UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
October 21, 2010
(Date of earliest event reported)
LABORATORY CORPORATION OF
AMERICA HOLDINGS
(Exact Name of Registrant as Specified in its Charter)
| Delaware | | 1-11353 | | 13-3757370 |
| (State or other jurisdiction of Incorporation) | | (Commission File Number) | | (I.R.S. Employer Identification No.) |
| 358 South Main Street, | | | | |
| Burlington, North Carolina | | 27215 | | 336-229-1127 |
| (Address of principal executive offices) | | (Zip Code) | | (Registrant’s telephone number including area code) |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| [ ] | Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| [ ] | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| [ ] | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| [ ] | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 7.01 | Regulation FD Disclosure |
Summary information of the Company dated October 21, 2010.
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
LABORATORY CORPORATION OF AMERICA HOLDINGS
Registrant
| | By: | /s/ F. SAMUEL EBERTS III |
| | | F. Samuel Eberts III |
| | | Chief Legal Officer and Secretary |
October 21, 2010
8-K Filed October 21, 2010
2
Introduction
This slide presentation contains forward-looking statements which are
subject to change based on various important factors, including without
limitation, competitive actions in the marketplace and adverse actions of
governmental and other third-party payors.
Actual results could differ materially from those suggested by these
forward-looking statements. Further information on potential factors that
could affect the Company’s financial results will be included in the
Company’s Form 10-K for the year ended December 31, 2009, and
subsequent SEC filings. The Company has no obligation to provide any
updates to these forward-looking statements even if its expectations
change.
3
Third Quarter Results
(In millions, except per share data)
| Three Months Ended Sep 30, | | | |
| 2010 | | 2009 | | +/(-) | |
Revenue | $ 1,276.5 | | $ 1,185.1 | | 7.7% | |
Adjusted Operating Income (1) | $ 250.1 | | $ 237.6 | | 5.3% | |
Adjusted Operating Income Margin (1) | 19.6% | | 20.0% | | (40) | bp |
Adjusted EPS (1) | $ 1.47 | | $ 1.22 | | 20.5% | |
| | | | | | |
Operating Cash Flow | $ 176.2 | | $ 246.4 | | -28.5% | |
Less: Capital Expenditures | $ (34.3) | | $ (22.7) | | 51.1% | |
Free Cash Flow | $ 141.9 | | $ 223.7 | | -36.6% | |
| | | | | | |
(1) See Reconciliation of non-GAAP Financial Measures (included herein) | | | | | |
4
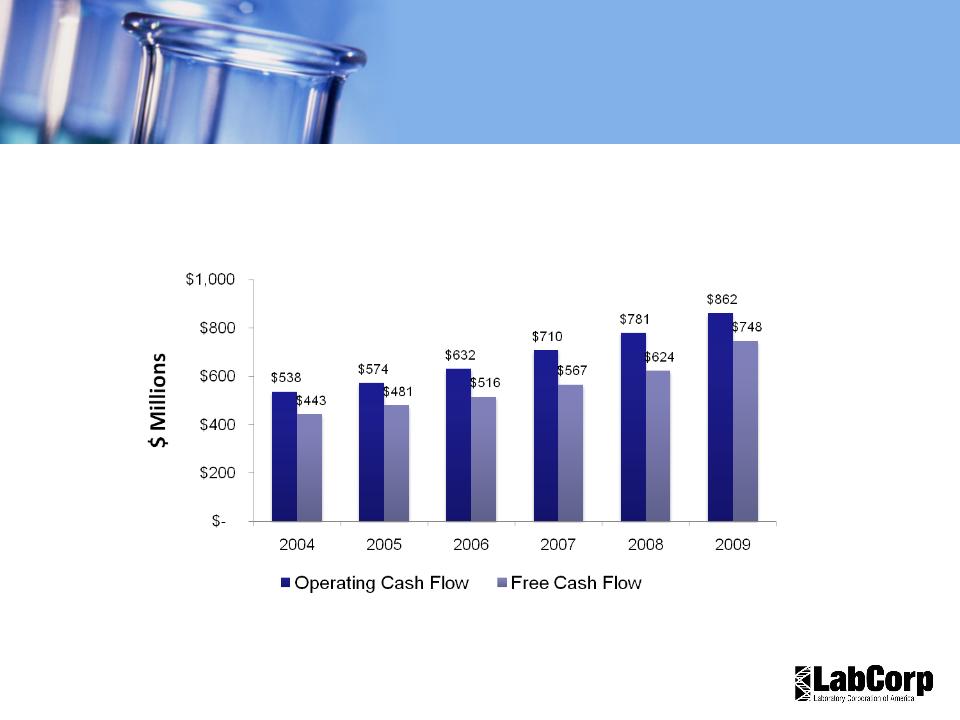
Cash Flow Trends
11% FCF CAGR
2004-2009
5
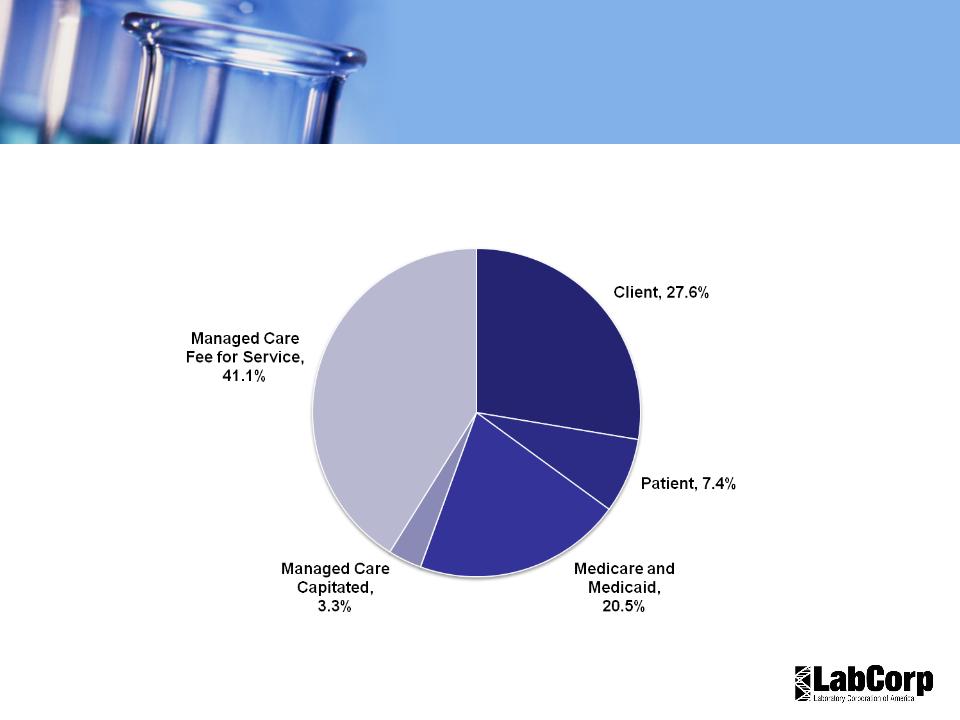
Revenue by Payer- US
2010 YTD
6
Revenue by Business Area - US
2010 YTD
7
Revenue by Payer
(in millions, except PPA)
| YTD Q3-2008 | | YTD Q3-2009 | | YTD Q3-2010 |
| Revenue | | | | Revenue | | | | Revenue | | |
| $'s | % | Accns | PPA | | $'s | % | Accns | PPA | | $'s | % | Accns | PPA |
Client | $ 895.7 | 28% | 26.844 | $ 33.37 | | $ 911.6 | 27% | 26.641 | $ 34.22 | | $ 967.3 | 28% | 26.030 | $ 37.16 |
Patient | 280.1 | 9% | 1.708 | $164.00 | | 256.8 | 8% | 1.596 | $160.91 | | $ 258.9 | 7% | 1.579 | $163.98 |
Third Party (Medicare/Medicaid) | 602.0 | 19% | 14.220 | $ 42.33 | | 677.9 | 20% | 14.960 | $ 45.31 | | $ 718.8 | 21% | 15.053 | $ 47.75 |
Managed Care: | | | | | | | | | | | | | | |
- Capitated | 135.5 | 4% | 11.409 | $ 11.88 | | 127.4 | 4% | 11.269 | $ 11.30 | | $ 116.0 | 3% | 10.300 | $ 11.26 |
- Fee for service | 1,282.4 | 40% | 28.352 | $ 45.23 | | 1,375.4 | 41% | 29.225 | $ 47.06 | | $1,439.9 | 41% | 29.809 | $ 48.30 |
Total Managed Care | 1,417.9 | 44% | 39.761 | $ 35.66 | | 1,502.8 | 45% | 40.494 | $ 37.11 | | $1,555.9 | 44% | 40.109 | $ 38.79 |
LabCorp Total - US | $3,195.6 | 100% | 82.533 | $ 38.72 | | $3,349.1 | 100% | 83.691 | $ 40.02 | | $3,500.9 | 100% | 82.771 | $ 42.30 |
| | | | | | | | | | | | | | |
LabCorp Total - Canada | $ 190.4 | | 5.957 | $ 31.97 | | $ 180.5 | | 6.855 | $ 26.33 | | $ 207.6 | | 6.873 | $ 30.21 |
| | | | | | | | | | | | | | |
LabCorp Total | $3,386.1 | | 88.490 | $ 38.26 | | $3,529.7 | | 90.546 | $ 38.98 | | $3,708.5 | | 89.644 | $ 41.37 |
8
Revenue by Business Area
(in millions, except PPA)
| YTD Q3-2008 | | YTD Q3-2009 | | YTD Q3-2010 |
| Revenue | | | | Revenue | | | | Revenue | | |
| $'s | % | Accns | PPA | | $'s | % | Accns | PPA | | $'s | % | Accns | PPA |
All Genomic | $ 486.7 | 15% | 6.539 | $ 74.43 | | $ 516.8 | 15% | 6.812 | $ 75.87 | | $ 547.1 | 16% | 6.702 | $ 81.64 |
Other Esoteric | 378.6 | 12% | 9.086 | 41.67 | | 452.8 | 14% | 10.607 | 42.69 | | 506.0 | 14% | 11.872 | 42.62 |
Histology | 241.9 | 8% | 1.921 | 125.94 | | 221.5 | 7% | 1.823 | 121.49 | | 219.1 | 6% | 1.764 | 124.20 |
All Genomic / Esoteric | 1,107.1 | 35% | 17.545 | 63.10 | | 1,191.1 | 36% | 19.242 | 61.90 | | 1,272.2 | 36% | 20.338 | 62.55 |
Core | 2,088.5 | 65% | 64.987 | 32.14 | | 2,158.0 | 64% | 64.449 | 33.48 | | 2,228.7 | 64% | 62.433 | 35.70 |
LabCorp Total - US | $ 3,195.6 | 100% | 82.533 | $ 38.72 | | $ 3,349.1 | 100% | 83.691 | $ 40.02 | | $ 3,500.9 | 100% | 82.771 | $ 42.30 |
| | | | | | | | | | | | | | |
LabCorp Total - Canada | $ 190.4 | | 5.957 | $ 31.97 | | $ 180.5 | | 6.855 | $ 26.33 | | $ 207.6 | | 6.873 | $ 30.21 |
| | | | | | | | | | | | | | |
LabCorp Total | $ 3,386.1 | | 88.490 | $ 38.26 | | $ 3,529.7 | | 90.546 | $ 38.98 | | $ 3,708.5 | | 89.644 | $ 41.37 |
9
Financial Guidance - 2010
Excluding the impact of restructuring and other special charges
and share repurchase activity after September 30, 2010,
guidance for 2010 is:
• Revenue growth(1): | Approximately 5.0% |
• Adjusted EPS(2): | $5.52 to $5.57 |
• Operating cash flow of approximately(3): | $870 Million |
• Capital expenditures of approximately: | $135 Million |
(1) Compared to previous guidance of 4.5% to 5.5%
(2) Compared to previous guidance of $5.40 to $5.55
(3) Operating cash flow guidance excludes any transition payments to UnitedHealthcare
(4) This guidance does not include any impact from the Genzyme Genetics acquisition
10
Supplemental Financial
Information
Laboratory Corporation of America |
Other Financial Information |
September 30, 2010 |
($ in millions) |
| | | | | | | |
| | Q1 10 | Q2 10 | Q3 10 | YTD 10 | | |
| | | | | | | |
Depreciation | | $ 32.2 | $ 32.0 | $ 32.2 | $ 96.3 | | |
Amortization | | $ 17.4 | $ 17.7 | $ 18.0 | $ 53.1 | | |
Capital expenditures | | $ 24.5 | $ 34.5 | $ 34.3 | $ 93.3 | | |
Cash flows from operations | | $ 232.0 | $ 216.2 | $ 176.2 | $ 624.4 | | |
Bad debt as a percentage of sales | | 5.05% | 4.80% | 4.80% | 4.88% | | |
Effective interest rate on debt: | | | | | | | |
Zero coupon-subordinated notes | | 2.00% | 2.00% | 2.00% | 2.00% | | |
5 1/2% Senior Notes | | 5.38% | 5.38% | 5.38% | 5.38% | | |
5 5/8% Senior Notes | | 5.75% | 5.75% | 5.75% | 5.75% | | |
Term loan | | 3.67% | 3.67% | 3.67% | 3.67% | | |
Revolving credit facility (weighted average) | | 0.58% | 0.70% | 0.61% | 0.61% | | |
Days sales outstanding | | 46 | 45 | 44 | 44 | | |
UnitedHeathcare transition payments - Billed | | $ 10.1 | $ 1.1 | $ - | $ 11.2 | | |
UnitedHeathcare transition payments - Paid | | $ 14.5 | $ 2.1 | $ 0.2 | $ 16.8 | | |
11
Reconciliation of non-GAAP
Financial Measures
Reconciliation of non-GAAP Financial Measures | | |
(In millions, except per share data) | | |
| | | | | | | |
| | | Three Months Ended Sep 30, |
Adjusted Operating Income | | 2010 | | 2009 | | |
| Operating income | | $ 235.3 | | $ 234.9 | | |
| Restructuring and other special charges (1) (2) | | $ 14.8 | | $ 2.7 | | |
| Adjusted operating income | | $ 250.1 | | $ 237.6 | | |
| | | | | | | |
Adjusted EPS | | | | | | |
| Diluted earnings per common share | | $ 1.34 | | $ 1.21 | | |
| Impact of restructuring and other special charges (1) (2) | | $ 0.13 | | $ 0.01 | | |
| Adjusted EPS | | $ 1.47 | | $ 1.22 | | |
| | | | | | | |
(1) During the third quarter of 2010, the Company recorded restructuring and other special charges of $21.8 million, consisting of $10.9 million in
professional fees and expenses associated with recent acquisitions; $7.0 million in bridge financing fees associated with the signing of an asset purchase
agreement for Genzyme Genetics; and $3.9 million in severance related liabilities associated with workforce reduction initiatives. The after tax impact of
these charges decreased net earnings for the three months ended September 30, 2010, by $13.4 million and diluted earnings per share by $0.13 ($13.4 million
divided by 104.1 million shares). | | |
(2) During the third quarter of 2009, the Company recorded a charge of approximately $2.7 million representing fees and expenses associated with its
acquisition of Monogram Biosciences. The after tax impact of this charge reduced net earnings for the three months ended September 30, 2009, by $1.6
million and diluted earnings per share by $0.01 ($1.6 million divided by 108.8 million shares). | | |