J., 07033 or by phoning (908) 740-4000. Moreover, Merck and Cubist file annual, quarterly and current reports and other information with the SEC. You may read and copy any reports or other information filed by Merck or Cubist at the SEC public reference room at 100 F Street, N.E., Washington, D.C., 20549. For further information on the SEC public reference room, please call 1-800-SEC-0330. Merck’s and Cubist’s filings with the SEC are also available to the public from commercial document-retrieval services and at the SEC’s website at www.sec.gov.
MERCK
Be well
3

Merck Announces the Acquisition of Cubist
Merck launched its global strategic initiative to sharpen Commercial and R&D focus in 2013
Initiative highlighted Hospital Acute Care as a core area of focus
Represents a unique leadership and growth opportunity for Merck
Segment has favorable market dynamics, significant unmet medical need, and opportunities to improve patient care
Acquisition of Cubist aligns with Merck’s strategic initiative
Strengthens Merck’s Hospital Acute Care portfolio, commercial position, and Acute Care operating model and capabilities
MERCK
Be well
4
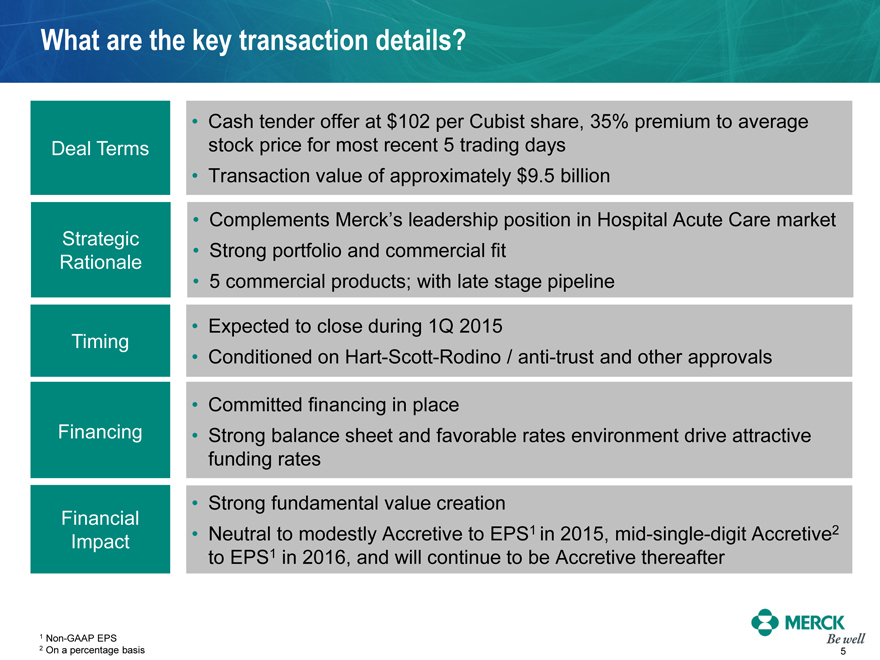
What are the key transaction details?
Deal Terms
Strategic Rationale
Timing
Financing
Financial Impact
Cash tender offer at $102 per Cubist share, 35% premium to average stock price for most recent 5 trading days
Transaction value of approximately $9.5 billion
Complements Merck’s leadership position in Hospital Acute Care market
Strong portfolio and commercial fit
5 commercial products; with late stage pipeline
Expected to close during 1Q 2015
Conditioned on Hart-Scott-Rodino / anti-trust and other approvals
Committed financing in place
Strong balance sheet and favorable rates environment drive attractive funding rates
Strong fundamental value creation
Neutral to modestly Accretive to EPS1 in 2015, mid-single-digit Accretive2 to EPS1 in 2016, and will continue to be Accretive thereafter
1 Non-GAAP EPS
2 On a percentage basis
MERCK
Be well
5

Merck Identified Hospital Acute Care as a Core Area of Focus
DIABETES
Total deaths from diabetes are projected to increase by 50% in the next 10 years
HOSPITAL ACUTE CARE
Antibiotic-resistant bacteria infects over 2 million Americans annually, resulting in 23,000 deaths
VACCINES
Cervical cancer is the second most common cancer in women worldwide
ONCOLOGY
Every year, 8 million people die from cancer worldwide
MERCK
Be well
6
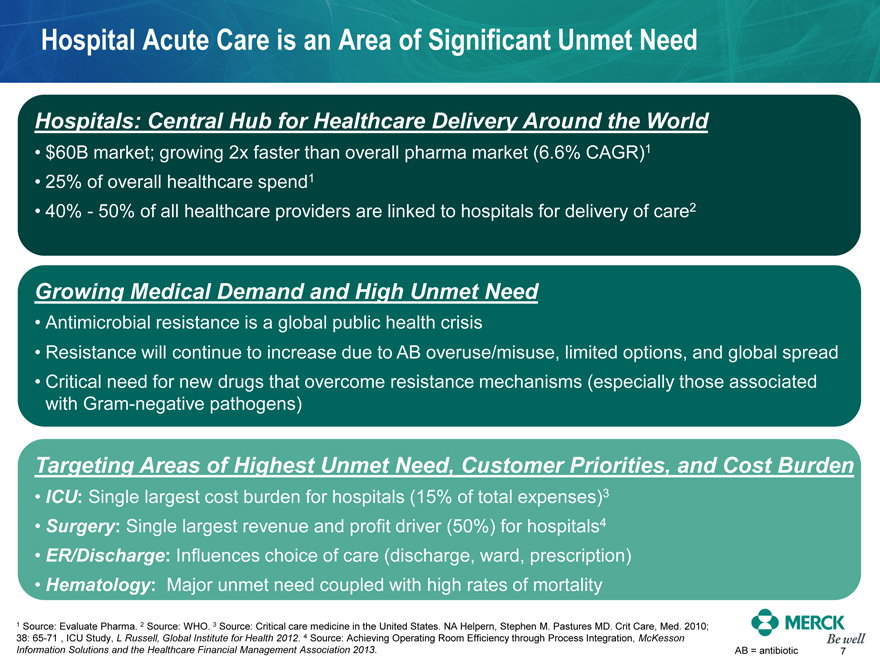
Hospital Acute Care is an Area of Significant Unmet Need
Hospitals: Central Hub for Healthcare Delivery Around the World
$60B market; growing 2x faster than overall pharma market (6.6% CAGR)1
25% of overall healthcare spend1
40% - 50% of all healthcare providers are linked to hospitals for delivery of care2
Growing Medical Demand and High Unmet Need
Antimicrobial resistance is a global public health crisis
Resistance will continue to increase due to AB overuse/misuse, limited options, and global spread
Critical need for new drugs that overcome resistance mechanisms (especially those associated with Gram-negative pathogens)
Targeting Areas of Highest Unmet Need, Customer Priorities, and Cost Burden
ICU: Single largest cost burden for hospitals (15% of total expenses)3
Surgery: Single largest revenue and profit driver (50%) for hospitals4
ER/Discharge: Influences choice of care (discharge, ward, prescription)
Hematology: Major unmet need coupled with high rates of mortality
1 Source: Evaluate Pharma. 2 Source: WHO. 3 Source: Critical care medicine in the United States. NA Helpern, Stephen M. Pastures MD. Crit Care, Med. 2010; 38: 65-71 , ICU Study, L Russell, Global Institute for Health 2012. 4 Source: Achieving Operating Room Efficiency through Process Integration, McKesson
Information Solutions and the Healthcare Financial Management Association 2013. AB = antibiotic
MERCK
Be well
7
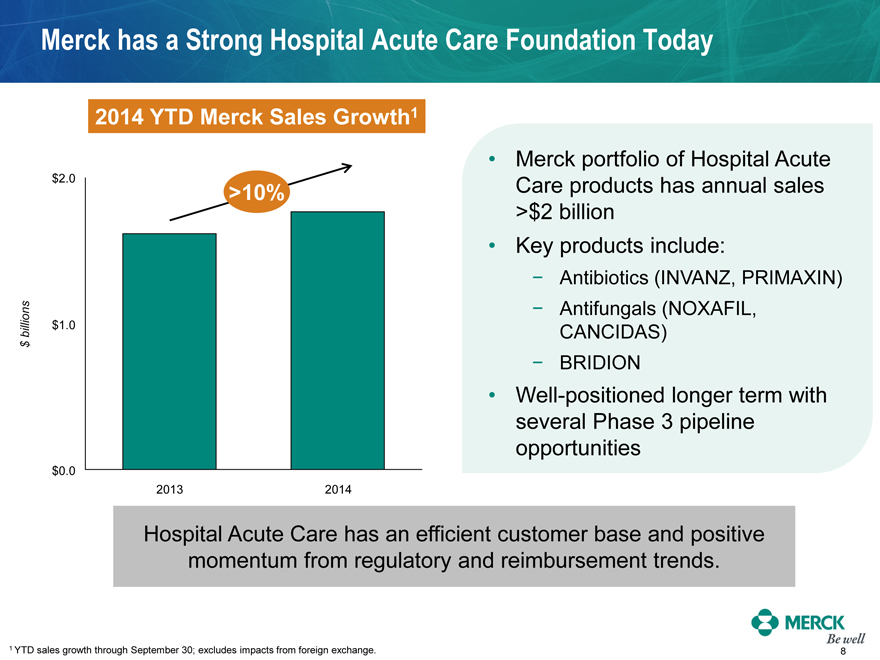
Merck has a Strong Hospital Acute Care Foundation Today
2014 YTD Merck Sales Growth1
$2.0
>10%
$ billions $1.0
$0.0
2013 2014
Merck portfolio of Hospital Acute Care products has annual sales
>$2 billion
Key products include:
- Antibiotics (INVANZ, PRIMAXIN)
- Antifungals (NOXAFIL, CANCIDAS)
- BRIDION
Well-positioned longer term with several Phase 3 pipeline opportunities
Hospital Acute Care has an efficient customer base and positive momentum from regulatory and reimbursement trends.
1 YTD sales growth through September 30; excludes impacts from foreign exchange.
MERCK
Be well
8

Acquisition Aligns with Merck’s Strategic Focus
Merck’s Strategic Focus
CUBIST
TAs where payers will pay for innovation Acute Care Market – Strong willingness to pay for innovation that improves outcomes and lowers costsü
Platform for growth Antibiotic-resistant bacteria infects over 2 million Americans annually; increasing proportions of resistant strainsü
Priority markets with greatest opportunity Focus on medicines that impact our top 10 markets, which account for ~70% of our revenue1ü
Global customers Focus on medicines that address the needs of our major customers, where our top 50 customers account for ~50% of our revenue1ü
1 Global Human Health MERCK
Be well
9

Merck Sees Significant Value in the Acquisition of Cubist
Market Leader in Treatment of Gram-positive and Gram-negative Infections
Biopharmaceutical company focused on the research, commercialization, and production of compounds for acute care treatment
5 commercial products
Gram-positive Gram-negative Complementary portfolio
in acute care
Once-A-Day
CUBICIN®
(daptomycin for injection) ZERBAXA™* ceftolozane/tazobactam for injection ENTEREG® (alvimopan)
ONCE-DAILY IV/ORAL
SIVEXTRO™
(tedizolid phosphate)200mg Attractive near-term commercial opportunity DIFICID® (fidaxomicin) tablets 200mg
Late stage pipeline with breadth in anti-infectives
ZERBAXA (ceftolozane/tazobactam) for cUTI / cIAI
Approved in the US
Company also submitted an MAA in August 2014, with a decision from EMA expected in 2H 2015
* Approved in US; under regulatory review in EU
MERCK
Be well
10
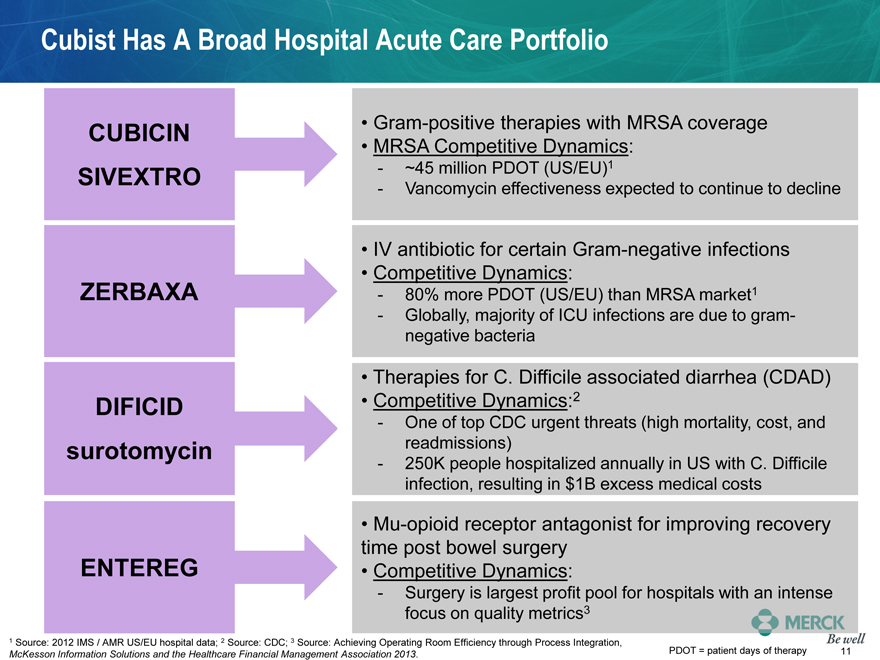
Cubist Has A Broad Hospital Acute Care Portfolio
CUBICIN Gram-positive therapies with MRSA coverage
MRSA Competitive Dynamics:
SIVEXTRO - ~45 million PDOT (US/EU)1
- Vancomycin effectiveness expected to continue to decline
IV antibiotic for certain Gram-negative infections
Competitive Dynamics:
ZERBAXA - 80% more PDOT (US/EU) than MRSA market1
- Globally, majority of ICU infections are due to gram-
negative bacteria
Therapies for C. Difficile associated diarrhea (CDAD)
DIFICID Competitive Dynamics:2
- One of top CDC urgent threats (high mortality, cost, and
surotomycin readmissions)
- 250K people hospitalized annually in US with C. Difficile
infection, resulting in $1B excess medical costs
Mu-opioid receptor antagonist for improving recovery
time post bowel surgery
ENTEREG Competitive Dynamics:
- Surgery is largest profit pool for hospitals with an intense
focus on quality metrics3
1 Source: 2012 IMS / AMR US/EU hospital data; 2 Source: CDC; 3 Source: Achieving Operating Room Efficiency through Process Integration,
McKesson Information Solutions and the Healthcare Financial Management Association 2013. PDOT = patient days of therapy
MERCK
Be well
11
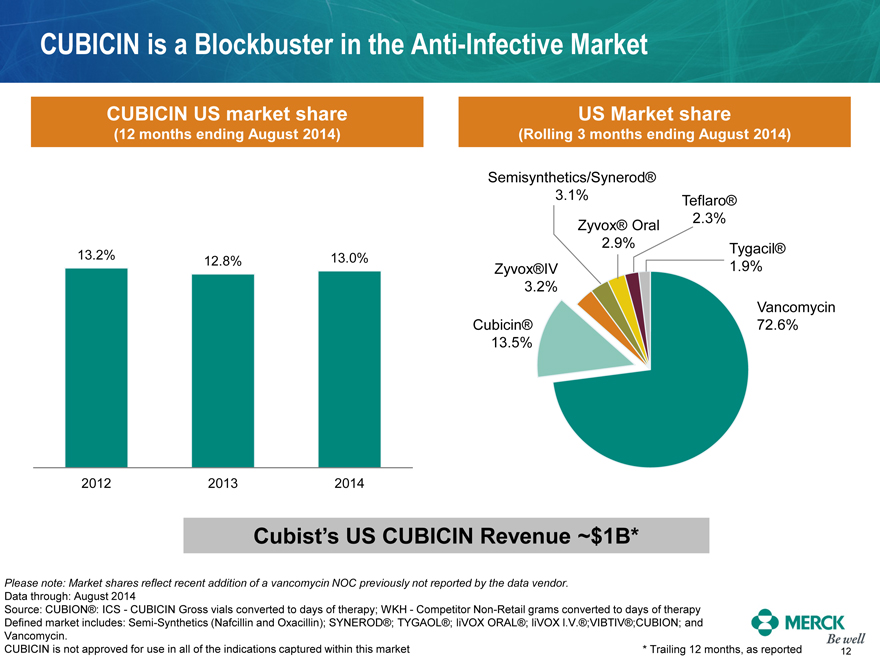
CUBICIN is a Blockbuster in the Anti-Infective Market
CUBICIN US market share
(12 months ending August 2014)
13.2% 12.8% 13.0%
2012 2013 2014
US Market share
(Rolling 3 months ending August 2014)
Semisynthetics/Synerod®
3.1%
Teflaro®
Zyvox® Oral
2.3%
2.9%
Tygacil®
Zyvox®IV
1.9%
3.2%
Vancomycin
Cubicin®
72.6%
13.5%
Cubist’s US CUBICIN Revenue ~$1B*
Please note: Market shares reflect recent addition of a vancomycin NOC previously not reported by the data vendor.
Data through: August 2014
Source: CUBION®: ICS - CUBICIN Gross vials converted to days of therapy; WKH - Competitor Non-Retail grams converted to days of therapy
Defined market includes: Semi-Synthetics (Nafcillin and Oxacillin); SYNEROD®; TYGAOL®; liVOX ORAL®; liVOX l.V.®;VIBTIV®;CUBION; and
Vancomycin.
CUBICIN is not approved for use in all of the indications captured within this market * Trailing 12 months, as reported
MERCK
Be well
12
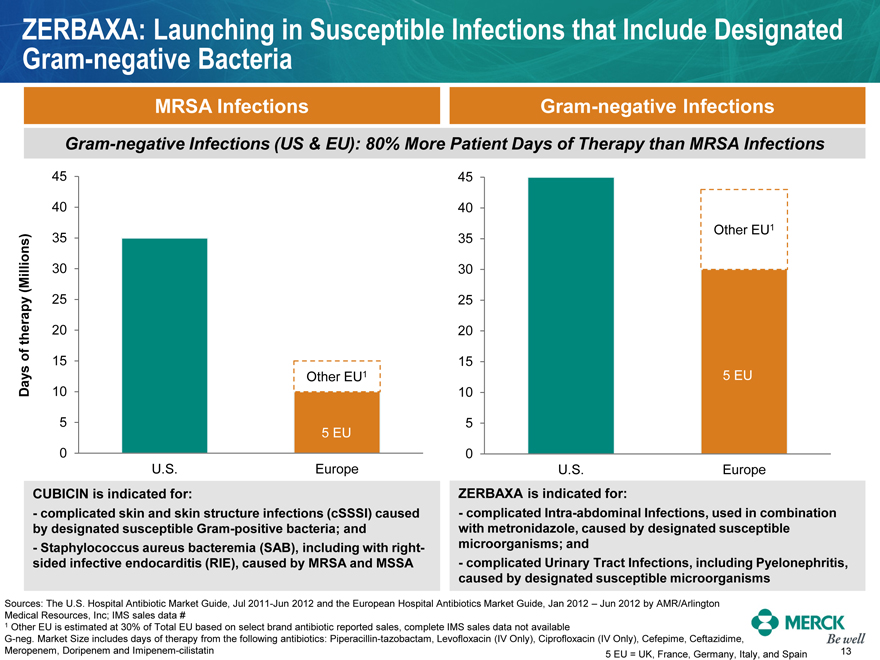
ZERBAXA: Launching in Susceptible Infections that Include Designated Gram-negative Bacteria
MRSA Infections Gram-negative Infections Gram-negative Infections (US & EU): 80% More Patient Days of Therapy than MRSA Infections Days of therapy (Millions)
45 40 35 30 25 20 15 10 5 0 U.S. Other EU1 5 EU Europe 45 40 35 30 25 20 15 5 EU 10 5 0 U.S. Other EU1 Europe
CUBICIN is indicated for: - complicated skin and skin structure infections (cSSSI) caused by designated susceptible Gram-positive bacteria; and - Staphylococcus aureus bacteremia (SAB), including with right- sided infective endocarditis (RIE), caused by MRSA and MSSA ZERBAXA is indicated for: - complicated Intra-abdominal Infections, used in combination with metronidazole, caused by designated susceptible microorganisms; and - complicated Urinary Tract Infections, including Pyelonephritis, caused by designated susceptible microorganisms Sources: The U.S. Hospital Antibiotic Market Guide, Jul 2011-Jun 2012 and the European Hospital Antibiotics Market Guide, Jan 2012 – Jun 2012 by AMR/Arlington Medical Resources, Inc; IMS sales data # 1 Other EU is estimated at 30% of Total EU based on select brand antibiotic reported sales, complete IMS sales data not available G-neg. Market Size includes days of therapy from the following antibiotics: Piperacillin-tazobactam, Levofloxacin (IV Only), Ciprofloxacin (IV Only), Cefepime, Ceftazidime,
Meropenem, Doripenem and Imipenem-cilistatin 5 EU = UK, France, Germany, Italy, and Spain MERCK Be well 13
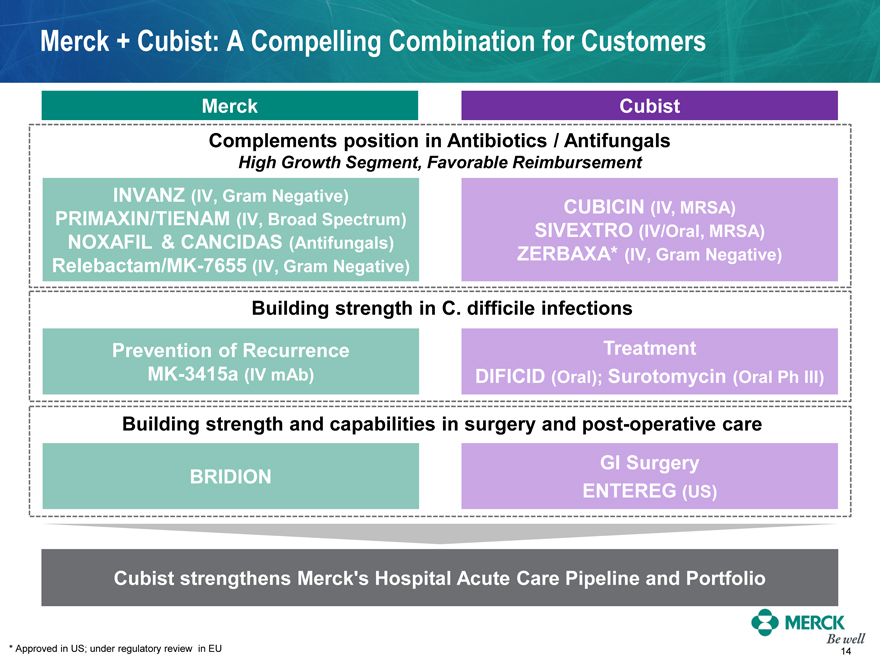
Merck + Cubist: A Compelling Combination for Customers
Merck
Cubist
Complements position in Antibiotics / Antifungals
High Growth Segment, Favorable Reimbursement
INVANZ (IV, Gram Negative)
PRIMAXIN/TIENAM (IV, Broad Spectrum)
CUBICIN (IV, MRSA)
SIVEXTRO (IV/Oral, MRSA)
NOXAFIL & CANCIDAS (Antifungals)
ZERBAXA* (IV, Gram Negative)
Relebactam/MK-7655 (IV, Gram Negative)
Building strength in C. difficile infections
Prevention of Recurrence
Treatment
MK-3415a (IV mAb)
DIFICID (Oral); Surotomycin (Oral Ph III)
Building strength and capabilities in surgery and post-operative care
BRIDION
GI Surgery
ENTEREG (US)
Cubist strengthens Merck’s Hospital Acute Care Pipeline and Portfolio
* Approved in US; under regulatory review in EU
MERCK
Be well
14
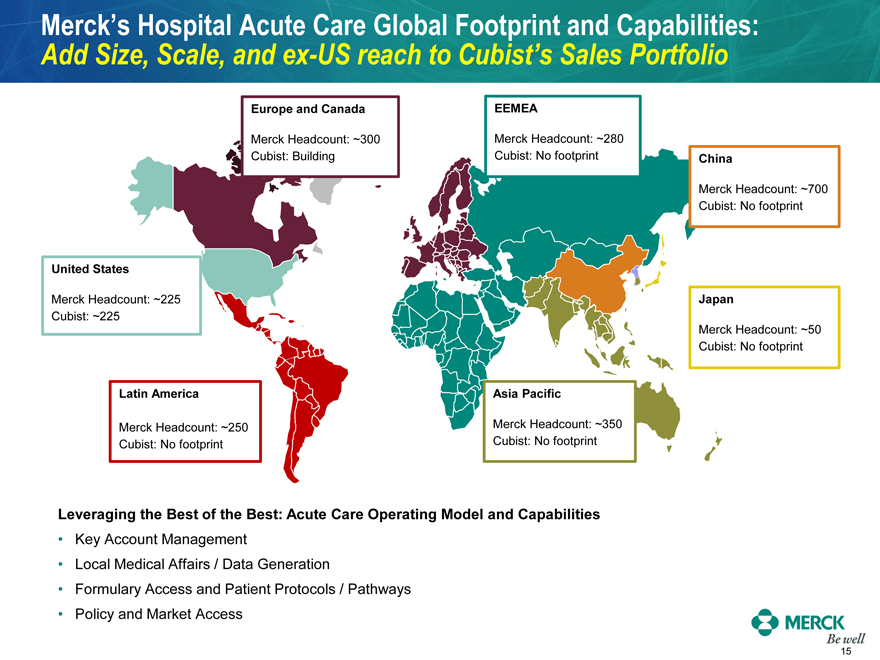
Merck’s Hospital Acute Care Global Footprint and Capabilities:
Add Size, Scale, and ex-US reach to Cubist’s Sales Portfolio
Europe and Canada
EEMEA Merck Headcount: ~300 Merck Headcount: ~280 Cubist: Building Cubist: No footprint China
Merck Headcount: ~700 Cubist: No footprint United States
Merck Headcount: ~225 Cubist: ~225 Japan
Merck Headcount: ~50 Cubist: No footprint Latin America Asia Pacific Merck Headcount: ~250 Merck Headcount: ~350
Cubist: No footprint Cubist: No footprint Leveraging the Best of the Best: Acute Care Operating Model and Capabilities
Key Account Management Local Medical Affairs / Data Generation Formulary Access and Patient Protocols / Pathways
Policy and Market Access MERCK Be well 15
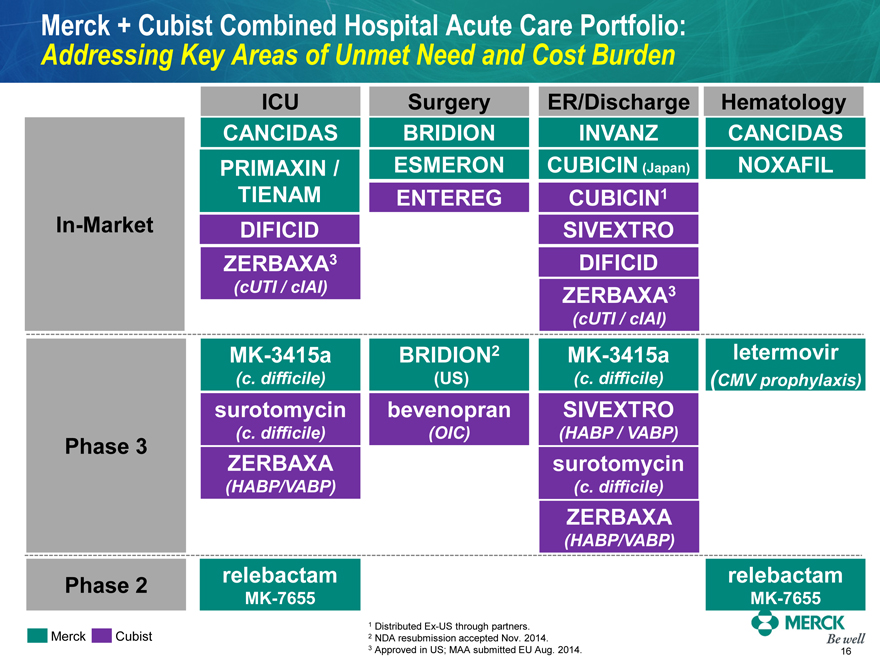
Merck + Cubist Combined Hospital Acute Care Portfolio:
Addressing Key Areas of Unmet Need and Cost Burden
| | | | | | | | |
In-Market (cUTI / cIAI) Phase 3 ZERBAXA (HABP/VABP) Phase 2 MK-7655 | | ICU CANCIDAS PRIMAXIN / TIENAM DIFICID ZERBAXA3 (cUTI / cIAI) MK-3415a (c. difficile) surotomycin (c. difficile) ZERBAXA (HABP/VABP) relebactam | | Surgery BRIDION ESMERON ENTEREG BRIDION2 (US) bevenopran (OIC) | | ER/Discharge INVANZ
CUBICIN (Japan) CUBICIN1
SIVEXTRO DIFICID
ZERBAXA3 MK-3415a (c.
difficile) SIVEXTRO (HABP /
VABP) surotomycin (c.
difficile) | | Hematology CANCIDAS
NOXAFIL letermovir (CMV
prophylaxis) relebactam MK-
7655 |
1 Distributed Ex-US through partners. Merck Cubist 2 NDA resubmission accepted Nov. 2014. 3 Approved in US; MAA submitted EU Aug. 2014. MERCK Be well 16
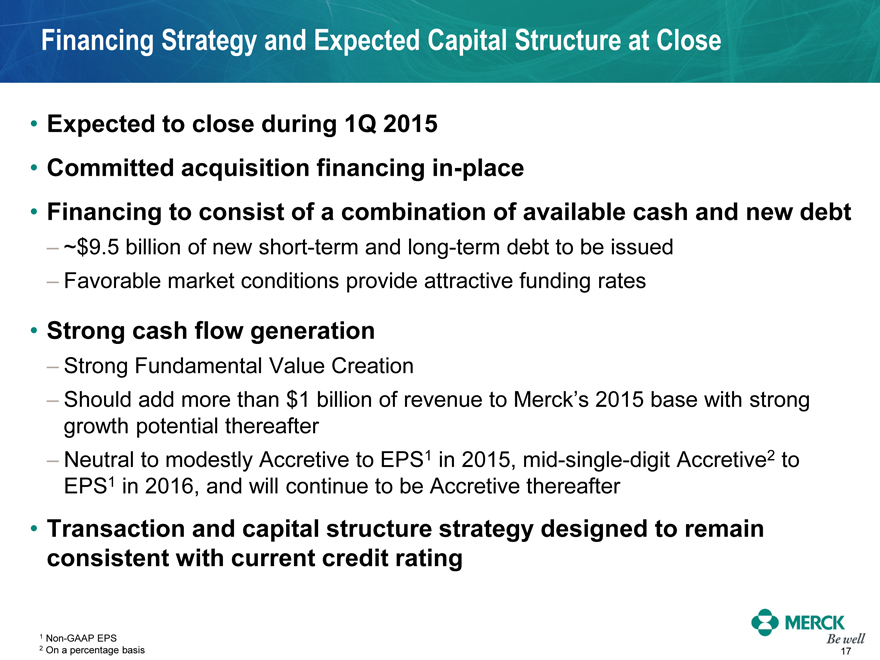
Financing Strategy and Expected Capital Structure at Close
Expected to close during 1Q 2015
Committed acquisition financing in-place
Financing to consist of a combination of available cash and new debt
- ~$9.5 billion of new short-term and long-term debt to be issued
- Favorable market conditions provide attractive funding rates
Strong cash flow generation
- Strong Fundamental Value Creation
- Should add more than $1 billion of revenue to Merck’s 2015 base with strong growth potential thereafter
- Neutral to modestly Accretive to EPS1 in 2015, mid-single-digit Accretive2 to EPS1 in 2016, and will continue to be Accretive thereafter
Transaction and capital structure strategy designed to remain consistent with current credit rating
1 Non-GAAP EPS
2 On a percentage basis
MERCK
Be well
17
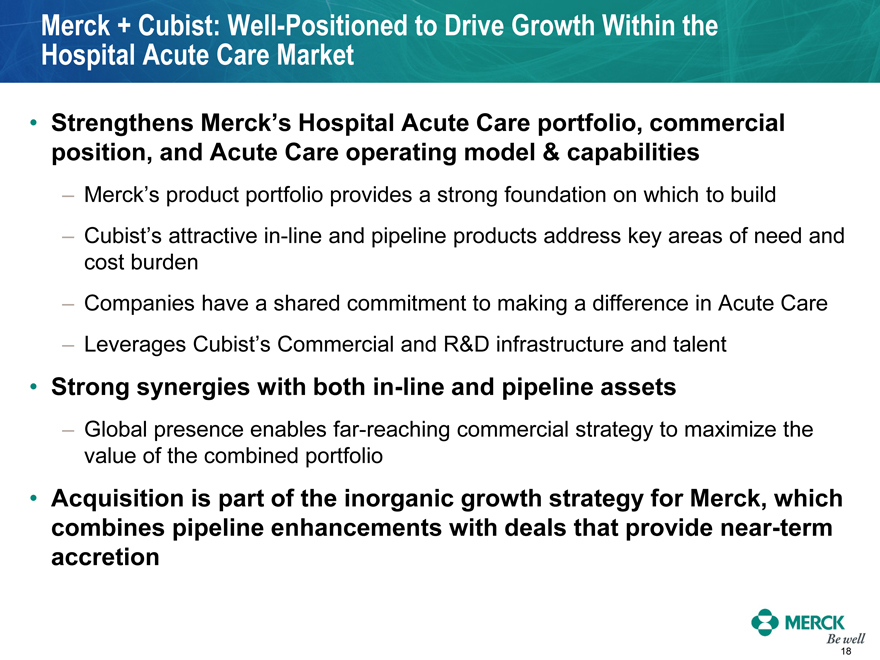
Merck + Cubist: Well-Positioned to Drive Growth Within the Hospital Acute Care Market
Strengthens Merck’s Hospital Acute Care portfolio, commercial position, and Acute Care operating model & capabilities
Merck’s product portfolio provides a strong foundation on which to build
Cubist’s attractive in-line and pipeline products address key areas of need and cost burden
Companies have a shared commitment to making a difference in Acute Care
Leverages Cubist’s Commercial and R&D infrastructure and talent
Strong synergies with both in-line and pipeline assets
Global presence enables far-reaching commercial strategy to maximize the value of the combined portfolio
Acquisition is part of the inorganic growth strategy for Merck, which combines pipeline enhancements with deals that provide near-term accretion
MERCK
Be well
18

















