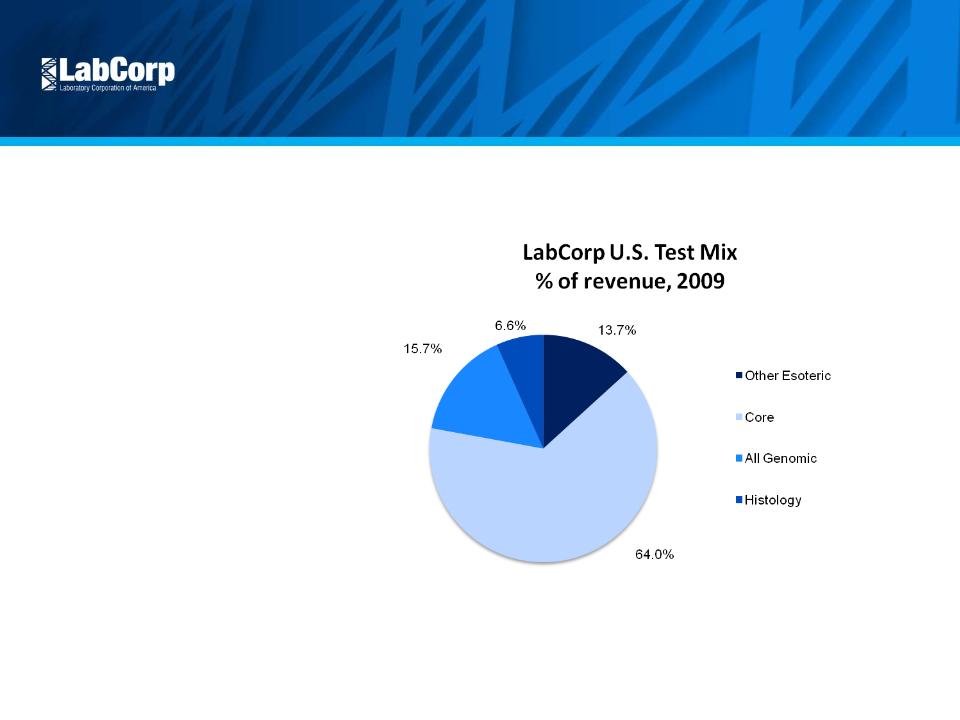
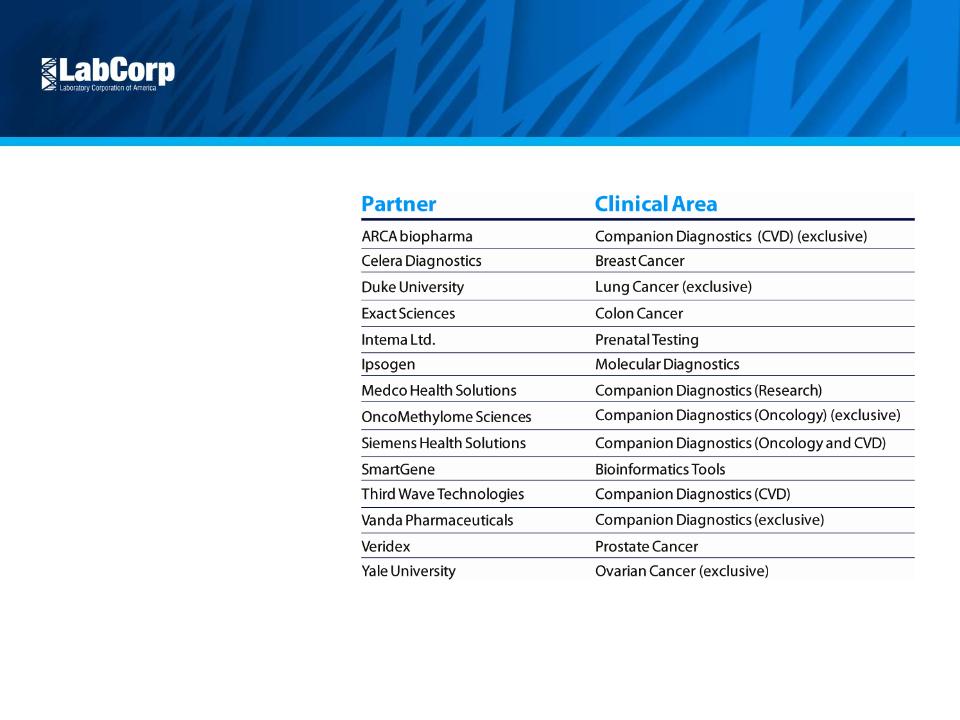
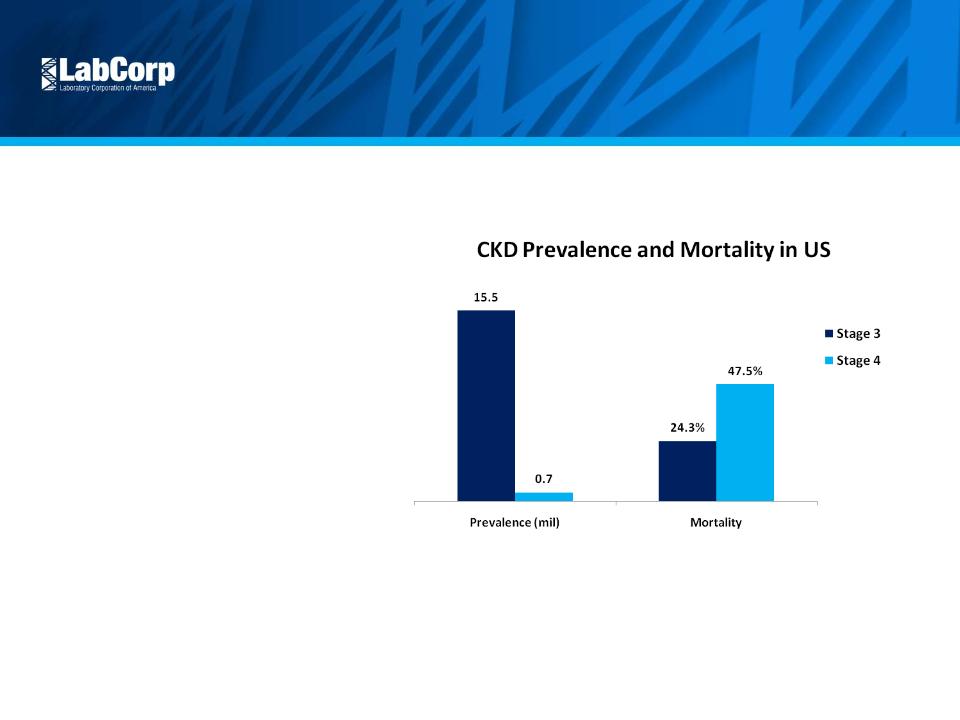
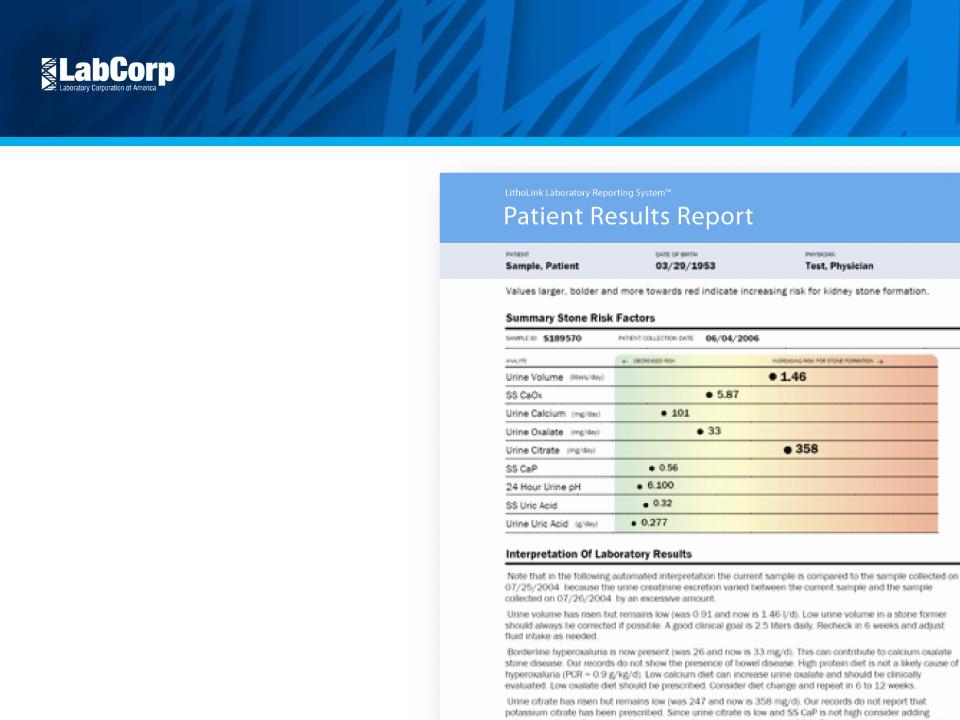
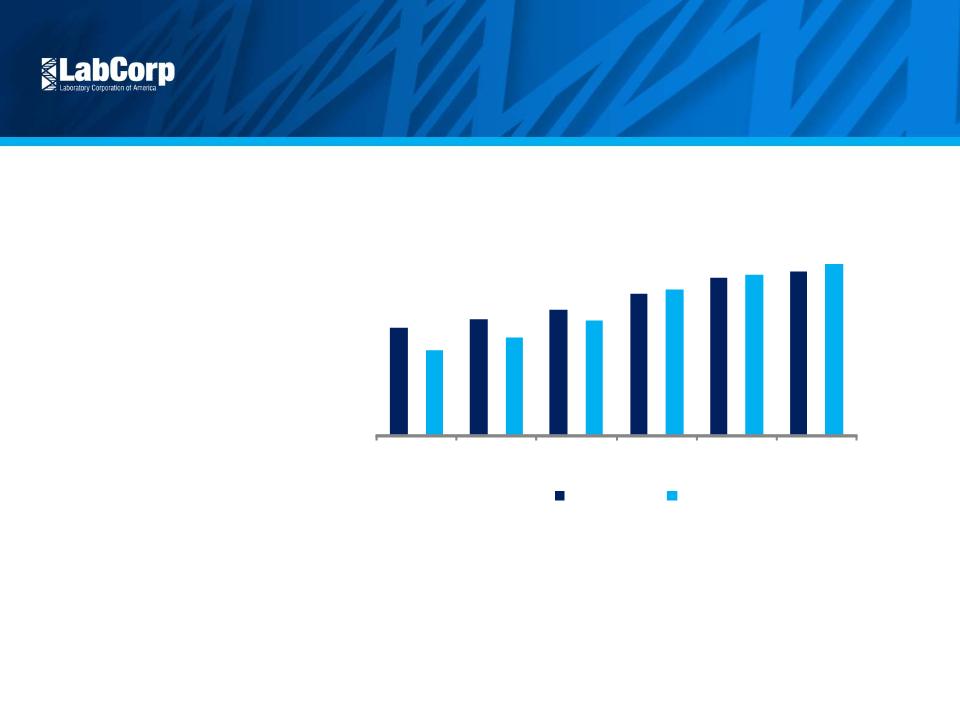
Continue Scientific Leadership
20
Develop and
Commercialize
Companion Diagnostics
• Invest in clinical trials
• Relationships with biotech and
pharma companies
• Promote key tests
• K-RAS
• HLA-B* 5701
• BRAF Gene Mutation Detection
• EGFR Mutation Analysis
• CYP 450 2C19
• Monogram Biosciences
• Trofile
• PhenoSense, PhenoSense GT
• HERmark
“K-RAS testing should be routinely conducted in
all colorectal cancer patients immediately after
diagnosis to ensure the best treatment strategies
for the individual Patient”
- Dr. Eric Van Cutsem, presenter at the June 2008 American
Society of Clinical Oncology meeting
FDA recommends genetic screening prior to
treatment with Abacavir
ROCKVILLE, Md -- July 24, 2008 -- The US Food and Drug Administration (FDA) has issued
an alert regarding serious, and sometimes fatal, hypersensitivity reactions (HSRs) caused by
abacavir (Ziagen) therapy in patients with a particular human leukocyte antigen (HLA) allele,
HLA-B* 5701.
Genetic tests for HLA-B*5701 are already available, and all patients should be screened for the
HLA-B*5701 allele before starting or restarting treatment with abacavir or abacavir-containing
medications.
“FDA has approved the expanded use of Selzentry…
to include adult patients with CCR5-tropic HIV-1
virus who are starting treatment for the first time.”
- ViiV Healthcare Press Release, November 20th, 2009